Abstract
Purpose of Review
In 2019, over 50 million Americans were expected to use wearables at least monthly. The technologies have varied capabilities, with many designed to monitor health conditions. We present a narrative review to raise awareness of wearable technologies that may be relevant to the field of neurology. We also discuss the implications of these wearables for our patients and briefly discuss issues related to researching new wearable technologies.
Recent Findings
There are a variety of wearables for neurologic conditions, e.g., stroke (for potential arrhythmia capture), epilepsy, Parkinson disease, and sleep. Research is being performed to capture the risk of neuropsychiatric relapse. However, data are limited and adherence to these wearables is often poorly studied.
Summary
The care of neurology patients may ultimately be improved with the use of wearable technologies. More research needs to examine efficacy and implementation strategies.

As of 2019, an estimated one quarter of US adults, 56.7 million people, use a wearable device at least monthly.1 The question arises, “How can neurologists who treat varied neurologic conditions as well as psychiatric comorbidities better understand these evolving technologies and consider integrating them into clinical practice?” Consider, for example, a neurologist treating a patient with migraine knows that because migraine is highly disabling,2 some patients will report a sedentary lifestyle and spend much time in bed with the curtains drawn to limit sunlight. However, the neurologist has counseled that patient to exercise because evidence shows that exercise is effective for migraine prevention. It would be advantageous for the neurologist to know if the patient has been adherent to the treatment recommendations.
The purpose of this review is to (1) raise awareness of wearable technologies that may be relevant to neurology, (2) discuss the implications of these applications for our patients, and (3) briefly discuss issues related to researching new wearable technologies.
Background on Wearable Technologies
A wearable device is emerging technology that enables continuous ambulatory monitoring of human vital signs during daily life.3 The device may capture various types of data and create a digital phenotype (See table 1 for definitions). Anticipated benefits include empowerment of individuals who will gain access to data recorded by the wearable device and improved clinical care via incorporation of that data into clinical practice. There is a scarcity of data showing whether use of wearables has positive benefits for neurologic conditions. Yet, the private sector has expressed an expanding interest in these devices. With more than $2.5 billion spent by the top-funded digital health companies, the biosensors market, including wearables, received the highest share of funding at $706 million (28% of total). Among these companies, 45% considered patients as the target end user, whereas 30% targeted health care providers, and 25% had a mix of targeted end users (e.g., patients, providers, or employers). Most such companies are quite new; approximately 60% were founded after 2006, and none were founded earlier than 1993.4
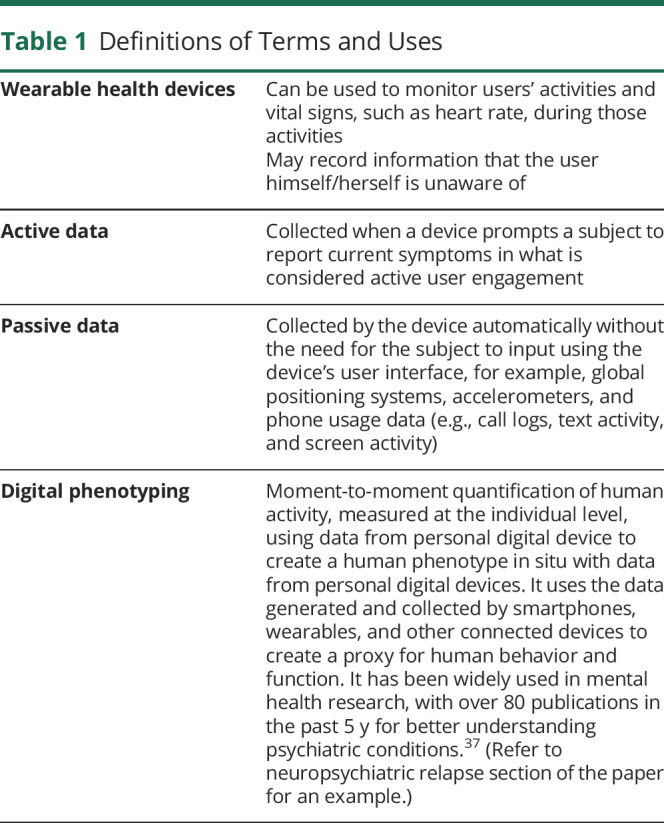
Table 1.
