Abstract
Objective
The pursuit of an effective therapeutic intervention for dementia has inspired interest in the class of medications known as tyrosine kinase inhibitors such as bosutinib.
Methods
Thirty-one patients with probable Alzheimer dementia or Parkinson spectrum disorder with dementia completed 12 months of bosutinib therapy and an additional 12 months of follow-up. The Clinical Dementia Rating scale (as estimated by the Quick Dementia Rating System [QDRS]) was the primary cognitive status outcome measure. Secondary outcome measures included the Repeatable Battery Assessment of Neuropsychological Status (RBANS) and the Montreal Cognitive Assessment. Cox regression methods were used to compare results with population-based estimates of cognitive decline.
Results
The present article reports on cognitive outcomes obtained at 12 months for 31 participants and up to 24 months for a 16-participant subset. Safety and tolerability of bosutinib were confirmed among the study population (Mage = 73.7 years, SDage = 14 years). Bosutinib was associated with less worsening in Clinical Dementia Rating (CDR) scores (hazard ratio = −0.62, p < 0.001, 95% confidence interval [CI]: −1.02 to −0.30) and less decline in RBANS performance (hazard ratio = −3.42, p < 0.001, 95% CI: −3.59 to −3.72) during the year of treatment than population-based estimates of decline. In the 24-month follow-up, wherein 16 patients were observed after 1 year postintervention, 31.2% of participants exhibited worsened CDR levels compared with their 12-month performances.
Conclusions
Results support an overall positive outcome after 1 year of bosutinib. Future studies should explore the relationship between tyrosine kinases and neurodegenerative pathology as well as related avenues of treatment.

To date, no therapy has proven to significantly halt clinical deterioration among patients with neurodegenerative conditions, including Alzheimer and Parkinson spectrum diseases.1 Existing literature demonstrates conversion rates from amnestic mild cognitive impairment (MCI) to dementia ranging from 15% to 29% annually, with expected progression to more advanced stages cited at 22%–36% each year.2–11 As many as 5.6% of patients with MCI have been reported to revert to normal cognition.10 Sustained improvement among patients with dementia is unexpected, although transient periods of lucidity may be observed.11
The pursuit of an effective intervention has inspired interest in tyrosine kinase inhibitors (TKIs). Tyrosine kinases such as Abl and Src have been associated with the neuronal destabilization seen in neurodegenerative conditions.12,13 Several preclinical studies have shown that TKIs can facilitate autophagy, promoting the elimination of abnormal protein deposits.12,14–16 In addition, TKI medications appear to reduce inflammation, possibly promoting facilitatory microglial responses.14,17 These effects may not require a crossing of the blood-brain barrier, as there appears to be reciprocal communication between neuroinflammation and systemic inflammatory factors.17–19 Bosutinib (Bosulif) is a dual Src/Abl kinase inhibitor with a record of safety and efficacy for treatment of chronic myeloid leukemia.20–24 The present article reports on an ongoing, open-label clinical trial using bosutinib for the investigational treatment of degenerative dementias. We hypothesize that 1 year of bosutinib therapy will yield reduced rates of clinical deterioration compared with population-based estimates of decline in neurodegenerative disease.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
This study (Clinical Trial Identifier #NCT02921477) was initially reviewed and approved by Quorum Institutional Review Board. This study has since been transferred to Advarra IRB and is listed under the protocol number Pro00036231. The most recent approval number is CR00173276.
Potential participants were recruited from Los Angeles neurology clinics. If potential participants endorsed interest, study staff provided overview information to those patients and their caregivers. Screening was then completed (as detailed below), and once candidacy was established, study staff briefed participants and their caregivers on the protocol, possible risks and benefits, follow-up procedures, costs, and examinations. Informed consent was reviewed in full with participants and caregivers and allowed time for any and all questions. Caregivers were included in the consenting process to verify commitment and adherence to follow-up procedures. All participants provided written, signed informed consent. Each participant was given a copy of their signed consent.
Participants
Currently, 60 participants between the ages of 45 and 89 years are enrolled in this study. This article reports on (1) the 31 participants who have completed the first year of study involvement and (2) the 16 participants who have completed the second year. Of the 31 participants, 16 were diagnosed with Parkinson disease spectrum disorders with dementia (PDD) (Mage = 71.8 years, SDage = 10.94 years; 4 females and 12 males), whereas the remaining 15 met the criteria for probable Alzheimer dementia (AD) with evidence of pathophysiologic process (Mage = 74.63 years, SDage = 9.59; 8 females and 7 males).8,10 Of the 16 participants who completed the second year, 8 were diagnosed with PDD (Mage = 75.66 years, SDage = 7.25 years; 1 female and 7 males), and 8 were diagnosed with AD (Mage = 79.75, SDage = 6.02 years; 4 females and 4 males).
Neurocognitive and Behavioral Performance Measures
The following measures were used to evaluate participant neurocognitive status throughout study involvement: the Quick Dementia Rating System (QDRS) used to estimate the Clinical Dementia Rating (CDR); Repeatable Battery for the Assessment of Neuropsychological Status (RBANS; versions A–D); Montreal Cognitive Assessment (MoCA; 7.1–7.3); Smell Test (Brief Smell Identification Test [B-SIT]); 9-Hole Pegboard Task; 25-foot Timed Gait Task; and Beck Depression Inventory-II (BDI-II).
