Abstract
Objective
Because there is no standard treatment to control dyskinesia in anti-NMDA receptor (NMDAR) encephalitis, we analyzed the therapeutic efficacy of high-dose diazepam in dyskinesia associated with NMDAR encephalitis.
Methods
We reviewed patients with NMDAR encephalitis with dyskinesia who were admitted to Seoul National University Hospital between November 2012 and July 2018. High-dose diazepam was administered orally or via a nasogastric tube 3–6 times a day. We assessed the treatment effect by comparing dyskinesia severity between the first day of the highest dose of diazepam and one week after the treatment.
Results
Among 68 patients with NMDAR encephalitis during the study period, 33 patients were treated with enteral diazepam (ranging from 6 to 180 mg) to control dyskinesia, along with immunotherapy. The severity of dyskinesia improved from average grade 2.4 ± 0.6 to 1.1 ± 0.7 after 1 week of the highest dose of diazepam (mean severity change −1.4 ± 0.6, 95% confidence interval −1.2 to −1.6; p < 0.001). No patients had serious adverse events except for mild sedation.
Conclusions
Dyskinesia in NMDAR encephalitis improved after treatment with enteral diazepam without significant side effects. This study suggests that enteral diazepam could be a treatment option for control dyskinesia in NMDAR encephalitis.
Classification of Evidence
This study provides Class IV evidence that for patients with dyskinesias associated with NMDAR encephalitis, enteral diazepam is effective and safe in dyskinesia control.
Anti-NMDA receptor (NMDAR) encephalitis is the most common autoimmune encephalitis caused by immunoglobulin G (IgG) antibodies against the NR1 subunit of NMDARs.1–4 Although the majority of patients have good prognosis, approximately 20%–33% of them remain disabled due to incomplete recovery or organ damage.4,5 Thus, more effective treatments and various immune therapies are necessary.4,6–12
Dyskinesia is one of the most troublesome symptoms of NMDAR encephalitis.13–15 NMDARs have inhibitory effects on dopaminergic neurons in the globus pallidus via the nucleus accumbens and ventral tegmental area.16 In NMDAR encephalitis, these inhibitory actions are decreased due to downregulation of NMDARs, and dopaminergic overactivation causes hyperkinetic movement disorders.16 Severe dyskinesia causes self-injuries, falls, and sometimes rhabdomyolysis.17 It also can cause acute renal failure due to rhabdomyolysis,18 requires anesthetics or neuromuscular blockers, and sometimes contributes to mortality.17 However, there is no effective treatment to control severe dyskinesia in NMDAR encephalitis.
Diazepam has muscle relaxant properties19,20 and has been used in some spasm-dominant disorders, such as neuroleptic malignant syndrome (NMS),21 stiff-person syndrome,22–24 and tetanus.25 Except for its sedative effects, diazepam has tolerable safety and broad therapeutic range.26,27 Based on several empirical treatments, we initially thought that patients with NMDAR encephalitis with dyskinesia seem to be responsive to enteral diazepam. Thus, we aimed to know whether enteral diazepam could control dyskinesia associated with NMDAR encephalitis effectively without significant side effects.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
We performed a retrospective analysis of the NMDAR encephalitis cohort from Seoul National University Hospital. This study was approved by the Seoul National University Hospital Institutional Review Board and followed the principles of the Declaration of Helsinki. Approval from an ethical standards committee to conduct this study was received. The information of patients is sufficiently anonymized.
Patient Enrollment
We retrospectively reviewed all patients diagnosed with NMDAR encephalitis who were admitted to Seoul National University Hospital between November 2012 and July 2018 and selected the study patients who had dyskinesia and received enteral administration of diazepam for more than 1 week to relieve the symptom.
All patients were diagnosed as NMDAR encephalitis by diagnostic criteria of definite NMDAR encephalitis,28 which included rapid onset (less than 3 months) of at least one of the following major symptoms; psychiatric symptoms or cognitive dysfunction, speech dysfunction, seizures, movement disorders, decreased level of consciousness, and autonomic dysfunction or central hypoventilation, confirmed by the presence of IgG anti-GluN1 antibodies in CSF, and exclusion of other disorders.3,5,28 Autoantibodies were detected by immunostaining of rat brain sections with patient serum (1:200) and CSF (1:20).3,29 The presence of NMDAR antibody was confirmed by cell-based immunocytochemistry (Euroimmun AG, Lübeck, Germany).
