Abstract
Background:
Early detection of gastric cancer (GC) has been the topic of major efforts in China. This study aimed to explore the risk factors associated with GC and to provide evidence for the selection of a high-risk population of GC.
Methods:
Based on the cancer screening cohort of the National Cancer Screening Program in Urban China, GC patients diagnosed by endoscopy and pathological examinations constituted the case group, and controls were 1:3 matched by sex and age (±5 years) individually. The variables were selected by univariable analysis of factors such as body mass index (BMI), dietary habits, lifestyle, stomach disease history, and family history of GC; and multivariable logistic regression was used to analyze the influencing factors of GC and to calculate the odds ratio (OR) of related factors and its 95% confidence interval (CI).
Results:
A total of 215 GC cases and 645 matched healthy controls were included in the final analysis, with a median age of 61 years for the case and control groups. Overall analysis showed that high educational level (above primary school) (OR = 0.362, 95% CI = 0.219–0.599, P < 0.001), overweight/obesity (BMI ≥24 kg/m2; OR = 0.489, 95% CI = 0.329–0.726, P < 0.001), cigarette smoking (OR = 3.069, 95% CI = 1.700–5.540, P < 0.001), alcohol consumption (OR = 1.661, 95% CI = 1.028–2.683, P = 0.038), history of stomach disease (OR = 6.917, 95% CI = 4.594–10.416, P < 0.001), and family history of GC in first-degree relatives (OR = 4.291, 95% CI = 1.661–11.084, P = 0.003) were significantly correlated with the occurrence of GC. Subgroup analyses by age and gender indicated that GC risk was still increased in the presence of a history of stomach disease. A history of chronic gastritis, gastric ulcer, or gastric polyposis was positively associated with GC, with adjusted ORs of 4.155 (95% CI = 2.711–6.368), 1.839 (95% CI = 1.028–3.288), and 2.752 (95% CI = 1.197–6.326).
Conclusions:
Subjects who smoke, drink, with history of stomach disease and family history of GC in first-degree relatives are the high-risk populations for GC. Therefore, attention should be paid to these subjects for GC screening.
Keywords: Risk factors, Gastric cancer, Case-control study, Cancer screening
Introduction
Gastric cancer (GC) is one of the most common types of upper gastrointestinal cancer in China. According to the latest report from the International Agency for Research on Cancer,[1] there were 479,000 new cases and 374,000 deaths due to GC in China in 2020, accounting for 44.0% and 48.6%, respectively, of the global new cases and deaths due to GC. In the past 10 years, although the incidence and mortality rates for GC have shown a downward trend in China, it still ranks fourth in the incidence of malignant tumors, and its mortality ranks third, which seriously threatens the lives and health of people in China.[1–2]
Because the clinical symptoms of early GC are not obvious, most patients are in the middle or late stages when they are diagnosed. The clinical treatment costs are high, with poor outcomes, and the survival period is brief. According to the latest data,[3] the 5-year survival rate for GC is 35.1% in China. When GC is discovered at an early stage and properly treated, the 5-year survival rate can exceed 90%, sometimes achieving complete remission. Therefore, prevention and timely diagnosis are crucial to improve the prognosis of GC. However, due to the multidimensional nature of GC, prevention is subject to accurate identification of the risk factors and underlying causes of this disease, as well as the management of these factors.[4]
In view of the increasing burden of cancer among urban populations in China, the Ministry of Finance and the previous Ministry of Health of China approved the urban cancer early diagnosis and treatment project in 2012 to carry out assessments of high-risk populations and clinical screening for six major types of cancers, including GC. Based on the screening population of this project, a case-control study was conducted to explore the risk factors affecting the occurrence of GC and provide a scientific basis for the design of GC screening strategies in China's urban population.
Methods
Ethical approval
The study was approved by the Ethics Committee of National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences, and Peking Union Medical College, Beijing, China (No.15-070/997). Informed consent was obtained from all participants before enrollment.
