Abstract
Background
Carbapenem-resistant Enterobacterales (CRE) are rapidly increasing worldwide in last two decades and lead few antibiotics for treatment. The molecular epidemiology of CRE in China was investigated to provide basis for clinical rational use of antibiotics and prevent its spread.
Methods
All CRE isolates in this study were collected from 11 hospitals from October 2015 to July 2018. The isolates were subjected to antimicrobial susceptibility tests, PCR molecular identification, pulsed-field gel electrophoresis, and multilocus sequence typing.
Results
Among the 399 CRE isolates, 51.6% (206/399) harbored carbapenemase genes. Three carbapenemase genes were detected, namely blaKPC-2, blaNDM-1, and blaIMP at rates of 29.8% (119/399), 17.5% (70/399), and 4.0% (16/399), respectively. In Klebsiella pneumoniae (350) and Escherichia coli (26), blaKPC-2 (33.4%, 117/350) and blaNDM-1 (61.5%, 16/26) were the predominant genes. The most common genes in the CRE isolates were blaKPC (85.5%) and blaNDM-1 (76.5%) from adults and children, respectively. Particularly, ST11 K. pneumoniae with blaKPC-2 harbored by IncFII plasmids were distributed in both general and primary hospitals, suggesting a clonal transmission pattern at these sites. In addition, the clonal distribution of ST2407 K. pneumoniae with blaNDM-1 located on IncX3 plasmids and blaIMP-38-positive ST307 K. pneumoniae were detected in a children’s hospital.
Conclusion
The distribution of carbapenemase genes differed among strains and age groups. Multiple carbapenemase genes in the CRE strains were clonally disseminated in the tested regions mediated by multiple plasmids. Therefore, CRE monitoring should be increased and measures should be adopted to prevent its transmission.
Keywords: carbapenem-resistant Enterobacterales, blaKPC-2, blaNDM-1, blaIMP, IncFII, ST11
Introduction
Enterobacterales are a large group of different types of bacteria that often cause infections in hospitals.1 Enterobacterales are constantly evolving new strategies to avoid the antibacterial effects of clinically applied antibiotics, leading to antibiotic resistance as the bacteria no longer respond to the antibiotics. Recently, carbapenem-resistant Enterobacterales (CRE) have been considered as one of major pathogenic strains that cause a series of severe infections in healthcare settings.2–4 CRE are also referred to as “superbugs” or “nightmare bacteria” due to limited amount of effective antibiotics available, which often lead to a high mortality.5,6 Therefore, this group of bacteria was identified as one of the world's leading critical pathogens in 2017 by the World Health Organization.7
Carbapenemase-producers are considered a main cause of CRE,8,9 such as KPC, NDM, IMP, and OXA-48.8 The clinical characteristics, detection rate, distribution, and molecular epidemiological characteristics of CRE are distinct among various geographical regions with different environmental conditions.10 The blaKPC gene is widely distributed worldwide with regional differences in its subtypes: blaKPC-2 in China, blaKPC-16 in Japan, and blaKPC-15 in Singapore. The blaOXA-48-like was initially limited to geographical areas surrounding Turkey but is currently found in several European countries (eg, France, Germany, Switzerland) and the United States.11 Recently, blaOXA-48-like was also found in China.12,13
Although there are many studies about CRE, they have mainly focused on general, primary, and community hospitals. In addition, studies have focused on understanding the dissemination of carbapenemases among CRE strains from adult patients, while only a few have investigated the distribution of CRE strains from children.14 In this study, the molecular characterization of CRE strains collected from several primary hospitals and general hospitals in the Hunan province of China was conducted. CRE strains collected from both adults and children were also compared and determined.
Materials and Methods
Ethics Statement and Study Subjects
This study was conducted using a protocol approved by the Ethics Committee of Hunan (Hunan Province, China) and according to the principles of the Declaration of Helsinki. Written informed consent was provided by the patients’ guardians prior to the study.
