Abstract
Introduction.
Children with chronic illnesses and medical complexity (CIMC) require frequent acute health care use thereby increasing medical care costs.
Purpose.
We evaluated parent/child perceptions of self-management, self-efficacy, and health related quality of life (HRQOL) in children with CIMC.
Methods.
Parents/children (n=32 pairs) completed three measures on self-management (PAM), HRQOL (PedsQL), and Self-Efficacy Scale (SES) prior to discharge from hospital.
Results.
Parents (56.3%) and children (40.6%) reported moderate levels of self-management. HRQOL was correlated with both self-management (r = 0.441, p=0.12) and self-efficacy (r = 0.464, p=0.008). At least 25% to 50% reported low subscale scores (<70) indicating having some problems with physical, emotional, social, and mental domains.
Conclusion.
Our findings support assessment of, not only physical, but also mental, emotional, and social needs in children with CIMC. We recommend development and testing strategies promoting self-management and self-efficacy to maximize HRQOL and improve health outcomes in children with CIMC
Keywords: Children, Chronic Illness, Medical Complexity, Self-Management, Self-Efficacy, Health-related Quality of Life
INTRODUCTION
Children with chronic illnesses have complex health care needs, leading to frequent acute health care use, high medical care costs, and ultimately poor health outcomes. Children with chronic illness and medical complexity (CIMC), are patients under the age of 18, who have three or more of the following: (a) medications and/or supportive therapies (nutrition, respiratory, dialyses); (b) chronic medical diagnoses for 12 months or more; and (d) technology dependent (central or long term peripheral line, tracheostomy, gastrostomy) (Cohen, et al., 2011). Although only a small portion (~0.5%) of all U.S. children have CIMC, their impact on healthcare expenditure is prominent, accounting for one-third of the child healthcare expenditure in the United States (Berry, Agrawal, Cohen, Kuo, 2013; Berry, 2015; Kuo, Cohen, Agrawal, Berry, Casey, 2011). Indeed, children with CIMC are at high risk for acute illness that requires inpatient hospitalizations and frequent readmissions (Berry et al., 2011).
Children with CIMC and their caregivers are performing hospital-level care at home, and become experts on child‟s management (Berry, 2015). Their caregivers expressed the need for more education and support from the healthcare team to increase their confidence in skills and ability to manage care (Nelson et al., 2016; Hudson et al., 2014). By equipping patients and caregivers with the tools necessary to identify and manage symptoms, we can shift health care utilization from costly acute care admissions to cost-effective primary care health services (Nelson et al., 2016). In order to facilitate early recognition of symptoms and minimize the recurrent episodes of acute illness and complications, research is needed to evaluate knowledge, skills, and disease management children with CIMC.
Self-management reflects the child and caregiver‟s active engagement in knowing about the disease, recognizing signs and symptoms, and decision-making abilities to seek help in preventing potential complications (Lorig and Holman, 2003). These tasks include medication management, maintenance of daily activities, and the emotional work of having a chronic illness (Lorig and Holman, 2003). When children were taught self-management skills, their outcomes were associated with improved disease-related outcomes (Lorig and Holman, 2003; Yarcheski, Mahon, Yarcheski, Cannella, 2004). An improvement in health outcomes of type-1 diabetics were reported indicating that increased self-management skills were associated with improvement in Hemoglobin A1c levels (McBroom & Enriquez, 2009). The importance of interventions that promote self-management has been well documented in children indicating improvement in symptoms, medication adherence, and ability to manage illness. (Burkhart, Rayens, Oakley, Abshire, Zhang, 2007; Lozano & Houtrow, 2017; McBroom & Enriquez, 2009). Self-management in children with CIMC can be burdensome for both parent and child (Christian, 2016). For example, management of type 1 diabetes mellitus (T1DM) requires extensive education, comfort, and familiarity with dosage that can vary based on the child‟s current physical state (Rankin et al, 2018). In adolescent cancer patients, self-management needs were identified as disease knowledge, care skills, social support, and accessible healthcare services (Stinson et al, 2016). To date, very little information is available about individual perceptions of self-management across several populations of children with CIMC.
Self-efficacy is defined as the confidence the patient has in their capabilities to manage the demands of their illness adequately (Bandura 1997; Bandura 1986). Self-efficacy is an important mediator to self-management that leads to improvement in health status and health-related quality of life (Lorig and Holman, 2003). Those will lower self-efficacy was associated with greater perception of disease burden (Rutten et al., 2016). Caregivers reported higher self-efficacy, which is correlated with high self-management abilities (Sawyer, Drew, Yeo, Britto, 2007). A systematic review on self-management interventions indicated that youth with asthma demonstrated improvements in self-efficacy after self-management education (Guevara, Wolf, Grum, Clark, 2003). Several reports in adolescents with type 1 diabetes (T1D M) showed that self-efficacy may be an important factor in maintaining glycemic control and other aspects of disease management (Ott, Greening, Palardy, Holderby, DeBell, 2000; Sawyer, Drew, Yeo, Britto, 2007). Adolescents with cystic fibrosis reported significantly higher perceived self-efficacy compared to all other conditions, such as adolescents with kidney/urological conditions (Cramm, Strating, Roebroeck, Nieboer, 2013).
Health-related quality of life (HRQOL) describes the overall well-being related to health status (Varni, Limbers, Burwinkle, 2007; Varni et al., 2003). HRQOL consists of four different elements: 1) physical (pain, fatigue, breathing difficulty, etc.), emotional (worry, fear, anxiety), social (relation with peers, communication, interaction with others, etc.), and mental health (memory, problem solving, ability to do math, etc.). HRQOL is one of the ten identified domains for promoting a healthy lifestyle for children with CIMC (Barnert et al., 2018). Chronic illnesses such as cystic fibrosis, T1DM, neuromuscular disorders, rheumatoid arthritis, kidney/urological conditions were found to have lower HRQOL in most domains (social, physical, emotional) (Cramm, Strating, Roebroeck, Nieboer, 2013).
Purpose.
The few studies that reported on self-management, and self-efficacy in children with CIMC were disease specific and relationships among self-management, self-efficacy, and HRQOL were not reported. We therefore, evaluated self-management, self-efficacy and their effects on HRQOL. More specifically, we (1) examined self-management levels (low, medium, high), (2) explored relationships between self-management and self-efficacy, and 3) identified factors (demographic and medical characteristics self-efficacy, self-management) that may influence HRQOL. We hypothesized that high levels of self-management is highly correlated with higher self-efficacy and higher HRQOL. Findings from the study will provide preliminary information for the development of interventions that will improve disease self-management, and self-efficacy, that would consequently improve health outcomes (preventable and recurrent acute episodes of illness, complications, etc.) and overall HRQOL.
