ABSTRACT
Background
Responses to dietary calcium (Ca) and supplemented phytase on prececal amino acid digestibility (pcAAD) in broiler chickens vary among studies. The variation may arise from the dietary acid-binding capacity (ABC) that influences the activity of enzymes in the digestive tract and from microbial activity.
Objective
This study aimed to investigate whether the ABC influences phytase effects on pcAAD and whether microbial activity contributes to this.
Methods
Male Ross 308 broiler chickens were provided 1 of 12 diets in 72 pens (15/pen) from day 16 of age until the end of the experiment on days 21 or 22. In a 3 × 2 × 2-factorial arrangement, the ABC was varied by replacing calcium carbonate (CaCO3) with Ca-formate or by adding formic acid to CaCO3-containing diets, and contained 5.6 or 8.2 g Ca/kg and 0 or 1500 phytase units/kg. The ileum content was collected for pcAAD measurement and microbial community composition was used to investigate whether changes in pcAAD are related to the microbiota.
Results
Three-factor ANOVA showed that reducing the ABC increased pcAAD (average 1.1 percentage points) and no significant interaction of the ABC with Ca concentration and phytase supplementation including 3-way interactions. Without phytase, increasing dietary Ca concentration decreased pcAAD (average 3.1 percentage points). Phytase supplementation increased pcAAD (average 2.1 and 5.0 percentage points at low and high Ca concentrations, respectively), to reach the same level for both Ca concentrations. Microbial functional predictions pointed towards an influence of the microbiota in the crop and ileum content on amino acid concentrations, as indicated by different relative abundances of predicted genes related to amino acid biosynthesis, degradation, and metabolism.
Conclusions
Dietary Ca concentrations but not the ABC modulates the effect of supplemented phytase on pcAAD in broiler chickens. The microbiota might contribute to differences in pcAAD by changing the amino acid composition of the digesta. The extent of this effect is still unknown.
Keywords: calcium, phytase, microbial functional predictions, amino acids, broiler chickens
The dietary calcium concentration influenced the response of amino acid digestibility to phytase supplementation. The ABC increased amino acid digestibility but did not interact with Ca and phytase supplementation.
Introduction
Increasing the nutrient utilization of farm animals reduces the nutrient input needed to produce animal-based food. The utilization of amino acids (AA) is of particular interest because feedstuffs rich in protein are a limited resource. Unutilized nitrogen (N) may act detrimentally on the environment after being excreted by animals. The prececal (pc) AA digestibility (pcAAD) is considered an adequate measure for evaluating dietary proteins. Formulating diets based on pcAAD is necessary to achieve high AA utilization in broiler chickens.
Phytases are widely used feed additives in nonruminant nutrition, primarily to support the cleavage of phytate to increase phosphorus (P) utilization. Some studies provide evidence that phytase supplementation can also increase pcAAD (1, 2), whereas others did not (3, 4). Inconsistent results may be related to dietary ingredients (5, 6), phytate concentrations (6), organic acids (7), or calcium (Ca) concentrations (1, 8), and consequences of phytase supplementation on pcAAD still remain unpredictable.
With regard to Ca, some studies described decreasing pc AA or crude protein digestibility when dietary Ca increased in the range of 2.8–31.5 g/kg dry matter in some studies (8–10), whereas other studies described no such effect for the range of 6.2–14.0 g/kg dry matter (1, 11, 12) (Ca concentrations were calculated assuming 88% dry matter in the diets if dry matter was not stated). Hence, the inconsistent Ca supplementation effect on pcAAD must have been caused by factors other than dietary Ca concentration per se.
Decreased pcAAD at higher dietary Ca concentration is usually explained by an increased pH in the digestive tract as this can reduce the efficacy of enzymes, including proteases and phytases (11, 13). Another explanation for decreased pcAAD at high Ca concentrations is seen in the formation of protein-phytate complexes. Binary phytate-protein complexes are primarily formed at pH below 4 in the proventriculus and gizzard whereas ternary Ca-phytate-protein complexes are formed at pH above 7 in the small intestine (14). Fewer phytate-protein complexes are probably formed in the proventriculus and gizzard when phytate is more degraded in anterior sections of the digestive tract, such as the crop.
Studies investigating pcAAD and P utilization usually used limestone as a Ca source. Limestone mainly consists of calcium carbonate (CaCO3), a compound with a high buffer and acid-binding capacity (ABC). Ca salts of organic acids like Ca-formate (CaF) are known to have a lower buffer capacity than CaCO3 (15). Hence, replacing CaCO3 by CaF might compensate for a pH increase in the digestive tract caused by increased dietary CaCO3 concentrations. The solubility of dietary Ca might also influence the intestinal pH. The Ca solubility of CaF was reported to be substantially higher than that of CaCO3 (16). Using CaF instead of CaCO3 might allow for higher Ca absorption in the proximal section of the small intestine and thus reduce the amount of Ca cations available for complex formation in the small intestine. An alternative way to decrease pH in the digestive tract is the addition of organic acids. Supplementation of organic acids, like formic acid, was reported to lower pH particularly in the crop, as reviewed by Kim et al. (17).
The present study aimed to investigate whether the dietary ABC interacts with dietary Ca concentration and supplemented phytase on pcAAD. The dietary ABC was varied by replacing CaCO3 with CaF or by adding formic acid to CaCO3-containing diets. Previous results on microbial functional predictions were used to identify whether changes in microbial functionality are associated with alterations in pcAAD. Reducing the ABC was hypothesized to influence the response in pcAAD to varying Ca concentrations and supplemented phytase.
Methods
The present study was part of a large experiment and companion data on growth, pH, phytate degradation, pc Ca and P digestibility, gut microbial characterization, and predicted microbial functionality were recently published (18). All details of the animal trial are reported there and presented briefly herein.
Animals and management
The experiment was conducted in accordance with German Animal Welfare Legislation following approval of the Regierungspräsidium Tübingen, Germany (approval no. HOH53-18TE). A total of 1110 unsexed Ross 308 broilers were obtained from a commercial hatchery (Brüterei Süd ZN der BWEBrüterei Weser-Ems GmbH & Co. KG) and distributed in 72 floor pens in groups of 15 animals (115 cm × 230 cm). The room temperature was 34°C at placement of the hatchlings and then continuously reduced to 26°C at the end of the experiment on day 22. Lighting was permanent for the first 3 d, and then 18 h of light and 6 h of darkness were provided daily. Experimental diets were provided from day 16; previously, a commercial starter diet (Deutsche Tiernahrung Cremer GmbH & Co. KG) was offered. Wood shavings as litter material were removed from the pens on day 16 and birds were then kept on perforated floors until the end of the experiment to avoid excreta intake. Birds were reallocated among pens on day 16 to achieve a similar group weight in all pens. The animals were inspected at least twice daily. Each dietary treatment was randomly assigned to 6 pens each in a randomized complete block design. Feed and water were available for ad libitum consumption throughout the experiment.
Experimental diets
Twelve experimental diets were prepared in a 3 × 2 × 2 factorial arrangement of treatments (Table 1). One factor, herein designated as “ABC”, was the use of CaCO3, addition of formic acid (Amasil 85, BASF SE; >85% wt/wt formic acid) to CaCO3-containing diets (CaCO3 + FA), and replacement of CaCO3 by CaF. CaCO3 and CaF were admixed to achieve dietary Ca concentrations of 5.6 g Ca/kg dry matter (“low”) or 8.2 g Ca/kg dry matter (“high”). Mass differences between diets were balanced using diatomaceous earth. The resulting 6 diets were supplemented with 1500 phytase units (FTU) phytase/kg (“+”, Natuphos E 5000 G, BASF SE) or left unsupplemented (“–”). Diets were based on corn, soybean meal, rapeseed meal, and sunflower meal and were formulated without a mineral P supplement (Supplementary Table 1). Titanium dioxide was added at a concentration of 5 g/kg as an indigestible marker to determine the digestibility of nutrients based on changes in the nutrient to marker ratios between the experimental diet and the digesta in the digestive tract. Diets were pelleted through a 3 mm die. Analyzed concentrations of P, Ca, phytate, phytase, crude protein, and AA are provided in Supplementary Table 2. Details of ingredient composition and analyzed concentrations of proximate nutrients, gross energy, inositol phosphate isomers, and particle size distribution of the diets as well as particle size distribution of CaCO3 and CaF are provided in the companion article (18). Experimental diets were provided from day 16 until the end of the experiment.
