Abstract
Background
Poor sleep is associated with adverse outcomes among postpartum women. Exercise may improve sleep, but this has not been well examined in the postpartum period.
Purpose
To examine the impact of a culturally modified, individually tailored lifestyle intervention on sleep outcomes among postpartum Latina women.
Methods
Estudio PARTO was a randomized controlled trial aimed at reducing Type 2 diabetes among Latina women with abnormal glucose tolerance in pregnancy. Participants were randomized to a lifestyle (i.e., diet and exercise; n = 70) or a health and wellness control intervention (n = 78) in late pregnancy (baseline). The Pittsburgh Sleep Quality Index (PSQI) was used to measure sleep quality (PSQI score), onset latency (minutes per night), duration (hours per night), efficiency (percentage of the time in bed asleep), and daytime dysfunction at baseline, 6 weeks, 6 months, and 12 months postpartum.
Results
Mean PSQI score (6.56 ± 3.87), sleep duration (6.84 ± 1.75 hr/night), and sleep efficiency (79.70% ± 18.10%) did not differ between the arms at baseline. Mixed-effects models indicated a greater decrease of 1.29 in PSQI score (i.e., improved sleep quality) in the lifestyle versus health and wellness arm (95% confidence interval [CI] = −2.50 to −0.08, p = .04) over follow-up. There was the suggestion of a smaller decrease in sleep duration (mean = 0.48 hr/night, 95% CI = −0.10 to 1.06, p = .10) in the lifestyle versus health and wellness arm. There were no statistically significant differences in other sleep outcomes between arms.
Conclusions
Findings suggest that lifestyle interventions improve sleep quality but not sleep duration, sleep onset latency, sleep efficiency, or daytime dysfunction in postpartum Latina women and, therefore, may hold promise for improving subsequent mental and physical health in this population.
Clinical Trials Registration
Keywords: Exercise, Women’s health, Physical activity, Sleep, Postpartum, Latina
Participation in sports and exercise was positively associated with postpartum sleep quality and duration among at risk Hispanic women and should be considered in future postpartum intervention programs.
Background
Sleep disruption is common among postpartum women and is associated with adverse maternal outcomes, including postpartum weight retention and postpartum depression [1, 2]. For example, in the Project VIVA study, short sleep duration at 6 months postpartum was associated with three times the risk of substantial (>5 kg) postpartum weight retention at 1 year postpartum [3]. The Child Health Promotion Project in Shanghai study found that persistently poor sleep quality (vs. improving sleep quality) from late pregnancy (~36–38 weeks gestation) to 36 weeks postpartum was associated with higher depressive symptoms at 36 weeks postpartum [4].
Sleep conveys a broad range of mental and physical health benefits. Healthy sleep is associated with positive emotion regulation and mood [5]. Acute bouts of sleep support emotional memory consolidation and reset the amygdala–prefrontal cortex activity to provide the appropriate levels of inhibition on behavior [6]. Sleep is likewise associated with immune health [7] and allows for the functioning of the glymphatic system, which supports brain health [8]. Thus, interventions that improve sleep in women during the postpartum period are essential for promoting short- and long-term maternal health.
The 2018 Guidelines for Physical Activity and Health noted that there is moderately strong evidence that physical activity can improve sleep quality in the general adult population [9]. A meta-analysis of acute and regular exercise interventions found that exercise had small to large effects on several domains of sleep (e.g., subjective sleep quality) in general adult populations [10]. Moreover, in a pooled analysis of 4 MsFlash Trials, exercise interventions were found to be less effective than cognitive behavioral therapy for insomnia but at least as effective as sleep medications at reducing symptoms of insomnia among perimenopausal and postmenopausal women [11]. Given that there are fewer concerns for adverse side effects of engaging in exercise compared to long-term use of sleep medications, exercise promotion may be an effective alternative for addressing poor sleep.
However, the majority of prior exercise interventions were conducted among nonpostpartum populations. Postpartum women have unique factors that disrupt their sleep, such as infant feeding and sleeping schedule, other caregiving demands, anxiety, or worry about the child, depression, or other mood disturbances, work/life balance, adjusting to new parental roles, and relationship stress [12–15]. Thus, it is unclear if exercise is an effective strategy for improving sleep in women during the postpartum period.
Also, the limited prior research among postpartum women was conducted among non-Latina populations [16, 17]. Therefore, research is needed to determine the effects of lifestyle modification on sleep among postpartum Latina women. Latina populations represent a growing demographic that are disproportionately affected by poor sleep and adverse maternal health outcomes [18, 19]. For example, Latinas are two-to-four times more likely to report poor sleep compared to whites, and racial/ethnic disparities in sleep have been recognized as likely contributors to racial/ethnic disparities in cardiometabolic health outcomes [20, 21]. In the Hispanic Community Health Study/Study of Latinos (HCHS/SOL), short sleep was most common in individuals of Puerto Rican heritage (25.6%) amongst all Latina subgroups [22]. The HCHS/SOL also found that stress related to acculturation and ethnic discrimination was associated with a higher prevalence of excessive daytime sleepiness, and ethnic discrimination was further associated with a higher prevalence of short and long sleep duration [23]. Moreover, Latina Americans have the highest age-adjusted prevalence of obesity in the USA (50.6%) [24] as well as higher proportions of excessive postpartum weight retention (i.e., 40%–60%) compared to non-Latina whites [25, 26]. In spite of these observations, due to socioeconomic circumstances, health literacy, and language barriers, Latina women have had limited access to interventions that promote healthy lifestyles. Lifestyle interventions that are appropriately tailored to Latina populations to increase their acceptability and effects have the potential to make a substantial impact on maternal health [27, 28].
The purpose of this secondary analysis was to examine the impact of a culturally and linguistically modified, individually tailored lifestyle intervention that addressed diet and exercise on sleep quality, sleep duration, and sleep efficiency among postpartum Latina women. We hypothesized that participation in the lifestyle intervention would be associated with improved sleep outcomes compared to a health and wellness comparison arm.
Methods
Study Population
Estudio PARTO (Proyecto pAra Reducir diabetes Tipo dOs; NCT01679210) was a randomized controlled trial conducted in the ambulatory obstetrical practices of Baystate Medical Center from 2013 to 2017. Baystate Medical Center is a large tertiary care facility in western Massachusetts that serves an ethnically and socioeconomically diverse population. The study design and participant recruitment and enrollment are described in more detail elsewhere [29, 30]. In brief, the overall goal of the trial was to test the efficacy of a culturally and linguistically modified, individually-tailored lifestyle intervention to reduce risk factors for Type 2 diabetes and cardiovascular disease postpartum (e.g., postpartum weight retention and physical activity) [31].