Sample Collection
A total of 399 CRE isolates were collected from 11 hospitals in Hunan province from October 2015 to July 2018 as follows: Xiangya Hospital of Central South University (n = 166), People’s Hospital of Ningxiang (n = 10), The Third Hospital of Changsha (n = 10), Hunan Want Hospital (n = 1), The First People’s Hospital of Xiangtan City (n = 55), Xiangtan City Central Hospital (n = 46), People’s Hospital of Xiangxi Autonomous Prefecture (n = 9), Shimen County People’s Hospital (n = 8), People’s Hospital of Shaoyang County (n = 6), Xinshao County People’s Hospital (n = 2), and Hunan Children’s Hospital (n = 86). All samples were collected from individual patients, and bacteria were recovered from colonization or infection sites: cerebral spinal fluid (n = 2), drainage fluid (n = 4), ascitic fluid (n = 3), blood (n = 14), pus (n = 2), bile (n = 1), pleural fluid (n = 1), catheter (n = 13), lower respiratory tract specimens (n = 294), urine (n = 33), secretion (n = 25), and puncture fluid (n = 7). These isolates were identified by matrix assisted laser desorption/ionization-time of flight-mass spectrometry (MALDI-TOF MS, Bruker Daltonics GmbH, Bremen, Germany). Briefly, one colony from an overnight culture was taken with a disposable loop and spotted onto the metal plate. The spots were covered with 1 μL α-cyano-4-hydroxy-cinnamic acid (HCCA) matrix (Bruker Daltonik GmbH, Bremen, Germany). Finally, bacterial samples on the microplate were analyzed with MALDI-TOF MS. MALDI Biotyper® (Bruker Daltonik GmbH, Bremen, Germany) software was used to classify bacteria at genus and species level.
Antimicrobial Susceptibility Testing
Antimicrobial susceptibility testing (AST) was performed with the VITEK-2 automated microbiology analyzer (BioMérieux, Marcy-l’Étoile, France) according to Clinical and Laboratory Standards Institute standards.15 The MIC of tigecycline was performed by broth microdilution method with the breakpoint established by the US Food and Drug Administration. Escherichia coli ATCC 25922 served as a strain for quality control (National Center for Clinical Laboratories, Beijing, China).
Detection of Carbapenemase Genes
The genomic DNA from Klebsiella pneumoniae strains was extracted from a bacterial culture with the boiling method.16 The carbapenemase genes (eg, blaKPC, blaNDM, blaVIM, blaIMP, blaOXA-48) were amplified by standard PCR procedures, as previously described.17 All of the PCR produces were sequenced and analyzed with BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi?CMD=Web&PAGE_TYPE=BlastHome).
Determination of Genetic Relatedness
Genetic correlations among different CRE isolates were measured with pulsed-field gel electrophoresis (PFGE). Briefly, cells from culture in the stationary phase were collected and embedded in agarose gel plugs (Lonza Rockland, Rockland, ME, USA). Then they were lysed in lysis buffer with proteinase K, followed by incubation with the XbaI restriction enzyme for 18 h at 37°C. Electrophoresis was performed for 19 h at 14°C with the Bio-Rad CHEF III system. Gels were stained using the GelRed nucleic acid stain (Biotium Inc., Fremont, CA, USA) and images were captured under ultraviolet light. Cluster analyses were conducted with BioNumerics software version 5.1 (Applied Maths, Kortrijk, Belgium). Isolates with ≥90% similarities were considered the same PFGE cluster.18,19 Isolates representing different PFGE clusters were selected for further analyses with multilocus sequence typing (MLST) with seven housekeeping genes (gapA, infB, mdh, pgi, phoE, rpoB, and tonB) following a standard protocol from the Pasteur MLST website.20
Whole-Genome Sequencing
The resistant plasmids were identified in CRE strains by whole-genome sequencing (WGS). The genomic DNA of bacteria was extracted with the DNeasy UltraClean Microbial Kit (QIAGEN, Hilden, Germany). Briefly, 10 μg DNA from each strain was used for the creation of Illumina paired-end libraries with 500 to 2000 bp insertion lengths of DNA. Then libraries were sequenced with the Illumina GA IIx sequencer (Illumina Inc., San Diego, CA, USA). Raw data were filtered and processed to remove reads as follows: 5 bp of ambiguous bases, 20 bp of low-quality bases, adapter contamination, and duplicated reads. The cleaned-out reads for each strain had about 100× genome coverage in depth. Genome assembly was conducted by SOAPdenovo version 1.05.21 The types of plasmids in the CRE isolates were identified by PlasmidFinder from the Centre for Genomic Epidemiology.22
Results
Isolates and Clinical Features
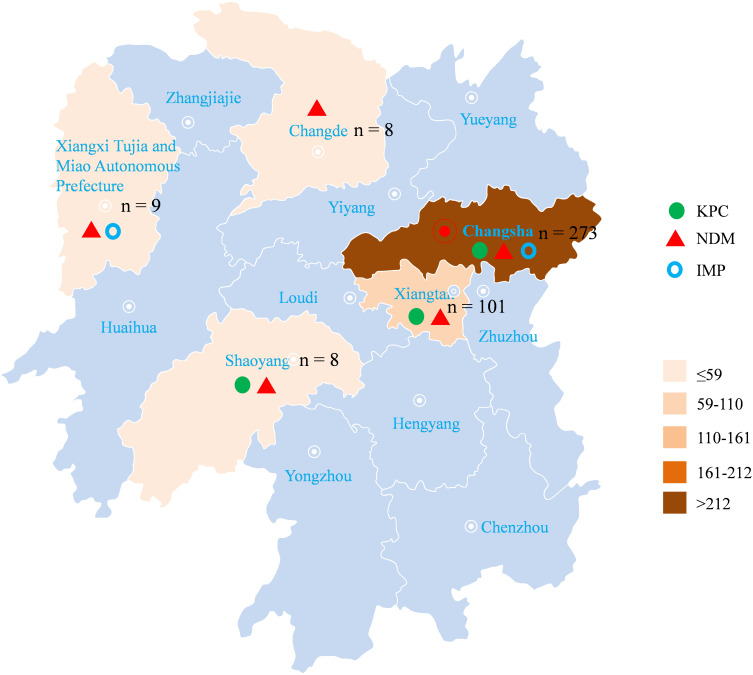
A total of 399 CRE isolates in this study were collected, and the main pathogen was K. pneumoniae, accounting for 87.7% (350/399), followed by E. coli (6.5%, 26/399), Enterobacter cloacae (3.8%, 15/399), and other strains (2.0%, 8/399). Eleven hospitals were located in five different areas of Hunan province: Changsha, Changde, Shaoyang, Xiangtan, and Xiangxi Tujia and Miao Autonomous Prefecture. (Figure 1).