Individual and Family Self-Management Theory
The Individual and Family Self-Management Theory (IFSMT) was used as a guide for selection of variables in the study (Ryan & Sawin 2009). The IFSMT has four constructs: (1) context (risk and protective factors), (2) process (self-management), (3) proximal outcomes and (4) distal outcomes (Ryan & Sawin 2009). Context refers to the condition, physical and social environment, and individual/family. Context in the study includes demographic (age, sex, ethnicity, primary language, city of residence), diagnosis, and reasons for admission. We included self-management and self-efficacy as part of the process, and HRQOL as the primary outcome. Embedded in the theory is the hypothesis that self-management and self-efficacy are essential factors that lead to improvement in HRQOL (Ryan & Sawin 2009).
METHODS
Design.
A cross-sectional survey design was used to examine child and parent perceptions of self-management, self-efficacy, and HRQOL. Participants completed the Patient Activation Measure (PAM), Self-Efficacy Scale (SES), and Pediatric Quality of Life (PedsQL) scales once during hospitalization.
Sample.
Children and parents/legal guardian were recruited from three acute care pediatric units in a large quaternary medical center in southwestern United States. Children and parents were eligible if 1) age is 8 to 17 years and the child has CIMC. CIMC defined as receiving: (1) multiple medications and supportive therapies for nutrition, respiratory, renal clearance), (2) technology dependent (central or peripheral line for long-term infusions, tracheostomy, mechanical ventilation, oxygen therapy, gastrostomy or jejunostomy), or (3) requiring extended hours of home nursing care (> 8 hrs/week). Children and parents were excluded if they were known to have cognitive impairment or neurologic delays that prevent completion of the outcome measures. Because the data collection tools were available only in English, those who are not able to read, write, or speak English were also excluded. Children whose parent or legal guardian was not available were not recruited. Continuous enrollment occurred during a 12-month period in order to get a representative sample that takes into account seasonal variations.
Procedures.
The lead registered nurse (RN) in the acute care pediatric units identified potential participants based on the criteria above, and distributed recruitment flyers, and requested permission for the research staff to explain the details of the study. The research staff explained the details of the study using the Study Information Sheet. Participants were allowed to ask questions and express concerns, discuss participation with family members, and ask if they agree and willing to being part of the study. Those who agreed to participate were read the Parent Consent & Permission form, and the assent form. Enrollment occurred after consent/assent procedures, which took place in the patient room or in a quiet private room within the pediatric unit. Data collection procedures occurred within the 24 to 48 hours prior to anticipated discharge, indicating readiness to go home. Both parents and child completed the measures, which took a total of 30 minutes. All materials and procedures were approved by the Institutional Review Board.
Outcome Measures.
The following three measures were used to collect parent and child perceptions of self-management, self-efficacy, and HRQOL.
Patient/Parent Activation Measure –
The Patient Activation Measure (PAM) evaluates the participant‟s reported knowledge, skills, and confidence for self-management of health condition (Hibbard, et al, 2004). The items represent four domains: 1) Believes Active Role as Important; 2) Confidence and Knowledge to Take Action; 3) Taking Action; 4) Staying the Course Under Stress. Both parent and child versions consisted of 10 items related to (1) taking responsibility of child‟s health, (2) the importance of taking an active role in child‟s health; (3) knowledge of medications; (4) confidence with being able to tell whether the child needs to go to the doctor, or ability take care of child‟s health problem; (5) with being able to talk about concerns with the doctor; 6) with following through on medical treatments at home; 7) being able to maintain life changes (exercise/physical activity, eating; 8) knowing how to prevent problems with one‟s health; 9) with figuring out solutions to potential problems; and 10) maintaining lifestyle changes, even during times of stress.
The PAM has a Likert response format ranging from strongly disagree to strongly agree. Data were sent to Insignia Health® for scoring, with scores ranging from 0 to 100, with higher scores indicating high level of self-management. PAM levels were used to determine how many and what percent of parent/child participants were categorized as having low (Levels 1, 2), medium (Level 3), and high (Level 4) self-management. Total Scores and Levels were used for analyses.
Level 1:
Disengaged and Overwhelmed, defined as parent of/or a child/adolescent that has a low level of knowledge and skills, passive in their illness management, has a lack of confidence, and has poor adherence to treatment. The perception is “My doctor is in charge of my health” (PAM scores <25).
Level 2:
Becoming Aware and Struggling, defined as parent/child having some knowledge but gaps in knowledge remain. The child‟s health is perceived as largely out of their control, but they can set simple goals. The perception is “I could be doing more” (PAM scores range from 25–50).
Level 3:
Taking Action, defined as parent/child have key information and is building self-management skills. They are striving for best practice and are goal oriented. The perspective is “I’m part of my health care team” (PAM scores range from 50–75)
Level 4:
Maintaining Behaviors and Pushing Further, defined as parent/child has adopted new behaviors, but may struggle in times of stress or change. They focus on maintaining a healthy lifestyle. The perspective is “I am my own advocate” (PAM scores > 75).
The reliability of the PAM indicated correlation coefficients between 0.84 to 0.89 in with a variety of chronic conditions, different ethnicities, educational level, and socioeconomic status (Hibbard, Stockard, Mahoney, Tusler, 2004). The PAM is correlated with increased patient participation or engaging in specific self-care and preventive behaviors, better health outcomes, and significantly lower rates of doctor office visits, emergency room visits, and hospital lengths of stay – all associated with self-management (Hibbard et al, 2004; Hibbard & Greene 2013; Mitchell et al., 2014; Stepleman et al., 2010). Construct validity of PAM was evident in several studies indicating positive outcomes (preventive behaviors, disease-specific self-management behaviors, patient engagement) with higher PAM scores (Fowles, Terry, Xi, Hibbard, Bloom, Harvey, 2009; Stepleman et al, 2010). There were no studies found in children using the PAM, we therefore examined using reliability analyses PAM, SES, PedsQL Scores with Cronbach α= 0.730 for child measures based on standardized items.
Parent/Child Self-Efficacy Scale.
The Sickle Cell Disease Self-Efficacy Scale (SCD-SES) (Edwards, et al, 2000; Clay & Telfair, 2007) was selected to be modified for the measurement of self-efficacy for parent/child SES-CIMC version, because there were a reasonable number of items (n=9) and generalizability, compared to other disease specific self-efficacy scale (e.g. diabetes, asthma, arthritis, etc.). The SCD-CIMC has nine items describing perceived ability to engage in daily functional activities despite having a, which was revised to state “despite having a chronic condition”. Items with the word “pain” were replaced with “signs and symptoms related to chronic condition”. Response categories for each item range from “not at all sure” to “very sure.” The measure provides an overall index of self-efficacy by summing item scores; higher scores indicate greater self-efficacy. Acceptable reliability (r=0.45, p<0.001) and validity (alpha=0.89) of the SCD-SES have been established in children with SCD-SES (Edwards, et al, 2000; Clay & Telfair, 2007). In our sample, internal consistency for parents ranged from α =0.235 (parents of children) to α=0.769 (parents of adolescents). Internal consistency reliability for children (α=0.755) and teens (α=0.825) was demonstrated for the SES measure in the sample.
Parent/Child PedsQL.
