Abstract
Background
Despite the key role of physical activity in the management of diabetes, many individuals with diabetes do not engage in the recommended levels of physical activity. However, our knowledge of the mechanisms underlying the relationship between diabetes and physical inactivity is limited.
Purpose
To investigate the associations between diabetes and the levels and evolution of physical activity across aging, and to determine whether physical, emotional, and cognitive factors mediate these associations.
Methods
Data from 105,622 adults aged 50–96 years from the Survey of Health, Ageing and Retirement in Europe (SHARE) were used in adjusted linear mixed models to examine whether diabetes was associated with physical activity levels and variations across aging. The potential mediators were subjective energy, muscle strength, physical and cognitive disability, sleep problems, depressive symptoms, and cognitive functions. The variables were measured up to seven times over a 13-year period.
Results
Individuals with diabetes demonstrated a lower level and a steeper decrease in physical activity across aging than individual without diabetes. Mediators explained ~53% and 94% of the association of diabetes with the level of physical activity and with the linear evolution of physical activity across aging, respectively. All mediators were significantly associated with physical activity. Physical and cognitive disability as well as depressive symptoms were the strongest mediators, while sleep was the lowest one.
Conclusions
These findings suggest that the etiology of physical inactivity in individuals with diabetes can result from several physical, emotional, and cognitive changes associated with the emergence of this disease.
Keywords: Diabetes, Physical activity, Disability, Emotions, Cognition, Aging, Depression
Individuals with diabetes engage in lower physical activity and show steeper decline across aging mainly because of physical, emotional, and cognitive changes associated with the disease.
Introduction
Physical activity is important not only for the prevention of diabetes, but also for its management [1–5]. Physical activity facilitates this management through improved regulation of the glycemic index and body weight and the reduction of comorbidities [4–7]. Moreover, physical activity reduces the risk of cardiovascular disease, which is at least twice higher in individuals with than without diabetes [8, 9]. Yet, many individuals with diabetes do not engage in the recommended levels of physical activity [10, 11]. Understanding the mechanisms underlying the relationship between diabetes and physical inactivity is thus warranted.
Physical inactivity in individuals with diabetes may be explained by at least three types of intertwined pathways: physical, emotional, and cognitive. For instance, regarding the physical pathway, previous studies showed that diabetes was associated with higher fatigue [12, 13], lower muscle strength [14–17], lower functional independence in activities of daily living (ADL) and instrumental activities of daily living (IADL) [18, 19], and more sleep impairment [20, 21]. Regarding the emotional pathway, previous studies showed that diabetes was associated with emotional distress [22–25] and depression [26–29]. Finally, regarding the cognitive pathway, numerous reviews showed that diabetes was associated with lower cognitive functioning [30–33].
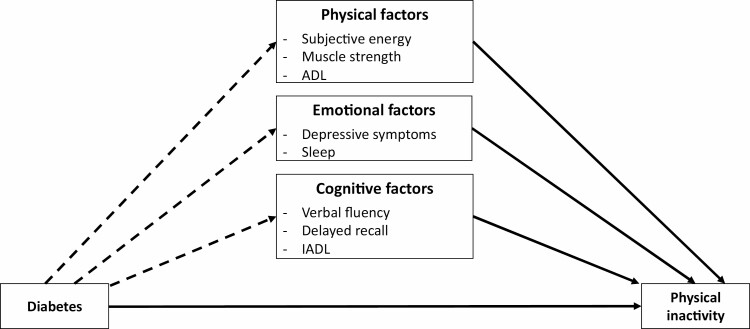
The association between diabetes and these physical, emotional, and cognitive conditions can reduce engagement in physical activity. This suggestion is supported by the literature showing that higher fatigue [34, 35], lower cognitive functioning [36–40], and higher depressive symptoms [41–43] are associated with lower physical activity. In sum, on the one hand, a great deal of research has shown that diabetes is associated with the impairments in physical, emotional, and cognitive functions. On the other hand, data support the effectiveness of these multiple impairments on physical activity, thereby suggesting that they could mediate the effect of diabetes on physical inactivity (Fig. 1).
Fig. 1.
Diagram of the performed analyses. Note. The arrows represent the associations tested in the present study including the direct effect of diabetes on physical activity level and evolution across aging (Model 1), as well as the mediator role of physical, emotional and cognitive factors on these associations. The dashed arrow represents the direct effects of diabetes on the physical, emotional and cognitive factors, which were not tested in this study. ADL, activities of daily living; IADL, instrumental activities of daily living.
To the best of our knowledge, only one large-scale cross-sectional international study has assessed the potential mediation of the associations between diabetes and physical activity by physical and emotional factors [11]. This study, conducted in low- and middle-income countries, showed that the negative association between diabetes and physical activity was partially mediated by physical factors (i.e., mobility, sleep/energy) and emotional factors (i.e., pain/discomfort, depression). However, despite its numerous strengths, this study relied on a cross-sectional design, thereby providing relatively weak evidence on the relationship between diabetes and the age-related decline in physical activity. Moreover, this study did not consider cognitive functioning as a potential mediator. Yet, diabetes has been associated with poor cognitive functions [30–33] and cognitive functions have been associated with lower physical activity [38]. As such, cognitive functioning should be considered as a potential mediator of the association between diabetes and physical inactivity. In sum, current evidence on the factors explaining the relationship between diabetes and physical inactivity is weak.
The objective of the present study was to examine the association between diabetes and the levels and evolution of physical activity across aging. We also assessed whether these associations were mediated by physical, emotional, and cognitive factors. We hypothesized that diabetes would be associated with lower levels of physical activity (H1) and a steeper decrease in physical activity over aging (H2). Based on previous results [11], we also hypothesized that physical, emotional, and cognitive factors would partly mediate these associations (H3).
Methods
Population and Design
Our analyses used data from the Survey of Health, Ageing and Retirement in Europe (SHARE) [44], a multidisciplinary longitudinal and cross-national database of microdata on health, socioeconomic status, and social and family networks of about 140,000 individuals aged 50 or older. SHARE comprises seven waves of data collected every 2 years between 2004 and 2017, with some participants having started the study at wave 1 and others later. In the current study, we used data collected at wave 1 (2004–2005), 2 (2006–2007), 4 (2010–2011), 5 (2013), 6 (2015), and 7 (2017). The survey was initially conducted in 11 countries (Austria, Belgium, Denmark, France, Germany, Greece, Italy, Spain, Sweden, Switzerland, and The Netherlands) and other countries joined SHARE across waves. Data were collected using computer-assisted personal interviews that increase the standardization of the procedure and improve measurement reliability between participants. Diabetes, physical activity, and the potential mediators were assessed at each of these waves. With the exception of grip strength and cognitive functions (i.e., delayed recall and verbal fluency), which were assessed using a handheld dynamometer and tests respectively, all the variables were measured using self-reported questionnaires. In wave 3 (2008–2009), retrospective life-course data related to early and adult-life socioeconomic conditions were collected. This wave was not used in the current study because it did not measure the predictors nor the outcome. For the current analysis, we included data for participants aged 50–96 years with at least one measure of physical activity and each potential mediator. SHARE (waves 1–4) was approved by the Ethics Committee of the University of Mannheim. SHARE (waves 4–8) was approved by the Ethics Council of the Max Plank Society.
Measures
Physical Activity
Low-to-moderate physical activity was derived from the question: “How often do you engage in activities that require a low or moderate level of energy such as gardening, cleaning the car, or doing a walk?” [45–47]. Participants answered on a four-point scale: 1, more than once a week; 2, once a week; 3, one to three times a month; 4, hardly ever, or never. In the statistical models, the variable was reversed so that higher values indicated higher physical activity.
Diabetes
Diabetes was derived from the question: “Has a doctor ever told you that you had / Do you currently have any of the conditions on this card?”; a card that includes diabetes or high blood sugar [46]. It was specified that this meant that a doctor had told the participants that they had this condition, and that they were either currently being treated for or bothered by this condition. If participants selected the option “Diabetes or high blood sugar,” they were included in the analyses as having diabetes.
