Abstract
Objective
Morvan syndrome is characterized by central, autonomic, and peripheral hyperexcitability due to contactin-associated protein 2 (CASPR2) antibody. Our objective was to study the clinical spectrum, electrophysiologic, autonomic, polysomnographic, and neuropsychological profile in patients with CASPR2–related Morvan syndrome.
Methods
Serum and CSF samples that were CASPR2 antibody positive from 2016 to 2019 were assessed. Among them, patients with Morvan syndrome diagnosed based on clinical and electrophysiologic basis were included.
Results
Fourteen (M:F = 10:4) patients with Morvan syndrome were included with age at onset of 37.1 ± 17.5 years. The clinical features were muscle twitching (12), insomnia (12), pain (11), paresthesias (9), hyperhidrosis (7), hypersalivation (6), double incontinence (3), spastic speech (2), dysphagia (2), behavioral disturbances (2), seizures (1), and cold intolerance (1). Neurologic examination revealed myokymia (12), hyperactive tendon reflexes (10), and tremor (6). EMG revealed neuromyotonia (12) and increased spontaneous activity (7). Autonomic function tests conducted in 8 patients revealed definite autonomic dysfunction (4), orthostatic hypotension (2), early dysfunction (1), and postural orthostatic tachycardia syndrome (1). Polysomnography findings in 6 patients revealed insomnia (3), absence of deep sleep (1), high-frequency beta activity (1), REM behavior disorder (1), and periodic leg movements (1). Neuropsychological evaluation showed subtle involvement of the left frontal and temporal lobe. Malignancy workup was negative. All patients were treated with steroids. There was complete neurologic resolution in follow-up with persistent neuropathic pain in 5 patients.
Conclusions
This study has contributed to the growing knowledge on CASPR2-related Morvan syndrome. It is important for an increased awareness and early recognition as it is potentially treatable by immunotherapy.

Augustine Marie Morvan described a rare disorder termed la choree fibrillaire (Morvan syndrome) associated with autonomic dysfunction and severe insomnia in 1890.1 It is characterized by central, autonomic, and peripheral hyperexcitability due to contactin-associated protein 2 (CASPR2) antibodies.2,3 CASPR2 is an axonal transmembrane protein of the neurexin superfamily expressed in the central and peripheral nervous system that binds to contactin-2. Its cytoplasmic domain is involved in the clustering of Kv1 potassium channels in the juxtaparanodal region, resulting in promotion of axon myelination.4 Mutations and polymorphisms of the CNTNAP2 gene that encodes CASPR2 are found to result in schizophrenia, epilepsy, and peripheral nerve hyperexcitability.5 The clinical spectrum of CASPR2 antibody–associated neurologic disorder is diverse, found in association with Morvan syndrome, epilepsy, pain, anterior horn cell disorder, and limbic encephalitis.6 Morvan syndrome is diagnosed when the following triad of clinical features is observed: (1) CNS: confusion, cognitive dysfunction, hallucinations, insomnia, and myoclonus; (2) autonomic symptoms: hyperhidrosis and fluctuations in blood pressure; and (3) peripheral nerve hyperexcitability: clinical or electrophysiologic evidence of spontaneous muscle overactivity in the form of painful cramps, fasciculations, myokymia, and neuromyotonia.2,3
In the past 2 decades, various solitary case reports and short case series of Morvan syndrome have been described. Initial reports of Morvan syndrome were found in association with voltage-gated potassium channel (VGKC) antibodies mainly CASPR2 and leucine-rich glioma-inactivated 1 (LGI-1) antibodies. But eventually Morvan syndrome is more commonly associated with only CASPR2 antibody involvement.7,8 Few cases of Morvan syndrome have occurred following exposure to indigenous drug use9 and heavy metal exposure.1 Malignancy of the thymus, lung, and prostate is reported in nearly 40% of Morvan syndrome.1–3 The present study has been conducted to further characterize Morvan syndrome with an emphasis on its clinical spectrum, imaging, autonomic, electrophysiologic profile, polysomnography (PSG), and neuropsychological findings.
