Abstract
Chronic kidney disease (CKD) and end stage renal disease (ESRD) are increasingly frequent and devastating conditions that have driven a surge in the need for kidney transplantation. A stark shortage of organs has fueled interest in generating viable replacement tissues ex vivo for transplantation. One promising approach has been self-organizing organoids, which mimic developmental processes and yield multicellular, organ-specific tissues. However, a recognized roadblock to this approach is that many organoid cell types fail to acquire full maturity and function. Here, we comprehensively assess the vasculature in two distinct kidney organoid models as well as in explanted embryonic kidneys. Using a variety of methods, we show that while organoids can develop a wide range of kidney cell types, as previously shown, endothelial cells (ECs) initially arise but then rapidly regress over time in culture. Vasculature of cultured embryonic kidneys exhibit similar regression. By contrast, engraftment of kidney organoids under the kidney capsule results in the formation of a stable, perfused vasculature that integrates into the organoid. This work demonstrates that kidney organoids offer a promising model system to define the complexities of vascular-nephron interactions, but the establishment and maintenance of a vascular network present unique challenges when grown ex vivo.
Keywords: Endothelial cell, Angioblast, Blood vessel, Cord, Kidney, RNAseq, Organoid
1. Introduction
Current treatment strategies for end stage renal disease (ESRD) are largely refinements of approaches developed almost a century ago, including dialysis, supportive care, and whole kidney transplantation. For most patients, kidney transplantation is the best option; however, demand greatly surpasses the supply of available organs. For those who do ultimately receive a transplant, the average wait time for a kidney is 3–5 years and the threat of immunologic rejection is ever present (Nankivell and Alexander, 2010). Recent technological advances are providing hope for alternative sources of functional renal tissue. Current work in stem cell and regenerative medicine has the potential to provide a historic turning point in treatment possibilities and its success could provide treatment for millions of individuals with chronic kidney disease that will progress to ESRD. The last half-decade has witnessed rapid advances in stem cell technology for kidney tissue differentiation. Critical proof-of-principle generation of kidney tissue ‘in a dish’ provides viable new experimental approaches with potential for clinical application.
Kidney tissue engineering and regeneration efforts have largely focused on epithelial and endothelial components, as these two cell types are responsible for much of renal filtration function. Beginning in 2015, several landmark studies established protocols to differentiate induced pluripotent stem cells (iPSCs) or embryonic stem cells (ESCs) into self-organizing organoids with the capacity to produce a wide range of kidney cell types (Morizane et al., 2015; Taguchi et al., 2014; Taguchi and Nishinakamura, 2015; Takasato et al., 2015, 2016a; Takasato and Little, 2017) Alternative techniques have also been explored, including decellularized kidney matrices and ‘organ-on-a-chip’ technologies. However, it has ultimately become clear that achieving conversion of ex vivo generated kidney tissue into patient kidneys will require a full understanding of how kidney tissue develops, specifically epithelial tubules and their closely-associated interstitium, which includes both blood vessels and stroma. These approaches that recapitulate normal kidney development hold a tangible promise for generating functional replacement tissue.
Despite these significant advances in tissue engineering and stem cell biology in the last decade, we have not yet achieved transplantable kidney tissue. In order to generate potentially functional replacement kidneys, the tissue must be scaled in size to fit the host and plumbed appropriately, allowing for blood filtration and urine excretion. One increasingly recognized obstacle in laboratory-created tissues, including organoids, is the difficulty of growing and maintaining a functional vasculature. Blood vessels are necessary for tissues to develop long-term and to achieve sizes beyond the limit of oxygen and nutrient diffusion. Lastly, growing evidence supports the necessity of vasculature in promoting differentiation and maturation of tissues, including the kidney (Rafii et al., 2016; Rocha et al., 2015; Rymer et al., 2014; van den Berg et al., 2018; Yao et al., 2017). In fact, numerous studies have recognized this importance and shown that mimicking flow can ameliorate organoid vascularization (Homan et al., 2019; Morizane and Bonventre, 2017; Zhang et al., 2017). Despite this progress, nascent organoid vasculature still falls short. Across published protocols, organoid blood vessels generated are immature and disorganized, and our understanding of their specification, formation, and dynamics is limited. The low and transient expression of endothelial genes in kidney organoids has been shown by qPCR, but the endothelial cells (ECs) themselves have not been analyzed throughout this process (Takasato et al., 2015). In addition, it is still unclear how kidney vasculature is specified in vivo, how its heterogeneity might impact kidney cell types, and the dynamics of EC growth during in vitro culture. As the existing analysis of organoid vasculature to date has been limited, a closer look at organoid blood vessels is warranted.
In this study, we assess the dynamics, maturity, and maintenance of blood vessels in renal organoids, cultured kidneys, and implanted organoids. Using organoids generated from either human embryonic stem cells (hESCs) or mouse embryo nephrogenic zone cells (NZCs), we explore vascular structures and their growth. We show that the vasculature in both types of organoids is transient and fundamentally defective. While epithelial kidney cell types display relatively normal specification and initial patterning, the vasculature largely fails to thrive. Organoid vessels display significantly lower density and complexity than equivalent normal midgestation kidney tissue and vessels largely fail to associate with kidney epithelium. Most significantly, organoid vessels regress over time in culture. To test whether vascular regression is precipitated by initial failure of EC specification during directed differentiation of hESCs, we examine vessels in cultured embryonic kidneys and NZC-derived organoids, both of which already contain ECs. We observe significant regression in all three systems, suggesting necessity of hemodynamic flow in vessel maintenance. When organoids are implanted under the kidney capsule of adult mice, however, they are vascularized by the host. Notably, implanted organoids display capillary tufts in the glomeruli. To identify differences between ECs in lab-generated kidney tissue versus normal embryonic kidney, we analyze publicly available single cell RNA sequencing (scRNA-seq) data comparing ECs in week 8–18 human embryonic kidneys to those in human ESC and iPSC kidney organoids (day 0–34). We find that early during culture, organoid ECs have a similar transcriptional signature to embryonic ECs, but progressively diverge over the course of culture. Self-organizing models of renal development, such as organoids, offer a promising tool for studying the kidney and for engineering replacement kidney tissue; however, our work underscores critical shortcomings of organoid vasculogenesis and demonstrates the importance of developing methods to promote, maintain, and properly identify blood vessels in cultured kidney tissues.
2. Materials and methods
2.1. Mice and embryo handling
Experiments were performed in accordance with protocols approved by the UT Southwestern Medical Center IACUC. For each experiment, female mice of 7–8 weeks of age were crossed with a male of 9–10 weeks of age. Plugs were checked and the embryos were collected at the desired time points for further analysis. Noon of the day on which the mating plug was observed was designated embryonic day (E) 0.5. Flk1-GFP endothelial reporter mice were acquired from Dr. Eli Keshet (available from Jackson Laboratory Kdrtm2.1Jrt/J, stock number 017006), (Takahashi et al., 1996). These mice were created by knocking in the eGFP cassette into the first exon of Kdr. The homozygous mice are embryonic lethal and are maintained as heterozygotes in a CD1 background. In addition, the following mice were used in the studies described: Foxd1-Cre (MGI Cat# 4437923, RRID:MGI:4437922 (Humphreys et al., 2010)), Six2Cre (MGI Cat# 3848504, RRID:MGI:3848504 (Kobayashi et al., 2008),), Hoxb7Cre (IMSR Cat# JAX:004692, RRID:IMSR_JAX:004692 (Yu et al., 2002)), Rosa26 YFP (IMSR Cat# JAX:006148, RRID:IMSR_JAX:006148 (Srinivas et al., 2001)), and the RosaDTRflox or terminator mouse was kindly provided by Lloyd Cantley (Guo et al., 2013).
2.2. Generation of human ESC organoids
The methods used were adapted from previously published protocols (Takasato et al., 2015, 2016a). H9 ESCs were generously provided by Dr. Jenny Hsieh and Dr. Vanesa Nieto-Estevez at University of Texas at San Antonio. Cells were validated free of mycoplasma with a Universal Mycoplasma Detection Kit (ATCC, 30–1012K). H9 ESCs were cultured on Matrigel-coated plates in mTeSR plus (STEMCELL, 05825) with 10 μM Rock inhibitor Y-27632 (ATCC) for the first 24 h of growth to limit cell death. For differentiation, ESCs were split at 1:12 ratio and cultured for 24 h in mTeSR plus and Rock inhibitor. The next day, day −7, the media was replaced with TeSR-E6 (STEMCELL, 05946) with 6 μM CHIR 99021 (R&D, 4423). Media was changed daily or every other day. On day −3, the media was replaced with TeSR-E6 with 200 ng/ml FGF9 (R&D, 273-F9–025). On day 0, the cells were collected using TrypLE Express (ThermoFisher, 12604013). Cells were distributed into an Ultra-Low Attachment 96 well U bottom plate (Corning, CLS7007–24 EA) with approximately 100,000 cells per well in TeSR-E6 with 200 ng/ml FGF9. Media was changed every 2–3 days. On day 5, FGF9 was no longer added. Organoids were also made by spotting 100,000 cells in 1 μl onto a 2.4 cm Transwell filter, which required a 1 h 6 μM CHIR pulse. (Unlike filter organoids, we found that submerged organoids did not require a CHIR pulse to differentiate. Additionally, the submerged format limited user error by accidental flooding of the Transwell which caused the organoids to die. Lastly, the 96 well plate format provided a more efficient use of media than Transwell filters in a 6-well plate (200 μl media per organoid vs. >300 μl per organoid) plus the additional media that was required for the 1 h CHIR pulse after aggregation.
2.3. Generation of mouse NZC derived kidney organoids
Nephrogenic zone cells (NZCs) were isolated using the protocol from Brown et al. (Brown et al. (2015). Briefly, kidneys were dissected from embryonic day 17.5 (E17.5) mice in PBS. Kidneys were cleaned and capsules were carefully removed ensuring no damage to the kidneys. The kidneys were combined and washed in HBSS (Gibco, 14175) on a nutator (Fisher Scientific, 260 100F) at 37 °C for 2 min. HBSS was removed and the kidneys were carefully submersed in a filtered 0.1% collagenase A (Roche, 11,088,793 001)/0.5% porcine pancreatin (Sigma, P1625) solution in PBS without calcium or magnesium (Corning, 20–031 CV). The kidneys were incubated in the collagenase/pancreatin enzyme digest on a nutator at 37 °C for 10 min. The enzyme was inactivated using 125 μl of fetal bovine serum (FBS). While avoiding contact with the kidneys, the supernatant was removed and centrifuged at 2000 RPM for 5 min using a microcentrifuge (Eppendorf Centrifuge 5415 D). The supernatant was discarded and the cell pellet was resuspended in 1 ml of AutoMacs buffer (Miltenyi Biotec, 130-091-221) and passed through a 30 μm pre-separation filter (Miltenyi Biotec, 130-041-407) that was previously primed with 4 ml of AutoMacs buffer. The filter was immediately washed with 500 μl of AutoMacs buffer and cells were counted from the resulting filtrate. If cells were to be plated in monolayer, cells were seeded onto a tissue culture plate pre-coated in 25% Matrigel (Fisher Scientific, 08-774-553)/DMEM F12 (Gibco, 11,320,033) at a cell density of between 5000 and 25,000 cells per cm2 similar to the method used by Brown et al. (Brown et al. (2015). Cells were cultured in APEL2 (STEMCELL, 05270) with 1% Penicillin-Streptomycin supplemented with 200 ng/ml FGF9 (R&D, 273-F9–025) and 10 μM Rock inhibitor Y27632 (Millipore, 688001). Media was changed every two days and the cells were passaged when after reaching 70% confluency using TrypLE dissociation solution (Life Technologies, 12,563,029). Cells can be maintained in monolayer no more than two passages.
If the collected NZCs were to be aggregated into organoids, cells were resuspended in AutoMacs buffer at a concentration of 500,000 cells/2 μl, accounting for the volume of the cells. 2 μl of the cell solution was carefully pipetted onto a Nuclepore Track-Etch Membrane (Whatman, #110409) which was floating in 1 ml APEL 2 (StemCell, 05270) with 1% Penicillin-Streptomycin and 8 μM CHIR. Organoids were incubated for in 37 °C at 5% CO2 for 1 h after which the 8 μM CHIR media was removed and replaced with 1 ml APEL 2 with 1% Penicillin-Streptomycin and 200 ng/ml human recombinant FGF9 (R&D, 273-F9–025). Except for Fig. 4J, where the organoid was treated with CHIR overnight. Organoids were cultured for 5 days after which the media was removed and replaced with 1 ml APEL 2 with 1% Penicillin-Streptomycin without any additional growth factors and cultured for an additional 5 days for a total of 10 days of growth. Throughout the culture period, media was replaced every two days.