The parent version (appropriate for age 8 to 12; 13 to 17 years) and child version (appropriate for age 8 to 12; 13 to 17 years) PedsQL Acute versions were used to collect parent and child perceptions of HRQOL (Varni & Limbers, 2009). The PedsQL was developed to assess both parent/child perceptions of HRQOL in children with different health conditions. It measures the core physical, mental, social, and cognitive health dimensions of HRQOL. Response items indicated the extent of a problem: (0 = never a problem; 1 = almost never a problem; 2 = sometimes a problem; 3 = often a problem; 4 = almost always a problem). Items are reverse-scored and linearly transformed from a 0 to 100 scale (0=100, 1=75, 2=50, 3=25, 4=0). The internal consistency reliability for child/self-report and parent proxy report ranged from 0.66 to 0.93; the parent/child concordance intercorrelations ranged from 0.305 to 0.944 (Varni, et al., 2001). The higher PedsQL scores indicate better Health Related Quality of Life (Varni, Seid, & Kurtin, 2001). The internal consistency reliability for our sample is Cronbach α= 0.802 for children/teens and Cronbach α= 0.842 for parents.
In addition to the three outcome measures, parents were also asked about demographics (age, sex, ethnicity, etc.) and medical information (diagnosis, reason for admission). A checklist was used to verify medical complexity, such as, medications, nutrition support, therapeutic interventions (respiratory, oxygen, dialyses), technology dependence (intravenous medications, parenteral nutrition support, central or peripheral lines, tracheostomy, ventilation, gastrostomy, jejunostomy, colostomy), and others indicating medical complexity.
Data Analyses.
All data were entered using SPSS (Chicago, IL, version 25.0). Descriptive statistics (frequencies, means, standard deviations) were used to describe the demographics, diagnoses, reasons for hospitalizations, self-management (PAM), self-efficacy (SES), HRQOL (PedsQL) and subscales (physical, emotional, social, mental). Pearson correlations were used to examine the relationships among the variables, and between parent and child ratings on PAM, SES, PedsQL, and subscales. T-tests and ANOVA were used to compare factors (age, sex, primary language, ethnicity, area of residence, diagnoses, reasons for hospitalizations, SES, PAM) that may have impacts on HRQOL.
RESULTS
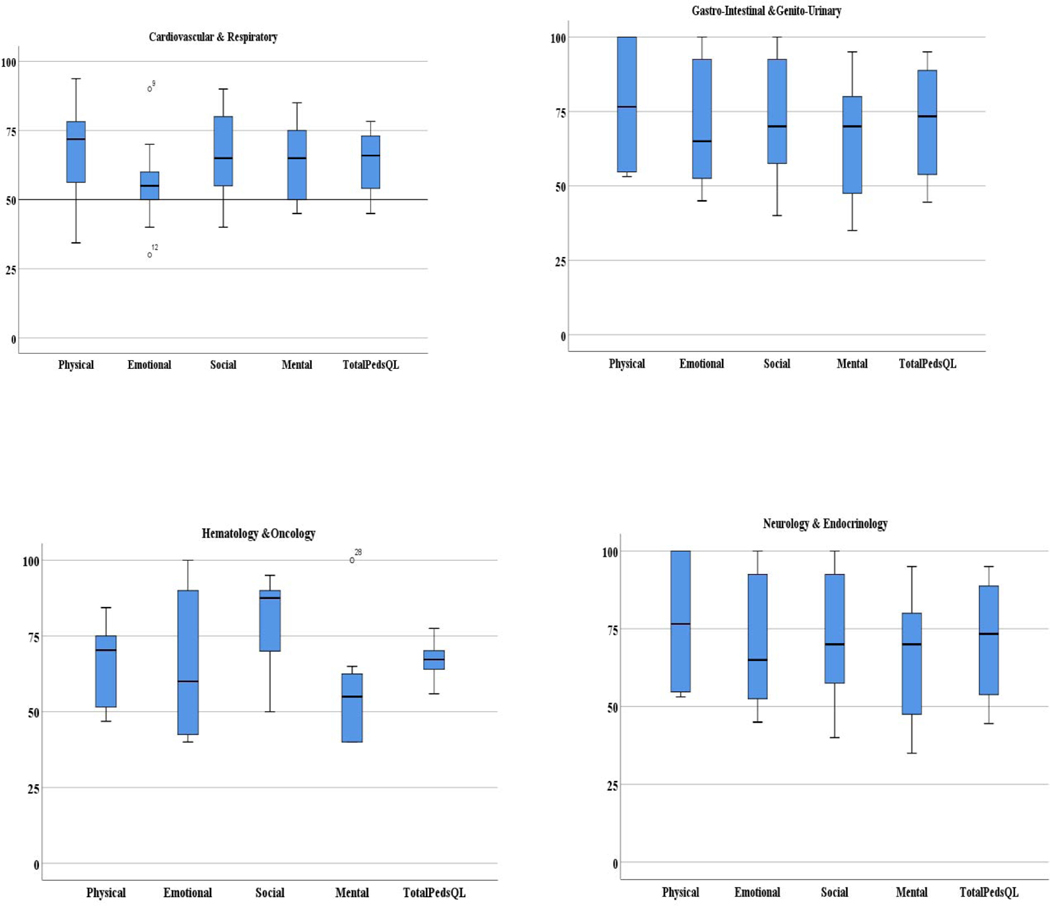
The participants were children (n=11; mean age 10.4 ± 1.4 years), adolescents (n= 21; 15.2 ± 1.5 years) and their parents or guardians (n=32). The majority of participants (Table 1) were adolescents (65.6%), Caucasian (68.8%), primary language spoken at home was English (81.3%), and resided outside the metropolitan Los Angeles area (65.6%). The most frequently reported diagnoses (Table 1) were gastrointestinal-genitourinary (28.1%), followed by neurology-endocrinology (25.0%), hematology-oncology (25.0%), and cardio-respiratory (21.9%). The most frequent reasons for hospitalization (Table 1) was for management of sign and symptoms (65.6%), and the others were for diagnostic or surgical procedures (28.1%), and medical treatments such as chemotherapy (6.3%).
Table 1.
Demographics (N=32)
| n (%) | |
|---|---|
| Age | |
| Children (10.4 ± 1.4 years) | 11 (34.4%) |
| Adolescent (15.2 ± 1.5 years) | 21 (65.6%) |
| Gender | |
| Male | 16 (50.0%) |
| Female | 16 (50.0%) |
| Race-Ethnicity | |
| Caucasian | 22 (68.8%) |
| Latinx | 5 (15.6%) |
| Other | 5 (15.6%) |
| Primary Language | |
| English Only | 26 (81.3%) |
| English/Other | 6 (18.8%) |
| Area of Residence | |
| Out of Metro LA | 21 (65.6%) |
| Metro LA | 9 (28.1%) |
| Out of State | 2 (6.3%) |
| Diagnoses | |
| Gastro-Intestinal-Genito-Urinary | 9 (28.1%) |
| Hematology-Oncology | 8 (25.0%) |
| Cardio-Respiratory | 8 (25.0%) |
| Neurology- Endocrine | 7 (21.9%) |
| Reason for Admission | |
| Signs & Symptoms | 21 (65.6%) |
| Procedures (Diagnostics/Surgeries) | 9 (28.1%) |
| Medical Treatments | 2 (6.3%) |
Note. Children—ages 8–12; Adolescents—ages 13–17; Latinx—gender inclusive term referring to those of Hispanic or Latino/a origin
Children/Adolescents
Self-Management.