Immunotherapy and Patient Management
We reviewed immunotherapy before and during the administration of diazepam. Immunotherapy was classified into first-line and second-line treatments. Traditional treatments, such as corticosteroid, IVIg, and plasmapheresis, were regarded as first-line immunotherapies, and other newer treatments, such as rituximab,6,7 tocilizumab,8,9 cyclophosphamide, bortezomib,10,11 aldesleukin,12 and mycophenolate mofetil, were regarded as second-line immunotherapies. All patients had systematic screening of cancer as anti-NMDAR encephalitis is known to be related to teratoma.1,3,30 Cancer screening was performed by chest CT, abdomen and pelvis CT, pelvis MRI, or whole-body PET.
Protocol of Diazepam Treatment
All patients received diazepam for more than 1 week. Diazepam was administered orally or via a nasogastric tube 3–6 times a day. The initial dose was chosen by the physician; then, the doses were slowly increased based on the physician's decision until the dyskinesia was controlled. After reaching the highest and effective dose, the dose was gradually tapered according to their clinical course.
Analysis of Dyskinesia and Adverse Events
The degree of dyskinesia severity was evaluated on the first day of diazepam at the highest dose, and after 1 week of the treatment by retrospective review of the medical records. We also assessed dyskinesia severity at 30 days after the diazepam started to evaluate response rates at the predefined interval after the initiation of diazepam and to know the characteristics of late responders who had no improvement in dyskinesia at 30 days.
Two expert neurologists (H.-R.S. and S.-T.L.) reviewed and graded the degree of dyskinesia. To evaluate the dyskinesia severity more objectively, we used the rating system, which is used in the Clinical Assessment Scale in Autoimmune Encephalitis (CASE), a novel scale that represents overall clinical severity in autoimmune encephalitis.31 In the CASE, the dyskinesia severity is categorized into 3 grades: grade 1 for mild degree, which does not affect daily activities, grade 2 for moderate degree, which interferes with daily activities, and grade 3 for severe degree causing secondary medical conditions such as rhabdomyolysis or self-injury.31 We additionally subclassified grade 3 patients who needed IV infusion of anesthetics or neuromuscular blockers due to severe dyskinesia as grade 3A. Rhabdomyolysis was defined as damage of skeletal muscle with elevation of serum creatine kinase (CK) above the normal range (>270 IU/L).
Clinical presentation of dyskinesia was classified into 4 groups as previous studies on movement disorders in NMDAR encephalitis13,14: (1) oromandibular, (2) limb dyskinesia (chorea/ballism), (3) catatonia/dystonia, and (4) tremor/myoclonus. Combined encephalitis symptoms other than dyskinesia were classified into another 6 groups: seizure, memory loss, psychosis, decreased mentality, autonomic dysfunction, and central hypoventilation, as described in previous studies.2,5,31
The treatment effect was assessed by comparing dyskinesia severity between the first day of treatment with the highest dose of diazepam and after 1 week of treatment. We also assessed the overall clinical severity at the same time using a modified Rankin Scale (mRS) score to compare change in dyskinesia severity with overall disease improvement.
Adverse events were defined as unintended and unfavorable events that developed during the diazepam treatment. Adverse events were collected from both medical records and laboratory tests and classified according to the Common Terminology Criteria for Adverse Events (CTCAE) v.4.03.32 Respiratory depression was defined by any increased demand of ventilator support in intubated patients.
Statistical Analysis
All results are presented as mean ± SD, number with percentage, or 95% confidence interval (CI). The paired-sample t test was performed to compare the severity of dyskinesia and mRS scores before and after treatment with diazepam, and a 2-tailed p value <0.05 was considered to be statistically significant. The analysis of variance was used to compare the highest dose of diazepam or the diazepam titration time according to the changes in dyskinesia severity. SPSS 25 was used for all statistical analyses.
Methods/Primary Research Question
Did the enteral diazepam, along with immunotherapy, improved dyskinesia associated with NMDAR encephalitis? This study provides Class IV evidence that enteral diazepam was effective and safe to control the dyskinesia of patients with NMDAR encephalitis because of its retrospective uncontrolled design.
Data Availability
Data of this study are based from electronic medical records of patients with autoimmune encephalitis in our institution. Baseline data are available to qualified investigators on request to the corresponding author.
Results
Clinical Characteristics of the Patients and Dyskinesia
Among the 68 patients with NMDAR encephalitis admitted to Seoul National University Hospital during the study period, 44 patients presented dyskinesia during their clinical course. Among the 44 patients with NMDAR encephalitis with dyskinesia, 33 patients were treated with enteral diazepam to control dyskinesia and were analyzed as the study population (figure 1, table 1, and table e-1, links.lww.com/CPJ/A224). In these 33 study patients, 27 patients (81.8%) were female, and the mean age of the patients was 25.6 ± 10.8 years (ranging from 1 to 58 years). Eighteen patients (54.5%) had teratomas: 17 had ovarian teratomas and 1 had mediastinal teratoma. All patients who were diagnosed with teratoma had tumor removal surgery. One patient was pregnant, and the pregnancy was terminated after the diagnosis of NMDAR encephalitis.