Definitions of Terms and Uses
Potential Neurologic Uses of Wearables and Applications
Below is a selection of various neurologic conditions and associated symptoms with wearables that may be applicable to our patient population. Some of these wearables are commercially available, and others are being studied to examine their feasibility/acceptability and to test their validity (table e-1 shows Food and Drug Administration [FDA] status, links.lww.com/CPJ/A216). However, as a disclaimer, additional study is necessary to know whether clinicians may rely on such wearables.
Stroke
Just as a “phone” is no longer just a device for talking to another person at a distance, a “watch” is no longer simply a source to tell time. Smartphones and smartwatches can detect the pulse and, in some cases, can even detect cardiac arrhythmia (e.g., atrial fibrillation or atrial flutter).5 This information might be helpful for patients with transient ischemic attacks or strokes potentially caused by cardiac arrhythmia.
Cognitive Impairment
For patients with cognitive impairment and diabetes, continuous monitoring of blood sugar is available with the Dexcom6 and the FreeStyle Libre7 in case patients cannot remember to check blood sugar and do not have a caregiver to remind them to do so.
Seizure Detection
Several devices have been developed for seizure detection. The Embrace smartwatch device can detect unusual or involuntary motor activity associated with tonic-clonic or convulsive seizures. It measures changes in the electric conductance of the skin that correlate to seizure activity in the brain.8 If the device detects seizure activity, it alerts the wearer, a caregiver, and/or a family member with a companion wearable device. The Embrace-2 is a second-generation version approved by the FDA for seizure detection in adults and for children aged 6 and older.9 Another similar device cleared by the FDA is the Brain Sentinel, which uses electrodes attached to the biceps, allowing detection of tonic-clonic seizures that either start in or spread to the motor cortex.8 SeizAlarm, another device, similarly allows people with simple partial seizures to alert emergency contacts or send a request for help via text, email, and phone when it detects seizure-like motion.8 The global positioning system (GPS) function of the phone or watch shares the user's location, and a diary function keeps track of the time and duration of the seizure. Finally, the Inspyre smartwatch app detects repetitive shaking motion and sends text and phone call alerts including the date, time, GPS location, and duration of the event.10
Of note, automatic detection of generalized tonic-clonic seizures with accelerometers and wrist-worn electrodermal sensors has proven effective with a sensitivity of more than 92%. These seizure detection devices can help with caregiver concerns regarding sudden unexpected death in epilepsy. Unfortunately, these devices may mistake some normal daily activities (e.g., hand clapping or teeth brushing) for generalized tonic-clonic seizures, creating the challenge of false alarms that can increase caregiver anxiety and cause unnecessary medical expenses and stress.11
Parkinson Disease
Wearable devices can measure many symptoms of Parkinson disease correlated with well-being and quality of life. Step activity counters, accelerometers, and sensor units attached to the lower back measure overall activity. Parkinson mPower is being used in a 2-year research study of Parkinson disease progression open to people 18+.12 In this study, an app uses interactive tasks and activities to monitor changes in key indicators of disease progression, including dexterity, tremors, mobility, balance, gait, and memory.