The mean self-management score was 66.06 ± 17.9. As illustrated in Table 2, some (28.1%) had significantly lower self-management (47.0 ± 9.8) compared to others (40.6%) who had moderate (62.9 ± 5.1) or high (31.3%) self-management scores (87.4 ± 10.0; p<.001). Self-management for different CIMC conditions (Table 3) ranged from 54.8 ± 14.4 (hematology, oncology) to 77.0 ± 14.4 (cardiac-respiratory). Self-management by reasons for admission ranged from 53.0 ± 4.2 (medical treatments) to 68.7 ± 22.5 (diagnostic-surgical procedures). Differences in self-management scores among diagnoses and reasons for hospitalization were not significant. Self-management scores were not significantly different by age, gender, ethnicity, language, or area of residence.
Table 2.
PAM Level and Self-Management, Self-Efficacy & HRQOL
| Low (Means ± SD) | Medium (Means ± SD) | High (Means ± SD) | ANOVA (F, p-value) | |
|---|---|---|---|---|
| CHILDREN | n=9 | n=13 | n=10 | |
|
| ||||
| Self-Management | 47.0 ± 9.8 | 62.9 ± 5.1 | 87.4 ±10.0 | 58.45,<0.001** |
| Self-Efficacy | 28.4 ± 3.9 | 32.9 ± 4.1 | 37.6 ± 7.1 | 7.38, 0.003** |
| HRQOL Total | 64.4 ± 10.6 | 64.7 ± 11.6 | 80.5 ± 11.3 | 6.82,0.004** |
| Physical | 68.8 ± 13.3 | 65.6 ± 18.7 | 87.8 ± 13.1 | 6.19,0.006** |
| Emotional | 55.0 ± 17.0 | 65.8 ± 23.6 | 76.0 ± 19.7 | 2.43, 0.106 |
| Social | 65.0 ± 10.0 | 70.4 ± 19.5 | 88.0 ± 11.8 | 6.29,0.005** |
| Mental | 68.9 ± 21.9 | 56.9 ± 15.4 | 70.0 ± 10.8 | 2.28, 0.120 |
|
| ||||
| PARENTS | n=4 | n=10 | n=18 | |
|
| ||||
| Self-Management | 39.0 ± 26.0 | 63.4 ± 7.1 | 88.2 ± 8.6 | 37.3, 0.00** |
| Self-Efficacy | 34.0 ± 5.4 | 31.4 ± 9.1 | 36.8 ± 6.2 | 1.84, 0.18 |
| HRQOL Total | 65.0 ± 20.6 | 65.7 ± 15.9 | 69.5 ± 10.3 | 0.35, 0.71 |
| Physical | 62.5 ± 19.3 | 74.1 ± 22.8 | 81.4 ± 12.8 | 2.16, 0.13 |
| Emotional | 60.0 ± 15.8 | 54.5 ± 21.0 | 55.8 ± 16.8 | 0.13, 0.88 |
| Social | 61.3 ± 30.7 | 67.5 ± 22.6 | 69.4 ± 17.1 | 0.26, 0.77 |
| Mental | 76.3 ± 21.4 | 73.5 ± 17.0 | 71.4 ± 17.3 | 0.14, 0.87 |
Significant (p<0.05)
Note. HRQOL—Pediatric Health Related Quality of Life—Child/Teen and Parent Scales
Table 3.
Self-Management, Self-Efficacy, HRQOL by Diagnosis
| GI-GU (n=9) Means ± SD | Card-Resp (n=8) Means ± SD | Heme-Onc (n=8) Means ± SD | Neuro-Endo (n=7) Means ± SD | ANOVA F, p value | |
|---|---|---|---|---|---|
| Self-Management | 65.4 ± 15.8 | 77.0 ± 14.4 | 54.8 ± 14.4 | 68.5 ± 21.7 | 2.22, 0.11 |
| Self-Efficacy | 31.3 ± 6.2 | 34.43 ± 6.3 | 31.6 ± 4.8 | 35.4 ± 7.3 | 0.86, 0.48 |
| HRQOL | |||||
| Total | 64.4 ± 11.6 | 76.8 ± 11.3 | 67.0 ± 6.3 | 71.4 ± 19.3 | 1.35, 0.28 |
| Physical | 68.8 ± 18.9 | 84.4 ± 12.0 | 65.6 ± 13.8 | 77.0 ± 22.5 | 1.76, 0.18 |
| Emotional | 56.7 ± 17.1 | 72.9 ± 22.0 | 65.6 ± 25.6 | 70.6 ± 22.3 | 0.90, 0.45 |
| Social | 68.3 ± 17.0 | 77.9 ± 15.0 | 80.0 ± 16.3 | 72.5 ± 21.5 | 0.74, 0.54 |
| Mental | 63.9 ± 14.1 | 72.1 ± 10.7 | 56.9 ± 20.0 | 65.6 ± 20.8 | 1.02, 0.40 |
Note. HRQOL—Pediatric Health Related Quality of Life—Child/Teen and Parent Scales; GI-GU: Gastrointestinal-Genitourinary; Card-Resp:Cardiac-Respiratory; HemeOnc:Hematology-Oncology; Neuro-Endo:Neurology-Oncology.
Self-Efficacy.
The mean self-efficacy score was 33.1 ± 6.2 (range 23–45). There were significant differences in self-efficacy by self-management levels (Table 2). Those with higher self-management level had higher self-efficacy (37.6 ± 7.1) compared to those with medium self-management level (32.9 ± 4.1) or low self-management level (28.4±3.9; p=0.003; Table 2). Self-efficacy scores (Table 3) ranged from 31.3 ± 6.2 (GI-GU) to 35.4 ± 7.3 (Neuro-Endo); no significant differences in self-efficacy across CIMC diagnoses were found. Self-efficacy was similar regardless of reason for admission (Table 4). Self-management and self-efficacy (Table 5) were highly correlated (r = 0.520, p=0.002). Self-efficacy scores were not significantly different by age, gender, ethnicity, primary language, or area of residence.
Table 4.