TABLE 1.
Description of 12 dietary treatments in a 3 × 2 × 2 experimental design
| Acid-binding capacity | Ca concentration (g/kg dry matter) | Phytase supplementation (FTU/kg) |
|---|---|---|
| CaCO3 | 5.6 | 0 |
| 1500 | ||
| 8.2 | 0 | |
| 1500 | ||
| CaCO3 + formic acid | 5.6 | 0 |
| 1500 | ||
| 8.2 | 0 | |
| 1500 | ||
| Ca-formate | 5.6 | 0 |
| 1500 | ||
| 8.2 | 0 | |
| 1500 |
See Table 2 for abbreviations.
Experimental procedures
Intake of the experimental diets in each pen was recorded from day 16 until slaughter. Half of the pens of each treatment were slaughtered on days 21 and 22, respectively. Two hours before slaughter, feed was withdrawn for 1 h in order to standardize feed intake prior to sampling and, in consequence, gut fill, thereby also counteracting possible diurnal feed intake patterns. The birds were killed by CO2 exposure after stunning with a gas mixture (35% CO2, 35% N2, and 30% O2). The posterior half of the section between Meckel's diverticulum and 2 cm anterior to the ileo-ceco-colonic junction, herein defined as the ileum, was removed. Digesta from ∼2 cm of this section was carefully stripped out for pH and microbiota analyses. Samples for DNA extraction were immediately frozen at –20°C. For AA analysis, digesta in the remaining part of the ileum was flushed out using ice-cold deionized water. Digesta samples were pooled on a pen basis, and immediately stored at –20°C until being freeze-dried.
Chemical analysis and DNA measurements
The AA concentrations of feed and digesta were analyzed according to Rodehutscord et al. (3) with modifications by Siegert et al. (19). In this assay, methionine (Met) and cysteine (Cys) were determined as methionine sulfone and cysteic acid. The amide residue in the side group of asparagine and glutamine is lost during acid hydrolysis and aspartic acid (Asp) and glutamic acid (Glu) are formed (20). Hence, aspartic acid together with asparagine (Asx) and Glu together with glutamine (Glx) were analyzed. Descriptions of sample preparation, analyses of fractions other than AA, and microbiota analyses and functional predictions are provided in the companion article (18). In brief, a DNA extraction kit (FastDNA™ Spin Kit, MP Biomedicals LLC) was used to extract DNA from crop and ileum digesta samples and DNA was quantified spectrophotometrically with a NanoDrop 2000 device (Thermo Fisher Scientific). The V1–2 region of the 16S rRNA gene was amplified and the first PCR product was used as a template in the second PCR with multiplexing and indexing primers. Pair-end sequencing was done with the 250 bp paired-end sequencing chemistry on an Illumina MiSeq platform. Raw reads were checked for quality, assembled, aligned, and possible chimeras were identified using the mothur pipeline tool. The data included 74,662 ± 3399 sequences per sample. Reads were clustered into operative taxonomic units (OTUs) and closest representatives were identified using seqmatch from the Ribosomal Database Project. Taxonomic assignation followed the defined confidence threshold cut-off value for each taxonomic level of Yarza et al. (21): genus (94.5%), family (86.5%), order (82.0%), class (78.5%), and phylum (75.0%). A species name was given if >97% similarity was observed with the closest representative sequence. All species names and taxonomy levels present in this article follow these rules. Functionality prediction was conducted using Tax4Fun2 for assignations relying on the SILVA database and using the KEGG hierarchy as a gene catalog of sequenced genomes.
Calculations and statistical analysis
The pcAAD was calculated on a pen basis using the following equation:
 |
(1) |
All traits including the functional prediction of the microbiota were statistically analyzed by 3-factor ANOVA using the MIXED procedure of the software package SAS (version 9.4; SAS Institute Inc.). Pens were the experimental unit for all studied traits. The following model was used:
 |
(2) |
where yijkl is the dependent trait and eijklis the residual error. The ABC (i = CaCO3, CaCO3 + FA, or CaF), Ca concentration (j = low or high), and phytase (k = without or with) were fixed effects. The random block effect (l = 1–6) included possible effects of location in the building and sampling on day 21 or 22 because 3 blocks were processed each day. Significant differences between 2 groups were determined using t-tests if P ≤ 0.05. No valid observation was excluded from evaluation. Normal distribution and homogeneity of variance were tested prior to statistical analysis. Pearson product-moment correlations were calculated using the CORR procedure of SAS.
Results
The Ca concentration × phytase interaction was significant (P ≤ 0.003) for pc digestibility of all AA (Table 2). Other interactions were not significant. The pc digestibility of all AA was significantly lower at the high compared with the low Ca concentration among diets without supplemented phytase (P ≤ 0.005), with an average difference of 3.1 percentage points. Phytase supplementation increased pcAAD by an average of 2.1 percentage points at the low Ca concentration and 5.0 percentage points at the high Ca concentration (P ≤ 0.005). Among the phytase-supplemented treatments, pcAAD was not significantly different for all AA (P ≥ 0.52) except for Cys, which was 1.3 percentage points lower in digestibility at the low compared with the high Ca concentration (P = 0.047). The main effect of the ABC was significant for the pc digestibility of all AA (P ≤ 0.029) except Cys (P = 0.24). Adding formic acid to CaCO3 increased pc digestibility of all AA (P ≤ 0.047) except Cys (P = 0.38) by an average of 1.1 percentage points. Replacing CaCO3 with CaF increased pc digestibility of all AA except for Asx, Cys, and histidine (His) (P ≤ 0.037) on average by 1.1 percentage points.
TABLE 2.
