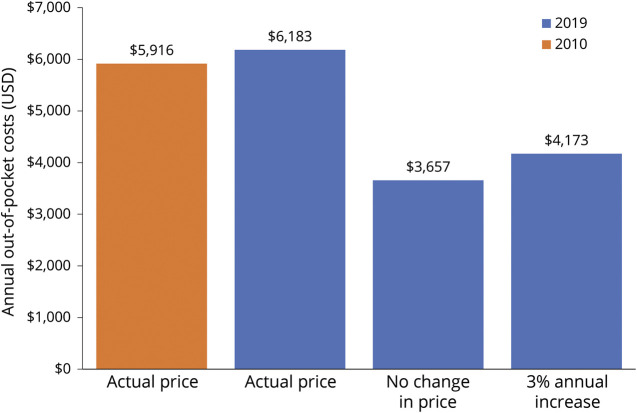Abstract
Objective
To determine whether closing the Part D coverage gap (donut hole) between 2010 and 2019 lowered patients' out-of-pocket costs for disease-modifying therapies (DMTs) for multiple sclerosis (MS).
Methods
Using nationwide Medicare Formulary and Drug Pricing Files, we analyzed Part D drug benefit design and DMT prices in 2010, 2016, and 2019. We calculated average monthly list prices for DMTs available in each year (4 DMTs in 2010, 11 DMTs in 2016, and 14 DMTs in 2019). We projected patients' annual out-of-pocket cost for each DMT alone under a standard Part D plan in that year. We estimated potential savings attributable to closing the coverage gap between 2010 and 2019 (beneficiaries' cost sharing dropped from 100% to 25%) under 3 scenarios: no increase in price, an inflation-indexed price increase (3% annually), and the observed price increase.
Results
Median monthly DMT prices rose from $2,804 to $5,987 to $7,009 over the years 2010, 2016, and 2019, respectively. Median projected annual out-of-pocket costs rose from $5,916 to $6,229 to $6,618. With unchanged or inflation-indexed DMT price changes, closing the coverage gap would have reduced annual out-of-pocket costs by $2,260 (38% reduction) and $1,744 (29% reduction), respectively. Despite having the lowest monthly price, generic glatiramer acetate had among the highest out-of-pocket costs ($6,731 to $6,939 a year) in 2019.
Conclusions
Medicare Part D beneficiaries can pay thousands of dollars yearly out of pocket for DMTs. Closing the Part D coverage gap did not reduce out-of-pocket costs for patients because of simultaneous increases in DMT prices.
Of the 720,000 Americans living with multiple sclerosis (MS), more than 1 in 4 are insured by Medicare.1,2 The majority of these individuals rely on the Medicare Part D drug benefit for access to medications, including coverage of expensive self-administered disease-modifying therapies (DMTs) priced at $80,000 annually or more.3,4 Despite Part D coverage, patients can face substantial costs to access DMTs because Part D imposes cost sharing with no limit on out-of-pocket costs.5
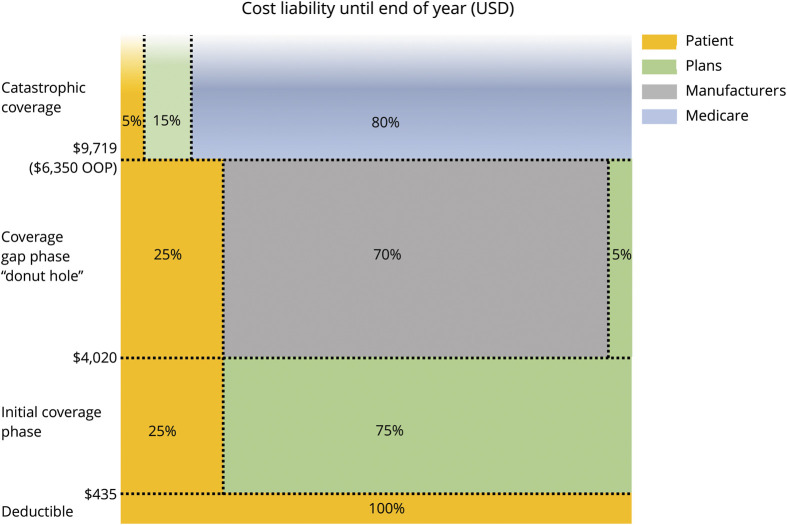
