Abstract
Objective
To investigate differences in pregnancy-related and perinatal outcomes in women with multiple sclerosis (MS) compared with the general population.
Methods
We conducted a cross-sectional study including pregnancies from January 1, 1997, to December 31, 2016, to women registered in the Danish Multiple Sclerosis Registry (the study cohort). Pregnancy-related and perinatal outcomes were compared with a randomly selected subcohort of pregnancies from the general population (the comparison cohort) using logistic regression adjusted for possible confounders.
Results
In total, 2,930 pregnancies were included in the study cohort and 56,958 pregnancies in the comparison cohort. No differences were found in pregnancy-related complications (preeclampsia/gestational diabetes or placenta complications), emergency caesarean section (c-section), instrumental delivery, low Apgar score, stillbirth, preterm birth, or congenital malformations. Elective c-section (odds ratio [OR] 1.89 [95% confidence interval (CI) 1.65–2.16]), induced delivery (OR 1.15 [95% CI 1.01–1.31]), and being born small for gestational age (SGA) (OR 1.29 [95 %CI 1.04–1.60]) had a higher prevalence in the study cohort, whereas the prevalence of signs indicating asphyxia was lower in the study cohort (OR 0.87 [95% CI 0.78–0.97]) relative to the comparison cohort.
Conclusion
We found a higher prevalence of elective c-sections, induced delivery, and infants being SGA among newborns to women with MS, whereas the prevalence of asphyxia was lower in the study cohort. There were no significant differences in severe adverse perinatal outcomes when comparing women with MS and their newborns with those of the general population.

Pregnancy and childbirth in women with multiple sclerosis (MS) are not considered high-risk clinical issues; however, some outcomes are still a matter of debate.
Women account for approximately 75% of patients with MS1 and are commonly diagnosed at age 20–40 years—their reproductive years.2 MS is a progressive, immune-mediated, neurologic disease, and its treatment is complex and requires careful consideration of several long-term and short-term factors, of which family planning should be included.
Today, women diagnosed with MS (wwMS) are not discouraged from having children, although the risk of pregnancy-related complications and adverse perinatal outcomes remains debated. Several studies have investigated pregnancy complications and perinatal outcomes in wwMS compared with the general population with diverging results and no affirmative conclusions.3–9 Some studies have reported no differences in outcomes related to either mother or child,6,10,11 whereas others reported differences in rates of induction of delivery3 and caesarean section (c-section),3,4,7 lower Apgar score,5 increased risk of assisted delivery,8 infections during pregnancy, and preterm delivery.9
Denmark has universal health care and nationwide population-based registers that provide excellent possibilities for epidemiologic research with high generalizability. The objective of this study was to investigate whether the prevalence of pregnancy-related outcomes in wwMS and perinatal outcomes in newborns of wwMS differed from those of the general population using nationwide register-based data from The Danish Multiple Sclerosis Registry (DMSR)12 and registers on pregnancy-related complications and perinatal outcomes.
Methods
Study Design and Study Population
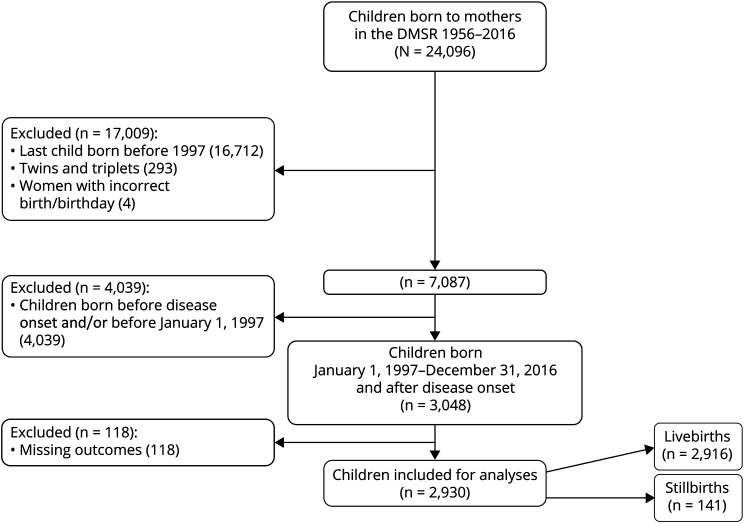
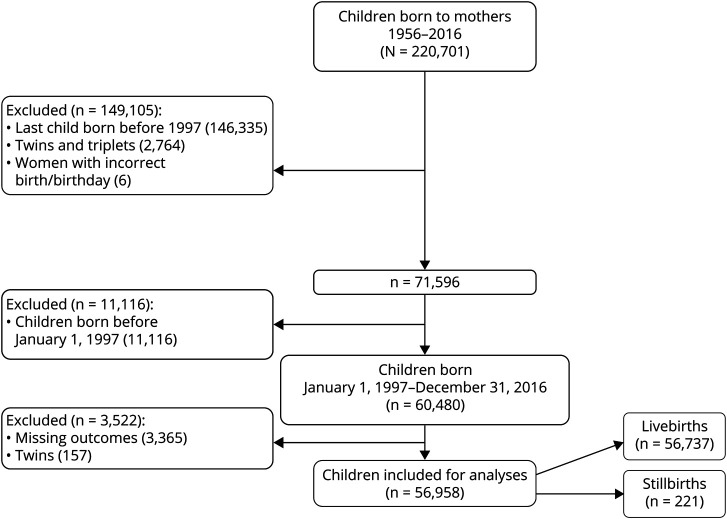
We conducted a cross-sectional study using nationwide data in which we included all births to women registered in the DMSR resulting in either live or stillbirth from January 1, 1997, to December 31, 2016, “the study cohort.” The DMSR12 provided data on wwMS confirmed by a neurologist according to the diagnostic criteria of the time.13–15 We compared pregnancy-related and perinatal outcomes in the study cohort with births from the general population, “the comparison cohort.” The comparison cohort consisted of a 5% random sample of women from the general population and their births randomly identified through the Danish Civil Registration System.16 Flowcharts of the study and comparison cohorts are illustrated in figures 1 and 2, respectively.
