Abstract
Objective
To compare survival among patients with different combinations of apraxia of speech (AOS) and agrammatic aphasia, including those with isolated AOS (primary progressive AOS, PPAOS), both AOS and agrammatic aphasia (AOS + progressive agrammatic aphasia [PAA]), and isolated agrammatic aphasia (PAA).
Methods
One hundred nine patients were recruited who had any combination of AOS and agrammatic aphasia (42 PPAOS, 56 AOS + PAA, and 11 PAA) and were followed longitudinally, with 57 patients having since died. Cox proportional hazard models were used to quantify the relative risk of death across diagnoses. Adjusted survival curves are presented based on this model. We also assessed the influence of AOS and aphasia severity on survival.
Results
PPAOS had the longest survival (median survival of 5.97 years from the baseline visit), followed by PAA (5.26 years) and then AOS + PAA (4.33 years). AOS + PAA had a greater risk of death than PPAOS, with a hazard ratio of 3.01 (lower/upper confidence interval = 1.66/5.46, p < 0.001). Risk of death did not differ between PAA and the other groups. All results accounted for age and time from onset to baseline visit. AOS severity, independent of syndromic diagnosis, was associated with greater risk of death, with a hazard ratio of 1.35 for a 1-point increase in severity. Aphasia severity was not associated with risk of death.
Conclusions
Individuals with PPAOS have better survival and reduced risk of death compared with individuals with AOS + PAA. This finding will help improve prognostic estimates for these patients and supports the value of distinguishing PPAOS from AOS + PAA.

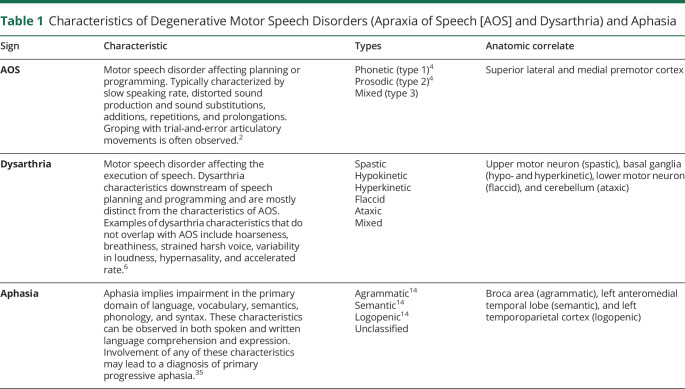
Primary progressive apraxia of speech (PPAOS) is a neurodegenerative syndrome that is defined by the presence of apraxia of speech (AOS), in the absence of other neurologic features, including aphasia, cognitive impairment, or parkinsonism.1 AOS (sometimes called aphemia or cortical dysarthria) is a disorder of motor planning and/or programming and affects the physical production of speech.2,3 The characteristic features of AOS include slow rate, articulatory distortions and distorted sound substitutions, and segmentation of syllables within or across words.4 It is distinguishable from dysarthria, which reflects problems with the neuromuscular control or execution of speech resulting from central or peripheral nervous system damage,5,6 and from aphasia, which reflects language processing deficits that typically cross language domains (e.g., semantics, syntax, and phonology) and modalities (e.g., spoken and written language comprehension and expression) (table 1). Patients with PPAOS can develop dysarthria (usually spastic and/or hypokinetic) and aphasia over the course of the disease, as well as parkinsonism, limb apraxia, and eventually cognitive impairment,7,8 although in some cases, the AOS remains the isolated neurologic feature for many years.7,9
Table 1.
