Abstract
Objective
Advances in medical discoveries have bolstered expectations of precise and complete care, but delivering on such a promise for complex, chronic neurologic care delivery requires solving last-mile challenges. We describe the iterative human-centered design and pilot process for multiple sclerosis (MS) NeuroShare, a digital health solution that brings practical information to the point of care so that clinicians and patients with MS can view, discuss, and make informed decisions together.
Methods
We initiated a comprehensive human-centered process to iteratively design, develop, and implement a digital health solution for managing MS in the routine outpatient setting of the nonprofit Sutter Health system in Northern California. The human-centered codesign process included 3 phases: discovery and design, development, and implementation and pilot. Stakeholders included Sutter Health's Research Development and Dissemination team, academic domain experts, neurologists, patients with MS, and an advisory group.
Results
MS NeuroShare went live in November 2018. It included a patient- and clinician-facing web application that launches from the electronic health record, visually displays a patient's data relevant to MS, and prompts the clinician to comprehensively evaluate and treat the patient. Both patients and clinicians valued the ability to jointly view patient-generated and other data. Preliminary results suggest that MS NeuroShare promotes patient-clinician communication and more active patient participation in decision-making.
Conclusions
Lessons learned in the design and implementation of MS NeuroShare are broadly applicable to the design and implementation of digital tools aiming to improve the experience of delivering and receiving high-quality care for complex, neurologic conditions across large health systems.

In our current era, there is a proliferation of data that should allow us to deliver more personalized care, but new knowledge is generated faster than we can act on it. In neurologic care, insights generated from research must be brought from academic centers to clinics and transformed into actionable information to enable patient-centered care. There are at least 4 challenges that hinder this process: (1) neurologists caring for patients may lack subspecialty up-to-date expertise, (2) suboptimal access to data (including patient-reported data) that are either inconsistently collected or must be extracted from the electronic health record (EHR) in a time-consuming process that drains the clinical encounter, (3) lack of efficient ways to process and share data with patients for optimal treatment decision-making,1 and (4) not accounting for health literacy and information retention demands on patients grappling with emotional topics or cognitive impairment.
Our ability to use new and highly personalized information is almost impossible with our current tools that struggle to harness the volume of data contained in EHRs in ways that do not induce burnout.2 Digital health solutions that use human-centered design3,4 to incorporate the patient and clinician voice and account for their workflows5,6 can augment the EHR to deliver high-quality and personalized care in routine practice.7–10 We describe our experience with NeuroShare, a digital health solution for delivering actionable information into the hands of neurologists, starting with multiple sclerosis (MS).11 Although digital tools have been developed to monitor function and deliver a comprehensive visualization of a patient's trajectory and treatment options in MS,12–14 to date, none works seamlessly in general care settings.
Methods
Overview
We used a human-centered codesign process,15 partnering with neurologists, patients, and technologists through 3 phases: (1) discovery and design to understand challenges in delivering and receiving MS care and to design a digital health solution, (2) development to build the digital health solution and integrate it into the EHR and clinician and patient workflows, and (3) implementation and pilot to test initial deployment of MS NeuroShare.
Setting
Sutter Health is a nonprofit health system serving more than 3 million patients in northern California. Its medical network includes 272 primary care clinics, 5,500 physicians, 25 neurology clinics, and 62 neurology clinicians (medical doctor [MD], doctor of osteopathy, and nurse practitioner). Sutter Health has used the Epic Systems (Verona, WI) EHR since 2001.
Teams and Stakeholders
The work was led by Sutter Health's Research, Development and Dissemination team, which has designed, developed, and evaluated 10 innovative digital health solutions deployed in primary, specialty, and inpatient care settings.16–19 The MS BioScreen team at the University of California San Francisco (UCSF) previously developed a clinician-facing tool (MS BioScreen) to put advanced visualization, contextualization, and prediction of key patient outcomes into the hands of clinicians and to allow both clinicians and patients to view these together, empowering patients to participate in their care.20 The team contributed their MS domain expertise, user experience for MS-related tools, and created an application programming interface (API) for a Sutter Health patient's MS course to be contextualized against a UCSF cohort. Sutter Health Neurologists (n = 3) and patients with MS (n = 10) participated in interactive codesign sessions for MS NeuroShare. The same neurologists who participated in the design, along with 1 additional clinician, piloted the solution with their panel of patients with MS. A stakeholder advisory group (SAG) comprised the aforementioned groups, 3 individuals living with MS, and the Northern California chapter president of the National Multiple Sclerosis Society. The SAG convened 6 times between January 2017 and February 2019 to review progress and support problem solving.