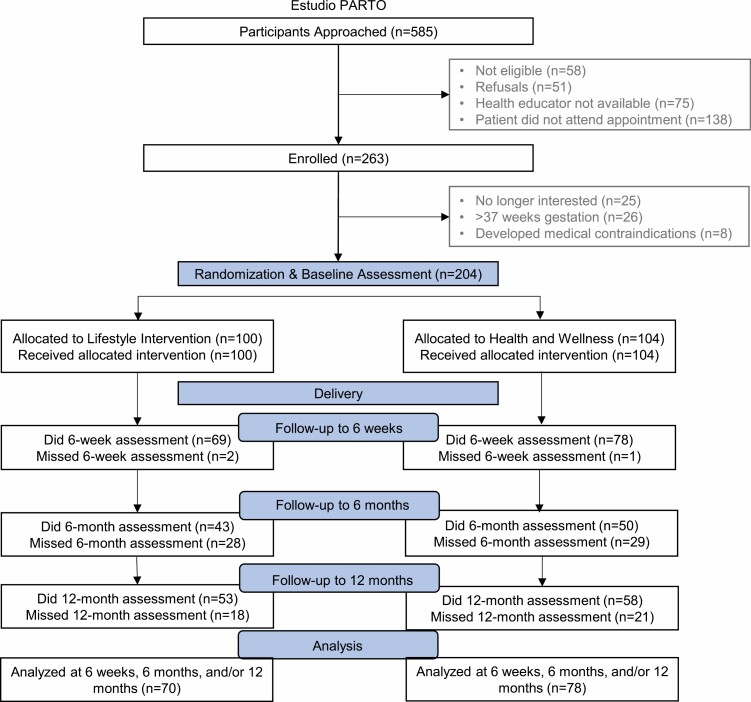
Eligibility criteria included women at elevated risk for developing Type 2 diabetes, defined by having plasma glucose concentration ≥135 mg/dL on the routine screen for gestational diabetes mellitus. Latina ethnicity was identified via self-report using the fixed category question, “Are you Latina or of Spanish or Latina origin or descent? (yes, no)” in the manner of the U.S. census. Exclusion criteria included women with a (a) history of Type 1 or Type 2 diabetes, heart disease, or chronic renal disease, (b) contraindications to postpartum participation in the trial’s intervention activities, including engagement in moderate-intensity physical activity, consumption of a low-fat/high-fiber diet (e.g., Crohn’s disease and ulcerative colitis), or (c) self-reported inability to read English or Spanish at a sixth-grade level. For this secondary analysis, we further restricted our sample to women with data on any sleep outcome at baseline and at least one follow-up visit. Figure 1 describes the participant flow.
Fig. 1.
Participant flow diagram; Estudio PARTO, 2013–2017.
Eligible women were recruited by bilingual/bicultural health educators at the time of routine screening for gestational diabetes mellitus (24–28 weeks gestation) and randomly assigned to either a lifestyle or health and wellness intervention (Fig. 1). Randomization was stratified by the results of the diagnostic 100 g oral glucose tolerance test using thresholds defined by the American Diabetes Association (ADA): (a) no glucose values meeting or exceeding the ADA thresholds or (b) one or more glucose values meeting or exceeding the ADA thresholds [32].
Procedures
The intervention design is described in more detail elsewhere [30]. Briefly, both intervention arms had an introductory phase (~29 weeks gestation to the time of birth) that was followed by an active phase (6 weeks postpartum–6 months postpartum) and then a maintenance phase (6 months–1 year postpartum). Both the introductory phase and the active phase were initiated with an in-person session, after which participants received weekly, biweekly, and monthly telephone booster calls and mailings. Tailoring questionnaires were administered to both intervention arms at 6 weeks, 6 months, and 1 year postpartum to inform culturally and motivationally tailored feedback. The diet tailoring questionnaire asked participants to report the frequency of consumption of high-calorie and lower-calorie foods and beverages that were relevant to Latina/Latinx populations. Participants were also asked to indicate their confidence level for decreasing their consumption of high-calorie foods and increasing low-calorie foods. The exercise tailoring questionnaire consisted of three measures: stages of change for physical activity [33], processes of change for physical activity [34], and self-efficacy for physical activity [35]. All print-based intervention materials were written at a sixth-grade level in Spanish or English depending upon participant preference. Trained bicultural and bilingual health interviewers, blinded to the intervention arm, conducted assessments at baseline and at 6 weeks, 6 months, and 12 months postpartum.
Lifestyle Intervention
The lifestyle intervention was designed by utilizing constructs from both the social cognitive theory [36] and the transtheoretical model [37] and took into account social, cultural, economic, and environmental resources and challenges relevant to Latina women [38, 39]. During the introductory phase, the goal of the lifestyle intervention was to optimize gestational weight gain for the remaining pregnancy period. In the active phase of the lifestyle intervention, the target was to achieve postpartum weight goals. Specifically, the health educator focused on a weekly weight loss goal of 1–2 pounds per week via increasing physical activity by 10% every week and healthy diets taking into account breastfeeding status.
To promote behavioral change, the health educators used motivational interviewing to identify and strengthen the participants’ motivation for change and increase knowledge and attitudes toward weight management during and after pregnancy and Type 2 diabetes prevention. In addition, health educators helped participants establish individual goals. Telephone booster calls involved the review of progress toward previous weight loss goals, problem-solving for challenges faced in achieving these goals, and the setting of new goals.
Health and Wellness Intervention
The health and wellness intervention served as the comparison arm. Participants randomized to this arm received the same number of in-person sessions, telephone calls, and mailings at the same time points as the lifestyle arm. However, this information consisted of general information available to the public from the American College of Obstetrician and Gynecologist (ACOG) and the American Academy of Pediatrics and did not target weight loss, exercise, or diet. In this manner, we controlled for contact time while keeping the content of the two interventions distinct.
Sleep Outcomes
The Pittsburgh Sleep Quality Index (PSQI) was used to measure habitual sleep and assess sleep habits and difficulties over the past month. Participants completed the PSQI at baseline, 6 weeks, 6 months, and 12 months postpartum. The PSQI is a commonly used questionnaire that has high sensitivity (89.6%) and specificity (86.5%) at distinguishing between good and poor sleepers [40]. Prior research demonstrated that the Spanish-language version of the PSQI has good construct validity [41].
The PSQI assesses seven components of sleep (i.e., subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medications, and daytime dysfunction over the last month), each scored on a scale of 0 to 3. Overall sleep quality is the sum of all components (possible range of 0–21), with higher scores indicating worse sleep quality. Sleep onset latency was assessed by asking women, “How long (minutes) has it taken you to fall asleep each night?” Sleep duration was assessed by asking women, “How many hours of actual sleep did you get at night?” during the past month. Sleep efficiency is defined as the percentage of time spent in bed asleep. We calculated time in bed by taking the difference in reported bedtime and wake time. We then divided the self-reported actual sleep time by the time in bed. Daytime dysfunction was calculated as the sum of two questions, both scored on a scale of 0–3, which asks about the frequency of trouble staying awake while driving, eating meals, or engaging in social activity and problems maintaining enthusiasm for getting things done.