Figure 1. Inclusion Flowchart of the Study Cohort.
Figure 2. Inclusion Chart of the Comparison Cohort.
Data Sources
The DMSR is a nationwide population-based registry established in 1956.17 The DMSR continuously collects information on patients diagnosed with MS: demographical, clinical, and paraclinical data such as date of onset and diagnosis, relapses, Expanded Disability Status Scale (EDSS), and disease-modifying therapy (DMT). Since 1996, it has been mandatory for all 14 neurologic departments in Denmark to regularly report follow-up data on all patients treated with DMT.18 Data from the DMSR were linked to national health registries by use of the unique 10-digit personal identification number which all Danish inhabitants have.
The Danish National Patient Register (DNPR) was established in 1977 and encompasses all contacts from public and private hospitals or outpatient clinics in Denmark. It contains demographic data, diagnosis, information on type of contact, and dates.19 The DNPR provided demographic information on the women included and abortions (both spontaneous and medically induced abortions). The Register of Legally Induced Abortions (RLIA) contains data on all induced abortions performed in Denmark including those from private physicians licensed to perform legal abortions,20 hence added data from private physicians not available from the DNPR. The Danish Medical Birth Register (DMBR)21 was established in 1973 and contains specific variables on pregnancy, delivery, and perinatal outcomes registered by health care professionals during pregnancy and birth. Diagnoses are based on the Danish Healthcare Classification System including International Classification of Disease codes (version 10) (ICD-10).21 The date of conception was defined as the birth date subtracted the gestational age in days. The Population's Education Register was established in 1981 and provides information on individual education achievements.22
Outcomes and Covariates
Outcomes
Pregnancy-related outcomes were defined as pregnancy-related complications, namely, preeclampsia (ICD-10: O14 with subcodes), gestational diabetes (ICD-10: O24.4 and O24.9), and placenta complications (placenta abruptio or placenta accreta) confirmed by a health care professional at birth.
Delivery mode was classified into spontaneous delivery (ICD-10: O80, O80.0, O80.1, O80.8, O80.9, and O83.8), emergency c-section (ICD-10: O82.1, a, b, and c), elective c-section (ICD-10: O82, O82.0, O82.2, O82.8, and O82.9), induced delivery (ICD-10: O80.2, O80.3, and O83.8a), or instrumental delivery (ICD-10: O81 with subcodes O83.2).
Perinatal Outcomes
Preterm birth was defined as delivery before gestational week 37 and stillbirth as birth of a dead fetus after completion of gestational week 22 in accordance with the literature.23 Small for gestational age (SGA) was defined as birth weight below the mean minus 2 times SDs as described by Sankilampi et al.24 and their standards were used as reference. SGA was calculated for each gestational week and separately for boys and girls.
A low Apgar score was defined as below 7. It is used as a quick assessment of the postnatal condition of the newborn and is defined as “reassuring of normality” if the score is above 6 points.25 Signs of asphyxia are partly based on components from the Apgar evaluation and partly from umbilical cord blood analysis.23
Congenital malformations are usually recorded at birth but may be retrospectively registered within the first year of life.21 A congenital malformation in this study was defined as a registration of at least one of the ICD-10 codes: Q00-99.