Figure 1. The Selection of Study Subjects Among the Patients With NMDAR Encephalitis.
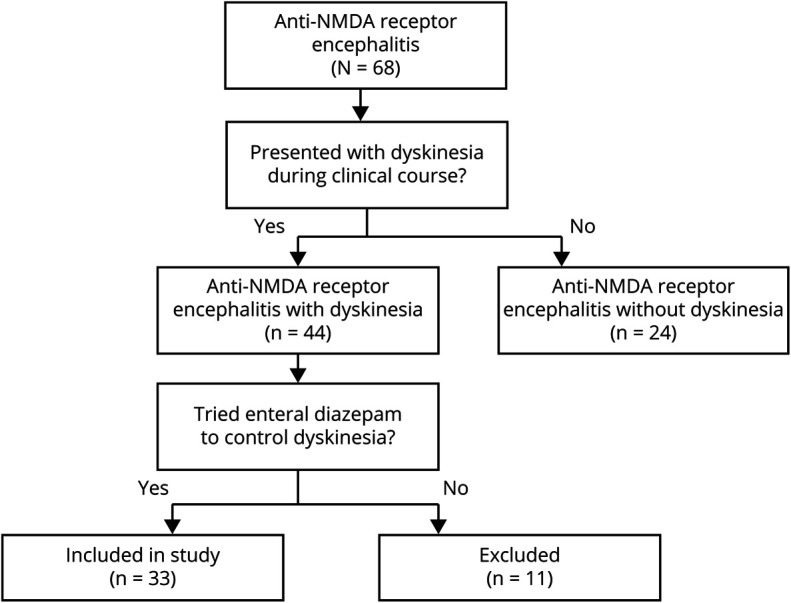
From patients with NMDAR encephalitis who were admitted to Seoul National University Hospital between November 2012 and July 2018 (N = 68), 44 patients presented dyskinesia during their clinical course. Among them, we reviewed 33 patients who had enteral diazepam to control dyskinesia. NMDAR = NMDA receptor.
Table 1.
Clinical Characteristics of the Study Patients
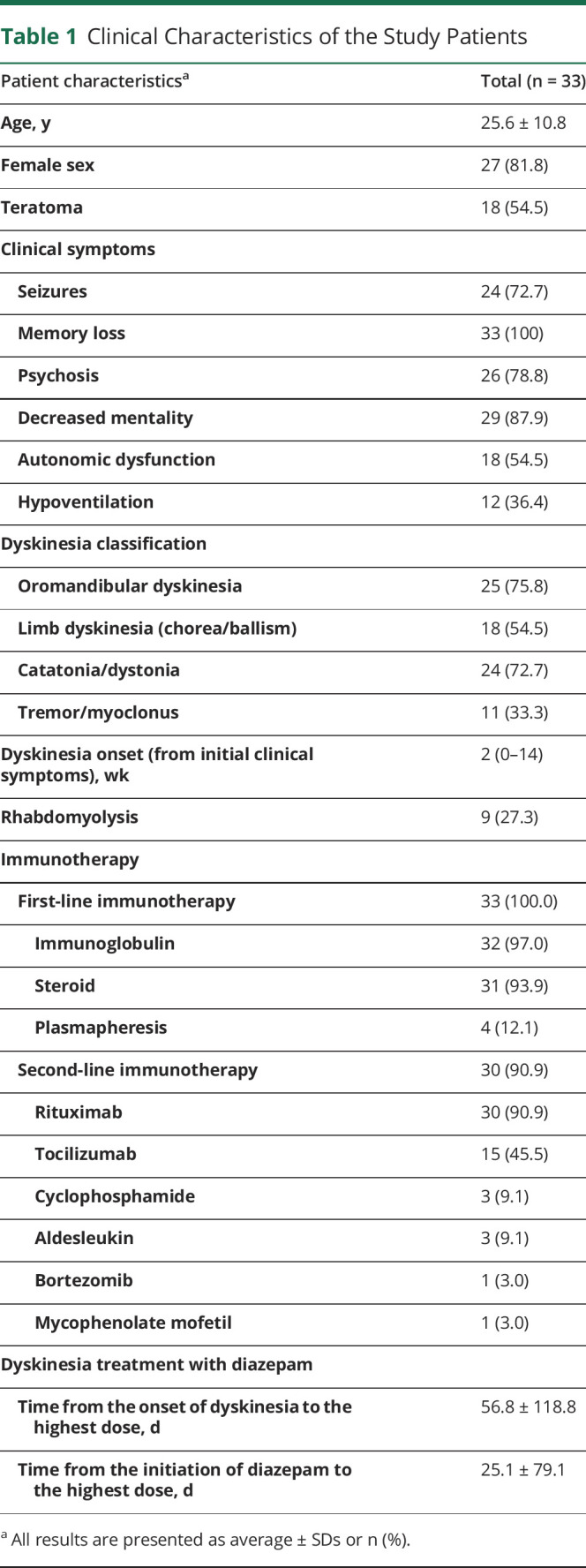
All patients had immunotherapy along with diazepam treatment, and all of them already had first-line immunotherapy when the dose of diazepam reached their highest. The average time interval from the initial NMDAR encephalitis symptom to immunotherapy was 16.2 ± 18.3 days (ranging from 4 to 70 days). Thirty patients (90.9%) also had second-line immunotherapy. In the first week of the highest dose of diazepam, 24 patients (72.7%) concomitantly received additional immunotherapy: 22 patients (66.7%) had second-line immunotherapy, 2 patients (6.1%) had high-dose IV steroid pulse, and 4 patients (12.1%) had IVIg. Among 18 patients with teratoma, 10 patients (30.3%) underwent teratoma removal surgery before the treatment with the highest dose of diazepam, and 1 had surgery during treatment with the highest dose of diazepam.