Study population
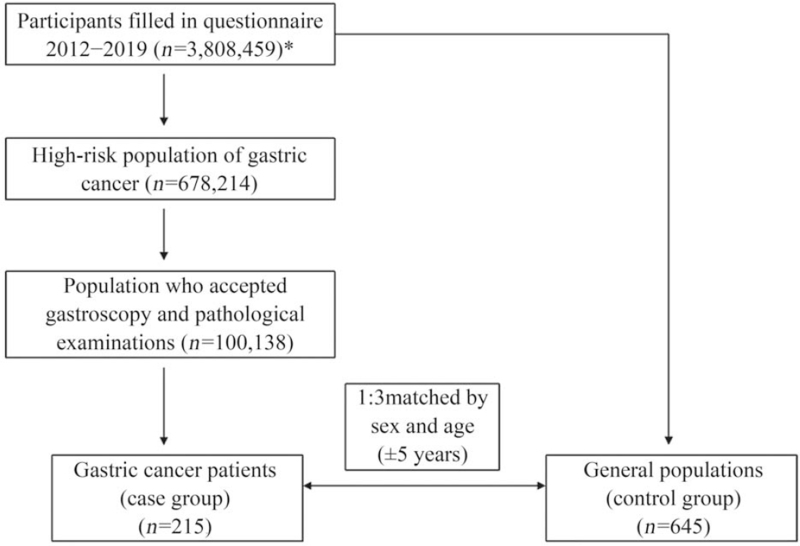
The study was based on a large-scale, multicenter cancer screening cohort in urban areas of China. Subjects who were 40 to 74 years old, had no serious organ dysfunction or mental illness, and had been living in the current location for at least 3 years were eligible to participate in the screening program. Altogether 3,808,459 participants were enrolled and completed the questionnaire in 22 provinces between September 2012 and December 2019. After filling the questionnaire, 678,214 high-risk individuals of GC were selected through the risk assessment model and they were recommended to go to designated hospitals for free gastroscopy. Among them, 100,138 subjects accepted gastroscopy and pathological examinations, and 215 GC patients were diagnosed. All endoscopic examinations were conducted by well-trained endoscopy doctors at local hospitals according to the protocol for cancer screening in urban areas of China. Then, pathologists independently read the biopsy slides. Control subjects were selected from the general population who filled in the questionnaire and individually matched at a ratio of 1:3 by sex and age (±5 years). There were 215 patients in the case group, and there were 645 subjects in the control group [Figure 1].
Figure 1.
Flowchart for the selection of the case and control subjects. ∗Participants came from 22 provinces, including Anhui, Beijing, Gansu, Guangdong, Guangxi, Hainan, Hebei, Henan, Heilongjiang, Hubei, Hunan, Jiangsu, Jiangxi, Liaoning, Inner Mongolia, Shandong, Shanxi, Shaanxi, Xinjiang, Yunnan, Zhejiang, and Chongqing.
Data collection
All study interviews were conducted face-to-face by highly trained investigators. A structured questionnaire consisting of five categories of questions was used for data collection. The questionnaires included the following categories: (1) general demographic information, including age, gender, height, weight, marital status, and educational level; (2) dietary habits, including the intake of fresh fruits and vegetables, preference of food temperature, taste preference of diet, and pickled foods; (3) lifestyle factors, including cigarette smoking, alcohol consumption, and physical activity; (4) history of stomach disease, including chronic gastritis, gastric ulcer, duodenal ulcer, gastric polyposis, remnant stomach, dysplasia, and intestinal metaplasia; and (5) family history of GC (FHGC) in first-degree relatives.
Regarding dietary habits, the intake of fruits and vegetables was measured as the intake frequency or the average personal intake in the last 2 years, which was calculated by dividing the total intake of household by the number of a family member and calculated by the weight of uncooked (fresh fruits were not peeled). Frequent fruits and vegetable intake was defined as >3 days/week or >2500 g/week for vegetables and >1250 g/week for fruits. Pickled food included salted fish, sauerkraut, and pickles, and frequent intake was defined as consuming >4 days/week. Participants were defined as smokers if they had smoked at least one cigarette per day during the previous 6 months. Alcohol consumption was defined as drinking alcoholic beverages at least once per week during the previous 6 months. Regular physical activity was defined as exercising for >30 min at least three times per week. History of stomach disease includes chronic gastritis, gastric ulcer, duodenal ulcer, gastric polyposis, remnant stomach, gastric mucosal intraepithelial neoplasia, and gastrointestinal epithelial metaplasia, which were examined at a formal medical institution with corresponding examination conditions and diagnostic capabilities, as well as confirmed by a practicing physician. FHGC in first-degree relatives was defined as having a first-degree relative at least (ie, a parent or child of the participant) who was diagnosed with stomach cancer. According to the standard recommended by the guidelines for the prevention and control of overweight and obesity in adults in China,[5] body mass index (BMI) < 18.5 kg/m2 means low weight, 18.5 to 23.9 kg/m2 means normal weight, and BMI ≥24.0 kg/m2 means overweight and obesity.