Covariates
Covariates were chosen based on existing knowledge from the literature. Calendar year of birth was categorized into 5 groups of 4-year intervals (1997–2000, 2001–2004, 2005–2008, 2009–2012, and 2013–2016) and introduced in the models to adjust for a possible cohort effect. Highest attained educational level of the mother was categorized into 4 groups (edu1, primary school equivalent to 9 years; edu2, secondary school equivalent to 10–12 years; edu3, vocational education/short or medium higher education equivalent to bachelor equivalent to 13–16 years; and edu4, longer higher education above bachelor equivalent to more than 16 years) as previous studies have shown an inverse association between educational level and adverse perinatal outcomes.26 Maternal age at birth was categorized into 4 groups (<25 years, 25–30 years, 31–35 years, and >35 years) as increased maternal age is associated with the risk of adverse outcome for both mother and neonate.27 Prior abortions and c-sections were adjusted for as binary variables (yes/no) as both have been shown to be associated with the risk of adverse perinatal outcomes.28 Parity, including prior stillbirth, was categorized into 3 groups defined by the index pregnancy (primipara, secundipara, or multipara).
Statistical Analyses
Baseline demographic and clinical characteristics are presented as frequencies with corresponding percentages for categorical variables. Continuous variables were summarized using mean and SD or median and interquartile range as appropriate.
Complete-cases analyses were used to estimate the prevalence of the pregnancy-related and perinatal outcomes by calculating the point prevalence for the full cohort.
We used logistic regression to calculate odds ratios (ORs) with 95% confidence intervals (CIs) for the association between MS and pregnancy/perinatal outcomes for both binary and multinomial outcomes using the SAS proc GEE (generalized estimating equations) procedure to account for the clustered nature of data. Analyses were performed both unadjusted and adjusted for clinically relevant covariates. Statistical significance was defined as p < 0.05. SAS Enterprise Guide version 7.15 was used for all statistical analyses.
We used backward elimination to identify possible predictors of those outcomes found to be significantly associated with MS in the main analysis. Only the study cohort consisting of births of wwMS was included in this analysis. We included all variables from the fully adjusted models plus exposure to DMT defined as maternal DMT treatment (both first- and second-line drugs) within 6 months before conception (DMT-exposed newborns). The significance level for staying in the model was set to p < 0.05.
