Abstract
Background
The workup for idiopathic normal pressure hydrocephalus (INPH) can be difficult to coordinate, and determining appropriate patients for ventriculoperitoneal shunting can be challenging. Therefore, we hypothesized that implementing a formalized protocol can improve patient selection for a shunt. In conjunction with neurology and neurosurgery, we instituted a standardized means of assessing patients whose presentation is concerning for INPH and compared their workup with similar patients seen without the Protocol (i.e., preprotocol [PP]) regarding baseline characteristics, assessment, and outcomes.
Methods
Twenty-six PP patients were compared with 40 Protocol patients on measures, including baseline deficits, workup, neurosurgical evaluation, and response to shunt.
Results
Average age was similar between groups, and the percentage of patients who had a decline in gait, cognition, and/or incontinence was not statistically different (p > 0.05). Significantly more Protocol patients underwent high-volume lumbar puncture (HVLP; 97.5%; PP, 61.5%; p < 0.001) and received formalized gait assessment with the Gait Scale (90%; PP, 0%, p < 0.001) and standardized cognitive testing (95%; PP, 38.5%; p < 0.001). Significantly more Protocol patients had no improvement after HVLP (33.3%; PP, 6.25%; p < 0.045); subsequently, fewer got shunted (57.5%; PP, 84.6%; p < 0.030). More Protocol patients who were shunted reported gait improvement (100%; PP, 72.7%; p = 0.009), although there was no difference in cognition (59.2%; PP, 82.6%; p = 0.108) or incontinence (18.2%; PP, 39.1%; p = 0.189).
Conclusions
Implementing an INPH Protocol leads to standardized and more extensive assessment and better patient selection for and subsequent outcomes from shunting, specifically regarding gait.

Idiopathic normal pressure hydrocephalus (INPH) is a neurologic disease with the triad of shuffling gait, cognitive impairment, and urinary incontinence, without elevated intracranial pressure.1 Treatment is placement of a ventriculoperitoneal shunt (VPS)1–3; it can improve symptoms,4–6 of which gait is often the most commonly improved.7
Diagnosing INPH is challenging because patients may not have the full triad, and comorbidities can explain some of the symptoms.4,8,9
Predicting which patients may respond to a shunt is difficult.10,11 High-volume lumbar puncture (HVLP) can assess the potential for response to VPS,1,12–16 with removal of at least 30–50 mL of CSF.16–19 Evaluation by medical professionals provides formal assessment after HVLP,20 such as measuring gait,17,21–24 although the predictive value is variable. Gait is correlated with improvement on a timed walk test 6 months after shunt placement.2 These assessments should be documented at baseline or at the time of CSF removal.25 Data about optimal timing for post-HVLP assessment varies17; however, assessment within the first 24 hours after HVLP may be appropriate.26
Because assessment of INPH varied within our institution, we devised a unified protocol to determine surgical candidacy, with pre- and post-HVLP (within 1–3 hours) evaluations, followed by an outpatient neurosurgical visit soon after HVLP. Sixteen months after protocol initiation, we performed a retrospective outcomes analysis. The hypothesis was standardized assessments, including HVLP, would improve predicting successful outcomes for VPS in patients suspected of having INPH.
Methods
Participants
Data were reviewed from patients who underwent routine clinical care of suspected INPH at Vanderbilt University Medical Center (VUMC) between 2007 and 2017. Patients who are evaluated using the Protocol were compared with those before initiation of the Protocol (preprotocol [PP] patients). Further inclusion criteria were outpatient evaluation by neurology, a clinical history concerning for INPH (whether the patient had cognitive decline, gait changes, ±incontinence, and ventricular enlargement on brain imaging), and outpatient evaluation by neurosurgery.
As part of a retrospective analysis, review of the electronic medical record identified PP patients who had the associated diagnostic code of INPH 2007–2015. Charts were reviewed, and patients evaluated by both neurology and neurosurgery were included. These patients also had symptoms within the INPH triad and documented ventriculomegaly on brain imaging. This was a convenience sample that can provide a comparable cohort to the Protocol patients.
Protocol patients were identified after initiation of the INPH Protocol in 2015–2017.