Characteristics of Degenerative Motor Speech Disorders (Apraxia of Speech [AOS] and Dysarthria) and Aphasia
However, AOS can also commonly coexist with agrammatic aphasia, with some patients presenting with both AOS and agrammatic aphasia (referred to here as AOS + progressive agrammatic aphasia [PAA]). Agrammatic aphasia is defined as a language disorder that affects language production, resulting in telegraphic speech, grammatical simplification, the omission of function words, and difficulty with syntax and verbs, among other deficits; comprehension of syntactically or grammatically complex sentences can also be impaired.10,11 Furthermore, although rare, patients can present with agrammatic aphasia in the absence of AOS (PAA12). Patients with AOS + PAA also often develop parkinsonism, limb apraxia, and cognitive dysfunction over time.12,13 However, patients with PAA do not tend to develop parkinsonism and limb apraxia, but instead show more rapid declines in aphasia.12
All 3 of these groups of patients could be subsumed under the diagnosis of nonfluent/agrammatic primary progressive aphasia (agPPA)14 because the current, widely used diagnostic criteria for agPPA require the presence of either agrammatic aphasia or AOS.14 Hence, many investigators include patients with PPAOS, AOS + PAA, and PAA within studies of agPPA. However, we have found different neuroimaging signatures across PPAOS, AOS + PAA, and PAA, and patterns of disease progression can differ across groups1,7,12,15; hence, we have proposed that these should be separate diagnostic entities. It will be important to determine whether this diagnostic classification has implications for patients in terms of survival and ultimate prognosis. Therefore, in this study, we aimed to compare survival across patients with PPAOS, AOS + PAA, and PAA using a large cohort of patients that has been followed for many years.
Methods
Participants
One hundred nine patients who presented with any combination of progressive AOS and/or agrammatic aphasia were recruited by the Neurodegenerative Research Group at Mayo Clinic, Rochester, MN, between January 1, 2010, and January 16, 2019. All patients were recruited from the Department of Neurology. Patients with concurrent illnesses that could account for the speech and/or language deficits, such as traumatic brain injury, stroke, or developmental syndromes, and patients meeting the criteria for another neurodegenerative disease, including the logopenic and semantic variants of PPA,14 were excluded. We applied the 2017 Movement Disorder Society clinical criteria for PSP16 using operational definitions we previously described17 to determine whether patients met the criteria for PSP, and none met possible or probable PSP criteria at presentation. Similarly, we applied the corticobasal syndrome (CBS) clinical criteria,18 and none met the criteria for probable CBS at presentation. At the first research visit, each patient underwent a thorough speech-language evaluation by a speech-language pathologist (J.R.D., H.M.C., E.A.S., or R.L.U.) and a neurologic evaluation, as previously described in detail.1
All patients were enrolled into 1 of 3 NIH-funded longitudinal studies and were followed with approximately yearly research visits. A total of 57 patients have since died. Patients who dropped out of the longitudinal study were contacted in October 2019 to determine current status. Hence, all patients, except 2 who could not be contacted, were censored (i.e., the last known date a patient was known to be alive) within the last year. Figure 1 shows the available follow-up and death/censor dates for each patient.
Figure 1. Swim Plot of Follow-up by Calendar Year.
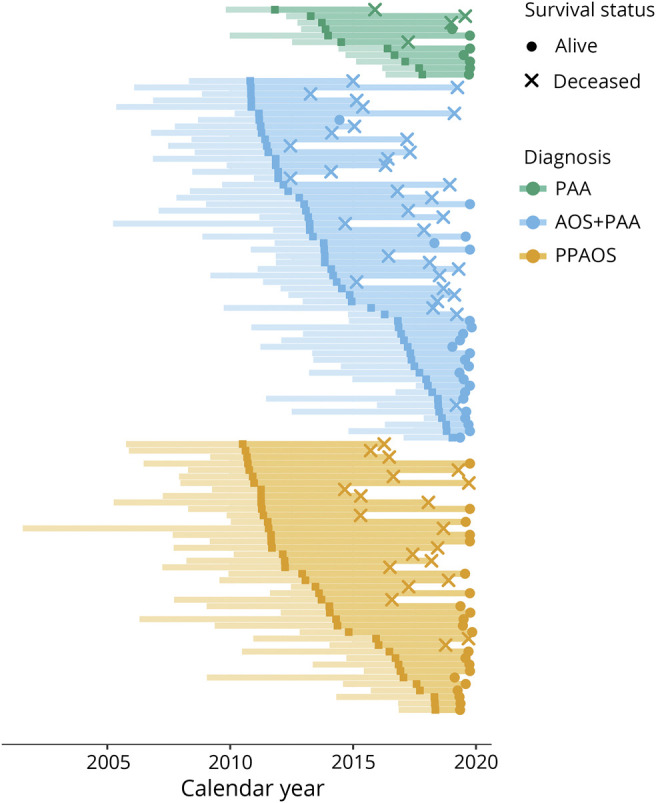
Each individual is represented by a horizontal segment in this plot, colored, and arranged by diagnosis group. The calendar date of the first visit is the dark square in each segment. Left of the dark point represents the time from onset to first visit, and right of the point represents follow-up from the first visit, thus the total length of the line is used in the time-to-event analyses. An × at the end of a segment indicates that the patient has died, and a • indicates a censor (i.e., the last known date a patient was alive). AOS = apraxia of speech; PAA = progressive agrammatic aphasia; PPAOS = primary progressive AOS.