Methods
The retrospective chart review was conducted at a tertiary neuropsychiatric hospital between 2016 and 2019. Patients with positive serum CASPR2 antibodies presenting with various neurologic manifestations were assessed. They were found to have Morvan syndrome (24), autoimmune encephalitis (4), anterior horn cell disorder (3), and other disorders (5). The patients with CASPR2-positive Morvan syndrome form the study population. Patients' data regarding the demographic profile (table 1), clinical features, and investigations (table 2) were retrieved from the case records. Written informed consent was obtained from all the patients where personal details were available (photographs, videos, and PSG).
Table 1.
Clinical Profile of CASPR2-Related Morvan Syndrome
Table 2.
Investigation Findings of Patients With CASPR2-Related Morvan Syndrome
Standard Protocol Approvals, Registrations, and Patient Consents
The study is a retrospective chart review. Hence, institutional ethical permission was not taken. Written informed consent was obtained from all patients (or guardians of patients) participating in the study (consent for research) before inclusion into the study.
Clinical Trial Registry
The study is a chart review of the clinical and investigative profile of patients with Morvan syndrome. There is no usage of any new medications. Hence, it does not warrant permission from the clinical trial registry.
Authorization for disclosure (consent to disclose) of any recognizable persons in photographs, videos, or other information that may be published in the Journal has been provided.
Immunologic Tests
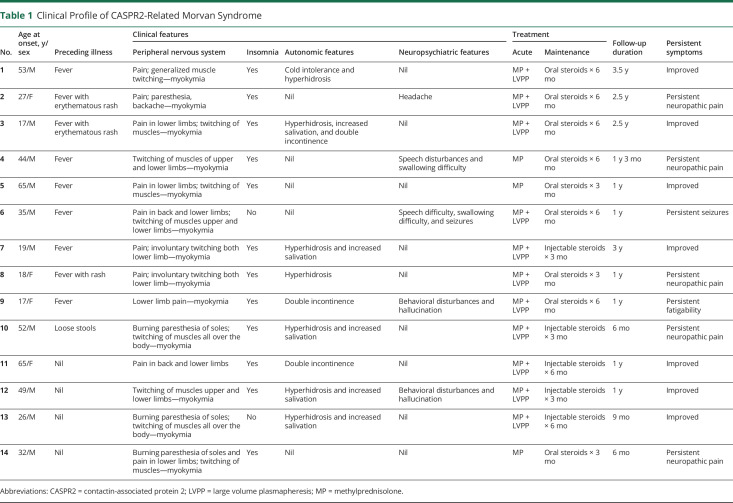
Patients' serum and CSF samples were tested for panel of neuropil antibodies associated with autoimmune encephalitis using the commercially available indirect immunofluorescence test—Euroimmun cell-based immunoassay. The patient's serum (1:10 dilution) or undiluted CSF sample is incubated with the substrates coated in the microtiter plates. The antigen-antibody complexes are detected with fluorescein-labeled anti-human antibodies and visualized with a fluorescence microscope (figure 1).
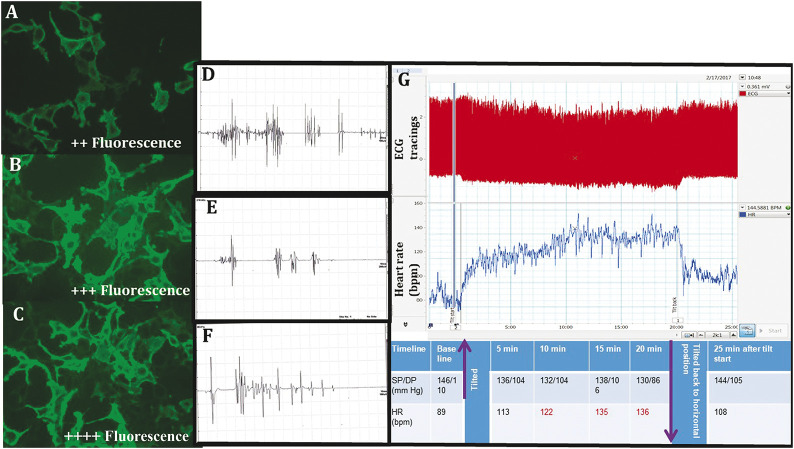
Figure 1. The CASPR2 Immunofluorescence, EMG Bursts, and Autonomic Function Observations.
