Abstract
PURPOSE:
Radiolabeled somatostatin receptor 2 (SSTR2) antagonists have shown higher tumor uptake and tumor-to-organ ratios than somatostatin agonists in preclinical models of NETs. We performed a phase I study to evaluate the safety and efficacy of SSTR2 antagonist 177Lu-satoreotide tetraxetan.
EXPERIMENTAL DESIGN:
Twenty patients with advanced SSTR2 positive NETs were treated with 177Lu-satoreotide tetraxetan. Patients first underwent a dosimetry study with 177Lu-satoreotide tetraxetan to determine the therapeutic activity that could be safely administered. This activity was split into two equal cycles to be delivered three months apart. The maximum activity was 7.4 GBq per cycle.
RESULTS:
Of 20 NET patients (1 lung, 7 small bowel, 9 pancreatic, 1 gastric, 1 rectal, 1 kidney; mean prior treatments: 3), 6 received one cycle of 177Lu- satoreotide tetraxetan and 14 received two cycles. Hematologic toxicity after cycle 1 was mild-moderate and reversed before cycle 2. However, grade 4 hematologic toxicity occurred in 4/7 (57%) patients after cycle 2 of 177Lu-satoreotide tetraxetan. The study was suspended, and the protocol modified to limit the cumulative absorbed bone marrow dose to 1Gy and to reduce prescribed activity for cycle 2 by 50%. The best overall response rate was 45% (5% complete response (1/20), 40% partial response (8/20)); with 40% stable disease (8/20) and 15% progression of disease (3/20). Median progression-free survival (PFS) was 21.0 months (95% CI: 13.6-NR).
CONCLUSION:
In this trial of heavily treated NETs, preliminary data are promising for the use of 177Lu-satoreotide tetraxetan. Additional studies are on-going to determine optimal therapeutic dose/schedule.
Keywords: Somatostatin receptor antagonists, 177Lu-satoreotide tetraxetan, neuroendocrine tumors
INTRODUCTION
Background
Recent publications of pivotal trials and drug approvals have redefined imaging and treatment for patients with progressive metastatic neuroendocrine tumors (NETs).1,2 Diagnostically, the Food and Drug Administration (FDA) approved 68Ga-DOTATATE PET for imaging of NETs, which will likely replace the traditional Octreoscan® imaging (i.e., 111In-DTPA-octreotide). Data suggest 68Ga-DOTATATE PET scan is more sensitive at detecting NETs than conventional cross-sectional and Octreoscan® imaging.2
The treatment of NETs includes the use of somatostatin analogs (i.e., octreotide and lanreotide) that target the SSTR for hormonal and disease control.3–5 The radiolabeled octreotate 177Lu-DOTATATE was recently approved by the FDA for treatment of somatostatin receptor-positive gastroenteropancreatic neuroendocrine tumors (GEP-NETs), including foregut, midgut, and hindgut neuroendocrine tumors in adults on the basis of the NETTER1 study.1 177Lu-DOTATATE and other radiolabeled somatostatin analogs provide a means of delivering targeted radiation with a high therapeutic index to tumors that over-express somatostatin receptors.6–11
68Ga-DOTATATE (i.e., the diagnostic agent) and 177Lu-DOTATATE (i.e., the therapeutic agent) are somatostatin receptor (SSTR) agonists. They bind the receptor as the natural ligand somatostatin, leading to internalization of the receptor–ligand complex.12 SSTR agonists can only bind to the somatostatin receptor when in its active state.13 In contrast, somatostatin antagonists can bind to the SSTR in its active or inactive state.12 SSTR antagonists have demonstrated significantly higher tumor uptake in mice than agonists.12 Furthermore, a substantially higher number of binding sites was shown by ex-vivo autoradiography of human NET samples.14
Consistent with these preclinical data, preliminary clinical data have also indicated higher tumor uptake and retention of SSTR antagonists as compared to agonists. In a head-to-head comparison of the SSTR antagonist 111In-DOTA-BASS and the agonist 111In-DTPA-octreotide, the antagonist demonstrated improved image contrast and visualized significantly more metastatic lesions than the agonist, although both ligands exhibited almost identical SSTR affinity.15 Tumor uptake of 111In-DOTA-BASS remained higher than that of 111In-octreotide until 24 h after injection,15 indicating that antagonists are well retained in the tumor tissue even though they do not lead to significant internalization of the SSTR. Similarly, PET/CT with the somatostatin antagonist 68Ga-satoreotide tetraxetan detected significantly more metastases than PET/CT with the SSTR agonist 68Ga-DOTATOC.16
Here we report the clinical results of a phase I study to assess the efficacy and safety of the novel therapeutic SSTR antagonist 177Lu-satoreotide tetraxetan (also called 177Lu-IPN01072 and previously known as 177Lu-OPS201 and 177Lu-DOTA-JR11)17 for therapy of patients with advanced and progressive metastatic NETs. 177Lu-satoreotide tetraxetan has a similar SSTR affinity profile as the clinically approved SSTR agonist 177Lu-DOTATATE (Lutathera®).18 A preliminary comparison of these two SSTR ligands in four patients with metastatic NETs has indicated higher tumor doses and tumor-to-kidney dose ratios for 177Lu-satoreotide tetraxetan than for 177Lu-DOTATATE.17
PATIENTS AND METHODS
The study was a phase I investigator-initiated study conducted at Memorial Sloan Kettering Cancer Center (MSK) reviewed by the institutional review board (ClinicalTrials.gov NCT02609737) and conducted under the auspices of an Investigational New Drug (IND) application acknowledged by the FDA. The study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines. All study participants provided written informed consent before study enrollment.
Patient Selection
Eligibility criteria included pathologically confirmed, well-differentiated NETs with unresectable measurable disease according to Response Evaluation Criteria in Solid Tumors (RECIST; version 1.1) on either computed tomography (CT) or magnetic resonance imaging (MRI), and progressive metastatic disease defined either by RECIST 1.1 or by increasing symptomatic disease (i.e., worsening hormonal symptoms or increased symptoms related to tumor burden). At least one metastasis had to show uptake of 111In-DTPA-octreotide on SPECT that was higher than physiologic radiotracer uptake by the liver. Patients were further required to have an Eastern Cooperative Oncology Group performance status ≤ 2, be 18 years or older, and have an estimated life expectancy of > 6 months. Adequate kidney and bone marrow function were required (creatinine ≤ 1.5 x upper limit of normal (ULN), absolute neutrophil count ≥ 1.5 × 10/L, hemoglobin ≥ 9.0 g/dL, platelets ≥ 200 × 10/L) and adequate hepatic function, defined as total bilirubin ≤ 1.25 X ULN, AST/ALT ≤ 2.5 X ULN with liver metastases. Patients with a history of > 20% bone marrow external beam radiotherapy and/or previous radioisotope therapy were excluded.
