Abstract
Nicotinic acetylcholine receptors (nAChRs) are pharmacological targets for the treatment of neuropathic pain, and the α6β4 subtype has been identified as particularly promising. Rat α6β4 nAChRs are less sensitive to some ligands than the human homologue potentially complicating the use of rodent α6β4 receptors for screening therapeutic compounds. We used molecular dynamics simulations coupled with functional assays to study the interaction between α-conotoxin PeIA and α6β4 nAChRs and to identify key ligand–receptor interactions that contribute to species differences in α-conotoxin potency. Our results show that human and rat α6β4 nAChRs have distinct ligand-binding motifs and show markedly different sensitivities to α-conotoxins. These studies facilitated the creation of PeIA-5667, a peptide that shows 270-fold higher potency for rat α6β4 nAChRs over native PeIA and similar potency for the human homologue. Our results may inform the design of therapeutic ligands that target α6β4 nAChRs for the treatment of neuropathic pain.
Graphical Abstract

INTRODUCTION
Nicotinic acetylcholine receptors (nAChRs) containing α6 subunits have been implicated in neuropathic pain. Wieskopf et al. have shown that mice lacking the α6 subunit gene (CHRNA6) are refractory to the analgesic effects of nicotine in models of neuropathic pain, and conversely, mice with a gain-of-function mutation in the α6 subunit show increased analgesic responses to nicotine.1 Rodent dorsal root ganglion (DRG) neurons abundantly express the α6β4* nAChR subtype (the asterisk indicates the known or potential presence of other subunits in native receptor complexes),1,2 and mice with decreased expression of α6β4* in DRG neurons show less analgesia in response to the nAChR agonist ABT-594 in sciatic nerve injury.3 In heterologous expression systems, α6β4 nAChRs have been shown to functionally couple to purinergic P2X receptors and negatively regulate receptor activation.4 Reduced P2X receptor activity by antagonists produces analgesia and is an active area of research for the treatment of neuropathic pain.5,6 In humans, transcriptomics and quantitative PCR studies of DRG neurons demonstrated the presence of mRNA transcripts for the α6 subunit,7,8 and the susceptibility to developing extended post-surgical pain in patients is associated with CHRNA6 expression levels.1 Thus, the α6β4 nAChR appears to be a promising and novel target for pharmacotherapy of neuropathic pain syndromes.9
Conotoxins are peptides found in the venom of carnivorous marine snails of the genus Conus and are used by these organisms for defense and to capture prey.10,11 Conotoxins are classified into various categories and frameworks according to their sequences. For example, α-conotoxin (α-Ctx) PeIA belongs to Framework I based on the presence of four Cys residues connected in pairs by disulfide bonds.12 PeIA is further classified as an α4/7 conotoxin having four residues in the first loop between Cys2 and Cys8 and seven in the second loop between Cys8 and Cys16. Native α-Ctxs and their synthetic analogues are antagonists of nAChRs.13 We recently showed that certain α-Ctxs are more potent on human than rat α6β4 nAChRs heterologously expressed in Xenopus oocytes.14 Sequence homology comparisons of the extracellular ligand-binding domain reveal that human and rat α6 subunits are highly conserved, and residues that are important for α-Ctx binding are strictly conserved between the two species.15-17 By contrast, residues of the β4 subunit that form the ligand-binding pocket show species divergence in their sequences at several positions (human/rat) notably 34 (Lys/Arg), 36 (Gln/Glu), 119 (Leu/Gln), 163 (Met/Lys), and 168 (Ser/Ile).
In this report, we used computational and functional studies to identify key molecular determinants that contribute to differences in α-Ctx binding modes for α6β4 nAChRs. Importantly, this information facilitated the creation of several PeIA analogues that show >250-fold increase in potency for inhibition of rat α6/α3β4 nAChRs and subnanomolar affinity for human α6/α3β4 nAChRs. These studies demonstrate that ligands with similar potencies for human and rat α6β4 nAChRs can be generated, and may, in turn, be useful in rodent models of human neuropathic pain to further our understanding of the role α6β4 nAChRs play in chronic pain conditions.
RESULTS
Molecular Dynamics Simulations Identify Interactions Between Specific Peptide and Receptor Residues.
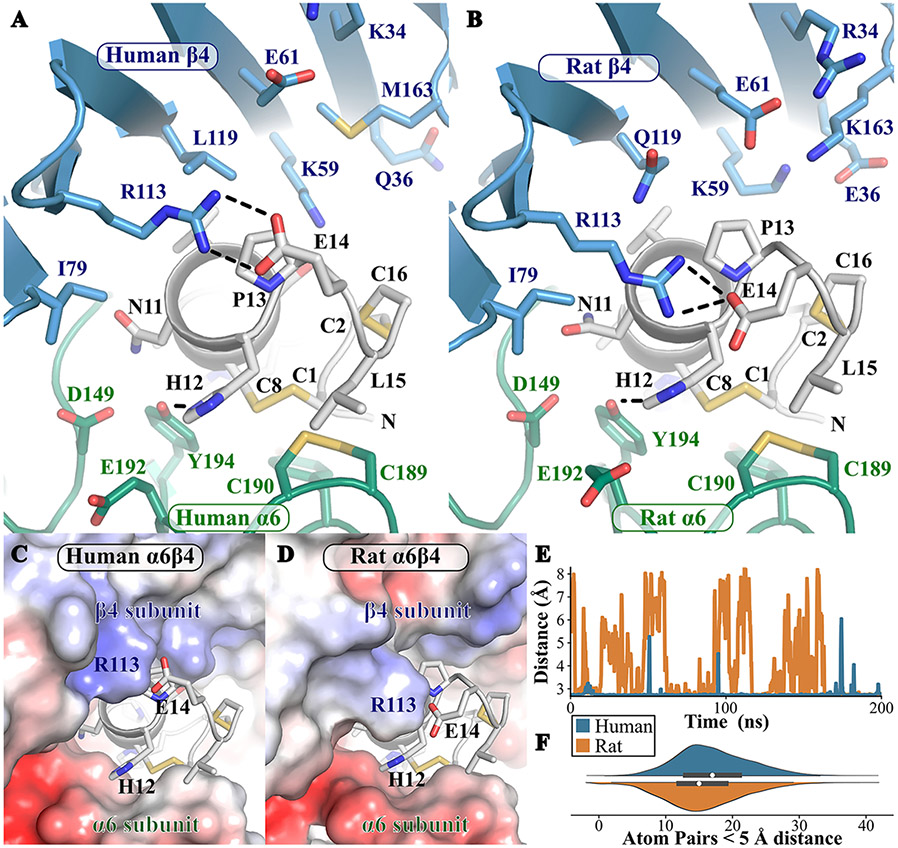
Recently, we showed that α-Ctxs PeIA and PnIA show higher potency for human over rat α6β4 nAChRs.16 To gain mechanistic insights into this species difference, we generated molecular models of the interactions between PeIA and both human and rat α6β4 nAChRs and studied the interactions at the ligand-binding interface during 200 ns molecular dynamics simulations. The molecular models of PeIA/human α6β4 and PeIA/rat α6β4 nAChRs are illustrated in Figure 1. During the molecular dynamics simulations, most pairs of residue interactions at the interface were similar for the two complexes, but several interactions were species-specific (Table 1). These species differences arise from either receptor positions occupied by nonconserved amino acids, specifically β4 subunit residues 36, 119, 163, and 168, or from conserved receptor residues establishing different interactions with PeIA. The majority of the different contact pairs (13 out of 19) were made by residues in the second loop of PeIA, suggesting that positions 9–16 are particularly important for creating different interactions with α6β4 nAChRs. In the first loop, i.e., positions 4 to 7, Ser4 is located at the front of the ligand-binding pocket and established contacts with several residues of the β4 subunit, including residue 168 that is nonconserved in human and rat. His5 is deeply buried and displayed very similar interactions with both human and rat α6β4 nAChRs. Pro6 occupies the bottom of the ligand-binding site and is typically conserved among α-Ctxs. Ala7 is deeply buried at the back of the pocket and displayed two additional interactions in the context of the human receptor that were not observed for rat α6β4 nAChRs. Only residue contact pairs present during at least half of the simulations are reported in Table 1, and we note that three of the contact pairs identified as species-specific were just above that duration threshold. The data reported in Table 1 are insufficient for evaluating the type of interactions, such as hydrogen bonding, as well as the strength of the interactions. Therefore, additional measurements were carried out, including computation of the electrostatic potential generated by the two receptors (Figure 1C,D), the evolution of distance between atoms, and computation of the number of atom contact pairs between two residues (Figure 1E,F). Electrostatic potential seems to be important as several nonconserved residues in human and rat β4 subunits involve charge differences. Positions 36 and 163 have charged residues Lys and Glu, respectively, in the rat β4 subunit, but in human β4, Met and Gln are present, respectively. As shown in Figure 1C,D, the principal subunit α6 of both receptors displays a negatively charged patch that establishes extensive contacts with His12, suggesting that this His residue is in a positively charged state. Residue β4 Arg113 formed a salt bridge (combination of charge and hydrogen bonding) with PeIA Glu14, as illustrated in Figure 1A,B. The positive charge potential on the surface of β4 Arg113 is weaker for the rat receptor. The strength of the salt bridge seems, accordingly, to be weaker during the simulation of the rat receptor compared to that of the human receptor: the evolution of the minimum distance between the oxygen atoms of the Glu14 side chain and the nitrogen atoms of the Arg113 side chain indicates that a salt bridge forms during 99% of the simulations with the human receptor but only 60% of the time in simulations with the rat receptor. This suggests that the salt bridge between Glu14 and Arg113 is more stable and therefore potentially more energetically stabilizing in the context of the human receptor than it is for the rat receptor. Finally, the side chain of PeIA Leu15 is packed against the vicinal disulfide bridge of α6 residues Cys189–Cys190, and the number of atom pair contacts was, on average, slightly higher during the simulations of human α6β4 than during those of the rat receptor suggesting better stabilization with the human α6 subunit created by Leu15.
Figure 1.
Molecular dynamics simulation of the complexes between PelA and human or rat α6β4 nAChRs. (A, B) Illustrative frames of the simulations of PeIA/human α6β4 nAChR (A) and PeIA/rat α6β4 nAChR (B). (C, D) Electrostatic potential generated by human (C) or rat (D) α6β4 nAChRs mapped on their molecular surface. (E) Evolution of the minimum distance between the oxygen atoms of PeIA Glu14 side chain and the nitrogen atoms of β4 Arg113 side chain. (F) Violin plot (box plot combined with kernel density estimate) of the number of atom pairs that are less than 5 Å apart between PeIA Leu15 and α6 Cys189–Cys190. In (A) and (B), α6 is in green, β4 is in blue, and PeIA is in white. The illustration focuses on the second loop of PeIA and its interactions with the receptor. Select hydrogen bonds are shown as black dashed lines. The molecular dynamics frame chosen for the illustration is the middle frame of the largest cluster from a 5-fold k-means clustering based on the Cα of the complex. In (C) and (D), the molecular surface of the α6β4 nAChR models, which are also shown in (A) and (B), was colored according to the electrostatic potential generated by the receptor from −5 kT/e (and red) in blue to 0 in white to +5 kT/e (and above) in blue. In (E) and (F), data for simulations of rat α6β4/PeIA or human α6β4/PeIA complexes are in blue and orange, respectively. In (F), the box plot represents the first and third quartiles, and the median is shown as a white disk.
Table 1.