Speech and Language Evaluation
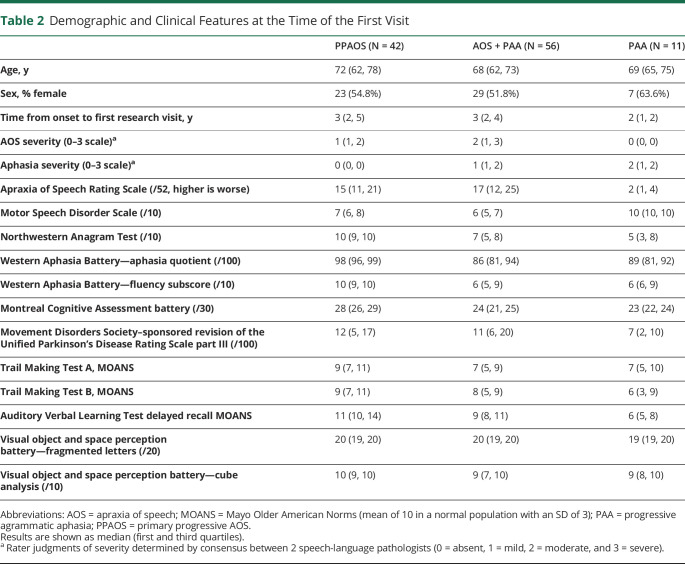
Motor speech was assessed using the Apraxia of Speech Rating Scale , which rates the presence and prominence of a number of clinical features associated with AOS,19 and a Motor Speech Disorders20 Scale, which rates the effect of any motor speech disorder (AOS or dysarthria) on communication function and speech intelligibility, independent of its specific features. Aphasia was assessed using the Northwestern Anagram Test,21 which assesses syntactic performance, and the Western Aphasia Battery (WAB).22 The WAB tests lexical content, fluency, repetition, naming, and language comprehension, and subscores are summed to create the Aphasia Quotient, an index of overall aphasia severity. Speech and language test scores and video recordings from each patient were reviewed by at least 2 speech-language pathologists, and the presence/absence and severity (0–4 scale) of agrammatism and of AOS were recorded separately, each by consensus, for each patient. In order for a patient to meet the criteria for having agrammatism, there had to be performance outside of the normal range on the Northwestern Anagram Test or function word omissions or syntactic errors had to be present during the WAB picture description task, in general conversation, in the narrative writing subtest of the WAB. AOS was identified by consensus, based on all spoken language tasks of the WAB plus additional speech tasks that included vowel prolongation, speech alternating motion rates, speech sequential motion rates, word and sentence repetition tasks, and a conversational speech sample. The designation of agrammatism was made independent of the motor speech characteristics of speech and vice versa. Patients were diagnosed at the first research visit as follows: PPAOS = AOS was present and aphasia was absent or equivocal (n = 42), PAA = agrammatic aphasia was present and AOS was absent or equivocal (n = 11), and AOS + PAA = both AOS and agrammatic aphasia were unequivocally present (n = 56) (see table 2 for demographic and clinical features). A total of 20 (48%) PPAOS; 4 (36%) PAA; and 33 (59%) AOS + PAA have since died.
