Abstract
Brachytherapy (BT) is an important local treatment of tumor and it can be applied to different anatomical sites either in a curative or palliative setting. BT can deliver large dose of radiation to the tumor while sparing the surrounding normal tissue which translates into a better therapeutic ratio compared to external beam radiotherapy. However, the evidence for the use of brachytherapy in the palliative setting is lacking in the literature. In this case report, we describe the brachytherapy technique and outcome of a patient with squamous cell carcinoma of the hypopharynx who underwent palliative brachytherapy to the hypopharynx and metastatic tumor at the right axilla.
Keywords: brachytherapy, axilla, hypopharynx, elderly, squamous cell carcinoma
Introduction
Brachytherapy (BT) has a long tradition in the treatment of malignancies and, in fact, radiation oncology did indeed start with brachytherapy before the advent of teletherapy equipment. However, with the technological advancements of external beam radiotherapy (EBRT), radiation oncologists lost many of the surgical procedural skills in BT over the last few decades. At present, there is a worrying trend of rapid decline in the use of BT even for anatomical sites with proven efficacy [1, 2]. Unlike EBRT, the literature on BT is limited even in the radical setting and, hence, unsurprisingly, the evidence of BT as a palliative treatment is scanty, with oesophageal and tracheal BT being an exception [3–5]. BT can safely deliver unparalleled large dose of radiation to the tumor while sparing the surrounding normal structure, thus improving the therapeutic ratio compared to EBRT modalities [6]. On top of the physical and radiobiological benefit, BT is also more economical when compared to intensity modulated radiation therapy (IMRT) [7]. In this manuscript we are describing a case of metastatic squamous cell carcinoma of the right axilla from hypopharynx who had good palliation and quality of life from BT treatment to both the metastatic and primary site.
Case presentation
This interesting patient is an 86-year-old male, smoker of 40 pack years with severe smoking-related lung disease. He quit smoking 20 years ago after being diagnosed with laryngeal cancer for which he underwent definitive EBRT and had been in disease remission since then. Despite the advanced age, he had been generally fit and independent till July 2017 when he presented with a right axillary mass. He underwent surgery to remove the lesion at another hospital and the exact nature of the mass, the diagnosis and clinical assessments that were made at that time is not known. Subsequently, there was a persistent non-healing ulcer at the excision site which was biopsy confirmed as poorly differentiated squamous cell carcinoma one month later.
A staging positron emission tomographic (PET-CT) scan then showed an increased uptake in the right axilla (standardized uptake value, SUV 8.6) and hypopharynx (SUV 7.6) with corresponding contrast enhancing lesions in the CT-images. Fine needle aspiration biopsy (FNAB) of the hypopharyngeal lesion confirmed it as squamous cell carcinoma. He was re-assessed in the same hospital and was deemed unfit for surgery due to his age and extent of the local disease. Afterwards, he had a course of EBRT to 66 Gy in 33 daily fractions to the axilla, which was completed with interruptions in the radiotherapy schedule due to intermittent flaring of his smoking-induced lung disease. Despite initial 4 months of local control after the course of radiotherapy, the axillary lesion recurred and was rapidly increasing in size. He was then started on chemotherapy with oral Capecitabine. However, the axillary lesion progressed on chemotherapy and the oral Capecitabine was discontinued after 3 cycles.