These patients were evaluated by outpatient neurology. The referring neurologist diagnosed INPH and made the referral for the INPH Protocol. Protocol evaluation was performed by a neurology nurse practitioner (NP), physical therapist (PT), and neurology resident. The NP or, if necessary, the resident performed the neurologic examination. The NP administered the Montreal Cognitive Assessment (MoCA).27 When the Protocol was first launched, the Mini-Mental State Examination (MMSE)28 was administered, which was replaced by the MoCA to reduce the practice effect by being able to give alternate versions before and after HVLP. The NP remained consistent across evaluations. The PT assessed gait, described below. There were 2 PTs who alternated the evaluations. A neurology resident, whose assignment varied from within the residency program, performed HVLP at the bedside. If bedside HVLP was unsuccessful, fluoroscopy performed HVLP. After HVLP, the NP administered another version of the MoCA, and the PT would perform gait assessment. Both objective (i.e., scores on cognitive and gait assessments) and subjective improvements (i.e., clinician and patient or caregiver report of improvement) were noted.
Standard Protocol Approvals, Registrations, and Patient Consents
Institutional Review Board approval was obtained within VUMC's ethical standards committee; as part of routine clinical care, written consent was not necessary.
Assessment Tools
Established cognitive screening tests (i.e., MMSE or MoCA) were reviewed for all patients.
For Protocol patients, standardized gait assessments were part of the diagnostic workup, including Steps (number of steps required for a 10-m walk, averaged over 3 trials), Time (number of seconds required for a 10-m walk, averaged over 3 trials), Gait Scale (attributing a point scale to aspects of gait, such as whether stride is wide-based, reduced foot-floor clearance), and Gait Score Total (a composite of these other scores).29,30 Other outcome measures included reported improvement after the HVLP and the number of days between the initial evaluation, HVLP, and neurosurgery appointments. Data reviewed regarding improvement after HVLP and placement of VPS included the assessments performed by the clinicians and patient and family report,7,31 which were considered collectively as either responding or not responding.
Post-HVLP assessments were performed approximately 1–3 hours after the HVLP was completed.32 Postshunt data were collected from the first neurosurgical visit as part of postoperative follow-up.
Imaging was not consistently available for PP patients. Protocol patients had imaging performed, usually computerized tomography of the head or magnetic resonance imaging of the brain. Imaging was reviewed when available, but because of the lack of comprehensive data across cohorts, no further analysis, such as measuring the ventricular size using the Evans ratio,7 was used.
Analytical Plan
Study data were collected and managed using Research Electronic Data Capture (REDCap) electronic data capture tools hosted at VUMC.33,34 REDCap is a secure, web-based software platform designed to support data capture for research studies, providing (1) an intuitive interface for validated data capture; (2) audit trails for tracking data manipulation and export procedures; (3) automated export procedures for seamless data downloads to common statistical packages; and (4) procedures for data integration and interoperability with external sources.
Descriptive statistics for baseline demographics (including age, sex, and clinical features of INPH) were calculated separately for Protocol and PP patients. Because of the small sample size, the Fisher exact test was used for categorical variables, and the Wilcoxon rank-sum test was used for continuous variables. Significance was set a priori at p < 0.05. Statistical analyses were conducted using Stata (software version 13.1).
Data Availability
Anonymized data not published within this article will be made available to qualified investigators on request and with approval by appropriate Institutional Review Board permission.
Results
Sample Characteristics
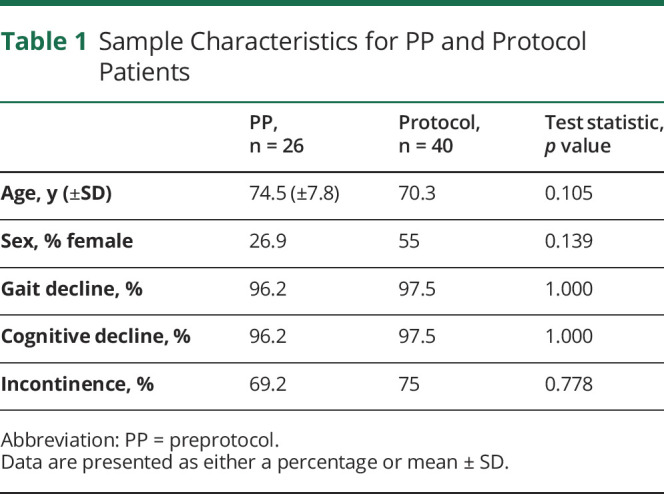
Twenty-six PP and 40 Protocol participants (table 1) were included, with no difference in basic demographics or clinical characteristics. Any patient who had reported change in gait, cognition, and/or incontinence by self-report or caregiver and/or documented on clinical examination was considered to have gait decline, cognitive decline, and incontinence, respectively.