Prececal amino acid digestibility (%) in broiler chickens fed differently acidified diets with different calcium concentrations without (–) and with (+) supplementation of 1500 FTU phytase/kg1
| ABC | Ca con-centration | Phytase | Ala | Arg | Asx | Cys | Glx | Gly | His | Ile | Leu | Lys | Met | Phe | Pro | Ser | Thr | Tyr | Val |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CaCO3 | low | – | 80.4 | 87.0 | 77.5 | 68.5 | 85.4 | 73.8 | 79.2 | 81.6 | 81.6 | 82.3 | 87.8 | 82.6 | 77.4 | 76.6 | 73.7 | 80.4 | 79.8 |
| + | 81.7 | 88.4 | 79.3 | 72.7 | 86.9 | 75.5 | 80.3 | 83.7 | 83.4 | 83.7 | 88.8 | 84.7 | 79.4 | 78.4 | 75.0 | 82.1 | 81.6 | ||
| high | – | 76.0 | 84.9 | 74.1 | 66.3 | 82.9 | 70.9 | 75.9 | 78.1 | 78.0 | 79.1 | 84.6 | 79.1 | 74.9 | 73.0 | 69.6 | 76.9 | 76.4 | |
| + | 81.9 | 88.5 | 79.7 | 74.5 | 87.4 | 75.9 | 80.8 | 83.8 | 84.0 | 83.9 | 89.1 | 85.0 | 80.1 | 79.0 | 75.3 | 82.7 | 81.8 | ||
| CaCO3 + formic acid | low | – | 81.4 | 87.6 | 78.0 | 68.7 | 86.0 | 74.5 | 79.9 | 82.4 | 82.6 | 83.0 | 88.7 | 83.5 | 78.4 | 77.6 | 74.5 | 81.3 | 80.8 |
| + | 84.0 | 89.7 | 81.2 | 74.3 | 88.4 | 77.8 | 82.1 | 85.7 | 85.8 | 85.6 | 90.5 | 86.6 | 81.6 | 80.7 | 77.6 | 84.3 | 83.9 | ||
| high | – | 77.6 | 86.1 | 75.2 | 67.5 | 83.6 | 72.0 | 77.4 | 79.0 | 79.1 | 80.4 | 85.4 | 80.1 | 76.1 | 74.2 | 71.2 | 77.8 | 77.4 | |
| + | 83.4 | 89.1 | 80.2 | 74.2 | 87.8 | 76.8 | 81.9 | 85.0 | 85.1 | 85.0 | 89.9 | 85.8 | 80.7 | 80.0 | 76.9 | 83.7 | 83.4 | ||
| Ca-formate | low | – | 81.4 | 87.6 | 78.1 | 68.7 | 86.1 | 75.0 | 79.4 | 83.0 | 82.6 | 83.0 | 88.7 | 83.5 | 78.4 | 77.4 | 74.7 | 81.6 | 81.3 |
| + | 82.9 | 88.8 | 80.0 | 71.5 | 87.5 | 76.2 | 81.4 | 84.5 | 84.5 | 84.5 | 90.0 | 85.3 | 80.3 | 79.7 | 76.4 | 83.0 | 82.5 | ||
| high | – | 78.4 | 85.9 | 75.1 | 67.5 | 84.1 | 72.0 | 77.2 | 80.1 | 80.4 | 80.6 | 86.8 | 81.0 | 76.8 | 74.5 | 71.7 | 78.5 | 78.4 | |
| + | 83.2 | 88.7 | 80.0 | 73.5 | 87.7 | 76.2 | 81.4 | 84.5 | 84.8 | 84.5 | 90.0 | 85.5 | 80.6 | 79.7 | 76.3 | 83.4 | 82.5 | ||
| SEM | 0.88 | 0.40 | 0.59 | 0.91 | 0.48 | 0.63 | 0.70 | 0.69 | 0.77 | 0.63 | 0.77 | 0.67 | 0.70 | 0.71 | 0.84 | 0.75 | 0.73 | ||
| Ca concentration × phytase2 | |||||||||||||||||||
| low | – | 81.0b | 87.4b | 77.8b | 68.7c | 85.8b | 74.4b | 79.5b | 82.3b | 82.3b | 82.8b | 88.4b | 83.2b | 78.1b | 77.2b | 74.3b | 81.1b | 80.6b | |
| + | 82.8a | 89.0a | 80.2a | 72.8b | 87.6a | 76.5a | 81.3a | 84.6a | 84.6a | 84.6a | 89.8a | 85.5a | 80.4a | 79.6a | 76.3a | 83.2a | 82.7a | ||
| high | – | 77.3c | 85.6c | 74.8c | 67.1d | 83.5c | 71.6c | 76.8c | 79.1c | 79.2c | 80.0c | 85.6c | 80.1c | 75.9c | 73.9c | 70.9c | 77.7c | 77.4c | |
| + | 82.8a | 88.8a | 80.0a | 74.0a | 87.6a | 76.3a | 81.4a | 84.4ab | 84.6a | 84.5a | 89.7a | 85.5a | 80.5a | 79.6a | 76.2a | 83.2a | 82.5a | ||
| SEM | 0.68 | 0.27 | 0.40 | 0.64 | 0.32 | 0.44 | 0.52 | 0.49 | 0.58 | 0.48 | 0.59 | 0.48 | 0.51 | 0.50 | 0.63 | 0.55 | 0.53 | ||
| ABC3 | |||||||||||||||||||
| CaCO3 | 80.0B | 87.2B | 77.6B | 70.5 | 85.7B | 74.0B | 79.1B | 81.8A | 81.8B | 82.3B | 87.6B | 82.8B | 77.9B | 76.7B | 73.4B | 80.5B | 79.9B | ||
| CaCO3 + formic acid | 81.6A | 88.1A | 78.7A | 71.2 | 86.5A | 75.2A | 80.3A | 83.0B | 83.1A | 83.5A | 88.6A | 84.0A | 79.2A | 78.1A | 75.0A | 81.8A | 81.3A | ||
| Ca-formate | 81.5A | 87.7A | 78.3AB | 70.3 | 86.4A | 74.8A | 79.8AB | 83.0B | 83.1A | 83.1A | 88.9A | 83.8A | 79.0A | 77.8A | 74.8A | 81.6A | 81.2A | ||
| SEM | 0.65 | 0.25 | 0.37 | 0.60 | 0.29 | 0.42 | 0.50 | 0.46 | 0.55 | 0.46 | 0.56 | 0.45 | 0.48 | 0.47 | 0.59 | 0.52 | 0.50 | ||
| ANOVA | |||||||||||||||||||
| ABC | 0.002 | 0.003 | 0.029 | 0.24 | 0.027 | 0.011 | 0.010 | 0.007 | 0.004 | 0.003 | 0.011 | 0.013 | 0.008 | 0.006 | 0.002 | 0.015 | 0.002 | ||
| Ca concentration | <0.001 | <0.001 | 0.723 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.003 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| Phytase | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| ABC × Ca concentration | 0.60 | 0.93 | 0.82 | 0.64 | 0.60 | 0.83 | 0.95 | 0.75 | 0.43 | 0.84 | 0.53 | 0.56 | 0.45 | 0.66 | 0.88 | 0.72 | 0.83 | ||
| ABC × phytase | 0.54 | 0.47 | 0.63 | 0.20 | 0.49 | 0.24 | 0.91 | 0.12 | 0.29 | 0.49 | 0.60 | 0.29 | 0.41 | 0.72 | 0.44 | 0.38 | 0.11 | ||
| Ca concentration × phytase | <0.001 | <0.001 | 0.003 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.003 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| ABC × Ca concentration × phytase | 0.71 | 0.44 | 0.44 | 0.40 | 0.57 | 0.46 | 0.53 | 0.86 | 0.64 | 0.65 | 0.68 | 0.69 | 0.63 | 0.66 | 0.64 | 0.78 | 0.91 | ||
Values are least square means, n = 6 pens/treatment with 15 birds/pen. Labeled means of the Ca concentration × phytase interaction in a column without a common lowercase superscript letter differ significantly, P ≤ 0.050; labeled means of the ABC effect in a column without a common uppercase superscript letter differ significantly, P ≤ 0.050; ABC, acid-binding capacity; Ala, alanine; Arg, arginine; Asx, aspartic acid/asparagine; Ca, calcium; CaCO3, calcium carbonate; Cys, cysteine; FTU, phytase units; Glx, glutamic acid/glutamine; Gly, glycine; His, histidine; Ile, isoleucine; Leu, leucine; Lys, lysine; Met, methionine; Phe, phenylalanine; Pro, proline; Ser, serine; Thr, threonine; Tyr, tyrosine; Val, valine.
Other 2-way interaction means not presented because not significant (P ≥ 0.11).
Other main effect means not presented because of the significant interaction (P ≤ 0.003) between Ca concentration and phytase.