Sleep Disorders
Sleep quality and quantity affects patients across many neurologic conditions. Wrist-worn actimetry sensors have been available for decades and are used in and validated for sleep studies. Sleep-monitoring devices contain one or more sensor types to measure physiologic tracking. Cardiac monitoring during sleep can serve as an alternative to wrist-worn devices, and there are mattress-based devices—considered “nearables”—to track thoracic movements associated with breathing.13
Importantly, many consumer-grade available sleep monitors make indirect claims about improving sleep; however, most such claims lack research-validation. Several newer devices with more direct interventions can potentially improve sleep quality. The 2breathe device combines a thoracic belt with an app to coach users to breathe in a way that induces sleep. Scent-delivery devices and sound technology devices are also used to promote and/or induce sleep.13
Suicide Prevention Applications
The risk of suicide is higher in some neurologic disorders. Patients with neurologic disorders have an adjusted incidence rate ratio of 1.8 (95% confidence interval [CI]: 1.7–1.8) with excess adjusted incidence rate ratios as high as 4.9 (95% CI, 3.1–7.7) for Huntington disease and 2.2 (95% CI, 1.9–2.6) for multiple sclerosis for suicide compared with those without a diagnosed neurologic disorder.14 Neurologists must be aware of suicide risks. Unfortunately, despite more than 50 years of research on the prediction of suicidal ideation and attempts, only 0.1% of that research has examined which factors predict suicidal behavior within 30 days.15 Known risk factors for suicidal behavior predict suicidal ideation but do not predict the transition from suicidal ideation to attempt(s). Research is ongoing to determine if analysis of vocal frequencies and other language parameters may identify differences among people with depression, suicidal ideation, suicidal intent, and conversion to suicidal behavior. People with depression have a reduced acoustic range of speech.16 Real-time facial motion monitoring may detect subtle expression changes in people with suicidal ideation. Measurement of other parameters (e.g., skin conductance and heart rate) could help determine other physiologic changes in people with suicidal ideation. Although this research is promising, researchers must critically evaluate the commercially available suicide prevention tools, which may lack evidence or clinical validation.
Neuropsychiatric Relapse Assessment
A neuropsychiatric research app, The Mind Lamp, attempts to assess the risk of relapse for specific mental health conditions.17 The app uses symptom surveys, cognitive tests, measures of physical activity, timestamps, and geolocation to create a digital phenotype and measure changes in that phenotype over time. For example, schizophrenia has a relapse rate of up to 40% for individuals discharged from hospital-based care. Relapse is associated with job loss, increased self-harm, difficulty with relationships, and increased risk of cognitive decline.18 The app uses active data from symptom surveys and cognitive testing and passive data from timestamps, geolocation, and physical activity to create a baseline profile or digital phenotype. (See table 1 for definitions of active and passive data.) The app may measure changes over time to develop models to predict relapse. If there is an anomaly or relapse risk is identified, the app can prompt users to complete interventions to hopefully reduce relapse risk.19
Issues With Wearables for Neuropsychiatric Conditions
There is a paucity of high-quality data for many wearables, a lack of accepted standards for evaluating them, and limited knowledge in implementation strategies.
Limited Efficacy Data
In a cross-sectional analysis of the 20 leading digital health companies with the most private equity funding, there were 156 publications on the products and services they offered, 104 of which were indexed in PubMed. The study results indicate that the 5 most disabling neuropsychiatric conditions (1. stroke, 2. migraine, 3. depression, 4. Alzheimer/dementia, and 5. anxiety)20,21 were not well studied. There were 8 studies for general mental health and 5 for depression; none of these studies evaluated clinical effectiveness. There were 9 for epilepsy, but only 2 of these evaluated clinical effectiveness.3
No Accepted Framework
The American Psychiatric Association created a framework for evaluating digital health tools based on disease burden, clinical effectiveness, population, condition, and risk factors.22 Although new frameworks are being developed, there is no widely accepted standard for evaluating wearables.23
Limited Implementation Strategies
A mixed-methods systematic review of wearables for epilepsy, Parkinson disease, and stroke24 found that some wearables were tested in laboratory (21), hospital (17), and free-living environments (28). However, few studies examine the adaptation of wearables in health care systems, and the creation of medical research and validation protocols cannot be developed as quickly as the pace at which mHealth tools are released. Wearables provide health care providers with massive amounts of data for analysis and adaptation lags behind patient needs.25
Lack of Financial Stability for Device Companies
Many medical device companies, particularly smaller companies, may depend on the success of a single product to succeed as a business entity. Furthermore, startup companies “may be unprofitable for years before either developing a viable product or going out of business.”26 Although innovation is obviously important regarding the creation of useful medical devices, users and health care providers need some assurances in the manufacturer's ability to survive as an ongoing business to support the device throughout the product's lifecycle. This issue is even more critical for devices that require the use of the manufacturer's computing resources or replacement/disposable parts only available from the original manufacturer.