Study Evaluations
Medical history, MRI or CT, blood counts, and chemistry values were documented at screening and follow-ups. Tumor response was monitored by CT and MR studies, acquired as part of routine standard clinical care, approximately three and six months after the last treatment cycle. Tumor response was classified according to RECIST 1.1. Adverse events (AEs) were graded using Common Terminology Criteria for Adverse Events Version 4 (CTCAE), and the relationship to 177Lu-satoreotide tetraxetan was assessed. Dose-limiting toxicity was defined as any 177Lu-satoreotide tetraxetan -related AE ≥ grade 3 (G3).
Statistics
Given that this was a first-in-human study with limited data on the dosimetry of 177Lu-satoreotide tetraxetan, the main goal was to define safety and tolerability and characterize the AE profile. Safety, tolerability, and AEs were summarized using descriptive statistics. Overall response rate was according to RECIST 1.1. The median point estimate and 95% confidence interval for progression-free survival (PFS) and overall survival (OS) were estimated using the Kaplan–Meier method. A stopping rule was incorporated into the trial that if we observed more than 1 excessive toxicity within the first 10 patients we would stop the study, otherwise we would proceed to enroll 10 more patients. If we saw more than 2 excessive toxicities within the 20 (cumulative including the 1 that might have been seen amongst the first 10) we would stop the study. Excessive toxicities were defined as treatment related toxicities leading to an unacceptable delay in completing the 2 cycles of therapy as well as any delayed or irreversible grade 3 (G3) or grade 4 (G4) toxicities.
Investigational Diagnostic and Therapeutic Agents
The 177Lu-satoreotide tetraxetan therapeutic agent was manufactured by the MSK Radiochemistry and Molecular Imaging Probes Core Facility in compliance with the requirements specified in the Chemistry, Manufacturing, and Controls section of an FDA-acknowledged IND (#128,082).
Treatment
The treatment schema (Fig 1) included imaging with the SSTR2 antagonist 68Ga-DOTA-JR11, which is chemically identical to 177Lu- satoreotide tetraxetan (previously known as 177Lu-DOTA-JR11) except for the different radioisotope (the positron emitter 68Ga rather than the beta emitter 177Lu). Only patients with 68Ga-DOTA-JR11 uptake greater than liver in at least one metastasis of more than 2 cm diameter were considered for therapy. Patients with 68Ga-DOTA-JR11-positive disease then underwent a dosimetry study with 177Lu- satoreotide tetraxetan to determine the therapeutic activity that could be safely administered, based on normal tissue-absorbed radiation dose criteria. Given the worldwide clinical experience with radiolabeled SSTR agonists, we expected the kidney and bone marrow to be the main dose-limiting organs. The absorbed dose limits were set at 23 Gy total dose (i.e., combined dosimetry and therapy) for kidney, as suggested by the QUANTEC guidelines for external beam radiotherapy, and 1.5 Gy for red marrow, based on experience with 131I therapy for thyroid cancer,19 131I-labeled antibodies,19 and 177Lu-labeled SSTR agonists.20 The administered activity for the pre-therapy dosimetry study was nominally set at 1.85 GBq (50 mCi). Thereafter, the balance of activity that was predicted to deliver the lower of the two absorbed dose limits was calculated. This was split into two equal amounts to be delivered three months apart. However, if the first treatment resulted in estimated absorbed doses that differed from the initial predictions, the second treatment could be decreased or increased to achieve the intended dose limits. Irrespective of these calculated activities, the maximum allowable activity of 177Lu- satoreotide tetraxetan was fixed at 7.4 GBq (200 mCi) per administration. The mass dose of satoreotide tetraxetan injected into the patient ranged from 50–100 µg for PET imaging, dosimetry, and both therapeutic administrations.
Figure 1.

Study Design Dose modification occurred after trial suspension. Trial was modified so that the dose of cycle 2 was reduced by an additional 50%.
The study was suspended when 4/7 (57%) patients unexpectedly developed G4 hematologic toxicity after the second therapeutic administration of 177Lu-satoreotide tetraxetan. When the study was restarted, the treatment schema was modified such that the prescribed activity for the second therapeutic cycle was reduced to 50% of what it otherwise would have been. A dose reduction of 50% was chosen arbitrarily to ensure safety.
Radiation Dose Calculations
Full details of the methodology and results of radiation dose estimation will be presented elsewhere. Briefly, radiation dose estimates were calculated based on the analysis of serial blood counts and planar scintigraphic images together with a single SPECT/CT scan of the upper abdomen. Volumes of interest (VOI) were generated to correspond to kidney, spleen, uninvolved liver, bone, and 1–5 index lesions for each patient. The activity concentrations in VOI and the corresponding areas under the curve were estimated. It was assumed that the activity concentration in red bone marrow was equal to that in blood.21 These data were used to provide input to the radiation dose estimation software package OLINDA/EXM 1.0.22 Radiation dose estimates to index lesions were generated, considering only the non-penetrating component (i.e., beta particles) of 177Lu emissions.
RESULTS
Demographics and Treatments
From December 2015 to July 2017, 20 patients (10 female, 10 male) with an average age of 58 years were enrolled in this prospective single-center study. Patient characteristics are summarized in Table 1. The most common primary tumor site was pancreas (45%). All patients had metastatic disease, with the most common sites being liver (95%), lymph nodes (75%), and bone (35%). Ten percent (2/10) were hormone secreting, both small bowel with carcinoid syndrome. Seventy-five percent of tumors were classified as intermediate grade, 20% were low-grade, and 5% high-grade. The median number of prior treatments was three. Prior to study enrollment, 60% of the patients had undergone surgical resection, 80% somatostatin analog therapy, 45% hepatic arterial embolization, 55% cytotoxic therapy, and 55% targeted therapy.
Table 1.
Patient Demographics
| Number of patients | 20 |
|---|---|
| Sex, M | 10 (50%) |
| Age, years, median (range) | 57.5 (22–73) |
| Time since diagnosis, months median (range) | 64 (14–143) |
| Primary site, n (%) | |
| Lung | 1 (5%) |
| Stomach | 1 (5%) |
| Pancreas | 9 (45%) |
| Small bowel | 7 (35%) |
| Rectum | 1 (5%) |
| Kidney | 1 (5%) |
| Functional (Hormone Secreting) | 2(10%) both small bowel carcinoid syndrome |
| Grading (GEP, WHO 2010), n (%) Well Differentiated | |
| G1 Low | 4 (20%) |
| G2 Intermediate | 15 (75%) |
| G3 High | 1 (5%) |
| Metastases, n (%) | 19 (95%) |
| Liver | 19 (95%) |
| Lymph nodes | 15 (75%) |
| Bone | 7 (35%) |
| Lung | 2 (10%) |
| Peritoneum | 3 (15%) |
| Other (pancreas, spleen, pleura) | 4 (20%) |
| Prior treatments, n (%) | |
| SSA | 16 (80%) |
| Surgery | 12 (60%) |
| Cytotoxic | 11 (55%) |
| TACE/TAE | 9 (45%) |
| Everolimus | 7 (35%) |
| Sunitinib | 3 (15%) |
| Cabozantinib | 1 (5%) |
| External beam radiation therapy | 1 (5%) |
Abbreviations: SSA, somatostatin analogs; TACE, trans-arterial chemoembolization; TAE, trans-arterial embolization.
