Abstract
Background:
Globally, colorectal cancer (CRC) imposes a substantial burden on healthcare systems and confers considerable medical expenditures. We aimed to evaluate the global and regional burden in epidemiological trends and factors associated with the incidence and mortality of CRC.
Methods:
We used data from the GLOBOCAN database to estimate CRC incidence and mortality worldwide in 2020 and their association with the human development index (HDI). Trends of age-standardized rates of incidence and mortality in 60 countries (2000–2019) were evaluated by Joinpoint regression analysis using data of Global Burden of Disease 2019. The association between exposure to country-level lifestyle, metabolic and socioeconomic factors obtained from the World Health Organization Global Health Observatory and World Bank DataBank data and CRC incidence and mortality was determined by multivariable linear regression.
Results:
CRC incidence and mortality varied greatly in the 60 selected countries, and much higher incidence and mortality were observed in countries with higher HDIs, and vice versa. From 2000 to 2019, significant increases of incidence and mortality were observed for 33 countries (average annual percent changes [AAPCs], 0.24–3.82) and 18 countries (AAPCs, 0.41–2.22), respectively. A stronger increase in incidence was observed among males (AAPCs, 0.36–4.54) and individuals <50 years (AAPCs, 0.56–3.86). Notably, 15 countries showed significant decreases in both incidence (AAPCs, −0.24 to −2.19) and mortality (AAPCs, −0.84 to −2.74). A significant increase of incidence among individuals <50 years was observed in 30 countries (AAPCs, 0.28–3.62). Countries with higher incidence were more likely to have a higher prevalence of alcohol drinking, higher level of cholesterol level, higher level of unemployment, and a poorer healthcare system.
Conclusions:
Some high-HDI countries showed decreasing trends in CRC incidence and mortality, whereas developing countries that previously had low disease burden showed significantly increased incidence and mortality trends, especially in males and populations ≥50 years, which require targeted preventive health programs.
Keywords: Colorectal cancer burden, Trend, Incidence, Mortality, Risk factor, GLOBOCAN, Global Burden of Disease
Introduction
Colorectal cancer (CRC) was the third most commonly diagnosed malignancy and the second leading cause of cancer-related deaths worldwide in 2020.[1] Due to widespread screening and treatment promotion, declining trends of CRC incidence and mortality over the past few decades have been reported from some developed countries[2]; however, CRC continues to dominate the cancer spectrum. In contrast, CRC incidence and mortality rates have been increasing markedly in some developing countries that previously had a relatively low disease burden.[2] Globally, CRC imposes a substantial burden on healthcare systems and confers considerable medical expenditures.
There are several CRC-associated modifiable risk factors, including consumption of processed meat,[3] smoking,[4,5] excessive alcohol consumption,[6] and obesity.[7,8] In addition to these established risk factors, research has been increasingly focused on population-level socioeconomic factors,[9] including gross domestic product (GDP), health expenditure, and unemployment, which have been reported to be associated with pulmonary disease,[10] hepatic disease,[11] and some other cancers,[12–14] but rarely in CRC. Therefore, understanding the trends in CRC incidence and mortality as well as the associated factors at the population level is crucial to guide appropriate strategies for controlling the disease through population-based preventive initiatives.
Previous studies that have reported trends of CRC incidence and mortality were limited by being restricted to certain populations,[15] neglecting sex- and age-related variations,[2] or lack of up-to-date data (until 2016).[16] Furthermore, few studies have comprehensively examined the association between CRC incidence and mortality and exposure to related factors at the national level. Thus, there is an urgent need to examine the global trends in CRC incidence and mortality using up-to-date data and to evaluate the associated factors at the national level.
In this study, we aimed to describe the global trends in age- and sex-specific CRC incidence and mortality for 60 countries from 2000 through 2019 using the up-to-date data from the Global Burden of Disease (GBD) 2019.[17] Furthermore, the association between the exposure to lifestyle, metabolic, and socioeconomic factors at a country level and CRC incidence and mortality was comprehensively investigated to identify factors that could help to design cancer control policy in the future.
Methods
Data collection on CRC incidence and mortality
The estimated incidence and mortality rates of CRC of 184 countries in 2020 were extracted from the GLOBOCAN database.[18] To assess the temporal trends, the CRC incidence and mortality data between 2000 and 2019 were retrieved from the GBD 2019.[19] The GBD estimation is based on a comprehensive integration of multisource data, including data from censuses, household surveys, civil registration and vital statistics, disease registries, disease notifications, and others. Overall, the GBD 2019 covered 204 countries and territories. However, to ensure data validity in this study, we only included the incidence and mortality rates from countries with a 3-star or higher level in the GBD dataset, which was defined by a “percent well-certified” ≥35%[20]; therefore, 60 countries were included in the final analysis (Table 1). Detailed information about the GBD 2019 is presented in Online Supplementary Methods section. The rates were adjusted by age and presented as age-standardized incidence rates (ASIR) and age-standardized mortality rates (ASMR) according to Segi standard population.[21]
Table 1.
Group categories for trends in CRC incidence and mortality in 2000–2019 among 60 countries.
| Group | Incidence | Mortality | Countries (AAPC of incidence, AAPC of mortality) |
| Group A | Increasing | Increasing | China (3.82, 1.18), Ecuador (3.02, 2.22), Costa Rica (2.64, 1.82), Romania (2.47, 1.41), Republic of Moldova (2.36,1.55), Mexico (2.28, 1.68), Chile (2.00, 0.77), Mauritius (1.79, 1.28), Grenada (1.77, 1.31), Panama (1.70, 1.00), Cuba (1.70, 0.89), Guatemala (1.64, 1.06), Serbia (1.61, 0.41), Croatia (1.54, 0.60), Barbados (1.52, 0.94), Fiji (1.38, 1.14), Philippines (1.28, 0.96), Saint Lucia (1.13, 0.47) |
| Group B | Increasing | Stable | Kuwait (1.62, 0.57), Antigua and Barbuda (1.42, 0.76), Colombia (1.08, −0.09), Spain (0.99, −0.07), Brazil (0.75, −0.01), Argentina (0.66, 0.06) |
| Group C | Increasing | Decreasing | Estonia (1.40, −0.43), Republic of Korea (1.39, −0.54), Puerto Rico (0.87, −0.34), Slovakia (0.82, −0.36), Latvia (0.65, −0.34), Poland (0.65, −0.23), Netherlands (0.54, −0.46), Finland (0.48, −0.56), Malta (0.24, −0.78) |
| Group D | Stable | Stable | Trinidad and Tobago (0.55, −0.14), Lithuania (0.31, −0.26), Guyana (0.28, 0.05) |
| Group E | Stable | Decreasing | Slovenia (−0.12, −1.42), Denmark (0.06, −1.33), United Kingdom (0.10, −0.97), Kyrgyzstan (−0.36, −0.84), Hungary (0.08, −0.77), Japan (0.09, −0.75), Greece (0.09, −0.57), Canada (0.05, −0.56), Uruguay (0.05, −0.48) |
| Group F | Decreasing | Decreasing | Austria (−2.19, −2.74), Luxembourg (−1.60. −2.33), Germany (−1.53, −2.51), Israel (−1.25, −2.22), Switzerland (−1.13, −1.45), Singapore (−0.85, −2.26), Australia (−0.78, −1.58), Iceland (−0.72, −1.22), United States of America (−0.69, −1.05), Belgium (−0.46, −1.25), France (−0.44, −1.33), New Zealand (−0.42, −1.22), Italy (−0.42, −1.11), Sweden (−0.38, −0.84), Norway (−0.24, −1.10) |
“Increasing” or “decreasing” were used when the AAPC was statistically significant (P < 0.05); otherwise, the term “stable” was used. AAPC: Average annual percent change; CRC: Colorectal cancer.
