Abstract
Background
A purpose of the study was to investigate the dosimetric impact of contrast media on dose calculation using average 4D contrast-enhanced computed tomography (4D-CECT) and delayed 4D-CT (d4D-CT) images caused by CT simulation contrast agents for stereotactic body radiation therapy (SBRT) of liver cases.
Materials and methods
Fifteen patients of liver SBRT treated using the volumetric modulated arc therapy (VMAT) technique were selected retrospectively. 4D-CECT, and d4D-CT were acquired with the Anzai gating system and GE CT. For all patients, gross target volume (GTV) was contoured on the ten phases after rigid registration of both the contrast and delayed scans and merged to generate internal target volume (ITV) on average CT images. Region of interest (ROI) was drawn on contrast images and then copied to the delayed images after rigid registration of two average CT datasets. The treatment plans were generated for contrast enhanced average CT, delayed average CT and contrast enhanced average CT with electron density of the heart overridden.
Results
No significant dosimetric difference was observed in plans parameters (mean HU value of the liver, total monitor units, total control points, degree of modulation and average segment area) except mean HU value of the aorta amongst the three arms. All the OARs were evaluated and resulted in statistically insignificant variation (p > 0.05) using one way ANOVA analysis.
Conclusions
Contrast enhanced 4D-CT is advantageous in accurate delineation of tumors and assessing accurate ITV. The treatment plans generated on average 4D-CECT and average d4D-CT have a clinically insignificant effect on dosimetric parameters.
Keywords: 4D-CECT, d4D-CT, SBRT, HCC, HU, VMAT
Introduction
Contrast-enhanced computed tomography (CECT) acquisition facilitates an accurate delineation of target volumes in hepatocellular carcinoma (HCC) [1], whereas an alternation in the hounsfield unit (HU) value due to contrast media will result in erroneous dose calculation [2]. This might result in overestimation or underestimation of the dose and unintended dose is delivered to the target and healthy tissues. Most of the authors have studied the dosimetric influence of contrast media in radiotherapy planning on three dimensional computed tomography (3D-CT) image datasets for various clinical sites [3–9]. Many authors have reported clinically insignificant effects, whereas few authors have suggested incorporating the effect of contrast media by assigning HU values to the contrast-enhanced structures or using non-contrast CT datasets for treatment planning.
Most commonly, triphasic 3D-CT scans along with a non-contrast four-dimensional computed tomography (4D-CT) are acquired for target delineation and planning for patients with HCC. This method is accessible to patients who cannot co-operate with the breath-hold technique. The triphasic CT takes advantage of HCC’s characteristic enhancement in the arterial phase, followed by a washout in the portal or delayed phases [10]. Internal target volume (ITV) is assessed using 4D-CT and an isotropic or anisotropic margin is added around the clinical target volume (CTV) based upon the target movement. However, there are limitations to this method; the 3D-CT may be acquired at any random phase, giving an ITV margin on a random phase will result in over treatment of the healthy tissues. Also, the 4D-CT derived geometric expansion may not cover the hysteresis of the liver. To tackle these problems, our department utilizes a synchronized intravenous contrast 4D planning CT (4D-CECT) followed by a delayed 4D planning CT (d4D-CT) [11–13]. This method allows for precise target delineation on a 4D-CT and dose calculation on the average phase of the 4D-CT. Synchronized 4D-CT contrast scans also avoid the need for triple-phase CT scans for target delineation, reducing the radiation exposure to the patient [11, 12]. Also, the day to day matching on a 4D cone beam computed tomography (CBCT) may be more accurate with the average phase of 4D-CT.
Earlier studies have quantified the effect of contrast media on dose distribution using 3D-CT image data sets. To the best of our knowledge, no study has assessed the impact of contrast media on dose calculation on a 4D-CECT. As contrast media can widely alter the dose distribution in the healthy liver, its dosimetric impact needs to be studied.
The present study aims to study the dosimetric impact of contrast media on dose calculation using average 4D-CECT and d4D-CT images caused by CT simulation contrast agents.