Statistical analysis
SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA) was used for all analyses. When the continuous variables met the criteria for normality and homogeneity of variance, they were expressed as mean ± standard deviation, and Student's t test was performed to compare the case and control groups. Otherwise, continuous variables were expressed as median (P25, P75) and compared using the Wilcoxon signed-rank test. The categorical variables were expressed as percentages and compared using the Chi-square or Fisher's exact test. To assess the risk factors for GC, conditional logistic regression was used to estimate the odds ratios (ORs) and 95% confidence intervals (CIs). All variables related to GC at a significance level according to the univariable conditional logistic analysis were included in the multivariable conditional logistic regression analysis. Moreover, subgroup analysis by age and gender was performed to evaluate the relationship between history of stomach disease and GC. All reported P-values were two-tailed, and P < 0.05 were considered statistically significant.
Results
The distributions of basic characteristics of the study participants are shown in Table 1. Because case and control groups were individually matched on age (±5 years) and gender, the median age of the two groups was 61 years old. Fifty-eight percent of the study participants were male, and approximately 99% of participants were married. Differences in educational level, BMI, taste preference of diet, pickled food intake, cigarette smoking, alcohol consumption, physical activity, history of stomach disease, and FHGC in first-degree relatives between the case and control groups were statistically significant (P < 0.05).
Table 1.
Comparison of basic characteristics of the case and control groups from the National Cancer Screening Program in Urban China.
| Parameters | Case group (n = 215) | Control group (n = 645) | Chi-square value | P value |
| Age, years | 61 (55, 66) | 61 (55, 66) | – | – |
| Gender | – | – | ||
| Male | 125 (58.14) | 375 (58.14) | ||
| Female | 90 (41.86) | 270 (41.86) | ||
| BMI (kg/m2)∗ | 17.284 | <0.001 | ||
| <24.0 | 105 (48.84) | 213 (33.02) | ||
| ≥24.0 | 110 (51.16) | 432 (66.98) | ||
| Educational level | 18.236 | <0.001 | ||
| Primary school and below | 59 (27.44) | 94 (14.57) | ||
| Above primary school | 156 (72.56) | 551 (85.43) | ||
| Marital status | –† | 0.050 | ||
| Unmarried | 3 (1.40) | 1 (0.16) | ||
| Married | 212 (98.60) | 644 (99.84) | ||
| Fruits and vegetables intake | 14.049 | <0.001 | ||
| Not frequent | 162 (75.35) | 395 (61.24) | ||
| Frequent | 53 (24.65) | 250 (38.76) | ||
| Preference of food temperature | 22.690 | <0.001 | ||
| Not hot | 127 (59.07) | 490 (75.97) | ||
| Hot | 88 (40.93) | 155 (24.03) | ||
| Taste preference of diet | 32.857 | <0.001 | ||
| Mild or low salt | 112 (52.09) | 472 (73.18) | ||
| High salt | 103 (47.91) | 173 (26.82) | ||
| Pickled food intake | 20.132 | <0.001 | ||
| Not frequent | 151 (70.23) | 543 (84.19) | ||
| Frequent | 64 (29.77) | 102 (15.81) | ||
| Cigarette smoking | 53.186 | <0.001 | ||
| No | 101 (46.98) | 477 (73.95) | ||
| Yes | 114 (53.02) | 168 (26.05) | ||
| Alcohol consumption | 50.192 | <0.001 | ||
| No | 94 (43.72) | 455 (70.54) | ||
| Yes | 121 (56.28) | 190 (29.46) | ||
| Physical activity | 12.308 | <0.001 | ||
| Not frequent | 136 (63.26) | 319 (49.46) | ||
| Frequent | 79 (36.74) | 326 (50.54) | ||
| History of stomach disease | 192.653 | <0.001 | ||
| No | 64 (29.77) | 521 (80.78) | ||
| Yes | 151 (70.23) | 124 (19.22) | ||
| FHGC‡ | 28.319 | <0.001 | ||
| No | 196 (91.16) | 636 (98.6) | ||
| Yes | 19 (8.84) | 9 (1.40) |
Data are presented as median (P25, P75) or n (%). BMI: Body mass index; FHGC: Family history of GC.