As shown in figure 1, under Part D, out-of-pocket costs are incurred year-round in 4 sequential phases—a deductible, coverage phase, coverage gap (nicknamed the donut hole), and catastrophic coverage. Patients move through each phase of the Part D benefit based on thresholds of total drug spending. For example, in 2020, patients with a standard Part D benefit will pay 100% out of pocket for the first $435 spent in the deductible phase, after which they will pay 25% of drug costs during the initial coverage phase. After reaching $4,020 in total drug spending, patients then enter the coverage gap. Historically, patients' largest out-of-pocket costs occurred during the coverage gap where they paid 100% of drug cost until reaching catastrophic coverage. To alleviate this financial burden, provisions of the Affordable Care Act and later legislation have gradually closed the coverage gap by reducing out-of-pocket liability from 100% in 2010 to 25% of drug price in 2019.6 Last, patients enter the catastrophic coverage phase when their total out-of-pocket spending reaches $6,350 (or about $9,719 in total drug spending), after which they pay 5% of drug costs until the end of the year.
Figure 1. Medicare Part D Standard Benefit Parameters (2020) for Branded Drugs.
Share of costs in coverage gap are born by manufacturers for branded drugs. For generic drugs, Part D plans pay 75% of drug costs (patients pay 25%). Dollar amounts reflect total drug spending. To reach catastrophic coverage, $6,350 out of pocket is approximately $9,719 in total drug costs.
Unfortunately, for beneficiaries with MS, the price of DMTs simultaneously increased 4-fold from 2006 to 2016.7 Thus, escalating DMT prices may have undermined the policy intent of lowering patients' out-of-pocket costs by closing the coverage gap.
The goal of this study was to evaluate the net effect of rising prices and closure of the Medicare Part D coverage gap on out-of-pocket expenses for self-administered DMTs for MS between 2010 and 2019.
Methods
We used Medicare Prescription Drug Plan Formulary, Pharmacy Network, and Pricing Information Files for the years 2010, 2016, and 2019 to analyze trends in self-administered DMT price and out-of-pocket costs for Part D plans nationwide. Files contain plans' drug benefit design and not patient claims. We chose 2010 as the final year in which patients paid 100% coinsurance during the gap. Because only 4 DMTs were available in 2010 files, we included 2016 data to capture baseline price and cost-sharing for 7 additional DMTs that became available subsequently. We selected 2019 as the first year in which patient cost-sharing during the gap first fell to 25% of drug price (i.e., the coverage gap closed), and 14 DMTs were available. We excluded infused DMTs because they are covered under Medicare Part B. We also excluded the oral DMTs (cladribine, siponimod, and diroximel fumarate) because their market entrance predated inclusion in the 2019 Q1 formulary file.