Privacy Implications and Regulatory Enforcement
Privacy Implications
Patients and physicians need to understand the privacy risks associated with wearables. Data collected and shared by devices may include personally identifiable information, pseudonymized information, and anonymous information. Unlike many other countries, the United States uses a sector-based approach to privacy in which the health care sector (e.g., doctors, hospitals, and insurers) must comply with the strict rules of the Health Insurance Portability and Accountability Act (HIPAA), whereas general consumer goods may be subject to far less strict privacy rules, frequently defined by the scope of the privacy policies for the device and its apps. Even if a wearable device claims to provide health-related benefits (e.g., monitoring heart rate and body temperature), HIPAA may not apply unless the device/app company has a direct contractual relationship with a health care provider or is actually offered to the patient by the health care provider.29 Researchers use commercially available wearables and may wish to specifically contract with the manufacturer to ensure that the research subject's information has stricter protections than those available to the general public under the provider's own privacy policy or ensure that data are only shared with the research institution and are subject to privacy rules for health care and research.28,29 Informed consent in research studies should include plain English explanations about how collected data may be used and shared. In addition, researchers and health care providers may want to explain to patients that most commercially available apps/devices are not subject to HIPAA's strict privacy rules even if the patient provides the data to a physician.27
Some employers provide their employees with mobile health device wellness programs that may allow employers to receive employee data (e.g., fatigue or sleep difficulties). We ask, “Do our patients (the potential employees) really want their employers to know that they are fatigued or that they are having trouble sleeping?” Although there may be advantages to health care providers and researchers having these data to better understand fatigue generally and in patients with conditions prone to fatigue (e.g., traumatic brain injuries, multiple sclerosis, or migraine), we also need to be aware of privacy and data ownership concerns. Of note, health information collected from a wearable device may be subject to HIPAA if the device is part of a workplace wellness program run through an employer's health plan. However, even the if HIPAA does not apply, the employer's collection and use of such data may be subject to a variety of other state and federal laws.30
Regulatory Enforcement
The FDA has the right to regulate devices that are (1) intended for use in the diagnosis, cure, mitigation, treatment, or prevention of disease; (2) intended to affect the structure or any function of the body (but not through chemical action); or (3) recognized as such in specific publications. Devices that fit this definition must go through FDA procedures for approval and regulation. However, the FDA has recognized, in “nonbinding” guidance, that it may not be practical for every app making medical claims to be subject to the FDA's approval and regulation.31 As stated by the FDA, “(s)ome software functions may meet the definition of a medical device, but because they pose a lower risk to the public, FDA intends to exercise enforcement discretion over these devices (meaning it will not enforce requirements under the FD&C Act).” For example, devices and apps that take measurements to deliver medication pose a higher risk and are more likely to be subject to strict regulatory oversight, whereas devices and apps that provide coaching to supplement a doctor's care are less likely to be subject to strict regulatory oversight. Similarly, even if a product is intended to “reduce the impact of certain chronic diseases or conditions,” the FDA will consider it a low-risk general wellness product if it both (1) relates to the role of a healthy lifestyle regarding that chronic disease or condition and (2) is noninvasive, not implanted, and does not involve a technology that poses a safety risk if regulatory controls are not applied.32 Based on these guidance documents, the clear implication is that safety risks are the priority in determining whether any such device should be subject to regulation. As wearables become more common and gain more health-related features, such guidance may change or become more specific.
Decision-making in Deciding to Use Wearables for Neurologic Conditions
Patients may not realize the benefit of the wearable, and those with depression or apathy may be less inclined to initiate discussion about its use. Thus, the recommendation to use wearables needs to be determined on a case-by-case basis and is not always shared decision-making.