The 68Ga-DOTA-JR11 PET/CT scans demonstrated high tracer uptake of the known metastases in all patients (peak standardized uptake value, SUV peak, 2.5–84.0, average 18.0). Tracer uptake by the liver was markedly lower (average SUV 1.1, range 0.7–1.9) 23. Therefore, all patients went on to the dosimetry study, and received one therapeutic dose of 177Lu- satoreotide tetraxetan. Fourteen patients received two therapeutic doses. Five of the six patients did not receive the second dose because of clinical or radiographic progression; the sixth patient sustained a PR with the first dose and opted to come off study and received radioembolization to the liver eight months after the first treatment dose.
The study was put on hold when 4/7 patients developed G4 hematologic toxicity after their second therapeutic dose, approximately 4–8 weeks after treatment. The hematologic toxicities thus occurred at the typical time after the second treatment and did not delay the treatment. Since all G4 toxicities were reversible and occurred after the completion of the therapy we did not meet the stopping rules to close the study. We did, however, place the study on hold which was approved by the IRB and FDA. None of the patients with G4 toxicities had been treated with cytotoxic therapy prior to study enrollment. At the time the study was placed on hold, 19 patients had received their first therapeutic dose. The study restarted after a delay of approximately one year, and the gap between treatments for the next 6 patients was a median of 80 weeks (70–85 weeks). The reason for the extended gap was that we prioritized the patients that did not have a measurable response and treated them first. These patients received no additional antineoplastic treatment while awaiting the second treatment dose. Those that achieved PR were withheld treatment until the other patients were treated. The final patient entered the study after the restart and had a gap between treatments of three months. Activities for all administrations of 177Lu-satoreotide tetraxetan with associated time gaps and best responses are shown in Supplementary Table 1.
Radiation Dose Estimates
Radiation dose estimates to normal tissues based on pre-therapeutic dosimetry administration are shown in Table 2. The normal tissues that received the highest radiation doses were spleen (mean 1.48 Gy/GBq; range 0.12–4.01), kidneys (1.03 Gy/GBq; 0.15–1.52), and liver (0.47 Gy/GBq; 0.24–1.13). The estimated radiation dose to the red bone marrow was a mean of 0.09 Gy/GBq (range 0.06–0.15). For index lesions, the estimated radiation doses on a per-patient average basis ranged from 1.6 to 11.9 Gy/GBq.
Table 2.
Radiation-absorbed Dose Estimates to Normal Tissues from 177Lu-satoreotide tetraxetan
| Target Organ | Mean | SD | Min | Max |
|---|---|---|---|---|
| Adrenals | 0.10 | 0.03 | 0.07 | 0.18 |
| Brain | 0.08 | 0.03 | 0.04 | 0.16 |
| Breasts | 0.08 | 0.03 | 0.05 | 0.16 |
| Gallbladder wall | 0.11 | 0.02 | 0.08 | 0.18 |
| LLI wall | 0.09 | 0.03 | 0.05 | 0.17 |
| Small intestine | 0.09 | 0.03 | 0.06 | 0.17 |
| Stomach wall | 0.09 | 0.03 | 0.06 | 0.17 |
| ULI wall | 0.09 | 0.03 | 0.06 | 0.17 |
| Heart wall | 0.09 | 0.03 | 0.03 | 0.18 |
| Kidneys | 1.03 | 0.34 | 0.15 | 1.52 |
| Liver | 0.47 | 0.25 | 0.24 | 1.13 |
| Lungs | 0.09 | 0.03 | 0.06 | 0.17 |
| Muscle | 0.08 | 0.03 | 0.05 | 0.17 |
| Ovaries | 0.09 | 0.03 | 0.05 | 0.17 |
| Pancreas | 0.10 | 0.03 | 0.07 | 0.18 |
| Red marrow | 0.09 | 0.03 | 0.06 | 0.15 |
| Osteogenic cells | 0.43 | 0.14 | 0.18 | 0.69 |
| Skin | 0.08 | 0.03 | 0.05 | 0.16 |
| Spleen | 1.48 | 0.93 | 0.12 | 4.01 |
| Testes | 0.08 | 0.02 | 0.05 | 0.10 |
| Thymus | 0.08 | 0.03 | 0.05 | 0.17 |
| Thyroid | 0.08 | 0.03 | 0.04 | 0.17 |
| Urinary bladder wall | 0.31 | 0.07 | 0.20 | 0.41 |
| Uterus | 0.09 | 0.03 | 0.05 | 0.17 |
| Total body | 0.14 | 0.02 | 0.10 | 0.19 |
Note: All values are based on pre-therapeutic dosimetry administration only and are expressed in units of Gy/GBq.
Disease Targeting
177Lu-satoreotide tetraxetan targeted disease with high uptake in all known sites of disease (liver, nodes, bone). Figure 2 shows a selection of patient images illustrating intense tumor uptake of 177Lu-satoreotide tetraxetan and prolonged intratumoral retention of activity. On a patient-average basis, the effective terminal half-life ranged from 66–117 h with a median of 87 h, corresponding to biological half-times of 111–430 h with a median of 188 h.
Figure 2.