For individuals with a standard Part D benefit design, out-of-pocket costs are incurred in all phases of their benefit and directly based on a drug's retail list price before any rebates or discounts. We averaged the 30-day list price reported across all Part D plans nationwide for each DMT in each year (2010, 2016, and 2019). To project annual out-of-pocket costs, we applied cost-sharing parameters (i.e., % paid out of pocket by patient) from a standard Part D plan for each benefit phase (deductible, coverage, coverage gap, and catastrophic) in each year (table legend and illustrated in figure 1). For each year, we applied the standard Part D deductible amount ($310, $360, and $415 in 2010, 2016, and 2019, respectively) and average cost-sharing amounts in the coverage phase specific for each DMTs (not reported but ranged from 28% to 30% coinsurance for all years). In 2010, 2016, and 2019, cost-sharing during the coverage gap for brand name drugs was 100%, 45%, and 25% and cost-sharing for generic drugs was 100%, 58%, and 37% of drug list price, respectively.8 Decreasing cost-share liability in the coverage gap between 2010 and 2019 was funded through increasing discounts provided by manufacturers or Part D plans. During the gap, manufacturers of branded drugs provide a discount, which counts toward a patient's total out-of-pocket costs to qualify for catastrophic coverage.8 For generic drugs, these discounts are borne by Part D plans and do not count toward a patient's total out-of-pocket costs. Finally, when total out-of-pocket spending exceeds a set threshold ($4,550, $4,850, and $5,100 in 2010, 2016, and 2019, respectively), patients enter the catastrophic coverage phase and pay 5% of drug price for the remainder of the year.
To estimate the potential patient savings attributable to closing the coverage gap, we projected annual out-of-pocket costs under 3 scenarios: (1) no increase in DMT price, (2) a medical care inflation rate-indexed increase in price (3% annually),9 and (3) the observed price increase. We used the median monthly DMT price in 2010 as the basis for our out-of-pocket cost projections.
Standard Protocol Approvals, Registrations, and Patient Consents
This study was approved by the University of Hawaii Institutional Review Board.
Data Availability
This study did not involve human subjects. The data are available for purchase from the Centers for Medicare and Medicaid Services (CMS). Further information on how to purchase is on the CMS website (cms.gov/Research-Statistics-Data-and-Systems/Files-for-Order/NonIdentifiableDataFiles/PrescriptionDrugPlanFormularyPharmacyNetworkandPricingInformationFiles).
Results
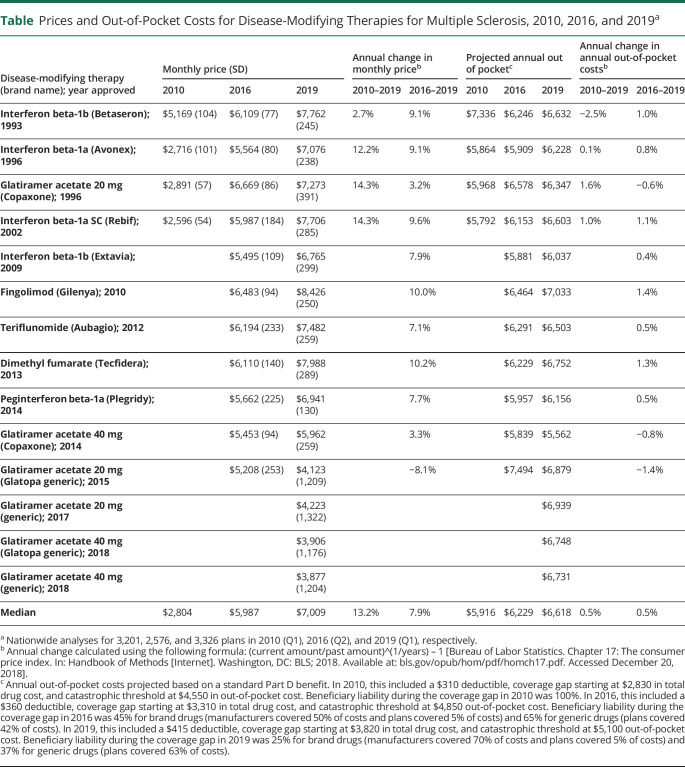
Median monthly price of DMTs rose from $2,804 to $5,987 to $7,009 for the years 2010, 2016, and 2019, respectively (table). This represented price increases of 13.2% annually (range 2.7%–14.3%) from 2010 to 2019 (4 DMTs) and 7.9% annually (range −8.1% to 10.2%) from 2016 to 2019 (11 DMTs). Generic glatiramer acetate, the only DMT with a generic formulation, had the lowest prices in 2019 ($3,877 to $4,223 per month). Similarly, both generic and brand name glatiramer acetate had the smallest annual price increases between 2016 and 2019 (range −8.1% to 3.3%). Brand name only DMTs such as fingolimod had the highest prices in 2019 ($8,426) and had the second largest annual increase (10.0%) behind dimethyl fumarate (10.2%) between 2016 and 2019.