Adherence
A mixed-methods systematic review of 56 studies about wearables for epilepsy, Parkinson disease, and stroke24 found that just 6 studies assessed adherence. Across the studies, adherence was variable. One large study (N = 408) assessing adherence to the use of a step activity monitor over 2 days had adherence rates between 61 and 68% for separate days. Only 53% of participants wore sensors for 2 consecutive days. Those who were older with better balance, self-efficacy, and walking endurance had more adherence.33 However, a stroke patient study in which participants wore accelerometers showed better adherence; participants wore them for 76–89% of waking hours in a 3-day measurement.34 A study assessing the acceptability of wrist-worn sensors in PD stated that 32 of 34 participants wore sensors for the full 7-day period, with 4% nonadherence time. All but one subject preferred the wearables to maintaining a symptom diary, 32 of 34 (94.1%) were willing to wear the sensors at home, and 29 of 34 (85.3%) stated they were willing to wear the sensors in public.35
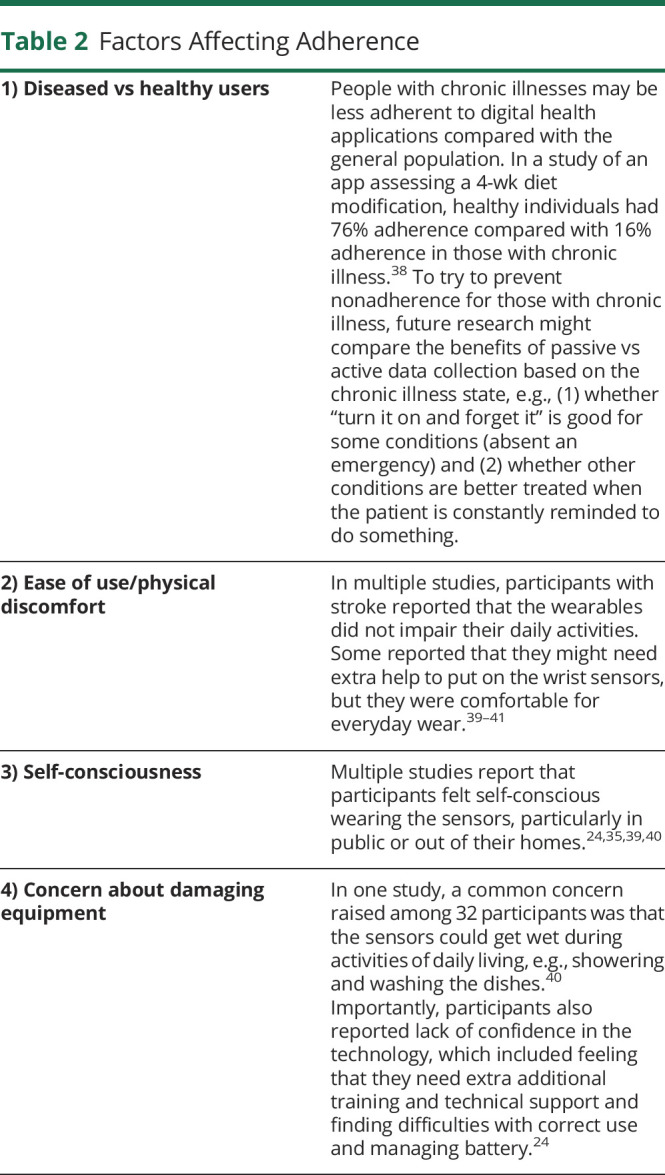
Factors Affecting Adherence
Several factors may affect adherence, including (1) diseased vs healthy users, (2) ease of use/physical discomfort, (3) self-consciousness, and (4) concern about damaging the equipment. Examples of how these factors affect adherence may be found in table 2. Importantly, adherence may be connected to lack of confidence in the technologies. Some participants reported that they needed additional training and technical support and reported difficulties with correct use and managing the battery.24
Table 2.
Factors Affecting Adherence
Issues Related to Researching Wearables
There are many commercial devices on the market, but few have been thoroughly validated. Research into the use of wearables for neurologic conditions is an important area of future work. Between 2014 and 2018, the NIH spent $138,110,270 on first-year grant dollars for studies with specialized smartphone intervention applications (including but not limited to wearables).27
Certain special considerations should be taken into account when conducting research using wearables. When adapting or modifying a known validated test or intervention to a wearable technology, this creates a new test or intervention that needs its own validation.36
The constant upgrades made by commercial companies to their devices also raise considerable concerns. Researchers may not know about changes made to the technology—e.g., consumer-grade Fitbits use algorithms for these measurements that change without warning—which also makes the use of Fitbits in research unfeasible (Oral communication from the Neuroscience in the Clinic Session Panel of the American Academy of Neurology Annual Meeting, May 4-10, 2019. Philadelphia, PA).