Fig. 4. NZC-derived kidney organoid generation.

A) Schematic and timeline of NZC-derived organoid protocol. A heterogeneous population of nephrogenic zone cells (NZCs), including stromal cells, nephron progenitor cells (NPCs), endothelial cells (ECs), and ureteric bud (UB) tip cells, are isolated from embryonic mouse kidneys. NZCs may be expanded on monolayer for up to 2 passages to increase the organoid yield or proceed directly to the aggregation step. Organoids are aggregated on a filter floating on APEL2 media then treated with a 1 h CHIR pulse before culture with 200 ng/ml FGF9 for 5 days before being switched to growth-factor free media. Mouse illustration from http://www.emouseatlas.org B) Immunofluorescence of an E17.5 mouse kidney with E-Cad+ UB, Six2+ NPCs, and CD31/EMCN + ECs. The nephrogenic zone is demarcated with a yellow dotted line. B′) After pancreatin and collagenase A digestion, the NPCs, ECs, and stromal cells of the nephrogenic zone are no longer present. C-D) Immunofluorescence showing the NZCs after monolayer culture. C) Foxd1Cre; YFP + stromal cells, Six2+ NPCs and D) Hoxb7Cre; YFP + UB cells. E) Brightfield images of NZC organoids 2–7 days after aggregation. F-J) Immunofluorescence of various kidney cell types present in NZC organoids. F) Whole mount immunofluorescence of an NZC organoid with E-Cad+ epithelium. 80 μm scale. G-J) Immunofluorescence of sectioned NZC organoids, oriented with the air-liquid interface on top displaying Aqp1+ proximal tubule and descending loop of Henle and NPHS1+ podocytes (G), LTL+ proximal tubule and Umod+ loop of Henle (H), Meis 1/2/3+ stroma (I), and Aqp3+ and pan-cytokeratin+ UB (J). GH 100 μm scale. DAPI marks nuclei. Representative immunofluorescence images shown in A-C were derived from n ≥3 organoids from at least 2 different litters.
2.4. Explant culture of embryonic kidneys
Flk1GFP embryos were dissected at E12.5. Kidneys were cultured on Nuclepore Track-Etch Membranes (Whatman, 110409) floating on DMEM/F12 medium with 10% FBS and 1% Penicillin-Streptomycin. For live imaging, kidneys were cut with a tungsten wire and cultured cut-side down on fibronectin-coated, glass-bottom plates (MatTek, P35G-1.5–14-C).
2.5. Histology and immunohistochemistry on sections
Kidneys and organoids isolated at the desired time points were fixed using 4% paraformaldehyde at 4 °C overnight (kidneys and NZC organoids) or 20 min at room temperature (hESC organoids) with gentle agitation. For paraffin histology, tissue was washed 3 times in PBS for 5 min, dehydrated in an ethanol series, xylene cleared, and embedded in paraffin. 10 μm sections from paraffin-embedded kidneys were stained by haematoxylin and eosin or immunofluorescence. Alternatively, tissue was processed through cryosectioning, where tissue was washed 3 times in PBS for 5 min, incubated overnight in 30% sucrose, and embedded in OCT (ThermoFisher Scientific, 23-730-571). OCT-embedded tissue was sectioned on a cryostat into 10 μm sections. Paraffin and frozen sections were baked at 60 °C for 10 min. Paraffin sections were deparaffinized in xylene then hydrated in an ethanol series to water. Both paraffin and frozen sections were then washed in 0.1% Triton-X/PBS and blocked in 5% normal donkey serum/0.1% Triton-X/PBS for a minimum of 1 h at room temperature. Sections were incubated in a variety of primary antibodies in blocking solution overnight at 4 °C (for dilutions, see Table S1) followed by 3 washes in 0.1% Triton-X/PBS. Sections were then incubated in secondary antibody at 1:500 in blocking solution for 1 h at room temperature, washed 3 times in PBS, incubated in DAPI, and mounted with anti-fade mounting media (Vectashield, H1000) or Prolong Gold Mounting Medium (Cell Signaling, 8961). Tissue was viewed and imaged by scanning laser confocal microscopy (NIKON A1R).
2.6. Whole-mount immunofluorescence
Fixed organoids were washed in PBS and permeabilized with 1% Triton-X 100 for 1.5 h before being blocked using CAS-Block (ThermoFisher Scientific, 008120) for 1.5 h. The tissues were incubated in primary antibodies in CAS block (for dilutions, see Table S1) overnight at 4 °C. Tissues were washed in PBS at least 5 times for 10 min each and incubated in secondary antibodies in CAS block for 1 h and then washed and subsequently incubated in DAPI for 1 h. Tissues were mounted on slides with ProLong Gold and visualized using a Nikon A1R confocal to take optical sections every 1 μm.
Whole kidneys were additionally cleared by incubating them in a 1:2 mixture of benzyl alcohol/benzyl benzoate (BABB) for at least 10 min. Kidneys were mounted in BABB and visualized using an LSM710 Meta Zeiss confocal to take optical sections every 2.5–3 μm as described in Daniel et al., 2018.
2.7. Vascular quantification
Whole mount organoids and embryonic kidneys stained for CD31 and Sox17 (organoids) or CD31 and EMCN (kidneys) were analyzed with Imaris. Volume of the organoids was automatically calculated. For the kidneys, due to extraneous tissue, a surface was created manually by using the contour setting to trace the kidney about every 5 slices through the z-stack. The volume of this surface was used to estimate the kidney volume. Then, the surface was used to create a mask of the CD31/EMCN channel so that blood vessel volume was only calculated within the kidney. Next, a surface of the masked CD31/EMCN channel was created. Surfaces were created automatically using the same parameters across all samples, including the absolute intensity and automatic thresholding. The sum of surface volumes of this channel were used as the estimation of blood vessel volume. Automatic surface calculations with Imaris fail when there is extremely low signal and/or high background. Therefore, the surface was adjusted by hand for quantification of the day 3, VEGF-treated organoid and background subtraction was used for the quantification of day 15 and 18 VEGF treated organoids (Fig. 3). Sox17+ nuclei were counted using the spots function on Imaris using a 6 μm estimated diameter with automatic quality thresholding and background subtraction.
Fig. 3. hESC-derived organoid vasculature regresses in culture.

A-F) Whole mount immunofluorescence of hESC organoids with CD31 and Sox17 on days 6–18. DAPI mark nuclei. 200 μm scale. Zoomed in areas are marked with a white box. G-H) Quantification of day 3–18 images by percent volume of CD31 (G) and Sox17+ nuclei (H). H–N) vasculature of VEGF-treated organoids by whole mount immunofluorescence (I-N) and quantified by percent volume of CD31 (O) and Sox17+ nuclei (P) compared to control treated data (G-H). Q-V) qPCR data of 6 different organoid experiments (by color) showing relative gene expression relative to day 0 before aggregation to day 15. Q) CD31, R) VEGFR2, S) Podxl, T) Meis1 and Col3a1 U) Six2, and V) E-Cad. W–Y) Cell death in organoids shown by cleaved caspase 3 (CC3), with CD31 and E-Cad. W) Whole mount immunofluorescence of a day 12 organoid. 200 μm scale. X–Y) Selected virtual slices of regions of (W). Arrows point to cell-specific CC3. 30 μm scales. DAPI marks nuclei. Representative immunofluorescence images were derived from n ≥3 organoids from at least 2 different experiments.
Branching of vasculature was quantified with angiotool, a free software developed by the NIH (Zudaire et al., 2011). The same whole mount organoids and E13.5 embryonic kidneys stained for CD31 and Sox17 (organoids) or CD31 and EMCN (kidneys) analyzed for Fig. 2D–F were analyzed for branching. Every 5th virtual slice was analyzed and the number of junctions and junction density were automatically calculated. For epithelial-endothelial contact, EC contact with E-Cad+ epithelial structures were counted by hand using FIJI. 10 μm sections of E15.5 kidneys stained by immunofluorescence for Flk1-GFP and E-Cad as well as 10 μm virtual sections of whole mount organoids stained for CD31 and E-Cad were analyzed. Brightness/contrast adjustments made for representative images were equally applied to all images in that set.
Fig. 2. hESC-derived organoid blood vessels are sparse and lack patterning.

A) Whole mount immunofluorescence of a day 12 organoid demonstrating a network of CD31+ ECs. B) Virtual slice of Sox17+VEGFR2+ ECs. 100 μm scale, inset 10 μm scale. C) Whole mount immunofluorescence of CD31+Sox17+ ECs in a day 12 hESC organoid (yellow arrow). CD31−Sox17+ cells are indicated with white arrows. 100 μm scale. D-F) Comparison of vascular volume by whole mount immunofluorescence staining of embryonic kidneys and hESC organoids. D) 3D view of an E13.5 mouse kidney stained with EMCN. 100μmscale. E) 3D view of an hESC organoid stained with CD31. 100 μm scale. F) A graph quantifying the percent volume of ECs in a whole mount image comparing 3 embryonic kidneys at E12.5, 13.5, and 14.5 with 9 organoids from 3 independent experiments. One-way ANOVA performed. **** adjusted p value < 0.0001. EC % volume is not significantly different between kidneys. G) E-Cad+ tubule surrounded by Flk-1 GFP + ECs in a E15.5 kidney. 10 μm scale. H) E15.5 glomerulus formed by a Flk-1 GFP+ capillary tuft surrounded by Podxl+ podocytes. 10 μm scale. I) Epithelial tubule (E-Cad, dotted line) is not surrounded by ECs (CD31) in the hESC organoid. 10 μm scale. J) ECs (CD31) did not invade hESC organoid glomerulus (Podxl). 10 μm scale. K) Immunofluorescence on a section of a day 12 hESC organoid stained for ECs with CD31. 100 μm scale. Yellow arrow (K′) points to a lumenized blood vessel. White arrow (K’‘) are cords without lumens. L) Immunofluorescence of a day 12 hESC organoid on section stained with CD31 and PDGFRβ. White arrow points to a single EC or EC cord surrounded by PDGFRβ+ cells. Yellow arrow points to a lumenized vessel without PDGFRβ coverage. 100 μm scale. DAPI marks nuclei. Representative immunofluorescence images were derived from n ≥3 organoids from at least 3 different experiments.
To analyze NZC organoid vascularity and variability, we visually assessed (two observers, one blinded) by EC whole mount antibody stain. We classified organoids into Class I (little), II, and III (lot) depending on the area of EC staining.
2.8. Quantitative PCR
At least 8 organoids were collected and RNA was isolated using the RNeasy Micro Plus kit (Qiagen, 74034). Up to 2 μg of total RNA was used to make cDNA with the SuperScript™ III Reverse Transcriptase kit (Invitrogen, 18080). Primers were selected from published sources and are available in Table S2. qPCR was performed in triplicate using SYBR green master mix (Applied Biosystems, 4,309,155) and 1 μl diluted cDNA (~2 ng) with an annealing temperature of 60 °C. The reaction was run with the QuantStudio 3 Real-Time PCR system (Applied Biosystems/Thermo Fisher, A28567). Gene expression levels were determined by PCR reactions (15 s at 95 °C and 1 min at 60 °C for 40 cycles). GAPDH was used as an internal control for gene expression and the ΔΔCt method was used to calculate fold change compared to day 0.
2.9. Organoid engraftment under the kidney capsule
hPSC organoids for engraftment were developed according to the protocol in Kumar Gupta et al. (2020) where two days after organoid aggregation, organoids cells are collected and mixed with newly differentiated kidney progenitor cells then reaggregated. 18 days after the initial differentiation began, organoids were implanted under the kidney capsule of an immunocompromised NOD SCID gamma (NSG) mouse. Organoids were harvested 3 weeks after engraftment. 100 μl of 1 μg/μl of Isolectin B4-FITC (Sigma) was injected into the retroorbital sinus of the host mouse 30 min before harvest in order to visualize perfused vasculature. Tissue was sectioned with a vibratome and analyzed by immunofluorescent staining. More detailed methods in (Kumar Gupta et al., 2020).
2.10. Single cell data analysis
Mouse embryonic kidney datasets published (Combes et al., 2018; England et al., 2020) are curated in the Gene Expression Omnibus (GEO) database under accession numbers GSE108291 and GSE155794, respectively. The GEO accession numbers for each human embryonic and organoid single cell RNA-seq dataset analyzed can be found in Tables S3 and S4, respectively. In addition, sequencing data for 8- and 9-week embryos published by (Young et al., 2018) was included.