Descriptive Characteristics
Demographic and behavioral characteristics were collected at the time of recruitment via standardized questionnaires. Sociodemographic characteristics included age, education, marital status, annual household income, living situation (e.g., with a spouse or partner), and the number of adults and children in the household. Information on prepregnancy and pregnancy cigarette smoking was collected using questions from the Pregnancy Risk Assessment Monitoring System [42]. Acculturation was assessed with the Psychological Acculturation Scale (PAS) [43]. PAS scores between 1 and <3 were categorized as low acculturation and scores 3 or greater were categorized as high acculturation.
The Perceived Stress Scale [44] is a 14-item scale used to assess how often participants experienced stress in the past month; scores range from 0 to 56, with higher scores indicating more perceived stress. The Edinburgh Postnatal Depression Scale was used to measure depression symptoms [45]. Scores range from 0 to 30, with higher scores indicating more depressive symptoms; probable minor depression was defined as a score ≥13 [45].
The Pregnancy Physical Activity Questionnaire (PPAQ) is a semiquantitative instrument, previously validated in this study population [46]. The PPAQ measures the duration and intensity of self-reported physical activities in the past month within several domains of activity (i.e., household/caregiving, occupational, transportation, and sports/exercise). The number of minutes spent in each reported activity was multiplied by its metabolic equivalent of task (MET) level and summed to arrive at an estimate of average weekly MET hours per week in each domain. Meeting ACOG guidelines for physical activity was defined as reporting at least 7.5 MET hours/week of sports/exercise-related activities.
Clinical characteristics of the pregnancy were abstracted from the medical record and included height, prepregnancy weight, parity, and gestational weight gain. Gestational weight gain was classified as below, within, or above recommendations based on the Institute of Medicine guidelines [25].
Statistical Analysis
SAS version 9.4 (SAS Institute Inc.) was used for all statistical analyses. All analyses were considered statistically significant at an alpha <0.05.
Two-sample t-tests, Fisher exact probability tests, and chi-square tests were used to explore differences in characteristics between intervention arms at baseline. Within-arm differences in mean changes from baseline (late pregnancy) to 6 weeks, 6 months, and 1 year postpartum, respectively, were assessed with paired t-tests.
We used an intent-to-treat analysis to evaluate the impact of the lifestyle intervention on postpartum sleep outcomes compared to the health and wellness comparison arm. Specifically, linear mixed models were used to evaluate differences in the changes from baseline in sleep outcomes during the first year postpartum between the intervention arms. These models accounted for the repeated measures of sleep with random subject effects with fixed effects for intervention arm and assessment time. The interaction between intervention arm and assessment time was assessed to determine whether there was evidence of heterogeneity in the intervention differences between the two follow-up assessment times (6 and 12 months); a nonsignificant finding (p > .10) was prespecified to justify dropping the interaction term from the model [47]. We calculated Hedges’ g to estimate the effect size of the intervention.
We then conducted several sensitivity analyses. We limited the analysis to women in the lifestyle intervention arm who were adherent with the intervention defined as: (a) meeting the ACOG guidelines for postpartum exercise and (b) returning ≥1 tailoring questionnaire during the postpartum period. We repeated the analysis limiting the sample to those with prepregnancy overweight or obesity as some prior studies have found that the effectiveness of interventions differs according to this characteristic. Finally, we compared those missing all sleep data to those with sleep data at any time point.
PARTO included a number of outcome variables, including postpartum weight loss, biomarkers of insulin resistance, and cardiovascular risk factors. The sample size was selected to ensure that there was adequate power across all outcome variables. Specifically, assuming a standard deviation of 4.5 kg for weight loss [48], a study of 102 women (randomly assigned to two groups) had 99% power at the 5% significance level to detect a differential 5% weight loss between groups (equivalent to 4 kg), assuming a mean baseline weight of 80 kg and allowing for a 33% dropout rate. A 5% weight loss was selected given its association with clinically meaningful health benefits [49].
Results
A total of 585 participants were approached and screened for eligibility (Fig. 1). Of these, 263 women met the eligibility criteria and were enrolled in the study. Fifty-nine were subsequently excluded prior to randomization because: (a) they were no longer interested (n = 25), (b) >37 weeks gestation (n = 26), or (c) developed other medical contraindications (n = 8). The remaining 204 women were randomized to the lifestyle intervention (n = 100) or to the health and wellness intervention (n = 104). Of these women, 56 chose not to participate in the study after delivery and were excluded due to missing sleep data at all three postpartum time points. Therefore, the final sample included 148 women: lifestyle intervention (n = 70) and health and wellness intervention (n = 78). There were no statistically significant differences in demographic, behavioral, or psychosocial characteristics of women with or without data on change in sleep outcomes.
Among participants in the final sample, there were no differences in any demographic, behavioral, or psychosocial characteristics between intervention arms at baseline (Table 1). The overall mean age of the sample was 27.8 ± 5.7 years and nearly 80% of the sample was overweight or obese prepregnancy. The majority of participants were not married but lived with a partner. Nearly 90% of participants had two or more adults in the household. Few participants reported currently smoking cigarettes or meeting ACOG guidelines for physical activity.
Table 1.
