Abstract
Objective
Studies indicate that the functional outcome evolves in the year after ischemic stroke onset. However, the traditional outcome measure in stroke trials is the modified Rankin Scale (mRS) at 90 days from onset. To determine mRS fluctuations in the first year after stroke, we examined data from 3 major stroke trials.
Methods
In a secondary analysis, we evaluated intrapatient mRS between 90 days and 1 year from stroke onset, the mRS shift (∆mRS = 1 year-day 90), and the trials' primary outcome at day 90 and 1 year
Results
We included 624 patients from the National Institute of Neurological Disorders and Stroke rt-PA Stroke Study, 587 from Albumin Treatment for Acute Ischaemic Stroke, and 611 from Interventional Management of Stroke III, for which the proportion of patients with a ∆mRS change between day 90 and 1 year was 36.5%, 41.7%, and 36.0%. However, the trials' primary outcomes did not differ at 1 year vs 90 days. Similar findings were seen in a second cohort where we pooled the trials and excluded patients with recurrent stroke or death during the follow-up. In those 1,314 patients, 544 (41.4%) had a ∆mRS change, of which 379 (28.9%) had improvement and 165 (12.5%) had worsening, apart from death.
Conclusion
We describe the patient-level spectrum of mRS change from day 90 to 1 year after ischemic stroke in 3 high-quality randomized trials. The patient-level shifts consisted of a sufficiently counterbalanced number of mRS improvements and declines, which masked clinical evolution occurring in over one-third of patients. These results may have important implications, both for clinical trial design and outcome adjudication in stroke research and duration of rehabilitative therapy.
After acute ischemic stroke, the most frequently used functional outcome measure is the modified Rankin Scale (mRS), an ordinal scale with 7 possible values. The mRS is typically measured at 90 days from stroke onset because of the presumed stability of stroke recovery at that point.1 However, functional outcomes continue to evolve after 90 days, either improving or deteriorating in some patients, while remaining stable in others.2 Although studies have shown that mRS in the month after stroke is predictive of the 90-day value and have reported group values of mRS at 1 year from onset,3 there is not enough data on patient-level mRS after 90 days from stroke onset. This is an important omission because later variations in recovery trajectory influence rehabilitation planning and prognosis and could offer insights on the biological mediators of durable recovery. We performed a secondary analysis of 3 high-quality randomized ischemic stroke trials to examine patient-level mRS shifts between day 90 and 1 year and determined if the shifts altered the trials' main results.
Methods
We included 3 trials where we had access to individual patient's mRS data at day 90 and 1 year to perform a secondary analysis of patients from the National Institute of Neurological Disorders and Stroke rt-PA Stroke Study (NINDS tPA) parts 1 and 2, High-dose Albumin Treatment for Acute Ischaemic Stroke (ALIAS) Part 2, and Interventional Management of Stroke III (IMS-III).4 Our primary outcome is the patient-level mRS score at both time points. The secondary outcomes are intrapatient mRS shift (∆mRS = 1 year-day 90) and the trials' primary outcomes: mRS 0–1 for NINDS tPA and ALIAS and mRS 0–2 for IMS III. We depicted patient-level mRS at day 90 and 1 year using spine plots and a Sankey diagram. We show the proportions of our secondary outcomes and compare them in the intervention vs control arms using the χ2 test. We performed our analysis in a second cohort where we pooled the trials and excluded patients with recurrent stroke or death during the follow-up. To further explore the predictors of functional outcome change from day 90 to 1 year, we used backward stepwise selection at a threshold of p < 0.05 and fit to improvement or worsening of the ∆mRS in the pooled cohort.
Standard Protocol Approvals, Registrations, and Patient Consents
Because the datasets used in this secondary analysis are all deidentified, we were not required to obtain Institutional Board Review approval. The use of this deidentified dataset also did not require patient consent.
Data Availability
All data used in this analysis is publicly available at ninds.nih.gov/Current-Research/Research-Funded-NINDS/Clinical-Research/Archived-Clinical-Research-Datasets.
