Abstract
Background
Fluoropyrimidine drugs (such as 5-fluorouracil and capecitabine) are used to treat different types of cancer. However, these drugs may cause severe toxicity in about 10% to 40% of patients. A deficiency in the dihydropyrimidine dehydrogenase (DPD) enzyme, encoded by the DPYD gene, increases the risk of severe toxicity. DPYD genotyping aims to identify variants that lead to DPD deficiency and may help to identify people who are at higher risk of developing severe toxicity, allowing their treatment to be modified before it begins. Recommendations for fluoropyrimidine treatment modification are available for four DPYD variants, which are the focus of this review: DPYD∗2A, DPYD∗13, c.2846A>T, and c.1236G>A. We conducted a health technology assessment of DPYD genotyping for patients who have planned cancer treatment with fluoropyrimidines, which included an evaluation of clinical validity, clinical utility, the effectiveness of treatment with a reduced fluoropyrimidine dose, cost-effectiveness, the budget impact of publicly funding DPYD genotyping, and patient preferences and values.
Methods
We performed a systematic literature search of the clinical evidence. We assessed the risk of bias of each included systematic review and primary study using the Risk of Bias in Systematic Reviews (ROBIS) tool and the Newcastle-Ottawa Scale, respectively, and we assessed the quality of the body of evidence according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Working Group criteria. We performed a systematic economic literature review and conducted cost-effectiveness and cost–utility analyses with a half-year time horizon from a public payer perspective. We also analyzed the budget impact of publicly funding pre-treatment DPYD genotyping in patients with planned fluoropyrimidine treatment in Ontario. To contextualize the potential value of DPYD testing, we spoke with people who had planned cancer treatment with fluoropyrimidines.
Results
We included 29 observational studies in the clinical evidence review, 25 of which compared the risk of severe toxicity in carriers of a DPYD variant treated with a standard fluoropyrimidine dose with the risk in wild-type patients (i.e., non-carriers of the variants under assessment). Heterozygous carriers of a DPYD variant treated with a standard fluoropyrimidine dose may have a higher risk of severe toxicity, dose reduction, treatment discontinuation, and hospitalization compared to wild-type patients (GRADE: Low). Six studies evaluated the risk of severe toxicity in DPYD carriers treated with a genotype-guided reduced fluoropyrimidine dose versus the risk in wild-type patients; one study also included a second comparator group of DPYD carriers treated with a standard dose. The evidence was uncertain, because the results of most of these studies were imprecise (GRADE: Very low). The length of hospital stay was shorter in DPYD carriers treated with a reduced dose than in DPYD carriers treated with a standard dose, but the evidence was uncertain (GRADE: Very low). One study assessed the effectiveness of a genotype-guided reduced fluoropyrimidine dose in DPYD∗2A carriers versus wild-type patients, but the results were imprecise (GRADE: Very low).
We found two cost-minimization analyses that compared the costs of the DPYD genotyping strategy with usual care (no testing) in the economic literature review. Both studies found that DPYD genotyping was cost-saving compared to usual care. Our primary economic evaluation, a cost-utility analysis, found that DPYD genotyping might be slightly more effective (incremental quality-adjusted life years of 0.0011) and less costly than usual care (a savings of $144.88 per patient), with some uncertainty. The probability of DPYD genotyping being cost-effective compared to usual care was 91% and 96% at the commonly used willingness-to-pay values of $50,000 and $100,000 per quality-adjusted life-year gained, respectively. Assuming a slow uptake, we estimated that publicly funding pre-treatment DPYD genotyping in Ontario would lead to a savings of $714,963 over the next 5 years.
The participants we spoke to had been diagnosed with cancer and treated with fluoropyrimidines. They reported on the negative side effects of their treatment, which affected their day-to-day activities, employment, and mental health. Participants viewed DPYD testing as a beneficial addition to their treatment journey; they noted the importance of having all available information possible so they could make informed decisions to avoid adverse reactions. Barriers to DPYD testing include lack of awareness of the test and the fact that the test is being offered in only one hospital in Ontario.
Conclusions
Studies found that carriers of a DPYD variant who were treated with a standard fluoropyrimidine dose may have a higher risk of severe toxicity than wild-type patients treated with a standard dose. DPYD genotyping led to fluoropyrimidine treatment modifications. It is uncertain whether genotype-guided dose reduction in heterozygous DPYD carriers resulted in a risk of severe toxicity comparable to that of wild-type patients. It is also uncertain if the reduced dose resulted in a lower risk of severe toxicity compared to DPYD carriers treated with a standard dose. It is also uncertain whether the treatment effectiveness of a reduced dose in carriers was comparable to the effectiveness of a standard dose in wild-type patients.
For patients with planned cancer treatment with fluoropyrimidines, DPYD genotyping is likely cost-effective compared to usual care. We estimate that publicly funding DPYD genotyping in Ontario may be cost-saving, with an estimated total of $714,963 over the next 5 years, provided that the implementation, service delivery, and program coordination costs do not exceed this amount.
For people treated with fluoropyrimidines, cancer and treatment side effects had a substantial negative effect on their quality of life and mental health. Most saw the value of DPYD testing as a way of reducing the risk of serious adverse events. Barriers to receipt of DPYD genotyping included lack of awareness and limited access to DPYD testing.
Objective
This health technology assessment evaluates the clinical validity, clinical utility, and cost-effectiveness of DPYD genotyping in patients who have planned cancer treatment with fluoropyrimidines. It also evaluates the effectiveness of a genotype-guided reduced dose in carriers of certain DPYD variants compared to patients treated with a standard dose; the budget impact of publicly funding DPYD genotyping; and the experiences, preferences, and values of people with cancers that can be treated with fluoropyrimidines.
Background
Fluoropyrimidines and Fluoropyrimidine-Associated Toxicity
Fluoropyrimidines are drugs frequently used to treat several different types of cancer, including colorectal, breast, head and neck, pancreatic, and gastric cancers.1,2 This group of drugs includes 5-fluorouracil (5-FU), capecitabine, and tegafur.3,4 Capecitabine and tegafur are prodrugs of 5-FU—that is, once absorbed, they are metabolized (converted) to 5-FU.4,5 Fluoropyrimidines can be used alone or as the core component in several combination treatment regimens1,3,6; they can also be combined with radiotherapy.7 5-FU and capecitabine are used for cancer treatment in Ontario, but tegafur is not; it has not been approved by Health Canada.
5-FU has a narrow therapeutic window: that is, the difference between the minimum efficacious dose and the maximum tolerable dose is small.8 Although fluoropyrimidines are important for treating several types of cancer,6 10% to 40% of the patients who receive them may experience severe toxicity,8 which can be fatal in up to 1% of patients.4,9,10 Fluoropyrimidine-associated adverse events can occur as early as the first cycle of chemotherapy4 and include hematologic (leukopenia, neutropenia, anemia, thrombocytopenia), gastrointestinal (mucositis, diarrhea, nausea, and vomiting), and dermatologic (hand-foot syndrome) reactions.1,11,12 These adverse events can lead to hospitalization, dose reduction, treatment delay, and treatment discontinuation.11–13
The National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) define the levels of toxicity using a scale of 1 to 514: grade 1, mild; grade 2, moderate; grade 3, severe but not immediately life-threatening; grade 4, an event with life-threatening consequences; grade 5, a fatal adverse event.14
Factors that can influence the risk of fluoropyrimidine-associated toxicity include the patient's age, sex, renal function, and performance status; the type and stage of cancer; and the type, mode, and duration of administration of the fluoropyrimidine.5,10,15,16 The type of cancer treatment regimen can also play a role, because fluoropyrimidines are often used in combination with other anticancer drugs that are associated with toxicity (e.g., platinum-based drugs or irinotecan).10,16 Genetic factors that affect people's ability to metabolize fluoropyrimidines may also affect the development of toxicity.5,15
The antitumour activity of 5-FU includes inhibition of DNA synthesis and repair, resulting in cell death, and incorporation into DNA and RNA, causing damage. To exert that activity, 5-FU requires intracellular conversion into cytotoxic metabolites (i.e., the body's cells convert it into active molecules that kill cancer cells).1 Approximately 80% of the dose is catabolized into inactive metabolites (i.e., broken down into simpler molecules that do not kill cancer cells) before being eliminated, and the rest is eliminated unchanged in the urine.1,3,16
The dihydropyrimidine dehydrogenase (DPD) enzyme is the first and rate-limiting enzyme in the catabolic pathway1,3; it converts 5-FU into inactive metabolites in the liver.2,17 If DPD activity is lower than normal, less 5-FU is converted to the inactive metabolite, and more of the active metabolite accumulates,2 increasing a person's risk of toxicity.1,3 Deficiency in DPD accounts for 20% to 60% of the toxicity patients experience.10,18
The goal of testing for DPD deficiency is to reduce the risk of severe toxicity by allowing the fluoropyrimidine dose to be adjusted or an alternative treatment to be recommended, depending on the level of deficiency.2,10 In patients with partial DPD deficiency, the aim of a lower fluoropyrimidine dose is to maintain plasma levels of 5-FU and its metabolites at the intended therapeutic level2 (similar to patients with normal DPD activity); decrease the risk of severe toxicity; and maintain treatment efficacy.2,10 To avoid underdosing, the fluoropyrimidine dose can be increased in subsequent treatment cycles if the patient experiences no toxicity or clinically tolerable toxicity.8
Uridine triacetate is an oral antidote used after an overdose of 5-FU or capecitabine (even if asymptomatic),19 or in cases of early-onset, severe, or life-threatening toxicity,10,20 to reduce the risk of death.19 Uridine competes with toxic 5-FU metabolites for incorporation into RNA, reducing cellular damage.20 It has not been approved by Health Canada, but it is available through the Health Canada Special Access Program.20 Because its use is limited to the 96 hours after the end of 5-FU administration10,20 and mostly in emergency situations of overdose, we did not consider uridine triacetate as an alternative to DPYD genotyping, and it will not be included as a comparator in this review.
Clinical Need and Target Population
DPYD Genotype and DPD Deficiency
The DPYD gene is located on chromosome 1p22 and encompasses 23 exons. The DPYD gene encodes the enzyme DPD.2
In human cells, each gene found on an autosomal (non-sex) chromosome has two alleles-one inherited from each parent. Variations in the DNA sequence of a gene can be heterozygous (present in only one of the two alleles), homozygous (the identical variant is present in both alleles), or double or compound heterozygous (different variants present in each of the two alleles).
Normal DPD activity is thought to be associated with the wild-type allele—that is, the non-variant form. The presence of at least one variant allele of the DPYD gene may result in a structural change in the DPD enzyme translated from that allele and lead to reduced or absent enzyme activity.2
In this report, we will use the term “carrier” to refer to people who carry one or more DPYD gene variants that predispose to toxicity; we will use “wild-type” to refer to the form of the gene that does not predispose to toxicity.
There is wide inter- and intraindividual variation in the activity of the DPD enzyme, and the effect of each DPYD variant on DPD enzyme activity varies.8 Several DPYD variants have been studied, but an association with DPD deficiency has been observed for four variants in particular2:
c.1905+1G>A (DPYD∗2A; IVS14+1G>A; rs3918290)
c.1679T>G (DPYD∗13; I560S; rs55886062)
c.2846A>T (D949V; rs67376798)
c.[1236G>A; 1129-5923C>G]
Of these four variants, DPYD∗2A and DPYD∗13 have the strongest effect on DPD activity, resulting in 50% (DPYD∗2A) and 60% to 68% (DPYD∗13) reductions in enzyme activity in heterozygous carriers.8,10 In homozygous carriers, 100%6 (DPYD∗2A) and 75% (DPYD∗13) reductions2,10 have been shown. Heterozygous carriers of the c.2846A>T and c.1236G>A variants display 20% to 30% (c.2846A>T) and 20% to 35% (c.1236G>A) reductions in enzyme activity, respectively.8,10 In homozygous carriers, 50% (c.2846A>T) and 20% to 70% (c.1236G>A)reductions have been observed.2,10
Partial DPD deficiency affects 5% to 7% of the Caucasian population,4,21 and 0.01% to 0.2% are estimated to have complete DPD deficiency.4 Approximately 5% to 8% of people with African ancestry have partial DPD deficiency.4,21 In Caucasians, the c.1236G>A variant is the most common of the four, affecting 2.6% to 6.3% of the population;2 the estimated prevalence of c.2846A>T, DPYD∗2A, and DPYD∗13 in Caucasians is 1.1%, 0.7%, and 0.1%, respectively.2 The estimated prevalence of homozygous DPYD∗2A carriers is 0.1% in Caucasians.6 In people of African ancestry, the DPYD∗2A and the c.2846A>T variants have an estimated prevalence of 0.1%,2 but another less extensively studied DPYD variant, c.557A>G (Y186C), is more prevalent in this population, at 3% to 5%.8,22
Alternative or Complementary Tests
The DPD enzyme converts endogenous uracil into dihydrouracil.6 Its activity can be measured directly in peripheral blood mononuclear cells8 and indirectly by measuring plasma uracil concentrations or the dihydrouracil:uracil (UH2:U) ratio.6,8 These phenotype tests can be used as an alternative or a complement to DPYD genotyping.8 Their limitations include lack of availability8; difficulty implementing them as routine tests23; issues with the interpretation of results; unclear validation for predictive use6; the fact that thresholds for dose adjustment may not be established24; and lack of standardization for some tests.2
Systemic 5-FU levels can be measured with therapeutic drug monitoring (pharmacokinetics), a technique that can also be used to ensure that 5-FU levels are within the therapeutic range and reduce the risk of adverse effects.6,8
Health Technology Under Review
DPYD genotyping is an assay that identifies specific germline variants in the DPYD gene. It aims to predict the level of DPD enzyme activity based on the expected effect of each variant on DPD function. Genotyping methods are faster and easier, and they may be less expensive than phenotype tests.2
According to a 2017 review from the Institut National de d'Excellence en Santé et en Services Sociaux (INESSS),25 the analytical validity of DPYD genotyping was accurate in two studies that compared results from a real-time polymerase chain reaction (PCR) assay with those from DNA sequencing. In one of the studies (which included 165 people), the results from a DPYD∗2A real-time PCR test were identical to those from DNA sequencing.25 In the second study (in which 568 people were tested for eight DPYD variants using real-time PCR), DNA sequencing validation confirmed that there was 100% agreement between the two tests.25
Guidance on DPYD Genotyping From Regulatory Agencies
Canadian 5-FU and capecitabine monographs state that patients with DPD deficiency are at risk of severe life-threatening toxicity when treated with these drugs. The use of 5-FU and capecitabine is contraindicated in patients with known complete absence of DPD activity, and they should be used with extreme caution in patients with partial DPD deficiency.26–29
Some Canadian fluoropyrimidine product monographs state that testing for DPD deficiency should be considered prior to treatment, based on local availability and current guidelines.26,28,29
In April 2020, the European Medicines Agency (EMA)16,24 recommended that testing for DPD deficiency be done before starting cancer treatment with 5-fluorouracil, capecitabine, or tegafur using phenotype or genotype tests. According to the EMA, the level of the available evidence does not allow for conclusive recommendations on the most suitable of the two test types.16 The EMA states that fluoropyrimidines are contraindicated in patients with complete DPD deficiency24; in patients with partial deficiency, a reduced starting dose should be considered.24 The EMA also states that therapeutic drug monitoring may improve clinical outcomes in patients who receive a continuous infusion of 5-FU.24
Guidelines for Treatment Based on DPYD Genotyping
We identified three pharmacogenetic guidelines on DPYD genotyping.4,8,30 The Dutch Pharmacogenetics Working Group (DPWG),4 the Clinical Pharmacogenetics Implementation Consortium (CPIC),8 and the Swiss Group of Pharmacogenomics and Personalised Therapy30 have proposed that treatment modifications for 5-FU and capecitabine be implemented before the start of treatment to reduce the risk of severe, potentially fatal toxicity in carriers of four DPYD variants (Table 1). The DPYD variants included in the guidelines are those for which sufficient evidence on an association with severe toxicity is available (DPYD∗2A, DPYD∗13, c.2846A>T, and c.1236G>A).4 The recommendations are based on the association between the DPYD genotype and DPD enzyme activity, therapeutic drug monitoring (5-FU pharmacokinetics), and severe fluoropyrimidine-associated toxicity.4,8 Based on the magnitude of their deleterious effect on DPD function, DPYD∗2A and DPYD∗13 are considered no-function variants and c.2846A>T and c.1236G>A are considered decreased-function variants.4,8,30
Table 1:
Pharmacogenetic Guidelines for Fluorouracil and Capecitabine Regimens
| Starting Dose Recommendation | |||
|---|---|---|---|
| Genotypea | CPIC8,21 | DPWG4 | SPT30 |
| Carrier of normal-function variants | Use label-recommended dosage and administration | No changes to standard dose | NR |
| Heterozygous carrier of 1 reduced-function or 1 no-function variant | 50% of full standard doseb Dose increase based on clinical judgment and ideally TDMc |
50% of standard dose Further dose titration may be done, guided by toxicity |
50% of standard dose Dose titration based on TDM should be favoured over toxicity-based titrationd |
| Heterozygous or homozygous carrier of 2 reduced-function variants | 50% of full standard doseb Dose increase based on clinical judgment and ideally TDMc |
Determined by DPD activity level (phenotype)e | 25% of standard dose (75% reduction) Dose titration based on TDM should be favoured over toxicity-based titrationd |
| Carrier of 1 reduced-function and 1 no-function variant | Avoid fluoropyrimidine-based regimens If no fluoropyrimidine-free regimen is suitable, 5-FU should be administered at a strongly reduced dosef with early TDMg |
Determined by DPD activity level (phenotype)e | No fluoropyrimidine chemotherapy recommended |
| Carrier of 2 no-function variants | Avoid 5-FU or 5-FU prodrug-based regimens | Avoid systemic and cutaneous administration of 5-FU or capecitabine; tegafur is not an alternative If these drugs cannot be avoided, DPD activity may be measured to adjust the dose |
No fluoropyrimidine chemotherapy recommended |
Abbreviations: 5-FU, 5-fluorouracil; CPIC, Clinical Pharmacogenetics Implementation Consortium; DPD, dihydropyrimidine dehydrogenase; DPWG, Dutch Pharmacogenetics Working Group; NR, not reported; SPT, Swiss Group of Pharmacogenomics and Personalised Therapy; TDM, therapeutic drug monitoring.
Based on DPYD variants DPYD∗2A, DPYD∗13, c.2846A>T, and c.1236G>A.
Increase dose in patients with no or clinically tolerable toxicity in first two cycles to maintain effectiveness; decrease dose if starting dose not tolerated to minimize toxicity.21
“To enable TDM-based dose titration, we generally recommend treating patients carrying a DPYD risk variant with an infusional 5-FU regimen and avoiding the use of the oral prodrug capecitabine. Only if the use of an infusional 5-FU regimen is not possible, should a prudent titration of capecitabine doses based on monitoring of toxicity and starting with the recommended reduced dose be considered.”30
When two different genetic variants are identified in one patient, they may be located on the same allele or on different alleles.4 Because the location of the variants results in differences in DPD function, and because genotyping methods cannot determine the allelic location of the variants, DPD function cannot be accurately predicted by genotype.4 The DPWG recommends performing a phenotype test to assess DPD activity in this situation.4
If available, a phenotyping test should be considered to estimate the starting dose. In the absence of phenotyping data, a dose of < 25% of the normal starting dose is estimated, assuming additive effects of alleles.21 No reports of the successful administration of low-dose 5-FU in DPYD poor metabolizers are available to date.21
Therapeutic drug monitoring should be done at the earliest point possible to immediately discontinue therapy if the drug level is too high.21
Predicted DPD activity can be expressed as the DPYD gene activity score, which ranges from 0 (no DPD enzyme activity) to 2 (normal DPD enzyme activity).4 Both the DPWG4 and the CPIC8 state that carriers of one no-function or reduced-function variant and one normal-function variant have a gene activity score of 1 to 1.5, and those with two normal-function variants have a gene activity score of 2. The CPIC states that carriers of two no-function variants or one no-function and one reduced-function variant have a gene activity score of 0 to 0.5, and carriers of two reduced-function variants have a gene activity score of 1 to 1.5.8 The CPIC considers patients with a gene activity score of 1 to 1.5 to be intermediate metabolizers, and those with a gene activity score of 0 to 0.5 to be poor metabolizers.8 The DPWG also considers carriers of two no-function variants to have a gene activity score of 0.4 However, in the presence of two reduced-function variants or one reduced-function and one no-function variant, the DPWG recommends assessment of DPD activity (phenotype testing) to guide treatment decisions, because enzyme activity cannot be predicted correctly with genotyping.4
All three guidelines note that further dose reduction may be required after the start of treatment, based on the development of toxicity.4,8,30
Some patients who carry reduced-function or no-function variants may tolerate normal doses of fluoropyrimidines.8 To avoid underdosing and maintain drug effectiveness, the CPIC recommends that patients with genotype-guided dose reductions who experience no or clinically tolerable toxicity in the first two chemotherapy cycles or who have subtherapeutic plasma 5-FU concentrations should have their dose increased in subsequent cycles.8 The CPIC also recommends follow-up 5-FU pharmacokinetic testing to avoid underdosing.8
The DPWG guideline noted that variants with a possible effect on DPD activity may be identified in the future, and that evidence for some variants is insufficient at present.4 For the DPYD variant c.557A>G (Y186C), which is more prevalent in people of African ancestry, one study showed an association with reduced DPD activity, but its association with toxicity was weak.8 Because the addition of other variants may affect the ability of DPYD genotyping to predict DPD enzyme activity, guidelines may be updated if new evidence becomes available.4
Guidelines on DPYD Genotyping From Clinical Associations
A consensus paper from scientific medical associations in Germany, Austria, and Switzerland proposed implementation of the EMA's recommendation on DPD deficiency.5 Before treatment with fluoropyrimidines, patients should undergo DPYD genotyping (DPYD∗2A, DPYD∗13, c.2846A>T, and c.1236G>A), and the genetic results should form the basis of recommendations for treatment.5 The consensus paper noted that treatment recommendations must be tailored to the individual disease situation and alternative available treatments, and that genetic testing may be supplemented with therapeutic drug monitoring.5 Although the group noted that the evidence base for phenotype tests was less extensive than for genotype tests, they considered pre-treatment measurement of plasma uracil or DPD activity in leukocytes to be alternatives to genotyping.5 They also stated that recommendations based on test results should be integrated into the treatment plan without causing delays.5
The European Society for Medical Oncology guidelines for localized colorectal cancer note that “DPD genotyping or phenotyping is strongly recommended before initiating fluoropyrimidine-based adjuvant therapy according to regulatory bodies.”31
The 2018 Guidelines of the Groupe de Pharmacologie Clinique Oncologique–UNICANCER on DPD Deficiency Screening recommend screening for DPD deficiency using both DPYD genotyping (four variants listed above) and phenotype tests (plasma uracil level) to guide decisions on dose reductions or the need for an alternative treatment.19
Health Technology Assessment Recommendations on DPYD Genotyping
In Quebec, INESSS32 recommended that prospective genotyping for DPYD variants DPYD∗2A, DPYD∗13, c.2846A>T, and c.1129-5923C>G (c.1236G>A) be included in the planning of cancer treatment with fluoropyrimidines.1,25 They noted that the association between the DPYD genotype and DPD activity is imperfect, but compared to phenotype testing, DPYD genotyping is accessible, fast, and inexpensive, and it may reduce the risk of severe toxicity in carriers.32 According to the experts consulted by INESSS,32 the results from DPYD genotyping are clinically important and could lead to a change in clinical conduct. Concerns raised by the experts included the fact that not all patients with a positive result developed severe toxicity and some patients with a negative result did, as well as the lower level of clinical evidence for the c.1236G>A variant.32
France's Haute Autorité de Santé (HAS) noted that the association between three DPYD variants (DPYD∗2A, DPYD∗13, and c.2846A>T) and severe toxicity has been demonstrated, but that the evidence was insufficient for an association between c.1236G>A and toxicity.10 The HAS concluded that, based on three variants and despite its proven association with toxicity, DPYD genotyping has a low sensitivity to detect DPD deficiency (i.e., only some patients with DPD deficiency can be identified by this test).10 As well, the variants currently identified are more common in Caucasian than non-Caucasian people.10 The HAS recommended that DPD deficiency be tested by determining plasma uracil concentrations in patients with planned fluoropyrimidine treatment, “as it is considered to be the most likely to be able to identify at least, and as far as possible, all patients with complete DPD deficiency.”10,33 As a consequence, the plasma uracil test was standardized across French laboratories, and thresholds for deficiency and treatment decisions were developed.10
Equity Considerations
Health inequities are differences in the distribution of health that may be avoidable, as well as unjust and unfair.34
The DPYD variants that have been more extensively studied and for which fluoropyrimidine dose adjustment is recommended are those that are more prevalent in the Caucasian population.10 Other DPYD variants with a potential effect on DPD activity that are more prevalent in other racial/ethnic groups have not been studied as extensively, so recommendations on the use of fluoropyrimidines in carriers of these variants are not available.10
DPYD genotyping is currently performed at one hospital in Ontario, so the test is not available to patients who are not receiving care at this hospital or who cannot be referred there.
Ethics Considerations
People with one no-function variant can be considered carriers of an inborn error of metabolism; they may wish to share this information with their offspring8 and other close relatives in case they are also carriers. People who are homozygous for no-function DPYD variants have complete DPD inactivity, a clinically heterogeneous autosomal-recessive disorder of pyrimidine metabolism; clinical presentation ranges from no symptoms to severe convulsive disorders with motor and mental impairment.8
Regulatory Information
DPYD genotyping using laboratory-developed tests is not subject to regulatory approval.
Ontario, Canadian, and International Context
DPYD genotyping was not publicly funded in Ontario at the time of writing of this report.
At the time of writing, one hospital conducted DPYD genotyping in patients with planned fluoropyrimidine-based treatment. The test was being done through a research program (Richard Kim, MD, email communication, November 9, 2020). A DPYD genotyping assay has also been developed and validated at another hospital in Ontario (Lei Fu, PhD, email communication, February 8, 2021), but the test was not in use at the time of writing.
At the time of publication of this report, Ontario had no provincial guideline for DPYD genotyping before chemotherapy (Lei Fu, PhD, email communication, February 8, 2021; Richard Kim, MD, email communication, January 12, 2021; John Lenehan, MD, email communication, February 12, 2021; Geoffrey Liu, MD, email communication, January 18, 2021; Michael Raphael, MD, email communication, February 10, 2021; Jason Yu, MD, email communication, January 17, 2021). Phenotype tests for DPD activity are not routinely done in Ontario given the challenges associated with implementing them as routine tests (Richard Kim, MD, email communication, January 12, 2021; John Lenehan, MD, email communication, February 12, 2021; Geoffrey Liu, MD, email communication, January 18, 2021; Jason Yu, MD, email communication, January 17, 2021).
In Quebec, DPYD genotyping for all four variants (DPYD∗2A, DPYD∗13, c.2846A>T, and c.1236G>A) is publicly funded; the test is performed in three laboratories in the province.35 We are uncertain whether other Canadian provinces are using DPYD genotyping and its funding status.
In 2020, the National Health Service in England, in response to an urgent policy request, recommended that all patients undergo DPYD genotyping (four variants mentioned above) before starting a fluoropyrimidine-based treatment.36 They also recommended the monitoring of prescribing decisions (e.g. dose adjustments) and patient toxicity to inform future updates to the recommendation.36
DPYD genotyping is publicly funded in Switzerland.37 In France, fluoropyrimidines cannot be prescribed without the results of a plasma uracil test.38
Expert Consultation
We engaged with experts in the specialty areas of pharmacogenetics, clinical oncology, and laboratory medicine to help inform our understanding of aspects of the health technology and our methodologies, and to contextualize the evidence.
PROSPERO Registration
This health technology assessment has been registered in PROSPERO, the international prospective register of systematic reviews (CRD42020176858), available at https://www.crd.york.ac.uk/PROSPERO.
Clinical Evidence
When planning this review, we considered the following to be out of scope:
Phenotype tests to measure DPD activity, as such tests are not currently done in Ontario given the limitations mentioned in the Background section
DYPD variants for which genotype-guided fluoropyrimidine dose recommendation guidelines were not available
The analytical validity of DPYD genotyping, because studies identified by a 2017 review25 have already demonstrated that the analytical validity of DPYD genotyping is accurate
The effectiveness of alternative chemotherapy treatments in DPYD carriers for whom fluoropyrimidines are considered contraindicated
Research Questions
What is the risk of severe fluoropyrimidine-associated toxicity in carriers of the DPYD variants under assessment (DPYD∗2A, DPYD∗13, c.2846A>T, and c.1236G>A) compared to patients with wild-type DPYD in those who have planned cancer treatment with fluoropyrimidines (clinical validity)?
Does pre-treatment DPYD genotyping for the variants under assessment lead to changes in treatment decision-making and/or decrease the risk of severe fluoropyrimidine-associated toxicity compared to no testing or other tests for DPD deficiency in patients who have planned cancer treatment with fluoropyrimidines (clinical utility)?
What is the effectiveness of treatment with fluoropyrimidines in patients who had their fluoropyrimidine dose adjusted before the start of treatment (because they carried at least one of the DPYD variants under assessment) compared to patients who did not have pre-treatment dose adjustment?
Methods
Clinical Literature Search
Because we identified relevant systematic reviews during scoping, we performed a systematic literature search for systematic reviews and health technology assessments that matched our research questions and PICOTS (population, intervention, comparator, outcomes, timing, and setting) to use them as a source of primary studies published until their literature search dates. We assessed eligible systematic reviews and health technology assessments using the Risk of Bias in Systematic Reviews (ROBIS) tool.39 We searched for systematic reviews and health technology assessments that had a low risk of bias and matched the scope of our review; we used recency and comprehensiveness as additional inclusion criteria. We permitted the selection of more than one report in case a single report did not cover the full scope of our review.
Then, we ran a systematic literature search to identify studies published since the searches for the selected systematic reviews were performed; we used the earliest search date among the selected two systematic reviews. We included primary studies identified from the selected systematic reviews and from the systematic literature search in our review.
We performed a clinical literature search on February 20, 2020, to retrieve systematic reviews and health technology assessments published from database inception until the search date. The health technology assessments we selected searched the literature from database inception (earliest search start date) until January 2018 (earliest search end date). We then performed a clinical literature search for primary studies on February 27, 2020, to retrieve studies published from January 2018 until the search date. We used the Ovid interface in the following databases: MEDLINE, Embase, the Cochrane Database of Systematic Reviews, the Health Technology Assessment database, and the National Health Service Economic Evaluation Database (NHS EED). We used the Cochrane Central Register of Controlled Trials exclusively in the search for primary studies.
A medical librarian developed the search strategies using controlled vocabulary (e.g., Medical Subject Headings) and relevant keywords. A methodological filter was used to limit retrieval to systematic reviews, meta-analyses, and health technology assessments in our first search. The final search strategies were peer-reviewed using the PRESS Checklist.40
We created database auto-alerts in MEDLINE and Embase and monitored them for the duration of the assessment period. We also performed a targeted grey literature search of health technology assessment agency websites as well as clinical trial and systematic review registries. See Appendix 1 for our literature search strategies, including all search terms.
Eligibility Criteria
STUDIES
Systematic Reviews
Inclusion Criteria
English-language full-text publications
Systematic reviews and health technology assessments published from database inception until February 20, 2020
Systematic reviews and health technology assessments that included a systematic review and had a low risk of bias as assessed by the ROBIS tool39
Reports whose research question and PICOTS matched or included the ones that were the focus of the present report
Reports that provided information about their literature search methods, including databases searched, search strategy, and search start and end dates
Reports that had prespecified eligibility criteria
Exclusion Criteria
Nonsystematic reviews, editorials, commentaries, conference abstracts, and letters
Primary Studies
Inclusion Criteria
English-language full-text publications
Studies published before January 2018 that were identified from the health technology assessments selected, and from January 2018 until February 27, 2020, that were identified through the primary studies literature search
Randomized controlled trials, prospective or retrospective comparative observational studies
Studies that provided information about fluoropyrimidine dose adjustments before and/or after the start of treatment
Exclusion Criteria
Animal and in vitro studies
Editorials, commentaries, case-reports (< 10 patients included), conferences abstracts, letters
PARTICIPANTS
Adult and pediatric patients who had planned cancer treatment with fluoropyrimidines, alone or in combination with other therapies
INTERVENTIONS
Clinical Validity
Exposure
Included: carriers of at least one of the variants under assessment (DPYD∗2A, DPYD∗13, c.2846A>T, c.1236G>A)
Excluded: carriers of other DPYD or other gene variants; those with DPD deficiency defined according to phenotype tests for DPD function (e.g., plasma uracil concentration or dihydrouracil:uracil [UH2:U] ratio)
Control
Included: wild-type patients (noncarriers of variants under assessment) defined by DPYD genotyping
Excluded: wild-type patients with an absence of DPD deficiency according to phenotype tests or other genetic tests
Clinical Utility
Intervention
Included: DPYD genotyping of the variants under assessment (DPYD∗2A, DPYD∗13, c.2846A>T, c.1236G>A) before the start of treatment; or carriers of at least one of the DPYD variants under assessment who received a genotype-guided fluoropyrimidine dose reduction
Excluded: treatment decisions based on testing for other DPYD or other gene variants; treatment decisions based on phenotype tests for DPD function (e.g., plasma uracil concentration or UH2:U ratio) or 5-FU pharmacokinetics assessment
Comparator
Included: patients with no testing; patients with phenotype tests for DPD function (e.g., plasma uracil concentration or UH2:U ratio) before the start of treatment, or 5-FU pharmacokinetics assessment after the start of treatment; or wild-type patients or DPYD carriers without a genotype-guided fluoropyrimidine dose reduction
Excluded: patients who received treatment with uridine triacetate
Fluoropyrimidine Treatment Effectiveness
Intervention
Included: carriers of at least one of the variants under assessment, with DPYD-genotyping-guided fluoropyrimidine dose adjustment before the start of treatment
Excluded: patients with no fluoropyrimidine dose adjustment before the start of treatment; patients with fluoropyrimidine dose adjustment based on criteria other than DPYD genotyping; patients undergoing alternative cancer treatment because of a contraindication to fluoropyrimidines
Comparator
Included: wild-type patients or DPYD carriers with no fluoropyrimidine dose adjustment before the start of treatment
Excluded: patients who had a fluoropyrimidine dose adjustment before the start of treatment
OUTCOME MEASURES
Clinical Validity
Severe fluoropyrimidine-related toxicity, overall and by type (hematological, gastrointestinal, dermatological)
Clinical sensitivity, specificity, and positive and negative predictive value of DPYD genotyping (three to four variants) for the prediction of fluoropyrimidine-associated toxicity
Toxicity-related changes to fluoropyrimidine-based treatment (i.e., dose reduction or increase, treatment delay and discontinuation)
Toxicity-related hospitalization
Toxicity-related mortality
Clinical Utility
Fluoropyrimidine dose reduction, increase, discontinuation; use of alternative treatment
Toxicity-related changes to fluoropyrimidine-based treatment (i.e., dose reduction or increase, treatment delay and discontinuation)
Severe fluoropyrimidine-related toxicity, overall and by type (hematological, gastrointestinal, dermatological)
Toxicity-related hospitalization
Toxicity-related mortality
Fluoropyrimidine Treatment Effectiveness
Treatment response
Disease progression
Overall survival
Progression-free survival
Literature Screening
A single reviewer conducted an initial screening of titles and abstracts using Covidence41 and then obtained the full texts of studies that appeared eligible for review according to the inclusion criteria. A single reviewer then examined the full-text articles and selected studies eligible for inclusion. A single reviewer also examined reference lists and consulted content experts for any additional relevant studies not identified through the search.
Data Extraction
We extracted relevant data on study characteristics and risk-of-bias items using a data form to collect information on the following:
Source (e.g., citation information, study type)
Methods (e.g., study design, study duration and years, participant allocation, reporting of missing data, reporting of outcomes, whether the study compared two or more groups)
Outcomes (e.g., outcomes measured, number of participants for each outcome, number of participants missing for each outcome, outcome definition and source of information, unit of measurement, time points at which the outcomes were assessed)
We contacted study authors to provide clarification as needed.
Data Presentation and Statistical Analysis
When assessing dichotomous outcomes such as severe toxicity, treatment modifications, hospitalization, and mortality, we extracted information on the number of patients from each group who experienced an event from the studies identified. Toxicity was graded using the National Cancer Institute Common Toxicity Criteria for Adverse Events (NCI-CTCAE)14 across studies and included common toxicities with fluoropyrimidine treatment (hematological, gastrointestinal, and dermatological). Severe toxicity was defined as grade 3 or higher; specifically for hand–foot syndrome, a toxicity grade of 2 or higher was considered severe in some studies because of its clinical relevance. When possible, we reported results separately for each DPYD variant under assessment and by combining carriers of any one of these variants into a single group. We used risk ratios as the effect measures for these dichotomous outcomes; we calculated risk ratios and 95% confidence intervals (CIs) based on information provided in the studies. We used the exact method (R exactmeta package42) to calculate the confidence interval because it does not rely on approximation to normal distribution and is therefore more suitable for sparse data, as was the case with the data reported in the studies.43 We calculated P-values using the Fisher exact test when risk ratios could not be calculated (e.g., in the case of zero events in one of the study groups).
The included studies also reported the clinical sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of DPYD genotyping for predicting severe fluoropyrimidine-related toxicity. We used the occurrence of severe toxicity as the reference standard; sensitivity was defined as the proportion of patients identified as carriers of a DPYD variant among those who experienced severe toxicity. We defined specificity as the proportion of wild-type patients among those who did not experience severe toxicity. When these outcomes were not reported in the studies, we calculated them based on the information provided: number of carriers of DPYD variants who experienced severe toxicity (true positive); number of wild-type patients without severe toxicity (true negative); number of wild-type patients with severe toxicity (false negative); number of DPYD variant carriers without severe toxicity (false positive). We defined PPV as the proportion of patients who experienced toxicity among those identified as DPYD variant carriers and NPV as the proportion of patients who did not experience toxicity among wild-type patients. We calculated sensitivity, specificity, and the corresponding 95% confidence intervals without continuity correction (modified Wilson method) using the Mada package in R.42
We used the prevalence of DPYD variant carriers reported in the studies to calculate the pooled prevalence and 95% confidence interval for each variant individually and combined, using the exact method in R (meta package).42
For effectiveness outcomes, we extracted the number of patients who experienced the outcomes of interest and hazard ratios comparing DPYD variant carriers and wild-type patients from the study.
We had originally planned subgroup analyses (type of cancer, type of fluoropyrimidine used, route of administration, and one or more factors relevant to this topic that may predispose patients to health inequities:34 place of residence, race/ethnicity, occupation, gender/sex, religion, education, socioeconomic status, and social capital, among others); however, the studies we identified did not provide sufficient information for us to conduct these analyses.
The study results are represented using forest plots. We judged heterogeneity by visual inspection of the forest plots. In the studies we identified, the data were sparse, so we were unable to perform tests of homogeneity (e.g., Cochran's Q test) because they rely on the large sample assumption. Instead, we based homogeneity assumptions on our knowledge of the distribution of rates of toxicity across populations. When appropriate, we performed fixed-effect meta-analyses in the absence of heterogeneity using the exact method in R (exactmeta and gplots packages).42
Critical Appraisal of Evidence
We assessed risk of bias using the Newcastle-Ottawa Scale (Appendix 3) for observational studies.44 We used the ROBIS risk of bias tool39 for systematic reviews. We used only domains 1 and 2 (study eligibility criteria and identification and selection of studies) of the ROBIS tool because we used the selected systematic reviews as a source of eligible studies (i.e., we did not use the results, synthesis, and conclusions sections of the reviews).
We evaluated the quality of the body of evidence for each outcome according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Handbook.45 The body of evidence was assessed based on the following considerations: risk of bias, inconsistency, indirectness, imprecision, and publication bias. The overall rating reflects our certainty in the evidence. For toxicity outcomes, we assessed the quality of the evidence for overall toxicity and for the most commonly reported types of toxicity (hematological, gastrointestinal, and dermatological) because we believed these would be most relevant for decision-making.
Results
Clinical Literature Searches
SYSTEMATIC REVIEWS
The database search of the clinical literature for systematic reviews yielded 128 citations published from database inception until February 20, 2020. We identified six additional studies from other sources. In total, we identified seven studies (four systematic reviews and three health technology assessments) that met our inclusion criteria.10,32,46–50 Figure 1 present the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram for the clinical literature search for systematic reviews.
Figure 1: PRISMA Flow Diagram—Clinical Search Strategy (Systematic Reviews).
Abbreviation: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses.
Source: Adapted from Moher et al.51
We identified four eligible systematic reviews published between 2013 and 2016 and three eligible health technology assessments published between 2016 and 2019.10,32,46–50 All seven publications evaluated the clinical validity of DPYD genotyping, but only one health technology assessment included all four DPYD variants under assessment in the current review.10 Two health technology assessments assessed the clinical utility of DPYD genotyping, including all four variants.10,32 One also assessed treatment effectiveness among DPYD variant carriers who had their fluoropyrimidine dose reduced as a result of genotyping.32
The systematic reviews and health technology assessments we identified had a generally low risk of bias, but none covered all of the research questions and DPYD variants that were the object of this review. Therefore, we selected two health technology assessments10,32 because they were recent and because together they covered all of the research questions and DPYD variants we assessed. Neither of the two health technology assessments planned to perform new meta-analyses.10,32 We complemented their literature search and performed de novo analyses when appropriate.
PRIMARY STUDIES
The database search of the clinical literature for primary studies yielded 355 citations published between January 2018 and February 27, 2020. We identified 19 additional primary studies (published up to 2018) from the selected health technology assessments and one from database auto-alerts. Overall, we identified 29 studies (all observational) that met our inclusion criteria; we used 25 to answer the clinical validity research question7,9,13,15,18,52–71; six to answer the clinical utility research question7,11,12,23,71,72; and one to answer the treatment effectiveness research question.11 (We used two studies7,71 for both the clinical validity and clinical utility questions, and one study11 for both the clinical utility and treatment effectiveness questions.) Figure 2 presents the PRISMA flow diagram for the clinical literature search for primary studies.
Figure 2: PRISMA Flow Diagram—Clinical Search Strategy (Primary Studies).
Abbreviation: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses.
Source: Adapted from Moher et al.51
Characteristics of Included Studies
CLINICAL VALIDITY
A total of 25 studies evaluated the risk of severe fluoropyrimidine-related toxicity in carriers of at least one of the DPYD variants under assessment and treated with a standard fluoropyrimidine dose, compared to wild-type patients.7,9,13,15,18,52–69,71 The studies were performed in Canada, Europe, the United States, and Bangladesh. Toxicity was graded using the National Cancer Institute Common Toxicity Criteria for Adverse Events (NCI-CTCAE)14 and included events that commonly occur with fluoropyrimidine treatment (hematological, gastrointestinal, and dermatological). Severe toxicity was defined as grade 3 or higher, although for hand–foot syndrome, toxicity grade 2 or higher was considered severe in some studies because of its clinical relevance. We relied on the investigators' judgment regarding the association between outcomes and fluoropyrimidine treatment.
Study samples ranged in size from 73 to 2,886 patients, and the number of DPYD carriers ranged from 1 to 85. Overall, 4 (16%) studies identified just one carrier, and 12 (48%) identified 10 carriers or more. Participants included patients with different types of cancers (e.g., colorectal, gastrointestinal, and breast) who had planned treatment with either 5-FU or capecitabine, alone or in combination with other chemotherapy drugs. In three studies, patients also received radiotherapy.7,62,71
Four studies included all four DPYD variants under assessment,7,56,57,61 nine studies assessed three variants,9,15,18,53,55,58–60,62 and the remainder evaluated one or two DPYD variants. DPYD∗2A was the most common variant assessed (20 studies),7,9,15,53–69 followed by c.2846A>T (16 studies),7,9,15,18,52,53,55–62,67,70 DPYD∗13 (13 studies),7,9,15,18,52,53,55–58,60–62 and c.1236G>A (9 studies).7,13,18,56,57,59,61,70,71
The timing of the evaluation of toxicity varied across studies, occurring in the first 1 to 2 cycles in 6 studies18,56,57,61,67,68 and the first three to four cycles in three studies.9,53,60 In the remaining studies, either the full duration of treatment was used or the period of evaluation was not reported.7,13,15,52,54,55,58,59,62–66,69–71
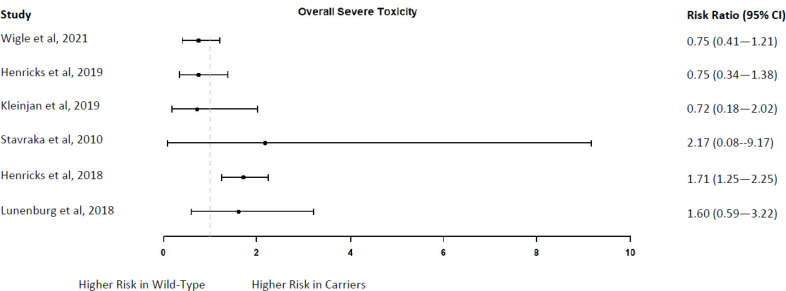
CLINICAL UTILITY
Six studies identified included carriers of one of the DPYD variants under assessment who received a genotype-guided reduced fluoropyrimidine dose from the start of treatment.7,11,12,23,71,72 These studies compared the outcomes of DPYD carriers with those of wild-type patients who received a standard fluoropyrimidine dose. The studies were performed in Canada and Europe.
We did not include a study by Deenen et al73 because its data were part of a larger, more recent study (Henricks et al, 201911) that we did include in this report.
One study (Lunenburg et al, 20187) also compared the outcomes of DPYD carriers treated with a genotype-guided reduced fluoropyrimidine dose with those of DPYD carriers treated with a standard dose. This study combined three separate databases to form the study groups.7
A study by Henricks et al11 also included a comparison group of DPYD∗2A carriers treated with a standard fluoropyrimidine dose, but this group represented a historical cohort based on results reported from other studies, rather than a direct patient comparison. Given that this historical cohort did not originate from the same population base as the reduced-dose cohort, there were differences in the distribution of important patient characteristics (sex, type of cancer, fluoropyrimidine used). Because there was no adjustment for such potential confounders, we did not include this comparison group in our report.
Another study by Henricks et al72 compared the risk ratio that they obtained in their study (for severe toxicity in DPYD carriers treated with a reduced dose versus wild-type patients) with the risk ratio from a previous meta-analysis that compared DPYD carriers treated with a standard dose versus wild-type patients. We did not include this comparison in our report because it was not a direct patient comparison.
Study samples ranged in size from 66 to 1,646 patients; 3 to 85 of those were DPYD carriers treated with a reduced fluoropyrimidine dose. Participants included patients with different types of cancers (e.g., colorectal, gastrointestinal, and breast) who had planned treatment with 5-FU or capecitabine, either alone or in combination with other chemotherapy drugs. In four studies, patients also received radiotherapy.7,11,71,72
Four studies included all four variants under assessment,7,12,71,72 one included three variants (DPYD∗2A, c.2846A>T, and DPYD∗13),23 and one study only included DPYD∗2A.11 One study identified five homozygous carriers (1 carrier of DPYD∗2A, 2 carriers of c.2846A>T, two carriers of c.1236G>A) and one compound heterozygous carrier (c.2846A>T/c.1236G>A); these patients were excluded from the study and treated with individualized regimens.72,74 The study by Wigle et al71 identified two compound heterozygous carriers (DPYD variants not reported); these patients were excluded from the study, and the treating oncologists were advised to use an alternative treatment instead of fluoropyrimidines. Results refer to heterozygous carriers unless otherwise specified.
The turnaround time for DPYD genotyping ranged from 2 working days to 1 week, based on two studies.23,72
Outcomes included the frequency of treatment modification, severe toxicity, and toxicity-related hospitalization and mortality. Toxicity was graded according to the NCI-CTCAE14 across studies and included toxicities that are common with fluoropyrimidine treatment (hematological, gastrointestinal, and dermatological). Severe toxicity was defined as grade 3 or higher, although for hand–foot syndrome, toxicity grade 2 or higher was considered severe in some studies because of its clinical relevance. Results for individual variants were provided in only one study,72 so we reported results for all DPYD variants assessed in each study as a single group. Additional information is provided in Appendix 2.
FLUOROPYRIMIDINE TREATMENT EFFECTIVENESS
One study assessed the effectiveness of fluoropyrimidine treatment in 37 DPYD∗2A carriers who received a genotype-guided reduced fluoropyrimidine dose compared to 37 wild-type patients who were treated with a standard dose and matched according to variables that were expected to affect treatment outcome.11
Risk of Bias in the Included Studies
CLINICAL VALIDITY
The included studies generally used appropriate methods for patient group selection (carriers and wild-type patients), exposure, and outcome ascertainment, and follow-up was adequate. However, no adjustment or matching was used when comparing the frequency of severe toxicity between study groups. Additional information is provided in Appendix 3.
CLINICAL UTILITY
The included studies generally used appropriate methods for patient group selection (carriers and wild-type patients), exposure, and outcome ascertainment, and follow-up was adequate. However, no adjustment or matching was used for the comparative groups. The study by Lunenburg et al7 reported an imbalance in the distribution of DPYD variants between reduced-dose carriers and standard-dose carriers: the two variants that were expected to have a weaker effect on DPD activity were overrepresented in the latter group. This may have led to an underestimate of the frequency of severe toxicity in the standard-dose group and consequently an underestimate in the difference between groups. Additional information is provided in Appendix 3.
FLUOROPYRIMIDINE TREATMENT EFFECTIVENESS
The included study used appropriate methods for patient group selection (carriers and wild-type), exposure, and outcome ascertainment, and follow-up was adequate. Patients were matched according to variables that were expected to affect the outcomes assessed. Additional information is provided in Appendix 3.
