Abstract
Objective
By surveying a multiple sclerosis (MS) population, we tested the hypothesis that influenza vaccine uptake would not meet public health targets and that vaccine misconceptions would contribute to lower than desired uptake.
Methods
In spring 2020, we surveyed participants in the North American Research Committee on Multiple Sclerosis Registry regarding vaccinations. Participants reported whether they had received hepatitis A, hepatitis B, pneumococcal, shingles, varicella, measles/mumps/rubella, tetanus, or influenza vaccines. Participants who had not received influenza vaccine last year reported the reasons. We summarized responses descriptively. Using multivariable logistic regression, we assessed participant characteristics associated with uptake of seasonal influenza vaccine.
Results
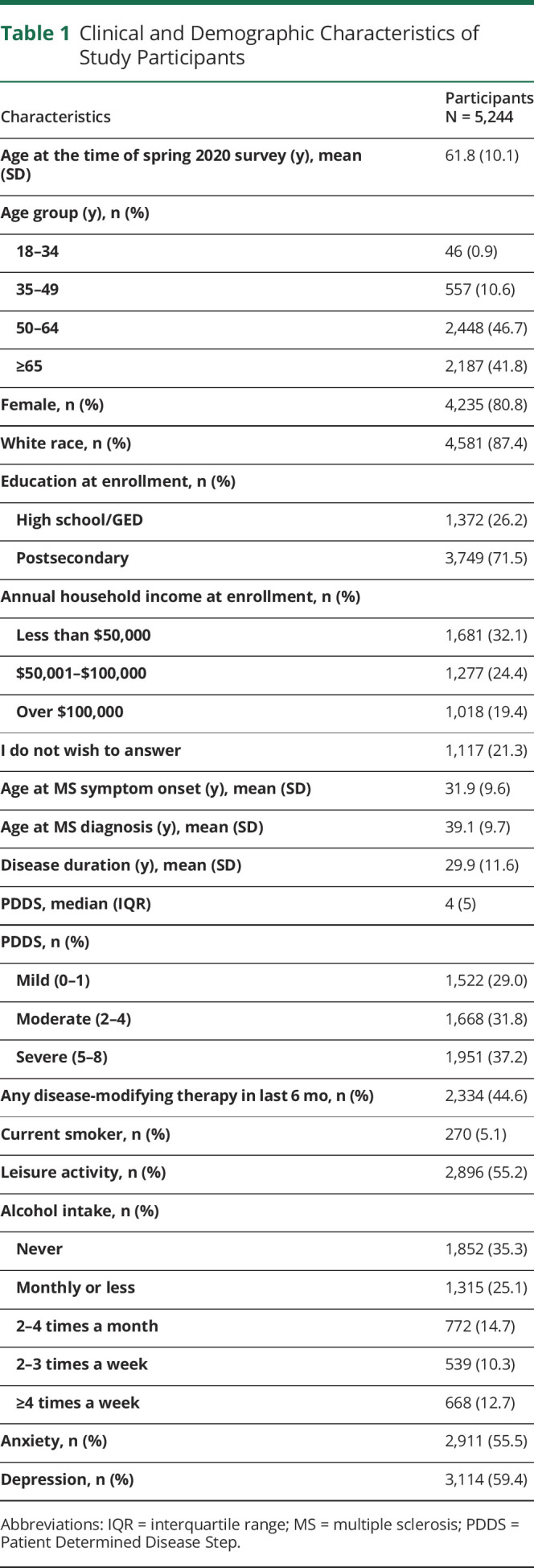
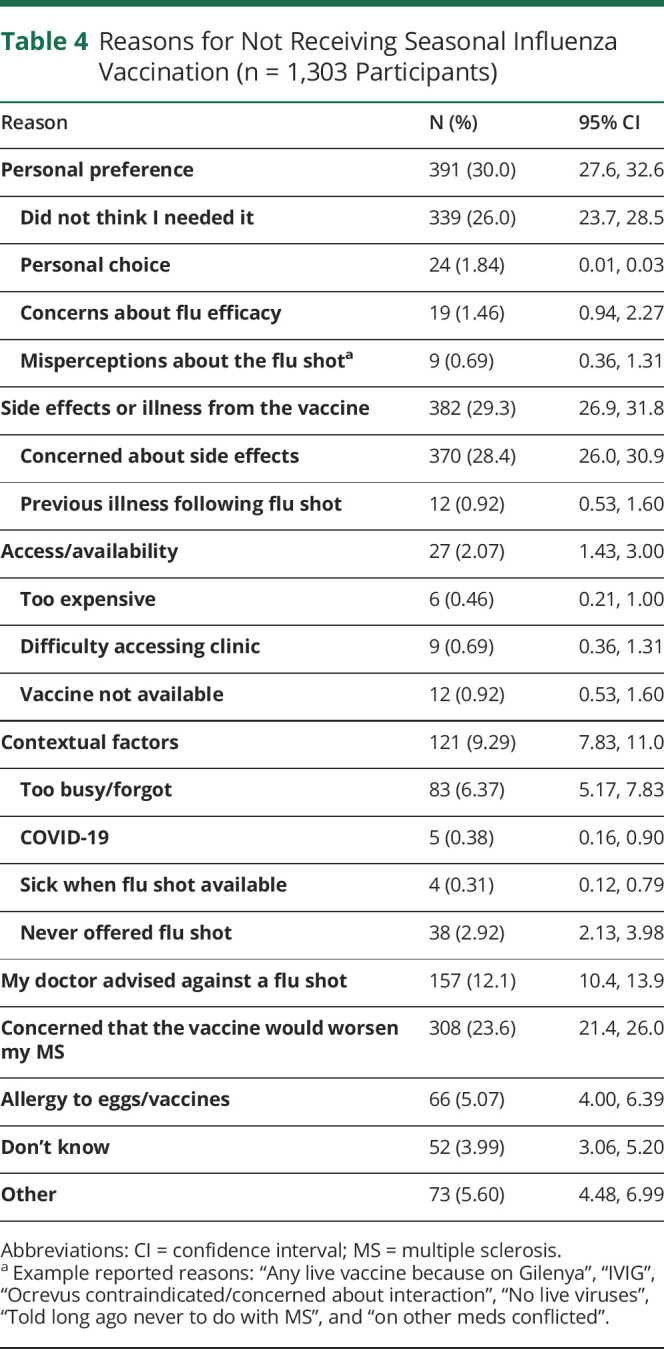
Of 5,244 eligible respondents, 80.8% were female, with a mean (SD) age of 61.8 (10.1) years. Overall, 43.0% (2,161/5,032) of participants reported that their neurologist had ever asked about their immunization history. The percentage of participants who received the seasonal flu vaccine last year ranged from 59.1% among those aged 18–24 years to 79.9% for persons aged ≥65 years. Among those who did not get the influenza vaccination, the most common reasons were personal preference (29.6%), concerns about possible adverse effects in general (29.3%), and concerns that the vaccine would worsen their MS (23.7%).
Conclusion
Vaccination uptake is lower than desired in the MS population compared with existing recommendations, including for seasonal influenza. Misconceptions about the safety of vaccination in the context of MS and personal preference appear to play important roles in vaccination choices, highlighting the importance of education about these issues.