Figure 1.
Distribution of CRE isolates in the Hunan province and proportion of carbapenemase genes.
Antimicrobial Susceptibility
In general, CRE have very high resistance rates to the most of the tested antibiotics. Among the 399 CRE strains, the resistance rates for imipenem and meropenem were 98.7% and 97.7%, respectively. The resistance rates for amikacin, gentamicin, ciprofloxacin, levofloxacin, and trimethoprim- sulfamethoxazole were 46.1%, 61.4%, 73.7%, 66.9%, and 46.1%, respectively. The resistance rate for tigecycline was 6.0%. (Table 1).
Table 1.
Antimicrobial Susceptibility Rates of Clinical CRE Strains (MICs, mg/L)
| Antimicrobial Agents | Number (%) of isolates | |
|---|---|---|
| R | S | |
| Piperacillin | 96.2 | 3.8 |
| Cefoperazone-sulbactam | 95.7 | 4.3 |
| Piperacillin-tazobactam | 94.7 | 5.3 |
| Cefazolin | 100.0 | 0 |
| Cefatriaxone | 98.5 | 1.5 |
| Ceftazidime | 98.0 | 2.0 |
| Cefepime | 98.2 | 1.8 |
| Imipenem | 98.7 | 1.3 |
| Meropenem | 97.7 | 2.3 |
| Amikacin | 46.1 | 53.9 |
| Gentamicin | 61.4 | 38.6 |
| Ciprofloxacin | 73.7 | 26.3 |
| Levofloxacin | 66.9 | 33.1 |
| Trimethoprim- sulfamethoxazole | 46.1 | 53.9 |
| Tigecycline | 6.0 | 94.0 |
Abbreviations: S, susceptible; R, resistant.
Distribution of Carbapenemase Genes
In all of the CRE isolates, 206 of 399 (51.6%) harbored carbapenemase genes, and three carbapenemase genes were detected, namely blaKPC-2, blaNDM-1 and blaIMP-38, with the positive rates of 29.8% (119/399), 17.5% (70/399), 4.0% (16/399), respectively. The positive rate of carbapenemase genes in K. pneumoniae was 48.9% (171/350), and blaKPC-2 was 33.4% (117/350), with detection of blaNDM-1 (12.3%, 43/350) and blaIMP (3.4%, 12/350), and one strain carried both blaKPC-2 and blaNDM-1 genes. Among the 26 E. coli strains, 22 harbored carbapenemase genes, mainly blaNDM (61.5%, 16/26), followed by blaKPC-2 (11.5%, 3/26)and blaIMP (11.5%, 3/26). The carbapenemase genes were detected in 11 of 15 E. cloacae strains, including 10 blaNDM-1-positive strains and one blaIMP-positive strain. These data suggest that the detection rates of carbapenemase genes differed among strains.
Importantly, three genes were detected in Changsha, while one to two genes were found in other areas, namely blaNDM-1 in Changde, blaKPC-2 and blaNDM-1 in Shaoyang, blaNDM-1 and blaIMP in Xiangxi Tujia and Miao Autonomous Prefecture, and blaKPC-2, and blaNDM-1 in Xiangtan. The blaNDM gene was detected in all five regions but the blaIMP gene was predominantly detected in isolates from Changsha, Xiangxi Tujia and Miao Autonomous Prefecture. The blaKPC-2 gene was found in isolates from Changsha, Shaoyang, and Xiangtan, suggesting that the carbapenemase genes and clonal distribution vary in different regions. (Figure 1).
