Abstract
Treatment of the novel Coronavirus Disease 2019 (COVID-19) remains a complicated challenge, especially among patients with severe disease. In recent studies, immunosuppressive therapy has shown promising results for control of the cytokine storm syndrome (CSS) in severe cases of COVID-19. However, it is well documented that immunosuppressive agents (e.g., corticosteroids and cytokine blockers) increase the risk of opportunistic infections. On the other hand, several opportunistic infections were reported in COVID-19 patients, including Aspergillus spp., Candida spp., Cryptococcus neoformans, Pneumocystis jiroveci (carinii), mucormycosis, Cytomegalovirus (CMV), Herpes simplex virus (HSV), Strongyloides stercoralis, Mycobacterium tuberculosis, and Toxoplasma gondii. This review is a snapshot about the main opportunistic infections that reported among COVID-19 patients. As such, we summarized information about the main immunosuppressive agents that were used in recent clinical trials for COVID-19 patients and the risk of opportunistic infections following these treatments. We also discussed about the main challenges regarding diagnosis and treatment of COVID-19-associated opportunistic infections (CAOIs).
Supplementary Information
The online version contains supplementary material available at 10.1007/s10238-021-00751-7.
Keywords: COVID-19, SARS-CoV-2, Opportunistic infection, Cytokine storm syndrome, Immunosuppressive therapy, Aspergillosis, Candidiasis, Mucormycosis, Cryptococcosis, Pneumocystis jirovecii pneumonia, Tuberculosis, Cytomegalovirus, Herpes simplex virus, Toxoplasmosis, Strongyloidiasis, Helminth infections
Introduction
The Coronavirus Disease 2019 (COVID-19) was emerged in Wuhan, China, in late 2019 and immediately spread to become pandemic [1, 2]. The disease is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and has diverse sequels in all ages [3, 4]. However, older individuals (> 60 years old), males, and patients who have comorbidities or coinfections, such as pulmonary diseases, chronic kidney disease, diabetes, hypertension, and cardiovascular diseases, have a higher risk of severe infection [1, 2, 5, 6]. Close contact and respiratory droplets are the main route of COVID-19 transmission through breathing, sneezing, coughing, and even normal speech [7–9].
Pulmonary system is the most commonly affected organ, and therefore, the main clinical manifestations of COVID-19 are respiratory signs, including cough, dyspnea, sore throat, and fever [10]. In the severe COVID-19 state, development of pneumonia with acute respiratory distress syndrome (ARDS), hypoxic respiratory failure, and/or death have been occurred. On the other hand, extrapulmonary organ involvement (e.g., cardiac, neurologic, endocrine, gastrointestinal, hepatic, renal, ocular, and dermatologic) associated with loss of smell or taste has significant health threat [10, 11].
Mutations of SARS-CoV-2 virus and new classification
Mutations have been occurred in all viruses, including SARS-CoV-2 over time. While most mutations have scanted or no influence on the viruses’ properties, some mutations may influence on the virus’s properties, such as speed of spread, disease severity, performance of vaccines, diagnostic tools, therapeutic medicines, or other public health measures (https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/). Accordingly, three classes of SARS-CoV-2 variants were developed, including variants of concern (VOC) (B.1.1.7 (Alpha), B.1.351 (Beta), B.1.617.2 (Delta), and P.1 (Gamma)), variants of interest (VOI) (B.1.525 (Eta), B.1.526 (Iota), B.1.617.1 (Kappa) and C.37 (Lambda)), and variant of high consequence (VOHC)). VOC has increase transmissibility, severity of disease, reduced effectiveness of diagnosis, treatment, and vaccination. VOI has an increased prevalence rate. There is no VOHC till now. Evidence suggests that VOHC has the following properties, including failure diagnostics, significant reduction in vaccine effectiveness, reduced susceptibility to approved therapeutics, and more severe clinical disease and hospitalization rate (https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-info.html#Consequence). There are some differences between the symptoms of the new variants of SARS-CoV-2 with the typical symptoms of COVID-19. For instance, the symptoms of the Delta variant in the UK were akin to a heavy cold; instead, the typical symptoms of the COVID-19, such as loss of taste and smell and shortness of breath, were less prevalent [12].
COVID-19 and the cytokine storm syndrome
Hyperinflammation and cytokine storm syndrome (CSS) are among the major causes of acute respiratory distress syndrome (ARDS), multiorgan failure, and death due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection [13–15]. An unexpected increase in the circulating levels of proinflammatory cytokines, especially interleukin-1 (IL-1), IL-6, gamma interferon (IFNγ) and tumor necrosis factor-α (TNF-α), leads to hyperinflammation and the CSS [16]. When CSS occurs, different immune cells (e.g., neutrophils, macrophages, and T cells) infiltrate into the site of infection, and consequently, a cascade of damage of the vascular barrier, diffuse alveolar damage and lung injury, multiorgan failure, and ultimately death occurs [17]. ARDS is one of the major consequences of CSS that directly link to severe sequel and mortality in acute COVID-19 patients [13, 16]. Therefore, therapeutic strategies to mitigate the risk of CSS and ARDS are essential.
Immunosuppressive therapy as a treatment option for COVID-19
Immunosuppressive therapy has been used to mitigate hyperinflammation and cytokine storm syndrome (CSS) in COVID-19 patients [18]. Administration of dexamethasone among hospitalized patients with COVID-19 resulted in lower 28-day mortality in patients who received oxygen or invasive mechanical ventilation [19]. The results of a recent meta-analysis revealed that administration of systemic corticosteroids (e.g., dexamethasone, hydrocortisone, and methylprednisolone) was associated with lower 28-day all-cause mortality compared with usual care or placebo [20]. Other studies reported the usefulness of Janus kinase (JAKs) inhibitors and inflammatory cytokine blockers, including IL-6, IL-1, TNFα, and granulocyte–macrophage colony-stimulating factor (GM-CSF) for CSS in COVID-19 patients (Table 1). Taken together, immunosuppressive therapy has shown to have an important therapeutic option for patients with severe COVID-19.
Table 1.
A snapshot on immunosuppressive agents that were used for “cytokine storm syndrome” in severe cases of COVID-19
| Agents | Drug names |
|---|---|
| Corticosteroids [20] |
Hydrocortisone [20] Methylprednisolone [20] |
| JAKs inhibitors [200] |
Tofacitinib [200] |
| IL-6 blockers | Tocilizumab [185, 186, 213] |
| IL-1 blockers | |
| TNF-α blockers | Infliximab [201, 202] |
| GM-CSF blockers | Mavrilimumab [207] |
JAKs: Janus kinases, IL: Interleukin, TNF-α: tumor necrosis factor-α, GM-CSF: granulocyte–macrophage colony-stimulating factor
Immunosuppressive therapy as a risk factor for opportunistic infection
Immunosuppressive therapy has been used for a number of autoimmune diseases to alter their immune status (Supplementary Table 1). However, it is an important risk factor for opportunistic infection [21, 22]. Previous studies among patients with Inflammatory Bowel Disease (IBD) revealed that the use of corticosteroids, infliximab, and azathioprine/6-mercaptopurine was significantly increased the odds of opportunistic infection (OR = 3.4, OR = 4.4, and OR = 3.1, respectively) [22]. As reviewed elsewhere, a number of opportunistic pathogens (e.g., Aspergillus spp., Candida spp., Cryptococcus neoformans, Pneumocystis jiroveci (carinii), mucormycosis, Cytomegalovirus (CMV), Herpes simplex virus (HSV), Strongyloides stercoralis, Mycobacterium tuberculosis, and Toxoplasma gondii) have been reported from patients who received immunosuppressive therapy for their underlying diseases, such as IBD, systemic lupus erythematosus (SLE), and rheumatoid arthritis (RA) [21] (Table 2).
Table 2.
A snapshot on most common opportunistic infections following immunosuppressive therapy
| Agents | Opportunistic infections |
|---|---|
| Corticosteroids |
Cryptococcosis: cutaneous infection [214, 215], meningitis [216, 217], disseminated infection [218], pneumonia [219] Pneumocystis jiroveci (carinii) pneumonia [220, 221] Candidiasis: oral candidiasis [226], esophageal candidiasis [227], disseminated [228] Mucormycosis: pulmonary [229, 230], disseminated [230], cutaneous [231, 232], gastric perforation [233], maxillary sinus [234] Strongyloidiasis: Severe [235–238], disseminated [239, 240], cerebral involvement [239], pulmonary strongyloidiasis [241], hyperinfection [242] Tuberculosis (pulmonary) [243] Cytomegalovirus (CMV) infection [244, 245] Toxoplasmosis: ocular toxoplasmosis [154–156], cerebral toxoplasmosis [157, 158] |
| JAKs inhibitors |
Toxoplasmosis: cerebral toxoplasmosis [159], toxoplasmic retinitis [160] Cryptococcosis: pneumonia and pulmonary infection [246–248], meningoencephalitis [249], meningitis [250] Aspergillosis: retinal necrosis [251] Tuberculosis (pulmonary) [252–255] CMV retinitis [256] |
| IL-6 blockers |
Cryptococcosis: disseminated [190], meningitis [191] Aspergillosis [192] Tuberculosis (pulmonary) [194] CMV pneumonitis [193] |
| IL-1 blockers | Aspergillosis [257] |
| TNF-α blockers |
Toxoplasmosis: toxoplasmic retinochoroiditis [161, 162], cerebral toxoplasmosis [163] Cryptococcosis: pneumonia and pulmonary infection [258, 259], disseminated infection [260, 261], meningitis [262, 263], cutaneous infection [264] Pneumocystis jiroveci (carinii) pneumonia [265–268] Aspergillosis: allergic bronchopulmonary [269], pulmonary [270], sinus aspergillosis [271], central nervous system aspergillosis [272], disseminated [273] Candidiasis: systemic candidiasis [274], arthritis [275], oral candidiasis [276] Mucormycosis: cutaneous [277], gastric perforation [233], disseminated [278], sinusitis [279], pulmonary [280] Strongyloidiasis: hyperinfection [281] |
| IL-17A blockers (Ixekizumab) | Toxoplasmosis: lymphadenopathy [164] |
COVID-19-associated opportunistic infections
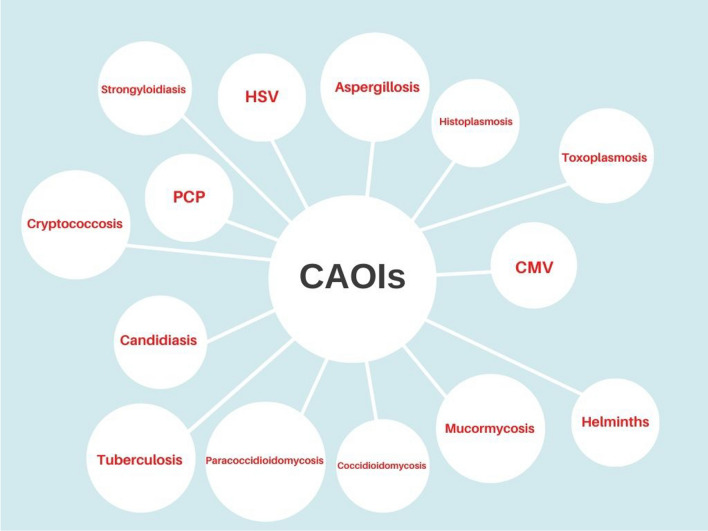
The increased case reports of opportunistic infections in COVID-19 patients raise an important concern, especially for patients with underlying diseases and who received immunosuppressive therapy. Among the opportunistic infections, fungal infections account for the most case reports in COVID-19 patients. Other reported pathogens were related to viral, bacterial, protozoa, and helminth infections (Fig. 1). This narrative review is a snapshot about the main opportunistic infection among COVID-19 patients.
Fig. 1.
A snapshot on the CAOIs. Created by https://www.canva.com/
Fungal infections
Fungal infections are among the major infections among immunocompromised patients [23]. A sharp increase in the incidence and mortality rates of fungal infections has been reported among COVID-19 patients, especially those who received immunosuppressive therapies or who have underlying conditions [24–28].
Aspergillosis
Aspergillosis is caused by a common mold, Aspergillus spp., that lives indoors and outdoors. Most people are exposed to Aspergillus spores and breathe them every day without getting sick; however, individuals with compromised immune systems or lung diseases have a higher risk of developing active infection [23, 29, 30]. Until the emergence of COVID-19, a number of cases with COVID-19-associated pulmonary aspergillosis (CAPA) have been reported worldwide [31–36]. Invasive pulmonary aspergillosis (IPA) is associated with high mortality rates among COVID-19 patients [37, 38]. The results of a multicentric retrospective cohort in France revealed that the overall mortality rate among COVID-19 patients with and without IPI was 71.4% versus 36.8%, p < 0.01. Treatment with azithromycin as well as with high-dose dexamethasone (which both of them have immunomodulatory properties) increased susceptibility to IPA among COVID-19 patients [39]. According to a recent review [31], data from 186 cases of CAPA were gathered worldwide. The data revealed that 97.8% (182) of the patients with CAPA were admitted to the intensive care unit (ICU), for ARDS (180; 96.8%) or mechanical ventilation (175; 94.1%). The overall mortality rate was 52.2% (97); which 33.0% of them were attributed to CAPA. The common underlying conditions included corticosteroid use (98; 52.7%), chronic cardiovascular disorders (94; 50.5%), renal disease (74; 39.8%), diabetes mellitus (64; 34.4%), obesity (47; 25.3%), chronic pulmonary disorders (40; 21.5%), and hematologic or oncologic disease (21; 11.3%) [31]. Collectively, the most important risk factors of CAPA were reported corticosteroid use and the presence of comorbidities [31, 38, 39]. Unfortunately, there is not an optimal diagnostic algorithm for diagnosing CAPA until now [38]. The most promising diagnostic modality for CAPA was included recovery of Aspergillus spp. on culture media of bronchoalveolar fluid (BALF) and tracheal aspirate and conventional Galactomannan (GM) testing from BALF [38]. Utilizing Aspergillus PCR and serologic biomarker testing are also useful and sensitive diagnostic tests for CAPA [38]. The first-line treatment of IPA is the approved drug voriconazole. Posaconazole is also approved for prophylaxis against invasive fungal infections [40]. Among the CAPA, patients who received voriconazole had a higher survival rate [35]. However, drug–drug interactions are the major challenge of voriconazole in the ICU setting [38, 41, 42]. Other treatments such as mold-active triazole and amphotericin B (AmB) have been used for CAPA as well [37]. Taken together, early diagnosis and treatment are the major challenges of aspergillosis among COVID-19 patients.
