Abstract
Interest in autonomic arousal in Autism Spectrum Disorder (ASD) is increasing; however, reliability of these measures in ASD is unknown and previously reported associations with social and cognitive abilities are inconsistent. This study assesses heart rate (HR) and heart rate variability (HRV) in preschoolers with ASD or typical development (TD) while they passively watched naturalistic videos. Measurement reliability, group differences, and the relationship with social and cognitive abilities were evaluated. 71 ASD and 66 TD children (2–4 years) provided cardiac data from two sessions. Test-retest intra-class correlations of HR and HRV over a three-week period were moderate to good in both groups. Groups did not differ in mean level of HR or HRV. Intra-individual variability of HR between video segments within a session was higher in the ASD group, but intra-class correlations of this metric were low. Higher HR related to better language skills in TD children, but not after accounting for age and non-verbal ability. Higher HRV related to better expressive and receptive language in ASD children after controlling for age and non-verbal ability. HR/HRV were not related to social or executive functioning skills and did not explain any additional variance in abilities at a 12-month follow-up visit. In summary, variation in language abilities is associated with HR in the TD group and HRV in the ASD group. Whilst preliminary, these results are promising for consideration of autonomic control as a biomarker for individual differences in ASD and may help us understand the mechanisms that contribute to communication skills.
Keywords: Autism Spectrum Disorder, autonomic control, biomarker, cognition, heart rate, language, reliability
Lay Abstract
Cardiac activity, such as heart rate and heart rate variability, is linked to a wide range of psychological functions. This study shows that there is an association between heart rate and heart rate variability and language skills in young children with Autism Spectrum Disorder (ASD). These results may help us understand what underlies individual differences in developmental abilities in young children with ASD.
Background
Autism Spectrum Disorder (ASD) is a neurodevelopmental disorder, characterised by difficulties with social communication and interaction, and the presence of restricted and repetitive behavioural patterns and atypical sensory responses (American Psychiatric Association, 2013). Although ASD is well described at a behavioural level, questions about the role of physiological functioning in the expression of ASD characteristics remain. Identifying physiological biomarkers could help to explain heterogeneity in ASD symptom profile, facilitate early diagnosis, and provide markers for assessing treatment response (McPartland, 2016). This may be particularly important in toddlerhood and early childhood where heterogeneity in developmental trajectory is maximal (Pickles et al., 2014). A biomarker is an objectively measured biological process which is clinically relevant such as related to diagnosis, clinical endpoints, or effect of intervention (Biomarkers Definitions Working Group, 2001; Strimbu & Tavel, 2010). Moreover, reliability of the marker over time is important for accuracy and measuring change. The search for biomarkers in ASD has commonly focussed on structural and functional differences in the brain, eye-tracking, and genetics (Klin et al., 2015; Bedford et al., 2017; Hazlett et al., 2017). Finding biomarkers related to the ASD diagnosis proves to be challenging due to heterogeneity of the disorder, which urges for a shift to identifying biomarkers related to dimensional measures of symptomatology (Loth et al., 2016; McPartland, 2016).
Autonomic activity as a candidate biomarker in toddlers with ASD
The autonomic nervous system (ANS) is a complex system of feedback pathways that link cardiac activity with central nervous system processes (e.g. Chambers & Allen, 2007). The ANS is important for maintaining homeostasis in the body through balanced activation of parasympathetic and sympathetic branches (Berntson et al., 1997). In brief, and as detailed in these papers (Malik et al., 1996; Berntson et al., 1997), the parasympathetic nervous system (PNS) is relatively more active during rest and can be measured through the high-frequency band of heart rate variability (HRV), which corresponds with respiration rate. Increase in parasympathetic activity leads to more vagal influence, suppressing the sinus node of the heart, which decreases heart rate (HR) and increases HRV. In contrast, the sympathetic branch activates to prepare for mobilisation (the ‘flight or fight’ response) and is reflected in increased HR due to the removal of the vagal break on the sinus node. Although other factors play a role in regulating the heart, HR and HRV can give us a peripheral, readily accessible index of the balance of parasympathetic and sympathetic arousal.
Cardiophysiological variables are objective, quantifiable characteristics of biological processes that could provide candidate biomarkers in toddlers with ASD. Cardiac activity and specifically HRV at baseline, when PNS is relatively more active, is considered to be a marker for a wide range of psychological functions and psychopathology and could therefore be equally important to consider in ASD in general (Beauchaine, 2015). It is related to self-regulation and the ability to adjust to the environment. Indeed, higher HRV at baseline has been associated with the change in HRV in response to stimuli (Richards & Casey, 1991; Patriquin, Scarpa, et al., 2013). Psychometric properties such as test-retest reliability are higher during baseline responses compared to task-related responses (Benevides & Lane, 2015). However, assessing traditional baseline physiology, often referred to as resting state, is challenging, especially in young children and individuals with ASD for whom compliance with behavioral requests to sit quietly with little external input is difficult.
In general, basic characteristics of measurement validity for cardiac activity during baseline activities such as calm viewing have not been explored in young children with ASD.
Critically, a candidate biomarker should have good test-retest reliability. Knowledge of measurement reliability during development is important if using measures across multiple time points to understand developmental change or response to treatment. HR and HRV have high test-retest reliability in children in the general population, though HRV reliability diminishes in a heterogeneous sample including children with a medical illness or condition (for meta-analysis see Weiner & McGrath, 2017).
As well, it is important to know the variability of HR and HRV measures within a session. This may provide additional information as to the source of any observed group differences and associations with skills, potentially contributing to heterogeneity in ASD and conflicting findings from previous research. Moreover, individual metrics of variability itself could potentially be a useful biomarker in ASD (Geurts et al., 2008). Intra-individual variability in performance may indicate whether physiological systems can accurately and consistently respond to environmental demands. Higher intra-individual variability has been reported in children with ASD, both behaviourally, in reaction time (6–13 years; Geurts et al., 2008), and biologically, in amplitude and latency of event-related potentials (7–16 years; Milne, 2011) and resting-state brain connectivity (7–30 years; Falahpour et al., 2016). Falahpour et al. (2016) hypothesized that these fluctuations may affect cognitive processing. Fluctuations in physiology over time could reflect difficulties with attention control, which could affect performance and might underlie cognitive and behavioural variability observed in ASD, although this has not yet been investigated.
Finally, we must consider whether cardiovascular measures provide biomarkers of ASD at the diagnostic level, and/or whether they relate to dimensional profiles in particular domains. For young toddlers calm viewing or rest likely requires endogenous control, and indeed PNS activity during rest or calm viewing is related to self-regulation (Thayer et al., 2009; Holzman & Bridgett, 2017) and cognitive functioning (Staton et al., 2009). Measures of PNS activity during calm viewing could, therefore, be an informative marker of individual differences in cognitive functioning, emotion regulation, social functioning, and language. First, differences in HR and HRV could stem from poor control of emotional responses, which may be related to limited executive functioning (EF) and emotion regulation skills (Thayer & Lane, 2000; Thayer et al., 2009; Beauchaine, 2015). Indeed, consistent lower HRV from 5 to 48 months during viewing of a toy or video clip is associated with more parent-reported withdrawal, pervasive developmental problems and oppositional defiant problems (Patriquin et al., 2015). Longitudinally, TD children who did not show an increase in HRV between 24 and 48 months, compared to children who did, had more parent-reported social communication and social cognition problems on the Social Responsiveness Scale at age 4 (Constantino & Gruber, 2005; Patriquin et al., 2014). Second, more vagal control is associated with more spontaneous social engagement and downstream effects of vagal nerve activity can be measured in HR and HRV (Porges, 2003, 2007; Geisler et al., 2013). Third, cardiac measures change during phases of attention (Richards, 2010). More vagal control during calm viewing could reflect stronger control of endogenous attention, and thus be important to domains that require focused attention like language learning (Mahurin-Smith et al., 2017). Indeed, higher fetal PNS activity predict better language skills at 2.5 years (DiPietro et al., 2007). Taken together, ANS indicators could provide biomarkers of functioning in a range of ASD-relevant domains.
