Abstract
Purpose of Review
Idiopathic intracranial hypertension (IIH) prevalence increased in conjunction with rising obesity rates. Here, we highlight the importance of weight management in IIH and introduce glucagon-like peptide 1 (GLP-1) receptor agonists (RAs) as potential treatment strategy for IIH.
Recent Findings
Weight gain is a risk factor for IIH, and weight loss (via any treatment strategy) plays a key role in IIH management. GLP-1 is an incretin secreted by the distal small intestine in response to a meal. GLP-1 RAs have been shown to improve glycaemic control (no hypoglycaemia) and lower body weight in patients with and without type 2 diabetes. The choroid plexus has been found to express GLP-1 receptors, and treatment with a GLP-1 RA significantly reduces CSF secretion in vitro and intracranial pressure (ICP) in rodents.
Summary
New research evaluating the pathophysiology of IIH supports GLP-1 RA as a potential treatment for IIH via weight loss dependent and independent mechanism to directly reduce ICP.

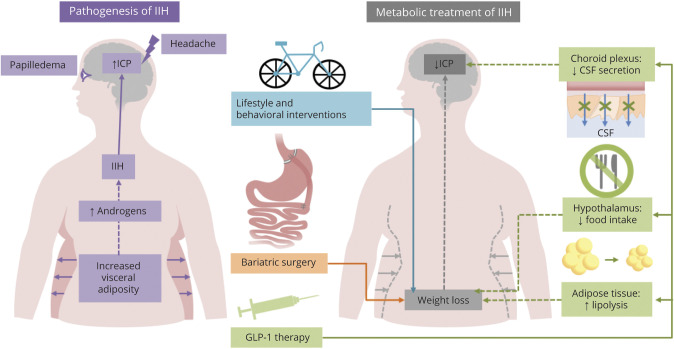
Idiopathic intracranial hypertension (IIH) is an increasingly observed condition characterized by raised intracranial pressure (ICP), papilloedema with the potential risk of permanent visual loss, and debilitating headache, which profoundly reduces the quality of life. 1,2 IIH predominately affects women of reproductive age and importantly has an established association with obesity. 3,4 The predominant symptom of IIH is headache, which is typically migraine-like. Obesity has also been shown to be a risk factor for headaches and migraine.5 Obesity is a complex disease, and the World Health Organization estimates that the rates have tripled since 1975. Similarly, the incidence of IIH has increased by more than 250% between 2005 and 2017 in women and correlates with the female obesity rate.4 The individual's weight threshold to develop IIH is not clear,6 although weight is not a reliable indicator of visceral adiposity. The population risk of developing IIH increases exponentially in those with a body mass index > 30 kg/m24 (figure) with weight gain (5%–15%)—a risk for both developing and experiencing a recurrence of IIH.7
Figure. Infogram Detailing Known Pathogenic Factors and Potential Targeted Treatments for IIH.
GLP-1 = glucagon-like peptide 1; ICP = intracranial pressure; IIH = idiopathic intracranial hypertension.
For more than half a century, weight loss has been identified as an effective treatment for IIH, with a reduction in body weight of between 3% and 15% resulting in disease remission.6–8 Application of a very low-calorie diet to induce weight loss (approximately 15%) resulted in significantly lowered ICP, improved papilloedema, and a 50% improvement in headache frequency and severity, with a concomitant reduction in analgesic use.8 Weight loss specifically from the truncal region was noted to be associated with disease remission in IIH, with truncal fat mass also correlating with ICP.9 These findings potentially implicate truncal adiposity in driving disease activity in IIH; however, the pathogenic role of obesity is not fully understood. A unique profile of androgen excess has been identified in IIH (distinct to that observed in those with simple obesity and polycystic ovarian syndrome), which drives dysregulation of cerebral spinal fluid dynamics in vitro.10 The androgen excess in IIH is not of ovarian origin and is more likely to be generated from the truncal adiposity, a known source of androgen synthesis. IIH is also associated with a twofold risk of cardiovascular disease independent of patient obesity.4 These studies imply that IIH is more than a disease of the CNS axis but a systemic metabolic disease.
