ABSTRACT
Some adipocytes are produced from bone marrow hematopoietic stem cells. In vitro studies previously indicated that these bone marrow-derived adipocytes (BMDAs) were generated from adipose tissue macrophage (ATM) that lose their hematopoietic markers and acquire mesenchymal markers prior to terminal adipogenic differentiation. Here we interrogated whether this hematopoietic-to-mesenchymal transition drives BMDA production In vitro. We generated transgenic mice in which the lysozyme gene promoter (LysM) indelibly labeled ATM with green fluorescent protein (GFP). We discovered that adipose stroma contained a population of LysM-positive myeloid cells that simultaneously expressed hematopoietic/myeloid markers (CD45 and CD11b), and mesenchymal markers (CD29, PDGFRa and Sca-1) typically found on conventional adipocyte progenitors. These cells were capable of adipogenic differentiation In vitro and In vitro, while other stromal populations deficient in PDGFRa and Sca-1 were non-adipogenic. BMDAs and conventional adipocytes expressed common fat cell markers but exhibited little or no expression of hematopoietic and mesenchymal progenitor cell markers. The data indicate that BMDAs are produced from ATM simultaneously expressing hematopoietic and mesenchymal markers rather than via a stepwise hematopoietic-to-mesenchymal transition. Because BMDA production is stimulated by high fat feeding, their production from hematopoietic progenitors may maintain adipocyte production when conventional adipocyte precursors are diminished.
KEYWORDS: Adipocyte, progenitor, haematopoietic stem cell, mesenchymal, myeloid
Introduction
Once believed to be a homogeneous and metabolically dormant tissue, adipose tissue is now recognized to play central roles in energy metabolism and inflammation, and exhibits substantial heterogeneity between different body locations, and even within the same depot. Most adipocytes are derived from mesenchymal stem cells via lineage-restricted progenitors that contribute to the adipocyte population of specific fat depots [1]. For example, adipocytes that reside in epididymal fat are derived from either Wilm’s Tumour 1 [2] or Pax-3-expressing [3] mesenchymal progenitors, while inguinal adipocytes arise from Prx-1-expressing precursors [2]. Likewise, brown adipocytes, noted for thermogenic lipid oxidation, are generated from Myf5-expressing skeletal muscle progenitors [4], while thermogenic beige adipocytes can arise from either smooth muscle cell progenitors [5] or via transdifferentiation from white adipocytes [6,7].
It was originally assumed that all adipocytes were solely derived from the mesenchymal lineage, but there is now substantial evidence that some adipocytes arise from a haematopoietic origin. Initial experiments demonstrated that green fluorescent protein (GFP)-expressing adipocytes were produced in the major adipose depots of wild type mice transplanted with bone marrow from donor mice ubiquitously expressing GFP [8–10]. Subsequent studies using either competitive bone marrow transplantation, or non-myeloablative lineage analysis models revealed that the bone marrow-derived adipocytes (BMDAs) were more abundant in gonadal rather than subcutaneous adipose tissue [8,9,11–13], and were generated from the haematopoietic lineage via myeloid intermediates [11–13]. Other laboratories have confirmed and extended these initial observations by demonstrating the production of both white [14–22] and beige/brown adipocytes [22] from bone marrow cells [5,14,18,19,22], HSCs [16,17,20,21] and myeloid cells [15] in mice. Moreover, our laboratory [11] and Ryden et al. [14,18,19] have also reported the production of BMDAs in human bone marrow transplant recipients.
We recently reported that adipose tissue macrophages (ATMs) cultured in 3-dimensional fibrin [9] or Matrigel [9,13] matrices undergo a ‘hematopoietic-to-mesenchymal’ conversion during which haematopoietic (CD45) and myeloid (CD11b) markers are lost and conventional mesenchymal adipocyte progenitor markers (e.g. CD29, Sca-1 and PDGFRalpha) were upregulated prior to adipogenic conversion. To determine whether this developmental transition occurs in vivo, we measured the expression of haematopoietic/myeloid and mesenchymal markers on adipose stromal cells indelibly labelled with a myeloid-specific gene promoter. We observed that a small percentage of adipose stromal cells arising from the myeloid lineage simultaneously expressed high levels of the haematopoietic/myeloid and mesenchymal progenitor markers and were capable of adipogenic conversion in vitro and in vivo. Subsequent experiments showed that mature BMDAs and conventional adipocytes expressed comparable levels of several common adipocyte markers, but minimal or no expression of either haematopoietic and mesenchymal progenitor markers, suggesting that those markers are lost simultaneously during adipogenic conversion. Thus, in contrast to our previous in vitro results, it appears that BMDA progenitors retain expression of both haematopoietic and mesenchymal progenitor markers until terminal adipogenic differentiation in vivo.
Results
BMDAs are generated from BM HSCs via the myeloid lineage
Competitive BM transplants were performed with either haematopoietic or mesenchymal progenitor cells constitutively expressing GFP, mixed with GFP naïve carrier cells. After 12 weeks, gonadal adipose tissue was isolated from the transplanted mice, digested with collagenase, and floating adipocytes were analysed by flow cytometry. GFP expressing (GFPPOS) BMDAs were detected in the adipocyte fraction from mice transplanted with GFP-labelled BM HSC but not labelled BM mesenchymal stem cells (Figure 1a). Backgating of the GFPPOS events in Figure 1a confirmed their large size (high forward scatter) and low internal complexity (low side scatter) common to unilocular adipocytes (Figure 1b). Images of GFPPOS cells from the adipocyte fraction of adipose tissue from mice transplanted with GFPPOS HSC confirmed their identity as unilocular adipocytes with a single nucleus and cytosolic GFP fluorescence (Figure 1c).
Figure 1.