Human-Centered Codesign Process
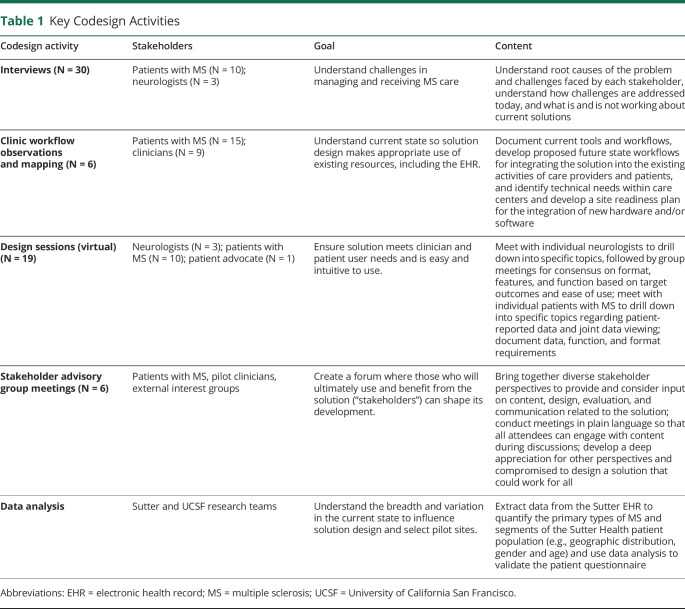
Phase 1: Discovery and Design (February 2017–June 2017)
We explored current challenges with in-clinic delivery of patient-centered care for MS, and the impact of these challenges on the time and quality of the encounter for both clinicians and patients (table 1). We then iteratively codesigned the MS NeuroShare questionnaire and clinic application requirements, designs, and workflows.
Table 1.
Key Codesign Activities
Phase 2: Development (July 2017–October 2018)
Using the phase I design, we developed MS NeuroShare using modern frameworks and approaches, such as APIs, to facilitate real-time data exchange between multiple data systems.
Phase 3: Implementation and Pilot (November 2018–February 2019)
MS NeuroShare was available to the pilot neurology clinicians (3 MDs and 1 nurse practitioner at 3 clinics) and their patients in November 2018. As an extension of the design phase, the pilot was used to collect information on usability and usefulness of MS NeuroShare when used in busy day-to-day practice.
We prepared and supported the sites and clinicians for MS NeuroShare implementation by putting the necessary hardware and workflow changes in place, providing virtual and on-site training, and providing onsite support during the 2-week “go-live” window and periodically thereafter. We then began to track MS NeuroShare use and elicited feedback on the experience of using it in real-world clinical encounters. We accessed usage logs to track indicators of adoption, including when, by whom, for whom, and for how long MS NeuroShare and the EHRs were accessed. We conducted qualitative interviews with the 4 pilot clinicians and with 14 patients for whom MS NeuroShare was used in an office encounter to assess facilitators, barriers, and the near-term effort required to realize the value of using MS NeuroShare in practice.
Standard Protocol Approvals, Registrations, and Patient Consents
Funding was provided by the California Initiative to Advance Precision Medicine, Sutter Health, and the Hilton Foundation. The stakeholder engagement aspect of the human-centered design process was approved as a quality improvement activity by the Sutter Health Institutional Review Board, and the final platform evaluation by clinicians and patients was approved by the Sutter Health Institutional Review Board as human research (approval # 2017.047EXP, IRBNet # 1026976-3).
Data Availability
Anonymized data are available from the corresponding author on reasonable request.