Self-Management, Self-Efficacy, and HRQOL by Reason for Hospitalization
| Sign-Symptoms n=21 Means ± SD | Procedures n=9 Means ± SD | Treatments n=2 Means ± SD | ANOVA F, P value | |
|---|---|---|---|---|
| Self-Management | 66.2 ± 16.5 | 68.7 ± 22.5 | 53.0 ± 4.2 | 0.62, 0.55 |
| Self-Efficacy | 33.0 ± 5.9 | 33.4 ± 7.7 | 33.0 ± 2.8 | 0.019, 0.98 |
| HRQOL | ||||
| Total | 71.8 ± 11.2 | 66.2 ± 17.7 | 60.9 ± 7.0 | 1.03, 0.37 |
| Physical | 73.4 ± 18.5 | 76.4 ± 18.8 | 60.9 ± 11.0 | 0.58, 0.57 |
| Emotional | 69.8 ± 19.8 | 58.3 ± 25.1 | 60.0 ± 28.3 | 0.95, 0.40 |
| Social | 79.1 ± 14.0 | 64.4 ± 20.4 | 70.0 ± 28.3 | 2.49, 0.10 |
| Mental | 65.0 ± 15.4 | 65.6 ± 21.3 | 52.5 ± 17.7 | 0.51, 0.61 |
Note. HRQOL—Pediatric Health Related Quality of Life—Child/Teen and Parent Scales
Table 5.
Relationships Among Self-Management, Self-Efficacy, and HRQOL
| Self-Management | Self-Efficacy | |
|---|---|---|
|
| ||
| CHILDREN | r, p-value | r, p-value |
| Self-Management | -- | |
|
| ||
| Self-Efficacy | 0.520, 0.002** | -- |
| Total HRQOL | 0.441, 0.012** | 0.464, 0.008** |
| Physical | 0.417, 0.018** | 0.290, 0.107 |
| Emotional | 0.298, 0.098 | 0.553, 0.001** |
| Social | 0.476, 0.006** | 0.393, 0.026** |
| Mental | 0.058, 0.753 | 0.025, 0.891 |
|
| ||
| PARENTS | ||
|
| ||
| Self-Management | -- | |
| SES | 0.178, 0.33 | -- |
| Total HRQOL | 0.145, 0.43 | −0.117, 0.52 |
| Physical | 0.374, 0.04** | −0.067, 0.71 |
| Emotional | −0.069, 0.71 | −0.444, 0.01** |
| Social | 0.115, 0.53 | −0.236, 0.19 |
| Mental | −0.097, 0.60 | −0.005, 0.98 |
Significant (p<0.05)
Note. HRQOL—Pediatric Health Related Quality of Life—Child/Teen and Parent Scales
Health Related Quality of Life.
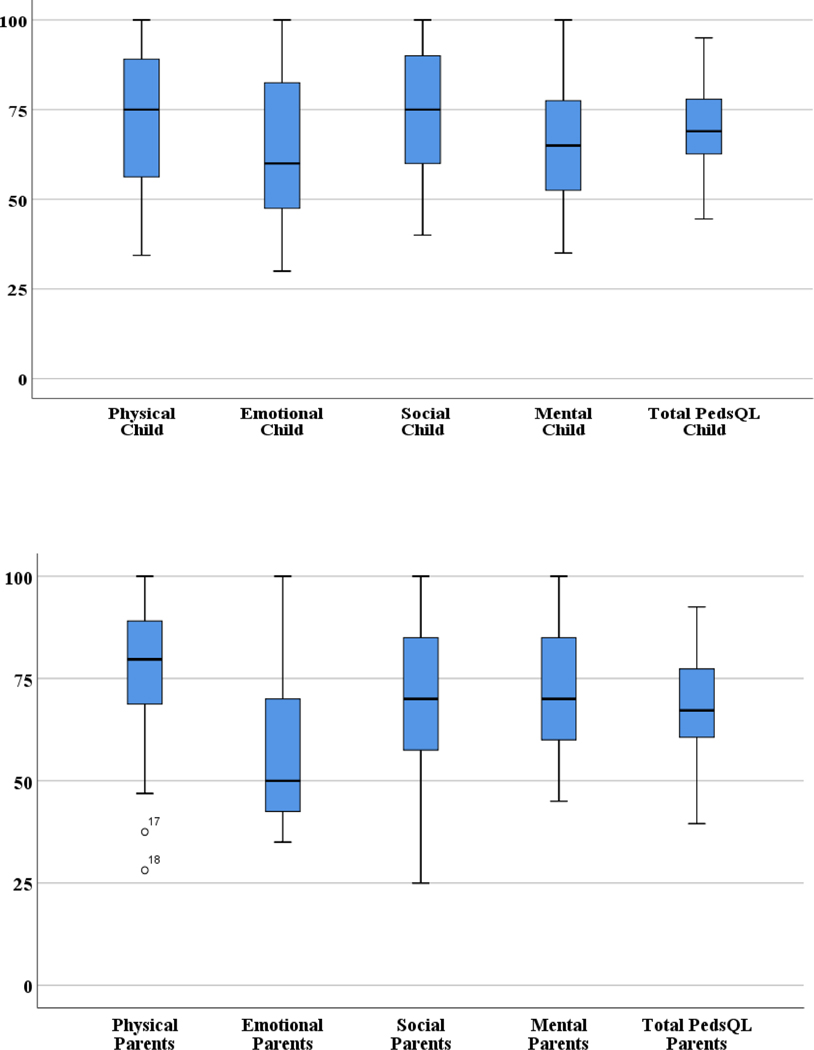
The mean total PedsQL score was 69.5 ± 13.2. The physical (73.4 ± 18.2) and social (74.4 ± 17.4) subscale scores were high (>70). The mean scores were low (<70) in the emotional (65.9 ± 21.7) and mental (64.4 ± 17.0) subscale scores (Figure 1). As the boxplots in Figure 1 indicated, at least 25% to 50% have low scores (<70) in all subscales, indicating problems with physical, emotional, social, mental functioning. PedsQL scores (Table 3) by diagnoses ranged from 64.4 ± 11.6 (GI-GU) to 76.8 ± 11.3 (Card-Resp). PedsQL scores by reason for hospitalization (Table 4) ranged from 60.9 ± 7.0 (medical treatments) to 71.8 ± 11.2 (signs-symptoms). Differences in HRQOL scores among diagnoses and reasons for hospitalization were not significant (Figure 2).
Figure 1.
Distribution of Parental and Child Perceptions of Health Related Quality of Life Score in Children with Chronic Illness & Medical Complexity
Figure 2.
Distribution of Child Perceptions of Health Related Quality of Life Score by Medical Diagnosis (Cardiology & Respiratory, Gastro-Intestinal & Genito-Urinary
The total HRQOL is highly correlated with both self-management (r = 0.441, p=0.12) and self-efficacy (r = 0.464, p=0.008), indicating that those with high HRQOL also had high self-management and self-efficacy (Table 3). The physical (r = 0.417, p = 0.018) and social (r = 0.476, p=0.006) subscale scores, were significantly correlated with self-management (Table 3). The emotional (r=0.553, p=0.001) and social (r=0.393, p=0.026) subscale scores were correlated with self-efficacy (Table 3).
There were significant differences in total HRQOL by self-management levels. Those with low (64.4 ± 10.6) and medium (64.7 ± 11.6) self-management levels, had significantly lower HRQOL, compared to those with high self-management level (80.5 ± 11.3, p = 0.004). There were also significant differences by self-management for physical (p = 0.006) and social (p=0.005) subscale scores, with HRQOL in these domains significantly higher as self-management level increased (Table 2).