Although the therapeutic impact of weight loss in IIH is clear, the degree of weight loss and optimal strategy necessary to induce remission is not established and may be patient-specific. A low salt diet is often cited, but there is minimal evidence in this population. Importantly, lifestyle and behavioral interventions typically result in 5% weight loss.11 Weight loss drugs may have a role, although similarly they may not achieve the degree of weight loss necessary to affect disease activity (e.g., orlistat in conjunction with a lifestyle intervention achieves an additional 2.5 kg weight loss above that achieved from lifestyle intervention alone).12 Maintenance of weight loss is also a challenge, with patients on average regaining one-third to one-half of the weight that was lost at one year and returning to their original weight within 5 years.13
Currently, bariatric surgery is the most effective method to achieve lasting weight reduction, with 15%–30% weight loss over 15–20 years depending on the surgical procedure.14 The beneficial effects of bariatric surgery in IIH have been highlighted in a number of cases studies. 15,16 However, detailed evaluation with prospective and randomized controlled trials leading to class one evidence is currently lacking. Intriguingly, bariatric surgery may offer patients with IIH sustained disease remission with a cost comparable with ventriculoperitoneal shunting with a programmable valve. The efficacy and evidence for bariatric surgery in the management of IIH will be assessed when a randomized controlled trial reports on evaluating bariatric surgery against a community weight loss program.17
A number of novel hormonal and metabolic therapeutic targets are emerging for IIH, the most promising being glucagon-like peptide 1 (GLP-1) receptor agonism. GLP-1 receptor agonists (RAs) (exenatide, liraglutide, lixisenatide, semaglutide, albiglutide, and dulaglutide) are widely used in the treatment of type 2 diabetes mellitus (they do not induce hypoglycaemia) and more recently to manage obesity.18 In the context of IIH pathophysiology, the use of GLP-1 RAs extends beyond the weight-modifying effects (figure). GLP-1 receptor has been identified in human and rodent choroid plexus epithelium, which, when stimulated with the GLP-1 RA exenatide, reduces CSF secretion through a cyclic adenosine monophosphate protein kinase—a signaling pathway with subsequent inhibition of Na+K+ adenosine triphosphatase ion channel activity (a key regulator of CSF secretion)—thereby reducing ICP. Pathologically elevated ICP can be reduced by nearly 50% in rats with intracranial hypertension treated with exenatide, with effects sustained over a week.19 This striking effect on ICP is not seen when rats are treated with clinically relevant doses of acetazolamide, the most commonly used medicine in IIH; of note, topiramate significantly reduces ICP in vivo.20
Mounting evidence demonstrates that weight gain and obesity are modifiable risk factors for IIH disease activity. Obesity is now recognized as a disease entity (World Health Organization, US Food and Drug Administration, and the American Medical Association) with growing realization that it is not driven by personal choice, but a relapsing and remitting disease determined by genetic, biological, and environmental factors interacting. Obesity stigma among healthcare professionals, the public, and policy makers and weight bias internalization have been associated with poorer patient outcomes, detrimental effects on mental health, and avoidance of health care.21 Current obstacles to management include a lack of sensitivity when discussing obesity, apprehension of negative connotations, concerns about deleterious effects on the doctor-patient relationship, and a lack of knowledge.22 Obesity management strategies in IIH are evolving with lifestyle and behavioral interventions, bariatric surgery, and novel therapies, such as GLP-1 RAs, which directly target CSF secretion and have weight-lowering effects. The pathophysiology detailed above necessitates the performance of effective research programs and well-designed trials in IIH to (1) test the effects of GLP-1 agonists for comorbid IIH and obesity, (2) establish ideal strategies to promote weight loss, and (3) examine the functional role and potential to therapeutically target androgen excess.
As the IIH rates are rising consistently with the global obesity epidemic, healthcare professionals and patients with IIH should not hesitate from assessment, discussion, and management of obesity because this is central to sustained remission of IIH.23
Appendix. Authors
Footnotes
Editorial, page 271
Study Funding
A.J. Sinclair is funded by a Sir Jules Thorn Award for Biomedical Science. A.A. Tahrani: none. S.P. Mollan.: none. No grants or funds were used in preparation of this manuscript.
Disclosure
S.P. Mollan: Invex therapeutics advisory board (2019). A.A. Tahrani reports personal fees and nonfinancial support from Novo Nordisk. A.S. Sinclair: Invex therapeutics, company director with salary and stock options (2019, 2020). Full disclosure form information provided by the authors is available
TAKE-HOME POINTS
→ Recent weight gain is a risk factor for development of IIH.
→ Weight loss has a key role in management of IIH.
→ GLP-1 is a gut neuropeptide, and GLP-1 analogues are successfully used in patients with and without type 2 diabetes mellitus to aide weight loss and improve long-term outcomes.
→ GLP-1 receptors have been identified in the choroid plexus, and antagonism reduces CSF secretion and reduces ICP in rodent models.
→ GLP-1 RAs may have a potential role in targeting obesity and ICP in IIH.
with the full text of this article at Neurology.org/cp.