Since subjects with BMI < 18.5 kg/m2 are few, therefore it was merged into the normal weight group and recorded as BMI < 24.0 kg/m2.
The variable marital status did not meet the application conditions of the chi-square test, which a grid theoretical frequency was <1, so Fisher's exact test was used.
FHGC in first-degree relatives.
All variables related to GC at a significance level of P < 0.05 according to the univariable analysis were included in the multivariate conditional logistic regression analysis. The ORs of major factors significantly and marginally associated with GC are shown in Table 2. Educational level was inversely associated with GC risk (above primary school vs. primary school and below: OR = 0.362, 95% CI = 0.219–0.599). Overweight/obesity (BMI ≥24 kg/m2) had a protective effect on GC risk compared with normal/lean weight, with an OR of 0.489 (95% CI = 0.329–0.726). The OR for smokers vs. never-smokers was 3.069 (95% CI = 1.700–5.540). The OR for drinkers vs. never-drinkers was 1.661 (95% CI = 1.028–2.683). GC risk was increased in the presence of a history of stomach disease (OR = 6.917, 95% CI = 4.594–10.416) and FHGC in first-degree relatives (OR = 4.291, 95% CI = 1.661–11.084).
Table 2.
Association between potential factors and GC in multivariate conditional logistic regression analysis.
| Parameters | β | Standard error | Wald χ2 | P-value | OR | 95% CI | |
| Educational level | |||||||
| Primary school and below vs. above primary school | −0.508 | 0.129 | 15.604 | <0.001 | 0.362 | 0.219–0.599 | |
| BMI (kg/m2) | |||||||
| ≥24 vs. <24 | −0.358 | 0.101 | 12.566 | <0.001 | 0.489 | 0.329–0.726 | |
| Fruits and vegetables intake | |||||||
| Frequent vs. not frequent | −0.104 | 0.114 | 0.843 | 0.359 | 0.812 | 0.520–1.267 | |
| Preference of food temperature | |||||||
| Hot vs. not hot | 0.037 | 0.110 | 0.113 | 0.737 | 1.077 | 0.699–1.657 | |
| Taste preference of diet | |||||||
| High salt vs. mild or low salt | −0.001 | 0.118 | <0.001 | 0.994 | 0.998 | 0.630–1.582 | |
| Pickled food intake | |||||||
| Frequent vs. not frequent | 0.020 | 0.128 | 0.024 | 0.877 | 1.040 | 0.629–1.720 | |
| Cigarette smoking | |||||||
| Yes vs. no | 0.561 | 0.151 | 13.839 | <0.001 | 3.069 | 1.700–5.540 | |
| Alcohol consumption | |||||||
| Yes vs. no | 0.254 | 0.122 | 4.293 | 0.038 | 1.661 | 1.028–2.683 | |
| Physical activity | |||||||
| Frequent vs. not frequent | −0.102 | 0.101 | 1.010 | 0.315 | 0.816 | 0.549–1.213 | |
| FHGC in first-degree relatives | |||||||
| Yes vs. no | 0.728 | 0.242 | 9.045 | 0.003 | 4.291 | 1.661–11.084 | |
| History of stomach disease | |||||||
| Yes vs. no | 0.967 | 0.104 | 85.773 | <0.001 | 6.917 | 4.594–10.416 | |
CI: Confidence interval; FHGC: Family history of GC; GC: Gastric cancer; OR: Odds ratio. Frequent fruits and vegetables intake: >3 days/week or >2500 g/week for vegetables and >1250 g/week for fruits. Frequent pickled food intake: >4 days/week. Frequent physical activity: >3 times/week and >30 minutes per time.