Each batch was processed independently using the scran Bioconductor package (Lun et al., 2016b). Unfiltered feature-barcode matrices were generating by running the CellRanger count pipeline for data provided in this format. Otherwise, provided gene expression matrices were used directly. Cells were called from empty droplets by testing for deviation of the expression profile for each cell from the ambient RNA pool (Lun et al., 2018). Cells with large mitochondrial proportions, i.e. more than 3 mean-absolute deviations away from the median, were removed. Cells were pre-clustered, a deconvolution method was applied to compute size factors for all cells (Lun et al., 2016a) and normalized log-expression values were calculated. Variance was partitioned into technical and biological components by assuming technical noise was Poisson-distributed and attributing any estimated variance in excess of that accounted for by a fitted Poisson trend to biological variation. The dimensionality of the data set was reduced by performing principal component analysis and discarding the later principal components for which the variance explained was less than variance attributable to technical noise.
A single set of features for batch correction were obtained by computing the average biological component of variation across batches and retaining those genes with a positive biological component. The batches were rescaled, and log-normalized expression values recomputed after the size factors were adjusted for systemic differences in sequencing depth between batches. Batch effects were corrected by matching mutual nearest neighbors in the high-dimensional expression space (Haghverdi et al., 2018). The resulting reduced-dimensional representation of the data was used for all subsequent embeddings including UMAP’s and diffusion maps.
Cells were clustered by building a shared nearest neighbor graph (Xu and Su, 2015) and executing the Walktrap algorithm (Pons and Matthieu., 2006). Differential gene expression analysis was performed using the two-part generalized linear model that concurrently models expression rate above background and expression mean implemented in MAST (Finak et al., 2015). A one-versus-all strategy was employed comparing each cluster to all other identified interstitial clusters.
Cell type labels were transferred from embryonic mouse to embryonic human cells as follows. First, the mouse and human embryonic cells were integrated and embedded together in a 50-dimensional diffusion map (Haghverdi et al., 2016). Next, the TensorFlow platform (Abadi et al., 2015) was utilized to train a sequential neural network with two hidden layers each containing 512 nodes, with dropout layers following each hidden layer, to classify the mouse embryonic cells by cell type as either epithelium, interstitium, endothelium, erythrocyte or leukocyte. The Adam optimizer was used with a focal sparse categorical cross entropy loss (Lin et al., 2017). Graph regularization (Bui et al., 2018) was employed. Five-fold cross validation was used during training. Next, clusters of the human embryonic cells, obtained independently as above, were assigned to one of the above cell types by a voting process where each cell’s mapped cell type determined its vote. These assigned cell types were then used to classify the human organoid cells in the same way.
3. Results
3.1. Kidney organoids generated from hESCs
To investigate blood vessels in kidney organoids, we used a previously published protocol (Takasato et al., 2016b) as a basis for differentiating hESCs. In this method, we differentiated hESCs in monolayer before collecting and aggregating cells to form organoids (Fig. 1A). We optimized the protocol by modifying the CHIR treatment length during hESC differentiation to achieve a maximal quantity of endothelial cells (ECs) while still generating a significant portion of renal structures (Fig. S1A–H). As described in (Takasato et al., 2016a), the ratio between nephrons and collecting duct in the organoids may be controlled by altering the length of CHIR treatment vs. FGF9 during the 7-day differentiation step (day −7 to day 0). A longer treatment of CHIR during hESC differentiation—and a subsequently shorter FGF9 treatment—results in organoids with a higher ratio of nephrons. Additionally, each cell line must be optimized for CHIR concentration and length to account for any variability in response to treatments (personal communication). We tested 4 μM, 6 μM, and 8 μM CHIR and we assessed the effects of CHIR/Fgf9 switch at day −4 or −3. Optimal organoid formation occurred with 6 μM conditions and are shown (Fig. S2A–H). A 4 day 6 μM CHIR treatment was selected as the standard method. After 7 days of hESC differentiation in monolayer (day −7 to day 0), we assessed differentiating cell types. E-cadherin (E-Cad)+ epithelium formed tubule-like structures (Fig. 1B’, arrows and 1C) surrounded by Meis1/2/3+ stromal cells and Six2+ nephron progenitor cells (NPCs) (Fig. 1B). Scattered VEGFR2+ angioblasts (with rare co-expression of Sox17, a marker of EC specification) were observed as well (Fig. 1D).
Fig. 1. hESC-derived kidney organoids display expected renal cell types.

A) Schematic and timeline of human embryonic stem cell (hESC) kidney organoid protocol. H9 ESCs were cultured in TeSR-E6 with 6 μM CHIR, with a switch to 200 ng/ml Fgf9 at day −3. On day 0, the differentiated cells were aggregated and placed into an ultra-low attachment 96-well plate with 200 ng/ml FGF9 for 5 days before being switched to growth-factor free media. B-D) Immunofluorescence of day 0 differentiated hESCs. 150 μm scale bars. B) Accumulations of cells with Meis1/2/3+ stroma surrounded by Six2+ NPCs. Cells in the middle forming tubule-like rings as seen by DAPI (arrows). C) Formation of E-Cad+ epithelial structures. D) VEGFR2+Sox17− angioblasts. E) Brightfield images of hESC organoids 2–7 days after aggregation. 0.5 mm scale bar. F-G) Immunofluorescence of day 12 hESC organoids demonstrating F) E-Cad+ epithelium and LTL+ proximal tubules and G) Podxl+ podocytes. H) Paraffin section of Meis1/2/3+ stroma. 100 μm scale bars. DAPI marks nuclei. N≥3 from at least 3 independent experiments.
Organoids were generated by collecting the mixed population of differentiated cells from monolayers and either pipetting droplets of approximately 100,000 cells onto filters or into ultra-low attachment 96-well plates in suspension (Fig. S1I). In the first instance, organoids were cultured on Transwell filters at the air-liquid interface (ALI), in a manner similar to typical mouse kidney explant culture (Fig. S2I, left) (Takasato et al., 2016a). These organoids were large in diameter (~2 mm) and displayed a flat pancake-shaped appearance (Fig. S1J; Fig. S2A). By contrast, when cells were cultured in low attachment plates, they self-aggregated and developed as rounded clusters (diameter of about 1 mm) submerged in media (Fig. 1E and Fig. S1I, right).
The low-attachment plate method with submerged organoids was chosen as it yielded more consistent organoids than Transwell organoids, as we found the latter more frequently and stochastically failed to form nephrons (Fig. S2B). Using the submerged organoid production method, we found that, as previously reported, self-organization into visible tubules could be observed after four days of culture (Fig. 1E) (Takasato et al., 2015).
3.2. hESC-derived kidney organoids contain specialized epithelium and stromal cells
We confirmed that hESC-derived kidney organoids produced the expected range of renal cell types in accordance with previous studies (Morizane et al., 2015; Takasato et al., 2015, 2016a). Using immunofluorescence, we showed that organoids developed NPCs, tubule epithelium, and stromal cells (Fig. 1F–H, Fig. S1A). Epithelial tubules stained for E-Cad; with a subset of Lotus Tetragonolobus Lectin (LTL)+ proximal tubules (Fig. 1F). However, in a deviation from normal kidney tissue, we found that LTL was not restricted to the apical surface of lumens as it is in vivo (Fig. F’). We also found scattered glomerulus-like structures of Podocalyxin (Podxl)+ podocytes (Fig. 1G). Between epithelial structures, we observed stromal cells identified by Meis1/2/3 (Fig. 1H). Hence, the hESC-derived organoid protocol altered to increase EC production still generated most expected kidney cell types, similar to previously reported protocols, but with some deviations from normal kidneys.
3.3. Vascular density in hESC-derived kidney organoids is low
We next examined the vascular structures in hESC-derived organoids at day 12, a stage previously reported to display staining for known endothelial markers (Takasato et al., 2014). Immunofluorescence for established EC markers CD31, Sox17, and VEGFR2 demonstrated the presence of positive cells at this stage (Fig. 2A–C). We found that Sox17 was the most reliable marker of ECs in the organoids. Although most presumptive ECs were Sox17+CD31+ (Fig. 2C, yellow arrow), a subset were Sox17+CD31− (Fig. 2C, white arrows). As seen by the 3D view of the organoid (Fig. 2A, C) and in optical slices (Fig. 2B), ECs organized into thin, linear aggregates with some interconnections, suggesting a primitive plexus. Therefore, by both marker expression and morphology, these vascular structures resembled blood vessels.
One striking and consistent observation, however, was that organoid vessels were distinctly sparse compared to kidneys in vivo. We compared the relative density by volume of vessels in normal embryonic kidneys to that in organoids and found that organoids had a significant reduction in ECs by whole mount immunofluorescent images (Fig. 2D–F). Despite selecting the most highly vascularized sections of the organoids for comparison, quantification showed hESC organoids contained less than 2% ECs by volume compared to more than an average of 20% for embryonic day 12.5 (E12.5), E13.5, and E14.5 kidneys (Fig. 2F). Together, these data showed that at day 12, the overall vascular mass in organoids was over 80% lower than in normal tissue.
3.4. Blood vessels in hESC organoids lack both patterning and lumens
We further analyzed organoid vessels to examine other aspects of renal vessels, including their physical association with kidney cell types, which is normally required for proper physiology. In the mouse embryonic kidney, the developing renal epithelium is surrounded by a plexus of vessels (Fig. 2G) and the glomeruli consist of a complex capillary tuft enveloped by podocytes (Fig. 2H) (Daniel et al., 2018; Vaughan and Quaggin, 2008). By striking contrast, we found that kidney organoid vasculature was largely not associated or aligned with the epithelium (Fig. 2I) and organoid podocytes were not vascularized (Fig. 2J). Instead, we often observed a loose plexus of ECs extending throughout the organoid, but not surrounding epithelial structures, and only occasionally associating with podocytes (Fig. 2J, arrow). Altogether, the organoid vessels appear unstructured. Furthermore, we find that organoids have reduced vascularity and patterning, as measured by a reduced number of blood vessel branch points and fewer points of EC-epithelial contact (Fig. S2C–D).
We further noted that most vascular structures in the organoids presented as either single cells or cords of single cells; most cords were not wider than an EC nucleus (Fig. 2B, K″). We assessed presence of lumens in these cords and found few identifiable open spaces (Fig. 2K’). In normal kidney tissue, capillary vessels express the sialomucin podocalyxin at their apical surface (Sawada et al., 1986), lining the lumen (Fig. S2E). This could be seen in some organoid vessels as well, although there was rarely clear apical localization (Fig. S2F–G).
Finally, we assessed whether organoid vessels associated with mural cells, such as smooth muscle cells and pericytes. We carried out staining for PDGFRβ and smooth muscle Actin (SMA), and while we observed PDGFRβ+ cells, they marked much of the stroma and did not always associate with vascular structures. Additionally, some of the organoid epithelium was also PDGFRβ+ (Fig. 2L). Rarely, PDGFRβ+SMA+ pericytes were observed associated with a blood vessel (Fig. S2H, inset, white arrow). Not only were these cells sparse, but they were not observed after day 9 of culture (data not shown). These observations point to pericytes largely missing from organoids.
3.5. Blood vessels in hESC-derived kidney organoids are transient
We found that the vasculature in organoids displayed high variability in overall mass and pattern depending on when they were assayed. Hence, we speculated that their development might change over time in culture. To elucidate the dynamic process of vessel formation in organoids, we examined vascular structures at multiple timepoints during organoid formation. Prior to organoid aggregation, we observed the presence of scattered endothelial progenitors, or angioblasts, at day 0. These cells were positive for known endothelial marker VEGFR2 (Fig. 1D). On day 3 after aggregation, Sox17+ ECs were scattered throughout the organoid, with some small CD31+ cords (data not shown). Over the course of culture and hESC differentiation, we found increasing numbers of identifiable ECs, peaking around day 6 (Fig. 3A–B, G–H, Q–R. By day 12, however, vascular structures were beginning to decline in number (Fig. 3E–F). By day 18, organoids displayed few cells that expressed endothelial markers, with CD31 staining being diffuse and punctate (Fig. 3E–F). Quantification of ECs at these stages demonstrate that EC numbers peak at days 6–9 and decline over time afterwards (Fig. 3G–H). Attempts to increase EC mass using VEGF increased vasculature but did not prevent regression (Fig. 3I–P). In fact, differentiated hESCs cultured in EGM-2 endothelial media (which includes VEGF) were unable to form nephrons and subsequently died, indicating that optimization of organoid culture specifically for ECs was sometimes detrimental to kidney cell types (data not shown). In contrast to the vasculature, Podxl (podocytes, Fig. 3S) and E-Cad (epithelium, Fig. 3V) levels increase or stay stable through day 15 of culture. The Six2+ nephron progenitors also decreases quickly (Fig. 3U). Interestingly, two markers of the stroma, Meis1 and Col3a1, show differential levels throughout time in culture, with Col3a1 increasing substantially (Fig. 3T). This indicates there may be an overgrowth of stroma in organoids (Fig. S3A–B). These data are consistent with published data (Takasato et al., 2015).