Candidiasis
Candida spp. is the second most numerous fungal infection worldwide [23]. The fungi are normally living on the skin, as well as inside the body, such as the throat, mouth, gut, and vagina. Oropharyngeal candidiasis is one of the most common infections in immunocompromised people, such as HIV/AIDS. Invasive candidiasis and candidemia are a serious and common infection in hospitalized patients (https://www.cdc.gov/fungal/diseases/candidiasis/invasive/index.html). C. albicans is the most common Candida species; however, the emerging species such as C. auris is a highly invasive and multidrug-resistant yeast causing complicated conditions [43, 44]. COVID-19-associated candidiasis (CAC) has been reported in a number of cases until now [43], and C. auris was the most reported species [43, 45–48]. Other reported Candida species were C. albicans, C. tropicalis, C. parapsilosis, C. orthopsilosis, and C. glabrata among COVID-19 patients [43, 49]. The coinfection of C. auris and COVID-19 in critically ill patients has been related to an above 50% mortality rate [50, 51]. Prolonged ICU stay, central venous catheters, and corticosteroid use were among the major risk factors for candidiasis in COVID-19 patients [43]. The diagnosis of invasive candidiasis remains challenging, mainly due to the low number of Candida in infected tissues or circulation [43]. Serologic tests with β-D-Glucan (BDG) and mannan antigen as well as molecular platforms are recommended tests for diagnosis of candidiasis [52–54]. In COVID-19 patients, the treatment of invasive candidiasis is similar to that of non-COVID-19 patients [43]. Echinocandins that are usually well tolerated are the choice drugs for invasive candidiasis, while voriconazole, fluconazole, liposomal amphotericin B, posaconazolem, and isavuconazole are the second line of treatment [43]. Early diagnosis and treatment of CAC are important for management of the disease; however, multidrug-resistant species are a major challenge for CAC management.
Mucormycosis
Mucormycosis is an invasive fungal infection caused by species belonging to Mucorales. Classically, immunosuppressive conditions such as uncontrolled diabetes mellitus (DM), neutropenia, and corticosteroid therapy are the major risk factors for mucormycosis [55]. Inhalation of spores is the main route of infection that usually causes pulmonary infection; however, cutaneous and soft tissue invasion primarily occurs after skin disruption because of traumatic injuries, surgery, or burns [55]. Rhino-orbito-cerebral infection classically develops in patients with uncontrolled DM. Gastrointestinal mucormycosis is a rare manifestation of the infection, which is mostly reported in neonates [55]. The mortality rates of mucormycosis varied from 40 to 80%, depending on the site of infection and underlying conditions [55]. The global prevalence of mucormycosis is ranging from 0.005 to 1.7 per million, while its prevalence is about 0.14 per 1000 in India [56, 57]. Diabetes mellitus, ketoacidosis, hematological malignancies, glucocorticoid use, or broad-spectrum antibiotics, transplantation, prolonged neutropenia, trauma, iron overload, illicit intravenous drug use, neonatal prematurity, and malnourishment are among the main predisposing factors of mucormycosis [58, 59]. Diagnosis of mucormycosis consists of clinical diagnosis, imaging modalities alongside with histopathology, cultures, and molecular techniques [59].
High-dose liposomal amphotericin B is the first-line treatment of mucormycosis, while isavuconazole (intravenous) and posaconazole (intravenous or delayed release tablets) are recommended with moderate strength [55].
COVID-19-associated mucormycoses (CAM) have been reported in a number of cases worldwide [60–63]. According to a recent systematic review, 101 cases (82 cases from India and 19 from the rest of the world) of CAM have been reported worldwide till now [60]. The infection was predominantly reported in males (78.9%) and in COVID-19 patients who had active (59.4%) or recovered (40.6%) infection. The most common clinical presentation of CAM was invasive infection in the nose and sinuses (88.9%), followed by rhino-orbital (56.7%) involvement. Preexisting DM was recorded in 80% of the cases, and corticosteroid therapy was seen in 76.3% of cases. The mortality rate was 30.7% of the cases [60]. The general management for mucormycosis included hyperglycemia control, early treatment with liposomal amphotericin B, and surgery [62], although treatment with amphotericin B and isavuconazole had only about 50% clinical improvement in CAM cases (reviewed in [58]). The major challenge of CAM is the late diagnosis of the disease and consequently treatment failure. Hence, early diagnosis of mucormycosis is of utmost importance in COVID-19 patients.
Cryptococcosis
Cryptococcus neoformans is an encapsulated yeast that causes subclinical infection in immunocompetent individuals. Humans can become infected by breathing fungal spores, and so, pulmonary infection is the major form of the disease. Corticosteroid or immunosuppressive therapy, DM, and malignancies are among the major risk factors of cryptococcosis [64]. Severe infections, cryptococcemia, meningoencephalitis, and pulmonary cryptococcosis have been reported in immunocompromised patients [23, 64, 65]. Serologic methods based on antigen screening and advanced molecular methods are sensitive and specific tests for the diagnosis of cryptococcosis [66, 67]. Antifungal agents, including amphotericin B, fluconazole, and 5-flucytosine, are the current approved treatment of cryptococcosis [68]. Till now, some cases of Cryptococcus infection were reported in COVID-19 patients [65, 69, 70], which one of them was a 60-year-old man who received tocilizumab plus corticosteroids and diagnosed with candidemia and cryptococcemia [65]. Despite antifungal therapy, the patient died within 10 days of cryptococcemia [65]. Meningoencephalitis due to C. neoformans was reported in a 73-year-old woman who received azithromycin and dexamethasone after COVID-19 infection [70]. Other cases were reported from COVID-19 patients with several underlying diseases who received immunosuppressive therapy (e.g., corticosteroids and tocilizumab) [24, 71–73]. Like other fungal infections, early detection and treatment can improve the outcome of infection in COVID-19 patients.
Pneumocystis pneumonia (PCP)
Pneumocystis jirovecii is another common respiratory opportunistic pathogen in individuals with immunocompromising conditions, which causes pneumocystis pneumonia (PCP). PCP spreads from person to person through the air. Some healthy adults have the fungus in their lungs asymptomatically, and they can spread the infection to other individuals. The choice treatment for PCP is trimethoprim/sulfamethoxazole (TMP/SMX), which also known as co-trimoxazole (https://www.cdc.gov/fungal/diseases/pneumocystis-pneumonia/index.html#symptoms). Coinfection of PCP and COVID-19 has been reported in some patients with COVID-19 till now [74–87], in which some of them received immunosuppressive therapies, such as corticosteroids [76, 81, 88, 89] and tocilizumab [88]. The first confirmed case of PCP and COVID-19 coinfection was diagnosed by autopsy and real-time PCR in a 52-year-old male chronic smoker and drinker with severe dyspnea. The patient and 17 h postdiagnosis [90]. Chong et al. [85] reviewed 12 cases of COVID-19 and PCP coinfection. Accordingly, all PCP infections were reported in critically ill patients with COVID-19, which most of them were immunosuppressed with HIV (58.3%, 7/12) or long-term exposure to immunosuppressants (91.7%, 11/12), such as high-dose corticosteroids. In both HIV and non-HIV patients, severe lymphocytopenia (< 1000 cells/ mm3) was encountered with absolute lymphocyte count (ALC) less than 900 and CD4+T cell count less than 200 cells/mm. Another laboratory finding among coinfected patients was elevation of serum lactate dehydrogenase (LDH) and β-D-glucan. The diagnostic samples were lower respiratory tract (LRT) specimens, including sputum, BAL, and endotracheal aspirate (ETA). The diagnosis was made by microscopic staining, immunofluorescence microscopy (IFM), or molecular methods. Sulfamethoxazole–trimethoprim are the most used therapy of the patients. The mortality rate was 42.9% (3/7) in the HIV group and 40% in non-HIV group [85]. Diagnosis of COVID-19 and PCP coinfection is challenging because there are clinical and radiological similarities between the two diseases [90], while, in some cases, PCP was misdiagnosed as COVID-19 in patients with underlying diseases [89, 91–94]. In these cases, differential diagnosis by laboratory tests should be considered alongside with clinical and radiological findings.
Endemic fungal infections
To date, scarce information is available regarding the coinfection of endemic fungal infections with CIVID-19. Histoplasmosis caused by Histoplasma capsulatum was reported in five cases of COVID-19 from Brazil [95–97] and Argentina [98, 99] in which three of them were HIV positive as well [96, 98, 99]. All cases had pulmonary infections and two of them developed disseminated histoplasmosis [98, 99]. All patients were successfully treated with the antifungal itraconazole.
Pulmonary coccidioidomycosis caused by Coccidioides immitis was reported in three cases of COVID-19 from the US. The infection was occurred in two Hispanic males (48 and 52 years) [100, 101] and a 48-year-old Hispanic female [102]. Two of them received dexamethasone before developing coccidioidomycosis [101, 102]. A 52-year-old male was died before receiving antifungals [101], while other two patients were treated with fluconazole that resulted in clinical improvement [100, 102].
Paracoccidioidomycosis coinfected with COVID-19 was reported in a 19-year-old Brazilian male with abdominal distension, disseminated cutaneous lesions, and multiple cervical, axillary, and inguinal lymph node enlargements [103]. The patient was treated with Amphotericin B lipid complex, but later developed a bloodstream infection that empirically received piperacillin–tazobactam [103].
Bacterial infections
Bacterial coinfections are associated with increased morbidity and mortality rates among patients with respiratory viral infections, including influenza and COVID-19 [104–106]. To date, numerous studies have been reported the association of COVID-19 with bacterial infections; many of them are classified as nosocomial or hospital-acquired infections. In this regard, the results of a living rapid review and meta-analysis revealed that bacterial coinfection and secondary bacterial infection were detected in 3.5% (95%CI 0.4–6.7%) and 14.3% (95%CI 9.6–18.9%) of COVID-19 patients, respectively. Critically ill patients reported to have more bacterial infection (8.1%, 95%CI 2.3–13.8%) [107]. A prospective cohort study analyzed data from 48 902 COVID-19 inpatients from 260 hospitals in England, Scotland, and Wales. The results have shown that the most common pathogens causing respiratory coinfections were Staphylococcus aureus and Haemophilus influenzae, which diagnosed ≤ 2 days after admission. S. aureus and Enterobacteriaceae were also the most common secondary respiratory infections. As such, the most frequent bloodstream infections were caused by Escherichia coli and S. aureus [108]. A retrospective study among 257 laboratory-confirmed COVID-19 patients in China (Jiangsu Province) revealed coinfection with 24 respiratory pathogens among the patients; Streptococcus pneumoniae, followed by Klebsiella pneumoniae and H. influenzae, was the most common bacterial coinfections [109]. A study among COVID-19 patients who admitted to ICU in Iran revealed that all patients had bacterial coinfections, including Acinetobacter baumannii (90%) and S. aureus (10%) strains. Antibiotics resistance was found in 17% of A. baumannii isolates. Methicillin-resistant S. aureus (MRSA) was detected in an isolate [110]. Mycoplasma pneumoniae, Pseudomonas aeruginosa, and Legionella pneumophila are other important bacterial pathogens that were detected among COVID-19 patients [104].
Tuberculosis
Tuberculosis (TB) remains as a significant public health problem worldwide. According to the estimations, a quarter of the world’s human population has latent TB infection [111]. Like COVID-19, TB can spread through the air from an infected person to others. The primary site of TB infection is the lungs; however, extrapulmonary infection is common, especially among immunocompromised patients [112]. Coinfection of TB with COVID-19 has been reported in a number of studies [113–117]. There are some similarities between the clinical symptoms of TB and COVID-19; hence, misdiagnosis of both infections has been reported [118]. However, studies have shown that TB infection can worsen outcome of the COVID-19 disease, leading to increased severity and mortality [114]. According to a recent meta-analysis, coinfection of M. tuberculosis with SARS-CoV-2 infection resulted in two times increased risk of mortality among COVID-19 patients (RR = 2.10; 95% CI, 1.75–2.51; I2 = 0%) [119]. Hence, strategies for prevention, screening, and treatment of TB should be considered in patients who suspected to COVID-19 infection.
Viral infections
Respiratory viral pathogens, including Influenza, Parainfluenza, Metapneumovirus, and Rhinovirus, were reported among COVID-19 patients in different studies [120–122]. However, some infections such as CMV and HSV are opportunistic pathogens that could reactivate after immunosuppression.
Cytomegalovirus (CMV)
CMV is a latent infection in immunocompetent individuals. The seroprevalence rate of CMV accounts about 80% in the elderly. CMV has been shown to manipulate the immune system by affecting T cell proliferation, decreasing in naïve T cell diversity, and increasing inflammatory cytokines (e.g., IL-6), leading to aging of the immune system (also called immunosenescence) [123, 124]. CMV infection has several sequels, mainly retinitis, pneumonitis, encephalitis/myelitis, neuropsychiatric disorder, and intrauterine infection [125–127]. Ganciclovir is an effective treatment option for CMV infection, especially for immunosuppressed individuals [128, 129]. To date, disseminated CMV infection [130], CMV myocarditis [131], CMV proctitis [132], pneumonitis [133, 134] as well as CMV viremia [135, 136] were reported among COVID-19 patients, in which some of them received corticosteroids [130, 132–136] or tocilizumab [135]. While CMV is latent in most individuals, reactivation of latent infection can lead to severe infections with fatal outcomes following immunosuppression in COVID-19 patients. Therefore, differential diagnosis and treatment should be considered in these patients.
Herpes simplex virus (HSV)
HSV is a contagious virus that categorizes into two types, including HSV-1 or oral herpes and HSV-2 or genital herpes. HSV-1 is generally responsible for cold sores and fever blisters around the mouth and lips; it can also cause genital herpes in some cases. HSV-2 is primarily causing sores around the genitals or rectum. HSV can be transmitted by direct contact. HSV-1 is transmitted through contact with oral secretions or sores on the skin and HSV-2 during sexual intercourse [137]. Sensory neurons are the main targets of HSV. Both HSV-1 and HSV-2 establish latent infections, and reactivation occurs after stress or immunosuppression [137]. Aciclovir is the standard therapy for HSV infections, which helps symptom control, but it could not eliminate the latent infection [137]. Reactivation of latent HSV and CMV infections has been reported among COVID-19 patients [138]. Seeßle et al. [139] found a high rate of HSV-1 reactivation (83.3%, 15 out of 18 patients) in invasively ventilated COVID-19 patients. Encephalitis due to HSV-1 was reported in a 73-year-old woman who suspected to prior COVID-19 infection [140]. Conjunctivitis was also reported in a 69-year-old male with COVID-19 infection [141]. Five cases of HSV-1 keratitis were reported in COVID-19 patients in Slovakia [142]. Fatal cases of acute liver failure due to HSV-1 infection were reported in two COVID-19 patients following tocilizumab and methylprednisolone prescriptions [143]. Coinfection of HSV and HIV infection [144] as well as with HSV and varicella zoster virus (VZV) infections [145] was reported in critically ill patients with COVID-19. Varicella-like exanthem was reported among 22 patients with COVID-19 in Italy [146]. While HSV has diverse symptoms and sequels, differential diagnosis and management of the infection could mitigate the outcome of COVID-19 patients.
Protozoa infections
Parasitic protozoa are a large group of unicellular pathogens, including blood and tissue protozoa (e.g., malaria, Leishmania, Toxoplasma, Trypanosoma, etc.) and intestinal and luminal protozoa (e.g., Entamoeba, Giardia, Cryptosporidium, Cyclospora, Blastocystis, Trichomonas vaginalis, etc.) [147]. However, intestinal protozoa and toxoplasmosis are an important opportunistic infections because their infections are usually latent or asymptomatic in healthy individuals, but severe infections could occur in immunocompromised patients [148, 149]. Reactivation of latent toxoplasmosis causes severe sequel in HIV/AIDS and immunocompromised patients (see the next subtitle) [149]. Intestinal protozoa can modulate immune responses through their direct interaction with host cells or by changing the gut microbiome composition [147]. To date, scarce information is available regarding the interaction of intestinal protozoa and COVID-19. Hence, there is an opportunity for researchers to investigate about the interaction of intestinal protozoa and COVID-19 in the clinical setting and experimental models. Nevertheless, opportunistic protozoa infection should not be neglected among COVID-19 patients, especially in endemic regions and in tropical and subtropical areas.