Table 3 shows the results of subgroup analysis of the association between a history of stomach disease and GC risk by age and gender. GC risk was increased in the presence of a history of stomach disease in different subgroups.
Table 3.
Subgroup analysis by age and gender for the case and control groups from the National Cancer Screening Program in Urban China.
| Case group (n = 215) | Control group (n = 645) | |||||||
| History of stomach disease | N | % | N | % | OR∗ | 95% CI | OR† | 95% CI |
| Male, age ≤60 years | ||||||||
| No | 15 | 28.85 | 126 | 80.77 | 1.000 | – | 1.000 | – |
| Yes | 37 | 71.15 | 30 | 19.23 | 9.870 | 4.821–20.206 | 5.691 | 2.327–13.917 |
| Male, age >60 years | ||||||||
| No | 22 | 30.14 | 188 | 85.84 | 1.000 | – | 1.000 | – |
| Yes | 51 | 69.86 | 31 | 14.16 | 14.314 | 7.451–27.500 | 11.031 | 5.075–23.973 |
| Female, age ≤60 years | ||||||||
| No | 12 | 27.27 | 98 | 74.24 | 1.000 | – | 1.000 | – |
| Yes | 32 | 72.73 | 34 | 25.76 | 7.189 | 3.365–15.360 | 8.622 | 3.170–23.448 |
| Female, age >60 years | ||||||||
| No | 15 | 32.61 | 109 | 78.99 | 1.000 | – | 1.000 | – |
| Yes | 31 | 67.39 | 29 | 21.01 | 8.135 | 3.737–17.710 | 5.396 | 2.141–13.603 |
∗Adjusted for age and gender. †Adjusted for age, gender, educational level, BMI, salt intake, pickled food intake, cigarette smoking, alcohol consumption, physical activity, and FHGC. BMI: Body mass index; CI: Confidence interval; FHGC: Family history of GC; OR: Odds ratio.
Stomach disease included chronic gastritis, gastric ulcer, duodenal ulcer, gastric polyposis, remnant stomach, dysplasia, and intestinal metaplasia. We did not analyze the association between a history of dysplasia, as well as intestinal metaplasia, and GC risk due to the limited number of patients in case group and subjects in the control group. However, a history of chronic gastritis, gastric ulcer, or gastric polyposis was positively associated with GC, with adjusted ORs of 4.155 (95% CI = 2.711–6.368), 1.839 (95% CI = 1.028–3.288), and 2.752 (95% CI = 1.197–6.326) [Table 4].
Table 4.
Association between specific stomach diseases and GC risk.
| Case group | Control group | |||||||
| History of stomach disease | N | % | N | % | OR∗ | 95% CI | OR† | 95% CI |
| Chronic gastritis | ||||||||
| No | 84 | 39.07 | 543 | 84.19 | 1.000 | – | 1.000 | – |
| Yes | 131 | 60.93 | 102 | 15.81 | 8.271 | 5.810–11.773 | 4.155 | 2.711–6.368 |
| Gastric ulcer | ||||||||
| No | 145 | 67.44 | 601 | 93.18 | 1.000 | – | 1.000 | – |
| Yes | 70 | 32.56 | 44 | 6.82 | 7.451 | 4.770–11.640 | 1.839 | 1.028–3.288 |
| Duodenal ulcer | ||||||||
| No | 179 | 83.26 | 627 | 97.21 | 1.000 | – | 1.000 | – |
| Yes | 36 | 16.74 | 18 | 2.79 | 7.172 | 3.952–13.018 | 1.857 | 0.873–3.949 |
| Gastric polyposis | ||||||||
| No | 181 | 84.19 | 631 | 97.83 | 1.000 | – | 1.000 | – |
| Yes | 34 | 15.81 | 14 | 2.17 | 9.269 | 4.730–18.165 | 2.752 | 1.197– 6.326 |
| Remnant stomach | ||||||||
| No | 208 | 96.74 | 643 | 99.69 | 1.000 | – | 1.000 | – |
| Yes | 7 | 3.26 | 2 | 0.31 | 10.883 | 2.238–52.931 | 1.300 | 0.194–8.709 |
∗Adjusted for age and gender. †Adjusted for age, gender, educational level, BMI, salt habit, pickled food, cigarette smoking, alcohol consumption, physical activity, and FHGC. CI: Confidence interval; BMI: Body mass index; FHGC: Family history of GC; GC: Gastric cancer; OR: Odds ratio.