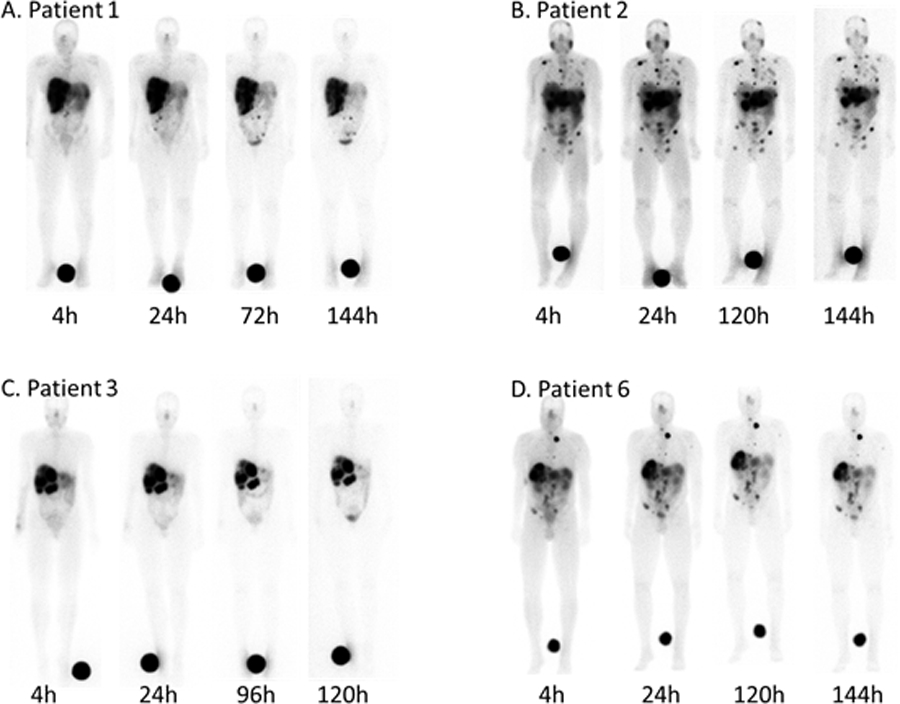
Planar whole-body scans of four patients with metastatic NETs after injection of 177Lu-satoreotide tetraxetan at the indicated times. Intense tracer uptake was observed in liver, lymph node, and osseous metastases.
Typically, disease that was visualized on 177Lu-satoreotide tetraxetan scans was also seen on the prior 68Ga-DOTA-JR11 PET scans. A detailed comparison of lesion targeting between 68Ga-DOTA-JR11 and 177Lu-satoreotide tetraxetan will be reported elsewhere.
Toxicity and Safety
Non-hematologic AEs that were considered possibly, probably, or definitely related to 177Lu-satoreotide tetraxetan treatment occurred in 14 patients (70%) and were all grade 1 or 2 G1 or G2). These included G2 nausea (5%) and G2 vomiting (5%), which were attributed to the concurrently infused amino acid solution and resolved once the infusions were complete. Other AEs included G1 hair loss (10%), G1 asthenia (5%), G1 tremor (5%), G2 diarrhea (10%), G1 facial edema (5%), G2 lower extremity edema (10%), and G2 mucositis (5%) (Table 3). There were no renal toxic effects during the observed time frame. One of the two hormone secreting patients required octreotide short acting octreotide to control the carcinoid syndrome immediately following the infusion but neither patient had an exacerbation of the carcinoid syndrome after the treatment.
Table 3.
Non-hematologic Toxicities Possibly, Probably, or Definitely Related to Treatment
| Toxicity | # of patients | Grade |
|---|---|---|
| Alopecia | 2 (10%) | 1 |
| Diarrhea | 2 (10%) | 2 |
| Dyspnea | 1 (5%) | 2 |
| Edema (face) | 1 (5%) | 2 |
| Edema (limbs) | 2 (10%) | 2 |
| Mucositis (oral) | 1 (5%) | 2 |
| Asthenia | 1 (5%) | 2 |
| Nausea | 1 (5%) | 2 |
| Tremor | 1 (5%) | 2 |
| Vomiting | 1 (5%) | 2 |
The maximum grades of hematological toxicity are detailed in Table 4. Subacute hematologic toxicity after cycle 1 was mild-moderate (G3/4 2/19 (10%) leukopenia that reversed before cycle 2). However, G4 thrombocytopenia occurred in 4 of the first 7 patients (57%) who received a second therapeutic administration of 177Lu-satoreotide tetraxetan. This toxicity started approximately 4–6 weeks after administration of the second therapeutic dose and lasted 7 to 16 weeks. Two of these patients also developed G3 anemia and three of these patients developed G3/4 neutropenia (Table 4). To examine any possible dose-response relationship with hematological toxicity, we calculated the projected total radiation doses to red bone marrow for the combined dosimetry and therapeutic administrations. These are based on the red marrow doses estimated from the pre-therapeutic dosimetry administration.
Table 4.
Maximum Grade of Hematological Toxicity After 177Lu-satoreotide tetraxetan possibly, probably, or definitely related to treatment (CTCAE 4.03) as well as administered activities of 177Lu-S satoreotide tetraxetan and associated time gaps. N/A indicates patients did not have a second therapeutic administration. Total RMD is the estimated total radiation absorbed dose to the red marrow for all 177Lu- satoreotide tetraxetan administrations combined, based on the red marrow dose estimates from the dosimetry administration. Best response to treatment per RECIST 1.1.
| Patient | Cycles (#) | Hb toxicity | WBC toxicity | PLT toxicity | ANC toxicity | Dosimetry (GBq) | D-T1 gap (days) | Therapy 1 (GBq) | T1-T2 gap (weeks) | Therapy 2 (GBq) | Total RMD (Gy) | Best Response |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 1 | 0 | 0 | 0 | 1.81 | 58 | 7.12 | 13 | 7.29 | 1 | SD |
| 2 | 2 | 3 | 4 | 4 | 4 | 1.23 | 21 | 7.18 | 11 | 7.28 | 1.54 | SD |
| 3 | 2 | 1 | 2 | 1 | 1 | 0.81 | 7 | 7.85 | 10 | 7.15 | 0.99 | PR |
| 4 | 2 | 1 | 2 | 0 | 2 | 1.98 | 14 | 7.28 | 12 | 7.3 | 1.08 | PR |
| 5 | 2 | 2 | 3 | 4 | 3 | 1.82 | 13 | 6.6 | 13 | 7.24 | 1.5 | CR |
| 6 | 2 | 2 | 2 | 4 | 2 | 1.95 | 8 | 7.33 | 13 | 7.32 | 1.71 | SD |
| 7* | 1 | 2 | 0 | 0 | 0 | 1.91 | 15 | 6.22 | N/A | N/A | 0.58 | PD |
| 8 | 2 | 3 | 3 | 4 | 3 | 1.99 | 28 | 5.65 | 12 | 4.86 | 1.44 | PR |
| 9* | 1 | 2 | 2 | 2 | 2 | 1.92 | 28 | 7.37 | N/A | N/A | 0.69 | PD |
| 10* | 1 | 0 | 0 | 1 | 0 | 1.93 | 20 | 7.37 | N/A | N/A | 0.78 | PR |
| 11 | 2 | 2 | 2 | 1 | 0 | 2.02 | 29 | 5.06 | 70 | 2.51 | 1.41 | SD |
| 12 | 2 | 2 | 2 | 1 | 2 | 1.88 | 21 | 6.16 | 74 | 3.62 | 1.42 | SD |
| 13 | 2 | 2 | 2 | 1 | 0 | 2.02 | 21 | 7.28 | 80 | 3.98 | 1.2 | PR |
| 14* | 1 | 2 | 2 | 1 | 0 | 2.01 | 29 | 6.29 | N/A | N/A | 1 | SD |
| 15* | 1 | 0 | 2 | 1 | 0 | 1.86 | 28 | 6.98 | N/A | N/A | 0.83 | SD |
| 16 | 2 | 1 | 0 | 0 | 0 | 2 | 29 | 7.28 | 84 | 4.02 | 0.74 | SD |
| 17 | 2 | 0 | 0 | 0 | 0 | 2.01 | 42 | 6.8 | 85 | 3.63 | 1.19 | PR |
| 18* | 1 | 1 | 0 | 0 | 0 | 1.96 | 7 | 4.96 | N/A | N/A | 0.8 | PD |
| 19 | 2 | 0 | 1 | 2 | 1 | 2.01 | 20 | 6.17 | 80 | 3.16 | 1.35 | PR |
| 20 | 2 | 0 | 1 | 0 | 0 | 1.83 | 20 | 7.25 | 11 | 3.9 | 0.88 | PR |
These six patients came off study after only one dose of treatment. All patients had either clinical or radiographic disease progression except for patient 10 who sustained a partial response; the patient opted to come off study instead of receiving the 2nd dose and was treated with radioembolization upon disease progression approximately 8 months later.