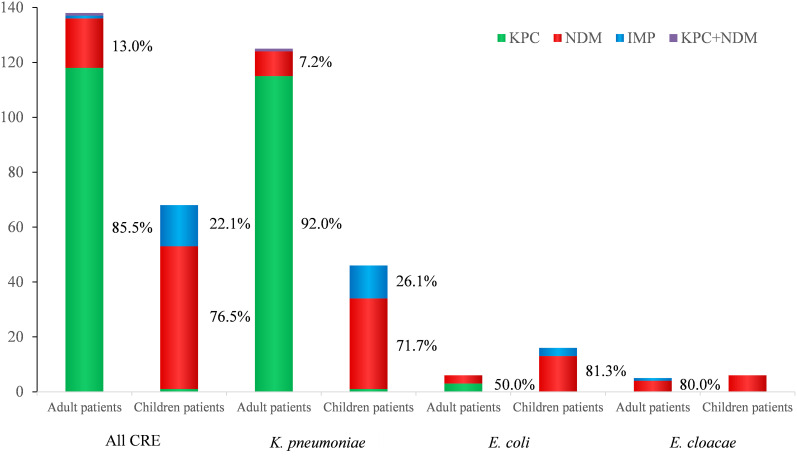
The differences in distribution of carbapenemase genes between adults and children were compared. The dominant CRE genes from adults and children were blaKPC-2 (85.5%) and blaNDM-1 (76.5%), respectively. In all of the K. pneumoniae strains, blaKPC-2 was also the major carbapenemase gene in adults, and blaNDM-1 was the main gene for children. In other identified CRE isolates, blaNDM-1 was more prevalent than blaKPC-2 in both adults and children. These results suggest that the carbapenemase genes differ between adults and children. (Figure 2).
Figure 2.
Distribution of carbapenemase among different CRE strains from adults and pediatric patients. The vertical axis represents the percent of each strain.
PFGE and Genetic Relatedness Analyses
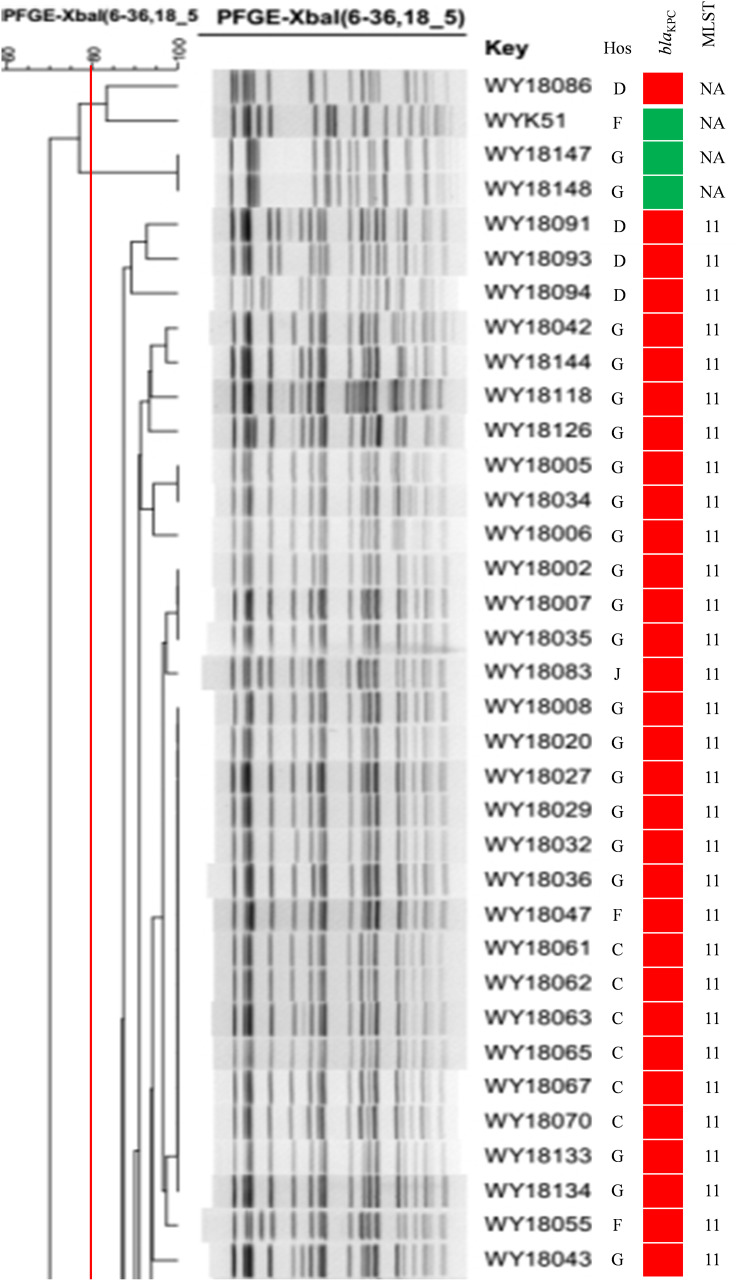
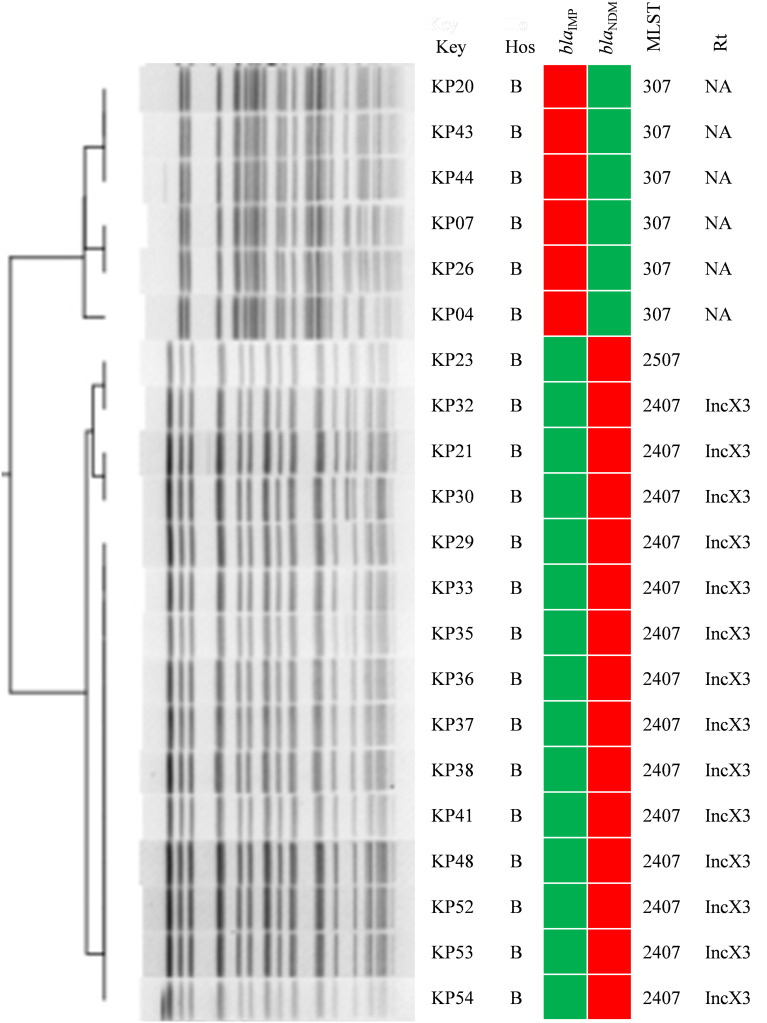
A variety of sequence types (STs) including ST11, ST2407, ST307, ST476, ST20, ST23, ST590, and others were determined, and the major one was ST11. The blaKPC-2-positive ST11 K. pneumoniae strains were detected in Xiangya Hospital of Central South University, the First People’s Hospital of Xiangtan City, People’s Hospital of Ningxiang, the Third Hospital of Changsha, and People’s Hospital of Shaoyang County. These strains belonged to the same PFGE type (Figure 3). In addition, blaNDM-1-positive ST2407 K. pneumoniae and six blaIMP-38-positive ST307 K. pneumoniae also showed a clonal transmission pattern in Hunan Children’s Hospital. (Figure 4).
Figure 3.
The blaKPC-2-positive ST11 K. pneumoniae in Hunan province (part). Red and green squares indicate the presence or absence of the indicated resistance genes, respectively. Isolates with ≥90% genetic similarity were classified in the same group.
Abbreviations: Hos, hospital; G, Xiangya Hospital of Central South University; F, The First People’s Hospital of Xiangtan City; C, People’s Hospital of Ningxiang; D, The Third Hospital of Changsha; J, People’s Hospital of Shaoyang County; NA, not detected.
Figure 4.
Clonal distribution of blaIMP-38-positive ST307 K. pneumoniae and blaNDM-1-positive ST2407 K. pneumoniae in Hunan Children’s Hospital. Red and green squares indicate the presence and absence of the indicated resistance genes, respectively.
Abbreviations: Hos, hospital; B, Hunan Children’s Hospital; Rt, replicon typing; NA, not detected.