The interval from the initial NMDAR encephalitis symptom onset to dyskinesia onset was mean 2.7 ± 3.3 weeks. The patients had a variety of combined symptoms of NMDAR encephalitis (table 1), including seizures, memory loss, psychosis, decreased mentality, autonomic dysfunction, and central hypoventilation. The clinical presentation of dyskinesia included all types: oromandibular dyskinesia, limb dyskinesia (chorea/ballism), catatonia/dystonia, and tremor/myoclonus.
The dyskinesia severity when the treatment with the highest dose of diazepam started was in the range of dyskinesia grade 1 (mild) to grade 3 (severe or causing secondary medical conditions). Three patients (9.1%) had IV infusion of anesthetics or neuromuscular blockers due to intractable dyskinesia and were classified as grade 3A. Nine patients (27.3%) developed rhabdomyolysis, and the peak CK level was mean 2159.8 ± 2564.7 IU/L (ranging from 374 to 6470 IU/L) among patients who had rhabdomyolysis. None of them had NMS or status epilepticus, which could also cause rhabdomyolysis.
Therapeutic Effects of Diazepam on Dyskinesia
The detailed treatment information is described in table 1 and table e-1 (links.lww.com/CPJ/A224). The starting dose of diazepam was mean 12.8 ± 7.8 mg/d, and the highest dose was mean 38.1 ± 41.8 mg/d (ranging from 6 to 180 mg/d, in divided doses). The average time from dyskinesia onset to the initiation of diazepam was 31.7 ± 63.0 days, and the average time from dyskinesia onset to reach the highest dose was 56.8 ± 118.8 days (ranging from 0 to 575 days). The average time to reach the highest dose from the initiation of diazepam (titration duration) was 25.1 ± 79.1 days (ranging from 0 to 428 days). The majority of the patients (25 patients, 75.8%) reached their highest dose within 10 days.
