Abstract
Objective Allogeneic and xenogeneic acellular dermal matrix (ADM) grafts have been used to treat periodontal soft tissue defects. The purpose of the current study was to compare the effect of human ADM (AlloDerm) and porcine ADM (Derma) on human primary gingival fibroblasts in vitro regarding the biocompatibility test.
Materials and Methods Gingival fibroblasts were obtained from healthy adult gingiva and seeded on AlloDerm or Derma ADM in 96-well plate. The control cells were grown on a surface-treated polystyrene cell-culture plate without matrix. The cells were cultured for 3, 7, and 14 days. The fibroblasts morphology was examined using inverted microscopy, and the cell viability of fibroblasts adherent to the dermal matrix was evaluated using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) cell viability assay after 3, 7, and 14 days in culture. The data were statistically evaluated by one-way analysis of variance. p -Value of 0.05 was considered significant.
Results Gingival fibroblasts adjacent to the AlloDerm and Derma matrices were healthy, attached to the well, and did not exhibit any cytopathic changes similar to control. There were no statistically significant differences in the cell viability between the gingival fibroblasts attached to Derma and AlloDerm on day 3 ( p = 0.841), day 7 ( p = 0.198), and day 14 ( p = 0.788).
Conclusion Considering this in vitro study’s limitations, both human and porcine ADM were compatible with the surrounding human primary gingival fibroblasts. No significant differences were observed in the cell viability between the gingival fibroblasts that were attached to Derma and AlloDerm.
Keywords: human and porcine acellular dermal matrix, gingival fibroblasts, cell viability
Introduction
Inadequate keratinized tissue and gingival recession are common deformities associated with mucogingival tissue. It is recommended to have a minimum of 2 mm keratinized gingiva (KG) of which at least 1 mm must be attached, to facilitate plaque control, improve patient comfort, and preserve periodontal health. 1 2 On the other hand, inadequate KG with less than optimum oral hygiene practice leads to gingival recession and its subsequences, hypersensitivity, root caries, and esthetic concerns that necessitate gingival augmentation and root coverage periodontal surgeries. 3 4
Several mucogingival procedures have been suggested to treat gingival recession and increase gingival thickness including autogenous free gingival graft (FGG) and subepithelial connective tissue graft (CTG). 5 The FGG is effective for gingival augmentation, while CTG is considered the gold standard and the most reliable for gingival recessions treatment. 6 Despite the high predictability and excellent esthetic results with autogenous soft tissue grafts, there are some obstacles. The limited availability, another site surgery, patient discomfort, and time-consuming are significant concerns regarding the harvesting of autogenous grafts that lead the patient seek alternative treatment options. 7
Many biomaterials have been introduced in periodontology to substitute and overcome the limited availability and morbidity of autogenous grafts. The ideal soft tissue substitute must be biocompatible, promote hemostasis, and granulation tissue formation without causing an adverse immune reaction, healing interruption, or infection. Acellular dermal matrix (ADM) graft is processed chemically to remove all cells of epidermis and dermis without damaging the basement membrane and the connective tissue matrix. This processing method is aimed to maintain the integrity of collagen and elastin matrices that act as a scaffolding where epithelial cells, fibroblasts, and blood vessels of surrounding tissues adhere and incorporate into the newly formed soft tissue and finally replaced by host tissues completely. 8 9 10
Several studies have demonstrated that ADM is efficient and equivalent to CTG in treating gingival recession in short- and long-term outcomes. 11 12 13 Human ADM (AlloDerm) allograft has been used in multiple periodontal surgical operations, including root coverage, 14 gingival augmentation, 15 ridge deformities repair, 15 melanin pigmentation removal, 16 and guided tissue regeneration. 17
Despite the success and comparable AlloDerm results to autogenous soft tissue grafts, it has some concerns. 18 The restricted use in some countries, the potential risk of disease transmission, and the high cost of human ADM require finding another source of soft tissue graft to avoid these limitations.
Recently, xenogeneic ADM (Derma) is prepared from porcine skin by removing all cellular components of epidermis and dermis while keeping the dermal extracellular matrix (ECM) intact. 19 Previous studies have showed that the porcine ADM could be used as a replacement for CTG in the treatment of single gingival recession combined with coronally advanced flap (CAF). 20 Other studies reported that the porcine dermal matrix could be used to augment the thickness of keratinized tissue. 19 21 Due to the unlimited availability of porcine dermal matrix, low cost, and patient comfort and acceptance, make it a good substitute replacing human ADM or CTG.