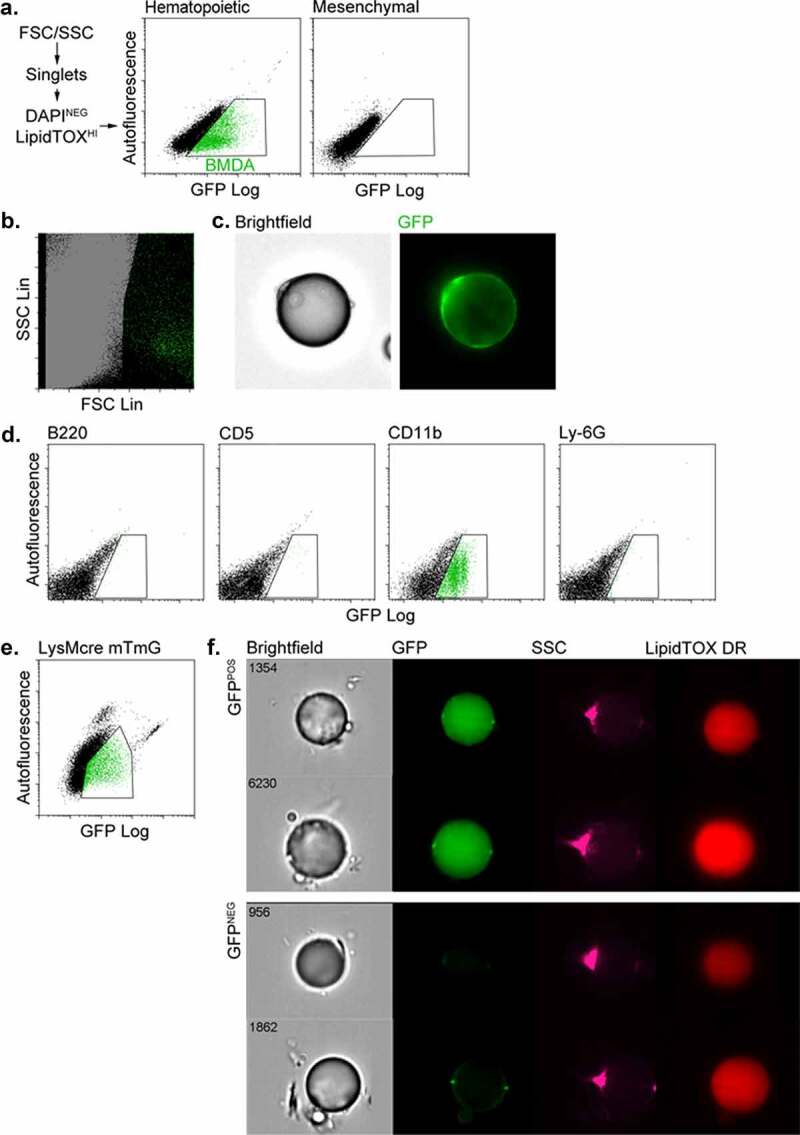
BMDAs are generated from HSCs via the myeloid lineage. (a) Competitive transplants were performed with either haematopoietic or mesenchymal progenitor cells constitutively expressing GFP, mixed with GFP-naïve carrier cells. After 8 weeks, free-floating adipocytes were prepared from adipose tissue from the transplanted mice and analysed by flow cytometry. GFPPOS BMDAs were detected in the adipocyte fraction of mice transplanted with GFP-labelled HSC (gated green events) but not labelled BM mesenchymal stem cells. (b) Backgating of the GFPPOS events in Figure 1a confirmed their large size (high forward scatter) and low internal complexity (low forward scatter) common to unilocular adipocytes. (c) Images of GFPPOS adipocytes from mice transplanted with GFPPOS HSC confirmed their identity as unilocular adipocytes with a single nucleus and cytosolic GFP fluorescence. (d) Haematopoietic sub-population cells were isolated from the BM of GFP-expressing mice with magnetic bead bearing antibodies to specific lineages (B220 = B lymphocyte, CD5 = T lymphocyte, CD11b = myeloid and Ly-6 G = granulocytes). The separated GFPPOS BM cells were combined with GFPNEG BM cells from wild type mice to ensure survival of recipients. After 8 weeks flow cytometry showed production of BMDAs only in mice transplanted with labelled (Cd11bPOS) myeloid cells. (e) Flow cytometry of free-floating adipocytes isolated from dual transgenic mice in which GFP expression (from an mTmG transgene) was controlled by the myeloid-specific lysozyme gene promoter (LysMcre). A population of GFPPOS adipocytes confirmed that some adipocytes were produced from the myeloid lineage. (f) Free-floating adipocytes were isolated from the LysMcre mTmG mice and subjected to imaging flow cytometry. Brightfield and fluorescence images confirmed the presence of GFPPOS unilocular (single LipidTOX stained lipid droplet) adipocytes in additional to GFPNEG adipocytes
Subsequent experiments employed competitive adoptive transfer to assess which haematopoietic linage(s) gave rise to BMDAs. GFPPOS BMDAs were only detected in mice transplanted with GFP-labelled BM myeloid cells, and no BMDAs were detected in mice transplanted with GFPPOS B or T lymphocytes or granulocytes (Figure 1d).
To confirm a myeloid origin for BMDAs and ensure their production was not an artefact of myeloablation, we generated mice in which myeloid cells were indelibly labelled with GFP (LysMcre-mTmG mice). GFP-expressing adipocytes were detected in these mice by flow cytometry (Figure 1e) or imaging flow cytometry (figure 1f) supporting a myeloid origin for BMDAs.
Some ATMs possess mesenchymal progenitor cell markers and are capable of adipogenesis
We previously reported that ATMs could generate adipocytes when propagated in three-dimensional fibrin or Matrigel matrices [9,13,23]. During this conversion the cells lost expression of pan-haematopoietic (CD45) and myeloid (CD11b) markers and acquired expression of mesenchymal markers (CD29, PDGFRalpha, Sca-1) traditionally found on conventional adipocyte progenitors [1,24,25]. This led us to ask whether a similar myeloid-to-mesenchymal conversion occurs in vivo during the production of BMDAs.
Flow cytometry analysis of adipose stroma from LysMcre-mTmG mice showed that a substantial proportion of stromal cells expressed GFP, and thus, were derived from the myeloid lineage (Figure 2a). The majority (~99%) of these cells also expressed the pan-haematopoietic marker CD45 and the myeloid marker CD11b (Figure 2b). The majority of these myeloid cells also expressed the mesenchymal marker CD29, with high (red events), low (blue events) or no (grey events) expression of a second mesenchymal progenitor marker, PDGFR (Figure 2c). CD45POS/CD11bPOS/CD29POS stromal cells expressing high levels of PDGFR also expressed the progenitor marker, Sca-1 (Figure 2d), and were capable of adipogenesis in culture when treated with adipogenic agents (Figure 2e), or spontaneously when implanted subcutaneously in mice (Figure 2h). Cells expressing CD45, CD11b, and CD29 with no or low PDGFR expression were plastic adherent but did not differentiate to adipocytes even when they were maintained in culture for several weeks and their media supplemented with adipogenic agents (figure 2f, g). A small number of stromal myeloid cells lacked CD45 and CD11b, and were also negative for CD29 and PDGFRalpha expression (Figure 2c, lower left quandrant). These cells did not adhere to plastic culture surfaces and were incapable of adipogenic conversion when plated in 3-D fibrin.
Figure 2.