The correlations between the pcAAD and the relative abundances of OTU1 (Lactobacillus johnsonii) [except for alanine (Ala), His, and Met] and OTU10 (Streptococcus alactolyticus) (except for Cys) in the ileum content were significant (Supplementary Table 3). The relative abundance in the ileum of these OTUs thereby ranged from 23.6–45.6% for OTU1 and 0.2–15.6% for OTU10 between treatments (18). Further correlations between relative abundances of OTU2 (L. gallinarum) and OTU13 (L. reuteri) in the crop content were significant for some AA. The relative abundances of these OTUs in the crop ranged from 11.1 to 28.7% and from 0.6 to 2.4% between treatments, respectively (18).
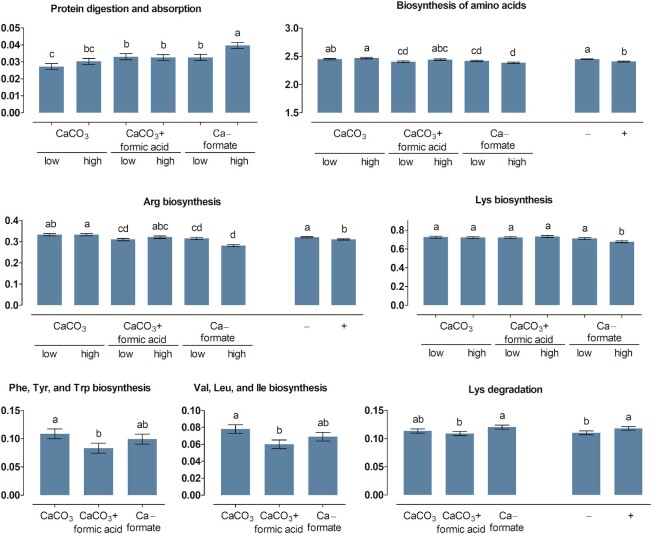
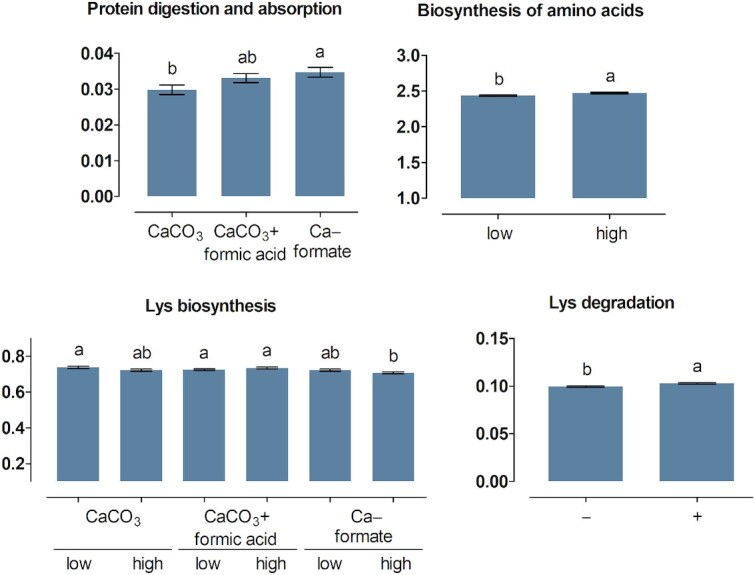
More KEGG pathways related to protein digestion and absorption (pathway no. 04974) and AA biosynthesis, AA degradation, and AA metabolism (pathways no. 00250 to 00400) of the microbiota were significantly influenced by treatments in the crop (Figures 1 and 2) than in the ileum content (Figures 3 and 4). The ABC × Ca concentration interaction was significant for the relative abundance of predicted genes related to protein digestion and absorption in the crop content (P = 0.037). Higher values were determined at the high compared with the low Ca concentration for CaF (P < 0.001), but not for CaCO3 and CaCO3 + FA (P ≥ 0.13). In the ileum content, genes encoding for protein digestion and absorption were more pronounced for CaF than for CaCO3 (P = 0.046), with CaCO3 + FA in-between.
FIGURE 1.
Percent of genes assigned to the metabolic pathways protein digestion and absorption as well as biosynthesis and degradation of amino acids listed in the KEGG database in the crop content of broiler chickens fed with differently acidified diets with low and high calcium concentrations without (–) and with (+) supplementation of 1500 FTU phytase/kg, values are least square means, n = 6 pens/treatment with 15 birds/pen, only significantly influenced pathways are presented (P ≤ 0.05), columns within a statistical comparison not sharing the same letter are significantly different (P ≤ 0.05), details of the statistical evaluations are shown in Supplementary Table 4. See Table 2 for abbreviations.
FIGURE 2.
Percent of genes assigned to metabolic pathways related to amino acid metabolism listed in the KEGG database in the crop content of broiler chickens fed with differently acidified diets with low and high calcium concentrations without (–) and with (+) supplementation of 1500 FTU phytase/kg, values are least square means, n = 6 pens/treatment with 15 birds/pen, only significantly influenced pathways are presented (P ≤ 0.05), columns within a statistical comparison not sharing the same letter are significantly different (P ≤ 0.05), details of the statistical evaluations are shown in Supplementary Table 4. See Table 2 for abbreviations.
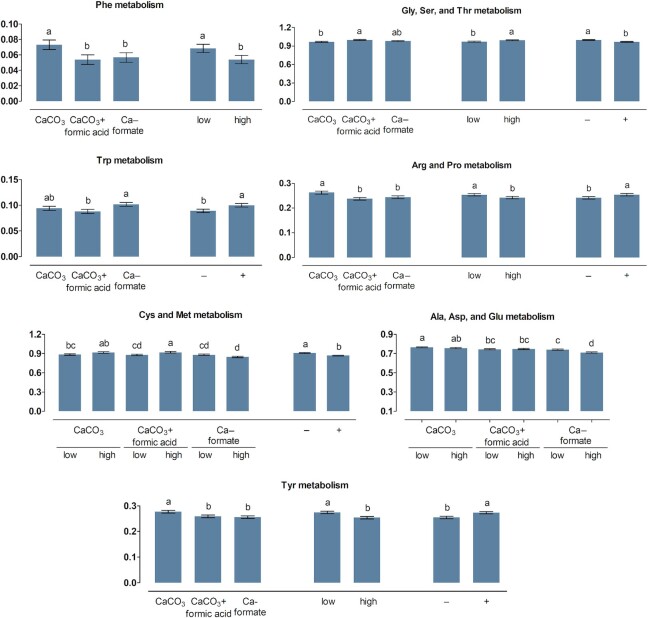
FIGURE 3.
Percent of genes assigned to the metabolic pathways protein digestion and absorption as well as biosynthesis and degradation of amino acids listed in the KEGG database in the ileum content of broiler chickens fed with differently acidified diets with low and high calcium concentrations without (–) and with (+) supplementation of 1500 FTU phytase/kg, values are least square means, n = 6 pens/treatment with 15 birds/pen, only significantly influenced pathways are presented (P ≤ 0.05), columns within a statistical comparison not sharing the same letter are significantly different (P ≤ 0.05), details of the statistical evaluations are shown in Supplementary Table 5. See Table 2 for abbreviations.