Distribution of participant characteristics according to intervention arm; Estudio PARTO, 2013–2017
| Total sample (N = 148) | Lifestyle intervention (N = 70) | Health and wellness intervention (N = 78) | |||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | p valuea | |
| Sociodemographic characteristics | |||||||
| Age, years | |||||||
| 16–19 | 11 | 7.4 | 4 | 5.7 | 7 | 9.0 | .22 |
| 20–24 | 32 | 21.6 | 11 | 15.7 | 21 | 26.9 | |
| 25–29 | 55 | 37.2 | 29 | 41.4 | 26 | 33.3 | |
| 30–34 | 28 | 18.9 | 17 | 24.3 | 11 | 14.1 | |
| 35–45 | 22 | 14.9 | 9 | 12.9 | 13 | 16.7 | |
| Number of children in the household | |||||||
| 0 | 30 | 20.3 | 15 | 21.4 | 15 | 19.2 | .80 |
| 1 | 61 | 41.2 | 30 | 42.9 | 31 | 39.7 | |
| 2 | 31 | 20.9 | 15 | 21.4 | 16 | 20.5 | |
| 3+ | 26 | 17.6 | 10 | 14.3 | 16 | 20.5 | |
| Number of adults in the household | |||||||
| 1 | 18 | 12.2 | 11 | 15.7 | 7 | 9.0 | .45 |
| 2 | 92 | 62.2 | 42 | 60.0 | 50 | 64.1 | |
| 3+ | 38 | 25.7 | 17 | 24.3 | 21 | 26.9 | |
| Married | |||||||
| No | 105 | 70.9 | 47 | 67.1 | 58 | 74.4 | .33 |
| Yes | 43 | 29.1 | 23 | 32.9 | 20 | 25.6 | |
| Living with a partner | |||||||
| No | 38 | 25.7 | 22 | 31.4 | 16 | 20.5 | .14 |
| Yes | 109 | 73.6 | 48 | 68.6 | 61 | 78.2 | |
| Education status | |||||||
| Less than high school | 34 | 23.0 | 13 | 18.6 | 21 | 26.9 | .47 |
| High school graduate or GED | 45 | 30.4 | 22 | 31.4 | 23 | 29.5 | |
| Post high school | 69 | 46.6 | 35 | 50.0 | 34 | 43.6 | |
| Language preference | |||||||
| English | 108 | 73.0 | 51 | 72.9 | 57 | 73.1 | .98 |
| Spanish | 40 | 27.0 | 18 | 27.1 | 21 | 26.9 | |
| Generations in the USA | |||||||
| Born outside continental USA | 66 | 44.6 | 34 | 48.6 | 32 | 41.0 | .17 |
| Parent born outside continental USA | 53 | 35.8 | 20 | 28.6 | 33 | 42.3 | |
| Grandparent outside continental USA | 19 | 12.8 | 12 | 17.1 | 7 | 9.0 | |
| All grandparents born in continental USA | 9 | 6.1 | 3 | 4.3 | 6 | 7.7 | |
| Acculturation level | |||||||
| Low (1 to <3) | 111 | 75.0 | 55 | 78.6 | 56 | 71.8 | .34 |
| High (≥3) | 37 | 25.0 | 15 | 21.4 | 22 | 28.2 | |
| Clinical characteristics | |||||||
| Prepregnancy body mass index, kg/m2 | |||||||
| <25 | 33 | 22.3 | 14 | 20.0 | 19 | 24.4 | .70 |
| 25 to <30 | 44 | 29.7 | 20 | 28.6 | 24 | 30.8 | |
| ≥30 | 71 | 48.0 | 36 | 51.4 | 35 | 44.9 | |
| Parity | |||||||
| 0 | 41 | 27.7 | 23 | 32.9 | 18 | 23.1 | .37 |
| 1 to 2 | 82 | 55.4 | 37 | 52.9 | 45 | 57.7 | |
| 3+ | 25 | 16.9 | 10 | 14.3 | 15 | 19.2 | |
| Adherence with IOM guidelines for gestational weight gain | |||||||
| Within guidelines | 32 | 21.6 | 15 | 21.4 | 17 | 21.8 | .99 |
| Below guidelines | 29 | 19.6 | 14 | 20.0 | 15 | 19.2 | |
| Above guidelines | 84 | 56.8 | 40 | 57.1 | 44 | 56.4 | |
| Behavioral characteristics | |||||||
| Prepregnancy smoking | |||||||
| None | 108 | 73.0 | 49 | 71.0 | 59 | 75.6 | .44 |
| ≤10 cigarettes/day | 40 | 27.0 | 21 | 30.4 | 19 | 24.4 | |
| Pregnancy smoking | |||||||
| None | 138 | 93.2 | 64 | 92.8 | 74 | 94.9 | .52 |
| ≤10 cigarettes/day | 10 | 6.8 | 6 | 8.7 | 4 | 5.1 | |
| Met ACOG physical activity guidelines | |||||||
| No | 97 | 65.5 | 44 | 63.8 | 53 | 67.9 | .52 |
| Yes | 51 | 34.5 | 26 | 37.7 | 25 | 32.1 | |
| Sports/exercise, MET hr/weekb | 3.8 | 9.6 | 3.1 | 9.3 | 4.0 | 9.6 | .51 |
| Psychosocial characteristics | |||||||
| Perceived stressa | 20.4 | 8.1 | 20.4 | 9.6 | 20.3 | 7.2 | .96 |
| Probable minor depression | 6.0 | 9.0 | 5.5 | 9.0 | 6.0 | 8.0 | .72 |
| No | 129 | 87.2 | 61 | 87.1 | 68 | 87.2 | .71 |
| Yes | 12 | 8.1 | 5 | 7.1 | 7 | 9.0 | |
ACOG American College of Obstetricians and Gynecologists; IOM Institute of Medicine; MET metabolic equivalent of task.
a p value for generation in the USA is from a Fisher’s Exact Test; p values from all other categorical variables are from chi-square test.
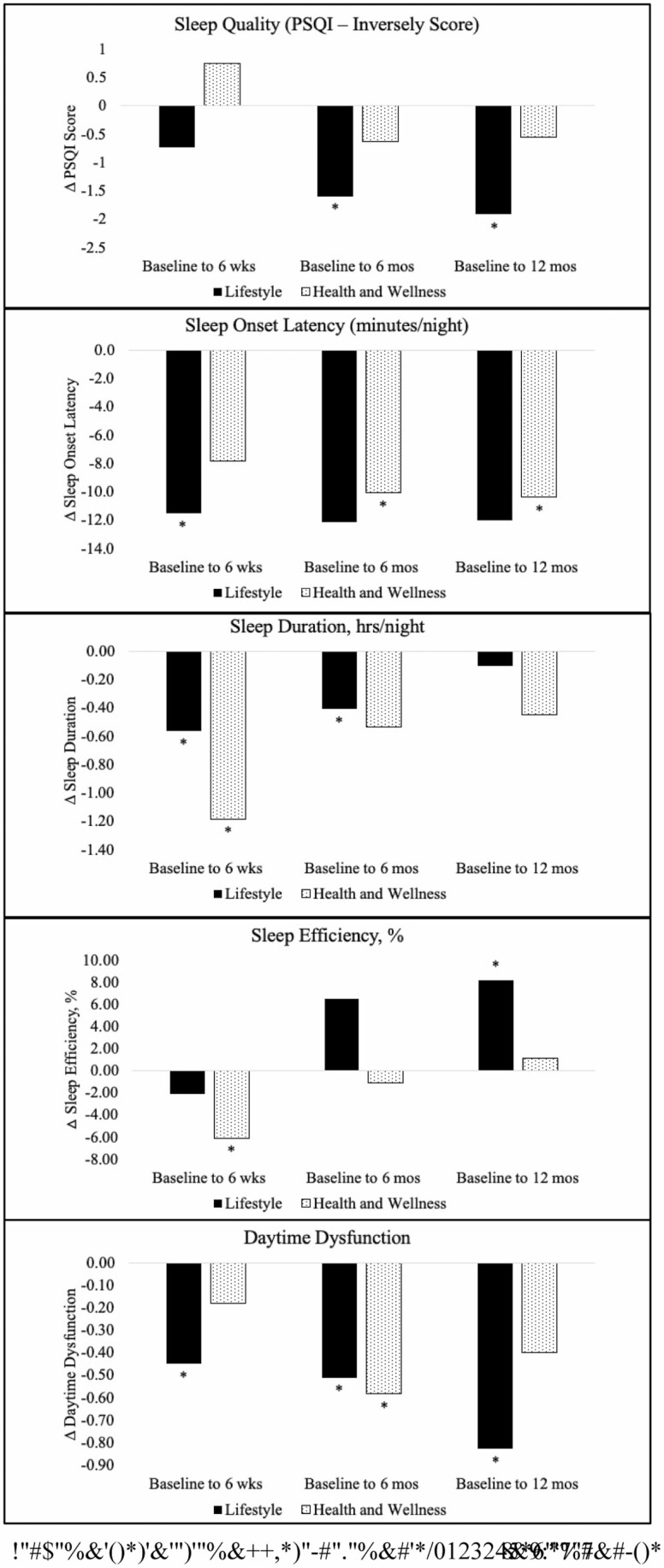
The intervention arms had similar sleep characteristics at baseline (Table 2). Both interventions arms reported changes in sleep characteristics from baseline to each follow-up assessment (Fig. 2; Table 1). At 6 weeks postpartum, sleep onset latency and sleep duration decreased in both arms. Daytime dysfunction decreased in the lifestyle arm only (mean change = −0.45, 95% CI = −0.83 to −0.07) and sleep efficiency decreased (mean change = 6.07%, 95% CI = 11.28 to −0.86) in the health and wellness arm only. There were no statistically significant changes in sleep quality in either arm.