Mediators
Subjective energy, grip strength, and ADL were included as physical factors. Depressive symptoms and sleep problems were included as emotional factors. IADL, verbal fluency, and delayed recall were included as cognitive factors. Of note, some measures can tap into several dimensions. For instance, IADL includes items capturing both physical (e.g., doing work around the house or garden) and cognitive processes (e.g., taking medications or saving money). Depression includes items tapping into both physical (e.g., subjective energy) and cognitive (e.g., concentration) dimensions. In other words, the measures predominantly target one dimension, but can also assess, albeit to a lesser extent, other dimensions.
Subjective energy was derived from the question: “In the last month, did you have too little energy to do things you wanted to do?” Participants answered yes or no.
Grip strength was measured twice in both hands, alternating between the hands, using a handheld dynamometer (Smedley, S Dynamometer, TTM, Tokyo, 100 kg). The mean of the maximum values obtained for each hand was used as an indicator of muscle strength [48–50]. When values for one hand were missing or were equal to 0, the measurement at this time point was excluded from the analysis. Then, consistent with previous literature, the cut-off for low muscle strength was computed based on gender and body mass index (BMI) quartiles [51, 52] following the Fried criterion (i.e., grip strength in the lowest 20% adjusted for gender and BMI). For men with BMI lower or equal to 24, between 24 and 26, between 26 and 28, and higher than 28 kg/m2, the cut-offs for low muscle strength were ≤26, 29, 30, and 32 kg, respectively. For women with BMI lower or equal to 23, between 23 and 26, between 26 and 28, and higher than 28 kg/m2, the cut-offs for low muscle strength were 17, 17.3, 18, and 21 kg, respectively.
Physical and cognitive disability was measured using the number of functional limitations in ADL and IADL, respectively. Functional dependence in ADL was derived from the index of Katz and Ford [53], which includes six activities: dressing, walking across a room, bathing or showering, eating, getting in or out of bed, and using the toilet. Functional dependence in IADL was based on the index of Lawton and Brody [54], which includes seven activities: using a map, preparing a meal, shopping for groceries, making telephone calls, taking medications, doing work around the house or garden, and managing money. In the analyses, participants were categorized as having “no ADL (IADL) limitations” or “1 or more ADL (IADL) limitations” [55].
Sleep problems were derived from the following question: “Have you had trouble sleeping recently?” Participants who answered “Trouble with sleep or recent change in pattern” were considered as having sleeping problems, whereas participants who answered “No trouble sleeping” were considered free of sleeping problems [56].
Depressive symptoms were measured using the EURO–D scale [57, 58]. This scale includes the following 12 items: depression, pessimism, wishing death, guilt, sleep, interest, irritability, appetite, fatigue, concentration, enjoyment, and tearfulness. Each item was coded 0 (symptom absent) or 1 (symptom present), generating an ordinal scale ranging from 0 to 12 [59–61].
Verbal fluency was derived from the verbal fluency test [62], in which participants name as many different animals as they can think of in 60 s. The score is the total number of correctly named animals [63]. Verbal fluency is thought to reflect executing functioning, which includes executive control or selective inhibition [64].
Delayed recall was captured using the 10-word delayed recall test [65], in which participants listen to a list of ten words that are read out loud by the interviewer. Participants are asked to recall as many words as possible immediately after reading the wordlist and after the verbal fluency tool place. The delayed recall score ranges from 0 to 10 based on the number of words that the respondent is able to recall [63]. The delayed recall is believed to reflect memory performance [66].
Covariates
The following covariates were used: gender (male, female), BMI, cardiovascular disease (a condition that included a heart attack including myocardial infarction or coronary thrombosis or any other heart problem including congestive heart failure; yes vs. no), hypertension (i.e., high blood pressure or hypertension), birth cohort [1919–1928, 1929–1938 (great depression), 1939–1945 (World War II), and post-1945], country of residence (Austria, Belgium, Croatia, Czech Republic, Denmark, Estonia, France, Germany, Greece, Hungary, Ireland, Israel, Italy, Luxembourg, Netherlands, Poland, Portugal, Slovenia, Spain, Sweden, Switzerland), education (primary, secondary, tertiary), household’s ability to make ends meet (easily, fairly easily, with difficulty, with great difficulty), and attrition [no dropout, dropout (participants did not respond to waves 6 and 7), death].
As prior research showed that these variables influence physical activity [38, 47, 67–70], they were added as covariates in the models to examine the independent effect of diabetes and the potential mediators on physical activity. In addition, the models were adjusted for cardiovascular diseases and hypertension because they are comorbidities of diabetes [71–74]. BMI, cardiovascular disease, and hypertension were used as time-varying predictors, while the other covariates were used as time-invariant predictors.
Statistical Analysis
Physical activity evolution across aging was estimated in an accelerated longitudinal design [75] using mixed-effects models [76]. These models account for the nested structure of the data (here, multiple observations within a single participant) and do not require an equal number of observations across participants, thereby allowing participants with missing observations to be included in the analyses. In other words, all participants were included in the analyses on the condition that they had participated in at least one wave. All models had a random intercept, a random linear slope, and a random quadratic slope for participants that estimated each participant’s engagement in physical activity and the rate of change of this engagement across aging. The quadratic effect of age was added to account for the potential accelerated (or decelerated) change of physical activity across aging.
The first model examined the level of physical activity and the change in physical activity across aging as a function of diabetes status, adjusting for prior covariates. Diabetes status was used as a time-varying variable. Therefore, participants can be included in the diabetic group after the baseline assessment. Age was centered at the midpoint of the sample’s age range (i.e., 73 years) and was then divided by 10. Thus, a 1-unit change in the coefficients yielded effects on the physical activity rate of change over a 10-year period. The first model included interaction terms between age (linear and quadratic) and diabetes to assess whether diabetes modified the evolution of physical activity across aging. A statistically significant interaction indicated that the rate of age-related physical activity change was different depending on whether individuals were diabetic or not.
The second model included all the time-varying mediators at the same time (i.e., subjective energy, grip strength, IADL, ADL, sleep problems, verbal fluency, delayed recall, and depressive symptoms), as well as their interactions with age. This model allowed to assess the overall percentage of the association between diabetes and physical activity that was explained when all the potential mediators were included together. In line with previous studies [47], the decrease in the percentage of the association between diabetes and physical activity between the first model and this second model testing the mediating variables was calculated as follows:
with b representing the coefficients of diabetes on physical activity. This percentage provided an estimate of the proportional influence of the potential mediating variables on the relationship between diabetes and physical activity. Finally, in a series of eight models, each potential mediator (i.e., subjective energy, grip strength, IADL, ADL, sleep problems, verbal fluency, delayed recall, and depressive symptoms), as well as their interactions with age, were tested separately. This one-by-one strategy allowed to assess the percentage of the association between diabetes and physical activity explained by a single mediator at a time. Statistical analyses were performed using the lme4 and lmerTest R packages [77–79]. An estimate of the effect size for fixed effects was reported using the marginal pseudo-R2, computed using the MuMin package [80].
To minimize the impact of reverse causation bias, we performed a sensitivity analysis where a time lag was introduced between the time-varying predictors (i.e., diabetes and the potential mediators) and the outcome (i.e., physical activity). Specifically, for a given wave (except for baseline), the time-varying predictors were assigned the value of the preceding wave.
Results
Table 1 summarizes the characteristics of the participants by diabetes status at baseline. The study sample consisted of 105,622 people (54.4% women, 11.1% with diabetes), aged between 50 and 96 years. Having diabetes at baseline (vs. not having diabetes) was associated with older age, male sex, higher BMI, higher cardiovascular disease, higher hypertension, higher depressive symptoms, higher sleep problems, higher dependence in ADL and IADL, longer delayed recall, lower subjective energy, lower muscle strength, lower verbal fluency, lower level of education, and lower income. During the study, 9,067 (8.6%) participants died and 30,118 (28.5%) dropped out for other reasons. On average, participants had two out of six complete waves (information on all predictors and outcomes). More specifically, the percentage of participants with one, two, three, four, five, and six measurement waves was 39.9% (n = 42,133), 28.1% (n = 29,728), 18.3% (n = 19,345), 5.7% (n = 6,056), 4.4% (n = 4,598), and 3.6% (n = 3,762), respectively. Descriptive statistics are provided in supplemental material. The likelihood of death was higher in individuals with than without diabetes at baseline (14.5% vs. 7.8%).
Table 1.
