Abstract
Objective
To identify the presence of unapproved pharmaceutical drugs in over-the-counter dietary supplements marketed to improve memory and cognitive function.
Methods
Supplements were identified by searching 2 supplement databases for products labeled as containing omberacetam, aniracetam, phenylpiracetam, or oxiracetam, 4 drugs not approved for human use in the United States. Products were purchased online and analyzed using nontargeted liquid chromatography-quadrupole time-of-flight mass spectrometry methods.
Results
In the 10 products tested, omberacetam and aniracetam were detected along with 3 additional unapproved drugs (i.e., phenibut, vinpocetine and picamilon). By consuming recommended serving sizes, consumers could be exposed to pharmaceutical-level dosages of drugs including a maximum of 40.6 ± 0.4 mg omberacetam (typical pharmacologic dose of 10 mg), 502 ± 0.8 mg of aniracetam (typical pharmacologic dose 200–750 mg), 15.4 ± 0.3 mg of phenibut (typical pharmacologic dose 250–500 mg), 4.3 ± 0.1 mg of vinpocetine (typical pharmacologic dose 5–40 mg), and 90.1 ± 0.7 mg of picamilon (typical pharmacologic dose 50–200 mg). Several detected drugs were not declared on the label, and several declared drugs were not detected in the products. For those products with drug quantities provided on the labels, 75% (9/12) of declared quantities were inaccurate. Consumers could be exposed to up to four-fold greater than pharmaceutical dosages and as many as 4 unapproved drugs when using individual products.
Conclusions
Over-the-counter cognitive enhancement supplements may contain multiple unapproved drugs. The health effects of consuming untested combinations of unapproved drugs at unpredictable dosages without clinician oversight in supplements are unknown.

Over-the-counter dietary supplements marketed to improve memory and mental focus, “nootropics,” have become increasingly popular in the United States (US) with hundreds of millions of US dollars in sales per year. The United States Food and Drug Administration (FDA) has recently raised concerns that these products may be unsafe and prevent patients from receiving appropriate diagnosis and treatment.1
Despite their popularity, the content and safety of cognitive enhancement supplements have received limited scrutiny. An emerging concern is the introduction of unapproved pharmaceutical drugs in nootropic supplements. Some products are openly labeled with drugs not approved for use in the US. In one recent example, the drug piracetam was detected in brain enhancement supplements.2 Piracetam belongs to the racetam family of drugs of which only levetiracetam has been approved by the FDA for use in the US. Piracetam, prescribed in many European countries, was found to be available in greater than pharmaceutical dosages in over-the-counter brain boosting supplements.2
In the current study, we analyzed supplements labeled as containing analogs of piracetam. These analogs have been studied in Russia, Japan, and elsewhere for the treatment of dementia, ischemic strokes, traumatic brain injury, and other neurologic conditions.3,4 Some are available as prescription drugs in other countries, but none have been approved by the FDA for use in the US.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
The current research did not involve either animal or human subjects; therefore, protocol approval, clinical trial registration and patient consents were not applicable.
Supplements listing omberacetam (Noopept), aniracetam, oxiracetam, or phenylpiracetam (all analogs of piracetam) as an ingredient in either the National Institute of Health's (NIH) Dietary Supplement Label Database (ods.od.nih.gov/Research/Dietary_Supplement_Label_Database.aspx) or the Natural Medicines Database (naturalmedicines.therapeuticresearch.com) in August 2019 were purchased online in September 2019. Supplements were excluded if the actual label on the purchased product did not list at least 1 of the 4 piracetam analogs.
The contents of the supplements were extracted in methanol and analyzed using nontargeted liquid chromatography-quadrupole time-of-flight mass spectrometry methods for the presence of unapproved synthetic drugs. The presence of each drug was subsequently confirmed by the use of reference standards. Quantitation was performed by the external standard method. Quantities were considered accurately labeled if they were within ±20% of the labeled quantity.
Data Availability
Additional detailed analytical methods for this study can be accessed in the supplementary material, links.lww.com/CPJ/A206, accompanying this paper.