Table 2.
Baseline sleep characteristics according to intervention arm; Estudio PARTO 2013–2017
| Total sample (N = 148) | Lifestyle intervention (N = 70) | Health and wellness intervention (N = 78) | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | p valuea | |
| PSQI score | 6.56 | 3.87 | 6.90 | 4.09 | 6.25 | 3.67 | .32 |
| Sleep onset latency | 29.9 | 38.9 | 32.3 | 42.8 | 27.7 | 35.1 | .48 |
| Sleep duration | 6.84 | 1.75 | 6.60 | 1.58 | 7.05 | 1.87 | .12 |
| Sleep efficiency | 79.7 | 18.1 | 79.7 | 18.6 | 79.7 | 17.8 | .98 |
| Daytime dysfunction | 1.26 | 1.48 | 1.31 | 1.47 | 1.22 | 1.50 | .69 |
PSQI Pittsburgh Sleep Quality Index; SD standard deviation.
a p value from two-sample t-test.
Fig. 2.
Within-group changes in sleep outcomes from baseline to each follow-up assessment
At 6 months postpartum, compared to baseline, sleep quality increased in the lifestyle arm (mean change = −1.60 PSQI global score, 95% CI = −2.82 to −0.38) but not in the health and wellness arm (mean change = −0.63 PSQI global score, 95% CI = −1.77 to −0.52; Table 3). Sleep onset latency, sleep duration, and daytime dysfunction decreased in both arms. There were no statistically significant changes in sleep efficiency in either arm.
Table 3.
Change in sleep outcomes over follow-up according to intervention arm; Estudio PARTO 2013–2017
| Change from baseline at 6 weeks | Change from baseline at 6 months | Change from baseline at 12 months | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | p valuea | 95% CI | Mean | SD | p valuea | 95% CI | n | Mean | SD | p valuea | 95% CI | |
| Lifestyle intervention | |||||||||||||
| Sleep quality | −0.73 | 4.47 | .19 | −1.83 to 0.37 | −1.60 | 3.81 | .01 | −2.82 to −0.38 | 44 | −1.91 | 5.41 | .02 | −3.53 to −0.26 |
| Sleep onset latency | −11.5 | 46.3 | .04 | −22.6 to −0.39 | −12.11 | 58.3 | .18 | −29.9 to 5.62 | 52 | −12.0 | 61.2 | .16 | −29.0 to 5.07 |
| Sleep duration | −0.56 | 2.18 | .04 | −1.08 to −0.03 | −0.41 | 1.32 | .05 | −0.81 to −0.01 | 53 | −0.10 | 1.96 | .70 | −0.64 to 0.44 |
| Sleep efficiency | −2.08 | 27.0 | .52 | −8.55 to 4.40 | 6.56 | 24.7 | .09 | −1.04 to 14.16 | 53 | 8.22 | 26.0 | .03 | 1.06 to 15.4 |
| Daytime dysfunction | −0.45 | 1.58 | .02 | −0.83 to −0.07 | −0.51 | 1.69 | .05 | −1.02 to −0.00 | 52 | −0.83 | 1.58 | <.01 | −1.27 to −0.39 |
| Health and wellness | |||||||||||||
| Sleep quality | 0.75 | 3.73 | .10 | −0.13 to 1.63 | −0.63 | 3.93 | .28 | −1.77 to 0.52 | 51 | −0.55 | 3.91 | .32 | −1.65 to 0.55 |
| Sleep onset latency | −7.81 | 35.6 | .06 | −15.84 to 0.23 | −10.1 | 25.5 | .01 | −17.3 to −2.84 | 58 | −10.3 | 36.3 | .03 | −19.9 to −0.80 |
| Sleep duration | −1.18 | 2.29 | <.01 | −1.70 to −0.67 | −0.54 | 2.11 | .08 | −1.13 to 0.06 | 58 | −0.44 | 2.23 | .14 | −1.03 to 0.14 |
| Sleep efficiency | −6.07 | 23.1 | .02 | −11.3 to −0.86 | −1.05 | 24.3 | .76 | −7.96 to 5.85 | 58 | 1.17 | 23.4 | .70 | −4.99 to 7.33 |
| Daytime dysfunction | −0.18 | 1.45 | .28 | −0.51 to 0.15 | −0.58 | 1.59 | .01 | −1.03 to −0.13 | 58 | −0.40 | 1.59 | .06 | −0.81 to 0.02 |
Bold indicates statistical significance.
CI confidence interval; SD standard deviation.
a p value from paired t-tests.
At 12 months postpartum, compared to baseline, sleep quality (mean change = −1.91 PSQI Global Score, 95% CI = −3.53 to −0.26) and sleep efficiency (mean change = 8.22%, 95% CI = 1.06 to 15.38) increased and daytime dysfunction decreased (mean = −0.82, 95% CI = −1.27 to −0.39) in the lifestyle intervention but not the health and wellness arm. Sleep duration decreased in the health and wellness (mean change = −0.44 hrs/night, 95% CI = −1.03 to 0.14) but not the lifestyle intervention arm.
Next, we used linear mixed models to compare differences in change between the intervention arms (Table 4). We observed no statistically significant arm by time interactions for any sleep outcome (all p > .10). After removing interaction terms, there was a statistically significant greater improvement over time in sleep quality in the lifestyle versus health and wellness arm (−1.29 PSQI global score, 95% CI = −2.50 to −0.08, p = .04). There was also a suggestion of a smaller decrease over time in sleep duration in the lifestyle versus health and wellness arm (0.48 hr/night, 95% CI = −0.10 to 1.06, p = .10). We did not observe statistically significant intervention effects for any other sleep outcome.
Table 4.