Results
Phase 1: Discovery and Design
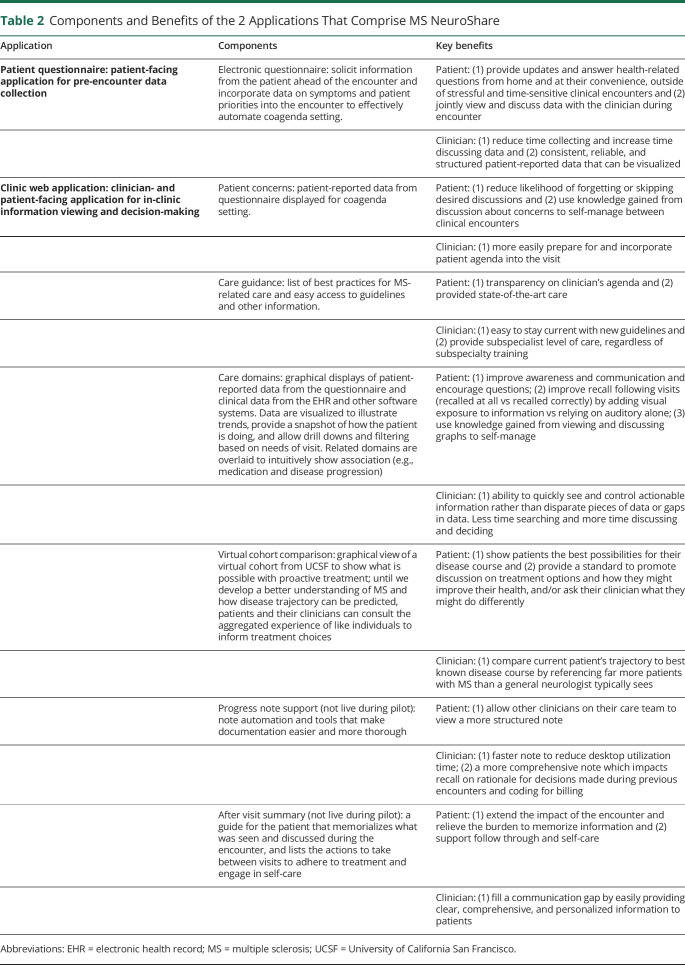
The qualitative work described in table 1 confirmed that in MS care, as in other domains of medicine, care delivery challenges include too much time required to find data, important data unavailable, and too little time for the clinician and patient to consider all data relevant for decision-making. We identified 3 clinician and patient goals (1) make complex encounters simple by curating, organizing, and emphasizing information for easy interpretation and translation into mutually agreed on postencounter actions, (2) integrate the patient's voice in the encounter agenda, and (3) enhance the patient's understanding and involvement in care. To accomplish these goals, MS NeuroShare required 2 applications—an electronic patient questionnaire (own unpublished work) and a clinic application (table 2) and supporting workflows.
Table 2.
Components and Benefits of the 2 Applications That Comprise MS NeuroShare
The questionnaire design started with a clinically validated questionnaire administered by clinicians to assess neurologic impairment in MS.21 Guided by over 8 hours of patient feedback and principles of health literacy,22 we modified the questionnaire so that patients with MS could easily complete it at home, making the experience more flexible (can be completed over several sessions), consistent (parallel rating structures within sections), nuanced (options to capture less consistent symptoms), and hopeful (language reminding patients that many symptoms may not now or ever apply). The revised questionnaire was then tested to ensure whether it remained clinically valid before being deployed.
Patient Workflow
Two weeks before an encounter, patients received a link to the questionnaire in an email that explained their responses would be securely sent to their clinician before their next visit. The responses flowed directly to MS NeuroShare, so the patient and clinician could view them together during the appointment. Time normally spent rushing to report symptoms and answer routine questions could now be repurposed for making shared decisions about treatment.