The Quick Dementia Rating System (QDRS) form consists of 10 categorical questions (5 cognitive and 5 functional), each with 5 detailed options depicting the level of impairment as 0 (normal), 0.5 (mild/inconsistent impairment), 1 (mild/consistent impairment), 2 (moderate impairment), or 3 (severe impairment). To ensure consistency, the individual identified as the caregiver agreed to complete the QDRS interview at each follow-up milestone. Based on Galvin's25 conversion table, total QDRS scores were converted to CDR scores ranging from 0 (normal aging), 0.5 (MCI), 1 (mild dementia), 2 (moderate dementia), and 3 (severe dementia).
The RBANS versions A, B, C, and D were administered at each follow-up time point to assess immediate memory, visuospatial skill, language, attention, and delayed memory.26 The 4 versions (A–D) were rotated after each testing session to minimize practice effects.
The MoCA was used to evaluate gross cognitive status.27,28 The MoCA tests frontal executive functions, language, orientation to time and place, visuospatial construction, attention, and immediate and delayed memory. MoCA scores range from 0 to 30 possible points; 26 or greater is considered to reflect normal cognitive status.29 Different versions of the MoCA were used to mitigate practice effects.
The Timed 25-Foot Walk Test (T25-FW) and the Rolyan Nine-Hole Pegboard Test (9-HPT) were used to evaluate gross motor functioning and fine motor dexterity, respectively. Both tests were particularly relevant for participants with PD.30 The Brief B-SIT, which has also been shown as a valid and reliable measure, was used to assess olfactory function before study entry.31
Advanced MRI Techniques
Each prospective subject underwent advanced magnetic resonance (MR) neuroimaging as part of the inclusion criteria. The multimodal MRI included volumetric measurement of the hippocampus, arterial spin labeling (ASL) perfusion scans, blood oxygen level–dependent sequences, and diffusion tensor imaging. These modalities have been shown to yield clinical indicators for various neurodegenerative subgroups and show sensitivity to disease progression.32–35
Participant scans were performed at 1 of 3 imaging centers in the Los Angeles/Santa Monica area. Imaging sequences were standardized, and data were harmonized among the locations. All MRIs were quality controlled before being processed and analyzed. Postprocessing was performed using FMRIB Software Library with identical parameters (fmrib.ox.ac.uk.fsl).36
Participants were required to do follow-up imaging at the same center where baseline scans were conducted. For each participant, the clinical information and imaging data were reviewed by a panel consisting of board-certified neurologists, board-certified psychiatrists, a neuropsychologist, and a neuroradiologist; diagnosis and clinical classification were determined by expert consensus.
Procedure
All participants received a neurocognitive examination, lumbar puncture, multimodal MRI, electrocardiogram, BDI, and bloodwork to evaluate their eligibility for this study. To qualify for inclusion, participants were required to show pathophysiologic evidence of AD or meet the criteria for PDD. Estimated CDR scores were required to fall between 0.5 and 2, a range equivalent to MCI and Moderate Dementia, respectively.25–38
The lumbar puncture, which assessed for Aβ-42 and Tau protein levels in CSF, has been shown to demonstrate sensitivity and specificity in identifying AD pathophysiology.37 The advanced MRI obtained before study entry was required to show evidence of a neurodegenerative process for study inclusion: disproportionate atrophy of mesial temporal structures including the hippocampus, decreased signal on ASL in the temporal and parietal regions, and/or reduced signal in the putamen. Other pathologic findings (e.g., stroke, cortical dysplasia, and neoplasm) that could be related to the patient's dementia presentation were exclusionary.
Patients with cognitive decline due to acute illness or vascular pathologies were excluded. Other exclusionary criteria included advanced terminal illness, advanced kidney, pulmonary, cardiac, or liver failure, definite or probable pregnancy, breastfeeding, and Major Depressive Disorder (MDD). The BDI-II, which has been demonstrated as a statistically valid and reliable measure of MDD, was administered as a screening method for depression.39,40 Inability to give informed consent was also a basis for exclusion.
Concurrent interventions and therapies were not considered exclusionary unless the study doctor deemed them a threat to patient safety when used in conjunction with the study protocol. Study participants were allowed to participate in additional therapies at the same site and under the purview of the study doctor. Concurrent interventions were recorded and are included in the Discussion section of this article. Attempts were made to harmonize concurrent treatments, which included a list of nutraceuticals (table 1). Future publications from this study group will focus on distinguishing effect sizes between different treatment modalities.
Table 1.