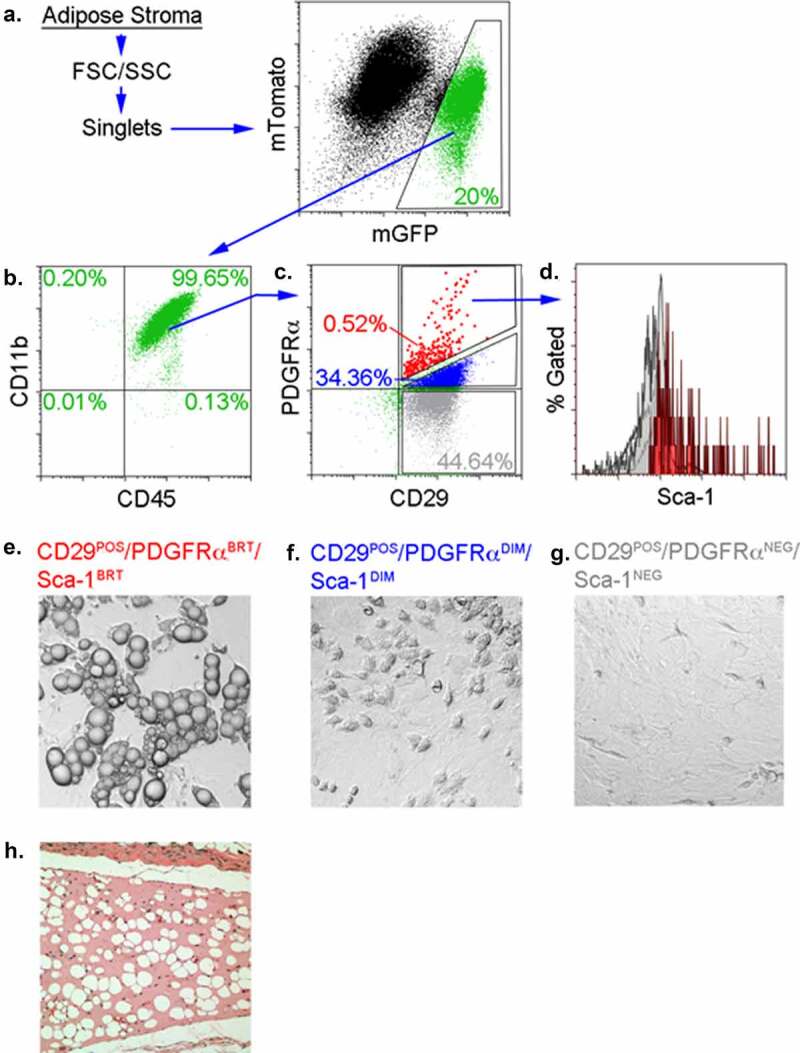
BMDAs are produced from ATM that simultaneously express haematopoietic/myeloid and mesenchymal progenitor markers. (a) Adipose tissue stromal cells from LysMcre-mTmG were separated from cell debris based on size (forward scatter, FSC) and internal complexity (side scatter, SSC), and single cells were isolated by singlet discrimination. LysMPOS cells were isolated based on their expression of membrane-bound GFP. (b) Approximately 99% of the LysMPOS cells in panel A expressed both the pan-haematopoietic marker CD45 and the myeloid marker CD11b. (c) The majority of cells in panel B also expressed the mesenchymal progenitor marker, CD29, but exhibited variable expression of PDGFRalpha (magnified red events = high expression, blue events = normal expression and grey events = no expression). Cells lacking both CD29 and PDGFR are displayed in as green events. (d) The magnified red events in panel c also expressed Sca-1, whereas other events in panel c (blue, grey and green events) were Sca-1NEG (overlapping light grey peaks in panel d). (e) LysMPOS stromal cells expressing CD29, Sca-1 and high levels of PDGFRalpha were plastic adherent and differentiated into adipocytes when treated with MDI. The percentage of gated cells is indicated is indicated in the gates of panels b-e in font colours matching each gate. (f & g) LysMPOS cells expressing CD29 and only modest (blue events in panel c or no expression (grey events in panel c) of PDGFR were also plastic adherent by incapable of adipogenic conversion even when treated with adipogenic agents. (h) Spontaneous adipogenic differentiation of LysMPOS/CD29POS/PDGFRPOS/Sca-1POS cells (red events in panel c) implanted subcutaneously in mice
LysMPOS cells in BM or the circulation, with or without mesenchymal markers, are incapable of adipogenic conversion
The repertoire of specific haematopoietic and mesenchymal markers on LysMPOS cells was also examined on BM or circulating cells from LysMcre mTmG mice. LysMPOS cells from BM exhibited a similar distribution of haematopoietic and mesenchymal markers as observed in adipose tissue stroma (Supplementary Fig. 1A). Most of the cells expressed CD29 with variable levels (none, low or high) of PDGFRalpha expression as observed in adipose tissue stroma. A small percentage of cells were negative for the expression of all four markers (green events). None of the BM or circulating populations were capable of adhesion to plastic, nor did they differentiate into adipocytes in 3D fibrin in vitro, or Matrigel in vivo (data not shown).
LysMPOS cells in the circulation displayed a similar distribution of CD29, Sca-1, CD45 and CD11b expression to that observed in adipose tissue stroma, although the abundance of PDGFRHIGH cells was less than was measured in adipose stroma or bone marrow (Supplementary Fig. 1B). Like the BM, none of the myeloid-derived cells were capable of adipogenic conversion in vitro or in vivo (data not shown).
PDGFR-expressing HSC generate adipocytes in adipose tissue
Because PDGFRalpha is a common mesenchymal marker for the adipocyte lineage and co-expressed with CD29 on putative BMDA precursors, we evaluated whether BMDAs were produced from progenitor cells expressing this marker. GFPPOS adipocytes were detected in the adipose tissue of mice transplanted with HSCs from donor mice in which GFP expression was controlled by the PDGFRalpha gene promoter (Figure 3a, b).
Figure 3.
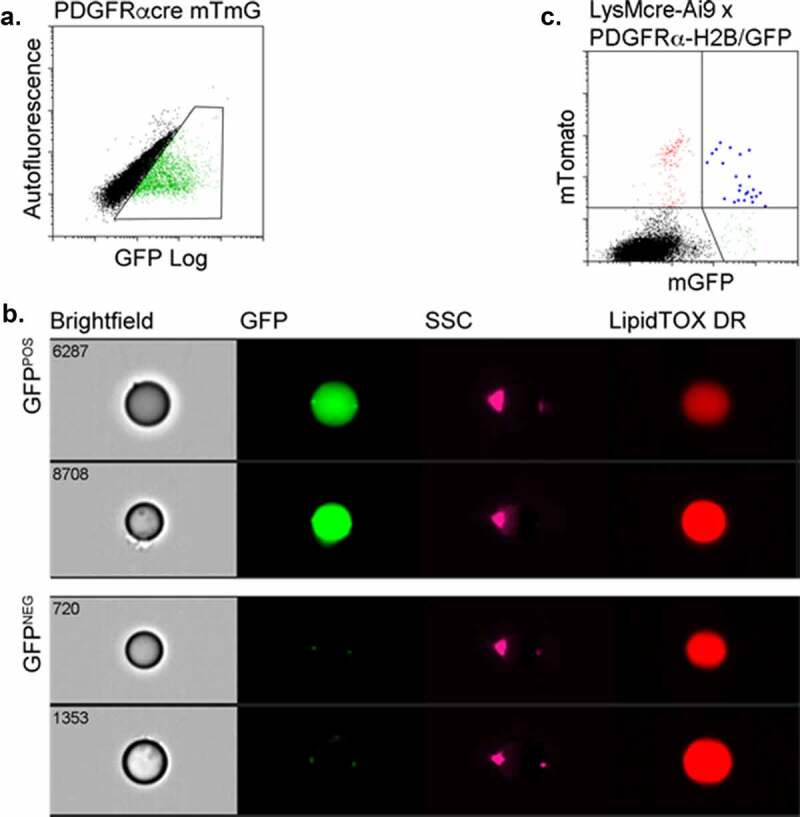
BMDAs are produced from HSCs expressing PDGFRalpha. (a & b) Wild-type mice were transplanted with HSCs from PDGFRcre-mTmG mice. After 12 weeks, adipose tissue was recovered, digested and analysed by flow cytometry. Adipocytes indelibly labelled with GFP by PDGFRalpha gene promoter activity were detected by conventional (a) or imaging (b) flow cytometry. (c) Triple transgenic donor mice were created in which mTomato expression from an Ai9 locus was controlled by cre recombinase expression regulated by the LysM gene promoter, and histone-stabilized GFP (H2B-GFP) expression was driven by the PDGFR gene promoter. HSCs from these mice were transplanted into wild-type recipients. After 8 weeks, adipocytes were isolated from the adipose tissue of the transplanted mice. Flow cytometry revealed the presence of adipocytes simultaneously expressing mTomato and H2B-GFP (blue events, upper right quadrant) indicating overlap between haematopoietic and mesenchymal developmental pathways in the production of these adipocytes
To verify the production of BMDAs from HSC via intermediates that express both myeloid and mesenchymal markers, we generated triple transgenic mice in which LysM promoter activity drove indelible mTomato expression from the Ai9 locus, and the PDGFRalpha gene promoter controlled expression of a chimeric protein composed of nuclear localized histone H2B linked to GFP. PDGFRalpha gene promoter-driven expression of H2B-GFP has previously been demonstrated in adipocyte progenitors [24,26]. In our experiments, flow cytometry of free-floating adipocytes from these mice revealed the presence of dual LysM/PDGFR-labelled adipocytes confirming their production from progenitors, sequentially or simultaneously, expressing these markers (Figure 3c). The relatively low number of the dual-labelled adipocytes likely reflects the low retention of the H2B-GFP label as PDGFRalpha gene promoter activity ceases as cells proliferate during their progression to mature adipocytes.