The following subanalyses were performed: (1) a complete-case analysis with further adjustment for prepregnancy body mass index (BMI) (binary; normal 15–24.9 and overweight 25–60) when this information was available after 2003 and (2) predictor models among a subgroup of the study cohort with an available EDSS score up to 2 years before conception. Only outcomes found to be statistically significantly associated with having MS in the main analyses were investigated.
Standard Protocol Approvals, Registrations, and Patient Consents
This study was approved by the Danish Data Protection Agency (j.nr.:2012-58-0004). Noninterventional register-based studies do not require ethical approval in Denmark.
Data Availability
Anonymized data will be shared on request from any qualified researcher under approval from the Danish Data Protection Agency.
Results
We included 2,930 births in the study cohort (2,916 live births and 14 stillbirths) of 1,953 individual women with onset of MS before conception (figure 1). The pregnancy-related complications and perinatal outcomes were compared with 56,958 births (56,737 live births and 221 stillbirths) of 32,767 individual women from the general population (figure 2). Each pregnancy was treated as an individual pregnancy in the descriptive statistics.
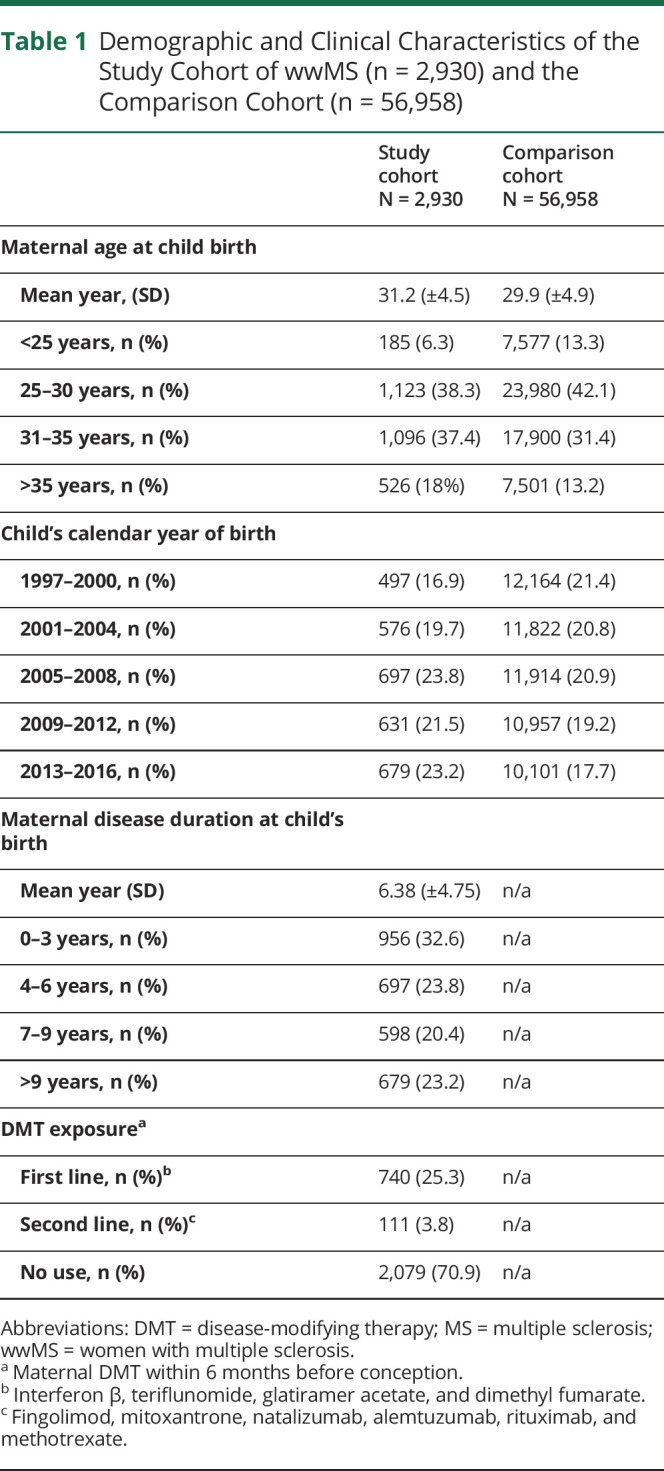
wwMS (mothers of the study cohort) were slightly older than mothers of the comparison cohort when giving birth (mean age of 31.2 years vs 29.9 years), respectively. wwMS had a mean disease duration of 6.38 years (±4.75) when giving birth, and their mean age at disease onset was 25.9 years (±5.4) as presented in table 1.
Table 1.
Demographic and Clinical Characteristics of the Study Cohort of wwMS (n = 2,930) and the Comparison Cohort (n = 56,958)
Pregnancy-Related Outcomes
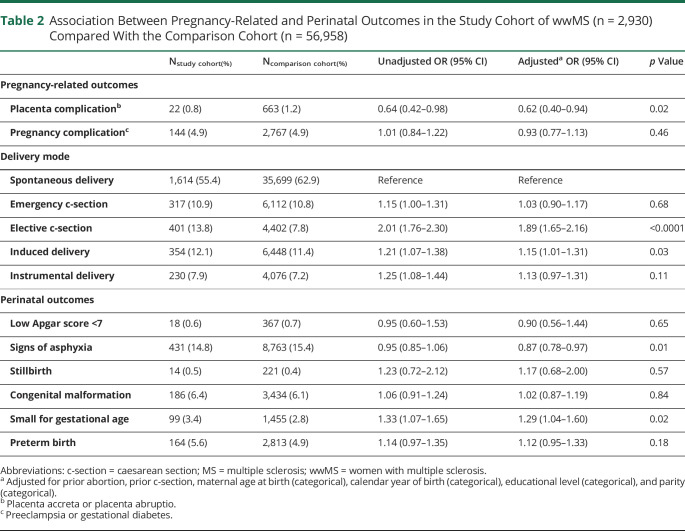
A lower odds of placenta complications was found in the study cohort (OR 0.62, 95% CI 0.40–0.94) relative to the comparison cohort, although the prevalence was very low in both cohorts (0.8% in the study cohort vs 1.2% in the comparison cohort) (table 2). No difference in odds of other pregnancy complications was found between the 2 cohorts (OR 0.93, 95% CI 0.77–1.13) (table 2).
Table 2.
Association Between Pregnancy-Related and Perinatal Outcomes in the Study Cohort of wwMS (n = 2,930) Compared With the Comparison Cohort (n = 56,958)
Delivery Mode–Related Outcomes
The prevalence of spontaneous deliveries was 55.4% and 62.9% in the study cohort and the comparison cohort, respectively (table 2). Compared with spontaneous delivery, the odds of elective c-section (OR 1.89, 95% CI 1.65–2.16) and induced delivery (OR 1.15, 95% CI 1.01–1.31) were higher in the study cohort.
Perinatal-Related Outcomes
The odds of signs of asphyxia were lower among newborns of wwMS (OR 0.87, 95% CI 0.78–0.97), whereas the odds of being born SGA was higher in the study cohort (OR 1.29, 95% CI 1.04–1.60) when compared with the comparison cohort (table 2).