List of Recommended Supplements
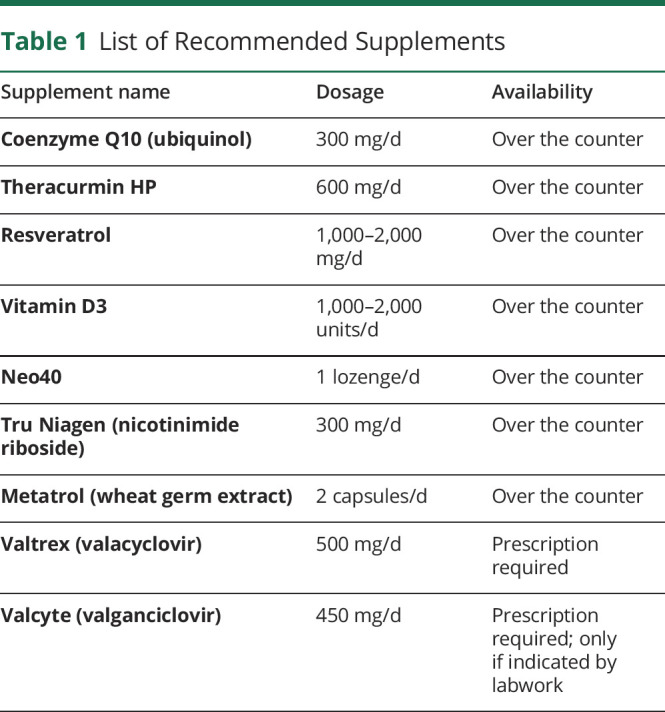
Once enrolled, participants were given the study medication (at no cost) and instructed to take 1 pill each morning with breakfast. All participants started at 100 mg daily for the first month. The dosage titration regimen was set to increase by 1 pill (100 mg) each month until a maximum daily dosage of 300 mg was achieved. Participants whose labs (detailed in the following paragraph) were within study guidelines with minimal to no side effects were cleared to increase to 200 mg daily by the second month. After 2 months, based on the same criteria, participants were permitted to increase to the maximum 300 mg daily dose. This dose was maintained for the duration of the first year unless labs or side effects necessitated a dose reduction. Participants whose transaminase levels or other tolerability issues precluded them from achieving the maximum study dose maintained the highest dose that was safe and tolerable for them. On achieving dose stabilization, participants were also allowed to use nutritional supplements according to a list (table 1). Compliance with nutritional supplementation was discussed at each visit, but there was no attempt to perform pill counts or other means of monitoring.
Participants obtained bloodwork semi-weekly during the first 3 months of medication to verify safety and tolerability. After reaching the maximum dose, participants were cleared to decrease the bloodwork frequency to monthly for the duration of the year. Each lab order included tests for vitamin B12, thyroid hormone (TSH, T4, and T3), bilirubin (direct and total), alkaline phosphatase, alanine transaminase, aspartate aminotransferase, potassium, magnesium, creatinine/creatinine clearance, and hemoglobin, as well as total counts of white blood cells, platelets, and absolute neutrophils. Additional labs were ordered at the discretion of study doctors based on tolerability, reported side effects, and patient history.
In addition, participants met routinely with study staff and doctors to assess tolerability and monitor for any potential side effects. Throughout the 2 years of study involvement, participants met with the doctor every month. Participants living out of state were permitted to meet this criterion through telemedicine visits so long as they tolerated the medication without side effects and had access to a local physician familiar with the study. All study participants were required to report to the primary study site for neuropsychological testing appointments.
Neuropsychological examinations, including the RBANS, MoCA, 9-HPT, and T25-FW tests, were repeated at 6, 12, 18, and 24 months. Functional neuroimaging was repeated twice after baseline scans: once upon completion of study medication and again at the 2-year mark.
Participants were not paid or reimbursed for participation. Consultation and follow-up visits were provided free of charge. Pfizer served as the study sponsor and provided medication at no cost to participants. Most other study-related costs were absorbed by the principle investigator; any costs that fell to the responsibility of the participant were clarified before and as a part of informed consent.
Statistical Analyses
Bootstrapped Cox proportional hazards regression analyses were performed to assess the impact of bosutinib on participants' cognitive performance compared with the expected rate of cognitive decline in neurodegenerative disease. The expected rate of decline was calculated as the average of reported values in existing literature, yielding a 22% conversion rate from MCI to dementia and a 29% progression rate to a more advanced stage of dementia.4–13 The expected rate of improvement for MCI was set at 5.6%, whereas the expected rate of improvement for dementia was set at 0%.9,10 All statistical analyses had an alpha value of 0.05 and were performed with IBM SPSS Statistics for Windows, version 26 (IBM Corp., Armonk, NY).
Data Availability
Data from this study will not be made publicly available due to ethical and privacy concerns. Anonymized data will be available on reasonable request from any qualified investigator.