Finally, analyzing data from wearables is complex, and there can be major concerns regarding data quality. Researchers' use of user-generated health data relies on participants' willingness to share data. If users limit data sharing or are nonadherent, or if their devices are frequently off, then there can be considerable uncertainty for data interpretation. In the mixed-methods systematic review, missing data reported in 12 studies were because of technical errors, e.g., device failures, disconnected sensors, data storage issues, and/or human factors (device removed or used incorrectly).24 Another issue with data quality can be seen with Fitbits, a popular activity monitor marketed as a health and wellness tool. In a comparison of Fitbits with research-grade activity monitors in 2018, a systematic review28 showed that Fitbit devices may not differentiate between time spent in bed and asleep. Fitbits overestimated time spent in higher intensity activities but underestimated faster-paced ambulation distances. Measurements were also unreliable for people with gait abnormalities.
Conclusion
In conclusion, the future is bright in that there is much funding available for wearable development in health care. However, there is a lack of evidence for many interventions. In addition, questions remain about who should own and have access to the generated data. There remain issues about how to transfer information to clinicians and researchers, which is very limited at present, and how to do this in ways that protect patients' privacy and other rights.
TAKE-HOME POINTS
→ There has been increasing interest in the development of wearables over time. Such wearables have been developed for a wide variety of conditions, including neurologic conditions.
→ Wearables may help with seizure detection, arrhythmia detection as part of a stroke workup, glucose monitoring for diabetes control that may help those with cognitive impairment and sleep, and other neurologic conditions.
→ More research on wearables is needed for studying their efficacy, effectiveness, and implementation in health care systems. Research is also needed to best understand who might be most likely to adhere to these new evolving wearable technologies.
→ Health care providers and patients should understand that the privacy of medical data collected by wearables is very context-dependent, and HIPAA's strict protections do not always apply. Health care providers must also be aware that the FDA exercises enforcement discretion for wearables and apps considered to be low-safety risks and that therefore many wearables may not have been subject to extensive safety or reliability testing for the use intended by the doctor.
Acknowledgment
Dr. Minen would like to acknowledge Dr. John Torous for his initial help in thinking about the content of the presentation on Wearables for the 2019 American Neuropsychiatric Association (ANPA) meeting upon which this review paper is based. Dr. Minen would also like to thank Dr. Anne Sydor for her help editing the manuscript.
Appendix. Authors
Study Funding
No targeted funding reported.
Disclosure
M.T. Minen reports that she has a NIH K23AT009706 grant funding to conduct app-based research and research support from the Doris Duke Fellowship to Retain Clinician Scientists. E.J. Stieglitz reports no disclosures relevant to the manuscript. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Wurmser Y. Wearables 2019: Advanced wearables pick up pace as fitness trackers slow. eMarketer 2019. Available at: https://www.emarketer.com/content/wearables-2019. Accessed April 1, 2020. [Google Scholar]
- 2.Steiner TJ, Stovner LJ, Vos T, Jensen R, Katsarava Z. Migraine is first cause of disability in under 50s: will health politicians now take notice? J Headache Pain 2018;19:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Safavi K, Mathews SC, Bates DW, Dorsey ER, Cohen AB. Top-funded digital health companies and their impact on high-burden, high-cost conditions. Health Aff (Millwood) 2019;38:115–123. [DOI] [PubMed] [Google Scholar]
- 4.Johnson J. What's holding wearables back? Brodeur Partners. 2015. Available at: https://www.brodeur.com/wp-content/uploads/2015/09/Wearable_white-paper_PDF.pdf. Accessed September 20, 2020.
- 5.Raja JM, Elsakr C, Roman S, et al. Apple watch, wearables, and heart rhythm: where do we stand? Ann Transl Med 2019;7:417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dexcom. Dexcom, Continuous Glucose Monitoring Web site. Available at: www.dexcom.com/get-started-cgm/40?sfc=701f30000018vibAAA&gclid=CjwKCAjw3c_tBRA4EiwAICs8CuypxHZEEJ1UmP9ElAC_4mcw2fvBmrsw4UxzbYIoIcq2eQG_ucq9LRoCOYYQAvD_BwE. Accessed November 6, 2019.