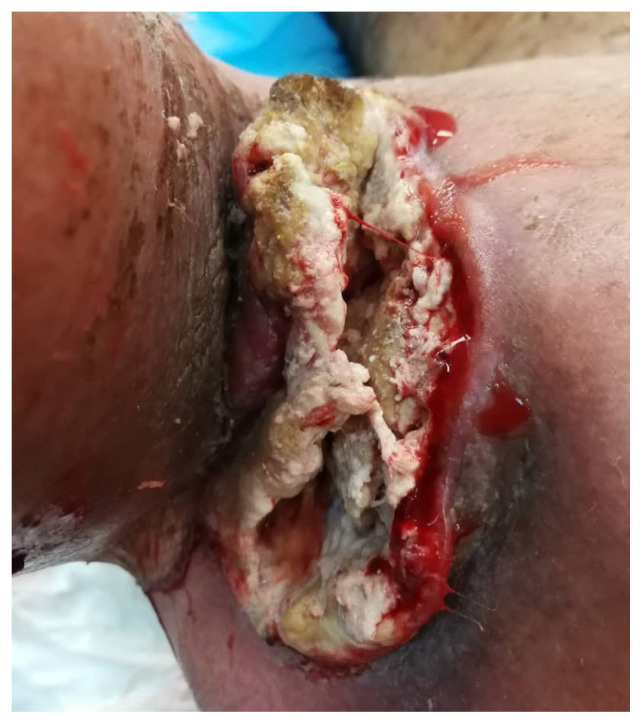
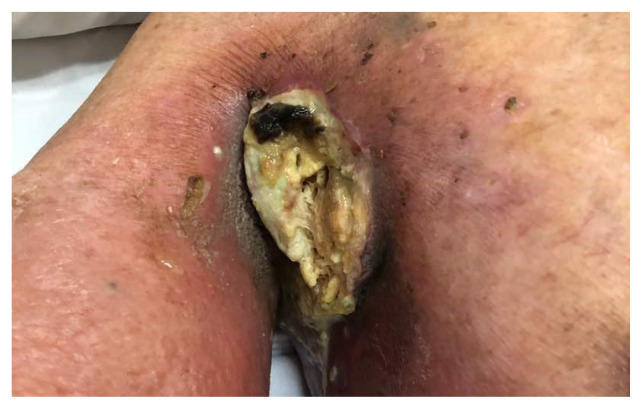
When the patient first presented to our centre one year after the initial diagnosis of hypopharyngeal carcinoma metastatic to the axilla, he was unable to move the right shoulder due to severe pain with pain score of 9/10. He suffered from significant lymphoedema and total loss of both motor function and sensation in the right upper limb. There was a 12 × 7 cm foul smelling fungating lesion at the right axilla that bled spontaneously with movement (Fig. 1). Patient’s general mobility was severely limited due to pain and he was wheelchair bound. His Eastern Cooperative Oncology group (ECOG) performance status was 3. However, he was asymptomatic for the hypopharyngeal lesion and was tolerating orally well.
Figure 1.
12 × 7 cm foul smelling fungating lesion at the right axilla
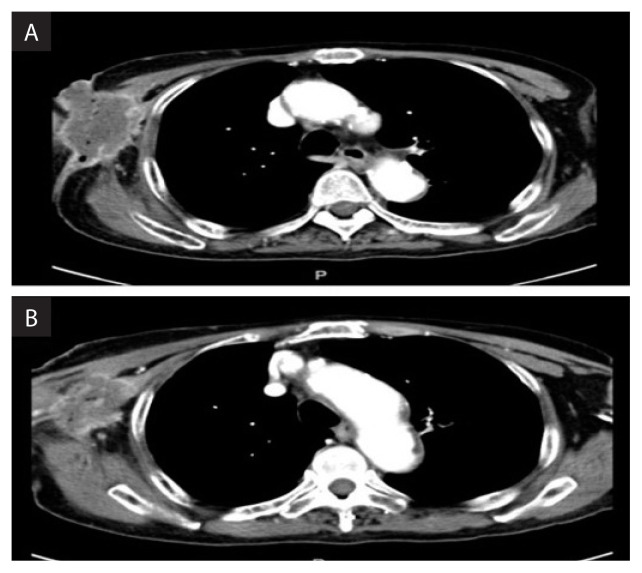
A repeat CT neck-thorax-abdomen-pelvis (NTAP) did not show any other metastatic disease; however, the local disease at the right axilla was extensive, involving the chest wall and right arm (Fig. 2A and 2B). The hypopharyngeal lesion was not eroding into the surrounding structures and there was no regional nodal involvement (staging according to AJCC 7th edition cT3N0M1).