Individuals with multiple sclerosis (MS) have an elevated risk for infection compared with individuals without MS1,2 and an elevated risk for postinfectious complications. For example, individuals with MS are more likely to be hospitalized and die of influenza than individuals without MS.2 Influenza infection also increases relapse risk.3 Several potentially serious infections, including influenza, can be prevented by vaccination. Vaccinations are of increasing concern as stronger immunomodulating therapies are used more widely for MS management, as these therapies may influence the need for and safety of vaccinations. Recent guidelines from the American Academy of Neurology focused on vaccinations,4 and the COVID-19 pandemic has heightened interest in vaccines in people living with MS.
Few studies have evaluated vaccination uptake in the MS population, and most have focused on influenza.5 In Israel, only one-third of respondents reported receiving seasonal flu vaccine in 2009–2010.5 In Manitoba, Canada, fewer than 40% of persons with MS received the influenza vaccine in 2015.6 In other populations with immune-mediated diseases, general and disease-specific barriers to vaccination have been reported,7 but relevant barriers in the MS population have not been reported. We aimed to determine uptake of seasonal influenza vaccine in a large sociodemographically diverse MS population, to investigate reasons for not obtaining the vaccine, and to determine lifetime uptake of other common vaccinations. We hypothesized that influenza vaccine uptake would not meet public health targets and that vaccine misconceptions would contribute to lower than desired uptake.
Methods
Study Population
The North American Research Committee on Multiple Sclerosis (NARCOMS) Registry is a self-report registry for persons with MS.8 At enrollment, participants report sociodemographic and clinical information, which is updated semiannually via survey. Surveys are completed either on paper or online.
Standard Protocols, Approvals, and Participant Consents
Participants permit use of their deidentified information for research purposes. At the time of the spring 2020 survey, the NARCOMS Registry was approved by the Institutional Review Board of Washington University at St. Louis.