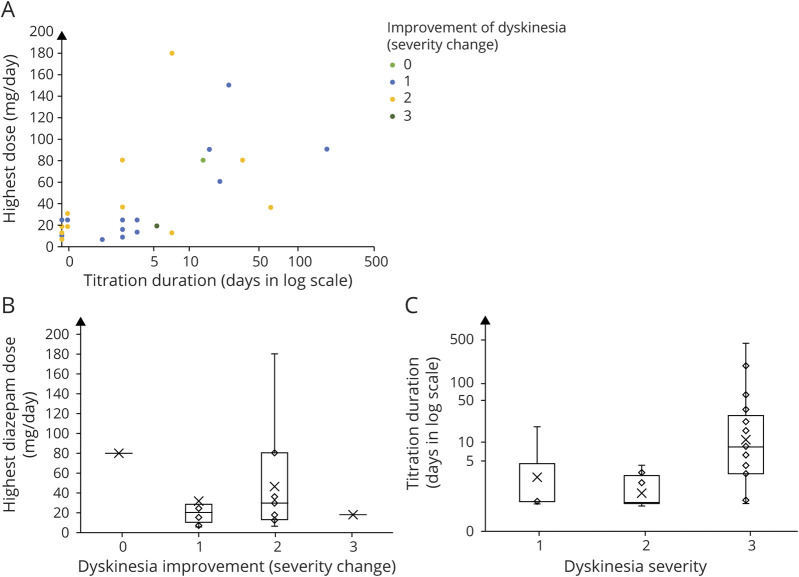
The titration schedule to reach the highest dose was highly variable and dependent on the clinical severity. In figure 2A, we visualized the association among titration duration (days), the highest dose of diazepam (mg), and the improvement of dyskinesia (severity change). For example, a 22-year-old woman with refractory dyskinesia started to use diazepam 4 mg 3 times a day, which was doubled every day for 3 days to reach 16 mg 3 times a day. The dose was increased to 20 mg 6 times a day at day 7 and finally reached the highest dose, 30 mg 6 times a day at day 8, which controlled the dyskinesia. Meanwhile, in a 50-year-old man, we started diazepam 5 mg once a day and increased it to 4 mg 3 times a day on the next day. Then, it reached the highest dose, 5 mg 3 times a day at day 5, which controlled the dyskinesia.
Figure 2. Dyskinesia Response According to the Diazepam Titration Duration and the Highest Dose of Diazepam.
(A) The scatter plot depicts the diazepam titration time, the highest dose of diazepam, and the change in dyskinesia severity. (B) We compared the highest dose of diazepam between different groups of dyskinesia severity change, and it showed no significant difference (p = 0.360). (C) The diazepam titration time to the highest dose was not varied between different dyskinesia severity groups at diazepam initiation (p = 0.890).
When we analyzed whether the dose of diazepam determines the improvement of dyskinesia, there was no difference in the highest dose of diazepam among the groups (p = 0.360) (figure 2B). The uptitration time to the highest dose was also not varied among different dyskinesia severity groups at diazepam initiation (p = 0.232) (figure 2C).
Twenty-nine patients (87.9%) maintained the highest dose of diazepam for more than a week, but 4 patients maintained the highest dose for less than a week (1, 4, 4, and 5 days each). Five patients (15.2%) also received additional drugs to control dyskinesia, such as trihexyphenidyl (anticholinergics) and baclofen (muscle relaxant), during the administration of high-dose diazepam. However, the dosage and regimen of additional drugs were not changed.
Then, we compared the severity of dyskinesia between 2 time points, at the initiation of treatment with the highest dose of diazepam and at 1 week of the treatment (figure 3). The mean severity of dyskinesia on the first day of the highest dose of diazepam was grade 2.4 ± 0.6, and after the 1-week treatment with the highest dose of diazepam, the severity improved to grade 1.1 ± 0.7 (mean severity change −1.4 ± 0.6, 95% CI −1.2 to −1.6; p < 0.001). On the first day of the highest dose of diazepam, 15 patients (45.5%) had moderate dyskinesia (grade 2), and 16 patients (48.5%) had severe one (grade 3). However, after the 1-week treatment with the highest dose of diazepam, the numbers of patients with grade 2 and 3 dyskinesia decreased to 5 (15.2%) and 1 (3.0%), respectively. The remaining 22 patients (66.7%) had only mild dyskinesia (grade 1), and 5 patients (15.2%) had no dyskinesia after the treatment. Although 1 patient still required anesthetics to control the dyskinesia (grade 3A) even after the 1 week of highest-dose diazepam administration, the dose of anesthesia required to control the dyskinesia was decreased after the treatment.
Figure 3. Changes in Dyskinesia Severity After Diazepam Treatment.
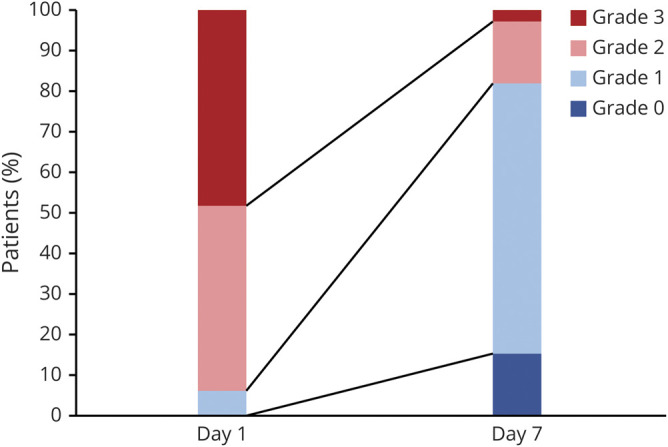
Bar graphs show the dyskinesia severity at the first day of the highest dose and at 1 week of maintenance treatment with the highest dose of diazepam. The high-dose enteral diazepam treatment significantly improved the dyskinesia (mean severity change −1.4 ± 0.6, 95% confidence interval −1.2 to −1.6; p < 0.001).