Table 1.
Sample Characteristics for PP and Protocol Patients
INPH Evaluation
The evaluation between Protocol and PP patients varied (table 2). PP were less likely to undergo lumbar puncture, had less CSF removed, and were less likely to have received bedside HVLP, being more likely to undergo fluoroscopically guided HVLP. Approximately 15.4% of PP patients underwent cisternogram, which was not a part of the Protocol. Some neurologists appeared to use cisternogram in place of HVLP, so analysis of PP patients included both of these together. With that, significantly more patients underwent CSF removal or study with the Protocol.
Table 2.
Lumbar Puncture/Cisternogram for PP and Protocol Patients

Formalized gait assessment was not consistently documented PP, so this was not included in the analysis (table 3). PT evaluation using Steps, Time, Gait Scale, and Gait Score Total was part of the INPH Protocol (90%) but was not done PP. Among Protocol patients undergoing PT evaluation, there was a significant difference in Steps (pre-HVLP 6.33, post-HVLP 5.81, p = 0.006) and Gait Score Total (pre-HVLP 22.37, post-HVLP 20.93, p = 0.009). The difference in Steps means that patients took fewer steps to walk the specified 10 m (5.81 steps, as opposed to 6.3 steps). Gait Score Total tallies change in speed and quality of gait, with a lower number indicating improvement.
Table 3.
Physical Therapist Assessment for Preprotocol and Protocol Patients

Cognitive testing (table 4) was more likely to occur in Protocol than PP patients (95% vs 38.5%, p < 0.001). For those who underwent HVLP, this included pre-HVLP testing (PP 37.5%, Protocol 97.5%, p < 0.001), post-HVLP testing (PP 6.25%, Protocol 82.1%, p < 0.001), and pre- and post-HVLP testing (PP 6.25%, Protocol 82.1%, p < 0.001). Looking at whether any kind of cognitive testing had been done, Protocol patients were still more likely to have had some sort of formalized cognitive screening test performed (95%) compared with PP (38.5%, p < 0.001). The only test administered in both Protocol and PP patients was the MMSE, which was typically only pre-HVLP in PP patients. Scores were comparable between groups. There was no significant difference in Protocol patients on comparing pre- and post-HVLP scores for either the MMSE or MoCA.
Table 4.
Cognitive Testing for PP and Protocol Patients
There was no significant difference in subjective improvement post-HVLP between PP and Protocol patients for gait or cognition. Fewer PP patients reported no improvement s/p HVLP (6.25%) as opposed to the Protocol patients (33.3%, p = 0.045).
Neurosurgical evaluation (table 5) as an outpatient approached significance on comparing PP patients (100%) with Protocol patients (87.5%, p = 0.066). The wait time between initial neurologic evaluation and neurosurgery, although shorter with the Protocol than with PP, was not statistically significant. The wait time between the HVLP and neurosurgical evaluation was significantly shorter with the Protocol (7.2 days) as opposed to PP (47.5 days, p < 0.001), between the initial neurologic evaluation and shunt (p = 0.048) and between the HVLP and the shunt (p = 0.033).
Table 5.
Neurosurgical Evaluation and Shunting for PP and Protocol Patients
Fewer Protocol patients were shunted (table 5; 57.5%) compared with PP (84.6%, p = 0.03). In neurosurgical follow-up, more Protocol patients had gait improvement (100%) compared with PP (72.7%, p = 0.009). There was no difference in incontinence or cognition. More patients reported any improvement after shunting with the Protocol (100%) as opposed to PP (77.3%, p = 0.022).
Discussion
This article is one of the few to assess implementation of a standardized and practical approach to evaluate patients with a presentation concerning INPH with HVLP and neurologic and neurosurgical evaluation.2,13,17 Using retrospective analysis, the Protocol demonstrates the benefits of incorporating a consistent assessment of INPH, making the evaluation of these patients more sensitive and specific. More Protocol patients underwent HVLP with a larger amount of CSF removed, with more patients identified without improvement after HVLP. Fewer Protocol patients were shunted compared with PP, with more Protocol than PP patients reporting improvement in gait after a shunt. Patient workup with HVLP and neurosurgical assessment also occurred over a shorter period. The initial hypothesis that the Protocol would improve patient care was demonstrated by the higher percentage of patients who underwent HVLP with more CSF removed and by the greater percentage of patients who reported improvement after VPS.