Clinical Validity
We assessed clinical validity by comparing the frequency of severe fluoropyrimidine-related toxicity (overall and by type) in carriers of DPYD variants (DPYD∗2A, DPYD∗13, c.2846A>T, c.1236G>A) treated with a standard fluoropyrimidine compared to wild-type patients. We also evaluated the frequency of treatment modifications and hospitalizations. Results are presented for the three to four DPYD variants as a group, and then separately for each variant.
We also calculated the clinical sensitivity, specificity, PPV, and NPV of DPYD genotyping to predict fluoropyrimidine-related toxicity using the occurrence of severe toxicity as the reference standard. However, the assumption that toxicity is the reference standard to calculate these parameters may not be satisfied, because DPD function is not the only factor that affects toxicity in patients treated with fluoropyrimidines; factors other than DPYD genotyping affect DPD function4; and other unknown DPYD variants may affect DPD function.
Given the clinical heterogeneity in terms of type of cancer, type of fluoropyrimidine, mode of administration, and combination regimens, we conducted meta-analyses of study results in only some cases.
Results refer to heterozygous carriers unless otherwise specified. Because fluoropyrimidine dosing regimens vary according to type of cancer and cancer stage, whether fluoropyrimidines are used alone or in combination, and the type of combination regimen, we have used the term “standard dose” to refer to the usual fluoropyrimidine dose in a given patient population to distinguish it from the genotype-guided reduced dose.
Although patients generally started treatment on a standard fluoropyrimidine dose, dose reductions were allowed in both DPYD carriers and wild-type patients according to the development of toxicity, clinical condition, and or other factors such as age.9,13,15,54–62,64,67,68 In some studies, the reduction was based on toxicity grade of less than 3,9,57,59,62,64,67 which may have prevented severe (grade ≥ 3) toxicity and led to an underestimate of severe toxicity. Additional information is provided in Appendix 2.
PATIENT CHARACTERISTICS
The mean age of patients in the included studies ranged from 47 to 67 years, and a large proportion were male (42% to 73%), except for two studies that included only women with breast cancer.56,68 Based on information from nine studies;9,13,18,53,57,58,60,67,71 67% to 100% of patients were of Caucasian origin; another study stated that patients were mostly Caucasian (numbers not provided).61 Additional information is provided in Appendix 4.
Colorectal cancer was the most common type of cancer, affecting all patients in 12 studies13,15,54,55,58,59,61,62,64,66,67,69 and 35% to 85% of patients in nine studies;7,18,52,53,57,60,63,65,71 other types of cancer included breast, gastrointestinal, esophageal, and head and neck cancers.7,9,13,15,18,52–69 The most common fluoropyrimidine used was 5-fluorouracil: 11 studies included only patients treated with 5-FU alone or in combination regimens13,15,54,55,58,62,64–67,69; four included only patients treated with capecitabine alone or in combination56,61,68,70; and in the remaining studies, 12% to 91% of patients were prescribed 5-FU.7,9,18,53,57,59,60,63,71 Three studies reported that none of the DPYD variants under assessment were present in non-Caucasian patients.12,53,57
Table 2 shows the prevalence of DPYD variant carriers identified in the included studies (Appendix 5).
Table 2:
Prevalence of the DPYD Variants in the Included Studies
| Variant | Prevalence Range, % | Pooled Prevalence, % (95% CI) |
|---|---|---|
| DPYD∗2A, heterozygous | 0.7 to 5.07,9,15,53–69 | 1.1 (0.9–1.4) |
| DPYD∗13, heterozygous | 0.0 to 0.67,9,15,52,53,55–58,60–62 | 0.2 (0.1–0.3) |
| c.2846A>T, heterozygous | 0.6 to 2.87,9,15,52,53,55–62,67,70 | 1.2 (1.0–1.5) |
| c.1236G>A, heterozygous | 1.7 to 8.17,13,56,57,59,61,70,71 | 4.0 (3.4–4.7) |
| Any of the 4 DPYD variants, heterozygous | 4.5 to 7.47,56,57,61 | 6.6 (5.6–7.7) |
| c.1236G>A, homozygous | 0.05 to 0.213,57,61 | 0.1 (0.03–0.3) |
| Compound heterozygous |
DPYD∗2A/c.2846A>T: 0.0358 to 0.467 DPYD∗2A/DPYD∗13: 0.2%9 DPYD∗13/c.1236G>A: 0.2%57 |
0.09 (0.04–0.3) |
Abbreviation: CI, confidence interval.
RISK OF SEVERE TOXICITY IN DPYD CARRIERS VERSUS WILD-TYPE PATIENTS
Carriers of Any of the 4 DPYD Variants
Overall Severe Toxicity
Of carriers of any of the DPYD variants under assessment who received a standard fluoropyrimidine dose, overall severe toxicity was reported in 23.5% to 100%, compared to 8.2% to 41.5% in wild-type patients, across seven studies.7,9,57–60,62 Two studies included all four DPYD variants under assessment,7,57 and five studies included three variants (DPYD∗2A, DPYD∗13, and c.2846A>T).
The results of six of the seven studies indicated a higher risk in DPYD carriers treated with a standard fluoropyrimidine dose compared to wild-type patients9,57–60,62; in the seventh study, the point estimate of the risk ratio (RR) was consistent with an increased risk in DPYD carriers, but the confidence interval included the possibility of a lower risk.7 Pooling the results of these seven studies yielded a risk ratio of 2.63 (95% CI 2.15–3.96; Figure 3); we decided that the range of effect estimates observed across studies warranted meta-analysis. This analysis did not include homozygous or compound heterozygous carriers; effects for these groups were analyzed separately.
Figure 3: Carriers of Any of the Four DPYD Variants Versus Wild-Type Patients—Risk of Overall Severe Toxicity.
Sources: Lunenburg et al,7 Froehlich et al,57 Toffoli et al,9 Lee et al,58 Jennings et al,59 Loganayagam et al,60 and Cellier et al.62
The GRADE quality of the evidence was low because the evidence came from observational studies (Appendix 3).
Severe Hematological Toxicity
Neutropenia was the most common hematological toxicity reported. Two (5.9%) DPYD carriers who received a standard fluoropyrimidine dose and 12 (1.6%) wild-type patients also treated with a standard dose had severe neutropenia (RR 3.69, 95% CI 0.53–16.97) in the study by Lunenburg et al,7 whereas six (35.3%) DPYD carriers and 38 (6.5%) wild-type patients had severe neutropenia (RR 5.43, 95% CI 2.15–11.53) in the study by Toffoli et al.9 The pooled risk ratio was 4.42 (95% CI 1.59–9.18; Figure 4); we decided that the range of effect estimates observed across studies warranted meta-analysis.
Figure 4: Carriers of Any of the Four DPYD Variants Versus Wild-Type Patients—Risk of Severe Neutropenia.
Figure 5: Carriers of Any of the Four DPYD Variants Versus Wild-Type Patients—Risk of Severe Diarrhea.
The GRADE quality of the evidence was low because the evidence came from observational studies (Appendix 3).
Severe Gastrointestinal Toxicity
Diarrhea was the most common gastrointestinal toxicity reported. Six (17.6%) DPYD carriers who received a standard fluoropyrimidine dose and 58 (7.5%) wild-type patients also treated with a standard dose had severe diarrhea (RR 2.35, 95% CI 0.94–4.81) in the study by Lunenburg et al,7 whereas six (35.3%) DPYD carriers and 34 (5.8%) wild-type patients had severe diarrhea (RR 6.09, 95% CI 2.37–12.66) in the study by Toffoli et al.9
The GRADE quality of the evidence was low because the evidence came from observational studies (Appendix 3).
Severe Dermatological Toxicity
Only the study by Lunenburg et al7 reported on severe dermatological toxicity. In that study, 24 (3.1%) wild-type patients treated with a standard fluoropyrimidine dose experienced severe hand-foot syndrome, but none of the DPYD carriers who received a standard fluoropyrimidine dose experienced this adverse event (P = .62).
The GRADE quality of the evidence was very low because the evidence came from observational studies, and because of imprecision (Appendix 3).
Carriers of the DPYD∗2A Variant
Overall Severe Toxicity
In one study,57 none of the four DPYD∗2A carriers identified experienced severe toxicity, but in the other 16 studies,9,15,54–56,58–65,67–69 46.2% to 100% of DPYD∗2A carriers treated with a standard fluoropyrimidine dose experienced severe toxicity. Among wild-type patients treated with a standard fluoropyrimidine dose, 3.3% to 57.5% experienced severe toxicity,9,15,54–60,62–65,67–69 except in one study where 85.2% of wild-type patients experienced severe toxicity.61
The authors of the study in which none of the DPYD∗2A carriers experienced toxicity commented that some patients had their treatment delayed or stopped as a result of grade 2 toxicity, and this may have prevented the occurrence of severe (grade ≥ 3) toxicity in these patients.57 According to the authors, most DPYD∗2A carriers in the study experienced grade 2 toxicity and required more treatment delays and cessation than wild-type patients, indicating a more clinically important toxicity profile in carriers that was not reflected in higher toxicity grades.57
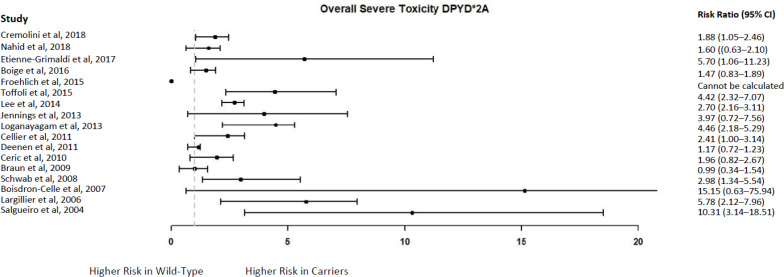
The point estimates of the risk ratio from 15 of the 16 studies9,15,54–56,58–63,65,67–69 indicated a higher risk in DPYD∗2A carriers compared to wild-type patients; however, with the exception of 8 studies,9,54,56,58,60,65,68,69 the confidence intervals also included the possibility of a lower risk in DPYD∗2A carriers (Figure 6; Appendix 5).
Figure 6: DPYD∗2A Carriers Versus Wild-Type Patients—Risk of Overall Severe Toxicity.
Sources: Cremolini et al,55 Nahid et al,54 Etienne-Grimaldi et al,56 Boige et al,15 Froehlich et al,57 Toffoli et al,9 Lee et al,58 Jennings et al,59 Loganayagam et al,60 Cellier et al,62 Deenen et al,61 Cerić et al,63 Braun et al,64 Schwab et al,65 Boisdron-Celle et al,67 Largillier et al68, and Salgueiro et al69
The GRADE quality of the evidence was low because the evidence came from observational studies (Appendix 3).
Severe Hematological Toxicity
Neutropenia was the most common hematological toxicity reported. Severe neutropenia occurred in 33% to 100% of DPYD∗2A carriers treated with a standard fluoropyrimidine dose and 2% to 36% of wild-type patients treated with a standard dose, across nine studies (Figure 7; Appendix 5).9,15,53–55,58,63,68,69
Figure 7: DPYD∗2A Carriers Versus Wild-Type Patients—Risk of Severe Neutropenia.
Sources: Maharjan et al,53 Cremolini et al,55 Nahid et al,54 Boige et al,15 Toffoli et al,9 Lee et al,58 Cerić et al,63 Largillier et al,68 and Salgueiro et al.69
The point estimates of the risk ratio indicated a higher risk in DPYD∗2A carriers compared to wild-type patients in all studies, but the confidence intervals of three studies15,53,55 also included the possibility of a lower risk in DPYD∗2A carriers. The results for other severe hematological toxicities followed a similar pattern. Additional details are provided in Appendix 5.
The GRADE quality of the evidence was low because the evidence came from observational studies (Appendix 3).
Severe Gastrointestinal Toxicity
Diarrhea was the most commonly reported gastrointestinal toxicity. Whereas two studies reported that none of the DPYD∗2A carriers treated with a standard fluoropyrimidine dose experienced severe diarrhea,55,69 in the other nine studies9,15,53,54,58,61,63,65,68 its frequency ranged from 12.0% to 100%. Among wild-type patients treated with a standard fluoropyrimidine dose, 1.4% to 27.5% experienced severe diarrhea.9,15,53–55,58,61,63,65,68,69
The point estimates of the risk ratio from nine studies indicated an increased risk in DPYD∗2A carriers compared to wild-type patients, 9,15,53,54,58,61,63,65,68 but the confidence intervals of three studies also included the possibility of a lower risk in DPYD∗2A carriers (Figure 8).54,58,65 Similar results were reported for other types of gastrointestinal toxicity (Appendix 5).
Figure 8: DPYD∗2A Carriers Versus Wild-Type Patients—Risk of Severe Diarrhea.
Sources: Maharjan et al,53 Cremolini et al,55 Nahid et al,54 Boige et al,15 Toffoli et al,9 Lee et al,58 Deenen et al,61 Cerić et al,63 Schwab et al,65 Largillier et al,68 and Salgueiro et al.69
The GRADE quality of the evidence was low because the evidence came from observational studies (Appendix 3).
Severe Dermatological Toxicity
Severe hand–foot syndrome occurred in 3 (42.9%) DPYD∗2A carriers treated with a standard fluoropyrimidine dose and 242 (43.2%) wild-type patients treated with a standard dose in the study by Deenen et al61 (RR 0.99, 95% CI 0.25–1.82). One carrier in the study by Largillier et al68 developed severe hand–foot syndrome (100.0%) compared to five (4.8%) wild-type patients (RR 20.83, 95% CI 5.55–35.60). In the study by Cellier et al,62 none of the patients, DPYD∗2A carriers or wild-type, experienced severe hand–foot syndrome.
The GRADE quality of the evidence was very low because the evidence came from observational studies, and because of imprecision (Appendix 3).
Carriers of the DPYD∗13 Variant
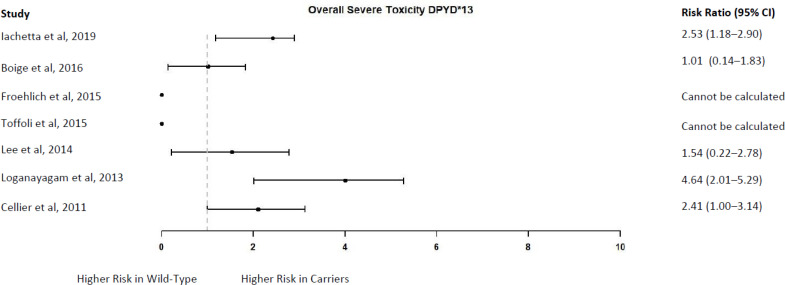
Overall Severe Toxicity
Two studies that each identified a single DPYD∗13 carrier reported that neither of these carriers experienced severe toxicity9,57; in five other studies, the frequency ranged from 50.0% to 100.0% in DPYD∗13 carriers who received a standard fluoropyrimidine dose.15,52,58,60,62 Among wild-type patients treated with a standard dose, 8.2% to 49.5% developed severe toxicity across the seven studies.9,15,52,57,58,60,62
The point estimates of the risk ratio ranged from 1.01 to 4.64 in five studies,15,52,58,60,62 but the confidence intervals of two studies also included the possibility of a lower risk in DPYD∗13 carriers (Figure 9).15,58,62 The risk ratio could not be calculated in the two studies in which no carriers DPYD∗13 experienced severe toxicity9,57; in both studies, according to the authors, the DPYD∗13 carriers either had a fluoropyrimidine dose reduction or a treatment delay as a result of a grade 2 toxicity, and this may have prevented the development of severe toxicity.9,57
Figure 9: DPYD∗13 Carriers Versus Wild-Type Patients—Risk of Overall Severe Toxicity.
Sources: Iachetta et al,52 Boige et al,15 Froehlich et al,57 Toffoli et al,9 Lee et al,58 Loganayagam et al, 60 and Cellier et al.62
The GRADE quality of the evidence was low because the evidence came from observational studies (Appendix 3).
Severe Hematological Toxicity
In one study, one (25.0%) DPYD∗13 carrier who received a standard fluoropyrimidine dose and 561 (36.4%) wild-type patients also treated with a standard dose developed severe neutropenia (RR 0.73, 95% CI 0.02–2.10).15 In a second study, no DPYD∗13 carriers had severe neutropenia, but 8 (1.4%) wild-type patients did (P = 1.00).9
The GRADE quality of the evidence was very low because the evidence came from observational studies, and because of imprecision (Appendix 3).
Severe Gastrointestinal Toxicity
Severe diarrhea was the most commonly reported gastrointestinal toxicity. In one study, two (50.0%) DPYD∗13 carriers treated with a standard fluoropyrimidine dose and 190 (12.3%) wild-type patients also treated with a standard dose developed severe diarrhea (RR 4.07, 95% CI 0.62–7.71).15 One (100.0%) DPYD∗13 carrier and 18 (22.0%) wild-type patients developed severe diarrhea in a second study (RR 4.55, 95% CI 1.72–6.32).62 In a third study, no DPYD∗13 carriers and 34 (5.8%) wild-type patients developed severe diarrhea (P = 1.00).9
The GRADE quality of the evidence was low because the evidence came from observational studies (Appendix 3).
Severe Dermatological Toxicity
None of studies identified reported on this outcome.
Carriers of the c.2846A>T Variant
Overall Severe Toxicity
In one study, none of the three c.2846A>T carriers treated with a standard fluoropyrimidine dose experienced severe toxicity,57 but in 12 studies the frequency in carriers varied between 60.0% and 100.0%.9,15,52,55,56,58–62,67,70 Among wild-type patients treated with a standard dose, 3.3% to 50.1% experienced severe toxicity.9,15,52,55–62,67,70
In the study by Froehlich et al,57 similar to what was reported for carriers of other DPYD variants, c.2846A>T carriers had their fluoropyrimidine treatment delayed or stopped as a result of grade 2 toxicity, and this may have prevented the development of severe (grade ≥ 3) toxicity.
The point estimates of the risk ratio from 12 of the 13 studies indicated a higher risk in c.2846A>T carriers versus wild-type patients,9,15,52,55–62,67,70 but the confidence intervals of four studies also included the possibility of no difference between groups or a lower risk in c.2846A>T carriers (Figure 10; Appendix 5).55,61,62,70
Figure 10: c.2846A>T Carriers Versus Wild-Type Patients—Risk of Overall Severe Toxicity.
Sources: Iachetta et al,52 Cremolini et al,55 Etienne-Grimaldi et al,56 Meulendijks et al,70 Boige et al,15 Froehlich et al,57 Toffoli et al,9 Lee et al,58 Jennings et al,59 Loganayagam et al,60 Cellier et al,61 Deenen et al,62 and Boisdron-Celle et al.67
The GRADE quality of the evidence was low because the evidence came from observational studies (Appendix 3).
Severe Hematological Toxicity
Severe neutropenia was the most commonly reported hematological toxicity. In one of the studies, none of the two c.2846A>T carriers treated with a standard fluoropyrimidine dose developed severe neutropenia53; in four other studies, severe neutropenia occurred in 20.0% to 61.9% of c.2846A>T carriers.9,15,55,58 Among the wild-type patients treated with a standard dose in the five studies, 6.5% to 36.0% experienced severe neutropenia.9,15,53,55,58
The point estimates of the risk ratio indicated a higher risk in carriers in four studies,9,15,55,58 but the confidence intervals of two studies also included the possibility of a lower risk in carriers (Figure 11).9,55 The risk ratio could not be calculated in one study, because no events occurred in c.2846A>T carriers, compared to 4 (7.7%) events in wild-type patients (P = 1.00).53 Similar results were reported for other types of hematological toxicities. Additional information is provided in Appendix 5.
Figure 11: c.2846A>T Carriers Versus Wild-Type Patients—Risk of Severe Neutropenia.
Sources: Cremolini et al,55 Boige et al,15 Toffoli et al,9 and Lee et al.58
The GRADE quality of the evidence was low because the evidence came from observational studies (Appendix 3).
Severe Gastrointestinal Toxicity
Severe diarrhea was the most commonly reported gastrointestinal toxicity. It was reported in 14.3% to 100% of c.2846A>T carriers treated with a standard fluoropyrimidine dose and 5.8% to 23.9% of wild-type patients treated with a standard dose, across six studies.9,15,53,55,58,61
The point estimates of the risk ratio indicated a higher risk in c.2846A>T carriers in all six studies, but the confidence intervals of two studies also included the possibility of a lower risk in carriers (Figure 12).15,55 Similar results were reported for other types of gastrointestinal toxicity. Additional information is provided in Appendix 5.
Figure 12: c.2846A>T Carriers Versus Wild-Type Patients—Risk of Severe Diarrhea.
Sources: Maharjan et al,53 Cremolini et al,55 Boige et al,15 Toffoli et al,9 Lee et al,58 and Deenen et al.61
The GRADE quality of the evidence was low because the evidence came from observational studies (Appendix 3).
Severe Dermatological Toxicity
One study reported that four (50.0%) c.2846A>T carriers treated with a standard fluoropyrimidine dose and 241 (43.1%) wild-type patients also treated with a standard dose experienced severe (grade ≥ 2) hand–foot syndrome (RR 1.16, 95% CI 0.40–1.91).61 Additional information is provided in Appendix 5.
The GRADE quality of the evidence was very low because the evidence came from observational studies, and because of imprecision (Appendix 3).
Carriers of the c.1236G>A Variant
Overall Severe Toxicity
The frequency of overall severe toxicity varied between 30.0% and 92.9% in heterozygous c.1236G>A carriers treated with a standard fluoropyrimidine dose and 8.2% and 85.0% of wild-type patients treated with a standard dose across six studies.13,57,59,61,70,71
One study reported a higher risk of overall severe toxicity in carriers versus wild-type patients (RR 5.12, 95% CI 2.54–9.87).57 However, the point estimates of the risk ratio were closer to 1 and the results were imprecise in five studies: 1.25 (0.91–1.61),13 1.82 (0.47–5.21),59 1.09 (0.91–1.19),61 0.83 (0.37–1.63),70 and 1.10 (0.67–1.62).71
Two homozygous c.1236G>A carriers identified in two studies experienced severe toxicity (100.0%), compared to 8.5%57and 32.3%13 in wild-type patients. The risk of severe toxicity in homozygous carriers was higher than in wild-type patients in both studies (RR 3.10, 95% CI 1.47–3.31;13 RR 11.76, 95% CI 4.73–14.9157). One study did not report results for the one homozygous c.1236G>A carrier identified.61
The GRADE quality of the evidence was low because the evidence came from observational studies (Appendix 3). Given the imprecision in the study results, the GRADE quality of the evidence was very low for heterozygous carriers (Appendix 3).
Severe Hematological Toxicity
Seventeen (22.1%) heterozygous c.1236G>A carriers treated with a standard fluoropyrimidine dose and 184 (9.8%) wild-type patients also treated with a standard dose experienced severe neutropenia in one study (RR 2.26, 95% CI 1.38–3.40).13 The one homozygous carrier identified in this study did not experience severe neutropenia.13
The GRADE quality of the evidence was low because the evidence came from observational studies (Appendix 3).
Severe Gastrointestinal Toxicity
Severe diarrhea was the most commonly reported gastrointestinal toxicity. In one study, 11 (14.3%) c.1236G>A heterozygous carriers treated with a standard fluoropyrimidine dose and 234 (12.5%) wild-type patients also treated with a standard dose experienced severe diarrhea (RR 1.14, 95% CI 0.61–1.92).13 A single homozygous carrier (100.0%) experienced severe nausea and/or vomiting compared to 88 (4.7%) wild-type patients (RR 21.3, 95% CI 9.29–25.49), but not severe diarrhea.13
A second study reported that 14 (50.0%) carriers and 125 (23.1%) wild-type patients experienced severe diarrhea (RR 2.16, 95% CI 1.35–3.34).61
The GRADE quality of the evidence was low because the evidence came from observational studies (Appendix 3).
Severe Dermatological Toxicity
One study reported that 26 (92.9%) heterozygous c.1236G>A carriers treated with a standard fluoropyrimidine dose and 459 (85.0%) wild-type patients also treated with a standard dose experienced severe (grade ≥3) hand–foot syndrome (RR 1.09, 95% CI 0.91–1.95).61
The GRADE quality of the evidence was very low because the evidence came from observational studies, and because of imprecision (Appendix 3).
Compound Heterozygous
All four compound heterozygous carriers treated with a standard fluoropyrimidine dose experienced severe toxicity; all four studies reported a higher risk compared to wild-type patients treated with a standard dose (Table 3).9,57,58,67
Table 3:
Compound Heterozygous Carriers Versus Wild-Type Patients—Risk of Overall Severe Toxicity
| Author, Year N (Carriers/Wild-Type) | Compound Heterozygous Genotype Identified | Overall Severe Toxicity, n (%) | RR (95% CI) |
|---|---|---|---|
| Froehlich et al, 201557 469 (1/468) | DPYD∗13/c.1236G>A | Carrier: 1 (100.0) Wild-type: 40 (8.5) |
11.76 (4.73–14.91) |
| Toffoli et al, 20159 586 (1/585) | DPYD∗2A/DPYD∗13 | Carrier: 1 (100.0) Wild-type: 84 (14.4) |
6.94 (3.07–8.34) |
| Lee et al, 201458 2,565 (1/2,564) | DPYD∗2A/c.2846A>T | Carrier: 1 (100.0) Wild-type: 834 (32.5) |
3.08 (1.47–3.25) |
| Boisdron-Celle et al, 200767 243 (1/242) | DPYD∗2A/c.2846A>T | Carrier: 1 (100.0) Wild-type: 8 (3.3) |
30.30 (9.10–52.26) |
Abbreviations: CI, confidence interval; RR, risk ratio.
In the study by Toffoli et al,9 the one compound heterozygous carrier experienced severe diarrhea (100.0%) compared to 34 (5.8%) wild-type patients (RR 17.2, 95% CI 6.75–22.20). The other three studies did not specify types of toxicity.57,58,67
The GRADE quality of the evidence was low because the evidence came from observational studies (Appendix 3).
DPYD GENOTYPING TO PREDICT SEVERE FLUOROPYRIMIDINE-RELATED TOXICITY
We used severe fluoropyrimidine-related toxicity as the reference standard to calculate the sensitivity, specificity, PPV, and NPV of DPYD genotyping: that is, when toxicity was observed among carriers of a DPYD variant, it was considered a true positive finding, and when toxicity was not reported among non-carriers it was considered a true negative.
The sensitivity of simultaneously genotyping the three to four DPYD variants assessed in this review ranged from 3.5% to 21.6%; the specificity ranged from 96.2% to 100.0%; the PPV ranged from 23.5% to 100.0%; and the NPV ranged from 50.5% to 91.5% (Table 4).
Table 4:
DPYD Genotyping to Detect Severe Toxicity (3–4 Variants)—Sensitivity, Specificity, PPV, and NPV
| Author, Year | Sensitivity, % (95% CI) | Specificity, % (95% CI) | PPV, % | NPV, % |
|---|---|---|---|---|
| Lunenburg et al, 20187 | 7.1 (3.6–13.4) | 96.2 (94.6–97.4) | 23.5 | 86.4 |
| Meulendijks et al, 201718 | 6.0 (NR)a | 95.0 (NR)a | 13.0a | 88.0a |
| Boige et al, 201615 | 3.5 (2.5–5.1) | 99.0 (98.0–99.5) | 77.8 | 50.5 |
| Froehlich et al, 201557 | 21.6 (12.5–34.6) | 95.3 (93.0–96.9) | 34.4 | 91.5 |
| Toffoli et al, 20159 | 11.6 (0.7–19.6) | 98.6 (97.2–99.3) | 61.1 | 85.6 |
| Lee et al, 201458 | 5.3 (4.0–7.0) | 99.4 (98.9–99.7) | 82.5 | 67.4 |
| Jennings et al, 201359 | 15.9 (7.9–29.4) | 96.2 (92.6–98.0) | 46.7 | 84.5 |
| Loganayagam et al, 201360 | 9.6 (5.3–16.8) | 100.0 (98.8–100.0) | 100.0 | 77.6 |
| Cellier et al, 201162 | 8.1 (2.8–21.3) | 100.0 (92.6–100.0) | 100.0 | 58.5 |
Abbreviations: CI, confidence interval; NPV, negative predictive value; NR, not reported; PPV, positive predictive value.
As reported by the authors.
TOXICITY-RELATED DOSE REDUCTION, TREATMENT DELAY, AND DISCONTINUATION
In one study, six (60.0%) carriers of DPYD∗2A, DPYD∗13, or c.2864A>T required a dose reduction, compared to 98 (23.6%) wild-type patients (RR 2.54, 95% CI 1.23–4.38); four (40.0%) carriers and 27 (6.4%) wild-type patients discontinued treatment due to toxicity (RR 6.25, 95% CI 2.08–16.41).60 Another study reported that 36.4% to 100.0% of carriers had their treatment delayed as a result of toxicity, but did not provide information for wild-type patients.9
Similar results were reported for individual DPYD variants (Appendix 5). Information was not provided for compound heterozygous or homozygous c.1236G>A carriers.
The GRADE quality of the evidence was low because the evidence came from observational studies (Appendix 3).
TOXICITY-RELATED HOSPITALIZATIONS
DPYD carriers treated with a standard fluoropyrimidine dose had a higher risk of hospitalization than wild-type patients in three studies7,60,67; however, the confidence interval of one study included the possibility of a lower risk in DPYD carriers.7 Two other studies reported the frequency of hospitalizations in DPYD carriers, but not in wild-type patients.68,69 Additional information is provided in Table 5.
Table 5:
DPYD Carriers Versus Wild-Type Patients—Toxicity-Related Hospitalization
| Author, Year N (Carrier/Wild-Type) DPYD Variants Included | Patients With Toxicity-Related Hospitalization, n (%) | RR (95% CI) | Number of Days in Hospital, Mean (Range) |
|---|---|---|---|
| Lunenburg et al, 20187 805 (34/771) DPYD∗2A, DPYD∗13, c.2846A>T, c.1236G>A |
Carrier: 6 (17.6) Wild-type: 60 (7.8) |
2.26 (0.69—5.14) | Carrier: 23 (6–36) Wild-type: 13 (1–76) |
| Loganayagam et al, 201360 430 (10/420) DPYD∗2A, DPYD∗13, c.2846A>T |
Carrier: 10 (100.0) Wild-type: 94 (22.4) |
4.46 (3.26–5.29) | Carrier: 7.1 Wild-type: 2.7 |
| Boisdron-Celle et al, 200767 243 (1/242) Compound heterozygous (DPYD∗2A/c.2846A>T) |
Carrier: 1 (100.0) Wild-type: 4 (1.7) |
58.82 (15.19–168.60) | Carrier: 40 Wild-type: NR |
| Largillier et al, 200668 105 (1/104) DPYD∗2A |
Carrier: 1 (100) Wild-type: NR |
Could not be calculated | Carrier: 7 Wild-type: NR |
| Salgueiro et al, 200469 73 (1/72) DPYD∗2A |
Carrier: 1 (100.0) Wild-type: NR |
Could not be calculated | NR |
Abbreviations: CI, confidence interval; NR, not reported; RR, risk ratio.
The GRADE quality of the evidence was low because the evidence came from observational studies (Appendix 3).
TOXICITY-RELATED MORTALITY
The frequency of fluoropyrimidine-related mortality ranged from 0.0% to 100.0% in heterozygous DPYD carriers treated with a standard dose and 0.0% to 2.0% in wild-type patients (Table 6). Two studies that included DPYD∗2A carriers compared the risk between groups and found a higher risk in carriers (RR 50.00, 95% CI 6.21–74.5363; RR 52.63, 95% CI 10.40–120.9068).
Table 6:
Heterozygous DPYD Carriers Versus Wild-Type Patients—Toxicity-Related Mortality
| Author, Year N (Carrier/Wild-Type) | Mortality (Any DPYD Variant, Heterozygous), n (%); Time of Occurrence |
|---|---|
| Wigle et al, 202171 1,388 (41/1,347) |
Carrier: 0 Wild-type: 10 (0.7); timing unclear |
| Cremolini et al, 201855 438 (5/433) |
Carrier: 1 (10.0); cycle 1 Wild-type: NR |
| Etienne-Grimaldi et al, 201756 243 (6/237) |
Carrier: 1 (16.7); cycle 1 Wild-type: 0 |
| Loganayagam et al, 201360 430 (10/420) |
Carrier: 0 Wild-type: NR |
| Deenen et al, 201161 604 (44/560) |
Carrier: 1 (2.3); cycle 3 Wild-type: NR |
| Cerić et al, 201063 50 (1/49) |
Carrier: 1 (100.0); cycle 1 Wild-type: 1 (2.0); timing unclear |
| Schwab et al, 200865 683 (13/670) |
Carrier: 0 Wild-type: 0 |
| Boisdron-Celle et al, 200767 252 (10/242) |
Carrier: 0 Wild-type: 1 (0.4); timing unclear |
| Largillier et al, 200668 105 (1/104) |
Carrier: 1 (100.0); treated until day 12 Wild-type: 2 (1.9); timing unclear |
Abbreviation: NR, not reported.
One study reported a death in the only homozygous c.1236G>A carrier identified, but mortality information was not reported for wild-type patients.57
Among four compound heterozygous carriers treated with a standard fluoropyrimidine dose identified in four studies,9,57,58,67 three (1 DPYD∗2A/DPYD∗13 and 2 DPYD∗2A/c.2846A>T) died as result of fluoropyrimidine-related toxicity.9,58,67 The toxicity occurred on the first cycle in one study9 but the timing was unclear in the other two.58,67 Only one of these studies reported mortality in both groups: one (100%) carrier and one (0.4%) wild-type patient (RR 250.00, 95% CI 32.04–341.82).67
The GRADE quality of the evidence was low because the evidence came from observational studies (Appendix 3).
Clinical Utility
Studies evaluating clinical utility should assess whether the test under evaluation (in this case, pre-treatment DPYD genotyping) allows treatment modifications to be implemented, resulting in improved treatment outcomes compared to not testing or to other tests (i.e., pre-treatment DPD activity measurement). However, the studies we identified were not designed to evaluate such a comparison.
Because fluoropyrimidine dosing regimens varied (by type and stage of cancer, by whether fluoropyrimidines were used alone or in combination, and by the type of combination regimen), we have used the term “standard dose” to refer to the usual fluoropyrimidine dose in a given patient population or a given regimen to distinguish it from the genotype-guided reduced dose.
Given the clinical heterogeneity in terms of type of cancer, type of fluoropyrimidine, dose, mode of administration, and combination regimens, we did not conduct meta-analyses of study results, except in some cases.
PATIENT CHARACTERISTICS
The mean age of patients in the included studies ranged from 58 to 65 years7,11,12,23,71,72; 35% to 59% were male,7,11,12,72 except for one study in women with breast cancer.23 Based on information from four studies,11,12,23,71 98% to 100% of patients were of Caucasian origin. Additional information is provided in Appendix 4.
Colorectal was the most common type of cancer in most studies (53% to 77% of patients),7,11,12,71,72 except for one study in patients with breast cancer.23 Capecitabine was the most common fluoropyrimidine prescribed: 100% in two studies12,23 and 52% to 90% in the remaining three.7,11,71,72 Concomitant radiotherapy was allowed in four studies.7,11,71,72
The prevalence of the DPYD variants was as follows:
TOXICITY-RELATED DOSE REDUCTION, INCREASE, AND DISCONTINUATION
In most studies, heterozygous DPYD∗2A and DPYD∗13 carriers started fluoropyrimidine treatment with a 50% dose reduction, and heterozygous c.2846A>T and c.1236G>A carriers started treatment with a 25% dose reduction, based on institutional and international guidelines. Dose increases were permitted according to the patient's tolerance.7,11,12,23,72 In the study by Wigle et al,71 DPYD∗2A, DPYD∗13, and c.2864A>T carriers started fluoropyrimidine treatment with a 50% dose reduction, and heterozygous c.1236G>A carriers started treatment with a 25% to 50% dose reduction. In the studies identified, wild-type patients generally received a standard fluoropyrimidine dose, although reductions were allowed.
In one of the studies, three (3.5%) DPYD carriers had their fluoropyrimidine dose reduced after the start of treatment, and one (1.2%) received a full dose for two cycles and experienced fatal toxicity.72
Dose increases occurred in 9.1% to 54.5% of DPYD carriers7,11,12,23,72 and 0.5% to 3.0% of wild-type patients who had had a previous dose reduction (Table 7).7,72
Table 7:
DPYD Carriers With a Genotype-Guided Reduced Fluoropyrimidine Dose Versus Wild-Type Patients—Treatment Modifications
| Author, Year N (Carrier/Wild-Type) | Initial Dose Reduction | Additional Dose Reductions, n (%) | Dose Increase, n (%) |
|---|---|---|---|
| Wigle et al, 202171 1,394 (47/1,347) |
Mean % of standard dose (SD) Carriers: 52 (18.0) Wild-type: 87.4 (15.2) |
NR | NR |
| Henricks et al, 201911 1,646 (40/1,606) |
Mean % of standard dose Carrier: 53 Wild-type: 92 |
Carrier: 7 (17.5) Wild-type: NR |
Carrier: 11 (27.5) Wild-type: NR |
| Kleinjan et al, 201912 185 (11/174) |
NR | Carrier: 2 (18.2); 1 because of severe toxicity after dose increase Wild-type: NR |
Carrier: 6 (54.5) Wild-type: |
| Stavraka et al, 201923 63 (2/61) |
Dose reductions, n (%) Carrier: 2 (100) Wild-type, n (%): 9 (14.8) |
Carrier: 0 Wild-type: NR |
Carrier: 2 (100); 1 (50) had grade 3 toxicity with increased dose and treatment was stopped Wild-type: 0 |
| Henricks et al, 201872 1,103 (85/1,018) |
Mean % of standard dose (range) Carrier: 69 (37–97) Wild-type: 94 (49–128) |
Carrier: 5 (5.9%); because of toxicity after dose increase | Carrier: 11 (12.9) Wild-type: 31 (3.0) |
| Lunenburg et al, 20187 827 (22a/771/34b) |
NR | Carrier: 2 (9.1) Wild-type: 34 (4.4) Carriera: 4 (11.8) |
Carrier: 2 (9.1) Wild-type: 4 (0.5) Carriera: NR |
Abbreviation: NR, not reported; SD, standard deviation.
One patient was excluded from the analyses.
DPYD carriers who received a standard dose.
None of the DPYD variant carriers in one study required dose reduction beyond what was applied pre-treatment23; in other studies, 5.9% to 18.2% of carriers7,11,12,72 and 4.4% of wild-type patients7 required further dose reductions as a result of toxicity (Table 7).
Treatment was discontinued due to toxicity in 17.5% to 50.0% of DPYD carriers treated with a genotype-guided reduced dose and 3.3% to 17.2% of wild-type patients.7,11,23,71,72 The point estimates indicated a similar risk of discontinuation between groups in two studies and a higher risk in DPYD carriers in three other studies. However, the study results were imprecise (Figure 13, Appendix 5).
Figure 13: DPYD Carriers With a Genotype-Guided Reduced Fluoropyrimidine Dose Versus Wild-Type Patients—Treatment Discontinuation.
Sources: Wigle et al,71 Henricks et al (2019),11 Stavraka et al,23 Henricks et al (2018),72 and Lunenburg et al.7
In the study by Lunenburg et al,7 four (18.2%) DPYD carriers treated with a genotype-guided reduced dose and six (17.6%) treated with a standard dose discontinued treatment (RR 1.03, 95% CI 0.28–4.03).
The GRADE quality of the evidence was very low because the evidence came from observational studies, and because of imprecision (Appendix 3).
SEVERE FLUOROPYRIMIDINE-RELATED TOXICITY
Overall Severe Toxicity
Overall severe toxicity occurred in 18% to 50% of carriers with a genotype-guided reduced dose compared to 14% to 38% of wild-type patients treated with a standard dose.7,11,12,23,71,72 The point estimates of the risk ratio indicated a lower risk of severe toxicity in DPYD carriers treated with a reduced dose compared to wild-type patients in three studies and a higher risk in DPYD carriers in three other studies. However, the confidence intervals included the possibility of both higher and lower risk in DPYD carriers in all but one study (Figure 14; Appendix 5).72 The authors of that study believed that the 25% fluoropyrimidine dose reduction applied in c.2846A>T and c.1236G>A carriers was not sufficient to prevent severe toxicity.72 A guideline that had previously recommended a 25% dose reduction in these patients has been updated to recommend a 50% dose reduction.21
Figure 14: DPYD Carriers With a Genotype-Guided Reduced Fluoropyrimidine Dose Versus Wild-Type Patients—Risk of Overall Severe Toxicity.
Sources: Wigle et al,71 Henricks et al (2019),11 Kleinjan et al,12 Stavraka et al,23 Henricks et al (2018),72 and Lunenburg et al.7
In the study by Lunenburg et al,7 five (22.7%) DPYD carriers treated with a genotype-guided reduced dose and eight (23.5%) treated with a standard dose experienced severe toxicity (RR 0.97, 95% CI 0.30–3.05).
Severe Toxicity by Type
Only the study by Henricks et al (2018)72 reported a higher risk of both severe hematological and severe gastrointestinal toxicity in DPYD carriers treated with a genotype-guided reduced dose compared to wild-type patients treated with a standard dose. The results of the other studies were imprecise, and the 95% confidence intervals included the possibility of both higher and lower risk in DPYD carriers (Figures 15 and 16; Appendix 5).7,11,12,23,71 For severe hand–foot syndrome, all five studies reported imprecise results, and the confidence intervals included the possibility of higher and a lower risk in DPYD carriers versus wild-type patients (Figure 17; Appendix 5).7,11,12,23,71,72
Figure 15: DPYD Carriers With a Genotype-Guided Reduced Fluoropyrimidine Dose Versus Wild-Type Patients—Risk of Severe Hematological Toxicity.
Sources: Wigle et al,71 Henricks et al (2019),11 Kleinjan et al,12 Stavraka et al,23 Henricks et al (2018),72 and Lunenburg et al.7
Figure 16: DPYD Carriers With a Genotype-Guided Reduced Fluoropyrimidine Dose Versus Wild-Type Patients—Risk of Severe Gastrointestinal Toxicity.
Sources: Wigle et al,71 Henricks et al (2019),11 Kleinjan et al,12 Stavraka et al,23 Henricks et al (2018),72 and Lunenburg et al.7
Figure 17: DPYD Carriers With a Genotype-Guided Reduced Fluoropyrimidine Dose Versus Wild-Type Patients—Risk of Severe Hand–Foot Syndrome.
Sources: Wigle et al,71 Henricks et al (2019),11 Kleinjan et al,12 Stavraka et al,23 Henricks et al (2018),72 and Lunenburg et al.7
In the study by Lunenburg et al,7 two (9.1%) DPYD carriers treated with a genotype-guided reduced fluoropyrimidine dose experienced severe hematological and severe gastrointestinal toxicity compared to four (11.8%) carriers who received a standard dose and experienced severe hematological toxicity and six (17.6%) carriers who received a standard dose and experienced severe gastrointestinal toxicity. The risk ratios were 0.77 (95% CI 0.12–5.72) and 0.52 (95% CI 0.08–2.28), respectively.
The GRADE quality of the evidence for the severe toxicity outcomes (DPYD carriers with a reduced dose vs. wild-type patients) was very low because the evidence came from observational studies, and because of imprecision (Appendix 3). As well, when comparing DPYD carriers who received a reduced dose with carriers who received a standard dose, the GRADE quality of the evidence was further downgraded because of risk of bias (Appendix 3).
TOXICITY-RELATED HOSPITALIZATIONS
Toxicity-related hospitalizations were reported in 15% to 50% of DPYD carriers who received a genotype-guided reduced fluoropyrimidine dose and in 0% to 14% of wild-type patients treated with a standard dose.7,11,12,23,72 The point estimates of four studies indicated a higher risk of treatment-related hospitalization in carriers compared to wild-type patients, but the confidence intervals also included the possibility of lower risk in DPYD carriers (Figure 18).7,11,12,72 The risk ratio could not be calculated in one study because none of the wild-type patients were hospitalized as a result of toxicity (compared to one [50.0%] carrier, P = .03). Additional information is provided in Appendix 5.
Figure 18: DPYD Carriers With a Genotype-Guided Reduced Fluoropyrimidine Dose Versus Wild-Type Patients—Risk of Hospitalization.
Sources: Henricks et al (2019),11 Kleinjan et al,12 Stavraka et al,23 Henricks et al (2018),72 and Lunenburg et al.7
The median number of days in hospital ranged from 4 to 6.5 in DPYD carriers treated with a genotype-guided reduced dose and 5 to 13 in wild-type patients, based on three studies.7,12,72 Lunenburg et al7 reported a similar risk of hospitalization between DPYD carriers who received a genotype-guided reduced fluoropyrimidine dose and DPYD carriers who received a standard dose (18.2% vs. 17.6%, respectively, RR 1.03, 95% CI 0.27—4.03). However, the length of stay in hospital was shorter in DPYD carriers treated with a reduced dose (mean 4 days, range 2–5) than in those treated with a standard dose (mean 23 days, range 6–36; P = .010).7
The GRADE quality of the evidence (DPYD carriers with a reduced dose vs. wild-type) was very low because the evidence came from observational studies, and because of imprecision (Appendix 3). As well, when comparing DPYD carriers who received a reduced dose with carriers who received a standard dose, the GRADE quality of the evidence was further downgraded because of risk of bias (Appendix 3).
TOXICITY-RELATED MORTALITY
The only fluoropyrimidine-related death reported in DPYD carriers who were treated with a reduced dose occurred after the patient had been wrongly prescribed a standard fluoropyrimidine dose for two cycles.72 Therefore, we could not determine what the outcome would have been if the patient had received a reduced dose. In wild-type patients, one study reported 2 (0.1%) deaths11 a second study reported three (0.3%) deaths,72 and a third study reported 10 (0.7%) deaths.71 Additional information is provided in Appendix 5.
Fluoropyrimidine Treatment Effectiveness
We evaluated fluoropyrimidine treatment effectiveness in carriers of the DPYD variants under assessment who were treated with a genotype-guided reduced dose and compared it with treatment effectiveness in wild-type patients treated with a standard dose. Outcomes included treatment response, disease progression, overall survival, and progression-free survival.
The authors did not provide baseline characteristics specific to the matched DPYD carriers and wild-type patients. However, based on the full cohort, 45% of patients were male, the median age was 61 years (range 21–91), 96% were Caucasian, and approximately half had colorectal cancer.11
Median survival was 27 months (range 1–83) in DPYD∗2A carriers and 24 months (range 0.7–97) in wild-type patients (hazard ratio 0.82, 95% CI 0.47–1.43).11 The median progression-free survival was 14 months (range 0.7–83) in DPYD∗2A carriers and 10 months (range 0.2–97) in wild-type patients (hazard ratio 0.83, 95% CI 0.47–1.50).11 Results for treatment response and disease progression followed a similar direction (Appendix 5).
The GRADE quality of the evidence for both outcomes was very low because the evidence came from observational studies, and because of imprecision (Appendix 3).
Experience With DPYD Genotyping in Canada
Wigle et al71 reported experiences with DPYD genotyping at an Ontario hospital between December 2013 and December 2019. Initially, testing included three variants (DPYD∗2A, DPYD∗13, and c.2864A>T), but in May 2018 a fourth variant (c.1236G>A) was added, based on its addition to the Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline in 2017.71 During the study period, a total of 1,845 patients were tested before they started cancer treatment with fluoropyrimidines.71 Among 1,394 patients who started treatment at the hospital before December 2019, nine (0.6%) DPYD∗2A carriers, one (0.07%) DPYD∗13 carrier, 19 (1.4%) c.2864A>T carriers, and 18 (1.3%) c.1236G>A carriers were identified and started treatment with a genotype-guided reduced fluoropyrimidine dose.71 Two (0.1%) compound heterozygous carriers were identified, and the treating oncologist was advised to prescribe an alternative treatment instead of fluoropyrimidines.71 Forty-one patients who received a standard fluoropyrimidine dose were retrospectively identified as c.1236G>A carriers when this variant was added to the panel.71
In Quebec, public funding is available for all four DPYD variants assessed in this review.35 The province's experience with DPYD∗2A, the first variant introduced, has been published.75 A total of 2,617 DPYD∗2A genotype tests were performed between March 2017 and August 2018.75 The test was performed at one site, and samples were sent from hospitals across the province; test results were available in a mean of 6 days, including transportation time between institutions.75 A total of 25 patients were identified as DPYD∗2A carriers: 24 (0.92%) heterozygous and one (0.038%) homozygous.75 All carriers identified were Caucasian, but most patients tested were Caucasian.75 DPYD genotyping allowed treatment modifications in all 14 carriers who were tested before the start of treatment: five patients received an alternative regimen, one refused treatment, and eight started treatment with a 50% to 75% fluoropyrimidine dose reduction.75 Some of these patients had their dose increased, but in two patients who reached a full dose, the dose had to be decreased because of grade 2 toxicity.75 According to the authors, none of the patients treated with a genotype-guided reduced fluoropyrimidine dose experienced severe (grade ≥ 3) toxicity or required treatment withdrawal because of toxicity.75 The authors concluded that pre-treatment DPYD genotyping was feasible in clinical practice and could prevent severe toxicities and hospitalizations without delaying treatment initiation.75
Ongoing Studies
We are aware of the following ongoing studies that have potential relevance to our research questions.