Pairs of Residues in Contact between PeIA and Human or Rat α6β4 during Molecular Dynamics Simulationsa
| subunit |
||||
|---|---|---|---|---|
| PeIA | human α6 | human β4 | rat α6 | rat β4 |
| Gly1 | Tyr187 (1.00) | Asp170 (0.99), bAsp171 (0.80) | Tyr187 (1.00) | Asp170 (0.99) |
| Cys2 | Tyr187 (1.00), Cys189 (1.00), Cys190 (0.98) | Tyr187 (1.00), Cys189 (0.99), Cys190 (0.97) | ||
| Cys3 | Trp57 (1.00), Lys59 (0.97), cSer168 (0.61) | Trp57 (1.00), Lys59 (0.94), cIle168 (1.00) | ||
| Ser4 | Ser38 (0.92), Trp57 (1.00), cSer168 (0.97), Asp170 (1.00), Asp171 (1.00) | Ser38 (0.81), Trp57 (1.00), cIle168 (1.00), Asp170 (1.00), Asp171 (1.00) | ||
| His5 | Tyr90 (0.98), Ser145 (0.83), Ile185 (0.90), Tyr187 (1.00), Tyr194 (1.00), Thr195 (0.95), Asp196 (0.99) | Trp57 (0.99), Leu121 (0.97), Asp171 (0.75) | Tyr90 (0.79), Ser145 (0.74), Ile185 (0.89), Tyr187 (1.00), Tyr194 (1.00), Thr195 (0.89), Asp19 (0.99) | Trp57 (0.96), Leu121 (0.98), Asp171 (0.76) |
| Pro6 | Tyr90 (1.00), Ser145 (0.89), Trp146 (1.00), Thr147 (0.93) | Trp57 (1.00), Leu121 (1.00) | Tyr90 (1.00), Ser145 (0.73), Trp146 (1.00), Thr147 (0.97) | Trp57 (0.90), Leu121 (1.00), bAsp171 (0.66) |
| Ala7 | bTyr90 (0.67), Ser145 (1.00), Trp146 (1.00), Thr147 (1.00), Tyr148 (1.00), Asp149 (0.87), Tyr194 (1.00) | bArg81 (0.58), Ile111 (0.88), Leu121 (0.96) | Ser145 (1.00), Trp146 (1.00), Thr147 (1.00), Tyr148 (1.00), Asp149 (0.88), Tyr194 (1.00) | Ile111 (0.98), Leu121 (0.94) |
| Cys8 | Tyr187 (1.00), Cys189 (0.55), Cys190 (0.98), Tyr194 (1.00) | Tyr187 (1.00), Cys189 (0.54), Cys190 (0.95), Tyr194 (1.00) | ||
| Ser9 | Trp57 (1.00), Lys59 (1.00), cLeu119 (0.96), Leu121 (1.00) | Trp57 (0.98), Lys59 (1.00), bGlu61 (0.50), cGln9 (0.62), Leu121 (1.00) | ||
| Val10 | Trp146 (0.68), Thr147 (1.00) | Lys59 (1.00), Gln60 (0.86), Ile79 (0.65), Ile111 (1.00), Arg113 (0.83), cLeu119 (1.00), Trp120 (1.00), Leu121 (1.00) | Trp146 (0.56), Thr147 (1.00) | Lys59 (1.00), Gln60 (0.91), bGlu61 (0.90), Ile79 (0.62), Ile111 (1.00), Arg113 (0.60), cGln119 (1.00), Trp120 (1.00), Leu121 (1.00) |
| Asn11 | Thr147 (1.00), Tyr148 (0.99), Asp149 (0.93), Glu192 (0.91), Tyr194 (1.00) | Ile79 (1.00), Arg81 (1.00), Ile111 (1.00), Arg113 (0.95), cLeu119 (0.75) | Thr147 (1.00), Tyr148 (0.99), Asp149 (0.90), Glu192 (0.91), Tyr194 (1.00) | Ile79 (1.00), Arg81 (1.00), Ile111 (1.00), Arg113 (0.84), cGln119 (0.74) |
| His12 | Asp149 (0.90), bCys189 (0.51), Cys190 (1.00), Glu191 (0.79), Glu192 (1.00), Tyr194 (1.00) | Arg113 (0.84) | Asp149 (0.56), Cys190 (0.99), Glu191 (0.62), Glu192 (1.00), Tyr194 (1.00) | bIle79 (0.70), Arg113 (0.69) |
| Pro13 | Lys59 (1.00), Glu61 (0.96), Ile111 (0.86), Arg113 (1.00), cLeu119 (1.00) | Lys59 (0.96), Glu61 (0.99), bIle79 (0.52), Ile111 (0.63), Arg113 (0.99), cGln119 (1.00), cLys163 (0.97) | ||
| Glu14 | Arg113 (1.00) | Arg113 (0.94), cLys163 (0.79) | ||
| Leu15 | Cys189 (1.00), Cys190 (0.98) | Cys189 (1.00), Cys190 (0.96) | ||
| Cys16 | Lys59 (1.00) | cGlu36 (0.54), Lys59 (0.86), cLys163 (0.96) | ||
The relative frequency of the contacts during the molecular dynamics simulations is indicated within parentheses. Only pairs of residues that were within 5 Å from each other for at least 50% of the simulations are listed.
Residues in bold indicate contact pairs that are specific for one of the two receptors and involve a conserved receptor residue.
Residues in bold and italics indicate contact pairs that involve a nonconserved receptor residue.
PeIA Analogues Show Differential Potencies for Human and Rat α6/α3β4 nAChRs.
α-Conotoxin MII and PeIA are α-4/7-framework peptides whose sequences differ by seven amino-acid residues (Figure 2A,B). The activity of PeIA has been extensively characterized, but the activity of MII on human and rat α6/α3β4 nAChRs had not been reported. When tested on α6/α3β4 nAChRs, we found that MII was ~30-fold more potent on human receptors over rat receptors (Figure 2C,D; Table 2). MII was also more potent than PeIA on both human and rat receptors. One or more of the residues that differ between the two peptides likely contributes to the higher potency of MII for human α6/α3β4 nAChRs. To help identify the ligand-binding interactions between PeIA and α6β4 nAChRs, we tested a panel of PeIA analogues where single amino-acid residues were replaced with those found in the homologous positions of MII.18 Most of these substitutions decreased the potency of PeIA for human α6/α3β4 nAChRs with the exception of A7V and S9H which altered the potency such that the resulting analogues [Val7]PeIA and [His9]PeIA showed equal potency (<3-fold difference) with MII (Figure 2C; Table 2). Similar results were found for rat α6/α3β4 nAChRs, but the magnitude of the effect that the A7V and S9H substitutions had on PeIA potency was much larger (~10-fold). In fact, the IC50 values of [Val7]PeIA and [His9]PeIA were lower than that of MII (Figure 2D; Table 2). Substitutions that caused a divergence (≥3-fold difference) in the potency of PeIA for human and rat α6/α3β4 nAChRs include H5N, A7V, S9H, and P13S. These results suggest that PeIA interacts differently at sites within the ligand-binding pocket of human and rat α6β4 nAChRs and are consistent with the results obtained by molecular dynamics simulations.
Figure 2.
Comparative structure–activity relationship studies of MII and PeIA reveal differential potencies for human and rat α6/α3β4 nAChRs. X. laevis oocytes expressing human or rat α6/α3β4 nAChRs were subjected to two-electrode voltage-clamp (TEVC) electrophysiology and the IC50 values for inhibition of ACh-evoked currents by the peptides determined as described in the Experimental Section. (A, B) Sequences of MII and PeIA showing the disulfide connectivity between Cys residues; residues that comprise the first loop are those between Cys2 and Cys8 whereas those between Cys3 and Cys16 form the second loop. Residues in green and red are those that differ between the two peptides. (C, D) MII and PeIA were more potent on human than rat α6/α3β4 nAChRs with MII being the most potent. Analogues of PeIA that had nonconserved amino acids substituted with those found in the homologous positions of MII were tested to identify residues that contributed to the higher potency of MII. Analogues [Val7]PeIA and [His9]PeIA showed minimal changes in activity but trended toward increased potency for human α6/α3β4 nAChRs compared to native PeIA. [Val7]PeIA, [His9]PeIA, and [Leu10]PeIA showed increased (>3-fold) potency for rat α6/α3β4 nAChRs. Note that [Val7]PeIA and [His9]PeIA were equally (<3-fold difference) potent as MII on human and rat α6/α3β4 nAChRs but the magnitude of the change was larger for rat. Also note that [Asn5]PeIA and [Ser13]PeIA were ~4- and ~3-fold less potent on human α6/α3β4 whereas little or no change (<3-fold) was found for rat. The “±” values and the error bars indicate the standard deviation (SD) of the data. The peptides are rank-ordered from top to bottom by potency; see Table 2 for values. In panel (C), curves for [His9]PeIA and [Asn5]PeIA were offset slightly to avoid overlap as well as those for [His9]PeIA, [Asn5]PeIA, and [Leu10]PeIA in panel (D).
Table 2.
IC50 Values for Inhibition of Human and Rat α6/α3β4 nAChRs by MII, PeIA, and PeIA Analoguesa
| human |
rat |
|||||
|---|---|---|---|---|---|---|
| peptide | IC50 (nM) | n | log change in PeIA IC50 | IC50 (nM) | n | log change in PeIA IC50 |
| MII | 1.49 (1.18–1.88) | 4 | 31.5 (29.2–33.9) | 4 | ||
| PeIA | 6.75 (5.54–8.25) | 4 | 1 | 130 (117–145) | 8 | 1 |
| [Asn5]PeIA | 29.8 (27.2–32.3) | 4 | 0.7 | 64.9 (54.7–77.0) | 4 | −0.3 |
| [Val7]PeIA | 3.52 (3.03–4.10) | 4 | −0.3 | 20.9 (17.1–25.5) | 4 | −0.9 |
| [His9]PeIA | 2.90 (2.35–3.57) | 4 | −0.4 | 18.3 (13.9–24.0) | 4 | −0.9 |
| [Leu10]PeIA | 9.64 (8.56–10.9) | 4 | 0.2 | 63.8 (56.5–72.1) | 4 | −0.3 |
| [Glu11]PeIA | 70.9 (58.2–86.3) | 4 | 1.0 | 2341 (1847–2968) | 4 | 1.2 |
| [Ser13]PeIA | 23.2 (21.7–24.9) | 4 | 0.5 | 119 (100–141) | 4 | −0.1 |
| [Asn14]PeIA | 16.2 (13.6–19.2) | 4 | 0.4 | 191 (174–209) | 4 | 0.1 |
Values within parentheses indicate the 95% confidence interval; log change in IC50 calculated relative to native PeIA; n indicates the number of oocytes tested.
Structure–Activity Relationship (SAR) Studies Using Ala-substituted Analogues of PeIA Reveal Differences in the Ligand-Binding Motifs of Human and Rat α6β4 nAChRs.
To further examine the interaction of PeIA with α6β4 nAChRs, we tested a series of PeIA analogues, where non-Cys residues were replaced with Ala and compared the IC50 values for human and rat α6/α3β4 nAChRs. Changes in PeIA potency for α6/α3β4 nAChRs resulting from Ala substitutions in the first loop (residues between Cys2 and Cys8) generally followed the same trends for both species. Substitution of Ser4 with Ala increased the IC50 value of PeIA for human α6/α3β4 by ~3-fold (Figure 3A; Table 3) but had no effect (<3-fold) for rat α6/α3β4 nAChRs (Figure 3B; Table 3). Substitution of His5 and Pro6 with Ala increased the IC50 of PeIA substantially (≥10-fold) for human α6/α3β4 nAChRs and rendered the peptide essentially inactive (IC50 >10 μM) on rat α6/α3β4 nAChRs. By contrast, Ala substitution of residues in the second loop (residues between Cys8 and Cys16) showed differential changes in PeIA potency for human and rat α6/α3β4 nAChRs. For human α6/α3β4, all Ala-substituted analogues, with the exception of [Ala11]PeIA, had IC50 values that increased by ~3 to 13-fold, but for rat α6/α3β4 nAChRs, only [Ala13]PeIA showed an increase in the IC50 value (~3-fold) (Figure 3C,D; Table 3). Analogues that showed divergence in the change (≥3-fold) in potency relative to native PeIA include [Ala4]PeIA, [Ala9]PeIA, [Ala10]PeIA, [Ala13]PeIA, [Ala14]PeIA, and [Ala15]PeIA.
Figure 3.