BMDAs express conventional adipocyte markers but exhibit minimal expression of haematopoietic and mesenchymal progenitor markers
BMDAs and conventional adipocytes from mouse gonadal adipose tissue were purified by flow cytometry. qRT-PCR was performed for a series of mature adipocyte markers, as well as mesenchymal and haematopoietic progenitor markers (Figure 4a). Both BMDAs and conventional adipocytes expressed common adipocyte markers including adiponectin, hormone-sensitive lipase (HSL), fatty acid synthase (FAS), C/EBPalpha and PPARgamma. However, leptin expression was significantly lower in BMDAs than conventional fat cells; a previously reported phenomenon [8,9]. The haematopoietic markers, CD45 and F4/80, were detected in both adipocyte populations at low levels, while CD11b was not detected in either population. The mesenchymal markers, CD29 and PDGFRalpha, were absent in conventional adipocytes and BMDAs. Because adiponectin expression is restricted solely to bona fide adipocytes [27], we wanted to further confirm its expression in BMDAs. Thus, we transplanted wild type mice with HSC from donors in which GFP expression was controlled by the adiponectin gene promoter (Figure 4b, c). AdiponectinPOS BMDAs were detected in these mice at levels comparable to that measured with other lineage labelling systems (e.g. LysMcre and PDGFRcre) used to identify BMDAs.
Figure 4.
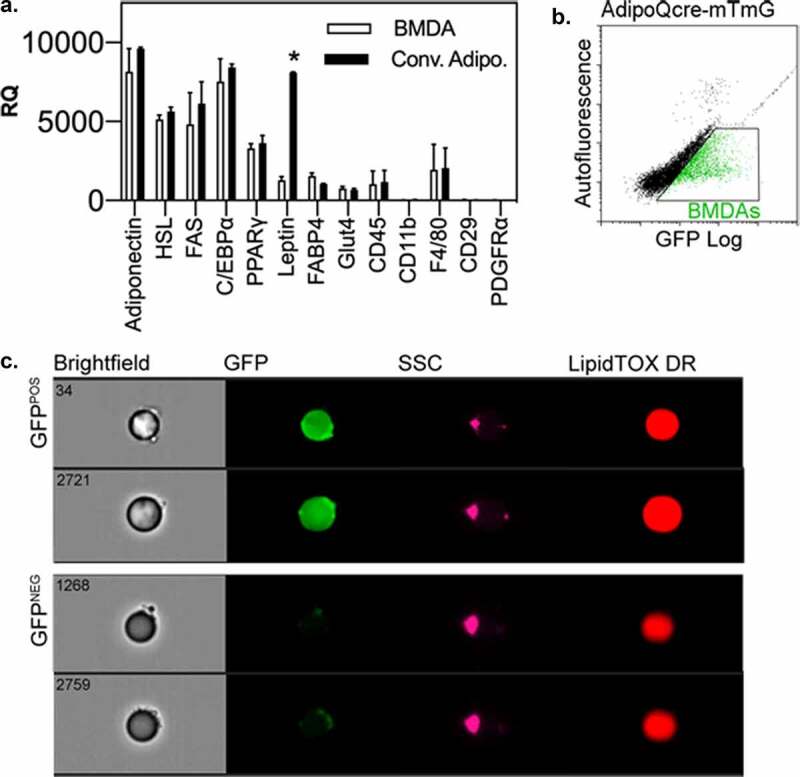
BMDAs express mature adipocytes markers, but not leptin. (a) RNA was isolated from flow cytometry purified, free floating adipocytes from wild type mice transplanted with HSCs from AdipoQcre-mTmG donors. Quantitative RT-PCR was performed with commercial primer sets. Expression of adipocyte-specific genes, with the exception of leptin, which was repressed in BMDAs, was similar between conventional and BMD adipocytes. Expression of mesenchymal and haematopoietic progenitor markers was absent or equally low in both populations. (b & c) Free floating adipocytes from wild type mice transplanted with HSCs from AdipoQcre-mTmG donors were analysed by conventional (b) and imaging (c) flow cytometry. GFPPOS events/adipocytes were detected among GFPNEG adipocytes by both methods
The repertoire of cell surface markers on buoyant BMDAs and conventional adipocytes, and the non-buoyant stromal fraction of adipose tissue were also examined by flow cytometry (Table 1). Both BMDAs and conventional adipocytes showed robust staining for CD36 (fatty acid transporter) and the lipid stain, LipidTOX, both of which were much lower or absent in adipose stromal cells. However, both BMDA and conventional adipocytes were devoid of haematopoietic and mesenchymal progenitor markers, and markers found on vascular endothelial, lymphatic endothelial, smooth muscle and neuronal cells, and a cadre of miscellaneous cell surface markers. Markers for skeletal muscle were not found in any adipose cell population. Interestingly, low-level staining for platelet/endothelial cell adhesion molecule-1 (PECAM-1) was detected on BMDAs but not conventional adipocytes. While originally detected on platelets and vascular endothelial cells, PECAM-1 is also expressed on monocytes [28] and neutrophils [29] in the vascular lumen where it may foster binding and transmigration of the these cells from the vascular lumen to the adipose mesenchyme. This may be crucial to the production of BMDAs from circulating myeloid cells.
Table 1.
Flow cytometry markers on BMDAs, conventional adipocytes, & stromal cells in wild type mice transplanted with HSCs from UbqC-GFP donor mice
| |
BMDA |
Conv WA |
SVF |
| Adipocyte | |||
| CD36/FAT | ++ | ++ | +/- |
| LipidTOX | ++ | ++ | +/- |
| GFP | + | - | +/- |
| Hematopoietic | |||
| Lin | - | - | +/- |
| CD45 | - | - | +/- |
| CD11b | - | - | +/- |
| Gr-1 | - | - | +/- |
| B220 | - | - | +/- |
| Thy-1 | - | - | +/- |
| Ter119 | - | - | +/- |
| Progenitor | |||
| CD29/Itg β1 | - | - | + |
| Sca-1 | - | - | + |
| c-Kit | - | - | + |
| CD34 | - | - | + |
| Vascular | |||
| Endothelial | |||
| PECAM1 | + | - | +/- |
| VE-Cadherin | - | - | +/- |
| Flk-1 | - | - | +/- |
| Lymphatic Endothelial | |||
| VEGFR-3 | - | - | +/- |
| LYVE1 | - | - | +/- |
| Skeletal | |||
| Muscle | |||
| αActinin | - | - | - |
| Desmin | - | - | - |
| Smooth Muscle | |||
| Itg α7 | - | - | - |
| Neuronal | |||
| NCAM | - | - | +/- |
| Misc | |||
| PDGFRα | - | - | +/- |
| PDGFRβ | - | - | +/- |
| Notch 4 | - | - | +/- |
| Itg alpha5 | - | - | +/- |
BMDA, bone marrow derived adipocyte
Conv WA, conventional white adipocyte
SVF, stromal vascular fraction
FAT, fatty acid transporter
VE, vascular endothelial
Itg, integrin
PDGFR, platelet-derived growth factor receptor
PECAM, platelet endothelial cell adhesion molecule
LYVE, lymphatic vessel endothelial receptor
FLK1, fetal liver kinase 1
Sca-1, stem cell antigen 1
VEGFR-3, vascular endothelial growth factor receptor 3
Lin, lineage
NCAM, neural cell adhesion molecule
c-kit, stem cell factor ligand
Discussion
While the majority of adipocytes in adipose depots arise from mesenchymal progenitor cells, results herein demonstrate that a subpopulation of adipocytes is produced from haematopoietic stem cells via circulating myeloid intermediates that co-express mesenchymal progenitor markers. This conclusion is further supported by adoptive transfer studies in which donor HSC ultimately gave rise to BMDAs indelibly labelled by either the PDGFRalpha (mesenchymal progenitor) or adiponectin (mature adipocyte) gene promoters. The three models presented here highlight the generation of BMDAs via the following pathway 1) HSCs that traffic to adipose tissue and 2) generate myeloid intermediates/adipose tissue macrophage, 3) some of which co-express mesenchymal markers. As these cells differentiate into BMDAs they express the canonical adipocyte marker, adiponectin. The LysM model was also used in non-transplant experiments confirming that BMDAs are not an artefact of myeloablation.