Table.
Prices and Out-of-Pocket Costs for Disease-Modifying Therapies for Multiple Sclerosis, 2010, 2016, and 2019a
As the coverage gap closed, the median projected annual out-of-pocket costs for DMTs rose overall, increasing from a median of $5,916 to $6,229 to $6,618 for the years 2010, 2016, and 2019 (table), respectively. This represented a median increase of 0.5% annually (range −2.5% to 1.6%) between 2010 and 2019 and a median 0.5% annual increase (range −1.4% to 1.4%) between 2016 and 2019 across the DMTs. Despite having among the lowest monthly prices in 2019, the 4 generic versions of glatiramer acetate had among the highest annual out-of-pocket costs ($6,731 to $6,939).
As summarized in figure 2, patients' out-of-pocket costs would have dropped from $5,916 to $3,657 (38% decrease) if the median DMT price had remained unchanged through 2019. If DMT prices increased at the inflation rate, patients' out-of-pocket costs would have dropped to $4,173 (29% decrease).
Figure 2. Projected Annual Out-of-Pocket Costs in 2010 and 2019 for Disease-Modifying Therapy (DMT) Based on Median DMT Price in Each Year.
The orange bar is 2010; the blue bars are 2019. For 2019, the projected annual out-of-pocket cost is given for 3 scenarios: (1) actual median DMT price for 2019 ($7,009), (2) DMT price remained stable (no change) from 2010 to 2019 ($2,804), and (3) DMT price increased at medical care inflation rate (3%) from 2010 to 2019 ($3,658).
Discussion
Our results confirm that patients' out-of-pocket expenditures for MS drugs continue to be high5,7,10 and demonstrate how escalating list prices have out-stripped well-meaning reforms meant to protect patients from excessive out-of-pocket costs. In this analysis of Part D plans nationwide, closing the coverage gap from 2010 to 2019 should have lowered patients' cost sharing by approximately one-third if drug prices remained stable or increased only at 3% annually. Instead, median DMT prices increased 8%–13.2% annually, and out-of-pocket costs remained near $6,000 a year. Because the coverage gap liability will remain at 25%, continued escalation in DMT list prices will only increase patients' out-of-pocket liability in the coming years.
Traditionally, the entry of generic formulations translates into reduced prices, lower cost sharing, and greater affordability and access for patients. In this study, generic versions of glatiramer acetate had the lowest prices and showed the least price increase over time. However, prices for these generic DMTs still neared or exceed $4,000 a month, though less costly than the $6,000 to $8,000 for brand name only DMTs. Paradoxically, patients' out-of-pocket costs were also very high for generic glatiramer acetate due to Part D design. Under Part D, manufacturers must provide discounts for branded drugs in the gap, and this counts toward patients' out-of-pocket costs in qualifying for catastrophic coverage. In contrast, generic drugs receive no manufacturer gap discounts. Consequently, beneficiaries using expensive generic drugs, like generic glatiramer acetate, remain in the coverage gap for a longer period of time, pay a higher cost-sharing amount, and incur more out-of-pocket expenses. Thus, the entry of additional generic DMTs in the future does not ensure the eventual affordability of DMTs for Medicare beneficiaries with MS.