Most patients (29 patients, 87.9%) had improvement in dyskinesia severity (1 point or more) within 30 days after the initiation of diazepam (30-day responder). They reached to their highest dose of diazepam at average 25.1 ± 79.1 days. The mean severity of dyskinesia at 30 days after the initiation of diazepam was 0.9 ± 1.1, and 16 patients (48.5%) were free of dyskinesia.
In contrast, 4 patients (12.1%) showed no improvement in the dyskinesia severity in 30 days after the diazepam (30-day nonresponder). All these were female, with ovarian teratoma and with severe dyskinesia (grade 3). When we compared the clinical characteristics between the 30-day responder and nonresponder, there were no difference in the time interval from the dyskinesia onset to the initiation of diazepam (30-day responder = 18.5 ± 19.6 days, 30-day nonresponder = 127.5 ± 157.6 days, p = 0.261) or in the time interval from the NMDAR encephalitis symptom onset to the immunotherapy (30-day responder = 17.2 ± 19.3 days, 30-day nonresponder = 9.0 ± 4.2 days, p = 0.408). In addition, the highest dose of diazepam was not different between the 2 groups (30-day responder = 35.4 ± 42.7 mg/d, 30-day nonresponder = 57.5 ± 32.4 mg/d, p = 0.330).
However, the overall clinical severity of NMDAR encephalitis, measured by the mRS, had no significant change after the 1-week treatment with the highest dose of diazepam (mean mRS change −0.1 ± 0.2, 95% CI −0.2 to 0.0; p = 0.160), suggesting that the dyskinesia improvement cannot be explained by the general clinical course improvement. The majority of patients (26 patients, 78.8%) had the same mRS scores during the 1 week of treatment with the highest dose of diazepam along with immunotherapy. After the long-term follow-up over a year, the overall clinical outcome was good. Most of the patients (31 patients, 93.9%) improved their functional disability to less than mRS score 3, and all patients recovered awareness and cognition after the long-term follow-up.
Safety and Side Effects of Diazepam
During the treatment with enteral diazepam, 16 patients (48.5%) had the side effect of sedation (CTCAE grades 1–2) (table e-1, links.lww.com/CPJ/A224). However, there were no severe adverse events (CTCAE grades 3–5), such as respiratory depression, hepatotoxicity, and aggravation of other NMDAR encephalitis symptoms even in very high doses (maximum 180 mg/d). After reaching their highest dose, diazepam was gradually tapered, and the duration of maintenance ranged from 11 days to over 1 year. There were no significant side effects during the maintenance period as well.
Discussion
This study suggests enteral diazepam as a symptomatic treatment of dyskinesia in NMDAR encephalitis. Dyskinesia severity was improved after treatment with enteral diazepam during immunotherapy. In addition, enteral diazepam had no significant side effect except mild sedation, even in very high doses.
Although the mechanism of action of diazepam in the treatment of dyskinesia induced by NMDAR encephalitis is unclear, it might be related to gamma aminobutyric acid (GABA) systemic modulation33 and muscle relaxation.19,20 GABAergic neurons express high concentrations of NMDARs,16 and the clinical presentation of NMDAR encephalitis, including dyskinesia, might result from inactivation of GABAergic neurons subsequent to depression of NMDARs.5,16 Benzodiazepines, including diazepam, bind to GABAA receptors and enhance the effect of GABAergic neurons.14,33 Diazepam might control the clinical symptoms of NMDAR encephalitis–induced dyskinesia by enhancing the effect of GABA. Among various kinds of benzodiazepines, diazepam has a potential as muscle relaxant19,20 and has been proven to be effective in other diseases, which presents with muscle spasm, such as NMS,21 stiff-person syndrome,22–24 and tetanus.25 The muscle relaxant effect of diazepam might be another possible mechanism of action in dyskinesia control.
High-dose enteral diazepam can be a symptomatic treatment for dyskinesia in NMDAR encephalitis, along with immunotherapy. There have been no standard treatments or guidelines to control dyskinesia in NMDAR encephalitis so far. Because severe dyskinesia in NMDAR encephalitis causes secondary medical problems such as self-injury and rhabdomyolysis,17 additional symptomatic treatments for dyskinesia are necessary in addition to immunotherapy. Effective control of dyskinesia could reduce morbidity and mortality in patients with NMDAR encephalitis. In our cohort, many patients had severe and refractory NMDAR, having high mRS scores, and intractable symptoms.