Figure 2AB.
Axial slice of CT showing extensive right axilla lesion involving the chest wall and right arm
After extensive discussion with the patient and his family members, taking into consideration his age, pre-existing severe smoking induced chronic lung disease and extent of the disease at the axilla, a mutual decision was made to have a good palliative procedure to improve the quality of life. Since the axillary lesion was unresectable and further high dose fractionated radiotherapy with EBRT could cause significant toxicity, palliative brachytherapy was suggested to which the patient and his family members agreed and consented. The risks of rib fracture at the site of treatment, chances of further worsening of lymphoedema, chronic non-healing ulcer at the axilla and surgical risks of brachial vessel rupture during the interstitial BT applicator insertion were explained in detail. Patient also understood that the nerve damage was probably permanent and may not improve with the expected axillary tumour shrinkage after BT.
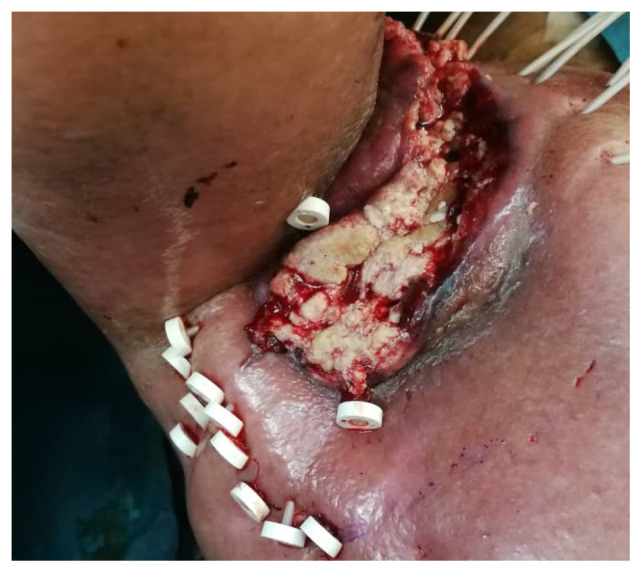
The procedure was performed under general anaesthesia and doppler ultrasound guidance was used to avoid puncturing the brachial artery. The patient was positioned semi-supine with his body rotated 30 degrees to the left lateral. Tumour edges were palpated clinically and marked on the skin. Under ultrasound guidance the deep-seated tumour areas and the course of the brachial artery were identified to be avoided. Introducers were inserted from the antero-medial to postero-lateral direction by puncturing the skin and tumour through and through under real-time ultrasound guidance. 6F flexible BT applicators with buttoned end OncoSmart Catheter System (Elekta, Stockholm, Sweden) were introduced through the introducer and fixed at the postero-lateral side. In total, 15 BT applicators were used to cover the marked tumour area sufficiently (Fig. 3). Care was taken to ensure the spacing between applicators was within 3 cm to reduce hotspots. The procedure was uneventful with minimal bleeding. Puncture sites were cleaned and covered with povidone-soaked gauze.
Figure 3.
15 applicators were used to cover the marked tumour area sufficiently
The patient was CT-simulated with CT markers inserted into the placed BT applicators, acquiring 2mm contrasted axial slices in the region of interest. Tumour was identified and contoured in each slice as gross tumor volume (GTV) and a 5 mm safety margin was expanded automatically as the planning target volume (PTV). Care was taken to avoid the shoulder joint in the PTV where possible without compromising GTV coverage. A total dose of 54 Gy to cover the PTV was prescribed.