Sample characteristics by diabetes status at baseline
| No diabetes (N = 93,916) | Diabetes (N = 11,706) | p value | |||
|---|---|---|---|---|---|
| Physical activity outcomes | |||||
| Low-to-moderate physical activity (1–4), SD | 3.5 | 1.0 | 3.2 | 1.2 | <.001 |
| Mediators | |||||
| Subjective energy | |||||
| Low level of energy | 30,349 | 32.3% | 5,256 | 44.9% | |
| Sufficient level of energy | 63,567 | 67.7% | 6,450 | 55.1% | <.001 |
| Muscle strength | |||||
| Low muscle strength | 10,782 | 11.5% | 2,773 | 23.7% | |
| Normal muscle strength | 83,134 | 88.5% | 8,933 | 76.3% | <.001 |
| ADL | |||||
| No | 86,718 | 92.3% | 9,752 | 83.3% | |
| Yes | 7,198 | 7.7% | 1,954 | 16.7% | <.001 |
| IADL | |||||
| No | 82,453 | 87.8% | 8,778 | 75.0% | |
| Yes | 11,463 | 12.2% | 2,928 | 25.0% | <.001 |
| Sleep problems | |||||
| No | 63,228 | 67.3% | 6,868 | 58.7% | |
| Yes | 30,688 | 32.7% | 4,838 | 41.3% | <.001 |
| Depressive symptoms | 2.3 | 2.2 | 3.0 | 2.5 | <.001 |
| Delayed recall | 3.8 | 2.1 | 3.2 | 2.0 | <.001 |
| Verbal fluency | 20.0 | 7.5 | 18.0 | 7.3 | <.001 |
| Covariates | |||||
| Age at baseline (years), SD | 63.1 | 9.6 | 66.8 | 9.3 | <.001 |
| Gender | |||||
| Female | 51,586 | 54.9% | 5,828 | 49.8% | |
| Male | 42,330 | 45.1% | 5,878 | 50.2% | <.001 |
| Body mass index (BMI) | 26.5 | 4.3 | 29.3 | 5.2 | <.001 |
| Cardiovascular disease | |||||
| No | 83,803 | 89.2% | 9,067 | 77.5% | |
| Yes | 10,113 | 10.8% | 2,639 | 22.5% | <.001 |
| Hypertension | |||||
| No | 62,423 | 66.5% | 4,549 | 38.9% | |
| Yes | 31,493 | 33.5% | 7,157 | 61.1% | <.001 |
| Education | |||||
| Primary | 20,732 | 22.1% | 3,781 | 32.3% | |
| Secondary | 52,474 | 55.9% | 6,188 | 52.9% | |
| Tertiary | 20,710 | 22.0% | 1,737 | 14.8% | <.001 |
| Satisfaction with income | |||||
| With great difficulty | 10,530 | 11.2% | 2,012 | 17.2% | |
| With some difficulty | 20,073 | 21.4% | 3,158 | 27.0% | |
| Fairly easily | 28,251 | 30.1% | 3,494 | 29.8% | |
| Easily | 35,062 | 37.3% | 3,042 | 26.0% | <.001 |
| Countries | |||||
| Belgium | 8,025 | 8.5% | 799 | 6.8% | |
| Austria | 5,004 | 5.3% | 556 | 4.8% | |
| Denmark | 4,971 | 5.3% | 360 | 3.1% | |
| France | 6,545 | 7.0% | 717 | 6.1% | |
| Germany | 6,912 | 7.4% | 919 | 7.9% | |
| Greece | 5,072 | 5.4% | 598 | 5.1% | |
| Israel | 2,472 | 2.6% | 615 | 5.3% | |
| Italy | 6,564 | 7.0% | 730 | 6.2% | |
| Netherlands | 5,260 | 5.6% | 453 | 3.9% | |
| Spain | 6,424 | 6.8% | 1,007 | 8.6% | |
| Sweden | 5,508 | 5.9% | 509 | 4.3% | |
| Switzerland | 3,929 | 4.2% | 261 | 2.2% | |
| Czech Republic | 6,496 | 6.9% | 1,212 | 10.4% | |
| Ireland | 691 | .7% | 70 | .6% | |
| Poland | 2,414 | 2.6% | 291 | 2.5% | |
| Estonia | 6,167 | 6.6% | 793 | 6.8% | |
| Hungary | 2,246 | 2.4% | 443 | 3.8% | |
| Portugal | 1,489 | 1.6% | 327 | 2.8% | |
| Slovenia | 4,222 | 4.5% | 552 | 4.7% | |
| Luxembourg | 1,626 | 1.7% | 217 | 1.9% | |
| Croatia | 1,879 | 2.0% | 277 | 2.4% | <.001 |
| Birth cohort | |||||
| After 1945 | 53,300 | 56.8% | 4,850 | 41.4% | |
| Between 1939 and 1945 | 18,059 | 19.2% | 2,793 | 23.9% | |
| Between 1929 and 1938 | 16,670 | 17.7% | 3,108 | 26.5% | |
| Between 1919 and 1928 | 5,887 | 6.3% | 955 | 8.2% | <.001 |
| Attrition | |||||
| No drop out | 59,304 | 63.2% | 7,133 | 60.9% | |
| Drop out | 27,246 | 29.0% | 2,872 | 24.6% | |
| Death | 7,366 | 7.8% | 1,701 | 14.5% | <.001 |
Note. Baseline = the first measurement occasion for each participant; SD = standard deviation; ADL = activities of daily living; IADL = instrumental activities of daily living. p values are based on the analysis of variance and chi-square tests for continuous and categorical variables, respectively, testing the effect of diabetes (vs. no diabetes) on these variables.
Associations Between Diabetes and Physical Activity
As hypothesized (H1), results showed that at the midpoint of the sample age range (i.e., 73 years), individuals with diabetes were less physically active than individuals without diabetes (b = −.13, 95% CI: −.15 to −.11, p < .001) (Table 2).
Table 2.
Associations between diabetes and physical activity (levels and trajectories)
| Outcome: physical activity | ||
|---|---|---|
| b (95% CI) | p | |
| Fixed effects | ||
| Intercept | 3.32 (3.30; 3.34) | <.001 |
| Age (10-year follow-up) | −.30 (−.32; −.29) | <.001 |
| Age2 (10-year follow-up) | −.11 (−.12; −.10) | <.001 |
| Diabetes (ref. no diabetes) | ||
| Having diabetes | −.13 (−.15; −.11) | <.001 |
| Age × diabetes (ref. no diabetes) | ||
| Age × having diabetes | −.04 (−.06; −.02) | <.001 |
| Age2 × diabetes (ref. no diabetes) | ||
| Age2 × having diabetes | −.01 (−.02; .01) | .222 |
| Random effects | ||
| Participants | ||
| Intercept | .32324 | |
| Age (10-year follow-up) | .22670 | |
| Age2 (10-year follow-up) | .01993 | |
| Covariance | ||
| Intercept − Age (10-year follow-up) | .39 | |
| Intercept − Age2 (10-year follow-up) | −.43 | |
| Age (10-year follow-up) − Age2 (10-year follow-up) | .66 | |
| Residuals | .58263 | |
Note. All models were adjusted for gender (male, female), body mass index (BMI), birth cohort, country of residence, education, household’s ability to make ends meet, and participants’ attrition. “Age (10-year follow-up)” and “Age (10-year follow-up) squared” estimated the linear and quadratic changes in the engagement in physical activity over a 10-year period.
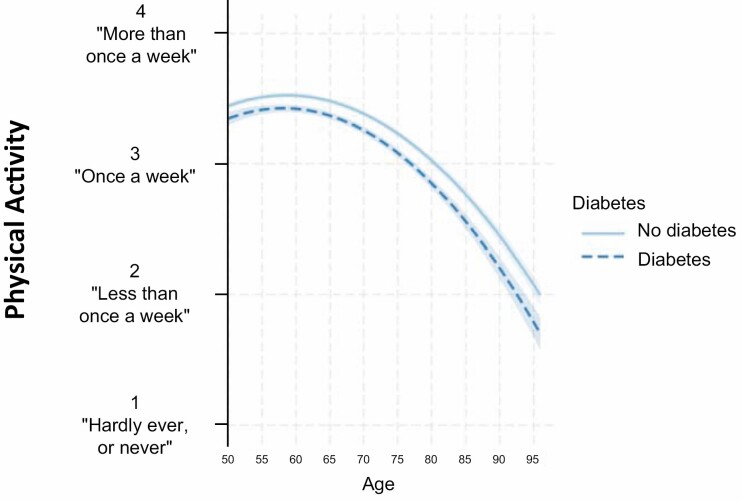
As hypothesized (H2), individuals with diabetes demonstrated a steeper linear decline of physical activity across aging compared to individuals without diabetes (b = −.04, 95% CI: −.06 to −.02, p < .001) (Table 2; Fig. 2). However, the association between diabetes and quadratic evolution of physical activity was not significant (p = .222).