Structure–activity relationship studies using Ala-substituted analogues of PeIA and α6/α3β4 nAChRs identify key positions that are important for PeIA activity. X. laevis oocytes expressing human or rat α6/α3β4 nAChRs were subjected to TEVC electrophysiology and the IC50 values for inhibition of ACh-evoked currents by PeIA analogues determined as described in the Experimental Section. (A, C) For human α6/α3β4 nAChRs, most analogues showed minor (≥3- to <5-fold) to substantial (≥10-fold) increases in IC50 values with the exception of [Ala11]PeIA, where no change (<3-fold) was observed. [Ala12]PeIA was essentially inactive (IC50 value >10 μM); the responses in the presence the peptide (10 μM) were 70 ± 2% (n = 4) of controls. In a separate set of experiments (data not shown), the responses in the presence of PeIA (10 μM) were compared to those for [Ala12]PeIA (10 μM) in the same oocytes. The ACh-evoked currents in the presence of PeIA were 0.6 ± 0.4% of control responses compared to 62 ± 12% for [Ala12]PeIA and were statistically different (p ≤ 0.0001); n = 4 for both. (B, D) By contrast, for rat α6/α3β4 nAChRs, analogues [Ala5]PeIA, [Ala6]PeIA, [Ala9]PeIA, [Ala12]PeIA, and [Ala13]PeIA showed minor (≥3 to <5-fold) to substantial (≥10-fold) changes in IC50 values. [Ala5]PeIA, [Ala6]PeIA, and [Ala12]PeIA were essentially inactive (IC50 values >10 μM); the responses in the presence of the peptides (10 μM) were 77 ± 16, 82 ± 14, and 96 ± 4% of control values, respectively; n = 4 for all. The peptides are rank-ordered from top to bottom by potency; see Table 3 for values. Data for PeIA were presented previously in Figure 2, and are shown here for ease of curve comparison. In (B) and (D), curves for [Ala9]PeIA, [Ala14]PeIA, and [Ala15]PeIA were offset slightly to avoid overlap. For analogues that inhibited the ACh-evoked currents by <50%, a pairwise comparison of the analogue’s activities with that of native PeIA was performed in a separate set of experiments by applying the peptides (10 μM) sequentially to the same oocyte. The responses in the presence of PeIA, [Ala5]PeIA, [Ala6]PeIA, and [Ala12]PeIA were 1.3 ± 0.4%, 78 ± 9% (p ≤ 0.0001), 82 ± 8% (p ≤ 0.0001), and 79 ± 8% (p ≤ 0.0001) of control values, respectively; (n = 4) for all; data not shown. The “±” values indicate the SD of the data for all experimental determinations; significance was determined with a Students’ t-test.
Table 3.
IC50 Values for Inhibition of Human and Rat α6/α3β4 nAChRs by PeIA- and Ala-Substituted Analoguesa
| human |
rat |
|||||
|---|---|---|---|---|---|---|
| peptide | IC50 (nM) | n | log change in IC50 |
IC50 (nM) | n | log change in IC50 |
| [Ala4] PeIA | 22.0 (17.8–27.4) | 4 | 0.5 | 175 (151–202) | 4 | 0.1 |
| [Ala5] PeIA | 7610 (5449–10 630) | −4 | 3.1 | >10 000 | 4 | >1.9 |
| [Ala6] PeIA | 1915 (1378–2662) | −4 | 2.5 | >10 000 | 4 | >1.9 |
| [Ala9] PeIA | 60.5 (55.1–66.4) | 4 | 1.0 | 399 (345–461) | 4 | 0.5 |
| [Ala10] PeIA | 22.1 (19.0–25.6) | 4 | 0.5 | 137 (111–168) | 4 | 0.1 |
| [Ala11] PeIA | 10.7 (8.84–12.8) | 4 | 0.2 | 56.6 (52.8–62.9) | 4 | −0.4 |
| [Ala12] PeIA | 16 060 (9586–26 920) | 4 | 3.4 | >10 000 | 4 | >1.9 |
| b [Ala13] PeIA | 73.3 (66.8–80.5) | 4 | 1.1 | 381 (317–456) | 4 | 0.5 |
| [Ala14] PeIA | 68.0 (61.8–74.9) | 4 | 1.0 | 230 (206–256) | 7 | 0.2 |
| [Ala15] PeIA | 89.4 (81.0–98.8) | 4 | 1.1 | 237 (211–265) | 4 | 0.3 |
Values within parentheses indicate the 95% confidence interval; log change in IC50 calculated relative to native PeIA.
Values from (16); n indicates the number of oocytes tested.
Activity of PeIA on α6β4 nAChRs Can Be Substantially Enhanced or Reduced by the Presence of Select Amino-Acid Residues in Key Positions Important for Ligand-Binding.
The SAR studies presented in Figures 2 and 3 coupled with the molecular dynamics simulations results identified several key interactions between PeIA and α6β4 nAChRs. In particular, the results shown in Figure 2 demonstrate that residues in positions 7 and 9 of PeIA can be substituted to increase the binding affinity of PeIA for α6/α3β4 nAChRs. To examine further the importance of these positions, and others, for binding of PeIA to α6β4 nAChRs as well as the divergence in activity on human and rat receptors, we tested an additional set of analogues with select positions individually substituted with several different amino acids. First, we synthesized five analogues where Ser4 was replaced with other polar or aliphatic amino acids. All of the resulting analogues including [Hse4]PeIA (Hse; homoserine) reduced the potency of PeIA for human α6/α3β4 nAChRs (Figure 4A; Table 4). Substitutions of Ser9 resulted in strikingly different changes in PeIA potency particularly the S9N and S9D substitutions. Human α6/α3β4 nAChRs were ~3-fold more sensitive to inhibition by [Asn9]PeIA but >200-fold less sensitive to [Asp9]PeIA than native PeIA (Figure 4B; Table 4). Higher IC50 values of ~5- and ~14-fold were also observed with [Tyr9]PeIA and [Arg9]PeIA, respectively. Substitution of Val10 with other aliphatic amino acids had very little effect (<2-fold) on the IC50 value of PeIA for human α6/α3β4 nAChRs, but Asp10 rendered PeIA essentially inactive (IC50 > 10 μM) (Figure 4C; Table 4). As shown in Figure 2C, substitution of Asn11 with Glu had a negative impact on the potency of PeIA for human α6/α3β4 nAChRs. Likewise, substitution with other negatively charged amino acids including Asp and Api (aminopimelic acid) negatively impacted PeIA potency resulting in increased IC50 values of ~18- and ~3-fold, respectively (Figure 4D; Table 4). However, the presence of positively charged Arg or Lys in position 11 decreased the IC50 value by ~3- and ~5-fold, respectively. PeIA potency was also affected by amino acids with minor differences in the side-chain substituent present in position 14. When Glu14 was substituted with Asp, very little change (<2-fold) was observed in the IC50 value of PeIA, but increasing the side-chain length by one (aminoadipic acid; Adi) or three carbons (amino-suberic acid; Asu) increased the IC50 value substantially by ~9- and ~15-fold, respectively (Figure 4E; Table 4). A minor increase (~3-fold) in the IC50 value of PeIA was also observed when γ-carboxy glutamate (Gla) was incorporated in position 14 and no change was observed with Gln (<2-fold). Positively charged Arg also negatively impacted the potency of PeIA and increased the IC50 value by ~7-fold. The importance of side-chain length was also examined for position 15 by substituting Leu with norleucine (Nle), Val, or Ile. Norleucine increased the IC50 value by ~3-fold, whereas very little change (<3-fold) was observed with Ile and Val (Figure 4E; Table 4).
Figure 4.
Presence of optimal amino-acid residues in key positions enhances binding of PeIA to human α6/α3β4 nAChRs. X. laevis oocytes expressing α6/α3β4 nAChRs were subjected to TEVC electrophysiology and the IC50 values for inhibition of ACh-evoked currents by PeIA analogues determined as described in the Experimental Section. (A) All analogues with substitutions of Ser4 showed minor (≥3- to <5-fold) to substantial (≥10-fold) losses of potency relative to native PeIA. (B) [Asn9]PeIA and [Thr9]PeIA showed minor (≥3- to <5-fold) increases in potency. (C) Analogues with substitutions of Val10 with other hydrophobic amino acids showed little change in activity (<3-fold). [Asp10]PeIA was essentially inactive (IC50 > 10 μM); the responses in the presence of the peptide (10 μM) were 89 ± 13% (n = 4) of control values. (D) [Arg11]PeIA and [Lys11]PeIA showed minor (≥3- to <5-fold) increases in potency. (E) Substitution of Glu14 with amino acids whose side-chain lengths were similar or shorter (Asp, Gla, and Gln) showed little change in activity (<3-fold), but those with longer side chains (Aad, Asu, and Arg) had IC values that were ~10-fold higher than that of native PeIA. (F) No change (<3-fold) in PeIA activity was found with Ile and Val substitutions of Leu15 but Arg and Nle decreased activity. The peptides are rank-ordered from top to bottom by potency; see Table 4 for values. Curves for [Thr4]PeIA, [Gln14]PeIA, [Asp14]PeIA, [Gla14]PeIA, and [Arg14]PeIA have been offset slightly to avoid overlap. Data for PeIA were presented previously in Figure 2, and are shown here for ease of curve comparison. For analogues that inhibited the ACh-evoked currents by <50%, a pairwise comparison of their activities with that of native PeIA was performed in a separate set of experiments by applying the peptides (10 μM) sequentially to the same oocyte. The responses in the presence of PeIA, [Thr4]PeIA, and [Asp10]PeIA were 0.2 ± 0.1%, 79 ± 1% (p ≤ 0.0001), and 86 ± 6% (p ≤ 0.0001) of control values, respectively; (n = 4) for all; data not shown. The “±” values indicate the SD of the data for all experimental determinations; significance was determined with a Students’ t-test.
Table 4.
IC50 Values for Inhibition of Human and Rat α6/α3β4 nAChRs by PeIA Analoguesa
| human |
rat |
|||||
|---|---|---|---|---|---|---|
| peptide | IC50 (nM) | n | log change in IC50 |
IC50 (nM) | n | log change in IC50 |
| [Val4] PeIA | 29.4 (26.6–32.5) | 4 | 0.6 | 1054 (853–1301) | 5 | 0.9 |
| [Ile4] PeIA | 36.5 (32.3–41.3) | 5 | 0.7 | 2145 (1745–2637) | 5 | 1.2 |
| [Leu4] PeIA | 86.2 (69.7–107) | 5 | 1.1 | 4150 (3339–5160) | 4 | 1.5 |
| [Hse4] PeIA | 26.5 (23.0–30.1) | 4 | 0.6 | 670 (571–786) | 4 | 0.7 |
| [Thr4] PeIA | >10 000 | 4 | >3.0 | >10 000 | 4 | >1.9 |
| [Tyr9] PeIA | 30.6 (27.6–33.9) | 4 | 0.7 | 86.9 (75.4–100) | 4 | −0.2 |
| [Asn9] PeIA | 2.20 (1.91–2.53) | 4 | −0.5 | 13.4 (12.2–15.2) | 4 | −1.0 |
| [Thr9] PeIA | 2.00 (1.57–2.46) | 4 | −0.5 | 94.6 (83.4–107) | 4 | −0.1 |
| [Asp9] PeIA | 1,415 (1,342–1,492) | 4 | 2.3 | >10 000 | 4 | >1.9 |
| [Arg9] PeIA | 96.8 (86.2–109) | 4 | 1.2 | 9750 (8522–11 150) | 4 | 1.9 |
| [Ile10] PeIA | 9.24 (8.45–10.1) | 4 | 0.1 | 308 (294–322) | 5 | 0.4 |
| [Nle10] PeIA | 13.4 (12.1–14.9) | 4 | 0.3 | 497 (427–579) | 4 | 0.6 |
| [Asp10] PeIA | >10 000 | 4 | >3.0 | >10 000 | 4 | >1.9 |
| [Arg10] PeIA | 271 (201–366) | 4 | 1.6 | 2159 (1721–2709) | 5 | 1.1 |
| [Arg11] PeIA | 2.24 (1.67–2.99) | 4 | −0.5 | 14.7 (13.0–16.2) | 4 | −0.9 |
| [Lys11] PeIA | 1.46 (1.28–1.66) | 4 | −0.7 | 44.5 (37.8–52.3) | 4 | −0.5 |
| [Asp11] PeIA | 124 (114–135) | 4 | 1.3 | 6607 (5158–8464) | 4 | 1.7 |
| [Api11] PeIA | 18.9 (14.8–24.1) | 4 | 0.4 | 275 (228–332) | 4 | 0.3 |
| [Gln14] PeIA | 12.6 (11.7–13.5) | 4 | 0.3 | 180 (171–190) | 4 | 0.1 |
| [Asp14] PeIA | 12.8 (10.2–15.7) | 4 | 0.3 | 474 (438–513) | 4 | 0.6 |
| [Gla14] PeIA | 18.2 (14.9–24.4) | 4 | 0.4 | 430 (374–495) | 4 | 0.5 |
| [Aad14] PeIA | 59.8 (54.5–65.7) | 4 | 0.9 | 771 (716–830) | 4 | 0.8 |
| [Asu14] PeIA | 103 (96.5–111) | 4 | 1.2 | 613 (531–707) | 4 | 0.7 |
| [Arg14] PeIA | 49.4 (42.2–57.8) | 4 | 0.9 | 1036 (891–1206) | 4 | 0.9 |
| [Arg15] PeIA | 31.9 (23.6–43.1) | 4 | 0.7 | 371 (337–408) | 4 | 0.5 |
| [Nle15] PeIA | 21.0 (18.8–23.5) | 4 | 0.5 | 284 (240–337) | 4 | 0.3 |
| [Val15] PeIA | 5.70 (5.07–6.42) | 4 | 0.1 | 59.8 (55.1–64.9) | 4 | −0.3 |
| [Ile15] PeIA | 2.84 (2.66–3.03) | 4 | −0.4 | 113 (89.8–142) | 4 | −0.1 |
Values within parentheses indicate the 95% confidence interval; log change in IC50 calculated relative to native PeIA; n indicates the number of oocytes tested.