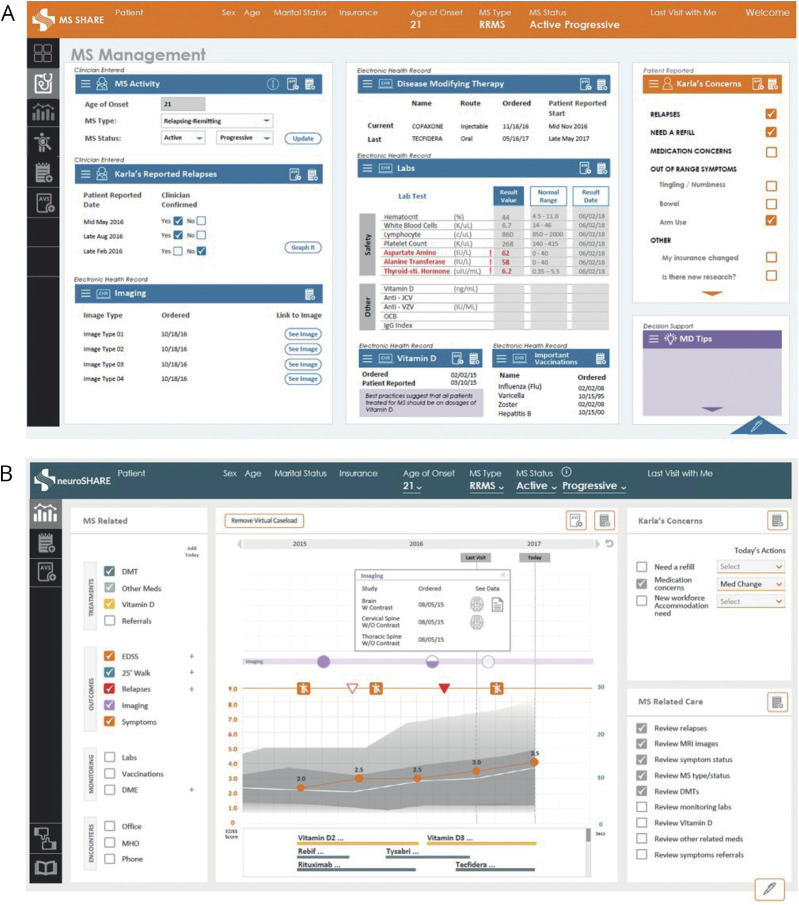
The clinic application design started with UCSF's pre-existing MS BioScreen to help clinicians envision possibilities. The first major design departed from MS BioScreen by including more clinical data drawn directly from the EHR. Subsequent versions became increasingly complex because the clinicians requested more diverse data and features, as demonstrated by 1 of the 7 tabs in the penultimate version (figure, A). However, clinicians believed that the tool had become too onerous and patients reported that it was too complicated, reducing its value to both groups. The agreed-on solution was a menu of data options and a canvas that could display a diversity of data in a common way, allowing clinicians to select, view, and drill down for data relevant to the current encounter. The final design scaled the number of tabs to 3, with 1 each for data (patient-reported and clinical), documentation, and an after-visit summary (figure, B).
Figure. Multiple Sclerosis (MS) NeuroShare Design, Iterative, and Final.
(A) Version 2 design of MS NeuroShare. (B) Final design version of MS NeuroShare (live as of November 2018).
Clinician Workflow
A link to MS NeuroShare was available in the EHR. When clicked, the MS NeuroShare application launched in a web browser, allowing simultaneous use of the application and the EHR.
Phase 2: Development
We developed custom APIs to drive questionnaire logic and to exchange information between the questionnaire, clinic application, EHR, imaging system, and a contextualization algorithm comparing the patient against UCSF MS research cohort data (modified from14). We integrated MS NeuroShare with the EHR using a hyperlink in the EHR's navigation menu. The link passed user credentials and key contextual information directly to the clinic application, so the clinician could immediately review information on the patient of interest.
Phase 3: Implementation and Pilot
During the 7-week pilot, clinicians opened MS NeuroShare for 122 of the 345 (35%) applicable patient encounters. Clinician-reported drivers of use were the ability to easily review images and laboratory test results and the desire to demonstrate MS NeuroShare to patients underscoring the importance of the patient-reported data and encouraging completion of the questionnaire in the future. Lack of time was the primary barrier to use, with MS NeuroShare less likely to be used on busy clinic days. Analysis of individual feature use showed that simple displays of data were used more regularly (e.g., laboratory values), whereas those requiring additional actions were used less often (e.g., clinician-entered Timed 25 Foot Walk).
Of the 266 patients sent an email containing a link to the questionnaire, 97 (36%) submitted complete questionnaires. Patients reported that the primary barrier to completion was lack of awareness that the email containing the questionnaire link had come from their clinician. Usage data affirmed that lack of awareness was a driver of low completion rates. Of patients who clicked on the questionnaire link, 90% completed and submitted it.
Although too early to measure the impact on the efficiency and quality of clinical care, both patients and clinicians reported favorable changes in the quality of communication, and in patients' understanding of the management plans, serving as early indicators that MS NeuroShare is changing the nature of the visit for both groups.