An increasing number of studies consider the role of the ANS in ASD (Benevides et al., 2015; Lydon et al., 2016). Toichi and Kamio (2003) suggest that autism is associated with hyper-arousal (higher sympathetic and lower parasympathetic activity) at rest. This is supported by studies reporting lower cardiac parasympathetic activity in school-aged children with ASD during resting conditions (Ming et al., 2005; Vaughan Van Hecke et al., 2009; Bal et al., 2010; Bujnakova et al., 2016). Low parasympathetic activity implies these children either perceive the situation as more stressful, are unable to regulate autonomic activity to rest, or remain in a mobilized state. On a dimensional level, Guy et al. (2014) reported that lower PNS activity during attention to a picture was associated with poorer socialisation skills in 12-year-old children with and without ASD. In 7 to 17-year-old children, lower HRV whilst sitting quietly was also found to be associated with slower emotion recognition in the ASD group, but not in the TD group, which showed faster emotion recognition overall compared to the ASD group (Bal et al., 2010).
In younger children, group differences between those with and without ASD have been less consistently observed. In two studies, 2 to 6-year-old children with ASD and TD did not show differences in HR or HRV at the group level (Sheinkopf et al., 2013; Zantinge et al., 2017). In 4 to 7-year-olds with ASD, HRV was lower in children with no verbal language and more cognitive delays (Patriquin, Lorenzi, et al., 2013). In the same cohort, higher HRV was associated with better receptive language skills (Patriquin, Scarpa, et al., 2013). Conversely, Watson et al. (2010) did not find an association between HRV and language or socialisation skills during non-speech stimulus processing in children with ASD (28–42 month). Together, these studies indicate that autonomic activity in young children may relate to dimensional variation in skills, though evidence is mixed.
Current Study
In the present study, we examine autonomic activity in a longitudinal study of 2 to 4-year-old toddlers with and without ASD. During this period, social communication abilities develop rapidly and children with ASD show large differences in language development (Pickles et al., 2014). Our primary goal is to assess the potential for HR and HRV to provide biomarkers for dimensional variation in behavioural phenotypes in preschool children with ASD. Because a good biomarker should show good reliability, we first assess the test-retest reliability of different measures of autonomic arousal over a 2 to 3-week period in children with ASD and TD during a non-social passive viewing battery. Specifically, we conduct analyses on average HR/HRV and intra-individual variability of HR and HRV across individual segments within an experimental session. Secondly, we look at group differences in both average levels and intra-individual variability of HR and HRV. In line with studies using similar age groups, we do not expect differences in average HR/HRV, but we do expect greater intra-individual variability for both measures in the ASD group. The third aim of the study is to explore the relation between autonomic arousal and individual differences in expressive and receptive language, socialisation skills and EF. Based on previous work, we expect lower HR and higher HRV to be associated with higher abilities in all children. Additionally, we expect lower HR and higher HRV to predict better scores in these same domains at a 12-month follow-up visit.
Methods
Participants
198 children (N = 110 ASD & 88 TD) between 2 and 4 years participated in the first session of the study. For all children, exclusion criteria were evaluated at screening and included serious medical or neurological conditions and motor impairments, vision or hearing loss, history of severe ear infections, birth weight below 1500 grams and a gestational age below 34 weeks.
To qualify for the ASD group, the child participant met criteria for ASD on the revised algorithm of the Autism Diagnostic Observation Schedule (ADOS; Gotham et al., 2007) and DSM-IV criteria (American Psychiatric Association, 2000) based on all available information (clinical records, parent report, and child observation). In addition, to exclude children with a more global developmental delay or neurological complications, children in the ASD group had scores >70 on the Vineland Adaptive Behavior Scale-II (VABS-II; Sparrow et al., 2005) motor standard score. Of note, 96% of children with ASD received a first-time diagnosis within the context of the study.
Children in the TD group were included if they additionally had no history of parent or pediatrician concerns regarding the child’s development, VABS-II Social, Communication, and Motor domain standard scores ≥ 80, and did not have a 1st, 2nd or 3rd degree relative with ASD.
Final Sample
Of the 198 children enrolled, 44 children (24 ASD & 20 TD) withdrew or were excluded after the first session based on additional medical information that might impact heart rate measurement (e.g. medication taken during pregnancy with potential impact on cardiovascular development) or inconsistent or missing clinical information. An additional 17 children were excluded because of no physiology data or issues with the data signal (15 ASD & 2 TD). Characteristics of the included vs. excluded children in the ASD group did not differ (Supplementary Material S1).
The final sample included 71 children with ASD and 66 TD children (Table 1). There were no gender differences; however, the ASD group was younger than the TD group, scored higher on the ADOS, were reported to have more difficulties with EF, and scored lower on all other standardized measures. All analyses were repeated including age and non-verbal ability as independent variables, because of significant differences between the two groups.
Table 1.
Descriptives of the TD and ASD groups
| TD (N = 66) | ASD (N = 71) | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Mean | SD | Range | Mean | SD | Range | t, χ2 | |
|
| |||||||
| Age (months) [range] † | 42.2 | 8.95 | 27–56 | 38.3 | 9.27 | 24–59 | t(135) = 2.56, p = .012 |
| Gender (Male : Female) | 55 : 11 | 64 : 7 | χ2(1) = 1.39, p = .239 | ||||
| Ethnicity (White : Other) | 55 : 11 | 40 : 31 | χ2(1) = 11.73, p = .001 | ||||
| ADOS total score | 3.2a | 1.80 | 0–7 | 16.4 | 4.60 | 7–28 | t(93.11) = −22.34, p < .001 |
| Mullen Scales of Early Learning | |||||||
| Visual Reception (t-score) | 58.8 | 11.53 | 34–80 | 37.4b | 15.62 | 20–68 | t(126.81) = 9.13, p < .001 |
| Preschool Language Scales | |||||||
| Auditory Comprehension (standard score) | 119.1c | 11.76 | 81–145 | 75.7 | 25.41 | 50–139 | t(100.56) = 12.93, p < .001 |
| Expressive Communication (standard score) | 119.3d | 13.40 | 93–145 | 80.0e | 19.73 | 50–131 | t(113.19) = 13.14, p <.001 |
| Vineland Adaptive Behaviour - II | |||||||
| Receptive Language (v-score) | 15.7 | 1.96 | 12–21 | 10.5 | 2.66 | 3–16 | t(128.48) = 13.26, p < .001 |
| Expressive Language (v-score) | 16.1 | 1.56 | 13–20 | 11.1 | 2.73 | 6–18 | t(112.79) = 13.21, p < .001 |
| Socialisation (standard score) | 96.7 | 7.60 | 80–112 | 74.7 | 10.29 | 50–106 | t(128.61) = 14.30, p < .001 |
| Behavior Rating Inventory of Executive Function | |||||||
| Global Executive Composite (t-score) | 45.8 | 10.39 | 31–74 | 64.8f | 16.10 | 32–97 | t(116.91) = −8.18, p < .001 |
| Number of available segments | 20.4 | 5.38 | 4–24 | 18.6 | 5.88 | 5–24 | t(135) = 1.93, p = .056 |
Age at baseline session 2
N = 63
N = 70
N = 65
N = 61
N = 65
N = 69
Protocol
This study was approved by the local Institutional Review Board. Participants were recruited from autism specialty clinics, birth-to-three centers, local pediatric clinics, affiliated hospitals, and local research registries. A guardian/parent of a participating child provided informed consent.
