Abstract
Objective
To conduct a pilot randomized controlled trial to determine whether participation in a group-based structured telehealth intervention increases physical activity in people with multiple sclerosis (MS).
Methods
In this parallel-arms trial, all study procedures were administered remotely. Adults diagnosed with MS (any subtype) were randomized to one of two 12-week (1 h/wk) active conditions: eFIT, online moderated structured groups; or eJournal, online independent journaling. For comparison, a treatment-as-usual (TAU; i.e., no eFIT/eJournal) group was enrolled. The primary outcome was feasibility (completion and adherence). The secondary efficacy outcomes included self-reported physical activity level (International Physical Activity Questionnaire, IPAQ).
Results
Participants were 37 adults with MS. The sample was diverse: 66.7% female; age range 23–64 years; 17.5% Hispanic, 12.5% Black; and progressive and relapsing-remitting disease subtypes. Regarding feasibility, 70.7% completed; average adherence was 74.9%. Physical activity in active groups increased by 34.2% (baseline IPAQ = 2,406.8 ± 1,959.7, follow-up = 3,229.4 ± 2,575.2) and decreased in the TAU group by 17.4% (baseline = 2,519.9 ± 1,500.1, follow-up = 2,081.2 ± 1,814.9); group × time interaction was not statistically significant [F(2,25) = 1.467, p = 0.250; partial η2 = 0.105].
Conclusions
Telehealth represents an accessible, acceptable vehicle to deliver targeted behavioral treatments to a neurologic population. eFIT may be an effective intervention for increasing physical activity, a historically intractable treatment target, in individuals with MS. In addition, these results provide evidence for feasibility of conducting fully remote clinical trial research.
Classification of Evidence
This study provides Class II evidence that for people with MS, participation in a group-based structured telehealth intervention compared with TAU resulted in a (non-significant) increase in self-reported physical activity level. The percentage of participants who completed follow-up questionnaires did not differ between groups. The trial was registered at ClinicalTrials.gov (NCT03829267).
Physical inactivity and sedentary behavior increase the risk of dying and lead to comorbidities, including obesity, type II diabetes, and some types of cancer.1 Growing evidence demonstrates the benefits of exercise on multiple levels, e.g., physical, cognitive, and psychological. Finding ways to motivate sustained participation in physical activity is a critical public health priority.2
Individuals with multiple sclerosis (MS) are at increased risk of sedentary behaviors due to common symptoms (e.g., physical disability, fatigue, and depression) that may deter engagement in exercise and physical activity. In addition, exercise is avoided by many because of overheating and exhaustion (i.e., Uhthoff phenomenon).3 However, the benefits of exercise for people with MS are manifold: increased muscle strength,4 improved balance,5 decreased fatigue6 and depressive symptoms,7 improved memory,8 and enhanced overall quality of life.9 Preclinical and clinical work suggest a possible disease-modifying effect of exercise.10 Finding ways to increase exercise participation in this population will deliver direct benefits on numerous levels.
Previously, we demonstrated feasibility and efficacy of a telehealth-delivered group-based 12-week intervention to reduce loneliness and depression in MS.11 In the present trial, participants were randomized to receive either eFIT or an active control condition, eJournal, involving 1-hour weekly structured online activities. A treatment-as-usual (TAU; i.e., no eFIT or eJournal) group (recruited separately) completed baseline and follow-up surveys. The primary outcome was feasibility (adherence and completion); the secondary outcome was efficacy, i.e., change in self-reported physical activity level from baseline to follow-up. The objective was to determine feasibility of the 12-week eFIT program, a telehealth, group-based targeted intervention to increase physical activity in people with MS.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
This study was approved by the Institutional Review Board at Columbia University Irving Medical Center (CUIMC). Informed consent was obtained from all participants via eConsent. The trial was registered at ClinicalTrials.gov (NCT03829267).