To determine how vessels were regressing, we examined cleaved caspase 3 (CC3) staining in organoids at day 12. We observed significant cell death, particularly in the center of the organoid (Fig. 3W, S.3C). The location of CC3 can be viewed by zoomed in virtual slices (Fig. 3X–Y, arrows). Most CC3 appears to be in the interstitium, some in ECs, and a minor amount in epithelium. These findings underscore that while ECs are present in kidney organoids, as reported in other studies, organoid vessels are generally sparse and transient, regressing relatively quickly over the course of culture.
3.6. Kidney organoids can be generated from mouse nephrogenic zone cells
After observing poor formation of vasculature in hESC-derived organoids, we reasoned that protocols designed to generate renal cell types may not be optimized to generate properly specified vascular precursors with the potential to differentiate properly. We hypothesized that blood vessels might develop better in organoids where ECs are sourced from tissue where kidney endothelium is already present. We therefore turned to an alternative method of organoid generation based on isolation and propagation of committed renal progenitors (Brown et al., 2015, 2019). In this method, we disaggregated actively proliferating cells from the cortex of the embryonic kidney, which contains ECs as well as nephron, ureteric bud, and stromal progenitor cells. As the cortex is the area of active nephrogenesis, it is referred to as the “nephrogenic zone” and the cells collected from this region as nephrogenic zone cells (NZCs).
Based on these methods, kidneys were isolated from mouse embryos at E17.5 and digested whole to release NZCs in the outer cortex (Fig. 4A). Only the most cortical cell layers, made up of nephron progenitor cells (NPCs), ureteric bud (UB) tips, ECs, and stromal cells, are released and isolated from the kidney using this technique (Fig. 4B). The differentiation potential of isolated NZCs was assessed using reporter mice with lineage tracer constructs in addition to immunofluorescence. Flk1-GFP was used to identify ECs; Hoxb7Cre; RYFP confirmed the UB lineage; Six2Cre; RYFP demonstrated the NPC lineage; and Foxd1Cre; RYFP marked the stromal progenitor cell lineage (S3D–G). After digestion, NZCs were either aggregated immediately to form organoids by spotting approximately 500,000 cells onto a floating membrane or cultured on monolayer to expand the population (Fig. 4A). NZCs could be maintained and passaged up to two times as a heterogeneous population of kidney progenitor cells (Fig. 4A, C–D). Similar to differentiated hESCs plated in monolayers, NZCs formed clusters of epithelial cells (Hoxb7-CreYFP+ UB cells) surrounded by Six2+ NPCs and stromal cells (Foxd1-CreYFP) (Fig. 4C and D).
As previously reported (Daniel et al., 2018; Munro et al., 2017), although the NPCs in the developing kidney organize into cap-like structures, which are themselves predominantly avascular, they are surrounded by a thin layer of interstitium and ECs. Hence the isolated NZCs contain a small number of ECs that we detected using Flk1-GFP one day after plating (Fig. S3H). However, ECs experienced limited growth in monolayer and became increasingly difficult to identify, likely not surviving in culture (Fig. S3H–I). In fact, culture of human umbilical vein endothelial cells (HUVECs) in kidney organoid media resulted in cell death (data not shown). Therefore, to maximize ECs in NZC-derived organoids, we carried out organoid aggregation immediately after digestion and collection of NZCs.
Like hESC-derived organoids, NZCs readily self-organized and differentiated into organoids, with tubule-like structures becoming visible in brightfield by day 4 (Fig. 4E). We next assessed the epithelial and stromal structures in NZC-derived organoids and found that NZC organoids contained ureteric and nephron epithelium, podocytes, and stromal cells (Fig. 4F–J). Whole mount immunofluorescence of NZC organoids showed a large number of E-Cad+ epithelial tubules and Podxl+ podocytes (Fig. 4F). Sectioned organoids (Fig. 4G–J) showed Aqp1+ proximal tubule and descending loop of Henle and NPHS1+ podocytes (Fig. 4G), LTL+ proximal tubule and Umod+ loop of Henle (Fig. 4H), a stromal population throughout the organoid marked by the protein Meis1/2/3 (Fig. 4I), and collecting duct marked by Aqp3 and pan-cytokeratin (Fig. 4J). These findings demonstrate the ability of embryonic NZCs to form organoids with a range of kidney cell types.
3.7. NZC-derived organoids contain intermediate ‘off-target’ vascular cell types
To analyze organoid vasculature, we generated NZC organoids from the kidneys of Flk1-GFP reporter mice. Interestingly, however, we identified discrepancies between vascular cell type specific reporters and immunofluorescence. In particular, we observed relatively few Flk1-GFP+ ECs in NZC organoids. By contrast, although CD31 could be seen in EC cords, it was also patchy throughout large regions of the organoid (Fig. 5A). We investigated these cells further and found that NZC organoids contained two distinct populations of presumptive ECs; one located on the filter side and one at the ALI. ECs along the ALI were rare but often organized into cords (Fig. S4A, yellow arrows), while those at the filter interface were abundant and disorganized (Fig. S4A, white arrows). Co-staining for the endothelial marker EMCN and the stromal marker Meis1/2/3 demonstrated that these more abundant cells were positive for both stromal and EC markers, while the cells at the organoid ALI exclusively expressed EC markers (Fig. S4B).
Fig. 5. NZC-derived kidney organoids develop sparse vasculature that regresses in culture.

A-D) Whole mount immunofluorescence of NZC organoids. A) A small Flk1-GFP+CD31+ vascular plexus at the air-liquid interface. E-Cad+ epithelium and additional CD31+ cells are present. 80 μm scale. B) Flk1-GFP+ ECs and Podxl+ podocytes visualized by 3D and section view. White arrow: podocytes. Yellow arrow: Cross section view of an EC cord lacking a lumen. 20 μm scale. C) Live images of Flk1-GFP in NZC organoids from days 1–7. Yellow arrows point to ECs. D) Endothelial cord is formed by polarized ECs expressing Podxl at the apical surface. No open lumen is visible. E) Three NZC organoids varying levels of vascularization from a single experiment scored as I, II, and III by level of vasculature. 150 μm scale bar. E′) Graph of EC score quantification of 244 organoids. DAPI marks nuclei. Representative immunofluorescence images shown in A-C were derived from n = 3 organoids from at least 2 different litters.
To trace the origins of these “endothelial” cells in organoids, we used genetic lineage tracing. Surprisingly, we found that NZC organoids displayed EC marker expression in cells that had originated from both stromal and NPC progenitors (Fig. S4C–D). Co-expression of stromal and EC markers led us to further investigate whether the double positive cells were due to aberrant expression of endothelial genes by stromal cells. We used the Foxd1Cre; RYFP/Terminator mouse to generate organoids derived solely from nephrogenic stromal cells (Fig. S4C) (Guo et al., 2013). This mouse contained a transgenic diptheria toxin receptor (DTR) flanked by LoxP sites at the Rosa26 locus. Following Cre recombination in Foxd1-expressing stromal progenitors, the DTR was deleted and those cells were resistant to diptheria toxin (DT). Following isolation, NZCs in culture were treated with DT in vitro to kill all non-stromal cells, resulting in an organoid made exclusively from stromal-lineage cells. Unexpectedly, these “stroma only” organoids contained CD31+ cells that were derived from the stromal lineage (Fig. S4C). These cells resembled the filter-side CD31 and EMCN-expressing cells that we deemed to not be true ECs. Similarly, we observed that NPCs, normally fated to become epithelium, also inappropriately expressed stromal and endothelial markers in wildtype organoids, as demonstrated by triple positive cells (YFP+ EMCN+ Meis1/2/3+) in Six2Cre; RosaYFP traced organoids (Fig. S4D). In accordance with observations above, these triple positive cells localized to the filter region of the organoid and did not appear to form nephrons or EC cords.
In addition to alternative progenitor lineages, cells in NZC organoids aberrantly co-expressed markers of multiple differentiated cell types. For instance, we observed organoid tubules that were double positive for cytokeratin, a marker of collecting duct, and LTL, a marker of proximal tubule (Fig. S4F). These two types of tubules normally originate from different cell lineages (UB and NPCs, respectively) and are not known to be co-expressed in any kidney cell types in vivo. We hypothesized that ectopic gene expression was an artifact of organoid culture. Therefore, we examined whether ectopic gene expression could also be observed in hESC organoids as well. Interestingly, immunofluorescence suggested that hESC organoids did not display an overlap in protein expression between ECs or NPCs and stromal markers (Fig. S4G–H). These data suggest that cells in organoids, particularly in NZCs, have relatively plastic identities or differentiation status. This underscores the importance of using multiple independent methods to validate cell type authenticity, including multiple molecular markers, cell and tissue morphology, and functionality tests.
3.8. Vasculature of NZC-derived organoids is scarce and deficient
We further analyzed vasculature structures in NZC-derived organoids to establish whether initial inclusion of bona fide ECs would yield a more robust organoid vasculature (Fig. 5A). Like the vasculature in hESC-derived organoids, on rare occasions, larger vessels developed that contained partial lumens (Fig. S5A–C). However, most vessels presented as EC cords, with no distinguishable central luminal space. We visualized the lack of lumens using virtual section view (Fig. 5B, B′ yellow arrow). In contrast to the EC cords of hESC organoids, some ECs did appear to polarize, suggesting an initial step towards lumen formation, as shown by the presence of Podxl and Icam2 at the apical membrane between ECs (Fig. 5D, S5A). Most NZC organoid vascular structures, however, largely failed to invade the glomerulus or form a capillary tuft within the podocytes (Fig. 5B, B’ white arrow).
Beyond morphological abnormalities, we found that ECs in organoids were highly variable in other respects, including overall vascular mass. To address the issue of EC mass, we analyzed a large number of organoids and systematically measured the vascular content based on immunofluorescence expression of EC markers and expected morphology. Fig. 5E shows three day 7 NZC organoids from one experiment with vastly different vascularization levels. These were classified as Class I, II or III as indicated, from low to high vasculature, as well as a score of 0 for no identifiable ECs. Over 200 NZC organoids were evaluated and scored, showing that NZC organoids were distributed across all four scores, with a skewing towards a score of 0 or 1 (Fig. 5E’).
In addition to variability, much like the hESC-derived organoids, NZC-derived organoids demonstrated substantial vascular regression over the course of their time in culture. The Flk1-GFP reporter allowed us to track ECs over time within a single organoid (Fig. 5C). On day 1, the day after NZC aggregation, single ECs could be identified as GFP+ punctae (day 1, arrows). These cells proliferated and elongated, forming cords by day 3. Shortly thereafter, however, the EC cords began to shrink, often leaving behind only a few ECs, or sometimes none at all (day 7, arrows). Cell death of NZC organoid vasculature is seen by CC3 immunofluorescence Fig. S3J–K. These data not only highlight the high level of variability in NZC organoid ECs, but it underscores the overall low vascularity of the organoids. These findings suggest that the paucity of ECs observed in ESC derived organoids is not an effect of inappropriate re-programming to an endothelial progenitor state but rather a consequence of culture conditions.
3.9. Vasculature of explanted embryonic kidneys
Based on studies showing the dependence of vascular maintenance on shear stress from blood flow, both in vivo and in organoid culture (Langille and O’Donnell 1986; Meeson et al., 1996; (Ando and Yamamoto, 2009; Homan et al., 2019; Korn and Augustin, 2015; Langille and O’Donnell, 1986; Meeson et al., 1996), we speculated that vessels in both hESC- and NZC-derived organoids were largely sparse and lacked lumens due to the absence of hemodynamic flow in culture. This idea has been tested in other settings, and experimental data suggests flow can potentiate kidney organoid vessels (Homan et al., 2019). To further examine this possibility, we explanted normal embryonic kidney tissues and cultured them on filters (Ihermann-Hella and Kuure, 2019; Rak-Raszewska et al., 2015) in the absence of flow. These organs have all the requisite progenitor cell types of the mature organ and also contain the proper 3D patterning, allowing us to directly test the impact of loss of blood flow on vascular maintenance.
As expected, the vascularity of explanted E12.5 embryonic kidneys at the start of ex vivo culture was much higher than that of either NZC or hESC organoids (Fig. S6C). However, over the 3–6 day course of culture, established blood vessels quickly regressed (Fig. S6A). A comparison of whole mount immunofluorescence of explanted kidneys with their age-matched embryonic kidneys (one day in vitro is roughly equal to half a day of in vivo development) showed that explanted kidneys at days 3 and 6 had significantly less vasculature (Fig. 6A–D, S6C–G). However, NPCs were maintained and the UB continued to branch (Fig. 6A–D, S6F–G). Live imaging of Flk1-GFP in E12.5 kidney explants showed the dynamic nature of these vessels, including regression (Fig. 6E–F, S6A–B). Areas of vessels, and sometimes entire branches, were observed to fall apart around day 3 (Fig. 6F, extended data video). Cleaved caspase 3 staining showed that ECs underwent programmed cell death to an extent not observed in normal embryonic kidney tissues (Fig. 6G–I). Additionally, there was increased death in the center of the explant in non-vascularized tissues, a phenomenon observed in organoids as well. To determine whether EC regression was due to a lack of necessary angiogenic signaling, we treated the explants with a high amount of VEGF (200 ng/ml) to promote EC growth. VEGF-treated explants did not exhibit differences in EC regression compared to controls (Fig. 6J and K). Together, these findings further suggest the possibility that blood flow is a key factor in the differentiation and maintenance of ex vivo blood vessels.