The changes in PeIA activity observed for human α6/α3β4 nAChRs were similar for rat α6/α3β4 nAChRs, but there were some notable differences. All analogues with substitutions of Ser4 showed reduced potency relative to native PeIA (Figure 5A; Table 4). [Asn9]PeIA showed a ~10-fold lower IC50 value for rat α6/α3β4 nAChRs, whereas [Thr9]PeIA and [Tyr9]PeIA showed no change (Figure 5B; Table 4) in contrast to the results obtained for the human receptor. Substitution of Ser9 with Arg resulted in a greater increase (~75-fold) in the IC50 value of PeIA for rat α6/α3β4 nAChRs compared to human (~14-fold). Analogues with substitutions of Val10 all showed similar increases in IC50 values except for [Nle10]PeIA for which a decrease in potency was observed (Figure 5C; Table 4). Analogues with substitutions of Asn11, Glu14, or Leu15 showed similar shifts in the IC50 values for human and rat α6/α3β4 nAChRs except for Asu14, which had less of an impact on the IC50 value of PeIA for rat α6/α3β4 (Figure 5D-F; Table 4).
Figure 5.
Presence of optimal amino-acid residues in key positions enhances binding of PeIA to rat α6/α3β4 nAChRs. X. laevis oocytes expressing rat α6/α3β4 nAChRs were subjected to TEVC electrophysiology and the IC50 values for inhibition of ACh-evoked currents by PeIA analogues determined as described in the Experimental Section. (A) All analogues with substitutions of Ser4 were moderately (≥5- to <10-fold) less potent on α6/α3β4 nAChRs than native PeIA. (B) [Asn9]PeIA was substantially (≥10-fold) more potent. [Asp9]PeIA was essentially inactive (IC50 > 10 μM); the responses in the presence of the peptide (10 μM) were 77 ± 6% (n = 4) of control values. (C) [Asp10]PeIA was also inactive (IC50 > 10 μM); the responses in the presence of the peptide (10 μM) were 95 ± 6% (n = 4) of control values. (D) [Arg11]PeIA and [Lys11]PeIA showed moderately (≥5- to <10-fold) increased potency. (E) Substitutions of Glu14 reduced PeIA activity by ~3- to ~10-fold except for Gln, which had no effect (<3-fold change in the IC50 value). (F) No changes (<3-fold) in PeIA activity were found with substitutions of Leu15. The peptides are rank-ordered from top to bottom by potency; see Table 4 for values. Data for [Tyr9]PeIA, [Arg14]PeIA, and [Aad14]PeIA have been offset slightly to avoid overlap. Data for PeIA were presented previously in Figure 2, and are shown here for ease of curve comparison. For analogues that inhibited the ACh-evoked currents by <50%, a pairwise comparison of their activities with that of native PeIA was performed by applying the peptides (10 μM) sequentially to the same oocyte. The responses in the presence of PeIA, [Thr4]PeIA, [Asp9]PeIA, and [Asp10]PeIA were 1.3 ± 0.4%, 93 ± 6% (p ≤ 0.0001), 86 ± 13% (p ≤ 0.0001), and 87 ± 13% (p ≤ 0.0001) of control values, respectively; (n = 4) for all; data not shown. The “±” values indicate the SD of the data for all experimental determinations; significance was determined with Students’ t-test.
Transmembrane Domain of the α6 Subunit Does Not Influence the Potency of PeIA, [Val7]PeIA, [Asn9]PeIA, [Arg11]PeIA, or [Ile15]PeIA for Rat α6β4 nAChRs.
To determine if the transmembrane domain of α3 present in the α6/α3 chimera influenced binding of PeIA to α6β4 nAChRs, we tested PeIA and four of its analogues on nonchimeric rat α6β4 nAChRs. We found that the IC50 values of PeIA, [Val7]PeIA, [Asn9]PeIA, [Arg11]PeIA, and [Ile15]PeIA were all similar (<2-fold difference) to the values obtained for rat α6/α3β4 nAChRs indicating that the α3 transmembrane domain did not substantially influence α-conopeptide binding with respect to α6β4 nAChRs (Figure 6).
Figure 6.
Potencies of PeIA and analogues for rat α6β4 nAChRs are similar to those for the α6/α3β4 chimera. X. laevis oocytes expressing rat α6β4 nAChRs were subjected to TEVC electrophysiology and the IC50 values for inhibition of ACh-evoked currents by PeIA and select analogues determined as described in the Experimental Section. Effects of the α3 transmembrane domain on ligand binding to α6β4 nAChRs were assessed by expressing nonchimeric α6 subunits with β4 subunits. PeIA, [Val7]PeIA, [Asn9]PeIA, [Arg11]PeIA, and [Ile15]PeIA were tested for inhibition of α6β4 and the IC50 values compared to those obtained for rat α6/α3β4 nAChRs (see Tables 2 and4). The IC50 value for inhibition of ACh-evoked currents by native PeIA was 222 (189–262) nM (n = 5). The IC50 values (nM) for [Val7]PeIA, [Asn9]PeIA, [Arg11]PeIA, and [Ile15]PeIA were 27.2 (22.9–32.2) (n = 4), 17.2 (14.9–20.1) (n = 4), 23.3 (20.1–27.1) (n = 4), and 49.7 (43.8–56.3) (n = 4), respectively. The IC50 values for inhibition of α6β4 and α6/α3β4 nAChRs were <2-fold different for all tested peptides. The peptides are rank-ordered from top to bottom by potency. Values within parentheses indicate the 95% CI of the IC50 estimate, and the error bars indicate the SD of the data. The curve for [Arg11]PeIA is shown offset slightly to avoid overlap.
Analogues of PeIA with Substitutions of Ala7, Ser9, Asn11, and Leu15 Show Increased Potency for α6/α3β4 nAChRs.
As shown in Figures 4-6, [Val7]PeIA, [Asn9]PeIA, [Arg11]PeIA, and [Ile15]PeIA were moderately to substantially more potent on human and rat α6/α3β4 nAChRs relative to native PeIA. To assess these observations further, we synthesized a series of PeIA analogues that included several of the substitutions that increased PeIA potency. The sequences of these new analogues are shown in Table 5.
Table 5.
Amino-Acid Sequences of PeIA and Analoguesa
| peptide | sequence |
|---|---|
| PeIA | GCCSHPACSVNHPELC |
| PeIA-5344 | GCCSHPVCHVRHPELC |
| PeIA-5640 | GCCSHPVCHVRHPEIC |
| PeIA-5652 | GCCSHPVCNVRHPELC |
| PeIA-5667 | GCCSHPVCNVRHPEIC |
| PeIA-5181 | GCCSHPVCRARHPALC |
| PeIA-5712 | GCCSHPVCRARHRALC |
Residues highlighted in red are those that differ from native PeIA. RP-HPLC chromatograms for each of the peptides are shown in SI Figure 2, and mass spectrometry values are provided in SI Table 1.
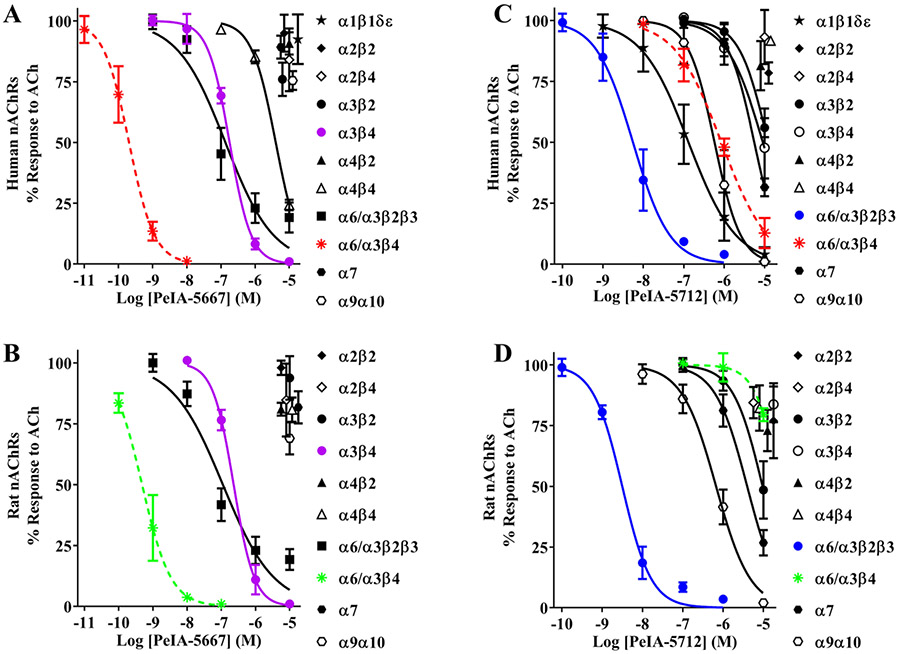
The first analogue, containing Val7, His9, and Arg11 (PeIA-5344), and the second, containing Val7, His9, Arg11, and Ile15 (PeIA-5640), showed substantially (~10 to 40-fold) increased potency for both human and rat α6/α3β4 nAChRs (Figure 7A,B; Table 6). The second pair containing Val7, Asn9, and Arg11 (PeIA-5652) and Val7, Asn9, Arg11 and, Ile15 (PeIA-5667) also showed substantially (~30 to 270-fold) increased potency for human and rat α6/α3β4 nAChRs. However, like each of the single-substituted peptides, all four of these analogues showed larger increases in potency for rat α6/α3β4 nAChRs compared to human. The presence of Asn9, instead of His9, in PeIA-5662 and PeIA-5667 had little additional impact on potency for human α6/α3β4 nAChRs, but Asn9 produced further gains in potency for rat receptors over PeIA-5344 and PeIA-5640. PeIA-5667 was ~270-fold more potent on rat α6/α3β4 nAChRs, relative to native PeIA, whereas for human α6/α3β4 nAChRs a smaller ~30-fold increase was observed.
Figure 7.