Study Design
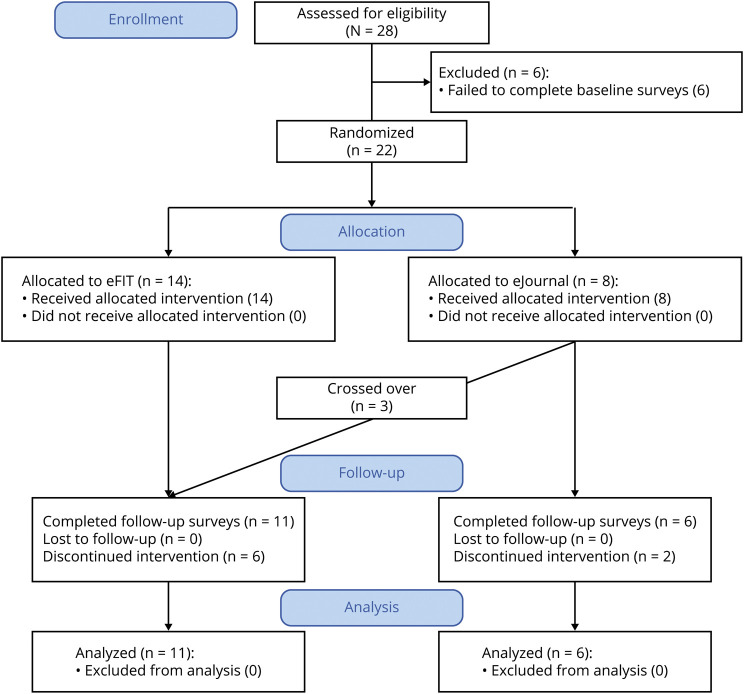
A phase I/II randomized clinical feasibility trial of a telehealth group-based structured behavioral intervention to increase physical activity (eFIT) with an active control condition (eJournal) was conducted (figure 1, depicting the flow diagram for active participants); a TAU (i.e., no eFIT or eJournal) group served as an inactive control condition. The primary research questions were as follows: (1) Is a group-based telehealth behavioral intervention to increase physical activity in MS feasible? and (2) Does participation in a group-based telehealth behavioral intervention in MS result in increased physical activity? These questions have been assigned a Class II classification of level of evidence, based on the pilot feasibility study design presented herein.
Figure 1. Flow Diagram for Active Participants of eFIT Intervention (n = 28).
Note that a treatment-as-usual group (N = 16) was recruited separately.
Remotely Delivered Clinical Trial Design Elements
Consistent with prior telehealth group-based interventions conducted by our group,11 all study procedures were conducted remotely. Baseline and follow-up surveys (including outcomes) were deployed using REDCap, electronic data capture software used for research databases and developed for secure remote data capture. The intervention was also conducted remotely, using zoom video-link software. All participants completed 12 weeks of internet-based activities for 1 h/wk. eFIT participants joined private facilitated video-link groups; the group size was limited to 6. eJournal participants completed independent weekly online structured journaling via REDCap that included no content related to accountability and no social aspect.
Participants
Participants were recruited into an internet-based intervention to increase physical activity. Recruitment and enrollment took place at CUIMC from April to December 2019. Inclusion criteria were as follows: participants were adults (aged ≥18 years) with a diagnosis of MS (based on the McDonald criteria12). The sample size was based on a prior feasibility trial of a telehealth group-based behavioral intervention conducted by our group.11 A total of 38 participants were enrolled. Of these, 22 were enrolled in the active portion of the study (eFIT or eJournal) and were allocated 1:1 via random number generator to condition. To prevent selection bias, allocation concealment was maintained in the following way: the allocation sequence was generated by a member of the study team (V.M.L.), and participant assignment to the intervention group was made by the research coordinator (S.B.). Despite this, 3 participants assigned to eJournal learned of the group-based intervention arm and requested to cross over to eFIT. These participants therefore completed both conditions, yielding a final active sample of 24. A separate sample of 16 participants were enrolled in a survey-only condition (TAU), in which baseline and follow-up surveys were administered at the same time points.
Safety
In week 1 of the active intervention, all participants completed the Physical Activity Readiness Questionnaire to ensure safety. At no point during the intervention was any specific program of exercise endorsed or required; instead, participants were encouraged to develop their own program of physical activities based on individual physical limitations or pragmatic considerations. The word exercise was omitted from recruitment materials; participants were invited to join the study on the basis of sharing an expressed goal to “increase physical activity.”
Treatment Intervention
I. eFIT. Each week, participants met in a group with a trained coach who guided them through accountability-building exercises and psychoeducational modules (presented via PowerPoint slides) focusing on benefits of physical activity for individuals with MS (e.g., exercise and fatigue, exercise and cognition, and exercise and mood). Their coach worked with them to set attainable goals and use accountability. In addition to receiving training in accountability on a conceptual level, participants learned logistics of incorporating accountability partners into their own lives beyond the group, while modeling accountability within the group. Safety was a key theme of all modules. Study personnel had access to an exercise physiologist (N.L.) to answer any questions/address concerns throughout the trial.