At entrance to the study, children and their parents were invited to the lab for three visits (Table 2): a diagnostic/cognitive visit (session 1) and two visits for cardiac measures and other experimental paradigms (sessions 2 & 3). Children were followed up during another lab visit 6 and 12-months after the first three sessions. Only the 12-month follow-up was considered in this analysis and data at this time-point was available for 57 children in each group.
Table 2.
Visit information
| Time point | Days since last visit (Mean) | ADOS | MSEL | PLS | VABS-II | BRIEF-P | HR & HRV |
|---|---|---|---|---|---|---|---|
| Baseline Session 1 | x | x | x | x | x | ||
| Baseline Session 2 | 13 | x | |||||
| Baseline Session 3 | 19 | x | |||||
| 12-month follow-up | 404 | x | x | x |
Note: This table only includes the protocol relevant to the analyses discussed in this article.
ADOS: Autism Diagnostic Observation Scale; MSEL: Mullen Scales of Early Learning; PLS: Preschool Language Scale (Session 1: version 4, 12-month follow up: version 5); VABS-II: Vineland Adaptive Behaviour Scale – II; BRIEF-P: Behavior Rating Intervention of Exectutive Function Preschool; HR: Heart rate; HRV: Heart Rate Variability
At the diagnostic/cognitive visit, all children received: (A) the ADOS (Lord et al., 2000) as a measure of autism symptoms; (B) the visual reception (VR) domain of the Mullen Scales of Early Learning (Mullen, 1995) as an index of non-verbal developmental level; and (C) the Preschool Language Scale (PLS-4; Zimmerman et al., 2002) as a measure of auditory comprehension and expressive verbal communication.
Parent report of additional child characteristics was available via the VABS-II (Sparrow et al., 2005) to assess adaptive communication and socialisation skills and Global Executive Composite of the Preschool version of the Behaviour Rating Inventory of Executive Functions (BRIEF-P; Gioia et al., 2003) to assess difficulties with EF. The PLS-4, BRIEF-P and VABS-II were completed at the initial time point, and the PLS-5, BRIEF-P, and VABS-II were completed at the six and twelve-month follow up. The BRIEF-P was provided in a paper version and completed in person or was mailed together with a return envelope. The VABS-II was completed as an interview over the phone.
For reasons of parsimony, we created a composite score for expressive and receptive language, based on the in-person PLS-4 and the parent-report instrument VABS-II. Receptive language was computed as the mean of the z-scores for the auditory comprehension standard score (PLS-4) and receptive language v-score (VABS-II). Expressive language was computed as the mean of the z-scores for the expressive communication standard score (PLS-4) and the expressive language v-score (VABS-II). For the children who had no data available on the PLS (1 child for receptive, 11 children for expressive language) we used the score of the VABS-II only. The same was done for the receptive and expressive language scales at the twelve-month follow up. All correlations between the receptive subscales and the expressive subscales of the PLS and VABS-II were above r =.63
PNS Protocol
During both sessions 2 and 3, children completed a computer-delivered attention battery at the onset of the session. The battery, approximately 20 minutes in length, included nine blocks alternating calm viewing and active attention tasks. The current study only includes the calm viewing blocks, which were fixed in order (block 1, 3, 6, and 9). The two physiology sessions (session 2 and 3) were 2 to 6 weeks apart (M = 19.2 days, range 10–43 days). Parents reported on the sleep times of the night before each session, current flu symptoms and recent vaccines. The experimenters measured the child’s body temperature, height and weight. As detailed in Supplementary Table S2, there was no relation to cardiophysiological variables. These variables are not further considered.
Calm Viewing Stimuli
Because traditional resting state (sitting calmly with no external stimulation) is difficult with young children, we used a “calm viewing” protocol. The children watched 4 × 90-second video of wildlife footage of baby animals accompanied by classical music. Nature clips are more often used as a resting state measure in studies with young participants (Patriquin, Lorenzi, et al., 2013).
Autonomic measures
HR data were collected using Biolab (Version 3.0.4, MindWare Technologies LTD), sampled at 500 Hz and with a 0.5 Hz low pass and 40 Hz high pass filter. The electrodes were placed in a lead-II position on the chest or the back if the child did not tolerate them on the chest. Digital markers indicating the beginning and end of each video were sent via E-Prime 2.0 (Psychology Software Tools, Inc). Identification of the R-peak in the electrocardiogram was done offline using the automatic peak detection in HRV 3.0.12 (MindWare Technologies LTD). All peaks were visually inspected for missed and miss-identified peaks by one of the authors [TB]. Data was segmented using the digital markers in MATLAB R2014a (The MathWorks, Inc) and missing peaks or ectopic beats were corrected by dividing the interbeat interval (IBI), up to a maximum of three consecutive missing peaks. 30-second segments with over ten percent or over three consecutive missing peaks were excluded from further analysis. The data was analysed in 30-second segments in Kubios (version 2.2; interpolation rate: 4 Hz, Fast Fourier Transformation window width: 128 samples, window overlap: 50%). The HRV frequency band was set to 0.24 to 1.04 to fit the respiration rate of the 2 to 4-year age range of the children included in the study (Porges et al., 1996; Skowron et al., 2014). All data included in the study had at least two out of three 30-second segments per calm viewing block and children had data on at least two blocks at session 2 and/or 3. 14 children in the TD group and 18 in the ASD group only had data from one session available due to no data or overall bad data signal. Of the remaining segments, more were excluded in the ASD group (181, 12.2%; 50 missing segments, 131 with missing peaks) compared to the TD group (70, 4.9%; 13 missing segments, 54 with missing peaks; t(105.46) = 3.32, p = .001). The number of available 30-second segments per child was higher in the TD group; this difference approached significance (Table 1).
Statistical Analysis
Analyses were run in Stata 14.2 (StataCorp, 2015). First, the HRV segments were log-transformed. Mean HR and HRV were computed by taking the average of all available segments per session. Second, the variability between segments within an individual was calculated by taking the average of the squared difference from the mean per 30-second segment for each participant. Intra-individual variability of HR and HRV were computed for each session separately. Third, to measure test-retest reliability, we calculated the intra-class correlation (ICC) of HR, HRV, intra-individual variability of HR (HRIIV) and of HRV (HRVIIV) between the two physiology sessions. Fourth, we used a t-test to look at group differences in mean HR and HRV, taking the average of all available segments over both sessions. Visual inspection and test for equality of variance were used to compare the distribution in both groups. Fifth, to look at the relationship between autonomic measures and cognitive and social abilities, we ran separate correlations per group between Receptive language, Expressive language, Socialisation or EF and HR/HRV. Sixth, to look at the relation between autonomic arousal and developmental abilities one year after the initial assessment, we ran four separate hierarchical regression analyses per group with Receptive language, Expressive language, Socialisation and EF at the 12-month follow-up as the dependent variables. In step one, we included the session 1 score for Receptive/Expressive language, Socialisation and EF as the dependent variable, respectively. In the second step, we added the HR/HRV variables.
Results
Test-retest reliability
Results per group for HR, HRV, HRIIV and HRVIIV are given in Table 3. 53 children in the ASD and 52 children in the TD group had data from both sessions available. The ICC for HR and HRV was moderate to good (Cicchetti & Sparrow, 1981) and the confidence intervals between the two groups overlapped. ICC for HRIIV and HRVIIV values were poor and all ICC comparisons for HRIIV and HRVIIV were significantly lower than HR/HRV.
Table 3.