Note: Patient numbers were assigned at the time of inclusion in the study and do not show the order in which patients were treated.
Abbreviations: Hb, hemoglobin; WBC, white blood cells; PLT, platelets; ANC, absolute neutrophil count;
We examined the relationship between the estimated red marrow radiation dose (for all administrations combined) and the appearance of hematologic toxicity. G4 thrombocytopenia was used as the toxicity endpoint, as this was the most common G4 toxicity observed; Furthermore, prior work had suggested the existence of a dose response for platelet toxicity by radionuclide therapy.19 All patients treated with two cycles of 177Lu-satoreotide tetraxetan within three months who received ≥ 1.44 Gy bone marrow dose developed G4 thrombocytopenia (and G3/4 neutropenia) (Fig 3). In contrast, no patient with ≤ 1.08 Gy bone marrow dose experienced G4 thrombocytopenia or neutropenia.
Figure 3.
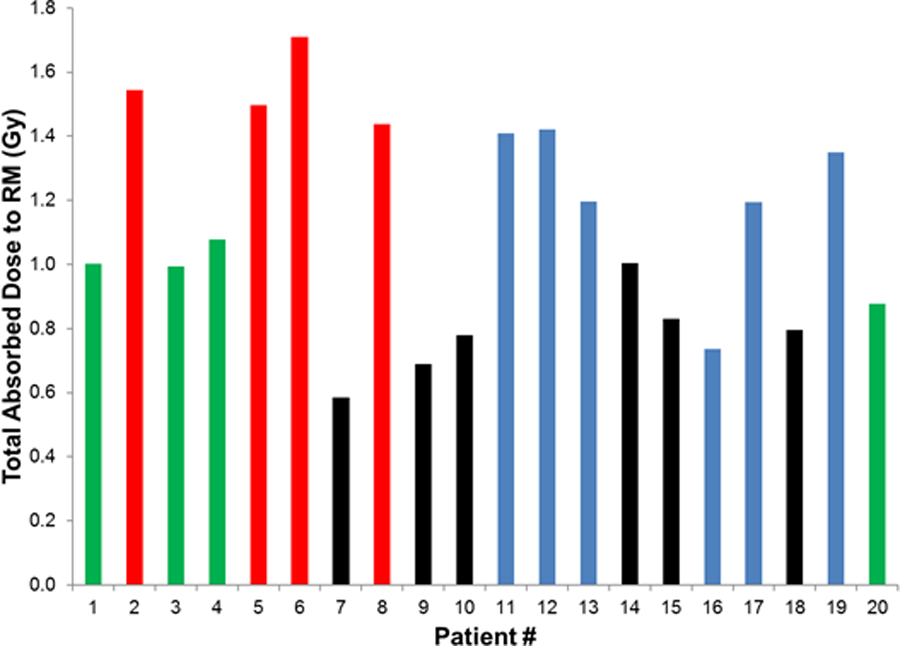
Relationship between estimated absorbed radiation dose to red marrow (all administrations combined) and development of G4 thrombocytopenia. Red: G4 thrombocytopenia after two cycles (3 months apart). Green: no G4 thrombocytopenia after two cycles (≤ 3 months apart). Blue: no G4 thrombocytopenia after two cycles (> 3 months apart). Black: no G4 thrombocytopenia after one cycle (did not have second cycle).
Myelodysplastic syndrome has not been observed in this trial to date. One patient with low counts subsequently underwent a bone marrow biopsy that revealed normal tri-lineage hematopoiesis and his counts subsequently recovered.
Efficacy
Overall response rate was 45% (1 CR (1/20, 5%), 40% PR (8/20)); 40% had SD (8/20), and 15% had PD (3/20). Supplemental Figure 4 shows an example of tumor response to 177Lu- satoreotide tetraxetan. Median follow-up was 32 months. Median PFS was 21.0 months (95% CI: 13.6-NR) and two-year PFS was 38% (20–73 months). Six deaths occurred, and median OS was not reached. Two-year overall survival was 85% (71–100) and three-year overall survival was 63% (43–94) (Figure 4). Responses were also seen in patients for whom the second treatment dose had been reduced and/or delayed (Table 4). Supplemental Figures 1, 2 and 3 show (as examples) the tumor response for Patients 19 and 20, who enrolled after the protocol was modified with dose reduction. Total red marrow dose was 1.35 and 0.88 Gy, respectively, and both achieved a partial sustained response.
Figure 4.

Progression-free survival and overall survival: Median follow-up of 32 months. Note: two patients (ID# 14,18) died early on had non-measurable disease (peritoneum and bone) are included in PFS at the time of death
DISCUSSION
In this phase I trial involving patients with progressive, heavily pretreated, well-differentiated, and mostly intermediate-grade NETs, 1–2 cycles of 177Lu-satoreotide tetraxetan resulted in a promising clinical response rate of 45%. Median PFS was 21.0 months (95% CI: 13.6-NR) and two-year PFS was 38% (20–73 months). Benefit was seen across all different types of gastroenteropancreatic NETs studied. High tumor uptake and slow washout resulting in high tumor radiation doses likely explain the high response rate with only 1–2 treatment cycles.23
No renal toxicity was observed, consistent with other PRRT studies with 177Lu-labeled somatostatin ligands.24,25 Hematologic toxicity, however, was unexpected and more severe than reported with other radionuclide therapies and none of these patients were heavily treated.26,27,28 G3 or G4 hematotoxicity occurs in about 10% of patients treated with 4 cycles of 7.4 GBq 177Lu-DOTATATE, a somatostatin receptor agonist with a similar affinity for SSTR2 as 177Lu-satoreotide tetraxetan.24 In about half of these patients, the hematotoxicity after 177Lu-DOTATATE was prolonged (> 6 months) or required transfusions. As in the present study, leukopenia and thrombocytopenia are more common after 177Lu-DOTATATE therapy than anemia. Dosimetry studies have indicated that the red marrow dose for 4 cycles of 177Lu-DOTATATE may exceed 2 Gy in up to 50% of patients.24,29 In contrast, we observed prolonged G4 thrombocytopenia and G3 leukopenia in 4 of 7 patients who received an estimated bone marrow radiation dose in the range of 1.5 Gy. After dose modification, there were no safety issues.