Table 2.
Demographic and Clinical Features at the Time of the First Visit
Neurologic and Neuropsychological Assessments
The neurologic battery included the Montreal Cognitive Assessment Battery23 to assess general cognitive function and the Movement Disorders Society–sponsored revision of the Unified Parkinson's Disease Rating Scale part III24 to assess parkinsonism. The neuropsychological battery included tests for processing speed (Trail Making Test A25), executive function (Trail Making Test B25), episodic memory (Auditory Verbal Learning Test26), visuoperceptual ability (Visual Object and Space Perception battery27 fragmented letters), and visuospatial ability (Visual Object and Space Perception battery cube analysis).
Standard Protocol Approvals/Patient Consents
The study was approved by the Mayo Institutional Review Board. All participants consented for enrollment into the study.
Statistical Methods
Cox proportional hazard models were used to quantify the relative risk across diagnoses (PPAOS, PAA, and AOS + PAA) while controlling for the effects of age at first research visit and time from patient-reported onset to first research visit. The Cox model evaluates how multiple factors simultaneously affect survival time (years from first research visit to death), reporting effects as hazard ratios. Hazard ratios are a ratio of the rates of death at a given time point and can be thought of in terms of cross-sectional relative risk. We include time from patient-reported illness onset to first research visit as a covariate rather than backdating our follow-up time to onset to avoid the problem of immortal time bias.28 From the results of the Cox model, we visualize expected survival to assist in contextualizing the hazard ratios. In separate Cox models, we also assessed the influence of aphasia and AOS severities (using the 0–4 qualitative scale) on survival in patients with AOS (PPAOS and AOS + PAA) and aphasia severity in patients with aphasia (PAA and AOS + PAA) while accounting for diagnosis, age at baseline, and time from onset to first research visit. To manage (1) collinearity between baseline AOS severity and time from onset to first research visit (Spearman correlation of 0.58), (2) any potential relationships between diagnostic groups and AOS or aphasia severity, and (3) to prevent overfitting (i.e., more predictors than generally would be used with the number of events in our data), an elastic net regularization29 was used in these second and third models. Elastic net regularization acts as a shrinkage estimator, helping to address the issue of multiple comparisons by introducing helpful bias (toward no effect). We report only these shrunken effect estimates in these secondary models, without p values or confidence intervals (CIs), as there is no widely accepted method of estimating whether uncertainty in the estimate arises from this helpful bias or within the independent variable itself.
Data Availability
Anonymized data will be shared by request from any qualified investigator.
Results
The expected survival curves visualizing the model results show that participants with PPAOS had longer survival than those with AOS + PAA (figure 2). PAA survival was not different than either PPAOS or AOS + PAA, possibly due to the small sample size, but the effect estimates suggest that PAA survival may be somewhere between PPAOS and AOS + PAA survival. Median survival estimates from the first research visit were 5.97 years (lower CI = 5.72, upper CI not reached) in PPAOS, 5.26 years (lower CI = 4.07, upper CI not reached) in PAA, and 4.33 years (lower CI = 4.19, upper CI = 5.40) in AOS + PAA. The Cox proportional hazard models showed that AOS + PAA had a greater risk of death than PPAOS, with a hazard ratio of 3.01 (lower CI = 1.66, upper CI = 5.46). At 5 years, this hazard ratio corresponds to an absolute risk reduction of 0.20 (lower CI = 0.04, upper CI = 0.33). The equivalent of the number needed to treat, often reported in clinical trials involving interventions, was 3.07 (lower CI = 2.78, upper CI = 3.48), or, in other words, 3 more PPAOS cases would be needed to expect one PPAOS case to survive 5 years longer than the expected survival of an AOS + PAA case.
Figure 2. Visualization of the Cox Model Results Depicting Expected Survival by Diagnosis Group, PPAOS, AOS + PAA, and PAA.