Predictor Analyses Among Births of Women With MS
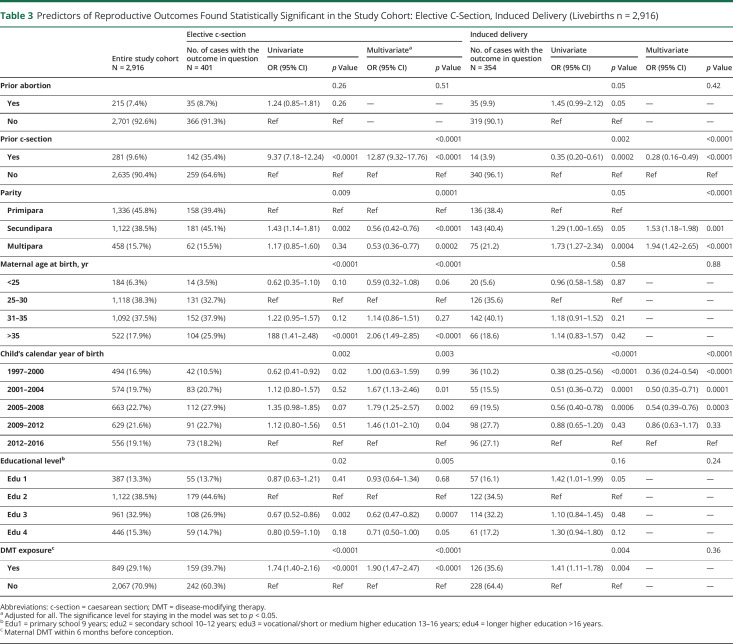
Maternal age above 35 years was associated with higher odds of elective c-section, as were previous c-section, DMT exposure (all p < 0.0001), and giving birth between 2001 and 2012 (p = 0.003). On the other hand, being secundipara or multipara and higher educational level were associated with lower odds of having an elective c-section (p = 0.0001 and p = 0.005, respectively) (table 3).
Table 3.
Predictors of Reproductive Outcomes Found Statistically Significant in the Study Cohort: Elective C-Section, Induced Delivery (Livebirths n = 2,916)
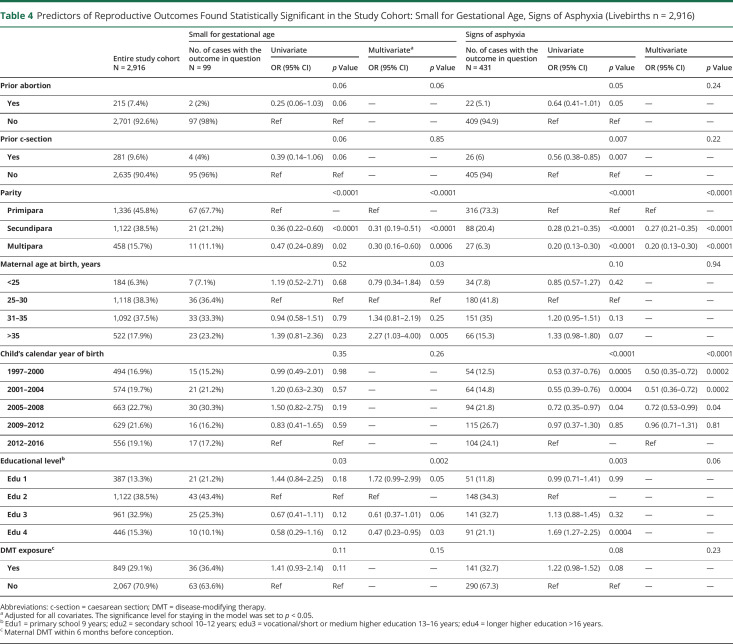
Regarding induced delivery, we found a higher odds in births of wwMS with 2 or more previous pregnancies, whereas previous c-section and giving birth before 2008 lowered the odds (all p < 0.0001) (table 3). Furthermore, 2 or more previous births and births before 2008 were associated with decreased odds of signs of asphyxia (both p < 0 .0001) (table 4).
Table 4.
Predictors of Reproductive Outcomes Found Statistically Significant in the Study Cohort: Small for Gestational Age, Signs of Asphyxia (Livebirths n = 2,916)
Maternal age above 35 years was strongly associated with higher odds of SGA newborns (p = 0.005) as was DMT exposure, although only in the univariate analysis and without reaching statistical significance (p = 0.11). Being secundipara or multipara (p < 0 .0001) and a higher maternal educational level (edu3 or 4, p = 0.001) were associated with lower odds for SGA (table 4).
Subanalyses
The subgroup analysis included only women with an available prepregnancy BMI (study cohort 90.2% and comparison cohort 88.7% of the eligible, respectively). It showed similar, although slightly less precise, results than the main analysis (table e-1, links.lww.com/CPJ/A243). Among births of wwMS, a BMI above 25 was associated with higher odds of elective c-section in the univariate model, but not in the multivariate model, whereas it remained statistically significant for induced delivery in both models (table e-2, e-3).
wwMS with an available EDSS (32.8%) had longer mean disease duration when giving birth (7.51 years [±4.27]) compared with those without (5.84 [±4.88]). Furthermore, 82.8% of those with an available EDSS were treated with DMT, whereas only 2.8% without available EDSS had been treated before conception (table e-4, links.lww.com/CPJ/A243). Other clinical characteristics were similar. Women with a higher EDSS score (≥3) showed increased odds of having elective c-section compared with those with an EDSS score of <3 (tables e-5 and e-6).