Discussion
In this study, based on a population in urban areas of China, cigarette smoking, alcohol consumption, history of stomach disease, and FHGC in first-degree relatives were identified as the factors to increase the risk of GC. However, overweight and obesity (BMI ≥24 kg/m2) and high educational level were inversely associated with the risk of GC.
Educational level and income, which are the most important determinants of social class, are directly related to the level of health.[6] Although educational attainment alone cannot directly affect GC risk, it can affect some GC risk factors, including Helicobacter pylori infection, which play a role in the development of GC. In our study, educational level was inversely associated with GC risk (above primary school vs. primary school and below: OR = 0.362, 95% CI = 0.219–0.599). The result of a meta-analysis conducted by Bonequi et al[7] showed that higher educational levels and GC had a significant inverse correlation and was associated with a 52% decrease in GC risk. These findings were consistent with the results of our study.
Our study observed that GC was associated with cigarette smoking, and the OR for smokers vs. never smokers was 3.069 (95% CI = 1.700–5.540). In 2004, a report jointly published by the Office of the Surgeon General (US) and the Office on Smoking and Health (US), stated that based on research evidence, smoking had a causal relationship with GC.[8] Many meta-analyses have revealed a positive association between cigarette smoking and GC.[9–11] Poorolajal et al[9] conducted a meta-analysis that included 77 studies published between 1985 and 2018; the results indicated that current smokers and former smokers were at higher risk of GC than non-smokers (OR = 1.610, 95% CI = 1.490–1.750 and OR = 1.430, 95% CI = 1.290–1.590, respectively).
Many studies have reported that alcohol consumption is another risk factor for GC.[6,12,13] The result of a meta-analysis performed by Poorolajal et al[9] revealed that GC risk was higher in drinkers than in never-drinkers (OR = 1.190, 95% CI = 1.100–1.290). Concerning the effects of alcohol consumption on GC risk, it can be said that alcohol created a cancer-stimulating mechanism that involved a chronic inflammatory response to the toxic effects of ethanol metabolites and cytokines and, thus, the increased exposure of nitrosamines.[14]
Chen et al[15] conducted a meta-analysis of 24 prospective studies with 41,791 cases published before 2013 to evaluate the association between GC and BMI. Overall, both overweight (BMI = 25–30 kg/m2) and obesity (BMI ≥30 kg/m2) showed a protective but non-significant association with stomach cancer [overweight: summary relative risks (SRR) = 1.01, 95% CI = 0.960–1.070; obesity: SRR = 1.06, 95% CI = 0.990–1.120]. However, we found that overweight and obesity (BMI ≥24 kg/m2) was the protective factor for GC compared with normal/low weight (OR = 0.489, 95% CI = 0.329–0.726). This discrepancy could be due to the small sample size or unavailability of data regarding H. pylori infection in the study participants.
Having a FHGC in first-degree relatives was also found to be a strong potential risk factor for GC in this study (OR = 4.291, 95% CI = 1.661–11.084). Having a first-degree relative with GC was a consistent risk factor for GC, although the magnitude of the OR associated with a positive family history varied depending on the ethnic group and geographic region, ranging from 2 to 10.[16,17]
Based on our study, having a history of stomach disease dramatically increased GC risk in fully adjusted regression models (OR = 6.917, 95% CI = 4.594–10.416). Furthermore, the elevated risk remained when a subgroup analysis was conducted for age and gender, with ORs ranging from 7 to 14 when adjusted for age and gender and from 5 to 11 when adjusted for all variables. For specific stomach diseases, chronic gastritis, gastric ulcer, and gastric polyposis showed increased GC risk in age- and gender-adjusted and fully adjusted regression models. An association between history of stomach disease and GC was reported in two previous studies.[18,19] In a cohort study, Sadjadi et al[18] found that a history of gastric ulcer was a strong risk determinant [hazard ratio (HR) = 9.00, 95% CI = 3.30–24.80] in fully adjusted Cox regression model. People with a history of atrophic gastritis had a higher risk for GC. Moreover, GC risk was positively correlated with atrophic gastritis severity (mild: HR = 2.08, 95% CI = 0.74–5.80; moderate: HR = 3.60, 95% CI = 1.14–11.34; marked: HR = 6.77, 95% CI = 1.62–28.43; Ptrend = 0.036), which is consistent with the results of our study.