Main effects model: differences in change in sleep outcomes from baseline over follow-up between intervention arms; Estudio PARTO 2013–2017
| Main effects modelsa (lifestyle vs. health and wellness arm) | |||||
|---|---|---|---|---|---|
| Intervention effect (β) | 95% CI | SE | p value | Hedge’s g | |
| PSQI score | −1.29 | −2.50 to −0.08 | 0.61 | .04 | −0.17 |
| Sleep onset latency | −1.72 | −14.3 to 10.9 | 6.36 | .79 | −0.23 |
| Sleep duration | 0.48 | −0.10 to 1.06 | 0.29 | .10 | 0.06 |
| Sleep efficiency | 4.61 | −2.58 to 11.8 | 3.64 | .21 | 0.61 |
| Daytime dysfunction | −0.29 | −0.72 to 0.15 | 0.22 | .20 | −0.04 |
CI confidence interval; PSQI Pittsburgh Sleep Quality Index; SE standard error.
aUsing mixed models.
We then conducted several sensitivity analyses. Findings were attenuated and no longer statistically significant after limiting the analysis to women who were adherent to the lifestyle intervention defined as meeting the ACOG guidelines for sports/exercise at any postpartum time point (44.3%) or after limiting the analysis to participants in either intervention arm who returned one or more tailoring questionnaires during the postpartum period (73% in the lifestyle arm and 83% in the health and wellness arm). When we repeated the main analysis among women with prepregnancy overweight or obesity (80% in the lifestyle arm, and 76% in the health and wellness arm), findings were also attenuated and no longer statistically significant. Finally, women missing sleep data did not differ from those in the final sample according to any of the sociodemographic, behavioral, or clinical variables (p > .05).
Discussion
In this randomized controlled trial of a culturally and linguistically modified, individually tailored lifestyle (i.e., diet, exercise, and weight loss) among postpartum Latina women, we found that the lifestyle intervention had small but statistically significant positive effects on sleep quality compared to the health and wellness comparison intervention during the 12 month postpartum period. The lifestyle intervention did not have a statistically significant effect on sleep duration, sleep onset latency, sleep efficiency, or daytime dysfunction compared to the health and wellness intervention.
The results of the current study are similar to the two prior lifestyle-based interventions that assessed sleep outcomes during the postpartum period [16]. For example, Ashrafinia et al. examined the effect of an 8 week home-based Pilates intervention versus education comparison arm on sleep outcomes (PSQI global score) among 40 primigravida Iranian women approximately 1 week postpartum [16, 17]. The Pilates intervention improved sleep quality (PSQI at baseline = 9.97 ± 2.21 vs. postintervention = 5.45 ± 1.51) compared to the comparison arm (PSQI at baseline = 10.40 ± 2.68 vs. postintervention = 8.35 ± 1.83) at approximately 2 months postpartum. The intervention arm also experienced statistically significant improvements in sleep duration, sleep efficiency, and daytime dysfunction compared to the comparison arm. There were no differences in change in sleep onset latency or sleep disturbances between the intervention arms. Similarly, Yang et al. examined the effects of 12 week aerobic exercise or usual care comparison arm on sleep quality (Postpartum Sleep Quality Scale) among 140 Taiwanese women at 6 weeks postpartum [17]. There was a suggestion that the intervention (vs. usual care control) led to the reduction in physical symptoms related to sleep efficiency at approximately 12 weeks postpartum (p = .06), but there was no corresponding improvement in daytime dysfunction. Differences in findings could be due to differences in the parameterization of sleep as well as differences in the study population.
While we observed positive effects on sleep quality, the lifestyle intervention did not have a statistically significant effect on sleep duration, sleep onset latency, sleep efficiency, or daytime dysfunction. Reasons for this could be due to measurement error in self-reported estimates of these constructs. The cognitive burden required to estimate how long it takes to fall asleep (sleep onset latency) and actual sleep time relative to total time in bed (sleep efficiency) is high and may reduce the precision in self-reported estimates [50]. Nondifferential misclassification of self-reported sleep measures would decrease the absolute difference in outcomes between the intervention arms [51]. The low rates of daytime dysfunction could also contribute to a lack of observed intervention effect. Specifically, the overall mean score for daytime dysfunction was 1.26 (possible score from 0 to 6), which indicates that participants were, on average, experiencing daytime dysfunction less than once per week. Given the relatively low burden of daytime dysfunction in this population, the lack of a statistically significant improvement in this construct is not unexpected.
Our study had several limitations. First, the study was limited by reliance on self-reported measures of sleep. However, the PSQI is one of the most widely used measures of self-reported sleep quality, which allows our findings to be compared to other studies [40]. Second, after delivery, the pressures of caring for a new baby dominate the early postpartum period and fatigue, work-related obstacles, and lack of time and motivation can be major barriers to engagement [29]. Consistent with these barriers, follow-up data on sleep outcomes were not available in 25%–30% of our sample who chose not to participate in the study after delivery, which, therefore, reduced the power to detect differences in change between groups. However, there were no statistically significant differences in demographic or behavioral characteristics of women with and without missing follow-up data on sleep outcomes. Additionally, linear mixed models can produce unbiased estimates when assuming data are missing at random.
Over the course of follow-up, we found that 72.7%–82.3% of women were adherent to the intervention in that they returned tailoring questionnaires, and 44.3% of women in the lifestyle intervention met ACOG recommendations for postpartum sports/exercise. Low levels of adherence during the postpartum period may be due to the fact that women with glucose intolerance in pregnancy do not perceive themselves as being at high risk for diabetes after delivery [52].
We did not collect information on physical symptoms (e.g., pain) and infant characteristics (e.g., feeding habits, nocturnal sleep duration, or sleep location), which are two of the strongest predictors of sleep quality postpartum [12]. However, the advantage of a randomized trial is that, when effective, it randomizes both known and unknown preintervention factors. Indeed, we did not observe baseline differences between study arms in parity or the number of children in the household. Therefore, it is likely that infant sleep would also be similarly randomized but, to the extent that it was not, it may have confounded our findings. Finally, we did not collect information on the participant’s specific country of origin. However, based on U.S. census data in Springfield, MA, the majority of women were primarily of Puerto Rican ancestry. Therefore, our results are likely generalizable to postpartum women of Puerto Rican ancestry with glucose intolerance in pregnancy.
Our study has several notable strengths. First, prior studies among postpartum women were conducted in predominantly healthy non-Latinas. In the current sample, women were Latina, of low socioeconomic status, and at risk for developing Type 2 diabetes postpartum. Latina women with low socioeconomic status experience several barriers to engaging in lifestyle interventions (e.g., health literacy and language barriers). Indeed, Gubrium et al. described the facilitators and barriers to implementing the current lifestyle intervention in the study population [29]. The health educators reported that the most important enablers of the intervention were its duration and flexibility, allowing time for health educators to build relationships with participants, serve as health advocates, and adapt the intervention to participants’ needs. They felt that their shared cultural background with the participants was critical to the success of the intervention. The health educators recommended that future interventions consider strategies for dealing with stress related to food insecurity, low health literacy (e.g., more basic information on healthy eating), challenges of recent immigrants, and engaging family members.