What are the implications of this study for neurologists? Nationally, more than 1 in 5 people report not being able to pay for a prescription drug because of cost.11 Among individuals with MS, a recent survey found that one-third of patients are personally affected by rising DMT prices, and more than half are very concerned about being able to afford their DMT in the next few years.12 Several studies using administrative claims data confirm that rising out-of-pocket costs have a detrimental effect on DMT adherence among individuals with MS.13–16 To address this issue, the American Academy of Neurology (AAN) has designated prescription drug pricing one of their top legislative and advocacy priorities for 2020.17 Neurologists should support the advocacy work of the AAN to seek lower drug prices. In addition, it is imperative that neurologists who prescribe MS DMTs are aware of the potential financial implications for patients who may have very high out-of-pocket costs despite having comprehensive insurance. This is particularly true of patients with prescription coverage via Medicare Part D.
Our study has limitations. Our analyses represent beneficiaries without low-income subsidies. In 2018, 28% of Part D enrollees qualified for a low-income subsidy.18 Projected annual out-of-pocket costs are based on use of a single DMT, and patients' actual out-of-pocket costs will reflect other medications that individuals with MS may take. List prices reported by Part D plans do not include manufacturer rebates, but under Part D, patients' cost sharing is based on prerebate list prices. Finally, our analysis is restricted to self-administered DMTs, which are covered through Medicare's Part D benefit. Infused DMTs (ocrelizumab, alemtuzumab, and natalizumab) are covered by Medicare Part B, which requires patients to pay a 20% coinsurance unless they purchase supplemental insurance (e.g., Medigap).19
For those prescribed expensive specialty medications such as DMTs for MS, out-of-pocket costs have increased substantially over the last several years.20 Closing the Part D coverage gap was intended to reduce this out-of-pocket burden. However, we found that escalating DMT prices have largely negated the intent of this policy, even despite the arrival of the first generic DMT. Considerable out-of-pocket savings could have been achieved for Medicare beneficiaries with MS if manufacturers had not increased prices as aggressively over the last decade.
For health conditions other than MS, the trend in rising prices of expensive specialty medications has caused patients' out-of-pocket costs to increase as well.21,22 Some argue that modernization of the Part D benefit design, including capping out-of-pocket costs, needs to be part of the solution.23,24 However, the exorbitant cost of many prescription drugs, including MS DMTs, remains the larger, more intractable problem. The US pharmaceutical market is a notoriously complex and purposely opaque system.25 Government-sanctioned monopolies and distorted incentives create an environment where normal economic forces do not effectively moderate the profit motive of the pharmaceutical industry and other firms in the drug distribution channel (e.g., pharmacy benefit managers).26,27 Several proposals in both the US House of Representatives and Senate are being considered to address rising drug prices. These include proposals to permit Medicare to directly negotiate with manufacturers and limit price increases to inflation or to prices paid in other selected countries.28,29 Although the current political landscape creates uncertainty, there is widespread public support for most, if not all, of the proposals under debate.29 It is incumbent on our elected officials to find common ground, forge compromise, and pass legislation to address the crisis of medication affordability for individuals with MS and other chronic conditions.
Appendix. Authors
Footnotes
Editorial, page 269
Study Funding
The study was funded by the National Multiple Sclerosis Society (Hartung and Johnston) and Hawaii Medical Service Association Endowed Chair in Health Services and Quality Research (Tseng and Chen).
Disclosure
D.M. Hartung reports receiving research support from AbbVie Pharmaceuticals. K.A. Johnston, D.N. Bourdette, and R. Chen report no disclosures. C.-W. Tseng is the Hawaii Medical Service Association Endowed Chair in Health Services and Quality Research. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
TAKE-HOME POINTS
→ Between 2010 and 2019, median prices for MS DMTs covered by the Medicare Part D drug benefit more than doubled from $2,804 to $7,009 per month.
→ Despite legislation to reduce patients' share of drug cost from 100% to 25% of drug cost (closing the Part D coverage gap), patients' projected annual out-of-pocket costs for MS DMTs increased from $5,916 to $6,618 because of escalating drug prices during the same time period.