Test-retest of autonomic measures between Session 2 and 3
| TD N = 52 |
ASD N = 53 |
|||
|---|---|---|---|---|
| Variable | ICC | 95% CI | ICC | 95% CI |
| HRmean | .72 | .57–.83 | .59 | .38–.74 |
| HRVmean | .60 | .39–.75 | .68 | .51–80 |
| HRIIV | .29 | .01–.52 | .29 | .03–.52 |
| HRVIIV | .33 | .06–.55 | .03 | −.24–.29 |
Note: based on singles measures, absolute agreement intra-class correlation (ICC), IIV = intra-individual variability
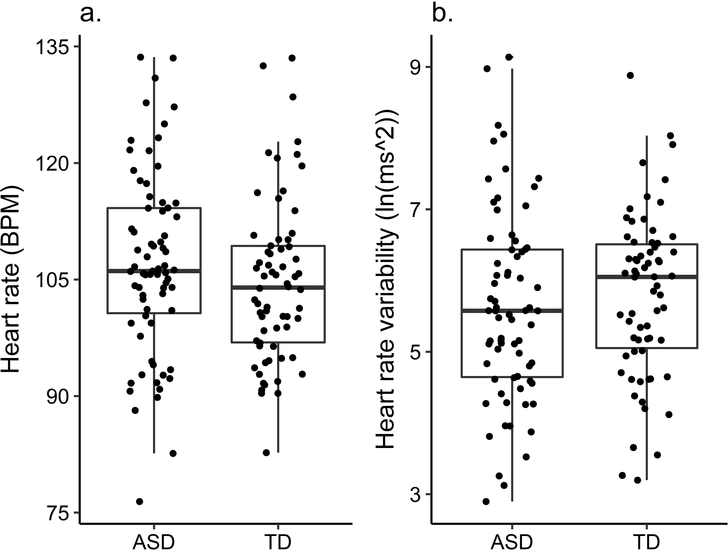
Group differences in mean HR and HRV
As seen in Figure 1, the ASD and TD group did not differ in their average HR (t(135) = −1.25, p = .214, d = −.21; ASD: M = 107.04, SD = 11.85, TD: M = 104.64, SD = 10.50) or HRV (t(135) = 0.80, p =.427, d = .14; ASD: M = 5.62, SD = 1.34, TD: M = 5.80, SD = 1.15). Visual inspection and the equality of variance test did not show any differences in the distribution of HR or HRV between the two groups. Including only children with all 24 segments (TD: 34; ASD: 24) did not change the results. Re-analysing the data including age, number of segments and VR as an independent variable in a regression did not change the results; only age was a significant predictor, older children had lower HR (β = −.519, p < .001) and higher HRV (β = .409, p < .001).
Figure 1.
Group differences in heart rate and heart rate variability
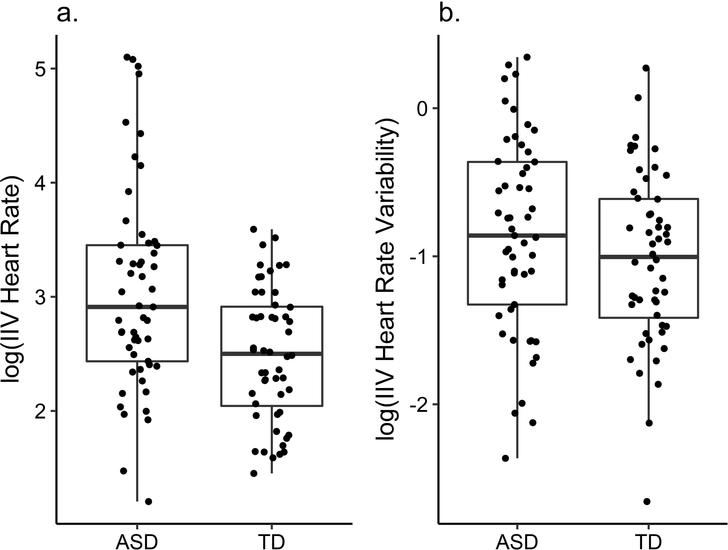
Intra-individual variability
We averaged intra-individual variability of the two sessions. HRIIV and HRVIIV scores were log-transformed because of skewness. The ASD group showed higher HRIIV (t(89.16) = 3.81, p < .001, d = −.74) than the TD group (ASD: M = 3.05, SD = .90; TD: M = 2.50, SD = .58; Figure 2). There was no difference of HRVIIV between groups (t(103) = 1.34, p = .184, d = −.26; ASD: M = −.86, SD = .66, TD: M = −1.02, SD = .58). Re-analysing the data as a regression including age, VR and number of segments as an independent variable did not change the results. Variability decreased with age (HRIIV: β = −.22, p = .021, HRVIIV: β = −.21, p = .038).
Figure 2.
Group differences in intra-individual variability (IIV) of heart rate and heart rate variability
Relation to language, social and executive abilities
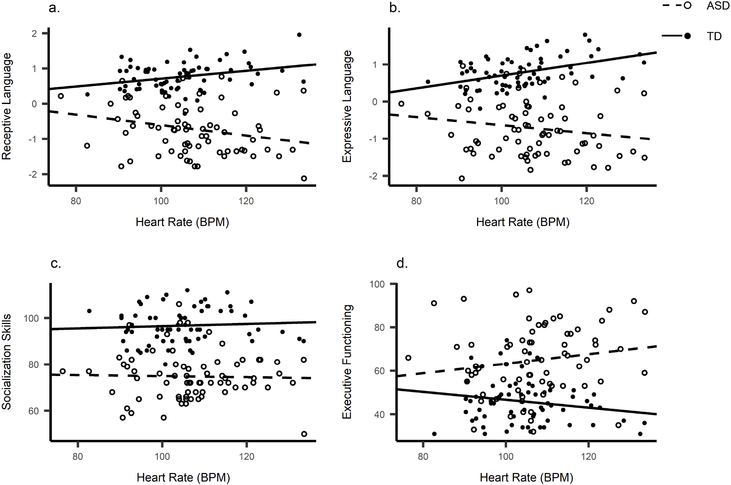
Heart Rate
Pearson correlations are reported in Table 4, Figure 3. HR correlated positively with Expressive and Receptive language in the TD group and negatively with Receptive language in the ASD group. The correlations did not remain significant after controlling for age and VR.
Table 4.
Pearson correlation (up right) and partial correlation† (bottom left) coefficients for TD and ASD group between cardiac measures and social and cognitive abilities
| TD | ASD | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| 1. | 2. | 3. | 4. | 5. | 6. | 1. | 2. | 3. | 4. | 5. | 6. | |
|
| ||||||||||||
| 1. Heart Rate | −.855*** | .432*** | .294* | .065 | −.184 | −.839*** | −.169 | −.242* | −.027 | .164 | ||
| 2. Heart Rate Variability | −.843*** | −.326** | −.331** | −.038 | .121 | −.788*** | .233 | .270* | .073 | −.147 | ||
| 3. Expressive Language | .242 | −.134 | .613*** | .155 | −.237 | −.163 | .301* | .832*** | .463*** | −.102 | ||
| 4. Receptive Language | .202 | −.235 | .531*** | .098 | −.314* | −.237 | .323** | .652*** | .461*** | −.330** | ||
| 5. Socialization Skills | −.180 | .101 | .016 | .059 | −.357** | −.071 | .128 | .391** | .409*** | −.365* | ||
| 6. Executive Functioning | −.029 | .030 | −.164 | −.318* | −250* | .174 | −.161 | .115 | −.225 | −.320** | ||
p < .05
p < .01
p < .001
partial correlations are controlled for age at session 2 and visual reception score
Figure 3.
Scatterplots between heart rate and (a) Receptive language, (b) Expressive language, (c) Socialisation and (d) Executive Functioning.