Toxoplasmosis
Like the latent CMV infection, toxoplasmosis is a latent infection in immunocompetent individuals, but reactivation of latent toxoplasmosis or acquired toxoplasmosis among immunocompromised patients can cause severe infections with various sequels, such as toxoplasmic encephalitis, disseminated infections, and death [150–153]. Combination of pyrimethamine and sulfadiazine is the choice drug for toxoplasmosis treatment among nonpregnant women [149]. Reactivation of latent toxoplasmosis has been reported in a number of patients who received immunosuppressive therapy, such as corticosteroids [154–158], JAKs inhibitors [159, 160], TNF-α blockers [161–163] and IL-17A blockers [164], and the most common sequels were ocular [154–156, 160–162] and cerebral [157–159, 163] toxoplasmosis. In a case–control study among 100 COVID-19 patients in Egypt, a significantly higher prevalence of toxoplasmosis was detected among mild to moderate COVID-19 patients than the healthy control group [165]. Indeed, T. gondii seropositive cases exhibited a significant increase in lymphocytic expression of programmed death-1 (PD-1), which is a marker that correlates with proinflammatory status. Due to the high prevalence rates of latent toxoplasmosis in humans, reactivation of latent infection should be considered following immunosuppressive therapy in COVID-19 patients [166].
Helminth infections
Helminths are among the major neglected tropical diseases (NTDs) worldwide. Most of the helminth infections are causing chronic infections with minor clinical symptoms [167, 168]. Nevertheless, helminth infections could alter the immune system toward type 2 immunity and anti-inflammatory conditions, leading to diminished the essential immune response against microbial pathogens and increased susceptibility and severity of infectious diseases [167–169]. Helminths can also cause malabsorption and compete for host nutrients [169]. Helminth infections can also mitigate vaccine efficacy by suppressing immune responses [169]. The outcome of COVID-19 patients may be worsened in individuals with helminth coinfections [169–171]. Further investigations are needed to demonstrate the interaction between helminths and SARS-CoV-2 infection.
Strongyloidiasis
Strongyloidiasis is a neglected tropical infection caused by the soil-transmitted helminth Strongyloides stercoralis. Strongyloidiasis is usually asymptomatic in immunocompetent individuals, while disseminating infection and hyperinfection syndrome are important sequels among immunocompromised patients [172, 173]. The infected stage of the parasite is the filariform larva, which can penetrate into the human skin when it contacts with contaminated soil; then, the larva migrates to the small intestine and transforms to the adult worm. Alternatively, consumption of raw vegetables contaminated with filariform larva could be a source of infection. Internal autoinfection is common, leading to hyperinfection syndrome. Disseminated strongyloidiasis usually occurs in patients with immunocompromising conditions leading to complicated conditions [172, 173]. Ivermectin is the choice drug for treatment of strongyloidiasis; also, it could be used as a preventive strategy for high-risk populations (e.g., HIV/AIDS and immunocompromised individuals) [174]. Disseminated strongyloidiasis is reported in two patients with COVID-19 up until now [175, 176], in which both of them received immunosuppressive agents (dexamethasone [176], methylprednisolone, and tocilizumab [175]). Therefore, patients who received immunosuppressive therapy are considered as a high-risk group and should be screened for S. stercoralis, especially in tropical areas in which the infection is high prevalence.
Discussion
Most of the opportunistic infections have no or at least minor clinical symptoms among immunocompetent individuals. Nevertheless, these infections have several sequels among immunocompromised patients, such as patients with cancer and malignancies [177–179], organ recipients' individuals [180], patients with autoimmunity who received immunosuppressive therapies [22] and HIV/AIDS patients with low CD4 T cell counts [21, 181]. The CSS and ARDS are among the major causes of multiorgan failure and death due to COVID-19 [182, 183]. Hence, immunosuppressive therapy with corticosteroids [19, 20, 184] and monoclonal antibodies has been used to mitigate the CSS in critically ill COVID-19 patients (Table 1). Administration of dexamethasone in hospitalized patients with COVID-19 resulted in a significant increase in the number of ventilator-free days [184] and a lower mortality rates [19] over 28 days. The results of a meta-analysis have shown that administration of systemic corticosteroids (e.g., dexamethasone, hydrocortisone, and methylprednisolone) in comparison with usual care or placebo was associated with in lower 28-day all-cause mortality [20]. Previous reports revealed that corticosteroid use increases the risk of different opportunistic infections (Table 2). A number of opportunistic infections have been reported among COVID-19 patients who received immunosuppressive therapies (Table 3). For instance, CAPA and CAM were associated with corticosteroid use in 52.7% [31] and 76.3% [60] of COVID-19 patients, respectively. Tocilizumab is an IL-6 blocker that is used in a number of trials for COVID-19 patients [185]. The results of two randomized, double-blind, placebo-controlled trials revealed that tocilizumab was associated with a reduced likelihood of mechanical ventilation or death in hospitalized patients with COVID-19 [186], but was not effective for prevention of intubation or death in moderately ill patients with COVID-19 [187]. The results of a meta-analysis demonstrated that administration of tocilizumab to standard therapy was associated with reduced need for mechanical ventilation and mortality rate in patients with severe COVID-19 [185]. IL-6 is produced in response to infections and tissue damage [188], although IL-6 inhibition was associated with an increased risk of secondary bacterial and fungal infections [189]. Likewise, disseminated cryptococcosis [190], meningitis [191], invasive aspergillosis [192], CMV pneumonitis [193], and tuberculosis [194] were reported in patients who received tocilizumab (Table 2). In COVID-19 patients, different cases with cryptococcosis [24, 65, 71–73], aspergillosis [88], strongyloidiasis [175], PCP [88], CMV [135], and HSV [143] infections were reported following tocilizumab use (Table 3). Inhibition of the JAKs pathway (ruxolitinib [195–197], baricitinib [198, 199], and tofacitinib [200]), TNFα (infliximab [201, 202]), IL-1 (Anakinra [203, 204] and Canakinumab [205, 206]), and GM-CSF (Mavrilimumab [207]) was also used for mitigating the CSS in COVID-19 patients (Table 1). These immunomodulatory agents can also increase the risk of opportunistic infections (Table 2), although there are not reported in COVID-19 patients till now.
Table 3.
Opportunistic infections in COVID-19 patients who received immunosuppressive therapying
| Agents | Opportunistic infections |
|---|---|
| Corticosteroids |
Candidiasis [43] Mucormycosis [60] Cryptococcosis [24, 65, 71–73] Pneumocystis jiroveci (carinii) pneumonia [76, 81, 88, 89] Histoplasma capsulatum [95, 96, 98] Coccidioides immitis [101, 102] HSV [143] |
| IL-6 blocker (Tocilizumab) |
Cryptococcosis [24, 65, 71–73] Pneumocystis jiroveci (carinii) pneumonia [88] Aspergillosis [88] Strongyloidiasis: [175] CMV: [135] HSV: [143] |
Some opportunistic infections are associated with alterations of the immune system, such as CMV [123] and helminth infections [167, 169]; hence, coinfection with these pathogens could mitigate essential immune responses against SARS-CoV-2 and probably diminish the immune response following vaccination [169].
Early detection and differential diagnosis of opportunistic infections are an important point and challenging issue in COVID-19 patients. Early detection can lead to early treatment and consequently lower sequel and better outcome of COVID-19 patients. Differential diagnosis should be considered especially for opportunistic respiratory infections, such as PCP [89, 91–94]. These infections could be misdiagnosed with COVID-19 in some cases due to some clinical and paraclinical similarities. In the COIVD-19 period, the diagnostic procedures are diverted to COVID-19. Although vigilance is needed in the diagnosis of COVID-19, the common disorders should not be negligible [208].
Reactivation of latent infection following immunosuppression is the major cause of severe infections in some opportunistic infections, such as HSV, CMV, TB, and toxoplasmosis [21, 149]. Prophylaxis with TMP-SMX can reduce the risk of some opportunistic infections, such as PCP, TB, toxoplasmosis, and different bacterial infections [21, 209, 210]. Hence, prophylactic strategies may be useful for COVID-19 patients, especially for those who received immunosuppressive therapy or who have underlying diseases.
Drug–drug interaction is a challenge for treatment of opportunistic infections in COVID-19 patients, especially those who hospitalized in ICU [211]. For example, drug–drug interactions are the major challenge of voriconazole in the ICU setting [38, 41, 42]. Concentrations of itraconazole and clindamycin are increased by lopinavir/ritonavir, while voriconazole, posaconazole, and clarithromycin increase lopinavir/ritonavir concentration [212]. Hence, the possibility of drug–drug interaction should consider when prescribing new medications in COVID-19 patients.
Mutations of the SARS-CoV-2 virus are an emerging challenge that could be associated with reduced effectiveness of diagnosis, treatment, and vaccination as well as increased transmissibility and severity of the disease (https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-info.html#Consequence). In these conditions, coinfection with opportunistic pathogens could be an additional challenge for the diagnosis, treatment, and management of COVID-19 patients.
To date, some clinical studies are registered in the clinicaltrials.gov regarding opportunistic infections and COVID-19. However, it seems that future studies are needed for standard diagnosis and new treatment options of these infections in COVID-19 patients (Table 4).
Table 4.
Clinical studies that registered in the clinicaltrials.gov regarding opportunistic infections and COVID-19
| Trial/phase | Title | Setting | Status | Estimated Primary Completion Date |
|---|---|---|---|---|
| NCT04818853 | COVID-19 Associated Pulmonary Aspergillosis (CAPA) and Other Invasive Fungal Infections (IFI) | Observational: Cohort | Recruiting | March 1, 2023 |
| NCT04240886 Phase 2 | A Phase 2, Open-Label Study to Evaluate the Safety and Efficacy of APX001 in the Treatment of Patients with Invasive Mold Infections Caused by Aspergillus Species or Rare Molds | Interventional | Recruiting | July 2021 |
| NCT04368221 | Characterization of Fungal Infections in COVID-19 Infected and Mechanically Ventilated Patients in ICU | Observational: Cohort | Completed | February 23, 2021 |
| NCT04707703 Phase 3 | Isavuconazole for the Prevention of SARS-CoV-2-associated Invasive Aspergillosis in Critically-Ill Patients | Interventional | Recruiting | January 2022 |
| NCT04935463 | Mucormycosis in COVID-19 | Observational: Cohort | Enrolling by invitation | June 1, 2022 |
| NCT04813328 | A Pilot Study of the Effects of Helminth Infection and SARS-CoV-2 Seropositivity on Immune Response and the Intestinal Microbiota in India | Observational | Not yet recruiting | April 2022 |
Limitations of the study
In this review, we summarized the main opportunistic infections among COVID-19 patients. However, one of the major limitations of this review is lots of case reports or case series with various settings, diagnosis, and treatment strategies. Hence, if there was a systematic review article regarding opportunistic infections, we selected these articles instead of each case report.
Conclusions and future perspectives
Taken together, the COVID-19 patients with underlying diseases or who received immunosuppressive therapy have an increased risk of CAOIs. Strategies for screening and early diagnosis, treatment, and prophylaxis could mitigate the risk of CAOIs in COVID-19 patients. Future investigations for generation of reliable algorithms and standardization of diagnostic procedures are highly needed for early and differential diagnosis of CAOIs in COVID-19 patients. As such, standard prophylactic strategies are needed in high-risk COVID-19 patients, such as those who received immunosuppressive therapy, patients with underlying diseases, patients who admitted to the ICU, and those who received invasive mechanical ventilation.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to apologize from authors whose works have not cited in this article because of space limitations.
Abbreviations
- COVID-19
Coronavirus Disease 2019
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- CAOIs
COVID-19-associated opportunistic infections
- CSS
Cytokine storm syndrome
- ARDS
Acute respiratory distress syndrome
- VOC
Variants of concern
- VOI
Variants of interest
- VOHC
Variant of high consequence
- IL
Interleukin
- IFNγ
Gamma interferon
- TNF-α
Tumor necrosis factor-α
- JAKs
Janus kinases
- GM-CSF
Granulocyte–macrophage colony-stimulating factor
- PCP
Pneumocystis jiroveci (carinii) Pneumonia
- CMV
Cytomegalovirus
- HSV
Herpes simplex virus
- IBD
Inflammatory bowel disease
- SLE
Systemic lupus erythematosus
- RA
Rheumatoid arthritis
- DM
Diabetes mellitus
- TB
Tuberculosis
- CAPA
COVID-19-associated pulmonary aspergillosis
- ICU
Intensive care unit
- IPA
Invasive pulmonary aspergillosis
- BALF
Bronchoalveolar fluid
- GM
Galactomannan
- BDG
β-D-Glucan
- CAC
COVID-19-associated candidiasis
- CAM
COVID-19-associated mucormycosis
- TMP/SMX
Trimethoprim/sulfamethoxazole
- ALC
Absolute lymphocyte count
- ETA
Endotracheal aspirate
- IFM
Immunofluorescence microscopy
- MRSA
Methicillin-resistant Staphylococcus aureus
- NTDs
Neglected tropical diseases
Authors' contributions
AA conceived the article. AA, SF, and AK analyzed the references. AA, SF, and AK wrote the draft of manuscript. All authors revised the initial manuscript and approved its final version.
Funding
None.
Declarations
Conflict of interest
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Falahi S, Kenarkoohi A. Sex and gender differences in the outcome of patients with COVID-19. J Med Virol. 2021;93(1):151–152. doi: 10.1002/jmv.26243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falahi S, Abdoli A, Kenarkoohi A. Claims and reasons about mild COVID- 19 infection in children. New Microbes New Infect. 2021 doi: 10.1016/j.nmni.2021.100864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shams M, Basati G, Kalvandi G, Abdoli A, Tavan H. Frequency of underlying diseases, symptoms and mortality rate of COVID-19: a systematic review and meta-analysis. Reviews in Medical Microbiology. 9000. 10.1097/MRM.0000000000000262.
- 6.Kenarkoohi A, Maleki M, Ghiasi B, Bastani E, Pakzad I, Bonyadi M, et al. Prevalence and clinical presentation of COVID-19 infection in hemodialysis patients. J Nephropathol 2021;10.34172/jnp.2021.xx.