One study is assessing the additional value of pre-treatment DPD phenotyping to guide dose individualization in wild-type patients. As a secondary objective, the authors are evaluating the effect of a 50% dose reduction on fluoropyrimidine-related toxicity in c.1236G>A and c.2846A>T carriers.76 The estimated study completion date was January 2021.76
Another study is evaluating the effect on toxicity of a fluoropyrimidine dose reduction based on pre-treatment genotyping and phenotyping according to the French guidelines compared to a dose reduction based on the literature.77 The expected study completion date is September 2021.77
Discussion
Our systematic review identified a large number of studies (n = 29), all in adult patients.7,9,11–13,15,18,23,52–69,71,72 The prevalence of each variant (DPYD∗2A 1.1%, DPYD∗13 0.2%, c.2846A>T 1.2%, c.1236G>A 4.0%) and of all four variants combined (6.6%) was consistent with what has been reported in the literature. Few homozygous carriers were identified (0.05%–0.2%), and of those, only for variant c.1236G>A.13,57,61 Compound heterozygous carriers constituted 0.03% to 0.4% of the patients in four studies.9,57,58,67
The frequency of severe toxicity varied considerably across studies, both in wild-type patients (3% to 89%) and DPYD variant carriers treated with a standard fluoropyrimidine dose (30% to 100%, except for two studies in which carriers had their dose reduced due to non-severe toxicity). Many factors can affect the risk of toxicity (e.g., age, sex, renal function, performance status, type, dose, and mode administration of fluoropyrimidines, combination with other cytotoxic chemotherapy drugs, and the timing of toxicity measurement).8,10,13,15,16 The studies we identified included patients that may have differed with respect to some of these factors, reflecting the population that may receive cancer treatment with fluoropyrimidines. We were unable to conduct subgroup analyses, but the point estimates from most included studies were consistent in suggesting an increased risk of severe toxicity in carriers of the DPYD variants under assessment who received a standard fluoropyrimidine dose compared to wild-type patients. However, we found wide variation in the point estimates of the risk ratios across studies, and wide confidence intervals in some studies. As stated by the Clinical Pharmacogenetics Implementation Consortium (CPIC), disease and treatment regimens may affect the risk of toxicity, but the association of DPYD variants with fluoropyrimidine-related toxicity has been fairly consistent across regimens.8
Death as a result of fluoropyrimidine-related toxicity is estimated to occur in approximately 0.1% to 1.0% of patients.4,9,10 The frequency of mortality in wild-type patients reported in five studies ranged from 0.0% to 2.0%56,63,65,67,68,71 and seemed to be consistent with the literature. In heterozygous DPYD carriers treated with a standard fluoropyrimidine dose, three studies reported no deaths,60,65,67 and five studies reported that 14.3% to 100% of carriers died as a result of toxicity.55,56,61,63,68 In four studies, three of four compound heterozygous carriers died as a result of fluoropyrimidine-related toxicity,9,57,58,67 and in the single study that reported mortality, the one homozygous c.1236G>A carrier died.57
Most studies did not directly assess the clinical utility of DPYD genotyping, because they were not specifically designed to compare pre-treatment DPYD genotyping with no testing or with other tests to measure DPD deficiency. Phenotype tests that measure DPD function have limitations such as issues with standardization, validation, and interpretation of results, because thresholds for treatment decisions have not been established.2,6 They are also difficult to implement in routine practice and involve a longer turnaround time compared to genotyping.23,72 At present, DPD function phenotype tests are not routinely done in Ontario, in part because of some these reasons (Richard Kim, MD, email communication, January 12, 2021; Leta Forbes, MD, email communication, January 8, 2021; John Lenehan, MD, email communication, February 12, 2021; Michael Raphael, MD, email communication, February, 10, 2021).
Strengths and Limitations
Our results were consistent with those of previous systematic reviews and built on the results of two health technology assessments, complementing the evidence base with nine additional studies. Limitations in the literature identified included the following:
Some study results were imprecise, partly because of the low prevalence of DPYD variants, leading to reduced statistical power to detect differences between groups
Some studies reported fluoropyrimidine dose reduction based on a toxicity grade lower than 3 without knowledge of DPYD genotyping results,9,57,59,62,64,67 and this may have prevented severe (grade ≥ 3) toxicity from occurring, leading to an underestimate of severe toxicity
Mostly Caucasian patients were included in the studies, and authors recommended that more research on the frequency and clinical relevance of these and other DPYD variants in other racial/ethnic groups be performed72
Clinical utility was assessed indirectly in most studies by comparing the outcomes of DPYD carriers who received a genotype-guided fluoropyrimidine dose reduction with wild-type patients who received a standard dose. One study retrospectively compared DPYD carriers who received a genotype-guided reduced fluoropyrimidine dose with DPYD carriers who received a standard dose.7 However, it was difficult to draw conclusions based on this study given the small number of carriers identified and imbalances in the distribution of DPYD variants between the two groups
We were unable to perform subgroup analyses based on factors that may affect the risk of severe toxicity (e.g., age, sex, cancer type, fluoropyrimidine type and dose, and combination with other cytotoxic chemotherapy drugs) for clinical validity, clinical utility, and fluoropyrimidine treatment effectiveness
Conclusions
Clinical Validity
Studies found that heterozygous carriers of any one of the variants under assessment (DPYD∗2A, DPYD∗13, c.2846A>T, c.1236G>A) treated with a standard fluoropyrimidine dose (assessed as a single group) may have a higher risk of severe fluoropyrimidine-related toxicity than wild-type patients treated with a standard dose. This may lead to dose reduction, treatment discontinuation, and hospitalization (GRADE: Low).
A similar trend was observed when each variant was evaluated separately, but the results of some of the studies were imprecise, especially for DPYD∗13, the variant with the lowest prevalence, and c.1236G>A (GRADE: Low to Very low, depending on the variant)
A similar trend was observed for compound heterozygous carriers and homozygous c.1236G>A carriers (GRADE: Low)
DPYD genotyping had a high clinical specificity to detect severe toxicity. However, because some of the wild-type patients also experienced severe toxicity, the clinical sensitivity of the test to detect severe toxicity was low. This may have been due to the fact that other unmeasured DPYD variants may also contribute to DPD deficiency, and that severe toxicity in patients treated with fluoropyrimidines may be explained by causes other than DPD deficiency
Clinical Utility
We were unable to adequately assess the clinical utility of DPYD genotyping because the studies identified were not specifically designed to evaluate this outcome. Six studies compared outcomes between heterozygous carriers of one of the DPYD variants under assessment who received a genotype-guided reduced fluoropyrimidine dose with wild-type patients who received a standard dose. One study also compared outcomes with DPYD carriers who received a standard dose.
DPYD genotyping led to changes in clinical conduct by permitting fluoropyrimidine treatment modifications (dose modifications or avoiding fluoropyrimidines)
Given the imprecision in study results, it is uncertain whether genotype-guided dose reduction in heterozygous DPYD carriers led to a risk of severe toxicity and toxicity-related hospitalization that was comparable to that of wild-type patients on a standard dose (GRADE: Very low)
One study compared DPYD carriers treated with a reduced fluoropyrimidine dose and DPYD carriers treated with a standard dose; however, given the imprecision in study results and imbalances in the distribution of DPYD variants between groups, it is uncertain whether genotype-guided dose reduction in heterozygous DPYD carriers led to a lower risk of severe toxicity and toxicity-related hospitalization than that of carriers treated with a standard dose (GRADE: Very low). Hospital length of stay was shorter in DPYD carriers treated with a reduced dose than in DPYD carriers treated with a standard dose, but the evidence was uncertain (GRADE: Very low)
Compound heterozygous and homozygous DPYD carriers were not represented in the clinical utility studies. According to some study authors and experts, DPYD genotyping is important for identifying these patients, because they are expected to have very low or even absent DPD activity; a full fluoropyrimidine dose could be life-threatening,72 even with the first dose (Richard Kim, MD, email communication, January 12, 2021; John Lenehan, MD, email communication, February 12, 2021; Michael Raphael, MD, email communication, February 10, 2021)
Fluoropyrimidine Treatment Effectiveness
It is uncertain whether the treatment effectiveness of a reduced dose in DPYD carriers is comparable to the treatment effectiveness of a standard dose in wild-type patients (GRADE: Very low).
Challenges in assessing the evidence included the low frequency of the DPYD variants under assessment, which led to imprecision in the results of some studies. The current evidence is based mostly on experience in Caucasian patients, and study authors pointed out that more research on the frequency and clinical relevance of these and other DPYD variants in other racial/ethnic groups is necessary.72
Economic Evidence
Research Question
What is the cost-effectiveness or cost impact of pre-treatment DPYD genotyping compared to usual care (no testing) or with other tests for DPD deficiency before the start of treatment in patients who have planned cancer treatment with fluoropyrimidines?
Methods
Economic Literature Search
We performed an economic literature search on February 20, 2020, to retrieve studies published from database inception until the search date. To retrieve relevant studies, we developed a search using the clinical search strategy with an economic and costing filter applied. In addition to the databases used for the clinical systematic review search, we also used the Ovid interface in the Cochrane Central Register of Controlled Trials.
We created database auto-alerts in MEDLINE and Embase and monitored them for the duration of the assessment period. We also performed a targeted grey literature search of health technology assessment agency websites, systematic review registries, and the Tufts Cost-Effectiveness Analysis Registry. See the Clinical Literature Search section, above, for further details on methods used. See Appendix 1 for our literature search strategies, including all search terms.
Eligibility Criteria
STUDIES
Inclusion Criteria
English-language full-text publications
Studies published from database inception until February 20, 2020
Cost–benefit analyses, cost-effectiveness analyses, cost-minimization analyses, cost–consequence analyses, cost–utility analyses, or cost analyses
Exclusion Criteria
Narrative reviews, letters/editorials, case reports, commentaries, conference abstracts, posters, and unpublished studies
POPULATION
Adult and pediatric patients who had planned cancer treatment with fluoropyrimidines, alone or in combination with other therapies
INTERVENTIONS
Inclusion Criterion
DPYD genotyping of the variants under assessment (DPYD∗2A, DPYD∗13, c.2846A>T, c.1236G>A) before the start of treatment
Carriers of DPYD variants under assessment who had their fluoropyrimidine dose reduced as a result of pre-treatment genotyping
Exclusion Criteria
Treatment decisions based on testing for other DPYD or other gene variants (e.g., TYMS, CES)
Treatment decisions based on either phenotypic measurement of dihydropyrimidine dehydrogenase (DPD) enzyme activity, such as plasma uracil concentration or dihydrouracil:uracil (UH2:U) ratio, or 5-fluorouracil (5-FU) pharmacokinetics assessment
COMPARATOR
Inclusion Criteria
No DPYD genotyping
Phenotypic tests for DPD deficiency (e.g., plasma uracil concentration or UH2:U ratio) before the start of treatment, or 5-FU pharmacokinetics assessment
Wild-type patients or carriers who did not have the fluoropyrimidine dose adjusted as a result of pre-treatment genotyping
Exclusion Criterion
Treatment with uridine triacetate (antidote for 5-FU toxicity), given its limited use
OUTCOME MEASURES
Costs
Health outcomes (e.g., quality-adjusted life-years [QALYs], number of adverse events)
Incremental costs and incremental effectiveness
Incremental cost-effectiveness ratios (ICER)
Literature Screening
A single reviewer conducted an initial screening of titles and abstracts using Covidence41 and then obtained the full texts of studies that appeared eligible for review according to the inclusion criteria. A single reviewer then examined the full-text articles and selected studies eligible for inclusion. The reviewer also examined reference lists for any additional relevant studies not identified through the search.
Data Extraction
We extracted relevant data on study characteristics and outcomes to collect information about the following:
Source (e.g., citation information, study type)
Methods (e.g., study design, analytic technique, perspective, time horizon, population, intervention, comparator)
Outcomes (e.g., health outcomes, costs, incremental cost-effectiveness ratios)
Study Applicability and Limitations
If an eligible study was identified, we determined the usefulness of each study for decision-making by applying a modified quality appraisal checklist for economic evaluations originally developed by the National Institute for Health and Care Excellence (NICE) in the United Kingdom to inform the development of NICE's clinical guidelines.78 We modified the wording of the questions to remove references to guidelines and to make it specific to Ontario. Next, we separated the checklist into two sections. In the first section, we assessed the applicability of each study to the research question (directly, partially, or not applicable). In the second section, we assessed the limitations (minor, potentially serious, or very serious) of the studies that we found to be directly applicable.
Results
Economic Literature Search
The database search of the economic literature yielded 158 citations published from database inception to February 20, 2020. We identified eight additional studies from other sources, for a total of 131 after removing duplicates. We excluded a total of 100 articles based on information in the title and abstract. We then obtained the full text of 31 potentially relevant articles for further assessment. Two studies met the inclusion criteria. Figure 19 presents the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram for the economic literature search.
Figure 19: PRISMA Flow Diagram—Economic Search Strategy.
Abbreviation: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses.
Source: Adapted from Moher et al, 2009.51
Overview of Included Economic Studies
We identified two cost-minimization analyses79,80 that met the inclusion criteria. The characteristics and results of the included studies are summarized in Table 8.
Table 8:
Summary of the Included Economic Studies
| Results | ||||||
|---|---|---|---|---|---|---|
| Author, Year, Country of Publication | Analytic Technique, Study Design, Perspective, Time Horizon | Population | Intervention and Comparator | Health Outcomes | Costs | Cost-Effectiveness |
| Deenen et al, 201679 Netherlands |
• Cost-minimization analysis • Decision-tree model • Health care payer perspective • Time horizon not specified |
• Cancer patients intended to undergo fluoropyrimidine-based treatment (n = 2,038) • Age (mean): 61 y • Male: 45% • Prevalence of DPYD variant carrier: 1.1% (22/2,038) |
• Pre-treatment DPYD genotyping for 1 variant (DPYD∗2A) • Usual care (no testing) |
Proportion of people experiencing severe toxicity (grade ≥ 3) • Pre-treatment DPYD genotyping: 23.18%a • Usual care: 23.72%a • Difference: –0.54% |
Total cost per patient (in 2014 Euros) • Pre-treatment DPYD genotyping: €2,772 • Usual care: €2,817 • Difference, deterministic: –€45 • Difference, probabilistic: –€44 (−;€331 to €74) |
Pre-treatment DPYD genotyping was slightly more effective and less costly |
| Henricks et al., 201980 Netherlands |
• Cost-minimization analysis • Decision-tree model • Health care payer perspective • Time horizon not specified |
• Cancer patients intended to undergo fluoropyrimidine-based treatment (n = 1,103) • Age (mean): 64 y • Male: 54% • Prevalence of DPYD variant carriers: 7.7% (85/1,103) |
• Pre-treatment DPYD genotyping for 4 variants (DPYD∗2A, c.2846A>T, c.1679T>G, and c.1236G>A) • Usual care (no testing) |
Proportion of people experiencing severe toxicity (grade ≥ 3) • Pre-treatment DPYD genotyping: 23.93%b • Usual care: 24.81%b • Difference, deterministic: –0.88% • Difference, probabilistic: –0.89% (−;1.79% to 0.04%) |
Total cost per patient (in 2019 Euros) • Pre-treatment DPYD genotyping: €2,599 • Usual care: €2,650 • Difference, deterministic: –€51 • Difference, probabilistic: –€52 (−;€176 to €38) |
Pre-treatment DPYD genotyping was cost-saving or cost neutral |
Calculated based on Appendix Table A21(prevalence of DPYD variant carriers and the probability of severe toxicity).
Calculated based on prevalence of DPYD variant carriers and the probability of severe toxicity.
Both studies79,80 were conducted in the Netherlands and used similar decision-tree models to compare the costs of a pre-treatment DPYD genotyping strategy with no genotyping. The analyses were conducted alongside two prospective observational studies.79,81 Both studies included patients who were intended to start on a fluoropyrimidine-based chemotherapy and both studies conducted DPYD genotyping before treatment initiation. Deenen et al,79 who screened patients for one variant (DPYD∗2A), identified 1.1% of patients (22 of 2,038) as heterozygous DPYD variant carriers. Henricks et al,81 who screened for four variants (DPYD∗2A, c.2846A>T, c.1679T>G, and c.1236G>A), identified 7.7% of patients (85 of 1,103) as heterozygous DPYD variant carriers. DPYD variant carriers were given a dose reduction and wild-type patients received the standard dose. The outcome measured was frequency of severe (grade ≥ 3) toxicities. Because a randomized trial comparing genotyping to no testing was considered unethical, both studies obtained toxicity data on DPYD variant carriers treated with standard-dose fluoropyrimidines from two different historical cohorts identified from the literature. Both analyses were conducted from a Dutch health care payer perspective and included only direct medical costs, such as costs for DPYD genotyping, fluoropyrimidine drug therapy, and treatment of severe toxicity. The cost of the DPYD genotyping test ranged from €75 per test for one variant79 to €100 per test for four variants.80
Both analyses found the DPYD genotyping strategy to be slightly less costly than usual care (no testing), with some uncertainty. The savings were attributed to a reduction in severe fluoropyrimidine-related toxicities. Deenen et al79 estimated the total treatment cost to be €2,772 per patient for DPYD genotyping and €2,817 per patient for no genotyping, resulting in an average cost savings of €44 per patient (range: −€74 to €331, in 2014 Euros). The result was most sensitive to the risk of hospitalization in DPYD variant carriers receiving standard dose, frequency of DPYD variant genotype, and genotyping cost. Henricks et al80 had very similar results. The estimated cost of the DPYD genotyping strategy and the no-genotyping strategy was €2,599 and €2,650 per patient, respectively. The average incremental cost was −€52 per patient (range: −€176 to €38, in 2019 Euros), indicating savings. The average incremental rate of severe toxicity was −0.89% (range: −1.79% to 0.04%), indicating improved safety. One-way sensitivity analysis showed that the most influential parameters were the frequency of DPYD variant genotypes, risk of hospitalization, and genotyping costs.
Applicability of the Included Studies
Appendix 7 provides the results of the quality appraisal checklist for economic evaluations applied to the included studies. Both studies were deemed partially applicable to our research question because they considered our population and intervention of interest. However, both studies were conducted from a Dutch health care payer perspective and were not directly applicable to the Canadian setting.
Discussion
Our literature review showed that the economic evidence for DPYD genotyping is still very limited. Only two studies met the inclusion criteria, and both were cost-minimization analyses. There is a lack of cost-effectiveness and cost–utility studies that evaluate the effect of DPYD genotyping on patients' survival or quality of life. The strength of the two cost-minimization analyses was that clinical and resource-use data on wild-type patients and DPYD variant carriers who received reduced doses were collected directly from two prospective observational studies.79,81 Although the studies are well conducted, there were some limitations. First, ethical considerations prevented randomized clinical trials from directly comparing pre-treatment DPYD genotyping with no genotyping. Instead, Deenen et al79 and Henricks et al72 assessed the impact of DPYD genotyping indirectly by comparing DPYD variant carriers who received reduced-dose fluoropyrimidines with historical controls (DPYD variant carriers who received a standard dose, identified from the literature). As a result, many confounding factors might have contributed to the observed toxicity outcomes (e.g., differences in age, sex, cancer type, and treatment regimens between the comparison groups). For example, in Deenen et al,79 historical controls were more often treated with 5-FU–based regimens than with capecitabine-based regimens, compared to the prospective patient cohort. Second, the impact of DPYD genotyping could have been slightly underestimated in both studies because they did not include any compound heterozygous or homozygous DPYD variant carriers (i.e., poor metabolizers). Although poor metabolizers are very rare, according to the literature and clinical experts, they may actually benefit the most from DPYD genotyping because standard-dose fluoropyrimidines could cause very severe or even life-threatening toxicities. Last, both studies were conducted in the Netherlands. Because practice patterns and unit prices may be different between the Netherlands and Canada, the study results may not be generalizable to the Ontario setting.
Excluded Studies
We also identified three cost studies and one cost–utility analysis in patients who received retrospective DPYD genotyping. These studies did not meet our inclusion criteria because the patients were genotyped after they had already received fluoropyrimidine treatment.82–85 The studies were also excluded because they did not compare a pre-treatment DPYD genotyping strategy to a no-genotyping strategy.
Cortejoso et al82 compared the cost of DPYD genotyping for three variants (DPYD∗2A, c.2846A>T, and c.1679T>G) with the cost of treating severe fluoropyrimidine-induced neutropenia in a Spanish hospital setting. Based on hospital records of 20 colorectal cancer patients who developed severe neutropenia, the average cost of treating a patient with severe neutropenia was estimated to be €3,044, and the cost of testing 1,000 patients was estimated to be €6,400. Therefore, the researchers concluded that DPYD genotyping would be cost-effective if 2.1 cases of neutropenia could be avoided for every 1,000 patients treated.
Another cost analysis, by Murphy et al,83 investigated the costs of two DPYD testing strategies in an Irish hospital setting: reactive DPYD screening (testing patients for DPYD variants after experiencing severe toxicity) and prospective screening. Over a period of 3 years, a total of 134 patients who received first-line fluoropyrimidine-based chemotherapy for colorectal cancer were included in the study. Thirty patients experienced severe toxicity (23%) and were subsequently tested for four DPYD variants (DPYD∗2A, c.2846A>T, c.1679T>G, and c.1601G>A). Of these, five (17% of those tested, 4.5% of total population) were heterozygous DPYD variant carriers. The total cost of toxicity-related hospitalization for these five patients was €232,061 (an average of €46,412 per case). However, the cost of conducting pre-treatment DPYD genotyping in all 134 patients would have been €23,718 (at €177 per test). The authors concluded that if 60% of patients identified as DPYD variant carriers were prevented from experiencing severe toxicity resulting in hospitalization, approximately €120,000 in costs could have been avoided over a 3-year period.
A third cost analysis was conducted by Toffoli et al84 on 550 colorectal cancer patients who were treated with fluoropyrimidine-based chemotherapy in Italy. Patients were retrospectively genotyped for four variants (DPYD∗2A, DPYD∗13, DPYD c.2846A>T and DPYD-HapB3). Their results showed that carriers of at least one DPYD variant had higher toxicity management costs (€2,972; 95% CI: €2,456–€3,505) than noncarriers (€825; 95% CI: €785–€864) and a higher risk for toxicity requiring hospitalization (odds ratio, 4.14; 95% CI: 1.87–9.14).
Lastly, Fragoulakis et al85 conducted a cost–utility analysis on patients who received fluoropyrimidine-based chemotherapy and were retrospectively genotyped for four DPYD variants (DPYD∗2A, DPYD∗13, DPYD c.2846A>T, and DPYD-HapB3) in Italy. They compared the costs, survival, and QALYs of two groups of patients: wild-type patients and DPYD-variant carriers. The results showed that wild-type patients had lower cost, slightly higher survival, and higher QALYs.
Conclusions
We found two cost-minimization analyses comparing pre-treatment DPYD genotyping with no genotyping in patients with planned fluoropyrimidine treatment. The studies suggested that, compared to no genotyping, pre-treatment DPYD genotyping may reduce severe fluoropyrimidine-related toxicity and lead to small cost savings.
Primary Economic Evaluation
We found two published cost-minimization analyses comparing pre-treatment DPYD genotyping with no testing in patients with planned fluoropyrimidine treatment. Although those studies addressed our research question, they were not directly applicable to the Canadian setting as both studies were conducted from a Dutch health care payer perspective. Also, they evaluated only costs and the frequency of severe toxicities and did not consider the impact of DPYD genotyping on patients' survival or quality of life. Owing to these limitations, we conducted a primary economic evaluation to estimate the cost-effectiveness of DPYD genotyping in Ontario.
Research Question
What is the cost-effectiveness of pre-treatment DPYD genotyping compared to usual care (no testing) in patients who have planned cancer treatment with fluoropyrimidines from the perspective of the Ontario Ministry of Health?
Methods
The information presented in this report follows the reporting standards set out by the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement.86
Type of Analysis
We conducted a probabilistic cost–utility analysis as it is the recommended reference case approach by the Canadian Agency for Drugs and Technologies in Health (CADTH) guidelines for economic evaluation. The effectiveness outcome is quality-adjusted life years (QALYs), which considers both patient's survival and quality of life (e.g., 1 QALY represents 1 year of perfect health). A generic outcome measure such as QALY allows decision-makers to make comparisons across different conditions and interventions. We also conducted a cost-effectiveness analysis with outcome expressed in natural units, such as the proportion of patients with severe fluoropyrimidine-related toxicities.
Target Population
The target population was adults who had planned fluoropyrimidine-based anticancer treatment. Based on 23 clinical validity studies identified from the clinical evidence review, the mean age of patients in clinical studies ranged from 47 to 67 years. Between 42% and 68% of patients were male, except for two studies that focused on women with breast cancer. Patients often had different types of cancer, with colorectal being the most common. Among the fluoropyrimidines, 5-fluorouracil (5-FU) was used more commonly.
We also obtained data on patients treated with fluoropyrimidines in Ontario (Ontario Health; Ontario Cancer Registry, Cancer Activity Level Reporting and Ontario Drug Benefit datasets; prepared March 2020). Compared to the clinical literature, the characteristics of the Ontario patient population were very similar (Table 9). The mean age was 63.6 years, and 50.8% were female. The most common types of cancer were colorectal, breast, and upper gastrointestinal. Similarly, a majority of the patients in Ontario received 5-FU.
Table 9:
Characteristics of the Ontario Population—Patients Who Received Fluoropyrimidines From 2014/2015 to 2018/2019 in Ontario (N = 38,225)
| Patient Characteristics | Mean Value |
|---|---|
| Age | 63.6 years |
| Sex | |
| Male | 49.2% |
| Female | 50.8% |
| Type of cancer | |
| Colorectal | 40% |
| Breast | 22% |
| Upper gastrointestinal | 10% |
| Other (e.g., pancreatic, prostate, skin, lymphoid) | 28% |
| Fluoropyrimidine prescribed | |
| 5-Fluorouracil alone | 66.8% |
| Capecitabine alone | 25.1% |
| Both | 8.1% |
Source: Data prepared in March 2020 by Ontario Health (Cancer Care Ontario) using Ontario Cancer Registry, Cancer Activity Level Reporting and Ontario Drug Benefit datasets.
Perspective
We conducted this analysis from the perspective of the Ontario Ministry of Health.
Intervention and Comparator
We compared pre-treatment DPYD genotyping with usual care (no testing). In the DPYD genotyping strategy, all patients with planned fluoropyrimidine treatment would receive an upfront DPYD genotyping test for the four most common and well-established risk variants (DPYD∗2A, DPYD∗13, c.2846A>T, c.1236G>A).6,8 The result of the DPYD genotyping test would be used to guide treatment decisions and dosing of fluoropyrimidines. Individuals without the variants (wild-type) were classified as DPYD normal metabolizers, and carriers of the variants were classified as DPYD intermediate or poor metabolizers. We assumed that the treatment decisions and dose adjustments made would follow the 2017 Clinical Pharmacogenetics Implementation Consortium guidelines (Table 10).8
Table 10:
Genotype Determination and Treatment Adjustments
| DPYD Genotype | Gene Activity Score | Likely Phenotype | Treatment Adjustment |
|---|---|---|---|
| Wild-type or noncarrier: • 2 normal-function variants |
2 | Normal metabolizer; normal DPD enzyme activity with “normal” risk for fluoropyrimidine toxicity | No dose adjustments; use label-recommended dose |
|
DPYD variant carrier: • 1 normal-function variant and 1 no-function variant • 1 normal-function variant and 1 decreased-function variant • 2 decreased-function variants |
1 or 1.5 | Intermediate metabolizer; reduced DPD enzyme activity and increased risk for severe or fatal toxicity when treated with fluoropyrimidine drugs | Reduced dose followed by titration of dose based on occurrence of toxicity |
|
DPYD variant carrier: • 2 no-function variant • 1 no-function variant and 1 decreased-function variant |
0 or 0.5 | Poor metabolizer; complete DPD deficiency and high risk for severe or fatal toxicity when treated with fluoropyrimidine drugs | Alternative chemotherapy regimen |
Abbreviation: DPD, dihydropyrimidine dehydrogenase.
Source: Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline, 2017.8
In the usual care strategy, no DPYD genotyping is conducted, and all patients receive standard-dose fluoropyrimidines. The dose may vary by patient depending on age, cancer type, disease stage, and other characteristics.87 We did not consider strategies using other measures of dihydropyrimidine dehydrogenase (DPD) deficiency or 5-FU pharmacokinetics assessment due to their complexity and implementation challenges (Aaron Pollett, MD, email communication, September 20, 2020; Leta Forbes, MD, email communication, September 30, 2020). We also did not consider treating severe fluoropyrimidine-related toxicity with uridine triacetate (i.e., an antidote), because this treatment is available only through the Special Access Program and, based on expert opinion (Aaron Pollett, MD, email communication, September 20, 2020; Leta Forbes, MD, email communication, September 30, 2020), is rarely used in practice, is reserved for emergency treatment, and should not be considered a surrogate for prospective genotyping of DPYD alleles.32
Time Horizon and Discounting
For the reference case analysis, we used a 6-month time horizon to capture the impact of DPYD genotyping on short-term costs and outcomes. No discounting was applied because the time horizon was less than 1 year. We chose a short time horizon because most toxicities associated with fluoropyrimidines resolve within a few months and have relatively short-term impacts on people's health (Jason Yu, MD, Geoffrey Liu, MD, Theodore Wigle, MD, Leta Forbes, MD, personal communication, June 21 to September 25, 2020). To capture the potential QALY loss due to rare but fatal toxicities, we assumed that death due to severe fluoropyrimidine-related toxicity would usually occur after the first or second cycle of chemotherapy.
We did not model the long-term impact of DPYD genotyping on cancer morbidity and mortality. These cancer-related outcomes are inherently connected to chemotherapy efficacy. We assumed that chemotherapy efficacy would be similar between DPYD carriers who received a reduced dose and wild-type patients. We based this assumption on the fact that DPYD genotyping-guided dose reduction aims to maintain plasma levels of 5-FU and its metabolites at the intended therapeutic levels (hence similar treatment efficacy). The clinical review identified a matched-pair analysis that compared overall survival and progression-free survival between 37 DPYD∗2A carriers who received a reduced fluoropyrimidine dose and 37 wild-type patients.11 In that study, Henricks et al11 found that, compared to wild-type patients, a reduced dose did not negatively affect overall survival (median 27 vs. 24 months, P = 0.47) or progression-free survival (median 14 vs. 10 months, P = 0.54). It is possible that DPYD genotyping may produce false-positive results. Reducing the fluoropyrimidine dose in patients who do not have DPD deficiency may cause them to be underdosed. However, to avoid underdosing, clinical guidelines recommend that doses be increased in subsequent cycles in people who experience no clinically tolerable toxicity in the first two chemotherapy cycles21 and that people should be monitored (e.g., using pharmacokinetic testing) to identify those who can tolerate higher 5-FU doses. Based on these considerations, we assumed that long-term treatment efficacy would be similar and therefore it was not modelled.
Model Structure
We developed a decision-tree model based on the treatment pathways in Ontario, clinical guidelines,8 and published economic studies.79,80 The model structure is depicted in Figure 20.
Figure 20: Decision Model Structure.
The decision tree compared DPYD genotyping with usual care. In the usual care strategy, no DPYD genotyping was conducted and all patients received a standard fluoropyrimidine dose. In the DPYD genotyping strategy, all patients received a DPYD genotyping test prior to the start of fluoropyrimidine treatment. Patients were then classified into one of three groups based on their test results: DPYD normal metabolizers, intermediate metabolizers, and poor metabolizers. Based on the 2017 Clinical Pharmacogenetics Implementation Consortium guidelines, DPYD normal metabolizers would receive standard-dose fluoropyrimidines, intermediate metabolizers would receive reduced-dose fluoropyrimidines, and poor metabolizers would receive an alternative to fluoropyrimidines (Table 10).
Each group of patients might experience severe (grade ≥ 3) or non-severe (grade 0–2) toxicities at different rates of occurrence. A small proportion of severe toxicities might be fatal. We calculated the costs of DPYD genotyping, fluoropyrimidine chemotherapy, and toxicity-related treatment for both strategies. We also calculated the total number of severe toxicities and patient QALYs. We did not model background mortality and cancer-related mortality because they were not expected to be affected by DPYD genotyping.
Main Assumptions
The main assumptions for the reference case analysis were:
Most patients would be heterozygous DPYD variant carriers because most patients in the included clinical studies were such carriers. Data from these studies were used to inform input parameters for DPYD intermediate metabolizers in the model
There are limited clinical data on DPYD poor metabolizers (compound heterozygous and homozygous carriers) treated with standard-dose fluoropyrimidines. This is likely because DPYD poor metabolizers are rare (approximately 0.19% of patients treated with fluoropyrimidines). Because these patients are expected to have a very low or even absent DPD activity, we assumed that if they were treated with standard-dose fluoropyrimidines, all would experience severe toxicities. We based this assumption on a few cases of compound heterozygous and homozygous carriers identified from the clinical evidence review (all of whom experienced severe toxicities) and consultation with clinical experts. We also assumed that, compared to intermediate metabolizers treated with a standard dose, a higher proportion of poor metabolizers would be hospitalized and their hospital length of stay would be longer (Brandon Meyers, MD, email communication, June 16, 2020)
Data are also lacking on DPYD poor metabolizers treated with an alternative regimen. We assumed that these patients could metabolize non-fluoropyrimidine drugs normally, and therefore the risks of overall severe toxicity, hospitalization, and death, as well as hospital length of stay, would be similar to those for DPYD wild-type patients. This assumption was validated by clinical experts as reasonable (Geoffrey Liu, MD, telephone communication, June 24, 2020)
Treatment-related hospitalization would occur only in patients with severe toxicities
Approximately 1% to 2% of DPYD tests would fail (e.g., issues with DNA extraction, reaction failure, etc.) and need to be re-run (Richard Kim, MD, email communication, February 20, 2020; Lei Fu, PhD, email communication, June 22, 2020)
DPYD genotype-guided dosing and treatment adjustments would follow the 2017 Clinical Pharmacogenetics Implementation Consortium guidelines8
Clinical Parameters
The clinical parameters used in the model are presented in Table 11.
Table 11:
Clinical Inputs Used in the Economic Model
| Model Parameter | Mean | Distribution | Reference |
|---|---|---|---|
| Prevalence of DPYD Phenotypes | |||
| Intermediate metabolizer Poor metabolizer Normal metabolizer |
6.60% 0.19% 93.21% |
Beta Beta — |
Clinical evidence review (pooled prevalence) Clinical evidence review (pooled prevalence) Calculated |
| Genotype Testing Parameters | |||
| Test failure | 1.5% | Betaa | Expert opinion |
| Probability of Overall Severe Toxicity in All Patients | |||
|
DPYD wild-type DPYD intermediate metabolizer, reduced dose DPYD intermediate metabolizer, standard dose DPYD poor metabolizer, alternative regimen DPYD poor metabolizer, standard dose |
13.62% 22.73% 23.53% 13.62% 100% |
Beta Beta Beta — — |
Lunenburg et al, 20187 Lunenburg et al, 20187 Lunenburg et al, 20187 Assumed to be similar to wild-type Lee et al, 201613; Froehlich et al, 201588; Toffoli et al, 20159; Lee et al, 201458; Boisdron-Celle et al, 200767 (4 compound heterozygous and 2 homozygous carriers received a standard dose; all had severe toxicity) |
| Probability of Treatment-Related Hospitalization in All Patients | |||
|
DPYD wild-type DPYD intermediate metabolizer, reduced dose DPYD intermediate metabolizer, standard dose DPYD poor metabolizer, alternative regimen DPYD poor metabolizer, standard dose |
7.8% 18.2% 17.6% 7.8% 66.7% |
Beta Beta Beta — — |
Lunenburg et al, 20187 Lunenburg et al, 20187 Lunenburg et al, 20187 Assume similar to that of wild-type Lee et al, 201613; Froehlich et al, 201513,57; Toffoli et al, 20159,57; Lee et al, 20149,58; Boisdron-Celle et al, 200767 (4 compound heterozygous and 2 homozygous carriers received a standard dose; all had severe toxicity; 4 were fatal); assumed all fatal severity led to hospitalization |
| Days of Hospitalization for Patients Who Were Hospitalized | |||
| DPYD wild-type | 13 | Normala | Lunenburg et al, 20187 |
| DPYD intermediate metabolizer, reduced dose | 4 | Normala | Lunenburg et al, 20187 |
| DPYD intermediate metabolizer, standard dose | 23 | Normala | Lunenburg et al, 20187 |
| DPYD poor metabolizer, alternative regimen | 13 | Normala | Assumed to be similar to that of wild-type |
| DPYD poor metabolizer, standard dose | 29 | Normala | Assume 25% longer than that of intermediate metabolizer |
| Probability of Death Among Patients With Severe Toxicities | |||
| DPYD wild-type | 1.3% | Beta | Henricks et al, 201872 |
| DPYD intermediate metabolizer, reduced dose | 1.3% | — | Assume similar to that of wild-type |
| DPYD intermediate metabolizer, standard dose | 14.3% | Beta | Deenen et al, 201673 |
| DPYD poor metabolizer, alternative regimen | 1.3% | — | Assumed to be similar to that of wild-type |
| DPYD poor metabolizer, standard dose | 66.7% | Beta | Lee et al, 201613; Froehlich et al, 201588; Toffoli et al, 20159; Lee et al, 201458; Boisdron-Celle et al, 200767 (4 compound heterozygous and 2 homozygous carriers received a standard dose; all had severe toxicity; 4 were fatal) |
| Average Number of Severe Toxicities per Patient Who Had Severe Toxicity | |||
| DPYD wild-type | 1.5b | Normala | Lunenburg et al, 20187 |
| DPYD intermediate metabolizer, reduced dose | 1.4b | Normala | Lunenburg et al, 20187 |
| DPYD intermediate metabolizer, standard dose | 2.1b | Normala | Lunenburg et al, 20187 |
| DPYD poor metabolizer, alternative regimen | 1.5 | — | Assumed to be similar to that of wild-type |
| DPYD poor metabolizer, standard dose | 2.1 | — | Assumed to be similar to that of intermediate metabolizers |
Assumed standard error to be 20% of mean.
Derived by dividing the total number of adverse events reported (which were broken down by type of adverse event) by the number of patients who experienced an adverse event.
PREVALENCE OF DPYD PHENOTYPES
The prevalence of DPYD phenotypes (i.e., normal, intermediate, and poor metabolizers) was estimated based on the prevalence of DPYD genotypes. Based on the pooled prevalence of DPYD variant carriers from the clinical evidence review, we estimated the proportion of DPYD intermediate and poor metabolizers to be 6.60% and 0.19%, respectively.
PROBABILITY OF OVERALL SEVERE TOXICITY AND HOSPITALIZATION
The clinical evidence review identified 23 studies that compared DPYD variant carriers treated with a standard dose versus wild-type patients and five studies that compared DPYD variant carriers treated with a reduced dose versus wild-type patients. Among these studies, only Lunenburg et al7 evaluated all four variants under assessment and included all three groups of patients (wild-type receiving standard dose, DPYD carrier receiving standard dose, and DPYD carrier receiving reduced dose) in the same study. Therefore, we selected this study to inform the reference case analysis.
Lunenburg et al7 reviewed the medical records of 828 patients who received fluoropyrimidine-based chemoradiation therapy from three cancer centres in the Netherlands and northern Italy. The study included 771 wild-type patients who received a standard dose, 34 DPYD variant carriers who received a standard dose, and 23 DPYD variant carriers who received a reduced dose (one patient was excluded from the statistical analysis because of a substantial dose increase). The probability of overall severe toxicity was 13.62% (105/771) in wild-type patients receiving standard dose, 23.53% (8/34) in DPYD variant carriers who received a standard dose, and 22.73% (5/22) in DPYD variant carriers who received a reduced dose. The probability of hospitalization was 7.8% (60/771), 17.6% (6/34), and 18.2% (4/22), respectively. Although the probabilities of treatment-related severe toxicity and hospitalization were similar in the two DPYD carrier groups, the mean duration of hospitalization was much shorter in the reduced-dose group (P = 0.010). For patients who were hospitalized, the mean hospital length of stay was 13 days in wild-type patients who received a standard dose, 23 days in DPYD variant carriers who received a standard dose, and 4 days in DPYD variant carriers who received a reduced dose. According to the study authors, there were two possible explanations for this observation. It is possible that treating physicians were responding to the potentially increased risk of toxicity in DYPD variant carriers by more rapidly hospitalizing patients with signs of potential toxicity. It is also possible that DPYD variant carriers given dose reductions recovered more quickly from toxicity episodes. All but two patients in the Lunenburg study were heterozygous carriers, so we used these data to inform input parameters for DPYD intermediate metabolizers in the model.
As mentioned before, the clinical evidence for DPYD poor metabolizers is very limited. To estimate the probability of overall toxicity and hospitalization in DPYD poor metabolizers, we made several assumptions. Studies included in the clinical evidence review reported only a few cases of patients who were DPYD poor metabolizers. There were four compound heterozygous and two homozygous DPYD variant carriers; all received standard-dose fluoropyrimidines, and all had severe toxicity (four were fatal). Therefore, we assumed that all DPYD poor metabolizers treated with a standard dose would experience severe toxicities and about two-thirds would be hospitalized. We also assumed that the hospital length of stay would be longer than for intermediate metabolizers.
PROBABILITY OF TREATMENT-RELATED DEATH
Since treatment-related mortality was not reported by Lunenburg et al,7 we estimated the probability of death among patients with severe toxicity from other studies. Henricks et al11 was a large observational study that conducted pre-treatment DPYD genotyping for four variants in a cohort of 1,103 patients with planned fluoropyrimidine treatment. Of these patients, 1,018 (92%) were wild-type and 85 (8%) were heterozygous DPYD variant carriers. Compound heterozygous and homozygous DPYD variant carriers were not included in the study. Severe toxicity was experienced by 23% (231/1,018) of the wild-type patients and 39% (33/85) of the DPYD variant carriers treated with a reduced dose. Three patients in the wild-type group (1.3%) died because of fluoropyrimidine-related toxicity, and no treatment-related death occurred in the DPYD variant carrier group (one patient died due to protocol violation). Because the lack of deaths in the carrier group may have been driven by the small sample size, we assumed that the probability of treatment-related death in DPYD intermediate metabolizers who received a reduced dose was the same as for wild-type patients (1.3%).
For DPYD intermediate metabolizers who received a standard dose, we obtained the probability of treatment-related death from Deenen et al.79 These authors selected a group of patients (n = 48) who were genotyped for DPYD∗2A and treated with a standard fluoropyrimidine dose. Of these 48 patients, 35 had severe toxicity and five died because of fluoropyrimidine-related toxicity. Therefore, we estimated the probability of treatment-related death among DPYD variant carriers who received a standard dose and experienced severe toxicity to be 14.3% (5/35).
For DPYD poor metabolizers, we assumed that those who received an alternative regimen would have a risk of treatment-related death similar to the wild-type patients. For those treated with a standard fluoropyrimidine dose, we used information from the studies included in the clinical evidence review, in which four of six patients with severe toxicity died (66.7%).
AVERAGE NUMBER OF TOXICITIES PER PERSON
Patients may experience more than one severe toxicity during their course of chemotherapy. This is evident from Lunenburg et al,7 where the total number of severe toxicities (the sum of the different types of toxicity) was greater than the number of patients who experienced severe toxicities. We calculated the average number of toxicities per person for wild-type patients and DPYD variant carriers who received a reduced dose and a standard dose, and we used these values to adjust the costs and utilities in patients who experience severe toxicities.
Utility Parameters
We considered patients' health-related quality of life over the course of their chemotherapy treatment. Utilities are numeric weights that represent a person's preference for a certain health state, such as being on chemotherapy for colorectal cancer. Utilities are often measured on a scale ranging from 0 (death) to 1 (perfect health). Disutilities represent the decrement in utility due to a particular symptom and are often expressed as negative values (e.g., −0.11 for a person experiencing an adverse event).
The utility and disutility parameters used in our model are presented in Table 12. We first estimated the baseline utility of patients with planned fluoropyrimidine treatment. Although each patient's quality of life may vary depending on their type of cancer, disease stage, and chemotherapy regimen, we calculated a weighted average value using utilities of the three most common cancers (colorectal, breast, and upper gastrointestinal) and used it as a proxy to represent the average baseline utility in this patient population. We obtained the utility of patients with metastatic colorectal cancer from Bennett et al.89 This study analyzed data from the EQ-5D (a health-related quality-of-life instrument) collected from two phase III randomized controlled trials comparing different chemotherapy regimens. For patients who received a regimen containing 5-FU, leucovorin, and oxaliplatin, the baseline utility was 0.756. We obtained the utility of patients with metastatic breast cancer from Lloyd et al.90 This study estimated the utility values of metastatic breast cancer plus six common toxicities using both the standard gamble method and the EQ-5D instrument. The utility of a breast cancer patient with stable disease and no toxicity was estimated to be 0.715. Last, we obtained the utility of gastric cancer from Curran et al.91 In this study, the EQ-5D instrument was used to estimate the utility of patients with advanced gastric cancer who received two fluoropyrimidine-based chemotherapy regimens. For patients who received irinotecan, folinic acid, and 5-FU, the mean utility was 0.76. For patients who received cisplatin and 5-FU, the mean utility was 0.66. As a result, the weighted average utility of the three cancers was estimated to be 0.744.
Table 12:
Utility Parameters
| Parameter | Utility/Disutility Value | Distribution | Source |
|---|---|---|---|
| Baseline utility of patients with planned fluoropyrimidine treatment | 0.744 | Betaa | Calculatedb |
| Colorectal cancer | 0.756 | — | Bennett et al, 201189 |
| Breast cancer | 0.715 | — | Lloyd et al, 200690 |
| Gastric cancer | 0.710c | — | Curran et al, 200991 |
| Average disutility associated with a severe toxicity | –0.131 | Betaa | Calculated |
| Gastrointestinal | –0.127d | — | Lloyd et al, 200690 |
| Hand-foot syndrome | –0.116 | — | Lloyd et al, 200690 |
| Hematological | –0.150e | — | Lloyd et al, 200690 |
Assumed standard error to be 20% of mean.
Weighted average of colorectal (55.6%), breast (30.5%), and gastric cancer (13.9%) utilities.
Average utility of gastric cancer patients receiving irinotecan, folinic acid with 5-fluourouracil (0.76), and cisplatin with 5-fluourouracil (0.66).
Average disutility of diarrhea and vomiting (−;0.103) and stomatitis (−;0.151).
Disutility of febrile neutropenia.
Next, we obtained disutility values for fluoropyrimidine-related severe toxicities (e.g., gastrointestinal, hand-foot syndrome, and hematological) from Lloyd et al.90 We applied the average disutility values to the proportion of patients expected to have severe toxicities.
For the reference case analysis, we assumed that the negative impact of severe toxicities on patients' quality of life (i.e., the disutility) would last about 4 weeks (Jason Yu, MD, email communication, June 21, 2020; Leta Forbes, MD, email communication, September 30, 2020). For sensitivity analysis, we assumed that disutility could last longer (up to 6 weeks). To account for the QALY loss from treatment-related death, we assumed that a fatal toxicity would occur after the first one to two cycles of chemotherapy.
Cost Parameters
We included direct medical costs related to:
Physician visits
DYPD genotyping tests
Treatment of fluoropyrimidine-related toxicities
Chemotherapy drugs
All costs are reported in 2020 Canadian dollars. When 2020 costs were not available, the health care component of the Canadian Consumer Price Index was used to adjust costs.92
PHYSICIAN VISIT
In the reference case analysis, we assumed that DPYD genotyping would not result in any additional physician visits. In a scenario analysis, we assumed that DPYD genotyping could lead to an additional visit with an oncologist before the start of chemotherapy (OHIP Schedule of Benefit A441, $70.90 for a complex medical specific re-assessment by a medical oncologist).93
DPYD GENOTYPING
We estimated the per-person cost of DPYD genotyping and considered costs related to:
Sample collection
Sample transportation
Sample processing (to extract DNA) and testing (to generate DPYD genotype)
Result interpretation and reporting
Detailed cost information is presented in Table 13. The cost of sample collection was obtained from the Schedule of Benefits for Laboratory Services in Ontario ($10.76 per sample).94 The cost of sample shipping and handling ($52.50 per sample) was estimated from the literature.95 For simplicity, we assumed that for a majority of the time, patient blood samples would be processed externally at a diagnostic laboratory. Shipping costs may vary from centre to centre. For example, a larger centre with more samples may have a lower cost per sample compared to a smaller centre with fewer samples. If DPYD genotyping is conducted on site (in a hospital laboratory, where there is also a cancer clinic), the shipping cost may be zero.
Table 13:
Cost Parameters Related to Testing
| Model Parameter | Mean Cost, $a | Distribution | Reference |
|---|---|---|---|
| Sample collection | 10.76 | Fixed | OHIP SOB for Laboratory Services (L700)94 |
| Shipping and handling | 56.11 | Normal | Tsiplova et al, 201795 |
| DPYD genotyping test | 100.00 | Normal | Expert opinionb (based on 4 samples per batch run using PCR; $10 for DNA extraction, $30 for reagent/supplies, and $60 for labour/personnel) |
| Total | 166.87 |
Abbreviations: OHIP, Ontario Health Insurance Program; SOB, Schedule of Benefits.
Costs in 2020 CAD.
Clinical expert opinion (Lei Fu, PhD, email communication, January 22, 2021).
We estimated the cost of DPYD genotyping based on consultations with the two laboratories in Ontario that are currently conducting DPYD genotyping through research programs. Based on consultation with laboratory experts, the current cost in Ontario is about $100 per test (Theodore Wigle, MD, Lei Fu, PhD, Harriet Feilotter, PhD, personal communication, April to June 2020). This estimate included the cost of DNA extraction ($10), reagents and supplies for genotyping ($30; based on four samples per batch run using PCR), and laboratory personnel ($60). The cost of results interpretation and reporting is included in the labour and personnel costs. The patient's predicted genotype is usually automated based on the genotyping results, and the Clinical Pharmacogenetics Implementation Consortium guideline is used for treatment recommendations. We conducted sensitivity analyses using different costs, because we expected the test cost to vary depending on how testing is implemented in the province. According to laboratory experts, the cost per test may change depending on the annual volume of tests conducted and number of samples per run (Lei Fu, PhD, email communication, January 22, 2021; Table 14). If there is only one sample in each run, the cost can be as high as $250 per test. When more samples are batched in a single run, the cost per test decreases substantially (e.g., $26 per test with 89 samples per run).
Table 14:
Different Scenarios for DPYD Genotyping Test Cost
| Model Parameter | DPYD Genotyping Cost Scenario | ||||||
|---|---|---|---|---|---|---|---|
| Number of samples per run | 1 | 2 | 4 | 10 | 17 | 41 | 89 |
| Reagents + extractiona | $100 | $65 | $40 | $22 | $20 | $17 | $15 |
| Labour cost per sampleb | $150 | $105 | $60 | $28 | $20 | $13 | $11 |
| Total cost per sample | $250 | $170 | $100 | $50 | $40 | $30 | $26 |
Costs per sample in 2020 CAD.