Results
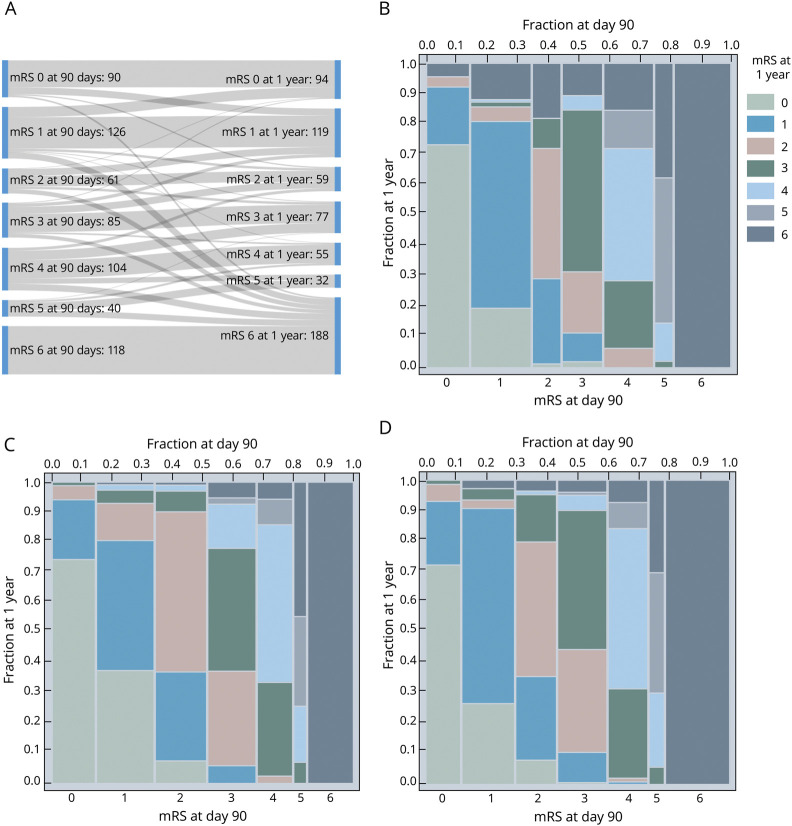
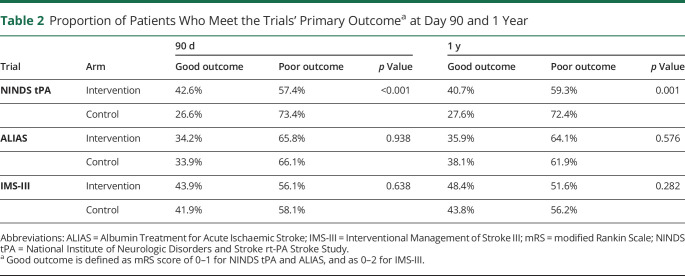
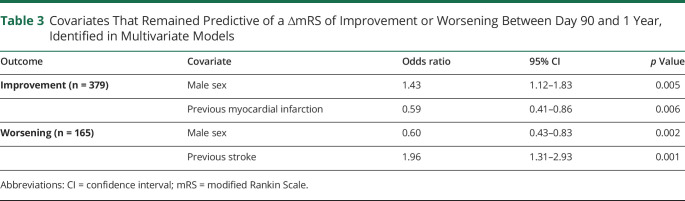
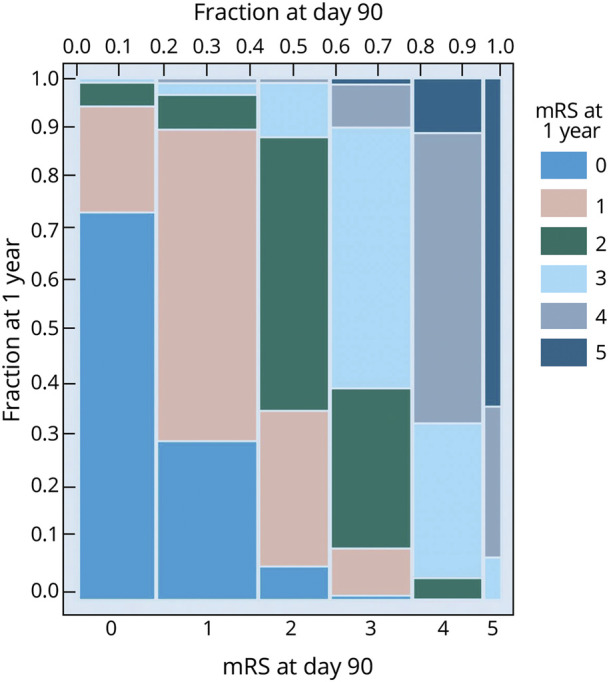
An mRS score at both day 90 and 1 year was available for 624 of 624 (100%) patients randomized in the NINDS tPA, 587 of 841 (69.8%) in ALIAS, and 611 of 656 (93.1%) in IMS-III. For the NINDS tPA trial, a Sankey diagram (figure 1A) illustrates the dynamic shifts between mRS strata from day 90 to 1 year. The spine plots in figure 1, B–D show the patient-level shifts in mRS between day 90 and 1 year, with distinct similarities among the 3 trials, which represent a wide range of acute ischemic stroke pathology. The proportion of patients who had a ∆mRS change between day 90 and 1 year was 36.5% for NINDS tPA, 41.7% for ALIAS, and 36.0% for IMS-III. We found the same pattern in the second cohort where we pooled the trials and excluded patients with recurrent stroke or death during the follow-up (figure 2).
Figure 1. Sankey Diagram and Spine Plots* for mRS at 90 Days and 1 Year.
(A-B) NINDS tpA trial, (C) ALIAS trial, and (D) IMS-III trial. *To help decipher a spine plot, consider in (B), for patients with 90 day mRS = 0 (see bottom x-axis), the proportion remaining a 0 at 1 year (color key) was approximately 75% (on the y-axis). The distribution of 90 day mRS scores can also be read from the plot as width across the top x-axis. ALIAS = Albumin Treatment for Acute Ischaemic Stroke; IMS-III = Interventional Management of Stroke III; mRS = modified Rankin Scale; NINDS tPA = National Institute of Neurologic Disorders and Stroke rt-PA Stroke Study.
Figure 2. Sankey Diagram and Spine Plot for mRS in the Pooled Second Cohort.