- 7.Falahi S, Kenarkoohi A. Transmission routes for SARS-CoV-2 infection: review of evidence. New Microbes New Infect. 2020;38:100778. doi: 10.1016/j.nmni.2020.100778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falahi S, Kenarkoohi A. COVID-19 reinfection: prolonged shedding or true reinfection? New Microbes New Infect. 2020;38:100812. doi: 10.1016/j.nmni.2020.100812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kenarkoohi A, Noorimotlagh Z, Falahi S, Amarloei A, Mirzaee SA, Pakzad I, et al. Hospital indoor air quality monitoring for the detection of SARS-CoV-2 (COVID-19) virus. Sci Total Environ. 2020;748:141324. doi: 10.1016/j.scitotenv.2020.141324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson KD, Harris C, Cain JK, Hummer C, Goyal H, Perisetti A. Pulmonary and extra-pulmonary clinical manifestations of COVID-19. Front Med. 2020 doi: 10.3389/fmed.2020.00526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26(7):1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burki TK. Lifting of COVID-19 restrictions in the UK and the Delta variant. The Lancet Respir Med. 2021 doi: 10.1016/s2213-2600(21)00328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang J, Jiang M, Chen X, Montaner LJ. Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: Review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J Leukoc Biol. 2020;108(1):17–41. doi: 10.1002/jlb.3covr0520-272r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lotfinejad P, Asadzadeh Z, Najjary S, Somi MH, Hajiasgharzadeh K, Mokhtarzadeh A, et al. COVID-19 infection: concise review based on the immunological perspective. Immunol Investig. 2020 doi: 10.1080/08820139.2020.1825480. [DOI] [PubMed] [Google Scholar]
- 16.Ragab D, Salah Eldin H, Taeimah M, Khattab R, Salem R. The COVID-19 cytokine storm; what we know so far. Front Immunol. 2020 doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahmoodpoor A, Nader ND. Immune responses to the novel coronavirus-2: Friend or Foe? Immunol Investig. 2020 doi: 10.1080/08820139.2020.1795191. [DOI] [PubMed] [Google Scholar]
- 18.Alijotas-Reig J, Esteve-Valverde E, Belizna C, Selva-O'Callaghan A, Pardos-Gea J, Quintana A, et al. Immunomodulatory therapy for the management of severe COVID-19. Beyond the anti-viral therapy: a comprehensive review. Autoimmun Rev. 2020;19(7):102569. doi: 10.1016/j.autrev.2020.102569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The RECOVERY Collaborative Group Dexamethasone in hospitalized patients with Covid-19. New England J Med. 2020;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis. JAMA. 2020;324(13):1330–41. 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed]
- 21.Fishman JA. Opportunistic infections–coming to the limits of immunosuppression? Cold Spring Harbor Perspect Med. 2013;3(10):a015669. doi: 10.1101/cshperspect.a015669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toruner M, Loftus EV, Harmsen WS, Zinsmeister AR, Orenstein R, Sandborn WJ, et al. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology. 2008;134(4):929–936. doi: 10.1053/j.gastro.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 23.Brown GD, Denning DW, Gow NAR, Levitz SM, Netea MG, Hidden WTC, Killers, Human fungal infections. Sci Transl Med. 2012;4(165):165rv13. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- 24.Cafardi J, Haas D, Lamarre T, Feinberg J. Opportunistic fungal infection associated with COVID-19. Open Forum Infect Dis. 2021 doi: 10.1093/ofid/ofab016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heard KL, Hughes S, Mughal N, Moore LS. COVID-19 and fungal superinfection. The Lancet Microbe. 2020;1(3):e107. doi: 10.1016/S2666-5247(20)30065-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhatt K, Agolli A, Patel MH, Garimella R, Devi M, Garcia E, et al. High mortality co-infections of COVID-19 patients: mucormycosis and other fungal infections. Discoveries. 2021;9(1):e126. doi: 10.15190/d.2021.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silva LN, de Mello TP, de Souza RL, Branquinha MH, Roudbary M, Dos Santos ALS. Fungal infections in COVID-19-positive patients: a lack of optimal treatment options. Curr Top Med Chem. 2020;20:1951–1957. doi: 10.2174/156802662022200917110102. [DOI] [PubMed] [Google Scholar]
- 28.Kula BE, Clancy CJ, Hong Nguyen M, Schwartz IS. Invasive mould disease in fatal COVID-19: a systematic review of autopsies. The Lancet Microbe. 2021 doi: 10.1016/S2666-5247(21)00091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Segal BH. Aspergillosis. N Engl J Med. 2009;360(18):1870–1884. doi: 10.1056/NEJMra0808853. [DOI] [PubMed] [Google Scholar]
- 30.Latgé J-P, Chamilos G. Aspergillus fumigatus and Aspergillosis in 2019. Clin Microbiol Rev. 2019;33(1):e00140–e218. doi: 10.1128/CMR.00140-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salmanton-García J, Sprute R, Stemler J, Bartoletti M, Dupont D, Valerio M, et al. COVID-19-associated pulmonary aspergillosis, march-august 2020. Emerg Infect Dis. 2021;27(4):1077–1086. doi: 10.3201/eid2704.204895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marr KA, Platt A, Tornheim JA, Zhang SX, Datta K, Cardozo C, et al. Aspergillosis complicating severe coronavirus disease. Emerg Infect Dis. 2021;27(1):18–25. doi: 10.3201/eid2701.202896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prattes J, Valentin T, Hoenigl M, Talakic E, Reisinger AC, Eller P. Invasive pulmonary aspergillosis complicating COVID-19 in the ICU - a case report. Med Mycol Case Rep. 2021;31:2–5. doi: 10.1016/j.mmcr.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blaize M, Mayaux J, Nabet C, Lampros A, Marcelin A-G, Thellier M, et al. Fatal invasive Aspergillosis and coronavirus disease in an immunocompetent patient. Emerg Infect Dis. 2020;26(7):1636–1637. doi: 10.3201/eid2607.201603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bartoletti M, Pascale R, Cricca M, Rinaldi M, Maccaro A, Bussini L, et al. Epidemiology of invasive pulmonary Aspergillosis among intubated patients with COVID-19: a prospective study. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lahmer T, Rasch S, Spinner C, Geisler F, Schmid RM, Huber W. Invasive pulmonary aspergillosis in severe COVID-19 pneumonia. Clin Microbiol Infect. 2020;27(1):147–148. doi: 10.1016/j.cmi.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Apostolopoulou A, Esquer Garrigos Z, Vijayvargiya P, Lerner AH, Farmakiotis D. Invasive pulmonary Aspergillosis in patients with SARS-CoV-2 infection: a systematic review of the literature. Diagnostics. 2020;10(10):807. doi: 10.3390/diagnostics10100807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arastehfar A, Carvalho A, van de Veerdonk FL, Jenks JD, Koehler P, Krause R, et al. COVID-19 associated pulmonary Aspergillosis (CAPA)—from immunology to treatment. J Fungi. 2020;6(2):91. doi: 10.3390/jof6020091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dellière S, Dudoignon E, Fodil S, Voicu S, Collet M, Oillic P-A, et al. Risk factors associated with COVID-19-associated pulmonary aspergillosis in ICU patients: a French multicentric retrospective cohort. Clin Microbiol Infect. 2021;27(5):7901.e1–e5. doi: 10.1016/j.cmi.2020.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karthaus M. Prophylaxis and treatment of invasive aspergillosis with voriconazole, posaconazole and caspofungin: review of the literature. Eur J Med Res. 2011;16(4):145–152. doi: 10.1186/2047-783x-16-4-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baniasadi S, Farzanegan B, Alehashem M. Important drug classes associated with potential drug–drug interactions in critically ill patients: highlights for cardiothoracic intensivists. Ann Intens Care. 2015;5(1):1–8. doi: 10.1186/s13613-015-0086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jenks JD, Mehta SR, Hoenigl M. Broad spectrum triazoles for invasive mould infections in adults: Which drug and when? Med Mycol. 2019;57:S168–S178. doi: 10.1093/mmy/myy052. [DOI] [PubMed] [Google Scholar]
- 43.Arastehfar A, Carvalho A, Nguyen MH, Hedayati MT, Netea MG, Perlin DS, et al. COVID-19-associated candidiasis (CAC): An underestimated complication in the absence of immunological predispositions? J Fungi. 2020;6:211. doi: 10.3390/jof6040211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Du H, Bing J, Hu T, Ennis CL, Nobile CJ, Huang G. Candida auris: epidemiology, biology, antifungal resistance, and virulence. PLoS Pathog. 2020;16(10):e1008921. doi: 10.1371/journal.ppat.1008921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prestel C, Anderson E, Forsberg K, Lyman M, de Perio MA, Kuhar D, et al. Candida auris outbreak in a COVID-19 specialty care Unit - Florida, July-August 2020. MMWR Morb Mortal Wkly Rep. 2021;70(2):56–57. doi: 10.15585/mmwr.mm7002e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Allaw F, Kara Zahreddine N, Ibrahim A, Tannous J, Taleb H, Bizri AR, et al. First Candida auris outbreak during a COVID-19 pandemic in a tertiary-care center in Lebanon. Pathogens. 2021;10(2):157. doi: 10.3390/pathogens10020157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Almeida JN, Francisco EC, Hagen F, Brandão IB, Pereira FM, Presta Dias PH, et al. Emergence of Candida auris in Brazil in a COVID-19 intensive care unit. J Fungi. 2021 doi: 10.3390/jof7030220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chowdhary A, Tarai B, Singh A, Sharma A. Multidrug-resistant Candida auris infections in critically Ill coronavirus disease patients, India, April–July 2020. Emerg Infect Dis. 2020;26(11):2694. doi: 10.3201/eid2611.203504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodriguez JY, Le Pape P, Lopez O, Esquea K, Labiosa AL, Alvarez-Moreno C. Candida auris: a latent threat to critically Ill patients with coronavirus disease 2019. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Villanueva-Lozano H, Treviño-Rangel RD, González GM, Ramírez-Elizondo MT, Lara-Medrano R, Aleman-Bocanegra MC, Guajardo-Lara CE, Gaona-Chávez N, Castilleja-Leal F, Torre-Amione G, Martínez-Reséndez MF. Outbreak of Candida auris infection in a COVID-19 hospital in Mexico. Clin Microbiol Infect. 2021;27(5):813–816. doi: 10.1016/j.cmi.2020.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Magnasco L, Mikulska M, Giacobbe DR, Taramasso L, Vena A, Dentone C, et al. Spread of carbapenem-resistant gram-negatives and Candida auris during the COVID-19 pandemic in critically Ill patients: One step back in antimicrobial Stewardship? Microorganisms. 2021;9(1):95. doi: 10.3390/microorganisms9010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clancy Cornelius J, Nguyen MH, Kraft Colleen S. Diagnosing invasive Candidiasis. J Clin Microbiol. 2018;56(5):01909–01917. doi: 10.1128/JCM.01909-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Avni T, Leibovici L, Paul M. PCR diagnosis of invasive candidiasis: systematic review and meta-analysis. J Clin Microbiol. 2011;49(2):665–670. doi: 10.1128/JCM.01602-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karageorgopoulos DE, Vouloumanou EK, Ntziora F, Michalopoulos A, Rafailidis PI, Falagas ME. β-D-Glucan assay for the diagnosis of invasive fungal infections: a meta-analysis. Clin Infect Dis. 2011;52(6):750–770. doi: 10.1093/cid/ciq206. [DOI] [PubMed] [Google Scholar]
- 55.Cornely OA, Alastruey-Izquierdo A, Arenz D, Chen SCA, Dannaoui E, Hochhegger B, et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European confederation of medical mycology in cooperation with the mycoses study group education and research consortium. Lancet Infect Dis. 2019;19(12):e405–e421. doi: 10.1016/S1473-3099(19)30312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Prakash H, Chakrabarti A. Global epidemiology of mucormycosis. J Fungi. 2019 doi: 10.3390/jof5010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Skiada A, Pavleas I, Drogari-Apiranthitou M. Epidemiology and diagnosis of mucormycosis: an update. J Fungi. 2020 doi: 10.3390/jof6040265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frías-De-León MG, Pinto-Almazán R, Hernández-Castro R, García-Salazar E, Meza-Meneses P, Rodríguez-Cerdeira C, et al. Epidemiology of systemic mycoses in the COVID-19 pandemic. J Fungi. 2021;7(7):556. doi: 10.3390/jof7070556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Skiada A, Pavleas I, Drogari-Apiranthitou M. Epidemiology and diagnosis of mucormycosis: an update. J Fungi. 2020;6(4):265. doi: 10.3390/jof6040265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Singh AK, Singh R, Joshi SR, Misra A. Mucormycosis in COVID-19: a systematic review of cases reported worldwide and in India. Diabetes Metabolic Syn Clin Res Rev. 2021 doi: 10.1016/j.dsx.2021.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ahmadikia K, Hashemi SJ, Khodavaisy S, Getso MI, Alijani N, Badali H, et al. The double-edged sword of systemic corticosteroid therapy in viral pneumonia: a case report and comparative review of influenza-associated mucormycosis versus COVID-19 associated mucormycosis. Mycoses. 2021 doi: 10.1111/myc.13256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Garg D, Muthu V, Sehgal IS, Ramachandran R, Kaur H, Bhalla A, et al. Coronavirus disease (Covid-19) associated Mucormycosis (CAM): case report and systematic review of literature. Mycopathologia. 2021;186(2):289–298. doi: 10.1007/s11046-021-00528-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ramaswami A, Sahu AK, Kumar A, Suresh S, Nair A, Gupta D, et al. COVID-19 associated Mucormycosis presenting to the emergency department – an observational study of 70 patients. QJM An Int J Med. 2021 doi: 10.1093/qjmed/hcab190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Setianingrum F, Rautemaa-Richardson R, Denning DW. Pulmonary cryptococcosis: a review of pathobiology and clinical aspects. Med Mycol. 2019;57(2):133–150. doi: 10.1093/mmy/myy086. [DOI] [PubMed] [Google Scholar]
- 65.Khatib MY, Ahmed AA, Shaat Said B, Mohamed AS, Nashwan AJ. Cryptococcemia in a patient with COVID-19: a case report. Clin Case Rep. 2021;9(2):853–855. doi: 10.1002/ccr3.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Greene G, Lawrence DS, Jordan A, Chiller T, Jarvis JN. Cryptococcal meningitis: a review of cryptococcal antigen screening programs in Africa. Exp Rev Anti Infect Ther. 2021;19(2):233–244. doi: 10.1080/14787210.2020.1785871. [DOI] [PubMed] [Google Scholar]