Types of Plasmid Replicon by WGS
The plasmid types harbored in the main clonal strains were analyzed, and the results showed that blaKPC-2 was carried by IncFII in ST11 K. pneumoniae , while blaNDM-1 was located on IncX3 in ST2407 K. pneumoniae. The six blaIMP-positive ST307 K. pneumoniae did not show any of the detected plasmid types. These results suggested that clonal transmission of carbapenemase genes was mediated by multiple types of plasmids. (Figures 3 and 4).
Discussion
CRE strains have gradually increased in adults and children during recent years, and become a serious pathogen causing nosocomial infection.23 However, the molecular characteristics of CRE strains from children and adults in hospitals are largely unclear. Therefore, a comprehensive molecular epidemiological study of CRE strains from 11 hospitals in Hunan province was conducted. The distribution of carbapenemase genes in this study differed among species and age groups. Multiple carbapenemase genes in the CRE strains with clonal dissemination were mediated by multiple plasmids. In particular, blaIMP-38-positive ST307 K. pneumoniae and blaNDM-1-positive ST2407 K. pneumoniae were distributed in Hunan Children’s Hospital at the same time.
Three carbapenemase genes were detected, blaKPC-2, blaNDM-1 and blaIMP, and K. pneumoniae harboring blaKPC-2 was predominantly found in China.24,25 The VIM-like carbapenemases were absent in China but present in other countries.26 The metalloenzyme (blaNDM-1 and blaIMP) was the primary carbapenemase genes in other Enterobacterales such as E. coli and E. cloacae. Six blaIMP-38-positive K. pneumoniae from Hunan Children’s Hospital were found, which were detected in clonal distribution in Xiangya Hospital of Central South University for the first time. In addition, carbapenemase genes in adults were largely serine enzyme genes (blaKPC-2), whereas metalloenzyme genes (blaNDM-1 and blaIMP) were mainly found in CRE from children, consistent with the study by Han et al24 showing different distributions of carbapenemase genes in different age groups. Ceftazidime-avibactam is an enzyme inhibitor activity against group A, and some D (OXA-48) serine carbapenemases. Therefore, the efficacy of ceftazidime-avibactam treatment against CRE might be significantly higher in adults than in children.
The ST11 blaKPC-positive K. pneumoniae belongs to the CG258 clone, which is prevalent worldwide.27–29 ST11 blaKPC-2-positive K. pneumoniae isolates with the same PFGE fingerprint found in our study were not only in several general hospitals but also in a primary hospital, indicating clonal transmission. Furthermore, the clonal distribution of blaNDM-1-positive ST2407 K. pneumoniae was detected in Hunan Children’s Hospital. Two previous studies have reported ST2407 K. pneumoniae.30,31 The clonal transmission of ST2407 K. pneumoniae from Hunan Children’s Hospital was reported for the first time in this study. The wide prevalence of blaIMP-38-positive ST307 K. pneumoniae strains also showed clonal distribution in the Children’s Hospital.
Plasmids are vital for the horizontal transfer of drug resistance genes. IncX3 is an important plasmid leading to the wide spread of blaNDM.32–34 The blaNDM-1-positive K. pneumoniae isolates were clonally distributed, which was mediated by IncX3 plasmids in Hunan Children’s Hospital. Several isolates with blaKPC-2 located on IncFII plasmids were distributed in multiple regions, indicating that IncFII-like plasmids might be involved in the clonal transmission of blaKPC-2 among K. pneumoniae.27
This study had some limitations. A different number of bacteria were isolated from different hospitals, which might be correlated with the low detection rate of CRE in primary hospitals. A variety of drug-resistant genes were clonally transmitted through plasmids, but it was very difficult to conduct an in-depth study due to the limitations of other factors such as the understanding of intestinal flora in the clonal population, the isolation conditions, and environment strains in the hospitals.
In conclusion, the distribution of carbapenemase genes differed among strains and age groups. The blaKPC-2, blaNDM-1, blaIMP-38 genes in CRE strains with clonal dissemination in our regions were mediated by multiple plasmids. Therefore, the timely measurement of CRE should be increased and effective measures should be adopted to prevent the transmission of bacteria.
Acknowledgments
We thank all of the teachers from the 11 hospitals for their contributions and help collecting the samples and identifying the CRE isolates.
Funding Statement
This study was supported by the Natural Science Foundation of Hunan Province (No. 2020JJ4886), the National Natural Science Foundation of China (No. 81702068), and the Science Foundation of Hunan Health Commission in Hunan province (No. 202111000066).
Data Sharing Statement
The datasets generated and analyzed during the present study are available from the corresponding author on reasonable request.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors have no conflicts of interest to declare.