Results
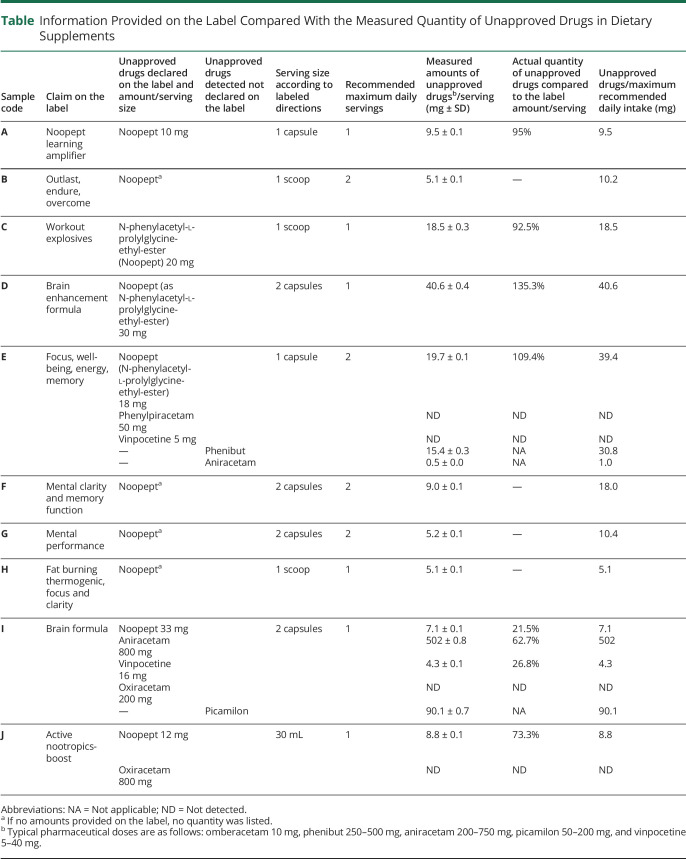
Twelve brands of supplements were purchased and, after the inspection of the labels, 2 were excluded because they did not list 1 of the 4 piracetam analogs as an ingredient on the actual label. Ten products were analyzed, and 8 were explicitly marketed to enhance mental function while 1 product was marketed to “outlast, endure, and overcome” and 1 as “workout explosives” (table).
Table.
Information Provided on the Label Compared With the Measured Quantity of Unapproved Drugs in Dietary Supplements
In total, 2 of the 4 piracetam analogs, omberacetam and aniracetam, were detected along with 3 additional unapproved drugs, vinpocetine, phenibut, and picamilon. Two of the 4 piracetam analogs, phenylpiracetam and oxiracetam, were listed on product labels but not detected in the products.
Three of the detected drugs were openly declared on product labels including omberacetam, a medication available in Russia used to treat traumatic brain injury, mood disorders, cerebral vascular disease, and other indications3; aniracetam, a drug approved for use to treat dementia in several countries including Italy, Argentina, and China4; and vinpocetine, a pharmaceutical drug available in Germany, Russia, and China used to treat acute stroke and cognitive impairment.5,6
Three unapproved drugs were detected in 2 products that were not declared on the label including aniracetam; phenibut, a prescription drug available in Russia used to treat anxiety, insomnia, alcohol withdrawal, and other indications7; and picamilon, a gamma-aminobutyric acid agonist used in Russia to treat cerebrovascular ischemia, mood disorders, and alcohol withdrawal.5
Omberacetam was present in all 10 products at dosages ±SD ranging from 5.1 ± 0.1 mg to 40.6 ± 0.4 mg compared with a typical pharmacologic dose of 10 mg. Other dosages of drugs detected included aniracetam 0.5 ± 0.0 mg to 502 ± 0.8 mg (typical pharmacologic dose 200–750 mg), picamilon 90.1 ± 0.7 mg (typical pharmacologic dose 50–200 mg), vinpocetine 4.3 ± 0.1 mg (typical pharmacologic dose 5–40 mg), and phenibut 15.4 ± 0.3 mg (typical pharmacologic dose 250–500 mg). In some cases, the quantity of a drug was listed on the label, but 75% (9/12) of declared quantities were inaccurate, with actual quantities ranging from 0% to 135% of quantity provided on the label.
Discussion
We detected 5 unapproved drugs in over-the-counter cognitive enhancement supplements sold in the US. Consumers might use these products in combination with prescription drugs or instead of seeking medical advice. Consumption of these products could expose people to amounts of these drugs four-fold greater than pharmaceutical dosages and combinations never tested in humans: one product combined 3 different drugs (omberacetam, phenibut, and aniracetam) and another product contained 4 different drugs (omberacetam, aniracetam, vinpocetine, and picamilon).
Furthermore, we found that consumers cannot obtain accurately labeled cognitive enhancement supplements by selecting supplements using the NIH's or Natural Medicines' supplement databases. Previous research found similar discrepancies between the information provided in the NIH's supplement database compared with the actual content of the supplement.8
Use of these cognitive enhancement supplements poses potentially serious health risks given the unpredictable dosing and lack of clinician supervision. The risks of using specific products are not known although these drugs have been associated with adverse effects including increased and decreased blood pressure, insomnia, agitation, dependence, sedation, hospitalization, and intubation.3–7
Our study does not address why these cognitive enhancement supplements contain both declared and undeclared drugs. The US law does not permit unapproved pharmaceuticals to be introduced as new ingredients into supplements, but the law places the burden of eliminating these products on the FDA. The FDA has previously attempted to remove some of these drugs from the supplement market. The FDA has issued a series of warning letters to companies selling picamilon, phenibut, omberacetam, and aniracetam supplements9,10 and a consumer warning that vinpocetine should not be consumed by women of childbearing age.11 Despite FDA warnings, these drugs remain openly listed as ingredients in the NIH's and Natural Medicines' supplement databases. This might be due to loopholes in the law that permits companies to sell supplements without informing the FDA and the lack of a system for the FDA to track products on the market.12 In addition, the FDA might not be using all enforcement tools available to remove these products from the marketplace.13 The FDA, for example, has not warned consumers about the presence of omberacetam or aniracetam in supplements, nor has the agency required mandatory recalls of these products. Our study also raises concerns regarding the quality and legality of supplements listed in the NIH's supplement database.