PeIA analogues with multiple optimal amino-acid residues show enhanced activity on α6/α3β4 nAChRs, while those with nonoptimal residues show decreased activity. X. laevis oocytes expressing human or rat α6/α3β4 nAChRs were subjected to TEVC electrophysiology, and the IC50 values for inhibition of ACh-evoked currents by PeIA analogues determined as described in the Experimental Section. (A, B) A series of six PeIA analogues were synthesized (see Table 5) and assessed for enhanced or decreased activity on α6/α3β4 nAChRs. Analogues PeIA-5344, PeIA-5640, PeIA-5652, and PeIA-5667 were ~16- to β34-fold more potent than native PeIA on human α6/α3β4 and ~43- to ~270-fold more potent on rat α6/α3β4 nAChRs. By contrast, PeIA-5181 and PeIA-5712 were ~8- and ~125-fold less potent on human α6/α3β4 and ~73- and >77-fold less potent on rat α6/α3β4 nAChRs, respectively. The response of rat α6/α3β4 nAChRs in the presence of PeIA-5712 (10 μM) was 80 ± 3% (n = 4) of control values. The “±” values and the error bars indicate the SD of the data; see Table 6 for IC50 values; data for PeIA were presented previously in Figure 2, and are shown here for ease of curve comparison. In a separate set of experiments, a pairwise comparison of the activity of PeIA-5712 with that of native PeIA on rat α6/α3β4 nAChRs was performed by applying the peptides (10 μM) sequentially to the same oocyte (data not shown). The responses in the presence of PeIA and PeIA-5712 were 1.3 ± 0.4% and 79 ± 6% (p ≤ 0.0001) of control values, respectively. The “±” values indicate the SD of the data (n = 4); significance was determined with Students’ t-test.
Table 6.
IC50 Values for Inhibition of Human and Rat α6/α3β4 nAChRs by PeIA Analogues with Optimized Ligand-Binding Motifsa
| human |
rat |
|||||
|---|---|---|---|---|---|---|
| peptide | IC50 (nM) | n | log IC50 ratio |
IC50 (nM) | n | log IC50 ratio |
| PeIA-5344 | 0.24 (0.19–0.30) | 5 | −1.4 | 1.73 (1.46–2.05) | 4 | −1.9 |
| PeIA-5640 | 0.41 (0.35–0.49) | 5 | −1.2 | 3.04 (2.51–3.69) | 4 | −1.8 |
| PeIA-5652 | 0.28 (0.23–0.35) | 4 | −1.4 | 1.81 (1.39–2.36) | 4 | −1.9 |
| PeIA-5667 | 0.20 (0.16–0.26) | 4 | −1.5 | 0.48 (0.37–0.63) | 4 | −2.4 |
| PeIA-5181 | 53.2 (49.4–57.3) | 4 | 0.9 | 9470 (6608–13 570) | 4 | 1.9 |
| PeIA-5712 | 853 (682–1065) | 4 | 2.1 | >10 000 | 4 | >2.0 |
Values within parentheses indicate the 95% confidence interval; log IC50 calculated relative to native PeIA; n indicates the number of oocytes tested.
The changes in affinity of PeIA resulting from these substitutions were, in part, due to altered dissociation kinetics. Figure 8 shows examples of ACh-evoked current traces from oocytes expressing either human or rat α6/α3β4 nAChRs. The oocytes were sequentially exposed to 5 min static bath applications of PeIA, then PeIA-5712, followed by PeIA-5667 and the response recovery monitored during wash-out of the peptide. Human α6/α3β4 nAChRs fully recovered from exposure to PeIA in about 10 min (Figure 8A) and full recovery from PeIA-5712 exposure occurred in <90 s. By contrast, at 10 min the responses had recovered to only ~2% after exposure to PeIA-5667 (Figure 8C). Similar results were found when the experiment was repeated for rat α6/α3β4 nAChRs except that the recovery rate was more rapid after exposure to PeIA with full recovery occurring in <180 s compared to the ~10 min required for human α6/α3β4 nAChRs. These results are consistent with the observed differences in IC50 values among the three peptides.
Figure 8.

PeIA, PeIA-5712, and PeIA-5667 show distinct dissociation kinetics for α6/α3β4 nAChRs. X. laevis oocytes expressing human or rat α6/α3β4 nAChRs were subjected to TEVC electrophysiology and the recovery rates of the ACh-evoked currents were examined after a 5 min static bath application (10 μM) of PeIA, PeIA-5712, and PeIA-5667. (A–C) Current traces from a single oocyte expressing human α6/α3β4 nAChRs before peptide application and after a 10 min wash-out period. The responses to ACh recovered to 99 ± 3% (n = 4) and 107 ± 8% (n = 4) after exposure to PeIA and PeIA-5712, respectively. By contrast, responses after exposure to PeIA-5667 recovered to only 2 ± 1% (n = 4) of control values. (D–F) Current traces from a single oocyte expressing rat α6/α3β4 nAChRs before peptide application (10 μM) and after a 10 min wash period. The responses to ACh after exposure to PeIA and PeIA-5712 recovered to 96 ± 8% (n = 5) and 103 ± 4% (n = 5), respectively, but to only 6 ± 2% (n = 5) after exposure to PeIA-5667. The “±” values and the error bars indicate the SD of the data. The scale bars above the peptides indicate a 5 min static bath application. Each trace is 30 s in duration with a 30 s gap between each response (reduced to 5 s in the figure for brevity).
The last two analogues, PeIA-5181 and PeIA-5712 (see Table 5 for sequences), were synthesized to further assess the importance of position 9 in PeIA as well as positions 10, 13, and 14, but in this case, these analogues contained amino-acid residues that were unfavorable for activity on α6/α3β4 nAChRs and included Arg9, Ala10, Arg13, and Ala14. PeIA-5181 displayed a ~5-fold higher IC50 value, relative to native PeIA, for human α6/α3β4 nAChRs and a ~70-fold higher value for rat α6/α3β4 nAChRs. PeIA-5712 is identical in sequence to PeIA-5181 with the exception of an Arg in position 13 instead of Pro. This substitution further reduced potency on human α6/α3β4 by ~16-fold, relative to PeIA-5181, whereas rat receptors were essentially insensitive (IC50 > 10 μM) to the peptide.
PeIA-5667 and PeIA-5712 Are Selective for α6/α3β4 and α6/α3β2β3 nAChRs, Respectively.
The selectivity profiles of PeIA-5667 and PeIA-5712 were assessed by testing them on a panel of ten human and rat nAChR subtypes. The IC50 values of PeIA-5667 for human α3β4, α4β4, and α6/α3β2β3 nAChRs were ~895-, >20 000-, and ~675-fold higher, respectively, than that for α6/α3β4 nAChRs (Figure 9A; Table 7). PeIA-5667 was essentially inactive (IC50 values >10 μM) on α1β1δε, α2β2, α2β4, α3β2, α4β2, α7, and α9α10 nAChRs. Similar results were found for rat nAChRs, but PeIA-5667 was also inactive (IC50 > 10 μM) on the α4β4 subtype (Figure 9B; Table 7). Next, we tested PeIA-5712 on the same panel of human and rat nAChR subtypes. PeIA-5712 was most potent on human α6/α3β2β3 with lower potency on α3β4, α7, and α9α10 subtypes (Figure 9C; Table 8) and essentially inactive (IC50 values >10 μM) on α2β2, α2β4, α3β2, α4β2, and α4β4 nAChRs. Like human α6/α3β2β3 nAChRs, the rat homolog was sensitive to low nM concentrations of PeIA-5712, and the IC50 values for α2β2, α2β4, α3β4, α4β2, α4β4, and α6/α3β4 were also >10 μM (Figure 9D; Table 8). Low potency was observed for rat α3β2, α7, and α9α10 nAChRs with IC50 values >2000, ~1275, and ~212-fold higher, respectively, than that for α6/α3β2β3 nAChRs.
Figure 9.
PeIA-5667 and PeIA-5712 are selective for α6/α3β4 and α6/α3β2β3 nAChRs, respectively. X. laevis oocytes expressing human or rat nAChRs were subjected to TEVC electrophysiology and the IC50 values for inhibition of ACh-evoked currents by PeIA analogues determined as described in the Experimental Section. The selectivity profiles of PeIA-5667 and PeIA-5712 were assessed by testing the peptides on a panel of nAChR subtypes. (A) PeIA-5667 was essentially inactive (IC50 values >10 μM) on human α1β1δε, α2β2, α2β4, α3β2, α4β2, α7, and α9α10 subtypes. The responses in the presence of the peptide (10 μM) were 93 ± 10% for α1β1δε, 95 ± 8% for α2β2, 84 ± 13% for α2β4, 76 ± 7% for α3β2, 91 ± 9% for α4β2, 89 ± 5% for α7, and 76 ± 4% for α9α10 nAChRs, compared to controls (n = 4 for all). Note that the IC50 value for α6/α3β4 (dashed red) was ~895-fold lower than that for α3β4 (purple) nAChRs. (B) Selectivity profile of PeIA-5667 for rat nAChRs was similar but was also inactive on the α4β4 subtype. The responses in the presence of PeIA-5667 (10 μM) were 98 ± 3% (n = 4) for α2β2, 85 ± 15% (n = 4) for α2β4, 94 ± 9% (n = 5) for α3β2, 81 ± 5% (n = 4) for α4β2, 81 ± 5% (n = 5) for α4β4, 82 ± 7% (n = 4) for α7, and 69 ± 7% (n = 4) for α9α10 nAChRs, compared to controls. The IC50 value for α6/α3β4 (dashed green) was ~489-fold lower than that for α3β4 (purple) nAChRs. (C) PeIA-5712 was most potent on α6/α3β2β3 (blue) and showed substantially (~150-fold) lower activity on human α6/α3β4 (dashed red) nAChRs. PeIA-5712 was essentially inactive (IC50 > 10 μM) on α2β2, α2β4, α4β2, and α4β4 subtypes; the responses in the presence of the peptide (10 μM) were 79 ± 4% for α2β2, 93 ± 11% for α2β4, 82 ± 10% for α4β2, and 92 ± 2% for α4β4, compared to controls (n = 4 for all). (D) For rat subtypes, PeIA-5712 was also most potent on α6/α3β2β3 (blue) and showed substantially (>3000-fold) lower activity on α6/α3β4 (dashed green) nAChRs. PeIA-5712 was essentially inactive on α2β2, α2β4, α3β4, α4β2, and α4β4 subtypes; the responses in the presence of the peptide (10 μM) were 77 ± 15% (n = 5) for α2β2, 85 ± 6% (n = 4) for α2β4, 84 ± 7% (n = 4) for α3β4, 73 ± 9% (n = 4) for α4β2, and 82 ± 9%(n = 4) for α4β4 compared to controls. The “±” values and the error bars indicate the SD of the data; see Table 7 for IC50 values. Data for PeIA-5667 and PeIA-5712 on α6/α3β4 nAChRs (dashed red) were presented previously in Figure 8, and are shown here for ease of curve comparison. Data points in (A–D) for single applications of peptide (10 μM) are shown staggered to avoid overlap.
Table 7.
Selectivity Profile of PeIA-5667 for Human and Rat nAChRsa
|
human
|
rat
|
|||||
|---|---|---|---|---|---|---|
| nAChR | IC50 (nM) | n | IC50 ratio | IC50 (nM) | n | IC50 ratio |
| α1β1δε | >10 000 | 4 | >50 000 | ND | ||
| α2β2 | >10 000 | 4 | >50 000 | >10 000 | 4 | >20 000 |
| α2β4 | >10 000 | 4 | >50 000 | >10 000 | 4 | >20 000 |
| α3β2 | >10 000 | 4 | >50 000 | >10 000 | 5 | >20 000 |
| α3β4 | 179 (160–201) | 4 | 895 | 230 (199–265) | 4 | 489 |
| α4β2 | >10 000 | 4 | >10 000 | >10 000 | 4 | >20 000 |
| α4β4 | 4015 (3620–4453) | −4 | 20 075 | >10 000 | 5 | >20 000 |
| α6/α3β2β3 | 135 (88.2–207) | 6 | 675 | 111 (67.0–184) | 4 | 227 |
| α7 | >10 000 | 4 | >50 000 | >10 000 | 4 | >20 000 |
| α9α10 | >10 000 | 4 | >50 000 | >10 000 | 4 | >20 000 |
Values within parentheses indicate the 95% confidence interval; IC50 ratio calculated relative to the IC50 value for α6/α3β4; n indicates the number of oocytes tested; ND, not determined.