When describing their experience viewing MS NeuroShare during their visit, several patients reported having viewed and discussed information not seen previously or participating in a different type of dialogue with their clinician, for example:
I think it was nice to be looking at the same thing my doctor is looking at. We have done that before looking at the scans, or the test results, but this was a friendlier environment for that. (Patient with MS)
Several patients articulated the value of a longitudinal view of their clinical trajectory and identified ways in which viewing information with their clinician directly impacted their understanding of their condition and ability to participate in managing the condition, for example:
Seeing the medication history impacted my understanding in a way. I mean, just to see through the years. MS is a disease of relapses and remissions, and so, showing the different medications, when I switched, and why I switched was kind of interesting because there's a new drug coming out that's a second generation of a drug I have tried. (Patient with MS)
Similarly, clinicians discussed the value of dialogue with patients around longitudinal data and described being able to display laboratory test results, imaging, and medications in a way that made it easier to provide patient education, for example:
The advantages that I see are that patients have a better understanding of what I'm talking about in terms of lab variations and MRI findings. So they're better educated, they understand what's happening with their disease. (NeuroShare Pilot Clinician)
Discussion
In the era of “death by a thousand [EHR] clicks,”1,2 our experience applying human-centered codesign principles to address problems in MS care delivery provides insights for how to build and evaluate disease-specific digital solutions that resolve, rather than reproduce, limitations of EHRs. Our MS NeuroShare pilot experience suggests that it was able to augment the EHR by streamlining data acquisition and introducing the patient voice and that it has the potential to change the quality of the visit, leading to improved patient-clinician communication and more active patient participation in decision-making.
Our first observation was that a codesign process that allowed clinicians the freedom to reimagine care delivery tools led to a design that became complex because it attempted to replicate, rather than augment, the EHR (figure, C). It took several design iterations to recognize the underlying need being expressed by clinicians, which was not a need for multiple displays of data to account for all the heterogeneity in MS, but rather the ability to see all the right data in a way that's flexible to the patient in front of them. When we landed on the true need, rather than the verbalized examples of challenging encounters, we modified the design to incorporate a menu of data domains and a canvas on which the data can be displayed. Our recommendation for future digital health projects that use a codesign process is to be intensely focused on identifying the underlying needs and less focused on creating designs that are responsive to specific examples.
Our second observation was that the design phase for a digital health solution must extend to the initial pilot test, when users engage with the actual, rather than conceptual, version of the solution. Users have adapted to their current workflow after years of practice, and despite their involvement in the design and stated enthusiasm for new solutions, adoption rates can be low initially because of the inertia of modifying established habits in a busy clinical practice. Until the point at which users are using a tool in a variety of applicable encounters, the design remains theoretical. We recommend that the early pilot period for a digital health solution be framed as a time to identify which features are used, when and why, and to adjust the design to refine and amplify the utility of those features. Although this may seem obvious, our own experience is that funders and other stakeholders often want to focus on clinically relevant outcomes, but these can only be measured reliably when the minimally valuable use case is validated in practice.
Our third observation was the importance of the impact of workflows on behavioral changes required for adoption of new technologies. Design teams (which often include nonclinicians) must recognize that new digital health solutions are deployed into environments in which both clinicians and patients are bombarded by electronic information (e.g., data and emails) and requests for input (e.g., satisfaction surveys). Against this background, our emailed questionnaire invitations to patients were most often dismissed as further digital noise from the health system. Although the fix was technically straightforward (address the email from the clinician, not the system), it involved lengthy reviews by legal and privacy groups to enact. This example represents one of the many challenges that design teams need to consider in collecting and delivering relevant data. In addition, new solutions designed to alleviate clinicians' workload can paradoxically trigger more and different kinds of work, such as investing extra time to educate patients about the use of the questionnaires and clinic application. The potential value of a new solution must be weighed against the time required to encourage its adoption. These tensions require careful consideration when designing workflows and setting expectations with clinicians, administrators, and funders that productivity dips may precede gains.
A limitation of MS NeuroShare is that it was designed within one health system. We attempted to mitigate this by choosing diverse clinician stakeholders and collaborating with an academic partner (UCSF) with deep disease-specific expertise. A second limitation is that we only have early pilot data to understand the impact of MS NeuroShare on quality and workflow efficiency—both of these measures will be further assessed in a planned series of evaluations. We nonetheless report these results because we believe them critical to creating a digital health solution that meets user needs, augments their existing capabilities, and can be easily adopted.