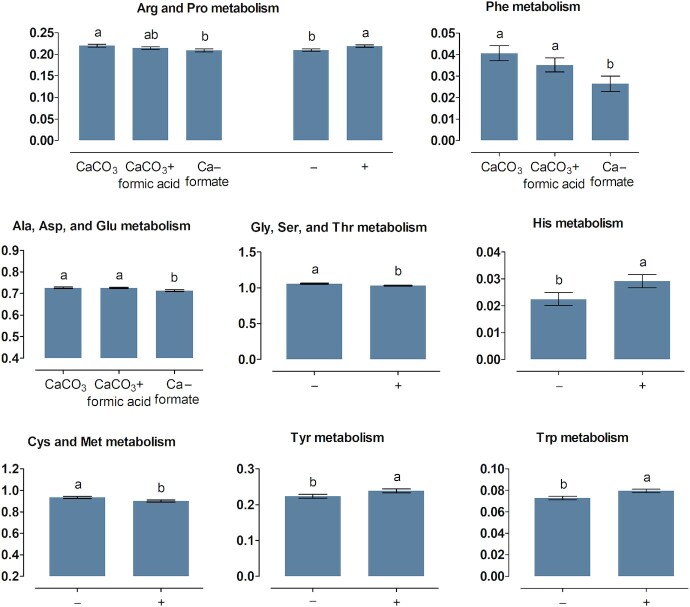
FIGURE 4.
Percent of genes assigned to metabolic pathways related to amino acid metabolism listed in the KEGG database in the ileum content of broiler chickens fed with differently acidified diets with low and high calcium concentrations without (–) and with (+) supplementation of 1500 FTU phytase/kg, values are least square means, n = 6 pens/treatment with 15 birds/pen, only significantly influenced pathways are presented (P ≤ 0.05), columns within a statistical comparison not sharing the same letter are significantly different (P ≤ 0.05), details of the statistical evaluations are shown in Supplementary Table 5. See Table 2 for abbreviations.
The relative abundance of genes related to the biosynthesis of lysine (Lys) and arginine (Arg) in the crop content was lower at the high compared with the low Ca concentration for CaF (P ≤ 0.007), with no difference between Ca concentrations for CaCO3 and CaCO3 + FA (P ≥ 0.16). Biosynthesis of phenylalanine (Phe)/tyrosine (Tyr)/tryptophan (Trp) and valine (Val)/leucine (Leu)/isoleucine (Ile) in the crop content was more pronounced for CaCO3 than for CaCO3 + FA (P ≤ 0.009), with CaF in-between. Arg biosynthesis and Lys degradation in the crop content decreased and increased upon phytase supplementation (P ≤ 0.032), respectively. Degradation of Lys in the crop content was more pronounced for CaF than for CaCO3 + FA (P = 0.011), with CaCO3 in-between.
The ABC × Ca concentration interaction was significant for the metabolism of Ala/Asp/Glu and Cys/Met in the crop content (P ≤ 0.007). Relative abundance of genes ranked CaCO3 > CaCO3 + FA > CaF for Ala/Asp/Glu metabolism and CaCO3 = CaCO3 + FA > CaF for Cys/Met metabolism. The Ala/Asp/Glu metabolism was decreased at the high compared with the low Ca concentration for CaF ( P < 0.001) but not for CaCO3 and CaCO3 + FA (P ≥ 0.41). The Cys/Met metabolism was more pronounced at the high compared with the low Ca concentration for CaCO3 + FA (P = 0.021) but not for CaCO3 and CaF (P ≥ 0.06). The relative abundance of genes encoding metabolism of Arg/proline (Pro), Tyr, and Phe in the crop content ranked CaCO3 > CaCO3 + FA ≅ CaF and the rank of relative abundance of genes encoding glycine (Gly)/serine (Ser)/threonine (Thr) was CaCO3 + FA > CaF > CaCO3. The Gly/Ser/Thr and the Arg/Pro metabolism were reduced at the high compared with the low Ca concentration (P ≤ 0.036). In the ileum content, the abundance of genes encoding Ala/Asp/Glu metabolism and Arg/Pro metabolism ranked CaCO3 ≅ CaCO3 + FA > CaF and CaCO3 > CaCO3 + FA > CaF, respectively. Phytase supplementation decreased the Gly/Ser/Thr metabolism in the crop and ileum content and the Cys/Met metabolism in the ileum content (P ≤ 0.002). The Arg/Pro, His, Tyr, and Trp metabolism pathways were increased upon phytase supplementation in the ileum content (P ≤ 0.020).
Discussion
The objective of this study was to investigate whether the dietary ABC, by replacing CaCO3 with CaF or adding formic acid to CaCO3-containing diets, affects Ca and phytase supplementation effects on pcAAD. Reducing the dietary ABC increased pcAAD but did not interact with Ca concentration and phytase supplementation. Hence, the hypothesis of this study is rejected. The results give evidence that phytase supplementation can compensate for a lowering effect of the high Ca concentration on pcAAD. An interaction between Ca concentrations and phytase supplementation was not determined in other studies (1, 2, 12) [except for 2 out of 15 AA in 1 study (12)]. This implies that unknown influences determined the different outcomes between studies. More research is therefore needed to better understand the conditions when dietary Ca affects pcAAD. For the present study, several mechanisms, including feed intake, microbial activity, and formation of phytate complexes may have contributed to the observed effects.
Influence of feed intake
Feed intake was reduced at the high Ca concentration without phytase (Figure 5A; Supplementary Figure 1), which likely influenced pcAAD because feed intake is one determinant of pcAAD in broiler chickens (22). Basal endogenous AA losses depended on feed intake (23, 24). Assuming that basal endogenous AA losses are a linear function of feed intake, the amount of basal endogenous AA losses at the high Ca concentration without phytase would account for 92% of the amount in the other treatments. The proportions of Asx, Cys, Glx, Ser, and Thr are high in endogenous AA losses (24, 25). Hence, lower feed intake might cause more pronounced differences in pc digestibility of those AA upon phytase supplementation compared with the other AA. However, differences in pc digestibility at the high Ca concentration upon phytase supplementation were about the same for all measured AA except for Cys (Table 2; Figure 6). Hence, influences other than basal endogenous AA losses must have caused the difference in pcAAD upon phytase supplementation at the high Ca concentration. Siegert et al. (22) proposed that feed intake might also influence pcAAD by altering the passage rate of the digesta through the digestive tract. As contractile activity seems to disperse the digesta along the length of the small intestine (26), a lower amount of digesta in the small intestine would facilitate mixing of the digesta and microbes contained therein. Pronounced mixing can increase pcAAD by increasing the encounter of digestive enzymes and the substrate and transport of absorbable AA to the enterocytes. Alternatively, pronounced mixing can decrease pcAAD by increasing epithelial cell abrasion and affect the unstirred water layer of the intestine. Feed intake was also reduced with increasing Ca concentrations in other studies investigating pcAAD (2, 8). A weak negative relation between dietary Ca and pcAAD existed in those studies, suggesting that feed intake affected pcAAD in those studies as it probably did in the present study.
FIGURE 5.