Fig. 6. Blood vessels of explanted embryonic kidneys regress in culture.

A-B) Whole mount immunofluorescence of explanted E12.5 kidneys after 3 (A) and 6 (B) days of culture. ECs are marked by Flk1-GFP, NPCs by Six2, and nephrons and ureteric bud by E-Cad. C-D) Whole mount immunofluorescence of C) E13.5 kidney stained with CD31 and EMCN for ECs and D) an E15.5 kidney stained for Flk1-GFP ECs E-G) Explanted E12.5 kidney on glass for live imaging. E) Brightfield and Flk1-GFP after 1–3 days of culture. F) Live imaging of Flk1-GFP over 12 h. G-I) Whole mount immunofluorescence of Flk1-GFP+ ECs, Cleaved Caspase 3 (CC3)+ apoptosing cells, and Phospho-histone H3 (pHH3)+ proliferating cells. J-K) Live images of explanted E12.5 kidneys in brightfield and Flk1-GFP. J) Control and K) treated with 200 ng/ml VEGF. 100 μm scale bars. DAPI marks nuclei. Representative immunofluorescence images were derived from n = 3 explants.
3.10. Organoid implantation ameliorates vascularization
Given that all kidney tissues, both from in vivo or in vitro sources, displayed regression of the vasculature upon culture, we asked whether providing flow would prevent loss of vascularity in organoids. Recent studies using bioengineered flow platforms show that providing flow improved maintenance of blood vessels (Homan et al., 2019). Engraftment of organoids under the kidney capsule of immunocompromised mice has been shown as an alternative method of introducing flow (van den Berg et al., 2018). We thus carried out implantation of hiPSC-derived organoids under the kidney capsule, as previously shown (Gupta et al., 2019). Organoids were found to be well-vascularized 3 weeks after implantation, with a total organoid age of 30 days, well after we would expect the vasculature to have regressed (Fig. 7).
Fig. 7. Implanted organoids display extensive vascularization.
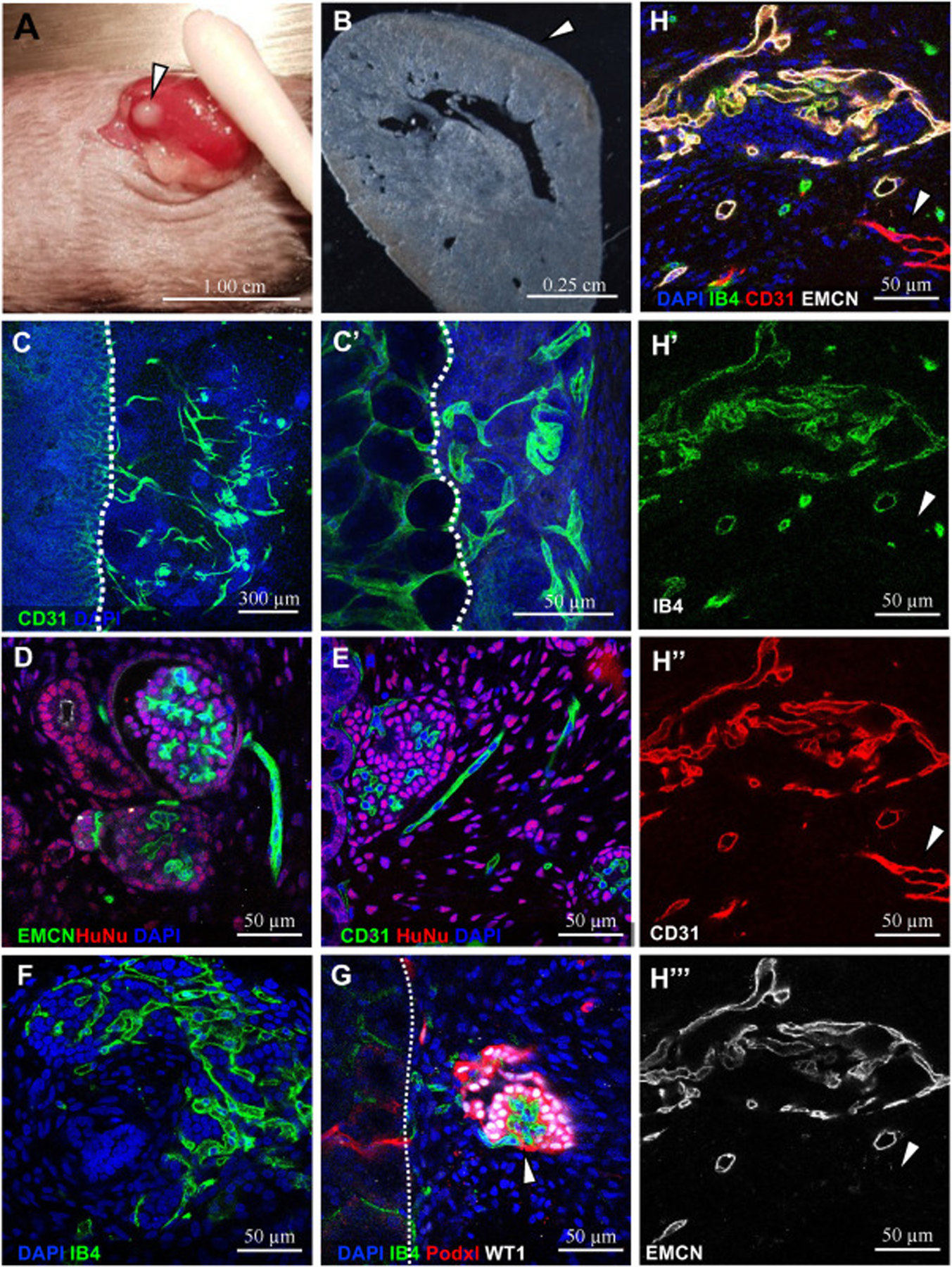
hiPSC organoids developed using methods from Kumar Gupta et al. (2020), 3 weeks after engraftment under the kidney capsule of NGS mice. A) Cluster of organoids under the kidney capsule after 3 weeks, arrow. 1 cm scale. B) Section of adult kidney showing growth of implanted organoids under the capsule, arrow. 2.5 mm scale. C) Mouse-specific antibody CD31+ vessels were visible at the interface between the host and organoid (dotted white line) 300 μm (C) and 50 μm (C′) scale. D) EMCN+ and E) CD31+ ECs in the organoid are HuNu−. F –H) Vessels display perfusion as per isolectin B4 (IB4) visible following injection into mouse host. G) IB4+ perfused capillary tuft in a Podxl+WT1+ glomerulus (arrow). H) Most perfused vessels are EMCN−CD31+ and non-perfused vessel (arrow) is EMCN−CD31+. D-H 50 μm scale. DAPI marks nuclei. Representative images shown here were derived from n = 6 NSG mice.
The implanted organoids grow and expand in mass at the surface of the kidney (Fig. 7A and B). We found that CD31+ blood vessels populated both the organoid and the interface between the host and organoid (Fig. 7C). To determine whether the vessels were derived from the host or the graft, we stained for Human Nuclear Antigen (HuNu). Surprisingly, the vasculature in the organoid was negative for HuNu, demonstrating that it was entirely populated from invading host vasculature (Fig. 7D and E). In addition, injection of isolectin B4 (IB4), which binds to perfused ECs upon systemic vascular injection, showed that blood flowing from the host was indeed reaching and penetrating the organoid (Fig. 7E and F). Unlike in the non-implanted organoids, we observed structures resembling glomerular capillary tufts within clusters of podocytes in the implanted organoids. Additionally, these capillaries were IB4+, suggesting circulation from host to well-developed glomeruli was occurring in implanted organoids (Fig. 7G).
These findings demonstrate that hPSC organoids have the capacity to form perfused, vascularized glomeruli, a major step in developing functional nephrons. However, this was not universal as we found a portion of unperfused vessels. While most vessels were EMCN+CD31+IB4+ triple positive, EMCN− CD31+ vessels were left unperfused (Fig. 7H, arrow, Fig. S7D). Conversely, these organoids only contained EMCN−CD31+ vasculature preceding implantation (Fig. S7C), demonstrating a difference in vessel maturation or identity upon perfusion. Therefore, further investigation into organoid vasculature heterogeneity and dynamics before and after engraftment will be of great interest to the field.
3.11. scRNA-seq of endothelial cells in organoids and human embryonic kidneys
To perform a comprehensive comparison of the vasculature in human embryonic kidneys (eECs) and human ESC and iPSC organoids (oECs), we compiled and compared scRNA-seq data from multiple published sources. GEO accession information for these data sets can be found in Tables 2 and 3 in the supplemental data. Uniform Manifold Approximation and Projection (UMAP) plots show the clustering of ECs in relation to the other cells in the embryonic kidneys (Fig. S8D) and organoids (Fig. S8A and C). First, we analyzed the percentage of ECs in the total cell population at each time point of organoid development (Fig. 8A–B). We discovered that there were two peaks where oECs were at their highest, at day 14 and day 25. It is important to note that these timepoints do not directly correspond with our protocol, for which the corresponding days would be day 7 and day 18 respectively. This is due to the days including the monolayer differentiation step. The two EC peaks do not indicate a transient death and reemergence of vasculature in organoids. Instead, they likely represent differences in the lifecycles oECs in organoids derived from ESCs versus iPSCs; organoids derived from the two cell types appear to generate different amounts of ECs that peak at different times. We inferred this because the clusters do not completely overlap, as seen in the UMAP in Fig. S8B. In fact, iPSC organoid ECs show an individual cluster that does not contain hESC ECs at all, meaning that there is a level of transcriptional divergence between organoids of different cell sources. Regardless of these differences, however, all organoids analyzed still demonstrate the pattern we observed where ECs emerge, proliferate for a short period, then regress.
Fig. 8. scRNA-seq comparing ECs from organoids and embryonic kidneys.

Analysis of scRNA-seq datasets of organoids and human embryonic kidney tissue from multiple publications. Organoids were differentiated using multiple protocols and ESC or PSC lines. Differentiation stages (days 0, 10, 12, 14, 16, 19, 21, 25, 26, 28, and 34 of organoid culture) do not correspond with methods used in precious figures. A) Histogram of EC percentage in organoid samples by day. B) UMAP of oECs color-coded by day. C. Heat map of 100 genes differentially expressed in eECs and oECs. Combines the top 50 genes differentially expressed in both eECs and OECs in comparison to other cells plus the top 25 genes highly expressed individually in eEC and oEC populations. Genes are grouped by cell expression similarity. Left to right, cells are grouped by gene expression similarity. eECs are indicated in green, and oECs are grey, with color coding above referring to stage. D) Differentially expressed endothelial genes in eECs and oECs.
Next, we assessed endothelial gene expression in the embryonic kidneys and PSC organoids. The heatmap in Fig. 8C illustrates this analysis. We find EC signature genes present in both eECs and oECs, including canonical endothelial genes such as EMCN, EGFL7, ANXA2, ARHGAP29, and COL41A. Surprisingly, we found an overall similarity of the eEC and oEC populations, showing that despite our findings of vascular deficiencies in organoids, oECs are transcriptionally similar to normal kidney ECs at early stages. On the other hand, oECs from day 14 organoids are more similar to the eECs than the oECs at later timepoints. This indicates that day 14 is both a peak in EC mass as well as in EC transcriptional similarity. Overall, there were not large differences between eECs at the different time points during the 8–18 week gestational period observed, although some time points display differential clustering (Fig. S8F). Our previous work (Daniel et al., 2018) described endothelial heterogeneity during mouse renal development. These data demonstrate similar findings, that most ECs have a baseline expression of a number of pan-endothelial genes, while displaying distinct individual profiles.