References
- 1.Mollan SP, Davies B, Silver NC, et al. Idiopathic intracranial hypertension: consensus guidelines on management. J Neurol Neurosurg Psychiatry 2018;89(10):1088–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mulla Y, Markey KA, Woolley RL, Patel S, Mollan SP, Sinclair AJ. Headache determines quality of life in idiopathic intracranial hypertension. J Headache Pain 2015;16:521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mollan SP, Aguiar M, Evison F, Frew E, Sinclair AJ. The expanding burden of idiopathic intracranial hypertension. Eye 2018;33:478–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adderley NJ, Subramanian A, Nirantharakumar K, et al. Association between idiopathic intracranial hypertension and risk of cardiovascular diseases in women in the United Kingdom. JAMA Neurol 2019;76(9):1088–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gelaye B, Sacco S, Brown WJ, Nitchie HL, Ornello R, Peterlin BL. Body composition status and the risk of migraine: a meta-analysis. Neurology 2017;88(19):1795–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daniels AB, Liu GT, Volpe NJ, et al. Profiles of obesity, weight gain, and quality of life in idiopathic intracranial hypertension (pseudotumor cerebri). Am J Ophthalmol 2007;143(4):635–641. [DOI] [PubMed] [Google Scholar]
- 7.Ko MW, Chang SC, Ridha MA, et al. Weight gain and recurrence in idiopathic intracranial hypertension: a case-control study. Neurology 2011;76:1564–1567. [DOI] [PubMed] [Google Scholar]
- 8.Sinclair AJ, Burdon MA, Nightingale PG, et al. Low energy diet and intracranial pressure in women with idiopathic intracranial hypertension: prospective cohort study. BMJ 2010;341:c2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hornby C, Botfield H, O'Reilly MW, et al. Evaluating the fat distribution in idiopathic intracranial hypertension using dual-energy x-ray absorptiometry scanning. Neuroophthalmology 2017;42(2):99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Reilly MW, Westgate CS, Hornby C, et al. A unique androgen excess signature in idiopathic intracranial hypertension is linked to cerebrospinal fluid dynamics. JCI Insight 2019;4(6):e125348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avenell A, Robertson C, Skea Z, et al. Bariatric surgery, lifestyle interventions and orlistat for severe obesity: the REBALANCE mixed-methods systematic review and economic evaluation. Health Technol Assess 2018;22(68):1–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dombrowski SU, Knittle K, Avenell A, Araújo-Soares V, Sniehotta FF. Long term maintenance of weight loss with non-surgical interventions in obese adults: systematic review and meta-analyses of randomised controlled trials. BMJ 2014;348:g2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fildes A, Charlton J, Rudisill C, Littlejohns P, Prevost AT, Gulliford MC. Probability of an obese person attaining normal body weight: cohort study using electronic health records. Am J Public Health 2015;105(9):e54–e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Courcoulas AP, King WC, Belle SH, et al. Seven-year weight trajectories and health outcomes in the Longitudinal Assessment of Bariatric Surgery (LABS) study. JAMA Surg 2018;153(5):427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun WYL, Switzer NJ, Dang JT, et al. Idiopathic intracranial hypertension and bariatric surgery: a systematic review. Can J Surg 2020;63(2):E123–E128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manfield JH, Yu KK, Efthimiou E, Darzi A, Athanasiou T, Ashrafian H. Bariatric surgery or non-surgical weight loss for idiopathic intracranial hypertension? A systematic review and comparison of meta-analyses. Obes Surg 2017;27(2):513–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ottridge R, Mollan SP, Mitchell J, et al. Randomised controlled trial of bariatric surgery versus a community weight loss programme for the sustained treatment of idiopathic intracranial hypertension: the Idiopathic Intracranial Hypertension Weight Trial (IIH:WT) protocol. BMJ Open 2017;7(9):e017426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghosh-Swaby OR, Goodman SG, Leiter LA, et al. Glucose-lowering drugs or strategies, atherosclerotic cardiovascular events, and heart failure in people with or at risk of type 2 diabetes: an updated systematic review and meta-analysis of randomised cardiovascular outcome trials. Lancet Diabetes Endocrinol 2020;8(5):418–435. [DOI] [PubMed] [Google Scholar]
- 19.Botfield HF, Uldall MS, Westgate CSJ, et al. A glucagon-like peptide-1 receptor agonist reduces intracranial pressure in a rat model of hydrocephalus. Sci Transl Med 2017;9(404):eaan0972. [DOI] [PubMed] [Google Scholar]
- 20.Scotton WJ, Botfield HF, Westgate CS, et al. Topiramate is more effective than acetazolamide at lowering intracranial pressure. Cephalalgia 2019:39(2):209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams O, Annandale E. Weight bias internalization as an embodied process: understanding how obesity stigma gets under the skin. Front Psychol 2019;10:953. doi: 10.3389/fpsyg.2019.00953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albury C, Strain WD, Brocq SL, et al. The importance of language in engagement between health-care professionals and people living with obesity: a joint consensus statement. Lancet Diabetes Endocrinol 2020;8(5):447–455. [DOI] [PubMed] [Google Scholar]
- 23.Mollan S, Hemmings K, Herd CP, Denton A, Williamson S, Sinclair AJ. What are the research priorities for idiopathic intracranial hypertension? A priority setting partnership between patients and healthcare professionals. BMJ Open 2019;9(3):e026573. [DOI] [PMC free article] [PubMed] [Google Scholar]