- 67.Perfect JR. Cryptococcosis. In: Mandell GL, Diamond RD, editors. Atlas of infectious diseases: fungal infections. London: Current Medicine Group; 2000. pp. 79–93. [Google Scholar]
- 68.Spadari CDC, Wirth F, Lopes LB, Ishida K. New approaches for Cryptococcosis treatment. Microorganisms. 2020;8(4):613. doi: 10.3390/microorganisms8040613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Passerini M, Terzi R, Piscaglia M, Passerini S, Piconi S. Disseminated cryptococcosis in a patient with metastatic prostate cancer who died in the coronavirus disease 2019 (COVID-19) outbreak. Cureus. 2020;12(5):8254–e. doi: 10.7759/cureus.8254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ghanem H, Sivasubramanian G. Cryptococcus neoformans Meningoencephalitis in an immunocompetent patient after COVID-19 infection. Case Rep Infect Dis. 2021;2021:5597473. doi: 10.1155/2021/5597473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thota DR, Ray B, Hasan M, Sharma K. Cryptococcal meningoencephalitis during convalescence from severe COVID-19 pneumonia. The Neurohospitalist. 2021 doi: 10.1177/19418744211009766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Passarelli VC, Perosa AH, de Souza Luna LK, Conte DD, Nascimento OA, Ota-Arakaki J, et al. Detected SARS-CoV-2 in Ascitic fluid followed by cryptococcemia: a case report. SN Compr Clin Med. 2020;2(11):2414–2418. doi: 10.1007/s42399-020-00574-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Woldie IL, Brown IG, Nwadiaro NF, Patel A, Jarrar M, Quint E, et al. Autoimmune hemolytic anemia in a 24-year-old patient with COVID-19 complicated by secondary cryptococcemia and acute necrotizing encephalitis: a case report and review of literature. J Med Cases. 2020;11(11):362–5. doi: 10.14740/jmc3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rubiano C, Tompkins K, Sellers SA, Bramson B, Eron J, Parr JB, et al. Pneumocystis and severe acute respiratory syndrome coronavirus 2 coinfection: a case report and review of an emerging diagnostic dilemma. Open Forum Infect Dis. 2021 doi: 10.1093/ofid/ofaa633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mang S, Kaddu-Mulindwa D, Metz C, Becker A, Seiler F, Smola S, et al. Pneumocystis jirovecii pneumonia and severe acute respiratory syndrome coronavirus 2 coinfection in a patient with newly diagnosed HIV-1 infection. Clin Infect Dis. 2020;72(8):1487–89. doi: 10.1093/cid/ciaa906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Viceconte G, Buonomo AR, Lanzardo A, Pinchera B, Zappulo E, Scotto R, et al. Pneumocystis jirovecii pneumonia in an immunocompetent patient recovered from COVID-19. Infect Dis. 2021;53(5):382–5. doi: 10.1080/23744235.2021.1890331. [DOI] [PubMed] [Google Scholar]
- 77.Jeican II, Inișca P, Gheban D, Tăbăran F, Aluaș M, Trombitas V, et al. COVID-19 and Pneumocystis jirovecii pulmonary coinfection—the first case confirmed through autopsy. Medicina. 2021;57(4):302. doi: 10.3390/medicina57040302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mouren D, Goyard C, Catherinot E, Givel C, Chabrol A, Tcherakian C, et al. COVID-19 and Pneumocystis jirovecii pneumonia: back to the basics. Respir Med Res. 2021;79:100814. doi: 10.1016/j.resmer.2021.100814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Menon AA, Berg DD, Brea EJ, Deutsch AJ, Kidia KK, Thurber EG, et al. A case of COVID-19 and Pneumocystis jirovecii coinfection. Am J Respir Crit Care Med. 2020;202(1):136–8. doi: 10.1164/rccm.202003-0766LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Coleman H, Snell LB, Simons R, Douthwaite ST, Lee MJ. Coronavirus disease 2019 and Pneumocystis jirovecii pneumonia: a diagnostic dilemma in HIV. AIDS. 2020;34(8):1258–60. doi: 10.1097/QAD.0000000000002571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alanio A, Dellière S, Voicu S, Bretagne S, Mégarbane B. The presence of Pneumocystis jirovecii in critically ill patients with COVID-19. J Infect. 2021;82(4):84–123. doi: 10.1016/j.jinf.2020.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bhat P, Noval M, Doub JB, Heil E. Concurrent COVID-19 and Pneumocystis jirovecii pneumonia in a severely immunocompromised 25-year-old patient. Int J Infect Dis. 2020;99:119–21. doi: 10.1016/j.ijid.2020.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mang S, Kaddu-Mulindwa D, Metz C, Becker A, Seiler F, Smola S, et al. Pneumocystis jirovecii pneumonia and severe acute respiratory syndrome coronavirus 2 coinfection in a patient with newly diagnosed HIV-1 infection. Clin Infect Dis. 2021;72(8):1487–9. doi: 10.1093/cid/ciaa906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Merchant EA, Flint K, Barouch DH, Blair BM. Co-infection with coronavirus disease 2019, previously undiagnosed human immunodeficiency virus, Pneumocystis jirovecii pneumonia and cytomegalovirus pneumonitis, with possible immune reconstitution inflammatory syndrome. IDCases. 2021;24:e01153. doi: 10.1016/j.idcr.2021.e01153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chong WH, Saha BK, Chopra A. Narrative review of the relationship between COVID-19 and PJP: does it represent coinfection or colonization? Infection. 2021 doi: 10.1007/s15010-021-01630-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zubair SM, Zaid HHM, Talha S. Pneumocystis jirovecii pneumonia as a sequela of COVID-19. J Biomed Res Environ Sci. 2021;2(6):425–428. doi: 10.37871/jbres1253. [DOI] [Google Scholar]
- 87.Neeraj G, Cherukuri B. An unusual case of severe Pneumocystis jirovecii pneumonia (pjp) presenting as “recurrent cytokine storm” following COVID-19 infection. Indian J Crit Care Med. 2021;25(SUPPL 1):S31–S32. [PubMed] [Google Scholar]
- 88.Cai S, Sun W, Li M, Dong L. A complex COVID-19 case with rheumatoid arthritis treated with tocilizumab. Clin Rheumatol. 2020;39(9):2797–802. doi: 10.1007/s10067-020-05234-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Barben J, Quipourt V, Vovelle J, Putot A, Manckoundia P. Not COVID-19, don’t overlook pneumocystis in patients on gefitinib! Curr Oncol. 2021;28(1):961–4. doi: 10.3390/curroncol28010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jeican II, Inișca P, Gheban D, Tăbăran F, Aluaș M, Trombitas V, et al. COVID-19 and Pneumocystis jirovecii pulmonary coinfection—the first case confirmed through autopsy. Medicina. 2021 doi: 10.3390/medicina57040302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kelly S, Waters L, Cevik M, Collins S, Lewis J, Wu M-S, et al. Pneumocystis pneumonia, a COVID-19 mimic, reminds us of the importance of HIV testing in COVID-19. Clin Med (Lond) 2020;20(6):590–592. doi: 10.7861/clinmed.2020-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Saifullah AAM, Omar MR, Bakhit NHDM, Mazian AN, Samad SZ, Sharil NS, et al. Pneumocystis jirovecii pneumonia mimicking COVID-19 pneumonia in a patient with newly diagnosed advanced HIV disease. Ulum Islamiyyah. 2021;1:107–16. doi: 10.33102/uij.vol33no1.299. [DOI] [Google Scholar]
- 93.Averyanov AV, Sotnikova AG, Lesnyak VN. Pneumocystis pneumonia mimicking COVID-19. J Clin Pract. 2020;1(2):87–92. [Google Scholar]
- 94.Schüßler M, Müller F, Rauschning D. Nicht alles Milchglas ist COVID-19–Pneumocystis jirovecii-Pneumonie als Differenzialdiagnose. DMW-Deutsche Medizinische Wochenschrift. 2021;146(09):603–7. doi: 10.1055/a-1391-4403. [DOI] [PubMed] [Google Scholar]
- 95.de Macedo PM, Freitas AD, Bártholo TP, Bernardes-Engemann AR, Almeida MD, Almeida-Silva F, et al. Acute pulmonary histoplasmosis following COVID-19: novel laboratorial methods aiding diagnosis. J Fungi. 2021 doi: 10.3390/jof7050346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Basso RP, Poester VR, Benelli JL, Stevens DA, Zogbi HE, Vasconcellos ICdS, et al. COVID-19-associated histoplasmosis in an AIDS patient. Mycopathologia. 2021;186(1):109–12. doi: 10.1007/s11046-020-00505-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stasiak CES, Nigri DH, Cardoso FR, Mattos RSdARd, Gonçalves Martins PA, Carvalho ARS, et al. Case report: incidental finding of COVID-19 infection after positron emission tomography/CT imaging in a patient with a diagnosis of histoplasmosis and recurring fever. The Am J Trop Med Hyg. 2021;104(5):1651–4. doi: 10.4269/ajtmh.20-0952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Messina FA, Marin E, Caceres DH, Romero M, Depardo R, Priarone MM, et al. Coronavirus disease 2019 (COVID-19) in a patient with disseminated histoplasmosis and HIV—a case report from argentina and literature review. J Fungi. 2020 doi: 10.3390/jof6040275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bertolini M, Mutti MF, Barletta JAE, Falak A, Cuatz D, Sisto A, et al. COVID-19 associated with AIDS-related disseminated histoplasmosis: a case report. Int J Std Aids. 2020;31(12):1222–4. doi: 10.1177/0956462420957518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shah AS, Heidari A, Civelli VF, Sharma R, Clark CS, Munoz AD, et al. The coincidence of 2 epidemics, coccidioidomycosis and SARS-CoV-2: a case report. J Investig Med High Impact Case Rep. 2020;8:2324709620930540. doi: 10.1177/2324709620930540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nielsen Marisa C, Reynoso D, Ren P, Burnham Carey-Ann D. The brief case: a fatal case of SARS-CoV-2 coinfection with coccidioides in texas—another challenge we face. J Clin Microbiol. 2021;59(8):e00163-21. doi: 10.1128/JCM.00163-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chang CC, Senining R, Kim J, Goyal R. An acute pulmonary coccidioidomycosis coinfection in a patient presenting with multifocal pneumonia with COVID-19. J Investig Med High Impact Case Rep. 2020;8:2324709620972244. doi: 10.1177/2324709620972244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.de Macedo PM, Freitas DFS, Varon AG, Lamas CdC, Ferreira LCF, Freitas AdA, et al. COVID-19 and acute juvenile paracoccidioidomycosis coinfection. PLOS Negl Trop Dis. 2020;14(8):e0008559. doi: 10.1371/journal.pntd.0008559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mirzaei R, Goodarzi P, Asadi M, Soltani A, Haa A, Jeda AS, et al. Bacterial co-infections with SARS-CoV-2. IUBMB Life. 2020;72(10):2097–111. doi: 10.1002/iub.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lansbury L, Lim B, Baskaran V, Lim WS. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect. 2020;81(2):266–275. doi: 10.1016/j.jinf.2020.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lai C-C, Wang C-Y, Hsueh P-R. Co-infections among patients with COVID-19: The need for combination therapy with non-anti-SARS-CoV-2 agents? J Microbiol Immunol Infect. 2020;53(4):505–512. doi: 10.1016/j.jmii.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Langford BJ, So M, Raybardhan S, Leung V, Westwood D, MacFadden DR, et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020;26(12):1622–9. doi: 10.1016/j.cmi.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Russell CD, Fairfield CJ, Drake TM, Turtle L, Seaton RA, Wootton DG, et al. Co-infections, secondary infections, and antimicrobial use in patients hospitalised with COVID-19 during the first pandemic wave from the ISARIC WHO CCP-UK study: a multicentre, prospective cohort study. The Lancet Microbe. 2021 doi: 10.1016/S2666-5247(21)00090-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhu X, Ge Y, Wu T, Zhao K, Chen Y, Wu B, et al. Co-infection with respiratory pathogens among COVID-2019 cases. Virus Res. 2020;285:198005. doi: 10.1016/j.virusres.2020.198005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sharifipour E, Shams S, Esmkhani M, Khodadadi J, Fotouhi-Ardakani R, Koohpaei A, et al. Evaluation of bacterial co-infections of the respiratory tract in COVID-19 patients admitted to ICU. BMC Infect Dis. 2020;20(1):646. doi: 10.1186/s12879-020-05374-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Houben RMGJ, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Med. 2016;13(10):e1002152–e. doi: 10.1371/journal.pmed.1002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Walzl G, McNerney R, du Plessis N, Bates M, McHugh TD, Chegou NN, et al. Tuberculosis: advances and challenges in development of new diagnostics and biomarkers. Lancet Infect Dis. 2018;18(7):e199–210. doi: 10.1016/S1473-3099(18)30111-7. [DOI] [PubMed] [Google Scholar]
- 113.Tadolini M, Codecasa LR, García-García J-M, Blanc F-X, Borisov S, Alffenaar J-W, et al. Active tuberculosis, sequelae and COVID-19 co-infection: first cohort of 49 cases. Eur Resp J. 2020 doi: 10.1183/13993003.01398-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gao Y, Liu M, Chen Y, Shi S, Geng J, Tian J. Association between tuberculosis and COVID-19 severity and mortality: a rapid systematic review and meta-analysis. J Med Virol. 2021;93(1):194–6. doi: 10.1002/jmv.26311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.LsAB Gadelha Farias, Gomes Moreira AL, Austregsilo Corra E, de Oliveira Landim, Lima CA, Lopes IMP, de Holanda PEL, et al. Case report: coronavirus disease and pulmonary tuberculosis in patients with human immunodeficiency virus: report of two cases. Am J Trop Med Hyg. 2020;103(4):1593–1596. doi: 10.4269/ajtmh.20-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.He G, Wu J, Shi J, Gamber M, Jiang X, Sun W, et al. COVID-19 in tuberculosis patients: a report of three cases. J Med Virol. 2020 doi: 10.1002/jmv.25943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rivas N, Espinoza M, Loban A, Luque O, Jurado J, Ns H-H, et al. Case report: COVID-19 recovery from triple infection with Mycobacterium tuberculosis, HIV, and SARS-CoV-2. The Am J Tro Med Hyg. 2020;103(4):1597–9. doi: 10.4269/ajtmh.20-0756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Visca D, Ong CWM, Tiberi S, Centis R, D’Ambrosio L, Chen B, et al. Tuberculosis and COVID-19 interaction: a review of biological, clinical and public health effects. Pulmonology. 2021;27(2):151–65. doi: 10.1016/j.pulmoe.2020.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sarkar S, Khanna P, Singh AK. Impact of COVID-19 in patients with concurrent co-infections: a systematic review and meta-analyses. J Med Virol. 2021;93(4):2385–95. doi: 10.1002/jmv.26740. [DOI] [PubMed] [Google Scholar]