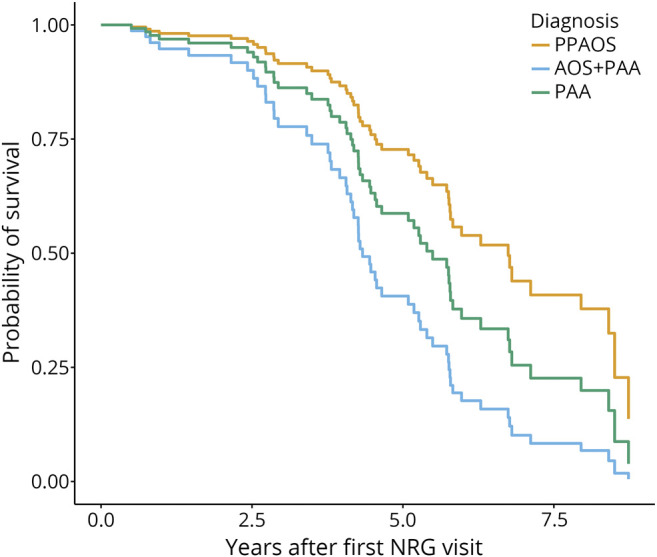
AOS = apraxia of speech; PAA = progressive agrammatic aphasia; PPAOS = primary progressive AOS.
Within participants who had AOS, increasing AOS severity was associated with a greater risk of death, with a hazard ratio of 1.35 for a 1-point increase in severity (on a 0–4 scale). However, even accounting for AOS severity, AOS + PAA was still associated with a greater risk of death than PPAOS with a hazard ratio of 1.66 in this second model. Within participants who had agrammatic aphasia, increasing aphasia severity was not associated with a greater risk of death (a hazard ratio of 1).
Discussion
This study demonstrates that participants given a diagnosis of PPAOS have better survival and lower risk of death than participants diagnosed with AOS + PAA. In fact, a participant with AOS + PAA was 3 times more likely to die before a participant with PPAOS. This finding is consistent with previous reports that have described participants with PPAOS who have survived for many years.9,30 The results also support the clinical validity and utility of a diagnosis of PPAOS and show that a diagnosis of PPAOS can provide essential and more accurate prognostic information to patients and families than a diagnosis of agPPA. The PAA group was much smaller than the other 2 groups, and hence, their survival estimates are less precise, and we cannot draw firm conclusions about how their survival compares with the other groups.
Worse survival in AOS + PAA concurs with the fact that these participants show a faster decline in performance on tests of cognition and functional ability, as well as, as expected, aphasia, compared with participants with PPAOS.12,13 At presentation, both PPAOS and AOS + PAA show atrophy on MRI and hypometabolism on FDG-PET, with PPAOS showing very focal involvement in the medial and lateral superior premotor cortex,1 whereas AOS + PAA shows additional more widespread involvement of the left inferior frontal gyrus (i.e., Broca area) likely reflecting the presence of agrammatism in this group.12,15 In patients with PPAOS that remain PPAOS over time, atrophy remains focal in the superior premotor cortex, whereas those that evolve into AOS + PAA develop atrophy of the inferior frontal gyrus.31 It may be argued that it is the focality (or lack thereof) or location of where the neurodegeneration is in the brain that accounts for worse prognosis. It could also be related to underlying pathology, although it is unknown whether the underlying pathology differs between PPAOS and AOS + PAA. Both syndromes are associated with 4-repeat tauopathies,9,32–34 including corticobasal degeneration, progressive supranuclear palsy, and globular glial tauopathy, but whether one pathology is more strongly associated with one clinical syndrome remains unknown. Although we consider PPAOS and AOS + PAA as separate entities, some may consider them part of a spectrum of disease, particularly given the fact that both arise from a 4-repeat tauopathy. Regardless, it is important to consider these entities as separate, given that communication interventions differ between the syndromes. For example, patients with PPAOS can continue to communicate with written language even when speech is severely affected or the patient is mute. On the other hand, aphasia will also affect writing and hence written language. The findings from this study give more credence to separating these syndromes by adding survival differences to differences in management.