The pooled cohort included 1,314 patients who did not have recurrent stroke or death within a year of the index stroke onset. mRS = modified Rankin Scale.
The full spectrum of mRS shifts is seen in table 1, demonstrating a substantial proportion of patients shifting up vs down. This is reflected in the trials' results, which do not change when using 1-year outcomes instead of day 90 (table 2). For example, the proportion of patients with a good outcome in the intervention vs control arms of the NINDS tPA trial was 42.6% vs 26.6% (p < 0.001) at 90 days and 40.7% vs 27.6% (p = 0.001) at 1 year. Thus, despite over a third of patients (36.5%) having a ∆mRS change in that trial from 90 days to 1 year, the magnitude of the shifts was balanced enough to maintain the trial's result.
Table 1.
Patient-Level Shifts in mRS Score Between Day 90 and 1 Year (∆mRS = mRS at 1 Year—mRS at 90 Days), Thus Negative Values Indicate Decline in Function
Table 2.
Proportion of Patients Who Meet the Trials' Primary Outcomea at Day 90 and 1 Year
Of the 1,314 patients in the pooled second cohort without recurrent stroke or death, 770 (58.6%) had a stable ∆mRS between day 90 and 1 year, whereas 544 (41.4%) had a ∆mRS change, of which 379 (28.9%) showed improvement and 165 (12.5%) worsening, apart from death. To predict the improvement or worsening of ∆mRS, we evaluated the following available shared baseline covariates in the pooled cohort: patient age, sex, race, weight, history of hypertension, diabetes, hyperlipidemia, congestive heart failure, atrial fibrillation, previous stroke, previous myocardial infarction, and admission NIH Stroke Scale score. The variables that remained associated with worsening or improvement are shown in table 3 and included sex and previous stroke or myocardial infarction.
Table 3.