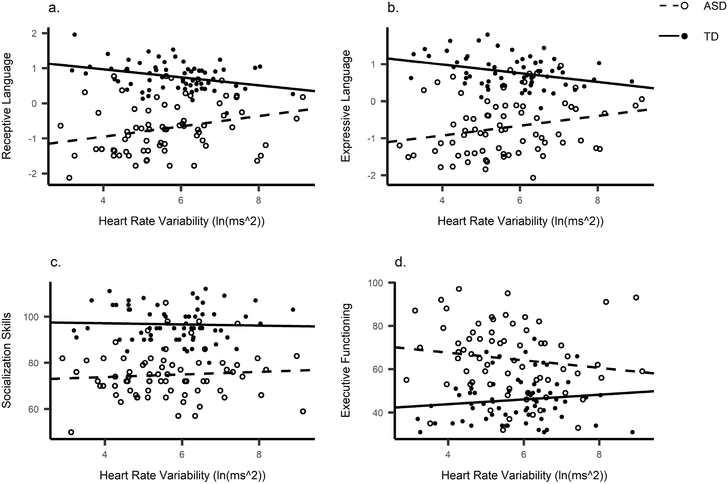
Heart Rate Variability
We performed the same correlations for HRV (Table 4, Figure 4). HRV and Receptive language correlated negatively in the TD group and positively in the ASD group. After controlling for age and VR, Receptive language remained significantly correlated in the ASD group (r = .323, p = .007). Expressive language also became significantly correlated to HRV in the ASD group (r = .301, p = .013). When adjusting α for four multiple comparisons per group (α = .0125), the correlation between Receptive language and HRV in ASD remained significant.
Figure 4.
Scatterplots between heart rate variability and (a) Receptive language, (b) Expressive language, (c) Socialisation and (d) Executive Functioning.
To further investigate the finding of opposite associations in both groups, we split the ASD group into a group with average and below average non-verbal skills (Supplementary Material S3). The ASD lower non-verbal group was younger compared to the ASD average non-verbal group. Receptive language remained significantly correlated with HR and HRV in the ASD lower non-verbal group, but not the ASD average non-verbal group. EF problems were also positively correlated with HR in the ASD lower non-verbal group.
Because of the observed group difference in intra-individual variability, we were interested to see if physiological intra-individual variability relates to Expressive language, Receptive language, Socialisation and EF. We repeated the correlations using the average HRIIV and HRVIIV of the two sessions. HRIIV related to Expressive language in the TD group (r = .28, p = .046), but not when corrected for multiple comparisons.
Relation to symptoms one year later
We ran four hierarchical regressions (Expressive and Receptive language, Socialization, EF) for each group. For most analyses, ability at baseline was a significant predictor of ability at the twelve-month follow up (Supplementary Tables S4 & S5). HR and HRV did not explain any additional variance in Language, Socialisation skills or EF abilities one year later.
Discussion
The purpose of this study was to look at the feasibility of physiological measures as a biomarker for categorical and dimensional variation in behavioural phenotypes in young children with and without ASD. Children passively watched naturalistic videos and cardiac data was collected during this calm viewing period. First, HR and HRV showed moderate reliability over a two to three-week interval in both groups. The reliability of within-session intra-individual variability in HR and HRV over this same interval was very low. Second, as expected and in-line with previous findings, we did not find group differences in HR or HRV between preschool-aged children with and without ASD, regardless of significant differences between the groups in cognitive ability. However, HRIIV was higher in the ASD than the TD group. Next, we looked at the association with behavioural phenotypes. After controlling for age and non-verbal skills, higher HRV during calm viewing related to better language skills in the ASD group. HR and HRV related only to concurrent abilities and did not explain additional variability in abilities one year later.
Biomarker Potential
The test-retest reliability of average HR and HRV during calm viewing in preschool-aged children suggests these measures are reliable across a two to three-week period-- the ICC ranged from fair to good overall. The overlapping confidence intervals of ICC indicates that the reliability of both measures does not differ between the two groups. These findings are comparable to test-retest reliability of HR/HRV in preschoolers (Doussard‐Roosevelt et al., 2003), brain measures in children (Somandepalli et al., 2015) and electrodermal activity in ASD (Schupak et al., 2016). The reliability of HR/HRV suggests that there are trait-like individual differences in regulation of arousal state in children with ASD and maybe promising for the use of cardiac activity as a biomarker for individual difference in preschoolers.
Our results suggest that cardiac activity during calm viewing is not suitable as a diagnostic biomarker of ASD. There were no group differences in either HR or HRV between ASD and TD. Additionally, groups show comparable within-group variation, highlighting the similarities between the ASD and TD group in autonomic arousal. The results remained the same when only including children who had data from all 24 segments. These findings are consistent with existing research in children with ASD in a similar age range (Sheinkopf et al., 2013; Zantinge et al., 2017) and provide further evidence that autonomic arousal during calm viewing is not a sensitive marker of ASD in young children. This implies that the ANS system functions typically during passive viewing of non-social stimuli, which is beneficial for future evaluation of possible atypical responses to social stimuli or cognitive tasks in the ASD population. Higher arousal at rest in older participants with ASD has been reported (Vaughan Van Hecke et al., 2009; Bal et al., 2010; Bujnakova et al., 2016), which might be a consequence of experience and life events.
To assess variability of the cardiac measures within a participant, we compared groups on intra-individual variability, i.e. the variation between the 30-second segments. The ASD group showed higher intra-individual variability in HR compared to the TD group. As HR is related to attention (Richards & Cronise, 2000), fluctuations in autonomic activity could impact information processing and may underly the variation in response observed in this group. There was, however, no relationship between intra-individual variability and (measured) developmental abilities. Also, the test-retest reliability of intra-individual variability was very low, making autonomic variability itself during calm viewing unsuitable as a biomarker at this age.
Relation to Ability
The next aim of the study was to relate physiological activity with developmental abilities. As expected, the ASD group scored significantly lower on Receptive and Expressive language, Socialisation and EF abilities compared to the TD group. After controlling for age and non-verbal abilities, the ASD group followed the hypothesized pattern of higher HRV relating to better Expressive and Receptive language skills. This is in line with the Polyvagal theory, suggesting that higher PNS activity is important for social engagement and language development (Porges, 2007). Of note, Klusek et al. (2013) found that in 4 to 15-year old children, pragmatic, but not expressive or receptive language, related to resting measures of HR and HRV. However, early trajectories of expressive and receptive language development are heterogeneous in ASD and stabilise after the age of 5 (Pickles et al., 2014). Therefore, autonomic arousal might play a larger role in language development in our sample, compared to older children, for whom language is more dependent on previously developed communication skills. Our results are in line with Patriquin, Scarpa, et al. (2013), who found a relation within the ASD group between HRV and receptive and expressive language delay (but see Watson et al., 2010).
Our findings suggest that higher parasympathetic activity during calm viewing is associated with better language development within preschool-aged children with ASD, making HR and HRV a possible target for or proxy outcome measure of intervention. It may be that calm viewing requires relatively high levels of endogenous attentional control, an important skill in many settings, including in education and learning. It will be important to vary the content of evoking stimuli, including integration with eye tracking to quantify visual attention, with the goal of developing a fuller understanding of the mechanisms underlying the association between autonomic control and language development.
The hypothesized relationship was not found between autonomic arousal and socialisation and EF skills, contradicting evidence from studies including older samples (Guy et al., 2014; Neuhaus et al., 2014). This suggests that this relationship is not present at preschool age during a passive, non-social situation. Both socialisation and EF in this study were based on parent report only, whereas autonomic measures and the combined language scores (in part) reflect behaviour during the sessions. Of note, the Social Affect calibrated severity score of the ADOS, also collected during the same session as the language measures, did not relate to HR or HRV (see Supplementary Material S6). As previously suggested, examining cardiac response during a social interaction may be more informative (Sheinkopf et al., 2013; Watson et al., 2010).