The severity of AOS was also associated with survival, with the relative risk of death increasing by 1.35 for every 1-point increase in AOS severity. This is possibly due to an accompanying emergence of dysphagia and immobility that has been reported.7 Hence, taking into account AOS severity at the first visit is also important to providing survival estimates for patients. We did not observe an increased hazard ratio for death with aphasia severity, perhaps because aphasia severity is less strongly related to dysphagia severity and immobility.12
The strengths of our study are that we studied a large cohort of over 100 patients with these relatively rare disorders that we have prospectively followed for many years and that all patients were well characterized and diagnosed by the same team of speech-language pathologists and neurologists. Furthermore, our analysis accounted for both age and the time from onset to first research visit, showing that our findings were robust even after accounting for these variables. Limitations are that not all of our patients have died and that our PAA cohort is small, limiting power and generalizability of our comparisons between PAA and the other 2 groups. Nevertheless, the study was well powered to demonstrate a difference in survival between PPAOS and AOS + PAA.
Appendix. Authors

Study Funding
Supported by the NIH (R01-DC012519, R01-DC010367, R01-DC14942 and R01-NS89757).
Disclosure
J.L. Whitwell receives funding from NIH grants R01-DC12519, R01-NS089757, R01-AG050603, R01-AG37491, and R01-DC14942. P. Martin reports no disclosures. J.R. Duffy receives funding from NIH grants R01-DC12519 and R01-DC14942. H.M. Clark receives funding from NIH grants R01-DC12519, R01-DC14942, and R01-NS089757. R.L. Utianski receives funding from NIH grant R01-DC14942. H. Botha receives funding from NIH grants R01-DC12519 and R01-AG62677. M.M. Machulda receives funding from NIH grants R01-DC12519 and R01-AG050603. E.A. Strand reports no disclosures. K.A. Josephs receives funding from NIH grants R01-NS089757, R01-AG37491, R01-DC12519, R01-DC14942, and R01-AG050603. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Josephs KA, Duffy JR, Strand EA, et al. Characterizing a neurodegenerative syndrome: primary progressive apraxia of speech. Brain 2012;135(pt 5):1522–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duffy J. Apraxia of Speech in degenerative neurologic disease. Aphasiology 2006;20:511–527. [Google Scholar]
- 3.Darley FL, Aronson AE, Brown JR. Motor Speech Disorders. Philadelphia: W.B. Saunders; 1975. [Google Scholar]
- 4.Utianski RL, Duffy JR, Clark HM, et al. Prosodic and phonetic subtypes of primary progressive apraxia of speech. Brain Lang 2018;184:54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darley FL, Aronson AE, Brown JR. Differential diagnostic patterns of dysarthria. J Speech Hear Res 1969;12:246–269. [DOI] [PubMed] [Google Scholar]
- 6.Duffy JR, Strand EA, Josephs KA. Motor speech disorders associated with primary progressive aphasia. Aphasiology 2014;28:1004–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Josephs KA, Duffy JR, Strand EA, et al. The evolution of primary progressive apraxia of speech. Brain 2014;137(pt 10):2783–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Utianski RL, Duffy JR, Clark HM, et al. Clinical progression in four cases of primary progressive apraxia of speech. Am J Speech Lang Pathol 2018;27:1303–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tetzloff KA, Duffy JR, Strand EA, et al. Clinical and imaging progression over 10 years in a patient with primary progressive apraxia of speech and autopsy-confirmed corticobasal degeneration. Neurocase 2018;24:111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ash S, McMillan C, Gunawardena D, et al. Speech errors in progressive non-fluent aphasia. Brain Lang 2010;113:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson CK, Ballard KJ, Tait ME, Weintraub S, Mesulam M. Patterns of language decline in non-fluent primary progressive aphasia. Aphasiology 1997;11:297–321. [Google Scholar]