Participant Characteristics
For this study, we used information from the enrollment and spring 2020 questionnaires. The information obtained from the enrollment questionnaire included birth date, sex, race, education level, region of residence, and ages at MS symptom onset and diagnosis. We categorized race as White or non-White. We categorized education level as high school/General Educational Development certificate and postsecondary (associate's degree, bachelor's degree, postgraduate education, and technical degree).
We obtained all the remaining information from the spring 2020 questionnaire, including annual household income, marital status, health behaviors, and disability status. Annual household income was reported as ≤$50,000, $50,001–$100,000, >$100,000, and “I do not wish to answer.” We categorized marital status as single (never married, divorced, widowed, or separated) and married (married or cohabiting). Health behaviors captured included smoking status, physical activity, and alcohol intake. Participants reported their current smoking status as no, yes some days, or yes every day, using a question from the Behavioral Risk Factor Surveillance Survey; we aggregated these responses to yes/no.9 They reported whether they had participated in any physical activity or exercise during the past month (yes/no). Participants also reported alcohol intake using 1 question from the Alcohol Use Disorders Identification Test.10 We modified the question to ask about alcohol intake over the past 6 months instead of the past 12 months. Response options were never, monthly or less, 2 to 4 times a month, 2 to 3 times a week, or 4 or more times a week.
Disease duration from symptom onset was categorized (<10, 10–19, and ≥20 years). Participants reported disability status using Patient Determined Disease Steps (PDDS), a single-item measure with response options ranging from 0 (normal) to 8 (bedridden). The PDDS correlates strongly with a physician-scored Expanded Disability Status Scale score.11 For this analysis, we categorized PDDS as mild (0–1), moderate (2–4), and severe (5–8).12 Based on the relevant items from SymptoMScreen, we classified participants as having any depression (yes/no) or anxiety (yes/no) symptoms.13 Participants reported use of disease-modifying therapies (DMTs) in the last 6 months. We grouped these as none or any.
Immunization
Participants reported whether their neurologist had ever asked about their immunization/vaccination history (yes, no, or don't know) and who they thought was responsible for determining which vaccinations they should receive (neurologist, primary care physician, participant, and other; multiple options were allowed).14 Participants were asked to report whether they had ever received vaccines for any of the following: hepatitis A, hepatitis B, pneumococcal, shingles, varicella, measles/mumps/rubella, and tetanus, where responses were yes, no, or don't know. For all vaccines except influenza, the time frame was ever receipt of the vaccine. We did not distinguish types of pneumococcal or zoster vaccines to avoid potential confusion by participants.
For influenza, we focused on the most recent influenza season to better assess current behaviors and attitudes. We adapted a questionnaire previously used in persons with inflammatory bowel disease (IBD).15 Participants who indicated that they had not received an influenza vaccine were asked to mention the reason, with potential responses being (1) never offered; (2) did not think I needed it; (3) allergy to eggs/vaccine; (4) concerned about side effects from the vaccine; (5) too expensive; (6) too busy/forgot; (7) my doctor advised against a flu shot; (8) concerned that the vaccine would worsen my MS; (9) vaccine not available; and (10) other (specify). Finally, participants were asked whether their neurologist had advised them to avoid certain vaccines and, if yes, to indicate which ones—the choices were the same as described earlier.
Statistical Analysis
We excluded individuals who did not report a confirmed diagnosis of MS, sex, or date of birth; those who did not live in the United States to reduce heterogeneity due to differences in health system delivery in other countries; and those with an age at symptom onset <16 years. Missing responses were not imputed.
First, we used descriptive statistics to summarize the characteristics of the respondents using means (SD), median (interquartile range), and frequency (percent) and their responses to the vaccination questions. Second, we summarized uptake of each vaccine queried overall and stratified by sex, age (18–34, 35–50, 51–64, and ≥65 years), disability status (mild, moderate, and severe), and DMT use (no as reference). Third, we summarized the reasons for not receiving influenza vaccine.
Finally, we examined factors associated with the uptake or nonuptake of the influenza vaccination using binary logistic regression. The models included sex (female as the reference group), age (≥65 years as the reference group), disease duration (<10 years as reference), race (White as reference), education level (high school as reference), income (<$50,000 as reference), current smoking status (no as reference), alcohol intake (never as reference), physical activity (inactive as reference), disability status (mild as reference), DMT use, depression (no as reference), and anxiety (no as reference). We used standard methods to assess model assumptions and assessed model fit using the Hosmer-Lemeshow goodness of fit statistic.16 We report the C-statistic for each model. Statistical analyses were conducted using SAS V9.4 (SAS Institute Inc., Cary, NC).