- 120.Davis B, Rothrock AN, Swetland S, Andris H, Davis P, Rothrock SG. Viral and atypical respiratory co-infections in COVID-19: a systematic review and meta-analysis. J Am College Emerg Phys Open. 2020;1(4):533–548. doi: 10.1002/emp2.12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kim D, Quinn J, Pinsky B, Shah NH, Brown I. Rates of co-infection between SARS-CoV-2 and other respiratory pathogens. JAMA. 2020;323(20):2085–2086. doi: 10.1001/jama.2020.6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ozaras R, Cirpin R, Duran A, Duman H, Arslan O, Bakcan Y, et al. Influenza and COVID-19 coinfection: report of six cases and review of the literature. J Med Virol. 2020;92(11):2657–65. doi: 10.1002/jmv.26125. [DOI] [PubMed] [Google Scholar]
- 123.Kadambari S, Klenerman P, Pollard AJ. Why the elderly appear to be more severely affected by COVID-19: the potential role of immunosenescence and CMV. Rev Med Virol. 2020;30(5):e2144. doi: 10.1002/rmv.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Poloni C, Szyf M, Cheishvili D, Tsoukas CM. Are the healthy vulnerable? Cytomegalovirus seropositivity in healthy adults is associated with accelerated epigenetic age and immune-dysregulation. J Infect Dis. 2021 doi: 10.1093/infdis/jiab365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gandhi MK, Khanna R. Human cytomegalovirus: clinical aspects, immune regulation, and emerging treatments. Lancet Infect Dis. 2004;4(12):725–738. doi: 10.1016/S1473-3099(04)01202-2. [DOI] [PubMed] [Google Scholar]
- 126.Tan BH. Cytomegalovirus treatment. Curr Treat Opt Infect Dis. 2014;6(3):256–270. doi: 10.1007/s40506-014-0021-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Abdoli A, Taghipour A, Pirestani M, Mofazzal Jahromi MA, Roustazadeh A, Mir H, et al. Infections, inflammation, and risk of neuropsychiatric disorders: the neglected role of “co-infection”. Heliyon. 2020;6(12):e05645. doi: 10.1016/j.heliyon.2020.e05645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Goodrich JM, Mori M, Gleaves CA, Du Mond C, Cays M, Ebeling DF, et al. Early treatment with ganciclovir to prevent cytomegalovirus disease after allogeneic bone marrow transplantation. N Engl J Med. 1991;325(23):1601–7. doi: 10.1056/NEJM199112053252303. [DOI] [PubMed] [Google Scholar]
- 129.Reed EC, Bowden RA, Dandliker PS, Lilleby KE, Meyers JD. Treatment of cytomegalovirus pneumonia with ganciclovir and intravenous cytomegalovirus immunoglobulin in patients with bone marrow transplants. Ann Intern Med. 1988;109(10):783–8. doi: 10.7326/0003-4819-109-10-783. [DOI] [PubMed] [Google Scholar]
- 130.Gozzi-Silva SC, Benard G, Alberca RW, Yendo TM, Teixeira FM, Oliveira LD, et al. SARS-CoV-2 infection and CMV dissemination in transplant recipients as a treatment for chagas cardiomyopathy: a case report. Trop Med Infect Dis. 2021 doi: 10.3390/tropicalmed6010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Oualim S, Elouarradi A, Hafid S, Naitelhou A, Sabry M. A misleading CMV myocarditis during the COVID-19 pandemic: case report. Pan Afr Med J. 2020;36:167. doi: 10.11604/pamj.2020.36.167.23922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Maillet F, Pourbaix A, le Pluart D, Sirmai L, Postolache SA, Couvelard A, et al. Cytomegalovirus proctitis as a complication of COVID-19 with immunosuppressive treatments. IDCases. 2021;24:e01111. doi: 10.1016/j.idcr.2021.e01111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shaikh AS, Shaim H, Caravedo MA, Ong KM, Reynoso D. A new viral coinfection: SARS-CoV-2 pneumonia and cytomegalovirus pneumonitis in a renal transplant recipient. Covid. 2021;1(1):115–9. doi: 10.3390/covid1010010. [DOI] [Google Scholar]
- 134.Amiya S, Hirata H, Shiroyama T, Adachi Y, Niitsu T, Noda Y, et al. Fatal cytomegalovirus pneumonia in a critically ill patient with COVID-19. Respirol Case Rep. 2021;9(7):e00801. doi: 10.1002/rcr2.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Amundson L, Boelts B, Kataria V, Spak C. Ganciclovir therapy for CMV viremia in a patient on VV ECMO with COVID-19 after treatment with tocilizumab. Infect Dis Clin Pract. 2021;29(3):e191–e192. doi: 10.1097/IPC.0000000000001035. [DOI] [Google Scholar]
- 136.Molaei H, Khedmat L, Nemati E, Rostami Z, Saadat SH. Iranian kidney transplant recipients with COVID-19 infection: clinical outcomes and cytomegalovirus coinfection. Transpl Infect Dis. 2021;23(1):e13455. doi: 10.1111/tid.13455. [DOI] [PubMed] [Google Scholar]
- 137.Whitley RJ, Roizman B. Herpes simplex virus infections. The Lancet. 2001;357(9267):1513–8. doi: 10.1016/S0140-6736(00)04638-9. [DOI] [PubMed] [Google Scholar]
- 138.Le Balc’h P, Pinceaux K, Pronier C, Seguin P, Tadié J-M, Reizine F, Herpes simplex virus and cytomegalovirus reactivations among severe COVID-19 patients. Crit Care. 2020;24(1):530. doi: 10.1186/s13054-020-03252-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Seeßle J, Hippchen T, Schnitzler P, Gsenger J, Giese T, Merle U. High rate of HSV-1 reactivation in invasively ventilated COVID-19 patients: Immunological findings. PLoS ONE. 2021;16(7):e0254129. doi: 10.1371/journal.pone.0254129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lovati C, Osio M, Pantoni L. Diagnosing herpes simplex-1 encephalitis at the time of COVID-19 pandemic. Neurol Sci. 2020;41(6):1361–64. doi: 10.1007/s10072-020-04461-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hernandez JM, Singam H, Babu A, Aslam S, Lakshmi S. SARS-CoV-2 infection (COVID-19) and herpes simplex virus-1 conjunctivitis: Concurrent viral infections or a cause-effect result? Cureus. 2021;13(1):e12592–e. doi: 10.7759/cureus.12592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Majtanova N, Kriskova P, Keri P, Fellner Z, Majtan J, Kolar P. Herpes simplex keratitis in patients with SARS-CoV-2 Infection: a series of five cases. Medicina. 2021;57(5):412. doi: 10.3390/medicina57050412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Busani S, Bedini A, Biagioni E, Serio L, Tonelli R, Meschiari M, et al. Two fatal cases of acute liver failure due to HSV-1 infection in COVID-19 patients following immunomodulatory therapies. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Alharthy A, Faqihi F, Noor A, Memish ZA, Karakitsos D. Co-infection of human immunodeficiency virus, herpes simplex virus-2 and SARS-CoV-2 with false-negative real-time polymerase chain reaction. Singapore Med J. 2020;1:10. doi: 10.11622/smedj.2020158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xu R, Zhou Y, Cai L, Wang L, Han J, Yang X, et al. Co-reactivation of the human herpesvirus alpha subfamily (herpes simplex virus-1 and varicella zoster virus) in a critically ill patient with COVID-19. Br J Dermatol. 2020;183(6):1145–7. doi: 10.1111/bjd.19484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Marzano AV, Genovese G, Fabbrocini G, Pigatto P, Monfrecola G, Piraccini BM, et al. Varicella-like exanthem as a specific COVID-19-associated skin manifestation: multicenter case series of 22 patients. J Am Acad Dermatol. 2020;83(1):280–5. doi: 10.1016/j.jaad.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wolday D, Tasew G, Amogne W, Urban B, Schallig HD, Harris V, et al. Interrogating the impact of intestinal parasite-microbiome on pathogenesis of COVID-19 in Sub-Saharan Africa. Front Microbiol. 2021;12:614522. doi: 10.3389/fmicb.2021.614522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rasti S, Hassanzadeh M, Hooshyar H, Momen-Heravi M, Mousavi SGA, Abdoli A. Intestinal parasitic infections in different groups of immunocompromised patients in Kashan and Qom cities, central Iran. Scand J Gastroenterol. 2017;52(6–7):738–41. doi: 10.1080/00365521.2017.1308547. [DOI] [PubMed] [Google Scholar]
- 149.Dalimi A, Abdoli A. Latent toxoplasmosis and human. Iran J Parasitol. 2012;7(1):1–17. [PMC free article] [PubMed] [Google Scholar]
- 150.Abdoli A. Neglected risk factors for HIV and Toxoplasma gondii co-infection. The lancet HIV. 2017;4(4):e152. doi: 10.1016/S2352-3018(17)30048-6. [DOI] [PubMed] [Google Scholar]
- 151.Abdoli A. Parasitic encephalitis in immunocompetent individuals. Lancet. 2019;394(10202):914–5. doi: 10.1016/S0140-6736(19)31776-3. [DOI] [PubMed] [Google Scholar]
- 152.Abdoli A, Barati M, Dalimi A, Pirestani M, Hoseini Shokouh SJ. Toxoplasmosis among patients with immunocompromising conditions: a snapshot. J Archiv Military Med. 2016;4(4):e41832. doi: 10.5812/jamm.41832. [DOI] [Google Scholar]
- 153.Rasti S, Hassanzadeh M, Soliemani A, Hooshyar H, Mousavi SGA, Nikoueinejad H, et al. Serological and molecular survey of toxoplasmosis in renal transplant recipients and hemodialysis patients in Kashan and Qom regions, central Iran. Ren Fail. 2016;38(6):970–3. doi: 10.3109/0886022X.2016.1172940. [DOI] [PubMed] [Google Scholar]
- 154.Nicholson DH, Wolchok EB. Ocular toxoplasmosis in an adult receiving long-term corticosteroid therapy. Arch Ophthalmol. 1976;94(2):248–54. doi: 10.1001/archopht.1976.03910030120009. [DOI] [PubMed] [Google Scholar]
- 155.Olson DJ, Parhiz AT, Wirthlin RS. Reactivation of latent toxoplasmosis following dexamethasone implant injection. Ophthal Surg Lasers Imag Retina. 2016;47(11):1050–2. doi: 10.3928/23258160-20161031-10. [DOI] [PubMed] [Google Scholar]
- 156.Oray M, Ozdal PC, Cebeci Z, Kir N, Tugal-Tutkun I. Fulminant ocular toxoplasmosis: the hazards of corticosteroid monotherapy. Ocul Immunol Inflamm. 2016;24(6):637–46. doi: 10.3109/09273948.2015.1057599. [DOI] [PubMed] [Google Scholar]
- 157.Pagalavan L, Kan FK. Cerebral toxoplasmosis in systemic lupus erythematosus following intravenous methylprednisolone. Med J Malaysia. 2011;66(1):68–70. [PubMed] [Google Scholar]
- 158.Pagalavan L. Cerebral toxoplasmosis: case report. Reactions. 2016;1602:173–221. [Google Scholar]
- 159.Safa G, Darrieux L. Cerebral toxoplasmosis after rituximab therapy. JAMA Intern Med. 2013;173(10):924–6. doi: 10.1001/jamainternmed.2013.374. [DOI] [PubMed] [Google Scholar]
- 160.Goldberg RA, Reichel E, Oshry LJ. Bilateral toxoplasmosis retinitis associated with ruxolitinib. N Engl J Med. 2013;369(7):681–3. doi: 10.1056/NEJMc1302895. [DOI] [PubMed] [Google Scholar]
- 161.de Paula Rodrigues KF, Faria e Arantes TE, Muccioli C, de Andrade Neto JL, Pinheiro MM, Incidence of toxoplasma retinochoroiditis in patients with ankylosing spondylitis after using TNF-α blockers. Parasitol Int. 2013;62(3):272–5. doi: 10.1016/j.parint.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 162.Lassoued S, Zabraniecki L, Marin F, Billey T. Toxoplasmic chorioretinitis and antitumor necrosis factor treatment in rheumatoid arthritis. Semin Arthritis Rheum. 2007;36(4):262–3. doi: 10.1016/j.semarthrit.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 163.Hill B, Wyatt N, Ennis D. Cerebral toxoplasmosis in a rheumatoid arthritis patient on immunosuppressive therapy. Cureus. 2020;12(6):e8547. doi: 10.7759/cureus.8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lobo Y, Holland T, Spelman L. Toxoplasmosis in a patient receiving ixekizumab for psoriasis. JAAD Case Rep. 2020;6(3):204. doi: 10.1016/j.jdcr.2020.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sharaf-El-Deen SA, Shalan FH, Agha MA, Brakat RM. Toxoplasma gondii as a possible risk factor for COVID-19 severity: a case-control study. Egyp J Med Microbiol. 2021;30(2):125–132. doi: 10.51429/ejmm30217. [DOI] [Google Scholar]
- 166.Roe K. The symptoms and clinical manifestations observed in COVID-19 patients/long COVID-19 symptoms that parallel toxoplasma gondii infections. J Neuroimmune Pharmacol. 2021 doi: 10.1007/s11481-021-09997-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Abdoli A, Ardakani HM. Helminth infections and immunosenescence: the friend of my enemy. Exp Gerontol. 2020;133:110852. doi: 10.1016/j.exger.2020.110852. [DOI] [PubMed] [Google Scholar]
- 168.Abdoli A, Pirestani M. Are pregnant women with chronic helminth infections more susceptible to congenital infections? Front Immunol. 2014 doi: 10.3389/fimmu.2014.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Abdoli A. Helminths and COVID-19 co-infections: a neglected critical challenge. ACS Pharmacol Transl Sci. 2020;3(5):1039–1041. doi: 10.1021/acsptsci.0c00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chacin-Bonilla L, Chacón-Fonseca N, Rodriguez-Morales AJ. Emerging issues in COVID-19 vaccination in tropical areas: impact of the immune response against helminths in endemic areas. Travel Med Infect Dis. 2021;42:102087. doi: 10.1016/j.tmaid.2021.102087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Paniz-Mondolfi AE, Ramírez JD, Delgado-Noguera LA, Rodriguez-Morales AJ, Sordillo EM. COVID-19 and helminth infection: beyond the Th1/Th2 paradigm. PLoS Negl Trop Dis. 2021;15(5):e0009402. doi: 10.1371/journal.pntd.0009402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Tamarozzi F, Martello E, Giorli G, Fittipaldo A, Staffolani S, Montresor A, et al. Morbidity associated with chronic Strongyloides stercoralis infection: a systematic review and meta-analysis. Am J Trop Med Hyg. 2019;100(6):1305–11. doi: 10.4269/ajtmh.18-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Buonfrate D, Requena-Mendez A, Angheben A, Muñoz J, Gobbi F, Van Den Ende J, et al. Severe strongyloidiasis: a systematic review of case reports. BMC Infect Dis. 2013;13(1):78. doi: 10.1186/1471-2334-13-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Stauffer WM, Alpern JD, Walker PF. COVID-19 and dexamethasone: a potential strategy to avoid steroid-related strongyloides hyperinfection. JAMA. 2020;324(7):623–4. doi: 10.1001/jama.2020.13170. [DOI] [PubMed] [Google Scholar]
- 175.Lier AJ, Tuan JJ, Davis MW, Paulson N, McManus D, Campbell S, et al. Case report: disseminated strongyloidiasis in a patient with COVID-19. Am J Trop Med Hyg. 2020;103(4):1590–1592. doi: 10.4269/ajtmh.20-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Marchese V, Crosato V, Gulletta M, Castelnuovo F, Cristini G, Matteelli A, et al. Strongyloides infection manifested during immunosuppressive therapy for SARS-CoV-2 pneumonia. Infection. 2020 doi: 10.1007/s15010-020-01522-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Katano H, Hishima T, Mochizuki M, Kodama Y, Oyaizu N, Ota Y, et al. The prevalence of opportunistic infections and malignancies in autopsied patients with human immunodeficiency virus infection in Japan. BMC Infect Dis. 2014;14(1):229. doi: 10.1186/1471-2334-14-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Klastersky J, Aoun M. Opportunistic infections in patients with cancer. Ann Oncol. 2004;15:iv329–iv35. doi: 10.1093/annonc/mdh947. [DOI] [PubMed] [Google Scholar]