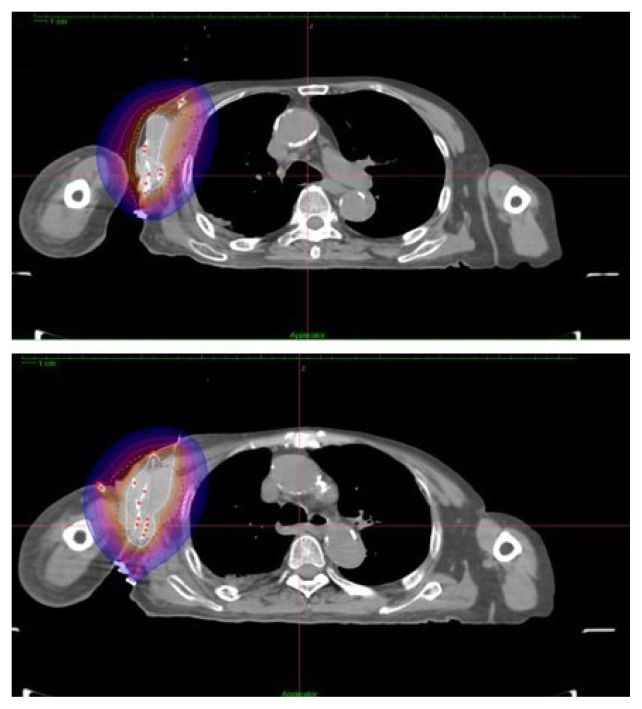
Brachytherapy dose planning to the target was performed using the Oncentra Masterplan V 4.5.3 brachytherapy treatment planning system (TPS) (Nucletron BV, Veenendaal, The Netherlands) which had an inverse planning capability. The target coverage was manipulated by adjusting the dwell time and dwell position of the Ir-192 source with an initial activity of 10 MCi. The 15 ProGuide plastic applicators of 240 mm in length (Elekta, Stockholm, Sweden) were reconstructed in the brachytherapy TPS. 500 normalisation points were created at the PTV surface. After achieving a good dose distribution (D90 of PTV receiving 7.23 Gy per fraction) with further manual graphical optimisation of the isodoses, the final plan was exported to the high dose rate (HDR) Nucletron remote after loader system (Fig. 4). The patient was treated with one fraction of 6 Gy daily for 9 consecutive days. Applicators were removed after completing the 9 days of treatment which was uneventful.
Figure 4.
Final isodose plan after optimization
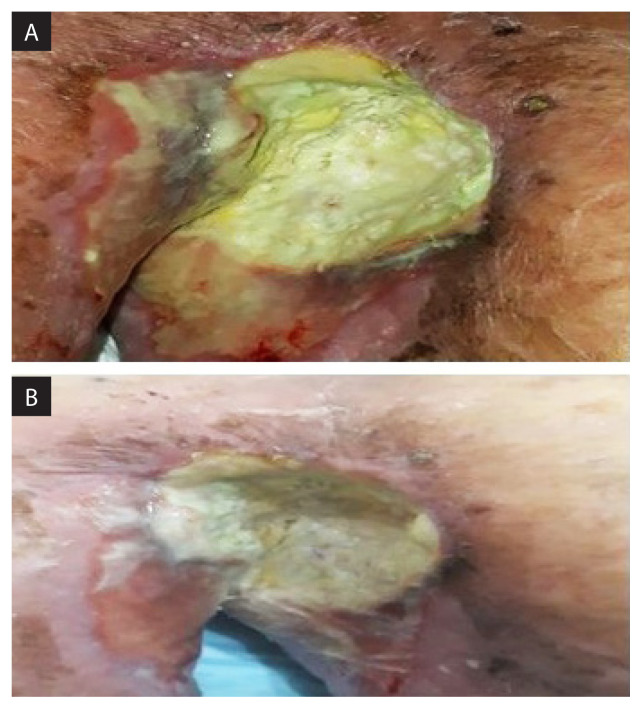
One week later, patient’s pain score had gone down to 3/10 from the pre-treatment score of 9/10. The axillary fungating mass had flattened and there was no more spontaneous bleeding (Fig. 5). At 1-month post treatment, the fungating lesion had completely resolved leaving a 5 × 4 cm of sloughy region. The patient was pain-free in the axilla and his ECOG performance status improved from ECOG-3 initially to ECOG-2.