(A–C) Strong cytoplasmic fluorescence for CASPR2 antibodies using cell-based immunofluorescence assay. Magnification ×40. (D–F) EMG Bursts. (D) EMG bursts consist of regular or irregular doublets, triplets, or multiplets (20–150 Hz). (E) Between bursts, there is electrical silence suggestive of myokymia. (F) Neuromyotonic discharges firing at higher frequencies (150–300 Hz) with abrupt ending and waxing amplitudes. (G) Representative image of the postural tachycardia syndrome (POTS) in one of our patients with Morvan syndrome. These are the raw recordings of patient's ECG tracings during the 60° head-up tilt procedure. The upper panel in red shows ECG tracings (these tracings are compressed over 25 minutes of tilt table test), and the lower panel is derived heart rate (HR; in beats per minute). The table depicts the HR and blood pressure in different time points during the tilt procedure, and purple arrows indicate starting and end of the tilt table procedure. HR values in red depicts (HR >120 bpm during tilting at 10–20 minutes after tilting without fall in blood pressure indicating POTS). CASPR2 = contactin-associated protein 2; DP = diastolic pressure; SP = systolic pressure.
Neuropsychological evaluation was performed in 6 patients. The various modalities tested were cognitive screening, processing and speed, focused attention, category fluency, verbal and visual working memory, visuospatial construction, visual and verbal learning, and memory.
Video PSG was acquired using machine and software from SOMNOmedics GmbH, Germany; electrodes were monopolar-based gold disc electrodes (Natus Neurology). Patients were connected with electroencephalography electrodes over scalp-Fp1, Fp2, F3, F4, C3, C4, T3, T4, O1, O2, and ground electrode at Fz and reference electrodes at FpZ. M1 and M2 electrodes were placed as a reference during offline analysis. Other electrodes were placed to record: electrooculogram, EMG (2 electrodes were placed over the chin), electrocardiogram, respiratory effort, and snoring. An airflow pulse oximeter measured the oxygen saturation. The sleep stages were scored over 30-second epochs and reported according to the guidelines laid down by the American Academy of Sleep Medicine 2014.10
Autonomic function tests were performed in 8 patients comprising conventional autonomic function tests (heart rate response to deep breathing, Valsalva maneuver, and orthostatic test) and time and frequency domain measures of heart rate variability (HRV). The pretest instructions with the details of cardiac autonomic function tests are described in earlier reports.11
Data Availability
The individual deidentified participant data will be shared if required on an SPSS sheet.
Results
Twenty-six patients were found to have neurologic disorders with positive serum CASPR2 antibodies with varying neurologic disorders (enumerated previously). The study population consisted of 14 (53.8%) patients with Morvan syndrome evaluated over a period of 3 years. None of the patients had exposure to any toxins or intake of indigenous medications. Male preponderance was seen (M:F = 10:4); the mean age at onset of illness was 37.1 ± 17.5 years with a median of 33.5 years. All the patients presented with 2- to 6-week history of the following symptoms (table 1): (1) peripheral nerve hyperexcitability (videos 1 and 2): muscle twitching (12), pain (11), and paresthesias (9); (2) CNS: insomnia (12), spastic speech (2), dysphagia (2), behavioral disturbances (2), and seizures (1); and (3) autonomic nervous system: hyperhidrosis (7), hypersalivation (6), double incontinence (3), and cold intolerance (1). Preceding infectious illness was reported in 10 patients. Neurologic examination revealed myokymia (12), hyperactive tendon reflexes (10), and postural tremor (6). Bifacial weakness, ataxic gait, pseudobulbar affect, polyminimyoclonus, and spastic speech were observed in 2 patients.