Fibroblasts play a significant role in normal wound healing. They migrate, adhere, and proliferate rapidly during soft tissue injury. Fibroblasts speed up the healing process through formation of collagen and deposition of ECM. 22 Additionally, fibroblasts synthesize and secrete various growth factors which are involved in wound healing, such as vascular endothelial growth factor, keratinocytes growth factors, transforming growth factor, platelets derived growth factors, and insulin-like growth factors. 23 Fibroblasts can adhere and spread successfully on the ADM allograft (AlloDerm), but their migration is limited. 24 It has been demonstrated that the porcine bilayer collagen matrix (Mucograft) is compatible with fibroblasts in vitro 25 and porcine dermal matrix (Mucoderm)-induced fibroblast proliferation. 26
Preliminary clinical data concerning the use of the porcine dermal matrix (Derma) have showed promising results. However, few studies have compared the porcine and human dermal matrix in vitro regarding biocompatibility. In this study, we hypothesize that there is no difference between the biocompatibility of human and porcine ADM with human primary gingival fibroblasts in vitro .
Materials and Methods
Acellular Dermal Matrix
In this in vitro study, two acellular dermal matrices, including human dermal matrix (AlloDerm; BioHorizons, Birmingham, Alabama, United States) and porcine dermal matrix (OsteoBiol Derma, Tecnoss, Giaveno, Italy), were prepared according to instructions of the manufacturers. Each matrix was put in a Petri dish, cut into 3 × 4 mm pieces, and transferred to a 96-well plate (SPL Life Sciences Co., Ltd.; Seoul, Korea).
Cell Culture
Gingival tissues were taken at Dental Teaching Hospital, Umm Al-Qura University, from healthy adult individual during crown lengthening surgical procedure after acquiring the signed informed consent.
The gingival fibroblasts were isolated from the healthy gingival tissues according to the method described by Mudalal et al. 27 The gingival tissues were washed with phosphate-buffered saline and incubated in dispase 1 mg/mL (Sigma, United States) overnight at 4°C to facilitate separation of the epithelial layer from the connective tissue. After removing the epithelial layer, the connective tissue was cut into small pieces with a scalpel blade and placed in a 25-mL flask. Culture medium was added to the flask and incubated in 37°C incubator with a humidified atmosphere containing 5% CO 2 .
The culture medium was composed of Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 100 U/mL penicillin, 100 µg/mL streptomycin, and 2.5 µg/mL amphotericin B (all from Gibco Thermo Scientific, United States).
Gingival fibroblasts at the second passage were seeded on Derma or AlloDerm dermal matrix at 10,000 cells/well in 96-well plate. The control is fibroblasts that were grown on a surface-treated polystyrene cell-culture plate without a matrix. The cells were incubated in a culture medium at 37°C in a humidified atmosphere of 5% CO 2 for 14 days. The medium was changed every 3 days. The cell viability of gingival fibroblasts adherent to the dermal matrix was assessed on days 3, 7, and 14 by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay.
Cell Viability MTT Assay
MTT assay measures cell proliferation and cytotoxicity. 28 29 When MTT is taken up by a viable cell, it is enzymatically reduced to formazan crystals, which is directly proportional to the number of these cells. Briefly, the culture medium was discarded, and the dermal matrix, along with the attached fibroblasts, was transferred to a new well in the same 96-well plate. MTT (ThermoFisher Scientific, United States) solution in culture medium (0.5 mg/mL) was added in each well and then incubated for 3 hours at 37°C. At the end of the incubation period, the MTT solution was discarded and formazan granules were dissolved by incubation in solvent solution (dimethyl sulfoxide:isopropanol at 1:1). After 30 minutes’ incubation, the solution was transferred to a new well, and the optical density was assessed by microplate spectrophotometer (SpectroStar Nano, BMG Laboratory) at 570 nm.
Statistical Analysis
The cell viability assays were performed in duplicate and the data were analyzed statistically in GraphPad Prism version 7 (GraphPad Software, Inc., San Diego, California, United States). The results were presented as mean ± standard error of the mean and analyzed by one-way analysis of variance. A p -value 0.05 was considered significant.