Current generation EHRs are not optimally designed to enhance the patient and clinician experience,1,24,25 resulting challenges to delivering patient-centered care. However, health systems that have already invested heavily in implementing and maintaining current EHR technologies have to balance the tension between EHR off-the-shelf functionality, and the desire of clinical and administrative stakeholders for customizations that can be quickly adapted as new knowledge and policies emerge. Although healthcare systems can request customizations, they are dependent on the EHR vendor's roadmap, which is often misaligned and implausibly long. We present the alternative approach of codesigning applications that sit on top of, and interact with, the EHR and that more easily support customization. This approach is gaining acceptance as a viable strategy for innovating in healthcare systems with EHRs.26–28 Our lessons learned in developing MS NeuroShare provide guidance to other organizations that seek to innovate on top of their EHR with digital health technology that patients and clinicians find engaging and are willing to adopt.
TAKE-HOME POINTS
→ Increasing amounts of data are available to support patient-centered neurology care, but there is a lack of tools to efficiently access or apply them.
→ Digital health solutions can help augment the EHR, but they must be designed to engage and meet the needs of both clinicians and patients to impact shared decision-making and quality of care.
→ The pilot phase must be considered an extension of the design phase—an opportunity to learn what features are actually used in busy day-to-day practices vs what features users anticipated using in an ideal world setting.
→ The potential value of a new solution must be weighed against the time required to encourage its adoption, and expectations should be set with clinicians, administrators, and funders that productivity dips may precede gains.
Acknowledgment
The authors are grateful to Joanna Cooper, MD, Leland Greenwald, MD, Lynn Jehle, NP, and Joseph Lacy, MD, and to participating patients who provided critical input during all phases of development.
Appendix. Authors
Study Funding
This study was supported by California Initiative to Advance Precision Medicine and National Multiple Sclerosis Society.
Disclosure
R. Bove has received research support from the National Multiple Sclerosis Society, the Hilton Foundation, the California Initiative to Advance Precision Medicine, the Sherak Foundation and Akili Interactive; she has also received personal compensation for consulting from Novartis, Sanofi Genzyme, Roche Genentech, and Pear Therapeutics. C.A. Bruce, C.K. Lunders, J.R. Pearce, J. Liu, and E. Schleimer have none to declare. S.L. Hauser serves on the Board of Directors for Neurona; Scientific Advisory Board for Alector, Annexon, Bionure, Molecular Stethoscope, and Symbiotix; he has also received nonfinancial support from F. Hoffmann-La Roche Ltd. and Novartis AG. W.F. Stewart serves as consultant to Amgen, Dr. Ready, Allergan, and Grisfol. J.B. Jones has received research support from the California Initiative to Advance Precision Medicine, Roche Genentech, AstraZeneca, Boehringer Ingelheim, and the Hilton Foundation. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Fry E, Schulte F. Death by a thousand clicks: where electronic health records went wrong. Fortune 2019. Available at: khn.org/news/death-by-a-thousand-clicks/. Accessed March 18, 2019. [Google Scholar]
- 2.Carayon P, Hoonakker P. Human factors and usability for health information technology: old and new challenges. Yearb Med Inform 2019;28:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.IDEO.org. Design kit: the human-centered design toolkit [online]. Available at: ideo.com/post/design-kit. Accessed November 18, 2020.