Materials and methods
The present study analyzes the dosimetric impact of contrast 4D-CT on the accuracy of dose distribution. Fifteen patients of HCC were randomly selected for this retrospective study with the characteristics as shown in Table 1. The details of the steps involved in the study are given in subsequent sections:
Table 1.
Patient characteristics along with planning target volume
| BCLC staging | TNM/AJCC staging | CP score | PTV [cc] | Age [yrs]/Gender | |
|---|---|---|---|---|---|
| Patient 1 | C | T4N0M0 | A5 | 772 | 52/M |
| Patient 2 | C | T4N0M0 | A6 | 213 | 48/M |
| Patient 3 | C | T4N0M0 | A5 | 896 | 67/M |
| Patient 4 | C | T4N1M0 | A6 | 477 | 56/M |
| Patient 5 | C | T3N1M1 | A6 | 306 | 74/M |
| Patient 6 | C | T4N0M0 | A5 | 176 | 65/M |
| Patient 7 | C | T4N0M0 | A6 | 241 | 55/M |
| Patient 8 | C | T4N0M0 | A5 | 898 | 59/F |
| Patient 9 | C | T4N0M0 | A6 | 151 | 57/M |
| Patient 10 | C | T4N0M0 | A6 | 313 | 64/F |
| Patient 11 | C | T4N0M0 | A5 | 118 | 49/M |
| Patient 12 | C | T4N1M0 | A6 | 513 | 65/M |
| Patient 13 | C | T4N0M0 | A6 | 663 | 50/F |
| Patient 14 | B | T2N0M0 | A6 | 89 | 69/F |
| Patient 15 | C | T4N0M0 | A5 | 159 | 52/M |
BCLC — Barcelona clinic liver cancer; AJCC — American Joint Committee on Cancer; CP score — Child Pugh Score; PTV — planning target volume
CT scan acquisition
All the patients were positioned with Blue-BAG™ (Elekta AB, Stockholm, Sweden) in a supine position. After ensuring normal renal function, 18 G to 20 G three-way IV cannula was inserted in the antecubital vein to ensure an adequate flow of contrast. The CT scans were acquired as per protocol approved by the department for routine clinical practice. All patients underwent 4D-CECT, and d4D-CT using 64-slice optima GE CT equipment (GE Medical Systems, USA) integrated with GE Discovery 710 Time of Flight (TOF) PET-CT scanner (GE Healthcare, Amersham, UK). A pressure sensor-based load cell device of the Anzai gating system (AZ-733V; Anzai Medical System, Tokyo, Japan) using an elastic belt wrapped around the patient’s abdomen to measure the respiratory waveform. The load cell was placed below the diaphragm level on the right side of the abdomen.
An automatic contrast injector Ulrich CT motion contrast injector (Ulrich medical® interface, Ulrich GmbH & Co. KG, Germany) was connected to the IV line, and 125 ml of Visipaque™ (iodixanol) containing 270 mg of iodine per ml was injected at a rate of 3ml/second. 4DCT was acquired from the carina to 6cm below the inferior-most point of the liver. A 140 kVp (tube potential), 140–160 mA (tube current), 0.5–1 sec/rotation speed, and a constant detector coverage of 2 cm (detector coverage is the length scanned by the CT scanner for a duration equal to the patient’s cine time) was applied. A delayed 4D-CT scan was taken after a gap of 5 minutes. The CT scans were acquired with 2.5mm slice thickness and eight images/rotation [11].
Contour delineation
For each patient, contrast and delayed 4D-CT scans were acquired. The 4D-CT datasets were imported into a treatment planning system (TPS; Monaco ver. 5.1, Elekta CMS, Maryland Heights, MO, USA), and the average CT dataset was generated in TPS. For all patients, gross tumor volume (GTV) was contoured on the ten phases after rigid registration of both the contrast and delayed scans. Areas that showed contrast enhancement and delayed washout were contoured as GTV and were merged to generate ITV on average CT images. For treatment planning, planning target volume (PTV) was generated by giving a population-specific 5 mm uniform margin around ITV to incorporate the set-up uncertainties. The GTV, CTV, and PTV, along with the other organs at risk (OAR), were delineated as mentioned in RTOG 1112 protocol14. ROI was drawn on contrast images and then copied to the delayed images after rigid registration of two average CT datasets. The same method was employed to analyze different CT datasets.