Results
Tolerability and Feasibility
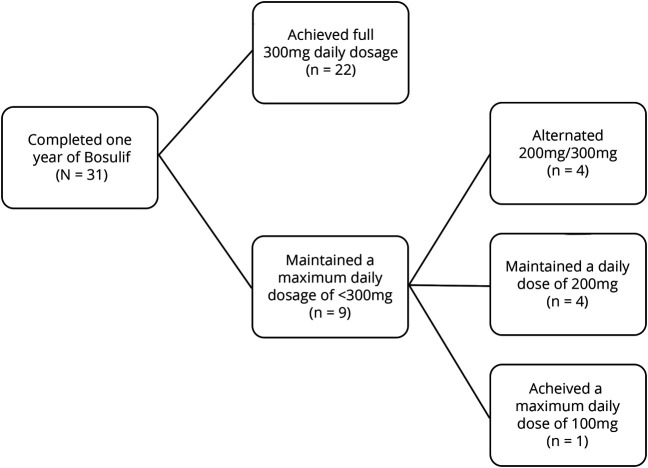
All participants were able to tolerate the drug without notable side effects. Four participants left the study for personal reasons unrelated to tolerability or medical justification. See the figure for a delineation of maximum dosage achieved among the 31 participants who have completed the year of bosutinib treatment. Body mass index (BMI) did not appear to be a primary limiting factor for maximum dose, as only 2 of the 9 participants who took 300 mg or less had a BMI less than the normal cutoff of 18.5 kg/m2. Per the study protocol, participants whose labwork indicated elevated transaminase levels required a reduction in dose.
Figure. Maximum Bosulif Dosage Achieved Among Study Participants.
A breakdown of maximum Bosulif dosages among all participants (total N = 31).
The only reported side effects were upset stomach, fatigue, and diarrhea, which occurred among less than 10% of participants. Overall, study participants tolerated the medication well. Bosutinib, therefore, has been confirmed as a safe agent among this population of older adults.
Cognitive Faculties at 12 Months of Bosutinib
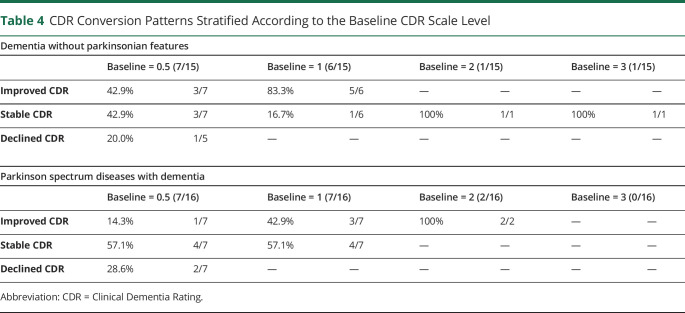
Estimated CDR scores were the primary cognitive outcome measure used in this study. Bosutinib was associated with less decline in the CDR scale level (hazard ratio = −3.5, p < 0.001, 95% confidence interval [CI]: −3.64 to −3.3) than the population-based estimate of expected cognitive deterioration. Bosutinib was also associated with an increased likelihood of maintaining a stable CDR scale level throughout the year of active treatment (hazard ratio = −0.62, p < 0.001, 95% CI: −1.02 to −0.30). Of the 31 participants who completed the year of bosutinib, 45.16% had an improved CDR score by at least 1 level from baseline at 12 months. An additional 45.16% had a stable CDR score at 12 months from baseline. Only 3 of 31 participants (9.68%) had a declined CDR scale level from baseline at 12 months (see tables 2 and 3 for a breakdown of results stratified by neurodegenerative condition type). There were no notable differences between groups stratified according to baseline CDR scores (table 4).
Table 2.
Twelve-Month CDR Score Conversions Per Neurodegenerative Type

Table 3.
CDR Scores From Baseline to 12 Months Per Condition
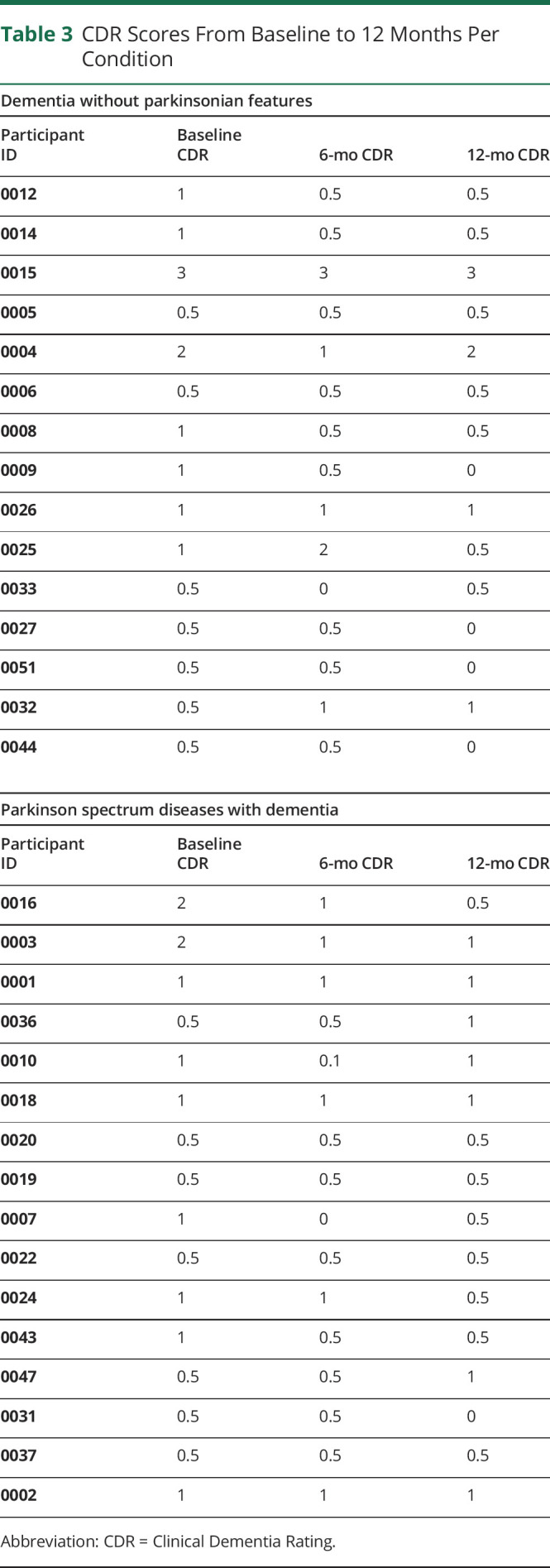
Table 4.