Figure 5.
One-week post treatment, the axillary fungating mass had flattened and there was no more spontaneous bleeding
Unfortunately, 2 months after brachytherapy, a repeat CT-NTAP showed liver and lung metastases, though the axillary lesion was much reduced in size in all dimensions with minimal contrast enhancing areas within the residual tumour. As the patient was unfit for any systemic therapy and asymptomatic at both the primary and metastatic sites, we opted for no active oncological intervention and he was referred to the palliative care team. Clinically, the axillary tumour site showed telangiactesia at the periphery and slough covered cavity with no significant pain or active bleeding (Fig. 6).
Figure 6.
The axillary tumour site showed telangiactesia at the periphery and slough covered cavity with no significant pain or active bleeding
Four months after the brachytherapy to the axilla, the patient presented to us again, this time with dysphagia tolerating liquid diet only. Laryngo-oesophagoscope showed near complete obstruction of the hypopharyngeal region by the tumour. Interestingly, the right axillary region did not show any clinical sign of local recurrence (Fig. 7A and B). The patient was unable to lie flat due to the pre-existing lung disease which made palliative EBRT to the neck region a difficult task. He was not keen on feeding percutaneous endoscopic gastrostomy (PEG) tube insertion. As such, we proceeded with palliative brachytherapy to the hypopharyx after informed consent from the patient.
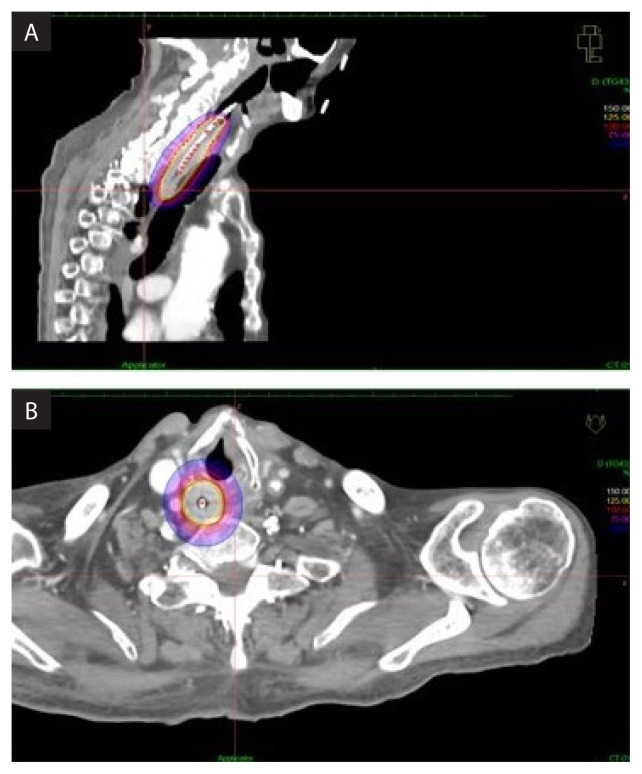
Figure 7A.