Data on human development index (HDI) and risk factor exposure
The HDI for 2019 was obtained from the Human Development Report 2020[22] published by the United Nations Development Program. The HDI is a comprehensive index defined by three key capabilities: a long and healthy life, access to knowledge, and a decent standard of living. Based on quartiles of their distributions of the component indicators averaged over 2004 to 2013, the cutoff points of the HDI for grouping countries were defined as follows: <0.550 for low, 0.550 to 0.699 for medium, 0.700 to 0.799 for high, and ≥0.800 for very high.
For the analyses of factors associated with CRC incidence and mortality rates, the age-adjusted prevalence of factors (mostly in 2015) for each country was extracted from the World Health Organization Global Health Observatory database[23] and from the World Bank DataBank,[24] including smoking, alcohol, physical inactivity, Universal Health Coverage (UHC), high cholesterol, GDP, out-of-pocket spend, unemployment, health expenditure, health system, and education indicators [Supplementary Table 1 and Supplementary Methods section].
Statistical analysis
The ASIR and ASMR were plotted for each country grouped by the HDI level, with generalized additive models applied subject to non-linear associations. To assess the trends in the incidence and mortality rates of CRC, we used the Joinpoint regression model to fit a single regression line over the entire time series.[25] A maximum of three joinpoints were specified based on the number of data points. To estimate the magnitude and direction of trends, the average annual percent change (AAPC) and the corresponding 95% confidence interval for each segment were calculated from 2000 to 2019.[26] The AAPC across the entire period represents the weighted average of the constituent segments, where the weights are proportional to the number of data points in the segment. With regard to the trends, the terms “increasing” or “decreasing” were used when the AAPC was statistically significant (P < 0.05); otherwise, the term “stable” was used. The Joinpoint regression analyses were conducted with Joinpoint Regression Program version 4.8.0.1 (National Cancer Institute, Bethesda, America).[27]
The associations between the prevalence of lifestyle, metabolic, and socioeconomic factors and the incidence and mortality rates of CRC for each country were examined using univariable and multivariable linear regression analyses. Moreover, time-lag analyses of 1-, 2-, 3-, and 4-year data were tested for possible downstream effects. Beta coefficients (β) were presented as the change in the age-standardized rate of incidence or mortality that is associated with a 1% increase of a specific related factor. All P values < 0.05 were considered statistically significant.
Results
CRC incidence and mortality in 2020
The CRC incidence and mortality in 2020 of the major countries are shown in Table 2 and Supplementary Table 2. Worldwide, approximately 1.9 million new CRC cases were diagnosed in 2020. The crude incidence rate was 24.8 per 100,000 and the ASIR was 19.5 per 100,000 of the population [Table 2]. Of the five continents, European countries generally had high incidence rates, wherein the ASIR exceeded 45 per 100,000 for Hungary, and the crude incidence rate exceeded 103 per 100,000 for Portugal. Nevertheless, the ASIR of CRC was relatively low in African countries, such as Guinea (3.3 per 100,000), and varied greatly in Asia, America, and Oceania. For instance, Japan had a relatively high ASIR of 38.5 per 100,000, whereas the ASIR in Bangladesh was only 3.8 per 100,000. China alone comprised nearly 29% (555,477 cases) of the global new CRC cases in 2020. The sex-specific distribution of CRC incidence was mostly similar worldwide.
Table 2.
Estimated incidence and mortality of CRC in the major countries in 2020, both sexes, all ages.∗
| Incidence | Mortality | ||||||
| Population | HDI in 2019 | New cases | Crude rate (1/105) | ASR (1/105) | Deaths | Crude rate (1/105) | ASR (1/105) |
| World | 1,931,590 | 24.8 | 19.5 | 935,173 | 12.0 | 9.0 | |