BMDAs arise from HSCs via the myeloid lineage
Previous in vitro studies demonstrated that some adipose tissue macrophage lost haematopoietic markers and acquired mesenchymal progenitor markers while cultured in three-dimensional matrices composed of fibrin or Matrigel [9,13,23]. When removed from the 3-dimensional matrices the haematopoietic marker-depleted and mesenchymal marker-enriched cells were capable of adipogenic differentiation in culture and in vivo. Our goal here was to determine whether this ‘hematopoietic-to-mesenchymal’ transition also occurred in vivo.
We created an adoptive transfer model in which HSCs from LysMcre reporter mice were transplanted into wild type recipients. Reporter-positive cells in adipose tissue identified 1) cells with an HSC origin, and 2) cells that currently or previously expressed the LysM myeloid marker. As anticipated, we found that the vast majority of LysMPOS cells in adipose tissue stroma were also positive for the haematopoietic/myeloid markers, CD45 and CD11b. A substantial portion of these cells were also CD29POS, but only a small number of LysMPOS cells simultaneously expressed high levels of PDGFRalpha and Sca-1. Only LysMPOS stromal cells simultaneously expressing both haematopoietic/myeloid and mesenchymal markers were capable of adipogenesis via the rapid, simultaneous loss of haematopoietic and mesenchymal markers, rather than the stepwise loss of haematopoietic markers prior to acquisition of mesenchymal features.
BMDAs are not simply lipid-engorged macrophage or foam cells
Previous flow cytometry studies and gene expression analysis of cell surface markers indicated that BMDAs, like conventional adipocytes, lack haematopoietic and mesenchymal progenitor markers [8,9]. Adipocytes generated from myeloid adipocyte progenitors in culture also lack CD11b and exhibit low expression of CD45 and F4/80, and are deficient in mesenchymal progenitor markers [23]. Here we showed that BMDAs also lack markers for other terminally differentiated lineages present in adipose tissue including vascular and lymphatic endothelial cells, skeletal and smooth muscle cells and neurons. However, they express the same markers as conventional adipocytes, including adiponectin, whose expression is restricted to bona fide adipocytes. Interestingly, leptin was reduced in BMDAs; a feature previously attributed to these cells [8,9].
BMDAs are also not lipid-engorged macrophage or foam cells. We have consistently failed to detect high-level expression of pan-leukocyte (CD45) or myeloid (CD11b, F4/80) markers on BMDAs by flow cytometry, gene microarray analysis or Q-RT-PCR [8,9,30,31]. BMDA phenotyping herein that shows no or very low expression of these markers on BMDAs amplifies this conclusion. Moreover, adiponectin gene expression, which is restricted to mature adipocytes [32], was detected in flow cytometry isolated BMDAs, whereas adiponectin expression suppresses foam cell formation [33,34]. Thus, BMDAs are not foam cells despite their myeloid origin.
Conventional adipocytes are generated from multiple mesenchymal lineages
Kirkland, et al. reported that human visceral and subcutaneous preadipocytes exhibit different capacities for adipogenic differentiation and survival [35–37]. They also noted substantial differences in global gene expression patterns between human preadipocytes from subcutaneous, mesenteric and omental fat [37], and epididymal and perirenal preadipocytes from rats [38]. These results showed that distinct adipocyte progenitors reside in the different regional fat depots and may explain the different metabolic characteristics in adipocytes from subcutaneous adipose tissue compared to visceral adipose tissue of humans [39,40].
Ultimately, fate-mapping studies provided unambiguous evidence that different adipocyte populations have distinct developmental origins. For example, Spiegelman, et al. used Myf5cre and Myh11cre-based models to demonstrate production of brown or beige adipocytes from skeletal and smooth muscle progenitor cells, respectively [4,5]. Recent studies indicate that a number of developmental lineages specified by Wt1 [2], Prx1 [2], Pax3 [3] and Sox10 [41] also contribute to adipose tissue development and heterogeneity between depots. Although the current studies link BMDAs to myeloid progenitors expressing CD45 and CD11b, additional studies are needed to explore the relationship between BMDA progenitors and depot-restricted mesenchymal lineage markers.
The production of adipocytes from haematopoietic lineages is supported by reports from other laboratories
Other groups have reported production of adipocytes from HSC or myeloid lineage progenitors. Guerrero-Juarez et al. [15] demonstrated the conversion of myeloid cells to fibroblasts having a dual-lineage gene expression signature in large excisional skin wounds. Additional experiments from the same study demonstrated the production of adipocytes indelibly labelled by the LacZ (myeloid), CD45 (pan-haematopoietic) and Resistin (adipocyte) gene promoters. LacZ expression was also noted in dermal papilla and sheath cells indicating that the lineage plasticity of myeloid cells extends beyond adipocytes.
This concept is supported by several reports from Ogawa and colleagues who, in addition to adipocytes [10], have demonstrated multilineage haematopoietic engraftment of fibroblast, myofibroblast and pericytes in various models [42–44]. Given the widespread contribution of HSC to multiple mesenchymal lineages these authors propose that mesenchymal progenitor cells are similar if not identical to HSC-derived fibroblasts. In support of this idea, they cited the presence of Mac1 or F4/80 expressing colonies in cultures of colony forming unit-fibroblast cells [45], and the ability of CD14POS PBMC to generate multiple mesenchymal lineages in vitro [46]. Streiter and colleagues [47] also reported the production of adipocytes from fibrocytes, which are circulating blood cells with features of both fibroblasts and HSCs. However, our current data refutes the idea that all mesenchymal progenitors and adipocytes are produced solely from HSC-derived fibroblasts as experiments using myeloablative and non-myeloablative lineage labelling models clearly reveal population of adipocytes produced from non-myeloid (LysMNEG) and non-HSC precursors, respectively.