Shows fasciculations noted in Patient No. 1 in bilateral thighs, calf muscles, shoulder and back region.Download Supplementary Video 1 (11.4MB, mp4) via http://dx.doi.org/10.1212/000978_Video_1
Shows fasciculations in the nasalis muscle in Patient No. 7.Download Supplementary Video 1 (4.8MB, mp4) via http://dx.doi.org/10.1212/000978_Video_2
Sensory manifestations in the form of paresthesias, pain, and numbness were noted in nearly 70% of patients. They did not have associated sensory abnormalities on both clinical and electrophysiologic assessment. Eight patients had severe insomnia; PSG was attempted in 6 patients, but 3 patients were unable to fall asleep in the laboratory and were uncooperative for PSG (table 2).
Hematologic and biochemical parameters were within normal limits. CSF analysis showed normal cell count, with elevated CSF protein in 4 patients. Autoimmune antinuclear profile revealed AMA-M2 positivity in 1 patient. Autoimmune profile revealed CASPR2 positivity in all the patients. In addition, LGI1 was positive in 1 patient, and anti-PNMA2 (Ma2/Ta) and Zic-4 were borderline positive in 1 patient. Neuropsychological abnormalities have been described in table 2, and none of them had dementia or cognitive impairment.
MRI of the brain was normal in all the patients with finding of incidental venous angioma in 1 patient. Malignancy workup was performed in all the cases with PET MRI (10) and/or CT-thorax and abdomen/ultrasound abdomen and pelvis (9); there was no evidence of malignancy. However, hypometabolism of basal ganglia and increased uptake in the muscles by fluorodeoxyglucose were seen in 1 patient. Nerve conduction studies were normal in all patients. EMG revealed neuromyotonia in 12 patients and increased spontaneous activity in 7 patients. The EMG bursts consisted of regular or irregular doublets, triplets, or multiplets (20–150 Hz) with electrical silence in between suggestive of neuromyotonia (table 2; figure 1).
Autonomic function tests were conducted in 8 patients. Among them, 4 patients had definite autonomic dysfunction, 1 had early dysfunction (based on involvement of heart rate–based tests in autonomic function tests or 2 tests showing borderline results), and 3 had normal autonomic functions with conventional autonomic function test. In terms of HRV, SD of normal-to-normal intervals, root mean square of SD, and total low frequency and high frequency powers were reduced in patients indicating decreased parasympathetic activity and increased sympathovagal balance. In the orthostatic or tilt table testing, 2 had orthostatic hypotension (fall of >20/10 mm Hg systolic/diastolic pressure), 1 had postural tachycardia syndrome (rise of heart rate >30 bpm or >120 bpm on standing without much change in blood pressure), and 4 had resting tachycardia (HR >100 per minute) (tables 2 and 3; figure 1).
Table 3.
Autonomic Variables in Patients With Morvan Syndrome Compared With Laboratory Normative Values
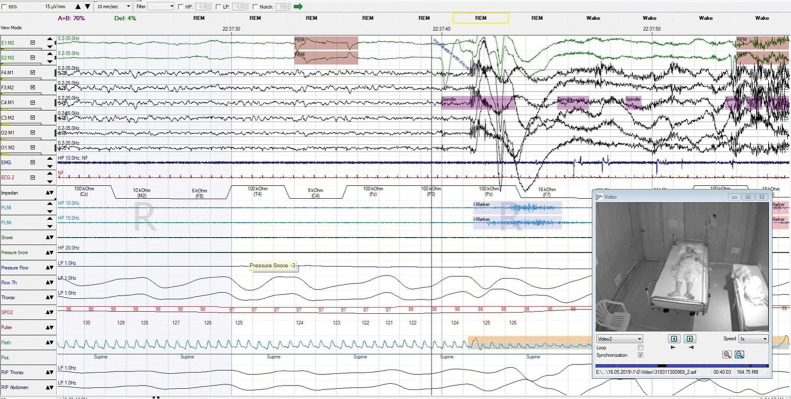
PSG was performed in 6 patients; 3 patients were not cooperative in view of behavioral disturbances and insomnia. Patients being uncooperative for PSG evaluation due to insomnia have been observed previously.12 PSG abnormalities in the rest were absence of deep sleep, high-frequency beta activity, REM behavior disorder (figures 2 and e-3, links.lww.com/CPJ/A219), and periodic leg movements (PLMs). One patient with seizures had epileptiform discharges recorded during PSG.