At $50 per hour.
Source: Clinical expert opinion (Lei Fu, PhD, email communication, January 22, 2021).
We did not consider capital/fixed costs related to purchasing, installing, and maintaining the testing equipment (e.g., a Sanger sequencer or a real-time PCR machine). If the test is publicly funded, samples could be sent to a few sites where there is existing infrastructure, equipment, and specialized personnel (such as a hospital or community laboratory). We also did not include costs related to overhead, test validation, licensing, accreditation, and personnel training because those one-time or ongoing costs are usually included in the budgets of the laboratories providing those tests.
TREATMENT OF FLUOROPYRIMIDINE-RELATED TOXICITIES
We estimated the average costs of treating fluoropyrimidine-related toxicities (Table 15). For patients who were hospitalized due to severe toxicity, we estimated the hospitalization cost by multiplying the duration of hospitalization by the average hospital cost per day. We obtained the average hospital cost per day from a 2016 report published by the Canadian Institute for Health Informatics.96
Table 15:
Cost of Treatment-Related Toxicities
| Model Parameter | Mean Cost, $ | SE | Distribution | Reference |
|---|---|---|---|---|
| Hospital cost per day (for patients with severe toxicities requiring hospitalization) | 1,232.10a | 246.42 | Gamma | CIHI 201696 |
| Non-hospitalization cost for non-severe toxicities (Grade 0–2)c | 144.90b | 28.98 | Gamma | Henricks et al, 201980 |
| Non-hospitalization cost for severe toxicities that did not require hospitalization (grade ≥ 3)c | 394.26d | 78.85 | Gamma |
Abbreviations: CIHI, Canadian Institute for Health Information; SE, standard error.
Inflated from 2015 to 2020 CAD.
Cost per event = €82, converted using purchasing power parity.97
Grade score based on the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) ratings system for level of toxicity.
Cost per event = €234, converted using purchasing power parity.97
Severe and non-severe toxicities that do not require hospitalization may require an extra follow-up visit, another blood draw, and/or a prescription for a common anti-diarrheal drug. Some patients may even need an emergency room or urgent care visit (Jason Yu, MD, email communication, June 21, 2020). To estimate these costs, we obtained the average non-hospitalization cost per event from a costing analysis conducted by Henricks et al80 and converted it to Canadian dollars.
CHEMOTHERAPY COSTS
We estimated the costs of 5-FU, capecitabine, and alternative chemotherapy drugs for DPYD poor metabolizers. Similar to previous economic analyses,79,80 we excluded costs that were unlikely to differ with DPYD genotyping. This included drug administration and clinical monitoring (e.g., costs of infusion clinics, consumables, nursing, and pharmacist time; Jason Yu, MD, email communication, June 21, 2020), as well as the costs of other drugs that may be used in combination with fluoropyrimidines in a chemotherapy regimen. We estimated chemotherapy drug costs per cycle using dosing information from the literature and unit prices from the Ontario Drug Benefit Formulary98 or other published sources99 (Table 16). We then calculated the weighted average cost of chemotherapy drugs using the estimated percentages of patients receiving 5-FU, capecitabine, and both in the target population (see Table 9, above). We then multiplied the per-cycle cost by the average number of cycles and the mean dose intensity.
Table 16:
Drug Acquisition Costs per 21-Day Cycle, Standard Dosing
| Drug | Dosing | Drug Costs | Reference | |||
|---|---|---|---|---|---|---|
| Starting Dose | Dose per Day or Cyclea | Cost per mg | Cost per Day | Cost per Cycle | ||
| Capecitabine | Low: 825 mg/m2 2× daily for 14 d High: 1,250 mg/m2 2× daily for 14 d |
3,135 mg/d 4,750 mg/d |
$1.5250 per pill (500 mg); $0.4575 per pill (150 mg) | $10.38b $16.47b |
$145.32b $230.58b Average: $187.95b |
Henricks et al, 201881; OH (CCO); ODB Formulary98 |
| 5-FU | Low: IV bolus, 500–600 mg/m2 on days 1 and 8 High: IV infusion, 200 mg/m2 on days 1–21 |
2,090 mg/cycle 7,980 mg/cycle |
$0.003 per mg | — — |
$6.27 $23.94 Average: $15.11 |
Henricks et al, 201881; OH (CCO); pCODR99 |
| Raltitrexed | 3 mg/m2 on day 1 | 5.7 mg/cycle | $324.62 per 2 mg | — | $925.17 | Alberta Blue Cross103; OH (CCO)102,104 |
Abbreviations: 5-FU, f-fluorouracil; CADTH, Canadian Agency for Drugs and Technologies in Health; ODB, Ontario Drug Benefit program; OH (CCO), Ontario Health (Cancer Care Ontario); pCODR, pan-Canadian Oncology Drug Review.
An 8% markup was included for oral drugs (CADTH costing guidance document).
We estimated the average per-cycle cost of 5-FU at the standard dose to be $15.11 per cycle. 5-FU is prescribed using a variety of dosing and administration schedules.87 Using Ontario Health (Cancer Care Ontario)'s dosing recommendations for 5-FU as an intravenous bolus or intravenous infusion,87 we identified the dosing schedules that resulted in the highest dose per cycle (i.e., 200 mg/m2 on days 1–21, or 4,200 mg/m2 per cycle) and the lowest dose per cycle (i.e., 500–600 mg/m2 days 1 and 8, or 1,100 mg/m2 per cycle).
We estimated the average cost of capecitabine at the standard dose to be $187.95 per cycle. We included an 8% markup because capecitabine is an oral drug.100 We calculated the average dose based on an average body surface area of 1.9 m2. Dosing information was from Ontario Health (Cancer Care Ontario)101 and the DPYD genotyping study by Lunenburg et al.7 For example, patients with advanced colorectal cancer usually receive a dose of 1,250 mg/m2 twice daily for 14 days, followed by a 7-day rest period. Patients with rectal cancer usually receive a dose of 825 mg/m2 twice daily.
We assumed that an alternative, fluoropyrimidine-free, chemotherapy would be given to patients who are poor metabolizers. This alternative chemotherapy may differ depending on cancer type and disease stage. For instance, raltitrexed may be used as an alternative for the treatment of colorectal cancer. For metastatic breast cancer, a different type of cytotoxic drug such as vinorelbine or gemcitabine may be used. In the adjuvant setting, if breast cancer patients were intolerant of fluoropyrimidines, it is likely that no other drugs would be given (Jason Yu, MD, email communication, June 21, 2020; Leta Forbes, MD, email communication, September 30, 2020). To estimate drug costs in the reference case analysis, we used raltitrexed for the typical cost, because colorectal cancer is the most common cancer in this patient population. We estimated the average cost of raltitrexed to be $925.17 per cycle based on dosing guidelines from Ontario Health (Cancer Care Ontario), which indicate that adult patients should receive 3 mg/m2 of raltitrexed in a 15-minute infusion.102 We obtained the cost of raltitrexed from the Alberta Blue Cross drug price list103 because there is limited information on the cost of raltitrexed in Ontario.
In the reference case analysis, we assumed that patients would undergo an average of five treatment cycles (Table 17).80 We also obtained the mean dose intensity from Lunenburg et al.7 Dose intensity is the amount of chemotherapy drugs received by the patient divided by the initial scheduled amount of chemotherapy.
Table 17:
Additional Chemotherapy Cost Parameters
| Model Parameter | Mean | Reference |
|---|---|---|
| Mean number of cycles | 5 | Henricks et al, 201980 |
| Mean dose Intensitya | ||
| DPYD wild-type | 97% | Lunenburg et al, 20187 |
| DPYD carrier receiving standard dose | 91% | Lunenburg et al, 20187 |
| DPYD carrier receiving reduced dose | 61% | Lunenburg et al, 20187 |
Dose intensity is the amount of chemotherapy drugs received by the patient divided by the initial scheduled amount of chemotherapy.
Internal Validation
Formal internal validation was conducted by the secondary health economist. This included testing the mathematical logic of the model and checking for errors and accuracy of parameter inputs and equations.
Analysis and Uncertainty
We conducted a reference case analysis and scenario analyses. Our reference case analysis adhered to CADTH guidelines105 when appropriate and represents the analysis with the most likely set of input parameters and model assumptions. Our scenario analyses explored how the results are affected by varying input parameters and model assumptions.
For the reference case, we conducted a probabilistic analysis to capture parameter uncertainty. When possible, we specified distributions around input parameters using the mean and standard error. Selected cost parameters were characterized by lognormal or normal distributions, and probabilities were characterized by beta distributions. We ran a total of 5,000 simulations and calculated the expected values of costs and outcomes for each strategy. We presented the probability of each strategy being cost-effective over a range of willingness-to-pay values plotted on a cost-effectiveness acceptability curve. We also examined additional structural and parameter uncertainty by conducting several scenario analyses. These analyses are summarized in Table 18.
Table 18:
Parameters for the Reference Case and Sensitivity Analyses
| Parameter | Reference Case | Sensitivity Analysis |
|---|---|---|
| Prevalence of DPYD intermediate metabolizers | Pooled prevalence from the clinical evidence review (based on 4 variants): 6.60% | Lower estimate: 5.6% Upper estimate: 7.7% |
| Prevalence of DPYD poor metabolizers | Pooled prevalence from the clinical evidence review (based on 4 variants): 0.19% | Lower estimate: 0% Upper estimate: 0.6% |
| Source of effectiveness and resource use estimate | Lunenburg et al, 20187 | Henricks et al, 201872 (see details in Table A21) |
| Probability of treatment-related hospitalization | Poor metabolizers receiving standard dose: 66.7% | Poor metabolizers receiving standard dose: 100% |
| Days of hospitalization | Poor metabolizers receiving standard dose: 28.75 days | 23 days 34.5 days |
| Impact of severe toxicities on quality of life | Assumed disutility would last for 4 weeks (28 days) | Assumed disutility would last for up to 6 weeks (42 days) |
| Alternative chemotherapy for poor metabolizers | Raltitrexed | No alternative drugs given |
| Cost of physician visit | Assumed no extra visit | Assumed 1 extra visit |
| Cost of DPYD genotyping (sample processing, sample testing, result interpretation, and reporting) | $100 per test | $50 and $150 per test |
Results
Reference Case Analysis
Results of the reference case analysis are presented in Table 19. The average total cost was $1,920.82 (95% CrI: $1,308.71 to $2,743.56) for the DPYD genotyping strategy and $2,065.70 (95% CrI: $1,340.67 to $3,060.75) for usual care, resulting in a cost difference of −$144.88 (95% CrI: −$543.10 to $101.91) per patient.
Table 19:
Reference Case Analysis Results
| Parameter | DPYD Genotyping, Mean (95% CrI) | Usual Care (No Testing), Mean (95% CrI) | Difference, Mean (95% CrI) |
|---|---|---|---|
| Average total costs Cost of DPYD genotyping test Cost of toxicities Cost of chemotherapy drugs Average proportion of patients with severe toxicities Average number of severe toxicities Average QALYs ICER ($/QALY) |
$1,920.82 ($1,308.71 to $2,743.56) $169.11 $1,396.85 $354.86 14.20% (11.76% to 16.92%) 0.21 (0.13 to 0.30) 0.3674 (0.1987 to 0.4781) |
$2,065.70 ($1,340.67 to $3,060.75) $0.00 $1,712.62 $353.08 14.42% (12.06% to 16.94%) 0.23 (0.15 to 0.32) 0.3663 (0.1978 to 0.4768) |
–$144.88 (−;$543.10 to $101.91) $169.11 –$315.77 $1.78 –0.22% (−;1.63% to 1.37%) –0.02 (−;0.05 to 0.02) 0.0011 (0.0003 to 0.0023) Dominant |
Abbreviations: CrI, credible interval; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life year.
The additional cost from DPYD genotyping ($169.11) was offset by cost reduction in the treatment of fluoropyrimidine-related toxicities (−;$315.77). The average proportion of patients with severe toxicities was 14.20% (95% CrI: 11.76% to 16.92%) in the DPYD genotyping group and 14.42% (95% CrI: 12.06% to 16.94%) in the usual care group. The average QALYs associated with the DPYD genotyping strategy and the usual care strategy were 0.3674 (95% CrI: 0.1987–0.4781) and 0.3663 (95% CrI: 0.1978–0.4768), resulting in a small QALY gain of 0.0011 (95% CrI: 0.0003–0.0023) over a half-year time horizon. Because DPYD genotyping is slightly more effective and less costly than usual care, it is considered a dominant strategy.
Figure 21 presents the cost-effectiveness acceptability curve, which shows the probability of each strategy being cost-effective across a range of willingness-to-pay values. At the commonly used willingness-to-pay values of $50,000 and $100,000 per QALY, DPYD genotyping is highly likely to be cost-effective (91% and 96% probability, respectively).
Figure 21: Cost-Effectiveness Acceptability Curve.
Scenario Analyses
Results of the scenario analyses are shown in Table 20. DPYD genotyping remained cost-saving and slightly more effective (better QALYs) in all scenarios. When we assumed a lower prevalence of intermediate metabolizers (scenario 1a) or poor metabolizers (scenario 2a) in the target population, the cost savings and QALY gained from DPYD genotyping became smaller. If a higher prevalence was assumed, the cost savings and QALY gained from DPYD genotyping increased (scenarios 1b and 2b). When we used effectiveness and resource use data from an alternative clinical study (scenario 3), the cost savings decreased slightly because there was a smaller difference in hospital length of stay between DPYD carriers who received a standard dose and DPYD carriers who received a reduced dose. When the probability of hospitalization in poor metabolizers treated with a standard dose was increased to 100% (versus 66.7% in the reference case), the DPYD genotyping resulted in larger cost savings (scenario 4). When we assumed a shorter (23 days) hospital length of stay in poor metabolizers treated with a standard dose (versus 29 days in the reference case), the cost savings became smaller (scenario 5a). When disutility due to severe toxicity was assumed to last longer (8 vs. 4 weeks), the QALY gained became slightly larger (0.0012; scenario 6). In the DPYD genotyping strategy, if no alternative drug was given to poor metabolizers (remove fluoropyrimidines from the chemotherapy regimen without replacement with another drug), the cost savings increased only slightly (scenario 7). If DPYD genotyping required an extra physician visit (e.g., to discuss the test result with the patient), the cost savings decreased by half to $75.89 per patient (scenario 8). Similarly, if DPYD genotyping test was more expensive ($150 instead of $100 per test), the cost savings decreased to $97.10 per patient (scenario 9b).
Table 20:
Scenario Analysis Results
| Scenario | Total Cost | Proportion of Patients With Severe Toxicity | Total QALYs | ||||||
|---|---|---|---|---|---|---|---|---|---|
| DPYD Genotyping | Usual Care | Difference | DPYD Genotyping | Usual Care | Difference | DPYD Genotyping | Usual Care | Difference | |
| Reference case | $1,920.82 | $2,065.70 | –$144.88 | 14.20% | 14.42% | –0.22% | 0.3674 | 0.3663 | 0.0011 |
| 1a: Prevalence of intermediate metabolizers, 5.6% | $1,937.26 | $2,041.28 | –$104.06 | 14.13% | 14.34% | –0.21% | 0.3743 | 0.3733 | 0.0010 |
| 1b: Prevalence of intermediate metabolizers, 7.7% | $1,901.37 | $2,079.76 | –$177.82 | 14.29% | 14.52% | –0.23% | 0.3728 | 0.3715 | 0.0012 |
| 2a: Prevalence of poor metabolizers, 0% | $1,923.02 | $2,032.54 | –$109.52 | 14.18% | 14.23% | –0.04% | 0.3677 | 0.3671 | 0.0007 |
| 2b: Prevalence of poor metabolizers, 0.6% | $1,941.42 | $2,164.32 | –$222.90 | 14.22% | 14.79% | –0.57% | 0.3694 | 0.3675 | 0.0020 |
| 3: Effectiveness and resource use based on Henricks et al, 201872 | $2,102.14 | $2,172.23 | –$70.08 | 23.75% | 24.64% | –0.90% | 0.3698 | 0.3680 | 0.0018 |
| 4: Probability of hospitalization in poor metabolizers, standard dose, 100% | $1,923.94 | $2,093.98 | –$170.04 | 14.22% | 14.44% | –0.22% | 0.3694 | 0.3683 | 0.0011 |
| 5a: Days of hospitalization in poor metabolizers, standard dose, 23 days | $1,923.94 | $2,062.82 | –$138.88 | 14.22% | 14.44% | –0.22% | 0.3694 | 0.3683 | 0.0011 |
| 5b: Days of hospitalization in poor metabolizers, standard dose, 34.5 days | $1,923.94 | $2,080.77 | –$156.83 | 14.22% | 14.44% | –0.22% | 0.3694 | 0.3683 | 0.0011 |
| 6: Assumed disutility would last for 8 weeks | $1,923.94 | $2,071.79 | –$147.85 | 14.22% | 14.44% | –0.22% | 0.3673 | 0.3661 | 0.0012 |
| 7: Assumed no alternative chemotherapy for poor metabolizers | $1,915.16 | $2,071.79 | –$156.63 | 14.22% | 14.44% | –0.22% | 0.3694 | 0.3683 | 0.0011 |
| 8: Assumed 1 extra physician visit | $1,995.90 | $2,071.79 | –$75.89 | 14.22% | 14.44% | –0.22% | 0.3694 | 0.3683 | 0.0011 |
| 9a: Cost of DPYD genotyping, $50 per test | $1,873.19 | $2,071.79 | –$198.60 | 14.22% | 14.44% | –0.22% | 0.3694 | 0.3683 | 0.0011 |
| 9b: Cost of DPYD genotyping, $150 per test | $1,974.69 | $2,071.79 | –$97.10 | 14.22% | 14.44% | –0.22% | 0.3694 | 0.3683 | 0.0011 |
Abbreviation: QALY, quality-adjusted life year.
Discussion
The results showed that pre-treatment DPYD genotyping could reduce severe fluoropyrimidine-related toxicity and save on cost, with some uncertainty. Overall, our results were consistent with findings in the literature. The two Dutch cost-minimization studies identified from the literature review (Deenen et al79 and Henricks et al80) also found that DPYD genotyping yielded a small cost saving (approximately €45 to €51 EUR per patient). We found a smaller reduction in the proportion of patients experiencing severe toxicity (−;0.22%, compared to the −0.54% found by Deenen et al79 when only DPYD∗2A was genotyped, and with the −0.88% found by Henricks et al80 when four variants were genotyped). This is because we used clinical data from a different observational study (Lunenburg et al7), which found a smaller reduction in the frequency of severe toxicity, but a shorter hospital length of stay in DPYD variant carriers receiving reduced dose.
COST OF DPYD GENOTYPE TESTING
The scenario analyses showed that results were sensitive to the cost of DPYD genotype testing. The cost of DPYD genotype testing may vary with many factors, such as the annual volume of tests conducted and number of samples per run (Table 21). In the reference case analysis, we used $100 per test based on expert opinion from two Ontario laboratories currently conducting the test under research programs. If DPYD genotyping is implemented at a larger scale in the province, the cost per test may go down further, although investment may be required to expand capacity. It is important to note that if a laboratory provides DPYD genotyping service to only one cancer center, the test volume may not be high enough to allow samples to be batched and the cost per test would be higher. In Quebec, DPYD∗2A was added to the provincial formulary in 2017. The variants DPYD∗13, c.2864A>T, and c.1236G>A were added in 2019 for patients who have planned cancer treatment with fluoropyrimidines. The experience with the first variant introduced has been published. According to the publication, the cost of genotyping for one variant, DPYD∗2A, is about $18.30 per test in Quebec (real-time PCR, tests run twice weekly at one laboratory). A centralized testing model was adopted, and the tests were run twice per week at one laboratory. This increase in throughput dramatically reduced the cost per sample while maintaining rapid turnaround time (about 6 days). Between March 2017 and August 2018, a total of 2,617 DPYD∗2A genotyping assays were performed, of which a majority (81%) were referred from 72 different hospitals in the province.75
Table 21:
Possible DPYD Genotyping Test Results and Potential Consequences
| Results | Usual Care Strategy: All Patients Receive Standard Dose | Pre-Treatment DPYD Genotyping Strategy: Patients Receive Dose Adjustment Based on Genotype Results |
|---|---|---|
| TP results (1.60%)a | By definition,b all patients experienced severe toxicity | Patients receive dose reduction so toxicity may be reduced (assumption required to estimate how much toxicity can be reduced) |
| FP results (5.19%)a | By definition,b all patients did not experience severe toxicity | Patients receive dose reduction unnecessarily. Because clinical guidelines recommended dose titration to avoid underdosing, assume no change in treatment efficacy. These patients do not experience additional severe toxicity (no change in toxicity) |
| FN results (12.69%)a | By definition,b all patients experienced severe toxicity | Patients receive standard dose as with usual care, so all patients would experience severe toxicity (no change in toxicity) |
| TN results (80.52%)a | By definition,b all patients did not experience severe toxicity | Patients receive standard dose as with usual care (no change in toxicity) |
Abbreviations: FN, false negative; FP, false positive; TN, true negative; TP, true positive.
The proportions of different test results are calculated using sensitivity/specificity derived from the Lunenburg study7 (sensitivity: 7.1% [8/113], specificity: 96.2% [666/692]).
The occurrence of severe toxicity was used as the reference standard to calculate sensitivity and specificity (i.e., using the DPYD genotyping test to predict severe toxicity in patients treated with a standard dose fluoropyrimidines). Sensitivity was defined as the proportion of patients identified as DPYD carriers among those who experienced severe toxicity. Specificity was defined as the proportion of wild-type patients among those who did not experience severe toxicity.
The cost of DPYD genotyping in other countries varied widely in the literature, from about €6.4082 to €17783 EUR per test. Cortejoso et al82 calculated a range of costs for DPYD genotyping in Spain based on the number of samples per run and the number of samples analyzed per year (not limited to DPYD testing) in the equipment used. The cost included reagents, equipment, and personnel (both clinical and laboratory). It was estimated that the per-patient cost of the DPYD genotype test could range from €43.1 to €3.5 EUR (1 sample per run, 100 samples per year to 20 samples per run, 2000 samples per year, respectively). In other studies, the reported costs of the DPYD genotype testing were higher (€75,73 €100,79 €177,83 and €12085 EUR). These costs were obtained through internal hospital data.
Because this is an active area of research, more DPYD variants could be added in the future, which might change the cost of testing, as well as the sensitivity and specificity of the test. The cost-effectiveness result may change accordingly. However, according to clinical experts, only minimal additional cost is expected if more variants are added. We expect the test to be more cost-effective if more people with increased risk of severe toxicity are identified as additional variants are added.
COST OF SEVERE TOXICITY
The cost of managing severe toxicity also varied in the literature, from about €7584 (nausea and vomiting) to €46,41283 (hospitalization related to gastrointestinal and hematologic toxicity) per event. Toffoli et al84 reported the average cost of managing fluoropyrimidine-related toxicity to be about €930 per patient. For more severe toxicity, the highest cost was estimated to be about €6,102 per episode (e.g., hospitalization for febrile neutropenia). Fragoulakis et al85 estimated the average cost of severe toxicity to be €1,150 in wild-type patients and €3,712 in carriers of any DPYD variant. Cortejoso et al82 estimated the mean cost of treating grade ≥ 3 fluoropyrimidine-induced neutropenia to be €3044.18 (range €17.45 to €14,103.25).82
COST OF CHEMOTHERAPY DRUGS
The results were not sensitive to the cost of chemotherapy drugs. Fluoropyrimidines are considered to be inexpensive, so the change in drug cost due to fluoropyrimidine dose reduction (DPYD intermediate metabolizers) would be very small. DPYD poor metabolizers may receive alternative chemotherapy and the new regimen could be more expensive than fluoropyrimidines, but the change in cost to the system would be small because the proportion of poor metabolizers is very small.
MODEL STRUCTURE
Similar to previous economic studies, we used a decision-tree model to estimate the impact of DPYD genotyping on costs and outcomes. The effect of DPYD genotyping was modelled using observed rates of toxicity in populations before and after genotyping from Lunenburg et al7 (which reflects clinical utility). An alternative modelling approach is to estimate the proportions of true positive (TP), false positive (FP), false negative (FN) and true negative (TN) results using sensitivity and specificity of the test (clinical validity), and then predict the rate of toxicity post-genotyping based on different types of test results. However, additional assumptions may be required to estimate the impact of different test results on health outcomes (Table 21). How the test impacts clinical results may depend on many factors, such as clinical implementation. Data on hospitalization and resource use are also only available by wild-type versus DPYD variant carriers, and not by each test result category. Based on these considerations, we did not use sensitivity and specificity explicitly in the current model, although these test characteristics were inherently incorporated (i.e., the rate of severe toxicity in the overall population before receiving genotype-guided treatment adjustment is the sum of the proportions of patients with TP and FN results).
Strengths and Limitations
Our analysis has several strengths. First, we considered the impact of DPYD genotyping on both cost and effectiveness (i.e., the proportion of patients experiencing severe toxicity and QALYs). By using QALYs as an outcome, we were able to capture the impact of DPYD genotyping on both survival (due to avoidance of fatal toxicity) and patients' quality of life. Second, instead of estimating clinical and resource use parameters of DPYD variant carriers receiving standard dose based on historical controls, we selected Lunenburg et al7 to inform the analysis because it included all three groups of patients in the same study (i.e., wild-type patients receiving standard dose, DPYD carriers receiving reduced dose, and DPYD carriers receiving standard dose). We considered that study to have less bias. Its results aligned well with the overall findings of the clinical evidence review. Finally, we included DPYD poor metabolizers in the analysis since DPYD genotyping is expected to be of greater importance for these patients than for intermediate metabolizers (i.e., heterozygous DPYD variant carriers).
There were some limitations to our analysis. Due to the lack of randomized clinical trial evidence, we relied on observational studies to estimate the impact of DPYD genotyping indirectly. The reference case analysis was based on a large observational study that compared the risks of severe toxicity in three groups of patients: wild-type patients receiving standard dose, DPYD carriers receiving standard dose, and DPYD carriers receiving reduced dose (Lunenburg et al7). All other clinical studies included only two groups. Clinical validity studies compared DPYD carriers receiving standard dose with wild-type patients, while clinical utility studies compared DPYD carriers receiving reduced dose with wild-type patients. Although Lunenburg et al7 was the only study that included all three groups of patients without using historical controls, it had several important limitations. First, although the authors included a large number of patients overall (n = 828), the sample sizes of DPYD carriers were very small (34 patients received a standard dose and 22 received a reduced dose), so it is difficult to draw firm conclusions from this comparison. Second, the distribution of different DPYD genotypes was not homogeneous across the two DPYD comparison groups. This might have affected the frequency of severe toxicity observed in the study. There was an overrepresentation of the variants that were expected to have the weakest effect on DPD in the DPYD carriers who received a standard dose (c.1236G>A and c.2846A>T). The authors speculated that more toxicity may have occurred in this group if DPYD variants were more equally distributed. Third, the study included patients from three different databases (some prospective and some retrospective), which could have led to biases. Finally, all patients included in the study received radiation therapy in addition to fluoropyrimidine-based regimens. According to the study authors, patients receiving radiation therapy usually receive lower fluoropyrimidine doses.
Furthermore, although it is a strength that we considered DPYD poor metabolizers in the analysis through modelling, there are very limited clinical data on these patients. We relied primarily on expert opinion to estimate the costs and outcomes associated with these patients. We assumed that if given standard-dose fluoropyrimidines, DPYD poor metabolizers would be more likely to have severe toxicities and hospitalizations, and even death. However, more research is needed to confirm those assumptions.
Finally, we did not model any potential long-term impacts of the test on cancer outcomes (e.g., differences in treatment efficacy associated with receiving reduced dose fluoropyrimidines or alternative treatment).
Conclusions
DPYD genotyping may be slightly more effective and less costly compared to usual care (no testing) because fewer patients would have severe fluoropyrimidine-related toxicity. At the commonly used willingness-to-pay values of $50,000 and $100,000 per QALY gained, DPYD genotyping is likely cost-effective compared to usual care (91% and 96% probability, respectively).
Budget Impact Analysis
Research Question
From the perspective of the Ontario Ministry of Health, what is the 5-year budget impact of publicly funding pre-treatment DPYD genotyping in patients who have planned cancer treatment with fluoropyrimidines in Ontario?
Methods
Analytic Framework
We estimated the budget impact of publicly funding pre-treatment DPYD genotyping using the cost difference between two scenarios: (1) current clinical practice without public funding for DPYD genotyping (the current scenario) and (2) anticipated clinical practice with public funding for DPYD genotyping (the new scenario). Figure 22 presents the budget impact model schematic.
Figure 22: Schematic Model of Budget Impact.
Key Assumptions
The following assumptions were made for the reference case analysis:
In the current scenario, we assumed that DPYD genotyping is not publicly funded
In the new scenario, we assumed there would be a slow uptake of pre-treatment DPYD genotyping in the next 5 years
Target Population
Our target population is patients who have planned cancer treatment with fluoropyrimidines (i.e., 5-fluorouracil [5-FU] and/or capecitabine). Both children and adults are included in the analysis, although the proportion of patients aged less than 18 years is very small (only 0.03%; Ontario Health Cancer Care Ontario; Ontario Cancer Registry, Cancer Activity Level Reporting and Ontario Drug Benefit datasets; prepared March 2020).
We obtained the number of patients administered fluoropyrimidines using data prepared by Ontario Health (Cancer Care Ontario) in March 2020. The data were obtained using the Ontario Cancer Registry to identify Ontario residents with a cancer diagnosis between April 1, 2009, and March 31, 2019. Then, using the Ontario Drug Benefit program and Cancer Activity Level Reporting, we identified the number of patients that were administered a fluoropyrimidine (i.e., 5-FU and/or capecitabine) between 2014/15 and 2018/19 (Table 22).
Table 22:
Number of Patients Who Received Fluoropyrimidines From 2014/15 to 2018/19 in Ontario
| Population | Fiscal Year | ||||
|---|---|---|---|---|---|
| 2014/15 | 2015/16 | 2016/17 | 2017/18 | 2018/19 | |
| Number of patients administered fluoropyrimidines | 8,099 | 7,429 | 7,398 | 7,533 | 7,766 |
Source: Data provided by Ontario Health (Cancer Care Ontario) in March 2020 using Ontario Cancer Registry, Cancer Activity Level Reporting and Ontario Drug Benefit datasets.
We estimated the target population for our budget impact analysis by extrapolating the data in Table 22 over the next 5 years. We used linear extrapolation and excluded the 2014/15 fiscal year, because it was likely higher than the following years because of changes made in 2014 to the Cancer Activity Level Reporting dataset (Ontario Health [Cancer Care Ontario], email communication, March 23, 2020). We assumed that all patients who had planned fluoropyrimidine treatment would be eligible for DPYD genotyping. We estimated that a total of 40,809 patients would be eligible for DPYD genotyping over the next 5 years (Table 23).
Table 23:
Number of Patients Projected to Receive Fluoropyrimidines From Year 1 to Year 5 in Ontario
| Population | Year of Analysis | |||||
|---|---|---|---|---|---|---|
| Year l | Year 2 | Year 3 | Year 4 | Year 5 | Total | |
| Number of patients who have planned cancer treatment with fluoropyrimidines | 7,933 | 8,047 | 8,162 | 8,276 | 8,391 | 40,809 |
Current Intervention Mix
Currently in Ontario, DPYD genotyping is used only in a research setting. Therefore, in our current scenario, we assumed that DPYD genotyping was not publicly funded and all patients were receiving usual care. Under usual care practices, all individuals receive standard-dose fluoropyrimidines without DPYD genotype testing.
Uptake of the New Intervention
The uptake of a new intervention such as DPYD genotyping could be limited by factors such as knowledge transfer, patterns of practice, and administrative barriers (Jason Yu, MD, email communication, June 21, 2020; Geoffrey Liu, MD, telephone communication, June 24, 2020). Based on the literature, international acceptance and implementation of DPYD genotyping as routine procedure for all patients with planned fluoropyrimidine treatment have been challenging. Therefore, we assumed the annual uptake rates for the reference case analysis to be 7%, 8%, 10%, 15%, and 20% in the next 5 years. We also explored the impact of higher uptake rates in sensitivity analyses (Table 24).
Table 24:
Uptake Rates of DPYD Genotyping in the Next 5 Years
| Uptake | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 |
|---|---|---|---|---|---|
| Uptake rates for reference case | 7% | 8% | 10% | 15% | 20% |
| Volume (N) | 555 | 644 | 816 | 1,241 | 1,678 |
| Intermediate uptake scenario | 10% | 20% | 30% | 40% | 50% |
| Rapid uptake scenario | 20% | 40% | 60% | 80% | 100% |
Source: Estimated based on clinical expert opinion and extrapolation from current volume trends.
Resources and Costs
The cost per person was derived from the primary economic evaluation. The costs included are:
DYPD genotyping (sample processing, testing, results interpretation, and reporting)
Treatment of toxicity (hospitalization and non-hospitalization costs)
Chemotherapy drugs
Physician visits
Internal Validation
The secondary health economist conducted formal internal validation. This process included checking for errors and ensuring the accuracy of parameter inputs and equations in the budget impact analysis.
Analysis
We conducted a reference case analysis and sensitivity analyses. Our reference case analysis represents the analysis with the most likely set of input parameters and model assumptions. In sensitivity analyses, we explored how the results are affected by varying input parameters and model assumptions.
Sensitivity analyses were conducted by varying:
Prevalence of DYPD intermediate and poor metabolizers
Uptake rate of DYPD genotyping test in years 1 to 5
Cost of DYPD genotyping
Results
Reference Case
The reference case results are presented in Table 25. In the current scenario, the cost of usual care is about $16 million to $17 million per year. In the new scenario, the cost of usual care would decrease each year as the uptake of DPYD genotyping increased. The total health care cost for the DPYD genotyping group would range from $1.067 million in year 1 to $3.223 million in year 5. The annual budget impact was estimated to be a savings of $80,453 in year 1, increasing to a savings of $243,137 in year 5, for a total savings of $714,963 over 5 years. The cost of providing DPYD genotype testing alone was estimated to be $93,907 in year 1, increasing to $283,797 in year 5, for a total cost of $834,527 over 5 years.
Table 25:
Budget Impact Analysis Results
| Scenario | Budget Impact, $ | |||||
|---|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Total | |
| Current Scenario | ||||||
| Usual care | $16,387,218 | $16,622,708 | $16,860,264 | $17,095,754 | $17,333,310 | $84,299,255 |
| New Scenario | ||||||
| Usual care DPYD genotyping |
$15,240,113 $1,066,652 |
$15,292,892 $1,236,549 |
$15,174,238 $1,567,775 |
$14,531,391 $2,384,509 |
$13,866,648 $3,223,525 |
$74,105,281 $9,479,010 |
| Budget impact Cost of DPYD genotype testing |
–$80,453 $93,907 |
–$93,268 $108,865 |
–$118,251 $138,026 |
–$179,854 $209,931 |
–$243,137 $283,797 |
–$714,963 $834,527 |
Sensitivity Analysis
The sensitivity analysis results are presented in Table 26. When we assumed a lower prevalence of intermediate metabolizers (scenario 1a) or poor metabolizers (scenario 2a) in the target population, the cost savings from DPYD genotyping became smaller. If we assumed a higher prevalence, the cost savings from DPYD genotyping increased. If DPYD genotyping required an extra physician visit (e.g., to discuss test results with patients), the cost savings decreased by half. Similarly, if DPYD genotype testing were assumed to be more expensive ($150 instead of $100 per test), the cost savings also decreased. If the uptake rates were higher, the cost savings would increase.
Table 26:
Budget Impact Sensitivity Analysis Results
| Scenario | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Total |
|---|---|---|---|---|---|---|
| 1a: Prevalence of Intermediate Metabolizers, 5.6% | ||||||
| Budget impact Cost of test |
–$57,763 $93,907 |
–$66,964 $108,865 |
–$84,901 $138,026 |
–$129,130 $209,931 |
–$174,566 $283,797 |
–$513,325 $834,527 |
| 1b: Prevalence of Intermediate Metabolizers, 7.7% | ||||||
| Budget impact Cost of test | –$99,062 $93,907 |
–$114,840 $108,865 |
–$145,602 $138,026 |
–$221,453 $209,931 |
–$299,374 $283,797 |
–$880,331 $834,527 |
| 2a: Prevalence of Poor Metabolizers, 0% | ||||||
| Budget impact Cost of test |
–$60,818 $93,907 |
–$70,505 $108,865 |
–$89,390 $138,026 |
–$135,958 $209,931 |
–$183,796 $283,797 |
–$540,467 $834,527 |
| 2b: Prevalence of Poor Metabolizers, 0.6% | ||||||
| Budget impact Cost of test |
–$123,779 $93,907 |
–$143,494 $108,865 |
–$181,931 $138,026 |
–$276,708 $209,931 |
–$374,071 $283,797 |
–$1,099,983 $834,527 |
| 3: Assume One Extra Physician Visit | ||||||
| Budget impact Cost of test |
–$42,142 $93,907 |
–$48,855 $108,865 |
–$61,941 $138,026 |
–$94,210 $209,931 |
–$127,359 $283,797 |
–$374,507 $834,527 |
| 4a: Cost of DPYD Genotype Testing, $50 Per Test | ||||||
| Budget impact Cost of test |
–$110,285 $66,142 |
–$127,851 $76,677 |
–$162,097 $97,216 |
–$246,542 $147,861 |
–$333,291 $199,887 |
–$980,065 $587,783 |
| 4b: Cost of DPYD Genotype Testing, $150 Per Test | ||||||
| Budget impact Cost of test |
–$53,921 $121,673 |
–$62,509 $141,053 |
–$79,253 $178,836 |
–$120,540 $272,001 |
–$162,953 $367,707 |
–$479,176 $1,081,270 |
| 5a: Intermediate Uptake | ||||||
| Budget impact Cost of test |
–$114,933 $134,154 |
–$233,170 $272,163 |
–$354,753 $414,078 |
–$479,611 $559,816 |
–$607,844 $709,493 |
–$1,790,311 $2,089,704 |
| 5b: Rapid Uptake | ||||||
| Budget impact Cost of test |
–$229,867 $268,307 |
–$466,340 $544,325 |
–$709,506 $828,157 |
–$959,222 $1,119,631 |
–$1,215,688 $1,418,987 |
–$3,580,622 $4,179,407 |
Discussion
The budget impact analysis showed that publicly funding pre-treatment DPYD genotyping in patients with planned fluoropyrimidine treatment may be cost-saving (a total savings of $714,963 over the next 5 years). We estimated the cost of providing the DPYD genotyping test itself to be about $834,527 over the next 5 years. According to laboratory experts, the per-test cost may depend on how testing is implemented. A centralized testing model would increase the throughput, which would reduce the cost per sample dramatically while maintaining the rapid turnaround time (< 1 week). Centralized testing would also reduce the number of trained personnel required and the need for validation at distributed sites.
Strengths and Limitations
Our analysis had several strengths. We estimated the size of the target population using real-world Ontario data from the Ontario Cancer Registry. We considered not only the cost of the DPYD genotyping test but also potential cost offsets related to fewer cases of severe toxicity. We also estimated the budget impact of several different scenarios by varying the prevalence, testing cost, and uptake rates. A limitation is that we did not estimate the costs related to implementation, service delivery, and program coordination, because these could vary substantially depending on how testing is implemented.
Conclusions
We estimated that publicly funding pre-treatment DPYD genotype testing may be cost-saving (a total of $714,963 saved over the next 5 years, provided that the implementation, service delivery, and program coordination costs do not exceed our estimated amounts). The cost of testing would be about $834,527 over the next 5 years.
Preferences and Values Evidence
Objective
The objective of this analysis was to explore the underlying values, needs, and priorities of those who have lived experience with fluoropyrimidine treatment, as well as the preferences and perceptions of those who have sought DPYD testing.
Background
Exploring patient preferences and values provides a unique source of information about people's experiences of a health condition and the health technologies or interventions used to manage or treat that health condition. It includes the impact of the condition and its treatment on the person with the health condition, their family and other caregivers, and the person's personal environment. Engagement also provides insights into how a health condition is managed by the province's health system.
Information shared from lived experience can also identify gaps or limitations in published research (e.g., outcomes important to those with lived experience that are not reflected in the literature).106–108 Additionally, lived experience can provide information and perspectives on the ethical and social values implications of health technologies or interventions.
Because the needs, preferences, priorities, and values of those with lived experience in Ontario are important to consider to understand the impact of the technology in people's lives, we may speak directly with people who live with a given health condition, including those with experience of the technology or intervention we are exploring.
For this analysis, we examined the preferences and values of people who have been treated with fluoropyrimidines and who sought DPYD testing for treatment purposes in three ways:
A review by the Canadian Agency for Drugs and Technologies in Health (CADTH) of the published qualitative evidence on patient preferences and values
A survey by the Quebec Institut National de d'Excellence en Santé et en Services Sociaux (INESSS) exploring the perspectives on DPYD testing of people with cancer
Direct engagement by Ontario Health through interviews with people who had lived experience with fluoropyrimidine treatment and those who sought DPYD testing
Qualitative Evidence
Ontario Health collaborated with CADTH to conduct this health technology assessment. DPYD testing is a type of pharmacogenomic test. Because the qualitative literature on DPYD testing is limited, CADTH conducted a review of the qualitative literature109 on patient perspectives of pharmacogenomic testing more broadly. We have summarized the key findings of this review below.
Key Findings
The rapid qualitative evidence synthesis included 13 primary studies that explored the views and understanding of patients and providers on pharmacogenomic testing
Patients and providers saw pharmacogenomic testing as beneficial. Although they sometimes wanted more information, most patients and providers said that pharmacogenomic testing helped them narrow their choices to the “best” medication so they could avoid adverse reactions
Patients and providers expressed worries about how pharmacogenomic testing would limit patient-centred care by limiting patients' choices of medications. They also raised concerns about having to select less effective or more expensive medications to avoid any potential adverse reactions flagged by the pharmacogenomic test results
Patients and providers raised concerns about the potential for genetic discrimination by insurers and employers, and about privacy and confidentiality. Limiting access to medical records, particularly electronic ones, appeared to be the primary mechanism by which patients and providers thought privacy and confidentially could be mitigated.
Pharmacogenomic test results can shape patient care over the life course. The potential for secondary findings from pharmacogenomic testing made patients worry about how results would affect them in the present and the future. The potential for pharmacogenomic test results to affect current and future family members also troubled patients and providers
The review found limited information about the use of and views on pharmacogenomic testing by disease or type of test. Findings point to the need for faster results from pharmacogenomic testing in life-limiting or rapidly progressing conditions. In areas such as mental health, pharmacogenomic testing was used less routinely, and generally applied when patients experienced adverse reactions or limited effectiveness. Providers and patients expected pharmacogenomic test results to be just one of several types of information they used for decision-making
Survey Evidence
In 2019 INESSS conducted a survey32 with cancer patients across Quebec who had been treated with fluoropyrimidines to ask them about their perspectives on fluoropyrimidine-based regimens and DPYD testing. We used some questions from the INESSS survey in our direct patient engagement interviews. We also requested and received the raw data from the survey to compare and contrast our results. The data shared by INESSS contained no information enabling the identification of patients who completed the survey.
Key Findings
The INESSS survey yielded 38 respondents
Several respondents indicated that they had not been informed about the potential risks associated with a standard fluoropyrimidine dose
Most patients preferred being offered DPYD testing, despite the fact that their chances of testing positive were approximately 1%
Direct Patient Engagement
Methods
PARTNERSHIP PLAN
The partnership plan for this health technology assessment focused on consultation to examine the experiences of people who received fluoropyrimidine treatment and those of their families and other caregivers. We engaged people via phone interviews.
We used a qualitative interview, as this method of engagement allowed us to explore the meaning of central themes in the experiences of people who have received fluoropyrimidine treatment, as well as those of their families and caregivers.110 The sensitive nature of exploring people's experiences of a health condition and their quality of life are other factors that support our choice of an interview methodology.
PARTICIPANT OUTREACH
We used an approach called purposive sampling,111–114 which involves actively reaching out to people with direct experience of the health condition and health technology or intervention being reviewed. We approached a variety of partner organizations, including Wellsprings, the University Health Network, Cancer Care Ontario, and London Health Sciences Centre, to spread the word about this engagement activity and to contact people who had been treated with fluoropyrimidines, family members, and caregivers, including those with experience of DPYD testing.
Inclusion Criteria
We sought to speak with people who had direct experience with fluoropyrimidines for the treatment of cancer, as well as their family or caregivers.
Exclusion Criteria
We did not set exclusion criteria.
Participants
For this project, we spoke with 16 people who had been treated with fluoropyrimidines and were living in Ontario, as well as one family member and caregiver. Of the 17 people we spoke with, three had experience with DPYD testing, and the others had experience with pathology testing.
APPROACH
At the beginning of the interview, we explained the role of Ontario Health, the purpose of this health technology assessment, the risks of participation, and how participants' personal health information would be protected. We gave this information to participants both verbally and in a letter of information (Appendix 9). We then obtained participants' verbal consent before starting the interview. With participants' consent, we audio-recorded and then transcribed the interviews.
Interviews lasted approximately 30 to 90 minutes. The interview was loosely structured and consisted of a series of open-ended questions. Questions were based on a list developed by the Health Technology Assessment International Interest Group on Patient and Citizen Involvement in Health Technology Assessment.115 Questions focused on the impact of cancer on people's quality of life, their experiences with treatments to manage or treat cancer, their experiences with the DPYD testing, and their perceptions of the benefits or limitations of DPYD testing. For family members and caregivers, questions focused on their perceptions of the impact of cancer and treatments on the quality of life of the person with cancer, as well as the impact of the person's health condition and treatments on the family members and caregivers themselves. See Appendix 10 for our interview guide.
DATA EXTRACTION AND ANALYSIS
We used a modified version of a grounded-theory methodology to analyze interview transcripts. The grounded-theory approach allowed us to organize and compare information on experiences across participants. This method consists of a repetitive process of obtaining, documenting, and analyzing responses while simultaneously collecting, analyzing, and comparing information.116,117 We used the qualitative data analysis software program NVivo118 to identify and interpret patterns in the data. The patterns we identified allowed us to highlight the impact of cancer and treatments on the people with cancer, family members, and caregivers we interviewed.
Results
DIAGNOSIS
Participants spoke about the varying symptoms they experienced, which led them to seek care and eventually to their cancer diagnosis. People had a variety of different experiences during their diagnosis journey. Some reported a positive, streamlined experience in which they were seen by a health care provider, sent for tests and diagnosed in a timely manner, and given treatment:
The stomach ache was not going away … I mentioned it to my family doctor, who immediately sent me for an ultrasound. Then the tumour that I had showed up immediately in the ultrasound.
I had a mammogram, and they thought they saw something, and then they did an ultrasound and another mammogram. They determined it was aggressive after it came back from a lab.
For a few participants, the search for a diagnosis was more difficult. Some were dismissed by physicians, some were misdiagnosed, and some did not have test results relayed in a timely manner. Two participants reported that it took years before they were given a proper diagnosis, during which time their cancer progressed. Patients expressed frustration with the process and felt they were not listened to or taken seriously until they advocated for themselves:
She showed me a pathology report from my Appendix 10 months earlier that showed it was adenocarcinoma. So I was quite shocked, by both the fact that I had the cancer, plus the fact that it had been let go for 10 months. And basically I had gone from stage one to stage three at that point.
For 3 to 4 years I fought to get a diagnosis. And by the time I started to have visible symptoms, which were rectal bleeding … I finally got a colonoscopy that was denied to me for 3 years. By that point, it had already gone into late stage three.
1 found it on my own and brought it to my family doctor's attention. We did an ultrasound and we just took it 6 months at a time, and we just watched the growth of it. I went through this for 2 years … I took matters in my own hands … I ended up finding a cancer doctor … and I was at stage four at that point.
Many described the cancer diagnosis as a shock—unexpected and life-changing. They had concerns about their family and what the future would look like. Some had suspected the cancer diagnosis but still found the news devastating:
My first diagnosis was difficult. I was a single mom with teenage boys and a daughter with challenges.
It was devastating to find out I had cancer. I kind of suspected it—I was almost expecting that answer from the way I felt and what I thought was going on in my body.
You hear the diagnosis of cancer. It was surreal, because you know 24 hours earlier, it didn't exist. It wasn't even in our vocabulary and now it was a stage four cancer.
A few participants said that they did not have a family history of cancer and highlighted their healthy lifestyle:
I'm a non-smoker, healthy lifestyle, active bodybuilder, into fitness. Probably the least likely person that you would expect to get colon cancer.
But being such an active, healthy person my entire life, this kind of blindsided all of us. I'm still shocked.
One patient felt relief at finding a diagnosis for their health concerns:
I actually felt better, because I had been struggling for the past year and had been asking for help to know what was going on and not getting anywhere … Well, at least I'm not crazy and there is really something wrong with me.
TREATMENT JOURNEY
Once participants had been accurately diagnosed, they faced another care journey for treatment. Interviewees spoke about treatment options, searching for second or third opinions, and selecting a treatment plan. People reported a combination of treatment options offered to them, including surgery, chemotherapy, radiation, and immunotherapy:
I had both breasts removed. That was when the doctor told me what stage I was at … And I had the surgery, and then we waited 10 days, I believe. Then we started chemotherapy right away.
The mastectomy was the only surgical option. And then I was 50–50 on both the chemo and radiation.
SIDE EFFECTS
All participants experienced side effects from their treatments including surgery, chemotherapy, and radiation. Participants noted that the side effects from their treatment had a significant effect on their quality of life. The most commonly reported side effects they experienced were vomiting, hair loss, diarrhea, brain fog, memory loss, fatigue, and neuropathy:
I had some hair loss … I really didn't have very many side effects. I was very lucky.
When you do your own research on the chemotherapy treatments, it doesn't tell you that you're going to have a little bit of a memory loss or a blockage, or you're going to feel like a car just hit you.