The benefits of this Protocol include incorporation of a multidisciplinary approach. Collaboration between neurology, neurosurgery, and PT leads to a comprehensive evaluation of INPH patients. Standardizing the assessment expedited patient work-up and patients who were shunted had better gait outcomes. As gait is the symptom that is most likely to improve after shunting,7,29,30 identifying which patients derived a gait benefit after HVLP improved determining shunt candidacy.1,12–16 Because we were more reliably able to determine who did or did not have gait improvement after HVLP, both by ensuring that HVLP was performed and that an appropriate amount of CSF removed, neurosurgery could identify who would and would not be a good candidate for VPS.
There are several limitations to Protocol implementation and result interpretation. This was a retrospective analysis of clinical outcomes, and because there had not been a standardized method of evaluating these patients, finding patients evaluated before the Protocol implementation was challenging. Patients could not be matched, such as by age, sex, and symptoms. There was selection bias with certain measures, such as cisternogram, and documentation, such as gait assessment, because of the retrospective design. Because we identified PP patients based on diagnostic codes, we may have missed INPH patients who were coded differently, potentially contributing to both selection and ascertainment bias. Because the diagnostic code was not used frequently, we extended the search over a decade to amass a comparable number of subjects. Making the department aware of the Protocol itself may also have led to referral bias.
Regarding interpretation of results, subjective improvement as noted by the patients, families, and clinicians was also used, which can be difficult to quantify and was not always consistently documented in the PP patients. Measures used could be converted more to a standardized scale to improve comparisons within and between patients.35 Although the gait assessments used by the PT have been verified in previous studies,30 the aggregated data need to be analyzed further to determine which specific measures predict response to a shunt. Cognitive testing is an important part of the INPH workup, but brief measures such as the MMSE and MoCA are also limited in their scope, particularly when administered within such a constrained period. Because cognitive testing was performed inconsistently before the Protocol, these cannot be equally compared.
The timing of the assessment is also something that could be explored. Per the Protocol, cognitive testing and gait assessment by the PT were performed typically within the next few hours after HVLP. Although within 24 hours is an acceptable timeframe,26 other studies have suggested that postponing the post-HVLP assessments even later, such as 3–8 hours, or even 3 days, after HVLP, shows the greatest sensitivity of response to suggest the possibility of improvement with a shunt.21,23,36
More objective means of quantifying patients' response to the HVLP would be beneficial.15 Both subjective and objective assessments were used to gauge symptomatic change.7,31 Future plans for analysis entail looking at the objective data obtained in the workup, such as CSF biomarkers,37,38 such as in Alzheimer disease,39 and elements of the gait assessment, to see whether these can predict who would respond to VPS. This includes portions of the gait assessment that we have been performing, and possibly incorporation of other measures, such as a quantitative assessment tool that performs gait analysis.24 Additional follow-up for the duration of symptom improvement postshunt would also be helpful, including years after the shunt insertion.31,40 All patient data are derived from the hospital electronic medical record and can be accessed in the future to determine the length of benefit or other occurrences (e.g., complications).
Implementation of an INPH Protocol to evaluate patients led to a more standardized assessment and criteria by which to judge improvement after HVLP, as well as more consistent evaluation with HVLP. Those patients who demonstrated improvement and underwent VPS also had better outcomes after surgery. Additional research is necessary to identify which measures are most predictive of positive VPS shunt outcomes, when to administer them, and how best to gauge improvement.
TAKE-HOME POINTS
→ A standardized protocol to evaluate patients with INPH provides more consistent and thorough evaluations of this population.
→ Formalized gait and cognitive testing and ensuring HVLPs were performed better identified who did and did not have symptomatic response after lumbar puncture, which led to better selection for shunt candidacy, with fewer patients being shunted.
→ Comparing INPH patients who were shunted, those who underwent a standardized protocol had better gait outcomes compared with those who did not.
Appendix. Authors

Study Funding
No targeted funding reported.