Table 8.
Selectivity Profile of PeIA-5712 for Human and Rat nAChRsa
| human |
rat |
|||||
|---|---|---|---|---|---|---|
| nAChR | IC50 (nM) | n | IC50 ratio | IC50 (nM) | n | IC50 ratio |
| α1β1δε | 131 (96.4–178) | 7 | 23 | ND | ||
| α2β2 | >10 000 | 4 | >1800 | >10 000 | 4 | >3185 |
| α2β4 | >10 000 | 4 | >1800 | >10 000 | 4 | >3185 |
| α3β2 | 12 200 (10 710–13 910) | 4 | 2218 | 9505 (7331–12 320) | 4 | 2070 |
| α3β4 | 9064 (6016–13 660) | 4 | 1648 | >10 000 | 4 | >3185 |
| α4β2 | >10 000 | 4 | >1800 | >10 000 | 4 | >3185 |
| α4β4 | >10 000 | 4 | >1800 | >10 000 | 4 | >3185 |
| α6/α3β2β3 | 5.55 (4.17–7.39) | 4 | 1 | 3.14 (2.59–3.81) | 4 | 1 |
| α7 | 5556 (4544–6793) | 4 | 1000 | 3942 (3209–4841) | 4 | 1275 |
| α9α10 | 576 (433–768) | 4 | 104 | 667 (539–825) | 4 | 212 |
Values within parentheses indicate the 95% confidence interval; IC50 ratio calculated relative to the IC50 value for α6/α3β2β3; n indicates the number of oocytes tested; ND, not determined.
DISCUSSION AND CONCLUSIONS
In an effort to elucidate differences in the ligand-binding motifs of human and rat α6β4 nAChRs, we performed molecular dynamics simulations of PeIA bound to α6β4 nAChRs. In these simulations, PeIA residues in positions 4, 9, 10, 11, 13, and 14 were shown to interact with nonconserved residues of the β4 subunit including those in positions 36, 119, 163, and 168. To functionally assess these interactions, we performed a series of electrophysiology experiments to determine α-conopeptide activity on human α6/α3β4 and rat α6/α3β4 or α6β4 nAChRs heterologously expressed in oocytes. First, we tested MII and found that, like PeIA, MII is substantially more potent on human α6/α3β4 nAChRs. We then used this finding to probe the influence of the positions suggested by molecular modeling to be important for activity by substituting single positions of PeIA with the amino acids found in the homologous positions of MII. With the exception of the Asn to Glu substitution at position 11, most of the substitutions examined caused only minor (3–5-fold) changes in the IC50 value of PeIA for human α6/α3β4 nAChRs. By contrast, analogues [Val7]PeIA, [His9]PeIA, and [Glu11]PeIA showed ~10-fold changes in IC50 values for rat α6/α3β4 nAChRs. We then carried out an Ala-scan of PeIA and determined that substitutions of positions 9, 13, 14, and 15 caused at least a 10-fold decrease in activity at human α6/α3β4 nAChRs but had less influence on potency for the rat receptor.
To further assess the molecular interactions predicted by molecular dynamics simulations, we then carried out a range of substitutions of positions 4, 9, 10, 11, 14, and 15 of PeIA with the intent to modulate the activity for both human and rat α6/α3β4 nAChRs. We designed variants of PeIA that incorporated several of the substitutions identified as increasing activity at both receptors and in some cases acting differentially depending on the receptor species. Iterative substitution of PeIA resulted in the creation of an analogue, PeIA-5667, with subnanomolar and equipotent activity for both human and rat α6/α3β4 nAChRs. Using the molecular models created for native PeIA, we discuss here the impact of the substitutions that facilitated the design of PeIA-5667, i.e., A7V, S9N, N11R, and L15I. We note that these substitutions seem to have additive effects as shown by the sum of the log change in IC50 (relative to the native peptide) for the single position variants compared to PeIA-5667: the sums are −1.7 and −2.9 compared to −1.5 and −2.4 for the human and rat receptors, respectively. This additivity suggests that the four substitutions used to create PeIA-5667 could be rationalized from the results obtained with each single-substituted analog.
The A7V substitution, which was suggested by homology with MII, led to increased activity at both receptors, albeit a larger increase was observed at the rat receptor. The molecular models suggest that Ala7 is at the interface with the receptor and contacts several positions of the α6 subunit including the side chain of Tyr194 and several backbone atoms from residues Ser145 to Asp149. Additionally, Ala7 was found to interact more frequently with Arg81 and Tyr90 in the context of human α6β4 than for the rat receptor. The A7V substitution introduces a longer side chain, which should increase the number of contacts at the peptide–receptor interface (van der Waals) for example with the side chains of Arg81 and Tyr90 in the rat α6 subunit. PeIA Ser9 established two stable hydrogen bonds with human β4 Lys59 during the molecular dynamics simulations of human α6β4 nAChRs, but these hydrogen bonds were not present in models of the rat receptor (SI Figure 1A). The 10-fold decrease in activity of [Ala9]PeIA at human α6β4, but not at the rat receptor, seems to correlate with the presence of these hydrogen bonds. The side chain of β4 Lys59 in the rat receptor is involved in a stable salt-bridge interaction with β4 Glu36 and is not conserved in the human receptor which possesses a noncharged Gln residue in position 36. The nature of residue 36 might therefore be at the origin of the differential interaction of PeIA Ser9 at the two species receptors because Lys59 preferentially interacts with Glu36 (SI Figure 1B). We note that an amino-acid residue at position 9 with a longer side chain than Ser could potentially reach the Lys59 nitrogen and establish a hydrogen bond even in the context of the rat receptor, thereby explaining the 10-fold specific increase in activity at the rat receptor resulting from the S9N substitution. We have previously shown that Ser9 of α-Ctx TxID can interact with β4 Lys59 but only in the context of rat α6β4 and not in that of the rat α3β4 subtype,19 suggesting that it is indeed possible for position 9 to interact with rat β4 Lys59. Substitution of Asn11 by negatively charged residues Asp or Glu led to at least a 10-fold decrease in activity at both species receptors, whereas substitution by positively charged residues Lys or Arg caused an increase in activity at both receptors. According to the molecular models, Asn11 is located in a negatively charged environment in the binding sites of both human and rat α6β4 nAChRs (Figure 1C,D) providing a simple explanation for the impact of the substitutions based on charge attraction/repulsion. Finally, with respect to position 15, PeIA Leu15 constantly interacted with the vicinal disulfide Cys189–Cys190 of the α6 subunit for both receptors during the molecular dynamics simulations. The L15A substitution resulted in a 10-fold decrease in activity at the human α6β4 nAChR but not at the rat receptor. It is interesting to note that Ala substitution of Glu14 led to a similar decrease in activity at both receptors. In the molecular models, a salt bridge established between Glu14 and β4 Arg113 was only stable in the context of the human receptor, providing an explanation for the species-dependent effect of the E14A substitution. Because the E14A and L15A substitutions have similar impact on activity, we propose that the L15A substitution could cause a change of conformation of the C-terminal residues Glu14 and Ala15 of loop 2, resulting in new packing interactions with Cys189–Cys190 but a loss of the PeIA Glu14- β4 Arg113 salt bridge in the context of the human receptor. Ile and Leu have different side-chain shapes but similar ability to interact through hydrophobic effects. Accordingly, the L15I substitution should preserve the interaction with the vicinal disulfide bond, and indeed resulted in only a modest change of activity of native PeIA.
A central goal of this work was to identify pharmacological differences in the ligand-binding motifs between human and rat α6β4 nAChRs, but also to generate ligands that can be used to study the role of this subtype in animal models of neuropathic pain. To this end, we created a series of analogues containing amino acids that enhance PeIA affinity for α6β4 nAChRs. Additionally, we synthesized two analogues with predicted lower affinity for α6β4 nAChRs by incorporating amino acids that were unfavorable for ligand binding to further highlight the importance of particular interactions between PeIA and α6β4 nAChRs. The first four analogues in Table 5 showed substantially increased potency for both human and rat α6/α3β4 nAChR. PeIA-5667, in particular, was ~30-fold more potent on human α6/α3β4 and ~270-fold more potent on rat α6/α3β4 nAChRs than native PeIA. PeIA-5712, by contrast, showed a substantial loss of potency on both human and rat α6/α3β4 nAChRs. Human α6/α3β4 nAChRS were ~125-fold less sensitive to PeIA-5712, compared to the native peptide, while rat receptors were essentially insensitive (IC50 > 10 μM) to the peptide. Next, we assessed the selectivity profiles of PeIA-5667 and PeIA-5712 on a panel of human and rat nAChR subtypes and found that PeIA-5667 was active on only two of the ten subtypes examined and include α3β4 and α6/α3β2β3 nAChRs (Figure 9A,B; Table 7). However, the IC50 values for human and rat α3β4 and α6/α3β2β3 nAChRs were >200-fold higher than that for α6/α3β4 nAChRs. PeIA-5712, by contrast, was most active on α6/α3β2β3 nAChRs and >100-fold less active on all other neuronal human and rat nAChR subtypes tested including α6/α3β4 (Figure 9CD; Table 8).
Several nAChRs have been implicated in neuropathic pain including α6β4, α7, and α9α10 subtypes.1,3,20-24 Notably, the most highly expressed subtypes in sensory DRG neurons are α3β4*, α6β4*, and α7.2,25 Previously, the most α6β4-selective α-conopeptide, [Ala5,Hyp6]BuIA, showed some preference for rat α6β4 but only 20-fold selectivity over the α3β4 subtype.26 α-Conopeptide [Ala11]MII is selective for rat α6β4 over α3β4 nAChRs (~328-fold) but shows equal affinity for α3β2 and higher affinity for α6/α3β2β3 nAChRs,27 two subtypes that may also be expressed by rat as well as human DRG neurons.2,7 PeIA-5667 was ~489-fold more potent on rat α6/α3β4 than α3β4 and >20 000-fold over α3β2, α7, and α9α10 nAChRs. Thus, PeIA-5667 possesses superior affinity and selectivity compared to previous α-conopeptides with activity on α6β4 nAChRs (Table 9).
Table 9.