Fig. 2.
Associations between diabetes and the evolution of physical activity across aging. Note. All models were adjusted for gender (male, female), body mass index (BMI), birth cohort, country of residence, education, household’s ability to make ends meet, and participants’ attrition. “Age (10-year follow-up)” and “Age2 (10-year follow-up)” estimated the linear and quadratic changes in the engagement in physical activity over a 10-year period, respectively.
Effects of All the Mediators
The model that included all the mediators explained 53.1% of the association between diabetes and physical activity level, 93.9% of the association between diabetes and linear evolution of physical activity, and 100% of the (although not significant) association between diabetes and the quadratic evolution of physical activity (Table 3). The association between diabetes and physical activity levels remained significant (b = −.06, 95% CI: −.08 to −.05, p < .001), which suggested that this association was partly explained by the mediators. The association between diabetes and linear evolution of physical activity was not significant (b = −.01, 95% CI: −.02 to .02, p = .797), which suggested that this association was fully explained by the mediators. Overall, the model including all the mediators explained 39.3% of the variance in physical activity.
Table 3.
Physical, emotional, and cognitive factors as mediators of the associations between diabetes and physical activity levels and evolution across aging (Age and Age2)
| Variables | b (95% CI) | p | % explained | ||
|---|---|---|---|---|---|
| Model without mediators | |||||
| Diabetes | Level | −.13 (−.15; −.11) | <.001 | ||
| Age (10-year follow-up) | −.04 (−.06; −.02) | <.001 | |||
| Age2 (10-year follow-up) | −.01 (−.02; .01) | .222 | |||
| Model including all the mediators | |||||
| Diabetes | Level | −.06 (−.08; −.05) | <.001 | 53.1% | |
| Age (10-year follow-up) | −.01 (−.02; .02) | .797 | 93.9% | ||
| Age2 (10-year follow-up) | .01 (−.01; .02) | .429 | All | ||
| Models including one mediator | |||||
| Depressive symptoms | Diabetes | Level | −.11 (−.12; −.09) | <.001 | 19.4% |
| Age (10-year follow-up) | −.03 (−.05; −.01) | .009 | 35.0% | ||
| Age2 (10-year follow-up) | −.01 (−.02; .01) | .571 | 54.2% | ||
| Depressive symptoms | Level | −.18 (−.18; −.17) | <.001 | ||
| Age (10-year follow-up) | −.06 (−.07; −.06) | <.001 | |||
| Age2 (10-year follow-up) | −.01 (−.01; −.01) | <.001 | |||
| Subjective energy | Diabetes | Level | −.12 (−.14; −.10) | <.001 | 9.6% |
| Age (10-year follow-up) | −.04 (−.06; −.02) | .001 | 13.2% | ||
| Age2 (10-year follow-up) | −.01 (−.02; .01) | .322 | 19.3% | ||
| Subjective energy | Level | −.22 (−.23; −.21) | <.001 | ||
| Age (10-year follow-up) | −.07 (−.08; −.05) | <.001 | |||
| Age2 (10-year follow-up) | −.01 (−.02; −.00) | .025 | |||
| Grip strength | Diabetes | Level | −.12 (−.14; −.10) | <.001 | 10.5% |
| Age (10-year follow-up) | −.04 (−.06; −.01) | .001 | 17.2% | ||
| Age2 (10-year follow-up) | −.01 (−.02; .01) | .458 | 39.6% | ||
| Grip strength | Level | −.32 (−.33; −.30) | <.001 | ||
| Age (10-year follow-up) | −.03 (−.04; −.01) | <.001 | |||
| Age2 (10-year follow-up) | −.03 (−.04; −.02) | <.001 | |||
| ADL | Diabetes | Level | −.10 (−.13; −.09) | <.001 | 16.9% |
| Age (10-year follow-up) | −.02 (−.04; −.00) | .035 | 48.4% | ||
| Age2 (10-year follow-up) | .00 (−.01; .01) | .952 | 95.2% | ||
| ADL | Level | −.58 (−.59; −.56) | <.001 | ||
| Age (10-year follow-up) | −.08 (−.10; −.06) | <.001 | |||
| Age2 (10-year follow-up) | .00 (−.01; .01) | .913 | |||
| IADL | Diabetes | Level | −.10 (−.11; −.08) | <.001 | 27.3% |
| Age (10-year follow-up) | −.02 (−.04; .00) | .082 | 58.1% | ||
| Age2 (10-year follow-up) | .00 (−.01; .01) | .941 | All | ||
| IADL | Level | −.58 (−.60; −.57) | <.001 | ||
| Age (10-year follow-up) | −.12 (−.13; −.10) | <.001 | |||
| Age2 (10-year follow-up) | −.02 (−.03; −.01) | .001 | |||
| Sleep | Diabetes | Level | −.13 (−.15; −.12) | <.001 | 2.6% |
| Age (10-year follow-up) | −.04 (−.06; −.02) | <.001 | 5.8% | ||
| Age2 (10-year follow-up) | −.01 (−.02; .01) | .294 | 14.2% | ||
| Sleep | Level | −.10 (−.11; −.09) | <.001 | ||
| Age (10-year follow-up) | −.04 (−.06; −.02) | <.001 | |||
| Age2 (10-year follow-up) | −.01 (−.02; −.00) | .020 | |||
| Verbal fluency | Diabetes | Level | −.12 (−.14; −.11) | <.001 | 7.0% |
| Age (10-year follow-up) | −.03 (−.05; −.01) | .002 | 21.6% | ||
| Age2 (10-year follow-up) | −.01 (−.02; .01) | .537 | 49.7% | ||
| Verbal Fluency | Level | .14 (.13; .15) | <.001 | ||
| Age (10-year follow-up) | .07 (.06; .07) | <.001 | |||
| Age2 (10-year follow-up) | .02 (.01; .02) | <.001 | |||
| Delayed recall | Diabetes | Level | −.13 (−.14; −.11) | <.001 | 3.8% |
| Age (10-year follow-up) | −.04 (−.06; −.02) | <.001 | 10.8% | ||
| Age2 (10-year follow-up) | −.01 (−.02; .00) | .325 | 19.5% | ||
| Delayed recall | Level | .07 (.06; .07) | <.001 | ||
| Age (10-year follow-up) | .04 (.03; .05) | <.001 | |||
| Age2 (10-year follow-up) | .01 (.01; .02) | <.001 |
Note. All models were adjusted for gender (male, female), body mass index (BMI), birth cohort, country of residence, education, household’s ability to make ends meet, and participants’ attrition. “Age (10-year follow-up)” and “Age2 (10-year follow-up)” estimated the effect of diabetes in the linear and quadratic evolution in the engagement in physical activity over a 10-year period. The percentage of the effect of diabetes on physical activity levels and evolution across aging explained by the variables were estimated based on the exacts estimates, not on the rounded values presented in the table. The model including all the mediators was meant to test the overall percentage of the association between diabetes and physical activity that was explained by all the potential mediators. The models including only one mediator at a time were meant to test the percentage of the association between diabetes and physical activity that was explained by this mediator of interest.
Effects of Each Mediator
Results of the models that included one mediator at a time further suggested that physical and cognitive disability (~16.9% for ADL; ~27.3% for IADL) and depressive symptoms (~19.4%) explained the largest part of the association between diabetes and the level of physical activity, followed by subjective energy (~9.6%) and muscle strength (~10.5%), then cognitive functions (~3.8% for delayed recall; ~7.0% for verbal fluency), and finally sleep (~2.6%). Regarding the factors explaining the link between diabetes and the linear evolution of physical activity across aging, results further revealed that physical and cognitive disability (~48.4% for ADL; ~58.1% for IADL) and depressive symptoms (~35.0%) explained the largest part of the effect of diabetes, followed by verbal fluency (~21.6%), muscle strength (~17.2%) and subjective energy (~13.2%), and finally by delayed recall (~10.8%) and sleep (~5.8%) (Table 3).