References
- 1.Gajdács M, Bátori Z, Ábrók M, Lázár A, Burián K. Characterization of resistance in Gram-negative urinary isolates using existing and novel indicators of clinical relevance: a 10-year data analysis. Life (Basel). 2020;10:16. doi: 10.3390/life10020016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lutgring JD. Carbapenem-resistant Enterobacteriaceae: an emerging bacterial threat. Semin Diagn Pathol. 2019;36:182–186. doi: 10.1053/j.semdp.2019.04.011 [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Wang Q, Yin Y, et al. Epidemiology of carbapenem-resistant Enterobacteriaceae infections: report from the China CRE Network. Antimicrob Agents Chemother. 2018;62. doi: 10.1128/aac.01882-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li J, Zou MX, Wang HC, et al. An outbreak of infections caused by a Klebsiella pneumoniae ST11 clone coproducing Klebsiella pneumoniae carbapenemase-2 and rmtB in a Chinese teaching hospital. Chin Med J (Engl). 2016;129:2033–2039. doi: 10.4103/0366-6999.189049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gajdács M. The concept of an ideal antibiotic: implications for drug design. Molecules. 2019;24(5):892. doi: 10.3390/molecules24050892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gajdács M, Albericio F. Antibiotic resistance: from the bench to patients. Antibiotics (Basel). 2019;8. doi: 10.3390/antibiotics8030129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization (WHO). Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. WHO; Geneva, Switzerland: 2017. Available from: https://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf
- 8.Kazmierczak KM, Karlowsky JA, de Jonge BLM, Stone GG, Sahm DF. Epidemiology of carbapenem resistance determinants identified in meropenem-nonsusceptible Enterobacterales collected as part of a global surveillance program, 2012 to 2017. Antimicrob Agents Chemother. 2021;65:e0200020. doi: 10.1128/aac.02000-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodman KE, Simner PJ, Tamma PD, Milstone AM. Infection control implications of heterogeneous resistance mechanisms in carbapenem-resistant Enterobacteriaceae (CRE). Expert Rev Anti Infect Ther. 2016;14:95–108. doi: 10.1586/14787210.2016.1106940 [DOI] [PubMed] [Google Scholar]
- 10.Gajdács M, Ábrók M, Lázár A, et al. Detection of VIM, NDM and OXA-48 producing carbapenem resistant Enterobacterales among clinical isolates in Southern Hungary. Acta Microbiol Immunol Hung. 2020;67(4):209–215. doi: 10.1556/030.2020.01181 [DOI] [PubMed] [Google Scholar]
- 11.Hansen GT. Continuous evolution: perspective on the epidemiology of carbapenemase resistance among Enterobacterales and other Gram-negative bacteria. Infect Dis Ther. 2021;10:75–92. doi: 10.1007/s40121-020-00395-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Ma W, Qin Q, et al. Nosocomial spread of OXA-232-producing Klebsiella pneumoniae ST15 in a teaching hospital, Shanghai, China. BMC Microbiol. 2019;19:235. doi: 10.1186/s12866-019-1609-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhuang Z, Lv L, Lu J, Lin J, Liu JH. Emergence of Klebsiella pneumoniae and Enterobacter cloacae producing OXA-48 carbapenemases from retail meats in China, 2018. J Antimicrob Chemother. 2019;74(12):3632–3634. doi: 10.1093/jac/dkz394 [DOI] [PubMed] [Google Scholar]
- 14.Li J, Yu T, Tao XY, et al. Emergence of an NDM-5-producing Escherichia coli sequence type 410 clone in infants in a children’s hospital in China. Infect Drug Resist. 2020;13:703–710. doi: 10.2147/idr.s244874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing. 30th ed. CLSI supplement M100. Wayne, PA: Clinical and Laboratory Standards Institute; 2020
- 16.Li J, Zou M, Dou Q, et al. Characterization of clinical extensively drug-resistant Pseudomonas aeruginosa in the Hunan province of China. Ann Clin Microbiol Antimicrob. 2016;15:35. doi: 10.1186/s12941-016-0148-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poirel L, Walsh TR, Cuvillier V, Nordmann P. Multiplex PCR for detection of acquired carbapenemase genes. Diagn Microbiol Infect Dis. 2011;70:119–123. doi: 10.1016/j.diagmicrobio.2010.12.002 [DOI] [PubMed] [Google Scholar]