The association between HR and language was in the opposite direction for the TD group-- higher HR was related to better language. Although the association became non-significant after controlling for age and non-verbal abilities, the direction contradicted the expectation. According to the Yerkes-Dodson law (Yerkes & Dodson, 1908), optimal arousal is related to task difficulty, following an inverted U-shape, with lower arousal more beneficial for more difficult tasks. The current study does not include a task as described in the Yerkes-Dodson law, but rather a calm viewing paradigm as a measure of resting state. However, children had to regulate their attention to the screen and the stimuli used could be perceived as more or less complex depending on the child’s developmental abilities. Although highly speculative, this may help explain why higher arousal relates to better language in the TD children. Children with better cognitive abilities might not need to allocate as much effort and attention to processing the stimuli and therefore show higher HR.
To provide an indirect test of the role of ability, we ran an additional analysis. We split the ASD group based on their non-verbal developmental level to probe the relationship within children with ASD and lower cognitive ability, which would be consistent with a U-shaped curve hypothesis. This was indeed the case-- the association with language remained significant only in the ASD group with lower non-verbal scores, not in the ASD group with average non-verbal skills. Although age differences in these two groups limit our interpretation of this finding, additional consideration of developmental abilities (and interaction with age) when looking at associations with PNS activity will be critical moving forward.
Identifying a true ‘resting state’ is challenging in young children, as they cannot be explicitly asked to ‘rest’ in the same way as adults. Thus, resting state data is typically collected with young children during a pre-determined putatively relaxing video with the potential for individual variability in engagement and interest (Valkenburg & Vroone, 2004). We also can not rule out the fact that some children were paying attention to the video because they understood from the context that they were ‘supposed to’, increasing the influence of top-down attentional control. Since sustained attention influences HR and HRV (Richards et al., 1991; Richards et al., 2000), group differences in the ability to interpret the pragmatics of the experimental context could influence our results. Our design does not allow us to dissect the contribution of differences in top-down processes, like interpretation of the situational pragmatics, versus bottom-up, the extent to which attention is naturally on the video. Therefore, it is unclear how this affects the relation between language and individual differences in autonomic control. This could also relate to the group differences in the direction of the relations, since children with ASD may be less likely to be able to ‘read’ the behavior of the examiner and react to their expectations. Future research could try to dissect these effects by comparing different methods of assessing resting-state in children.
Finally, there was no relation between autonomic activity and later developmental scores over a 12-month period for either Expressive or Receptive language, Socialisation skills or EF. Fetal HR and HRV are related to language at 2.5 years, such that higher prenatal parasympathetic activity related to better language skills in toddlers (DiPietro et al., 2007). Also, higher HRV in new-borns relates to better social abilities in three-year-olds (Doussard-Roosevelt et al., 1997). This suggests that while autonomic activity in infancy may relate to socio-communicative abilities later in life, autonomic variability during calm, non-social periods in preschool children does not contribute to variability in language skills over a 12-month period once language has started to develop. Changes in HRV from baseline to both social and non-social conditions at 10 months is associated with expressive and receptive language at respectively 2 and 3 years, which in turn relates to social competence and EF at 4 years (Whedon et al., 2018). Similarly, evaluating the autonomic response to non-social and social stimuli including language in preschoolers might provide a better predictor of later socio-cognitive skills than trait levels of PNS activity.
Limitations
The strengths of this study include the confirmed ASD diagnosis, using clinical judgement and gold-standard diagnostic instruments, and the large sample size compared to other physiology studies in ASD research. Data on cardiac activity during rest was collected during multiple segments over two days, creating a more stable and less context-dependent measure compared to a single short resting episode as done in the majority of studies. One limitation is that more segments were excluded in the ASD group, compared to the TD group. This could be due to more movement or poorer tolerance of the sensors in the ASD group. Although we controlled for number of segments in the analyses, we cannot rule out that this might potentially confound the results. Both groups, however, were comparable in terms of HR and HRV suggesting that if the ASD group showed more movement overall during the resting videos, this did not result in higher HR. There were no differences over time between the groups in the number of missing segments, suggesting that children with ASD were not becoming more fatigued with the battery compared to the TD group (Supplementary Material S7). Further, more artefacts in the data could also have led to lower HRV due to insertion of missing beats. Deleting segments with any missing peaks did however not change the results (Supplementary Material S8).
A second limitation is that we did not control for any interventions, although a substantial proportion of the ASD group (90%) received some kind of treatment between baseline and follow-up, most commonly speech or occupational therapy. However, there was substantial heterogeneity in the duration, nature and intensity of interventions experienced. This means that our design does not allow us to determine the extent to which interventions influenced the relation between early physiology and later language development. Since these were all interventions naturally accessed in the community, our results are likely generalizable to other samples with a similar access to services; but may not generalise to less well-served samples who might receive less intervention in this time-window. Future research designed to ask how specific interventions influence trajectories would be required to answer this question.
Another limitation is that developmental abilities of the ASD group were lower than the TD group. Because age correlates with cardiac indices, groups were matched on chronological, rather than developmental age. Our results suggest that the associations between PNS and socio-cognitive abilities might vary depending on the child’s developmental level. Our results could therefore relate to developmental abilities, rather than specifically ASD symptoms. Including a non-ASD group that is both chronologically and developmentally matched on age with the ASD group may provide further understanding of the role of autonomic arousal in ASD specifically.
Conclusions
In this study, we establish that there is good reliability of autonomic measures in the ASD group, comparable to the TD group. The results of this study show that HR and HRV do not differentiate children with ASD at the diagnostic level and are therefore not a biomarker for ASD. They may provide promising markers of language level within groups of toddlers with ASD. Higher parasympathetic activity during passive viewing of non-social stimuli relates to better expressive and receptive language abilities within ASD. These results underline that further research should focus on the association with developmental skills, rather than group differences alone. Research concentrating on earlier time points might provide insight into the role of cardiac arousal during the early development of social and cognitive skills in ASD, creating opportunities for intervention.
Supplementary Material
Acknowledgements
We thank the children and families participating in the SPARCS study. Funding for this study was provided by NICHD (R01 HD064820, Webb). TB’s PhD studies were supported by a grant from the European Commission’s Horizon 2020 Program under grant agreement no 642990 (Brainview). EJHJ and TC were supported by the Innovative Medicines Initiative Joint Undertaking under grant agreement no 115300 (EU-AIMS), resources of which are composed of financial contribution from the European Union’s Seventh Framework Programme (FP7/2007 - 2013) and EFPIA companies’ in kind contribution.
Footnotes
Conflict of Interest Statement
The authors declare that they have no conflict of interest.
Compliance with Ethical Standards
The study was approved by the Seattle Children’s Institutional Review Board and was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Informed written consent was obtained from a custodial parent of the child participants included in the study.