Figure 2. An Epoch of Polysomnogram Showing the Episode of REM Sleep Behavior Disorder With Vocalization and Loss of REM Atonia.
Treatment and Follow-up
Once the diagnosis was confirmed, all the patients received steroids; 11 patients in addition received plasmapheresis. Steroids were given for a period of 3–6 months (table 1). Follow-up duration ranged from 6 months to 3.5 years. Complete neurologic and autonomic resolution was observed in all patients over 2–3 months; neuropathic pain persisted in 5 patients and fatigability in 1 patient. Of interest, none of our patients were found to have neither autoimmune disorders nor malignancy at admission or at follow-up. All of them showed excellent response to first-line immunotherapy. Three of them had repeat evaluation of CASPR2 antibodies at the end of 12 months, and only 1 patient had persistent positivity.
Discussion
Morvan13 syndrome consists of constellation of symptom complex of multiple, irregular contractions of the muscles, cramps, weakness, hyperhidrosis, insomnia, and delirium. It has been found in association with thymoma, tumors (prostate adenoma and carcinoma of the sigmoid colon),2 autoimmune diseases, and myasthenia gravis suggesting an autoimmune or paraneoplastic etiology.14,15 Heavy metal intoxication with gold, mercury, and manganese has also been implicated to cause Morvan syndrome.2,16 It was later recognized that patients with Morvan syndrome have VGKC complex antibodies, which were mostly directed against CASPR2.17
The largest case series of CASPR2-related Morvan syndrome was reported in 2012.18 This was an observation of clinical and serologic profile of 23/29 patients who were identified by referral correspondence from various parts of the world.18 All the patients had neuromyotonia and neuropsychiatric manifestations in the form of insomnia, confusion, amnesia, hallucinations, and dysautonomia. Association with tumor was found in 41.4%; 11 of them being thymoma, and 1 patient had small-cell lung carcinoma.18 Thirty-eight cases of CASPR2-associated neurologic disorders (11 of them had Morvan syndrome) from 2 neuroimmunology centers from Barcelona and Rotterdam have been described.5
In the current study, we have described 14 patients with CASPR2 antibody–related Morvan syndrome from a single center. It is the second largest case series till date. This study has given a detailed description of the neuropsychiatric features, electrophysiology, autonomic, neuropsychological, and polysomnographic evaluation. Similar to previous literature, all our patients were predominantly men; all had clinical triad of neuropsychiatric symptoms, autonomic features, and peripheral nerve hyperexcitability. MRI of the brain was normal, and EMG showed myokymia and neuromyotonic discharges. This repository of symptoms is consistent with the features previously recognized with CAPR2 autoantibody,6,9,18–22 except that there were few salient features noted in our study cohort: (1) none of our patients experienced any relapse even with a long follow-up of 1–3.5 years in 78% of patients. (2) None of them were found in association with other autoimmune disorders or malignancy. (3) None of the patient's required second line of immunomodulation, all of them showed excellent response to steroids; few had required additional plasmapheresis in the acute period. (4) Comprehensive description of neuropsychological, autonomic, and polysomnographic features. (5) Ataxia was not a feature in our cohort, although previous studies have reported high incidence of cerebellar ataxia (∼35%).23 (6) Few patients were febrile at the onset of illness and had either exanthematous rash/loose stools. These symptoms could have been part of the autonomic symptoms or as a parainfectious phenomenon, but virologic studies were not contributory. A possible hypothesis of the benign nature of illness in our patients without evidence of any malignancy could have been a parainfectious etiology. Possible parainfectious nature was observed by Singh et al.24 in 4 patients with Morvan syndrome over a period of 6 months. Two of them had preceding fever. They were all from a restricted geographical distribution identified between May and November 2017 (summer and winter seasons), and a possible viral trigger for the immune-mediated condition was considered.
PET scan was normal in 8 patients, whereas 2 patients had subtle changes in the form of hypometabolism in basal ganglia and increased uptake in the limb muscles. Previously, 2 patients with Morvan syndrome with decreased metabolism in the left inferior frontal and temporal lobe have been described.12 These findings are in contrast with other neurologic disorders. Patients with limbic encephalitis show hypermetabolism in medial temporal lobes,25 and changes in the thalamus have been implicated in fatal familial insomnia.26 Neuropsychological evaluation did not reveal any evidence of cognitive impairment or dementia. Three of them had subtle involvement of the left frontal and temporal lobe.