Discussion
In this nationwide population-based study, we investigated whether the prevalence of pregnancy complications and perinatal outcomes of newborns to wwMS differed from the general population using 20 years of follow-up data. We found no differences in pregnancy-related complications but higher odds of having an elective c-section and induced delivery among wwMS. The newborns of wwMS had lower odds of demonstrating signs of asphyxia but higher odds of being SGA when compared with the general population.
Among births of wwMS alone, independent predictors with elective c-section were prior c-section; giving birth between 2001 and 2012; exposure to DMT; maternal age above 35 years; and prepregnancy EDSS ≥3. Prepregnancy BMI above 24.9 and being secundipara or multipara were associated with a higher odds of induced delivery, whereas giving birth between 1997 and 2012 and prior c-section showed a decreased odds with the same mode of delivery. Children of mothers older than 35 years had a higher odds of being SGA, whereas secundipara or multipara and a higher educational level were associated with lower odds of being SGA.
Worldwide low birth weight is defined as an absolute weight <2,500 g at birth but disregards the gestational age. SGA takes gestational week, sex, and singleton/twins into consideration because these contribute importantly to the birth weight. The birth weight is important because low birth weight increases the risk of death and developing chronic, neurologic disabilities, and academic achievements.29,30 Only limited data are available on whether DMT exposure before or during the pregnancy affects the fetus and to which extent, nevertheless, it is documented that several DMTs can cross the placental barrier.31 Whether the birth weight is affected by DMT remains controversial. A recent register study using data from the Swedish and Finnish medical birth registers compared 643 newborns exposed to interferon β within 3 months or later before the last menstrual period with 1,166 unexposed newborns and found no difference in birth weight.32 Contrarily, a Portuguese multicenter cohort included 97 births, of which 65 (67%) were exposed to DMT mainly in the first trimester (mean 8 weeks ± 9.2 weeks) and found a nonsignificant trend toward lower birth weight in newborns exposed to DMT (p = 0.054).6
In our study cohort, 851 (29%) of the newborns were exposed to DMT. On average, exposed newborns were 116 g lighter than those unexposed (3,378 vs 3,494 g) and their median gestational age correspondingly lower (39 vs 40 weeks) (data not shown). SGA occurred in 4.2% (n = 36) of the exposed newborns compared with 3% among those unexposed. We found a slightly increased odds of the newborn being SGA if exposed to DMT in the univariate analysis, although not reaching the significance level.
Two other register studies including 181 and 649 births, respectively, both demonstrated a higher prevalence of SGA (8.8% and 13.5%, respectively) in newborns of wwMS compared with our prevalence of 3.4%.4,10 The English study did not find any difference in OR,10 whereas the Norwegian study found a 1.45 higher odds of being SGA (p = 0.003) among newborns of wwMS.4 Neither of the 2 adjusted for DMT in their analyses and the definition of SGA is different—both in between the 2 former studies, and from ours, which complicates the comparability of the results.
To date, most women have been advised to discontinue treatment when planning a pregnancy as evidence related to the possible effect of DMT during pregnancy on the fetus is sparse. Several studies from the past 10 years have found no differences in serious pregnancy-related complications or severe adverse perinatal outcomes such as stillbirth or major congenital malformations in newborns of wwMS, although not taking DMT exposure into consideration.3,4,9–11 A few studies that included fetal DMT exposure in the analysis reached the same conclusions, although most of the studies are based on small sample sizes, different DMTs, and different definitions of exposure.5–8 On the contrary, a few studies have demonstrated an increased risk of congenital malformations in newborns exposed to second-line DMTs with high efficacy during the pregnancy.33,34
On average, elective c-sections comprised 8.3% of the total number of deliveries in Denmark from 2006 to 2016.21 This corresponds with the percentage found in our comparison cohort (7.8%), whereas the corresponding number was 13.8% in the study cohort. The higher prevalence of elective c-sections among wwMS most likely explains the corresponding lower odds of asphyxia.35 A Finnish and a Portuguese study7,8 similarly reported an increased prevalence of elective c-sections (11.5% and 14.3%, respectively) among wwMS. A systematic review and meta-analysis reported the range of cesarean deliveries among wwMS to be 9–41%,36 although not distinguishing between emergency and elective c-sections.
The frequency of induced delivery in Denmark also increased in the period 2000–2012 from approximately 12%–25% of the total deliveries per year with a steep increase in 2010.37
The proportion of induced deliveries in our study cohort comprised 12.1% of the total deliveries in the included study period 1997–2016, which corresponds with the nationwide range.
Possible explanations of the increased prevalence of elective c-section and induced delivery among wwMS could include MS-related symptoms such as neuromuscular perineal weakness, spasticity, or fatigue that might affect the birth. Any of these could accelerate exhaustion and lead to delivery complications, which could prompt the clinician and women to take extra precautions.
In our study, women with an EDSS score of ≥3 had a higher odds of having an elective c-section, supporting the importance of MS-related symptoms on clinical decision making.