II. eJournal. Participants in the eJournal condition received a link each week to an online structured journaling activity that they were required to engage in for an hour. In this condition, participants received the same psychoeducational modules, although their materials omitted the accountability content. eJournal prompts that aligned with the modules were provided, e.g., “Reflect on what it would take for you to make a lasting change in your life with regard to physical activity.”
Primary Outcomes
Feasibility outcomes were evaluated as follows: completion was defined as submission of completed REDCap surveys at 12-week follow-up; adherence (active treatment groups only) was defined as number of eFIT sessions attended or eJournal entries completed. Both outcomes were assessed after completion of the 12-week intervention.
Efficacy measures were administered via REDCap at baseline and follow-up. The primary efficacy outcome was self-reported physical activity quantified as total metabolic equivalents (MET) minutes score on the International Physical Activity Questionnaire (IPAQ). The secondary efficacy outcomes were loneliness (total score, UCLA Loneliness Scale) and depression (total score, Patient Health Questionnaire, PHQ-9 [8-item version, omitting the suicidality item which would not be appropriate for inclusion in a remote survey, for safety reasons]).13
Statistical Analysis
For efficacy, we used linear mixed factor analysis of variance to evaluate the effect of the intervention over time on primary and secondary efficacy outcomes. Group (eFIT, eJournal, and TAU) and change in physical activity (IPAQ total MET minutes: T2 − T1) were the between-subject variables, and time was the repeated-measures variable. Partial eta squared was calculated for observed effects. The same statistical approach was used to evaluate secondary efficacy outcomes of loneliness (UCLA Loneliness Scale) and depression (PHQ-9).
Data Availability
Data will be shared with qualified researchers upon reasonable request.
Results
Sample Demographics
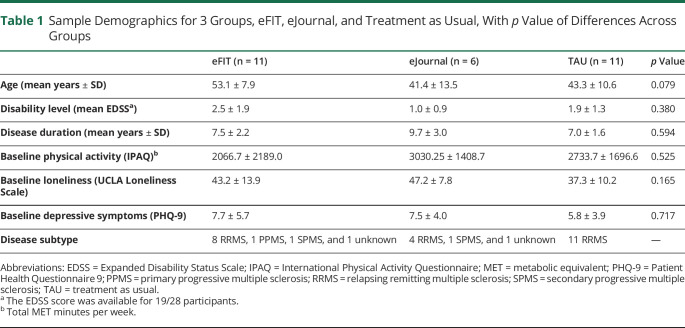
The sample was 66.7% female; age range: 23–64 years (mean 44.1 ± 11.3); racial/ethnic composition: 65% Caucasian, 17.5% Hispanic, and 12.5% Black. Disease phenotypes included 2 primary progressive, 2 secondary progressive, and the remainder relapsing-remitting MS. Disability as measured with the Expanded Disability Status Scale ranged from 0 to 6.0; the mean EDSS score did not differ across groups. Disease duration ranged from 1 to 19 years; mean disease duration did not differ across groups. Clinical variables are provided in table 1, as well as demographics for the 3 groups (eFIT, eJournal, and TAU), and baseline levels of physical activity, loneliness, and depression.
Table 1.
Sample Demographics for 3 Groups, eFIT, eJournal, and Treatment as Usual, With p Value of Differences Across Groups
Feasibility
In the active group, 21 participants completed initial surveys and began the intervention; 3 crossed over from eJournal to eFIT at their request, yielding an active sample of 24. Of these, 17 completed the intervention: 11/17 (70.8%) in eFIT; 6/8 (69%) in eJournal. In the TAU group, 11/16 (68.8%) participants completed follow-up surveys. Overall adherence across active groups was 74.9% (eFIT, 69.3%; eJournal, 80.5%).