Treatment planning
All the treatment plans were generated on average CT images using Monaco TPS for delivery with Versa HD (Elekta, Stockholm, Sweden) equipped with Agility MLC 80 leaf pairs for volumetric modulated arc therapy (VMAT) with a maximum dose rate of 600 MU/min using 6MV photon energy. Segment shape optimization (SSO) was used for the optimization of VMAT plans with minimum segment width of 0.5 cm and medium fluence smoothing followed by final dose calculation using the Monte Carlo dose calculation algorithm with 2 mm grid size and 3% Monte Carlo. The treatment planning was done with a very tight margin of 0–1 mm around the target using auto jaw tracking. All patients were planned with the VMAT technique (previous work has suggested that VMAT is superior to modified dynamic conformal arc) using two partial arcs (60–180 degrees) for a prescription dose in the range of 7–10 Gy/fraction using optimized planning parameters recommended by Thaper et al. [15–17]. Complementary collimator angles of 30 degrees and 330 degrees were used to reduce the tongue and groove effect, and accumulative effects of transmission through multileaf collimator (MLC). The dose-volume constraints of PTV and OAR, as mentioned in RTOG 1112, were achieved [14].
Analysis of treatment plans
Three types of treatment plans were generated, as mentioned in Table 2. The treatment plans were optimized on arterial average CT (Dart-reopt), delayed average CT (Ddel-reopt), arterial average CT with an electron density of heart override (Dhart-reopt). Dhart-reopt was evaluated as HU value of the heart is significantly higher in a contrast-enhanced phase and can cause uncertainties in dose calculations, especially if the tumor is located near the upper dome of the diaphragm. The beam characteristics and optimization parameters of the radiotherapy plan generated in one CT data set were copied and applied to the other CT datasets. All statistical analyses were performed using SPSS for Windows (Version 20.0 IBM Corporation, Armonk, NY). The mean and standard deviations of the plan parameters and OAR doses were calculated for each group. Degree of modulation (DoM), total control points (CP), mean HU of the liver and aorta, average segment area (SA), total monitor units (MU), mean liver dose (MLD), mean kidney dose, mean spleen dose, 0.5cc of the esophagus, duodenum, stomach, small bowel, large bowel, spinal cord and dose to 30cc for the heart was evaluated. One way ANOVA was performed using the tukey s-b method with p > 0.05 as a meaningless level of statistical tests.
Table 2.
Quantitative analysis of organ at risk (OAR) doses (mean, standard deviation and p-value)
| Dart-reopt | Dhart-reopt | Ddel-reopt | p-value | |