Data Availability
The data sets generated and analyzed during this study are held by the NARCOMS Registry (narcoms.org).
Results
The spring 2020 survey was distributed to 10,210 participants, of whom 6,385 (62.5%) responded. Compared with nonresponders, responders were more likely to be White (86.7% vs 82.6%, p < 0.001), female (80.9% vs 78.7%, p = 0.009), older (62.0 vs 59.0 years), and with a higher level of education (postsecondary 72.7% vs 68.9%, p < 0.001). However, most of these differences were statistically different but not clinically meaningful. After applying exclusion criteria, 5,244 participants constituted the final sample (figure e1, links.lww.com/CPJ/A292). Most participants were aged ≥51 years, White, and female, with moderate or severe disability (table 1).
Table 1.
Clinical and Demographic Characteristics of Study Participants
Vaccination Uptake
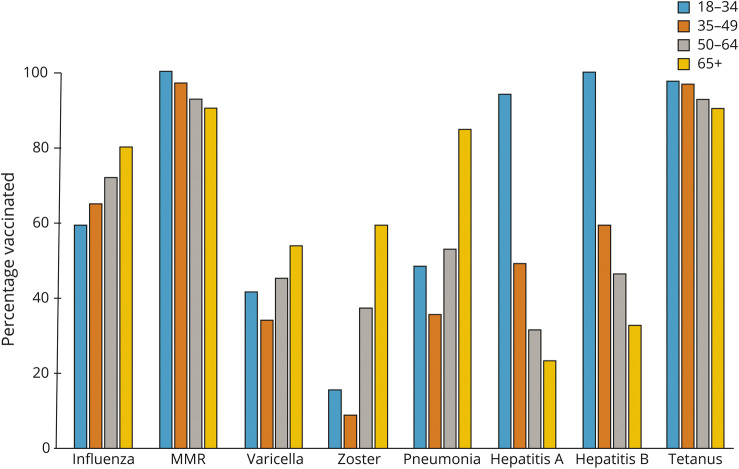
Of the vaccinations queried, the most commonly received were tetanus, followed by measles, mumps, rubella (MMR), and the flu shot (table 2). Vaccination uptake varied by age, and these age-related patterns differed by vaccine (figure 1). The percentage of participants who received the seasonal flu vaccine last year increased with age, reaching 79.9% for persons aged ≥65 years. Uptake of zoster (59.1%) and pneumonia (84.7%) vaccines was also highest among the oldest age group, consistent with the indications for those vaccines. However, the reverse pattern was observed for hepatitis A and B vaccinations, where the highest uptake was among those aged 18–34 years (hepatitis A: 93.9%, hepatitis B: 100%). We observed less variation in vaccination uptake according to disability status (figure e2, links.lww.com/CPJ/A292). Overall, 43.0% (2,161/5,032) of participants reported that their neurologist had ever asked about their immunization history, 39.1% indicated that they had not been asked, and 18.0% were uncertain.
Table 2.
Lifetime Uptake of Vaccines (n, %)

Figure 1. Percentage of Participants Vaccinated Stratified by Age.
Factors Associated With Receiving Influenza Vaccination
On univariate logistic regression, several demographic factors and health behaviors were associated with the likelihood of influenza vaccination. Compared with persons aged ≥65 years, those younger than 65 years had lower odds of being vaccinated (table 3). Postsecondary rather than high school education, a higher level of annual household income, a higher level of alcohol intake, being physically active, and using any DMT were also associated with increased odds of being vaccinated. In contrast, being male, symptoms of depression or anxiety, and currently smoking were associated with reduced odds of being vaccinated. Race, disability status, and disease duration were not associated with being vaccinated.
Table 3.
ORs (95% CIs) for the Association Between Participant Characteristics and Receipt of the Influenza Vaccination (n = 4,697)

On multivariable analysis, postsecondary education, household income >$100,000 vs <$50,000 remained associated with increased odds of being vaccinated. Compared with no alcohol intake, alcohol intake 2–4 times per month was associated with increased odds of being vaccinated, as was physical activity. Younger age and currently smoking continued to be associated with reduced odds of being vaccinated. Sex and symptoms of depression or anxiety were no longer associated with the odds of being vaccinated. Of MS characteristics, only use of a DMT was associated with vaccination. Participants reporting any use of a DMT had 41% increased odds of receiving influenza vaccination.