- 4.Matheson GO, Pacione C, Shultz RK, Klügl M. Leveraging human-centered design in chronic disease prevention. Am J Prev Med 2015;48:472–479. [DOI] [PubMed] [Google Scholar]
- 5.Karmali KN, Davies P, Taylor F, Beswick A, Martin N, Ebrahim S. Promoting patient uptake and adherence in cardiac rehabilitation. Cochrane Database Syst Rev 2014:CD007131. [DOI] [PubMed] [Google Scholar]
- 6.Yu CH, Parsons JA, Mamdani M, et al. A web-based intervention to support self-management of patients with type 2 diabetes mellitus: effect on self-efficacy, self-care and diabetes distress. BMC Med Inform Decis Mak 2014;14:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altman M, Huang TTK, Breland JY. Design thinking in health care. Prev Chronic Dis 2018;15:E117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cristancho-Lacroix V, Moulin F, Wrobel J, et al. A web-based program for informal caregivers of persons with Alzheimer's disease: an iterative user-centered design. JMIR Res Protoc 2014;3:e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Velardo C, Shah SA, Gibson O, et al. Digital health system for personalised COPD long-term management. BMC Med Inform Decis Mak 2017;17:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trail-Mahan T, Heisler S, Katica M. Quality improvement project to improve patient satisfaction with pain management: using human-centered design. J Nurs Care Qual 2016;31:105–112; quiz 113–114. [DOI] [PubMed] [Google Scholar]
- 11.Hauser SL, Goodin DS. Multiple sclerosis and other demyelinating diseases. In: Harrison's Principles of Internal Medicine [online]. New York: McGraw Hill; 2012. Available at: https://accessmedicine.mhmedical.com/content.aspx?bookid=1130§ionid=79756278. Accessed December 12, 2014. [Google Scholar]
- 12.Pelletier D, Rennert T, Chang M, Decunto S, Cen S. myMS; a comprehensive patient-centered mobile app to monitor MS at home (P3.2-003). Neurolgy 2019;92(15 suppl):P3.2-003. [Google Scholar]
- 13.Midaglia L, Mulero P, Montalban X, et al. Adherence and satisfaction of smartphone- and smartwatch-based remote active testing and passive monitoring in people with multiple sclerosis: nonrandomized interventional feasibility study. J Med Internet Res 2019;21:e14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gourraud PA, Henry RG, Cree BA, et al. Precision medicine in chronic disease management: the multiple sclerosis BioScreen. Ann Neurol 2014;76:633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyon AR, Koerner K. User‐centered design for psychosocial intervention development and implementation. Clin Psychol Sci Pract 2016;23:180–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones JB, Liang S, Husby HM, et al. CM-SHARE: development, integration, and adoption of an electronic health record–linked digital health solution to support care for diabetes in primary care. Clin Diabetes 2019;37:338–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones JB, Bruce CA, Shah NR, Taylor WF, Stewart WF. Shared decision making: using health information technology to integrate patient choice into primary care. Transl Behav Med 2011;1:123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newman ED, Lerch V, Jones J, Stewart W. Touchscreen questionnaire patient data collection in rheumatology practice: development of a highly successful system using process redesign. Arthritis Care Res (Hoboken) 2012;64:589–596. [DOI] [PubMed] [Google Scholar]
- 19.Jones JB, Shah NR, Bruce CA, Stewart WF. Meaningful use in practice using patient-specific risk in an electronic health record for shared decision making. Am J Prev Med 2011;40:S179–S186. [DOI] [PubMed] [Google Scholar]
- 20.Gourraud PA, Henry RG, Cree BA, et al. Precision medicine in chronic disease management: the multiple sclerosis BioScreen. Ann Neurol 2014;76:633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodin DS. A questionnaire to assess neurological impairment in multiple sclerosis. Mult Scler 1998;4:444–451. [DOI] [PubMed] [Google Scholar]
- 22.Shoemaker SJ, Wolf MS, Brach C. Development of the Patient Education Materials Assessment Tool (PEMAT): a new measure of understandability and actionability for print and audiovisual patient information. Patient Educ Couns 2014;96:395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gawande A. Why doctors hate their computers. The New Yorker, November 5, 2018.
- 24.Tai-Seale M, Olson CW, Li J, et al. Electronic health record logs indicate that physicians split time evenly between seeing patients and desktop medicine. Health Aff (Millwood) 2017;36:655–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arndt BG, Beasley JW, Watkinson MD, et al. Tethered to the EHR: primary care physician workload assessment using EHR event log data and time-motion observations. Ann Fam Med 2017;15:419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mandel JC, Kreda DA, Mandl KD, Kohane IS, Ramoni RB. SMART on FHIR: a standards-based, interoperable apps platform for electronic health records. J Am Med Inform Assoc 2016;23:899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bloomfield RA Jr, Polo-Wood F, Mandel JC, Mandl KD. Opening the Duke electronic health record to apps: implementing SMART on FHIR. Int J Med Inform 2017;99:1–10. [DOI] [PubMed] [Google Scholar]
- 28.Kasthurirathne SN, Mamlin B, Kumara H, Grieve G, Biondich P. Enabling better interoperability for healthcare: lessons in developing a standards based application programing interface for electronic medical record systems. J Med Syst 2015;39:182. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data are available from the corresponding author on reasonable request.