| Africa | 66,198 | 4.9 | 8.4 | 42,875 | 3.2 | 5.6 | |
| Cyprus | 0.887 | 541 | 44.8 | 24.3 | 268 | 22.2 | 10.7 |
| Mauritius | 0.804 | 372 | 29.3 | 17.8 | 169 | 13.3 | 7.9 |
| Libya | 0.724 | 904 | 13.2 | 15.7 | 551 | 8.0 | 10.2 |
| Botswana | 0.735 | 81 | 3.4 | 4.5 | 47 | 2.0 | 2.6 |
| The Republic of the Gambia | 0.496 | 38 | 1.6 | 3.7 | 29 | 1.2 | 3.2 |
| Guinea | 0.477 | 202 | 1.5 | 3.3 | 154 | 1.2 | 2.6 |
| Asia | 1,009,400 | 21.8 | 17.6 | 506,449 | 10.9 | 8.6 | |
| Japan | 0.919 | 148,505 | 117.4 | 38.5 | 59,912 | 47.4 | 11.6 |
| Singapore | 0.938 | 3558 | 60.8 | 33.0 | 1808 | 30.9 | 16.2 |
| China | 0.761 | 555,477 | 38.4 | 23.9 | 286,162 | 19.8 | 12.0 |
| Tajikistan | 0.668 | 291 | 3.1 | 4.7 | 186 | 2.0 | 3.2 |
| Bangladesh | 0.632 | 5664 | 3.4 | 3.8 | 3435 | 2.1 | 2.3 |
| Bhutan | 0.654 | 28 | 3.6 | 3.8 | 18 | 2.3 | 2.5 |
| Europe | 519,820 | 69.4 | 30.4 | 244,824 | 32.7 | 12.3 | |
| Hungary | 0.854 | 9793 | 101.4 | 45.3 | 4880 | 50.5 | 20.2 |
| Portugal | 0.864 | 10,501 | 103.0 | 39.4 | 4320 | 42.4 | 13.0 |
| Croatia | 0.851 | 3706 | 90.3 | 36.3 | 2320 | 56.5 | 19.6 |
| Slovakia | 0.860 | 4821 | 88.3 | 43.9 | 2584 | 47.3 | 21.0 |
| Albania | 0.795 | 387 | 13.4 | 7.7 | 212 | 7.4 | 3.8 |
| Sierra Leone | 0.452 | 222 | 2.8 | 5.0 | 174 | 2.2 | 4.1 |
| Americas | 315,518 | 30.8 | 20.7 | 133,422 | 13.0 | 8.1 | |
| Uruguay | 0.817 | 2123 | 61.1 | 32.0 | 1080 | 31.1 | 14.3 |
| Canada | 0.929 | 25,510 | 67.6 | 31.2 | 9523 | 25.2 | 9.9 |
| Barbados | 0.814 | 147 | 51.2 | 25.1 | 101 | 35.1 | 16.1 |
| Belize | 0.716 | 20 | 5.0 | 6.2 | 19 | 4.8 | 6.0 |
| Guatemala | 0.663 | 769 | 4.3 | 5.7 | 495 | 2.8 | 3.6 |
| Plurinational State of Bolivia | 0.718 | 627 | 5.4 | 5.7 | 387 | 3.3 | 3.4 |
| Oceania | 20,654 | 48.4 | 29.8 | 7603 | 17.8 | 9.3 | |
| Australia | 0.944 | 16,240 | 63.7 | 33.1 | 5603 | 22.0 | 8.9 |
| New Zealand | 0.931 | 3404 | 70.6 | 33.8 | 1435 | 29.8 | 12.3 |
| North Macedonia | 0.774 | 948 | 45.5 | 26.1 | 503 | 24.1 | 13.0 |
| Papua New Guinea | 0.555 | 596 | 6.7 | 11.3 | 346 | 3.9 | 6.9 |
| Fiji | 0.743 | 85 | 9.5 | 10.9 | 49 | 5.5 | 6.8 |
| Solomon Islands | 0.567 | 27 | 3.9 | 6.7 | 16 | 2.3 | 4.2 |
| Vanuatu | 0.609 | 10 | 3.3 | 5.2 | 9 | 2.9 | 4.8 |
Data are all from GLOBOCAN 2020 (https://gco.iarc.fr/today/home). ASR: Age-standardized rates; CRC: Colorectal cancer; HDI: Human development index.
Globally, there were 935,000 CRC-related deaths in 2020. The crude mortality rate was 12.0 per 100,000, and the ASMR was 9.0 per 100,000. Slovakia's ASMR was 21.0 per 100,000, which was the highest in the world. The CRC-related mortality rate was low in Africa and Oceania, such as in Botswana (2.6 per 100,000) and Solomon Islands (4.2 per 100,000), and varied greatly in Asia and America. For instance, the highest ASMR in Asia was observed in Singapore (38.5 per 100,000), whereas the ASIR in Bangladesh was only 2.3 per 100,000. The largest number of deaths (286,162) occurred in China, which accounted for 30.60% of the global CRC-related deaths.
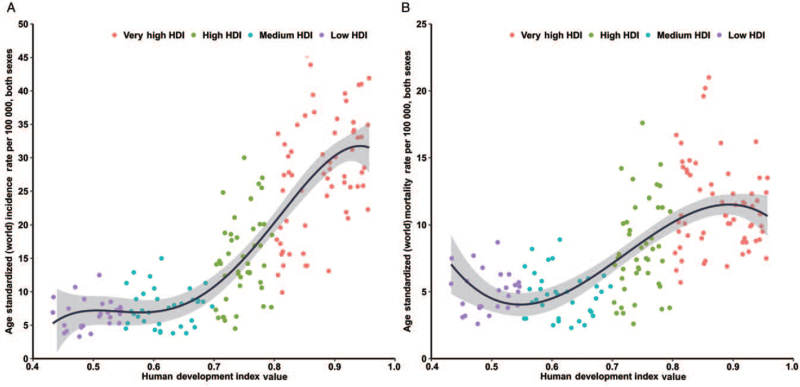
Through ecological correlation analysis, we found that the HDI was positively correlated with CRC incidence and mortality. Incidence rates in countries with very high HDI were nearly five times greater than those in countries with a low HDI, and the bulk of correlation was stronger than that between the HDI and mortality [Figure 1]. The top 20 incidence rates were all observed in countries with very high HDI and were highest in Hungary (ASIR, 45.3 per 100,000), followed by Slovakia (ASIR, 43.9 per 100,000), and Norway (ASIR, 41.9 per 100,000). The highest mortality rates were typically observed in high-HDI countries, and the highest was Slovakia (ASMR, 21.0 per 100,000), followed by Hungary (ASMR, 20.2 per 100,000), and Croatia (ASMR, 19.6 per 100,000). In contrast, the lowest ASMR was observed in Bangladesh, which is a medium-HDI country.
Figure 1.
Correlation between age-standardized CRC incidence (left panel) and mortality (right panel) and HDI in both genders combined (GLOBOCAN 2020). CRC: Colorectal cancer; HDI: Human development index.