There are several limitations in this study. This is a single-center retrospective study. The treatment protocol of enteral diazepam was not controlled, and the dyskinesia severity was graded by an unblinded reviewer. Because of the retrospective design, the dose or titration duration might be affected by confoundings, such as clinical severity, medical complications other than dyskinesia, and physician's preference or attention to dyskinesia. Especially, the highest dose was determined by the clinical responses, which might be the reason of the excellent response rate in all doses without dose-response relationship. In addition, because doses and titration schedule were highly variable, we could not confirm the dose-response or the titration speed-response relationships. Also, because all study patients received concomitant immunotherapy for NMDAR encephalitis, it is difficult to know whether dyskinesia is improved by overall clinical improvement due to immunotherapy.
However, after the 1-week treatment of highest dose of diazepam, dyskinesias improved significantly, whereas the mRS score, the overall clinical severity, did not change during the period. The majority of the patients achieved their highest dose within 10 days, suggesting that the effect of immunotherapy is limited in the improvement. In addition, we completely analyzed and selected patients who were treated with enteral diazepam to control dyskinesia in NMDAR encephalitis by reviewing all records of patients with autoimmune encephalitis in our institution. Our institution is the referral medical center in the entire national area to diagnose and treat autoimmune encephalitis. Because the relatively low prevalence of NMDAR encephalitis among general populations and appropriate treatment is crucial in the acute phase of this disease, the prospective and randomized double-blinded trials are difficult to conduct, even if it is necessary. Further clinical trials are needed in diverse patient populations before recommending diazepam for the management of severe dyskinesias in NMDAR encephalitis. We suggest that enteral diazepam could be an attractive option as a symptomatic treatment for dyskinesia in NMDAR encephalitis.
TAKE-HOME POINTS
→ Dyskinesia is one of the troublesome symptoms of NMDAR encephalitis, and there has been no symptomatic treatment to control dyskinesia in the disease.
→ We tried enteral diazepam to control dyskinesia in NMDAR encephalitis at high doses (ranging from 6 to 180 mg/d) along with immunotherapy, and the dyskinesia severity was improved.
→ The high-dose diazepam was well tolerated in NMDAR encephalitis.
Appendix. Authors
Footnotes
Class of Evidence: NPub.org/coe
Study Funding
This study was supported by the National Research Foundation of Korea (NRF) grants funded by the Ministry of Science, ICT and Future Planning, Republic of Korea (2019R1A2C1009003). H.-R. Shin and S.-T. Lee were supported by Lee SeungMoon research fund of Seoul National University Hospital (3020170130).
Disclosure
The authors report no disclosures relevant to the manuscript Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Dalmau J, Graus F. Antibody-mediated encephalitis. N Engl J Med 2018;378:840–851. [DOI] [PubMed] [Google Scholar]
- 2.Irani SR, Bera K, Waters P, et al. N-methyl-d-aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain 2010;133:1655–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Irani SR, Vincent A. NMDA receptor antibody encephalitis. Curr Neurol Neurosci Rep 2011;11:298–304. [DOI] [PubMed] [Google Scholar]
- 4.Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol 2013;12:157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol 2008;7:1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dale RC, Brilot F, Duffy LV, et al. Utility and safety of rituximab in pediatric autoimmune and inflammatory CNS disease. Neurology 2014;83:142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee WJ, Lee ST, Byun JI, et al. Rituximab treatment for autoimmune limbic encephalitis in an institutional cohort. Neurology 2016;86:1683–1691. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka T, Narazaki M, Kishimoto T. Anti-interleukin-6 receptor antibody, tocilizumab, for the treatment of autoimmune diseases. FEBS Lett 2011;585:3699–3709. [DOI] [PubMed] [Google Scholar]
- 9.Lee WJ, Lee ST, Moon J, et al. Tocilizumab in autoimmune encephalitis refractory to rituximab: an institutional cohort study. Neurotherapeutics 2016;13:824–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scheibe F, Prüss H, Mengel AM, et al. Bortezomib for treatment of therapy-refractory anti-NMDA receptor encephalitis. Neurology 2017;88:366–370. [DOI] [PubMed] [Google Scholar]