- 179.Abdoli A, Barati M, Pirestani M, Dalimi A. Screening of toxoplasmosis in cancer patients: a concern. Trop Doct. 2018;49(1):31–4. doi: 10.1177/0049475518801618. [DOI] [PubMed] [Google Scholar]
- 180.Garrido RSJ, Aguado JM, Díaz-Pedroche C, Len O, Montejo M, Moreno A, et al. A review of critical periods for opportunistic infection in the new transplantation era. Transplantation. 2006;82(11):1457–1462. doi: 10.1097/01.tp.0000245676.43979.86. [DOI] [PubMed] [Google Scholar]
- 181.Banerjee U. Progress in diagnosis of opportunistic infections in HIV/AIDS. Indian J Med Res. 2005;121(4):395–406. [PubMed] [Google Scholar]
- 182.Mokhtari T, Hassani F, Ghaffari N, Ebrahimi B, Yarahmadi A, Hassanzadeh G. COVID-19 and multiorgan failure: A narrative review on potential mechanisms. J Mol Histol. 2020;51(6):613–28. doi: 10.1007/s10735-020-09915-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yazdanpanah F, Hamblin MR, Rezaei N. The immune system and COVID-19: Friend or foe? Life Sci. 2020;256:117900. doi: 10.1016/j.lfs.2020.117900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Tomazini BM, Maia IS, Cavalcanti AB, Berwanger O, Rosa RG, Veiga VC, et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA. 2020;324(13):1307–1316. doi: 10.1001/jama.2020.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Aziz M, Haghbin H, Abu Sitta E, Nawras Y, Fatima R, Sharma S, et al. Efficacy of tocilizumab in COVID-19: a systematic review and meta-analysis. J Med Virol. 2021;93(3):1620–30. doi: 10.1002/jmv.26509. [DOI] [PubMed] [Google Scholar]
- 186.Salama C, Han J, Yau L, Reiss WG, Kramer B, Neidhart JD, et al. Tocilizumab in patients hospitalized with Covid-19 pneumonia. N Engl J Med. 2021;384(1):20–30. doi: 10.1056/NEJMoa2030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Stone JH, Frigault MJ, Serling-Boyd NJ, Fernandes AD, Harvey L, Foulkes AS, et al. Efficacy of tocilizumab in patients hospitalized with covid-19. N Engl J Med. 2020;383(24):2333–44. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Velazquez-Salinas L, Verdugo-Rodriguez A, Rodriguez LL, Borca MV. The role of interleukin 6 during viral infections. Front Microbiol. 2019 doi: 10.3389/fmicb.2019.01057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kimmig LM, Wu D, Gold M, Pettit NN, Pitrak D, Mueller J, et al. IL-6 inhibition in critically Ill COVID-19 patients is associated with increased secondary infections. Front Med. 2020;7:583897. doi: 10.3389/fmed.2020.583897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Nishioka H, Takegawa H, Kamei H. Disseminated cryptococcosis in a patient taking tocilizumab for Castleman's disease. J Infect Chemother. 2018;24(2):138–41. doi: 10.1016/j.jiac.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 191.Shtessel M. Cryptococcal meningitis: case report. Reactions. 2014;1508:36–45. [Google Scholar]
- 192.Honda H, Kida H, Yoshida M, Tomita T, Fujii M, Ihara S, et al. Recurrent allergic bronchopulmonary aspergillosis in a patient with rheumatoid arthritis treated with etanercept and tocilizumab. Mod Rheumatol. 2011;21(6):660–4. doi: 10.1007/s10165-011-0449-0. [DOI] [PubMed] [Google Scholar]
- 193.van Duin D, Miranda C, Husni E. Cytomegalovirus viremia, pneumonitis, and tocilizumab therapy. Emerg Infect Dis. 2011;17(4):754. doi: 10.3201/eid1706.101057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Reisinger AC, Hermann J, Vagena FR, Hackl G, Eller P. Tuberculosis sepsis after tocilizumab treatment. Clin Microbiol Infect. 2020;26(11):1493–1494. doi: 10.1016/j.cmi.2020.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.La Rosée F, Bremer HC, Gehrke I, Kehr A, Hochhaus A, Birndt S, et al. The Janus kinase 1/2 inhibitor ruxolitinib in COVID-19 with severe systemic hyperinflammation. Leukemia. 2020;34(7):1805–15. doi: 10.1038/s41375-020-0891-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Cao Y, Wei J, Zou L, Jiang T, Wang G, Chen L, et al. Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): a multicenter, single-blind, randomized controlled trial. J Allergy Clin Immunol. 2020;146(1):137–46.e3. doi: 10.1016/j.jaci.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Vannucchi AM, Sordi B, Morettini A, Nozzoli C, Poggesi L, Pieralli F, et al. Compassionate use of JAK1/2 inhibitor ruxolitinib for severe COVID-19: a prospective observational study. Leukemia. 2020 doi: 10.1038/s41375-020-01018-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Cantini F, Niccoli L, Nannini C, Matarrese D, Natale MED, Lotti P, et al. Beneficial impact of Baricitinib in COVID-19 moderate pneumonia; multicentre study. J Infect. 2020 doi: 10.1016/j.jinf.2020.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Titanji BK, Farley MM, Mehta A, Connor-Schuler R, Moanna A, Cribbs SK, et al. Use of Baricitinib in patients with moderate and severe COVID-19. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Luo W, Li Y-X, Jiang L-J, Chen Q, Wang T, Ye D-W. Targeting JAK-STAT signaling to control cytokine release syndrome in COVID-19. Trends Pharmacol Sci. 2020;41(8):531–43. doi: 10.1016/j.tips.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Stallmach A, Kortgen A, Gonnert F, Coldewey SM, Reuken P, Bauer M. Infliximab against severe COVID-19-induced cytokine storm syndrome with organ failure—a cautionary case series. Crit Care. 2020;24(1):444. doi: 10.1186/s13054-020-03158-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Farrokhpour M, Rezaie N, Moradi N, Ghaffari Rad F, Izadi S, Azimi M, et al. Infliximab and intravenous gammaglobulin in hospitalized severe COVID-19 patients in intensive care unit. Arch Iran Med. 2021;24(2):139–43. doi: 10.34172/aim.2021.22. [DOI] [PubMed] [Google Scholar]
- 203.Cavalli G, De Luca G, Campochiaro C, Della-Torre E, Ripa M, Canetti D, et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. The Lancet Rheumatol. 2020;2(6):e325–e331. doi: 10.1016/S2665-9913(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Huet T, Beaussier H, Voisin O, Jouveshomme S, Dauriat G, Lazareth I, et al. Anakinra for severe forms of COVID-19: a cohort study. The Lancet Rheumatol. 2020;2(7):e393–e400. doi: 10.1016/S2665-9913(20)30164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ucciferri C, Auricchio A, Di Nicola M, Potere N, Abbate A, Cipollone F, et al. Canakinumab in a subgroup of patients with COVID-19. The Lancet Rheumatol. 2020;2(8):e457–8. doi: 10.1016/S2665-9913(20)30167-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sheng CC, Sahoo D, Dugar S, Prada RA, Wang TKM, Abou Hassan OK, et al. Canakinumab to reduce deterioration of cardiac and respiratory function in SARS-CoV-2 associated myocardial injury with heightened inflammation (canakinumab in Covid-19 cardiac injury: the three C study) Clin Cardiol. 2020 doi: 10.1002/clc.23451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.De Luca G, Cavalli G, Campochiaro C, Della-Torre E, Angelillo P, Tomelleri A, et al. GM-CSF blockade with mavrilimumab in severe COVID-19 pneumonia and systemic hyperinflammation: a single-centre, prospective cohort study. The Lancet Rheumatol. 2020;2(8):e465–73. doi: 10.1016/S2665-9913(20)30170-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Yousefzai R, Bhimaraj A. Misdiagnosis in the COVID-19 Era: when zebras are everywhere, don’t forget the horses. JACC Case Rep. 2020;2(10):1614–9. doi: 10.1016/j.jaccas.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Vilchèze C, Jacobs WR., Jr The combination of sulfamethoxazole, trimethoprim, and isoniazid or rifampin is bactericidal and prevents the emergence of drug resistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2012;56(10):5142–8. doi: 10.1128/AAC.00832-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Forgacs P, Wengenack NL, Hall L, Zimmerman SK, Silverman ML, Roberts GD. Tuberculosis and trimethoprim-sulfamethoxazole. Antimicrob Agents Chemother. 2009;53(11):4789–93. doi: 10.1128/AAC.01658-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hodge C, Marra F, Marzolini C, Boyle A, Gibbons S, Siccardi M, et al. Drug interactions: a review of the unseen danger of experimental COVID-19 therapies. J Antimicrob Chemother. 2020;75(12):3417–24. doi: 10.1093/jac/dkaa340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Surmelioglu N, Demirkan K. COVID-19 drug interactions. J Critical Intens Care. 2020;11:43–45. doi: 10.37678/dcybd.2020.2409. [DOI] [Google Scholar]
- 213.Kewan T, Covut F, Al–Jaghbeer MJ, Rose L, Gopalakrishna KV, Akbik B, Tocilizumab for treatment of patients with severe COVID–19: a retrospective cohort study. EClin Med. 2020;24:100418. doi: 10.1016/j.eclinm.2020.100418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Hafner C, Linde HJ, Vogt T, Breindl G, Tintelnot K, Koellner K, et al. Primary cutaneous cryptococcosis and secondary antigenemia in a patient with long-term corticosteroid therapy. Infection. 2005;33(2):86–9. doi: 10.1007/s15010-005-4095-3. [DOI] [PubMed] [Google Scholar]
- 215.Vandersmissen G, Meuleman L, Tits G, Verhaeghe A, Peetermans WE. Cutaneous cryptococcosis in corticosteroid-treated patients without aids. Acta Clin Belg. 1996;51(2):111–7. doi: 10.1080/17843286.1996.11718496. [DOI] [PubMed] [Google Scholar]
- 216.Collins JV, Tong D, Bucknall RG, Warin AP. Cryptococcal meningitis as a complication of systemic lupus erythematosus treated with systemic corticosteroids. Postgrad Med J. 1972;48(555):52–5. doi: 10.1136/pgmj.48.555.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Chhakchhuak M, Agarwal J. Solitary pelvic ectopic kidney and limb anomalies: rare variant of acrorenal syndrome. Indian J Med Microbiol. 2005;23(3):207–8. doi: 10.4103/ijn.IJN_178_19. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 218.Sato S, Kambe E, Tamai Y. Disseminated cryptococcosis in a patient with multiple myeloma treated with daratumumab, lenalidomide, and dexamethasone. Intern Med. 2019;58(6):843–847. doi: 10.2169/internalmedicine.1726-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kamiya H, Ishikawa R, Moriya A, Arai A, Morimoto K, Ando T, et al. Disseminated cryptococcosis complicated with bilateral pleural effusion and ascites during corticosteroid therapy for organizing pneumonia with myelodysplastic syndrome. Intern Med. 2008;47(22):1981–6. doi: 10.2169/internalmedicine.47.0898. [DOI] [PubMed] [Google Scholar]
- 220.Yamaguchi T, Nagai Y, Morita T, Kiuchi D, Matsumoto M, Hisahara K, et al. Pneumocystis pneumonia in patients treated with long-term steroid therapy for symptom palliation: a neglected infection in palliative care. The Am J Hospice Palliative Care. 2014;31(8):857–61. doi: 10.1177/1049909113504238. [DOI] [PubMed] [Google Scholar]
- 221.Cooley L, Dendle C, Wolf J, Teh BW, Chen SC, Boutlis C, et al. Consensus guidelines for diagnosis, prophylaxis and management of Pneumocystis jirovecii pneumonia in patients with haematological and solid malignancies, 2014. Intern Med J. 2014;44(12b):1350–63. doi: 10.1111/imj.12599. [DOI] [PubMed] [Google Scholar]
- 222.Gustafson TL, Schaffner W, Lavely GB, Stratton CW, Johnson HK, Hutcheson RH., Jr Invasive Aspergillosis in renal transplant recipients: correlation with corticosteroid therapy. J Infect Dis. 1983;148(2):230–8. doi: 10.1093/infdis/148.2.230. [DOI] [PubMed] [Google Scholar]
- 223.Basich JE, Graves TS, Baz MN, Scanlon G, Hoffmann RG, Patterson R, et al. Allergic bronchopulmonary aspergillosis in corticosteroid-dependent asthmatics. J Allergy Clin Immunol. 1981;68(2):98–102. doi: 10.1016/0091-6749(81)90165-2. [DOI] [PubMed] [Google Scholar]
- 224.Palmer LB, Greenberg HE, Schiff MJ. Corticosteroid treatment as a risk factor for invasive aspergillosis in patients with lung disease. Thorax. 1991;46(1):15–20. doi: 10.1136/thx.46.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Wang H, Ding Y, Li X, Yang L, Zhang W, Kang W. Fatal aspergillosis in a patient with SARS who was treated with corticosteroids. N Engl J Med. 2003;349(5):507–8. doi: 10.1056/nejm200307313490519. [DOI] [PubMed] [Google Scholar]
- 226.Fukushima C, Matsuse H, Tomari S, Obase Y, Miyazaki Y, Shimoda T, et al. Oral candidiasis associated with inhaled corticosteroid use: comparison of fluticasone and beclomethasone. Ann Allergy Asthma Immunol. 2003;90(6):646–51. doi: 10.1016/S1081-1206(10)61870-4. [DOI] [PubMed] [Google Scholar]
- 227.Simon MR, Houser WL, Smith KA, Long PM. Esophageal candidiasis as a complication of inhaled corticosteroids. Ann Allergy Asthma Immunol. 1997;79(4):333–8. doi: 10.1016/S1081-1206(10)63024-4. [DOI] [PubMed] [Google Scholar]
- 228.Botas CM, Kurlat I, Young SM, Sola A. Disseminated candidal infections and intravenous hydrocortisone in preterm infants. Pediatrics. 1995;95(6):883–7. doi: 10.1542/peds.95.6.883. [DOI] [PubMed] [Google Scholar]
- 229.Hoang K, Abdo T, Reinersman JM, Lu R, Higuita NIA. A case of invasive pulmonary mucormycosis resulting from short courses of corticosteroids in a well-controlled diabetic patient. Med Mycol Case Rep. 2020;29:22–24. doi: 10.1016/j.mmcr.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Huang YQ, Tremblay J-A, Chapdelaine H, Luong M-L, Carrier FM. Pulmonary mucormycosis in a patient with acute liver failure: a case report and systematic review of the literature. J Crit Care. 2020;56:89–93. doi: 10.1016/j.jcrc.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 231.Person B, Bahouth H, Brauner E, Ben-Ishay O, Bickel A, Kluger YS. Surgical emergencies confounded by H1N1 influenza infection - a plea for concern. World J Emerg Surg. 2010;5(1):6. doi: 10.1186/1749-7922-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Hanley B, Naresh KN, Roufosse C, Nicholson AG, Weir J, Cooke GS, et al. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. The Lancet Microbe. 2020;1(6):e245–e253. doi: 10.1016/S2666-5247(20)30115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Devlin SM, Hu B, Ippoliti A. Mucormycosis presenting as recurrent gastric perforation in a patient with Crohn's disease on glucocorticoid, 6-mercaptopurine, and infliximab therapy. Dig Dis Sci. 2007;52(9):2078–81. doi: 10.1007/s10620-006-9455-z. [DOI] [PubMed] [Google Scholar]