Overall, the results of the sensitivity analysis were consistent with the main analysis. However, the percentages of the associations between diabetes and the levels and evolution of physical activity across aging explained by the mediators were weaker. Specifically, the model that included all the mediators explained 30.6% (vs. 53.1%) of the association between diabetes and physical activity level, and 49.4% (vs. 93.9%) of the association between diabetes and the linear evolution of physical activity (see supplemental material).
Discussion
Main Findings
Because of the key role of physical activity in the management of diabetes, a better understanding of the factors explaining inactive lifestyles in this population is warranted. The objective of this study was to investigate the association between diabetes and physical activity across aging, and to determine which factors explained this association. Results of this large-scale longitudinal international study among older Europeans (N = 105,622; 21 countries) showed that having diabetes was associated with a lower level of physical activity and a steeper linear decline of physical activity across aging. Moreover, we identified mediators that explained ~53% of the association between diabetes and physical activity levels and ~94% of the association between diabetes and linear physical activity trajectories. Particularly, physical and cognitive disability and depressive symptoms were the strongest mediators of these associations, but all mediators explained at least a small part of the associations. Hence, our study lends support for the suggestion that physical, emotional, and cognitive conditions related to diabetes can explain the low levels of physical activity in individuals with diabetes.
Comparison with Other Studies
Our findings support previous studies showing a negative association between diabetes and the level of physical activity [8, 9] and extend these results by revealing the negative association between diabetes and linear evolution of physical activity across aging. In other words, our study adds to the existing literature by showing that diabetes is associated not only with lower levels of physical activity, but also with a steeper decline. Our results further revealed that the association between diabetes and physical activity level and evolution in old age is explained by physical, emotional, and cognitive factors. These findings are consistent with previous literature showing the mediating role of physical and emotional factors on the association between diabetes and physical activity [11]. However, to the best of our knowledge, our study is the first to assess multiple types of mediators in a large-scale longitudinal design, and to assess the influence of these mediators not only on the level, but also on the evolution of physical activity across aging. Overall, the fact that physical, emotional, and cognitive factors explained a high percentage of the associations between diabetes and physical activity suggests that they contribute to the inactive lifestyles of individuals with diabetes.
Our findings showed that higher functional dependence in physical (ADL) and cognitive activities of daily living (IADL), as well as depressive symptoms, are the largest mediators of the associations between diabetes and physical activity level. These three variables were followed by subjective energy and muscle strength, by cognitive functioning, and finally by sleep. These results are consistent with the strength of the reduction observed in a previous study [11], although some differences can be noted. For example, the percentage of reduction by depressive symptoms is about three times larger in our study than in the one of Vancampfort et al. [11] (~19.4% vs. 7%), while the effect of sleep is about four times lower in our study (~2.6% vs. 11%). At least two factors can explain these discrepancies. First, the previous study relied on cross-sectional data, which cannot accurately disentangle the effect of age from the effects of other factors. Second, Vancampfort et al. [11] involved individuals from low- and middle-income (LMI) countries only, while our study involved Europeans. Yet, the association between diabetes and physical activity may differ in LMI countries because of multiple factors including the disease profiles [81], the knowledge regarding the health benefits associated with regular physical activity [82], environmental factors such as working conditions or access to facilities [83], and non-optimal chronic conditions treatments [84, 85]. Finally, in our study, sleep problems and depression were assessed using scales that were different from the ones in Vancampfort et al. [11]. For example, while we assessed depression with the EURO–D scale [57, 58] and treated the depression score as a continuous variable, Vancampfort et al. [11] used the DSM-IV algorithm and treated depression as a categorical variable (i.e., presence or absence of depressive symptoms in the previous 12 months) [86]. These different scales and approaches may explain some slight discrepancies in the results. Yet, it is important to note that the mediating role of depression observed in the current study is consistent with the findings from Vancampfort et al. [11].
The variables included in the models were considered as potential mediators in the association of diabetes with physical activity, since previous studies observed that diabetes is associated with the impairments in physical, emotional, and cognitive functions, impairments which, in turn, are associated with lower physical activity levels. Although a wide range of mediators was tested, a substantial part of the association between diabetes and physical activity remained unexplained in the fully adjusted model (i.e., ~50%). Moreover, diabetes remained directly associated with physical activity after accounting for all the tested mediators. Therefore, considering factors that were not assessed in our study may further improve our understanding of the relationship between diabetes and physical activity. One of these factors could be motivation as it has been found to explain physical activity in individuals with diabetes [87–89]. Likewise, factors related to diabetes complications such as neuropathy, nephropathy, or foot damage, may reduce the engagement in physical activity as they have been associated with sensorimotor impairments and painful movements [90–92].
Our study extends previous literature at least in two ways. First, it assesses whether cognitive factors explained the relationships between diabetes and physical activity level. Results showed that this was the case (~7.0% for verbal fluency; 3.8% for delayed recall), albeit in a modest way relative to the other factors. This result confirms that cognitive functioning, which can be required to counteract the human automatic tendency to effort minimization [38, 93], can also influence the lack of engagement in physical activity in older individuals with diabetes. Second, our study shows that physical, emotional, and cognitive factors not only explain the link between diabetes and physical activity level, but also between diabetes and changes in physical activity across aging. As for the levels, functional dependence in physical and cognitive ADL, as well as depressive symptoms, remained the largest mediators, while sleep remained the lowest one. Of note, verbal fluency plays a stronger role in explaining the evolution of physical activity across aging (~21.6% for a linear change) compared with physical activity level (~7.0%). This finding is in line with previous studies showing that verbal fluency, an indicator reflecting executive functioning [64], seems especially relevant to explain the evolution of physical activity across aging [38].
Strengths and Weaknesses
This study has several strengths. The first strength is to rely on a large-scale longitudinal design. Second, we applied an analytical model well-suited to examine the association of diabetes with not only physical activity level, but also physical activity evolution (linear and quadratic) over 46 years, from age 50 to 96. Third, we investigated a wide range of potential mediators of the association between diabetes and physical activity that are linked to multiple dimensions of health (physical, emotional, and cognitive).
However, several limitations of this study should be considered. The first limitation is related to the measure of diabetes that is self-reported and did not allow to disentangle between type 1 and type 2 diabetes. Yet, the mechanisms underpinning the links between diabetes and physical activity could be different depending on the type of diabetes in this older population. Of note, because the prevalence of diagnosed type 2 diabetes is higher than the prevalence of diagnosed type 1 diabetes in the general population, most of individuals with diabetes in our sample had likely type 2 diabetes [94, 95]. Yet, future studies should rely on an objective diagnostic allowing to differentiate between type 1 and type 2 diabetes. Likewise, the question used to assess the diabetic status was worded to identify whether the participants have had or currently had diabetes or high blood sugar (i.e., Has a doctor ever told you that you had/do you currently have diabetes or high blood sugar?). Accordingly, this question captures the occurrence of diabetes across the study duration, but not the potential resolution of type 2 diabetes. Second, our study was based on a self-reported measure of physical activity that can over or under-estimate the observed levels of physical activity [96]. Nevertheless, this measurement bias can hardly explain the observed associations between diabetes, mediators, and physical activity. Using a device-based measure of physical activity, such as accelerometer, remains however needed in future studies. Third, attrition is selective, which is inevitable in long-term prospective studies. Yet, we adjusted for attrition in all our analyses, and mixed-effects models allowed us to include participants with only one wave participation, thereby leading to a less severe selection bias. Fourth, subjective energy was measured with a binary variable (yes vs. no) targeting the average level of perceived energy over the last month. As such, this variable is thought to reflect a general and stable state of energy perception rather than short-term fluctuations of perceived energy. Hence, answering this question can be difficult for participants who exhibit daily or weekly fluctuations in the level of subjective energy. To accurately assess the potential mediating role of subjective energy between diabetes and physical inactivity, future studies should rely on a measure with a shorter timeframe (e.g., over the last week). Likewise, adopting a daily assessment approach to examine daily fluctuations and how these fluctuations impact physical activity could be particularly meaningful [97]. Fifth, motivational predictors of physical activity were not included in SHARE, while these variables have been found to predict physical activity in individuals with diabetes [87–89]. Finally, our analyses rely on correlational data. Therefore, we cannot infer a causal relationship between predictors and physical activity. However, the results of the sensitivity analysis that included a time lag between the predictors and the outcome to reduce the risk for a potential reverse causation bias were consistent with those of the main analyses.