Selectivity Profiles of Select α-Conopeptides that Target Rat α6-Containing nAChRsa
| IC50 values (nM) for nAChR subtypes | |||||||
|---|---|---|---|---|---|---|---|
| α-conopeptide | α3β2 | α3β4 | α4β2 | α4β4 | α6/α3β2β3 | α6/α3β4 | α7 |
| PeIA | b9.7 (8.1–11.7) | b1500 (1300–1700) | c>10 000 | ND | b11.1 (8.2–15.0) | d130 (117–145) | c1800 (1396–2206) |
| PeIA-5667 | d>10 000 | d230 (199–265) | d>10 000 | d>10 000 | d111 (67.0–187) | d0.49 (0.39–0.66) | d>10 000 |
| PeIA-5712 | d9505 (7331–12 320) | d>10 000 | d>10 000 | d>10 000 | d3.14 (2.59–3.81) | d>10 000 | d3941(3209–4841) |
| PeIA-5069 | e>10 000 | e>10 000 | e>10 000 | e>10 000 | e2.16 (1.98–2.35) | e43.8 (37.4–51.2) | e>10 000 |
| [Ala9,Ala15]MII | f4850 (340–6630) | f7800 (5300–11 500) | f>10 000 | f>10 000 | f2.40 (1.68–3.43) | f269 (153–476) | f>10 000 |
| [Ala5,Hyp6]BuIA | g>10 000 | g1200 (969–1390) | g>10 000 | g>10 000 | g>10 000 | g58.1 (50.2–67.3) | g>10 000 |
Effective treatment of chronic pain syndromes remains difficult and elusive. Current therapies, including nerve ablation and pharmacotherapy are, at best, only partially effective and often produce significant side effects.28,29 The widespread use of opioid drugs to treat pain has led to increased prevalence of dependence and abuse, precipitating a public health crisis. New analgesics that do not interact with the opioid system are needed. Nicotinic acetylcholine receptors that contain the α6 subunit have been shown to be essential for the analgesic response to nicotine and other compounds in rodent models of neuropathic pain and may represent a promising molecular target for novel drug therapies.1,3 Rodents are used to study the role of α6β4 nAChRs in neuropathic pain, but species variability in terms of expression patterns and receptor sensitivities to ligands can confound cross-species translation of experimental findings. Here, we demonstrate that human and rat α6β4 nAChRs show significant differences in sensitivity to α-conopeptides. Understanding these differences at the molecular level may be important and helpful for designing and testing new analgesic compounds in rodent models. To this end, we have mapped the chemical space of the α6β4 nAChR ligand-binding site using molecular dynamics simulations coupled with functional SAR studies using analogues of PeIA and show that despite high sequence homology, human and rat α6β4 nAChRs have pharmacologically distinct ligand-binding motifs. The high selectivity of PeIA-5667 and PeIA-5712 should make them useful pharmacological tools for dissecting the roles of α6-containing nAChRs in neuropathic pain and particularly for identifying different sensory neurons subtypes that express α6β4 nAChRs.
EXPERIMENTAL SECTION
Molecular Dynamics Simulation and Modeling.
Initial models of PeIA bound to the ligand binding domain of human α6β4 nAChR were built by homology using Modeller 9v2430 and the crystal structures of human α3β4 (PDB: 6PV7)31 and that of PeIA bound to the ACh binding-protein (AChBP) from Aplysia californica (PDB: 5JME).16 A total of 100 models were generated and the model with the lowest DOPE score32 was considered for further analysis using molecular dynamics simulations. Water molecules were first transferred from the high-resolution structure of α-Ctx ImI/AChBP complex (PDB: 2BYP) to the PeIA/human α6β4 homology model. The system was then solvated in an octahedron box in which 18 000 water molecules were added using tleap from the AMBER20 simulation package.33 At the same time, sodium and chloride ions were also added to reach 150 mM NaCl concentration. The system was energy minimized over 10 000 steps of steepest descent algorithm and then equilibrated in two steps: (1) raising the temperature to 300 K under constant volume and then equilibration of pressure to 1 atmosphere while constraining the positions of all proteins; and (2) progressively releasing the positions restraints on the side-chain atoms and then on the backbone atoms over a total of 22 ns simulation time. A molecular dynamics simulation of 200 ns was then carried out without position restraints with the exception of the backbone atoms of the three loops from each subunit that are at the interface with the transmembrane domains, as observed in the crystal structure of the α3β4 subtype (PDB: 6PV7). These loops are located at a distance from the α-Ctx binding sites, and restraining them is necessary to maintain the integrity of the ligand-binding domain in the absence of the transmembrane domain. During the molecular dynamics simulation, the Monte Carlo barostat (pressure relaxation time of 1.0 ps) and the Langevin thermostat (damping coefficient of 2.0 ps−1) were employed to maintain the temperature to 300 K and the pressure to 1 atmosphere, respectively. All bonds involving hydrogen atoms were restrained using the SHAKE algorithm, and the mass of the hydrogen atoms was repartitioned to enable the use of a 4.0 fs time step.34 All molecular simulations were carried out using the ff14SB force field35 and pmemd from the AMBER20 package. The molecular dynamics simulations were analyzed using cpptraj from the AMBER20 package. Molecular models of the complex between PeIA and the rat α6β4 nAChR were obtained by mutating, using Modeller 9v24, the model of the human α6β4 nAChR obtained after the 200 ns molecular dynamics simulation and then carrying similar energy minimization, equilibration, and molecular dynamics simulations as for the PeIA/human α6β4 nAChR complex.
Peptide Synthesis.
For simplicity, native peptides discovered in Conus venom or whose sequence was predicted from a Conus cDNA library are referred to as α-Ctxs. Synthetic analogues of α-Ctxs are referred to here as α-conopeptides or simply peptides for brevity. Synthesis of the new peptides described herein was performed using Fmoc solid-phase synthesis techniques; detailed synthesis methods for these as well as for previously described peptides are described elsewhere.18,36,37 The masses of the peptides were verified by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF), and correct folding of the peptides, purity, and yield were determined by reverse-phase high-performance liquid chromatography (RP-HPLC). Results of these analyses can be found in SI Table 1. All peptides had purity levels ≥95%, as determined by RP-HPLC.
Oocyte Electrophysiology.
Protocols (No. 17–07020) for obtaining oocytes from X. laevis frogs were approved by the University of Utah’s Institutional Animal Care and Use Committee. Frogs were purchased from Xenopus1 (Dexter, MI) and maintained by university personnel in an AAALAC accredited facility. Oocytes were obtained from frogs anesthetized with 0.4% wt/vol Tricaine-S (Thermo Fisher Scientific, Waltham, MA) and were sacrificed after removal of the ovarian lobes.
Methods for the preparation of cRNA constructs for expression of nAChRs in X. laevis oocytes have been previously described.16 Clones for human α1, β1, δ, and ε subunits were obtained from A.G. Engel (Mayo Clinic, Rochester, MN). Clones for human α3, α4, α6, α7, β2, β3, and β4 were provided by J. Garrett (Cognetix, Salt Lake City, UT) and the α9 and α10 clones by L.R. Lustig (University of California San Francisco, San Francisco, CA). The α6/α3 construct was provided by J.M. Lindstrom (University of Pennsylvania, Philadelphia, PA). Clones for α3, α4, and α7 subunits were provided by S. Heinemann (Salk Institute, La Jolla, CA), β2, β3, and β4 by C. Luetje (University of Miami, Miami, FL), and α9 and α10 by A.B. Elgoyhen (Universidad de Buenos Aires, Buenos Aires, Argentina). The α6 clone and the α6/α3 construct were provided by J. Garett. The α6/α3 constructs were used because injection of oocytes with cRNAs encoding human or rat α6 subunits results in few or no functional receptors when coexpressed with β2 and β3 subunits.38,39 Furthermore, expression of functional human α6β4 nAChRs failed across multiple oocyte donors (data not shown). Functional rat α6β4 nAChRs do express, but at low levels, and were used for some experiments to assess and compare IC50 values of rat α6β4 with α6/α3β4 nAChRs. Previous comparisons of α-conopeptide activity on α6β4 and α6/α3β4 nAChRs indicate similar IC50 values.18 For the purposes of this work, α6/α3 refers to the chimeric expression construct used here for functional studies of α6/α3β2β3 and α6/α3β4 nAChRs in oocytes. The nonchimeric rat α6 subunit expression construct is used for the study of α6β4 nAChRs in oocytes, but “α6β4” is also used to describe conceptual ideas, for example, the expression of native α6β4* nAChRs in neurons.
Stage IV–V oocytes were injected with equal ratios cRNAs encoding cloned human or rat nAChR subunits and subjected to two-electrode voltage-clamp (TEVC) electrophysiology 1–5 days after injection. For expressing human α1β1δε nAChRs, the oocytes were injected in the nucleus with equal ratios of cDNAs coding each subunit. The concentrations of ACh used were 100 μM for α1β1δε, β2-containing subtypes, α9α10 and 300 μM for all others. Acetylcholine (ACh) was applied at 60 s intervals for 1 s. For the assessment of peptide activity, the oocytes were continuously perfused with frog saline (control solution) and pulsed with ACh until a stable baseline-response was observed. The saline was then switched to one containing peptide and the ACh responses monitored for changes in amplitude. The ACh responses in the presence of peptide were normalized to the average of three responses in control solution. Peptides were applied in this manner for concentrations up to 1 μM. For concentrations >1 μM, peptides were applied in a static bath for 5 min and normalized to the ACh response after a 5 min bath application of control solution.
Statistical Analysis.
All statistical analyses were performed using Prism 8 (GraphPad Software, San Diego, CA). The estimated IC50 values for inhibition of ACh-evoked currents by peptides, were obtained by nonlinear regression using a four-parameter logistic equation and presented with the corresponding 95% CI to evaluate the precision of the IC50 estimate. The error bars indicate the SD of the data points obtained at each concentration and are provided to assess the variance of the data. The IC50 values of all PeIA analogues were compared to that of native PeIA to evaluate the effect of amino-acid residue substitution. To qualitatively compare changes in PeIA activity as a result of these substitutions, the IC50 values were classified as follows: analogues with values that were <3-fold different from that of native PeIA were considered to show very little, if any, change in activity; analogues with values ≥3- to ≤5-fold were considered to show minor changes in activity; values >5- to <10-fold were considered to show moderate changes in activity; and changes in activity ≥10-fold were considered substantial. Analogues that produced ≤50% inhibition of the ACh responses after a 10 μM application were considered to be essentially inactive relative to native PeIA. To compare the activities of these analogues with that of native PeIA, percent response values were obtained from oocytes sequentially exposed to PeIA (10 μM) and the analogues (10 μM) in the same oocytes and the values compared with a Student’s t-test. Differences were considered significant if the p value was ≤0.05. Data were analyzed for normality using a Shapiro–Wilk test.
Supplementary Material
ACKNOWLEDGMENTS
Funding for this study was provided by NIH grants R35 GM136430 and R01 GM103801 to J.M.M.; D.J.C. is an Australian Research Council Laureate Fellow (FL150100146), and work in his lab is supported by grant CE200100012 from the Australian Research Council. The authors declare no competing financial interests.
ABBREVIATIONS USED
- ACh
acetylcholine
- α-Ctx
α-conotoxin
- MALDI-TOF
matrix-assisted laser desorption/ionization time-of-flight mass spectrometry
- nAChRs
nicotinic acetylcholine receptors
- RP-HPLC
reverse-phase high-performance liquid chromatography
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jmedchem.0c01973.
Molecular models of the interactions of PeIA Ser9 with human or rat α6β4 nAChRs (Figure 1); chromatographic analysis of peptide purity by RP-HPLC (Figure 2); and quantitative analysis of peptide yield, purity, and mass (Table 1) (PDF)
The authors declare no competing financial interest.
Contributor Information
Arik J. Hone, School of Biological Sciences, University of Utah, Salt Lake City, Utah 84112, United States; MIRECC, George E. Whalen Veterans Affairs Medical Center, Salt Lake City, Utah 84148, United States.
Quentin Kaas, Institute for Molecular Bioscience, Australian Research Council Centre of Excellence for Innovations in Peptide and Protein Science, The University of Queensland, Brisbane, Queensland 4072, Australia.
Ireland Kearns, School of Biological Sciences, University of Utah, Salt Lake City, Utah 84112, United States.
Fuaad Hararah, School of Biological Sciences, University of Utah, Salt Lake City, Utah 84112, United States.
Joanna Gajewiak, School of Biological Sciences, University of Utah, Salt Lake City, Utah 84112, United States.
Sean Christensen, School of Biological Sciences, University of Utah, Salt Lake City, Utah 84112, United States.
David J. Craik, Institute for Molecular Bioscience, Australian Research Council Centre of Excellence for Innovations in Peptide and Protein Science, The University of Queensland, Brisbane, Queensland 4072, Australia.
J. Michael McIntosh, School of Biological Sciences and Department of Psychiatry, University of Utah, Salt Lake City, Utah 84112, United States; George E. Whalen Veterans Affairs Medical Center, Salt Lake City, Utah 84148, United States.