Efficacy
Comparing change in physical activity across all 3 groups (eFIT, eJournal, and TAU) revealed a main effect of group that failed to reach the level of significance despite a large effect size [F(2,25) = 2.031, p = 0.152; partial η2 = 0.140]. The interaction of group × time [F(2,25) = 1.467, p = 0.250; partial η2 = 0.105] was also nonsignificant but showed a medium effect size. Participants in both active conditions showed increased physical activity from baseline to follow-up: in eFIT, a 20.2% increase (baseline 2,066.7 ± 2,189.0, follow-up 2,484.1 ± 1,933.5) and in eJournal, a 51.7% increase (baseline 3,030.3 ± 1,408.7, follow-up 4,595.83 ± 3,206.5). The combined active groups (eFIT + eJournal) showed an average increase by 34.2% (baseline IPAQ = 2,406.8 ± 1,959.7, follow-up = 3,229.4 ± 2,575.2). In contrast, the TAU group showed a 17.4% decrease (baseline: 2,519.86 ± 1,500.1, follow-up: 2,081.2 ± 1,814.9; figure 2). Note that when the crossover participants were excluded from analyses, the pattern of results was unchanged. This was also the case when crossover participant data were included for their first condition (eJournal) only. Although the group × time interaction was not statistically significant in this small pilot study, the pattern of findings is encouraging insofar as it points to a benefit of participation for increasing physical activity and helps to refine power calculation for a phase III trial.
Figure 2. Efficacy Outcomes: Results of Repeated Measures ANOVA Comparing 3 Groups, eFIT, eJournal, and TAU, on Pre- and Post-physical Activity Level (IPAQ, Total MET Minutes).
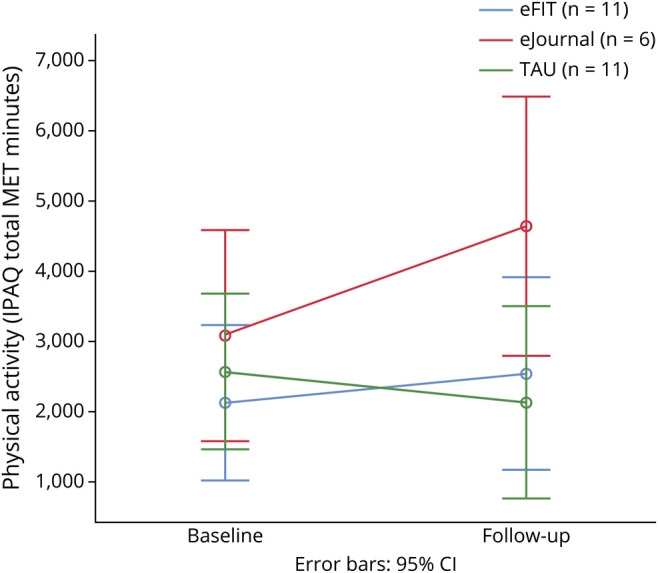
ANOVA = analysis of variance; CI = confidence interval; IPAQ = International Physical Activity Questionnaire; MET = metabolic equivalent; TAU = treatment as usual.
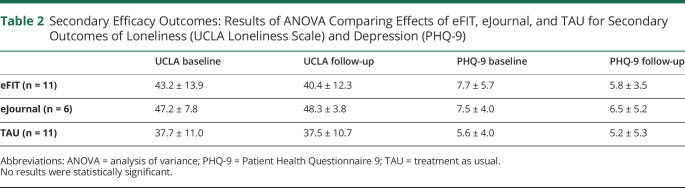
Regarding secondary outcomes, none of the results reached the level of statistical significance. However, the eFIT group showed decreased loneliness at follow-up, whereas eJournal had worse loneliness and TAU was unchanged at follow-up [F(2,25) = 0.702, p = 0.505, partial η2 = 0.053]. All 3 groups showed decreased depression at follow-up, [F(2,25) = 0.424, p = 0.659, partial η2 = 0.033]; please see table 2.
Table 2.
Secondary Efficacy Outcomes: Results of ANOVA Comparing Effects of eFIT, eJournal, and TAU for Secondary Outcomes of Loneliness (UCLA Loneliness Scale) and Depression (PHQ-9)
Qualitative Findings
Anecdotal feedback from eFIT participants was conveyed in the groups. At the conclusion of the intervention, feedback from eFIT participants was overwhelmingly positive: 100% of participants expressed a desire to continue meeting with their groups. Participants of eFIT reported finding the group-based format beneficial for learning how others with MS use technology to support their physical activity goals. Others enjoyed the structured activities in eFIT such as the coach-guided psychoeducational modules; particular favorites included a module on sleep hygiene (1 member began keeping a sleep diary during the trial). One participant noted that reflecting on personal goals with the group was motivating. There were unanticipated positive developments. One member joined eFIT meetings while riding his stationary cycle with an iPad propped on the handles. He enjoyed the company of the group; the group found it motivating to join him while he exercised. Perhaps the most impactful group experience was the participant who shared that he had not walked independently for years until the past week when he walked in the swimming pool in his MS aquatics class: “My family said they never saw me smile that big.” When asked whether eFIT was the reason he joined the class, he responded, “It was something I was planning to do for a long time, but the group got me there quicker than I would've on my own.”