Results
Morphology of Gingival Fibroblasts
The morphology of gingival fibroblasts was examined by inverted microscopy. The gingival fibroblasts grew out from gingival connective tissue after 10 days of culturing ( Fig. 1A ). The morphology of gingival fibroblasts adjacent to the Derma or AlloDerm matrix was compared with the control cells grown on surface-treated polystyrene cell-culture plate without matrix. The gingival fibroblasts next to the dermal matrix, were healthy, attached to the well, and did not exhibit any cytopathic changes similar to control ( Fig. 1 ).
Fig. 1.
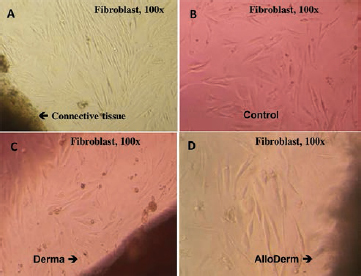
The morphology of primary human gingival fibroblasts at 100× magnification. ( A ) Gingival fibroblast growing from gingival connective tissue after 10 days of culturing. ( B ) The gingival fibroblasts at the second passage after 3 days of culturing (control). The gingival fibroblasts adjacent to Derma ( C ) or AlloDerm ( D ) acellular dermal matrix ( ADM ) on day 3 appear similar to control. The control fibroblasts are grown on surface-treated polystyrene cell-culture plate without ADM matrix.
Cell Viability Assay
The cell viability of fibroblasts adherent to Derma or AlloDerm dermal matrix was assessed using MTT cell viability assay after 3, 7, and 14 days in culture ( Fig. 2 ). The MTT assays showed no significant differences in the cell viability between the gingival fibroblasts attached to Derma and AlloDerm on day 3 ( p = 0.841), on day 7 ( p = 0.198), and on day 14 ( p = 0.788).
Fig. 2.
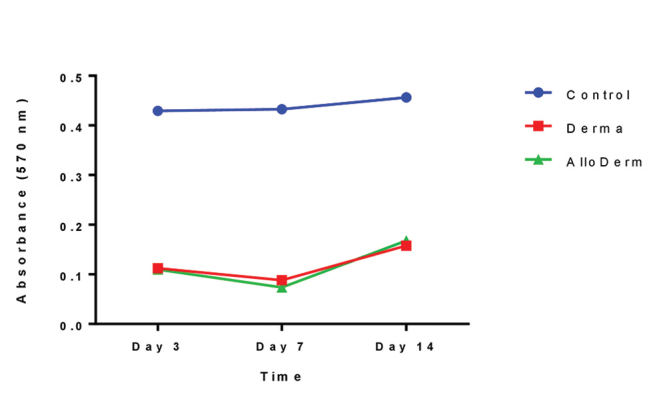
MTT cell viability of gingival fibroblasts adherent to Derma or AlloDerm acellular dermal matrix. Gingival fibroblasts were seeded on Derma or AlloDerm acellular dermal matrix ( ADM ) in 96-well plate. Gingival fibroblasts were grown on surface-treated polystyrene cell-culture plate without a matrix. The cell viability of gingival fibroblasts adherent to the Derma or AlloDerm dermal matrix did not show any statistically significant difference. There is no statistically significant difference between Derma and AlloDerm on day 3 ( p = 0.841), on day 7 ( p = 0.198), and on day 14 ( p = 0.788). The data from one representative experiment of three independent experiments presented as mean ± standard error of the mean (SEM).
Discussion
The autogenous soft tissue graft is a reliable treatment for root coverage and keratinized gingival augmentation procedures. Due to limited quantity and patient’s morbidity, there was a need for substitutes to treat soft tissue defects. Decellularization is the method by which the cellular components of tissues that elicit an adverse host response are removed while preserving the components of ECM. The decellularized ECM provides an immune-compatible substitute to autogenous tissue graft. Tissue decellularization maintains the original ultrastructure and structure of the ECM and provides nonimmunogenic matrices. 30 This matrix forms scaffolds for fibroblasts, epithelial, and other cells as well as the blood vessels from the surrounding tissue to ingrowth and incorporates into the newly formed soft tissue. 19 Human and porcine ADM have been used to substitute and overcome the shortage of autogenous grafts, reduce surgical time, and minimize postoperative pain. 7 31 32
In this study, the gingival fibroblasts adjacent to Derma or AlloDerm matrix were healthy, attached to the well, and did not exhibit any cytopathic changes like control cells which were grown on the cell-culture plate without matrix. This result showed that human and porcine ADM did not release any cytotoxic substance into the surrounding tissues.