Strengths of this study are the population-based nationwide long-time follow-up data of the DMSR, the nearly complete national coverage of reproductive registries, thereby eliminating recall bias. Furthermore, all Danish inhabitants have access to universal health care, which is government funded through taxation, hence providing equal access to all citizens increasing the generalizability of our findings. A limitation of the study is the lack of data on maternal smoking as the risk of being SGA increases due to maternal smoking.38 A rather large proportion of our cohorts had missing data on BMI (study cohort 27% vs comparison cohort 32%); however, the subgroup analysis showed similar results as the main analysis indicating that data were missing at random, and hence, the generalizability to the full cohort is acceptable. Finally, the sample size of the selected population of newborns exposed to DMT within 6 months before conception that experienced an outcome was too small to stratify on either first-line or second-line DMT or perform any separate statistical analyses.
In this large Danish cohort, newborns of wwMS were more frequently delivered by elective c-section or induced delivery. More children of wwMS were born SGA but showed less sign of asphyxia compared with the general population.
Acknowledgment
The authors thank Scleroseforeningen (The Danish MS Society) for their support.
Appendix. Authors
Study Funding
No targeted funding reported.
Disclosure
J.B. Andersen has received travel and congress participation funds from Merck. T.I. Kopp served on scientific advisory board and received speaker honoraria from Novartis. F. Sellebjerg has served on scientific advisory boards, been on the steering committees of clinical trials, served as a consultant, received support for congress participation, received speaker honoraria, or received research support for his laboratory from Biogen, Merck, Novartis, Roche, Sanofi Genzyme, and Teva. M. Magyari has served on scientific advisory board for Biogen, Sanofi, Teva, Roche, Novartis, and Merck; has received honoraria for lecturing from Biogen, Merck, Novartis, Sanofi, and Genzyme; and has received research support and support for congress participation from Biogen, Genzyme, Teva, Roche, Merck, and Novartis. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Koch-Henriksen N, Sørensen PS. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol 2010;9:520–532. [DOI] [PubMed] [Google Scholar]
- 2.Koch-Henriksen N, Thygesen LC, Stenager E, Laursen B, Magyari M. Incidence of MS has increased markedly over six decades in Denmark particularly with late onset and in women. Neurology 2018;90:1954–1963. [DOI] [PubMed] [Google Scholar]
- 3.Fong A, Chau CT, Quant C, Duffy J, Pan D, Ogunyemi DA. Multiple sclerosis in pregnancy: prevalence, sociodemographic features, and obstetrical outcomes. J Matern Neonatal Med 2018;31:382–387. [DOI] [PubMed] [Google Scholar]
- 4.Dahl J, Myhr KM, Daltveit AK, Hoff JM, Gilhus NE. Pregnancy, delivery, and birth outcome in women with multiple sclerosis. Neurology 2005;65:1961–1963. [DOI] [PubMed] [Google Scholar]
- 5.Yalcin SE, Yalcin Y, Yavuz A, Akkurt MO, Sezik M. Maternal and perinatal outcomes in pregnancies with multiple sclerosis: a case-control study. J Perinat Med 2017;45:455–460. [DOI] [PubMed] [Google Scholar]
- 6.Novo A, Castelo J, de Sousa A, et al. Pregnancy outcomes in Portuguese women with multiple sclerosis: the PREGNIMS study. Mult Scler Relat Disord 2019;28:172–176. [DOI] [PubMed] [Google Scholar]
- 7.Jesus-Ribeiro J, Correia I, Martins AI, et al. Pregnancy in multiple sclerosis: a Portuguese cohort study. Mult Scler Relat Disord 2017;17:63–68. [DOI] [PubMed] [Google Scholar]
- 8.Jalkanen A, Alanen A, Airas L. Pregnancy outcome in women with multiple sclerosis: results from a prospective nationwide study in Finland. Mult Scler 2010;16:950–955. [DOI] [PubMed] [Google Scholar]
- 9.Macdonald SC, McElrath TF, Hernández-Díaz S. Pregnancy outcomes in women with multiple sclerosis. Am J Epidemiol 2019;188:57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldacre A, Pakpoor J, Goldacre M. Perinatal characteristics and obstetric complications in mothers with multiple sclerosis: record-linkage study. Mult Scler Relat Disord 2017;12:4–8. [DOI] [PubMed] [Google Scholar]
- 11.Van Der Kop ML, Pearce MS, Dahlgren L, et al. Neonatal and delivery outcomes in women with multiple sclerosis. Ann Neurol 2011;70:41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koch-Henriksen N, Magyari M, Laursen B. Registers of multiple sclerosis in Denmark. Acta Neurol Scand 2015;132:4–10. [DOI] [PubMed] [Google Scholar]
- 13.Allison RS, Millar JH. Prevalence of disseminated sclerosis in Northern Ireland. Ulster Med J 1954;23(suppl 2):1–27. [PMC free article] [PubMed] [Google Scholar]
- 14.Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol 1983;13:227–231. [DOI] [PubMed] [Google Scholar]