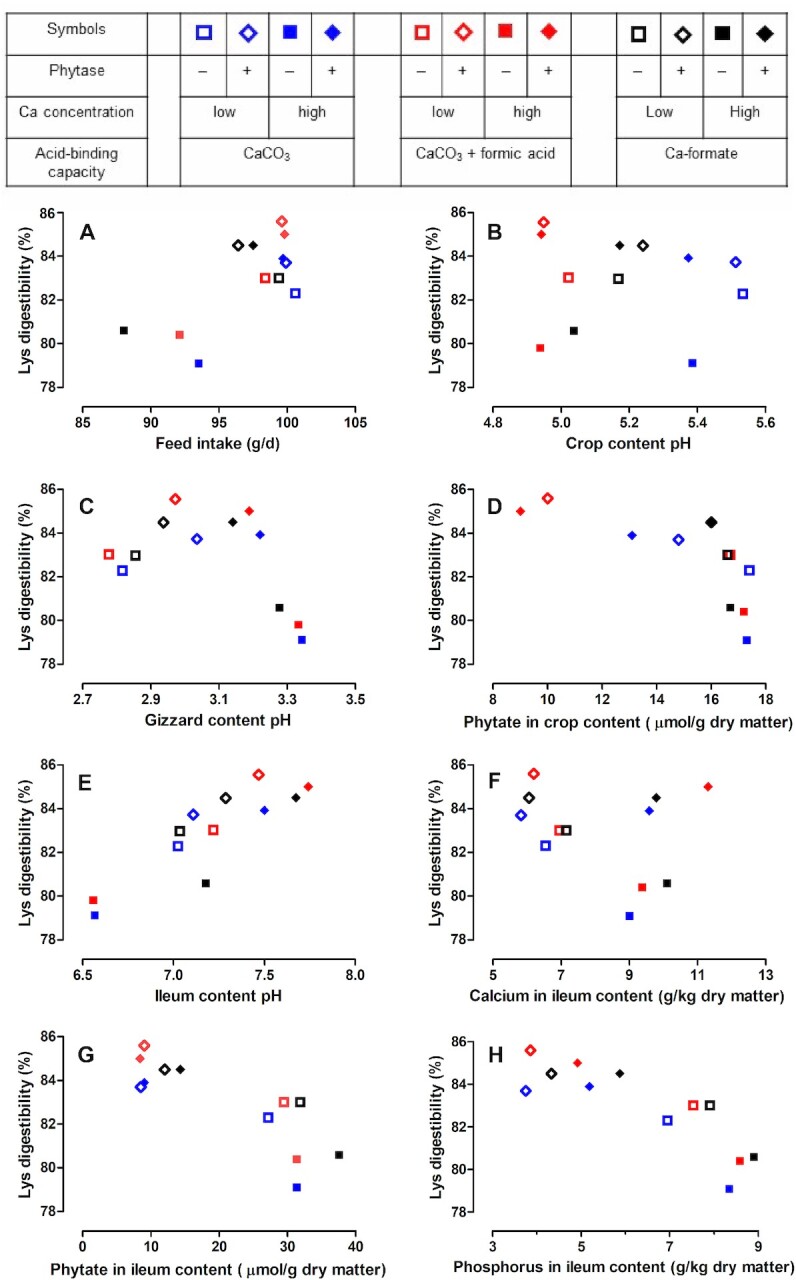
Relation between prececal lysine digestibility and feed intake (A), crop content pH (B), gizzard content pH (C), phytate concentrations in the crop content (D), ileum content pH (E), and calcium (F), phytate (G), and phosphorus (H) concentrations in the ileum content. Symbols represent least square means of prececal lysine digestibility and traits presented in the companion article (18), n = 6 pens/treatment with 15 birds/pen; relations for the prececal digestibility of other amino acids presented in Supplementary Figures 1–8. See Table 2 for abbreviations.
FIGURE 6.
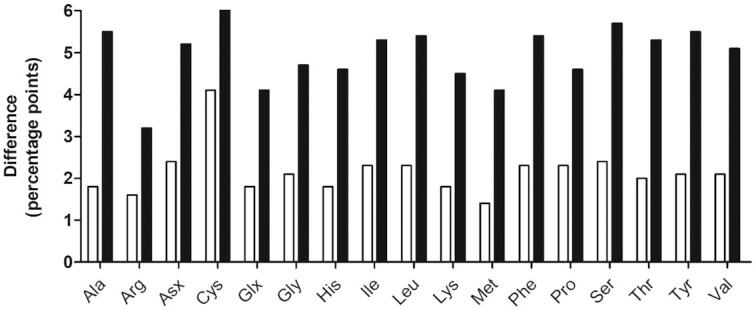
Effect of phytase supplementation on the prececal amino acid digestibility at a low and high Ca concentration (unfilled and filled columns, respectively). All differences were significant (P ≤ 0.050). Values are least square means, n = 6 pens/treatment with 15 birds/pen; see Table 2 for abbreviations.
At the low Ca concentration, the increase in pcAAD upon phytase supplementation was not associated with differences in feed intake and phytase caused a more pronounced increase in pc digestibility of Cys compared with the other AA. This is consistent with results from other studies that also found a more pronounced increase in pc digestibility of Cys than of other AA upon phytase supplementation (1, 5, 27).
Influence of the microbiota
The positive correlations between the pc digestibility of most AA and the relative abundance of OTU1 (L. johnsonii) in the ileum content may indicate that the differences in digestibility were in some part caused by L. johnsonii or the relative abundance of L. johnsonii was a function of different digestibility. Either way, effects may include interactions of L. johnsonii with other microorganisms. The genome sequence of an L. johnsonii strain revealed a higher number of proteases and peptide transporters compared with other Lactobacillus strains, probably because L. johnsonii has a low AA synthesis capability and therefore requires available AA from the environment (28). Thus, the relative abundance of L. johnsonii may have increased because more AA were present in an absorbable form without the action of L. johnsonii proteases. Alternatively, the proteases secreted by L. johnsonii may have enabled L. johnsonii to grow more than other microorganisms, thereby causing the higher relative abundance. If so, the contribution of L. johnsonii to pcAAD may be relevant given the relative abundances in the ileum ranging from about a quarter to half of all measured OTUs. We did not find any literature providing causative explanations for the significantly negative correlations between pcAAD and the relative abundances of OTU10 (S. alactolyticus). Perhaps, the negative correlation is a consequence of the positive correlations with the relative abundance of L. johnsonii. Relative abundances of other OTUs need to decrease when the relative abundance of L. johnsonii increases to the observed extent.
Reducing the dietary ABC increased the abundance of predicted genes encoding for protein digestion and absorption by microbes in the crop and ileum content. A higher AA absorption by microorganisms probably reduced their need for AA biosynthesis. This can explain the decreased abundances of genes encoding for AA biosynthesis when the dietary ABC was reduced.
Changes in the functional prediction of AA metabolism indicate that AA concentrations in the crop and ileum content (including microbial matter) were influenced but the amount of synthesized, degraded, and metabolized AA remains unknown. The AA absorbed by animals can originate from feed protein and de novo synthesized AA by the microbiota, which are absorbed when microbial protein is broken down up to the end of the small intestine (29). It has previously been shown in pigs that absorption of essential AA synthesized de novo by the microbiota can be considerable (30). Such an effect would be supported by reverse peristalsis when microbes from the hindgut are moved up to the anterior small intestine. The microbiota can increase AA digestibility when microbial protein is more digestible than the ingested protein and vice versa. Hence, the extent of the influence of the microbiota composition on pcAAD should be a subject of future investigations.
Influence of phytate complexes
Phytate degradation in the crop probably increased pcAAD by diminishing the formation of binary protein-phytate complexes, which can reduce pcAAD (14). Such binary protein-phytate complexes can partly explain differences in pcAAD between diets without or with supplemented phytase. Binary protein-phytate complexes are mainly formed in the proventriculus and gizzard because formation is maximized under 2 conditions: a pH below 4 and a pH below the isoelectric point of the protein (14). In the present experiment, the pH was between 4.9 and 5.5 in the crop (Figure 5B; Supplementary Figure 2) and between 2.8 and 3.3 in the gizzard (Figure 5C; Supplementary Figure 3). These values are below the isoelectric point of the diet, which was estimated at a pH of 5.5 based on the values for corn, soybean meal, rapeseed meal, and sunflower meal described in the literature (14), assuming no interactions between feedstuffs. Supplementing phytase reduced phytate concentrations in the crop content from 16.6–17.4 to 9.0–16.0 µmol/g dry matter in treatments without and with supplemented phytase, respectively (Figure 5D; Supplementary Figure 4). Hence, less phytate entered the proventriculus in phytase-supplemented treatments, which reduces the probability of the formation of binary protein-phytate complexes. Lower phosphorylated inositol phosphates may have also formed complexes with proteins, but the potential of lower inositol phosphates for complex formation is supposed to be low (31). The different magnitude of the effect of phytase supplementation on pcAAD between Ca concentrations, however, appears unlikely to be caused by binary protein-phytate complexes. Phytate concentrations in the crop were not influenced by Ca concentrations (18). This results in the same amount of phytate entering the proventriculus irrespective of the Ca concentrations and, thus, no different probability of binary protein-phytate complex formation.