The diarrhea kicked in fairly bad … and I had cartoon vomiting, is how I like to describe it.
A few participants experienced more severe side effects, such as myocardial infarction, blood clots, neuropathy, or severe diarrhea. Because of the severity of their side effects, they needed a reduction in their dosage or longer periods between treatments. In a few cases, the side effects led to hospitalizations:
So last week after I finished my 11 days of diarrhea, I called my doctor and I said, “You know, I don't think I can take the treatment this week. Can I have an extra week just to recover a bit from that before I start again for another?”
After the myocardial infarction, I had an emergency angiogram, a cardiogram, and various other tests, and was admitted for a while for that. So after that my chemo had to be changed.
A few participants mentioned latent side effects (such as trouble with cognitive function and neuropathy) that were still present years after their chemotherapy treatment:
For about 2½ years now I've been experiencing changes in my brain. I'm much more forgetful than I used to be. I can't concentrate like I used to. I can't multitask.
I still do have neuropathy in my feet. And that started once I had finished the treatment.
QUALITY OF LIFE
The side effects from treatment had a substantial impact on participants' quality of life. A majority mentioned that the side effects of chemotherapy limited their ability to do simple day-to-day tasks, especially household chores and errands. They had to get assistance from friends or family, or hire help:
I needed to be on bed rest for 48 hours until the chemo was out. That had a huge impact, because I basically just had to lie still and not do anything.
I had to hire a cleaning lady temporarily to come in and clean my house. I had family members dropping off dinners for my husband and me. When I was able to eat, I pretty much lived on soup broth.
He couldn't do very much for himself. Basically, I was doing almost everything for him.
Participants also noted that they had difficulty exercising. It was physically challenging for them because of the side effects of fatigue, neuropathy, and reduced lung capacity. Going for walks became difficult, and one person needed assistance:
I like to get out and walk and try to exercise. Not a stroll, but go for a good physical walk. Right now, as the treatments have come to a close, my lung capacity is not allowing that.
If I was to go out for a walk or something, I need somebody with me to hold me up, or I would use a walker.
When you stand up it feels like your foot's on fire. You tend not to try to do a lot of stuff.
One participant spoke about being unable to attend her son's wedding:
I had to cancel my son's wedding destination for me and my husband. He had to get married without us. I had to cancel family events. I had to cancel my vacation.
EMPLOYMENT
Those interviewed described challenges with employment. Often, people took a leave of absence during their treatment because they were unable to meet the demands of their job while experiencing the side effects of their treatment. A few tried to schedule tasks and work duties around treatment, knowing that chemotherapy would affect their cognitive function. Others were unable to hold employment altogether because of neuropathy and a decline in cognitive function:
Between the brain fog and the struggle with neuropathy—my hands and feet—that affect my day-to-day activities. I already got a year to retirement. I'm probably just going to end up staying on disability until I hit retirement.
I did 3 weeks' work in 3 days. It was a lot, but I knew I would have chemo brain.
I did take a medical leave of absence from my job. I would not have been able to do the job for the demands physically, mentally, and also the risk of being in an [environment] full of germs.
MENTAL HEALTH
Participants spoke about the emotional burden they felt. Patients reported struggling with feelings of depression, isolation, anxiety, and frustration. For some, the emotional toll was from getting a cancer diagnosis; others struggled with worry about experiencing adverse reactions to treatment:
It's very hard for someone who gets a diagnosis not to wander off into the black areas.
I was incredibly anxious … in the end I got admitted to the oncology ward, and they monitored me there. And it was all fine, but there was a lot of anxiety leading up to that.
You've seen people on chemo before, and sometimes the worst things happened—you know, all the hair falling out, and the severe vomiting, and things like that, and you hope you don't get that. But it's always in the back of your mind that you could be that severe. That definitely causes you a lot of anxiety.
For others, the side effects from their cancer treatments limited their ability to do simple tasks:
I'm very frustrated with myself when I can't do things. I'll try and do something I think I can do and just struggle … And I get very upset with myself, very angry about it.
Others tried to have a positive outlook on their situation:
I'm a very positive person. I have to be, because otherwise life would be miserable. I would be miserable. So no point in doing that.
I know I had “poor me” days, but I'm the kind of person that says, okay, you've got 20 minutes to get over it and move on. And basically, that's what I did.
DPYD TESTING
Toxicity From Fluoropyrimidines
A majority of participants emphasized that they were aware of the common side effects of fluoropyrimidines but were not aware of the possible level of toxicity. Participants noted that toxicity was not mentioned in the resources they were given, or in conversations with their care team:
I mean, they give you lists of side effects and things like that, but I don't recall the use of the word “toxicity.”
No, I was totally unaware. I just figured like everyone else going through chemo, I could possibly feel nauseous, be throwing up, have diarrhea … But no, I never knew it could be toxic.
I never knew there could be such a reaction to a drug that it could be to the point of being dangerous … I mean, side effects are uncomfortable. Yeah. But to the point of really being a health hazard.
DPYD Testing in Ontario
At the time of completing this health technology assessment, DPYD testing was conducted through at one hospital in Ontario. Of the 17 participants, three had direct experience with DPYD testing. They said that the process was a simple blood test, and they felt it was just one of many the tests they took as part of their care journey. Because DPYD testing is commonly done before starting chemotherapy treatment, two of the three participants were not aware of their results, because they had just had their test done:
I don't have the results of the test. The results went to the doctor. I'm interested in knowing. I'll find out tomorrow when I start chemo.
Only one participant knew their test result, and it was negative. This participant had researched and asked for a DPYD test after having an adverse reaction to their chemotherapy treatment:
I requested DPYD testing because, again, the cardiac thing is a bit iffy as to whether it's classic DPYD deficiency. The cardiac stuff is not well represented in the literature as much as the classic symptoms.
Non-users of DPYD Testing
Most of the participants did not have direct experience with DPYD testing. We gave participants basic information about the test, including the logistics of the test, the type of information it can provide, and how the information would be used to inform the treatment path. We then asked for their thoughts on DPYD testing and whether such a test would be of value to them.
Participants who had no direct experience with DPYD testing were open to testing and commented about its perceived benefits. Most participants acknowledged that the test would give them more information before they started treatment. They thought it would aid in their decision-making, especially because of the toxic nature of chemotherapy. The review of the qualitative literature109 illustrated this as well, reporting that “Some providers and patients described that they saw test results as useful in that they provided more information.”
People also spoke about how DPYD testing might prepare their care team to face any risks associated with fluoropyridines or avoid those risks altogether. They spoke about the importance of being as informed as possible:
My care team would have been forearmed with the knowledge if there was an issue that was beyond the normal parameters of what should be occurring. Medication could have been adjusted.
If you can get treatment—the correct treatment—to a patient initially, and cure or provide a curative response, or at least improve the quality of life right at the beginning, it's going to cost less than having to take care of somebody in our system for 5 or 10 years.
I don't know how I'm going to react to any of the drugs that I'm going to be given, but if I can alleviate one of the problems I could possibly have, then absolutely I would do it.
The INESSS survey32 contained the question, “Do you believe that this test should be offered to patients who are candidates for fluoropyrimidine treatment, knowing that about 1% of individuals will have a positive result for this test (this proportion could reach 7% with the inclusion of the three proposed genetic variations)?” (INESSS survey, raw data; February 12, 2021). We asked our interviewees the same question. A majority of the participants we interviewed agreed that DPYD testing should be offered even if only 1% of individuals received positive results:
I think it's important to test everybody so that you know if you are in the 1%. You'd want to know.
Cancer itself is such a horrible thing for people to have to go through, that even if it's only 1%, everybody should have that chance and not have to experience those side effects.
I think knowing what the possible side effects are and hearing the horror stories of what some people have to go through, I think if there was a way to help those patients … how many patients stop chemotherapy because they can't handle the chemotherapy? I think it should be offered.
A couple of participants did not completely agree that DPYD testing should be offered if only 1% of people tested positive, although both took into account the different perspectives and were not able to give a definite answer:
No, because it is just 1%, and how many sail through without any big worries. That said, if I was in that 1% I'd feel a lot differently.
1% is pretty low, in my opinion, if you're someone like myself who doesn't have a history of reactions to a lot of drugs. On the other hand, everybody's circumstances are different, and I
don't think money should be a barrier for a solution for a cure or better outlook on life … I'm of two minds.
BARRIERS TO DPYD TESTING
Awareness of DPYD Testing
Most of the non-users of DPYD testing were not aware of the test and said that this option was never brought up during their care journey:
There was no discussion of the testing that you're talking about … I never heard of it before.
No, I didn't even know it existed.
A few participants expressed frustrations over not being made aware of DPYD testing before they started their fluoropyrimidine treatment and talked about how important the results from this test could have been to them:
I'm just kind of wondering why I was not made aware of it. Why an oncologist or cancer clinic wouldn't be making that information available to people that this testing is available in case you want to take advantage of it.
To me, it's really important. And I don't know why every oncologist wouldn't want to send their patients to find out before they start giving them the medication.
Four and a half years with Folfox and what I went through with it, and at no point was I ever offered any testing.
Access to DPYD Testing
Participants were asked if they would have been willing to travel within Ontario, to get DPYD testing if it were offered to them. All interviewees said that they would have been open to travelling to get tested before they started their chemotherapy treatment:
The cost of getting there and the cost of staying overnight, because you can't drive up and back [from where I am]. But I really wouldn't mind at all.
It's 2 hours [to the test facility]. That's not a big deal for us. And if we decided it was something that was worthwhile, we would do it no matter what.
One of the participants who had experience with DPYD testing reflected on how surprised they were to be asked to travel for the test, especially when they lived in Toronto:
He told me about the blood test [location], which to me sounded like a crazy thing to do, because I had to go all the way to [that location] … I presume that there are enough doctors in Toronto or enough labs in Toronto who could do the test.
A couple of patients said that they would have been able to get to London to get DPYD testing, but that others might not have access to the same resources:
I would first ask why can't they just ship my blood over there and get it tested. But if that's logistically not possible, then yes, I would. But I'm reasonably healthy, I have the time, and I'm not old. I don't know how different it would be for someone older.
Most often than not, if a doctor said, “Go do it,” you [would] do it, but maybe not. Maybe people can't get there.
ETHICAL CONCERNS
Storage of Genetic Data
The qualitative literature review109 found that “patients and providers expressed worries around who would have access to patients' genetic information and the potential for it to be accessed and misused by unauthorized persons. These concerns around confidentiality and privacy were often raised in reference to fears of discrimination by insurers.”
In contrast, when we asked interviewees their opinions about the storage of genetic information, most were not concerned about the privacy and confidentiality of their genetic data. Participants noted that because the data were used for health purposes to benefit the patient, and because a health care organization was storing it, they had trust that their information would not be given to third parties such as insurance companies:
There's a code of ethics in every profession … and most of them adhere to the code of ethics of their profession.
You just end up weighing privacy concerns over health concerns or life and death concerns, and I guess for me, life and death always trumps privacy.
Sharing Information With Relatives
Participants were also asked their opinions about sharing their test results with relatives. All interviewees were open to sharing their results and noted that it would provide their loved ones with more information so they could make more informed decisions about their health:
I wouldn't have any problem sharing it with my children or my brother and sister. Anything that might be helpful in their decisions about their life, about having children, or about how it might even affect their lifestyle and their choices. Any kind of information is good as long as it doesn't depress you.
I would actually want them to know that, as it would have implications for them if they were needing to have chemotherapy. But my personal opinion is, yes, I would. I would believe in it, but I understand people's hesitation in sharing that with family members.
Absolutely. I have shared my genetic report, I have shared all my data with all of my cousin, and my sister.
Preferences and Values Evidence Discussion
We compared the findings from different types of recruitment and engagement with the results from our direct patient engagement. This included CADTH's qualitative literature rapid review109 on pharmacogenomics and the survey results from INESSS32 exploring patient perspectives on DPYD testing.
Participants in our direct patient engagement discussed and reflected on their diagnosis, the impacts of living with cancer, and their treatment journey. Interviewees spoke about their struggles with the side effects of from their cancer treatment. They also shared the burden of their condition and its disruption of their quality of life, including its effects on their daily life, mental health, and employment.
Participants also discussed their perspectives on DPYD testing. They mentioned a lack of awareness among cancer patients about the toxicity of fluoropyrimidines and the existence of the DPYD test. Respondents to the INESSS survey further reinforced the message that lack of awareness was a barrier. A majority of the people we interviewed said that they were not offered DPYD testing as an option, although they were very open to it and willing to take the test. Most noted that they would have made travel within Ontario to the health care facility the test is available at, to take the test if it were offered. Most of the people we interviewed spoke about the significance of having DPYD test results before starting their fluoropyrimidine treatment to provide guidance on dosage and reduce the risk of adverse effects. This finding was also reflected in the CADTH qualitative literature review.109 Participants we interviewed noted that the fact that DPYD testing was offered only at one health care facility in Ontario would be a barrier to those who did not have the resources to get there.
In contrast to the findings of the qualitative literature review,109 the patients and caregivers we interviewed were not concerned about privacy, confidentiality, or the storage of their genetic data, and interviewees were not reluctant to share their test results with relatives. Because we spoke to only a small number of interviewees, this finding may or may not reflect the views of all patients.
The applicability of our results is limited because of the small number of participants with direct experience of DPYD testing, and because most of those who had had the test were not yet aware of their results and their potential effect on treatment. As well, most participants lived in large urban areas and we presumed that they had high socioeconomic status, because no one mentioned resource barriers when asked about travelling within Ontario to access the DPYD test.
Preferences and Values Evidence Conclusions
Participants we interviewed who had been treated with fluoropyrimidines described the substantial impact of cancer and the treatment side effects on their quality of life and mental health. Most reflected on the perceived value of DPYD testing to reduce the risk of serious adverse events as a result of fluoropyrimidine treatment—a finding that was also reflected in the INESSS survey results32 and the qualitative literature review.109 Barriers included lack of awareness and limited access to DPYD testing.
Conclusions of the Health Technology Assessment
Studies found that carriers of any one of the variants under assessment treated with a standard fluoropyrimidine dose may have a higher risk of severe toxicity versus wild-type patients treated—this may lead to dose reduction, treatment discontinuation and hospitalization. DPYD genotyping led to changes in clinical conduct by allowing fluoropyrimidine treatment modifications. It was uncertain whether the genotype-guided dose reduction in heterozygous DPYD carriers led to a risk of severe toxicity and hospitalization that was either comparable to wild-type patients, or lower than DPYD carriers, treated with a standard dose. The length of hospital stay was shorter in carriers treated with a reduced versus standard dose, but the evidence was uncertain. It is uncertain whether the treatment effectiveness of a reduced dose in carriers is comparable to that in wild-type patients.
For patients with planned fluoropyrimidine treatment, DPYD genotyping is likely cost-effective compared to usual care (no testing). Publicly funding DPYD genotyping in Ontario may be cost-saving, with an estimated saving of $714,963 over the next 5 years, provided that the costs of implementation, service delivery, and program coordination do not exceed this amount.
Participants we interviewed who had been treated with fluoropyrimidines described the substantial impact of cancer and the treatment side effects on their quality of life and mental health. Most reflected on the perceived value of DPYD testing to prevent serious adverse events as a result of fluoropyrimidine treatment. Barriers expressed by patients to accessing the test included lack of awareness and limited access to DPYD testing.
Acknowledgments
This report was developed by a multidisciplinary team from Ontario Health. The clinical epidemiologist was Vania Costa, the medical librarian was Corinne Holubowich, the health economists were Chunmei Li and Lindsey Falk, the health economics associate was Selena Hussain, the secondary health economist was Shawn Xie, and the patient and public partnership analyst was Jigna Mistry.
The medical editors were Tim Maguire and Jeanne McKane. Others involved in the development and production of this report were Jamie Turnbull, Claude Soulodre, Susan Harrison, Saleemeh Abdolzahraei, Elisabeth Smitko, Sarah McDowell, Vivian Ng, David Wells, Andrée Mitchell, Charles de Mestral, and Nancy Sikich.
We would like to thank the following people for lending their expertise to the development of this report:
Richard Kim, London Health Sciences Centre
John Bartlett, Ontario Institute for Cancer Research
Lei Fu, Sunnybrook Health Sciences Centre
Shinya Ito, Hospital for Sick Children, Toronto
John Lenehan, London Health Sciences Centre
Geoffrey Liu, Princess Margaret Cancer Centre
Jason Yu, Royal Victoria Regional Health Centre
Leta Forbes, Ontario Health Clinical Institutes and Quality Programs
Aaron Pollett, Ontario Health Clinical Institutes and Quality Programs
Brandon Meyers, Juravinski Cancer Centre
Harriet Feilotter, Kingston Health Sciences Centre
Michael Raphael, Sunnybrook Health Sciences Centre
Tracy Stockley, University Health Network
Wendy Ungar, SickKids Research Institute
We also thank our lived experience participants who generously gave their time to share their stories with us for this report.
The statements, conclusions, and views expressed in this report do not necessarily represent the views of those we consulted.
Abbreviations
- CI
Confidence interval
- DPD
Dihydropyrimidine dehydrogenase
- EMA
European Medicines Agency
- GRADE
Grading of Recommendations Assessment, Development, and Evaluation
- ICER
Incremental cost-effectiveness ratio
- NCI-CTCAE
National Cancer Institute Common Terminology Criteria for Adverse Events
- NICE
The National Institute for Health and Care Excellence
- PCR
Polymerase chain reaction
- QALY
Quality-adjusted life-year
- RR
Relative risk
- SD
Standard deviation
Glossary
- Adverse event
An adverse event is an unexpected medical problem that happens during treatment for a health condition. Adverse events may be caused by something other than the treatment.
- Allele
A gene is the basic unit of heredity (humans have approximately 20,000 genes). An allele is one of two or more versions of a gene. An individual inherits two alleles for each gene—one from each parent.
- Budget impact analysis
A budget impact analysis estimates the financial impact of adopting a new health care intervention on the current budget (i.e., the affordability of the new intervention). It is based on predictions of how changes in the intervention mix will impact the level of health care spending for a specific population. Budget impact analyses are typically conducted for a short-term period (e.g., 5 years). The budget impact, sometimes referred to as the net budget impact, is the estimated cost difference between the current scenario (i.e., the anticipated amount of spending for a specific population without using the new intervention) and the new scenario (i.e., the anticipated amount of spending for a specific population following the introduction of the new intervention).
- Chromosome
A chromosome is a highly organized molecule of DNA in the cells of living organisms. Chromosomes are made up of genes (see alleles, above) that contain the codes for creating molecules that are required by an organism to function and survive. Cells of the body that contain two sets of chromosomes are called diploid.
- Cost–benefit analysis
A cost–benefit analysis is a type of economic evaluation that expresses the effects of a health care intervention in terms of a monetary value so that these effects can be compared with costs. Results can be reported either as a ratio of costs to benefits or as a simple sum that represents the net benefit (or net loss) of one intervention over another. The monetary valuation of the different intervention effects is based on either prices that are revealed by markets or an individual or societal willingness-to-pay value.
- Cost–consequence analysis
A cost–consequence analysis is a type of economic evaluation that estimates the costs and consequences (i.e., the health outcomes) of two or more health care interventions. In this type of analysis, the costs are presented separately from the consequences.
- Cost-effective
A health care intervention is considered cost-effective when it provides additional benefits, compared with relevant alternatives, at an additional cost that is acceptable to a decision-maker based on the maximum willingness-to-pay value.
- Cost-effectiveness acceptability curve
In economic evaluations, a cost-effectiveness acceptability curve is a graphical representation of the results of a probabilistic analysis. It illustrates the probability of health care interventions being cost-effective over a range of willingness-to-pay values. Willingness-to-pay values are plotted on the horizontal axis of the graph, and the probability of the intervention of interest and its comparator(s) being cost-effective at corresponding willingness-to-pay values is plotted on the vertical axis.
- Cost-effectiveness analysis
Used broadly, “cost-effectiveness analysis” may refer to an economic evaluation used to compare the benefits of two or more health care interventions with their costs. It may encompass several types of analysis (e.g., cost-effectiveness analysis, cost–utility analysis). Used more specifically, “cost-effectiveness analysis” may refer to a type of economic evaluation in which the main outcome measure is the incremental cost per natural unit of health (e.g., life-year, symptom-free day) gained.
- Cost-minimization analysis
In economic evaluations, a cost-minimization analysis compares the costs of two or more health care interventions. It is used when the intervention of interest and its relevant alternative(s) are determined to be equally effective.
- Cost–utility analysis
A cost–utility analysis is a type of economic evaluation used to compare the benefits of two or more health care interventions with their costs. The benefits are measured using quality-adjusted life-years, which capture both the quality and quantity of life. In a cost–utility analysis, the main outcome measure is the incremental cost per quality-adjusted life-year gained.
- Decision tree
A decision tree is a type of economic model used to assess the costs and benefits of two or more alternative health care interventions. Each intervention may be associated with different outcomes, which are represented by distinct branches in the tree. Each outcome may have a different probability of occurring and may lead to different costs and benefits.
- Discounting
Discounting is a method used in economic evaluations to adjust for the differential timing of the costs incurred and the benefits generated by a health care intervention over time. Discounting reflects the concept of positive time preference, whereby future costs and benefits are reduced to reflect their present value. The health technology assessments conducted by Ontario Health use an annual discount rate of 1.5% for both future costs and future benefits.
- Disutility
A disutility is a decrease in utility (i.e., a decrease in preference for a particular health outcome) typically resulting from a particular health condition (e.g., experiencing a symptom or complication).
- Dominant
A health care intervention is considered dominant when it is more effective and less costly than its comparator(s).
- DPD deficiency
DPD deficiency is a condition in which the activity of the DPD enzyme in the body is reduced or absent. One expression of this condition is in the reduced ability to process the fluoropyrimidine class of chemotherapy drugs, sometimes leading to toxic reactions requiring adjustment of chemotherapy treatment and potentially hospitalization and death.
- EQ-5D
The EQ-5D is a generic health-related quality-of-life classification system widely used in clinical studies. In economic evaluations, it is used as an indirect method of obtaining health state preferences (i.e., utility values). The EQ-5D questionnaire consists of five questions relating to different domains of quality of life: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. For each domain, there are three response options: no problems, some problems, or severe problems. A newer instrument, the EQ-5D-5L, includes five response options for each domain. A scoring table is used to convert EQ-5D scores to utility values.
- Exon
An exon is the portion of a gene that codes for amino acids, which are compounds that combine to form proteins.
- Genotyping
Testing to determine whether a genetic variant (genotype) that underlies an observable characteristic (phenotype) of a person is present.
- Health-related quality of life
Health-related quality of life is a measure of the impact of a health care intervention on a person's health. It includes the dimensions of physiology, function, social life, cognition, emotions, sleep and rest, energy and vitality, health perception, and general life satisfaction.
- Health state
A health state is a particular status of health (e.g., sick, well, dead). A health state is associated with some amount of benefit and may be associated with specific costs. Benefit is captured through individual or societal preferences for the time spent in each health state and is expressed in quality-adjusted weights called utility values. In a Markov model, a finite number of mutually exclusive health states are used to represent discrete states of health.
- Heterozygous
Having two different alleles for a particular gene.
- Homozygous
Having the same pair of alleles for a particular gene.
- Incremental cost
The incremental cost is the additional cost, typically per person, of a health care intervention versus a comparator.
- Incremental cost-effectiveness ratio (ICER)
The incremental cost-effectiveness ratio (ICER) is a summary measure that indicates, for a given health care intervention, how much more a health care consumer must pay to get an additional unit of benefit relative to an alternative intervention. It is obtained by dividing the incremental cost by the incremental effectiveness. Incremental cost-effectiveness ratios are typically presented as the cost per life-year gained or the cost per quality-adjusted life-year gained.
- Ministry of Health perspective
The perspective adopted in economic evaluations determines the types of costs and health benefits to include. Ontario Health develops health technology assessment reports from the perspective of the Ontario Ministry of Health. This perspective includes all costs and health benefits attributable to the Ministry of Health, such as treatment costs (e.g., drugs, administration, monitoring, hospital stays) and costs associated with managing adverse events caused by treatments. This perspective does not include out-of-pocket costs incurred by patients related to obtaining care (e.g., transportation) or loss of productivity (e.g., absenteeism).
- Negative predictive value (NPV)
A test performance characteristic, defined as the proportion of persons who do not have the disease among those who have a negative diagnostic test result; it is calculated as follows: true negatives ÷ (true negatives + false negatives).
- One-way sensitivity analysis
A one-way sensitivity analysis is used to explore uncertainty in the results of an economic evaluation. It is done by varying one model input (i.e., a parameter) at a time between its minimum and maximum values to observe the potential impact on the cost-effectiveness of the health care intervention of interest.
- Phenotype
The observable characteristics (e.g., appearance, development, behaviour) resulting from a person's genetic profile (their genotype).
- Positive predictive value (PPV)
A test performance characteristic, defined as the proportion of persons with a positive result in a diagnostic test who have the disease; it is calculated as follows: true positives ÷ (true positives + false positives).
- Probabilistic analysis
A probabilistic analysis (also known as a probabilistic sensitivity analysis) is used in economic models to explore uncertainty in several parameters simultaneously and is done using Monte Carlo simulation. Model inputs are defined as a distribution of possible values. In each iteration, model inputs are obtained by randomly sampling from each distribution, and a single estimate of cost and effectiveness is generated. This process is repeated many times (e.g., 10,000 times) to estimate the number of times (i.e., the probability) that the health care intervention of interest is cost-effective.
- Quality-adjusted life-year (QALY)
The quality-adjusted life-year (QALY) is a generic health outcome measure commonly used in cost–utility analyses to reflect the quantity and quality of life-years lived. The life-years lived are adjusted for quality of life using individual or societal preferences (i.e., utility values) for being in a particular health state. One year of perfect health is represented by one quality-adjusted life-year.
- Reference case
The reference case is a preferred set of methods and principles that provide the guidelines for economic evaluations. Its purpose is to standardize the approach of conducting and reporting economic evaluations, so that results can be compared across studies.
- Scenario analysis
A scenario analysis is used to explore uncertainty in the results of an economic evaluation. It is done by observing the potential impact of different scenarios on the cost-effectiveness of a health care intervention. Scenario analyses include varying structural assumptions from the reference case.
- Sensitivity analysis
Every economic evaluation contains some degree of uncertainty, and results can vary depending on the values taken by key parameters and the assumptions made. Sensitivity analysis allows these factors to be varied and shows the impact of these variations on the results of the evaluation. There are various types of sensitivity analysis, including deterministic, probabilistic, and scenario.
- Standard gamble
In economic evaluations, standard gamble is a direct method of measuring people's preferences for various health states. In a standard gamble, respondents are asked about their preference for either (a) remaining in a certain health state for the rest of their life, or (b) a gamble scenario in which there is a chance of having optimal health for the rest of one's life but also a chance of dying immediately. Respondents are surveyed repeatedly, with the risk of immediate death varying each time (e.g., 75% chance of optimal health, 25% chance of immediate death) until they are indifferent about their choice. The standard gamble is considered the gold standard for eliciting preferences as it incorporates individual risk attitudes, unlike other methods of eliciting preferences.
- Time horizon
In economic evaluations, the time horizon is the time frame over which costs and benefits are examined and calculated. The relevant time horizon is chosen based on the nature of the disease and health care intervention being assessed, as well as the purpose of the analysis. For instance, a lifetime horizon would be chosen to capture the long-term health and cost consequences over a patient's lifetime.
- Uptake rate
In instances where two technologies are being compared, the uptake rate is the rate at which a new technology is adopted. When a new technology is adopted, it may be used in addition to an existing technology, or it may replace an existing technology.
- Utility
A utility is a value that represents a person's preference for various health states. Typically, utility values are anchored at 0 (death) and 1 (perfect health). In some scoring systems, a negative utility value indicates a state of health valued as being worse than death. Utility values can be aggregated over time to derive quality-adjusted life-years, a common outcome measure in economic evaluations.
- Willingness-to-pay value
A willingness-to-pay value is the monetary value a health care consumer is willing to pay for added health benefits. When conducting a cost–utility analysis, the willingness-to-pay value represents the cost a consumer is willing to pay for an additional quality-adjusted life-year. If the incremental cost-effectiveness ratio is less than the willingness-to-pay value, the health care intervention of interest is considered cost-effective. If the incremental cost-effectiveness ratio is more than the willingness-to-pay value, the intervention is considered not to be cost-effective.
Appendices
Appendix 1: Literature Search Strategies
Clinical Evidence Search
Clinical Literature Search for Systematic Reviews
Search date: February 20, 2020
Databases searched: Ovid MEDLINE, Embase, Cochrane Database of Systematic Reviews, CRD Health Technology Assessment Database, and NHS Economic Evaluation Database
Database segments: EBM Reviews – Cochrane Database of Systematic Reviews <2005 to February 11, 2020>, EBM Reviews – Health Technology Assessment <4th Quarter 2016>, EBM Reviews – NHS Economic Evaluation Database <1st Quarter 2016>, Embase <1980 to 2020 Week 07>, Ovid MEDLINE(R) ALL <1946 to February 19, 2020>
Search Strategy:
-
1
Fluorouracil/ (171324)
-
2
(5 FU∗ or 5FU∗ or fluorouracil∗ or fluoro uracil∗ or fluoruracil∗ or fluouracil∗ or fluoroplex∗ or fluracedyl∗ or flurodex∗ or floxuridin∗ or fluoroblastin∗ or fluoxan∗ or flurablastin∗ or fluracil∗ or fluracilium∗ or fluril∗ or fluro uracil∗ or fluroblastin∗ or adrucil∗ or carac∗ or efudex∗ or efudix∗ or haemato FU∗ or neofluor∗ or onkofluor∗ or ribofluor∗).ti,ab,kf. (117421)
-
3
Capecitabine/ (32280)
-
4
(capecitabin∗ or fluorocytidin∗ or xeloda∗ or apecitab∗ or ecansya∗).ti,ab,kf. (18633)
-
5
(Fluoropyrimidin∗ or (fluoro adj3 pyrimidinedion∗)).ti,ab,kf. (8608)
-
6
Tegafur/ (11704)
-
7
(tegafur∗ or florafur∗ or fluorofur∗ or futraful∗ or ftorafur∗ or sunfural∗ or uftoral∗ or utefos∗).ti,ab,kf. (5175)
-
8
or/1-7 (234811)
-
9
Dihydropyrimidine Dehydrogenase Deficiency/ (471)
-
10
“Dihydrouracil Dehydrogenase (NADP)”/ (3232)
-
11
((dihydropyrimidin∗ adj2 dehydrogenas∗) or DPD∗ or ?DPYD∗).ti,ab,kf. (13245)
-
12
(Dihydropyrimidinas∗ or DHP or DHPDHASE∗).ti,ab,kf. (6254)
-
13
((dihydrothymin∗ or dihydrouracil) adj2 dehydrogenas∗).ti,ab,kf. (100)
-
14
((Pyrimidin∗ adj2 familial) or thymine uraciluri∗).ti,ab,kf. (48)
-
15
(c?1679?T∗ or c?2846?A∗ or c?1129?∗ or c?1236?G∗ or c?1905∗ or I560S∗ or D949V∗ or E412E∗).ti,ab,kf. (296)
-
16
((dihydrouracil or DHU) adj3 (uracil or U) adj3 ratio∗).ti,ab,kf. (94)
-
17
or/9-16 (20288)
-
18
8 and 17 (4127)
-
19
exp Animals/ not Humans/ (16500883)
-
20
18 not 19 (3014)
-
21
limit 20 to english language [Limit not valid in CDSR; records were retained] (2628)
-
22
21 use coch,clhta,cleed (1)
-
23
(Systematic Reviews or Meta Analysis).pt. (110870)
-
24
Systematic Review/ or Systematic Reviews as Topic/ or Meta-Analysis/ or exp Meta-Analysis as Topic/ or exp Technology Assessment, Biomedical/ (597167)
-
25
((systematic∗ or methodologic∗) adj3 (review∗ or overview∗)).ti,ab,kf. (406428)
-
26
(meta analy∗ or metaanaly∗ or met analy∗ or metanaly∗ or meta review∗ or metareview∗ or health technolog∗ assess∗ or HTA or HTAs or (technolog∗ adj (assessment∗ or overview∗ or appraisal∗))).ti,ab,kf. (408046)
-
27
(evidence adj (review∗ or overview∗ or synthes#s)).ti,ab,kf. (15235)
-
28
(review of reviews or overview of reviews).ti,ab,kf. (1428)
-
29
umbrella review∗.ti,ab,kf. (725)
-
30
GRADE Approach/ (357)
-
31
((pool∗ adj3 analy∗) or published studies or published literature or hand search∗ or handsearch∗ or manual search∗ or ((database∗ or systematic∗) adj2 search∗) or reference list∗ or bibliograph∗ or relevant journals or data synthes∗ or data extraction∗ or data abstraction∗).ti,ab,kf. (432364)
-
32
(medline or pubmed or medlars or embase or cinahl or web of science or ovid or ebsco∗ or scopus).ab. (454968)
-
33
cochrane.ti,ab,kf. (192787)
-
34
(meta regress∗ or metaregress∗).ti,ab,kf. (18895)
-
35
(((integrative or collaborative or quantitative) adj3 (review∗ or overview∗ or synthes∗)) or (research adj3 overview∗)).ti,ab,kf. (25228)
-
36
(cochrane or (health adj2 technology assessment) or evidence report or systematic review∗).jw. (64004)
-
37
((comparative adj3 (efficacy or effectiveness)) or relative effectiveness or ((indirect or indirect treatment or mixed-treatment) adj comparison∗)).ti,ab,kf. (43199)
-
38
or/23-37 (1195660)
-
39
21 and 38 (76)
-
40
39 use medall (31)
-
41
or/22,40 (32)
-
42
fluorouracil/ (171324)
-
43
(5 FU∗ or 5FU∗ or fluorouracil∗ or fluoro uracil∗ or fluoruracil∗ or fluouracil∗ or fluoroplex∗ or fluracedyl∗ or flurodex∗ or floxuridin∗ or fluoroblastin∗ or fluoxan∗ or flurablastin∗ or fluracil∗ or fluracilium∗ or fluril∗ or fluro uracil∗ or fluroblastin∗ or adrucil∗ or carac∗ or efudex∗ or efudix∗ or haemato FU∗ or neofluor∗ or onkofluor∗ or ribofluor∗).tw,kw. (119326)
-
44
capecitabine/ (32280)
-
45
(capecitabin∗ or fluorocytidin∗ or xeloda∗ or apecitab∗ or ecansya∗).tw,kw. (20145)
-
46
fluoropyrimidine/ (3900)
-
47
fluoropyrimidine derivative/ (2009)
-
48
(Fluoropyrimidin∗ or (fluoro adj3 pyrimidinedion∗)).tw,kw. (8769)
-
49
tegafur/ (11704)
-
50
(tegafur∗ or florafur∗ or fluorofur∗ or futraful∗ or ftorafur∗ or sunfural∗ or uftoral∗ or utefos∗).tw,kw. (5366)
-
51
or/42-50 (236382)
-
52
dihydropyrimidine dehydrogenase deficiency/ (471)
-
53
dihydropyrimidine dehydrogenase/ (3478)
-
54
((dihydropyrimidin∗ adj2 dehydrogenas∗) or DPD∗ or ?DPYD∗).tw,kw,dv. (13362)
-
55
(Dihydropyrimidinas∗ or DHP or DHPDHASE∗).tw,kw,dv. (6337)
-
56
((dihydrothymin∗ or dihydrouracil) adj2 dehydrogenas∗).tw,kw,dv. (110)
-
57
((Pyrimidin∗ adj2 familial) or thymine uraciluri∗).tw,kw,dv. (49)
-
58
(c?1679?T∗ or c?2846?A∗ or c?1129?∗ or c?1236?G∗ or c?1905∗ or I560S∗ or D949V∗ or E412E∗).tw,kw,dv. (296)
-
59
((dihydrouracil or DHU) adj3 (uracil or U) adj3 ratio∗).tw,kw,dv. (95)
-
60
or/52-59 (20531)
-
61
51 and 60 (4219)
-
62
(exp animal/ or nonhuman/) not exp human/ (10570073)
-
63
61 not 62 (4011)
-
64
limit 63 to english language [Limit not valid in CDSR; records were retained] (3557)
-
65
Systematic review/ or “systematic review (topic)”/ or exp Meta Analysis/ or “Meta Analysis (Topic)”/ or Biomedical Technology Assessment/ (578482)
-
66
(meta analy∗ or metaanaly∗ or health technolog∗ assess∗ or systematic review∗).hw. (573556)
-
67
((systematic∗ or methodologic∗) adj3 (review∗ or overview∗)).tw,kw. (418056)
-
68
(meta analy∗ or metaanaly∗ or met analy∗ or metanaly∗ or meta review∗ or metareview∗ or health technolog∗ assess∗ or HTA or HTAs or (technolog∗ adj (assessment∗ or overview∗ or appraisal∗))).tw,kw. (435063)
-
69
(evidence adj (review∗ or overview∗ or synthes#s)).tw,kw. (15631)
-
70
(review of reviews or overview of reviews).tw,kw. (1628)
-
71
umbrella review∗.tw,kw. (765)
-
72
((pool∗ adj3 analy∗) or published studies or published literature or hand search∗ or handsearch∗ or manual search∗ or ((database∗ or systematic∗) adj2 search∗) or reference list∗ or bibliograph∗ or relevant journals or data synthes∗ or data extraction∗ or data abstraction∗).tw,kw. (457621)
-
73
(medline or pubmed or medlars or embase or cinahl or web of science or ovid or ebsco∗ or scopus).ab. (454968)
-
74
cochrane.tw,kw. (196339)
-
75
(meta regress∗ or metaregress∗).tw,kw. (19823)
-
76
(((integrative or collaborative or quantitative) adj3 (review∗ or overview∗ or synthes∗)) or (research adj3 overview∗)).tw,kw. (26126)
-
77
(cochrane or (health adj2 technology assessment) or evidence report or systematic review∗).jw. (64004)
-
78
((comparative adj3 (efficacy or effectiveness)) or relative effectiveness or ((indirect or indirect treatment or mixed-treatment) adj comparison∗)).tw,kw. (44866)
-
79
or/65-78 (1215984)
-
80
64 and 79 (128)
-
81
80 use emez (94)
-
82
41 or 81 (126)
-
83
82 use medall (31)
-
84
82 use coch (0)
-
85
82 use clhta (1)
-
86
82 use cleed (0)
-
87
82 use emez (94)
-
88
remove duplicates from 82 (97)
Clinical Literature Search Update of Primary Studies from 2018–Present
Search date: February 27, 2020
Databases searched: Ovid MEDLINE, Embase, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, CRD Health Technology Assessment Database, and NHS Economic Evaluation Database
Database segments: EBM Reviews – Cochrane Central Register of Controlled Trials <January 2020>, EBM Reviews – Cochrane Database of Systematic Reviews <2005 to February 21, 2020>, EBM Reviews – Health Technology Assessment <4th Quarter 2016>, EBM Reviews – NHS Economic Evaluation Database <1st Quarter 2016>, Embase <1980 to 2020 Week 08>, Ovid MEDLINE(R) ALL <1946 to February 26, 2020>
Search Strategy:
-
1
Fluorouracil/ (176301)
-
2
(5 FU∗ or 5FU∗ or fluorouracil∗ or fluoro uracil∗ or fluoruracil∗ or fluouracil∗ or fluoroplex∗ or fluracedyl∗ or flurodex∗ or floxuridin∗ or fluoroblastin∗ or fluoxan∗ or flurablastin∗ or fluracil∗ or fluracilium∗ or fluril∗ or fluro uracil∗ or fluroblastin∗ or adrucil∗ or carac∗ or efudex∗ or efudix∗ or haemato FU∗ or neofluor∗ or onkofluor∗ or ribofluor∗).ti,ab,kf. (128574)
-
3
Capecitabine/ (33465)
-
4
(capecitabin∗ or fluorocytidin∗ or xeloda∗ or apecitab∗ or ecansya∗).ti,ab,kf. (22073)
-
5
(Fluoropyrimidin∗ or (fluoro adj3 pyrimidinedion∗)).ti,ab,kf. (9745)
-
6
Tegafur/ (12284)
-
7
(tegafur∗ or florafur∗ or fluorofur∗ or futraful∗ or ftorafur∗ or sunfural∗ or uftoral∗ or utefos∗).ti,ab,kf. (5825)
-
8
or/1-7 (250907)
-
9
Dihydropyrimidine Dehydrogenase Deficiency/ (473)
-
10
“Dihydrouracil Dehydrogenase (NADP)”/ (3267)
-
11
((dihydropyrimidin∗ adj2 dehydrogenas∗) or DPD∗ or ?DPYD∗).ti,ab,kf. (13677)
-
12
(Dihydropyrimidinas∗ or DHP or DHPDHASE∗).ti,ab,kf. (6333)
-
13
((dihydrothymin∗ or dihydrouracil) adj2 dehydrogenas∗).ti,ab,kf. (101)
-
14
((Pyrimidin∗ adj2 familial) or thymine uraciluri∗).ti,ab,kf. (48)
-
15
(c?1679?T∗ or c?2846?A∗ or c?1129?∗ or c?1236?G∗ or c?1905∗ or I560S∗ or D949V∗ or E412E∗).ti,ab,kf. (308)
-
16
((dihydrouracil or DHU) adj3 (uracil or U) adj3 ratio∗).ti,ab,kf. (98)
-
17
or/9-16 (20802)
-
18
8 and 17 (4269)
-
19
exp Animals/ not Humans/ (16503427)
-
20
18 not 19 (3156)
-
21
limit 20 to english language [Limit not valid in CDSR; records were retained] (2734)
-
22
limit 21 to yr=“2018 −Current” (243)
-
23
22 use medall,coch,cctr,clhta,cleed (126)
-
24
fluorouracil/ (176301)
-
25
(5 FU∗ or 5FU∗ or fluorouracil∗ or fluoro uracil∗ or fluoruracil∗ or fluouracil∗ or fluoroplex∗ or fluracedyl∗ or flurodex∗ or floxuridin∗ or fluoroblastin∗ or fluoxan∗ or flurablastin∗ or fluracil∗ or fluracilium∗ or fluril∗ or fluro uracil∗ or fluroblastin∗ or adrucil∗ or carac∗ or efudex∗ or efudix∗ or haemato FU∗ or neofluor∗ or onkofluor∗ or ribofluor∗).tw,kw. (130532)
-
26
capecitabine/ (33465)
-
27
(capecitabin∗ or fluorocytidin∗ or xeloda∗ or apecitab∗ or ecansya∗).tw,kw. (23603)
-
28
fluoropyrimidine/ (3905)
-
29
fluoropyrimidine derivative/ (2013)
-
30
(Fluoropyrimidin∗ or (fluoro adj3 pyrimidinedion∗)).tw,kw. (9910)
-
31
tegafur/ (12284)
-
32
(tegafur∗ or florafur∗ or fluorofur∗ or futraful∗ or ftorafur∗ or sunfural∗ or uftoral∗ or utefos∗).tw,kw. (6027)
-
33
or/24-32 (252546)
-
34
dihydropyrimidine dehydrogenase deficiency/ (473)
-
35
dihydropyrimidine dehydrogenase/ (3513)
-
36
((dihydropyrimidin∗ adj2 dehydrogenas∗) or DPD∗ or ?DPYD∗).tw,kw,dv. (13796)
-
37
(Dihydropyrimidinas∗ or DHP or DHPDHASE∗).tw,kw,dv. (6416)
-
38
((dihydrothymin∗ or dihydrouracil) adj2 dehydrogenas∗).tw,kw,dv. (111)
-
39
((Pyrimidin∗ adj2 familial) or thymine uraciluri∗).tw,kw,dv. (49)
-
40
(c?1679?T∗ or c?2846?A∗ or c?1129?∗ or c?1236?G∗ or c?1905∗ or I560S∗ or D949V∗ or E412E∗).tw,kw,dv. (308)
-
41
((dihydrouracil or DHU) adj3 (uracil or U) adj3 ratio∗).tw,kw,dv. (99)
-
42
or/34-41 (21047)
-
43
33 and 42 (4363)
-
44
(exp animal/ or nonhuman/) not exp human/ (10577094)
-
45
43 not 44 (4155)
-
46
limit 45 to english language [Limit not valid in CDSR; records were retained] (3665)
-
47
limit 46 to yr=“2018 −Current” (356)
-
48
47 use emez (229)
-
49
23 or 48 (355)
-
50
49 use medall (113)
-
51
49 use emez (229)
-
52
49 use coch (0)
-
53
49 use cctr (13)
-
54
49 use clhta (0)
-
55
49 use cleed (0)
-
56
remove duplicates from 49 (248)
Economic Evidence Search
Economic Evaluation and Cost Effectiveness Search
Search date: February 20, 2020
Databases searched: Ovid MEDLINE, Embase, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, Centre for Reviews and Dissemination (CRD) Health Technology Assessment Database, and National Health Service (NHS) Economic Evaluation Database
Database segments: EBM Reviews – Cochrane Central Register of Controlled Trials <January 2020>, EBM Reviews – Cochrane Database of Systematic Reviews <2005 to February 11, 2020>, EBM Reviews – Health Technology Assessment <4th Quarter 2016>, EBM Reviews – NHS Economic Evaluation Database <1st Quarter 2016>, Embase <1980 to 2020 Week 07>, Ovid MEDLINE(R) ALL <1946 to February 19, 2020>