Trend analysis for the incidence and mortality of CRC in 60 countries in 2000 to 2019
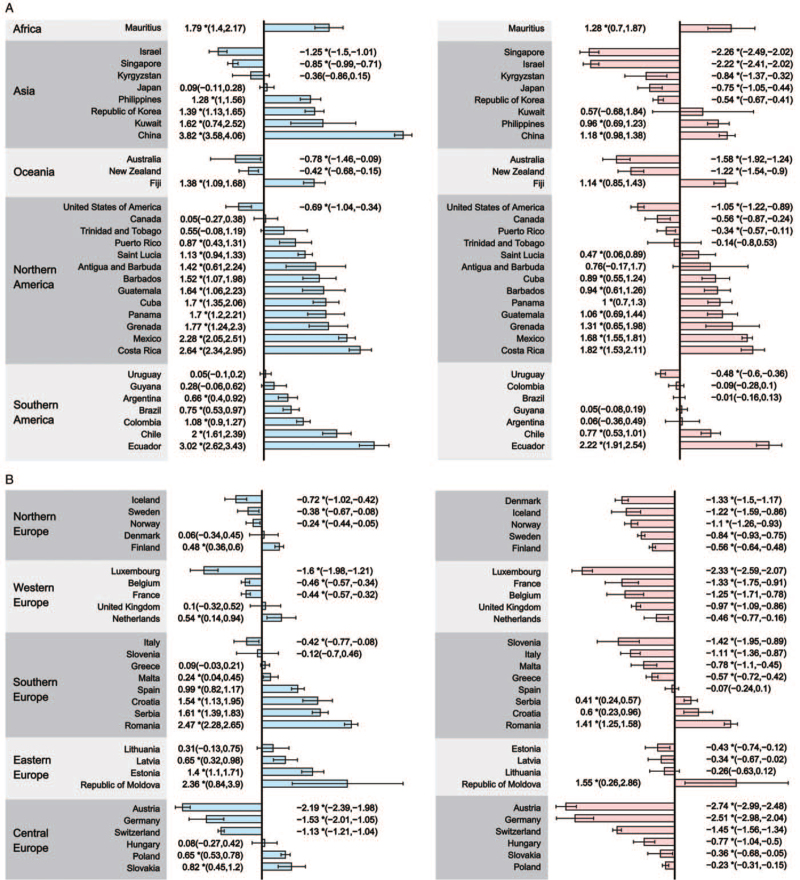
The detailed results of the trend analyses of CRC in ASIR and ASMR for the 60 selected countries are shown in Figure 2 and Supplementary Tables 3 and 4.
Figure 2.
The AAPC of the incidence (left panel) and mortality (right panel) of CRC in both genders, all ages. (A) AAPC of countries in Africa, Asia, Oceania, Northern America, and Southern America; (B) AAPC of countries in Northern Europe, Western Europe, Southern Europe, Eastern Europe, and Central Europe. ∗P values < 0.050. AAPC: Average annual percent change; CRC: Colorectal cancer.
An increasing incidence of CRC was observed in 33 countries in 2000 to 2019, with the AAPCs ranging from 0.24 (Malta) to 3.82 (China). Meanwhile, increasing mortality trends were observed in 18 of the 33 countries, with the AAPCs ranging from 0.41 (Serbia) to 2.22 (Ecuador); particularly, these 18 countries were mostly from Eastern Europe, Latin America, and Asia [Table 1, Group A]. An increasing incidence but decreasing mortality rate of CRC was observed in nine, mostly European, countries, including Estonia, Finland, Latvia, Malta, the Netherlands, Poland, Puerto Rico, Republic of Korea, and Slovakia [Table 1, Group C]. Among these nine countries, the largest decline in mortality was seen in Malta (AAPC, −0.78), followed by Finland (AAPC, −0.56) and the Republic of Korea (AAPC, −0.54). The other six countries had relatively stable trends in mortality, with AAPC showing no statistically significant difference [Table 1, Group B].
Twelve countries, mostly from Europe and Latin America, presented a stable CRC incidence trend from 2000 to 2019: Trinidad and Tobago (Latin America), Lithuania (Eastern Europe), and Guyana (Latin America) had steady trends in both CRC incidence and mortality rates over the past two decades [Table 1, Group D]. Among the remaining nine countries, the largest decreases were seen in countries from Europe, including Slovenia (AAPC, −1.42), Denmark (AAPC, −1.33), and the United Kingdom (AAPC, −0.97; Table 1, Group E).
Decreases in both CRC incidence and mortality from 2000 through 2019 were seen in 15 countries [Table 1, Group F], all of which had very high HDI, such as Australia, Germany, and the United States. The decreasing incidences of CRC were markedly apparent in Austria (AAPC, −2.19) and Luxembourg (AAPC, −1.60), and the greatest declines in CRC mortality were seen in Austria (AAPC, −2.74) and Germany (AAPC, −2.51).
Incidence and mortality trends among males and females
The results of the sex-stratified trend analyses are shown in Supplementary Tables 5 to 8 and Supplementary Figures 1 and 2. With regard to the CRC incidence rates, 31 countries had an increasing trend in both male (AAPCs, 0.36–4.54) and female populations (AAPCs, 0.16–2.82). The largest increasing incidence of CRC in males was observed in China (AAPC, 4.54), followed by Costa Rica (AAPC, 3.40) and Ecuador (AAPC, 3.11). Similarly, the largest increasing incidence in females was observed in Ecuador (AAPC, 2.82), followed by China (AAPC, 2.68) and Grenada (AAPC, 2.66). Furthermore, Kuwait, Greece, and Malta had increasing trends in males alone, whereas Trinidad and Tobago, the United Kingdom, and Grenada only had increasing trends in females. Decreasing CRC trends in males were observed in 11, mostly very high HDI, countries, such as Austria (AAPC, −2.22) and Germany (AAPC, −1.35). With regard to females, 13 countries, including Austria (AAPC, −2.14), Iceland (AAPC, −2.09), and Germany (AAPC, −1.87), showed a decreasing CRC incidence trend. Significantly, no opposite trend between males and females was observed in the 60 countries included in this analysis.
Compared with the CRC incidence trends, mortality presented a decreasing trend in most countries. In males, increasing mortality trends were observed in 22 countries (AAPCs, 0.11–2.50), with the largest increase in Costa Rica (AAPC, 2.50; Latin America), followed by other American countries such as Ecuador (AAPC, 2.38) and Mexico (AAPC, 2.29). Simultaneously, most of these countries showed increasing CRC mortality trends in females, with the AAPC ranging from 1.97 (Ecuador) to 0.15 (China). Decreasing CRC mortality trends in males were observed in 27 of the 60 countries, with the greatest decreasing mortality trend observed in Austria (AAPC, −2.89), Luxembourg (AAPC, −2.45), and Singapore (AAPC, −238), most of which are very high-HDI and high-HDI countries. The decline in trend was observed in 36 of all 60 countries in females, with Germany (AAPC, −2.83), Austria (AAPC, −2.71), and Israel (AAPC, −2.49) presenting the maximum decrease.