|---|---|---|---|---|
| MLD | 15.95±4.38 | 15.91±4.33 | 15.77±4.9 | 0.992 |
| RK (Mean) | 6.24±5.78 | 6.16±5.81 | 5.82±5.16 | 1.000 |
| Eso (0.5 cc) | 15.86±6.71 | 16.55±6.63 | 15.68±6.41 | 0.989 |
| Duo (0.5 cc) | 20.07±14.11 | 20.28±14.19 | 18.44±13.44 | 0.998 |
| Sto (0.5 cc) | 20.55±8.86 | 20.29±8.83 | 19.76±8.72 | 0.969 |
| Heart (30 cc) | 11.28±7.30 | 11.11±7.07 | 11.15±6.90 | 0.975 |
| SB (0.5 cc) | 16.26±13.86 | 15.62±13.89 | 15.9±14.66 | 0.998 |
| LB (0.5 cc) | 23.52±13.73 | 23.47±13.71 | 23.25±13.92 | 1.000 |
| Cord (0.5 cc) | 13.05±5.84 | 13.08±5.88 | 12.64±5.37 | 1.000 |
| Spleen (Mean) | 3.12±1.64 | 3.37±1.76 | 3.39±1.82 | 0.947 |
MLD — mean liver dose; RK — right kidney; Eso — esophagus; Duo — duodenum; Sto — stomach; SB — small bowel; LB — large bowel
Results
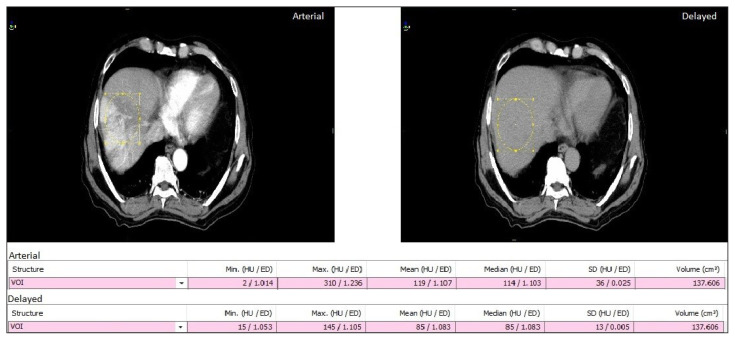
Figure 1 represents a typical axial tomographic slice of average arterial and delayed CT’s reconstructed from the 4D-CT dataset of a single patient. Enhancement in the average arterial phase is visible in the aorta and liver of the axial slice. Also, the mean HU value is 100 and 82 in average contrast and average delayed CT image for a particular VOI, respectively.
Figure 1.
Typical axial slice of computed tomography (CT) dataset of average arterial phase and average delayed phase for a typical patient
Table 3 summarizes the quantitative analysis of the mean HU value of the liver and aorta, MU, CP, DoM and SA of the treatment plan averaged over all the patients. The variation in HU value of the aorta in Dart-reopt is significantly different from Ddel-reopt (p < 0.005). However, the other parameters evaluated in Table 2 showed statistically insignificant variation (p > 0.05).
Table 3.
Quantitative analysis of plan parameters averaged over all the patients (mean and standard deviation) along with the statistical significance
| Dart-reopt | Dhart-reopt | Ddel-reopt | p-value | |
|---|---|---|---|---|
| MU | 3784.52 ± 780.46 | 3720.00 ± 808.12 | 3874.197 ± 887.03 | 0.885 |
| HU (liver) | 89.64 ± 21.42 | 89.64 ± 21.42 | 81.00 ± 7.86 | 0.354 |
| HU (aorta) | 281.57 ± 96.50 | 281.57 ± 96.50 | 90.64 ± 11.88 | < 0.005 |
| CP | 248.79 ± 54.73 | 236.71 ± 47.88 | 237.28 ± 42.74 | 0.762 |
| DoM | 4.93 ± 1.03 | 4.85 ± 1.02 | 5.02 ± 1.02 | 0.900 |
| SA | 17.63 ± 7.53 | 18.13 ± 7.93 | 17.45 ± 7.11 | 0.970 |
MU — monitor units; HU — hounsfield unit; CP — control points; DoM — degree of modulation; SA — segment area
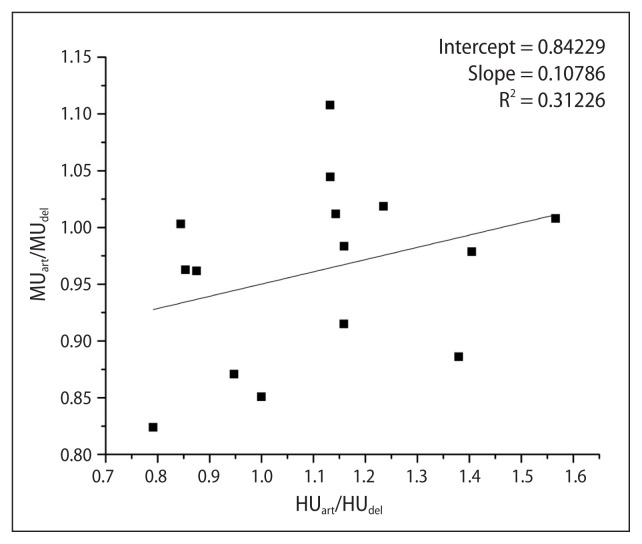
Figure 2 represents the scatter plot of the normalized HU and MU values w.r.t corresponding to the values in the delayed phase. A positive correlation was observed; however, it was non-significant (p > 0.05).