- 11.Shin YW, Lee ST, Kim TJ, Jun JS, Chu K. Bortezomib treatment for severe refractory anti-NMDA receptor encephalitis. Ann Clin Transl Neurol 2018;5:598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim JA, Lee ST, Moon J, Jun JS, Chu K. New feasible treatment for refractory autoimmune encephalitis: low-dose interleukin-2. J Neuroimmunol 2016;299:107–111. [DOI] [PubMed] [Google Scholar]
- 13.Varley JA, Webb AJS, Balint B, et al. The Movement disorder associated with NMDAR antibody-encephalitis is complex and characteristic: an expert video-rating study. J Neurol Neurosurg Psychiatry 2019;90:724–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohammad SS, Fung VS, Grattan-Smith P, et al. Movement disorders in children with anti-NMDAR encephalitis and other autoimmune encephalopathies. Mov Disord 2014;29:1539–1542. [DOI] [PubMed] [Google Scholar]
- 15.Rubio-Agustí I, Dalmau J, Sevilla T, Burgal M, Beltrán E, Bataller L. Isolated hemidystonia associated with NMDA receptor antibodies. Mov Disord 2011;26:351–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masdeu JC, Dalmau J, Berman KF. NMDA receptor internalization by autoantibodies: a reversible mechanism underlying psychosis? Trends Neurosci 2016;39:300–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim JA, Lee ST, Kim TJ, et al. Frequent rhabdomyolysis in anti-NMDA receptor encephalitis. J Neuroimmunol 2016;298:178–180. [DOI] [PubMed] [Google Scholar]
- 18.Bosch X, Poch E, Grau JM. Rhabdomyolysis and acute kidney injury. N Engl J Med 2009;361:62–72. [DOI] [PubMed] [Google Scholar]
- 19.Kita M, Goodkin DE. Drugs used to treat spasticity. Drugs 2000;59:487–495. [DOI] [PubMed] [Google Scholar]
- 20.Friend DG. Pharmacology of muscle relaxants. Clin Pharmacol Ther 1964;5:871–878. [Google Scholar]
- 21.Kontaxakis VP, Christodoulou GN, Markidis MP, Havaki-Kontaxaki BJ. Treatment of a mild form of neuroleptic malignant syndrome with oral diazepam. Acta Psychiatr Scand 1988;78: 396–398. [DOI] [PubMed] [Google Scholar]
- 22.Meinck HM, Thompson PD. Stiff man syndrome and related conditions. Mov Disord 2002;17:853–866. [DOI] [PubMed] [Google Scholar]
- 23.Howard FM. A new and effective drug in the treatment of the stiff-man syndrome: preliminary report. Proc Staff Meet Mayo Clin 1963;38:203–212. [PubMed] [Google Scholar]
- 24.Cohen L. Stiff-man syndrome. JAMA 1966;195:222. [DOI] [PubMed] [Google Scholar]
- 25.Okoromah CAN, Lesi AFE. Diazepam for treating tetanus. Cochrane Database of Systematic Reviews 2004, Issue1.Art.No.:CD003954. DOI: 10.1002/14651858.CD003954.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calcaterra NE, Barrow JC. Classics in chemical neuroscience: diazepam (Valium). ACS Chem Neurosci 2014;5:253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riss J, Cloyd J, Gates J, Collins S. Benzodiazepines in epilepsy: pharmacology and pharmacokinetics. Acta Neurol Scand 2008;118:69–86. [DOI] [PubMed] [Google Scholar]
- 28.Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol 2016;15:391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lancaster E, Lai M, Peng X, et al. Antibodies to the GABA(B) receptor in limbic encephalitis with seizures: case series and characterisation of the antigen. Lancet Neurol 2010;9:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tüzün E, Zhou L, Baehring JM, Bannykh S, Rosenfeld MR, Dalmau J. Evidence for antibody-mediated pathogenesis in anti-NMDAR encephalitis associated with ovarian teratoma. Acta Neuropathol 2009;118:737–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim JA, Lee ST, Moon J, et al. Development of the clinical assessment scale in autoimmune encephalitis. Ann Neurol 2019;85:352–358. [DOI] [PubMed] [Google Scholar]
- 32.National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAEv5.0). Bethesda: Cancer Therapy Evaluation Program (CTEP); 2018. Available at: ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50. Accessed March 9, 2018. [Google Scholar]
- 33.Walters RJ, Hadley SH, Morris KD, Amin J. Benzodiazepines act on GABAA receptors via two distinct and separable mechanisms. Nat Neurosci 2000;3:1274–1281. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data of this study are based from electronic medical records of patients with autoimmune encephalitis in our institution. Baseline data are available to qualified investigators on request to the corresponding author.