In the current study, MTT assay was used to determine the viability of gingival fibroblasts on human and porcine ADM. After seeding the cells on ADM, the matrix, along with adherent cells, moved to a new well to determine the cell viability of only adherent cells and exclude gingival fibroblasts grown on polystyrene adjacent to the ADM. No statistically significant differences in the cell viability between the gingival fibroblasts attached to Derma and AlloDerm were observed. The cell viability of cells adherent to the dental matrix was not compared with control cells because the number of cells in control wells was greater than the cells adherent to the matrix as the matrix membrane did not cover the entire well’s surface. Also, control fibroblasts were grown on the surface-treated polystyrene cell-culture plate, which facilitates cell adhesion and proliferation. The results of this study suggest that the biocompatibility of porcine Derma is equivalent to human AlloDerm.
Several studies have demonstrated similarities between human and porcine acellularized dermal matrix. Ge et al 33 have shown a strong resemblance between porcine and human ADM histologically, including scaffold structure, collagen structure, and arrangement in vitro . In addition, it has been demonstrated that fibroblasts attach and spread on the outer surface of human and porcine ADM 24 25 and fibroblasts adherence on the porcine and the human ADM was similar, but fibroblasts infiltration was better in human ADM than porcine ADM. 34 Regarding cell viability, there were no significant differences in cell viability of human fibroblasts and human lymphocytes assay between human ADM and porcine ADM. 33
Additionally, in vivo studies have shown that no difference was observed in adhesion and neovascularization between porcine and human ADM. However, human ADM has more cellular and vascular infiltration and tissue ingrowth into the original tissue compared with porcine ADM. 35 36
Human and porcine ADM have been used to substitute CTG for root coverage and gingival augmentation procedures. Regarding the recession coverage procedure, human ADM provided adequate root coverage comparable to CTG, 37 38 while porcine ADM showed less root coverage than CTG. 39 However, other studies have shown that porcine ADM was comparable to CTG when combined with CAF. 40 41 Regarding the increase in KG thickness, the porcine ADM showed an adequate increase in KG tissue comparable to CTG 42 while the human ADM was inferior to CTG. 15 38 However, other studies have shown that human ADM was comparable to CTG when combined with CAF. 43
The current study has some limitations worth noting. This experiment was conducted on isolated cells grown in cell culture, and the result represents only the response of these cells without considering other surrounding factors such as cell–cell interaction, recipient site, and host defense mechanisms. Another limitation of this study was that fibroblasts infiltration into the ADM histologically was not investigated.
Conclusion
Considering this in vitro study’s limitations, both human and porcine ADM were compatible with the surrounding human primary gingival fibroblasts and there was no significant difference in the cell viability between the gingival fibroblasts attached to Derma and AlloDerm.
Acknowledgments
This study was performed in the Dental College Research Laboratory, Umm Al-Qura University. We are grateful to Dr. Ahmed Dardir for providing the gingival specimen and Professor Ibrahim Hamoda for reviewing the manuscript.
Funding Statement
Funding None.
Footnotes
Conflict of InterestEthical Approval None declared.
The research proposal was approved by the ethical committee of dental college, Umm Al-Qura University (acceptance number: 189–20).