It appears unlikely that ternary protein-Ca-phytate complexes reduced pcAAD although the pH in the ileum suggests that ternary protein-Ca-phytate complexes might have been formed in the small intestine. The probability of ternary protein-Ca-phytate complex formation increases with pH in the small intestine (14). However, a positive relation between ileum pH and pcAAD irrespective of phytase was found in the present study (Figure 5E; Supplementary Figure 5). Further, the formation of protein-Ca-phytate complexes would reduce Ca digestibility but a relation between pcAAD and the Ca concentration in the ileum content did not exist (Figure 5F; Supplementary Figure 6). Further, it cannot be ruled out that binary Ca-phytate complexes formed in the small intestine exerted some influence on pcAAD by making the formation of ternary protein-Ca-phytate complexes less likely (13). The presence of such binary Ca-phytate complexes is more likely the higher the pH in the small intestine is. This would be in line with the observations of a positive relation between pH in the small intestine and pcAAD (Figure 5E; Supplementary Figure 5) and higher pcAAD when phytate concentrations in the ileum were lower (Figure 5G; Supplementary Figure 7). However, formation of binary Ca-phytate complexes would have reduced pc Ca digestibility and pc phytate degradation but relations between these traits and ileum pH were not determined (18).
Influence of chaotropic and kosmotropic agents
A less recognized mechanism possibly contributing to pcAAD is the consequence of phosphate and Ca acting as kosmotropic and chaotropic agents, respectively (14). Being a kosmotropic agent, phosphate can reduce protein solubility by stabilizing the hydrogen-bonding network and, hence, inducing stabilization and aggregation of undissolved proteins (32). Selle et al. (14) hypothesized that phosphate exerts kosmotropic effects when bound to the inositol ring of phytate and after being hydrolyzed from phytate. This entails kosmotropic effects of lower inositol phosphate isomers and unabsorbed (hydrolyzed) phosphate and might explain the closer relation between pcAAD and the P concentration in the ileum (Figure 5H; Supplementary Figure 8) than the phytate concentration in the ileum (Figure 5G; Supplementary Figure 7), respectively. As CO32– is a strongly kosmotropic agent (14), replacing CaCO3 with CaF had a chaotropic effect. Chaotropic agents promote protein solubility and protein denaturation (30). This may provide an explanation for the higher pcAAD for CaF than for CaCO3. Depending on how complete the reaction of CaCO3 with formic acid to CaF in the animals is, this may also provide an explanation for the higher pcAAD of CaCO3 + FA compared with CaCO3. Chaotropicity or kosmotropicity can affect the consequences of pH on biological reactions (33). Possibly, relations between pcAAD and the pH of the crop (Figure 5B; Supplementary Figure 2), gizzard (Figure 5C; Supplementary Figure 3), and ileum content (Figure 5E; Supplementary Figure 5) were blurred by different chaotropicity or kosmotropicity. However, none of the traits we measured can provide evidence of whether kosmotropic and chaotropic agents influenced pcAAD to a relevant extent.
In conclusion, the present study showed that phytase supplementation increased pcAAD and compensated a decreasing effect of the high Ca concentration on pcAAD. Reducing the dietary ABC by replacing CaCO3 with CaF or adding formic acid to CaCO3-containing diets increased pcAAD but no interaction of the dietary ABC with Ca concentration and phytase supplementation was determined. Several mechanisms probably contributed to the observed effects on pcAAD. Decreased feed intake caused by high Ca concentrations most likely contributed to differences in pcAAD; further possible influences include the formation of protein-phytate complexes and chaotropic/kosmotropic agents. The predicted microbiota functionality indicates that AA biosynthesis, degradation, and metabolism were different between treatments. The consequence thereof on AA concentrations in the content of the digestive tract and, thus, pcAAD is unknown and warrants further investigation.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—WS, JK, VS, DF, and MR: designed the study; WS, JK, VS, DB-M: conducted the research; DF: provided essential materials; WS, JK, and DB-M: analyzed the data and performed statistical analysis; WS, JK, VS, AC-S, and MR: wrote the manuscript and hold primary responsibility for the final content; and all authors: read and approved the final manuscript.
Notes
Presented in part at the annual meeting of the Society of Nutrition Physiology, Göttingen, Germany, 16–18 March, 2021. WS, JK, DB-M, DF, AC-S, MR. Influence of calcium level and source, acidification, and phytase supplementation on precaecal amino acid digestibility and intestinal microbiota of broiler chickens. Proc Soc Nutr Physiol 2021;27:66.
This study was supported by BASF SE, Germany.
The funding source had no influence on this study, including study design, data analysis, and interpretation.
Author disclosures: The authors report no conflicts of interest.
Supplemental Tables 1–5 and Supplemental Figures 1–8 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/cdn/.
WS and JK contributed equally to this manuscript.
Abbreviations used: AA, amino acid; ABC, acid-binding capacity; Arg, arginine; Asx, aspartic acid together with asparagine; Ca, calcium; CaCO3, calcium carbonate; CaCO3 + FA, calcium carbonate plus formic acid; CaF, calcium formate; Cys, cysteine; FTU, phytase units; Glu, glutamic acid; Glx, glutamic acid together with glutamine; Gly, glycine; His, histidine; Ile, isoleucine; Lys, lysine; Met, methionine; N, nitrogen; OTU; operative taxonomic unit; P, phosphorus; pc, prececal; pcAAD, prececal amino acid digestibility; Phe, phenylalanine; Ser, serine; Thr, threonine; Trp, tryptophan; Tyr, tyrosine; Val, valine.
Contributor Information
Wolfgang Siegert, Email: inst450@uni-hohenheim.de, Institute of Animal Science, University of Hohenheim, Stuttgart, Germany.
Jochen Krieg, Institute of Animal Science, University of Hohenheim, Stuttgart, Germany.
Vera Sommerfeld, Institute of Animal Science, University of Hohenheim, Stuttgart, Germany.
Daniel Borda-Molina, Institute of Animal Science, University of Hohenheim, Stuttgart, Germany.
Dieter Feuerstein, BASF SE, Lampertheim, Germany.
Amélia Camarinha-Silva, Institute of Animal Science, University of Hohenheim, Stuttgart, Germany.
Markus Rodehutscord, Institute of Animal Science, University of Hohenheim, Stuttgart, Germany.