The reasons for the unexpected severity of hematotoxicity are currently unclear. At the time the protocol was designed, 1.5 Gy was considered a relatively low (and safe) limit for red marrow, especially since treatment was to be delivered over multiple cycles. Hematopoiesis did eventually recover in all patients and no patient developed myelodysplastic syndrome in 32 months of follow-up. A bone marrow aspirate performed in one patient illustrated normal tri-lineage cells and failed to identify an etiology.
The hematotoxicity is likely caused by ionizing radiation11, because there was a relationship between the estimated red marrow radiation dose and the appearance of hematologic toxicity—all patients who received two therapeutic administrations three months apart of ≥ 1.44 Gy, and no patients who received doses ≤ 1.08 Gy, experienced G4 thrombocytopenia.
Furthermore, the amount of peptide administered per cycle was low (<=100 µg) and all patients received the same peptide mass. No patients developed hematotoxicity once a 50% reduced amount of 177Lu was administered with the same peptide mass. This suggests that a purely pharmacologic effect of the SSTR antagonist is unlikely; however, somatostatin and other neuropeptides are involved in the regulation of hematopoiesis30–32,33 and the possibility of some unknown radiation damage potentiating interaction cannot be excluded.
Increased binding capacity of 177Lu- satoreotide tetraxetan to SSTRs, including bone marrow stem cells, could have increased the radiation exposure of progenitor cells, thereby causing prolonged bone marrow suppression. Two manuscripts studied the effects of peptide receptor radiotherapy on the bone marrow. Forrer et al compared radioactivity of the blood to the bone marrow aspirates in 15 patients after treatment with 177Lu-DOTATATE. They noted that the radioactivity in the bone marrow was identical to that in the blood. There was no significant binding of the radiopharmaceutical to the whole marrow.21 However, Sigrid et al showed that SSTRs are specifically expressed on immature (CD34+) human and murine hematopoietic progenitors and that somatostatin acts as a chemoattractant for these cells.34 Flow cytometric analysis showed that SSTR expression was restricted to CD34+ cells and that the CD34+/CD117+ subset, the most immature population of CD34+ cells, showed the highest SSTR expression.34 Since these cells represent only a small fraction of the total bone marrow, high uptake of 177Lu- satoreotide will not be detectable on whole-body imaging studies. We therefore hypothesize, because of the short path length of the beta particles emitted by 177Lu, that SSTR2-positive stem cells may receive a higher radiation dose than the average for the whole bone marrow. Since clinical dosimetry studies only determine the average dose to the bone marrow, this may explain why we observed severe hematotoxicity despite the low average bone marrow dose. These results illustrate that macroscopic bone marrow radiation dose limits for one form of PRRT do not necessarily apply to other types of PRRT even if the target and radionuclide are the same. We believe these are important findings to consider when testing future radiolabeled therapies. In particular, phase I clinical trials with novel molecular targeting vectors may require a more conservative approach to starting levels of radiation dose/administered activity and toxicity monitoring. Our results show that severe toxicity can occur even when radiation dose estimates are within limits previously considered safe and that low-level marrow toxicity for the first treatment cycle does not guarantee the same for second or subsequent cycles even with a 12-week gap between cycles. However, estimated radiation dose to red marrow did seem to be a reliable guide to toxicity, albeit with tolerance doses less than expected from clinical experience with 177Lu-DOTATE.
Limitations of our study for assessment of PFS and OS include the extensive delay in treating a subgroup of patients with the second therapeutic dose, with some patients having scans that were quite delayed, which could have inflated the PFS. We therefore interpret the very long PFS in this group of patients with heavily pretreated, mostly intermediate-grade NETs with caution. Additionally, our patient sample size is small, which is, however, typical for this stage of early drug development. Additionally, extended follow-up was not reported to evaluate the longer-term safety profile of 177Lu-satoreotide tetraxetan but is ongoing.
CONCLUSION
In summary, this phase I study indicates that 177Lu-satoreotide tetraxetan can deliver high radiation doses to NETs with favorable tumor-to-normal organ dose ratios. Preliminary data on tumor response rates and PFS are encouraging and overall support a potential therapeutic role for radiolabeled somatostatin antagonists in the treatment of NETs. However, the initial treatment schedule of this trial was associated with more severe hematotoxicity than expected from SSTR2 agonists at the same or higher red marrow dose. Treatment with reduced activity and longer treatment intervals drastically reduced hematologic toxicity while preserving activity.
Supplementary Material
Suppl. Figure 1 Metastatic pancreatic NET (Patient 19). Patient received 2 doses of 177Lu- satoreotide tetraxetan, the second dose at 50% reduction (A) 68Ga-DOTA-JR11 whole body MIP and (B) fused PET/CT image of the liver pre-treatment. (C) T2-weighted MRI of liver metastasis before treatment and CT images of the liver metastasis at 3 months (D), 9 months (E), and 16 months (F) post treatment. The SSTR positive metastasis (red arrow) shows marked shrinkage that persists for 16 months.
Suppl. Figure 2 Patient 19 who received 2 doses of 177Lu- satoreotide tetraxetan, the second dose at 50% reduction (A) 68Ga-DOTA-JR11 whole body MIP and (B) fused PET/CT image of the lung pre-treatment. (C) CT of lung metastasis before treatment and CT images of the lung metastasis at 3 months (D), 9 months (E), and 16 months (F) post treatment. There is marked shrinkage of the metastasis (red arrows) and complete resolution at 16 months.
Suppl. Figure 3 Patient 20 who received a 50% reduced second dose of 177Lu- satoreotide tetraxetan. CT images show multiple liver metastases pre-treatment (A) that are unchanged 18 months post-treatment (B). Best response: PR; SD for 18 months.
Suppl. Figure 4 Example of tumor targeting and response after two doses of 177Lu-satoreotide tetraxetan. Pre-therapeutic 68Ga-JR11 PET/CT shows intense tracer uptake by liver metastases of a G2 pancreatic NET and low uptake by normal organs. Axial PET (A), axial PET/CT (B), and maximum intensity projection of PET (C). MRI of the liver shows metastases corresponding to the lesions on 68Ga-JR11 PET/CT prior to therapy (D). After two cycles of therapy, there is a marked reduction/resolution of the metastases (E).