Covariates That Remained Predictive of a ∆mRS of Improvement or Worsening Between Day 90 and 1 Year, Identified in Multivariate Models
Discussion
Our analysis of 3 major acute ischemic stroke trials shows that over a third of the patients had shifts in mRS between day 90 and 1 year. Previous studies have described long-term functional outcomes after acute ischemic stroke,5–7 which we expand on by describing patient-level trajectories in the year after stroke onset. In addition, long-term functional outcome in patients with similar pathology, such as intracranial hemorrhage and traumatic brain injury, have been previously described without the patient-level trajectories.8–10 Our selection of these 3 trials was based off their having recorded mRS at 1 year from stroke onset, and the findings were consistent across a diverse selection of acute ischemic stroke patients with a broad range of stroke severity. Despite continued changes in functional outcome for a large proportion of patients, the trials' results were stable because the magnitude of the shifts was sufficiently counterbalanced.
Recovery after stroke depends on several factors, including patient demographics, comorbidities, stroke interventions such as tPA or endovascular thrombectomy, poststroke rehabilitation, recurrent stroke, secondary complications of stroke, and genetic variations.11 The trajectory of an individual patient's poststroke recovery should influence rehabilitative efforts.12 Further study of genetic determinants of recovery and its evolution could lead to novel therapeutic interventions. Using high-quality data from clinical trials with standardized, robust measurements of functional outcome, we show that over a third of stroke patients will have changes of their functional outcome between day 90 and 1 year, a degree of granularity that is not captured by focusing on group averages. Our preliminary analysis of patient characteristics that could account for improvement or worsening showed potential associations with patient sex and history of stroke or myocardial infarction. However, these datasets were not specifically designed to answer this question and future research would need to collect more detailed data on other important mediators including socioeconomic factors, intensity of rehabilitation, incident disease, and medications. Measuring the individual behavioral domains that contribute to function/disability could also provide greater insights.13 However, collecting more granular recovery and outcome data also involves specialized instruments that require time and training, which will be challenging to implement in large scale clinical trials.
The major limitation of our study is that we cannot account for patients who were lost to follow-up between day 90 and 1 year and for patients that died between 90 days and 1 year because we do not have any measure of their change in mRS before death. We also cannot fully explore why some patients remained stable and others had a change between day 90 and 1 year. We selected the 3 trials in this analysis because they recorded mRS at 1 year, which is not routinely performed, and this introduced a selection bias. The ALIAS trial had an almost 30% rate of loss to follow-up by the 1-year visit, so our study's results from that trial have inherent bias. Nonetheless, our results are sufficiently consistent to warrant additional study of stroke recovery after 90 days from onset. Such research may benefit from the addition of more detailed data collection and analyzing recovery trajectories in specific domains over time with tests such as detailed motor function evaluation, comprehensive neurocognitive assessment, or patient-reported psychosocial outcomes.10,14,15 Continuing to measure recovery in stroke patients after 90 days could produce actionable insights into the most effective rehabilitative care.
We describe the patient-level spectrum of change in the mRS from day 90 to 1 year after ischemic stroke in 3 high-quality randomized clinical trials of stroke interventions. Although these shifts did not change the trials' overall results, focusing solely on 90-day binary outcomes masks later clinical evolution occurring in over one-third of patients. These results may have important implications, both for clinical trial design and outcome adjudication in stroke research and duration of rehabilitative therapy.
Acknowledgment
The authors thank NIH/NINDS and the NINDS tPA, ALIAS, and IMS-III Investigators for making their datasets publicly available.
Appendix. Authors
Study Funding
A. de Havenon: NIH-NINDS K23NS105924. A. Lindgren: Swedish Research Council (2019-01757), Swedish Government, Swedish Heart and Lung Foundation, Region Skåne, Lund University, Skåne University Hospital, Sparbanksstiftelsen Färs och Frosta, and Freemasons Lodge of Instruction Eos Lund. B.B. Worrall: Australian-American Fulbright Commission. J.J. Majersik: NIH/NINDS U24NS107228. R. Braun: NIH/NICHD (K12HD093427).