REFERENCES
- (1).Wieskopf JS; Mathur J; Limapichat W; Post MR; Al-Qazzaz M; Sorge RE; Martin LJ; Zaykin DV; Smith SB; Freitas K; Austin JS; Dai F; Zhang J; Marcovitz J; Tuttle AH; Slepian PM; Clarke S; Drenan RM; Janes J; Al Sharari S; Segall SK; Aasvang EK; Lai W; Bittner R; Richards CI; Slade GD; Kehlet H; Walker J; Maskos U; Changeux JP; Devor M; Maixner W; Diatchenko L; Belfer I; Dougherty DA; Su AI; Lummis SC; Imad Damaj M; Lester HA; Patapoutian A; Mogil JS The nicotinic alpha6 subunit gene determines variability in chronic pain sensitivity via cross-inhibition of P2X2/3 receptors. Sci. Transl. Med 2015, 7, No. 287ra72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Hone AJ; Meyer EL; McIntyre M; McIntosh JM Nicotinic acetylcholine receptors in dorsal root ganglion neurons include the alpha6beta4* subtype. FASEB J. 2012, 26, 917–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Knowland D; Gu S; Eckert WA 3rd; Dawe GB; Matta JA; Limberis J; Wickenden AD; Bhattacharya A; Bredt DS Functional alpha6beta4 acetylcholine receptor expression enables pharmacological testing of nicotinic agonists with analgesic properties. J. Clin. Invest 2020, 130, 6158–6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Limapichat W; Dougherty DA; Lester HA Subtype-specific mechanisms for functional interaction between alpha6beta4* nicotinic acetylcholine receptors and P2X receptors. Mol. Pharmacol 2014, 86, 263–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Marucci G; Dal Ben D; Buccioni M; Marti Navia A; Spinaci A; Volpini R; Lambertucci C Update on novel purinergic P2X3 and P2X2/3 receptor antagonists and their potential therapeutic applications. Expert Opin. Ther. Pat 2019, 29, 943–963. [DOI] [PubMed] [Google Scholar]
- (6).Bernier LP; Ase AR; Seguela P P2X receptor channels in chronic pain pathways. Br. J. Pharmacol 2018, 175, 2219–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Zhang X; Hartung JE; Friedman RL; Koerber HR; Belfer I; Gold MS Nicotine Evoked Currents in Human Primary Sensory Neurons. J. Pain 2019, 20, 810–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Ray P; Torck A; Quigley L; Wangzhou A; Neiman M; Rao C; Lam T; Kim JY; Kim TH; Zhang MQ; Dussor G; Price TJ Comparative transcriptome profiling of the human and mouse dorsal root ganglia: an RNA-seq-based resource for pain and sensory neuroscience research. Pain 2018, 159, 1325–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Grant S; Lester HA Proteins for increased surface expression of the alpha6beta4 nicotinic acetylcholine receptor: nothing but good news? J. Clin. Invest 2020, 130, 5685–5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Prashanth JR; Dutertre S; Lewis RJ Pharmacology of predatory and defensive venom peptides in cone snails. Mol. BioSyst 2017, 13, 2453–2465. [DOI] [PubMed] [Google Scholar]
- (11).Jin AH; Muttenthaler M; Dutertre S; Himaya SWA; Kaas Q; Craik DJ; Lewis RJ; Alewood PF Conotoxins: Chemistry and Biology. Chem. Rev 2019, 119, 11510–11549. [DOI] [PubMed] [Google Scholar]
- (12).McIntosh JM; Plazas PV; Watkins M; Gomez-Casati ME; Olivera BM; Elgoyhen AB A novel alpha-conotoxin, PeIA, cloned from Conus pergrandis, discriminates between rat alpha9alpha10 and alpha7 nicotinic cholinergic receptors. J. Biol. Chem 2005, 280, 30107–30112. [DOI] [PubMed] [Google Scholar]
- (13).Giribaldi J; Dutertre S alpha-Conotoxins to explore the molecular, physiological and pathophysiological functions of neuronal nicotinic acetylcholine receptors. Neurosci. Lett 2018, 679, 24–34. [DOI] [PubMed] [Google Scholar]
- (14).Hone AJ; McIntosh JM; Azam L; Lindstrom J; Lucero L; Whiteaker P; Passas J; Blazquez J; Albillos A alpha-Conotoxins Identify the alpha3beta4* Subtype as the Predominant Nicotinic Acetylcholine Receptor Expressed in Human Adrenal Chromaffin Cells. Mol. Pharmacol 2015, 88, 881–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Azam L; Yoshikami D; McIntosh JM Amino acid residues that confer high selectivity of the alpha6 nicotinic acetylcholine receptor subunit to alpha-conotoxin MII[S4A,E11A,L15A]. J. Biol. Chem 2008, 283, 11625–11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Hone AJ; Talley TT; Bobango J; Huidobro Melo C; Hararah F; Gajewiak J; Christensen S; Harvey PJ; Craik DJ; McIntosh JM Molecular determinants of alpha-conotoxin potency for inhibition of human and rat alpha6beta4 nicotinic acetylcholine receptors. J. Biol. Chem 2018, 293, 17838–17852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Pucci L; Grazioso G; Dallanoce C; Rizzi L; De Micheli C; Clementi F; Bertrand S; Bertrand D; Longhi R; De Amici M; Gotti C Engineering of alpha-conotoxin MII-derived peptides with increased selectivity for native alpha6beta2* nicotinic acetylcholine receptors. FASEB J. 2011, 25, 3775–3789. [DOI] [PubMed] [Google Scholar]
- (18).Hone AJ; Ruiz M; Scadden M; Christensen S; Gajewiak J; Azam L; McIntosh JM Positional scanning mutagenesis of alpha-conotoxin PeIA identifies critical residues that confer potency and selectivity for alpha6/alpha3beta2beta3 and alpha3beta2 nicotinic acetylcholine receptors. J. Biol. Chem 2013, 288, 25428–25439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Wu Y; Zhangsun D; Zhu X; Kaas Q; Zhangsun M; Harvey PJ; Craik DJ; McIntosh JM; Luo S alpha-Conotoxin [S9A]TxID Potently Discriminates between alpha3beta4 and alpha6/alpha3beta4 Nicotinic Acetylcholine Receptors. J. Med. Chem 2017, 60, 5826–5833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Hone AJ; McIntosh JM Nicotinic acetylcholine receptors in neuropathic and inflammatory pain. FEBS Lett. 2018, 592, 1045–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Hone AJ; Servent D; McIntosh JM alpha9-containing nicotinic acetylcholine receptors and the modulation of pain. Br. J. Pharmacol 2018, 175, 1915–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Bagdas D; Wilkerson JL; Kulkarni A; Toma W; AlSharari S; Gul Z; Lichtman AH; Papke RL; Thakur GA; Damaj MI The alpha7 nicotinic receptor dual allosteric agonist and positive allosteric modulator GAT107 reverses nociception in mouse models of inflammatory and neuropathic pain. Br. J. Pharmacol 2016, 173, 2506–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Mohammadi S; Christie MJ alpha9-nicotinic acetylcholine receptors contribute to the maintenance of chronic mechanical hyperalgesia, but not thermal or mechanical allodynia. Mol. Pain 2014, 10, 1744–8069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Romero HK; Christensen S; Di Cesare Mannelli L; Gajewiak J; Ramachandra R; Elmslie KS; Vetter DE; Ghelardini C; Ladonato SP; Mercado JL; Olivera BM; McIntosh JM Inhibition of α9α10 nicotinic acetylcholine receptors prevents chemotherapy-induced neuropathic pain. Proc. Natl. Acad. Sci. U.S.A 2017, 114, E1825–E1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Genzen JR; Van Cleve W; McGehee DS Dorsal root ganglion neurons express multiple nicotinic acetylcholine receptor subtypes. J. Neurophysiol 2001, 86, 1773–1782. [DOI] [PubMed] [Google Scholar]
- (26).Azam L; Maskos U; Changeux JP; Dowell CD; Christensen S; De Biasi M; McIntosh JM alpha-Conotoxin BuIA[T5A;P6O]: a novel ligand that discriminates between alpha6beta4 and alpha6beta2 nicotinic acetylcholine receptors and blocks nicotine-stimulated norepinephrine release. FASEB J. 2010, 24, 5113–5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).McIntosh JM; Azam L; Staheli S; Dowell C; Lindstrom JM; Kuryatov A; Garrett JE; Marks MJ; Whiteaker P Analogs of alpha-conotoxin MII are selective for alpha6-containing nicotinic acetylcholine receptors. Mol. Pharmacol 2004, 65, 944–952. [DOI] [PubMed] [Google Scholar]
- (28).Berta T; Qadri Y; Tan PH; Ji RR Targeting dorsal root ganglia and primary sensory neurons for the treatment of chronic pain. Expert Opin. Ther. Targets 2017, 21, 695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Byrd D; Mackey S Pulsed radiofrequency for chronic pain. Curr. Pain Headache Rep 2008, 12, 37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Webb B; Sali A Comparative Protein Structure Modeling Using MODELLER. Curr. Protoc. Protein Sci 2016, 86, 2.9.1–2.9.37. [DOI] [PubMed] [Google Scholar]
- (31).Gharpure A; Teng J; Zhuang Y; Noviello CM; Walsh RM Jr.; Cabuco R; Howard RJ; Zaveri NT; Lindahl E; Hibbs RE Agonist Selectivity and Ion Permeation in the alpha3beta4 Ganglionic Nicotinic Receptor. Neuron 2019, 104, 501–511 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Shen MY; Sali A Statistical potential for assessment and prediction of protein structures. Protein Sci. 2006, 15, 2507–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Case DA; Belfon K; Ben-Shalom IY; Brozell SR; Cerutti DS; Cheatham TE III; Cruzeiro VWD; Darden TA; Duke RE; Giambasi G; Gilson MK; Gohlke H; Goetz AW; Harris R; Izadi S; Izmailov SA; Kasavajhala K; Krasny R; Kurtzman T; Lee TS; LeGrand S; Li P; Lin C; Liu J; Luchko T; Luo R; Man V; Merz KM; Miao Y; Mikhailovskii O; Monard G; Nguyen H; Onufriev A; Pan F; Pantano S; Qi R; Roe DR; Roitberg A; Sagui C; Schott-Verdugo S; Shen J; Simmerling CL; Skrynnikov NR; Smith J; Swails J; Walker RC; Wang J; Wilson L; Wolf RM; Wu X; Xiong Y; Xue DM; York DM; Kollman. AMBER; University of California: San Francisco, 2020. [Google Scholar]
- (34).Hopkins CW; Le Grand S; Walker RC; Roitberg AE Long-Time-Step Molecular Dynamics through Hydrogen Mass Repartitioning. J. Chem. Theory Comput 2015, 11, 1864–1874. [DOI] [PubMed] [Google Scholar]
- (35).Maier JA; Martinez C; Kasavajhala K; Wickstrom L; Hauser KE; Simmerling C ff14SB: Improving the Accuracy of Protein Side Chain and Backbone Parameters from ff99SB. J. Chem. Theory Comput 2015, 11, 3696–3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Hone AJ; Fisher F; Christensen S; Gajewiak J; Larkin D; Whiteaker P; McIntosh JM PeIA-5466: A Novel Peptide Antagonist Containing Non-natural Amino Acids That Selectively Targets alpha3beta2 Nicotinic Acetylcholine Receptors. J. Med. Chem 2019, 62, 6262–6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Hone AJ; Scadden M; Gajewiak J; Christensen S; Lindstrom J; McIntosh JM alpha-Conotoxin PeIA-[S9H,V10A,E14N] potently and selectively blocks alpha6beta2beta3 versus alpha6beta4 nicotinic acetylcholine receptors. Mol. Pharmacol 2012, 82, 972–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Kuryatov A; Olale F; Cooper J; Choi C; Lindstrom J Human alpha6 AChR subtypes: subunit composition, assembly, and pharmacological responses. Neuropharmacology 2000, 39, 2570–2590. [DOI] [PubMed] [Google Scholar]
- (39).Dowell C; Olivera BM; Garrett JE; Staheli ST; Watkins M; Kuryatov A; Yoshikami D; Lindstrom JM; McIntosh JM Alpha-conotoxin PIA is selective for alpha6 subunit-containing nicotinic acetylcholine receptors. J. Neurosci 2003, 23, 8445–8452. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.