Figure 2.
Scatter plot of normalized monitor units (MU) versus normalized hounsfield units (HU) of the dose calculated on average arterial CT w.r.t. average delayed CT
Figure 3 represents the percentage variation of Dart-reopt and Dhart-reopt in MU, CP, SA w.r.t. to Ddel-reopt. Average segment area is higher for both Dart-reopt (1.31%) and Dhart-reopt (3.42%) in comparison to Ddel-reopt, though the difference was non-significant (p > 0.05, Tab. 2). Similarly, the mean variation in MU was found to be −1.30% and −3.39% for Dart-reopt and Dhart-reopt, respectively, w.r.t. Ddel-reopt (p > 0.05, Tab. 2). The total control points were 4.28% higher in Dart-reopt and almost similar in Dhart-reopt (−0.33%) w.r.t. Ddel-reopt with p-value being greater than 0.05 (Tab. 2).
Figure 3.
Percentage variation of Dart-reopt and Dhart-reopt in monitor units (MU), control points (CP), segment area (SA) w.r.t. to Ddel-reopt
Table 3 summarizes the quantitative analysis of OAR doses of the treatment plan corresponding to treatment plans calculated on three arms of the study. The dose-volume parameters evaluated are according to the RTOG 1112 protocol. All the OAR evaluated has shown statistically insignificant variation (p > 0.05, Tab. 3) using one way ANOVA analysis. PTV doses were normalized to the same value for all the plans.
Discussion
The effect of IV contrast agent on dose calculations has been studied by several authors using 3D-CT images. Xiao et al. reported a non-significant difference in dosimetric parameters for treatment planning on hepatic arterial phase, porto-venous phase, and non-contrast CT [18]. Sahbaee et al. reported a 35% increase in the dose of the liver for specific contrast media injection protocol [19]. Shibamoto et al. also suggested that contrast media have an impact on dose distribution for upper-abdominal irradiation especially when beams pass through the liver, spleen, or kidneys [20]. None of the authors studied the dosimetric comparison of treatment planning on 4D-CECT and d4D-CT images in liver cases.
In this study, the effect of the IV contrast agent on dose calculations on average 4D-CT images in radiation treatment planning was evaluated. For the purpose, the two sets of CTs (average contrast and average non-contrast) were taken in the same position and setting of scan acquisition parameters. As the 4D-CT acquisition takes a comparatively longer time as compared to conventional 3D-CT, the enhancement on average CT may be different from contrast 3D-CT. Hence, the evaluation of its effect on dose calculation was necessary as the treatment planning process makes use of HU values of CT images to calculate the dose which is altered with the use of IV contrast.
Considering Ddel-reopt as the reference, the parameters can be compared with the doses achieved from doses calculated at other CT datasets. The treatment planning on Dart-reopt and Dhart-reopt resulted in −1.30% and −3.39% average change in MU, respectively, w.r.t Ddel-reopt. However, it is to be noted that the difference was statistically non-significant (p > 0.05) (Tab. 2). These findings are consistent with the findings of Xiao et al. which found that there is a non-significant difference in dosimetric parameters (MU) for planning on arterial, porto-venous, and non-contrast CT images. However, the study of Xiao et al. was performed for 3D images only and had an inherent limitation of image acquisition at any random phase of the respiratory cycle which may alter the position of tumor and OARs in different CT datasets owing to uncertainty in results quoted.