CDR Conversion Patterns Stratified According to the Baseline CDR Scale Level
MoCA scores were used to assess for changes in gross cognitive status (MCID ≥ 2 points).41 Of note, 22.6% of participants demonstrated clinically meaningful improvement on MoCA scores at 12 months. An additional 25.8% yielded stable MoCA scores from baseline at 12 months. Of note, 51.6% had reduced performance on the MoCA at 12 months. Bosutinib was associated with less decline in RBANS performance than the population-based estimate of decline (hazard ratio = −3.42, p < 0.001, 95% CI: −3.59 to −3.72). Assessments based on RBANS Total Index scores revealed clinically meaningful improvement in 22.6% of participants, clinically stable scores in 58.1%, and clinically meaningful decline in 19.4% (MCID ≥ 8 points).42
Absolute Risk Reduction and Numbers Needed to Treat at 12-Month Study Mark
Using population-based estimates of decline among patients with neurodegenerative disease, absolute risk reduction and numbers needed to treat were calculated for (1) conversion of MCI to dementia and (2) progression to a more advanced stage of dementia.4–7,10,11 Absolute risk reduction was calculated to be 34.5%–167% for participants enrolled with a baseline CDR equivalent to MCI; for participants enrolled with a baseline CDR equivalent to mild or moderate dementia, absolute risk reduction was calculated to be 27.8%–245%. Numbers needed to treat were calculated to be 5.27–20 for participants enrolled with MCI. Numbers needed to treat for participants enrolled with mild to moderate dementia were calculated to be 3.85–8.33.
Based on reports of spontaneous recovery among 5.6% of patients with MCI who do not receive bosutinib therapy compared with participants in this study with MCI who did receive bosutinib therapy, absolute risk reduction was calculated to be 35.4% with a corresponding number needed to treat of 2.83.10
Cognitive Faculties at 6 Months Post-Bosutinib (18-Month Mark)
At the time of this publication, 26 participants have exceeded the 18-month mark, and 16 have surpassed the 24 months of study involvement. Of the 26 participants measured at 18 months, 30.8% yielded improved CDR scores compared with their performance at the 12-month mark. Of note, 38.4% exhibited stable CDR scores, and 30.8% showed worsened CDR scores. When comparing the baseline CDR score with the CDR score at the 18 month-mark, there was no longer a difference (statistical trend that did not withstand correction for multiple comparisons) in likelihood of dementia worsening between study participants and the population-based estimate of decline without intervention (hazard ratio = −0.53, p = 0.1, 95% CI: −1.15 to 0.31). However, participants were more likely to be at an improved CDR at the 24-month mark from their baseline CDR than was expected based on the population-based estimate of decline (hazard ratio = −2.05, p < 0.001, 95% CI: −3.62 to −1.04).
Regarding MoCA scores, at 18 months, 20% participants showed clinical improvement compared with scores at 12 months, 36% demonstrated clinically stable scores from 12 months, and the remaining 44% showed clinical decline from 12 months. RBANS Total Index Score results at the 18-month mark revealed that 32% of participants had clinically meaningful improvement from 12 months, 36% who yielded clinically stable scores from 12 months, and the remaining 32% exhibited clinically meaningful reduction in scores from 12 months. When comparing the RBANS score at 18 months with baseline scores, both participants and population-based estimates were equally likely to show decline (hazard ratio = −0.38, p = 0.28, 95% CI: −1.02 to 0.5); however, study participants were more likely to continue to show cognitive benefits than population-based estimates (hazard ratio = −1.82, p < 0.001, 95% CI: −3.34 to −0.66).
Cognitive Faculties at 1 Year Post-Bosutinib (24-Month Mark)
At the time of this publication, 16 participants have completed the 24 months of study involvement. CDR scores among this patient subset show 18.8% with clinically meaningful improvement from 12 months, 50% with clinical stability, and 31.2% with clinically meaningful decline. When comparing the baseline CDR score with the CDR score at 24 months, there was no longer a difference in likelihood of dementia worsening between participants and the population-based estimate of decline (hazard ratio = 0.1, p = 0.88, 95% CI: −3.13 to 0.97). However, study participants were more likely to earn an improved CDR score at the 24-month mark compared with baseline than population-based estimates of expected decline (hazard ratio = −0.69, p < 0.002, 95% CI: −3.1 to −0.14).