Multiple genes that encode ribosomal proteins such as RPS28 and RPL36 were identified in the eEC set. This indicates that the two sets of cells are so transcriptionally similar that housekeeping genes are being marked as upregulated. Interestingly, despite filtering out cells with high mitochondrial gene expression—indicative of cellular stress—the most consistently expressed gene in the ECs was HSPB1. This could mean ECs are particularly sensitive to the stress induced by the cell extraction process (Adam et al., 2017; O’Flanagan et al., 2019). In addition, a significant number of differentially expressed canonical EC genes in the eECs were omitted from the heatmap analysis due to discrepancies in quality control for the oEC population. These include PLVAP, APLNR, CDH5, TIE1, VEGFR1 and VEGFR2 (Fig. 8D). Consequently, most of the genes included in the heatmap were not known to be EC-specific. This could further illustrate the insufficiencies of organoid vasculature, but further analysis will be necessary to determine this definitively. Altogether, the data support the observations that organoid vasculature first develops normally and begins to mature but then degrades over time.
4. Discussion
In this study, we report in detail limitations of cultured kidney tissue blood vessel formation and maintenance. We analyze the vasculature generated using two distinct kidney organoid protocols––one which generates kidney cell types via directed differentiation of hESCs, and the other which isolates primary cells from the nephrogenic zone, including ECs, from developing mouse kidneys. In both cases, the endothelium is initially present and vascular cords form. However, in both protocols, we identify deviations from normal blood vessel development. First, organoid blood vessel morphology is abnormal in that vessels form few continuous lumens, indicating roadblocks in their functional differentiation. Second, organoid ECs can express non-endothelial markers (in NZC-derived organoids), such as stromal marker Meis1/2/3. This calls into question both their identity and our ability to recognize bona fide blood vessels without careful analysis of multiple markers. Third, while they are dynamic, similar to developing in vivo vessels, they are not stable, and we find that the vascular network in organoids inevitably regresses over time in culture. We propose that this is due to the absence of blood flow, as even cultures of intact embryonic kidneys exhibit this regression. Organoids implanted under the kidney capsule, by contrast, maintain complex vascular networks perfused by blood. Interestingly, these perfused organoid vessels are entirely derived from the host mouse vessels and display arteriovenous defects, as vessels associated with glomeruli stain for the venous marker (vessels entering and exiting the glomeruli are normally arterial). Lastly, transcriptomic analysis of ECs from hPSC organoids are relatively similar in gene expression to human embryonic kidney ECs early during their culture. It is likely that regression of organoid vessels over the course of culture exacerbates transcriptional differences observed at later stages.
While organoids have extraordinary potential for developmental biology studies and for personalized medicine, this study paints a cautionary tale regarding our ability to evaluate the vasculature of tissues generated ex vivo. Reliable blood vessel identification relies on a combination of standard vascular features, including multiple EC markers, vessel morphology, and patterning relative to other tissue structures. Although organoid ECs display some expected characteristics, we find that all three of these parameters are mostly abnormal in organoids.
4.1. Vascular deficiencies and regression in kidney organoids
We propose that in order for organoid systems to prove useful in therapeutic applications as functional ex vivo-grown tissue, they must contain blood vessels that are mature, perfused, and organized along the nephron. We show that organoid blood vessels display fundamental defects in both morphology and differentiation status. Despite source of origin or protocol used, organoid blood vessels regress over time in static culture significantly more rapidly than other organoid cell types. Therefore, methods have to be developed to further optimize, expand, and maintain blood vessels for them to perdure in culture, especially for the time it takes for a human kidney to develop in vivo (Hinchliffe et al., 1991; McMahon, 2016). In addition to limited vasculature, we find that NZC organoids have strikingly variable EC content. While hESC organoids were more consistent, they have noticeable batch variability, as noted in previous studies (Phipson et al., 2019). These are additional considerations that must be taken into account in organoid research and support the importance of large-batch analysis of scRNA-seq data that utilizes data from multiple datasets and labs.
Our findings point to some critical limitations of kidney organoid vasculature, which may prove applicable across different fields of organoid studies. In fact, cerebral organoids do not appear to contain ECs (Lancaster et al., 2013), although groups are trying to address this deficiency by adding exogenous ECs or expressing vascular transcription factors (Cakir et al., 2019; Shi et al., 2020a). Based on our findings, this could be due to the long culture times of these organoids of 75 days or more. Intestinal organoids, previously thought to not develop a significant vasculature as well, have recently been shown to contain a small population of ECs that arises and quickly disappears, similar to what we observe (Holloway et al., 2020; Spence et al., 2011). Previous studies in kidney organoids have also described the presence of varying amounts of vasculature. Some publications do not show any ECs, but most have at least a small amount of vasculature (Freedman et al., 2015; Kumar Gupta et al., 2020; Morizane et al., 2015; Takasato et al., 2015).
The reason for the dearth of vasculature, and the key to solving it, have thus far been unknown. However, the improvement of organoid vascularization has been tackled by multiple labs using a variety of methods. For instance, there has been progress in creating vascularized cerebral organoids by the addition of HUVECs or ECs differentiated from iPSCs in addition to engraftment (Pham et al., 2018; Shi et al., 2020b). HUVECs expressing ectopic ETV2 have been shown to have a remarkable ability to adapt tissue-specific identity and form stable, perfusable vessels that can colonize organoids (Palikuqi et al., 2020). Another recent study found a notable improvement in human intestinal organoid vasculature presence and maintenance through specific growth factor addition (Holloway et al., 2020). One study demonstrated that VEGF addition to kidney organoids appeared to drastically increase the endothelial population by immunofluorescence, but transcriptionally these cells appeared in the stromal cluster, raising the possibility of parallels with our observations of off-target cell types in NZC organoids and the importance of multi-marker EC verification (Czerniecki et al., 2018). We found that the addition of VEGF in static culture is not sufficient to block or reverse vascular regression in explants, although it may increase vasculature in the short term.
Another tantalizing approach to address vascular deficiencies might involve hypoxia, a known inducer of angiogenesis. It is known that embryonic in vivo blood vessels initially undergo vasculogenesis under markedly hypoxic conditions. In our experiments, we note that submerged organoids tend to fare better than organoids grown at the air-liquid interface, suggesting that elevated oxygen levels present in standard cell culture incubators may suppress vessel growth. However, investigation into the effects of hypo- and hyperoxia in kidney explant culture have thus far not demonstrated a clear benefit (Loughna et al., 1998; Rymer et al., 2014). Future studies will be needed to investigate this line of inquiry.
Together, our experiments highlight the complexities in blood vessel growth and maintenance in cultured tissues; the solution to improving ex vivo vasculature is therefore unlikely to rely on a single factor. In vivo, beyond hypoxia and VEGF signaling, there are many factors that regulate vascular stability, such as the ECM and factors provided by pericytes, and macrophages. In fact, we observed few pericytes (PDGFRβ, SMA antibody) and could not detect macrophages (data not shown, NG2 or F4–80 antibody), suggesting these cell types do not differentiate in organoids. While we cannot rule out the possibility that disruption of the normal 3D cellular environment of the kidney, likely inherent in any organoid model, might impact vascular maturation, we argue that kidney cell types self-organize relatively efficiently and cannot fully explain the vascular abnormalities observed. Additionally, these factors—ECM, pericytes, and macrophages—are already present in kidney explants, which still experience extensive vascular regression (Hoeffel et al., 2015). Therefore, we propose that hemodynamic flow is likely a prerequisite for maintaining vasculature in kidney organoids. Once a stable organoid vasculature has been achieved, the field may focus on the other important aspects of endothelium in the kidney. These studies show that while organoids may have defective vasculature, we are already making progress toward understanding and improving vascularization of cultured kidney tissue.
4.2. Implantation of organoids for perfusion
Why are blood vessels in vitro generally fragile and unstable? Our study suggests that one possibility is that the absence of blood flow results in vessel regression. Blood vessels are known to be sensitive to changes in hemodynamic stress (Ando and Yamamoto, 2009), so we tested whether tissues cultured in the absence of flow would lose their vascularity. We show that hESC-derived organoid vessels regress after about 12 days in culture. Similarly, despite containing ECs derived from an existing, functional vascular network, NZC-derived organoids experience an even more rapid loss of vessels in under a week. This may be due to the initially smaller number of ECs or perhaps the angioblasts produced through hESC differentiation are more resilient in culture than ECs from pre-formed vessels. To further test how acutely lack of blood flow impairs blood vessels, we challenged the established vasculature of embryonic kidneys explanted and cultured ex vivo and observed rapid regression and apoptotic cell death in static culture. Therefore, regardless of whether vessels formed de novo via vasculogenesis in organoids or via angiogenesis in embryonic kidneys, ECs regress in static culture. Our observations support recent studies that ameliorate organoid vasculature using microfluidics or engraftment (Homan et al., 2019; Kloth et al., 1993; Van den Berge et al., 2018).
We show that organoid implantation under the kidney capsule resulted in higher levels of vascularization than expected at the comparable 30-day culture time. Remarkably, capillaries even invade into podocyte clusters and form perfused, glomeruli-like capillary tufts. Strikingly, all the vasculature in the implanted organoid appeared to be primarily derived from the host. It was shown in a lymph-node engraftment model that this is progressive over 8 weeks (Kloth et al., 1993). In contrast, other studies have shown anastomosis of host and organoid vasculature (van den Berg et al., 2018). The real challenge ahead is to optimize transplantation efficiency and better understand the behavior and qualities of ECs in engrafted organoids and how to promote anastomosis with host vessels. Further analysis of the heterogeneity and maturity of vessels growing after implantation, as well as their arteriovenous differentiation, will be of particular interest to the fields of vascular biology and tissue engineering alike. Not only is vasculature maintained in the organoid upon implantation and perfusion, but organoids display improved maturation of epithelium (Homan et al., 2019; Kumar Gupta et al., 2020; van den Berg et al., 2018). Is this due to increased oxygenation, nutrient availability, signals from the ECs themselves, or a combination thereof? We hypothesize that ECs produce angiocrine signals that are important for kidney development and maturation, but this remains to be answered.
4.3. Blood vessel organization and patterning
In addition to the challenge of maintaining ECs in cultured organoids, optimization of kidney vessel patterning and regional specialization will be needed. In our previous work (Daniel et al., 2018), we described the spatial patterning of embryonic kidney vasculature. Recent studies further emphasize the striking heterogeneity of the vasculature along the differentiated nephron (Barry et al., 2019). While the full array of mechanisms that maintain the physical relationship between ECs and the developing nephron is not yet fully understood, it is likely that EC-nephron crosstalk has a role in ensuring proper differentiation of both tissues. This specificity is likely important for renal development and maintenance due to paracrine signals produced by ECs. Called ‘angiocrine signaling,’ ECs have been observed to influence tissue growth, maintenance, and patterning in many tissues including the lung, brain, pancreas, and liver (Jakab and Augustin, 2020; Magenheim et al., 2011; Rafii et al., 2016; Rocha et al., 2015). Additionally, EC heterogeneity, both phenotypically and transcriptionally, is necessary for nephron function and adaptations to the environment and stress (Aird, 2007; Barry et al., 2019; Ding et al., 2011; Dumas et al., 2020; Garlanda and Dejana, 1997; Kumar Gupta et al., 2020; Molema and Aird, 2012; Nolan et al., 2013; Ottone et al., 2014). Although active angiocrine signals in the kidney have yet to be identified, there are indications that blood vessels are involved in NPC maintenance and disruption of vasculature through Sox17 and Sox18 deletion results in medullary hypoplasia (Matsui et al., 2006; Rymer et al., 2014). As shown in intestinal organoids (Holloway et al., 2020), scRNA-seq data can provide clues to receptor-ligand pathways active in ECs and surrounding cells. Future analysis will seek to identify pathways possibly altered in organoids.
It is clear that nephron vasculature must be properly organized and differentiated into an arterial-capillary-venous system, as well as distinct renal EC types, to assure function of the kidney as a whole. However, even in the most highly vascularized organoids, the ECs are not uniformly distributed, have defective branching, and do not closely associate with nephrons or podocytes. In hESC organoids, we noticed heterogeneity in the vasculature that may be an indicator of a progression towards vascular maturity, with VEGFR2 marking angioblasts, the emergence of Sox17 expression indicating a slightly more mature EC (Matsui et al., 2006), then CD31 only appearing once cords begin to form. This is demonstrated by the VEGFR2+Sox17− ECs on monolayer on day 0, indicating that there are only immature angioblasts present. 3 days after aggregation, ECs are mostly CD31−Sox17+, but as the organoid develops, CD31+Sox17+ cords form. A similar hierarchy was described in Homan et al. (2019). Some organoid vessels also demonstrated maturity by the presence of Podxl and the emergence of lumens. A deeper understanding of endothelial maturity, identity, and heterogeneity is possible via scRNA-seq data analysis, which promises to move this field forward.