Search Strategy:
-
1
Fluorouracil/ (176163)
-
2
(5 FU∗ or 5FU∗ or fluorouracil∗ or fluoro uracil∗ or fluoruracil∗ or fluouracil∗ or fluoroplex∗ or fluracedyl∗ or flurodex∗ or floxuridin∗ or fluoroblastin∗ or fluoxan∗ or flurablastin∗ or fluracil∗ or fluracilium∗ or fluril∗ or fluro uracil∗ or fluroblastin∗ or adrucil∗ or carac∗ or efudex∗ or efudix∗ or haemato FU∗ or neofluor∗ or onkofluor∗ or ribofluor∗).ti,ab,kf. (128498)
-
3
Capecitabine/ (33412)
-
4
(capecitabin∗ or fluorocytidin∗ or xeloda∗ or apecitab∗ or ecansya∗).ti,ab,kf. (22042)
-
5
(Fluoropyrimidin∗ or (fluoro adj3 pyrimidinedion∗)).ti,ab,kf. (9736)
-
6
Tegafur/ (12278)
-
7
(tegafur∗ or florafur∗ or fluorofur∗ or futraful∗ or ftorafur∗ or sunfural∗ or uftoral∗ or utefos∗).ti,ab,kf. (5823)
-
8
or/1-7 (250717)
-
9
Dihydropyrimidine Dehydrogenase Deficiency/ (472)
-
10
“Dihydrouracil Dehydrogenase (NADP)”/ (3262)
-
11
((dihydropyrimidin∗ adj2 dehydrogenas∗) or DPD∗ or ?DPYD∗).ti,ab,kf. (13670)
-
12
(Dihydropyrimidinas∗ or DHP or DHPDHASE∗).ti,ab,kf. (6332)
-
13
((dihydrothymin∗ or dihydrouracil) adj2 dehydrogenas∗).ti,ab,kf. (101)
-
14
((Pyrimidin∗ adj2 familial) or thymine uraciluri∗).ti,ab,kf. (48)
-
15
(c?1679?T∗ or c?2846?A∗ or c?1129?∗ or c?1236?G∗ or c?1905∗ or I560S∗ or D949V∗ or E412E∗).ti,ab,kf. (308)
-
16
((dihydrouracil or DHU) adj3 (uracil or U) adj3 ratio∗).ti,ab,kf. (97)
-
17
or/9-16 (20794)
-
18
8 and 17 (4266)
-
19
exp Animals/ not Humans/ (16500893)
-
20
18 not 19 (3153)
-
21
limit 20 to english language [Limit not valid in CDSR; records were retained] (2731)
-
22
21 use coch,clhta,cleed (1)
-
23
economics/ (255908)
-
24
economics, medical/ or economics, pharmaceutical/ or exp economics, hospital/ or economics, nursing/ or economics, dental/ (852651)
-
25
economics.fs. (430085)
-
26
(econom∗ or price or prices or pricing or priced or discount∗ or expenditure∗ or budget∗ or pharmacoeconomic∗ or pharmaco-economic∗).ti,ab,kf. (929299)
-
27
exp “costs and cost analysis”/ (592564)
-
28
(cost or costs or costing or costly).ti. (273338)
-
29
cost effective∗.ti,ab,kf. (342723)
-
30
(cost∗ adj2 (util∗ or efficacy∗ or benefit∗ or minimi∗ or analy∗ or saving∗ or estimate∗ or allocation or control or sharing or instrument∗ or technolog∗)).ab,kf. (225084)
-
31
models, economic/ (13318)
-
32
markov chains/ or monte carlo method/ (84845)
-
33
(decision adj1 (tree∗ or analy∗ or model∗)).ti,ab,kf. (44747)
-
34
(markov or markow or monte carlo).ti,ab,kf. (136012)
-
35
quality-adjusted life years/ (41946)
-
36
(QOLY or QOLYs or HRQOL or HRQOLs or QALY or QALYs or QALE or QALEs).ti,ab,kf. (78715)
-
37
((adjusted adj1 (quality or life)) or (willing∗ adj2 pay) or sensitivity analys∗s).ti,ab,kf. (128516)
-
38
or/23-37 (2641736)
-
39
21 and 38 (104)
-
40
39 use medall,cctr (33)
-
41
or/22,40 (34)
-
42
fluorouracil/ (176163)
-
43
(5 FU∗ or 5FU∗ or fluorouracil∗ or fluoro uracil∗ or fluoruracil∗ or fluouracil∗ or fluoroplex∗ or fluracedyl∗ or flurodex∗ or floxuridin∗ or fluoroblastin∗ or fluoxan∗ or flurablastin∗ or fluracil∗ or fluracilium∗ or fluril∗ or fluro uracil∗ or fluroblastin∗ or adrucil∗ or carac∗ or efudex∗ or efudix∗ or haemato FU∗ or neofluor∗ or onkofluor∗ or ribofluor∗).tw,kw. (130452)
-
44
capecitabine/ (33412)
-
45
(capecitabin∗ or fluorocytidin∗ or xeloda∗ or apecitab∗ or ecansya∗).tw,kw. (23571)
-
46
fluoropyrimidine/ (3900)
-
47
fluoropyrimidine derivative/ (2009)
-
48
(Fluoropyrimidin∗ or (fluoro adj3 pyrimidinedion∗)).tw,kw. (9901)
-
49
tegafur/ (12278)
-
50
(tegafur∗ or florafur∗ or fluorofur∗ or futraful∗ or ftorafur∗ or sunfural∗ or uftoral∗ or utefos∗).tw,kw. (6025)
-
51
or/42-50 (252354)
-
52
dihydropyrimidine dehydrogenase deficiency/ (472)
-
53
dihydropyrimidine dehydrogenase/ (3508)
-
54
((dihydropyrimidin∗ adj2 dehydrogenas∗) or DPD∗ or ?DPYD∗).tw,kw,dv. (13789)
-
55
(Dihydropyrimidinas∗ or DHP or DHPDHASE∗).tw,kw,dv. (6415)
-
56
((dihydrothymin∗ or dihydrouracil) adj2 dehydrogenas∗).tw,kw,dv. (111)
-
57
((Pyrimidin∗ adj2 familial) or thymine uraciluri∗).tw,kw,dv. (49)
-
58
(c?1679?T∗ or c?2846?A∗ or c?1129?∗ or c?1236?G∗ or c?1905∗ or I560S∗ or D949V∗ or E412E∗).tw,kw,dv. (308)
-
59
((dihydrouracil or DHU) adj3 (uracil or U) adj3 ratio∗).tw,kw,dv. (98)
-
60
or/52-59 (21039)
-
61
51 and 60 (4360)
-
62
(exp animal/ or nonhuman/) not exp human/ (10570089)
-
63
61 not 62 (4152)
-
64
limit 63 to english language [Limit not valid in CDSR; records were retained] (3662)
-
65
Economics/ (255908)
-
66
Health Economics/ or Pharmacoeconomics/ or Drug Cost/ or Drug Formulary/ (131026)
-
67
Economic Aspect/ or exp Economic Evaluation/ (464763)
-
68
(econom∗ or price or prices or pricing or priced or discount∗ or expenditure∗ or budget∗ or pharmacoeconomic∗ or pharmaco-economic∗).tw,kw. (955437)
-
69
exp “Cost”/ (592564)
-
70
(cost or costs or costing or costly).ti. (273338)
-
71
cost effective∗.tw,kw. (355233)
-
72
(cost∗ adj2 (util∗ or efficac∗ or benefit∗ or minimi∗ or analy∗ or saving∗ or estimate∗ or allocation or control or sharing or instrument∗ or technolog∗)).ab,kw. (236617)
-
73
Monte Carlo Method/ (67433)
-
74
(decision adj1 (tree∗ or analy∗ or model∗)).tw,kw. (48599)
-
75
(markov or markow or monte carlo).tw,kw. (141087)
-
76
Quality-Adjusted Life Years/ (41946)
-
77
(QOLY or QOLYs or HRQOL or HRQOLs or QALY or QALYs or QALE or QALEs).tw,kw. (82603)
-
78
((adjusted adj1 (quality or life)) or (willing∗ adj2 pay) or sensitivity analys∗s).tw,kw. (149458)
-
79
or/65-78 (2267662)
-
80
64 and 79 (157)
-
81
80 use emez (124)
-
82
41 or 81 (158)
-
83
82 use medall (32)
-
84
82 use emez (124)
-
85
82 use cctr (1)
-
86
82 use coch (0)
-
87
82 use clhta (1)
-
88
82 use cleed (0)
-
89
remove duplicates from 82 (125)
Grey Literature Search
Performed: February 20–25, 2020
Websites searched:
HTA Database Canadian Repository, Alberta Health Evidence Reviews, BC Health Technology Assessments, Canadian Agency for Drugs and Technologies in Health
(CADTH), Institut national d'excellence en santé et en services sociaux (INESSS), Institute of Health Economics (IHE), McGill University Health Centre Health Technology Assessment Unit, Centre Hospitalier de l'Universite de Quebec-Universite Laval, Health Technology Assessment Database, Epistemonikos, National Institute for Health and Care Excellence (NICE), Agency for Healthcare Research and Quality (AHRQ) Evidence-based Practice Centers, Australian Government Medical Services Advisory Committee, Council of Australian Governments Health Technologies, Centers for Medicare & Medicaid Services Technology Assessments, Institute for Clinical and Economic Review, Ireland Health Information and Quality Authority Health Technology Assessments, Washington State Health Care Authority Health Technology Reviews, Health Technology Wales, Oregon Health Authority Health Evidence Review Commission, Veterans Affairs Health Services Research and Development, Italian National Agency for Regional Health Services (AGENAS), Australian Safety and Efficacy Register of New Interventional Procedures −Surgical (ASERNIP-S), Belgian Health Care Knowledge Centre, Ludwig Boltzmann Institute for Health Technology Assessment, Ministry of Health Malaysia Health Technology Assessment Section, Swedish Agency for Health Technology Assessment and Assessment of Social Services, PROSPERO, EUnetHTA, Tuft's Cost-Effectiveness Analysis Registry, SickKids Paediatric Economic Database Evaluation (PEDE) database
Keywords used:
DPYD, DPD, Dihydropyrimidine Dehydrogenase, Fluorouracil, Fluoropyrimidine, 5FU, toxicity
Clinical results (included in PRISMA): 5
Economic results (included in PRISMA): 5
Ongoing HTAs (PROSPERO/EUnetHTA/MSAC): 4
Ongoing RCTs (clinicaltrials.gov): 91
Appendix 2: Characteristics of Included Studies
Table A1:
Characteristics of Included Studies: Clinical Validity
| Author, Year N Country | Study Design Methods Study Period | Variants Evaluated | Participants | Intervention Control | Dose Adjustments | Outcomes |
|---|---|---|---|---|---|---|
| Wigle et al, 202171 N = 1,388 Canada |
Patients identified retrospectively (c.1236G>A) Retrospective genotyping (c.1236G>A); pre-treatment genotyping (wild-type) 2013–2019 |
c.1236G>A | • Different cancer types • Fluoropyrimidine-based regimen, alone or in combination with other drugs or radiotherapy |
DPYD carriers Wild-type |
Standard fluoropyrimidine dose |
Grade ≥ 3 toxicity (NCI-CTCAE v5.0) Treatment discontinuation Treatment-related mortality |
| Iachetta et al, 201952 N = 366 Italy |
Retrospective patient selection from database Retrospective genotyping 2011–2016 |
c.2846A>T DPYD∗13 |
• Different cancer types • Fluoropyrimidine-based chemoradiation therapy • Not carrying DPYD∗2A • Controls were matched by primary tumour location, stage, and age |
DPYD carriers Wild-type | Information on dose reduction not provided | Toxicity (NCI-CTCAE, v4.0) |
| Maharjan et al, 201953 N = 113 United States |
Retrospective patient selection from database Prospective genotyping 2011–2018 |
DPYD∗2A c.2846A>T DPYD∗13 |
• Different cancer types • Fluoropyrimidine-based regimens • Patients genotyped for DPYD; decision to genotype was at the investigator's discretion and may have been associated with the risk of developing toxicity |
DPYD carriers Wild-type | DPYD∗2A and c.2846A>T received full-dose chemotherapy | Toxicity (NCI-CTCAE v5.0) |
| Cremolini et al, 201855 N = 443 Italy |
Based on patients included in RCT Prospective genotyping Period NR |
DPYD∗2A DPYD∗13 c.2846A>T |
• Metastatic colorectal cancer • 5-FU-based regimens |
DPYD carriers Wild-type |
Dose modified before start of therapy based on prespecified adverse events, not based on genotyping | Severe toxicity grade ≥ 3 |
| Lunenburg et al, 20187 N = 828 Netherlands |
Retrospective patient selection (3 databases) Prospective or retrospective genotyping depending on database 1993–2017 depending on database |
DPYD∗2A c.2846A>T DPYD∗13 c.1236G>A |
• Different cancer types • Fluoropyrimidine-based chemoradiation therapy |
DPYD carriers Wild-type |
Standard fluoropyrimidine dose | Grade ≥ 3 toxicity (NCI-CTCAE v3.0 and v4.03) Treatment modification Treatment-related hospitalization Length of stay |
| Nahid et al, 201854 N = 161 Bangladesh |
Prospective recruitment Unclear whether genotyping was prospective or retrospective Period NR |
DPYD∗2A | • Colorectal cancer • 5-FU-based regimens • WHO performance status < 3 |
DPYD carriers Wild-type |
Dose reductions allowed | Toxicity (NCI-CTCAE, v3.0) Treatment modification |
| Etienne-Grimaldi et al, 201756 N = 243 France |
Prospective recruitment Pre-treatment genotyping and phenotype test 2009–2011 |
DPYD∗2A c.2846A>T DPYD∗13 c.1236G>A |
• Women > 18 y old • Advanced breast cancer • Capecitabine alone or in combination with an antiangiogenic drug • Available DPYD genotyping results |
DPYD carriers Wild-type |
Dose at investigator's discretion | Grade ≥ 3 toxicity (NCI-CTCAE v3) Sensitivity, specificity, PPV, NPV |
| Meulendijks et al, 201718 N = 550 Netherlands |
Prospective recruitment Serum collected before start of treatment Period NR |
c.2846A>T DPYD∗13 c.1236G>A |
• Different cancer types • Fluoropyrimidine-based chemoradiation therapy • Excluded DPYD∗2A carriers and patients undergoing chemoradiotherapy |
DPYD carriers Wild-type |
NR | Sensitivity, specificity, PPV, NPV (grade ≥ 3; NCI-CTCAE v3.0) |
| Meulendijks et al, 201770 N = 185 Netherlands |
Based on patients included in 3 clinical studies Retrospective genotyping (sample collected for studies) 2003–2014 |
c.2846A>T c.1236G>A |
• Advanced gastric cancer • Capecitabine-based regimens • WHO performance status 0–2 |
DPYD carriers Wild-type |
Dosing according to study protocol | Grade ≥ 3 toxicity (NCI-CTCAE v 3.0 and 4.3) |
| Boige et al, 201615 N = 1,545 France |
Based on patients included in RCT Retrospective genotyping Period NR |
DPYD∗2A DPYD∗13 c.2846A>T |
• Resected stage III colorectal cancer • 5-FU-based regimens |
DPYD carriers Wild-type |
Some patients had dose adjustment, not based on DPYD genotyping | Grade ≥ 3 toxicity (NCI-CTCAE v3.0) |
| Lee et al, 201613 N = 1,953 United States |
Based on patients included in RCT Retrospective genotyping (sample collected for RCT) Period NR |
DPYD variants of hapB3 | • Caucasian patients • Stage III colon cancer • 5-FU-based regimens • With available DNA sample • Excluded: Carriers or unknown status for DPYD∗2A, c.2846A>T, and DPYD∗13 |
DPYD carriers Wild-type |
Dose adjustment according to study guidelines (age and presence of toxicity, KRAS carrier status, cetuximab) | Grade ≥ 3 toxicity (NCI-CTCAE v3) |
| Froehlich et al, 201557 N = 500 Switzerland |
Retrospective patient selection from database Unclear if genotyping was prospective or retrospective 2006–2013 |
DPYD∗2A DPYD∗13 c.2846A>T c.1236G>A |
• Different cancer types • 5-FU- or capecitabine-based regimens |
DPYD carriers Wild-type |
Dose reduction related to toxicity (grade ≥ 2) after 1st or 2nd cycles | Toxicity (NCI-CTCAE v3.0) Treatment modification |
| Toffoli et al, 20159 N = 603 Italy |
Retrospective patient selection from database Genotyping on previously collected sample 2006–2013 |
DPYD∗2A DPYD∗13 c.2846A>T |
• Solid tumours • Fluoropyrimidine-based regimens (≥ 3 cycles unless interrupted due to toxicity) |
DPYD carriers Wild-type | Some patients had dose adjustment because of toxicity | Grade ≥ 3 toxicity Sensitivity, specificity, PPV, NPV for severe toxicity Treatment modification |
| Lee et al, 201458 N = 2,594 United States |
Based on patients included in RCT Genotyping on previously collected blood sample Period NR |
DPYD∗2A DPYD∗13 c.2846A>T |
• Resected stage III colon cancer • 5-FU-based regimen, within 10 wk of surgery • Only KRAS wild-type patients included |
DPYD carriers Wild-type |
Dose reduction in some patients because of toxicity, according to study guidelines | Grade ≥ 3 toxicity (NCI-CTCAE v3) Sensitivity, specificity, PPV, NPV Treatment modification |
| Jennings et al, 201359 N = 254 United Kingdom |
Retrospective patient selection from database Genotyping after start of treatment (email communication with author) 2008–2011 |
DPYD∗2A DPYD∗13 c.2846A>T c.1236G>A |
• Colorectal cancer • 5-FU or capecitabine alone or in combination as adjuvant, neoadjuvant, or palliative • WHO performance status 0–2 |
DPYD carriers Wild-type |
Dose modification and treatment withdrawal allowed as standard hospital protocol; not based on genotyping (email communication with author) | Grade ≥ 3 toxicity (NCI-CTCAE v4.0) Delays and dose reductions due to adverse events |
| Loganayagam et al, 201360 N = 430 United Kingdom |
Retrospective patient selection from database Unclear if prospective or retrospective genotyping Period NR |
DPYD∗2A DPYD∗13 c.2846A>T |
• Different cancer types • Fluoropyrimidine-based regimens • WHO performance status < 2 |
DPYD carriers Wild-type |
Dose reduction allowed before start of treatment because of comorbidities and after start of treatment because of toxicity | Grade ≥ 3 toxicity (NCI-CTCAE) Sensitivity, specificity, PPV, NPV Treatment modification Hospitalization |
| Cellier et al, 201162 N = 85 France |
Based on patients included in study Prospective testing 2002–2004 |
DPYD∗2A DPYD∗13 c.2846A>T |
• Locally advanced rectal cancer • Tegafur-uracil + leucovorin + radiotherapy • WHO performance status 0–2 |
DPYD carriers and phenotype test Wild-type and phenotype test |
Dose reduction or interruption not based on test results but on toxicity (grade 2–4) Careful monitoring of diarrhea and neutropenia if DPD deficiency identified; both clinician and patient informed of the risk, but no dose change |
Toxicity (WHO criteria for chemotherapy and early radiotherapy) |
| Deenen et al, 201161 N = 568 Netherlands |
Based on patients included in RCT Genotyping after treatment started Period NR |
DPYD∗2A DPYD∗13 c.2846A>T c.1236G>A |
• Metastatic colorectal cancer • Capecitabine-based regimens |
DPYD carriers Wild-type |
Dose modifications allowed because of toxicity | Capecitabine-related grade ≥ 3 toxicity (NCI-CTCAE v3) Treatment modifications |
| Cerić et al, 201063 N = 50 Bosnia-Herzegovina |
Data prospectively collected Genotyping performed after treatment started 2006–2007 |
DPYD∗2A | • Different cancer types • Fluoropyrimidine-based regimens |
DPYD carriers Wild-type |
NR | Grade 3–4 toxicity (NCI-CTCAE) |
| Braun et al, 200964 N = 1,181 United Kingdom |
Based on patients included in RCT Blood sample collection for RCT 2000–2003 |
DPYD∗2A | • Metastatic colorectal cancer • 5-FU alone or in combination |
DPYD carriers Wild-type |
Ongoing grade ≥ 2 toxicity at start of cycle: 1 wk treatment delay Grade ≥ 3 toxicity or 2 delays after grade 2 toxicity: 20% dose reduction (all drugs) |
Grade ≥ 3 toxicity (NCI-CTCAE v2.0) Delay and/or dose reduction because of toxicity |
| Schwab et al, 200865 N = 683 Germany |
Data prospectively collected Blood samples collected before treatment Period NR |
DPYD∗2A | • Colon, other GI, breast, or cancer of unknown primary • 5-FU alone or in combination with folinic acid or levamisole |
DPYD carriers Wild-type | NR | 5-FU-related toxicity (WHO criteria) Sensitivity, specificity, PPV, NPV for severe toxicity |
| Sulzyc-Bielicka et al, 200866 N = 252 Poland |
Data prospectively collected Unclear if prospective or retrospective genotyping 1998–2005 |
DPYD∗2A | • Colorectal cancer | DPYD carriers Wild-type | NR | Toxicity (NCI-CTCAE v2.0) |
| Boisdron-Celle et al, 200767 N = 252 France |
Data prospectively collected Blood samples collected before treatment; genotyping results not provided to treating physician Period NR |
DPYD∗2A c.2846A>T |
• Advanced colorectal carcinomas or in adjuvant setting • 5-FU-leucovorin • WHO performance status < 2 • Age < 80 y |
DPYD carriers Wild-type |
No pre-treatment dose adjustment Dose adjustment allowed starting at 2nd cycle based on 5-FU plasma concentration in previous cycle |
Grade ≥ 3 toxicity Treatment modifications based on toxicity and 5-FU levels |
| Largillier et al, 200668 N = 105 France |
Data prospectively collected Unclear if prospective or retrospective genotyping 2003–2004 |
DPYD∗2A | • Advanced breast cancer • Capecitabine alone |
DPYD carriers Wild-type |
Dose reduction at 2nd and 3rd cycle in some patients | Grade 3–4 toxicity (NCI-CTCAE) |
| Salgueiro et al, 200469 N = 73 Portugal |
Data prospectively collected Unclear if prospective or retrospective genotyping Period NR |
DPYD∗2A | • Colorectal cancer • Adjuvant 5-FU-based chemotherapy |
DPYD carriers Wild-type |
NR | Grade ≥ 3 toxicity (NCI-CTCAE) |
Abbreviations: 5-FU, 5-fluorouracil; DPD, dihydropyrimidine; GI, gastrointestinal; NCI-CTCAE, National Cancer Institute Common Terminology Criteria for Adverse Events; NPV, negative predictive value; NR, not reported; PPV, positive predictive value; RCT, randomized controlled trial; WHO, World Health Organization.
Table A2:
Characteristics of Included Studies: Clinical Utility
| Author, Year N Country | Study Design Methods Study Period | Variants Evaluated | Participants | Intervention Control | Outcomes |
|---|---|---|---|---|---|
| Wigle et al, 202171 N = 1,394 Canada |
Pre-treatment genotyping Patients identified prospectively 2013–2019 |
DPYD∗2A c.2846A>T DPYD∗13 c.1236G>A |
• Planned fluoropyrimidine-based regimen, alone or in combination with other drugs or radiotherapy • Different cancer types |
Intervention • Heterozygous DPYD∗2A, DPYD∗13, and c.2846A>T carriers: 50% dose reduction before start of treatment • Heterozygous c.1236G>A: 25% to 50% initial dose reduction before start of treatment • Dose increase allowed after 2 cycles based on tolerance for heterozygous carriers • Homozygous or compound heterozygous carriers: avoidance of fluoropyrimidines Control • Wild-type • Fluoropyrimidine dose as per standard of care |
Grade ≥ 3 toxicity (NCI-CTCAE v5.0) Treatment discontinuation Treatment-related mortality |
| Henricks et al, 201911 N = 1,732 Netherlands |
Pre-treatment genotyping Patients identified prospectively and retrospectively 2007–2015 |
DPYD∗2A | • Planned fluoropyrimidine-based regimen, alone or in combination with other drugs or radiotherapy • Different cancer types |
Intervention • Heterozygous DPYD∗2A carriers: 50% dose reduction before start of treatment • Dose increase allowed after 2 cycles based on tolerance Control • Wild-type • Standard fluoropyrimidine dose |
Grade ≥ 3 (NCI-CTCAE) Hospitalization for treatment-related toxicity Treatment discontinuation Treatment-related death |
| Kleinjan et al, 201912 N = 184 Netherlands |
Pre-treatment genotyping Patients identified retrospectively through databases of laboratories performing DPYD testing and hospital pharmacy database of genotype-guided dose adjustment 2006–2017 |
DPYD∗2A c.2846A>T DPYD∗13 c.1236G>A |
• Capecitabine-based regimens • Receiving ≥ 1 cycle • Different cancer types • Those who underwent DPYD genotyping (all patients as part of standard care) |
Intervention • Heterozygous DPYD∗2A and DPYD∗13 carriers: 50% dose reduction • Heterozygous c.2846A<T and c.1236G>A carriers: 25% dose reduction • After 1–2 cycles dose could be further reduced, maintained, or increased (by 15%, up to standard dose) based on capecitabine-related toxicity • Treatment stopped in case of disease progression; outcomes of subsequent treatment NR Control • Wild-type • Treated according to standard practice; dose reductions allowed according to age and comorbidities • Treatment stopped in case of disease progression; outcomes of subsequent treatment NR |
Grade ≥ 3 toxicity (NCI-CTCAE v5.0): except for hand-foot syndrome (grade ≥ 2) Treatment-related hospitalizations |
| Stavraka et al, 201923 N = 66 United Kingdom |
Retrospective patient identification Pre-treatment DPYD genotyping 2014–2017 |
DPYD∗2A c.2846A>T DPYD∗13 |
• Planned capecitabine with or without lapatinib treatment • Consecutive metastatic breast cancer |
Intervention • DPYD carriers • Heterozygous DPYD∗2A carriers: 50% dose reduction • Heterozygous c.2846A<T carriers: 25% dose reduction • Subsequent dose increase allowed Control • Wild-type • Dose modification if needed |
Toxicity (NCI-CTCAE v4.0) Toxicity-related hospitalizations Treatment decisions |
| Henricks et al, 201872 N = 1,103 Netherlands |
Prospective patient identification Pre-treatment DPYD genotyping 2015–2017 |
DPYD∗2A c.2846A>T DPYD∗13 c.1236G>A |
• Planned fluoropyrimidine-based regimen + radiotherapy • Different cancer types • Adults (≥ 18 y old) • WHO performance status 0–2 • Excluded: homozygous and compound heterozygous DPYD variant carriers |
Intervention • Heterozygous DPYD∗2A and DPYD∗13 carriers: 50% dose reduction • Heterozygous c.2846A<T and c.1236G>A carriers: 25% dose reduction • Dose increase allowed after 1st 2 cycles if treatment well tolerated at physician's discretion Control • Wild-type • Dose reduction and increase allowed at physician's discretion |
Grade ≥ 3 toxicity (NCI-CTCAE v4.03) Treatment-related hospitalization Treatment discontinuation |
| Lunenburg et al, 20187 Netherlands N = 828 |
Patients originated from 3 different cohorts Genotyping prospectively and retrospectively depending on variant and cohort 1993–2017 depending on database |
DPYD∗2A c.2846A>T DPYD∗13 c.1236G>A |
• Planned fluoropyrimidine-based chemoradiation therapy • Different cancer types |
Intervention • DPYD carriers on pre-treatment testing • Dose reduction 25% or 50% (≥ 50% for some DPYD∗2A carriers) • Dose increase permitted Control • DPYD variant carriers (retrospective testing) • Wild-type • Standard fluoropyrimidine chemoradiation dose used • Dose reduction and increase allowed at treating physician's discretion |
Toxicity (NCI-CTCAE grade ≥ 3) Dose reductions and increases Treatment delay and discontinuation Treatment-related hospitalizations Length of stay |
Abbreviations: DPD, dihydropyrimidine; NCI-CTCAE; National Cancer Institute Common Terminology Criteria for Adverse Events; NR = not reported; PK = pharmacokinetic; WHO, World Health Organization.
Appendix 3: Critical Appraisal of Clinical Evidence
Table A3:
Risk of Biasa Among Systematic Reviews (ROBIS Tool)
| Phase 2 | Phase 3 | ||||
|---|---|---|---|---|---|
| Author, Year | Study Eligibility Criteria | Identification and Selection of Studies | Data Collection and Study Appraisalb | Synthesis and Findingsb | Risk of Bias in the Reviewc |
| INESSS, 201932 | Low | Low | NA | NA | Low |
| HAS, 201810 | Low | Low | NA | NA | Low |
| Campbell et al, 201646 | Low | Low | NA | NA | Low |
| Meulendijks et al, 201547 | Lowd | Lowe | NA | NA | Low |
| Li et al, 201448 | Lowd | Lowe | NA | NA | Low |
| Terrazzino et al, 201349 | Lowd | Lowe | NA | NA | Low |
| Technology Evaluation Center, 201050 | Lowd | Lowe | NA | NA | Low |
Abbreviations: HAS, Haute Autorité de Santé; INESS, Institut National de d'Excellence en Santé et en Services Sociaux; NA, not applicable; ROBIS, Risk of Bias in Systematic Reviews.
Possible risk-of-bias levels: low, high, unclear.
We used only domains 1 and 2 (study eligibility criteria and identification and selection of studies) of the tool because we were using the selected systematic reviews to generate a list of included studies (i.e., the results synthesis and conclusions sections were not used).
Based on the domains assessed.
Did not search the grey literature, but this may not necessarily have affected the risk of bias; for this reason, the risk of bias for this domain was considered low.
Lack of a double reviewer could have led to missing relevant studies, but we could not determine whether this occurred; this did not warrant increasing the risk of bias.
Table A4:
Risk of Biasa Among Cohort Studies
| Selection | Comparability | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|
| Author, Year | Representativeness of the Exposed Cohort | Selection of the Non-exposed Cohort | Ascertainment of Exposure | Demonstration That Outcome of Interest Was Not Present at Start of Study | Comparability of Cohorts (Design or Analysis) | Assessment of Outcome | Was Follow-Up Long Enough for Outcomes to Occur | Adequacy of Follow-up of Cohorts |
| Clinical Validity | ||||||||
| Wigle et al, 202171 | A (∗) | A (∗) | A (∗) | A (∗) | Not controlledb | A (∗) | A (∗) | A (∗) |
| Iachetta et al, 201952 | A (∗) | A (∗) | A (∗) | A (∗) | Not controlledb | A (∗) | A (∗) | A (∗) |
| Maharjan et al, 201953 | Cc | A (∗) | A (∗) | B | Not controlledb | B (∗) | A (∗) | A (∗) |
| Cremolini et al, 201855 | A (∗) | A (∗) | A (∗) | A (∗) | Not controlledb | A (∗) | A (∗) | A (∗) |
| Lunenburg et al, 20187 | A (∗) | A (∗) | A (∗) | A (∗) | Not controlledb | B(∗)d | A (∗) | A (∗) |
| Nahid et al, 201854 | A (∗) | A (∗) | A (∗) | A (∗) | Not controlledb | A (∗) | A (∗) | A (∗) |
| Etienne-Grimaldi et al, 201756 | A (∗) | A (∗) | A (∗) | A (∗) | Not controlledb | A (∗) | A (∗) | A (∗) |
| Meulendijks et al, 201718 | A (∗) | A (∗) | A (∗) | A (∗) | Not controlledb | A (∗) | A (∗) | A (∗) |
| Meulendijks et al, 201770 | A (∗) | A (∗) | A (∗) | A (∗) | Not controlledb | A (∗) | A (∗) | A (∗) |
| Boige et al, 201615 | A (∗) | A (∗) | A (∗) | A (∗) | Not controlledb | A (∗) | A (∗) | A (∗) |
| Lee et al, 201613 | A (∗) | A (∗) | A (∗) | A (∗) | Not controlledb | A (∗) | A (∗) | A (∗) |
| Froehlich et al, 201557 | A (∗) | A (∗) | A (∗) | A (∗) | Not controlledb | A (∗) | A (∗) | A (∗) |
| Toffoli et al, 20159 | A (∗) | A (∗) | A (∗) | A (∗) | Not controlledb | A (∗) | A (∗) | A (∗) |
| Lee et al, 201458 | A (∗) | A (∗) | A (∗) | A (∗) | Not controlledb | A (∗) | A (∗) | A (∗) |
| Jennings et al, 201359 | A (∗) | A (∗) | A (∗) | A (∗) | Not controlledb | A (∗) | A (∗) | A (∗) |
| Loganayagam et al, 201360 | A (∗) | A (∗) | A (∗) | A (∗) | Not controlledb | A (∗) | A (∗) | A (∗) |
| Cellier et al, 201162 | A (∗) | A (∗) | A (∗) | A (∗) | Not controlledb | A (∗) | A (∗) | A (∗) |
| Deenen et al, 201161 | A (∗) | A (∗) | A (∗) | A (∗) | Not controlledb | A (∗) | A (∗) | A (∗) |
| Cerić et al, 201063 | A (∗) | A (∗) | A (∗) | A (∗) | Not controlledb | A (∗) | A (∗) | A (∗) |
| Braun et al, 200964 | A (∗) | A (∗) | A (∗) | A (∗) | Not controlledb | A (∗) | A (∗) | A (∗) |
| Schwab et al, 200865 | A (∗) | A (∗) | A (∗) | A (∗) | Not controlledb | A (∗) | A (∗) | A (∗) |
| Sulzyc-Bielicka et al, 200866 | A (∗) | A (∗) | A (∗) | A (∗) | Not controlledb | A (∗) | A (∗) | A (∗) |
| Boisdron-Celle et al, 200767 | A (∗) | A (∗) | A (∗) | A (∗) | Not controlledb | A (∗) | A (∗) | A (∗) |
| Largillier et al, 200668 | A (∗) | A (∗) | A (∗) | A (∗) | Not controlledb | A (∗) | A (∗) | A (∗) |
| Salgueiro et al, 200469 | A (∗) | A (∗) | A (∗) | A (∗) | Not controlledb | A (∗) | A (∗) | A (∗) |
| Clinical Utility | ||||||||
| Wigle et al, 202171 | A (∗) | A (∗) | A (∗) | A (∗) | Not controlledb | A (∗) | A (∗) | A (∗) |
| Henricks et al, 201911 | A (∗) | A (∗) or B | A (∗) | A (∗) | Not controlledb | B (∗) | A (∗) | A (∗) |
| Kleinjan et al, 201912 | A (∗) | A (∗) | A (∗) | A (∗) | Not controlledb | B (∗) | A (∗) | A (∗) |
| Stavraka et al, 201923 | A (∗) | A (∗) | A (∗) | A (∗) | Not controlledb | B (∗) | A (∗) | A (∗) |
| Henricks et al, 201872 | A (∗) | A (∗) | A (∗) | A (∗) | Not controlledb | A (∗) | A (∗) | A (∗) |
| Lunenburg et al, 20187 | A (∗) | A (∗) | A (∗) | A (∗) | Not controlledb | B (∗) | A (∗) | A (∗) |
Risk of bias assessed using the Newcastle-Ottawa Scale.44 The ratings range from A to B, A to C, or A to D depending on the parameter and the level of quality for each parameter; A is the highest level of quality. The scale then uses a “star system” to rate each domain overall. For the selection and outcome domains, a star (∗) is given if the rating is either A or A/B, depending on the parameter. For the comparability domain, a star may be given depending on the degree of control for confounders.44
Analysis comparing the frequency of toxicity in carriers vs. wild-type patients was not controlled for potential confounders.
Decision to genotype was at the investigator's discretion and may have been associated with the risk of developing toxicity.
Some differences in types of toxicity collected from each of the three databases included.
Table A5:
GRADE Evidence Profile for the Comparison of DPYD Variant Carriers Who Received a Standard Fluoropyrimidine Dose and Wild-Type Patients (Severe Toxicity)
| Number of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| Overall Severe Toxicity, Carriers of One of the DPYD Variants Under Assessment | |||||||
| 7 (observational)7,9,57–60,62 | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | NA | ⊕⊕ Low |
| Severe Neutropenia, Carriers of One of the DPYD Variants Under Assessment | |||||||
| 2 (observational)7,9 | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | NA | ⊕⊕ Low |
| Severe Diarrhea, Carriers of One of the DPYD Variants Under Assessment | |||||||
| 2 (observational)7,9 | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | NA | ⊕⊕ Low |
| Severe Hand–Foot Syndrome, Carriers of One of the DPYD Variants Under Assessment | |||||||
| 1 (observational)7 | No serious limitations | Could not be evaluated | No serious limitations | Serious limitations (−;1)a | Undetected | NA | ⊕ Very low |
| Overall Severe Toxicity, DPYD∗2A Carriers | |||||||
| 17 (observational)9,15,54–65,67–69 | No serious limitations | No serious limitationsb | No serious limitations | No serious limitationsc | Undetected | NA | ⊕⊕ Low |
| Severe Neutropenia, DPYD∗2A Carriers | |||||||
| 9 (observational)9,15,53–55,58,63,68,69 | No serious limitations | No serious limitationsb | No serious limitations | No serious limitationsc | Undetected | NA | ⊕⊕ Low |
| Severe Diarrhea, DPYD∗2A Carriers | |||||||
| 11 (observational)9,15,53–55,58,61,63,65,68,69 | No serious limitations | No serious limitationsb | No serious limitations | No serious limitationsc | Undetected | NA | ⊕⊕ Low |
| Severe Hand–Foot Syndrome, DPYD∗2A Carriers | |||||||
| 3 (observational)61,62,68 | No serious limitations | Serious limitations (−;1)d | No serious limitations | No serious limitationsc | Undetected | NA | ⊕ Very low |
| Overall Severe Toxicity, DPYD∗13 Carriers | |||||||
| 7 (observational)9,15,52,57,58,60,62 | No serious limitations | No serious limitationsb | No serious limitations | No serious limitations | Undetected | NA | ⊕⊕ Low |
| Severe Neutropenia, DPYD∗13 Carriers | |||||||
| 2 (observational)9,15 | No serious limitations | No serious limitations | No serious limitations | Serious limitations (−;1)a | Undetected | NA | ⊕ Very low |
| Severe Diarrhea, DPYD∗13 Carriers | |||||||
| 3 (observational)9,15,62 | No serious limitations | No serious limitationsb | No serious limitations | No serious limitations | Undetected | NA | ⊕⊕ Low |
| Overall Severe Toxicity, c.2846A>T Carriers | |||||||
| 13 (observational)9,15,52,55–62,67,70 | No serious limitations | No serious limitationsb | No serious limitations | No serious limitationsc | Undetected | NA | ⊕⊕ Low |
| Severe Neutropenia, c.2846A>T Carriers | |||||||
| 5 (observational)9,15,53,55,58 | No serious limitations | No serious limitationsb | No serious limitations | No serious limitationsc | Undetected | NA | ⊕⊕ Low |
| Severe Diarrhea, c.2846A>T Carriers | |||||||
| 6 (observational)9,15,53,55,58,61 | No serious limitations | No serious limitationsb | No serious limitations | No serious limitationsc | Undetected | NA | ⊕⊕ Low |
| Severe Hand–Foot Syndrome, c.2846A>T Carriers | |||||||
| 1 (observational)61 | No serious limitations | Could not be evaluated | No serious limitations | Serious limitations (−;1)a | Undetected | NA | ⊕ Very low |
| Overall Severe Toxicity, c.1236G>A Carriers, Heterozygous | |||||||
| 6 (observational)13,57,59,61,70,71 | No serious limitations | No serious limitations | No serious limitations | Serious limitations (−;1)e | Undetected | NA | ⊕ Very low |
| Overall Severe Toxicity, c.1236G>A Carriers, Homozygous | |||||||
| 2 (observational)13,57 | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | NA | ⊕⊕ Low |
| Severe Neutropenia, c.1236G>A Carriers, Heterozygous | |||||||
| 1 (observational)13 | No serious limitations | Could not be evaluated | No serious limitations | No serious limitations | Undetected | NA | ⊕⊕ Low |
| Severe Diarrhea, c.1236G>A Carriers, Heterozygous | |||||||
| 2 (observational)13,61 | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | NA | ⊕⊕ Low |
| Severe Nausea and/or Vomiting, c.1236G>A Carriers, Homozygous | |||||||
| 1 (observational)13 | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | NA | ⊕⊕ Low |
| Severe Hand–Foot Syndrome, c.1236G>A Carriers, Heterozygous | |||||||
| 2 (observational)13,71 | No serious limitations | Could not be evaluated | No serious limitations | Serious limitations (−;1)a | Undetected | NA | ⊕ Very low |
| Overall Severe Toxicity, Compound Heterozygous | |||||||
| 4 (observational)9,57,58,67 | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | NA | ⊕⊕ Low |
| Severe Diarrhea, Compound Heterozygous | |||||||
| 1 (observational)9 | No serious limitations | Could not be evaluated | No serious limitations | No serious limitations | Undetected | NA | ⊕⊕ Low |
Abbreviations: GRADE, Grading of Recommendations Assessment, Development, and Evaluation; NA, not applicable.
Insufficient statistical power to detect a difference between groups.
Decision not to downgrade based on the consistency in the direction of effect. Despite the range of point estimates obtained from the studies, they were consistent in their direction of effect and the variation in magnitude observed would not have affected the overall conclusion.
Not downgraded because in most studies the 95% confidence interval excluded the possibility of no effect or lower risk in carriers vs. wild-type patients.
Substantial inconsistency in the direction of effect among studies.
Insufficient statistical power to detect a difference between groups in most studies.
Table A6:
GRADE Evidence Profile for the Comparison of DPYD Variant Carriers Who Received a Standard Fluoropyrimidine Dose and Wild-Type Patients (Toxicity-Related Dose Reduction, Treatment Discontinuation, Hospitalization, and Mortality)
| Number of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| Toxicity-Related Dose Reduction, Carriers of One of the DPYD Variants Under Assessment | |||||||
| 1 (observational)60 | No serious limitations | Could not be evaluated | No serious limitations | No serious limitations | Undetected | NA | ⊕⊕ Low |
| Toxicity-Related Dose Reduction, DPYD∗2A Carriers | |||||||
| 2 (observational)58,60 | No serious limitations | No serious limitations | No serious limitations | Serious limitations (−;1)a | Undetected | NA | ⊕ Very low |
| Toxicity-Related Dose Reduction, DPYD∗13 Carriers | |||||||
| 2 (observational)58,60 | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | NA | ⊕⊕ Low |
| Toxicity-Related Dose Reduction, c.2846A>T Carriers | |||||||
| 2 (observational)58,60 | No serious limitations | No serious limitations | No serious limitations | Serious limitations (−;1)a | Undetected | NA | ⊕ Very low |
| Treatment Discontinuation, Carriers of One of the DPYD Variants Under Assessment | |||||||
| 1 (observational)60 | No serious limitations | Could not be evaluated | No serious limitations | No serious limitations | Undetected | NA | ⊕⊕ Low |
| Treatment Discontinuation, DPYD∗2A Carriers | |||||||
| 3 (observational)58,60,68 | No serious limitations | No serious limitations | No serious limitations | No serious limitationsb | Undetected | NA | ⊕⊕ Low |
| Treatment Discontinuation, DPYD∗13 Carriers | |||||||
| 2 (observational)58,60 | No serious limitations | No serious limitations | No serious limitations | Serious limitations (−;1)a | Undetected | NA | ⊕ Very low |
| Treatment Discontinuation, c.2846A>T Carriers | |||||||
| 2 (observational)58,60 | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | NA | ⊕⊕ Low |
| Treatment Discontinuation, c.1236G>A Carriers | |||||||
| 1 (observational)71 | No serious limitations | No serious limitations | No serious limitations | Serious limitations (−;1)a | Undetected | NA | ⊕ Very low |
| Hospitalization, Carriers of One of the DPYD Variants Under Assessment | |||||||
| 3 (observational)7,60,67 | No serious limitations | Could not be evaluated | No serious limitations | No serious limitations | Undetected | NA | ⊕⊕ Low |
| Hospitalization, Compound Heterozygous | |||||||
| 1 (observational)67 | No serious limitations | Could not be evaluated | No serious limitations | No serious limitations | Undetected | NA | ⊕⊕ Low |
| Mortality, Carriers of One of the DPYD Variants Under Assessment | |||||||
| 2 (observational)63,68 | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | NA | ⊕⊕ Low |
| Mortality, Compound Heterozygous | |||||||
| 1 (observational)67 | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | NA | ⊕⊕ Low |
Abbreviations: GRADE, Grading of Recommendations Assessment, Development, and Evaluation; NA, not applicable.
Insufficient statistical power to detect a difference between groups.
Not downgraded because in most studies the 95% confidence interval excluded the possibility of no effect or lower risk in carriers vs. wild-type patients.
Table A7:
GRADE Evidence Profile for the Comparison of DPYD Variant Carriers Who Received a Genotype-Guided Reduced Fluoropyrimidine Dose and Wild-Type Patients or DPYD Variant Carriers who Received a Standard Dose (Severe Toxicity, Treatment Discontinuation, and Hospitalization)
| Number of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| Overall Severe Toxicity, DPYD Variants Under Assessment vs. Wild-Type Patients | |||||||
| 6 (observational)7,11,12,23,71,72 | No serious limitations | No serious limitationsa | No serious limitations | Serious limitations (−;1)b | Undetected | NA | ⊕ Very low |
| Overall Severe Toxicity, DPYD Variants Under Assessment vs. DPYD Carriers Who Received a Standard Fluoropyrimidine Dose | |||||||
| 1 (observational)7 | Serious limitations (−;1)c | Could not be evaluated | No serious limitations | Serious limitations (−;1)b | Undetected | NA | ⊕ Very low |
| Severe Hematological Toxicity, DPYD Variants Under Assessment vs. Wild-Type Patients | |||||||
| 6 (observational)7,11,12,23,71,72 | No serious limitations | No serious limitations | No serious limitations | Serious limitations (−;1)b | Undetected | NA | ⊕ Very low |
| Severe Hematological Toxicity, DPYD Variants Under Assessment vs. DPYD Carriers Who Received a Standard Fluoropyrimidine Dose | |||||||
| 1 (observational)7 | Serious limitations (−;1)c | Could not be evaluated | No serious limitations | Serious limitations (−;1)b | Undetected | NA | ⊕ Very low |
| Severe Gastrointestinal Toxicity, DPYD Variants Under Assessment vs. Wild-Type Patients | |||||||
| 6 (observational)7,11,12,23,71,72 | No serious limitations | No serious limitationsa | No serious limitations | Serious limitations (−;1)b | Undetected | NA | ⊕ Very low |
| Severe Gastrointestinal Toxicity, DPYD Variants Under Assessment vs. DPYD Carriers Who Received a Standard Fluoropyrimidine Dose | |||||||
| 1 (observational)7 | Serious limitations (−;1)c | Could not be evaluated | No serious limitations | Serious limitations (−;1)b | Undetected | NA | ⊕ Very low |
| Severe Hand–Foot Syndrome, DPYD Variants Under Assessment vs. Wild-Type Patients | |||||||
| 6 (observational)7,11,12,23,71,72 | No serious limitations | No serious limitationsa | No serious limitations | Serious limitations (−;1)b | Undetected | NA | ⊕ Very low |
| Treatment Discontinuation, DPYD Variants Under Assessment vs. Wild-Type Patients | |||||||
| 5 (observational)7,11,23,71,72 | No serious limitations | No serious limitationsa | No serious limitations | Serious limitations (−;1)b | Undetected | NA | ⊕ Very low |
| Hospitalization, DPYD Variants Under Assessment vs. Wild-Type Patients | |||||||
| 5 (observational)7,11,12,23,72 | No serious limitations | No serious limitations | No serious limitations | Serious limitations (−;1)b | Undetected | NA | ⊕ Very low |
| Hospitalization, DPYD Variants Under Assessment vs. DPYD Carriers Who Received a Standard Fluoropyrimidine Dose | |||||||
| 1 (observational)7 | Serious limitations (−;1)c | No serious limitations | No serious limitations | Serious limitations (−;1)b | Undetected | NA | ⊕ Very low |
Abbreviations: GRADE, Grading of Recommendations Assessment, Development, and Evaluation; NA, not applicable.
Some inconsistency in the direction of point estimates; however, given the wide confidence intervals, this was considered to be due to imprecision. As a result, we downgraded the evidence for this outcome.
Insufficient statistical power to detect a difference between groups.
Downgraded because of the imbalance in the distribution of DPYD variants between the reduced-dose carrier and standard-dose carrier groups; the two variants that were expected to have a weaker effect on dihydropyrimidine (DPD) activity were overrepresented in the latter group. This may have led to an underestimate in the frequency of severe toxicity in the standard-dose group and an underestimate in the difference between groups.
Table A8:
GRADE Evidence Profile for the Comparison of DPYD Variant Carriers Who Received a Genotype-Guided Reduced Fluoropyrimidine Dose and Wild-Type Patients (Survival)
| Number of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| Overall Survival, DPYD∗2A Carriers | |||||||
| 1 (observational)11 | No serious limitations | Could not be evaluated | No serious limitations | Serious limitations (−;1)a | Undetected | NA | ⊕ Very low |
| Progression-Free Survival, DPYD∗2A Carriers | |||||||
| 1 (observational)11 | No serious limitations | Could not be evaluated | No serious limitations | Serious limitations (−;1)a | Undetected | NA | ⊕ Very low |
Abbreviations: GRADE, Grading of Recommendations Assessment, Development, and Evaluation; NA, not applicable.
Insufficient statistical power to detect a difference between groups.
Appendix 4: Clinical Evidence Review—Patient Characteristics
Table A9:
Characteristics of Patients Included in Clinical Validity Studies
| Author, Year N (Carrier/Wild-Type) | Age, y | Male, n (%) | Race/Ethnicity, n (%) | Type of Cancer, n (%) | Treatment Regimen, n (%) |
|---|---|---|---|---|---|
| Wigle et al, 202171 1,388 (41/1,347) |
Mean ± SD Carriers: 66 ± 10 Wild-type: 64 ± 12 |
Carriers: 28 (68.3) Wild-type: 742 (55.1) |
White Carriers: 40 (97.6) Wild-type: 1,267 (94.1) Other Carriers: 1 (2.4) Wild-type: 32 (2.4) Unknown Carriers: 0 Wild-type: 48 (3.6) |
Colorectal Carriers: 21 (51.2) Wild-type: 779 (57.8) Breast Carriers: 1 (2.4) Wild-type: 89 (6.6) Gastroesophageal Carriers: 5 (12.2) Wild-type: 189 (14.0) Pancreas Carriers: 2 (4.9) Wild-type: 106 (7.9) Other Carriers: 12 (29.3) Wild-type: 184 (13.7) |
5-FU Carriers: 19 (46.3) Wild-type: 643 (47.7) Capecitabine Carriers: 22 (53.7) Wild-type: 704 (52.3) Patients treated with capecitabine who also received radiotherapy Carriers: 11 (26.8) Wild-type: 277 (20.6) |
| Iachetta et al, 201952 366 (2/364) |
Median (range) Carriers: 67 (32–84) Wild-type: 65 (22–88) |
Carriers: 68 (46) Wild-type: 125 (57) |
NR |
Colorectal Carriers: 66 (45.2) Wild-type: 118 (53.6) Breast Carriers: 4 (3) Wild-type: 9 (4) Gastroesophageal Carriers: 61 (41.8) Wild-type: 74 (33.6) Other Carriers: 15 (10.3) Wild-type: 19 (8.6) |
Both 5-FU or capecitabine used; detailed information not provided |
| Maharjan et al, 201953 113 (5/108) |
Median (range) 59 (21–90) | 62 (54.9) | Caucasian: 75 (66.4) African American: 35 (31.0) Other: 3 (2.6) |
Colorectal: 79 (69.9) Breast: 0 Gastric: 23 (20.4) Other: 11 (9.7) |
5-FU: 79 (69.9) Capecitabine: 34 (30.1) |
| Cremolini et al, 201855 443 (10/433) |
Median (range) 61 (29–75) | 235 (53.0) | NR | Colorectal: 443 (100.0) | 5-FU: 443 (100.0) |
| Lunenburg et al, 20187 805 (34/771) |
Median (range) Carriers: 64 (45–79) Wild-type: 63 (23–88) |
Carriers: 20 (58.8) Wild-type: 432 (56.0) |
NR |
Colorectal Carriers: 22 (64.7) Wild-type: 554 (71.9) Gastrointestinal Carriers: 12 (35.3) Wild-type: 169 (21.9) Other Carriers: 0 (0.0) Wild-type: 48 (6.3) |
5-FU Carriers: 5 (14.7) Wild-type: 103 (13.4) Capecitabine Carriers: 29 (85.3) Wild-type: 668 (86.6) Number of patients with concomitant radiotherapy not provided |
| Nahid et al, 201854 161 (8/153) |
Median (range) 47 (25–75) | 97 (60.2) | NR | Colorectal: 161 (100.0) | 5-FU: 161 (100.0) |
| Etienne-Grimaldi et al, 201756 243 (6/237) |
Mean (range) 61.2 (30–88) | 0 (0.0) | NR | Breast: 243 (100.0) | Capecitabine: 243 (100.0) |
| Meulendijks et al, 201718 550 (30/520) |
Median (range) 61 (30–88) | 232 (42.2) | Caucasian: 521 (94.7) Other: 29 (5.3) |
Colorectal: 190 (34.5) Breast: 175 (31.8) Gastric: 126 (22.9) Other: 59 (10.7) |
5-FU: 70 (12.7) Capecitabine: 480 (87.3) |
| Meulendijks et al, 201770 185 (5/180) |
Mean (range) 59 (22–77) | 135 (73.0) | NR | Advanced gastric: 185 (100.0) | Capecitabine: 185 (100.0) |
| Boige et al, 201615 1,545 (36/1,509) |
Median (range) 60 (19–75) | 1,135 (73.5) | NR | Colon: 1,545 (100.0) | 5-FU: 1,545 (100.0) |
| Lee et al, 201613 1,953 (78/1,875) |
Mean ± SD 57 ± 11 | 1,069 (54.7) | Caucasian: 1,953 (100.0) | Colon: 1,953 (100.0) | 5-FU: 1,953 (100.0) |
| Froehlich et al, 201557 500 (32/468) |
n (%) 18–49 y: 47 (12.1) 50–69 y: 260 (66.8) > 69 y: 82 (21.1) |
230 (59.1) | Caucasian: 493 (98.6) | Colorectal: 275 (55.0) Esophageal: 97 (19.4) Breast: 26 (5.2) Gastric: 46 (9.2) Head and neck: 31 (6.2) Other: 25: (5.0) |
5-FU: 397 (79.4) Capecitabine: 103 (20.6) |
| Toffoli et al, 20159 603 (18/585) |
Median (range) 62 (17–99) | 310 (51.4) | Caucasian: 603 (100.0) | NR | 5-FU: 546 (90.5) Capecitabine: 55 (9.1) Other: 2 (0.4) |
| Lee et al, 201458 2,594 (62/2,532) |
Median (range) 58 (19–86) | 1,385 (53.4) | Caucasian: 2,248 (87.8) Black/African American: 170 (6.6) Asian: 121 (4.7) Other: 22 (0.9) Missing: 33 (12.7) |
Colon: 2,619 (100.0) | 5-FU: 2,619 (100.0) |
| Jennings et al, 201359 253 (15/238) |
Median (range) 67 (23–88) | 145 (57.3) | NR | Colorectal: 253 (100.0) | 5-FU: 94 (37.1) Capecitabine: 159 (62.9) |
| Loganayagam et al, 201360 430 (10/420) |
Mean (range) 62 (20–83) | 247 (57.4) | Caucasian: 364 (84.7) Afro-Caribbean: 50 (11.6) South Asian: 12 (2.8) Southeast Asian: 4 (0.9) |
Colorectal: 364 (84.7) Gastrointestinal: 62 (14.4) Other: 4 (0.9) |
5-FU: 186 (43.3) Capecitabine: 244 (56.7) |
| Cellier et al, 201162 85 (3/82) |
Median (range) 67 (25–81) | 56 (65.9) | NR | Rectal: 85 (100.0) | 5-FU + radiotherapy: 85 (100.0) |
| Deenen et al, 201161 568 (44/524) |
Median (range) 63 (31–83) | 345 (60.7) | Mostly Caucasian as reported by the authors, but figures not provided | Colorectal: 568 (100.0) | Capecitabine: 568 (100.0) |
| Cerić et al, 201063 50 (1/49) |
NR | 27 (54.0) | NR | Colorectal: 41 (82.0) Breast: 3 (6.0) Gastric: 3 (6.0) Other: 3 (6.0) |
5-FU: 42 (84.0) Capecitabine: 8 (16.0) |
| Braun et al, 200964 750 (7/743) |
Median (range) 64 (27–85) | 803 (68.0) | NR | Colorectal: 750 (100.0) | 5-FU: 750 (100.0) |
| Schwab et al, 200865 683 (13/670) |
NR | 383 (56.1) | NR | Colon: 470 (68.8) Breast: 32 (4.7) Gastric: 66 (9.7) Other: 28 (4.1) Unclear: 87 (12.7) |
5-FU: 683 (100.0) |
| Sulzyc-Bielicka et al, 200866 252 (1/251) |
62 (SD NR) | 146 (57.9) | NR | Colorectal: 252 (100.0) | 5-FU: 252 (100.0) |
| Boisdron-Celle et al, 200767 252 (10/242) |
Mean ± SD 67 ± 11 | 140 (56) | Caucasian: 252 (100.0) | Colorectal: 252 (100.0) | 5-FU: 252 (100.0) |
| Largillier et al, 200668 105 (1/104) |
Mean (range) 61 (33–84) | 0 | NR | Breast: 105 (100.0) | Capecitabine: 105 (100.0) |
| Salgueiro et al, 200469 73 (1/72) |
Mean (range) 59 (31–85) | 34 (46.6) | NR | Colorectal: 73 (100.0) | 5-FU: 73 (100.0) |
Abbreviations: 5-FU, 5-fluorouracil; NR = not reported; SD, standard deviation.