Many studies have revealed that diet and dietary habits (eg, inadequate fresh fruit and vegetable intake and excessive salt and pickled food intake) are the risk factors of GC.[6,9] The anti-carcinogenic effects of fruits and vegetables may be attributed to the antioxidant effect of their vitamin content, especially vitamin C and beta-carotene. Antioxidants neutralize reactive oxygen free radicals, which cause DNA damage.[20] Pickled food may increase the risk of GC because they contain large amounts of salt and key nutrients are lost in food under acidic and oxygenic conditions.[21,22] Furthermore, pickled vegetables are considered to be a possible source of nitrosamines, which may contribute to GC. Excessive salt consumption might act as a gastric mucosa stimulant, leading to atrophic gastritis, increased DNA synthesis, and cell proliferation, thereby providing the basis for the development of GC.[13] Our study showed that these dietary habits may increase GC risk, but the association was non-significant (P > 0.05). A possible explanation was the inaccurate calculation of dietary frequency and the existence of confounding factors.
The strengths of this study are that it is a case-control study, based on the large-scale cancer screening cohort of the National Cancer Screening Program in Urban China, as well as the relationship between the history of different types of gastric diseases and the risk of GC has been explored. One of the limitations of this study is that the risk factors for different types of GC, including cardia and non-cardia cancer, may be different. Therefore, focusing on the risk factors for each type of GC is suggested in future studies.
This study found that cigarette smoking, alcohol consumption, FHGC in first-degree relatives, and history of stomach disease, including chronic gastritis, gastric ulcer, and gastric polyposis, increased GC risk. Individuals with these potential risk factors should therefore be considered a high-risk population in urban areas of China.
Acknowledgments
The authors thank all the patients and subjects who participated in the study and also we thank all the collaborators who contributed to its success.
Funding
This study was supported by grants from the National Science & Technology Fundamental Resources Investigation Program of China (No. 2019FY101105), the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (No. 2019PT320027), and the National Key Public Health Program of China (Cancer Screening Program in Urban China).
Conflicts of interest
None.
Footnotes
How to cite this article: Zhang R, Li H, Li N, Shi JF, Li J, Chen HD, Yu YW, Qin C, Ren JS, Chen WQ, He J. Risk factors for gastric cancer: a large-scale, population-based case-control study. Chin Med J 2021;134:1952–1958. doi: 10.1097/CM9.0000000000001652
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016; 66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Zeng H, Chen W, Zheng R, Zhang S, Ji JS, Zou X, et al. Changing cancer survival in China during 2003-15: apooled analysis of 17 population-based cancer registries. Lancet Glob Health 2018; 6:e555–e567. doi: 10.1016/S2214-109X(18)30127-X. [DOI] [PubMed] [Google Scholar]
- 4.Yoon H, Kim N. Diagnosis and management of high risk group for gastric cancer. Gut Liver 2015; 9:5–17. doi: 10.5009/gnl14118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Department of Disease Control, Ministry of Health of the People's Republic of China. Guidelines for the prevention and control of overweight and obesity in Chinese adults (Trial). Beijing: People's Medical Publishing House; 2003. [Google Scholar]
- 6.Yusefi AR, Bagheri Lankarani K, Bastani P, Radinmanesh M, Kavosi Z. Risk factors for gastric cancer: a systematic review. Asian Pac J Cancer Prev 2018; 19:591–603. doi: 10.22034/APJCP.2018.19.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonequi P, Meneses-González F, Correa P, Rabkin CS, Camargo MC. Risk factors for gastric cancer in Latin America: a meta-analysis. Cancer Causes Control 2013; 24:217–231. doi: 10.1007/s10552-012-0110-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. U.S. Department of Health and Human Services. The health consequences of smoking: a report of the surgeon general. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2004. [Google Scholar]