Conclusion
Diabetes is associated with lower engagement in physical activity and steeper decline of this engagement across aging. Physical, emotional, and cognitive factors explain a substantial part of these relationships. Our findings suggest that the etiology of physical inactivity in individuals with diabetes can result from several physical, emotional, and cognitive changes associated with the emergence of this disease. In such a vicious circle, physical inactivity and other diabetic-related conditions reinforce each other. As physical activity is essential to diabetes management, a better understanding of the mechanisms underlying this vicious circle can help health professionals to break it.
Supplementary Material
Acknowledgments
This paper uses data from SHARE Waves 1, 2, 3 (SHARELIFE), 4, 5, and 6 (DOIs: 10..6103/SHARE.w1.600, 10..6103/SHARE.w2.600, 10.6103/SHARE.w3.600, 10.6103/SHARE.w4.600, 10.6103/SHARE.w5.600, and 10.6103/SHARE.w6.600). The SHARE data collection was primarily funded by the European Commission through FP5 (QLK6-CT-2001-00360), FP6 (SHARE-I3: RII-CT-2006–062193, COMPARE: CIT5-CT-2005–028857, SHARELIFE: CIT4-CT-2006–028812) and FP7 (SHARE-PREP: no. 211909, SHARE-LEAP: no. 227822, SHARE M4: no. 261982). Additional funding from the German Ministry of Education and Research, the Max Planck Society for the Advancement of Science, the U.S. National Institute on Aging (U01_AG09740-13S2, P01_AG005842, P01_AG08291, P30_AG12815, R21_AG025169, Y1-AG-4553-01, IAG_BSR06-11, OGHA_04-064, HHSN271201300071C) and from various national funding sources is gratefully acknowledged (see www.share-project.org). B.C. is supported by an Ambizione grant (PZ00P1_180040) from the Swiss National Science Foundation (SNSF).
Contributor Information
Boris Cheval, Swiss Center for Affective Sciences, University of Geneva, Switzerland; Laboratory for the Study of Emotion Elicitation and Expression (E3Lab), Department of Psychology, University of Geneva, Switzerland.
Silvio Maltagliati, Univ. Grenoble Alpes, SENS, Grenoble, France.
Stefan Sieber, Swiss NCCR “LIVES – Overcoming Vulnerability: Life Course Perspectives,” University of Geneva, Switzerland.
David Beran, Division of Tropical and Humanitarian Medicine, University of Geneva and Geneva University Hospitals, Switzerland.
Aïna Chalabaev, Univ. Grenoble Alpes, SENS, Grenoble, France.
David Sander, Swiss Center for Affective Sciences, University of Geneva, Switzerland; Laboratory for the Study of Emotion Elicitation and Expression (E3Lab), Department of Psychology, University of Geneva, Switzerland.
Stéphane Cullati, Population Health Laboratory, University of Fribourg, Switzerland; Department of Readaptation and Geriatrics, University of Geneva, Switzerland.
Matthieu P Boisgontier, School of Rehabilitation Sciences, Faculty of Health Sciences, University of Ottawa, Ottawa, ON, Canada; Bruyère Research Institute, Ottawa, ON, Canada.
Compliance with Ethical Standards
Authors’ Statement of Conflict of Interest and Adherence to Ethical Standards All authors declare that they have no conflict of interests.
Authors’ Contributions B.C. designed the study. B.C. analyzed the data. B.C. and M.P.B. drafted the manuscript. All authors critically appraised the manuscript, worked on its content, and approved its submitted version.
Ethical Approval This study was part of the SHARE study, approved by the relevant research ethics committees in the participating countries
Informed Consent All participants provided written informed consent.
Data Availability This SHARE dataset is available at http://www.share-project.org/data-access.html.
References
- 1. Hayes C, Kriska A. Role of physical activity in diabetes management and prevention. J Acad Nutr Diet. 2008;108:S19–S23. [DOI] [PubMed] [Google Scholar]
- 2. Arne M, Janson C, Janson S, et al. Physical activity and quality of life in subjects with chronic disease: Chronic obstructive pulmonary disease compared with rheumatoid arthritis and diabetes mellitus. Scand J Prim Health Care. 2009;27:141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Petersen KF, Befroy D, Dufour S, et al. Mitochondrial dysfunction in the elderly: Possible role in insulin resistance. Science. 2003;300:1140–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sigal RJ, Kenny GP, Wasserman DH, Castaneda-Sceppa C, White RD. Physical activity/exercise and type 2 diabetes: A consensus statement from the American Diabetes Association. Diabetes Care. 2006;29:1433–8. [DOI] [PubMed] [Google Scholar]
- 6. Toledo FG, Menshikova EV, Ritov VB, et al. Effects of physical activity and weight loss on skeletal muscle mitochondria and relationship with glucose control in type 2 diabetes. Diabetes. 2007;56:2142–7. [DOI] [PubMed] [Google Scholar]
- 7. Boulé NG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: A meta-analysis of controlled clinical trials. JAMA. 2001;286:1218–27. [DOI] [PubMed] [Google Scholar]
- 8. Buse JB, Ginsberg HN, Bakris GL, et al. Primary prevention of cardiovascular diseases in people with diabetes mellitus: A scientific statement from the American Heart Association and the American Diabetes Association. Circulation. 2007;115:114–26. [DOI] [PubMed] [Google Scholar]
- 9. Orchard TJ, Temprosa M, Goldberg R, et al. The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: The Diabetes Prevention Program randomized trial. Ann Intern Med. 2005;142:611–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nelson KM, Reiber G, Boyko EJ. Diet and exercise among adults with type 2 diabetes: Findings from the third national health and nutrition examination survey (NHANES III). Diabetes Care. 2002;25:1722–8. [DOI] [PubMed] [Google Scholar]
- 11. Vancampfort D, Koyanagi A, Ward PB, et al. Chronic physical conditions, multimorbidity and physical activity across 46 low-and middle-income countries. Int J Behav Nutr Phys Act. 2017;14:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fritschi C, Quinn L. Fatigue in patients with diabetes: A review. J Psychosom Res. 2010;69:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Drivsholm T, de Fine Olivarius N, Nielsen ABS, Siersma V. Symptoms, signs and complications in newly diagnosed type 2 diabetic patients, and their relationship to glycaemia, blood pressure and weight. Diabetologia. 2005;48:210–4. [DOI] [PubMed] [Google Scholar]
- 14. Park SW, Goodpaster BH, Strotmeyer ES, et al. Decreased muscle strength and quality in older adults with type 2 diabetes: The health, aging, and body composition study. Diabetes. 2006;55:1813–8. [DOI] [PubMed] [Google Scholar]
- 15. Sayer AA, Dennison EM, Syddall HE, Gilbody HJ, Phillips DI, Cooper C. Type 2 diabetes, muscle strength, and impaired physical function: The tip of the iceberg? Diabetes Care. 2005;28:2541–2. [DOI] [PubMed] [Google Scholar]
- 16. Leenders M, Verdijk LB, van der Hoeven L, et al. Patients with type 2 diabetes show a greater decline in muscle mass, muscle strength, and functional capacity with aging. J Am Med Dir Assoc. 2013;14:585–92. [DOI] [PubMed] [Google Scholar]
- 17. Bianchi L, Volpato S. Muscle dysfunction in type 2 diabetes: A major threat to patient’s mobility and independence. Acta Diabetol. 2016;53:879–89. [DOI] [PubMed] [Google Scholar]
- 18. Wu JH, Haan MN, Liang J, Ghosh D, Gonzalez HM, Herman WH. Diabetes as a predictor of change in functional status among older Mexican Americans: A population-based cohort study. Diabetes Care. 2003;26:314–9. [DOI] [PubMed] [Google Scholar]
- 19. Wong E, Backholer K, Gearon E, et al. Diabetes and risk of physical disability in adults: A systematic review and meta-analysis. Lancet Diabetes Endo. 2013;1:106–14. [DOI] [PubMed] [Google Scholar]
- 20. Barone MT, Menna-Barreto L. Diabetes and sleep: A complex cause-and-effect relationship. Diabetes Res Clin Pract. 2011;91:129–37. [DOI] [PubMed] [Google Scholar]