Our studies and those of other laboratories, now raise the question of why adipocytes, long considered solely a mesenchymal lineage, are produced from HSCs. Results from Guerrero-Juarez, et al. [15], support the contention that localized production of adipocytes from circulating myeloid cells in skin lesions provides for the regulated storage and release of energy during wound healing. Similarly, Xiong et al. [20,21] reported the production of adipocytes from HSCs in tumours and their contribution to tumour growth and cancer cell migration. Thus, BMDA production may support localized energy storage and release in tissues wherein the production of conventional adipocytes is restricted or absent. Our previous studies have noted an increase in BMDA production in conventional adipose depots in animals fed a high-fat diet or treated with adipogenic thiazolidinediones [8]. Interestingly, both conditions reduce the abundance of conventional adipocyte progenitors in adipose tissue [48–52]. Therefore, we postulate that the generation of BMDAs may serve to maintain new adipocyte production even when the production of conventional adipocytes is diminished.
Likewise, leptin-diminished BMDAs may have been crucial to the contribution of adipose tissue to metabolic regulation during early human evolution. Leptin participates in energy metabolism via its roles in short-term feeding behaviour and long-term energy expenditure [53]. During early human evolution, a period characterized by the restricted availability of dietary calories, the production of leptin-diminished BMDAs may have provided a solution for long-term energy storage without depletion of energy stores due to the penalty of leptin-induced energy expenditure.
Summary
BMDAs are produced from HSCs via the myeloid lineage. In this model, some ATM acquire mesenchymal progenitor markers prior to terminal adipogenesis. Haematopoietic and mesenchymal markers appear to be lost simultaneously prior to or during adipogenic conversion, and not in a stepwise haematopoietic-to-mesenchymal transition. BMDAs express conventional adipocyte markers including adiponectin, but have very low leptin gene expression and do not express haematopoietic or mesenchymal progenitor markers.
Materials and methods
Animal Models
All procedures and treatments were approved by the Institutional Animal Care and Use Committee at the University of Colorado Anschutz Medical Campus. Mice were purchased from The Jackson Laboratory and included wild-type C57Bl/6 J (cat. no. 000664); C57Bl/6-Tg (UBC-GFP)30Scha/J (UbqC-GFP, cat. no. 004353); B6.129P2-Lyz2tm1(cre)Ifo/J (LysMcre, cat. no. 018956); C57BL/6-Tg (Pdgfra-cre)1 Clc/J, (PDGFRcre, cat. no. 013148); B6; FVB-Tg(Adipoq-cre)1Evdr/J, (AdipoQcre, cat. no. 010803); B6.129 (Cg)-Gt (ROSA)26Sortm4(ACTB-tdTomato,EGFP)Luo/J) (mT/mG, cat. no. 007676); B6.129S4-Pdgfratm11(EGFP)Sor/J (PDGFR-H2B/GFP, cat. no. 007669) and B6.Cg-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J (Ai9, cat.no. 007909).
Materials
PCR Primers: Mouse genotyping was performed with the validated primers sets listed in Supplementary Table 1. Pre-optimized primers for qRT-PCR for the genes listed in Supplementary Table 2 were purchased from Integrated DNA Technologies (Coralville, IA) or Qiagen (Germantown, MD) as indicated.
Flow Cytometry Antibodies and Reagents: Flow cytometry antibodies recognizing murine cell surface markers used throughout his paper, and their vendor are listed in Supplementary Table 3.
Miscellaneous Reagents and Equipment: Cell culture reagents, chemical compounds, genetic reagents, major equipment, software and other materials are described in Supplementary Table 4.
Adoptive Transfer
Conventional BM transplant: Recipient mice were irradiated with a split dose of 600 rads using an X ray source. Immediately following irradiation, mice were injected in the retroorbital venous plexus with 5 × 106 bone marrow cells from the donor mice indicated in each experiment. At 8 weeks post-transplant, mice were assessed for chimerism by flow cytometry analysis of peripheral blood. Over 95% of peripheral blood cells expressed fluorescent lineage markers in the transplanted mice. The mice were maintained in a climate-controlled room with alternating 12-hour periods of light and dark and were fed ad libitum.
Competitive adoptive transfer: Competitive BM transplants were performed with GFP-expressing BM separated into haematopoietic stem cell (LinNEG/Sca-1POS/KitPOS) or mesenchymal progenitor cell (LinNEG/CD45NEG/CD24POS/PDGFRPOS) subpopulations based on the expression of specific cell surface markers by antibody-labelled magnetic bead separation. Two rounds of magnetic separation were performed to improve separation efficiency, which was greater than 90% [9]. The separated labelled BM cells were combined with marker-negative BM cells from wild type mice to ensure survival of recipients. Engraftment of the labelled cell subpopulations was greater than 90% for all transplants as determined by flow cytometry of PBMCs.
Fractionation of Adipose Tissue and Preparation of PBMCs and bone marrow cells
Adipocyte and stromal fractions were prepared by collagenase digestion and flotation/differential centrifugation as previously described [8]. PBMCs were isolated from defibrinated blood by centrifugation over Ficol-Paque medium. Approximately 2 ml of blood were mixed with an equal volume of PBS and gently layered over 3 ml of Ficol-Paque. The tubes were centrifuged at 500 x g for 15 minutes and the supernatant carefully removed. The PBMCs layered on top of the Ficol-Paque were recovered, washed once with PBS and resuspended in PBS or culture medium as necessary.
Bone marrow cells were collected from the same animals by dislocation and removal of the hind leg. The lower leg and tissue were dissected from the femurs and the epiphyses cut off. Using a 27 gauge needle the marrow was flushed with 5 ml cold HBSS + 2% FBS into a collection tube then filtered through a 100 µm cell strainer. The cells were pelleted and washed with HBSS + 2% FBS.
Sorted myeloid cells were pellet at 300 g x 10 minutes and resuspend in MesenCult Basal medium plus Stem Cell Stimulatory Supplements and replated at 40,000 cells directly into one well of 96 well plate.
Flow Cytometry
Staining of Adipocytes with DAPI and LipidTOX Deep Red: Buoyant adipocytes resulting from collagenase digestion and flotation/differential centrifugation were transferred to a clean tube and fixed with 4% paraformaldehyde in PBS for 20 minutes at room temperature. The fat cells were then transferred to another clean tube and fresh PBS added with gentle mixing. After the adipocytes accumulated at the top of the liquid, they were transferred to another fresh tube and washed with PBS two more times.
The cells were then incubated in a 1:200 dilution of LipidTox DR for 20 minutes at room temperature. DAPI was added to a final concentration of 3.75 ng/ml and incubation continued for another 10 minutes. The cells were washed and then examined by fluorescence microscopy or flow cytometry.
Conventional flow cytometry and sorting: Adipose stroma, BM cells, PBMC or adipocytes were stained with antibodies to the cell surface markers indicated in the figure legends. Gating strategies included exclusion of red blood cells with Ter199, and doublet discrimination. Controls included unstained cells and cell suspensions incubated with APC- or PE-conjugated isotype matched control antibodies. Cells were analysed or sorted using a Legacy Moflo cell sorter with Summit 4.3 software.
Imaging flow cytometry: Speed Beads were added to each sample of free-floating adipocytes for synchronization of the detectors and flow parameters. Data was acquired on an ImageStream X cytometer for brightfield (BF), side scatter (SSC), GFP fluorescence, and LipidTOX fluorescence using LED trans-illumination, 2 mW 785 nm and 100 mW 488 nm lasers, respectively. Gating parameters were determined post-hoc by IDEAS software with preliminary gating to separate intact cells from cell debris and synchronization beads. Images of cells with best focal quality were gated using a metric for image clarity (Gradient RMS of the BF image). Single colour controls were used to create a compensation matrix that was applied to all sample files. Following data acquisition, adipocytes were observed in the brightfield imagery. Approximately 25 events of each type were used to create ‘true’ populations and IDEAS software was used to compare the mean value for 25 gating features between the ‘true’ populations. The Rd values for each feature were ranked from highest to lowest, and the features with highest Rd were selected for further gating to distinguish the true populations.