Further, Ramm et al. reported that an increase in the number of incident beams lessens the effect of contrast on dose calculation [2]. As VMAT includes a large number of incident beams, the change in MU between doses of different CT datasets is minimal. Mean HU values of the liver were found to be 8.64 units higher for Dart-reopt than Ddel-reopt. It is also to be emphasized that mean variation in HU values of the liver in arterial and delayed CT datasets is also non-significant, however, value is lower as compared to the value of other parameters (p = 0.354, Tab. 2). The observations are in contrast to the results reported by Shibamoto et al. which quoted an increase of 51 ± 17 HU of enhanced liver CT over non-enhanced. It can be attributed to the use of a small cohort of patients and variability in the enhancement of HCC. Also, ten phases of CT scan are acquired according to cine time and respiratory rate (generally in the order of ~ 4–5 sec), generating an average CT results in averaging of HU values of all ten phases which might result in a lower HU value than the expected value of the liver in the arterial phase. Further, the HU value of the aorta at an axial slice of the upper border of the liver was evaluated and a significant difference was observed (p < 0.05, Tab. 2).
We also analyzed the dosimetric parameters of the critical organs as mentioned in the RTOG 1112 protocol. Three arms were compared as shown in table 3 and the difference in all the critical organs doses was statistically non-significant (p > 0.05). One of the limitations of the study is that the small cohorts of patients are used in this study. Another limitation is that only rigid registration is considered while registering two average CTs, however, deformable image registration may give more accurate results as there may be a residual shift between OAR due to a little bit change in the respiratory pattern of the patient.
Conclusion
SBRT is a challenging treatment modality for moving tumors, especially where the liver deforms continuously during treatment. Contrast enhanced 4D-CT is advantageous in accurate delineation of tumors and assessing accurate ITV. The treatment plans generated on average 4D-CECT and average d4D-CT has a clinically insignificant effect on dosimetric parameters. In our opinion, dose calculation can be performed on contrast-enhanced average CT images while overriding the electron densities of the heart for the VMAT technique.
Footnotes
Conflict of interest
None declared.
Funding
None declared.
References
- 1.Méndez Ro, Brunner TB, Kirichenko AV. Alternate Fractionation for Hepatic Tumors. Springer; Cham: 2017. pp. 173–201. [Google Scholar]
- 2.Ramm U, Damrau M, Mose S, et al. Influence of CT contrast agents on dose calculations in a 3D treatment planning system. Phys Med Biol. 2001;46(10):2631–2635. doi: 10.1088/0031-9155/46/10/308. [DOI] [PubMed] [Google Scholar]
- 3.Lee FKH, Chan CCL, Law CK. Influence of CT contrast agent on dose calculation of intensity modulated radiation therapy plan for nasopharyngeal carcinoma. J Med Imaging Radiat Oncol. 2009;53(1):114–118. doi: 10.1111/j.1754-9485.2009.02046.x. [DOI] [PubMed] [Google Scholar]
- 4.Lees J, Holloway L, Fuller M, et al. Effect of intravenous contrast on treatment planning system dose calculations in the lung. Australas Phys Eng Sci Med. 2005;28(3):190–195. doi: 10.1007/BF03178715. [DOI] [PubMed] [Google Scholar]
- 5.Liauw SL, Amdur RJ, Mendenhall WM, et al. The effect of intravenous contrast on intensity-modulated radiation therapy dose calculations for head and neck cancer. Am J Clin Oncol. 2005;28(5):456–459. doi: 10.1097/01.coc.0000170796.89560.02. [DOI] [PubMed] [Google Scholar]