- 21. Trento M, Broglio F, Riganti F, et al. Sleep abnormalities in type 2 diabetes may be associated with glycemic control. Acta Diabetol. 2008;45:225–9. [DOI] [PubMed] [Google Scholar]
- 22. Perrin N, Davies M, Robertson N, Snoek F, Khunti K. The prevalence of diabetes-specific emotional distress in people with Type 2 diabetes: A systematic review and meta-analysis. Diabet Med. 2017;34:1508–20. [DOI] [PubMed] [Google Scholar]
- 23. Pouwer F. Should we screen for emotional distress in type 2 diabetes mellitus? Nat Rev Endocrinol. 2009;5:665. [DOI] [PubMed] [Google Scholar]
- 24. Fisher L, Skaff M, Mullan J, Arean P, Glasgow R, Masharani U. A longitudinal study of affective and anxiety disorders, depressive affect and diabetes distress in adults with type 2 diabetes. Diabet Med. 2008;25:1096–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Snoek FJ, Bremmer MA, Hermanns N. Constructs of depression and distress in diabetes: Time for an appraisal. Lancet Diabetes Endo. 2015;3:450–60. [DOI] [PubMed] [Google Scholar]
- 26. Mezuk B, Eaton WW, Albrecht S, Golden SH. Depression and type 2 diabetes over the lifespan: A meta-analysis. Diabetes Care. 2008;31:2383–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tabák AG, Akbaraly TN, Batty GD, Kivimäki M. Depression and type 2 diabetes: A causal association? Lancet Diabetes Endo. 2014;2:236–45. [DOI] [PubMed] [Google Scholar]
- 28. Chen P-C, Chan Y-T, Chen H-F, Ko M-C, Li C-Y. Population-based cohort analyses of the bidirectional relationship between type 2 diabetes and depression. Diabetes Care. 2013;36:376–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nouwen A, Winkley K, Twisk J, et al. Type 2 diabetes mellitus as a risk factor for the onset of depression: A systematic review and meta-analysis. Diabetologia. 2010;53:2480–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Awad N, Gagnon M, Messier C. The relationship between impaired glucose tolerance, type 2 diabetes, and cognitive function. J Clin Exp Neuropsychol. 2004;26:1044–80. [DOI] [PubMed] [Google Scholar]
- 31. Allen KV, Frier BM, Strachan MW. The relationship between type 2 diabetes and cognitive dysfunction: Longitudinal studies and their methodological limitations. Eur J Pharmaco. 2004;490:169–75. [DOI] [PubMed] [Google Scholar]
- 32. Strachan MW, Reynolds RM, Marioni RE, Price JF. Cognitive function, dementia and type 2 diabetes mellitus in the elderly. Nat Rev Endocrinol. 2011;7:108. [DOI] [PubMed] [Google Scholar]
- 33. Xue M, Xu W, Ou Y-N, et al. Diabetes mellitus and risks of cognitive impairment and dementia: A systematic review and meta-analysis of 144 prospective studies. Ageing Res Rev. 2019;55:100944. [DOI] [PubMed] [Google Scholar]
- 34. Garber CE, Friedman JH. Effects of fatigue on physical activity and function in patients with Parkinson’s disease. Neurology. 2003;60:1119–24. [DOI] [PubMed] [Google Scholar]
- 35. Murphy SL, Smith DM, Clauw DJ, Alexander NB. The impact of momentary pain and fatigue on physical activity in women with osteoarthritis. Arthritis Care Res. 2008;59:849–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cheval B, Tipura E, Burra N, et al. Avoiding sedentary behaviors requires more cortical resources than avoiding physical activity: An EEG study. Neuropsychologia. 2018;119:68–80. [DOI] [PubMed] [Google Scholar]
- 37. Cheval B, Rebar AL, Miller MM, et al. Cognitive resources moderate the adverse impact of poor neighborhood conditions on physical activity. Prev Med. 2019;126:105741. [DOI] [PubMed] [Google Scholar]
- 38. Cheval B, Orsholits D, Sieber S, Courvoisier DC, Cullati S, Boisgontier M. Relationship between decline in cognitive resources and physical activity. Health Psychol. 2020;39:519–28. [DOI] [PubMed] [Google Scholar]
- 39. Daly M, McMinn D, Allan JL. A bidirectional relationship between physical activity and executive function in older adults. Front Hum Neurosci. 2015;8:1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cheval B, Bacelar M, Daou M, et al. Higher inhibitory control is required to escape the innate attraction to effort minimization. Psychol Sport Exerc. 2020;51:101781. [Google Scholar]
- 41. Roshanaei-Moghaddam B, Katon WJ, Russo J. The longitudinal effects of depression on physical activity. Gen Hosp Psychiatry. 2009;31:306–15. [DOI] [PubMed] [Google Scholar]
- 42. Vancampfort D, Stubbs B, Sienaert P, et al. What are the factors that influence physical activity participation in individuals with depression? A review of physical activity correlates from 59 studies. Psychiatr Danub. 2015;27:0–224. [PubMed] [Google Scholar]
- 43. Schuch F, Vancampfort D, Firth J, et al. Physical activity and sedentary behavior in people with major depressive disorder: A systematic review and meta-analysis. J Affect Disord. 2017;210:139–50. [DOI] [PubMed] [Google Scholar]
- 44. Börsch-Supan A, Brandt M, Schröder M. SHARELIFE—One century of life histories in Europe. Adv Life Course Res. 2013;18:1–4. [DOI] [PubMed] [Google Scholar]
- 45. Lindwall M, Larsman P, Hagger MS. The reciprocal relationship between physical activity and depression in older European adults: A prospective cross-lagged panel design using SHARE data. Health Psychol. 2011;30:453–62. [DOI] [PubMed] [Google Scholar]
- 46. de Souto Barreto P, Cesari M, Andrieu S, Vellas B, Rolland Y. Physical activity and incident chronic diseases: A longitudinal observational study in 16 European Countries. Am J Prev Med. 2017;52:373–8. [DOI] [PubMed] [Google Scholar]
- 47. Cheval B, Sieber S, Guessous I, et al. Effect of early-and adult-life socioeconomic circumstances on physical inactivity. Med Sci Sports Exerc. 2018;50:476–85. [DOI] [PubMed] [Google Scholar]
- 48. Cheval B, Boisgontier MP, Orsholits D, et al. Association of early-and adult-life socioeconomic circumstances with muscle strength in older age. Age Ageing. 2018;47:398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cheval B, Chabert C, Sieber S, et al. Association between adverse childhood experiences and muscle strength in older age. Gerontology. 2019;65:474–84. [DOI] [PubMed] [Google Scholar]
- 50. Leong DP, Teo KK, Rangarajan S, et al. Prognostic value of grip strength: Findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet. 2015;386:266–73. [DOI] [PubMed] [Google Scholar]
- 51. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis Report of the European Working Group on Sarcopenia in Older People A. J. Cruz-Gentoft et al. Age Ageing. 2010;39:412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci. 2001;56:M146-M57. [DOI] [PubMed] [Google Scholar]
- 53. Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged: The index of ADL: A standardized measure of biological and psychosocial function. JAMA. 1963;185:914–9. [DOI] [PubMed] [Google Scholar]
- 54. Lawton MP, Brody EM. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–86. [PubMed] [Google Scholar]
- 55. Landös A, von Arx M, Cheval B, et al. Childhood socioeconomic circumstances and disability trajectories in older men and women: A European cohort study. Eur J Public Health. 2019;29:50–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. van de Straat V, Cheval B, Schmidt RE, et al. Early predictors of impaired sleep: A study on life course socioeconomic conditions and sleeping problems in older adults. Aging Ment Health. 2020;24:322–32. [DOI] [PubMed] [Google Scholar]
- 57. Copeland JR, Beekman AT, Braam AW, et al. Depression among older people in Europe: The EURODEP studies. World Psychiatry. 2004;3:45. [PMC free article] [PubMed] [Google Scholar]
- 58. Prince MJ, Reischies F, Beekman AT, et al. Development of the EURO–D scale–A European Union initiative to compare symptoms of depression in 14 European centres. Br J Psychiatry. 1999;174:330–8. [DOI] [PubMed] [Google Scholar]