REFERENCES
- American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorder (4th ed., Text Revision). Washington, DC: Author. [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: Author. [Google Scholar]
- Bal E, Harden E, Lamb D, Van Hecke AV, Denver JW, & Porges SW (2010). Emotion recognition in children with autism spectrum disorders: relations to eye gaze and autonomic state. Journal of Autism and Developmental Disorders, 40(3), 358–370. doi: 10.1007/s10803-009-0884-3 [DOI] [PubMed] [Google Scholar]
- Beauchaine TP (2015). Respiratory sinus arrhythmia: a transdiagnostic biomarker of emotion dysregulation and psychopathology. Current Opinion in psychology, 3, 43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford R, Gliga T, Shephard E, Elsabbagh M, Pickles A, Charman T, et al. (2017). Neurocognitive and observational markers: prediction of autism spectrum disorder from infancy to mid-childhood. Molecular Autism, 8(1), 49. doi: 10.1186/s13229-017-0167-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benevides TW, & Lane SJ (2015). A review of cardiac autonomic measures: considerations for examination of physiological response in children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 45(2), 560–575. doi: 10.1007/s10803-013-1971-z [DOI] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT Jr., Eckberg DL, Grossman P, Kaufmann PG, Malik M, et al. (1997). Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology, 34(6), 623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x [DOI] [PubMed] [Google Scholar]
- Biomarkers Definitions Working Group. (2001). Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clinical Pharmacology & Therapeutics, 69(3), 89–95. doi: 10.1067/mcp.2001.113989 [DOI] [PubMed] [Google Scholar]
- Bujnakova I, Ondrejka I, Mestanik M, Visnovcova Z, Mestanikova A, Hrtanek I, et al. (2016). Autism spectrum disorder is associated with autonomic underarousal. Physiological Research, 65(Supplementum 5), S673–S682. [DOI] [PubMed] [Google Scholar]
- Chambers AS, & Allen JJ (2007). Cardiac vagal control, emotion, psychopathology, and health. Biological Psychology, 74(2), 113–115. [DOI] [PubMed] [Google Scholar]
- Cicchetti DV, & Sparrow SA (1981). Developing criteria for establishing interrater reliability of specific items: applications to assessment of adaptive behavior. American Journal of Mental Deficiency. [PubMed] [Google Scholar]
- Constantino J, & Gruber C (2005). Social Responsiveness Scale (SRS). Los Angeles: Western Psychological Services. [Google Scholar]
- DiPietro JA, Bornstein MH, Hahn CS, Costigan K, & Achy‐Brou A (2007). Fetal heart rate and variability: stability and prediction to developmental outcomes in early childhood. Child Development, 78(6), 1788–1798. doi: 10.1111/j.1467-8624.2007.01099.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doussard-Roosevelt JA, Porges SW, Scanlon JW, Alemi B, & Scanlon KB (1997). Vagal Regulation of Heart Rate in the Prediction of Developmental Outcome for Very Low Birth Weight Preterm Infants. Child Development, 68(2), 173–186. doi: 10.1111/j.1467-8624.1997.tb01934.x [DOI] [PubMed] [Google Scholar]
- Doussard‐Roosevelt JA, Montgomery LA, & Porges SW (2003). Short‐term stability of physiological measures in kindergarten children: Respiratory sinus arrhythmia, heart period, and cortisol. Developmental Psychobiology, 43(3), 230–242. [DOI] [PubMed] [Google Scholar]
- Falahpour M, Thompson WK, Abbott AE, Jahedi A, Mulvey ME, Datko M, et al. (2016). Underconnected, But Not Broken? Dynamic Functional Connectivity MRI Shows Underconnectivity in Autism Is Linked to Increased Intra-Individual Variability Across Time. Brain Connectivity, 6(5), 403–414. doi: 10.1089/brain.2015.0389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler FC, Kubiak T, Siewert K, & Weber H (2013). Cardiac vagal tone is associated with social engagement and self-regulation. Biological Psychology, 93(2), 279–286. [DOI] [PubMed] [Google Scholar]
- Geurts HM, Grasman RP, Verté S, Oosterlaan J, Roeyers H, van Kammen SM, et al. (2008). Intra-individual variability in ADHD, autism spectrum disorders and Tourette’s syndrome. Neuropsychologia, 46(13), 3030–3041. [DOI] [PubMed] [Google Scholar]
- Gioia GA, Espy KA, & Isquith PK (2003). Behavior Rating Inventory of Executive Function Preschool version. Florida: Psychological Assessment Resources. [Google Scholar]
- Gotham K, Risi S, Pickles A, & Lord C (2007). The Autism Diagnostic Observation Schedule: revised algorithms for improved diagnostic validity. Journal of Autism and Developmental Disorders, 37(4), 613–627. doi: 10.1007/s10803-006-0280-1 [DOI] [PubMed] [Google Scholar]
- Guy L, Souders M, Bradstreet L, DeLussey C, & Herrington JD (2014). Brief report: emotion regulation and respiratory sinus arrhythmia in autism spectrum disorder. Journal of Autism and Developmental Disorders, 44(10), 2614–2620. doi: 10.1007/s10803-014-2124-8 [DOI] [PubMed] [Google Scholar]
- Hazlett HC, Gu H, Munsell BC, Kim SH, Styner M, Wolff JJ, et al. (2017). Early brain development in infants at high risk for autism spectrum disorder. Nature, 542(7641), 348–351. doi: 10.1038/nature21369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzman JB, & Bridgett DJ (2017). Heart rate variability indices as bio-markers of top-down self-regulatory mechanisms: A meta-analytic review. Neuroscience & Biobehavioral Reviews, 74, 233–255. doi: 10.1016/j.neubiorev.2016.12.032 [DOI] [PubMed] [Google Scholar]
- Klin A, Shultz S, & Jones W (2015). Social visual engagement in infants and toddlers with autism: early developmental transitions and a model of pathogenesis. Neuroscience & Biobehavioral Reviews, 50. doi: 10.1016/j.neubiorev.2014.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klusek J, Martin GE, & Losh M (2013). Physiological arousal in autism and fragile X syndrome: group comparisons and links with pragmatic language. American Journal on Intellectual and Developmental Disabilities, 118(6), 475–495. doi: 10.1352/1944.7558-118.6.475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH Jr., Leventhal BL, DiLavore PC, et al. (2000). The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders, 30(3), 205–223. doi: 10.1023/a:1005592401947 [DOI] [PubMed] [Google Scholar]
- Loth E, Spooren W, Ham LM, Isaac MB, Auriche-Benichou C, Banaschewski T, et al. (2016). Identification and validation of biomarkers for autism spectrum disorders. Nature Reviews Drug Discovery, 15(1), 70–73. doi: 10.1038/nrd.2015.7 [DOI] [PubMed] [Google Scholar]
- Lydon S, Healy O, Reed P, Mulhern T, Hughes BM, & Goodwin MS (2016). A systematic review of physiological reactivity to stimuli in autism. Developmental Neurorehabilitation, 19(6), 335–355. doi: 10.3109/17518423.2014.971975 [DOI] [PubMed] [Google Scholar]
- Mahurin-Smith J, DeThorne LS, & Petrill SA (2017). Longitudinal Associations Across Prematurity, Attention, and Language in School-Age Children. Journal of Speech, Language, and Hearing Research, 60(12), 3601–3608. doi: 10.1044/2017_JSLHR-L-17-0015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik MJ, Bigger AT, Camm J, Kleiger R, E., Malliani A, Moss A, J., et al. (1996). Heart rate variability; Standards of measurement, physiological interpretation, and clinical use. European Heart Journal, 17(3), 354–381. doi: 10.1093/oxfordjournals.eurheartj.a014868 [DOI] [PubMed] [Google Scholar]
- McPartland JC (2016). Considerations in biomarker development for neurodevelopmental disorders. Current Opinion in Neurology, 29(2), 118–122. doi: 10.1097/wco.0000000000000300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne E (2011). Increased intra-participant variability in children with autistic spectrum disorders: evidence from single-trial analysis of evoked EEG. Frontiers in Psychology, 2, 51. doi: 10.3389/fpsyg.2011.00051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming X, Julu PO, Brimacombe M, Connor S, & Daniels ML (2005). Reduced cardiac parasympathetic activity in children with autism. Brain & Development, 27(7), 509–516. doi: 10.1016/j.braindev.2005.01.003 [DOI] [PubMed] [Google Scholar]