Disclosure
No conflict of interest. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Adams RD, Fisher CM, Hakim S, Ojemann RG, Sweet WH. Symptomatic occult hydrocephalus with normal cerebrospinal-fluid pressure. N Engl J Med 1965;273:117–126. [DOI] [PubMed] [Google Scholar]
- 2.Halperin JJ, Kurlan R, Schwalb JM, Cusimano MD, Gronseth G, Gloss D. Practice guideline: idiopathic normal pressure hydrocephalus: response to shunting and predictors of response: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2015;85:2063–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toma AK, Papadopoulos MC, Stapleton S, Kitchen ND, Watkins LD. Systematic review of the outcome of shunt surgery in idiopathic normal-pressure hydrocephalus. Acta Neurochir (Wien) 2013;115:1977–1980. [DOI] [PubMed] [Google Scholar]
- 4.Williams MA, Malm J. Diagnosis and treatment of idiopathic normal pressure hydrocephalus. Continuum (Minneap Minn) 2016;22:579–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas G, McGirt MJ, Woodworth GF, et al. Baseline neuropsychological profile and cognitive response to cerebrospinal fluid shunting for idiopathic normal pressure hydrocephalus. Dement Geriatr Cogn Disord 2005;20:163–168. [DOI] [PubMed] [Google Scholar]
- 6.Katzen H, Ravdin LD, Assuras S, et al. Postshunt cognitive and functional improvement in idiopathic normal pressure hydrocephalus. Neurosurgery 2011;68:416–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGirt MJ, Woodworth G, Coon AL, Thomas G, Williams MA, Rigamonti D. Diagnosis, treatment, and analysis of long-term outcomes in idiopathic normal-pressure hydrocephalus. Neurosurgery 2005;57:699–705; discussion 699-705. doi: 10.1093/neurosurgery/57.4.699. [DOI] [PubMed] [Google Scholar]
- 8.Malm J, Graff-Radford NR, Ishikawa M, et al. Influence of comorbidities in idiopathic normal pressure hydrocephalus—research and clinical care. A report of the ISHCSF task force on comorbidities in INPH. Fluids Barriers CNS 2013;10:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stolze H, Kuhtz-Buschbeck JP, Drücke H, Jöhnk K, Illert M, Deuschl G. Comparative analysis of the gait disorder of normal pressure hydrocephalus and Parkinson's disease. J Neurol Neurosurg Psychiatry 2001;70:289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergsneider M, Black PML, Klinge P, Marmarou A, Relkin N. INPH guidelines, part IV: surgical management of idiopathic normal-pressure hydrocephalus. Neurosurgery 2005;57:S29–S39. [DOI] [PubMed] [Google Scholar]
- 11.Tans JTJ, Boon AJW. How to select patients with normal pressure hydrocephalus for shunting. Acta Neurochir Suppl 2002;81:3–5. [DOI] [PubMed] [Google Scholar]
- 12.Kubo Y, Kazui H, Yoshida T, et al. Validation of grading scale for evaluating symptoms of idiopathic normal-pressure hydrocephalus. Dement Geriatr Cogn Disord 2007;25:37–45. [DOI] [PubMed] [Google Scholar]
- 13.Gallia GL, Rigamonti D, Williams MA. The diagnosis and treatment of idiopathic normal pressure hydrocephalus. Nat Clin Pract Neurol 2006;2:375–381. [DOI] [PubMed] [Google Scholar]
- 14.Kahlon B, Sundbärg G, Rehncrona S. Comparison between the lumbar infusion and CSF tap tests to predict outcome after shunt surgery in suspected normal pressure hydrocephalus. J Neurol Neurosurg Psychiatry 2002;73:721–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marmarou A, Young HF, Aygok GA, et al. Diagnosis and management of idiopathic normal-pressure hydrocephalus: a prospective study in 151 patients. J Neurosurg 2005;102:987–997. [DOI] [PubMed] [Google Scholar]
- 16.Damasceno BP, Carelli EF, Honorato DC, Facure JJ. The predictive value of cerebrospinal fluid tap-test in normal pressure hydrocephalus. Arq Neuropsiquiatr 1997;55:179–185. [DOI] [PubMed] [Google Scholar]
- 17.Marmarou A, Bergsneider M, Klinge P, Relkin N, Black PML. INPH guidelines, part III: the value of supplemental prognostic tests for the preoperative assessment of idiopathic normal-pressure hydrocephalus. Neurosurgery 2005;57(3 suppl):S17–S28. [DOI] [PubMed] [Google Scholar]
- 18.Mori E, Ishikawa M, Kato T, et al. Guidelines for management of idiopathic normal pressure hydrocephalus: second edition. Neurol Med Chir (Tokyo) 2012;52:775–809. [DOI] [PubMed] [Google Scholar]