References
- 1.Sommerfeld V, Schollenberger M, Kühn I, Rodehutscord M. Interactive effects of phosphorus, calcium, and phytase supplements on products of phytate degradation in the digestive tract of broiler chickens. Poult Sci. 2018;97:1177–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amerah AM, Plumstead PW, Barnard LP, Kumar A. Effect of calcium level and phytase addition on ileal phytate degradation and amino acid digestibility of broilers fed corn-based diets. Poult Sci. 2014;93:906–15. [DOI] [PubMed] [Google Scholar]
- 3.Rodehutscord M, Kapocius M, Timmler R, Dieckmann A. Linear regression approach to study amino acid digestibility in broiler chickens. Br Poult Sci. 2004;45:85–92. [DOI] [PubMed] [Google Scholar]
- 4.Sebastian S, Touchburn SP, Chavez ER, Lague PC. Apparent digestibility of protein and amino acids in broiler chickens fed a corn-soybean diet supplemented with microbial phytase. Poult Sci. 1997;76:1760–9. [DOI] [PubMed] [Google Scholar]
- 5.Siegert W, Zuber T, Sommerfeld V, Krieg J, Feuerstein D, Kurrle U, Rodehutscord M. Prececal amino acid digestibility and phytate degradation in broiler chickens when using different oilseed meals, phytase and protease supplements in the feed. Poult Sci. 2019;98:5700–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krieg J, Siegert W, Berghaus D, Bock J, Feuerstein D, Rodehutscord M. Phytase supplementation effects on amino acid digestibility depend on the protein source in the diet but are not related to InsP6 degradation in broiler chickens. Poult Sci. 2020;99:3251–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centeno C, Arija I, Viveros A, Brenes A. Effects of citric acid and microbial phytase on amino acid digestibility in broiler chickens. Br Poult Sci. 2007;48:469–79. [DOI] [PubMed] [Google Scholar]
- 8.Wilkinson SJ, Bradbury EJ, Thomson PC, Bedford MR, Cowieson AJ. Nutritional geometry of calcium and phosphorus nutrition in broiler chicks. The effect of different dietary calcium and phosphorus concentrations and ratios on nutrient digestibility. Animal. 2014;8:1080–8. [DOI] [PubMed] [Google Scholar]
- 9.Adedokun SA, Pescatore AJ, Ford MJ, Ao T, Jacob JP. Investigating the effect of dietary calcium levels on ileal endogenous amino acid losses and standardized ileal amino acid digestibility in broilers and laying hens. Poult Sci. 2018;97:131–9. [DOI] [PubMed] [Google Scholar]
- 10.Akter MM, Graham H, Iji PA. Influence of different levels of calcium, non-phytate phosphorus and phytase on apparent metabolizable energy, nutrient utilization, plasma mineral concentration and digestive enzyme activities of broiler chickens. J Appl Anim Res. 2017;46:278–86. [Google Scholar]
- 11.Mutucumarana RK, Ravindran V, Ravindran G, Cowieson AJ. Influence of dietary calcium concentration on the digestion of nutrients along the intestinal tract of broiler chickens. J Poult Sci. 2014;51:392–401. [Google Scholar]
- 12.Walk CL, Bedford MR, McElroy AP. Influence of limestone and phytase on broiler performance, gastrointestinal pH, and apparent ileal nutrient digestibility. Poult Sci. 2012;91:1371–8. [DOI] [PubMed] [Google Scholar]
- 13.Selle PH, Cowieson AJ, Ravindran V. Consequences of calcium interactions with phytate and phytase for poultry and pigs. Livest Sci. 2009;124:126–41. [Google Scholar]
- 14.Selle PH, Cowieson AJ, Cowieson NP, Ravindran V. Protein-phytate interactions in pig and poultry nutrition: a reappraisal. Nutr Res Rev. 2012;25:1–17. [DOI] [PubMed] [Google Scholar]
- 15.Lawlor PG, Lynch PB, Caffrey PJ, O'Reilly JJ, O'Connell MK. Measurements of the acid-binding capacity of ingredients used in pig diets. Ir Vet J. 2005;58:447–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rumble JR, Lide DR, Bruno TJ. CRC Handbook of Chemistry and Physics: A Ready-Reference Book of Chemical and Physical Data. 99th ed.Boca Raton, FL: CRC Press; 2018. [Google Scholar]
- 17.Kim JW, Kim JH, Kil DY. Dietary organic acids for broiler chickens. A review. Rev Colomb Cienc Pecu. 2015;28:109–23. [Google Scholar]
- 18.Krieg J, Borda-Molina D, Siegert W, Sommerfeld V, Chi Y-P, Taheri HR, Feuerstein D, Camarinha-Silva A, Rodehutscord M. Effects of calcium level and source, formic acid, and phytase on phytate degradation and the microbiota in the digestive tract of broiler chickens. Anim Microbiome. 2021;3:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siegert W, Boguhn J, Maurer HP, Weiss J, Zuber T, Möhring J, Rodehutscord M. Effect of nitrogen fertilisation on the amino acid digestibility of different triticale genotypes in caecectomised laying hens. J Sci Food Agric. 2017;97:144–50. [DOI] [PubMed] [Google Scholar]
- 20.Fontaine J. Amino acid analysis in feeds. In: D'Mello JPFeditor. Amino Acids In Animal Nutrition. 2nd ed. Wallingford, Oxon, UK: CABI Pub; 2003. p. 15–40. [Google Scholar]
- 21.Yarza P, Yilmaz P, Pruesse E, Glöckner FO, Ludwig W, Schleifer K-H, Whitman WB, Euzéby J, Amann R, Roselló-Móra R. Uniting the classification of cultured and uncultured bacteria and archaea using 16S rRNA gene sequences. Nat Rev Microbiol. 2014;12:635–45. [DOI] [PubMed] [Google Scholar]
- 22.Siegert W, Ganzer C, Kluth H, Rodehutscord M. Effect of amino acid deficiency on precaecal amino acid digestibility in broiler chickens. J Anim Physiol Anim Nut. 2019;103:723–37. [DOI] [PubMed] [Google Scholar]
- 23.Adedokun SA, Adeola O, Parsons CM, Lilburn MS, Applegate TJ. Factors affecting endogenous amino acid flow in chickens and the need for consistency in methodology. Poult Sci. 2011;90:1737–48. [DOI] [PubMed] [Google Scholar]
- 24.Adeola O, Xue PC, Cowieson AJ, Ajuwon KM. Basal endogenous losses of amino acids in protein nutrition research for swine and poultry. Anim Feed Sci Tech. 2016;221:274–83. [Google Scholar]
- 25.Kluth H, Rodehutscord M. Effect of inclusion of cellulose in the diet on the inevitable endogenous amino acid losses in the ileum of broiler chicken. Poult Sci. 2009;88:1199–205. [DOI] [PubMed] [Google Scholar]
- 26.Lentle RG, de Loubens C. A review of mixing and propulsion of chyme in the small intestine: fresh insights from new methods. J Comp Physiol B. 2015;185:369–87. [DOI] [PubMed] [Google Scholar]
- 27.Borda-Molina D, Zuber T, Siegert W, Camarinha da Silva A, Feuerstein D, Rodehutscord M. Effects of protease and phytase supplements on small intestinal microbiota and amino acid digestibility in broiler chickens. Poult Sci. 2019;98:2906–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pridmore RD, Berger B, Desiere F, Vilanova D, Barretto C, Pittet A-C, Zwahlen M-C, Rouvet M, Altermann E, Barrangou Ret al. The genome sequence of the probiotic intestinal bacterium Lactobacillus johnsoniiNCC 533. Proc Natl Acad Sci USA. 2004;101:2512–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Metges CC. Contribution of microbial amino acids to amino acid homeostasis of the host. J Nutr. 2000;130:1857S–64S. [DOI] [PubMed] [Google Scholar]
- 30.Torrallardona D, Harris CI, Fuller MF. Pigs' gastrointestinal microflora provide them with essential amino acids. J Nutr. 2003;133:1127–31. [DOI] [PubMed] [Google Scholar]
- 31.Yu S, Cowieson A, Gilbert C, Plumstead P, Dalsgaard S. Interactions of phytate and myo-inositol phosphate esters (IP1-5) including IP5 isomers with dietary protein and iron and inhibition of pepsin. J Anim Sci. 2012;90:1824–32. [DOI] [PubMed] [Google Scholar]
- 32.Moelbert S, Normand B, Los Rios P de. Kosmotropes and chaotropes: modelling preferential exclusion, binding and aggregate stability. Biophys Chem. 2004;112:45–57. [DOI] [PubMed] [Google Scholar]
- 33.Timson DJ. The roles and applications of chaotropes and kosmotropes in industrial fermentation processes. World J Microb Bot. 2000;36:89. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.