Table A10:
Characteristics of Patients Included in Clinical Utility Studies
| Author, Year N (Carriers/Wild-Type) | Age, y | Male, n (%) | Race/Ethnicity, n (%) | Type of Cancer, n (%) | Treatment Regimens, n (%) |
|---|---|---|---|---|---|
| Wigle et al, 202171 1,394 (47/1,347) |
Mean ± SD Carriers: 62 ± 13 Wild-type: 64 ± 12 |
Carriers: 22 (46.8) Wild-type: 742 (55.1) |
White Carriers: 45 (95.7) Wild-type: 1,267 (94.1) Other Carriers: 1 (2.1) Wild-type: 32 (2.4) Unknown Carriers: 1 (2.1) Wild-type: 48 (3.6) |
Colorectal Carriers: 21 (51.2) Wild-type: 779 (57.8) Breast Carriers: 1 (2.4) Wild-type: 89 (6.6) Gastroesophageal Carriers: 5 (13.4) Wild-type: 189 (14.0) Pancreas Carriers: 2 (4.9) Wild-type: 106 (7.9) Other Carriers: 12 (29.3) Wild-type: 184 (13.7) |
5-FU Carriers: 24 (51.1) Wild-type: 643 (47.7) Capecitabine Carriers: 23 (48.9) Wild-type: 704 (52.3) Patients treated with capecitabine who also received radiotherapy Carriers: 11 (23.4) Wild-type: 277 (20.6) |
| Henricks et al, 201911 1,646 (40/1,606) |
Median (range) Carriers: 62 (34–91) Wild-type: 61 (21–89) |
Carriers: 14 (35) Wild-type: 720 (45) |
Caucasian Carriers: 39 (98) Wild-type: 1,540 (96) Southeast Asian Carriers: 1 (2.5) Wild-type: 14 (0.9) African Carriers: 0 (0.0) Wild-type: 21 (1.3) Other Carriers: 0 (0.0) Wild-type: 31 (1.9) |
Colorectal Carriers: 13 (32.5) Wild-type: 854 (53.2) Breast Carriers: 15 (37.5) Wild-type: 369 (23.0) Gastric Carriers: 2 (5.0) Wild-type: 227 (14.1) Other Carriers: 10 (25.0) Wild-type: 156 (14.1) |
5-FU Carriers: 2 (5.0) Wild-type: 168 (10.5) Capecitabine Carriers: 38 (95.0) Wild-type: 1,438 (89.5) Patients treated with either 5-FU or capecitabine who also received radiotherapy Carriers: 12 (30.0) Wild-type: 490 (30.5) |
| Kleinjan et al, 201912 185 (11/174) |
Median (range) Carriers: 65 (36–77) Wild-type: 67 (32–85) |
Carriers: 5 (45.5) Wild-type: 92 (52.9) |
White Carriers: 11 (100) Wild-type: 171 (98.3) Other Carriers: 0 Wild-type: 3 (1.7) |
Colorectal Carriers: 8 (72.7) Wild-type: 134 (77.0) Breast Carriers: 1 (9.1) Wild-type: 21 (12.1) Other Carriers: 2 (18.2) Wild-type: 19 (10.9) |
Capecitabine: 185 (100.0) |
| Stavraka et al, 201923 63 (2/61) |
Median (range) 58 (28–85) | 2 (3.0) | NR | Metastatic: 63 (100.0) | Capecitabine: 63 (100.0) |
| Henricks et al, 201872 1,103 (85/1,018) |
Median (range) Carriers: 63 (54–71) Wild-type: 64 (56–71) |
Carriers: 48 (52.9) Wild-type: 545 (53.5) |
White Carriers: 84 (98.8) Wild-type: 964 (94.7) Black Carriers: 0 (0.0) Wild-type: 19 (1.9) Asian Carriers: 1 (1.2) Wild-type: 23 (2.3) Other Carriers: 0 (0.0) Wild-type: 12 (1.2) |
Colorectal Carriers: 56 (65.9) Wild-type: 648 (63.7) Breast Carriers: 10 (11.8%) Wild-type: 131 (12.9%) Gastric Carriers: 6 (7.1) Wild-type: 57 (5.6%) Other Carriers: 13 (15.3) Wild-type: 182 (17.9%) |
5-FU Carriers: 17 (20.0) Wild-type: 171 (16.8) Capecitabine Carriers: 68 (80.0) Wild-type: 847 (83.2) Patients treated with either 5-FU or capecitabine who also received radiotherapy Carriers: 24 (28.2) Wild-type: 303 (29.8) |
| Lunenburg et al, 20187 828 (23/771/34a) |
Median (range) Carriers: 66 (50–78) Carriersa: 64 (45–79) Wild-type: 63 (23–88) |
Carriers: 13 (56.5) Carriersa: 20 (58.8) Wild-type: 432 (56.0) |
NR |
Colorectal Carriers: 18 (78.3) Carriersa: 22 (64.7) Wild-type: 554 (71.9) Gastrointestinal Carriers: 4 (17.3) Carriersa: 12 (35.3) Wild-type: 169 (21.9) Other Carriers: 1 (4.3) Carriersa: 0 (0.0) Wild-type: 48 (6.3) |
5-FU Carriers: 3 (13.0) Carriersa: 5 (14.7) Wild-type: 103 (13.4) Capecitabine Carriers: 20 (87.0) Carriersa: 29 (85.3) Wild-type: 668 (86.6) Radiotherapy All patients received radiotherapy |
Abbreviations: 5-FU, 5-fluorouracil; NR = not reported; SD, standard deviation.
DPYD carriers who received a standard dose.
Appendix 5: Clinical Evidence Review—Study Results
Table A11:
Prevalence of DPYD Variants (Results for Heterozygous Carriers Unless Otherwise Specified)
| Author, Year N | DPYD∗2A, n (%) | DPYD∗13, n (%) | c.2846A>T, n (%) | c.1236G>A, n (%) | Compound heterozygous, n (%) | Combined (3 variantsa), n (%) | Combined (4 variants), n (%) |
|---|---|---|---|---|---|---|---|
| Wigle et al, 202171 1,397 |
9 (0.6) | 1 (0.07) | 19 (1.4) | 59 (4.2) | 2 (0.14) Variants not specified |
29 (2.1) | 88 (6.3) |
| Iachetta et al, 201952 366 |
NA | 2 (0.6) | 5 (1.4) | NA | NA | NA | NA |
| Kleinjan et al, 201912 185 |
4 (2.2) | 0 | 1 (0.5) | 6 (3.2) | 0 | 5 (2.7) | 11 (5.9) |
| Maharjan et al, 201953 113 |
3 (2.7) | 0 | 2 (1.8) | NA | NA | 5 (4.4) | NA |
| Stavraka et al, 201923 66 |
1 (1.5) | 0 | 2 (3.0) | NA | 0 | 3 (4.5) | NA |
| Cremolini et al, 201855 443 |
5 (1.1) | 0 | 5 (1.1) | NA | NA | 10 (2.2) | NA |
| Henricks et al, 201872 1,103 |
16 (1.5) | 1 (0.09) | 17 (1.5) | 51 (4.6) | NA | 33 (3.1) | 85 (7.7) |
| Lunenburg et al, 20187 828 |
13 (1.6) | 1 (0.1) | 10 (1.2) | 31 (3.7) Ht 2 (0.2) Hm |
NA | 24 (2.9) | 57 (6.972,74) |
| Nahid et al, 201854 161 |
8 (5.0) | NA | NA | NA | NA | NA | NA |
| Etienne-Grimaldi et al, 201756 243 |
3 (1.2) | 1 (0.4) | 3 (1.2) | 4 (1.6) | 0 | 7 (2.9) Ht | 11 (4.5) Ht |
| Meulendijks et al, 201770 185 |
NA | NA | 5 (2.3) | 15 (8.1) | NA | NA | NA |
| Boige et al, 201615 1,545 |
11 (0.7) | 4 (0.3) | 21 (1.4) | NA | NA | 36 (2.4) | NA |
| Lee et al, 201613 1,953 |
NA | NA | NA | 77 (3.9) Ht 1 (0.05) Hm |
NA | NA | NA |
| Froehlich et al, 201557 500 |
4 (0.8) | 2 (0.4) | 3 (0.6) | 22 (4.4) Ht 1 (0.2) Hm |
DPYD∗13/c.1236G>A 1 (0.2) |
9 (1.8) | 31 (6.4) |
| Toffoli et al, 20159 603 |
11 (1.8) | 1 (0.2) | 5 (0.8) | NA |
DPYD∗2A/DPYD∗13 1 (0.2) |
18 (3.0) | NA |
| Lee et al, 201458 2,886 |
27 (0.9) | 4 (0.1) | 32 (1.1) | NA |
DPYD∗2A/c.2846A>T 1 (0.03) |
64 (2.2) | NA |
| Jennings et al, 201359 253 |
3 (1.2) | NA | 2 (0.8) | 10 (3.9) | NA | NA | NA |
| Loganayagam et al, 201360 430 |
4 (0.9) | 1 (0.2) | 5 (1.1) | NA | 0 | 10 (2.2) | NA |
| Cellier et al, 201162 85 |
1 (1.2) | 1 (1.2) | 1 (1.2) | NA | NA | 3 (3.5) | NA |
| Deenen et al, 201161 568 |
7 (1.2) | 0 | 8 (1.4) | 27 (4.8) Ht 1 (0.2) Hm |
NA | NA | 42 (7.4) |
| Cerić et al, 201063 50 |
1 (2.0) | NA | NA | NA | NA | NA | NA |
| Braun et al, 200964 750 |
7 (0.9) | NA | NA | NA | NA | NA | NA |
| Schwab et al, 200865 683 |
13 (1.9) | NA | NA | NA | NA | NA | NA |
| Sulzyc-Bielicka et al, 200866 252 |
1 (0.4) | NA | NA | NA | NA | NA | NA |
| Boisdron-Celle et al, 200767 252 |
2 (0.8) | NA | 7 (2.8) | NA |
DPYD∗2A/c.2846A>T 1 (0.4) |
NA | NA |
| Largillier et al, 200668 105 |
1 (1.0) | NA | NA | NA | NA | NA | NA |
| Salgueiro et al, 200469 73 |
1 (1.4) | NA | NA | NA | NA | NA | NA |
Abbreviations: Hm, homozygous; Ht, heterozygous; NA, not applicable.
DPYD∗2A, DPYD∗13, and c.2846A>T.
Table A12:
Fluoropyrimidine Dose Reduction, Treatment Delay, and Discontinuation Due to Toxicity
| Author, Year N (Carrier/Wild-Type) | DPYD∗2A, n (%) | DPYD∗13, n (%) | c.2846A>T, n (%) | c.1236G>A, n (%) | Compound heterogeneous, n (%) | Combined (3–4 variants), n (%) |
|---|---|---|---|---|---|---|
| Wigle et al, 202171 1,388 (41/1,347) |
NR | NR | NR |
Treatment discontinuation Carriers: 7 (17.1) Wild-type: 232 (17.2) RR (95% CI): 0.99 (0.44–1.83) |
NR | NR |
| Nahid et al, 201854 161 (8/153) |
Dose reduction Carriers: 8 (100.0) Wild-type: NR Treatment delay NR Treatment discontinuation Carriers: 4 (50.0) Wild-type: NR |
NR | NR | NR | NR | NR |
| Froehlich et al, 201557 500 (32/468) |
Any treatment modificationa Carriers: 2 (50.0) |
Any treatment modificationa Carriers: 2 (100.0) |
Any treatment modificationa Carriers: 1 (33.3) |
Any treatment modificationa Carriers: 12 (50.0) |
NR |
Any treatment modificationa Carriers (4 variants): 16 (48.5) Carriers (3 variants: DPYD∗2A, DPYD∗13, c.2846A>T): 5 (55.6) |
| Toffoli et al, 20159 DPYD∗2A 596 (11/585) DPYD∗13 586 (1/585) c.2846A>T 590 (5/585) DPYD∗2A/ DPYD∗13 586 (1/585) |
Dose reduction Carriers: 1 (9.1) Wild-type: NR Treatment delay Carriers: 4 (36.4) Wild-type: NR Treatment discontinuation Carriers: 6 (54.5) Wild-type: NR |
Dose reduction Carriers: 1 (100) Wild-type: NR Treatment delay Carriers: 1 (100) Wild-type: NR Treatment discontinuation Carriers: 0 Wild-type: NR |
Dose reduction Carriers: 1 (20.0) Wild-type: NR Treatment delay Carriers: 3 (60.0) Wild-type: NR Treatment discontinuation Carriers: 1 (20.0) Wild-type: NR |
Not evaluated |
Dose reduction Carriers: 0 Wild-type: NR Treatment delay Carriers: 0 Wild-type: NR Treatment discontinuation Carriers: 1 (100); fatal toxicity Wild-type: NR |
NR |
| Lee et al, 201458 DPYD∗2A 2,589 (25/2,564) DPYD∗13 2,568 (4/2,564)b c.2846A>T 2,589 (27/2,562) DPYD∗2A/ c.2846A>T 2,565 (1/2,564)b |
Dose reduction Carriers: 20 (80.0) Wild-type: 1884 (74.3) RR (95% CI): 1.08 (0.82–1.26) Treatment delay NR Treatment discontinuation Carriers: 11 (44.0) Wild-type: 653 (25.5) RR (95% CI): 1.73 (1.00–2.51) |
Dose reduction Carriers: 3 (75.0) Wild-type: 1904 (74.3) RR (95% CI): 1.01 (0.27–1.23) Treatment delay NR Treatment discontinuation Carriers: 3 (75.0) Wild-type: 1,921 (74.9) RR (95% CI): 1.00 (0.27–1.32) |
Dose reduction Carriers: 20 (74.1) Wild-type: 1,884 (73.5) RR (95% CI): 1.01 (0.74–1.20) Treatment delay NR Treatment discontinuation Carriers: 8 (29.6) Wild-type: 656 (25.6) RR (95% CI): 1.16 (0.57–1.91) |
NR | NR | NR |
| Loganayagam et al, 201360 DPYD∗2A 424 (4/420) DPYD∗13 421 (1/420) c.2846A>T 425 (5/420) |
Dose reduction Carriers: 2 (50.0) Wild-type: 98 (23.3) RR (95% CI): 2.15 (0.34–4.58) Treatment delay NR Treatment discontinuation Carriers: 2 (50.0) Wild-type: 27 (6.4) RR (95% CI): 7.81 (1.36–23.30) |
Dose reduction Carriers: 1 (100.0) Wild-type: 98 (23.3) RR (95% CI): 4.29 (1.93–5.06) Treatment delay NR Treatment discontinuation Carriers: 0 Wild-type: 27 (6.4) P = 1.00 |
Dose reduction Carriers: 3 (60.0) Wild-type: 98 (23.3) RR (95% CI): 2.58 (0.73–4.58) Treatment delay NR Treatment discontinuation Carriers: 2 (40.0) Wild-type: 27 (6.4) RR (95% CI): 6.25 (1.06–20.44) |
NR | None observed | 3 variants (DPYD∗2A, DPYD∗13, c.2846A>T) Dose reduction Carriers: 6 (60.0) Wild-type: 98 (23.3) RR (95% CI): 2.58 (1.23–4.38) Treatment delay NR Treatment discontinuation Carriers: 4 (40.0) Wild-type: 27 (6.4) RR (95% CI): 6.25 (2.08–16.41) |
| Deenen et al, 201161 DPYD∗2A 567 (7/560) DPYD∗13 561 (1/560) c.2846A>T 568 (8/560) c.1236G>A 568 (28/540) |
Mean dose reduction Carriers: 50% Wild-type: 10% Treatment delay NR Treatment discontinuation NR |
No carrier identified |
Mean dose reduction Carriers: 25% Wild-type: 10% |
NR | NR | NR |
| Braun et al, 200964 750 (7/743) |
Dose reduction or delay Carriers: 4 (57.1) Wild-type: 265 (35.7) RR (95% CI): 1.60 (0.56–2.54) Treatment discontinuation NR |
NR | NR | NR | NR | NR |
| Largillier et al, 200668 105 (1/104) |
Dose reduction NR Treatment delay NR Treatment discontinuation Carriers: 1 (100); death Wild-type: 15 (14.4); severe toxicity (13), death (2) RR (95% CI): 6.94 (2.43–11.51) |
NR | NR | NR | NR | NR |
| Salgueiro et al, 200469 73 (1/72) |
Dose reduction NR Treatment delay NR Treatment discontinuation Carriers: 1 (100); 2nd cycle due to grade 4 febrile neutropenia and grade 3 gastrointestinal toxicity Wild-type: NR |
NR | NR | NR | NR | NR |
Abbreviations: CI, confidence interval; NR, not reported; RR, risk ratio.
Can include dose reduction, therapy delay, or withdrawal due to toxicity.
Denominator not provided; we assumed that it would be the same as for DPYD∗2A.
Table A13:
Clinical Validity Study Results—Overall Severe (Grade ≥ 3) Toxicity (All Variants)
| Author, Year N (Carriers/Wild-Type) | DPYD∗2A, n (%) | DPYD∗13, n (%) | c.2846A>T, n (%) | c.1236G>A, n (%) | Compound heterozygous, n (%) | Combined (3–4 variants), n (%) |
|---|---|---|---|---|---|---|
| Wigle et al, 202171 1,388 (41/1,347) |
NR | NR | NR | Carriers: 14 (34.1) Wild-type: 418 (31.0) RR (95% CI): 1.10 (0.67–1.62) |
NR | NR |
| Iachetta et al, 201952 DPYD∗13: 366 (2/364) c.2846A>T: 366 (5/361) |
NR | Carriers: 2 (100.0) Wild-type: 144 (39.6) RR (95% CI): 2.53 (1.18–2.90) Time of occurrence, median cycle Carriers: 2 Wild-type: 8 P < .0001 |
Carriers: 5 (100.0) Wild-type: 141 (39.1) RR (95% CI): 2.56 (1.36–2.92) Time of occurrence, median cycle Carriers: 1 Wild-type: 8 P < .0001 |
NR | NR | NR |
| Cremolini et al, 201855 DPYD∗2A: 438 (5/433) DPYD∗13: 433 (0/433) c.2846A>T: 438 (5/433) |
Carriers: 4 (80.0) Wild-type: 217 (50.1) RR (95% CI): 1.60 (0.63–2.10) |
NR | Carriers: 4 (80.0) Wild-type: 217 (50.1) RR (95% CI): 1.60 (0.63–2.10) |
NR | NR | NR |
| Lunenburg et al, 20187 805 (34/771) |
NR | NR | NR | NR | NR |
4 variants under study Carriers: 8 (23.5) Wild-type: 105 (13.6) RR (95% CI): 1.72 (0.82–3.06) |
| Nahid et al, 201854 161 (8/153) |
Carriers: 7 (87.5) Wild-type: 71 (46.4) RR (95% CI): 1.88 (1.05–2.46) |
NR | NR | NR | NR | NR |
| Etienne-Grimaldi et al, 201756 DPYD∗2A: 242 (3/239) c.2846A>T: 242 (3/239) |
Carriers: 2 (66.7) Wild-type: 28 (11.7) RR (95% CI); 5.70 (1.06–11.23) |
NR | Carriers: 2 (66.7) Wild-type: 28 (11.7) RR (95% CI): 5.70 (1.06–11.23) |
NR | NR | NR |
| Meulendijks et al, 201770 c.2846A>T: 185 (5/180) c.1236G>A: 185 (15/170) |
NR | NR | Carriers: 3 (60.0) Wild-type: 85 (47.0) RR (95% CI): 1.28 (0.35–2.20) |
Carriers: 6 (40.0) Wild-type: 82 (48.0) RR (95% CI): 0.83 (0.37–1.63) |
NR | NR |
| Lee et al, 201613 c.1236G>A: 1,953 (77 Ht/1 Hm/1,875) |
NR | NR | NR | Carriers (Ht): 31 (40.3) Carriers (Hm): 1 (100.0) Wild-type: 606 (32.3) RR (95% CI; Ht): 1.25 (0.91–1.61) RR (95% CI; Hm): 3.10 (1.47–3.31) |
NR | NR |
| Boige et al, 201615 DPYD∗2A: 1,545 (11/1,534) DPYD∗13 = 1,544 (4/1,540) c.2846A>T: 1,545 (21/1,524) Combined: 1,545 (36/1,509) |
Carriers: 8 (72.7) Wild-type: 757 (49.3) RR (95% CI): 1.47 (0.83–1.89) |
Carriers: 2 (50.0) Wild-type: 763 (49.5) RR (95% CI): 1.01 (0.14–1.83) |
Carriers: 18 (85.7) Wild-type: 747 (49.0) RR (95% CI): 1.75 (1.33–2.04) |
NR | NR | Could not be calculated because the number of events in wild-type patients for the combined analysis was unknown |
| Froehlich et al, 201557 DPYD∗2A: 472 (4/468) DPYD∗13: 469 (1/468) c.2846A>T: 471 (3/468) c.1236G>A: 480 (22/468) c.1236G>A: 469 (1/468) DPYD∗13/c.1236G>A: 469 (1/468) Combined: 498 (30/468; excludes compound heterozygous and homozygous) |
Carriers: 0 Wild-type: 40 (8.5) RR could not be calculated P = 1.00 |
Carriers: 0 Wild-type: 40 (8.5) RR could not be calculated P = 1.00 |
Carriers: 0 Wild-type: 40 (8.5) RR could not be calculated P = 1.00 |
Heterozygous Carriers: 9 (40.9) Wild-type: 40 (8.5) RR (95% CI): 4.81 4.79 (2.42–8.93) Homozygous Carriers: 1 (100.0) Wild-type: 38 (8.5) RR (95% CI): 11.76 (4.73–14.91) |
DPYD∗13/c.1236G>A Carriers: 1 (100.0) Wild-type: 40 (8.5) RR (95% CI): 11.76 (4.73–14.91) |
4 variants under study (excludes compound heterozygous and homozygous) Grade ≥ 3 Carriers: 9 (30.0) Wild-type: 38 (8.2) RR (95% CI): 3.66 (1.73–7.02) |
| Toffoli et al, 20159 DPYD∗2A: 596 (11/585) DPYD∗13: 586 (1/585) c.2846A>T: 590 (5/585) DPYD∗2A/ DPYD∗13 : 586 (1/585) Combined: 602 (17/585; excludes compound heterozygous) |
Carriers: 7 (63.6) Wild-type: 84 (14.4) RR (95% CI): 4.42 (2.32–7.07) |
Carriers: 0 Wild-type: 84 (14.4) RR could not be calculated P = 1.00 |
Carriers: 3 (60.0) Wild-type: 84 (14.4) RR (95% CI): 4.42 (1.19–7.87) |
NR | Carriers: 1 (100); fatal Wild-type: 84 (14.4) RR (95% CI): 6.94 (3.07– 8.34) |
3 variants: DPYD∗2A, DPYD∗13, c.2846A>T (excludes compound heterozygous) Carriers: 10 (58.8) Wild-type: 84 (14.4) RR (95% CI): 4.08 (2.36–6.41) |
| Lee et al, 201458 DPYD∗2A: 2,589 (25/2,564) DPYD∗13 = 2,568 (4/2,564)a c.2846A>T: 2,589 (27/2,562) DPYD∗2A/ c.2846A>T: 2,565 (1/2,564)a Combined: 2,618 (56/2,562; excludes compound heterozygous) |
Carriers: 22 (88.0) Wild-type: 834 (32.5) RR (95% CI): 2.70 (2.16–3.11) |
Carriers: 2 (50.0) Wild-type: 834 (32.5) RR (95% CI): 1.54 (0.22–2.78) |
Carriers: 22 (81.5) Wild-type: 835 (32.6) RR (95% CI): 2.50 (1.94–2.98) |
NR |
DPYD∗2A/ c.2846A>T Carriers: 1 (100.0) Wild-type: 834 (32.5)a RR (95% CI): 3.08 (1.47–3.25) |
3 variants: DPYD∗2A, DPYD∗13, c.2846A>T (excludes compound heterozygous) Carriers: 46 (82.5) Wild-type: 835 (32.6) RR (95% CI): 2.53 (2.14–2.89) |
| Jennings et al, 201359 DPYD∗2A: 253 (3/250) c.2846A>T: 253 (2/251) c.1236G>A: 253 (10/243) Combined (carrier/wild-type): 253 (15/238) |
Carriers: 2 (66.7) Wild-type: 42 (16.8) RR (95% CI): 3.97 (0.72–7.56) |
Not evaluated | Carriers: 2 (100.0) Wild-type: 42 (16.7) RR (95% CI): 5.99 (2.63–7.60) |
Carriers: 3 (30.0) Wild-type: 41 (16.8) RR (95% CI): 1.79 (0.47–5.21) |
NR | 3 variants: DPYD∗2A, c.2846A>T, c.1236G>A Carriers: 7 (46.7) Wild-type: 37 (15.5) RR (95% CI): 3.01 (1.41–6.00) |
| Loganayagam et al, 201360 DPYD∗2A: 424 (4/420) DPYD∗13: 421 (1/420) c.2846A>T: 425 (5/420) Combined: 430 (10/420) |
Carriers: 4 (100.0) Wild-type: 94 (22.4) RR (95% CI): 4.46 (2.18–5.29) |
Carriers: 1 (100.0) Wild-type: 94 (22.4) RR (95% CI): 4.46 (2.01–5.29) |
Carriers: 5 (100.0) Wild-type: 94 (22.4) RR (95% CI): 4.46 (2.40–5.29) |
NR | NR | 3 variants: DPYD∗2A, DPYD∗13, c.2846A>T Carriers: 10 (100.0) Wild-type: 94 (22.4) RR (95% CI): 4.46 (3.26–5.29) |
| Cellier et al, 201162 DPYD∗2A: 83 (1/82) DPYD∗13: 83 (1/82) c.2846A>T: 83 (1/82) Combined: 85 (3/82) |
Carriers: 1 (100.0) Wild-type: 34 (41.5) RR (95% CI): 2.41 (1.00–3.14) |
Carriers: 1 (100.0) Wild-type: 34 (41.5) RR (95% CI): 2.41 (1.00–3.14) |
Carriers: 1 (100.0) Wild-type: 34 (41.5) RR (95% CI): 2.41 (1.00–3.14) |
NR | NR | 3 variants: DPYD∗2A, DPYD∗13, c.2846A>T Carriers: 3 (100) Wild-type: 34 (41.5) RR (95% CI): 2.41 (1.11–3.14) |
| Deenen et al, 201161 DPYD∗2A: 567 (7/560) DPYD∗13: 561 (1/560) c.2846A>T: 568 (8/560) c.1236G>A: 568 (28/540) |
Carriers: 7 (100.0) Wild-type: 477 (85.2) RR (95% CI): 1.17 (0.72–1.23) |
NR | Carriers: 7 (87.5) Wild-type: 478 (85.4) RR (95% CI): 1.02 (0.59–1.19) |
Carriers: 26 (92.9) Wild-type: 459 (85.0) RR (95% CI): 1.09 (0.91–1.19) |
NR | NR |
| Cerić et al, 201063 DPYD∗2A: 50 (1/49) |
Carriers: 1 (100.0) Wild-type: 25 (51.0) RR (95% CI): 1.96 (0.82–2.67) |
NR | NR | NR | NR | NR |
| Braun et al, 200964 DPYD∗2A: 750 (7/743) | Carriers: 4 (57.1) Wild-type: 427 (57.5) RR (95% CI): 0.99 (0.34–1.54) |
NR | NR | NR | NR | NR |
| Schwab et al, 200865 DPYD∗2A: 683 (13/670) |
Carriers: 6 (46.2) Wild-type: 104 (15.5) RR (95% CI): 2.98 (1.34–5.54) |
NR | NR | NR | NR | NR |
| Boisdron-Celle et al, 200767 DPYD∗2A: 244 (2/242) c.2846A>T: 249 (7/242) Compound heterozygous: 243 (1/242) |
Carriers: 1 (50.0) Wild-type: 8 (3.3) RR (95% CI): 15.15 (0.63–75.94) |
NR | Carriers: 5 (71.4) Wild-type: 8 (3.3) RR (95% CI): 21.64 (8.30–52.26) |
NR |
DPYD∗2A/ c.2846A>T Carriers: 1 (100.0) Wild-type: 8 (3.3) RR (95% CI): 30.30 (9.10–52.26) |
NR |
| Largillier et al, 200668 105 (1/104) 1st cycle |
Carriers: 1 (100.0) Wild-type: 18 (17.3) RR (95% CI): 5.78 (2.12–7.96) |
NR | NR | NR | NR | NR |
| Salgueiro et al, 200469 DPYD∗2A: 73 (1/72) |
Carriers: 1 (100.0) Wild-type: 7 (9.7) RR (95% CI): 10.31 (3.14–18.51) |
NR | NR | NR | NR | NR |
Abbreviations: CI, confidence interval; Hm, homozygous; Ht, heterozygous; NR, not reported; RR, risk ratio.
Denominator not reported specifically for this group; assumed it would be the same as wild-type for other variants in this study.
Table A14:
Clinical Validity Study Results—Severe (Grade ≥ 3) Hematological Toxicity (DPYD∗2A)
| Author, Year N (Carriers/Wild-Type) | Neutropenia, n (%) | Leukopenia, n (%) | Anemia | Thrombocytopenia | Febrile Neutropenia | Overall Hematological |
|---|---|---|---|---|---|---|
| Maharjan et al, 201953 DPYD∗2A: 55 (3/52) |
Carriers: 1 (33.3) Wild-type: 4 (7.7) RR (95% CI): 4.32 (0.20–38.56) |
NR | Carriers: 0 Wild-type: 0 RR could not be calculated |
Carriers: 0 Wild-type: 0 RR could not be calculated |
Carriers: 0 Wild-type: 0 RR could not be calculated |
NR |
| Cremolini et al, 201855 DPYD∗2A: 438 (5/433) |
Carriers: 4 (80.0) Wild-type: 156 (36.0) RR (95% CI): 2.22 (0.90–3.04) |
NR | Carriers: 0 Wild-type: 6 (1.4) RR could not be calculated P = 1.00 |
Carriers: 1 (20.0) Wild-type: 5 (1.2) RR (95% CI): 16.67 (0.77–143.35) |
Carriers: 1 (20.0) Wild-type: 34 (7.9) RR (95% CI): 2.53 (0.09–16.45) |
Carriers: 4 (80.0) Wild-type: 163 (37.6) RR (95% CI): 2.13 (0.86–2.90) |
| Nahid et al, 201854 161 (8/153) |
Carriers: 5 (62.5) Wild-type: 40 (26.1) RR (95% CI): 2.39 (1.04–4.42) |
Carriers: 1 (12.5) Wild-type: 15 (9.8) RR (95% CI): 1.28 (0.05–10.46) |
Carriers: 4 (50.0) Wild-type: 31 (20.3) RR (95% CI): 2.46 (0.88–5.86) |
Carriers: 2 (25.0) Wild-type: 11 (7.2) RR (95% CI): 3.47 (0.60–15.85) |
NR | NR |
| Boige et al, 201615 1,545 (11/1,534) |
Carriers: 7 (63.7) Wild-type: 555 (36.2) RR (95% CI): 1.76 (0.90–2.43) |
NR | NR | NR | NR | Carriers: 7 (63.7) Wild-type: 610 (39.7) RR (95% CI): 1.60 (0.82–2.21) |
| Toffoli et al, 20159 596 (11/585) |
Carriers: 5 (45.5) Wild-type: 38 (6.5) RR (95% CI): 7.00 (2.81–15.14) |
Carriers: 1 (9.1) Wild-type: 8 (1.4) RR (95% CI): 6.50 (0.28–63.87) |
NR | Carriers: 1 (9.1) Wild-type: unclear RR could not be calculated |
NR | Carriers: 5 (45.5) Wild-type: 45 (7.7) RR (95% CI): 5.91 (2.40–12.82) |
| Lee et al, 201458 2,589 (25/2,564) |
Carriers: 16 (64.0) Wild-type: 288 (11.2) RR (95% CI): 5.71 (3.89–7.94) |
Carriers: 2 (8.0) Wild-type: 47 (1.8) RR (95% CI): 4.44 (0.67–14.79) |
NR | Carriers: 1 (4.0) Wild-type: 8 (0.3) RR (95% CI): 13.33 (0.53–130.48) |
Carriers: 2 (8.0) Wild-type: 42 (1.6) RR (95% CI): 5.00 (0.75–16.74) |
NR |
| Cerić et al, 201063 50 (1/49) |
Carriers: 1 (100.0) Wild-type: 13 (26.5) RR (95% CI): 3.77 (1.38–5.47) |
NR | NR | NR | NR | NR |
| Schwab et al, 200865 683 (13/670) |
NR | Carriers: 4 (30.8) Wild-type: 28 (4.2) RR (95% CI): 7.33 (2.44–19.45) |
NR | NR | NR | Carriers: 4 (30.8) Wild-type: 28 (4.2) RR (95% CI): 7.33 (2.44–19.45) |
| Sulzyc-Bielicka et al, 200866 252 (1/251) |
Not evaluated | Not evaluated | NR | NR | NR | Carriers: 1 (100.0) Wild-type: 3 (1.2) RR (95% CI): 83.33 (17.94–221.92) |
| Largillier et al, 200668 105 (1/104) |
Carriers: 1 (100.0) Wild-type: 2 (1.9) RR (95% CI): 52.63 (10.40–120.90) |
NR | NR | NR | NR | NR |
| Salgueiro et al, 200469 73 (1/72) |
Carriers: 1 (100.0) Wild-type: 5 (6.9) RR (95% CI): 14.49 (3.78–25.19) |
NR | Carriers: 0 Wild-type: 1 (1.4) RR could not be calculated P = 1.00 |
Carriers: 0 Wild-type: 1 (1.4) RR could not be calculated P = 1.00 |
Carriers: 1 (100.0) Wild-type: 0 RR could not be calculated P = .01 |
Carriers: 1 (100.0) Wild-type: 5 (6.9) RR (95% CI): 14.49 (3.78–25.19) |
Abbreviations: C, carrier; CI, confidence interval; NR, not reported; RR, risk ratio.
Table A15:
Clinical Validity Study Results—Severe (Grade ≥ 3) Gastrointestinal Toxicity (DPYD∗2A)
| Author, Year N (Carriers/Wild-Type) | Mucositis and Stomatitis, n (%) | Nausea, n (%) | Vomiting, n (%) | Diarrhea, n (%) | Overall Gastrointestinal, n (%) |
|---|---|---|---|---|---|
| Maharjan et al, 201953 DPYD∗2A: 55 (3/52) |
Carriers: 0 Wild-type: 2 (3.8) RR could not be calculated P = 1.00 |
Carriers: 0 Wild-type: 3 (5.8) RR could not be calculated P = 1.00 |
Carriers: 0 Wild-type: 2 (3.8) RR could not be calculated P = 1.00 |
Carriers: 3 (100.0) Wild-type: 10 (19.2) RR (95% CI): 5.21 (1.96–9.37) |
NR |
| Cremolini et al, 201855 438 (5/433) |
Carriers: 2 (40.0) Wild-type: 26 (6.0) RR (95% CI): 6.67 (1.14–21.94) |
Carriers: 0 Wild-type: 14 (3.2) RR could not be calculated P = 1.00 |
Carriers: 0 Wild-type: 18 (4.2) RR could not be calculated P = 1.00 |
Carriers: 0 Wild-type: 64 (14.8) RR could not be calculated P = 1.00 |
Carriers: 2 (40) Wild-type: 96 (22.2) RR (95% CI): 1.83 (0.28–4.40) |
| Nahid et al, 201854 161 (8/153) |
Carriers: 1 (12.5) Wild-type: 8 (5.2) RR (95% CI): 2.40 (0.10–20.63) |
Carriers: 3 (37.5) Wild-type: 18 (11.8) RR (95% CI): 3.18 (0.88–10.07) |
Carriers: 3 (37.5) Wild-type: 22 (14.4) RR (95% CI): 2.60 (0.70–7.42) |
Carriers: 5 (62.5) Wild-type: 42 (27.5) RR (95% CI): 2.27 (0.99–4.18) |
NR |
| Boige et al, 201615 1,545 (11/1,534) |
Carriers: 0 Wild-type: 73 (4.8) RR could not be calculated P = 1.00 |
Carriers: 0 Wild-type: 34 (2.2) RR could not be calculated P = 1.00 |
NR | Carriers: 5 (45.5) Wild-type: 187 (12.2) RR (95% CI): 3.73 (1.51–6.91) |
Carriers: 5 (45.5) Wild-type: 264 (17.2) RR (95% CI): 2.65 (1.06–4.36) |
| Toffoli et al, 20159 596 (11/585) |
Carriers: 0 Wild-type: 7 (1.2) RR could not be calculated P = 1.00 |
Nausea and vomiting Carriers: 1 (9.1) Wild-type: 7 (1.2) RR (95% CI): 7.58 (0.32–82.00) |
Carriers: 3 (27.3) Wild-type: 34 (5.8) RR (95% CI): 4.71 (1.16–14.43) |
NR | |
| Lee et al, 201458 2,589 (25/2,564) |
Carriers: 3 (12.0) Wild-type: 107 (4.2) RR (95% CI): 2.86 (0.71–7.39) |
Nausea and vomiting Carriers: 5 (20.0) Wild-type: 124 (4.8) RR (95% CI): 4.17 (1.54–8.42) |
Carriers: 3 (12.0) Wild-type: 305 (11.9) RR (95% CI): 1.01 (0.24–2.51) |
NR | |
| Deenen et al, 201161 567 (7/560) |
NR | NR | NR | Carriers: 5 (71.4) Wild-type: 134 (23.9) RR (95% CI): 2.99 (1.35–4.44) |
NR |
| Cerić et al, 201063 50 (1/49) |
Carriers: 1 (100.0) Wild-type: 6 (12.2) RR (95% CI): 8.20 (2.43–15.04) |
NR | NR | Carriers: 1 (100.0) Wild-type: 12 (24.5) RR (95% CI): 4.08 (1.45–5.94) |
NR |
| Schwab et al, 200865 683 (13/670) |
Carriers: 4 (30.8) Wild-type: 48 (7.2) RR (95% CI): 4.28 (1.38–10.88) |
NR | NR | Carriers: 3 (23.1) Wild-type: 56 (8.4) RR (95% CI): 2.75 (0.72–8.28) |
NR |
| Largillier et al, 200668 105 (1/104) |
Carriers: 1 (100.0) Wild-type: 1 (1.0) RR (95% CI): 100.00 (13.32–150.75) |
Nausea and vomiting Carriers: 0 Wild-type: 6 (5.8) RR could not be calculated |
Carriers: 1 (100.0) Wild-type: 5 (4.8) RR (95% CI): 20.83 (5.56–35.60) |
NR | |
| Salgueiro et al, 200469 73 (1/72) |
Carriers: 0 Wild-type: 2 (2.8) RR could not be calculated P = 1.00 |
Nausea and vomiting Carriers: 0 Wild-type: 0 |
NR | Carriers: 0 Wild-type: 1 (1.4) RR could not be calculated P = 1.00 |
Carriers: 0 Wild-type: 3 (4.2) RR could not be calculated P = 1.00 |
Abbreviations: CI, confidence interval; NR, not reported; RR, risk ratio.
Table A16:
Clinical Validity Study Results—Severe (Grade ≥ 3) Hematological Toxicity (c.2846A>T)
| Author, Year N (Carriers/Wild-Type) | Neutropenia, n (%) | Leukopenia, n (%) | Anemia, n (%) | Thrombocytopenia, n (%) | Febrile Neutropenia, n (%) | Overall Hematological, n (%) |
|---|---|---|---|---|---|---|
| Maharjan et al, 201953 54 (2/52) |
Carriers: 0 Wild-type: 4 (7.7) RR could not be calculated P = 1.00 |
NR | Carriers: 0 Wild-type: 0 |
Carriers: 0 Wild-type: 0 |
Carriers: 0 Wild-type: 0 |
Carriers: 0 Wild-type: 4 (7.7) RR could not be calculated P = 1.00 |
| Cremolini et al, 201855 438 (5/433) |
Carriers: 3 (60.0) Wild-type: 156 (36.0) RR (95% CI): 1.67 (0.46–2.75) |
NR | Carriers: 0 Wild-type: 6 (1.4) RR could not be calculated P = 1.00 |
Carriers: 0 Wild-type: 5 (1.2) RR could not be calculated P = 1.00 |
Carriers: 1 (20.0) Wild-type: 34 (7.9) RR (95% CI): 2.53 (0.87–16.45) |
Carriers: 3 (60.0) Wild-type: 163 (37.6) RR (95% CI): 1.60 (0.44–2.56) |
| Meulendijks et al, 201770 c.2846A>T: 185 (5/180) |
NR | NR | NR | NR | NR | Carriers: 2 (40.0) Wild-type: 58 (32.0) RR (95% CI): 1.25 (020–3.38) |
| Boige et al, 201615 1,545 (21/1,524) |
Carriers: 13 (61.9) Wild-type: 549 (36.0) RR (95% CI): 1.72 (1.11–2.26) |
NR | NR | NR | NR | Carriers: 16 (76.2) Wild-type: 601 (39.4) RR (95% CI): 1.93 (1.38–2.38) |
| Toffoli et al, 20159 590 (5/585) |
Carriers: 1 (20.0) Wild-type: 38 (6.5) RR (95% CI): 3.08 (0.10–19.69) |
NR | NR | NR | NR | Carriers: 1 (20.0) Wild-type: 45 (7.7) RR (95% CI): 2.60 (0.09–13.90) |
| Lee et al, 201458 2,589 (27/2,562) |
Carriers: 15 (55.6) Wild-type: 289 (11.3) RR (95% CI): 4.92 (3.22–6.72) |
Carriers: 4 (14.8) Wild-type: 46 (1.8) RR (95% CI): 8.22 (2.54–20.07) |
NR | Carriers: 3 (11.1) Wild-type: 6 (0.2) RR (95% CI): 55.50 (10.69–223.31) |
Carriers: 2 (7.4) Wild-type: 42 (1.6) RR (95% CI): 4.63 (0.70–15.65) |
NR |
Abbreviations: CI, confidence interval; NR, not reported; RR, risk ratio.