Attitudes Regarding Influenza Vaccination
Of the 1303 participants who did not get the influenza vaccination in the last flu season and who reported their reasons for not doing so, the most common reasons were related to personal preference (29.6%) and concerns about possible adverse effects in general (29.3%), followed closely by concerns that the vaccine would worsen their MS (23.7%) (table 4). The personal preference category was dominated by the perception that the individual did not need it (339/391, 86.7%). However, misconceptions that the influenza vaccine (by injection) was contraindicated with several disease-modifying therapies were also reported.
Table 4.
Reasons for Not Receiving Seasonal Influenza Vaccination (n = 1,303 Participants)
When asked who is responsible for determining what vaccinations you should receive, two-thirds of participants responded themselves (2,659, 66.9%), followed by their primary care providers (2,302, 57.9%) and their neurologists (1,636, 41.2%). A very small percentage of participants indicated that someone other than these 3 choices was responsible (88, 2.2%). Advice to avoid particular vaccines was infrequent, being reported most often for Zoster (362, 6.9%), followed by influenza nasal mist (287, 5.5%), other (273, 5.2%), influenza shot (132, 2.5%), pneumonia (76, 1.5%), varicella (48, 0.9%), MMR (39, 0.7%), hepatitis (24, 0.5%), and tetanus (14, 0.3%).
Discussion
In this large cross-sectional study, we found that in the most recent fall/winter season, multiple demographic factors and health behaviors were associated with the likelihood of receiving an influenza vaccination, including age, higher level of education, higher level of income, smoking, and physical activity. Only one of the MS characteristics we examined, use of a disease-modifying therapy, was associated with receiving an influenza vaccination. Among those who did not receive a vaccination, half had concerns regarding adverse effects and misconceptions about the possible impact of the vaccine on their MS. Although these were infrequent, access issues were also reported. Uptake of the other vaccinations queried varied substantially with age.
The most recent American Academy of Neurology guidelines recommend that people with MS follow all local vaccine standards unless there is a specific contraindication and receive the influenza vaccination annually unless there is a specific contraindication.9 In the United States, tetanus vaccination is recommended for all adults every 10 years. Vaccination to prevent shingles is recommended for all adults aged ≥50 years; and pneumococcal vaccine is recommended for all adults aged ≥65 years, and for immunocompromised adults aged ≥19 years. These targets were not met among our study participants even when we considered the age-specific nature of some of these recommendations.
In the 2018-19 season, 39.0% of US adults aged 18–64 years received the influenza vaccine, whereas 68.1% of adults aged 65 years and older received the vaccine.17 This appears lower than among our participants. However, uptake was higher among adults with high-risk conditions than among adults without high-risk conditions, possibly due to more frequent health system contacts. In other immune-mediated diseases such as IBD, uptake reportedly ranges from 6% to 80%7 and in rheumatoid arthritis varies from 26.6% to 85%.18,19 Comparable prior work regarding uptake of immunizations in people with MS is limited, focusing mainly on influenza vaccination. A Norwegian immunization register-based study reported that of 6,755 persons with MS, 60.7% received the pandemic (H1N1) vaccine in 2009–2010.20 A study of 101 persons with MS followed in Tel Aviv, Israel, found that 37.6% of participants received the seasonal flu vaccine in 2009/10, and 34.7% received the H1N1 flu vaccine, whereas 23.7% received both vaccines.5 A recent population-based study in Manitoba, Canada, found that approximately 40% of persons with MS received an influenza vaccination in 2015. Similar to our findings in the NARCOMS cohort, the uptake of the influenza vaccine was highest among those aged ≥65 years at 60%.6
We found that several factors were associated with uptake of the influenza vaccine. In the Manitoba, Canada, study, older age, higher socioeconomic status, and use of disease-modifying therapies were associated with increased uptake of the vaccine by people with MS, consistent with our findings.6 In contrast to our findings showing that sex was not associated with uptake, that study also found that female sex was associated with increased uptake. Another study showed that a postsecondary education and being female are associated with greater awareness of vaccine-preventable diseases.21
About 1 in 2 participants who did not get seasonal influenza vaccine reported a fear of general adverse effects or of adverse effects on their MS as the reason. One in 10 was advised against getting a flu shot by their physicians. Multiple factors may contribute to the decision not to get a vaccine, but these findings highlight important misconceptions about influenza vaccination and MS among patients and physicians. In the aforementioned Israeli study, the influenza vaccines were well tolerated. Only 5/49 participants who received at least 1 vaccine reported fever or flu-like symptoms, and no neurologic symptoms occurred after receiving vaccine.5 There is no convincing evidence that influenza vaccination causes exacerbations of MS.9 The inactivated influenza shot is not contraindicated, even in persons with MS who receive immunosuppressive therapies. Although the response to vaccination may be attenuated in individuals taking some therapies,22-25 some benefit may still be achieved. Our findings are generally concordant with those in other populations. In a 2015/2016 survey of the Canadian general population, commonly reported reasons for not getting the influenza vaccine included a lack of perceived susceptibility to influenza, lack of perceived severity of infection, and not believing in the vaccine's effectiveness.26 In populations with immune-mediated diseases other than MS, additional factors cited include not receiving information about vaccination from a physician, concerns about adverse effects, lack of awareness about indications for vaccination, and misconceptions about the effects on their disease.15,27 For example, in 1 study, 18% of persons with IBD who did not get vaccinated reported not doing so because it could be harmful for their IBD.7 In the previously mentioned Israeli study, cited reasons for not getting these vaccinations included fear of non-neurologic adverse effects (72.7%) or that their treating physician had recommended against it because of their MS (11.7%). This latter observation highlights the potential role of physician barriers to vaccination,28,29 including gaps in knowledge about safety of and recommendations for vaccinations in specific populations.