Discussion
These results provide evidence for the feasibility of conducting a completely remote RCT of a targeted behavioral intervention in a neurologic population. Participation in a structured, 12-week telehealth intervention of eFIT resulted in a (non-statistically significant) increase in self-reported physical activity for individuals with MS, a clinical population at elevated risk of sedentary behaviors and decreased engagement in physical activity. Exercise is beneficial for people with MS; however, a necessary precondition for engaging in exercise is will and sufficient motivation to initiate behavior. Finding ways of sustaining motivation until a pattern of behavior is established is the best way to incorporate meaningful change. As eusocial animals, we are driven by sociobiologically programmed behaviors such as competition, cooperation, and attraction.14 Accountability leverages innate social mechanisms to incite behavior change; put simply, by expressing goals outwardly, we become more likely to achieve them. This concept is the foundational bedrock underlying one of the best known behavioral interventions, Alcoholics Anonymous.15 For our study, we developed a structured group-based accountability telehealth intervention targeting physical activity in adults with MS called eFIT. Small groups of individuals with a shared goal of increasing physical activity were brought together in 1-hour weekly private, facilitated, video-link group meetings for 12 weeks. Groups were led by a trained moderator who provided structured content in the form of psychoeducational modules focusing on benefits of exercise tailored to MS, tools for engaging accountability partners, and activities to model accountability. Positive results of this trial support the social element of the group-based format as a potential mechanism underlying favorable results of targeted behavioral interventions.
Telehealth is rapidly taking its place as a scalable, accessible, and acceptable modality for the delivery of tailored treatments to clinical populations. Recent internet-based interventions to increase physical activity have been successfully implemented in MS,16–19 but these studies took an approach that was passive, with participants viewing online materials in self-directed programs. Nonetheless, psychoeducational materials delivered to patients with MS via telehealth did result in increased physical activity in a prior study,18 consistent with our findings of increased physical activity with a similar (medium) effect size albeit not statistically significant. MS researchers have highlighted the need for future telehealth approaches with an interactive component.20 The reliance on peer accountability that constitutes the foundation of eFIT leverages social motivational tools such as cooperation, competition, and accountability to strengthen the adoption of healthy behaviors in individuals with a chronic neurologic condition for whom exercise holds many benefits.21,22 Structured content was provided to promote adoption and maintenance of healthy exercise behaviors; accountability partner training was provided to support sustainability of behavior change beyond the 12-week intervention period. Future work to evaluate sustainability of benefits of eFIT is warranted.
Several strengths of the study should be highlighted. We did not limit our sample to patients with lower levels of disability or nonprogressive phenotypes. Because there was no specified program of exercise but rather participants were supported in developing their own safe, customized program, we were able to safely include individuals with all disease subtypes and all levels of disability. Anecdotally, wheelchair-dependent patients expressed high overall satisfaction with the intervention as did fully mobile individuals. Moreover, the focus of eFIT increased the acceptability of our treatment: individuals were able to connect with a supportive community in a way that they may have resisted in the past. One participant stated: “I'm not a support group type person, but this appealed to me because the focus is on physical activity.” Another participant reported eFIT as the first time in her 12-years since being diagnosed that she had ever spoken with other people who have MS. Given findings of decreased loneliness and depression in the active groups, our results are encouraging and suggest that benefits of eFIT are seen on multiple levels.