- 7.Continuous glucose monitoring system. FreeStyle Libre System Web site. Available at: www.freestylelibre.us/. Accessed April 1, 2020. [Google Scholar]
- 8.Samson K. New wearable medical tech tracks more than just steps. Brain&Life. 2015. Available at: https://www.brainandlife.org/articles/wristbands-smartwatches-and-other-wearable-devices-allow-for-more-real/. Accessed October 27, 2020. [Google Scholar]
- 9.Duffy S. Seizure monitoring smartwatch cleared for use in children. MPR The Right Dose of Information Web site. Available at: www.empr.com/home/news/seizure-monitoring-smartwatch-cleared-for-use-in-children/. Accessed November 10, 2019. [Google Scholar]
- 10.About the SmartWatch inspyre™ by smart monitor. Seizure & Epilepsy Alert app—smart-monitor Web site. Available at: smart-monitor.com/about-smartwatch-inspyre-by-smart-monitor/. Accessed March 31, 2020. [Google Scholar]
- 11.Meritam P, Ryvlin P, Beniczky S. User-based evaluation of applicability and usability of a wearable accelerometer device for detecting bilateral tonic-clonic seizures: a field study. Epilepsia 2018;59(1 suppl):48–52. [DOI] [PubMed] [Google Scholar]
- 12.van Uem JM, Isaacs T, Lewin A, et al. A viewpoint on wearable technology-enabled measurement of wellbeing and health-related quality of life in Parkinson's disease. J Parkinsons Dis 2016;6:279–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bianchi MT. Sleep devices: wearables and nearables, informational and interventional, consumer and clinical. Metab Clin Exp 2018;84:99–108. [DOI] [PubMed] [Google Scholar]
- 14.Erlangsen A, Stenager E, Conwell Y, et al. Association between neurological disorders and death by suicide in Denmark. JAMA 2020;323:444–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franklin JC, Ribeiro JD, Fox KR, et al. Risk factors for suicidal thoughts and behaviors: a meta-analysis of 50 years of research. Psychol Bull 2017;143:187–232. [DOI] [PubMed] [Google Scholar]
- 16.Vahabzadeh A, Sahin N, Kalali A. Digital suicide prevention: can technology become a game-changer? Innov Clin Neurosci 2016;13:16–20. [PMC free article] [PubMed] [Google Scholar]
- 17.Torous J, Rodriguez J, Powell A. The new digital divide for digital BioMarkers. Digit Biomark 2017;1:87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Emsley R, Chiliza B, Asmal L, Harvey BH. The nature of relapse in schizophrenia. BMC Psychiatry 2013;13:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barnett I, Torous J, Staples P, Sandoval L, Keshavan M, Onnela JP. Relapse prediction in schizophrenia through digital phenotyping: a pilot study. Neuropsychopharmacology 2018;43:1660–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Institute for Health Metrics and Evaluation. GBD results tool. 2016. GHDx Health Data Web site. Available at: gbd2016.healthdata.org/gbd-search/. Accessed June 12, 2018. [Google Scholar]
- 21.Gopal A, Sahyoun G, Stieglitz E, Torous J, Minen M. The functionality, evidence and privacy issues around smartphone apps for the top neuropsychiatric conditions: a comprehensive study Neurology 2019;92(15 suppl):P3.9-–064.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torous JB, Chan SR, Gipson SYT, et al. A hierarchical framework for evaluation and informed decision making regarding smartphone apps for clinical care. Psychiatr Serv 2018;69:498–500. [DOI] [PubMed] [Google Scholar]
- 23.Coravos A, Doerr M, Goldsack J, et al. Modernizing and designing evaluation frameworks for connected sensor technologies in medicine. NPJ Digit Med 2020;3:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johansson D, Malmgren K, Alt Murphy M. Wearable sensors for clinical applications in epilepsy, Parkinson's disease, and stroke: a mixed-methods systematic review. J Neurol 2018;265:1740–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bradway M, Carrion C, Vallespin B, et al. mHealth assessment: conceptualization of a global framework. JMIR Mhealth Uhealth 2017;5:e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Report to congress: medicare and the health care delivery system; chapter 7, an overview of the medical device industry. MedPac. 2017:207–237. Available at: http://www.medpac.gov/docs/default-source/reports/jun17_reporttocongress_sec.pdf. Accessed October 27, 2020. [Google Scholar]