References
- 1.Lang N P, Löe H. The relationship between the width of keratinized gingiva and gingival health. J Periodontol. 1972;43(10):623–627. doi: 10.1902/jop.1972.43.10.623. [DOI] [PubMed] [Google Scholar]
- 2.Wennström J, Lindhe J. Role of attached gingiva for maintenance of periodontal health. Healing following excisional and grafting procedures in dogs. J Clin Periodontol. 1983;10(02):206–221. doi: 10.1111/j.1600-051x.1983.tb02208.x. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy J E, Bird W C, Palcanis K G, Dorfman H S. A longitudinal evaluation of varying widths of attached gingiva. J Clin Periodontol. 1985;12(08):667–675. doi: 10.1111/j.1600-051x.1985.tb00938.x. [DOI] [PubMed] [Google Scholar]
- 4.Pradeep K, Rajababu P, Satyanarayana D, Sagar V. Gingival recession: review and strategies in treatment of recession. Case Rep Dent. 2012;2012:563421. doi: 10.1155/2012/563421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thoma D S, Benić G I, Zwahlen M, Hämmerle C H, Jung R E. A systematic review assessing soft tissue augmentation techniques. Clin Oral Implants Res. 2009;20 04:146–165. doi: 10.1111/j.1600-0501.2009.01784.x. [DOI] [PubMed] [Google Scholar]
- 6.Chambrone L, Sukekava F, Araújo M G, Pustiglioni F E, Chambrone L A, Lima L A. Root-coverage procedures for the treatment of localized recession-type defects: a Cochrane systematic review. J Periodontol. 2010;81(04):452–478. doi: 10.1902/jop.2010.090540. [DOI] [PubMed] [Google Scholar]
- 7.Barros R R, Novaes A B, Grisi M F, Souza S L, Taba M J, Palioto D B. A 6-month comparative clinical study of a conventional and a new surgical approach for root coverage with acellular dermal matrix. J Periodontol. 2004;75(10):1350–1356. doi: 10.1902/jop.2004.75.10.1350. [DOI] [PubMed] [Google Scholar]
- 8.Wainwright D J. Use of an acellular allograft dermal matrix (AlloDerm) in the management of full-thickness burns. Burns. 1995;21(04):243–248. doi: 10.1016/0305-4179(95)93866-i. [DOI] [PubMed] [Google Scholar]
- 9.Wong A K, Schonmeyer B H, Singh P, Carlson D L, Li S, Mehrara B J. Histologic analysis of angiogenesis and lymphangiogenesis in acellular human dermis. Plast Reconstr Surg. 2008;121(04):1144–1152. doi: 10.1097/01.prs.0000302505.43942.07. [DOI] [PubMed] [Google Scholar]
- 10.Wei P-C, Laurell L, Lingen M W, Geivelis M. Acellular dermal matrix allografts to achieve increased attached gingiva. Part 2. A histological comparative study. J Periodontol. 2002;73(03):257–265. doi: 10.1902/jop.2002.73.3.257. [DOI] [PubMed] [Google Scholar]
- 11.Kroiss S, Rathe F, Sader R, Weigl P, Schlee M. Acellular dermal matrix allograft versus autogenous connective tissue grafts for thickening soft tissue and covering multiple gingival recessions: a 5-year preference clinical study. Quintessence Int. 2019;50(04):278–285. doi: 10.3290/j.qi.a42160. [DOI] [PubMed] [Google Scholar]
- 12.Muthuraj T S, Bagchi S, Bandyopadhyay P, Mallick S, Ghosh P, Renganath M J. A randomized split mouth clinical study to compare the clinical outcomes of subepithelial connective graft and acellular dermal matrix in Miller’s Class I recession coverage therapy. J Indian Soc Periodontol. 2020;24(04):342–347. doi: 10.4103/jisp.jisp_609_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balaji V R, Ramakrishnan T, Manikandan D et al. Management of gingival recession with acellular dermal matrix graft: a clinical study. J Pharm Bioallied Sci. 2016;8 01:S59–S64. doi: 10.4103/0975-7406.191970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Novaes A B, Jr, Grisi D C, Molina G O, Souza S L, Taba M, Jr, Grisi M F. Comparative 6-month clinical study of a subepithelial connective tissue graft and acellular dermal matrix graft for the treatment of gingival recession. J Periodontol. 2001;72(11):1477–1484. doi: 10.1902/jop.2001.72.11.1477. [DOI] [PubMed] [Google Scholar]