The isodoses were normalized and adjusted graphically as to have the 100% isodose covering 1 cm depth laterally from the from the centre of the applicator; B. Due to the geographical position of the tumor, 0.5 cm safety margin superiorly and 1.5 cm inferiorly of the gross tumor volume (GTV) was assigned
Nucleotron (Veenendaal, The Netherlands) intra luminal applicator was inserted up to the lower oesophagus after a mild dilatation of the hypopharynx and upper oesophagus by the surgeon. Patient was CT-simulated in a semi-supine position (30-degree elevation) with intravenous contrast and CT-marker in situ. The tumour was marked as GTV in each of the 2 mm CT slices. A dose of 12 Gy in a single fraction was prescribed to cover the GTV using the Oncentra Masterplan V 4.5.3 brachytherapy TPS. Then, the isodoses were normalized and adjusted graphically to have the 100% isodose covering 1 cm depth laterally from the centre of the applicator (Fig. 7A). Due to the geographical position of the tumor, 0.5 cm safety margin superiorly and 1.5 cm inferiorly of the GTV (Fig. 7B) was assigned. The dwell time and position of the Ir-192 source was assigned automatically by the brachytherapy inverse planning TPS. The patient was treated with Nucleotron (Veenendaal, The Netherlands) HDR after loader brachytherapy system and the intraluminal applicator was removed immediately after the procedure.
Two weeks after the brachytherapy to hypopharynx, the patient was tolerating semi-solid diet and his dysphagia score had improved from grade 4 to 2. There were symptoms of grade 1 (Common Terminology Criteria for Adverse Events, CTC-AE 4.0) pharyngitis and oesophagitis. His axillary region was pain-free with no active bleeding. There were no clinical signs of recurrence. The upper limb lymphoedema had also improved. Unfortunately, patient’s general condition deteriorated thereafter, most likely due to the progression of the visceral disease and two weeks later he succumbed to his disease.
Discussion
HDR brachytherapy (HDRBT) is an important radiation therapy modality that can be used in the surface, intracavitary, intraluminal or interstitial application. The main advantage of HDR brachytherapy is that it allows high dose of radiation to be delivered to a pre-defined target volume while achieving a good sparing the adjacent normal tissue [3]. Despite being the first radiation modality to be used clinically, it’s usage has dropped in recent times compared to historically available data [1, 2]. Many radiation oncologists consider brachytherapy as an invasive procedure as it requires certain surgical skills that come with proper training. The emphasis on BT is also lacking in most radiation oncology residency programs, except for gynaecological and prostate cancers [8]. Furthermore, with the advent of technology, such as the highly complex modern linear accelerator (LINAC), which are readily available in many radiotherapy centres, less emphasis was given to BT. This phenomenon continues despite most radiation oncologists agreeing on the superiority of BT dose conformality whilst sparing the normal tissues compared to other available EBRT technology [6].
The goal of palliative radiotherapy is to alleviate symptoms in the shortest amount of time, maintain an optimal function and quality-of-life while minimizing toxicity and patient inconvenience [9]. A wide range of symptoms from advanced cancer is treated with palliative radiotherapy such as pain, bleeding, dysphagia, dyspnoea and to relieve neurological symptoms. Clinical trials have demonstrated that hypofractionated treatment provides equivalent symptomatic benefit to longer courses, with limited toxicity [3, 10]. Though these hypofractionated palliative radiotherapy can be delivered via EBRT or BT, evidence for palliative BT is still lacking in the literature as compared to palliative EBRT.
Studies have revealed improved local control with dose escalation, however the tolerance of the normal surrounding structures is a major issue in EBRT [11]. Due to the physical nature of radiation from the Ir-192 radioactive source average radiation dose received by the tumor is much higher than the prescribed dose at the periphery of the TV [12]. Another distinct feature of HDRBT is that the radiation doses within the vicinity of an implant are higher than the maximum dose levels generally accepted in EBRT protocols, and yet they are well tolerated because of the dose-volume relationship (small volumes can tolerate very high doses of radiation). These physical and radiobiological properties of BT gives it a great unexplored potential for tumors in many organ sites, e.g. overcoming radio resistance and focused radiation in critical organs such as the liver and in re-irradiation. Thus, by enhancing the local tumour control, BT, when used alone or in addition to EBRT, has a great potential in achieving the goals of palliation.