- 15.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the Diagnosis of Multiple Sclerosis. Ann Neurol 2015;28:193–205. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt M, Pedersen L, Sørensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol 2014;29:541–549. [DOI] [PubMed] [Google Scholar]
- 17.Koch-Henriksen N, Magyari M, Laursen B. Registers of multiple sclerosis in Denmark. Acta Neurol Scand 2015;132:4–10. [DOI] [PubMed] [Google Scholar]
- 18.Magyari M, Koch-Henriksen N, Sørensen PS. The Danish Multiple Sclerosis Treatment Register. Clin Epidemiol 2016;8:549–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmidt M, Schmidt SAJ, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol 2015;7:449–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tølbøll Blenstrup L, Knudsen LB. Danish registers on aspects of reproduction. Scand J Public Health 2011;39:79–82. [DOI] [PubMed] [Google Scholar]
- 21.Bliddal M, Broe A, Pottegård A, Olsen J, Langhoff-Roos J. The Danish Medical Birth Register. Eur J Epidemiol 2018;33:27–36. [DOI] [PubMed] [Google Scholar]
- 22.Jensen VM, Rasmussen AW. Danish education registers. Scand J Public Health 2011;39:91–94. [DOI] [PubMed] [Google Scholar]
- 23.Sundhedsdatastyrelsen. esundhed.dk. 2019. Available from: end2019.esundhed.dk/sundhedsregistre/MFR/Sider/MFR06A.aspx. Accessed March 5, 2020. [Google Scholar]
- 24.Sankilampi U, Hannila ML, Saari A, Gissler M, Dunkel L. New population-based references for birth weight, length, and head circumference in singletons and twins from 23 to 43 gestation weeks. Ann Med 2013;45:446–454. [DOI] [PubMed] [Google Scholar]
- 25.American Academy of Pediatrics Committee on Fetus and Newborn. Use and abuse of the Apgar score. Pediatrics 1996;98:141–142. [PubMed] [Google Scholar]
- 26.Bilsteen JF, Andresen JB, Mortensen LH, Hansen AV, Andersen AMN. Educational disparities in perinatal health in Denmark in the first decade of the 21st century: a register-based cohort study. BMJ Open 2018;8:e023531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lean SC, Derricott H, Jones RL, Heazell AEP. Advanced maternal age and adverse pregnancy outcomes: a systematic review and meta-analysis. PLoS One 2017;12:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fitzpatrick KE, Kurinczuk JJ, Bhattacharya S, Quigley MA. Planned mode of delivery after previous cesarean section and short-term maternal and perinatal outcomes: a population-based record linkage cohort study in Scotland. PLOS Med 2019;16:e1002913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cutland CL, Lackritz EM, Mallett-Moore T, et al. Low birth weight: case definition & guidelines for data collection, analysis, and presentation of maternal immunization safety data. Vaccine 2017;35:6492–6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blencowe H, Krasevec J, de Onis M, et al. National, regional, and worldwide estimates of low birthweight in 2015, with trends from 2000: a systematic analysis. Lancet Glob Health 2019;7:e849–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palmeira P, Quinello C, Silveira-Lessa AL, Zago CA, Carneiro-Sampaio M. IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol 2012;2012:985646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burkill S, Vattulainen P, Geissbuehler Y, et al. The association between exposure to interferon-beta during pregnancy and birth measurements in offspring of women with multiple sclerosis. PLoS One 2019;14:e0227120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hellwig K, Thiel S, Meinl I, Gold R, Kümpfel T. Long-term exposure to natalizumab during pregnancy - a prospective case series from the German Multiple Sclerosis and Pregnancy Registry. ECTRIMS Online Library 2018; Abstract 204. [Google Scholar]
- 34.Karlsson G, Francis G, Koren G, et al. Pregnancy outcomes in the clinical development program of fingolimod in multiple sclerosis. Neurology 2014;82:674–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karlström A, Lindgren H, Hildingsson I. Maternal and infant outcome after caesarean section without recorded medical indication: findings from a Swedish case-control study. BJOG 2013;120:479–486. [DOI] [PubMed] [Google Scholar]
- 36.Finkelsztejn A, Brooks JBB, Paschoal FM, Fragoso YD. What can we really tell women with multiple sclerosis regarding pregnancy? A systematic review and meta-analysis of the literature. BJOG 2011;118:790–797. [DOI] [PubMed] [Google Scholar]
- 37.Hedegaard M, Lidegaard Ø, Skovlund CW, Mørch LS, Hedegaard M. Reduction in stillbirths at term after new birth induction paradigm: results of a national intervention. BMJ Open 2014;4:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ko TJ, Tsai LY, Chu LC, et al. Parental smoking during pregnancy and its association with low birth weight, small for gestational age, and preterm birth offspring: a birth cohort study. Pediatr Neonatol 2014;55:20–27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared on request from any qualified researcher under approval from the Danish Data Protection Agency.