Suppl. Figure 5 Maximum decrease from baseline in the size of tumors in patients treated with 177Lu- satoreotide tetraxetan. Patient ID is noted above each response. Two patients (ID#14, 18), progressed with non-measurable disease (bone, peritoneum) and one patient (ID# 9) developed a new liver metastasis.
Translational Relevance.
Peptide receptor radiotherapy (PRRT) with somatostatin receptor 2 (SSTR2) agonists is an effective and safe therapy for progressive metastatic neuroendocrine tumors (NETs). Preclinical studies have suggested that radiolabeled somatostatin receptor antagonists deliver higher radiation doses to NETs than agonists and therefore may represent an even more effective PRRT. Herein, we performed a phase I study to evaluate the safety and efficacy of SSTR2 antagonist 177Lu-satoreotide tetraxetan. In a heavily treated population (mean number of therapies was 3), the overall response rate was 45%. However, despite bone marrow radiation doses that are safe for radiolabeled SSTR2 agonists, there was an unexpected high rate of hematologic toxicity. These results illustrate that macroscopic bone marrow radiation dose limits for one form of PRRT do not necessarily apply to other types of PRRT even if the target and radionuclide are the same. We believe these are important findings to consider when testing future PRRTs.
ACKNOWLEDGMENTS
This study was supported in part by the Geoffrey Beene Cancer Research Center at MSK and the MSK Radiochemistry and Molecular Imaging Probe Core, which is funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748. We gratefully acknowledge funding by Caring for the Carcinoid/NETRF. The precursor used in this study was provided by OctreoPharm/Ipsen. Ipsen reviewed this manuscript for scientific accuracy but had no input into the content. SK was supported in part by the NIH/NCI Paul Calabresi Career Development Award for Clinical Oncology K12 CA184746.
We gratefully acknowledge Rashid Ghani and members of the Nuclear Medicine Pharmacy; nuclear medicine nurses Ann Longing and Louise Harris for their help in patient management; RSAs Alicia Lashley, Hanh Pham, and Martha Ziolkowska and Clinical Research Manager Bolorsukh Gansukh for their excellent support with patient flow and protocol management; and the radiation safety officers and nuclear medicine technologists for their excellent technical assistance.
Footnotes
Disclosures
Diane Reidy-Lagunes is on advisory boards for Novartis, Ipsen and AAA; She has received research support from Novartis, Merck, and Ipsen
Neeta Pandit-Taskar- Consulting/ honoraria/ advisory board for Actinium Pharma, Progenics, Medimmune/Astrazeneca. Conducted research supported by Imaginab, Genentech, Janssen.
Joseph A. O’Donoghue -served as a consultant to Janssen Pharmaceuticals, Inc.
Simone Krebs- NONE
Kevin D. Staton-NONE
Serge K. Lyashchenko-NONE
Jason S. Lewis-NONE
Nitya Raj receives research support from Novartis and Xencor, Inc.
Mithat Gönen- NONE
Christian Lohrmann-NONE
Lisa Bodei is a consultant for Advance Accelerator Applications (AAA) and Ipsen, receives research grants from AAA.
Wolfgang Weber is on advisory boards and receives compensation from Bayer, Blue Earth Diagnostics, Endocyte and Pentixapharm. He has received research support from BMS, Ipsen and Piramal.
REFERENCES
- 1.Strosberg J, El-Haddad G, Wolin E, et al. : Phase 3 Trial of (177)Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med 376:125–135, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sadowski SM, Neychev V, Millo C, et al. : Prospective Study of 68Ga-DOTATATE Positron Emission Tomography/Computed Tomography for Detecting Gastro-Entero-Pancreatic Neuroendocrine Tumors and Unknown Primary Sites. J Clin Oncol 34:588–96, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rinke A, Muller HH, Schade-Brittinger C, et al. : Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol 27:4656–63, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Caplin ME, Pavel M, Ruszniewski P: Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med 371:1556–7, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Kvols LK, Moertel CG, O’Connell MJ, et al. : Treatment of the malignant carcinoid syndrome. Evaluation of a long-acting somatostatin analogue. N Engl J Med 315:663–6, 1986 [DOI] [PubMed] [Google Scholar]
- 6.Kwekkeboom DJ, de Herder WW, Kam BL, et al. : Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0,Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol 26:2124–30, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Bodei L, Cremonesi M, Grana CM, et al. : Peptide receptor radionuclide therapy with (1)(7)(7)Lu-DOTATATE: the IEO phase I-II study. Eur J Nucl Med Mol Imaging 38:2125–35, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Horsch D, Ezziddin S, Haug A, et al. : Effectiveness and side-effects of peptide receptor radionuclide therapy for neuroendocrine neoplasms in Germany: A multi-institutional registry study with prospective follow-up. Eur J Cancer 58:41–51, 2016 [DOI] [PubMed] [Google Scholar]
- 9.Imhof A, Brunner P, Marincek N, et al. : Response, survival, and long-term toxicity after therapy with the radiolabeled somatostatin analogue [90Y-DOTA]-TOC in metastasized neuroendocrine cancers. J Clin Oncol 29:2416–23, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Krenning EP, Kwekkeboom DJ, Bakker WH, et al. : Somatostatin receptor scintigraphy with [111In-DTPA-D-Phe1]- and [123I-Tyr3]-octreotide: the Rotterdam experience with more than 1000 patients. Eur J Nucl Med 20:716–31, 1993 [DOI] [PubMed] [Google Scholar]
- 11.Bodei L, Kidd M, Paganelli G, et al. : Long-term tolerability of PRRT in 807 patients with neuroendocrine tumours: the value and limitations of clinical factors. Eur J Nucl Med Mol Imaging 42:5–19, 2015 [DOI] [PubMed] [Google Scholar]
- 12.Ginj M, Zhang H, Waser B, et al. : Radiolabeled somatostatin receptor antagonists are preferable to agonists for in vivo peptide receptor targeting of tumors. Proc Natl Acad Sci U S A 103:16436–41, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perrin MH, Sutton SW, Cervini LA, et al. : Comparison of an agonist, urocortin, and an antagonist, astressin, as radioligands for characterization of corticotropin-releasing factor receptors. J Pharmacol Exp Ther 288:729–34, 1999 [PubMed] [Google Scholar]