The optimal radiation dose and fractionation for palliative BT is unknown [13]. There is also little evidence in the literature on the optimal radiation doses for BT with regard to tumour histology, unlike in the setting of fractionated EBRT. Our patient had initially received a total of 66 Gy in 2Gy per fraction of EBRT to the right axillary mass. The fact that the tumour in the right axilla recurred within 4 months of EBRT points towards a more radioresistant clones. The good tumour response with BT in this patient can be attributed to the large dose per fraction that was used. Experience with Stereotactic Radiation Therapy (SRT) showed that a larger fractional dose may have a strong role in overcoming radio-resistance [14]. In this patient, the expected survival was less than a year and aim of the treatment was to achieve a good local control and improve the quality of life with minimal emphasis on the long-term side effects associated with the large fractional dose delivered. Furthermore, the patient already had a complete neurological deficit of the right upper limb. The immediate improvement in pain symptoms and bleeding as early as 1 week after BT and complete tumor resolution in the right axilla at 4 months confirms the efficacy of this short course of HDRBTThe palliative treatment options for the hypopharyngeal tumour at that point was PEG-tube for feeding, which the patient refused, palliative EBRT and BT. Stenting was not feasible given the location of the tumour. As the tumour was quite circumferential around the upper oesophagus and compounded by the fact that the patient can’t lie flat for EBRT, intraluminal oesophageal BT was deemed the best option for him to improve dysphagia. In a 2004 Lancet paper, Homs et al. showed that single dose of brachytherapy (12 Gy) caused a better improvement in long term dysphagia scores than a metal stent placement. Quality-of-life scores were also in favour of brachytherapy as compared to stent placement [3]. Similar findings were also shown by Berqguist et al. in a study that randomised 65 patients with advanced cancer of the oesophagus to either an expandable stent placement or HDR endoluminal brachytherapy to a total dose of 21 Gy divided into 3 equal fractions, given over 2–4 weeks [12]. Palliative brachytherapy with or without stent placement should therefore always be considered in all patients with a life expectancy exceeding 3 months with stent insertion as the sole treatment reserved for patients with an extremely short life expectancy where the immediacy of symptomatic relief offered by this procedure clearly dominates. Our patient had a good palliation and was able to tolerate orally after the single dose of 12 Gy palliative intraluminal BT to the hypopharynx and upper oesophagus for the final 1 month of his life.
Despite the advances in systemic antineoplastic therapies, such as newer generation cytotoxic chemotherapy, molecular targeted therapy and immunotherapy, failure to control the tumour at its primary or metastatic site/s remains the predominant source of morbidity and often mortality in many cancer patients. This patient was not fit for aggressive systemic treatments in view of his age, co-morbidities and performance status. As his symptoms were localized to the tumor at the hypopharynx and right axilla, any attempt at an aggressive systemic therapy would have been of doubtful benefit. A very effective, simple and minimally invasive procedure which BT is, gave him a good quality of life for 6 months after the initial brachytherapy to the axilla.
In experienced hands, BT offers an elegant and minimally invasive way to safely deliver relatively large dose of radiation to the tumour while sparing the adjacent tissue. Good tumour response, as seen in this patient, is not uncommon for BT. More patients will benefit if the use of BT is further expanded and not limited to just a few organ sites in the palliative setting. Brachytherapy is an important trade and weapon owned by radiation oncologists that needs resurrection for the benefit of cancer patients.
Conclusions
Brachytherapy is a well-tolerated treatment option in selected patients with advanced cancer. It is a safe and effective treatment modality in experienced hands for palliation of advanced cancer, yielding a good local control with acceptable treatment-related side effects. In this case, the patient expectedly had a relatively good quality of life in terms of pain control, bleeding and dysphagia.
Acknowledgement
We would like to thank the all the staff for the help.
Footnotes
Conflict of interest
The authors have no conflict of interest to disclose.
Funding
All authors have no financial disclosure. No financial support for this case report.