- 19.Hashimoto M, Ishikawa M, Mori E, Kuwana N. Diagnosis of idiopathic normal pressure hydrocephalus is supported by MRI-based scheme: a prospective cohort study. Cerebrospinal Fluid Res 2010;7:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feick D, Sickmond J, Liu L, et al. Sensitivity and predictive value of occupational and physical therapy assessments in the functional evaluation of patients with suspected normal pressure hydrocephalus. J Rehabil Med 2008;40:715–720. [DOI] [PubMed] [Google Scholar]
- 21.Schniepp R, Trabold R, Romagna A, et al. Walking assessment after lumbar puncture in normal-pressure hydrocephalus: a delayed improvement over 3 days. J Neurosurg 2017;126:148–157. [DOI] [PubMed] [Google Scholar]
- 22.Stolze H, Kuhtz-Buschbeck JP, Drücke H, et al. Gait analysis in idiopathic normal pressure hydrocephalus—which parameters respond to the CSF tap test? Clin Neurophysiol 2000;111:1678–1686. [DOI] [PubMed] [Google Scholar]
- 23.Malm J, Kristensen B, Karlsson T, Fagerlund M, Elfverson J, Ekstedt J. The predictive value of cerebrospinal fluid dynamic tests in patients with th idiopathic adult hydrocephalus syndrome. Arch Neurol 1995;52:783–789. [DOI] [PubMed] [Google Scholar]
- 24.Cutlip RG, Mancinelli C, Huber F, Dipasquale J. Evaluation of an instrumented walkway for measurement of the kinematic parameters of gait. Gait Posture 2000;12:134–138. [DOI] [PubMed] [Google Scholar]
- 25.Behrens A, Eklund A, Elgh E, Smith C, Williams MA, Malm J. A computerized neuropsychological test battery designed for idiopathic normal pressure hydrocephalus. Fluids Barriers CNS 2014;11:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Virhammar J, Cesarini KG, Laurell K. The CSF tap test in normal pressure hydrocephalus: evaluation time, reliability and the influence of pain. Eur J Neurol 2012;19:271–276. [DOI] [PubMed] [Google Scholar]
- 27.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–699. [DOI] [PubMed] [Google Scholar]
- 28.Folstein MF, Folstein SE, McHugh PR. Mini-mental state: a practical method for grading the mental state of patients for clinicians. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 29.Boon AJW, Tans JTJ, Delwel EJ, et al. Dutch normal-pressure hydrocephalus study: prediction of outcome after shunting by resistance to outflow of cerebrospinal fluid. J Neurosurg 1997;87:687–693. [DOI] [PubMed] [Google Scholar]
- 30.Ravdin LD, Katzen HL, Jackson AE, Tsakanikas D, Assuras S, Relkin NR. Features of gait most responsive to tap test in normal pressure hydrocephalus. Clin Neurol Neurosurg 2008;110:455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petersen RC, Mokri B, Laws ER. Surgical treatment of idiopathic hydrocephalus in elderly patients. Neurology 1985;35:307–311. [DOI] [PubMed] [Google Scholar]
- 32.Tsakanikas D, Relkin N. Normal pressure hydrocephalus. Semin Neurol 2007;27:58–65. [DOI] [PubMed] [Google Scholar]
- 33.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hellström P, Klinge P, Tans J, Wikkelsø C. A new scale for assessment of severity and outcome in iNPH. Acta Neurol Scand 2012;126:229–237. [DOI] [PubMed] [Google Scholar]
- 36.Walchenbach R, Geiger E, Thomeer RTWM, Vanneste JAL. The value of temporary external lumbar CSF drainage in predicting the outcome of shunting on normal pressure hydrocephalus. J Neurol Neurosurg Psychiatry 2002;72:503–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schirinzi T, Sancesario GM, Di Lazzaro G, et al. Cerebrospinal fluid biomarkers profile of idiopathic normal pressure hydrocephalus. J Neural Transm (Vienna) 2018;125:673–679. [DOI] [PubMed] [Google Scholar]
- 38.Jeppsson A, Zetterberg H, Blennow K, Wikkelsø C. Idiopathic normal-pressure hydrocephalus pathophysiology and diagnosis by CSF biomarkers. Neurology 2013;80:1385–1392. [DOI] [PubMed] [Google Scholar]
- 39.Graff-Radford NR. Alzheimer CSF biomarkers may be misleading in normal-pressure hydrocephalus. Neurology 2014;83:1573–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klassen BT, Ahlskog JE. Normal pressure hydrocephalus: how often does the diagnosis hold water? Neurology 2011;77:1119–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available to qualified investigators on request and with approval by appropriate Institutional Review Board permission.