Twenty-four-month MoCA scores showed clinically meaningful improvement among 50% of participants from the 12-month mark, clinical stability among 43.8%, and a clinically meaningful decline in 1 subject (6.2%). RBANS Total Index scores at 24 months revealed 31.2% of participants with clinically meaningful improvement, 37.5% with clinically stable scores, and 31.2% with clinically meaningful worsening from 12 months. When comparing baseline RBANS scores with RBANS scores at the 24-month mark, study participants were equally likely to show decline as population-based estimates (hazard ratio = −0.24, p = 0.6, 95% CI: −1.02 to 1.58); however, participants were more likely to continue to show cognitive benefits (hazard ratio = −1.90, p < 0.001, 95% CI: −3.53 to −0.61).
Discussion
These results bolster findings from previously reported studies and justify further investigation of bosutinib as a therapeutic agent for dementia.13,28 The data suggest an overall positive outcome among participants after a year of bosutinib, with a worsening in condition following treatment discontinuation. By 6 months post-bosutinib, patient deterioration more than tripled and continued to decline by the 24-month mark (gauged by the CDR scale). Moreover, this observation was consistent among all participants, regardless of how severe the dementia was at baseline (table 4). Even so, study participants demonstrated increased likelihood of maintaining stable or improved cognitive performance (gauged by the CDR and total RBANS scores) than would be expected based on population-based estimates of decline in neurodegenerative disease.
Future studies should include a larger study population and extended follow-up to assess durability beyond 2 years, as this study demonstrates a well-documented positive response among participants thus far. The open-label nature of this study did not preclude participants from pursuing other treatments, including nutraceuticals, dietary adjustments, focused ultrasound therapy, and/or regenerative approaches (e.g., exosomes).43,44 Twenty-seven participants also underwent focused transcranial ultrasound as part of a separate study.45 Preliminary comparative analyses of groups suggest that transcranial ultrasound did not yield the same degree of therapeutic benefit unless it was paired with bosutinib treatment.43 Co-occurring interventions make it challenging to interpret direct relationships between the study medication and outcome results. However, given the likelihood of multisystem involvement in degenerative dementia, studies designed with a multimodal approach may yield improved clinical efficacy. The multifaceted treatment approach enacted in this study clinic was built around the intention to address the multisystem failures associated with Alzheimer and Parkinson spectrum diseases.45–47 Results from combined interventions will be addressed in future publications. An extension of this open-label study will delay any co-occurring interventions for 6 months to better clarify the independent contribution of bosutinib to the overall effect. Additional review of outcome measures obtained for this study, including neuroimaging and motor functioning, will also be included in future publications.
Future research should explore the usefulness of TKIs such as bosutinib in treating other degenerative conditions, such as dementia with Lewy bodies (Clinical Trials Identifier: NCT03888222). Other studies may be organized to evaluate the density of protein aggregates with Tau PET scans before and after bosutinib treatment. Inflammatory serologic markers evaluated before and after bosutinib treatment may help clarify the mechanism(s) of action. It will also be important to further elucidate the optimal dosage and duration of a treatment plan involving bosutinib for neurodegenerative conditions. TKI agents with better blood-brain barrier penetration may also be considered. Depending on licensing requirements for new indications of bosutinib, phase II and III study designs may be expedited to improve the accessibility of this medicine for all those seeking a therapeutic option for dementia.
Acknowledgment
The authors thank Daniel Franc, MD (Los Angeles Brain Science Project) for his contributions to the initial study design and Pfizer for providing the study medication at no cost to participants.
Appendix. Authors
Study Funding
No targeted funding reported.
Disclosure
The authors report no disclosures relevant to the manuscript. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
TAKE-HOME POINTS
→ TKI medications, such as bosutinib (Bosulif), have been posited as a potentially therapeutic intervention for neurodegenerative dementias.
→ Thirty-one participants with neurodegenerative conditions completed 1 year of bosutinib therapy and underwent cognitive status evaluations every 6 months for 2 years from baseline.
→ Safety and tolerability of bosutinib were confirmed among this population of older adults.
→ Results from neurocognitive evaluations support findings from other ongoing studies and warrant further investigation of bosutinib as a therapeutic agent for dementia.
→ Future studies should involve a larger sample size, obtain extended follow-up beyond 2 years, and clarify possible mechanisms of action.