Studies in other immune-mediated disease populations have highlighted interventions that can improve vaccination rates. For example, in an IBD clinic, a vaccine questionnaire was distributed to patients during the influenza season, and any outstanding vaccinations were offered at the visit.30 This increased uptake of the influenza vaccination from 54% in the prior year to 81% and uptake of pneumococcal vaccine in the prior 5 years from 31% to 54%. This approach is potentially feasible for improving vaccinations in people with MS, too. Provider recommendation is strongly associated with uptake of the seasonal influenza vaccine31; thus, recommendations at the time of routine clinic visits may be helpful.
Study limitations should be considered. The response rate was 62%, similar to the mean response rate of 60% in the medical literature.32 Responders differed with respect to race, sex, age, and educational status compared with nonresponders, although some of these differences were quite small. NARCOMS participants are volunteers, creating a potential selection bias, which may limit the generalizability of our findings to the entire MS population. We relied on self-reported vaccination status; however, in a cohort with IBD, the positive predicted value of self-reported influenza vaccination was 96.7% and that of pneumococcal vaccination was 96.4%. The positive predictive value of other vaccinations is lower.33 Our questionnaire asked about multiple reasons for not getting the influenza vaccination but did not explicitly ask about lack of efficacy; thus, we may have underestimated the frequency of this concern. Although we asked participants to report reasons for not obtaining the influenza vaccination, we did not capture reasons for not obtaining other optional vaccinations such as those for hepatitis or Zoster, and these would also be important to guide policy. Study strengths included the large sample size and evaluation of a broad range of demographic factors, health behaviors, and clinical characteristics with vaccination uptake.
Vaccination uptake is lower than desired in the MS population compared with public health recommended targets, including for seasonal influenza vaccination. This places people with MS at risk for preventable infections and related hospitalizations and complications. Misconceptions about the safety of vaccination in the context of MS appear to play an important role in the choice to not obtain vaccinations, highlighting the importance of education about these issues and consistent recommendations by health care providers.
Appendix. Authors

Study Funding
NARCOMS is a project of the Consortium of Multiple Sclerosis Centers (CMSC). NARCOMS is funded in part by the CMSC and the Foundation of the CMSC. The study was also supported in part by the Waugh Family Chair in Multiple Sclerosis and a Research Manitoba Chair (to R.A.M). The funding sources had no role in the study design, collection, analysis or interpretation of the data, or in the decision to submit the article for publication.
Disclosure
R.A. Marrie: receives research funding from the CIHR, MS Society of Canada, MS Scientific Research Foundation, National MS Society, Crohn's and Colitis Canada, the US Department of Defense, The Arthritis Society, Biogen Idec, Roche, and the CMSC; and is supported by the Waugh Family Chair in Multiple Sclerosis. L. Kosowan reports no disclosures relevant to the manuscript. G. Cutter: data/safety monitoring committees for AMO, BioLineRx, BrainStorm Cell Therapeutics, Galmed, Horizon, Hisun, Merck, Merck/Pfizer, OPKO Biologics, Neurim, Novartis, Orphazyme, Sanofi, Reata, Receptos/Celgene, Teva, NHLBI (Protocol Review Committee), and NICHD (OPRU oversight committee) and consulting/advisory boards for Biogen, Click Therapeutics, Genzyme, Genentech, GW, Klein Buendel, MedImmune, MedDay, Novartis, Osmotica, Perception Neuroscience, Recursion, Roche, Somahlution, and TG Therapeutics. R.J. Fox: consulting fees from AB Science, Actelion, Biogen, Celgene, EMD Serono, Genentech, Immunic, Novartis, Sanofi, and TG Therapeutics; advisory committees for Actelion, Biogen, Immunic, Novartis, and Sanofi; and research grant funding from Novartis. A. Salter: journal editor/member of editorial advisory board for Circulation: Cardiovascular Imaging. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
TAKE-HOME POINTS
→ Seasonal influenza vaccination uptake is lower than recommended among persons with multiple sclerosis (MS).