- 15.Wei P C, Laurell L, Geivelis M, Lingen M W, Maddalozzo D. Acellular dermal matrix allografts to achieve increased attached gingiva. Part 1. A clinical study. J Periodontol. 2000;71(08):1297–1305. doi: 10.1902/jop.2000.71.8.1297. [DOI] [PubMed] [Google Scholar]
- 16.Novaes A B, Jr, Pontes C C, Souza S L, Grisi M F, Taba M., Jr The use of acellular dermal matrix allograft for the elimination of gingival melanin pigmentation: case presentation with 2 years of follow-up. Pract Proced Aesthet Dent. 2002;14(08):619–623. [PubMed] [Google Scholar]
- 17.Novaes A B, Jr, Souza S L. Acellular dermal matrix graft as a membrane for guided bone regeneration: a case report. Implant Dent. 2001;10(03):192–196. doi: 10.1097/00008505-200107000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Moslemi N, Mousavi J azi, M, Haghighati F, Morovati S P, Jamali R. Acellular dermal matrix allograft versus subepithelial connective tissue graft in treatment of gingival recessions: a 5-year randomized clinical study. J Clin Periodontol. 2011;38(12):1122–1129. doi: 10.1111/j.1600-051X.2011.01789.x. [DOI] [PubMed] [Google Scholar]
- 19.Fickl S, Nannmark U, Schlagenhauf U, Hürzeler M B, Kebschull M. Porcine dermal matrix in the treatment of dehiscence-type defects–an experimental split-mouth animal trial. Clin Oral Implants Res. 2015;26(07):799–805. doi: 10.1111/clr.12355. [DOI] [PubMed] [Google Scholar]
- 20.Fickl S, Jockel-Schneider Y, Lincke T, Bechtold M, Fischer K R, Schlagenhauf U. Porcine dermal matrix for covering of recession type defects: a case series. Quintessence Int. 2013;44(03):243–246. doi: 10.3290/j.qi.a29053. [DOI] [PubMed] [Google Scholar]
- 21.Verardi S, Orsini M, Lombardi T et al. Comparison between two different techniques for peri-implant soft tissue augmentation: porcine dermal matrix graft versus tenting screw. J Periodontol. 2019 doi: 10.1002/JPER.19-0447. [DOI] [PubMed] [Google Scholar]
- 22.Erdag G, Sheridan R L. Fibroblasts improve performance of cultured composite skin substitutes on athymic mice. Burns. 2004;30(04):322–328. doi: 10.1016/j.burns.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 23.Naughton G, Mansbridge J, Gentzkow G. A metabolically active human dermal replacement for the treatment of diabetic foot ulcers. Artif Organs. 1997;21(11):1203–1210. doi: 10.1111/j.1525-1594.1997.tb00476.x. [DOI] [PubMed] [Google Scholar]
- 24.Rodrigues A Z, Oliveira P T, Novaes A B, Jr, Maia L P, Souza S L, Palioto D B. Evaluation of in vitro human gingival fibroblast seeding on acellular dermal matrix. Braz Dent J. 2010;21(03):179–189. doi: 10.1590/s0103-64402010000300001. [DOI] [PubMed] [Google Scholar]
- 25.Lima R S, Peruzzo D C, Napimoga M H, Saba-Chujfi E, Dos Santos-Pereira S A, Martinez E F. Evaluation of the biological behavior of Mucograft® in human gingival fibroblasts: an in vitro study. Braz Dent J. 2015;26(06):602–606. doi: 10.1590/0103-6440201300238. [DOI] [PubMed] [Google Scholar]
- 26.Pabst A M, Happe A, Callaway A et al. In vitro and in vivo characterization of porcine acellular dermal matrix for gingival augmentation procedures. J Periodontal Res. 2014;49(03):371–381. doi: 10.1111/jre.12115. [DOI] [PubMed] [Google Scholar]
- 27.Mudalal M, Sun X, Li X, Zhou Y. The evaluation of leukocyte-platelet rich fibrin as an anti-inflammatory autologous biological additive. A novel in vitro study. Saudi Med J. 2019;40(07):657–668. doi: 10.15537/smj.2019.7.24302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mosmann T.Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays J Immunol Methods 198365(1-2)55–63. [DOI] [PubMed] [Google Scholar]
- 29.Carmichael J, DeGraff W G, Gazdar A F, Minna J D, Mitchell J B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 1987;47(04):936–942. [PubMed] [Google Scholar]
- 30.Fu R H, Wang Y C, Liu S P et al. Decellularization and recellularization technologies in tissue engineering. Cell Transplant. 2014;23(04/05):621–630. doi: 10.3727/096368914X678382. [DOI] [PubMed] [Google Scholar]