References
- 1.Prince M, Wimo A, Guerchet MM, Ali GC, Wu Y-T, Prina M. World Alzheimer Report 2015: The Global Impact of Dementia: An Analysis of Prevalence, Incidence, Costs and Trends. London, England: Alzheimer's Disease International; 2015. [Google Scholar]
- 2.Owen AM. Cognitive dysfunction in Parkinson's disease: the role of frontostriatal circuitry. Neuroscientist 2004;10:525–537. [DOI] [PubMed] [Google Scholar]
- 3.Dewey RB, Taneja A, McClintock SM, et al. Motor symptoms at onset of Parkinson disease and risk for cognitive impairment and depression. Cogn Behav Neurol 2012;25:115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maioli F, Coveri M, Pagni P, et al. Conversion of mild cognitive impairment to dementia in elderly subjects: a preliminary study in a memory and cognitive disorder unit. Arch Gerontol Geriatr 2007;44:233–241. [DOI] [PubMed] [Google Scholar]
- 5.Amieva H, Letenneur L, Dartigues J-F, et al. Annual rate and predictors of conversion to dementia in subjects presenting mild cognitive impairment criteria defined according to a population-based study. Demen Geriatr Cogn Disord 2009;18:87–93. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell AJ, Shiri-Feshki M. Rate of progression of mild cognitive impairment to dementia—meta-analysis of 41 robust inception cohort studies. Acta Psychiatr Scand 2009;119:252–265. [DOI] [PubMed] [Google Scholar]
- 7.Schmidtke K, Hermeneit S. High rate of conversion to Alzheimer's disease in a cohort of amnestic MCI patients. Int Psychogeriatr 2008;20:96–108. [DOI] [PubMed] [Google Scholar]
- 8.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grande G, Cucumo V, Cova I, et al. Reversible mild cognitive impairment: the role of comorbidities at baseline evaluation. J Alzheimers Dis 2016;51:57–67. [DOI] [PubMed] [Google Scholar]
- 11.Mashour GA, Frank L, Batthynany A, et al. Paradoxical lucidity: a potential paradigm shift for the neurobiology and treatment of severe dementias. Alzheimers Dement 2019;15:1107–1114. [DOI] [PubMed] [Google Scholar]
- 12.Nygaard HB, Wagner AF, Bowen GS, et al. A phase 1b multiple ascending dose study of the safety, tolerability, and central nervous system availability of AZD0530 (saracatinib) in Alzheimer's disease. Alzheimers Res Ther 2015;7:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamamoto A, Suzuki T, Sakaki Y. Isolation of hNap1BP which interacts with human Nap1 (NCKAP1) whose expression is down-regulated in Alzheimer's disease. Gene 2001;271:159–169. [DOI] [PubMed] [Google Scholar]
- 14.Lonskaya I, Hebron ML, Selby ST, Turner RS, Moussa CE. Nilotinib and bosutinib modulate pre-plaque alterations of blood immune markers and neuro-inflammation in Alzheimer's disease models. Neuroscience 2015;24:316–327. [DOI] [PubMed] [Google Scholar]
- 15.Hebron ML, Lonskaya I, Moussa CE. Nilotinib reverses loss of dopamine neurons and improves motor behavior via autophagic degradation of alpha-synuclein in Parkinson's disease models. Hum Mol Genet 2013;22:3315–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lonskaya I, Hebron M, Chen W, Schacter J, Moussa C. Tau deletion impairs intracellular beta-amyloid-42 clearance and leads to more extracellular plaque deposition in gene transfer models. Mol Neurodegener 2014;9:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Z, Trapp BD. Microglia and neuroprotection. J Neurochem 2016;136:10–17. [DOI] [PubMed] [Google Scholar]
- 18.Sominsky L, De Luca S, Spencer SJ. Microglia: key players in neurodevelopment and neuronal plasticity. Int J Biochem Cell Biol 2018;94:56–60. [DOI] [PubMed] [Google Scholar]
- 19.Skaper SD. Chapter 2—impact of inflammation on the blood-neural barrier and blood-nerve interface: from review to therapeutic preview. Int Rev Neurobiol 2017;137:29–45. [DOI] [PubMed] [Google Scholar]
- 20.Levinson NM, Boxer SG. Structural and spectroscopic analysis of the kinase inhibitor bosutinib and an isomer of bosutinib binding to the Abl tyronsine kinase domain. PLoS One 2012;7:e29828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cortes JE, Kantarjian HM, Brümmendorf TH, et al. Safety and efficacy of bosutinib (SKI-606) in chronic phase Philadelphia chromosome-positive chronic myeloid leukemia patients with resistance or intolerance to imatinib. Blood 2011;118:4567–4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cortes JE, Gambacorti-Passerini C, Deininger MW, et al. Bosutinib versus imatinib for newly diagnosed chronic myeloid leukemia: results from the randomized BFORE trial. J Clin Oncol 2018;36:231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carneiro BA, Kaplan JB, Giles FJ. Tyrosine kinase inhibitor therapy in chronic myeloid leukemia: update on key adverse events. Expert Rev Hematol 2015;8:457–479. [DOI] [PubMed] [Google Scholar]
- 24.Pagan F, Valadez E, Torres-Yaghi Y, et al. Nilotinib improves motor skills, cognition and autonomic function in open label phase 1 clinical trial in Parkinson's disease with dementia and dementia with Lewy body. Neurology 2016;86. [Google Scholar]