Another advantage to our intervention is that participants performed self-selected activities. Self-selected activities are likely more sustainable over time than prescribing structured exercises. The use of a high-reach, low-cost design strategy may facilitate the translation of such interventions into clinical practice in underserved and minority populations [29].
In conclusion, we found that a lifestyle intervention that addressed diet and exercise was an effective, low-cost, and translatable approach to improving sleep quality in postpartum Latina women. These findings add to the sparse body of prior lifestyle interventions aimed at improving sleep among postpartum populations. Our findings are significant in demonstrating that a lifestyle intervention can be impactful on sleep during the postpartum period despite the unique barriers to making lifestyle changes and improving sleep, which are characteristic of this time period. Sleep provides broad benefits to mental (e.g., emotional regulation and mood) and physical (e.g., weight retention) health. Therefore, postpartum interventions that address sleep have the potential to impact maternal health during this critical period [1, 2].
Acknowledgments
Funding: This work was funded by the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (DK064902).
Contributor Information
Marquis Hawkins, Department of Epidemiology, University of Pittsburgh, Public Health, Pittsburgh, PA, USA.
Bess Marcus, Department of Behavioral and Social Sciences, Brown University School of Public Health, Providence, RI, USA.
Penelope Pekow, Department of Biostatistics and Epidemiology, University of Massachusetts, Amherst, MA, USA.
Milagros C Rosal, Division of Preventive and Behavioral Medicine, Department of Medicine, University of Massachusetts Medical School, Worcester, MA, USA.
Katherine L Tucker, Department of Biomedical and Nutritional Sciences, University of Massachusetts Lowell, Lowell, MA, USA.
Rebecca M C Spencer, Department of Psychological and Brain Sciences, University of Massachusetts, Amherst, MA, USA.
Lisa Chasan-Taber, Department of Biostatistics and Epidemiology, University of Massachusetts, Amherst, MA, USA.
Compliance With Ethical Standards
Authors’ Statement of Conflict of Interest and Adherence to Ethical Standards The authors declare that they have no conflict of interest.
Authors’ Contributions M.H. contributed to the data analysis and interpretation, drafting the article; B.M. contributed to the conceptual design and interpretation; P.P. contributed to the conceptual design, analysis and interpretation; M.C.R. contributed to the conceptual design and interpretation; K.L.T. contributed to the conceptual design and interpretation; R.M.C.S. contributed to the conceptual design and interpretation; L.C.T. contributed to the conceptual design, data collection, data analysis and interpretation; All authors provided critical revisions of the article and approval of the final published version.
Ethical Approval This study was approved by the Institution Review Board of the University of Massachusetts-Amherst and Baystate Medical Center.
Informed Consent All women included in the study were required to sign a written informed consent as approved by the Institution Review Board of the University of Massachusetts-Amherst and Baystate Medical Center prior to randomization.
References
- 1. Christian LM, Carroll JE, Teti DM, Hall MH. Maternal sleep in pregnancy and postpartum part i: Mental, physical, and interpersonal consequences. Curr Psychiatry Rep. 2019;21:20. [DOI] [PubMed] [Google Scholar]
- 2. Tomfohr LM, Buliga E, Letourneau NL, Campbell TS, Giesbrecht GF. Trajectories of sleep quality and associations with mood during the perinatal period. Sleep. 2015;38:1237–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gunderson EP, Rifas-Shiman SL, Oken E, et al. Association of fewer hours of sleep at 6 months postpartum with substantial weight retention at 1 year postpartum. Am J Epidemiol. 2008;167:178–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang G, Deng Y, Jiang Y, et al. Trajectories of sleep quality from late pregnancy to 36 months postpartum and association with maternal mood disturbances: A longitudinal and prospective cohort study. Sleep. 2018;41. [DOI] [PubMed] [Google Scholar]
- 5. Gruber R, Cassoff J. The interplay between sleep and emotion regulation: Conceptual framework empirical evidence and future directions. Curr Psychiatry Rep. 2014;16:500. [DOI] [PubMed] [Google Scholar]
- 6. Walker MP. The role of sleep in cognition and emotion. Ann N Y Acad Sci. 2009;1156:168–197. [DOI] [PubMed] [Google Scholar]
- 7. Cohen S, Doyle WJ, Alper CM, Janicki-Deverts D, Turner RB. Sleep habits and susceptibility to the common cold. Arch Intern Med. 2009;169:62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rasmussen MK, Mestre H, Nedergaard M. The glymphatic pathway in neurological disorders. Lancet Neurol. 2018;17:1016–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. 2018 Physical Activity Guidelines Advisory Committee. 2018 Physical Activity Guidelines Advisory Committee Scientific Report. Washington, DC: U.S. Department of Health and Human Services; 2018. [Google Scholar]
- 10. Kredlow MA, Capozzoli MC, Hearon BA, Calkins AW, Otto MW. The effects of physical activity on sleep: A meta-analytic review. J Behav Med. 2015;38:427–449. [DOI] [PubMed] [Google Scholar]
- 11. Guthrie KA, Larson JC, Ensrud KE, et al. Effects of pharmacologic and nonpharmacologic interventions on insomnia symptoms and self-reported sleep quality in women with hot flashes: A pooled analysis of individual participant data from four MsFLASH Trials. Sleep. 2018;41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ko SH, Chen CH, Wang HH, Su YT. Postpartum women’s sleep quality and its predictors in Taiwan. J Nurs Scholarsh. 2014;46:74–81. [DOI] [PubMed] [Google Scholar]
- 13. Doering J, Durfor SL. The process of “persevering toward normalcy” after childbirth. MCN Am J Matern Child Nurs. 2011;36:258–265. [DOI] [PubMed] [Google Scholar]
- 14. Troxel WM, Robles TF, Hall M, Buysse DJ. Marital quality and the marital bed: Examining the covariation between relationship quality and sleep. Sleep Med Rev. 2007;11:389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zambrano DN, Mindell JA, Reyes NR, Hart CN, Herring SJ. “It’s Not All About My Baby’s Sleep”: A qualitative study of factors influencing low-income african american mothers’ sleep quality. Behav Sleep Med. 2016;14:489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ashrafinia F, Mirmohammadali M, Rajabi H, et al. The effects of Pilates exercise on sleep quality in postpartum women. J Bodyw Mov Ther. 2014;18:190–199. [DOI] [PubMed] [Google Scholar]
- 17. Yang CL, Chen CH. Effectiveness of aerobic gymnastic exercise on stress, fatigue, and sleep quality during postpartum: A pilot randomized controlled trial. Int J Nurs Stud. 2018;77:1–7. [DOI] [PubMed] [Google Scholar]
- 18. U.S. Census Bureau. 2008 National Population Projections Tables. Available at https://www.census.gov/data/tables/2008/demo/popproj/2008-summary-tables.html. Accessibility verified December 1, 2019.