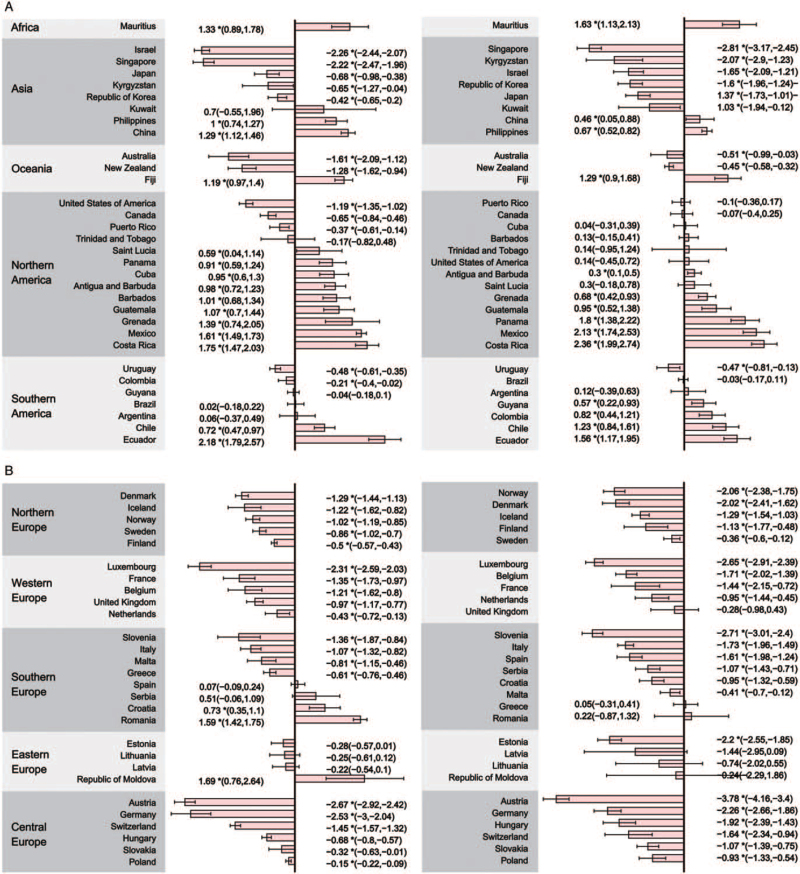
Incidence and mortality trends among younger and older individuals
The age-stratified incidence and mortality trends of the population younger or older than 50 years are shown in Supplementary Tables 9 to 12. Among the population aged 50 or older, the CRC incidence increased in 32 countries (AAPCs, 0.56–3.86) [Figure 3]. The most marked increase was observed in China (AAPC, 3.86), Ecuador (AAPC, 3.11), and Romania (AAPC, 2.66). Among these abovementioned 32 countries, 25 had an increasing incidence in the population that was younger than 50 years (AAPCs, 0.28–3.62), including China (AAPC, 3.62), Costa Rica (AAPC, 3.33), and Mexico (AAPC, 2.78). Moreover, 13 countries, which were all very high-HDI and high-HDI countries, showed a decreasing CRC incidence trend among the population aged 50 or older (AAPCs, −0.37 to −2.09). The greatest decline in the incidence trend was seen in Austria (AAPC, −2.09), followed by Luxembourg (AAPC, −1.59) and Germany (AAPC, −1.58). Among the population younger than 50 years, 13 countries, all of which were Asian and European countries, showed a decreasing trend (AAPCs, −0.17 to −2.88). Although many countries reported an increasing incidence trend, especially among those 50 years or older, some developed countries, such as the United States, had a decreasing incidence among adults aged 50 or older (AAPC, −0.88) but had an increasing trend in the population younger than 50 years (AAPC, 0.56).
Figure 3.
The AAPC of the incidence of CRC in both genders, 50 years or older (left panel) and younger than 50 years (right panel). (A) AAPC of countries in Africa, Asia, Oceania, Northern America, and Southern America; (B) AAPC of countries in Northern Europe, Western Europe, Southern Europe, Eastern Europe, and Central Europe.∗P values < 0.050. AAPC: Average annual percent change; CRC: Colorectal cancer.
With regard to CRC mortality, the most rapid increase in mortality trends among the population aged 50 or older was observed in Ecuador (AAPC, 2.18), followed by Costa Rica (AAPC, 1.75) and the Republic of Moldova (AAPC, 1.69) [Figure 4]. Only 14 countries showed an increasing trend among the population younger than 50 years, with the AAPC ranging from 0.30 (Antigua and Barbuda) to 2.36 (Costa Rica). Particularly, 31 countries, most of which were Asian or European countries, showed a decreasing trend in both cohorts.
Figure 4.
The AAPC of the mortality of CRC in both genders, 50 years or older (left panel) and younger than 50 years (right panel). (A) AAPC of countries in Africa, Asia, Oceania, Northern America, and Southern America; (B) AAPC of countries in Northern Europe, Western Europe, Southern Europe, Eastern Europe, and Central Europe. ∗P values < 0.050. AAPC: Average annual percent change; CRC: Colorectal cancer.
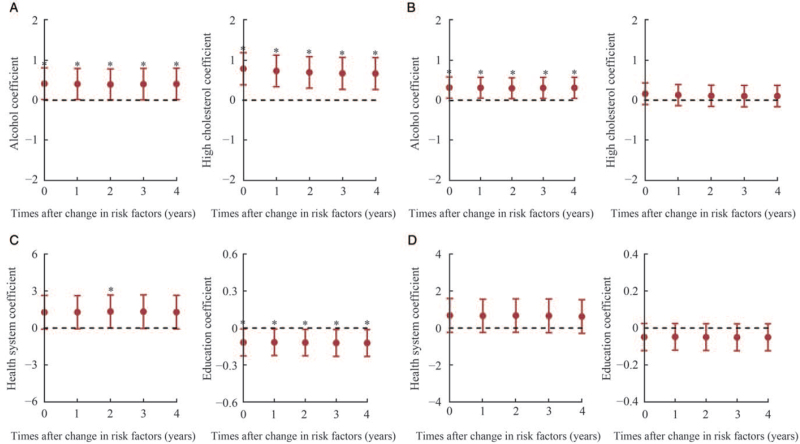
Association with lifestyle, metabolic, and socioeconomic factors
Ecological analyses to examine the association between the incidence and mortality of CRC and exposure to related factors at the country level were conducted, and the results are shown in Figure 5, Supplementary Tables 13 to 18, and Supplementary Figures 3 and 4. Overall, countries with higher ASIR of CRC were associated with higher prevalence of alcohol consumption (β = 0.412, P = 0.041), higher level of cholesterol (β = 0.785, P < 0.001), lower level of education (β = −0.116, P = 0.038), and poorer health system (β = 1.342, P = 0.050). Regarding the mortality, countries with higher ASMR were more likely to have higher alcohol consumption (β = 0.315, P = 0.020).
Figure 5.
Time-lag analyses of changes in associated factors for CRC incidence and mortality in both genders. CRC: Colorectal cancer.
For the sex-stratified analysis, four factors including alcohol (β = 0.436, P = 0.012), high cholesterol (β = 0.975, P < 0.001), unemployment (β = 0.455, P = 0.018), and health system (β = 1.960, P = 0.037) were associated with the ASIR among males. Alcohol (β = 0.410, P = 0.001) and unemployment (β = 0.307, P = 0.022) were associated with the ASMR. Time-lag analysis showed that the pattern was not changed throughout 4 years for all factors, except for mortality with regard to the health system [Supplementary Figure 3].