- Mullen E (1995). Manual for the Mullen scales of early learning: Circle Pines, MN: American Guidance Service. [Google Scholar]
- Neuhaus E, Bernier R, & Beauchaine TP (2014). Brief report: social skills, internalizing and externalizing symptoms, and respiratory sinus arrhythmia in autism. Journal of Autism and Developmental Disorders, 44(3), 730–737. doi: 10.1007/s10803-013-1923-7 [DOI] [PubMed] [Google Scholar]
- Patriquin MA, Lorenzi J, & Scarpa A (2013). Relationship between respiratory sinus arrhythmia, heart period, and caregiver-reported language and cognitive delays in children with autism spectrum disorders. Applied Psychophysiology and Biofeedback, 38(3), 203–207. doi: 10.1007/s10484-013-9225-6 [DOI] [PubMed] [Google Scholar]
- Patriquin MA, Lorenzi J, Scarpa A, & Bell MA (2014). Developmental trajectories of respiratory sinus arrhythmia: associations with social responsiveness. Developmental Psychobiology, 56(3), 317–326. doi: 10.1002/dev.21100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patriquin MA, Lorenzi J, Scarpa A, Calkins SD, & Bell MA (2015). Broad implications for respiratory sinus arrhythmia development: associations with childhood symptoms of psychopathology in a community sample. Developmental Psychobiology, 57(1), 120–130. doi: 10.1002/dev.21269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patriquin MA, Scarpa A, Friedman BH, & Porges SW (2013). Respiratory sinus arrhythmia: a marker for positive social functioning and receptive language skills in children with autism spectrum disorders. Developmental Psychobiology, 55(2), 101–112. doi: 10.1002/dev.21002 [DOI] [PubMed] [Google Scholar]
- Pickles A, Anderson DK, & Lord C (2014). Heterogeneity and plasticity in the development of language: A 17‐year follow‐up of children referred early for possible autism. Journal of Child Psychology and Psychiatry, 55(12), 1354–1362. [DOI] [PubMed] [Google Scholar]
- Porges SW (2003). The Polyvagal Theory: phylogenetic contributions to social behavior. Physiology & Behavior, 79(3), 503–513. doi: 10.1016/s0031-9384(03)00156-2 [DOI] [PubMed] [Google Scholar]
- Porges SW (2007). The polyvagal perspective. Biological Psychology, 74(2), 116–143. doi: 10.1016/j.biopsycho.2006.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW, Doussard-Roosevelt JA, Portales AL, & Greenspan SI (1996). Infant regulation of the vagal “brake” predicts child behavior problems: a psychobiological model of social behavior. Developmental Psychobiology, 29(8), 697–712. doi: [DOI] [PubMed] [Google Scholar]
- Richards JE (2010). The development of attention to simple and complex visual stimuli in infants: Behavioral and psychophysiological measures. Developmental review : DR, 30(2), 203–219. doi: 10.1016/j.dr.2010.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JE, & Casey BJ (1991). Heart rate variability during attention phases in young infants. Psychophysiology, 28(1), 43–53. [DOI] [PubMed] [Google Scholar]
- Richards JE, & Cronise K (2000). Extended visual fixation in the early preschool years: Look duration, heart rate changes, and attentional inertia. Child Development, 71(3), 602–620. [DOI] [PubMed] [Google Scholar]
- Schupak BM, Parasher RK, & Zipp GP (2016). Reliability of Electrodermal Activity: Quantifying Sensory Processing in Children With Autism. The American Journal of Occupational Therapy, 70(6), 7006220030p7006220031–7006220030p7006220036. doi: 10.5014/ajot.2016.018291 [DOI] [PubMed] [Google Scholar]
- Sheinkopf SJ, Neal-Beevers AR, Levine TP, Miller-Loncar C, & Lester B (2013). Parasympathetic response profiles related to social functioning in young children with autistic disorder. Autism Research and Treatment, 2013, 868396. doi: 10.1155/2013/868396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skowron EA, Cipriano-Essel E, Gatzke-Kopp LM, Teti DM, & Ammerman RT (2014). Early adversity, RSA, and inhibitory control: evidence of children’s neurobiological sensitivity to social context. Developmental Psychobiology, 56(5), 964–978. doi: 10.1002/dev.21175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somandepalli K, Kelly C, Reiss PT, Zuo X-N, Craddock RC, Yan C-G, et al. (2015). Short-term test–retest reliability of resting state fMRI metrics in children with and without attention-deficit/hyperactivity disorder. Developmental Cognitive Neuroscience, 15(Supplement C), 83–93. doi: 10.1016/j.dcn.2015.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow S, Cicchetti D, & Balla D (2005). Vineland adaptive behavior scales: (Vineland II), survey interview form/caregiver rating form. Livonia, MN: Pearson Assessments. [Google Scholar]
- StataCorp. (2015). Stata Statistical Software: Release 14. College Station, TX: StataCorp LP. [Google Scholar]
- Staton L, El‐Sheikh M, & Buckhalt JA (2009). Respiratory sinus arrhythmia and cognitive functioning in children. Developmental Psychobiology, 51(3), 249–258. doi:doi: 10.1002/dev.20361 [DOI] [PubMed] [Google Scholar]
- Strimbu K, & Tavel JA (2010). What are Biomarkers? Current Opinion in HIV and AIDS, 5(6), 463–466. doi: 10.1097/COH.0b013e32833ed177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, Hansen AL, Saus-Rose E, & Johnsen BH (2009). Heart rate variability, prefrontal neural function, and cognitive performance: the neurovisceral integration perspective on self-regulation, adaptation, and health. Annals of Behavioral Medicine, 37(2), 141–153. doi: 10.1007/s12160-009-9101-z [DOI] [PubMed] [Google Scholar]
- Thayer JF, & Lane RD (2000). A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders, 61(3), 201–216. [DOI] [PubMed] [Google Scholar]
- Toichi M, & Kamio Y (2003). Paradoxical autonomic response to mental tasks in autism. Journal of Autism and Developmental Disorders, 33(4), 417–426. [DOI] [PubMed] [Google Scholar]
- Valkenburg PM, & Vroone M (2004). Developmental Changes in Infants’ and Toddlers’ Attention to Television Entertainment. Communication Research, 31(3), 288–311. doi: 10.1177/0093650204263435 [DOI] [Google Scholar]
- Vaughan Van Hecke A, Lebow J, Bal E, Lamb D, Harden E, Kramer A, et al. (2009). Electroencephalogram and heart rate regulation to familiar and unfamiliar people in children with autism spectrum disorders. Child Development, 80(4), 1118–1133. doi: 10.1111/j.1467-8624.2009.01320.x [DOI] [PubMed] [Google Scholar]
- Watson LR, Baranek GT, Roberts JE, David FJ, & Perryman TY (2010). Behavioral and physiological responses to child-directed speech as predictors of communication outcomes in children with autism spectrum disorders. Journal of Speech, Language, and Hearing Research, 53(4), 1052–1064. doi: 10.1044/1092-4388(2009/09-0096) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner OM, & McGrath JJ (2017). Test-Retest Reliability of Pediatric Heart Rate Variability: A Meta-Analysis. Journal of Psychophysiology, 31(1), 6–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whedon M, Perry NB, Calkins SD, & Bell MA (2018). Cardiac vagal regulation in infancy predicts executive function and social competence in preschool: Indirect effects through language. Developmental Psychobiology. doi:doi: 10.1002/dev.21636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerkes RM, & Dodson JD (1908). The relation of strength of stimulus to rapidity of habit‐formation. Journal of Comparative Neurology, 18(5), 459–482. [Google Scholar]
- Zantinge G, van Rijn S, Stockmann L, & Swaab H (2017). Psychophysiological responses to emotions of others in young children with autism spectrum disorders: Correlates of social functioning. Autism Research, 1–11. doi: 10.1002/aur.1794 [DOI] [PubMed] [Google Scholar]
- Zimmerman I, Steiner V, & Pond R (2002). Preschool Language Scale (4th ed.). San Antonio, TX: The Psychological Corporation. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.