While children reported higher social scores (79.6 ± 15.4) compared to adolescents (71.7 ± 18.2), and lower mental scores (59.1 ± 16.1) than adolescents 67.1 ± 17.2), the difference was not significant. Similarly, children also reported lower physical (71.0 ± 20.8) and emotional (64.6 ± 18.5) compared to adolescents (74.7 ± 17.0 and 66.7 ± 23.6, respectively), the difference was not significant. HRQOL did not differ by gender, ethnicity, primary language, or area of residence.
Parents
Self-Management.
Parents reported an overall high mean score for self-management of their child‟s condition (74.3 ± 20.0). More than half (56.3%) reported high self-management (88.2 ± 8.6) compared to others (31.3%) who had moderate (63.4 ± 7.1) or low (12.5%) self-management scores (39.0 ± 26.0, F=37.3, p < 0.001). Self-management scores did not significantly differ by age, diagnoses (Table 3) nor reason for hospitalization (Table 4). No significant differences were found in self-management scores by age, gender, ethnicity, language, and city of residence.
Self-Efficacy.
The parent mean self-efficacy score was 34.8 ± 7.4 (range 9–44). There were no significant differences in self-efficacy score by self-management levels. Parents with higher self-management level had higher self-efficacy (36.8 ± 6.2) compared to those with moderate (31.4 ± 9.1) or low self-efficacy (34.0 ± 5.4) (Table 2). Parent self-efficacy scores did not significantly differ by demographics (age, gender, primary language, areas of residence), by diagnoses, or by reason for hospitalization. However, self-efficacy scores were significantly different by ethnicity, with the highest self-efficacy scores in Caucasian parents (36.5 ± 6.1), compared to Latinx (35.6 ± 4.4), and other ethnicity category (26.2 ± 9.9; F=5.1, p=0.01)
Health Related Quality of Life.
The parent mean total PedsQL score was 67.8 ± 13.3. The physical (76.8 ± 17.8) and mental (72.7 ± 17.2) subscale scores were high (>70). However, the mean scores were low (<70) in the emotional (55.9 ± 17.6) and d of (67.8 ± 20.2) subscale scores. Similar to child ratings, at least 25% to 50% reported low scores for their child (<70) in all subscales, indicating parents noted problems in the physical, emotional, social and mental domains of the child‟s HRQOL.
Self-management was significantly correlated with physical HRQOL (r=0.37, p=0.04), and self-efficacy with emotional HRQOL (r=−0.44, p=0.01) (Table 3). There were no significant differences in total HRQOL by self-management levels (F=0.348, p=0.71), and in all subscales (Table 3). Parent HRQOL did not differ by demographics (gender, ethnicity, primary language, area of residence) or diagnoses and reasons for hospitalization.
DISCUSSION
We evaluated self-management, self-efficacy and factors that impact HRQOL. Our findings indicated wide variations in parent and child report of self-management levels. We did not find other studies that reported self-management; the other reports were predominantly adults (Hibbard et al. 2005; Pennarola et al., 2012; Hibbard & Cunningham, 2008). These findings support assessment of self-management levels prior to discharge, and provide coaching to promote self-management in children with low and moderate self-management Future studies need to explore strategies that would promote self-management prior to discharge. We also found that parent/child participants had medium self-efficacy scores, which were consistent with findings in sickle cell disease (Crosby et al., 2017), celiac disease (Haas, Martin, Park, 2017), and hematopoietic stem cell transplant patients (Pennarola et al., 2012). Because self-management and self-efficacy are significantly correlated, teaching strategies to promote self-efficacy, may also promote self-management, strategies for promoting self-efficacy and self-management in children need to be developed and tested as previously reported in adults (Bonsaksen T, Lerdal A, Fagermoen MS., 2012; Jerant A, Moore M, Lorig K, Franks P., 2008).
We found high correlations between self-management and self-efficacy in both parent and child ratings, which is consistent with other reports (Guevara, Wolf, Grum, Clark, 2003; Ott, Greening, Palardy, Holderby, DeBell, 2000; Sawyer, Drew, Yeo, Britto, 2007). Some patients were requiring coaching prior to discharge to increase knowledge, skills, and confidence in disease and symptom management (Hibbard et al., 2005). Future studies are needed to test effective strategies that further promote self-management and self-efficacy in children with CIMC.
Our findings indicated that parents and child ratings of child‟s HRQOL were high. However, the emotional and mental score ratings were lower compared to physical and social scores. The lower mental scores indicate that children had problems often or almost always with paying attention in class, forgetting things, keeping up with schoolwork, and missing school. Those who reported lower emotional scores indicated had problems often or almost always with being afraid, worried, sad, and having trouble with sleeping. Parent ratings of child social scores were also low, indicating that their child had often or almost always have problems with relating to peers, being teased, keeping up with peers, and unable to do things that others in same are able to. Others also reported lower mental and emotional aspects of HRQOL (Varni et al., 2007). Therefore, health care providers need to assess the mental, emotional, and social well-being of children with CIMC and discuss with parents strategies for improving them. Future studies are needed to develop and test educational and psychosocial interventions that would improve the mental, emotional, and social well-being in children with CIMC.
Limitations.
Several limitations affect the generalizability of these findings, particularly the small sample size representing the different chronic illness and medical complexity. Children may have significant differences based on the CIMC diagnoses and treatment protocols, that may have affected their self-management, self-efficacy, and HROQL. Our preliminary findings support the need to assess and develop strategies that promotes self-efficacy, self-management that could consequently lead to better HRQOL and disease related outcomes. Future studies with larger sample representing the different CIMC diagnosis are needed.
We excluded patients younger than 8 years old and patients with cognitive or neurological impairments. These patients most likely have different HRQOL. Because we used only the English version, findings may not be generalized to those were not able to speak, read, write, and understand English. Lastly, we conducted the study in one setting with highly complex patients, which may have specific characteristics that differ from other inpatient acute care settings. In addition, children with CIMC at home may have different levels of self-management, self-efficacy, and HRQOL. Future studies are needed to examine self-management, self-efficacy, and HRQOL among diverse settings including home.
CONCLUSION
Our findings indicate that high self-efficacy is highly correlated with self-management and HRQOL in CIMIC. These findings provide preliminary evidence in support of the Individual and Family Self-Management Theory that guided this study. Health care providers need to pay attention not only to the physical needs, but also the mental, emotional, and social needs of children with CIMC. Future studies are needed to develop and test strategies that would promote self-management, self-efficacy, to maximize HRQOL improved health outcomes in children with CIMC. Educational and technology based interventions (Jacob, et al., 2012; Jacob, et al., 2013a; Jacob, et al., 2013b) that facilitated self-monitoring by children, remote monitoring by health care providers, and increase communications between child and health care providers may be tested to increase self-efficacy, self-management, and overall HRQOL in children with CIMC.
Supplementary Material
HIGHLIGHTS.
Children with chronic illnesses have complex health care needs requiring frequent hospitalizations due to acute exacerbations of symptoms or preventable complications.
Caregivers are performing hospital-level care at home and need ongoing education and support to increase self-management, self-efficacy that would lead to improved health related quality of life.