- 18.Tenover FC, Arbeit RD, Goering RV, et al. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Huang ZY, Yu T, et al. Isolation and characterization of a sequence type 25 carbapenem-resistant hypervirulent Klebsiella pneumoniae from the mid-south region of China. BMC Microbiol. 2019;19:219. doi: 10.1186/s12866-019-1593-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diancourt L, Passet V, Verhoef J, Grimont PA, Brisse S. Multilocus sequence typing of Klebsiella pneumoniae nosocomial isolates. J Clin Microbiol. 2005;43:4178–4182. doi: 10.1128/jcm.43.8.4178-4182.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sui W, Zhou H, Du P, et al. Whole genome sequence revealed the fine transmission map of carbapenem-resistant Klebsiella pneumonia isolates within a nosocomial outbreak. Antimicrob Resist Infect Control. 2018;7:70. doi: 10.1186/s13756-018-0363-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carattoli A, Zankari E, García-Fernández A, et al. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob Agents Chemother. 2014;58:3895–3903. doi: 10.1128/aac.02412-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aguilera-Alonso D, Escosa-García L, Saavedra-Lozano J, Cercenado E, Baquero-Artigao F. Carbapenem-resistant Gram-negative bacterial infections in Children. Antimicrob Agents Chemother. 2020;64. doi: 10.1128/aac.02183-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han R, Shi Q, Wu S, et al. Dissemination of carbapenemases (KPC, NDM, OXA-48, IMP, and VIM) among carbapenem-resistant Enterobacteriaceae isolated from adult and children patients in China. Front Cell Infect Microbiol. 2020;10:314. doi: 10.3389/fcimb.2020.00314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang M, Li J, Lu Y, et al. Expanding of ST11 carbapenemase-producing Klebsiella pneumoniae subclones in a Chinese Hospital, Shenzhen, China. Infect Drug Resist. 2021;14:1415–1422. doi: 10.2147/idr.s299478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tóth Á, Makai A, Jánvári L, Damjanova I, Gajdács M, Urbán E. Characterization of a rare bla(VIM-4) metallo-β-lactamase-producing Serratia marcescens clinical isolate in Hungary. Heliyon. 2020;6:e04231. doi: 10.1016/j.heliyon.2020.e04231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fu P, Tang Y, Li G, Yu L, Wang Y, Jiang X. Pandemic spread of bla(KPC-2) among Klebsiella pneumoniae ST11 in China is associated with horizontal transfer mediated by IncFII-like plasmids. Int J Antimicrob Agents. 2019;54:117–124. doi: 10.1016/j.ijantimicag.2019.03.014 [DOI] [PubMed] [Google Scholar]
- 28.Machulska M, Baraniak A, Żak I, et al. KPC-2-producing Klebsiella pneumoniae ST11 in a children’s hospital in Poland. Pol J Microbiol. 2017;66:401–404. doi: 10.5604/01.3001.0010.4884 [DOI] [PubMed] [Google Scholar]
- 29.Oteo J, Pérez-Vázquez M, Bautista V, et al. The spread of KPC-producing Enterobacteriaceae in Spain: WGS analysis of the emerging high-risk clones of Klebsiella pneumoniae ST11/KPC-2, ST101/KPC-2 and ST512/KPC-3. J Antimicrob Chemother. 2016;71:3392–3399. doi: 10.1093/jac/dkw321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qiao F, Wei L, Feng Y, et al. Handwashing sink contamination and carbapenem-resistant Klebsiella infection in the intensive care unit: a prospective multicenter study. Clin Infect Dis. 2020;71:S379–s385. doi: 10.1093/cid/ciaa1515 [DOI] [PubMed] [Google Scholar]
- 31.Patil S, Chen X, Wen F. Exploring the phenotype and genotype of multi-drug resistant Klebsiella pneumoniae harbouring bla(CTX-M) group extended-spectrum β-lactamases recovered from paediatric clinical cases in Shenzhen, China. Ann Clin Microbiol Antimicrob. 2019;18:32. doi: 10.1186/s12941-019-0331-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu W, Wang X, Qin J, Liang W, Shen Z. Dissemination and stability of the bla (NDM-5)-carrying IncX3-type plasmid among multiclonal Klebsiella pneumoniae isolates. mSphere. 2020;5. doi: 10.1128/mSphere.00917-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Q, Zhou J, Wu S, et al. Characterization of the IncX3 plasmid producing bla(NDM-7) from Klebsiella pneumoniae ST34. Front Microbiol. 2020;11:1885. doi: 10.3389/fmicb.2020.01885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pérez-Vázquez M, Sola Campoy PJ, Ortega A, et al. Emergence of NDM-producing Klebsiella pneumoniae and Escherichia coli in Spain: phylogeny, resistome, virulence and plasmids encoding blaNDM-like genes as determined by WGS. J Antimicrob Chemother. 2019;74:3489–3496. doi: 10.1093/jac/dkz366 [DOI] [PubMed] [Google Scholar]