- 19. Bryant AS, Worjoloh A, Caughey AB, Washington AE. Racial/ethnic disparities in obstetric outcomes and care: Prevalence and determinants. Am J Obstet Gynecol. 2010;202:335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kingsbury JH, Buxton OM, Emmons KM. Sleep and its relationship to racial and ethnic disparities in cardiovascular disease. Curr Cardiovasc Risk Rep. 2013;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Makarem N, St-Onge MP, Liao M, Lloyd-Jones DM, Aggarwal B. Association of sleep characteristics with cardiovascular health among women and differences by race/ethnicity and menopausal status: Findings from the American Heart Association Go Red for Women Strategically Focused Research Network. Sleep Health. 2019;5:501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dudley KA, Weng J, Sotres-Alvarez D, et al. Actigraphic sleep patterns of U.S. Hispanics: The Hispanic community health study/study of Latinos. Sleep. 2017;40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Alcántara C, Patel SR, Carnethon M, et al. Stress and sleep: Results from the Hispanic community health study/study of Latinos sociocultural ancillary study. SSM Popul Health. 2017;3:713–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity among adults and youth: United States, 2015–2016. NCHS Data Brief. 2017:1–8. [PubMed] [Google Scholar]
- 25. Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines. Weight Gain During Pregnancy: Reexamining the Guidelines. In: Rasmussen KM, Yaktine AL, eds. Washington, DC: National Academies Press (US); 2009. [PubMed] [Google Scholar]
- 26. Martin CL, Tate DF, Schaffner A, et al. Acculturation influences postpartum eating, activity, and weight retention in low-income Hispanic women. J Womens Health (Larchmt). 2017;26:1333–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McCurley JL, Crawford MA, Gallo LC. Prevention of type 2 diabetes in U.S. hispanic youth: A systematic review of lifestyle interventions. Am J Prev Med. 2017;53:519–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Office of Minority Health, U.S. DHHS. National Standards for Culturally and Linguistically Appropriate Services in Health Care: Final Report, 2001. Washington, DC: IQ Solutions, Inc; 2001. [Google Scholar]
- 29. Gubrium A, Leckenby D, Harvey MW, Marcus BH, Rosal MC, Chasan-Taber L. Perspectives of health educators and interviewers in a randomized controlled trial of a postpartum diabetes prevention program for Latinas: A qualitative assessment. BMC Health Serv Res. 2019;19:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chasan-Taber L, Marcus BH, Rosal MC, et al. Estudio Parto: postpartum diabetes prevention program for Hispanic women with abnormal glucose tolerance in pregnancy: A randomised controlled trial—study protocol. BMC Pregnancy Childbirth. 2014;14:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Burkart S, Marcus BH, Pekow P, et al. The impact of a randomized controlled trial of a lifestyle intervention on postpartum physical activity among at-risk hispanic women: Estudio PARTO. PLoS One. 2020;15:e0236408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. American Diabetes Association. 13. Management of diabetes in pregnancy: Standards of medical care in diabetes-2018. Diabetes Care. 2018, 41:S137–S143. [DOI] [PubMed] [Google Scholar]
- 33. Marcus BH, Selby VC, Niaura RS, Rossi JS. Self-efficacy and the stages of exercise behavior change. Res Q Exerc Sport. 1992;63:60–66. [DOI] [PubMed] [Google Scholar]
- 34. Marcus BH, Rossi JS, Selby VC, Niaura RS, Abrams DB. The stages and processes of exercise adoption and maintenance in a worksite sample. Health Psychol. 1992;11:386–395. [DOI] [PubMed] [Google Scholar]
- 35. Marcus BH, Bock BC, Pinto BM, Forsyth LH, Roberts MB, Traficante RM. Efficacy of an individualized, motivationally-tailored physical activity intervention. Ann Behav Med. 1998;20:174–180. [DOI] [PubMed] [Google Scholar]
- 36. Bandura A. The anatomy of stages of change. Am J Health Promot. 1997;12:8–10. [DOI] [PubMed] [Google Scholar]
- 37. Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: Toward an integrative model of change. J Consult Clin Psychol. 1983;51:390–395. [DOI] [PubMed] [Google Scholar]
- 38. Marquez DX, Bustamante EE, Bock BC, Markenson G, Tovar A, Chasan-Taber L. Perspectives of Latina and non-Latina white women on barriers and facilitators to exercise in pregnancy. Women Health. 2009;49:505–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Neighbors CJ, Marquez DX, Marcus BH. Leisure-time physical activity disparities among Hispanic subgroups in the United States. Am J Public Health. 2008;98:1460–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. [DOI] [PubMed] [Google Scholar]
- 41. Zhong QY, Gelaye B, Sánchez SE, Williams MA. Psychometric properties of the Pittsburgh Sleep Quality Index (PSQI) in a cohort of Peruvian pregnant women. J Clin Sleep Med. 2015;11:869–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Williams LM, Morrow B, Lansky A, et al. ; Centers for Disease Control and Prevention . Surveillance for selected maternal behaviors and experiences before, during, and after pregnancy. Pregnancy Risk Assessment Monitoring System (PRAMS), 2000. MMWR Surveill Summ. 2003;52:1–14. [PubMed] [Google Scholar]
- 43. Tropp LR, Erkut S, Coll CG, Alarcón O, Vázquez García HA. Psychological acculturation: Development of a new measure for Puerto Ricans on the U.S. Mainland. Educ Psychol Meas. 1999;59:351–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 45. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–786. [DOI] [PubMed] [Google Scholar]
- 46. Chasan-Taber L, Schmidt MD, Roberts DE, Hosmer D, Markenson G, Freedson PS. Development and validation of a pregnancy physical activity questionnaire. Med Sci Sports Exerc. 2004;36:1750–1760. [DOI] [PubMed] [Google Scholar]
- 47. Kleinbaum D, Kupper L, Nizam A, Rosenberg E. Applied Regression Analysis and Other Multivariable Methods. 5th ed. Boston, MA: Cengage Learning; 2013. [Google Scholar]
- 48. Bertz F, Brekke HK, Ellegård L, Rasmussen KM, Wennergren M, Winkvist A. Diet and exercise weight-loss trial in lactating overweight and obese women. Am J Clin Nutr. 2012;96:698–705. [DOI] [PubMed] [Google Scholar]
- 49. Department of Health. Start Active, Stay Active: a report on physical activity for health from the four home countries. Available at https://www.bhf.org.uk/informationsupport/support/healthy-living/staying-active. Accessibility verified December 1, 2020.
- 50. Girschik J, Fritschi L, Heyworth J, Waters F. Validation of self-reported sleep against actigraphy. J Epidemiol. 2012;22:462–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rothman K, Greenland S, Lash T. Modern Epidemiology. 3rd ed. Philadelphia, PA: Lippincott Willams & Wilkins; 2012. [Google Scholar]
- 52. Kim C, McEwen LN, Piette JD, Goewey J, Ferrara A, Walker EA. Risk perception for diabetes among women with histories of gestational diabetes mellitus. Diabetes Care. 2007;30:2281–2286. [DOI] [PubMed] [Google Scholar]