In Vivo Adipogenesis
Flow cytometry-purified adipose tissue stromal cells were gently resuspended in 200 ul of low growth factor Matrigel at 4°C. Female athymic mice were lightly anesthetized and the Matrigel/cell suspensions were injected subcutaneously into the abdomen anterior of the thigh. After 4 weeks the solid Matrigel plugs and adjacent skin and subcutaneous muscle were removed. The plugs were fixed overnight in 4% paraformaldehyde, and then sliced in half with a razor blade. The halves were oriented in paraffin blocks with the cut surfaces up for sectioning. Five um sections were stained with haematoxylin and eosin for histological examination.
Microscopy
Microscopy was performed on a Nikon TE2000-U inverted epifluorescent microscope with a motorized, remote focus/Z-axis stage controller. Brightfield and fluorescent images were captured to a desktop computer with either a DS-Fi2 colour camera or a DS-QiMc black & white camera both managed by a DS-U3 PC-based camera control unit. Image data was acquired with NIS-Elements software.
PCR
Conventional PCR: DNA was extracted from flow-sorted adipocytes using DNeasy Blood and Tissue Kit reagents and columns. Genomic DNA was amplified with RED Extract-n-AMP reagent in reactions containing 500 nM of each of the appropriate forward and reverse primers. A touch-down protocol was used in which the annealing temperature was lowered from 70°C to 60°C in 0.5°C increments per cycle. Annealing was carried out for 45 seconds and the other parameters included denaturation at 94°C for 1 minute and elongation at 72°C for 2 minutes. Following the series of touchdown cycles, an additional 32 cycles were performed with the 60°C annealing conditions. PCR was performed with denaturation at 94°C for 1 minute, annealing at 63°C for 45 seconds and elongation at 72°C for 2 minutes for 31 cycles.
Supplementary Material
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Eastern Colorado VA Geriatric Research, Education and Clinical Center (GRECC), Aurora, CO. This research was funded by U.S. National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases Grants R01DK109547 (to D.J.K.) and U54 AG062319 (to D.J.K. and W.M.K.); and K01 DK109053 (to K.M.G). This research was also supported in part by NIH National Center for Advancing Translational Sciences Colorado Clinical and Translational Science Awards Grant UL1 TR001082 and NIH National Cancer Institute Centers for Common Disease Genomics Grant P30 CA046934.
Funding Statement
This material is the result of work supported with resources and the use of facilities at the Eastern Colorado VA Geriatric Research, Education and Clinical Center (GRECC), Aurora, CO. This research was funded by U.S. National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases Grants R01DK109547 (to D.J.K.) and U54 AG062319 (to D.J.K. and W.M.K.); and K01 DK109053 (to K.M.G). This research was also supported in part by NIH National Center for Advancing Translational Sciences Colorado Clinical and Translational Science Awards Grant UL1 TR001082 and NIH National Cancer Institute Centers for Common Disease Genomics Grant P30 CA046934.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary Material
Supplemental data for this article can be accessed here.
References
- [1].Rodeheffer MS, Birsoy K, Friedman JM.. Identification of white adipocyte progenitor cells in vivo. Cell. 2008;135(2):240–249. [DOI] [PubMed] [Google Scholar]
- [2].Chau YY, Hastie N. Wt1, the mesothelium and the origins and heterogeneity of visceral fat progenitors. Adipocyte. 2015;4(3):217–221. Hastie, N.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mohsen-Kanson T, Hafner AL, Wdziekonski B, et al. Differentiation of human induced pluripotent stem cells into brown and white adipocytes: role of Pax3. Stem Cells. 2014;32(6):1459–1467. [DOI] [PubMed] [Google Scholar]
- [4].Kajimura S, Seale P, Kubota K, et al. Initiation of myoblast to brown fat switch by a PDRM16-C/EBP-beta transcriptional complex. Nature. 2009;460(7259):1154–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Long JZ, Svensson KJ, Tsai L, et al. A smooth muscle-like origin for beige adipocytes. Cell Metab. 2014May6;19(5):810–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Giralt M, Villarroya F. White, brown, beige/brite: different adipose cells for different function? Endocrinology. 2013;154(9):2992–3000. [DOI] [PubMed] [Google Scholar]
- [7].Maurer S, Harms M, Boucher J. The clorful versatility of adipocytes: white-to-brown transdifferentiation and its therapeutic potential in humans. FEBS J. 2021 Jun;288(12):3628–3646. [DOI] [PubMed] [Google Scholar]
- [8].Crossno JT Jr., Majka SM, Grazia T, et al. Rosiglitazone Promotes Differentiation of Bone Marrow-Derived Circulating Progenitor Cells to Multilocular Adipocytes in Adipose Tissue. J Clin Invest. 2006;116(12):3220–3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Majka SM, Fox KE, Psilas JC, et al. De novo generation of white adipocytes from the myeloid lineage via mesenchymal intermediates is age, adipose depot, and gender specific. Proc Natl Acad Sci U S A. 2010Aug17;107(33):14781–14786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sera Y, LaRue AC, Moussa O, et al. Hematopoietic stem cell origin of adipocytes. Exp Hematol. 2009Sep;37(9):1108–1120. e1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gavin KM, Gutman JA, Kohrt WM, et al. De novo generation of adipocytes from circulating progenitor cells in mouse and human adipose tissue. FASEB J. 2016Mar;30(3):1096–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gavin KM, Sullivan TM, Kohrt WM, et al. Ovarian Hormones Regulate the Production of Adipocytes From Bone Marrow-Derived Cells. Front Endocrinol (Lausanne). 2018May;28;9:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Majka SM, Miller HL, Sullivan T, et al. Adipose lineage specification of bone marrow-derived myeloid cells. Adipocyte. 2012Oct1;1(4):215–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Arner P, Ryden M. The contribution of bone marrow-derived cells to the human adipocyte pool. Adipocyte. 2017;6(3):187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Guerrero-Juarez CF, Dedhiq PH, Jin S, et al. Single-cell analysis reveals fibroblast heterogeneity and myeloid-derived adipocyte progenitors in murine skin wounds. Nat Commun. 2019;10(1):650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hu T, Kitano A, Luu V, et al. Bmi1 suppresses adipogenesis in the hematopoietic stem cell niche. Stem Cell Reports. 2019;13(3):545–558. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ogawa M, LaRue AC, Mehotra M. Plasticity of hematopoietic stem cells. Best Pract Res Clin Haematol. 2015;28(2–3):73–80. [DOI] [PubMed] [Google Scholar]
- [18].Ryden M. On the origin of human adipocytes and the contribution of bone marrow-derived cells. Adipocyte. 2016;5(3):312–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ryden M, Uzunel M, Hard JL, et al. Transplanted Bone Marrow-Derived Cells Contribute to Human Adipogenesis. Cell Metab. 2015Sep1;22(3):408–417. [DOI] [PubMed] [Google Scholar]