- 59. Baranyi G, Sieber S, Pearce J, et al. A longitudinal study of neighbourhood conditions and depression in ageing European adults: Do the associations vary by exposure to childhood stressors? Prev Med. 2019;126:105764. [DOI] [PubMed] [Google Scholar]
- 60. von Arx M, Cheval B, Sieber S, et al. The role of adult socioeconomic and relational reserves regarding the effect of childhood misfortune on late-life depressive symptoms. SSM-Population Health. 2019;8:100434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cheval B, Orsholits D, Sieber S, et al. Early-life socioeconomic circumstances explain health differences in old age, but not their evolution over time. J Epidemiol Community Health. 2019;73:703–11. [DOI] [PubMed] [Google Scholar]
- 62. Rosen WG. Verbal fluency in aging and dementia. J Clin Exp Neuropsychol. 1980;2:135–46. [Google Scholar]
- 63. Aartsen MJ, Cheval B, Sieber S, et al. Advantaged socioeconomic conditions in childhood are associated with higher cognitive functioning but stronger cognitive decline in older age. Proc Natl Acad Sci USA. 2019;116:5478–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lezak MD, Howieson DB, Loring DW, Fischer JS. Neuropsychological Assessment. USA: Oxford University Press; 2004. [Google Scholar]
- 65. Harris S, Dowson J. Recall of a 10-word list in the assessment of dementia in the elderly. Br J Psychiatry. 1982;141:524–7. [DOI] [PubMed] [Google Scholar]
- 66. Zhao Q, Lv Y, Zhou Y, Hong Z, Guo Q. Short-term delayed recall of auditory verbal learning test is equivalent to long-term delayed recall for identifying amnestic mild cognitive impairment. PLoS One. 2012;7:e51157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Beenackers MA, Kamphuis CB, Giskes K, et al. Socioeconomic inequalities in occupational, leisure-time, and transport related physical activity among European adults: A systematic review. Int J Behav Nutr Phys Act. 2012;9:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kirk MA, Rhodes RE. Occupation correlates of adults’ participation in leisure-time physical activity: A systematic review. Am J Prev Med. 2011;40:476–85. [DOI] [PubMed] [Google Scholar]
- 69. Cheval B, Darrous L, Choi K, et al. Physical activity and general cognitive functioning: A Mendelian Randomization study. bioRxiv. 2020. [Google Scholar]
- 70. Trost SG, Pate RR, Sallis JF, et al. Age and gender differences in objectively measured physical activity in youth. Med Sci Sports Exerc. 2002;34:350–5. [DOI] [PubMed] [Google Scholar]
- 71. De Boer IH, Bangalore S, Benetos A, et al. Diabetes and hypertension: A position statement by the American Diabetes Association. Diabetes Care. 2017;40:1273–84. [DOI] [PubMed] [Google Scholar]
- 72. Ferrannini E, Cushman WC. Diabetes and hypertension: The bad companions. Lancet. 2012;380:601–10. [DOI] [PubMed] [Google Scholar]
- 73. Kannel WB, McGee DL. Diabetes and cardiovascular disease: The Framingham study. JAMA. 1979;241:2035–8. [DOI] [PubMed] [Google Scholar]
- 74. Martín-Timón I, Sevillano-Collantes C, Segura-Galindo A, del Cañizo-Gómez FJ. Type 2 diabetes and cardiovascular disease: Have all risk factors the same strength? World J Diabetes. 2014;5:444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Duncan SC, Duncan TE, Hops H. Analysis of longitudinal data within accelerated longitudinal designs. Psychol Methods. 1996;1:236. [Google Scholar]
- 76. Boisgontier MP, Cheval B. The ANOVA to mixed model transition. Neurosci Biobehav Rev. 2016;68:1004–5. [DOI] [PubMed] [Google Scholar]
- 77. Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. 2015. 2015;67:48. [Google Scholar]
- 78. R Core Team. R: A language and environment for statistical computing. Vienna, Austria, 2017. Available at https://www.R-project.org/. [Google Scholar]
- 79. Kuznetsova A, Brockhoff PB, Christensen RHB. ImerTest: Tests in Linear Mixed Effects Models. R package version 2.0-33.2016. Available at https://CRAN.R-project.org/package=lmerTest.
- 80. Barton K. MuMIn: Multi-model inference. R package version 1.42.1. .https://CRANR-projectorg/package=MuMIn. 2018.
- 81. Collaborators G, Forouzanfar M, Alexander L, et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:2287–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pengpid S, Peltzer K, Kassean HK, Tsala JPT, Sychareun V, Müller-Riemenschneider F. Physical inactivity and associated factors among university students in 23 low-, middle-and high-income countries. Int J Public Health. 2015;60:539–49. [DOI] [PubMed] [Google Scholar]
- 83. Atkinson K, Lowe S, Moore S. Human development, occupational structure and physical inactivity among 47 low and middle income countries. Prev Med Rep. 2016;3:40–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Chow CK, Teo KK, Rangarajan S, et al. Prevalence, awareness, treatment, and control of hypertension in rural and urban communities in high-, middle-, and low-income countries. JAMA. 2013;310:959–68. [DOI] [PubMed] [Google Scholar]
- 85. Patel V, Araya R, Chatterjee S, et al. Treatment and prevention of mental disorders in low-income and middle-income countries. Lancet. 2007;370:991–1005. [DOI] [PubMed] [Google Scholar]
- 86. Cifuentes M, Sembajwe G, Tak S, Gore R, Kriebel D, Punnett L. The association of major depressive episodes with income inequality and the human development index. Social Sci Med. 2008;67:529–39. [DOI] [PubMed] [Google Scholar]
- 87. Plotnikoff RC, Lippke S, Trinh L, Courneya KS, Birkett N, Sigal RJ. Protection motivation theory and the prediction of physical activity among adults with type 1 or type 2 diabetes in a large population sample. Br J Health Psychol. 2010;15:643–61. [DOI] [PubMed] [Google Scholar]
- 88. Plotnikoff RC, Lippke S, Courneya K, Birkett N, Sigal R. Physical activity and diabetes: An application of the theory of planned behaviour to explain physical activity for Type 1 and Type 2 diabetes in an adult population sample. Psychol Health. 2010;25:7–23. [DOI] [PubMed] [Google Scholar]
- 89. Plotnikoff RC, Lubans DR, Penfold CM, Courneya KS. Testing the utility of three social-cognitive models for predicting objective and self-report physical activity in adults with type 2 diabetes. Br J Health Psychol. 2014;19:329–46. [DOI] [PubMed] [Google Scholar]
- 90. De Groot M, Anderson R, Freedland KE, Clouse RE, Lustman PJ. Association of depression and diabetes complications: A meta-analysis. Psychosomatic Med. 2001;63:619–30. [DOI] [PubMed] [Google Scholar]
- 91. Young M, Boulton A, MacLeod A, Williams D, Sonksen P. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia. 1993;36:150–4. [DOI] [PubMed] [Google Scholar]
- 92. Boyko EJ, Ahroni JH, Stensel V, Forsberg RC, Davignon DR, Smith DG. A prospective study of risk factors for diabetic foot ulcer. The Seattle Diabetic Foot Study. Diabetes Care. 1999;22:1036–42. [DOI] [PubMed] [Google Scholar]
- 93. Cheval B, Radel R, Neva JL, et al. Behavioral and neural evidence of the rewarding value of exercise behaviors: A systematic review. Sports Med. 2018;48:1389–404. [DOI] [PubMed] [Google Scholar]
- 94. Xu G, Liu B, Sun Y, et al. Prevalence of diagnosed type 1 and type 2 diabetes among US adults in 2016 and 2017: Population based study. BMJ. 2018;362:k1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Zhou B, Lu Y, Hajifathalian K, et al. Worldwide trends in diabetes since 1980: A pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387:1513–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Prince SA, Adamo KB, Hamel ME, Hardt J, Gorber SC, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: A systematic review. Int J Behav Nutr Phys Act. 2008;5:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Dunton GF. Sustaining health-protective behaviors such as physical activity and healthy eating. JAMA. 2018;320:639–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.