An additional strength is the diversity and heterogeneity of our sample, supporting the results as more generalizable: the gender of our sample was 33.3% male, age ranged from 23 to 64 years, race/ethnicity included 17.5% Hispanic and 12.5% Black, the EDSS score ranged from 0 to 6.0, and disease phenotype included relapsing-remitting, secondary progressive, and primary progressive patients. This diversity bolsters acceptability, accessibility, and generalizability of the benefits gained from eFIT and for telehealth interventions more generally for many individuals with MS, not just those who are younger, have lower levels of disability, and are Caucasian, i.e., those patients who currently constitute the overwhelming majority of participants represented in the MS exercise literature.23
Finally, our trial provides proof-of-concept evidence for feasibility of conducting clinical trial research using completely remote methods. It also highlights the feasibility of developing targeted treatments that can be deployed via telehealth, with preliminary efficacy evidence that provides encouragement for a shift in prioritizing telehealth as a novel vehicle for treatment delivery.
Our study has limitations. Although a medium effect size for increase in physical activity after participating in the active intervention was shown, failure to reach statistical significance was likely the result of insufficient power in this pilot study. A large-scale phase III trial to establish efficacy is warranted; such a study should also include an objective measure of physical activity, e.g., accelerometer, in addition to the subjective measure used here. Also, given that both eFIT and eJournal groups showed (nonsignificant) increases in physical activity, an intervention combining elements of both may be optimal. In addition, although baseline differences across groups did not reach statistical significance here, the eFIT group was older (>11 years) and had much lower physical activity level (∼32%) at baseline than the eJournal group. Although it is unclear how or whether this affected results, a large-scale follow-up trial should use stratified sampling to ensure well-matched groups. Finally, adequate control for level of participation (i.e., time, quality, and effort) across active groups of the intervention (eFIT and eJournal) is an area for improvement in future studies. Although participants in the eJournal condition were asked to engage in independent journaling activities for 1 h/wk (consistent with time commitment of eFIT participants), future studies should incorporate digital monitoring tools as a step toward ensuring equivalent effort across conditions.
A recent consensus article highlighted key priorities for MS exercise research, one of which was the need for strategies to support adherence.24 In line with this, we propose eFIT as an accountability-based telehealth solution leveraging the strength of social support as an effective strategy to support adherence to exercise by encouraging initiation and maintenance of healthy exercise behaviors for individuals with MS.
Acknowledgment
The authors thank the individuals with MS who participated in the trial for their enthusiastic support of this research.
Appendix. Authors
Footnotes
Class of Evidence: NPub.org/coe
Infographic: http://links.lww.com/CPJ/A312
Study Funding
The study was not funded directly from any outside source. As a NeuroNEXT fellow, Dr. Leavitt received funding from the National Institutes of Health (NINDS), 5U24NS107168-02.
Disclosure
V.M. Leavitt has received grant funding from the National Institutes of Health, the Department of Defense Congressionally Directed Medical Research Programs, and the National Multiple Sclerosis Society and has received compensation for consulting from Healios, LLC. She is the cofounder and Chief Scientific Officer of eSupport Health, PBC. C.S. Riley reports consulting or advisory work with Biogen Idec, Celgene, Genentech/Roche, Genzyme, and TG Therapeutics. P. De Jager is on the advisory board for the following: Celgene, Roche, Biogen, and Genzyme, and has sponsored research agreements with Roche and Biogen. I. Aguerre, N. Lee, and S. Bloom report no disclosures relevant to the manuscript. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Thorp AA, Owen N, Neuhaus M, Dunstan DW. Sedentary behaviors and subsequent health outcomes in adults. Am J Prev Med 2011;41:207–215. [DOI] [PubMed] [Google Scholar]
- 2.U.S. Department of Health and Human Services. Physical Activity Guidelines for Americans. 2nd ed. Washington; 2018. Available at: https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf. Accessed February 1, 2021. [Google Scholar]