In our cohort, we found autonomic dysfunction in the majority (5/8) of patients as assessed by cardiovascular autonomic function tests.10 Furthermore, HRV-based measures indicated decreased vagal and increased sympathovagal imbalance.27,28 Our study used these comprehensive cardiovascular autonomic functions as measures of autonomic dysfunction in a series of patients with Morvan syndrome. Autonomic functions in 1 patient12 had demonstrated impaired cardiovagal and cardiovascular adrenergic function along with reduced quantitative sudomotor axon reflex test response in the foot. A study by Liguori et al.8 suggested that antibodies to VGKC in the CNS or peripheral involvement of the antibodies on neurohormone secretion may be responsible for the CNS and autonomic involvement. Furthermore, we need future studies to investigate the progression of the disease and severity of cardiac autonomic involvement to use these measures in prognostication of this syndrome.
Clinical evidence of sleep disturbances in Morvan syndrome has been reported in 31%–48%.29,30 In the current study, 85.7% of patients had sleep abnormalities. In our study, PSG was attempted in 6 patients but only 3 patients cooperated for full PSG study. There was difficulty in performing PSG due to restlessness, insomnia, and behavioral changes resulting in non-cooperation of the patients as observed in previous studies.12 Three patients had prolonged latency, absence of deep sleep, REM behavior disorder, and PLMs. Insomnia is a cardinal feature with varied manifestations ranging from absent or decreased sleep (agrypnia excitata), status dissociatus to reversal of circadian changes.31 Previous polysomnographic studies have shown prolonged absence of all sleep cycles,32 absence of slow-wave REM sleep, reduced total sleep time, frequent awakenings, theta activity interspersed with faster rhythms, and periods of REM sleep without atonia.12 A solitary patient with insomnia in the early stages has been reported previously.3 Ten months into the illness, he developed REM behavior disorder along with occasional PLMs. Subfamilies of VGKCs are implicated in the regulation of sleep-wake cycle in experimental animal studies.33,34 These subunits are expressed in mice's thalamic reticular nucleus, which play a vital role in synchronized sleep generation. The animal studies have also shown marked reduction in sleep time with VGKC mutated flies33 and Kv3.1-Kv3.3 VGKC subunit knock-out mice.34 Extrapolating the sleep abnormalities from published literature, we hypothesize that the potassium channels may be directly involved in the thalamus and limbic system, resulting in secondary functional interruption of the thalamolimbic circuit and sleep changes.
This study has contributed to the growing knowledge on CASPR2-related Morvan syndrome. The major strengths of this study are the description of clinical features, long-term therapeutic outcome, and underrecognized description of neuropsychology, autonomic, and polysomnographic features. Follow-up study of CASPR2 antibodies, neuropsychology, autonomic and polysomnographic evaluation would have added more insight into the knowledge of the illness, which is a major limitation that needs to be addressed in future. It is important for an increased awareness and early recognition of this potentially treatable rare neuroimmunologic disorder. Further studies are required to address the range of autonomic, neuropsychological, and sleep abnormalities that can pave way for improved management of Morvan syndrome, resulting in better quality of life.
TAKE-HOME POINTS
→ Morvan syndrome is characterized by central, autonomic, and peripheral hyperexcitability due to CASPR2 antibody.
→ Autonomic function tests revealed definite autonomic dysfunction in half of the patients. The rest had orthostatic hypotension, early autonomic dysfunction, and postural orthostatic tachycardia syndrome.
→ PSG findings revealed insomnia, absence of deep sleep, high-frequency beta activity, REM behavior disorder, and periodic leg movements.
→ Neuropsychological evaluation showed subtle involvement of the left frontal and temporal lobe affection.
→ All patients showed complete improvement with steroids.
Appendix. Authors
Study Funding
No targeted funding reported.