- 9.Poorolajal J, Moradi L, Mohammadi Y, Cheraghi Z, Gohari-Ensaf F. Risk factors for stomach cancer: a systematic review and meta-analysis. Epidemiol Health 2020; 42:e2020004.doi: 10.4178/epih.e2020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ordóñez-Mena JM, Schöttker B, Mons U, Jenab M, Freisling H, Bueno-de-Mesquita B, et al. Quantification of the smoking-associated cancer risk with rate advancement periods: meta-analysis of individual participant data from cohorts of the CHANCES consortium. BMC Med 2016; 14:62.doi: 10.1186/s12916-016-0607-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.La Torre G, Chiaradia G, Gianfagna F, De Lauretis A, Boccia S, Mannocci A, et al. Smoking status and gastric cancer risk: an updated meta-analysis of case-control studies published in the past ten years. Tumori 2009; 95:13–22. doi: 10.1177/030089160909500103. [DOI] [PubMed] [Google Scholar]
- 12.Cai L, Zheng ZL, Zhang ZF. Risk factors for the gastric cardia cancer: a case-control study in Fujian Province. World J Gastroenterol 2003; 9:214–218. doi: 10.3748/wjg.v9.i2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fang X, Wei J, He X, An P, Wang H, Jiang L, et al. Landscape of dietary factors associated with risk of gastric cancer: a systematic review and dose-response meta-analysis of prospective cohort studies. Eur J Cancer 2015; 51:2820–2832. doi: 10.1016/j.ejca.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 14.Bartsch H, Nair J. Accumulation of lipid peroxidation-derived DNA lesions: potential lead markers for chemoprevention of inflammation-driven malignancies. Mutat Res 2005; 591:34–44. doi: 10.1016/j.mrfmmm.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y, Liu L, Wang X, Wang J, Yan Z, Cheng J, et al. Body mass index and risk of gastric cancer: a meta-analysis of a population with more than ten million from 24 prospective studies. Cancer Epidemiol Biomarkers Prev 2013; 22:1395–1408. doi: 10.1158/1055-9965.EPI-13-0042. [DOI] [PubMed] [Google Scholar]
- 16.La Vecchia C, Negri E, Franceschi S, Gentile A. Family history and the risk of stomach and colorectal cancer. Cancer 1992; 70:50–55. doi: 10.1002/1097-0142(19920701)70:1<50::aid-cncr2820700109>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 17.Bernini M, Barbi S, Roviello F, Scarpa A, Moore P, Pedrazzani C, et al. Family history of gastric cancer: a correlation between epidemiologic findings and clinical data. Gastric Cancer 2006; 9:9–13. doi: 10.1007/s10120-005-0350-7. [DOI] [PubMed] [Google Scholar]
- 18.Sadjadi A, Derakhshan MH, Yazdanbod A, Boreiri M, Parsaeian M, Babaei M, et al. Neglected role of hookah and opium in gastric carcinogenesis: a cohort study on risk factors and attributable fractions. Int J Cancer 2014; 134:181–188. doi: 10.1002/ijc.28344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu Y, Fan Y, Jiang Y, Wang Y, Liu H, Wei M. Analysis of risk factors associated with precancerous lesion of gastric cancer in patients from eastern China: a comparative study. J Cancer Res Ther 2013; 9:205–209. doi: 10.4103/0973-1482.113351. [DOI] [PubMed] [Google Scholar]
- 20.Akyön Y. Effect of antioxidants on the immune response of Helicobacter pylori. Clin Microbiol Infect 2002; 8:438–441. doi: 10.1046/j.1469-0691.2002.00426.x. [DOI] [PubMed] [Google Scholar]
- 21.Yalim S, Ozdemir Y. Effects of preparation procedures on ascorbic acid retention in pickled hot peppers. Int J Food Sci Nutr 2003; 54:291–296. doi: 10.1080/09637480120092116. [DOI] [PubMed] [Google Scholar]
- 22.Kim HJ, Lim SY, Lee JS, Park S, Shin A, Choi BY, et al. Fresh and pickled vegetable consumption and gastric cancer in Japanese and Korean populations: a meta-analysis of observational studies. Cancer Sci 2010; 101:508–516. doi: 10.1111/j.1349-7006.2009.01374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]