- 234.Odessey E, Cohn A, Beaman K, Schechter L. Invasive mucormycosis of the maxillary sinus: extensive destruction with an indolent presentation. Surg Infect. 2008;9(1):91–98. doi: 10.1089/sur.2006.039. [DOI] [PubMed] [Google Scholar]
- 235.Cruz T, Reboucas G, Rocha H. Fatal strongyloidiasis in patients receiving corticosteroids. N Engl J Med. 1966;275(20):1093–1096. doi: 10.1056/nejm196611172752003. [DOI] [PubMed] [Google Scholar]
- 236.Fardet L, Généreau T, Poirot J-L, Guidet B, Kettaneh A, Cabane J. Severe strongyloidiasis in corticosteroid-treated patients: case series and literature review. J Infect. 2007;54(1):18–27. doi: 10.1016/j.jinf.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 237.Fardet L, Généreau T, Cabane J, Kettaneh A. Severe strongyloidiasis in corticosteroid-treated patients. Clin Microbiol Infect. 2006;12(10):945–7. doi: 10.1111/j.1469-0691.2006.01443.x. [DOI] [PubMed] [Google Scholar]
- 238.Civantos F, Robinson MJ. Fatal Strongyloidiasis following corticosteroid therapy. Am J Dig Dis. 1969;14(9):643–51. doi: 10.1007/BF02239276. [DOI] [PubMed] [Google Scholar]
- 239.Neefe LI, Pinilla O, Garagusi VF, Bauer H. Disseminated strongyloidiasis with cerebral involvement: a complication of corticosteroid therapy. Am J Med. 1973;55(6):832–8. doi: 10.1016/0002-9343(73)90265-9. [DOI] [PubMed] [Google Scholar]
- 240.Thomas M, Costello S. Disseminated strongyloidiasis arising from a single dose of dexamethasone before stereotactic radiosurgery. Int J Clin Pract. 1998;52(7):520. [PubMed] [Google Scholar]
- 241.Berger R, Kraman S, Paciotti M. Pulmonary strongyloidiasis complicating therapy with corticosteroids. Am J Trop Med Hyg. 1980;29(1):31–4. doi: 10.4269/ajtmh.1980.29.31. [DOI] [PubMed] [Google Scholar]
- 242.Al Maslamani MA, Al Soub HA, Al Khal ALM, Al Bozom IA, Abu Khattab MJ, Chacko KC. Strongyloides stercoralis hyperinfection after corticosteroid therapy: a report of two cases. Ann Saudi Med. 2009;29(5):397–401. doi: 10.4103/0256-4947.55172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Castellana G, Castellana M, Castellana C, Castellana G, Resta E, Carone M, et al. Inhaled corticosteroids and risk of tuberculosis in patients with obstructive lung diseases: a systematic review and meta-analysis of non-randomized studies. Int J Chron Obstruct Pulmon Dis. 2019;14:2219. doi: 10.2147/COPD.S209273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Troselj-Vukic B, Milotic I, Milotic F, Crnic-Martinovic M, Grahovac B. Cytomegalovirus reactivation after low-dose steroid treatment for hemolytic anemia in a patient with primary Epstein-Barr virus infection. Wien Klin Wochenschr. 2007;119(13):435–437. doi: 10.1007/s00508-007-0821-4. [DOI] [PubMed] [Google Scholar]
- 245.Chiba S, Ikeda S, Agatsuma Y, Nakao T, Saheki Y. Active infection with cytomegalovirus and herpes group viruses in children receiving corticosteroid therapy. Tohoku J Exp Med. 1972;106(3):265–70. doi: 10.1620/tjem.106.265. [DOI] [PubMed] [Google Scholar]
- 246.Hirano A, Yamasaki M, Saito N, Iwato K, Daido W, Funaishi K, et al. Pulmonary cryptococcosis in a ruxolitinib-treated patient with primary myelofibrosis. Respir Med Case Rep. 2017;22:87–90. doi: 10.1016/j.rmcr.2017.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Wysham NG, Sullivan DR, Allada G. An opportunistic infection associated with Ruxolitinib, a Novel Janus Kinase 1,2 Inhibitor. Chest. 2013;143(5):1478–9. doi: 10.1378/chest.12-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Seminario-Vidal L, Cantrell W, Elewski BE. Pulmonary cryptococcosis in the setting of tofacitinib therapy for psoriasis. J Drugs Dermatol. 2015;14(8):901–2. [PubMed] [Google Scholar]
- 249.Chen CC, Chen YY, Huang CE. Cryptococcal meningoencephalitis associated with the long-term use of ruxolitinib. Ann Hematol. 2016;95(2):361–2. doi: 10.1007/s00277-015-2532-7. [DOI] [PubMed] [Google Scholar]
- 250.Tsukui D, Fujita H, Suzuki K, Hirata K. A case report of cryptococcal meningitis associated with ruxolitinib. Medicine. 2020;99(13):e19587. doi: 10.1097/MD.0000000000019587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Moruno-Rodríguez A, Sánchez-Vicente JL, Rueda-Rueda T, Lechón-Caballero B, Muñoz-Morales A, López-Herrero F. Invasive aspergillosis manifesting as retinal necrosis in a patient treated with ruxolitinib. Archivos de la Sociedad Española de Oftalmología (English Edition) 2019;94(5):237–41. doi: 10.1016/j.oftale.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 252.Patil VR, Chandrakala S, Wasekar NP, Jijina F, Mohite AB. Ruxolitinib-associated tuberculosis–a rare complication of a novel drug! Int J Med Sci Publ Health. 2017;6(3):652–655. doi: 10.5455/ijmsph.2017.0846912092016. [DOI] [Google Scholar]
- 253.Khalid F, Damlaj M, AlZahrani M, Abuelgasim KA, Gmati GE. Reactivation of tuberculosis following ruxolitinib therapy for primary myelofibrosis; case series & literature review. Hematol Oncol Stem Cell Ther. 2020;16;S1658–3876(20):30032–30037. doi: 10.1016/j.hemonc.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 254.Neethu C, James J, Prabhu R. Reactivation of a common infection following treatment with a novel agent for an uncommon disease-ruxolitinib associated tuberculosis: two cases. J Pharm Sci Res. 2017;9(12):2437–9. [Google Scholar]
- 255.Winthrop K, Park S, Gul A, Cardiel M, Gomez-Reino J, Tanaka Y, et al. Tuberculosis and other opportunistic infections in tofacitinib-treated patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75(6):1133–8. doi: 10.1136/annrheumdis-2015-207319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Yanagisawa K, Ogawa Y, Hosogai M, Todokoro D, Mitsui T, Yokohama A, et al. Cytomegalovirus retinitis followed by immune recovery uveitis in an elderly patient with rheumatoid arthritis undergoing administration of methotrexate and tofacitinib combination therapy. J Infect Chemother. 2017;23(8):572–5. doi: 10.1016/j.jiac.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 257.Chandrakumaran A, Malik M, Stevens MP, Abbate A. A case report of locally invasive Aspergillus fumigatus infection in a patient on canakinumab. Eur Heart J Case Rep. 2018 doi: 10.1093/ehjcr/yty098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Shrestha RK, Stoller JK, Honari G, Procop GW, Gordon SM. Pneumonia due to Cryptococcus neoformans in a patient receiving infliximab: possible zoonotic transmission from a pet Cockatiel. Respir Care. 2004;49(6):606–8. [PubMed] [Google Scholar]
- 259.Hirai F, Matsui T, Ishibashi Y, Higashi D, Futami K, Haraoka S, et al. Asymptomatic pulmonary cryptococcosis in a patient with Crohn's disease on infliximab: case report. Inflamm Bowel Dis. 2011;17(7):1637–8. doi: 10.1002/ibd.21564. [DOI] [PubMed] [Google Scholar]
- 260.True DG, Penmetcha M, Peckham SJ. Disseminated cryptococcal infection in rheumatoid arthritis treated with methotrexate and infliximab. J Rheumatol. 2002;29(7):1561–63. [PubMed] [Google Scholar]
- 261.Kozic H, Riggs K, Ringpfeil F, Lee JB. Disseminated Cryptococcus neoformans after treatment with infliximab for rheumatoid arthritis. J Am Acad Dermatol. 2008;58(5):S95–6. doi: 10.1016/j.jaad.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 262.Muñoz P, Giannella M, Valerio M, Soria T, Díaz F, Longo JL, et al. Cryptococcal meningitis in a patient treated with infliximab. Diagn Microbiol Infect Dis. 2007;57(4):443–6. doi: 10.1016/j.diagmicrobio.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 263.Kluger N, Poirier P, Guilpain P, Baixench M-T, Cohen P, Paugam A. Cryptococcal meningitis in a patient treated with infliximab and mycophenolate mofetil for Behcet's disease. Int J Infect Dis. 2009;13(5):e325. doi: 10.1016/j.ijid.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 264.Chiriac A, Mares M, Mihaila D, Solovan C, Moldovan C, Stolnicu S, et al. Primary cutaneous cryptococcosis during infliximab therapy. Dermatol Ther. 2017;30(1):e12405. doi: 10.1111/dth.12405. [DOI] [PubMed] [Google Scholar]
- 265.Kaur N, Mahl TC. Pneumocystis jiroveci (carinii) pneumonia after infliximab therapy: a review of 84 cases. Dig Dis Sci. 2007;52(6):1481–4. doi: 10.1007/s10620-006-9250-x. [DOI] [PubMed] [Google Scholar]
- 266.Seddik M, Meliez H, Seguy D, Viget N, Cortot A, Colombel JF. Pneumocystis Jiroveci (Carinii) pneumonia following initiation of infliximab and azathioprine therapy in a patient With Crohn's disease. Inflamm Bowel Dis. 2004;10(4):436–7. doi: 10.1097/00054725-200407000-00017. [DOI] [PubMed] [Google Scholar]
- 267.Mori S, Imamura F, Kiyofuji C, Ito K, Koga Y, Honda I, et al. Pneumocystis jiroveci pneumonia in a patient with rheumatoid arthritis as a complication of treatment with infliximab, anti-tumor necrosis factor α neutralizing antibody. Mod Rheumatol. 2006;16(1):58–62. doi: 10.1007/s10165-005-0454-2. [DOI] [PubMed] [Google Scholar]
- 268.Harigai M, Koike R, Miyasaka N. Pneumocystis pneumonia associated with infliximab in Japan. N Engl J Med. 2007;357(18):1874–6. doi: 10.1056/NEJMc070728. [DOI] [PubMed] [Google Scholar]
- 269.Judson MA. Allergic bronchopulmonary aspergillosis after infliximab therapy for sarcoidosis: a potential mechanism related to T-helper cytokine balance. Chest. 2009;135(5):1358–59. doi: 10.1378/chest.08-2106. [DOI] [PubMed] [Google Scholar]
- 270.Rosa FGD, Shaz D, Campagna AC, Dellaripa PF, Khettry U, Craven DE. Invasive pulmonary aspergillosis soon after therapy with infliximab, a tumor necrosis factor-alpha–neutralizing antibody: A possible healthcare-associated case? Infect Control Hosp Epidemiol. 2003;24(7):477–82. doi: 10.1086/502250. [DOI] [PubMed] [Google Scholar]
- 271.Guivarc'h M, Ordioni U, Catherine J-H, Campana F, Camps J, Bukiet F. Implications of endodontic-related sinus aspergillosis in a patient treated by infliximab: a case report. J Endod. 2015;41(1):125–9. doi: 10.1016/j.joen.2014.09.022. [DOI] [PubMed] [Google Scholar]
- 272.Bourne E-L, Dimou J. Invasive central nervous system aspergillosis in a patient with Crohn’s disease after treatment with infliximab and corticosteroids. J Clin Neurosci. 2016;30:163–4. doi: 10.1016/j.jocn.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 273.Alderson JW, Van Dinter Jr TG, Opatowsky MJ, Burton EC. Disseminated aspergillosis following infliximab therapy in an immunosuppressed patient with Crohn's disease and chronic hepatitis C: a case study and review of the literature. Medscape Gen Med. 2005;7(3):7. [PMC free article] [PubMed] [Google Scholar]
- 274.Belda A, Hinojosa J, Serra B, Garcia L, Merino C, Moles J. Systemic candidiasis and infliximab therapy. Gastroenterol Hepatol. 2004;27(6):365–7. doi: 10.1016/S0210-5705(03)70477-4. [DOI] [PubMed] [Google Scholar]
- 275.Miyamoto H, Miura T, Morita E, Morizaki Y, Uehara K, Ohe T, et al. Fungal arthritis of the wrist caused by Candida parapsilosis during infliximab therapy for rheumatoid arthritis. Mod Rheumatol. 2012;22(6):903–6. doi: 10.3109/s10165-012-0594-0. [DOI] [PubMed] [Google Scholar]
- 276.Kaur N, Mahl TC. CASE REPORT: Pneumocystis jiroveci pneumonia with oral candidiasis after infliximab therapy for Crohn's disease. Dig Dis Sci. 2004;49(9):1458–60. doi: 10.1023/B:DDAS.0000042246.58984.98. [DOI] [PubMed] [Google Scholar]
- 277.Gadadhar H, Hawkins S, Huffstutter JE, Panda M. Cutaneous mucormycosis complicating methotrexate, prednisone, and infliximab therapy. JCR J Clin Rheumatol. 2007;13(6):361–2. doi: 10.1097/RHU.0b013e31815d3ddd. [DOI] [PubMed] [Google Scholar]
- 278.Keijzer A, Valk P, Ossenkoppele GJ, Loosdrecht AA. Mucormycosis in a patient with low risk myelodysplasia treated with anti-TNF-alpha. Haematologica. 2006;91(12_Suppl):139–140. doi: 10.3324/%x. [DOI] [PubMed] [Google Scholar]
- 279.Wall GC, Leman BI. Mucormycosis in a Crohn’s disease patient treated with infliximab. Digestion. 2009;80(3):182–4. doi: 10.1159/000230676. [DOI] [PubMed] [Google Scholar]
- 280.Wright AJ, Steiner T, Bilawich AM, English JC, Ryan CF. Pulmonary mucormycosis in a patient with crohn disease on immunosuppressive medications including infliximab. Can J Infect Dis Medi Microbiol. 2013;24:363250. doi: 10.1155/2013/363250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Krishnamurthy R, Dincer HE, Whittemore D. Strongyloides stercoralis hyperinfection in a patient with rheumatoid arthritis after anti-TNF-α therapy. JCR J Clin Rheumatol. 2007;13(3):150–2. doi: 10.1097/RHU.0b013e3180690933. [DOI] [PubMed] [Google Scholar]
- 282.Puri AS, Desai D, Sood A, Sachdeva S. Infliximab-induced tuberculosis in patients with UC: experience from India—a country with high prevalence of tuberculosis. J Gastroenterol Hepatol. 2017;32(6):1191–94. doi: 10.1111/jgh.13669. [DOI] [PubMed] [Google Scholar]
- 283.Wang Q, Wen Z, Cao Q. Risk of tuberculosis during infliximab therapy for inflammatory bowel disease, rheumatoid arthritis, and spondyloarthropathy: a meta-analysis. Exp Ther Med. 2016;12(3):1693–704. doi: 10.3892/etm.2016.3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Agarwal A, Kedia S, Jain S, Gupta V, Bopanna S, Yadav DP, et al. High risk of tuberculosis during infliximab therapy despite tuberculosis screening in inflammatory bowel disease patients in India. Intest Res. 2018;16(4):588. doi: 10.5217/ir.2018.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Haerter G, Manfras BJ, de Jong-Hesse Y, Wilts H, Mertens T, Kern P, et al. Cytomegalovirus retinitis in a patient treated with anti—tumor necrosis factor alpha antibody therapy for rheumatoid arthritis. Clin Infect Dis. 2004;39(9):e88–e94. doi: 10.1086/425123. [DOI] [PubMed] [Google Scholar]
- 286.Helbling D, Breitbach TH, Krause M. Disseminated cytomegalovirus infection in Crohn's disease following anti-tumour necrosis factor therapy. Eur J Gastroenterol Hepatol. 2002;14(12):1393–5. doi: 10.1097/00042737-200212000-00018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.