- 12.Tetzloff KA, Duffy JR, Clark HM, et al. Progressive agrammatic aphasia without apraxia of speech as a distinct syndrome. Brain 2019;142:2466–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tetzloff KA, Duffy JR, Clark HM, et al. Longitudinal structural and molecular neuroimaging in agrammatic primary progressive aphasia. Brain 2017;141:302–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology 2011;76:1006–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Josephs KA, Duffy JR, Strand EA, et al. Syndromes dominated by apraxia of speech show distinct characteristics from agrammatic PPA. Neurology 2013;81:337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoglinger GU, Respondek G, Stamelou M, et al. Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria. Mov Disord 2017;32:853–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whitwell JL, Stevens CA, Duffy JR, et al. An evaluation of the progressive supranuclear palsy speech/language variant. Mov Disord Clin Pract 2019;6:452–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armstrong MJ, Litvan I, Lang AE, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology 2013;80:496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strand EA, Duffy JR, Clark HM, Josephs K. The Apraxia of Speech Rating Scale: a tool for diagnosis and description of apraxia of speech. J Commun Disord 2014;51:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yorkston KM, Strand EA, Miller R, Hillel A, Smith K. Speech deterioration in amyotrophic lateral sclerosis: implications for the timing of intervention. J Med Speech Lang Pathol 1993;1:35–46. [Google Scholar]
- 21.Weintraub S, Mesulam MM, Wieneke C, Rademaker A, Rogalski EJ, Thompson CK. The northwestern anagram test: measuring sentence production in primary progressive aphasia. Am Journal Alzheimers Dis Other Demen 2009;24:408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kertesz A. Western Aphasia Battery (Revised). San Antonio, TX: PsychCorp; 2007. [Google Scholar]
- 23.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–699. [DOI] [PubMed] [Google Scholar]
- 24.Goetz CG, Tilley BC, Shaftman SR, et al. Movement disorder society-sponsored revision of the unified Parkinson's disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 2008;23:2129–2170. [DOI] [PubMed] [Google Scholar]
- 25.Strauss E, Sherman E, Spreen O. Trail making test. In: Strauss E, Sherman EMS, Spreen O, editors. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. Oxford: Oxford University Press; 2006:655–677. [Google Scholar]
- 26.Schmidt M. Rey Auditory Verbal Learning Test: A Handbook. Los Angeles: Western Psychological Services; 1996. [Google Scholar]
- 27.Warrington EK, James M. The Visual Object and Space Perception Battery: VOSP. London: Pearson; 1991. [Google Scholar]
- 28.Shariff SZ, Cuerden MS, Jain AK, Garg AX. The secret of immortal time bias in epidemiologic studies. J Am Soc Nephrol 2008;19:841–843. [DOI] [PubMed] [Google Scholar]
- 29.Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw 2010;33:1–22. [PMC free article] [PubMed] [Google Scholar]
- 30.Tebartz van Elst LH, Juengling FD, Kassubek J, et al. On the role of quantitative brain imaging in the differential diagnosis of speech disorders. Psychiatry Clin Neurosci 2002;56:111–115. [DOI] [PubMed] [Google Scholar]
- 31.Whitwell JL, Duffy JR, Machulda MM, et al. Tracking the development of agrammatic aphasia: a tensor-based morphometry study. Cortex 2017;90:138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Josephs KA, Duffy JR, Strand EA, et al. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain 2006;129(pt 6):1385–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Josephs KA, Boeve BF, Duffy JR, et al. Atypical progressive supranuclear palsy underlying progressive apraxia of speech and nonfluent aphasia. Neurocase 2005;11:283–296. [DOI] [PubMed] [Google Scholar]
- 34.Santos-Santos MA, Mandelli ML, Binney RJ, et al. Features of patients with nonfluent/agrammatic primary progressive aphasia with underlying progressive supranuclear palsy pathology or corticobasal degeneration. JAMA Neurol 2016;73:733–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mesulam MM. Primary progressive aphasia: differentiation from Alzheimer's disease. Ann Neurol 1987;22:533–534. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared by request from any qualified investigator.