Our study has several limitations. We tested only supplements labeled as containing 1 of the 4 piracetam analogs in 2 databases at one point in time. Our results are not generalizable to all dietary supplements labeled as containing a piracetam analog. In addition, our study does not provide a comprehensive survey of all unapproved drugs marketed in brain enhancement dietary supplements.
The presence of these 5 unapproved drugs in supplements, including at supratherapeutic dosages, suggests serious risks to consumers and weaknesses in the regulatory framework under which supplements are permitted to be introduced in the US. Until the regulatory framework is reformed,12 clinicians should advise patients that supplements marketed as cognitive enhancement products may contain unpredictable combinations of unapproved drugs.
Acknowledgment
The authors thank Patricia Redd, MLS, of Cambridge Health Alliance, and Paul Bain, PhD, of Harvard Medical School, for their expert assistance in obtaining obscure references, John Travis, BS, of NSF International, for thoughtful comments on an earlier version of the manuscript, and Kay Negishi, MD, of Massachusetts General Hospital, and Cristina Avonto, PhD, of University of Mississippi, for assistance with translations from Japanese and Italian, respectively.
Appendix. Authors
Study Funding
No targeted funding reported.
Disclosure
P.A. Cohen has collaborated in research with NSF International, received compensation from UptoDate, and received research support from Consumers Union and PEW Charitable Trusts. B. Avula, Y.H. Wang, and I. Zakharevich report no disclosures relevant to the manuscript. I. Khan reported receiving grants from the US Food and Drug Administration, the National Institute of Health, and the US Department of Agriculture. I. Khan is also the coordinator of the International Conference on the Science of Botanicals which receives support for conference‐related expenses from multiple supplement‐related companies. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.US Food and Drug Administration. FDA takes action against 17 companies for illegally selling products claiming to treat Alzheimer's disease. 2019. Available at: www.fda.gov/news-events/press-announcements/fda-takes-action-against-17-companies-illegally-selling-products-claiming-treat-alzheimers-disease. Accessed September 6, 2020.
- 2.Cohen PA, Zakharevich I, Gerona R. Presence of piracetam in cognitive enhancement dietary supplements. JAMA Intern Med 2020;180:458–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neznamov GG, Teleshova ES. Comparative studies of noopept and piracetam in the treatment of patients with mild cognitive disorder in organic brain diseases of vascular and traumatic origin. Neurosci Beh Physiol 2009;39:311–321. [DOI] [PubMed] [Google Scholar]
- 4.Nakamura K. Aniracetam: its novel therapeutic potential in cerebral dysfunctional disorders based on recent pharmacological discoveries. CNS Drug Rev 2002;8:70–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avula B, Chittiboyina AG, Sagi S, et al. Identification and quantification of vinpocetine and picamilon in dietary supplements sold in the United States. Drug Test Anal 2016;8:334–343. [DOI] [PubMed] [Google Scholar]
- 6.Cohen PA. Vinpocetine: an unapproved drug sold as a dietary supplement. Mayo Clinic Proc 2015;90:1455. [DOI] [PubMed] [Google Scholar]
- 7.McCabe DJ, Bangh SA, Arens AM, Cole JB. Phenibut exposures and clinical effects reported to a regional poison center. Amer J Emerg Med 2019;37:2066–2071. [DOI] [PubMed] [Google Scholar]
- 8.Cohen PA, Sharfstein J, Kamugisha A, Vanhee C. Analysis of ingredients of supplements in the National Institutes of Health Supplement Database marketed as containing a novel alternative to anabolic steroids. JAMA Netw Open 2020;3:e202818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.US Food and Drug Administration. Picamilon in Dietary Supplements. 2017. Available at: fda.gov/food/dietary-supplement-products-ingredients/picamilon-dietary-supplements. Accessed September 6, 2020. [Google Scholar]
- 10.US Food and Drug Administration. Phenibut in Dietary Supplements. 2019. Available at: fda.gov/food/dietary-supplement-products-ingredients/phenibut-dietary-supplements. Accessed September 6, 2020. [Google Scholar]
- 11.US Food and Drug Administration. Statement on Warning for Women of Childbearing Age About Possible Safety Risks of Dietary Supplemetns Containing Vinpocetine. 2019. Available at: fda.gov/news-events/press-announcements/statement-warning-women-childbearing-age-about-possible-safety-risks-dietary-supplements-containing. Accessed September 6, 2020 [Google Scholar]
- 12.Cohen PA, Bass S. Injecting safety into supplements—modernizing the dietary supplement law. N Engl J Med 2019;381:2387–2389. [DOI] [PubMed] [Google Scholar]
- 13.Cohen PA. The FDA and adulterated supplements—dereliction of duty. JAMA Netw Open 2018;1:e183329. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Additional detailed analytical methods for this study can be accessed in the supplementary material, links.lww.com/CPJ/A206, accompanying this paper.