Among females in different countries, higher ASIR of CRC was associated with a higher level of UHC (β = 0.160, P = 0.025) and higher level of cholesterol (β = 0.521, P = 0.004). Higher education was associated with the lower ASMR (β = −0.096, P = 0.021). Time-lag analysis showed a similar trend [Supplementary Figure 4]. However, there was no significant association between the prevalence of any studied risk factors and ASMR at the country level (P > 0.05).
Discussion
In this study, we carried out a comprehensive analysis to examine the overall, sex- and age-specific trends in CRC incidence and mortality in 60 countries, and the results showed that 15 countries with relatively high HDI had decreasing trends in both incidence and mortality for CRC in 2000 to 2019, whereas 33 countries had contrastingly increasing trends. Furthermore, in some very high-HDI countries, CRC incidence increased among the population younger than 50 years, whereas decreasing trends were found in the population of individuals aged 50 or older. Notably, we identified that the exposure of some factors at a country level was associated with the CRC incidence and mortality, including smoking, alcohol, physical inactivity, obesity, blood cholesterol, unemployment, and healthy system.
The decline in the CRC incidence in some countries with relatively high HDI could be largely attributed to the implementation of screening programs and population-level changes toward healthier lifestyle choices.[28] Evidence from randomized controlled trials and cohort studies has demonstrated the effectiveness of screening by endoscopy or fecal occult blood test in reducing the burden of CRC. A meta-analysis by Brenner et al[29] showed that screening by colonoscopy could yield a reduction in CRC incidence and mortality by 49% and 47%, respectively, compared with no screening. Population-based screening by fecal immunochemical test also showed significant reduction of both incidence of advanced-stage CRC and deaths from CRC.[30] Particularly, the introduction of population-based screening in developed countries over the past two decades contributed to the decreasing CRC incidence and mortality in these regions.[31] Taking the United States as an example, CRC screening recommendations were first released in 1997,[32] and the uptake rate of screening with any recommended test increased from 38% in 2000 to 66% in 2018.[33] Accordingly, the CRC incidence (AAPC, −0.69) and mortality (AAPC, −1.05) decreased greatly from 2000 to 2019 in the United States, which confirmed the effect of screening programs on reducing the CRC burden. Therefore, for countries with high burden of CRC, implementation of population-based CRC screening programs targeting high-risk population might be a feasible and effective way in reducing the incidence and mortality of CRC. However, the overall screening strategies should be defined according to the country's actual condition.
Although some high-HDI countries have shown decreasing trends in overall CRC incidence, increasing trends in incidence among the population younger than 50 years deserve further attention. Thirty countries were experiencing increasing incidence trends of early onset CRC (typically defined as CRC diagnosed in individuals younger than 50 years). Genetic factors were thought to play an important role in early onset CRC,[34] but do not explain most of the causation. A review indicated that the presence of many risk factors, including socioeconomic factors, infections, tobacco, alcohol, antibiotics, diet, metabolic factors, hormones, gut microbiota, and so forth, may explain this alarming trend.[35] Although alcohol and smoking seem to be associated with early onset CRC, this link is demonstrated mostly in the relatively older early onset CRC population.[36] Accordingly, lifestyle changes and the increasing prevalence of obesity might be contributory factors with regard to the phenomenon of early onset CRC.[37–39] As the current recommended starting age of screening was 50 years in most of the guidelines, whether younger ones should undertake screening deserve further study and such evidence is still rather sparse.
The increasing trends in CRC incidence and mortality in formerly low-burden countries partly reflected the changes in lifestyle and diet. The results of a modeling study showed that adult alcohol per-capita consumption increased by 104% (58%–150%) in the southeast Asia region between 1990 and 2017.[40] Besides, China Kadoorie Biobank study indicated that among current regular drinkers, the proportion engaging in heavy episodic drinking increased in both rural (29% vs. 33%) and urban (31% vs. 36%) areas from 2004 to 2014.[41] Accordingly, the CRC incidence increased rapidly in many Southeast Asia countries including China during the past decades. Shifts toward an increased intake of animal-derived foods and a more sedentary lifestyle might lead to decreased physical activity and an increased prevalence of obesity, which are independently associated with CRC risk.[42] In addition, increases in CRC mortality rates might be attributed to the poor health system. Survival from CRC depends heavily on the stage at diagnosis and, therefore, the increasing trend of CRC in low-HDI countries cannot be hindered because of the lack of population-based screening programs.
The findings of the ecological analyses were generally consistent with the results of previous cohort studies. We found that an increase in CRC incidence in males was associated with high prevalence of total alcohol assumption at the country level. Moreover, such associations were observed for high cholesterol as well as for some socioeconomic factors. The association between CRC risk and increased cholesterol has been proved,[43,44] and the treatment of dyslipidemia with statins could reduce the CRC risk.[45] Furthermore, an ecological study found that the rise of unemployment was associated with an increase in mortality from CRC in both sexes.[9] Nevertheless, UHC seemed to reinforce this effect in our study, which is contrary to the findings reported in the literature.[9] To explain this discrepancy, more detailed analyses on mediating variables and interactive variables of UHC with CRC incidence would be desirable. Moreover, population with higher education had an associated higher CRC incidence, but no significant difference was found in the CRC mortality, which was consistent with the report from an earlier study.[46] Nevertheless, the CRC incidence and the prevalence of related factors might have been underrated in low-HDI countries due to the possibility of underestimation.
There are several major strengths of our analysis. First, this study analyzed the trends in overall, age- and sex-specific incidence and mortality of CRC using the most up-to-date data. Second, we explored the association between CRC incidence and mortality and related factors, especially socioeconomic factors which have not been assessed on a global scale previously.
However, our study also has several limitations. First, the assessment of the CRC trend used data from GBD 2019, which was based on estimation and modeling; nonetheless, many previous studies have verified the validity of GBD data in predicting the incidence and mortality of cancer.[17,20,47] Second, we did not conduct analyses to illustrate the variation of incidence and mortality for colon or rectal cancer separately, as such data were not available for the GBD dataset. Further analysis should be conducted using other data sources. Third, we cannot exclude the possibility of ecological fallacy for the analysis with regard to the association between the risk factors at the country level and the CRC incidence and mortality rates.
In this analysis of data from 60 countries, we found that some high-HDI countries showed decreasing trends in the incidence and mortality of CRC, whereas significantly increased CRC incidence and mortality trends were observed in developing countries that previously had a low disease burden, especially among men and in populations aged 50 or older. More preventive efforts and targeted screening programs are recommended for these high-risk populations to further reduce the incidence and mortality of CRC.