By equipping patients and caregivers with the tools necessary to identify and manage symptoms, health care utilization can be shifted from costly acute recurrent hospitalizations and most cost-effective health services that would lead to optimal health outcomes.
ACKNOWLEDGEMENT
Funding was received by Lilian Bravo and Mary Killela from the National Institutes of Health, National Institutes of Nursing Research T32NR007091-24: Interventions for Preventing and Managing Chronic Illness (MPIs: S. Santacroce/J. Leeman) while this work was being conducted. The authors are grateful for the nurses at UCLA Mattel Children‟s who facilitated access to patients. The authors are also grateful for the parents and participants who willingly agreed to complete data collection procedures. We also acknowledge the contributions of research students enrolled in Research Apprentice Course during the study period.
Authors Lilian Bravo and Mary Killela received support from the National Institutes of Health, National Institutes of Nursing Research T32NR007091-24: Interventions for Preventing and Managing Chronic Illness (MPIs: S. Santacroce/J. Leeman) while this work was being conducted.
All authors approved the content of the manuscript and have contributed significantly to research involved, and the writing of the manuscript.
Support from the National Institutes of Health, National Institutes of Nursing Research T32NR007091-24: Interventions for Preventing and Managing Chronic Illness (MPIs: S. Santacroce/J. Leeman) for two authors while this work was being conducted.
Footnotes
Ethical Statement
We the undersigned declare that this manuscript is original, has not been published before and is not currently being considered for publication elsewhere.
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed.
We further confirm that the order of authors listed in the manuscript has been approved by all of us.
We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
We further confirm that any aspect of the work covered in this manuscript that has involved human patients has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
We understand that the Corresponding Author is the sole contact for the Editorial process (including Editorial Manager and direct communications with the office). She is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs. We confirm that we have provided a current, correct email address which is accessible by the Corresponding Author.
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Lilian Bravo, University of North Carolina at Chapel Hill, Carrington Hall CB#7460, Chapel Hill, North Carolina 27599-7460.
Mary K. Killela, University of North Carolina at Chapel Hill, University of North Carolina at Chapel Hill, Carrington Hall CB#7460, Chapel Hill, North Carolina 27599-7460.
Beck L. Reyes, UCLA Adolescent Epilepsy Center, 300 UCLA Medical Plaza Driveway Suite B200, Los Angeles, CA 90095.
Karla Marie Banhan Santos, UCLA School or Nursing, 700 Tiverton Avenue, Factor Building 5-942, Los Angeles, CA 90095.
Vanessa Torres, Lucile Packard Children‟s Hospital Stanford, 725 Welch Rd, Palo Alto, CA 94304.
Chai-Chih Huang, UCLA Mattel Children’s Hospital, 757 Westwood Plaza, Suite 5356B, MC: 7404030; Los Angeles, CA 90095.
Eufemia Jacob, UCLA School of Nursing, 700 Tiverton Avenue, Factor Building 5-942, Los Angeles, CA 90095.
REFERENCES
- Barlow JH, & Ellard DR (2006). The psychosocial well- being of children with chronic disease, their parents and siblings: An overview of the research evidence base. Child: care, health and development, 32(1), 19–31. [DOI] [PubMed] [Google Scholar]
- Barnert ES, Coller RJ, Nelson BB, Thompson LR, Klitzner TS, Szilagyi M, … & Chung PJ (2018). A healthy life for a child with medical complexity: 10 domains for conceptualizing health. Pediatrics, 142(3), e20180779. [DOI] [PubMed] [Google Scholar]
- Berry JG, Agrawal RK, Cohen E, & Kuo DZ (2013). The landscape of medical care for children with medical complexity. Overland Park, KS: Children‟s Hospital Association, 7 [Google Scholar]
- Berry JG (2015). What children with medical complexity, their families, and healthcare providers deserve from an ideal healthcare system. system, 3(8). [Google Scholar]
- Bonsaksen T, Lerdal A, Fagermoen MS. (2012). Factors associated with self-efficacy in persons with chronic illness. Scand J Psychol, 53(4):333–9. [DOI] [PubMed] [Google Scholar]
- Burkhart PV, Rayens MK, Oakley MG, Abshire DA, & Zhang M (2007). Testing an intervention to promote children’s adherence to asthma self- management. Journal of Nursing Scholarship, 39(2), 133–140. [DOI] [PubMed] [Google Scholar]
- Christian B (2016). Translational research–Balancing the demands of chronic illness caregiving and self-management for children, adolescents, and their parents. Journal of pediatric nursing, 31(4), 449–452. [DOI] [PubMed] [Google Scholar]
- Cohen E, Kuo DZ, Agrawal R, Berry JG, Bhagat SK, Simon TD, & Srivastava R (2011). Children with medical complexity: an emerging population for clinical and research initiatives. Pediatrics, 127(3), 529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramm JM, Strating MM, Roebroeck ME, & Nieboer AP (2013). The importance of general self-efficacy for the quality of life of adolescents with chronic conditions. Social indicators research, 113(1), 551–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosby LE, Joffe NE, Peugh J, Ware RE, & Britto MT (2017). Pilot of the chronic disease self-management program for adolescents and young adults with sickle cell disease. Journal of Adolescent Health, 60(1), 120–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashiff C, Riley BH, Abdullatif H, & Moreland E (2011). Parents’ experiences supporting self-management of middle adolescents with type 1 diabetes mellitus. Pediatric nursing, 37(6). [PubMed] [Google Scholar]
- Fowles JB, Terry P, Xi M, Hibbard J, Bloom CT, & Harvey L (2009). Measuring self-management of patients‟ and employees‟ health: further validation of the Patient Activation Measure (PAM) based on its relation to employee characteristics. Patient education and counseling, 77(1), 116–122. [DOI] [PubMed] [Google Scholar]
- Gannoni AF, & Shute RH (2010). Parental and child perspectives on adaptation to childhood chronic illness: A qualitative study. Clinical Child Psychology and Psychiatry, 15(1), 39–53. [DOI] [PubMed] [Google Scholar]
- Guevara JP, Wolf FM, Grum CM, & Clark NM (2003). Effects of educational interventions for self management of asthma in children and adolescents: systematic review and meta-analysis. Bmj, 326(7402), 1308–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas K, Martin A, & Park KT (2017). Text Message Intervention (TEACH) Improves Quality of Life and Patient Activation in Celiac Disease: A Randomized Clinical Trial. The Journal of pediatrics, 185, 62–67.e2. doi: 10.1016/j.jpeds.2017.02.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbard JH, Stockard J, Mahoney ER, & Tusler M (2004). Development of the Patient Activation Measure (PAM): conceptualizing and measuring activation in patients and consumers. Health services research, 39(4p1), 1005–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbard JH, Mahoney ER, Stockard J, & Tusler M (2005). Development and testing of a short form of the patient activation measure. Health services research, 40(6p1), 1918–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbard JH, & Greene J (2013). What the evidence shows about patient activation: better health outcomes and care experiences; fewer data on costs. Health affairs, 32(2), 207–214. [DOI] [PubMed] [Google Scholar]