→ In winter 2019/2020, seasonal influenza vaccination uptake ranged from 59.1% among persons with MS aged 18–24 years to 79.9% for those aged ≥65 years.
→ The most common reasons for not getting influenza vaccine were personal preference, concerns about general adverse effects, and concerns that the vaccine would worsen MS.
→ Misconceptions about the safety of vaccination in the context of MS highlight the importance of education about these issues.
References
- 1.Wijnands JM, Kingwell E, Zhu F, et al. Infection-related health care utilization among people with and without multiple sclerosis. Mult Scler J. 2017;23:1506-1516. [DOI] [PubMed] [Google Scholar]
- 2.Montgomery S, Hillert J, Bahmanyar S. Hospital admission due to infections in multiple sclerosis patients. Eur J Neurol. 2013;20:1153-1160. [DOI] [PubMed] [Google Scholar]
- 3.De Keyser J, Zwanikken C, Boon M. Effects of influenza vaccination and influenza illness on exacerbations in multiple sclerosis. J Neurol Sci. 1998;159:51-53. [DOI] [PubMed] [Google Scholar]
- 4.Farez MF, Correale J, Armstrong MJ, et al. Practice guideline update summary: vaccine-preventable infections and immunization in multiple sclerosis. Neurology. 2019;93:584-594. [DOI] [PubMed] [Google Scholar]
- 5.Auriel E, Gadoth A, Regev K, Karni A. Seasonal and H1n1v influenza vaccines in MS: safety and compliance. J Neurol Sci. 2012;314:102-103. [DOI] [PubMed] [Google Scholar]
- 6.Marrie RA, Walld R, Bolton JM, et al. Uptake of influenza vaccination in inflammatory bowel disease, multiple sclerosis and rheumatoid arthritis: a cohort study. CMAJ Open. 2020;9(2):E510-E521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malhi G, Rumman A, Thanabalan R, et al. Vaccination in inflammatory bowel disease patients: attitudes, knowledge, and uptake. J Crohn's Colitis. 2015;9:439-444. [DOI] [PubMed] [Google Scholar]
- 8.Marrie RA, Cutter G, Tyry T, Campagnolo D, Vollmer T. Validation of the NARCOMS Registry: diagnosis. Mult Scler. 2007;13:770-775. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System Survey Questionnaire. U.S. Department of Health and Human Services; 1995. [Google Scholar]
- 10.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The Audit Alcohol Consumption Questions (audit-C): an effective brief screening test for problem drinking. Ambulatory care quality improvement project (acquip). Alcohol use Disorders identification test. Arch Intern Med. 1998;158:1789-1795. [DOI] [PubMed] [Google Scholar]
- 11.Marrie RA, Goldman MD. Validity of performance scales for disability assessment in multiple sclerosis. Mult Scler. 2007;13:1176-1182. [DOI] [PubMed] [Google Scholar]
- 12.Marrie RA, Salter A, Tyry T, Cutter GR, Cofield S, Fox RJ. High hypothetical interest in physician-assisted death in multiple sclerosis. Neurology. 2017;88:1528-1534. [DOI] [PubMed] [Google Scholar]
- 13.Green R, Kalina J, Ford R, Pandey K, Kister I. SymptoMScreen: a tool for rapid assessment of symptom severity in Ms across multiple domains. Appl Neuropsychol Adult. 2017;24:183-189. [DOI] [PubMed] [Google Scholar]
- 14.Hammami MB, Pandit P, Salamo RT, Odufalu FD, Schroeder K. Health Maintenance and vaccination of patients with inflammatory bowel disease: practice and perception of responsibility of gastroenterologists vs primary care providers. Ochsner J. 2019;19:210-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waszczuk K, Waszczuk E, Szenborn L. Can we better protect patients with inflammatory bowel disease against infections - patient Attitude and personal immunization knowledge. Acta Gastroenterol Belg. 2018;81:257-261. [PubMed] [Google Scholar]
- 16.Hosmer DW, Lemeshow S. Applied Logistic Regression. John Wiley & Sons; 1989. [Google Scholar]
- 17.Centers for Disease Control and Prevention National Center for Immunization and Respiratory Diseases. Influenza vaccination coverage* by age group, adults 18 Years and older, United States, behavioral risk factor surveillance system (brfss), 2018-19 season. 2019. Accessed November 9, 2020, cdc.gov/flu/fluvaxview/coverage-1819estimates.htm.