Funding
This work was supported by grants from the Natural Science Foundation of Beijing Municipality (No. 7202169), the Beijing Nova Program of Science and Technology (No. Z191100001119065), and the CAMS Innovation Fund for Medical Sciences (No. 2017-I2M-1-006).
Conflicts of interest
None.
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
How to cite this article: Lu B, Li N, Luo CY, Cai J, Lu M, Zhang YH, Chen HD, Dai M. Colorectal cancer incidence and mortality: the current status, temporal trends and their attributable risk factors in 60 countries in 2000–2019. Chin Med J 2021;134:1941–1951. doi: 10.1097/CM9.0000000000001619
Bin Lu and Na Li contributed equally to this work.
Supplemental digital content is available for this article.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017; 66:683–691. doi: 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 3.Händel MN, Rohde JF, Jacobsen R, Nielsen SM, Christensen R, Alexander DD, et al. Processed meat intake and incidence of colorectal cancer: a systematic review and meta-analysis of prospective observational studies. Eur J Clin Nutr 2020; 74:1132–1148. doi: 10.1038/s41430-020-0576-9. [DOI] [PubMed] [Google Scholar]
- 4.Cheng J, Chen Y, Wang X, Wang J, Yan Z, Gong G, et al. Meta-analysis of prospective cohort studies of cigarette smoking and the incidence of colon and rectal cancers. Eur J Cancer Prev 2015; 24:6–15. doi: 10.1097/cej.0000000000000011. [DOI] [PubMed] [Google Scholar]
- 5.Botteri E, Iodice S, Bagnardi V, Raimondi S, Lowenfels AB, Maisonneuve P. Smoking and colorectal cancer: a meta-analysis. JAMA 2008; 300:2765–2778. doi: 10.1001/jama.2008.839. [DOI] [PubMed] [Google Scholar]
- 6.McNabb S, Harrison TA, Albanes D, Berndt SI, Brenner H, Caan BJ, et al. Meta-analysis of 16 studies of the association of alcohol with colorectal cancer. Int J Cancer 2020; 146:861–873. doi: 10.1002/ijc.32377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kyrgiou M, Kalliala I, Markozannes G, Gunter MJ, Paraskevaidis E, Gabra H, et al. Adiposity and cancer at major anatomical sites: umbrella review of the literature. BMJ 2017; 356:j477.doi: 10.1136/bmj.j477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fang X, Wei J, He X, Lian J, Han D, An P, et al. Quantitative association between body mass index and the risk of cancer: a global meta-analysis of prospective cohort studies. Int J Cancer 2018; 143:1595–1603. doi: 10.1002/ijc.31553. [DOI] [PubMed] [Google Scholar]
- 9.Maruthappu M, Watkins J, Noor AM, Williams C, Ali R, Sullivan R, et al. Economic downturns, universal health coverage, and cancer mortality in high-income and middle-income countries, 1990-2010: a longitudinal analysis. Lancet 2016; 388:684–695. doi: 10.1016/s0140-6736(16)00577-8. [DOI] [PubMed] [Google Scholar]
- 10.Grønseth R, Erdal M, Tan WC, Obaseki DO, Amaral AFS, Gislason T, et al. Unemployment in chronic airflow obstruction around the world: results from the BOLD study. Eur Respir J 2017; 50:1700499.doi: 10.1183/13993003.00499-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li W, Kemos P, Salciccioli JD, Marshall DC, Shalhoub J, Alazawi W. Socioeconomic factors associated with liver-related mortality from 1985 to 2015 in 36 developed countries. Clin Gastroenterol Hepatol 2020; S1542-3565:31156–31163. doi: 10.1016/j.cgh.2020.08.047. [DOI] [PubMed] [Google Scholar]
- 12.Maruthappu M, Painter A, Watkins J, Williams C, Ali R, Zeltner T, et al. Unemployment, public-sector healthcare spending and stomach cancer mortality in the European Union, 1981-2009. Eur J Gastroenterol Hepatol 2014; 26:1222–1227. doi: 10.1097/meg.0000000000000201. [DOI] [PubMed] [Google Scholar]
- 13.Maruthappu M, Watkins JA, Waqar M, Williams C, Ali R, Atun R, et al. Unemployment, public-sector health-care spending and breast cancer mortality in the European Union: 1990-2009. Eur J Public Health 2015; 25:330–335. doi: 10.1093/eurpub/cku167. [DOI] [PubMed] [Google Scholar]
- 14.Trewin CB, Hjerkind KV, Johansson ALV, Strand BH, Kiserud CE, Ursin G. Socioeconomic inequalities in stage-specific breast cancer incidence: a nationwide registry study of 1.1 million young women in Norway, 2000-2015. Acta Oncol 2020; 59:1284–1290. doi: 10.1080/0284186x.2020.1753888. [DOI] [PubMed] [Google Scholar]
- 15.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JWW, Comber H, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 2013; 49:1374–1403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 16.Wong MCS, Huang J, Lok V, Wang J, Fung F, Ding H, et al. Differences in incidence and mortality trends of colorectal cancer worldwide based on sex, age, and anatomic location. Clin Gastroenterol Hepatol 2021; 19:955–966.e61. doi: 10.1016/j.cgh.2020.02.026. [DOI] [PubMed] [Google Scholar]
- 17.GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020; 396:1204–1222. doi: 10.1016/s0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Data Visualization Tools for Exploring the Global Cancer Burden in 2020. International Agency for Research on Cancer, WHO; 2020. Available from: https://gco.iarc.fr/today/home/. [Accessed April 11, 2021] [Google Scholar]
- 19. Global Burden of Disease Study 2019 (GBD 2019) Data Resources. GBD 2019 Diseases and Injuries Collaborators; 2020. Available from: http://ghdx.healthdata.org/gbd-results-tool/. [Accessed April 11, 2021] [Google Scholar]
- 20.GBD 2017 Colorectal Cancer Collaborators. The global, regional, and national burden of colorectal cancer and its attributable risk factors in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol 2019; 4:913–933. doi: 10.1016/s2468-1253(19)30345-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Segi M, Fujisaku S, Kurihara M. Geographical observation on cancer mortality by selected sites on the basis of standardised death rate. Gan 1957; 48:219–225. [PubMed] [Google Scholar]
- 22. Human Development Report 2020. The Next Frontier: Human development and the Anthropocene. New York: United Nations Development Programme (UNDP); 2020:347–350. [Google Scholar]