References
- 1.Martin JM, Handorf EA, Kutikov A, et al. The rise and fall of prostate brachytherapy: use of brachytherapy for the treatment of localized prostate cancer in the National Cancer Data Base. Cancer. 2014;120(14):2114–2121. doi: 10.1002/cncr.28697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gill BS, Lin JF, Krivak TC, et al. National Cancer Data Base analysis of radiation therapy consolidation modality for cervical cancer: the impact of new technological advancements. Int J Radiat Oncol Biol Phys. 2014;90(5):1083–1090. doi: 10.1016/j.ijrobp.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 3.Homs MYV, Steyerberg EW, Eijkenboom WMH, et al. Single-dose brachytherapy versus metal stent placement for the palliation of dysphagia from oesophageal cancer: multicentre randomised trial. Lancet. 2004;364(9444):1497–1504. doi: 10.1016/S0140-6736(04)17272-3. [DOI] [PubMed] [Google Scholar]
- 4.Skowronek J. Current status of brachytherapy in cancer treatment — short overview. J Contemp Brachytherapy. 2017;9(6):581–589. doi: 10.5114/jcb.2017.72607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skowronek J, Piotrowski T, Młynarczyk W, et al. Advanced tracheal carcinoma — a therapeutic significance of HDR brachytherapy in palliative treatment. Neoplasma. 2004;51(4):313–318. [PubMed] [Google Scholar]
- 6.Hass P, Mohnike K, Kropf S, et al. Comparative analysis between interstitial brachytherapy and stereotactic body irradiation for local ablation in liver malignancies. Brachytherapy. 2019;18(6):823–828. doi: 10.1016/j.brachy.2019.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Hayes JH, Ollendorf DA, Pearson SD, et al. Observation versus initial treatment for men with localized, low-risk prostate cancer: a cost-effectiveness analysis. Ann Intern Med. 2013;158(12):853–860. doi: 10.7326/0003-4819-158-12-201306180-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marcrom SR, Kahn JM, Colbert LE, et al. Brachytherapy Training Survey of Radiation Oncology Residents. Int J Radiat Oncol Biol Phys. 2019;103(3):557–560. doi: 10.1016/j.ijrobp.2018.10.023. [DOI] [PubMed] [Google Scholar]
- 9.Syadwa AS, Anita ZB. Palliative radiotherapy for advanced Cancer: Are we giving it to the right patient at the right time? Med J Malaysia. 2018;73(4):190–196. [PubMed] [Google Scholar]
- 10.Lutz ST, Chow EL, Hartsell WF, et al. A review of hypofractionated palliative radiotherapy. Cancer. 2007;109(8):1462–1470. doi: 10.1002/cncr.22555. [DOI] [PubMed] [Google Scholar]
- 11.Shasha D, Harrison LB. The role of brachytherapy for palliation. Semin Radiat Oncol. 2000;10(3):222–239. doi: 10.1053/srao.2000.6723. [DOI] [PubMed] [Google Scholar]
- 12.Bergquist H, Wenger U, Johnsson E, et al. Stent insertion or endoluminal brachytherapy as palliation of patients with advanced cancer of the esophagus and gastroesophageal junction. Results of a randomized, controlled clinical trial. Dis Esophagus. 2005;18(3):131–139. doi: 10.1111/j.1442-2050.2005.00467.x. [DOI] [PubMed] [Google Scholar]
- 13.Fuccio L, Mandolesi D, Farioli A, et al. Brachytherapy for the palliation of dysphagia owing to esophageal cancer: A systematic review and meta-analysis of prospective studies. Radiother Oncol. 2017;122(3):332–339. doi: 10.1016/j.radonc.2016.12.034. [DOI] [PubMed] [Google Scholar]
- 14.Rühle A, Andratschke N, Siva S, et al. Is there a role for stereotactic radiotherapy in the treatment of renal cell carcinoma? Clin Transl Radiat Oncol. 2019;18:104–112. doi: 10.1016/j.ctro.2019.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]