→ Had DMT prices remained unchanged or increased at the rate of inflation, closing the coverage gap would have reduced patients' annual out-of-pocket costs by 29%–38%, respectively.
References
- 1.Wallin MT, Culpepper WJ, Campbell JD, et al. The prevalence of MS in the United States: a population-based estimate using health claims data. Neurology 2019;92:e1029–e1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Multiple Sclerosis Society. Medicare. Available at: nationalmssociety.org/Living-Well-With-MS/Work-and-Home/Insurance-and-Financial-Information/Health-Insurance/Medicare. Accessed November 4, 2019. [Google Scholar]
- 3.Hartung DM, Bourdette D. Addressing the rising prices of disease-modifying therapies for multiple sclerosis. JAMA Neurol Epub 2019 Aug 26. [DOI] [PubMed]
- 4.Hartung DM, Bourdette DN, Ahmed SM, Whitham RH. The cost of multiple sclerosis drugs in the US and the pharmaceutical industry: too big to fail? Neurology 2015;84:2185–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hartung DM, Johnston KA, Irwin A, Markwardt S, Bourdette DN. Trends in coverage for disease-modifying therapies for multiple sclerosis in Medicare Part D. Health Aff (Millwood) 2019;38:303–312. [DOI] [PubMed] [Google Scholar]
- 6.Donohue JM, Huskamp HA. Doughnuts and discounts—changes to Medicare Part D under the Bipartisan Budget Act of 2018. N Engl J Med 2018;378:1957–1960. [DOI] [PubMed] [Google Scholar]
- 7.San-Juan-Rodriguez A, Good CB, Heyman RA, Parekh N, Shrank WH, Hernandez I. Trends in prices, market share, and spending on self-administered disease-modifying therapies for multiple sclerosis in Medicare Part D. JAMA Neurol 2019;76:1386–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Announcement of Calendar Year (CY) 2019 Medicare Advantage Capitation Rates and Medicare Advantage and Part D Payment Policies and Final Call Letter. Baltimore, MD: Centers for Medicare & Medicaid Services; 2018. [Google Scholar]
- 9.Bureau of Labor Statistics [online]. Available at: bls.gov/data/. Accessed October 12, 2019. [Google Scholar]
- 10.Callaghan BC, Reynolds E, Banerjee M, et al. Out-of-pocket costs are on the rise for commonly prescribed neurologic medications. Neurology 2019;92:e2604–e2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gallup-West Health National Healthcare Study. Millions in U.S. Lost Someone Who Couldn't Afford Treatment. 2019. Available at: news.gallup.com/poll/268094/millions-lost-someone-couldn-afford-treatment.aspx. Accessed March 18, 2020. [Google Scholar]
- 12.National Multiple Sclerosis Society. Quantifying the Effect of the High Cost of DMTs: Market Research Report. 2019. Available at: nationalmssociety.org/NationalMSSociety/media/MSNationalFiles/Advocacy/NMSS-Research-Report-Full-Access-to-MS-Medications.pdf. Accessed March 18, 2020. [Google Scholar]
- 13.Li P, Hu T, Yu X, et al. Impact of cost-sharing increases on continuity of specialty drug use: a quasi-experimental study. Health Serv Res 2018;53(suppl 1):2735–2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romley J, Goldman D, Eber M, Dastani H, Kim E, Raparla S. Cost-sharing and initiation of disease-modifying therapy for multiple sclerosis. Am J Manag Care 2012;18:460–464. [PubMed] [Google Scholar]
- 15.Dor A, Lage MJ, Tarrants ML, Castelli-Haley J. Cost sharing, benefit design, and adherence: the case of multiple sclerosis. Adv Health Econ Health Serv Res 2010;22:175–193. [DOI] [PubMed] [Google Scholar]
- 16.Shao H, Stoecker C, Monnette AM, Shi L. Cost sharing of disease-modifying treatments (DMTs) as policy lever to improve DMTs' access in multiple sclerosis. Value Health 2018;21:1083–1089. [DOI] [PubMed] [Google Scholar]