- [20].Xiong Y, McDonald LT, Russell DL, et al. Hematopoietic stem cell-derived adipocytes and fibroblasts in the tumor microevironment. World J Stem Cells. 2015;135(2):253–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Xiong Y, Russell DL, MacDonald LT. et al. Hematopoietic stem cell-derived adipocytes promote tumor growth and cancer cell migration. Int J Cancer Res Mol Mech. 2017; 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yoneshiro T, Shin W, Machida K, et al. Differentiation of bone marrow-derived cells toward thermogenic adipocytes in white adipose tissue induced by the beta3 adrenergic stimulation. Faseb J. 2019;33(4):5196–5207. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- [23].Gavin KM, Majka SM, Kohrt WM, et al. Hematopoietic-to-mesenchymal transition of adipose tissue macrophages is regulated by integrin b1 and fabricated fibrin matrices. Adipocyte. 2017;6(3):234–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Berry R, Rodeheffer MS. Characterization of the adipocyte cellular lineage in vivo. Nat Cell Biol. 2013;15(3):302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Rivera-Gonzalez GC, Shook BA, Andrae J, et al. Skin adipocyte stem cell self-renewal is regulated by a PDGFA/AKT-signaling axis. Cell Stem Cell. 2016;19(6):738–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Jeffery E, Berrry R, Church CD, et al. Characterization of Cre recombinase models for the study of adipose tissue. Adipocyte. 2014;3(3):206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Eguchi J, Wang X, Yu S, et al. Transcriptional control of adipose lipid handling by IRF1. Cell Metab. 2011;13(3):249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sandig M, Korvemaker ML, Ionescu CV, et al. Transendothelial migration of monocytes in rat aorta: disruption of F-actin, alpha-actin, LFA-1 and PECAM-1. Biotech Histochem. 1999;74(6):276–293. [DOI] [PubMed] [Google Scholar]
- [29].Christofidou-Solomidou M, Nakada MT, Williams J, et al. Neutrophil platelet endothelial cell adhesion molecule-1 participates in neutrophil recruitment at inflammatory sites and is down-regulated after leukocyte extravagation. J Immunol. 1997;158:4872–4878. [PubMed] [Google Scholar]
- [30].Majka SM, Miller HL, Helm KM, et al. Analysis and isolation of adipocytes by flow cytometry. In: MacDougald OA, editor Methods in Adipose Tissue Biology. Methods in Enzymology. Vol. 537, Amsterdam (Netherlands): Elsevior Press; 2014. p. 281–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Majka SM, Miller HL, Sullivan T, et al. Adipose Lineage Specification of Bone Marrow-Derived Myeloid Cells. Adipocyte. 2012;1(4):215–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kostopoulas CG, Spiroglou SG, Varakis JN, et al. Adiponectin/T-cadherin and apelin/APJ expression in human arteries and periadventitial fat: implication of local adipokine signaling in atherosclerosis. Cardiovasc Pathol. 2014;223:131–138. [DOI] [PubMed] [Google Scholar]
- [33].Tian L, Luo N, Zhu X, et al. Adiponectin-AdipoR1/2-APPL1 signaling axis suppresses human goam cell formation: differential ability of AdipoR1 and AdipoR2 to regulate inflammatory cytokine responses. Athersclerosis. 2012;221(1):66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wang M, Wang D, Zhang Y, et al. Adiponectin increases macrophages cholesterol efflux and suppresses foam cell formation in patients with type 2 diabetes mellitus. Atherosclerosis. 2013;229(1):62–70. [DOI] [PubMed] [Google Scholar]
- [35].Tchkonia T, Giorgadze N, Pirtskhalava T, et al. Fat depot origin affects adipogenesis in primary cultured and cloned human preadipocytes. Am J Physiol Regulatory Integrative Comp Physiol. 2001;282(5):R1286–R1296. [DOI] [PubMed] [Google Scholar]
- [36].Tchkonia T, Giorgadze N, Pirtskhalava T, et al. Fat depot-specific charactersitics are retained in strains derived from single human preadipocytes. Diabetes. 2006;55(9):2571–2578. [DOI] [PubMed] [Google Scholar]
- [37].Tchkonia T, Lenburg M, Thomou T, et al. Identification of depot-specific human fat cell progenitors through distinct expression profiles and developmental gene patterns. Am J Physiol Endocrinol Metab. 2007;292(1):E298–E307. [DOI] [PubMed] [Google Scholar]
- [38].Cartwright MJ, Schlauch K, Lenburg ME, et al. Aging, depot origin, and preadipocyte gene expression [Comparative Study Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. J Gerontol A Biol Sci Med Sci. 2010Mar;65A(3):242–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Engfeldt P, Arner P. Lipolysis in human adipocytes: effects of cell size, age and regional differences. Horm Metab Res Suppl. 1988;19:26–29. [PubMed] [Google Scholar]
- [40].Bahceci M, Gokalp D, Bahceci S, et al. The correlation between adiposity and adiponectin, tumor necrosis factor-a, interleukin-6 and high sensitivity C-reactive protein. Is adipocyte size associated with inflammation in adults? J Endocrinol Invest. 2007;30(3):210–214. [DOI] [PubMed] [Google Scholar]
- [41].Billon N, Iannarelli P, Monteiro MC, et al. The generation of adipocytes by the neural crest. Development. 2007Jun;134(12):2283–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Masuya M, Drake CJ, Fleming PA, et al. Hematopoietic origin of glomerular mesangial cells. Blood. 2003;101(6):2215–2218. [DOI] [PubMed] [Google Scholar]
- [43].Hess DC, Abe T, Hill WD, et al. Heamtopoietic origin of microglial and perivascular cells in brain. Exp Neurol. 2004;186(2):134–144. [DOI] [PubMed] [Google Scholar]
- [44].Lang H, Ebihara Y, Schmiedt RA, et al. Contribution of bone marrow hematopoietic stem cells to adult mouse inner ear: mesenchymal cells and fibrocytes. J Comp Neuro. 2006;496(2):187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Penn PE, Jiang DZ, Fei RG, et al. Further characterization of murine bone marrow stromal cells. Blood. 1993;81(5):1205–1213. [PubMed] [Google Scholar]
- [46].Kuwana M, Okazaki Y, Kodama H, et al. Human circulating CD14+ monocytes as a source of progenitors that exhibit mesenchymal cell differentiation. J Leukoc Biol. 2003;74(5):833–845. [DOI] [PubMed] [Google Scholar]
- [47].Hong KM, Buridick MD, Phillips RJ, et al. Characterization of human fibrocytes as circulating adipocyte progenitors and the formation of human adipose tissue in SCID mice. FASEB J. 2005;19(14):2029–2031. [DOI] [PubMed] [Google Scholar]
- [48].Gustafson B, Gogg S, Hedjazifar S, et al. Inflammation and impared adipogenesis in hypertrophic obesity in man. Am J Physiol Endocrinol Metab. 2009;297(5):E999–E1003. [DOI] [PubMed] [Google Scholar]
- [49].Hedbacker K, Lu Y-S, Dallner O, et al. Limitation of adipose tissue by the number of embryonic progenitor cells. eLife. 2020;9:e53075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Isakson P, Hammarstedt A, Gustafson B, et al. Impaired preadipocyte differentiation in human abdominal obesity: role of Wnt, tumor necrosis factor-alpha, and inflammation. Diabetes. 2009;58(7):1550–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Tchoukalova Y, Koutsari C, Jensen MD. Committed subcutaneous preadipocytes are reduced in human obesity. Diabetologia. 2007;50(1):151–157. [DOI] [PubMed] [Google Scholar]
- [52].Tchoukalova YD, Votruba SB, Tchkonia T, et al. Regional differences in cellular mechanisms of adipose tissue gain with overfeeding. Proc Natl Acad Sci USA. 2010;107(42):18226–18231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444(7121):847–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