- 6.Heydarheydari S, Farshchian N, Haghparast A. Influence of the contrast agents on treatment planning dose calculations of prostate and rectal cancers. Rep Pract Oncol Radiother. 2016;21(5):441–446. doi: 10.1016/j.rpor.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weber D, Rouzaud M, Miralbell R. Bladder opacification does not significantly influence dose distribution in conformal radiotherapy of prostate cancer. Radiother Oncol. 2001;59(1):95–97. doi: 10.1016/s0167-8140(01)00306-1. [DOI] [PubMed] [Google Scholar]
- 8.Izmirli M, Çakır T, Avcu S, et al. Impact of contrast agents on dose algorıthms of planning systems. Int J Radiat Res. 2016;14(1):25–30. doi: 10.18869/acadpub.ijrr.14.1.25.. [DOI] [Google Scholar]
- 9.Zabel-du Bois A, Ackermann B, Hauswald H, et al. Influence of intravenous contrast agent on dose calculation in 3-D treatment planning for radiosurgery of cerebral arteriovenous malformations. Strahlenther Onkol. 2009;185(5):318–324. doi: 10.1007/s00066-009-1927-6. [DOI] [PubMed] [Google Scholar]
- 10.Hennedige T, Venkatesh SK. Imaging of hepatocellular carcinoma: diagnosis, staging and treatment monitoring. Cancer Imaging. 2013;12:530–547. doi: 10.1102/1470-7330.2012.0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta A, Kumar R, Yadav HP, et al. Feasibility of 4D CT simulation with synchronized intravenous contrast injection in hepatocellular carcinoma. Rep Pract Oncol Radiother. 2020;25(2):293–298. doi: 10.1016/j.rpor.2019.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Helou J, Karotki A, Milot L, et al. 4DCT Simulation With Synchronized Contrast Injection in Liver SBRT Patients. Technol Cancer Res Treat. 2016;15(1):55–59. doi: 10.1177/1533034615572341. [DOI] [PubMed] [Google Scholar]
- 13.Beddar AS, Briere TM, Balter P, et al. 4D-CT imaging with synchronized intravenous contrast injection to improve delineation of liver tumors for treatment planning. Radiother Oncol. 2008;87(3):445–448. doi: 10.1016/j.radonc.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 14.Dawson L, Zhu A, Knox J, et al. Radiation Therapy Oncology Group RTOG 1112 Randomized Phase III Study of Sorafenib Versus Stereotactic Body Radiation Therapy Followed By Sorafenib in Hepatocellular Carcinoma. 2013 [Google Scholar]
- 15.Thaper D, Kamal R, Singh G, et al. Dosimetric comparison of dynamic conformal arc integrated with segment shape optimization and variable dose rate versus volumetric modulated arc therapy for liver SBRT. Rep Pract Oncol Radiother. 2020;25(4):667–677. doi: 10.1016/j.rpor.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thaper D, Singh G, Kamal R, et al. Impact of dose heterogeneity in target on TCP and NTCP for various radiobiological models in liver SBRT: different isodose prescription strategy. Biomed Phys Eng Express. 2021;7(1):015020. doi: 10.1088/2057-1976/abd3f0. [DOI] [PubMed] [Google Scholar]
- 17.Thaper D, Oinam AS, Kamal R, et al. Interplay effect modeling in stereotactic body radiotherapy treatment of liver cancer using volumetric modulated arc therapy. Phys Eng Sci Med. 2021;44(1):123–134. doi: 10.1007/s13246-020-00961-5. [DOI] [PubMed] [Google Scholar]
- 18.Xiao J, Li Y, Jiang Q, et al. Hepatic arterial phase and portal venous phase computed tomography for dose calculation of stereotactic body radiation therapy plans in liver cancer: a dosimetric comparison study. Radiat Oncol. 2013;8:264. doi: 10.1186/1748-717X-8-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sahbaee P, Abadi E, Segars WP, et al. The Effect of Contrast Material on Radiation Dose at CT: Part II. A Systematic Evaluation across 58 Patient Models. Radiology. 2017;283(3):749–757. doi: 10.1148/radiol.2017152852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shibamoto Y, Naruse A, Fukuma H, et al. Influence of contrast materials on dose calculation in radiotherapy planning using computed tomography for tumors at various anatomical regions: a prospective study. Radiother Oncol. 2007;84(1):52–55. doi: 10.1016/j.radonc.2007.05.015. [DOI] [PubMed] [Google Scholar]