- 17.American Academy of Neurology. Priority Issue: Drug Pricing. Available at: aan.com/policy-and-guidelines/policy/priority-issues/priority-issue-3. Accessed March 18, 2020. [Google Scholar]
- 18.Medicare Payment Advisory Commission (MedPAC). The Medicare Prescription Drug Program (Part D): Status Report. Available at: medpac.gov/docs/default-source/reports/mar19_medpac_ch14_sec.pdf. Accessed January 28, 2019. [Google Scholar]
- 19.Cubanski J, Swoope C, Boccuti C, et al. A Primer on Medicare: Key Facts about the Medicare Program and the People It Covers. San Francisco, CA: The Henry J. Kaiser Family Foundation. Available at: kff.org/medicare/report/a-primer-on-medicare-key-facts-about-the-medicare-program-and-the-people-it-covers. Accessed January 28, 2019. [Google Scholar]
- 20.Trish E, Xu J, Joyce G. Growing number of unsubsidized part D beneficiaries with catastrophic spending suggests need for an out-of-pocket cap. Health Aff (Millwood) 2018;37:1048–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dusetzina SB, Huskamp HA, Keating NL. Specialty drug pricing and out-of-pocket spending on orally administered anticancer drugs in Medicare Part D, 2010 to 2019. JAMA 2019;321:2025–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tseng CW, Masuda C, Chen R, Hartung DM. Impact of higher insulin prices on out-of-pocket costs in Medicare Part D. Diabetes Care 2020;43:e50–e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dusetzina SB, Keating NL, Huskamp HA. Proposals to redesign Medicare Part D: easing the burden of rising drug prices. N Engl J Med 2019;381:1401–1404. [DOI] [PubMed] [Google Scholar]
- 24.Daniel H, Bornstein SS; Health and Public Policy Committee of the American College of Physicians. Policy recommendations for public health plans to stem the escalating costs of prescription drugs: a position paper from the American College of Physicians. Ann Intern Med 2019;171:825–827. [DOI] [PubMed] [Google Scholar]
- 25.National Academies of Sciences, Engineering, and Medicine. Making Medicines Affordable: A National Imperative. Washington, DC: The National Academies Press; 2018. Available at: 10.17226/24946. Accessed February 4, 2020. [DOI] [Google Scholar]
- 26.Hartung DM, Alley L, Johnston KA, Bourdette DN. Qualitative study on the price of drugs for multiple sclerosis. Gaming the system. Neurology 2020;94:e368–e75. [DOI] [PubMed] [Google Scholar]
- 27.Dusetzina SB, Oberlander J. Advancing legislation on drug pricing: is there a path forward? N Engl J Med 2019;381:2081–2084. [DOI] [PubMed] [Google Scholar]
- 28.Freed M, Cubanski J, Neuman T. A Look at Recent Proposals to Control Drug Spending by Medicare and its Beneficiaries. San Francisco, CA: The Henry J. Kaiser Family Foundation; 2019. Available at: kff.org/medicare/issue-brief/a-look-at-recent-proposals-to-control-drug-spending-by-medicare-and-its-beneficiaries. Accessed February 4, 2020. [Google Scholar]
- 29.Cubanski J, Freed M, Neuman T. Ten Charts on Proposals to Lower Prescription Drug Costs. San Francisco, CA: The Henry J. Kaiser Family Foundation; 2020. Available at: kff.org/slideshow/ten-charts-on-proposals-to-lower-prescription-drug-costs. Accessed February 4, 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not involve human subjects. The data are available for purchase from the Centers for Medicare and Medicaid Services (CMS). Further information on how to purchase is on the CMS website (cms.gov/Research-Statistics-Data-and-Systems/Files-for-Order/NonIdentifiableDataFiles/PrescriptionDrugPlanFormularyPharmacyNetworkandPricingInformationFiles).