- 25.Galvin JE. The quick dementia rating system (QDRS): a rapid dementia staging tool. Alzheimers Dement (Amst) 2015;1:249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Randolph CI, Tierney MC, Mohr E, Chase TN. The repeatable battery for the assessment of neuropsychological status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol 1998;20:310–319. [DOI] [PubMed] [Google Scholar]
- 27.Breton A, Casey D, Arnaoutoglou NA. Cognitive tests for the detection of mild cognitive impairment (MCI), the prodromal stage of dementia: meta-analysis of diagnostic accuracy studies. Geriatr Psychiatry 2018;34:233–242. [DOI] [PubMed] [Google Scholar]
- 28.Smith T, Gildeh N, Holmes C. The Montreal Cognitive Assessment: validity and utility in a memory clinic setting. Can J Psychiatry 2007;52:329–332. [DOI] [PubMed] [Google Scholar]
- 29.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–699. [DOI] [PubMed] [Google Scholar]
- 30.Motl RW, Cohen JA, Benedict R, et al. ; Multiple Sclerosis Outcome Assessments Consortium. Validity of the timed 25-foot walk as an ambulatory performance outcome measure for multiple sclerosis. Mult Scler 2017;23:704–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El Rassi E, Mace JC, Steele TO, et al. Sensitivity analysis and diagnostic accuracy of the Brief Smell Identification Test in patients with chronic rhinosinusitis. Int Forum Allergy Rhinol 2016;6:287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burnham S, Bourgeat P, Doré V, et al. The cognitive and brain volumetric trajectories of healthy elderly controls with either Alzheimer's pathology, neurodegeneration (SNAP), or both. Alzheimers Dement 2015;11:123. [Google Scholar]
- 33.Zheng W, Cui B, Han Y, et al. Disrupted regional cerebral blood flow functional activity and connectivity in Alzheimer's disease: a combined ASL perfusion and resting state fMRI study. Front Neurosci 2019;13:738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mishra VR, Sreenivasan KR, Zhuang X, Yang Z, Cordes D, Walsh RR. Influence of analytic techniques on comparing DTI-derived measurements in early stage Parkinson's disease. Heliyon 2019;5:e01481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gorges M, Müller H, Liepelt-Scarfone I, et al. ; LANDSCAPE Consortium. Structural brain signature of cognitive decline in Parkinson's disease: DTI-based evidence from the Landscape study. Ther Adv Neurol Disord 2019;12:1756286419843447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuhn TP, Becerra SA, Duncan JD, et al. Translating state-of-the-art brain MRI techniques into clinical practice: clinical utility validation of multimodal neuroimaging pipeline for differentiating dementia subtypes. (under review).
- 37.Adamczuk K, Schaeverbeke J, Vanderstichele HM, et al. Diagnostic value of cerebrospinal fluid Aβ ratios in preclinical Alzheimer's disease. Alzheimers Res Ther 2015;7:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berman SE, Koscik RL, Clark LR, et al. Use of the quick dementia rating system (QDRS) as an initial screening measure in a longitudinal cohort at risk for Alzheimer's disease. J Alzheimers Dis Rep 2017;1:9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Visser M, Leentjens AF, Marinus J, Stiggelbout AM, van Hilten JJ. Reliability and validity of the Beck depression inventory in patients with Parkinson's disease. Mov Disord 2006;21:668–672. [DOI] [PubMed] [Google Scholar]
- 40.Erford BT, Johnson E, Bardoshi G. Meta-analysis of the English version of the Beck depression inventory—second edition. Meas Eval Couns Develop 2017;49:3–33. [Google Scholar]
- 41.Wong GK, Mak JS, Wong A, et al. Minimum clinically important difference of Montreal Cognitive Assessment in aneurysmal subarachnoid hemorrhage patients. J Clin Neurosci 2017;46:41–44. [DOI] [PubMed] [Google Scholar]
- 42.O'Connell ME, Gould B, Ursenbach J, Enright J, Morgan DG. Reliable change and minimum clinically important difference (MCID) of the repeatable battery for the assessment of neuropsychology status (RBANS) in a heterogenous dementia sample: support for reliable change methods but not the MCID. Appl Neuropsychol Adult 2019;26:268–274. [DOI] [PubMed] [Google Scholar]
- 43.Nicodemus NE, Becerra SA, Kuhn TP, et al. Focused transcranial ultrasound for treatment of neurodegenerative dementia. J Neurodegener Disord 2019;3:51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yeo RWY, Lai RC, Tan KH, Lim SK. Exosome: a novel and safer therapeutic refinement of mesenchymal stem cell. Exosomes Microvesicles 2014;1. [Google Scholar]
- 45.Chakroborty S, Stutzmann GE. Calcium channelopathies and Alzheimer's disease: insight into therapeutic success and failures. Eur J Pharmacol 2014;739:83–95. [DOI] [PubMed] [Google Scholar]
- 46.Wolters EC, Braak H. Parkinson's disease: premotor clinico-pathological correlations. In: Riederer P, Reichmann H, Youdim MBH, Gerlach M, editors. Parkinson's Disease and Related Disorders. Journal of Neural Transmission. Supplement a, vol 70. Vienna: Springer; 2006. [DOI] [PubMed] [Google Scholar]
- 47.Engelender S, Isacson O. The threshold theory for Parkinson's disease. Trends Neurosci 2017;40:4–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data from this study will not be made publicly available due to ethical and privacy concerns. Anonymized data will be available on reasonable request from any qualified investigator.