- 31.Fu J H, Su C Y, Wang H L. Esthetic soft tissue management for teeth and implants. J Evid Based Dent Pract. 2012;12 03:129–142. doi: 10.1016/S1532-3382(12)70025-8. [DOI] [PubMed] [Google Scholar]
- 32.Schmitt C M, Moest T, Lutz R, Wehrhan F, Neukam F W, Schlegel K A. Long-term outcomes after vestibuloplasty with a porcine collagen matrix (Mucograft®) versus the free gingival graft: a comparative prospective clinical trial. Clin Oral Implants Res. 2016;27(11):e125–e133. doi: 10.1111/clr.12575. [DOI] [PubMed] [Google Scholar]
- 33.Ge L, Zheng S, Wei H. Comparison of histological structure and biocompatibility between human acellular dermal matrix (ADM) and porcine ADM. Burns. 2009;35(01):46–50. doi: 10.1016/j.burns.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 34.Armour A D, Fish J S, Woodhouse K A, Semple J L. A comparison of human and porcine acellularized dermis: interactions with human fibroblasts in vitro. Plast Reconstr Surg. 2006;117(03):845–856. doi: 10.1097/01.prs.0000204567.28952.9d. [DOI] [PubMed] [Google Scholar]
- 35.Campbell K T, Burns N K, Rios C N, Mathur A B, Butler C E. Human versus non-cross-linked porcine acellular dermal matrix used for ventral hernia repair: comparison of in vivo fibrovascular remodeling and mechanical repair strength. Plast Reconstr Surg. 2011;127(06):2321–2332. doi: 10.1097/PRS.0b013e318213a053. [DOI] [PubMed] [Google Scholar]
- 36.Ngo M D, Aberman H M, Hawes M L, Choi B, Gertzman A A. Evaluation of human acellular dermis versus porcine acellular dermis in an in vivo model for incisional hernia repair. Cell Tissue Bank. 2011;12(02):135–145. doi: 10.1007/s10561-011-9245-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harris R J. A comparative study of root coverage obtained with an acellular dermal matrix versus a connective tissue graft: results of 107 recession defects in 50 consecutively treated patients. Int J Periodontics Restorative Dent. 2000;20(01):51–59. [PubMed] [Google Scholar]
- 38.Tal H, Moses O, Zohar R, Meir H, Nemcovsky C. Root coverage of advanced gingival recession: a comparative study between acellular dermal matrix allograft and subepithelial connective tissue grafts. J Periodontol. 2002;73(12):1405–1411. doi: 10.1902/jop.2002.73.12.1405. [DOI] [PubMed] [Google Scholar]
- 39.McGuire M K, Scheyer E T. Xenogeneic collagen matrix with coronally advanced flap compared to connective tissue with coronally advanced flap for the treatment of dehiscence-type recession defects. J Periodontol. 2010;81(08):1108–1117. doi: 10.1902/jop.2010.090698. [DOI] [PubMed] [Google Scholar]
- 40.Cardaropoli D, Tamagnone L, Roffredo A, Gaveglio L. Treatment of gingival recession defects using coronally advanced flap with a porcine collagen matrix compared to coronally advanced flap with connective tissue graft: a randomized controlled clinical trial. J Periodontol. 2012;83(03):321–328. doi: 10.1902/jop.2011.110215. [DOI] [PubMed] [Google Scholar]
- 41.Jepsen K, Jepsen S, Zucchelli G et al. Treatment of gingival recession defects with a coronally advanced flap and a xenogeneic collagen matrix: a multicenter randomized clinical trial. J Clin Periodontol. 2013;40(01):82–89. doi: 10.1111/jcpe.12019. [DOI] [PubMed] [Google Scholar]
- 42.Lorenzo R, García V, Orsini M, Martin C, Sanz M. Clinical efficacy of a xenogeneic collagen matrix in augmenting keratinized mucosa around implants: a randomized controlled prospective clinical trial. Clin Oral Implants Res. 2012;23(03):316–324. doi: 10.1111/j.1600-0501.2011.02260.x. [DOI] [PubMed] [Google Scholar]
- 43.de Souza S L, Novaes A B, Jr, Grisi D C, Taba M, Jr, Grisi M F de, de Andrade P F. Comparative clinical study of a subepithelial connective tissue graft and acellular dermal matrix graft for the treatment of gingival recessions: six- to 12-month changes. J Int Acad Periodontol. 2008;10(03):87–94. [PubMed] [Google Scholar]