- 3.Leavitt VM, Blanchard AR, Guo C-Y, Gelernt E, Sumowski JF, Stein J. Aspirin is an effective pretreatment for exercise in multiple sclerosis: a double-blind randomized controlled pilot trial. Mult Scler J 2018;24:1511–1513. [DOI] [PubMed] [Google Scholar]
- 4.Petajan JH, Gappmaier E, White AT, Spencer MK, Mino L, Hicks RW. Impact of aerobic training on fitness and quality of life in multiple sclerosis. Ann Neurol 1996;39:432–441. [DOI] [PubMed] [Google Scholar]
- 5.Gunn H, Markevics S, Haas B, Marsden J, Freeman J. Systematic review: the effectiveness of interventions to reduce falls and improve balance in adults with multiple sclerosis. Arch Phys Med Rehabil 2015;96:1898–1912. [DOI] [PubMed] [Google Scholar]
- 6.Alvarenga-Filho H, Sacramento PM, Ferreira TB, et al. Combined exercise training reduces fatigue and modulates the cytokine profile of T-cells from multiple sclerosis patients in response to neuromediators. J Neuroimmunol 2016;293:91–99. [DOI] [PubMed] [Google Scholar]
- 7.Cooney GM, Dwan K, Greig CA, et al. Exercise for depression. Cochrane Database Syst Rev 2013;9:CD004366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leavitt VMM, Cirnigliaro C, Cohen A, et al. Aerobic exercise increases hippocampal volume and improves memory in multiple sclerosis: preliminary findings. Neurocase 2014;20:695–697. [DOI] [PubMed] [Google Scholar]
- 9.Motl RW. Benefits, safety, and prescription of exercise in persons with multiple sclerosis. Expert Rev Neurother 2014;14:1429–1436. [DOI] [PubMed] [Google Scholar]
- 10.Dalgas U, Stenager E. Exercise and disease progression in multiple sclerosis: can exercise slow down the progression of multiple sclerosis? Ther Adv Neurol Disord 2012;5:81–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leavitt V, Riley C, De Jager P; eSupport Bloom S. Feasibility trial of telehealth support group participation to reduce loneliness in MS. Mult Scler 2020;26:1797–1800. [DOI] [PubMed] [Google Scholar]
- 12.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011;69:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amtmann D, Kim J, Chung H, et al. Comparing CESD-10, PHQ-9, and PROMIS depression instruments in individuals with multiple sclerosis. Rehabil Psychol 2014;59:220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson EO. Sociobiology. Abridged. Cambridge: The Belknap Press of Harvard University Press; 1980. [Google Scholar]
- 15.Kelly JF, Greene MC, Bergman BG. Recovery benefits of the “therapeutic alliance” among 12-step mutual-help organization attendees and their sponsors. Drug Alcohol Depend 2016;162:64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dlugonski D, Motl RW, McAuley E. Increasing physical activity in multiple sclerosis: replicating internet intervention effects using objective and self-report outcomes. J Rehabil Res Dev 2011;48:1129–1136. [DOI] [PubMed] [Google Scholar]
- 17.Dlugonski D, Motl RW, Mohr DC, Sandroff BM. Internet-delivered behavioral intervention to increase physical activity in persons with multiple sclerosis: sustainability and secondary outcomes. Psychol Health Med 2012;17:636–651. [DOI] [PubMed] [Google Scholar]
- 18.Motl RW, Dlugonski D, Wójcicki TR, McAuley E, Mohr DC. Internet intervention for increasing physical activity in persons with multiple sclerosis. Mult Scler 2011;17:116–128. [DOI] [PubMed] [Google Scholar]
- 19.Pilutti LA, Dlugonski D, Sandroff BM, Klaren R, Motl RW. Randomized controlled trial of a behavioral intervention targeting symptoms and physical activity in multiple sclerosis. Mult Scler 2014;20:594–601. [DOI] [PubMed] [Google Scholar]
- 20.Motl RW, Sandroff BM, Wingo BC, et al. Phase-III, randomized controlled trial of the behavioral intervention for increasing physical activity in multiple sclerosis: project BIPAMS. Contemp Clin Trials 2018;71:154–161. [DOI] [PubMed] [Google Scholar]
- 21.Backus D. Increasing physical activity and participation in people with multiple sclerosis: a Review. Arch Phys Med Rehabil 2016;97(suppl 7):S210–S217. [DOI] [PubMed] [Google Scholar]
- 22.Sasaki J, Motl R, Cutter G, Marrie R, Tyry T, Salter A. National estimates of self-reported sitting time in adults with multiple sclerosis. Mult Scler J Exp Transl Clin 2018;4:205521731875436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barclay A, Paul L, MacFarlane N, McFadyen AK. The effect of cycling using active-passive trainers on spasticity, cardiovascular fitness, function and quality of life in people with moderate to severe Multiple Sclerosis (MS); a feasibility study. Mult Scler Relat Disord 2019;34:128–134. [DOI] [PubMed] [Google Scholar]
- 24.Dalgas U, Hvid LG, Kwakkel G, et al. Moving exercise research in multiple sclerosis forward (the MoXFo initiative): developing consensus statements for research. Mult Scler J 2020;26:1303–1308. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be shared with qualified researchers upon reasonable request.