4.4. Evaluating organoid vasculature
How similar or different are organoid vessels from in vivo kidney vessels? How can we recognize properly differentiated kidney ECs? Our scRNA-seq transcriptional analysis combining numerous datasets identifies striking similarity between organoid ECs and human embryonic kidney ECs. Therefore, while not entirely differentiated or patterned, organoid ECs show a potential to become functional kidney ECs. This is corroborated by a pseudotime analysis of scRNA-seq of day 10, 12, and 14-day organoids suggesting maturation of immature ECs into arterial and venous-like fates (Low et al., 2019). Notably, day 14 organoids are much more like the eECs, while the day 26+ oECs have much lower similarity and EC gene expression in general. This further highlights our point that while vasculature may be present initially in organoids, it degrades both physically and transcriptionally.
The investigation into organoid vasculature through scRNA-seq brings about the often debated subject—what defines an endothelial cell? How can we definitively identify an angioblast or an endothelial cell within an organoid or cultured kidney tissue? How can we conclusively determine whether it contributes to a functional blood vessel? We have shown that gene expression of a few canonical markers is not sufficient, yet it is a method universally relied upon. ScRNA-seq is a powerful tool that reveals insights into cell fate and differentiation beyond what can be learned through classic methods such as antibody staining or quantitative PCR. However, we show that transcriptional signatures, particularly in organoids, may not provide definitive identification of bona fide ECs. Our study demonstrates that to validate blood vessels and other cell types, expression of multiple genes along with morphological features and ultimately function must be assessed to properly recognize vessels within engineered tissues.
We put forth that additional efforts will be required in future organoid studies to ensure that regionally specific, organotypic vasculature must differentiate in conjunction with their associated epithelia. The presence of ECs alone does not ensure functional engineered tissue vascularization. Further analysis, characterization, and understanding of organoid endothelium will be needed, and this may be facilitated using gene lists generated from scRNA-seq analysis. These types of efforts will likely provide needed insight into the successes and deficiencies of organoid vasculature and give direction for its advancement.
4.5. The promise of organoids
An explosion in organoid research has resulted in related publications increasing 10-fold within the last decade alone. With dozens of protocols developed for generating various tissue types, many of these studies still largely ignore the associated vasculature despite its central importance for organ survival and function. Additionally, our understanding of blood vessels and ECs themselves is experiencing a renaissance, particularly in the light of scRNA-seq which has provided new insights into organotypic EC heterogeneity. We propose that a more dedicated focus on the vasculature during organ development is essential and that directing more attention to blood vessels in organoid cultures and laboratory generated tissues will be crucial to achieving functional tissues for transplantation.
Our work highlights the importance of context during vessel formation and maintenance, both in vivo and in engineered tissues (Homan et al., 2019). Further work will be needed to generate regionally specific blood vessels, which we propose actively support epithelial development and the maintenance of a functional endothelial-epithelial association throughout the filtration process. While endothelial crosstalk has been implicated in the development and maintenance of multiple tissues (Cleaver and Melton, 2003), there is still much to be discovered in the kidney on that front. Bioinformatics and scRNA-seq data will provide more insight into the insufficiencies of organoid vasculature and provide candidate targets to try to support maturation and maintenance.
In this study, we have systematically investigated blood vessels in kidney ex vivo model systems, uncovering underappreciated (or previously unevaluated) challenges in the use of organoids and cultured tissues with respect to their vasculature. Organoids ultimately have the capability to form tissue-specific, mature vasculature, but we must develop ways to overcome the vascular deficiencies in ex vivo kidney tissues we have described. Together, our investigation into organoid vasculature underscores the engineering challenges ahead and cautions the field to properly evaluate in vitro ECs, as they are particularly susceptible to misidentification, immaturity, and regression.
Supplementary Material
Acknowledgements
We are grateful to the entire ReBuilding the Kidney (RBK) consortium for discussion on every aspect of this work. Specifically, we want to thank Dr. Melissa Little, Dr. Jessica Vanslambrouck, and Dr. Sara Howden for their invaluable advice regarding kidney organoids, as well as Drs. Jennifer Lewis and Kimberly Homan for discussions regarding hemodynamic flow and organoids. We also thank Drs. Jenny Hsieh, Vanesa Nieto-Estevez and Jun Wu for providing us with H9 ESCs and training and Dr. Janet Rossant for the Flk1-eGFP mouse line. We also thank the members of the Cleaver lab, including Haley Barlow, Dr. Caitlin Braitsch, Dr. Edward Daniel, and Dr. Xiaowu Gu for discussions and critical reading of the manuscript.
Funding
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases DK106743, DK079862 to O.C.; National Institute of Heart, Lung and Blood HL113498 to O.C.; a Center for Regenerative Science and Medicine (CRSM) predoctoral fellowship to A.R.; an American Society of Nephrology Pre-Doctoral Fellowship Award to A.R and an F31 DK124046. Deposited in PMC for release after 12 months.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ydbio.2021.04.009.
References
- Abadi, et al. https://arxiv.org/pdf/1603.04467.pdf arXiv:1603.04467.
- Adam M, Potter AS, Potter SS, 2017. Psychrophilic proteases dramatically reduce single-cell RNA-seq artifacts: a molecular atlas of kidney development. Development 144, 3625–3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aird WC, 2007. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ. Res 100, 174–190. [DOI] [PubMed] [Google Scholar]
- Ando J, Yamamoto K, 2009. Vascular mechanobiology: endothelial cell responses to fluid shear stress. Circ. J 73, 1983–1992. [DOI] [PubMed] [Google Scholar]
- Barry DM, McMillan EA, Kunar B, Lis R, Zhang T, Lu T, Daniel E, Yokoyama M, Gomez-Salinero JM, Sureshbabu A, Cleaver O, Di Lorenzo A, Choi ME, Xiang J, Redmond D, Rabbany SY, Muthukumar T, Rafii S, 2019. Molecular determinants of nephron vascular specialization in the kidney. Nat. Commun 10, 5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AC, Gupta AK, Oxburgh L, 2019. Long-term culture of nephron progenitor cells ex vivo. Methods in molecular biology (Clifton, N.J: 63–75, 1926. [DOI] [PubMed] [Google Scholar]
- Brown AC, Muthukrishnan SD, Oxburgh L, 2015. A synthetic niche for nephron progenitor cells. Dev. Cell 34, 229–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui TD, Ravi S, Ramavajjala V, 2018. Neural graph learning: training neural networks using graphs. In: Paper Presented at the Proceedings of the Eleventh ACM International Conference on Web Search and Data Mining. Marina Del Rey, CA, USA. [Google Scholar]
- Cakir B, Xiang Y, Tanaka Y, Kural MH, Parent M, Kang YJ, Chapeton K, Patterson B, Yuan Y, He CS, Raredon MSB, Dengelegi J, Kim KY, Sun P, Zhong M, Lee S, Patra P, Hyder F, Niklason LE, Lee SH, Yoon YS, Park IH, 2019. Engineering of human brain organoids with a functional vascular-like system. Nat. Methods 16, 1169–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleaver O, Melton DA, 2003. Endothelial signaling during development. Nat. Med 9, 661–668. [DOI] [PubMed] [Google Scholar]
- Combes AN, Wilson S, Phipson B, Binnie BB, Ju A, Lawlor KT, Cebrian C, Walton SL, Smyth IM, Moritz KM, Kopan R, Oshlack A, Little MH, 2018. Haploinsufficiency for the Six2 gene increases nephron progenitor proliferation promoting branching and nephron number. Kidney Int. 93, 589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerniecki SM, Cruz NM, Harder JL, Menon R, Annis J, Otto EA, Gulieva RE, Islas LV, Kim YK, Tran LM, Martins TJ, Pippin JW, Fu H, Kretzler M, Shankland SJ, Himmelfarb J, Moon RT, Paragas N, Freedman BS, 2018. High-throughput screening enhances kidney organoid differentiation from human pluripotent stem cells and enables automated multidimensional phenotyping. Cell Stem Cell 22, 929–940 e924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel E, Azizoglu DB, Ryan AR, Walji TA, Chaney CP, Sutton GI, Carroll TJ, Marciano DK, Cleaver O, 2018. Spatiotemporal heterogeneity and patterning of developing renal blood vessels. Angiogenesis 21, 617–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding BS, Nolan DJ, Guo P, Babazadeh AO, Cao Z, Rosenwaks Z, Crystal RG, Simons M, Sato TN, Worgall S, Shido K, Rabbany SY, Rafii S, 2011. Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization. Cell 147, 539–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas SJ, Meta E, Borri M, Goveia J, Rohlenova K, Conchinha NV, Falkenberg K, Teuwen LA, de Rooij L, Kalucka J, Chen R, Khan S, Taverna F, Lu W, Parys M, De Legher C, Vinckier S, Karakach TK, Schoonjans L, Lin L, Bolund L, Dewerchin M, Eelen G, Rabelink TJ, Li X, Luo Y, Carmeliet P, 2020. Single-cell RNA sequencing reveals renal endothelium heterogeneity and metabolic adaptation to water deprivation. J. Am. Soc. Nephrol 31, 118–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England AR, Chaney CP, Das A, Patel M, Malewska A, Armendariz D, Hon GC, Strand DW, Drake KA, Carroll TJ, 2020. Identification and characterization of cellular heterogeneity within the developing renal interstitium. Development 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finak G, McDavid A, Yajima M, Deng J, Gersuk V, Shalek AK, Slichter CK, Miller HW, McElrath MJ, Prlic M, Linsley PS, Gottardo R, 2015. MAST: a flexible statistical framework for assessing transcriptional changes and characterizing heterogeneity in single-cell RNA sequencing data. Genome Biol. 16, 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman BS, Brooks CR, Lam AQ, Fu H, Morizane R, Agrawal V, Saad AF, Li MK, Hughes MR, Werff RV, Peters DT, Lu J, Baccei A, Siedlecki AM, Valerius MT, Musunuru K, McNagny KM, Steinman TI, Zhou J, Lerou PH, Bonventre JV, 2015. Modelling kidney disease with CRISPR-mutant kidney organoids derived from human pluripotent epiblast spheroids. Nat. Commun 6, 8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlanda C, Dejana E, 1997. Heterogeneity of endothelial cells. Specific markers. Arterioscler. Thromb. Vasc. Biol 17, 1193–1202. [DOI] [PubMed] [Google Scholar]
- Guo JK, Shi H, Koraishy F, Marlier A, Ding Z, Shan A, Cantley LG, 2013. The Terminator mouse is a diphtheria toxin-receptor knock-in mouse strain for rapid and efficient enrichment of desired cell lineages. Kidney Int. 84, 1041–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta AK, Coburn JM, Davis-Knowlton J, Kimmerling E, Kaplan DL, Oxburgh L, 2019. Scaffolding kidney organoids on silk. J Tissue Eng Regen Med 13, 812–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghverdi L, Buttner M, Wolf FA, Buettner F, Theis FJ, 2016. Diffusion pseudotime robustly reconstructs lineage branching. Nat. Methods 13, 845–848. [DOI] [PubMed] [Google Scholar]
- Haghverdi L, Lun ATL, Morgan MD, Marioni JC, 2018. Batch effects in single-cell RNA-sequencing data are corrected by matching mutual nearest neighbors. Nat. Biotechnol 36, 421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinchliffe SA, Sargent PH, Howard CV, Chan YF, van Velzen D, 1991. Human intrauterine renal growth expressed in absolute number of glomeruli assessed by the disector method and Cavalieri principle. Laboratory investigation. a journal of technical methods and pathology 64, 777–784. [PubMed] [Google Scholar]
- Hoeffel G, Chen JM, Lavin Y, Low D, Almeida FF, See P, Beaudin AE, Lum J, Low I, Forsberg EC, Poidinger M, Zolezzi F, Larbi A, Ng LG, Chan JKY, Greter M, Becher B, Samokhvalov IM, Merad M, Ginhoux F, 2015. C-Myb(+) erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity 42, 665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway EM, Wu JH, Czerwinski M, Sweet CW, Wu A, Tsai YH, Huang S, Stoddard AE, Capeling MM, Glass I, Spence JR, 2020. Differentiation of human intestinal organoids with endogenous vascular endothelial cells. Dev. Cell 54, 516–528 e517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homan KA, Gupta N, Kroll KT, Kolesky DB, Skylar-Scott M, Miyoshi T, Mau D, Valerius MT, Ferrante T, Bonventre JV, Lewis JA, Morizane R, 2019. Flowenhanced vascularization and maturation of kidney organoids in vitro. Nat. Methods 16, 255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Valerius MT, McMahon AP, Duffield JS, 2010. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am. J. Pathol 176, 85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihermann-Hella A, Kuure S, 2019. Mouse ex vivo kidney culture methods. Methods in molecular biology (Clifton, N.J: 23–30, 1926. [DOI] [PubMed] [Google Scholar]