- 14.Cescato R, Waser B, Fani M, et al. : Evaluation of 177Lu-DOTA-sst2 antagonist versus 177Lu-DOTA-sst2 agonist binding in human cancers in vitro. J Nucl Med 52:1886–90, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Wild D, Fani M, Behe M, et al. : First clinical evidence that imaging with somatostatin receptor antagonists is feasible. J Nucl Med 52:1412–7, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Nicolas GP, Schreiter N, Kaul F, et al. : Sensitivity Comparison of (68)Ga-OPS202 and (68)Ga-DOTATOC PET/CT in Patients with Gastroenteropancreatic Neuroendocrine Tumors: A Prospective Phase II Imaging Study. J Nucl Med 59:915–921, 2018 [DOI] [PubMed] [Google Scholar]
- 17.Wild D, Fani M, Fischer R, et al. : Comparison of Somatostatin Receptor Agonist and Antagonist for Peptide Receptor Radionuclide Therapy: A Pilot Study. J Nucl Med 55:1248–1252, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Fani M, Braun F, Waser B, et al. : Unexpected sensitivity of sst2 antagonists to N-terminal radiometal modifications. J Nucl Med 53:1481–9, 2012 [DOI] [PubMed] [Google Scholar]
- 19.O’Donoghue JA, Baidoo N, Deland D, et al. : Hematologic toxicity in radioimmunotherapy: dose-response relationships for I-131 labeled antibody therapy. Cancer Biother Radiopharm 17:435–43, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Kam BL, Teunissen JJ, Krenning EP, et al. : Lutetium-labelled peptides for therapy of neuroendocrine tumours. Eur J Nucl Med Mol Imaging 39Suppl 1:S103–12, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forrer F, Krenning EP, Kooij PP, et al. : Bone marrow dosimetry in peptide receptor radionuclide therapy with [177Lu-DOTA(0),Tyr(3)]octreotate. Eur J Nucl Med Mol Imaging 36:1138–46, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stabin MG, Sparks RB, Crowe E: OLINDA/EXM: the second-generation personal computer software for internal dose assessment in nuclear medicine. J Nucl Med 46:1023–7, 2005 [PubMed] [Google Scholar]
- 23.Krebs S, Pandit-Taskar N, Reidy D, et al. : Biodistribution and radiation dose estimates for (68)Ga-DOTA-JR11 in patients with metastatic neuroendocrine tumors. Eur J Nucl Med Mol Imaging 46:677–686, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bergsma H, Konijnenberg MW, van der Zwan WA, et al. : Nephrotoxicity after PRRT with (177)Lu-DOTA-octreotate. Eur J Nucl Med Mol Imaging 43:1802–11, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cremonesi M, Ferrari ME, Bodei L, et al. : Correlation of dose with toxicity and tumour response to (90)Y- and (177)Lu-PRRT provides the basis for optimization through individualized treatment planning. Eur J Nucl Med Mol Imaging 45:2426–2441, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sabet A, Ezziddin K, Pape UF, et al. : Long-term hematotoxicity after peptide receptor radionuclide therapy with 177Lu-octreotate. J Nucl Med 54:1857–61, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Bergsma H, Konijnenberg MW, Kam BL, et al. : Subacute haematotoxicity after PRRT with (177)Lu-DOTA-octreotate: prognostic factors, incidence and course. Eur J Nucl Med Mol Imaging 43:453–63, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bodei L, Kwekkeboom DJ, Kidd M, et al. : Radiolabeled Somatostatin Analogue Therapy Of Gastroenteropancreatic Cancer. Semin Nucl Med 46:225–38, 2016 [DOI] [PubMed] [Google Scholar]
- 29.Sandstrom M, Garske-Roman U, Granberg D, et al. : Individualized dosimetry of kidney and bone marrow in patients undergoing 177Lu-DOTA-octreotate treatment. J Nucl Med 54:33–41, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Yamazaki K, Allen TD: Ultrastructural morphometric study of efferent nerve terminals on murine bone marrow stromal cells, and the recognition of a novel anatomical unit: the “neuro-reticular complex”. Am J Anat 187:261–76, 1990 [DOI] [PubMed] [Google Scholar]
- 31.Afan AM, Broome CS, Nicholls SE, et al. : Bone marrow innervation regulates cellular retention in the murine haemopoietic system. Br J Haematol 98:569–77, 1997 [DOI] [PubMed] [Google Scholar]
- 32.Miyan JA, Broome CS, Afan AM: Coordinated host defense through an integration of the neural, immune and haemopoietic systems. Domest Anim Endocrinol 15:297–304, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Broome CS, Whetton AD, Miyan JA: Neuropeptide control of bone marrow neutrophil production is mediated by both direct and indirect effects on CFU-GM. Br J Haematol 108:140–50, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Sigrid PM O, van Hennik, Antonissen, Lichtenauer-Kaligis, Hofland, Lamberts, Bloweberg, Touw: Somatostatin is a seclective chemoattractan for primitive CD34+ hematopoietic progenitor cells. Experimental Hematology 30:116–125, 2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Suppl. Figure 1 Metastatic pancreatic NET (Patient 19). Patient received 2 doses of 177Lu- satoreotide tetraxetan, the second dose at 50% reduction (A) 68Ga-DOTA-JR11 whole body MIP and (B) fused PET/CT image of the liver pre-treatment. (C) T2-weighted MRI of liver metastasis before treatment and CT images of the liver metastasis at 3 months (D), 9 months (E), and 16 months (F) post treatment. The SSTR positive metastasis (red arrow) shows marked shrinkage that persists for 16 months.
Suppl. Figure 2 Patient 19 who received 2 doses of 177Lu- satoreotide tetraxetan, the second dose at 50% reduction (A) 68Ga-DOTA-JR11 whole body MIP and (B) fused PET/CT image of the lung pre-treatment. (C) CT of lung metastasis before treatment and CT images of the lung metastasis at 3 months (D), 9 months (E), and 16 months (F) post treatment. There is marked shrinkage of the metastasis (red arrows) and complete resolution at 16 months.
Suppl. Figure 3 Patient 20 who received a 50% reduced second dose of 177Lu- satoreotide tetraxetan. CT images show multiple liver metastases pre-treatment (A) that are unchanged 18 months post-treatment (B). Best response: PR; SD for 18 months.
Suppl. Figure 4 Example of tumor targeting and response after two doses of 177Lu-satoreotide tetraxetan. Pre-therapeutic 68Ga-JR11 PET/CT shows intense tracer uptake by liver metastases of a G2 pancreatic NET and low uptake by normal organs. Axial PET (A), axial PET/CT (B), and maximum intensity projection of PET (C). MRI of the liver shows metastases corresponding to the lesions on 68Ga-JR11 PET/CT prior to therapy (D). After two cycles of therapy, there is a marked reduction/resolution of the metastases (E).
Suppl. Figure 5 Maximum decrease from baseline in the size of tumors in patients treated with 177Lu- satoreotide tetraxetan. Patient ID is noted above each response. Two patients (ID#14, 18), progressed with non-measurable disease (bone, peritoneum) and one patient (ID# 9) developed a new liver metastasis.