- 18.Krasselt M, Ivanov JP, Baerwald C, Seifert O. Low vaccination rates among patients with rheumatoid arthritis in a German outpatient clinic. Rheumatol Int. 2017;37(2):229-37. [DOI] [PubMed] [Google Scholar]
- 19.Subesinghe S, Rutherford AI, Ibrahim F, Harris H, Galloway J. A large two-centre study in to rates of influenza and pneumococcal vaccination and infection burden in rheumatoid arthritis in the UK. BMC Musculoskeletal Disorders. 2016;17:322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghaderi S, Berg-Hansen P, Bakken IJ, Magnus P, Trogstad L, Haberg SE. Hospitalization following influenza infection and pandemic vaccination in multiple sclerosis patients: a nationwide population-based registry study from Norway. Eur J Epidemiol. 2020;35:355-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu PJ, O'Halloran A, Kennedy ED, et al. Awareness among adults of vaccine-preventable diseases and recommended vaccinations, United States, 2015. Vaccine. 2017;35:3104-3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bar-Or A, Calkwood JC, Chognot C, et al. Effect of ocrelizumab on vaccine responses in patients with multiple sclerosis: the veloce study. Neurology. 2020;95:e1999-e2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bar-Or A, Freedman MS, Kremenchutzky M, et al. Teriflunomide effect on immune response to influenza vaccine in patients with multiple sclerosis. Neurology. 2013;81:552-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olberg HK, Eide GE, Cox RJ, et al. Antibody response to seasonal influenza vaccination in patients with multiple sclerosis receiving immunomodulatory therapy. Eur J Neurol. 2018;25:527-534. [DOI] [PubMed] [Google Scholar]
- 25.Kappos L, Mehling M, Arroyo R, et al. Randomized trial of vaccination in fingolimod-treated patients with multiple sclerosis. Neurology. 2015;84:872-879. [DOI] [PubMed] [Google Scholar]
- 26.Farmanara N, Sherrard L, Dube E, Gilbert NL. Determinants of non-vaccination against seasonal influenza in Canadian adults: findings from the 2015-2016 influenza immunization coverage survey. Can J Public Health. 2018;109:369-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wasan SK, Calderwood AH, Long MD, Kappelman MD, Sandler RS, Farraye FA. Immunization rates and vaccine beliefs among patients with inflammatory bowel disease: an opportunity for improvement. Inflamm Bowel Dis. 2014;20:246-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nichol KL, Zimmerman R. Generalist and subspecialist physicians' knowledge, attitudes, and practices regarding influenza and pneumococcal vaccinations for elderly and other high-risk patients: a nationwide survey. Arch Intern Med. 2001;161:2702-2708. [DOI] [PubMed] [Google Scholar]
- 29.Yeung JH, Goodman KJ, Fedorak RN. Inadequate knowledge of immunization guidelines: a missed opportunity for preventing infection in immunocompromised ibd patients. Inflamm Bowel Dis. 2011;18:34-40. [DOI] [PubMed] [Google Scholar]
- 30.Parker S, Chambers White L, Spangler C, et al. A quality improvement project significantly increased the vaccination rate for immunosuppressed patients with ibd. Inflamm Bowel Dis. 2013;19:1809-1814. [DOI] [PubMed] [Google Scholar]
- 31.Lu PJ, Srivastav A, Amaya A, et al. Association of provider recommendation and offer and influenza vaccination among adults aged ≥18 years—United States. Vaccine. 2018;36:890-898. [DOI] [PubMed] [Google Scholar]
- 32.Asch DA, Jedrziewski MK, Christakis NA. Response rates to mail surveys published in medical journals. J Clin Epidemiol. 1997;50:1129-1136. [DOI] [PubMed] [Google Scholar]
- 33.Rolnick SJ, Parker ED, Nordin JD, et al. Self-report compared to electronic medical record across eight adult vaccines: do results vary by demographic factors? Vaccine. 2013;31:3928-3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated and analyzed during this study are held by the NARCOMS Registry (narcoms.org).