- 27.Hansen WB, Scheier LM. Specialized smartphone intervention apps: review of 2014 to 2018 NIH funded grants. JMIR Mhealth Uhealth 2019;7:e14655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feehan LM, Geldman J, Sayre EC, et al. Accuracy of fitbit devices: systematic review and narrative syntheses of quantitative data. JMIR Mhealth Uhealth 2018;6:e10527:e10527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Health App Use Scenarios & HIPAA. 2016. Available at: hipaaqsportal.hhs.gov/community-library/accounts/92/925889/Public/OCR-health-app-developer-scenarios-2-2016.pdf. Accessed November 6, 2019. [Google Scholar]
- 30.OCR. HIPAA Privacy and Security and Workplace Wellness Programs. 2015. HHS.gov, U.S. Department of Health & Human Services Web site. Available at: www.hhs.gov/hipaa/for-professionals/privacy/workplace-wellness/index.html. Accessed May 15, 2020. [Google Scholar]
- 31.FDA. Policy for Device Software Functions and Mobile Medical Applications: Guidance for Industry and Food and Drug Administration Staff. 2019. Available at: www.fda.gov/media/80958/download. Accessed November 6, 2019. [Google Scholar]
- 32.Contains nonbinding recommendations general wellness: Policy for low risk devices guidance for industry and food and drug administration staff. 2019. Food & Drug Administration Web site. General Wellness: Policy for Low Risk Devices. Available at: https://www.fda.gov/media/90652/download. Accessed April 3, 2020. [Google Scholar]
- 33.Barak S, Wu SS, Dai Y, Duncan PW, Behrman AL, Locomotor experience applied post-stroke (LEAPS) investigative team. Adherence to accelerometry measurement of community ambulation poststroke. Phys Ther 2014;94:101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uswatte G, Foo WL, Olmstead H, Lopez K, Holand A, Simms LB. Ambulatory monitoring of arm movement using accelerometry: an objective measure of upper-extremity rehabilitation in persons with chronic stroke. Arch Phys Med Rehabil 2005;86:1498–1501. [DOI] [PubMed] [Google Scholar]
- 35.Fisher JM, Hammerla NY, Rochester L, Andras P, Walker RW. Body-worn sensors in Parkinson's disease: evaluating their acceptability to patients. Telemed J E Health 2016;22:63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Torous J, Larsen ME, Depp C, et al. Smartphones, sensors, and machine learning to advance real-time prediction and interventions for suicide prevention: a review of current progress and next steps. Curr Psychiatry Rep 2018;20:51. [DOI] [PubMed] [Google Scholar]
- 37.Huckvale K, Venkatesh S, Christensen H. Toward clinical digital phenotyping: a timely opportunity to consider purpose, quality, and safety. NPJ Digit Med 2019;2:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shaw RJ, Steinberg DM, Bonnet J, et al. Mobile health devices: will patients actually use them? J Am Med Inform Assoc 2016;23:462–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simone LK, Sundarrajan N, Luo X, Jia Y, Kamper DG. A low cost instrumented glove for extended monitoring and functional hand assessment. J Neurosci Methods 2007;160:335–348. [DOI] [PubMed] [Google Scholar]
- 40.Cancela J, Pastorino M, Tzallas AT, et al. Wearability assessment of a wearable system for Parkinson's disease remote monitoring based on a body area network of sensors. Sensors (Basel) 2014;14:17235–17255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mountain G, Wilson S, Eccleston C, et al. Developing and testing a telerehabilitation system for people following stroke: issues of usability. J Eng Des 2010;21:223–236. [Google Scholar]