Table A17:
Clinical Validity Study Results—Severe (Grade ≥ 3) Gastrointestinal Toxicity (c.2846A>T)
| Author, Year N (Carriers/Wild-Type) | Mucositis, n (%) | Nausea, n (%) | Vomiting, n (%) | Diarrhea, n (%) | Overall Gastrointestinal, n (%) |
|---|---|---|---|---|---|
| Maharjan et al, 201953 54 (2/52) |
Carriers: 0 Wild-type: 2 (3.8) RR could not be calculated P = 1.00 |
Carriers: 0 Wild-type: 3 (5.8) RR could not be calculated P = 1.00 |
Carriers: 0 Wild-type: 2 (3.8) RR could not be calculated P = 1.00 |
Carriers: 2 (100) Wild-type: 10 (19.2) RR (95% CI): 5.21 (1.67–9.37) |
NR |
| Cremolini et al, 201855 438 (5/433) |
Stomatitis Carriers: 2 (40.0) Wild-type: 26 (6.0) RR (95% CI): 6.67 (1.14–21.94) |
Carriers: 0 Wild-type: 14 (3.2) RR could not be calculated P = 1.00 |
Carriers: 0 Wild-type: 18 (4.2) RR could not be calculated P = 1.00 |
Carriers: 2 (40.0) Wild-type: 64 (14.8) RR (95% CI): 2.70 (0.43–7.26) |
Carriers: 4 (80.0) Wild-type: 96 (22.2) RR (95% CI): 3.60 (1.49–5.32) |
| Meulendijks et al, 201770 c.2846A>T: 185 (5/180) |
NR | NR | NR | NR | Carriers: 0 Wild-type: 25 (14.0) RR could not be calculated P = 1.00 |
| Boige et al, 201615 1,545 (21/1,524) |
Carriers: 5 (23.8) Wild-type: 68 (4.5) RR (95% CI): 5.29 (2.00–11.07) |
Carriers: 0 Wild-type: 34 (2.2) RR could not be calculated |
NR | Carriers: 3 (14.3) Wild-type: 189 (12.4) RR (95% CI): 1.15 (0.28–2.83) |
Carriers: 7 (33.3) Wild-type: 262 (17.2) RR (95% CI): 1.94 (0.91–3.26) |
| Toffoli et al, 20159 590 (5/585) |
Stomatitis Carriers: 0 Wild-type: 7 (1.2) RR could not be calculated P = 1.00 |
Nausea and vomiting Carriers: 0 Wild-type: 8 (1.4) RR could not be calculated P = 1.00 |
Carriers: 2 (40.0) Wild-type: 34 (5.8) RR (95% CI): 6.90 (1.15–22.19) |
NR | |
| Lee et al, 201458 2,589 (27/2,562) |
Mucositis/stomatitis Carriers: 2 (7.4) Wild-type: 106 (4.1) RR (95% CI): 1.80 (0.26–5.76) |
Nausea and vomiting Carriers: 2 (7.4) Wild-type: 127 (5.0) RR (95% CI): 1.48 (0.21–4.77) |
Carriers: 9 (33.3) Wild-type: 299 (11.7) RR (95% CI): 2.85 (1.49–4.57) |
NR | |
| Deenen et al, 201161 568 (8/560) |
NR | NR | NR | Carriers: 5 (62.5) Wild-type: 134 (23.9) RR (95% CI): 2.62 (1.13–4.27) |
NR |
Table A18:
Clinical Utility Study Results—Severe (Grade ≥ 3) Toxicity
| Author, Year N (Carriers/Wild-Type) | Hematological Toxicity, n (%) | Gastrointestinal Toxicity, n (%) | Hand–Foot Syndrome, n (%) | Overall Toxicity, n (%) | Treatment-Related Mortality, n (%) | Toxicity-Related Hospitalizations, n (%) |
|---|---|---|---|---|---|---|
| Wigle et al, 202171 1,394 (47/1,347) |
Carriers: 6 (12.8) Wild-type: 157 (11.7) RR (95% CI): 1.09 (0.44–2.18) By genotype DPYD∗2A: 2 (22.2) DPYD∗13: 0 c.2846A>T: 2 (10.5) c.1236G>A: 2 (11.1) |
Carriers: 6 (12.8) Wild-type: 167 (12.4) RR (95% CI): 1.03 (0.42–2.05) By genotype DPYD∗2A: 2 (22.2) DPYD∗13: 0 c.2846A>T: 2 (10.5) c.1236G>A: 2 (11.1) |
Carriers: 1 (2.1) Wild-type: 35 (2.6) RR (95% CI): 0.81 (0.03–4.68) By genotype DPYD∗2A: 0 DPYD∗13: 0 c.2846A>T: 1 (5.3) c.1236G>A: 0 |
Carriers: 11 (23.4) Wild-type: 418 (31.0) RR (95% CI): 0.75 (0.41–1.21) By genotype DPYD∗2A: 3 (33.3) DPYD∗13: 0 c.2846A>T: 5 (26.3) c.1236G>A: 3 (16.7) |
Carriers: 0 Wild-type: 10 (0.7) RR could not be calculated P > .99a |
NR |
| Henricks et al, 201911 1,646 (40/1,606) DPYD∗2A |
Carriers: 4 (10.0) Wild-type: 158 (10.0) RR (95% CI): 1.00 (0.32–2.35) |
Carriers: 4 (10.0) Wild-type: 150 (9.3) RR (95% CI): 1.08 (0.34–2.48) |
Carriers: 2 (5.0) Wild-type: 84 (5.2) RR (95% CI): 0.96 (0.14–3.21) |
Carriers: 7 (17.5) Wild-type: 372 (23.2) RR (95% CI): 0.75 (0.34–1.38) |
Carriers: 0 Wild-type: 2 (0.1) RR could not be calculated P > .99 |
Carriers: 6 (15.0) Wild-type: 179 (11.1) RR (95% CI): 1.35 (0.55—2.64) |
| Kleinjan et al, 201912 185 (11/174) 4 variants |
Carriers: 1 (9.1) Wild-type: 6 (3.4) RR (95% CI): 2.68 (0.11–28.11) |
Diarrhea Carriers: 2 (18.2) Wild-type: 34 (19.5) RR (95% CI): 0.93 (0.15–3.93) |
Carriers: 0 Wild-type: 35 (20.1) RR could not be calculated P = .13 |
Carriers: 3 (27.3) Wild-type: 66 (37.9) RR (95% CI): 0.72 (0.18—2.02) Cycle of 1st toxicity, median (range) Carriers: 2 (1–2) Wild-type: 2 (1–6) P = .33 Toxicity after dose increase Carriers: 1 (9.1) Wild-type: NA |
NR | Carriers: 2 (18.2) Wild-type: 20 (11.5) (diarrhea in both groups) RR (95% CI): 1.58 (0.26–7.39) Days in hospital, median (range) Carriers: 6.5 (6–7) Wild-type: 8 (1–34) P = .62 |
| Stavraka et al, 201923 63 (2/61) 3 variants, but none had DPYD∗13 |
Carriers: 1 (50.0) Wild-type: 0 RR could not be calculated P = .03 |
Carriers: 1 (50.0) Wild-type: 5 (8.2) RR (95% CI): 6.10 (0.27–35.23) |
Carriers: 1 (50.0) Wild-type: 9 (14.8) RR (95% CI): 3.38 (0.14–16.79) |
Carriers: 1 (50.0) Wild-type: 14 (23.0) RR (95% CI): 2.17 (0.08–9.17) |
Carriers: 0 Wild-type: 0 RR could not be calculated |
Carriers: 1 (50.0) Wild-type: 0 RR could not be calculated P = .03 |
| Henricks et al, 201872 1,103 (85/1,018) 4 variants DPYD∗2A: 16 DPYD∗13: 1 c.2846A>T: 17 c.1236G>A: 51 |
Carriers: 13 (15.3) Wild-type: 65 (6.4) RR (95% CI): 2.39 (1.29–4.09) By genotype DPYD∗2A: 2 (12.5) DPYD∗13: 0 c.2846A>T: 4 (23.5) c.1236G>A: 7 (13.7) |
Carriers: 17 (20.0) Wild-type: 86 (8.4) RR (95% CI): 2.38 (1.41–3.73) By genotype DPYD∗2A: 2 (12.5) DPYD∗13: 0 c.2846A>T: 4 (23.5) c.1236G>A: 11 (21.6) |
Carriers: 1 (1.2) Wild-type: 36 (3.5) RR (95% CI): 0.34 (0.01–1.92) By genotype DPYD∗2A: 0 DPYD∗13: 0 c.2846A>T: 1 (5.9) c.1236G>A: 0 |
Carriers: 33 (38.8) Wild-type: 231 (22.7) RR (95% CI): 1.71 (1.25–2.25) By genotype DPYD∗2A: 5 (31.3) DPYD∗13: 0 c.2846A>T: 8 (47.1) c.1236G>A: 20 (39.2) |
Carriers: could not be determined (1 death in a carrier who was wrongly treated with a full dose for 2 cycles) Wild-type: 3 (0.3) P = .55 |
Carriers: 16 (18.8) Wild-type: 140 (13.8) RR (95% CI): 1.36 (0.82—2.13) P = 0.21 Days in hospital, median (IQR) Carriers: 5 (3–7) Wild-type: 5 (3–10) |
| Lunenburg et al, 20187 827 (22b/771/34c) 4 variants |
Carriers: 2 (8.7) Wild-type: 22 (2.9) Carriersc: 4 (11.8) RR (95% CI) vs. wild-type: 3.00 (0.49–11.66) RR (95% CI) vs. Carriersc: 0.74 (0.12–5.72) |
Carriers: 2 (8.7) Wild-type: 62 (8.0) Carriersc: 6 (17.6) RR (95% CI) vs. wild-type: 1.08 (0.16–3.52) RR (95% CI) vs. carriersc: 0.49 (0.08–2.28) |
Grade ≥ 2 Carriers: 1 (4.3) Wild-type: 24 (3.1) Carriersc: NR RR (95% CI) vs. wild-type: 1.40 (0.05–8.27) |
Carriers: 5 (22.7) Wild-type: 105 (13.6) Carriersc: 8 (23.5) RR (95% CI) vs. wild-type: 1.60 (0.59–3.22) RR (95% CI) vs. carriersc: 0.97 (0.30–3.05) |
NR | Carriers: 4 (18.2) Wild-type: 60 (7.8) Carriersc: 6 (17.6) RR (95% CI) vs. wild-type: 2.24 (0.69–5.14) RR (95% CI) vs. carriersc: 1.03 (0.27—4.03) Days in hospital, median (range) Carriers: 4 (2–5) Carriersc: 23 (6–36) Wild-type: 13 (1–76) P = .01 (carriers vs. carriersc) P = NR (carriers vs. wild-type) |
Abbreviations: CI, confidence interval; NA, not applicable; NR, not reported; RR, risk ratio.
Calculated by study authors based on the Wilcoxon–Mann–Whitney test.71
One patient was excluded from the analyses.
DPYD carriers who received a standard dose.
Table A19:
Fluoropyrimidine Treatment Effectiveness Study Results—Treatment Response and Survival
| Author, Year N (Carriers/Wild-Type) | Treatment Response, n (%) | Disease Progression, n (%) | Overall Survival, n (%) | Progression-Free Survival, n (%) |
|---|---|---|---|---|
| Henricks et al, 201911 74 (37/37) DPYD∗2A |
Disease controlled Carriers: 12 (60) Wild-type: 10 (48) Complete response Carriers: 0 Wild-type: 1 (5) Partial response Carriers: 4 (20) Wild-type: 6 (29) Stable disease Carriers: 8 (40) Wild-type: 3 (14) P > .99 (overall for treatment response and progressive disease) |
Carriers: 8 (40) Wild-type: 11 (30) P > .99 (overall for treatment response and progressive disease) |
Median (range), months Carriers: 27 (1—83) Wild-type: 24 (0.7—97) HR: 0.82 (95% CI 0.47—1.43) P = .47 |
Median (range), months Carriers: 14 (0.7—83) Wild-type: 10 (0.2—97) HR: 0.83 (95% CI 0.47—1.50) P = .54 |
Abbreviations: CI, confidence interval; HR, hazard ratio; NA, not applicable; NR, not reported.
Appendix 6: Selected Excluded Studies—Clinical Evidence
For transparency, we provide a list of studies that readers might have expected to see but that did not meet the inclusion criteria, along with the primary reason for exclusion.
| Citation | Primary Reason for Exclusion |
|---|---|
| Systematic Reviews | |
| Leung HW, Chan AL. Association and prediction of severe 5-fluorouracil toxicity with dihydropyrimidine dehydrogenase gene polymorphisms: A meta-analysis. Biomed Rep. 2015;3(6):879-83 | Focused on a subpopulation of Asian patients |
| Rosmarin D, Palles C, Church D, Domingo E, Jones A, Johnstone E, et al. Genetic markers of toxicity from capecitabine and other fluorouracil-based regimens: investigation in the QUASAR2 study, systematic review, and meta-analysis. J Clin Oncol. 2014;32(10):1031-9 | Evaluated the association between severe and non-severe toxicity and did not provide information on fluoropyrimidine-related toxicity in DPYD carriers vs. wild-type patients |
| Primary Studies | |
| Amstutz U, Farese S, Aebi S, Largiadèr CR. Dihydropyrimidine dehydrogenase gene variation and severe 5-fluorouracil toxicity: a haplotype assessment. Pharmacogenomics. 2009;10(6):931-44 | Results of this study were part of a more recent and more complete publication included in this report |
| Boisdron-Celle M, Capitain O, Faroux R, Borg C, Metges JP, Galais MP, et al. Prevention of 5-fluorouracil-induced early severe toxicity by pre-therapeutic dihydropyrimidine dehydrogenase deficiency screening: Assessment of a multiparametric approach. Semin Oncol. 2017 Feb;44(1):13-23 | Results for DPYD genotyping alone could not be separated |
| Deenen MJ, Meulendijks D, Cats A, Sechterberger MK, Severens JL, Boot H, et al. Upfront genotyping of DPYD∗2A to individualize fluoropyrimidine therapy: a safety and cost analysis. J Clin Oncol. 2016;34(3):227-34 | Results of this study were part of a more recent and more complete publication included in this report |
Appendix 7: Applicability of Studies Included in the Economic Literature Review
Table A20:
Assessment of the Applicability of Studies Included in the Economic Literature Review
| Author, Year, Country of Publication | Is the study population similar to the question? | Are the interventions similar to the question? | Is the health care system studied sufficiently similar to Ontario? | Were the perspectives clearly stated? If yes, what were they? | Are all direct effects included? Are all other effects included where they are material? | Are all future costs and outcomes discounted? If yes, at what rate? | Is the value of health effects expressed in terms of quality-adjusted life-years? | Are costs and outcomes from other sectors fully and appropriately measured and valued? | Overall Judgmenta |
|---|---|---|---|---|---|---|---|---|---|
| Deenen et al, 201679 Netherlands |
Yes | Yes | Yes | Yes, health care payer | Nob | NA | No | No | Partially |
| Henricks et al, 201980 Netherlands |
Yes | Yes | Yes | Yes, health care payer | Nob | NA | No | No | Partially |
Note: Response options for all items were “yes,” “partially,” “no,” “unclear,” and “NA” (not applicable).
Overall judgment may be “directly applicable,” “partially applicable,” or “not applicable.”
Effects on patient's survival and quality of life were not included.
Appendix 8: Alternative Model Parameters Used in the Economic Scenario Analysis
For this scenario analysis, we used alternative clinical studies (Henricks et al, 201880,81) to inform some of the clinical and resource use model parameters. Other parameters and/or assumptions not shown in the table remain the same as in the reference case analysis.
Table A21:
Alternative Model Parameters Used in the Economic Scenario Analysis
| Model Parameter | Scenario Analysis (Henricks et al 201880,81) | Reference Case Analysis (Lunenburg et al 20187) |
|---|---|---|
| Probability of Severe Toxicity | ||
|
DPYD wild-type DPYD intermediate, reduced dose DPYD intermediate, standard dose |
22.69% 38.82% 50.15% |
13.62% 22.73% 23.53% |
| Probability of Hospitalizationa | ||
|
DPYD wild-type DPYD intermediate, reduced dose DPYD intermediate, standard dose |
13.56% (ward); 0.88% (ICU) 16.47% (ward); 2.35% (ICU) 23.5% (ward); 3.10% (ICU) |
7.8% 18.2% 17.6% |
| Hospital Length of Stay (days) | ||
|
DPYD wild-type DPYD intermediate, reduced dose DPYD intermediate, standard dose |
7.99 (ward); 3.11 (ICU) 5.79 (ward); 1 (ICU) 13.1 (ward); 7 (ICU) |
13 4 23 |
| Cost of Hospitalization | ||
| ICU per dayb | $3,899 | NA |
Abbreviations: ICU, intensive care unit; NA, not applicable.
For all patients.
Source for ICU cost per day: Canadian Institute for Health Information, 2016.96
Appendix 9: Letter of Information
Appendix 10: Interview Guide
Author contributions
This report was developed by a multidisciplinary team from Ontario Health. The clinical epidemiologist was Vania Costa, the medical librarian was Corinne Holubowich, the health economists were Chunmei Li and Lindsey Falk, the health economics associate was Selena Hussain, the secondary health economist was Shawn Xie, and the patient and public partnership analyst was Jigna Mistry.
Key Messages
What Is This Health Technology Assessment About?
Fluoropyrimidines (for example, 5-fluorouracil and capecitabine) are medications used to treat different types of cancer. An enzyme in the body called dihydropyrimidine dehydrogenase (or DPD) is needed to break down fluoropyrimidines. A deficiency in this enzyme, which may be caused by a variation in a gene called DPYD, increases the risk of some patients developing severe toxicity if they are treated with fluoropyrimidines. A test called DPYD genotyping can identify variants in the DPYD gene. Therefore, this test may be able to identify people who are at a higher risk of developing severe toxicity, allowing their treatment plan to be modified before treatment begins, for example by reducing the fluoropyrimidine dose or choosing an alternative treatment.
This health technology assessment looked at how valid, clinically useful, and cost-effective DPYD genotyping is in people who have planned cancer treatment with fluoropyrimidines. It also looked at how effective a reduced fluoropyrimidine dose is in lowering the risk of severe toxicity, the budget impact of publicly funding DPYD genotyping, and the experiences, preferences, and values of people who have planned cancer treatment with fluoropyrimidines.
What Did This Health Technology Assessment Find?
Carriers of a DPYD variant who were treated with a standard fluoropyrimidine dose had a higher risk of severe toxicity than non-carriers. The results of DPYD genotyping led physicians to change people's fluoropyrimidine treatment plans. We are not certain if reducing the treatment dose in carriers of a DPYD variant leads to a risk of severe toxicity that is similar to that of people without a DPYD variant. We are also uncertain if reducing the treatment dose in carriers of a DPYD variant leads to a lower risk of severe toxicity than in carriers who are treated with a standard dose.
For people with planned fluoropyrimidine treatment, DPYD genotyping is likely cost-effective compared to usual care (no testing). Publicly funding DPYD genotyping in Ontario may be cost-saving, with an estimated saving of $714,963 over the next 5 years, provided that the costs of implementation, service delivery, and program coordination do not exceed this amount.
People treated with fluoropyrimidines described the impact of cancer and treatment adverse effects on their quality of life and mental health. Barriers to DPYD testing included lack of awareness and limited access.
Contributor Information
Ontario Health (Quality):
Vania Costa, Corinne Holubowich, Chunmei Li, Lindsey Falk, Selena Hussain, Shawn Xie, and Jigna Mistry
About Us
Ontario Health is an agency of the Government of Ontario. Our mandate is to connect and coordinate our province's health care system in ways that have not been done before to help ensure that Ontarians receive the best possible care. We work to support better health outcomes, patient experiences, provider experiences and value for money spent.
For more information about Ontario Health, visit ontariohealth.ca.
References
- (1).Amstutz U, Froehlich TK, Largiader CR. Dihydropyrimidine dehydrogenase gene as a major predictor of severe 5-fluorouracil toxicity. Pharmacogenomics. 2011;12(9):1321–36. [DOI] [PubMed] [Google Scholar]
- (2).Henricks LM, Lunenburg CA, Meulendijks D, Gelderblom H, Cats A, Swen JJ. Translating DPYD genotype into DPD phenotype: using the DPYD gene activity score. Pharmacogenomics. 2015;16(11):1277–86. [DOI] [PubMed] [Google Scholar]
- (3).Henricks LM, Opdam FL, Beijnen JH, Cats A, Schellens JHM. DPYD genotype-guided dose individualization to improve patient safety of fluoropyrimidine therapy: call for a drug label update. Ann Oncol. 2017;28(12):2915–22. [DOI] [PubMed] [Google Scholar]
- (4).Lunenburg C, van der Wouden CH, Nijenhuis M, Crommentuijn-van Rhenen MH, de Boer-Veger NJ, Buunk AM. Dutch Pharmacogenetics Working Group (DPWG) guideline for the gene-drug interaction of DPYD and fluoropyrimidines. Eur J Hum Genet. 2020;28(4):508–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Wörmann B, Bokemeyer C, Burmeister T, Köhne CH, Schwab M, Arnold D. Dihydropyrimidine dehydrogenase testing prior to treatment with 5-fluorouracil, capecitabine, and tegafur: a consensus paper. Oncol Res Treat. 2020;43(11):628–36. [DOI] [PubMed] [Google Scholar]
- (6).Wigle TJ, Tsvetkova EV, Welch SA, Kim RB. DPYD and fluorouracil-based chemotherapy: mini review and case report. Pharmaceutics. 2019;11(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Lunenburg C, Henricks LM, Dreussi E, Peters FP, Fiocco M, Meulendijks D. Standard fluoropyrimidine dosages in chemoradiation therapy result in an increased risk of severe toxicity in DPYD variant allele carriers. Eur J Cancer. 2018;104:210–8. [DOI] [PubMed] [Google Scholar]
- (8).Amstutz U, Henricks LM, Offer SM, Barbarino J, Schellens JHM, Swen JJ. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for dihydropyrimidine dehydrogenase genotype and fluoropyrimidine dosing: 2017 update. Clin Pharmacol Ther. 2018;103(2):210–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Toffoli G, Giodini L, Buonadonna A, Berretta M, De Paoli A, Scalone S. Clinical validity of a DPYD-based pharmacogenetic test to predict severe toxicity to fluoropyrimidines. Int J Cancer. 2015;137(12):2971–80. [DOI] [PubMed] [Google Scholar]
- (10).Haute Autorité de Santé, Institut National du Cancer. Recherche de déficit en dihydropyrimidine déshydrogenase en vue de prévenir certaines toxicités sévères survenant sous traitement comportant des fluoropyrimidines (5-fluorouracile) [Internet]. 2018 [cited 2021. Feb 8]. Available from: https://www.has-sante.fr/upload/docs/application/pdf/2018-12/recherche_dun_deficit_en_dihydropyrimidine_deshydrogenase_visant_a_prevenir_certaines_toxicites_severes_associees_aux_traite.pdf
- (11).Henricks LM, van Merendonk LN, Meulendijks D, Deenen MJ, Beijnen JH, de Boer A. Effectiveness and safety of reduced-dose fluoropyrimidine therapy in patients carrying the DPYD∗2A variant: a matched pair analysis. Int J Cancer. 2019;144(9):2347–54. [DOI] [PubMed] [Google Scholar]
- (12).Kleinjan JP, Brinkman I, Bakema R, van Zanden JJ, van Rooijen JM. Tolerance-based capecitabine dose escalation after DPYD genotype-guided dosing in heterozygote DPYD variant carriers: a single-center observational study. Anticancer Drugs. 2019;30(4):410–5. [DOI] [PubMed] [Google Scholar]
- (13).Lee AM, Shi Q, Alberts SR, Sargent DJ, Sinicrope FA, Berenberg JL. Association between DPYD c.1129-5923 C>G/hapB3 and severe toxicity to 5-fluorouracil-based chemotherapy in stage III colon cancer patients: NCCTG N0147 (Alliance). Pharmacogenet Genomics. 2016;26(3):133–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).National Cancer Institute. Common terminology criteria for adverse events (CTCAE): version 5.0 [Internet]. Wash. D.C.: US Department of Health and Human Services; 2017. [cited 2021 Jan 8]. Available from: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5×11.pdf [Google Scholar]
- (15).Boige V, Vincent M, Alexandre P, Tejpar S, Landolfi S, Le Malicot K. DPYD genotyping to predict adverse events following treatment with fluorouracil-based adjuvant chemotherapy in patients with stage III colon cancer: a secondary analysis of the PETACC-8 randomized clinical trial. JAMA Oncol. 2016;2(5):655–62. [DOI] [PubMed] [Google Scholar]
- (16).European Medicines Agency. Fluorouracil and fluorouracil-related substances (capecitabine, tegafur and flucytosine) containing medicinal products. Assessment report EMA/274404/2020 [Internet]. 2020. [cited 2020 Nov 16]. Available from: https://www.ema.europa.eu/en/documents/referral/fluorouracil-fluorouracil-related-substances-article-31-referral-assessment-report_en.pdf
- (17).KNMP. General background text pharmacogenetics – dihydropyrimidine dehydrogenase (DPD) [Internet]. The Hague: KNMP; 2019. [cited 2019 Dec 16]. Available from: https://www.knmp.nl/downloads/g-standaard/farmacogenetica/english-background-information/DPD-english-2019.pdf [Google Scholar]
- (18).Meulendijks D, Henricks LM, Jacobs BAW, Aliev A, Deenen MJ, de Vries N. Pretreatment serum uracil concentration as a predictor of severe and fatal fluoropyrimidine-associated toxicity. Br J Cancer. 2017;116(11):1415–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Loriot MA, Ciccolini J, Thomas F, Barin-Le-Guellec C, Royer B, Milano G. Dihydropyrimidine dehydrogenase (DPD) deficiency screening and securing of fluoropyrimidine-based chemotherapies: update and recommendations of the French GPCO-Unicancer and RNPGx networks. Bull Cancer. 2018;105(4):397–407. [DOI] [PubMed] [Google Scholar]
- (20).Lipman B, Pasetka M. Dihydropyrimidine dehydrogenase (DPD) deficiency. Hot Spot: the newsletter of the Rapid Response Radiotherapy Program at Sunnybrook's Odette Cancer Centre. 2018;20(3):4–5. [Google Scholar]
- (21).Clinical Pharmacogenetics Implementation Consortium (CPIC). Guideline for fluoropyrimidines and DPYD [Internet]. 2018. [cited 2020 Oct 21]. Available from: https://cpicpgx.org/guidelines/guideline-for-fluoropyrimidines-and-dpyd/
- (22).Caudle KE, Thorn CF, Klein TE, Swen JJ, McLeod HL, Diasio RB. Clinical Pharmacogenetics Implementation Consortium guidelines for dihydropyrimidine dehydrogenase genotype and fluoropyrimidine dosing. Clin Pharmacol Ther. 2013;94(6):640–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Stavraka C, Pouptsis A, Okonta L, DeSouza K, Charlton P, Kapiris M. Clinical implementation of pre-treatment DPYD genotyping in capecitabine-treated metastatic breast cancer patients. Breast Cancer Res Treat. 2019;175(2):511–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).European Medicines Agency. EMA recommendations on DPD testing prior to treatment with fluorouracil, capecitabine, tegafur and flucytosine. EMA/229267/2020. 30 April 2020 [Internet]. 2020. [Oct 20, 2020]. Available from: https://www.ema.europa.eu/en/documents/press-release/ema-recommendations-dpd-testing-prior-treatment-fluorouracil-capecitabine-tegafur-flucytosine_en.pdf
- (25).Institut National d'Excellence en Sante et en Service Sociaux (INESSS). Genotypage de la mutation DPYD∗2A du gene de la dihydropyrimidine desydrogenase (DPYD) par PCR en temps reel (Reference-2016.02.001): avis d'evaluation [Internet]. Quebec, Qc: INESSS; 2017. [cited 2021 Jan 7]. Available from: https://www.inesss.qc.ca/fileadmin/doc/INESSS/Analyse_biomedicale/Fevrier_2017/01-Genotypage_mutation_DPYD-2A.pdf?sword_list%5B0%5D=dpyd&no_cache–1 [Google Scholar]
- (26).Cancer Care Ontario. Fluorouracil drug monograph [Internet]. 2020. [cited 2021 Jan 6]. Available from: https://www.cancercareontario.ca/en/drugformulary/drugs/monograph/43831
- (27).Cancer Care Ontario. Capecitabine product monograph [Internet]. 2019. [cited 2020 Dec 27]. Available from: https://www.cancercareontario.ca/en/drugformulary/drugs/capecitabine
- (28).Product monogaph: PrACH-CAPECITABINE [Internet]. 2019. [cited 2020 Nov 23]. Available from: https://pdf.hres.ca/dpd_pm/00053877.PDF
- (29).Generic Medical Partners Inc. Product monograph: Prfluorouracil injection 50 mg/mL [Internet]. Toronto: General Medical Partners; 2020. [cited 2020 Nov 23]. Available from: https://pdf.hres.ca/dpd_pm/00055477.PDF [Google Scholar]
- (30).Hamzic S, Aebi S, Joerger M, Montemurro M, Ansari M, Amstutz U. Fluoropyrimidine chemotherapy: recommendations for DPYD genotyping and therapeutic drug monitoring of the Swiss Group of Pharmacogenomics and Personalised Therapy. Swiss Med Wkly. 2020;150:w20375. [DOI] [PubMed] [Google Scholar]
- (31).Argilés G, Tabernero J, Labianca R, Hochhauser D, Salazar R, Iveson T. Localised colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(10):1291–305. [DOI] [PubMed] [Google Scholar]
- (32).Institut National d'Excellence en Sante e en Services Sociaux (INESSS). Traitement a base de fluoropyrimidines [Internet]. Quebec, Qc: INESSS; 2019. [cited 2020 Oct 21]. Available from: https://www.inesss.qc.ca/fileadmin/doc/INESSS/Rapports/Oncologie/INESSS_DPYD_Traitements.pdf [Google Scholar]
- (33).Haute Autorité de Santé. Screening for dihydropyrimidine dehydrogenase deficiency to decrease the risk of severe toxicities related to fluoropyrimidines (5-fluorouracil or capecitabine) [Internet]. 2018. [cited 2021 Jan 29]. Available from: https://www.hassante.fr/upload/docs/application/pdf/2019-01/inahta_brief_dpd_5fu.pdf
- (34).O'Neill J, Tabish H, Welch V, Petticrew M, Pottie K, Clarke M. Applying an equity lens to interventions: using PROGRESS ensures consideration of socially stratifying factors to illuminate inequities in health. J Clin Epidemiol. 2014;67(1):56–64. [DOI] [PubMed] [Google Scholar]
- (35).Quebec ministere de la sante e services sociaux. Répertoire des procédures suprarégionales de biologie médicale [Internet]. Québec City: Gouvernement du Québec; c2021. [cited 2020 Nov 16]. Available from: https://www.msss.gouv.qc.ca/repertoires/biomed/fiche.php?id–65036 [Google Scholar]
- (36).NHS England. Clinical commissioning urgent policy statement: pharmacogenomic testing for DPYD polymorphisms with fluoropyrimidine therapies [URN 1869] (200603P) [Internet]. Redditch (UK): National Health Servoce; 2020. [cited 2021 Jan 5]. Available from: https://www.england.nhs.uk/wp-content/uploads/2020/11/1869-dpyd-policy-statement.pdf [Google Scholar]
- (37).Pharmacogenetic testing information [Internet]. Geneva: Swiss Society of Clinical Pharmacology and Toxicology (SSCPT); c2020. [cited 2021 Jan 18]. Available from: https://clinpharm.ch/en/pharmacogenetics_spt/swiss_group_of_personalised_therapy_and_pharmacogenomics/pharmacogenetic_testing_information [Google Scholar]
- (38).Agence Nationale de Securite du Medicament et des Produits de Sante. Fluoropyrimidine-based chemotherapy [Internet]. 2019. [cited 2021 Jan 18]. Available from: https://www.ansm.sante.fr/S-informer/Points-d-information-Points-d-information/Chimiotherapies-a-base-de-5-FU-ou-capecitabine-recherche-obligatoire-du-deficiten-DPD-avant-tout-traitement-Point-d-Information
- (39).Whiting P, Savović J, Higgins JP, Caldwell DM, Reeves BC, Shea B. ROBIS: a new tool to assess risk of bias in systematic reviews was developed. J Clin Epidemiol. 2016;69:225–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–6. [DOI] [PubMed] [Google Scholar]
- (41).Covidence systematic review software [Internet]. Melbourne (Australia): Veritas Health Innovation; [Available from: https://www.covidence.org/home [Google Scholar]
- (42).The R Foundation. R statistical computing software version 3.5.126. R Foundation. Vienna. 2020. Available at: http://www.R-project.org.
- (43).Tian L, Cai T, Pfeffer MA, Piankov N, Cremieux PY, Wei LJ. Exact and efficient inference procedure for meta-analysis and its application to the analysis of independent 2 × 2 tables with all available data but without artificial continuity correction. Biostatistics. 2009;10(2):275–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Internet]. Ottawa (ON): Ottawa Hospital Research Institute; 2018. [cited 2018 Oct]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [Google Scholar]
- (45).Schünemann H, Brozek J, Guyatt G, Oxman A, editors. GRADE handbook [Internet]. Hamilton (ON): GRADE Working Group; 2013. [cited 2017 Dec]. Available from http://gdt.guidelinedevelopment.org/app/handbook/handbook.html [Internet]. [Google Scholar]
- (46).Campbell JM, Bateman E, Peters M, Bowen JM, Keefe DM, Stephenson MD. Fluoropyrimidine and platinum toxicity pharmacogenetics: an umbrella review of systematic reviews and meta-analyses. Pharmacogenomics. 2016;17(4):435–51. [DOI] [PubMed] [Google Scholar]
- (47).Meulendijks D, Henricks LM, Sonke GS, Deenen MJ, Froehlich TK, Amstutz U. Clinical relevance of DPYD variants c.1679T>G, c.1236G>A/HapB3, and c.1601G>A as predictors of severe fluoropyrimidine-associated toxicity: a systematic review and meta-analysis of individual patient data. Lancet Oncol. 2015;16(16):1639–50. [DOI] [PubMed] [Google Scholar]
- (48).Li Q, Liu Y, Zhang HM, Huang YP, Wang TY, Li DS. Influence of DPYD genetic polymorphisms on 5-fluorouracil toxicities in patients with colorectal cancer: a meta-analysis. Gastroenterol Res Pract. 2014;2014:827989. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- (49).Terrazzino S, Cargnin S, Del Re M, Danesi R, Canonico PL, Genazzani AA. DPYD IVS14+1G>A and 2846A>T genotyping for the prediction of severe fluoropyrimidine-related toxicity: a meta-analysis. Pharmacogenomics. 2013;14(11):1255–72. [DOI] [PubMed] [Google Scholar]
- (50).Pharmacogenetic testing to predict serious toxicity from 5-fluorouracil (5-FU) for patients administered 5-FU-based chemotherapy for cancer. Technol Eval CentAssess Program Exec Summ. 2010;24(13):1–3. [PubMed] [Google Scholar]
- (51).Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(6):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Iachetta F, Bonelli C, Romagnani A, Zamponi R, Tofani L, Farnetti E. The clinical relevance of multiple DPYD polymorphisms on patients candidate for fluoropyrimidine based-chemotherapy. an Italian case-control study. Br J Cancer. 2019;120(8):834–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Maharjan AS, McMillin GA, Patel GK, Awan S, Taylor WR, Pai S. The prevalence of DPYD 9A(c.85T>C) genotype and the genotype-phenotype correlation in patients with gastrointestinal malignancies treated with fluoropyrimidines: updated analysis. Clin Colorectal Cancer. 2019;18(3):e280–e6. [DOI] [PubMed] [Google Scholar]
- (54).Nahid NA, Apu MNH, Islam MR, Shabnaz S, Chowdhury SM, Ahmed MU. DPYD∗2A and MTHFR C677T predict toxicity and efficacy, respectively, in patients on chemotherapy with 5-fluorouracil for colorectal cancer. Cancer Chemother Pharmacol. 2018;81(1):119–29. [DOI] [PubMed] [Google Scholar]
- (55).Cremolini C, Del Re M, Antoniotti C, Lonardi S, Bergamo F, Loupakis F. DPYD and UGT1A1 genotyping to predict adverse events during first-line FOLFIRI or FOLFOXIRI plus bevacizumab in metastatic colorectal cancer. Oncotarget. 2018;9(8):7859–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Etienne-Grimaldi MC, Boyer JC, Beroud C, Mbatchi L, van Kuilenburg A, Bobin-Dubigeon C. New advances in DPYD genotype and risk of severe toxicity under capecitabine. PLoS ONE. 2017;12(5):e0175998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Froehlich TK, Amstutz U, Aebi S, Joerger M, Largiader CR. Clinical importance of risk variants in the dihydropyrimidine dehydrogenase gene for the prediction of early-onset fluoropyrimidine toxicity. Int J Cancer. 2015;136(3):730–9. [DOI] [PubMed] [Google Scholar]
- (58).Lee AM, Shi Q, Pavey E, Alberts SR, Sargent DJ, Sinicrope FA. DPYD variants as predictors of 5-fluorouracil toxicity in adjuvant colon cancer treatment (NCCTG N0147). J Natl Cancer Inst. 2014;106(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Jennings BA, Loke YK, Skinner J, Keane M, Chu GS, Turner R. Evaluating predictive pharmacogenetic signatures of adverse events in colorectal cancer patients treated with fluoropyrimidines. PLoS ONE. 2013;8(10):e78053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Loganayagam A, Arenas Hernandez M, Corrigan A, Fairbanks L, Lewis CM, Harper P. Pharmacogenetic variants in the DPYD, TYMS, CDA and MTHFR genes are clinically significant predictors of fluoropyrimidine toxicity. Br J Cancer. 2013;108(12):2505–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Deenen MJ, Tol J, Burylo AM, Doodeman VD, de Boer A, Vincent A. Relationship between single nucleotide polymorphisms and haplotypes in DPYD and toxicity and efficacy of capecitabine in advanced colorectal cancer. Clin Cancer Res. 2011;17(10):3455–68. [DOI] [PubMed] [Google Scholar]
- (62).Cellier P, Leduc B, Martin L, Vié B, Chevelle C, Vendrely V. Phase II study of preoperative radiation plus concurrent daily tegafur-uracil (UFT) with leucovorin for locally advanced rectal cancer. BMC cancer. 2011;11:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Cerić T, Obralić N, Kapur-Pojskić L, Macić D, Beslija S, Pasić A. Investigation of IVS14 + 1G > A polymorphism of DPYD gene in a group of Bosnian patients treated with 5-fluorouracil and capecitabine. Bosn J Basic Med Sci. 2010;10(2):133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Braun MS, Richman SD, Thompson L, Daly CL, Meade AM, Adlard JW. Association of molecular markers with toxicity outcomes in a randomized trial of chemotherapy for advanced colorectal cancer: the FOCUS trial. J Clin Oncol. 2009;27(33):5519–28. [DOI] [PubMed] [Google Scholar]
- (65).Schwab M, Zanger UM, Marx C, Schaeffeler E, Klein K, Dippon J. Role of genetic and nongenetic factors for fluorouracil treatment-related severe toxicity: a prospective clinical trial by the German 5-FU Toxicity Study Group. J Clin Oncol. 2008;26(13):2131–8. [DOI] [PubMed] [Google Scholar]
- (66).Sulzyc-Bielicka V, Bińczak-Kuleta A, Pioch W, Klsadny J, Gziut K, Bielicki D. 5-Fluorouracil toxicity-attributable IVS14 + 1G > A mutation of the dihydropyrimidine dehydrogenase gene in Polish colorectal cancer patients. Pharmacol Rep. 2008;60(2):238–42. [PubMed] [Google Scholar]
- (67).Boisdron-Celle M, Remaud G, Traore S, Poirier AL, Gamelin L, Morel A. 5-Fluorouracil-related severe toxicity: a comparison of different methods for the pretherapeutic detection of dihydropyrimidine dehydrogenase deficiency. Cancer Lett. 2007;249(2):271–82. [DOI] [PubMed] [Google Scholar]
- (68).Largillier R, Etienne-Grimaldi MC, Formento JL, Ciccolini J, Nebbia JF, Ginot A. Pharmacogenetics of capecitabine in advanced breast cancer patients. Clin Cancer Res. 2006;12(18):5496–502. [DOI] [PubMed] [Google Scholar]
- (69).Salgueiro N, Veiga I, Fragoso M, Sousa O, Costa N, Pellon ML. Mutations in exon 14 of dihydropyrimidine dehydrogenase and 5-fluorouracil toxicity in Portuguese colorectal cancer patients. Genet Med. 2004;6(2):102–7. [DOI] [PubMed] [Google Scholar]
- (70).Meulendijks D, Rozeman EA, Cats A, Sikorska K, Joerger M, Deenen MJ. Pharmacogenetic variants associated with outcome in patients with advanced gastric cancer treated with fluoropyrimidine and platinum-based triplet combinations: a pooled analysis of three prospective studies. Pharmacogenomics J. 2017;17(5):441–51. [DOI] [PubMed] [Google Scholar]
- (71).Wigle TJ, Povitz BL, Medwid S, Teft WA, Legan RM, Lenehan J. Impact of pretreatment dihydropyrimidine dehydrogenase genotype-guided fluoropyrimidine dosing on chemotherapy associated adverse events. Clin Transl Sci. 2021. Feb 23 [epub ahead of print]. doi:10.1111/cts.12981. [DOI] [PMC free article] [PubMed]
- (72).Henricks LM, Lunenburg C, de Man FM, Meulendijks D, Frederix GWJ, Kienhuis E. DPYD genotype-guided dose individualisation of fluoropyrimidine therapy in patients with cancer: a prospective safety analysis. Lancet Oncol. 2018;19(11):1459–67. [DOI] [PubMed] [Google Scholar]
- (73).Deenen MJ, Meulendijks D, Cats A, Sechterberger MK, Severens JL, Boot H. Upfront genotyping of DPYD∗2A to individualize fluoropyrimidine therapy: a safety and cost analysis. J Clin Oncol. 2016;34(3):227–34. [DOI] [PubMed] [Google Scholar]
- (74).Henricks LM, Kienhuis E, Man FMd, Veldt AAMvd, Hamberg P, Kuilenburg ABPv. Treatment algorithm for homozygous or compound heterozygous DPYD variant allele carriers with low-dose capecitabine. JCO Precis Oncol. 2017(1):1–10. [DOI] [PubMed] [Google Scholar]
- (75).Jolivet C, Nassabein R, Soulières D, Weng X, Amireault C, Ayoub JP. Implementing DPYD∗2A genotyping in clinical practice: the Quebec, Canada, experience. The oncologist. 2020. [DOI] [PMC free article] [PubMed]
- (76).Cats A. Improving the safety of fluoropyrimidine-based chemotherapy (Alpe2U) (NCT04194957) [Internet]. 2020. [cited 2020 Oct 21]. Available from: https://clinicaltrials.gov/ct2/show/study/NCT04194957
- (77).Lambaerts A. Implementation of pre-emptive geno- and phenotyping in 5-fluorouracil- or capecitabine-treated patients (NCT04269369) [Internet]. 2020. [cited 2020 Oct 21]. Available from: https://clinicaltrials.gov/ct2/show/NCT04269369
- (78).National Institute for Health and Care Excellence. Appendix I: quality appraisal checklist—economic evaluations. 2012. [cited 2016 Jan]. In: Methods for the development of NICE public health guidance, 3rd ed [Internet]. London: The Institute. Available from: https://www.nice.org.uk/process/pmg4/chapter/appendix-i-quality-appraisal-checklist-economic-evaluations [PubMed]
- (79).Deenen MJ, Meulendijks D, Cats A, Sechterberger MK, Severens JL, Boot H. Upfront genotyping of DPYD 2A to individualize fluoropyrimidine therapy: a safety and cost analysis. J Clin Oncol. 2016;34(3):227–34. [DOI] [PubMed] [Google Scholar]
- (80).Henricks LM, Lunenburg C, de Man FM, Meulendijks D, Frederix GWJ, Kienhuis E. A cost analysis of upfront DPYD genotype-guided dose individualisation in fluoropyrimidine-based anticancer therapy. Eur J Cancer. 2019;107:60–7. [DOI] [PubMed] [Google Scholar]
- (81).Henricks LM, Lunenburg C, de Man FM, Meulendijks D, Frederix GWJ, Kienhuis E. DPYD genotype-guided dose individualisation of fluoropyrimidine therapy in patients with cancer: a prospective safety analysis. Lancet Oncol. 2018;19(11):1459–67. [DOI] [PubMed] [Google Scholar]
- (82).Cortejoso L, Garcia-Gonzalez X, Garcia MI, Garcia-Alfonso P, Sanjurjo M, Lopez-Fernandez LA. Cost-effectiveness of screening for DPYD polymorphisms to prevent neutropenia in cancer patients treated with fluoropyrimidines. Pharmacogenomics. 2016;17(9):979–84. [DOI] [PubMed] [Google Scholar]
- (83).Murphy C, Byrne S, Ahmed G, Kenny A, Gallagher J, Harvey H. Cost implications of reactive versus prospective testing for dihydropyrimidine dehydrogenase deficiency in patients with colorectal cancer: a single-institution experience. Dose Response. 2018;16(4):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Toffoli G, Innocenti F, Polesel J, De Mattia E, Sartor F, Dalle Fratte C. The genotype for DPYD risk variants in patients with colorectal cancer and the related toxicity management costs in clinical practice. Clin Pharmacol Ther. 2019;105(4):994–1002. [DOI] [PubMed] [Google Scholar]
- (85).Fragoulakis V, Roncato R, Fratte CD, Ecca F, Bartsakoulia M, Innocenti F. Estimating the Effectiveness of DPYD Genotyping in Italian Individuals Suffering from Cancer Based on the Cost of Chemotherapy-Induced Toxicity. American Journal of Human Genetics. 2019;104(6):1158–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)—explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health. 2013;16(2):231–50. [DOI] [PubMed] [Google Scholar]
- (87).Cancer Care Ontario. 5-Fluorourpyrimidine – Provider monograph [Internet]. 2020. [Available from: https://www.cancercareontario.ca/en/drugformulary/drugs/monograph/43831
- (88).Froehlich TK, Amstutz U, Aebi S, Joerger M, Largiadèr CR. Clinical importance of risk variants in the dihydropyrimidine dehydrogenase gene for the prediction of early-onset fluoropyrimidine toxicity. Int J Cancer. 2015;136(3):730–9. [DOI] [PubMed] [Google Scholar]
- (89).Bennett L, Zhao Z, Barber B, Zhou X, Peeters M, Zhang J. Health-related quality of life in patients with metastatic colorectal cancer treated with panitumumab in first- or second-line treatment. Br J Cancer. 2011;105(10):1495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (90).Lloyd A, Nafees B, Narewska J, Dewilde S, Watkins J. Health state utilities for metastatic breast cancer. Br J Cancer. 2006;95(6):683–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (91).Curran D, Pozzo C, Zaluski J, Dank M, Barone C, Valvere V. Quality of life of palliative chemotherapy naive patients with advanced adenocarcinoma of the stomach or esophagogastric junction treated with irinotecan combined with 5-fluorouracil and folinic acid: results of a randomised phase III trial. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2009;18(7):853–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (92).Statistics Canada. Table 18-10-0004-01: consumer price index, monthly, not seasonally adjusted [Internet]. Ottawa (ON): Statistics Canada; 2018. [cited 2018 Apr 30]. Available from: http://www5.statcan.gc.ca/cansim/a26?lang=eng&id=3260020 [Google Scholar]
- (93).Ministry of Health. Schedule of Benefits: Physician Services Under the Health Insurance Act [Internet]. Toronto (ON) 2020. Available from: http://www.health.gov.on.ca/en/pro/programs/ohip/sob/physserv/sob_master20200306.pdf
- (94).Ministry of Health. Schedule of benefits for laboratory services [Internet]. Toronto (ON)Queen's Printer for Ontario; 2020. Available from: http://www.health.gov.on.ca/en/pro/programs/ohip/sob/lab/lab_mn2020.pdf [Google Scholar]
- (95).Tsiplova K, Zur RM, Marshall CR, Stavropoulos DJ, Pereira SL, Merico D. A microcosting and cost-consequence analysis of clinical genomic testing strategies in autism spectrum disorder. Genet Med. 2017;19(11):1268–75. [DOI] [PubMed] [Google Scholar]
- (96).Canadian Institute for Health Information. Care in Canadian ICUs [Internet]. Ottawa (ON): CIHI Ottawa; August 2016. Available from: https://secure.cihi.ca/free_products/ICU_Report_EN.pdf [Google Scholar]
- (97).Ogranisation for Economic Co-operation and Development (OECD). Purchasing power parities (PPP) [Internet]. 2020. [cited 2020 March]. Available from: https://data.oecd.org/conversion/purchasing-power-parities-ppp.htm
- (98).Ministry of Health. Ontario drug benefit formulary/comparative drug index [Internet]. Toronto (ON): Queen's Printer for Ontario. 2020. Available from: https://www.formulary.health.gov.on.ca/formulary [Google Scholar]
- (99).The pan-Canadian Oncology Drug Review. Final economic guidance report: panitumumab (Vectibix) for left-sided metastatic colorectal cancer [Internet]. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2018. Available from: https://cadth.ca/sites/default/files/pcodr/pcodr_panitumumab_vectibix_ls_mcrc_fn_egr%20.pdf [Google Scholar]
- (100).Ministry of Health and Long-Term Care. Ontario drug benefit formulary/comparative drug index [Internet]. Toronto: Queen's Printer for Ontario; 2017. Available from: http://www.health.gov.on.ca/en/pro/programs/drugs/formulary42/edition_42.pdf [Google Scholar]
- (101).Cancer Care Ontario. Capecitabine: drug formulary [Internet]. 2020. [updated November 2019; cited 2020 March]. Available from: https://www.cancercareontario.ca/en/drugformulary/drugs/capecitabine
- (102).Cancer Care Ontario. RALT (Raltitrexed) regimen monograph [Internet]. Toronto: Queen's Printer for Ontario; 2017. Available from: https://www.cancercareontario.ca/sites/ccocancercare/files/RALT_GI_COL_A.pdf [Google Scholar]
- (103).Alberta Blue Cross. Products and pricing on the Alberta Blue Cross drug price list (ABCDPL) [Internet]. Edmonton (AB): ABC Benefits Corp.; 2014. Available from: https://www.ab.bluecross.ca/dbl/pdfs/ABCDPL_2014_11_17.pdf [Google Scholar]
- (104).Members of the Gastrointestinal Cancer Disease Site Group. Use of raltitrexed (Tomudex) in the management of metastatic colorectal cancer [Internet]. Toronto: Cancer Care Ontario; 2011. Sep 15. Available from: https://www.cancercareontario.ca/sites/ccocancercare/files/guidelines/full/pebc2-17f.pdf [Google Scholar]
- (105).Canadian Agency for Drugs and Technologies in Health. Guidelines for the economic evaluation of health technologies: Canada. 4th ed [Internet]. Ottawa (ON): The Agency; 2017. [cited 2018 Jan]. Available from: https://www.cadth.ca/sites/default/files/pdf/guidelines_for_the_economic_evaluation_of_health_technologies_canada_4th_ed.pdf [Google Scholar]
- (106).Barham L. Public and patient involvement at the UK National Institute for Health and Clinical Excellence. The patient. 2011;4(1):1–10. [DOI] [PubMed] [Google Scholar]
- (107).Messina J, Grainger DL. A pilot study to identify areas for further improvements in patient and public involvement in health technology assessments for medicines. The patient. 2012;5(3):199–211. [DOI] [PubMed] [Google Scholar]
- (108).Ontario Health Technology Advisory Committee Public Engagement Subcommittee. Public engagement for health technology assessment at Health Quality Ontario—final report from the Ontario Health Technology Advisory Committee Public Engagement Subcommittee [Internet]. Toronto (ON): Queen's Printer for Ontario; 2015 Apr. [cited 2018 Apr 30]. Available from: http://www.hqontario.ca/Portals/0/documents/evidence/special-reports/report-subcommittee-20150407-en.pdf [Google Scholar]
- (109).Smith A, Losak H. Pharmacogenomic testing for medication selection: a rapid qualitative review. (CADTH rapid response report: summary with critical appraisal) [Internet]. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2020. [cited 2020 Dec 17]. Available from: https://www.cadth.ca/sites/default/files/rr/2020/RC1246%20PxGx%20Qualitative%20FINAL.pdf [PubMed] [Google Scholar]
- (110).Kvale S. Interviews: an introduction to qualitative research interviewing. Thousand Oaks (CA): Sage; 1996. [Google Scholar]
- (111).Kuzel AJ. Sampling in qualitative inquiry. In: Miller WL, Crabtree BF, editors. Doing qualitative research. Thousand Oaks (CA): Sage; 1999. p. 33–45. [Google Scholar]
- (112).Morse J. Emerging from the data: cognitive processes of analysis in qualitative research. In: Morse J, editor. Critical issues in qualitative research methods. Thousand Oaks (CA): Sage; 1994. p. 23–41. [Google Scholar]
- (113).Patton MQ. Qualitative research and evaluation methods. 3rd ed.Thousand Oaks (CA): Sage; 2002. [Google Scholar]
- (114).Strauss AL, Corbin JM. Basics of qualitative research: techniques and procedures of developing a grounded theory. 2nd ed.Thousand Oaks (CA): Sage; 1998. [Google Scholar]
- (115).Health Technology Assessment International. Introduction to health technology assessment [Internet]. Edmonton (AB): Health Technology Assessment International; 2015. [cited 2018 Apr 30]. Available from: http://www.htai.org/fileadmin/HTAi_Files/ISG/PatientInvolvement/v2_files/Resource/PCISG-Resource-Intro_to_HTA__KFacey__Jun13.pdf [Google Scholar]
- (116).Strauss AL, Corbin JM. Grounded theory research: procedures, canons, and evaluative criteria. Qual Sociol. 1990;13(1):3–21. [Google Scholar]
- (117).Strauss AL, Corbin JM. Grounded theory methodology: an overview. In: Denzin NK, Lincoln YS, editors. Handbook of qualitative research. Thousand Oaks (CA): Sage; 1994. p. 273–85. [Google Scholar]
- (118).TreeAge Pro decision analysis software. TreeAge Software, Inc. Williamstown (MA) [Internet]. Available from: http://www.treeage.com