- 23. Global Health Observatory Data Repository. Geneva: World Health Organization; 2021. Available from: https://www.who.int/data/gho/. [Accessed April 11, 2021] [Google Scholar]
- 24. World Bank Development Indicators. World Bank; 2021. Available from: https://databank.worldbank.org/home.aspx/. [Accessed April 11, 2021] [Google Scholar]
- 25.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med 2000; 19:335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 26.Clegg LX, Hankey BF, Tiwari R, Feuer EJ, Edwards BK. Estimating average annual per cent change in trend analysis. Stat Med 2009; 28:3670–3682. doi: 10.1002/sim.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Joinpoint Trend Analysis Software, Version 4.8.0.1. NCI DoCCaPS; 2021. Available from: https://surveillance.cancer.gov/joinpoint/. [Last accessed on April 11, 2021] [Google Scholar]
- 28.Arnold M, Abnet CC, Neale RE, Vignat J, Giovannucci EL, McGlynn KA, et al. Global burden of 5 major types of gastrointestinal cancer. Gastroenterology 2020; 159:335–349.e15. doi: 10.1053/j.gastro.2020.02.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brenner H, Stock C, Hoffmeister M. Effect of screening sigmoidoscopy and screening colonoscopy on colorectal cancer incidence and mortality: systematic review and meta-analysis of randomised controlled trials and observational studies. BMJ 2014; 348:g2467.doi: 10.1136/bmj.g2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiu HM, Jen GHH, Wang YW, Fann JCY, Hsu CY, Jeng YC, et al. Long-term effectiveness of faecal immunochemical test screening for proximal and distal colorectal cancers. Gut 2021; doi: 10.1136/gutjnl-2020-322545. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Navarro M, Nicolas A, Ferrandez A, Lanas A. Colorectal cancer population screening programs worldwide in 2016: an update. World J Gastroenterol 2017; 23:3632–3642. doi: 10.3748/wjg.v23.i20.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winawer SJ, Fletcher RH, Miller L, Godlee F, Stolar MH, Mulrow CD, et al. Colorectal cancer screening: clinical guidelines and rationale. Gastroenterology 1997; 112:594–642. doi: 10.1053/gast.1997.v112.agast970594. [DOI] [PubMed] [Google Scholar]
- 33. National Center for Health Statistics, Division of Health Interview Statistics. National Health Interview Survey Public Use Data File 2018. Centers for Disease Control and Prevention; 2019. Available from: https://www.cdc.gov/asthma/nhis/2018/data.htm/. [Accessed April 11, 2021] [Google Scholar]
- 34.Pearlman R, Frankel WL, Swanson B, Zhao W, Yilmaz A, Miller K, et al. Prevalence and spectrum of germline cancer susceptibility gene mutations among patients with early-onset colorectal cancer. JAMA Oncol 2017; 3:464–471. doi: 10.1001/jamaoncol.2016.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wild CP, Scalbert A, Herceg Z. Measuring the exposome: a powerful basis for evaluating environmental exposures and cancer risk. Environ Mol Mutagen 2013; 54:480–499. doi: 10.1002/em.21777. [DOI] [PubMed] [Google Scholar]
- 36.Koo JE, Kim KJ, Park HW, Kim HK, Choe JW, Chang HS, et al. Prevalence and risk factors of advanced colorectal neoplasms in asymptomatic Korean people between 40 and 49 years of age. J Gastroenterol Hepatol 2017; 32:98–105. doi: 10.1111/jgh.13454. [DOI] [PubMed] [Google Scholar]
- 37.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K, et al. Body fatness and cancer - viewpoint of the IARC Working Group. N Engl J Med 2016; 375:794–798. doi: 10.1056/NEJMsr1606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Renehan AG, Zwahlen M, Egger M. Adiposity and cancer risk: new mechanistic insights from epidemiology. Nat Rev Cancer 2015; 15:484–498. doi: 10.1038/nrc3967. [DOI] [PubMed] [Google Scholar]
- 39.Roberts DL, Dive C, Renehan AG. Biological mechanisms linking obesity and cancer risk: new perspectives. Annu Rev Med 2010; 61:301–316. doi: 10.1146/annurev.med.080708.082713. [DOI] [PubMed] [Google Scholar]
- 40.Manthey J, Shield KD, Rylett M, Hasan OSM, Probst C, Rehm J. Global alcohol exposure between 1990 and 2017 and forecasts until 2030: a modelling study. Lancet 2019; 393:2493–2502. doi: 10.1016/s0140-6736(18)32744-2. [DOI] [PubMed] [Google Scholar]
- 41.Im PK, Millwood IY, Guo Y, Du H, Chen Y, Bian Z, et al. Patterns and trends of alcohol consumption in rural and urban areas of China: findings from the China Kadoorie Biobank. BMC Public Health 2019; 19:217.doi: 10.1186/s12889-019-6502-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Siegel RL, Miller KD, Sauer AG, Fedewa SA, Butterly LF, Anderson JC, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin 2020; 70:145–164. doi: 10.3322/caac.21601. [DOI] [PubMed] [Google Scholar]
- 43.Jun SY, Brown AJ, Chua NK, Yoon JY, Lee JJ, Yang JO, et al. Reduction of squalene epoxidase by cholesterol accumulation accelerates colorectal cancer progression and metastasis. Gastroenterology 2021; 160:1194–1207.e28. doi: 10.1053/j.gastro.2020.09.009. [DOI] [PubMed] [Google Scholar]
- 44.Zhang X, Zhao XW, Liu DB, Han CZ, Du LL, Jing JX, et al. Lipid levels in serum and cancerous tissues of colorectal cancer patients. World J Gastroenterol 2014; 20:8646–8652. doi: 10.3748/wjg.v20.i26.8646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poynter JN, Gruber SB, Higgins PDR, Almog R, Bonner JD, Rennert HS, et al. Statins and the risk of colorectal cancer. N Engl J Med 2005; 352:2184–2192. doi: 10.1056/NEJMoa043792. [DOI] [PubMed] [Google Scholar]
- 46.Savijärvi S, Seppä K, Malila N, Pitkäniemi J, Heikkinen S. Trends of colorectal cancer incidence by education and socioeconomic status in Finland. Acta Oncol 2019; 58:1557–1563. doi: 10.1080/0284186x.2019.1652340. [DOI] [PubMed] [Google Scholar]
- 47.Deng Y, Zhao P, Zhou L, Xiang D, Hu J, Liu Y, et al. Epidemiological trends of tracheal, bronchus, and lung cancer at the global, regional, and national levels: a population-based study. J Hematol Oncol 2020; 13:98.doi: 10.1186/s13045-020-00915-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.