Disclosure
The authors report no disclosures relevant to the manuscript. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Masood W, Sitammagari KK. Morvan syndrome (Morvan fibrillary chorea, MFC). In: StatPearls (Internet). Treasure Island: StatPearls Publishing; 2020. [Google Scholar]
- 2.Haug BA, Schoenle PW, Karch BJ, Bardosi A, Holzgraefe M. Morvan's fibrillary chorea. A case with possible manganese poisoning. Clin Neurol Neurosurgery 1989;91:53–59. [DOI] [PubMed] [Google Scholar]
- 3.Lotan I, Djaldetti R, Hellman MA, Benninger F. Atypical case of Morvan's syndrome. J Clin Neurosci 2016;25:132–134. [DOI] [PubMed] [Google Scholar]
- 4.Poliak S, Gollan L, Salomon D, et al. Localization of Caspr2 in myelinated nerves depends on axon–glia interactions and the generation of barriers along the axon. J Neurosci 2001;21:7568–7575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Sonderen A, Schreurs MWJ, Wirtz PW, Sillevis Smitt PAE, Titulaer MJ. From VGKC to LGI1 and CASPR2 encephalitis: the evolution of a disease entity over time. Autoimmun Rev 2016;15:970–974. [DOI] [PubMed] [Google Scholar]
- 6.van Sonderen A, Ariño H, Petit-Pedrol M, et al. The clinical spectrum of Caspr2 antibody-associated disease. Neurology 2016;87:521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee EK, Maselli RA, Ellis WG, Agius MA. Morvan's fibrillary chorea: a paraneoplastic manifestation of thymoma. J Neurol Neurosurg Psychiatry 1998;65:857–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liguori R, Vincent A, Clover L, et al. Morvan's syndrome: peripheral and central nervous system and cardiac involvement with antibodies to voltage-gated potassium channels. Brain 2001;124(pt 12):2417–2426. [DOI] [PubMed] [Google Scholar]
- 9.Panagariya A, Kumar H, Mathew V, Sharma B. Neuromyotonia: clinical profile of twenty cases from northwest India. Neurol India 2006;54:382. [DOI] [PubMed] [Google Scholar]
- 10.Sateia MJ, Buysse DJ, Krystal AD, Neubauer DN, Heald JL. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med 2017;13:307–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sathyaprabha TN, Satishchandra P, Netravathi K, Sinha S, Thennarasu K, Raju TR. Cardiac autonomic dysfunctions in chronic refractory epilepsy. Epilepsy Res 2006;72:49–56. [DOI] [PubMed] [Google Scholar]
- 12.Josephs KA, Silber MH, Fealey RD, Nippoldt TB, Auger RG, Vernino S. Neurophysiologic studies in Morvan syndrome. J Clin Neurophysiol 2004;21:440–445. [DOI] [PubMed] [Google Scholar]
- 13.Morvan AM. De la chorée fibrillaire. Gazette Hebdomadaire de Mèdicine et de Chirurgie 1890;27:173–200. [Google Scholar]
- 14.Halbach M, Hömberg V, Freund HJ. Neuromuscular, autonomic and central cholinergic hyperactivity associated with thymoma and acetylcholine receptor-binding antibody. J Neurol 1987;234:433–436. [DOI] [PubMed] [Google Scholar]
- 15.Madrid A, Gil-Peralta A, Gil-Neciga E, Gonzalez JR, Jarrin S. Morvan's fibrillary chorea: remission after plasmapheresis. J Neurol 1996;243:350–353. [DOI] [PubMed] [Google Scholar]
- 16.Petiot P, Charles N, Vial C, McGregor B, Aimard G. Complications neurologiques des sels d'or: discussion nosologique à propos d'un cas. Rev Neurol 1993;149:562–565. [PubMed] [Google Scholar]
- 17.Vincent A, Bien CG, Irani SR, Waters P. Autoantibodies associated with diseases of the CNS: new developments and future challenges. Lancet Neurol 2011;10:759–772. [DOI] [PubMed] [Google Scholar]
- 18.Irani SR, Pettingill P, Kleopa KA, et al. Morvan syndrome: clinical and serological observations in 29 cases. Ann Neurol 2012;72:241–255. [DOI] [PubMed] [Google Scholar]