Disclosure
A. Lindgren has received personal fees from Bayer, AstraZeneca, BMS-Pfizer, and Portola outside this work. S.C. Cramer serves as a consultant for AbbVie, Constant Therapeutics, MicroTransponder, Neurolutions, Regenera, SanBio, Stemedica, FUJIFILM Toyama Chemical Co., Biogen, and TRCare. B.B. Worrall is a deputy editor for Neurology®. J.J. Majersik is an associate editor for Stroke and a reviewer for UpToDate. A. de Havenon receives research funding from AMAG and Regeneron. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
TAKE-HOME POINTS
→ In the NINDS tPA, ALIAS part 2, and IMS-III trials, the proportion of patients with a change in their mRS score between day 90 and 1 year was 36.5%, 41.7%, and 36.0%.
→ The trials' primary outcomes did not differ when measured at 1 year instead of 90 days because the patient-level shifts consisted of a sufficiently counterbalanced number of both mRS improvements and declines.
→ Focusing on the primary outcome alone would have masked clinical evolution occurring in over one-third of patients, which has important implications, both for stroke research and rehabilitative therapy.
References
- 1.Quinn TJ, Dawson J, Walters MR, Lees KR. Reliability of the modified Rankin Scale: a systematic review. Stroke 2009;40:3393–3395. [DOI] [PubMed] [Google Scholar]
- 2.Hankey GJ, Spiesser J, Hakimi Z, Bego G, Carita P, Gabriel S. Rate, degree, and predictors of recovery from disability following ischemic stroke. Neurology 2007;68:1583–1587. [DOI] [PubMed] [Google Scholar]
- 3.Ovbiagele B, Lyden PD, Saver JL; VISTA Collaborators. Disability status at 1 month is a reliable proxy for final ischemic stroke outcome. Neurology 2010;75:688–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Available at: www.ninds.nih.gov/sites/default/files/NINDS_Data_Request_Form_508C.pdf. Accessed September 2, 2020.
- 5.Dhamoon Mandip S, Moon YP, Paik Myunghee C, et al. Long-term functional recovery after first ischemic stroke. Stroke 2009;40:2805–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huybrechts KF, Caro JJ. The Barthel Index and modified Rankin Scale as prognostic tools for long-term outcomes after stroke: a qualitative review of the literature. Curr Med Res Opin 2007;23:1627–1636. [DOI] [PubMed] [Google Scholar]
- 7.van den Berg LA, Dijkgraaf MGW, Berkhemer OA, et al. Two-year outcome after endovascular treatment for acute ischemic stroke. N Engl J Med 2017;376:1341–1349. [DOI] [PubMed] [Google Scholar]
- 8.Fleminger S, Ponsford J. Long term outcome after traumatic brain injury. BMJ 2005;331:1419–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson DA, Nakaji P, Albuquerque FC, McDougall CG, Zabramski JM, Spetzler RF. Time course of recovery following poor-grade SAH: the incidence of delayed improvement and implications for SAH outcome study design: clinical article. J Neurosurg 2013;119:606–612. [DOI] [PubMed] [Google Scholar]
- 10.Saulle MF, Schambra HM. Recovery and rehabilitation after intracerebral hemorrhage. Semin Neurol 2016;36:306–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindgren A, Maguire J. Stroke recovery genetics. Stroke 2016;47:2427–2434. [DOI] [PubMed] [Google Scholar]
- 12.National Clinical Guideline Centre (UK). Stroke Rehabilitation: Long Term Rehabilitation after Stroke [online]. London: Royal College of Physicians (UK); 2013. Available at: ncbi.nlm.nih.gov/books/NBK247494/. Accessed February 3, 2020. [PubMed] [Google Scholar]
- 13.Cramer SC, Koroshetz WJ, Finklestein SP. The case for modality-specific outcome measures in clinical trials of stroke recovery-promoting agents. Stroke 2007;38:1393–1395. [DOI] [PubMed] [Google Scholar]
- 14.See J, Dodakian L, Chou C, et al. A standardized approach to the Fugl-Meyer assessment and its implications for clinical trials. Neurorehabil Neural Repair 2013;27:732–741. [DOI] [PubMed] [Google Scholar]
- 15.Samra SK, Giordani B, Caveney AF, et al. Recovery of cognitive function after surgery for aneurysmal subarachnoid hemorrhage. Stroke 2007;38:1864–1872. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used in this analysis is publicly available at ninds.nih.gov/Current-Research/Research-Funded-NINDS/Clinical-Research/Archived-Clinical-Research-Datasets.