- Jakab M, Augustin HG, 2020. Understanding Angiodiversity: Insights from Single Cell Biology, vol. 147. Development. [DOI] [PubMed] [Google Scholar]
- Kloth S, Aigner J, Brandt E, Moll R, Minuth WW, 1993. Histochemical markers reveal an unexpected heterogeneous composition of the renal embryonic collecting duct epithelium. Kidney Int. 44, 527–536. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Valerius MT, Mugford JW, Carroll TJ, Self M, Oliver G, McMahon AP, 2008. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell 3, 169–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn C, Augustin HG, 2015. Mechanisms of vessel pruning and regression. Dev. Cell 34, 5–17. [DOI] [PubMed] [Google Scholar]
- Kumar Gupta A, Sarkar P, Wertheim JA, Pan X, Carroll TJ, Oxburgh L, 2020. Asynchronous mixing of kidney progenitor cells potentiates nephrogenesis in organoids. Commun Biol 3, 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA, 2013. Cerebral organoids model human brain development and microcephaly. Nature 501, 373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langille BL, O’Donnell F, 1986. Reductions in arterial diameter produced by chronic decreases in blood flow are endothelium-dependent. Science 231, 405–407. [DOI] [PubMed] [Google Scholar]
- Lin T, Goyal P, Girshick R, He K, Dollár P, 2017. Focal loss for dense object detection. In: Paper Presented at the 2017 IEEE International Conference on Computer Vision (ICCV). [Google Scholar]
- Loughna S, Yuan HT, Woolf AS, 1998. Effects of oxygen on vascular patterning in Tie1/LacZ metanephric kidneys in vitro. Biochem. Biophys. Res. Commun 247, 361–366. [DOI] [PubMed] [Google Scholar]
- Low JH, Li P, Chew EGY, Zhou B, Suzuki K, Zhang T, Lian MM, Liu M, Aizawa E, Rodriguez Esteban C, Yong KSM, Chen Q, Campistol JM, Fang M, Khor CC, Foo JN, Izpisua Belmonte JC, Xia Y, 2019. Generation of human PSC-derived kidney organoids with patterned nephron segments and a de novo vascular network. Cell Stem Cell 25, 373–387 e379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lun AT, Bach K, Marioni JC, 2016a. Pooling across cells to normalize single-cell RNA sequencing data with many zero counts. Genome Biol. 17, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lun AT, McCarthy DJ, Marioni JC, 2016b. A step-by-step workflow for low-level analysis of single-cell RNA-seq data with Bioconductor. F1000Res 5, 2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lun ATL, Riesenfeld S, Andrews T, Gomes T, Marioni JC, 2018. Distinguishing cells from empty droplets in droplet-based single-cell RNA sequencing data. Genome Biol. 20, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magenheim J, Ilovich O, Lazarus A, Klochendler A, Ziv O, Werman R, Hija A, Cleaver O, Mishani E, Keshet E, Dor Y, 2011. Blood vessels restrain pancreas branching, differentiation and growth. Development 138, 4743–4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T, Kanai-Azuma M, Hara K, Matoba S, Hiramatsu R, Kawakami H, Kurohmaru M, Koopman P, Kanai Y, 2006. Redundant roles of Sox17 and Sox18 in postnatal angiogenesis in mice. J. Cell Sci 119, 3513–3526. [DOI] [PubMed] [Google Scholar]
- McMahon AP, 2016. Development of the mammalian kidney. Curr. Top. Dev. Biol 117, 31–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeson A, Palmer M, Calfon M, Lang R, 1996. A relationship between apoptosis and flow during programmed capillary regression is revealed by vital analysis. Development 122, 3929–3938. [DOI] [PubMed] [Google Scholar]
- Molema G, Aird WC, 2012. Vascular heterogeneity in the kidney. Semin. Nephrol 32, 145–155. [DOI] [PubMed] [Google Scholar]
- Morizane R, Bonventre JV, 2017. Generation of nephron progenitor cells and kidney organoids from human pluripotent stem cells. Nat. Protoc 12, 195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morizane R, Lam AQ, Freedman BS, Kishi S, Valerius MT, Bonventre JV, 2015. Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat. Biotechnol 33, 1193–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro DAD, Hohenstein P, Davies JA, 2017. Cycles of vascular plexus formation within the nephrogenic zone of the developing mouse kidney. Sci. Rep 7, 3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nankivell BJ, Alexander SI, 2010. Rejection of the kidney allograft. N. Engl. J. Med 363, 1451–1462. [DOI] [PubMed] [Google Scholar]
- Nolan DJ, Ginsberg M, Israely E, Palikuqi B, Poulos MG, James D, Ding BS, Schachterle W, Liu Y, Rosenwaks Z, Butler JM, Xiang J, Rafii A, Shido K, Rabbany SY, Elemento O, Rafii S, 2013. Molecular signatures of tissue-specific microvascular endothelial cell heterogeneity in organ maintenance and regeneration. Dev. Cell 26, 204–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Flanagan CH, Campbell KR, Zhang AW, Kabeer F, Lim JLP, Biele J, Eirew P, Lai D, McPherson A, Kong E, Bates C, Borkowski K, Wiens M, Hewitson B, Hopkins J, Pham J, Ceglia N, Moore R, Mungall AJ, McAlpine JN, Team CIGC, Shah SP, Aparicio S, 2019. Dissociation of solid tumor tissues with cold active protease for single-cell RNA-seq minimizes conserved collagenase-associated stress responses. Genome Biol. 20, 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottone C, Krusche B, Whitby A, Clements M, Quadrato G, Pitulescu ME, Adams RH, Parrinello S, 2014. Direct cell-cell contact with the vascular niche maintains quiescent neural stem cells. Nat. Cell Biol 16, 1045–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palikuqi B, Nguyen DT, Li G, Schreiner R, Pellegata AF, Liu Y, Redmond D, Geng F, Lin Y, Gomez-Salinero JM, Yokoyama M, Zumbo P, Zhang T, Kunar B, Witherspoon M, Han T, Tedeschi AM, Scottoni F, Lipkin SM, Dow L, Elemento O, Xiang JZ, Shido K, Spence JR, Zhou QJ, Schwartz RE, De Coppi P, Rabbany SY, Rafii S, 2020. Adaptable haemodynamic endothelial cells for organogenesis and tumorigenesis. Nature 585, 426–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham MT, Pollock KM, Rose MD, Cary WA, Stewart HR, Zhou P, Nolta JA, Waldau B, 2018. Generation of human vascularized brain organoids. Neuroreport 29, 588–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phipson B, Er PX, Combes AN, Forbes TA, Howden SE, Zappia L, Yen HJ, Lawlor KT, Hale LJ, Sun J, Wolvetang E, Takasato M, Oshlack A, Little MH, 2019. Evaluation of variability in human kidney organoids. Nat. Methods 16, 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons PL, Matthieu, 2006. Computing communities in large networks using random walks. J. Graph Algorithm Appl 10, 191–218. [Google Scholar]
- Rafii S, Butler JM, Ding BS, 2016. Angiocrine functions of organ-specific endothelial cells. Nature 529, 316–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rak-Raszewska A, Hauser PV, Vainio S, 2015. Organ in vitro culture: what have we learned about early kidney development? Stem Cell. Int 2015, 959807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha AS, Vidal V, Mertz M, Kendall TJ, Charlet A, Okamoto H, Schedl A, 2015. The angiocrine factor Rspondin3 is a key determinant of liver zonation. Cell Rep. 13, 1757–1764. [DOI] [PubMed] [Google Scholar]
- Rymer C, Paredes J, Halt K, Schaefer C, Wiersch J, Zhang G, Potoka D, Vainio S, Gittes GK, Bates CM, Sims-Lucas S, 2014. Renal blood flow and oxygenation drive nephron progenitor differentiation. Am. J. Physiol. Ren. Physiol 307, F337–F345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada H, Stukenbrok H, Kerjaschki D, Farquhar MG, 1986. Epithelial polyanion (podocalyxin) is found on the sides but not the soles of the foot processes of the glomerular epithelium. Am. J. Pathol 125, 309–318. [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Sun L, Wang M, Liu J, Zhong S, Li R, Li P, Guo L, Fang A, Chen R, Ge WP, Wu Q, Wang X, 2020a. Vascularized human cortical organoids (vOrganoids) model cortical development in vivo. PLoS Biol. 18, e3000705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi YC, Sun L, Wang MD, Liu JW, Zhong SJ, Li R, Li P, Guo LJ, Fang A, Chen RG, Ge WOP, Wu Q, Wang XQ, 2020b. Vascularized human cortical organoids (vOrganoids) model cortical development in vivo. PLoS Biol. 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM, Shroyer NF, Wells JM, 2011. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470, 105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F, 2001. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol 1, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi A, Kaku Y, Ohmori T, Sharmin S, Ogawa M, Sasaki H, Nishinakamura R, 2014. Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell 14, 53–67. [DOI] [PubMed] [Google Scholar]
- Taguchi A, Nishinakamura R, 2015. Nephron reconstitution from pluripotent stem cells. Kidney Int. 87, 894–900. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Imanaka T, Takano T, 1996. Spatial and temporal pattern of smooth muscle cell differentiation during development of the vascular system in the mouse embryo. Anat. Embryol 194, 515–526. [DOI] [PubMed] [Google Scholar]
- Takasato M, Er PX, Chiu HS, Little MH, 2016a. Generation of kidney organoids from human pluripotent stem cells. Nat. Protoc 11, 1681–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasato M, Er PX, Chiu HS, Maier B, Baillie GJ, Ferguson C, Parton RG, Wolvetang EJ, Roost MS, Chuva de Sousa Lopes SM, Little MH, 2015. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 526, 564–568. [DOI] [PubMed] [Google Scholar]
- Takasato M, Er PX, Chiu HS, Maier B, Baillie GJ, Ferguson C, Parton RG, Wolvetang EJ, Roost MS, Lopes SM, Little MH, 2016b. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 536, 238. [DOI] [PubMed] [Google Scholar]
- Takasato M, Little MH, 2017. Making a kidney organoid using the directed differentiation of human pluripotent stem cells. Methods in molecular biology (Clifton, N.J: 1597, 195–206. [DOI] [PubMed] [Google Scholar]
- Takasato M, Maier B, Little MH, 2014. Recreating kidney progenitors from pluripotent cells. Pediatr. Nephrol 29, 543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg CW, Ritsma L, Avramut MC, Wiersma LE, van den Berg BM, Leuning DG, Lievers E, Koning M, Vanslambrouck JM, Koster AJ, Howden SE, Takasato M, Little MH, Rabelink TJ, 2018. Renal subcapsular transplantation of PSC-derived kidney organoids induces neo-vasculogenesis and significant glomerular and tubular maturation in vivo. Stem Cell Reports 10, 751–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Berge K, Perraudeau F, Soneson C, Love MI, Risso D, Vert JP, Robinson MD, Dudoit S, Clement L, 2018. Observation weights unlock bulk RNA-seq tools for zero inflation and single-cell applications. Genome Biol. 19, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan MR, Quaggin SE, 2008. How do mesangial and endothelial cells form the glomerular tuft? J. Am. Soc. Nephrol 19, 24–33. [DOI] [PubMed] [Google Scholar]
- Xu C, Su Z, 2015. Identification of cell types from single-cell transcriptomes using a novel clustering method. Bioinformatics 31, 1974–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Guihard PJ, Wu X, Blazquez-Medela AM, Spencer MJ, Jumabay M, Tontonoz P, Fogelman AM, Bostrom KI, Yao Y, 2017. Vascular endothelium plays a key role in directing pulmonary epithelial cell differentiation. J. Cell Biol 216, 3369–3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MD, Mitchell TJ, Vieira Braga FA, Tran MGB, Stewart BJ, Ferdinand JR, Collord G, Botting RA, Popescu DM, Loudon KW, Vento-Tormo R, Stephenson E, Cagan A, Farndon SJ, Del Castillo Velasco-Herrera M, Guzzo C, Richoz N, Mamanova L, Aho T, Armitage JN, Riddick ACP, Mushtaq I, Farrell S, Rampling D, Nicholson J, Filby A, Burge J, Lisgo S, Maxwell PH, Lindsay S, Warren AY, Stewart GD, Sebire N, Coleman N, Haniffa M, Teichmann SA, Clatworthy M, Behjati S, 2018. Single-cell transcriptomes from human kidneys reveal the cellular identity of renal tumors. Science 361, 594–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Carroll TJ, McMahon AP, 2002. Sonic hedgehog regulates proliferation and differentiation of mesenchymal cells in the mouse metanephric kidney. Development 129, 5301–5312. [DOI] [PubMed] [Google Scholar]
- Zhang YS, Pi Q, van Genderen AM, 2017. Microfluidic bioprinting for engineering vascularized tissues and organoids. J. Vis. Exp 126, e55957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zudaire E, Gambardella L, Kurcz C, Vermeren S, 2011. A computational tool for quantitative analysis of vascular networks. PloS One 6, e27385. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