- 19.Sunwoo JS, Lee ST, Byun JI, et al. Clinical manifestations of patients with CASPR2 antibodies. J Neuroimmunol 2015;281:17–22. [DOI] [PubMed] [Google Scholar]
- 20.Nagappa M, Mahadevan A, Sinha S, et al. Fatal Morvan syndrome associated with myasthenia gravis. Neurologist 2017;22:29–33. [DOI] [PubMed] [Google Scholar]
- 21.Sharma S, Sharma P. Morvan syndrome: after scrotal sac drainage and chemical instillation in hydrocele. Neurol India 2013;61:300. [DOI] [PubMed] [Google Scholar]
- 22.Kamble N, Netravathi M, Saini J, et al. Clinical and imaging characteristics of 16 patients with autoimmune neuronal synaptic encephalitis. Neurol India 2015;63:687–696. [DOI] [PubMed] [Google Scholar]
- 23.Bastiaansen AEM, Sonderen AV, Titulaer MJ. Autoimmune encephalitis with anti-leucine-rich glioma-inactivated 1 or anti-contactin-associated protein-like 2 antibodies (formerly called voltage gated potassium channel-complex antibodies). Curr Opin Neurol 2017;30:302–309. [DOI] [PubMed] [Google Scholar]
- 24.Singh R, Das P, Kaur U, et al. Morvan's syndrome—is a pathogen behind the curtain? Neurol Sci 2018;39:1965–1969. [DOI] [PubMed] [Google Scholar]
- 25.Fakhoury T, Abou-Khalil B, Kessler RM. Limbic encephalitis and hyperactive foci on PET scan. Seizure 1999;8:427–431. [DOI] [PubMed] [Google Scholar]
- 26.Perani D, Cortelli P, Lucignani G, et al. [18F] FDG PET in fatal familial insomnia: the functional effects of thalamic lesions. Neurology 1993;43:2565–2569. [DOI] [PubMed] [Google Scholar]
- 27.Task force of the European Society of Cardiology and the North American Society of Pacing and Electro Physiology. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Circulation 1996;93, 1043–1065. [PubMed] [Google Scholar]
- 28.Udupa K, Sathyaprabha TN, Thirthalli J, et al. Alteration of cardiac autonomic functions in patients with major depression: a study using heart rate variability measures. J Affect Disord 2007;100:137–141. [DOI] [PubMed] [Google Scholar]
- 29.Binks SNM, Klein CJ, Waters P, Pittock SJ, Irani SR. LGI1, CASPR2 and related antibodies: a molecular evolution of the phenotypes. J Neurol Neurosurg Psychiatry 2018;89:526–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balint B, Vincent A, Meinck HM, Irani SR, Bhatia KP. Movement disorders with neuronal antibodies: syndromic approach, genetic parallels and pathophysiology. Brain 2018;141:13–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahowald MW, Schenck CH. Status dissociatus—a perspective on states of being. Sleep 1991;14:69–79. [DOI] [PubMed] [Google Scholar]
- 32.Fischer-Perroudon C, Trillet M, Mouret J, et al. Polygraphic and metabolic studies of persistent insomnia with hallucinations: apropos of an antomo-clinical study of a case of Morvan's fibrillar chorea. Rev Neurol (Paris) 1974;130:111–125. [PubMed] [Google Scholar]
- 33.Cirelli C, Bushey D, Hill S, et al. Reduced sleep in Drosophila shaker mutants. Nature 2005;434:1087–1092. [DOI] [PubMed] [Google Scholar]
- 34.Baldelli L, Provini F. Fatal familial insomnia and Agrypnia Excitata: autonomic dysfunctions and pathophysiological implications. Auton Neurosci 2019;218:68–86. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Shows fasciculations noted in Patient No. 1 in bilateral thighs, calf muscles, shoulder and back region.Download Supplementary Video 1 (11.4MB, mp4) via http://dx.doi.org/10.1212/000978_Video_1
Shows fasciculations in the nasalis muscle in Patient No. 7.Download Supplementary Video 1 (4.8MB, mp4) via http://dx.doi.org/10.1212/000978_Video_2
Data Availability Statement
The individual deidentified participant data will be shared if required on an SPSS sheet.