- Hibbard JH, Greene J, & Overton V (2013). Patients with lower activation associated with higher costs; delivery systems should know their patients‟„scores‟. Health affairs, 32(2), 216–222. [DOI] [PubMed] [Google Scholar]
- Hudson SM, Newman SD, Hester WH, Magwood GS, Mueller M, & Laken MA (2014). Factors influencing hospital admissions and emergency department visits among children with complex chronic conditions: a qualitative study of parents‟ and providers‟ perspectives. Issues in comprehensive pediatric nursing, 37(1), 61–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janson SL, McGrath KW, Covington JK, Cheng SC, & Boushey HA (2009). Individualized asthma self-management improves medication adherence and markers of asthma control. Journal of Allergy and Clinical Immunology, 123(4), 840–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerant A, Moore M, Lorig K, Franks P. (2008). Perceived control moderated the self-efficacy-enhancing effects of a chronic illness self-management intervention. Chronic Illn,;4(3):173–82. [DOI] [PubMed] [Google Scholar]
- Jones V, Whitehead L, & Crowe MT (2016). Self-efficacy in managing chronic respiratory disease: Parents‟ experiences. Contemporary nurse, 52(2–3), 341–351. [DOI] [PubMed] [Google Scholar]
- Law GU, Tolgyesi CS, & Howard RA (2014). Illness beliefs and self-management in children and young people with chronic illness: a systematic review. Health Psychology Review, 8(3), 362–380. [DOI] [PubMed] [Google Scholar]
- Lorig KR, Sobel DS, Stewart AL, Brown BW Jr, Bandura A, Ritter P, … & Holman HR (1999). Evidence suggesting that a chronic disease self-management program can improve health status while reducing hospitalization: a randomized trial. Medical care, 5–14. [DOI] [PubMed] [Google Scholar]
- Lorig KR, & Holman HR (2003). Self-management education: history, definition, outcomes, and mechanisms. Annals of behavioral medicine, 26(1), 1–7. [DOI] [PubMed] [Google Scholar]
- Lozano P, & Houtrow A (2018). Supporting self-management in children and adolescents with complex chronic conditions. Pediatrics, 141(Supplement 3), S233–S241. [DOI] [PubMed] [Google Scholar]
- McBroom LA, & Enriquez M (2009). Review of family-centered interventions to enhance the health outcomes of children with type 1 diabetes. The Diabetes Educator, 35(3), 428–438. [DOI] [PubMed] [Google Scholar]
- Mitchell SE, Gardiner PM, Sadikova E, Martin JM, Jack BW, Hibbard JH, & Paasche-Orlow MK (2014). Patient activation and 30-day post-discharge hospital utilization. Journal of general internal medicine, 29(2), 349–355. doi: 10.1007/s11606-013-2647-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BB, Coller RJ, Saenz AA, Chung PJ, Kaplan A, Lerner CF, & Klitzner TS (2016). How avoidable are hospitalizations for children with medical complexity? Understanding parent perspectives. Academic pediatrics, 16(6), 579–586. [DOI] [PubMed] [Google Scholar]
- Newman S, Steed L, & Mulligan K (2004). Self-management interventions for chronic illness. The Lancet, 364(9444), 1523–1537. [DOI] [PubMed] [Google Scholar]
- Ott J, Greening L, Palardy N, Holderby A, & DeBell WK (2000). Self-efficacy as a mediator variable for adolescents’ adherence to treatment for insulin-dependent diabetes mellitus. Children’s Health Care, 29(1), 47–63. [Google Scholar]
- Pennarola BW, Rodday AM, Mayer DK, Ratichek SJ, Davies SM, Syrjala KL, … & Guinan EC (2012). Factors associated with parental activation in pediatric hematopoietic stem cell transplant. Medical Care Research and Review, 69(2), 194–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin D, Harden J, Barnard K, Bath L, Noyes K, Stephen J, & Lawton J (2018). Ran. BMC endocrine disorders, 18(1), 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutten LJF, Hesse BW, Sauver JLS, Wilson P, Chawla N, Hartigan DB, … & Arora NK (2016). Health self-efficacy among populations with multiple chronic conditions: the value of patient-centered communication. Advances in therapy, 33(8), 1440–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan P, & Sawin KJ (2009). The individual and family self-management theory: Background and perspectives on context, process, and outcomes. Nursing outlook, 57(4), 217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattoe JN, Bal MI, Roelofs PD, Bal R, Miedema HS, & van Staa A (2015). Self-management interventions for young people with chronic conditions: a systematic overview. Patient Education and Counseling, 98(6), 704–715. [DOI] [PubMed] [Google Scholar]
- Sawyer SM, Drew S, Yeo MS, & Britto MT (2007). Adolescents with a chronic condition: challenges living, challenges treating. The Lancet, 369(9571), 1481–1489. [DOI] [PubMed] [Google Scholar]
- Stepleman L, Rutter MC, Hibbard J, Johns L, Wright D, & Hughes M (2010). Validation of the patient activation measure in a multiple sclerosis clinic sample and implications for care. Disability and rehabilitation, 32(19), 1558–1567. [DOI] [PubMed] [Google Scholar]
- Siembida EJ, Kadan-Lottick NS, Moss K, & Bellizzi KM (2018). Adolescent cancer patients‟ perceived quality of cancer care: The roles of patient engagement and supporting independence. Patient education and counseling, 101(9), 1683–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinson JN, Sung L, Gupta A, White ME, Jibb LA, Dettmer E, & Baker N (2012). Disease self-management needs of adolescents with cancer: perspectives of adolescents with cancer and their parents and healthcare providers. Journal of Cancer Survivorship, 6(3), 278–286. [DOI] [PubMed] [Google Scholar]
- Varni JW, Burwinkle TM, & Lane MM (2005). Health-related quality of life measurement in pediatric clinical practice: an appraisal and precept for future research and application. Health and quality of life outcomes, 3(1), 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varni JW, Seid M, & Kurtin PS (2001). PedsQL™ 4.0: Reliability and validity of the Pediatric Quality of Life Inventory™ Version 4.0 Generic Core Scales in healthy and patient populations. Medical care, 39(8), 800–812. [DOI] [PubMed] [Google Scholar]
- Varni JW, Limbers CA, & Burwinkle TM (2007). Impaired health-related quality of life in children and adolescents with chronic conditions: a comparative analysis of 10 disease clusters and 33 disease categories/severities utilizing the PedsQL™ 4.0 Generic Core Scales. Health Qual Life Outcomes, 5(1), 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varni JW, & Limbers CA (2009). The pediatric quality of life inventory: measuring pediatric health-related quality of life from the perspective of children and their parents. Pediatric Clinics of North America, 56(4), 843–863. [DOI] [PubMed] [Google Scholar]
- Yarcheski A, Mahon NE, Yarcheski TJ, & Cannella BL (2004). A meta- analysis of predictors of positive health practices. Journal of Nursing Scholarship, 36(2), 102–108 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.