Abstract
Background:
Postural tachycardia syndrome (POTS) is a common childhood disease that seriously affects the patient's physical and mental health. This study aimed to investigate whether pre-treatment baseline left ventricular ejection fraction (LVEF) and left ventricular fractional shortening (LVFS) values were associated with symptom improvement after metoprolol therapy for children and adolescents with POTS.
Methods:
This retrospective study evaluated 51 children and adolescents with POTS who received metoprolol therapy at the Peking University First Hospital between November 2010 and July 2019. All patients had completed a standing test or basic head-up tilt test and cardiac echocardiography before treatment. Treatment response was evaluated 3 months after starting metoprolol therapy. The pre-treatment baseline LVEF and LVFS values were evaluated for correlations with decreases in the symptom score after treatment (ΔSS). Multivariable analysis was performed using factors with a P value of <0.100 in the univariate analyses and the demographic characteristics.
Results:
A comparison of responders and non-responders revealed no significant differences in demographic, hemodynamic characteristics, and urine specific gravity (all P > 0.050). However, responders had significantly higher baseline LVEF (71.09% ± 4.44% vs. 67.17% ± 4.88%, t = −2.789, P = 0.008) and LVFS values (40.00 [38.00, 42.00]% vs. 36.79% ± 4.11%, Z = −2.542, P = 0.010) than the non-responders. The baseline LVEF and LVFS were positively correlated with ΔSS (r = 0.378, P = 0.006; r = 0.363, P = 0.009), respectively. Logistic regression analysis revealed that LVEF was independently associated with the response to metoprolol therapy in children and adolescents with POTS (odds ratio: 1.201, 95% confidence interval: 1.039–1.387, P = 0.013).
Conclusions:
Pre-treatment baseline LVEF was associated with symptom improvement after metoprolol treatment for children and adolescents with POTS.
Keywords: Children, Left ventricular ejection fraction, Left ventricular fractional shortening, Metoprolol, Postural tachycardia syndrome
Introduction
Postural tachycardia syndrome (POTS) is a common form of orthostatic intolerance (OI) and is associated with excessive orthostatic tachycardia.[1–3] The incidence of POTS is approximately 6.80% among children and adolescents,[4] and this condition seriously affects their physical health, psychological health, and quality of life.[5,6] Beta-blockers are important treatment option for POTS in children and adolescents,[7,8] but they only improve symptoms in approximately 57.10% to 57.89% of children.[9,10] Therefore, to better guide personalized therapy, it would be useful to identify pre-treatment indexes that can predict symptom improvement in children and adolescents with POTS.
Previous research has suggested that the poor efficacy of metoprolol treatment for POTS is related to its complex pathogenesis, which can involve autonomic dysfunction, high catecholamine status, excessive vasodilation, and low central blood volume. Furthermore, previous studies have indicated that the efficacy of metoprolol treatment (a beta-adrenoceptor blocker) for POTS in children and adolescents was related to plasma concentrations of norepinephrine,[11] copeptin,[12] and C-type natriuretic peptide,[13] as well as heart rate (HR) variability from a 24-h dynamic electrocardiography (Holter) assessment.[14] However, these indicators can be unstable and relatively difficult to evaluate, which limits their clinical use. Therefore, stable, non-invasive, and easily evaluable indicators are needed to predict symptom improvement in response to metoprolol treatment for POTS.
Left ventricular ejection fraction (LVEF) and left ventricular fractional shortening (LVFS) are relatively stable echocardiography parameters that reflect the contractile function of the left ventricle. Furthermore, these markers reflect the state of catecholamine and sympathetic activity,[15,16] and can be easily evaluated in a non-invasive manner. Previous studies have also shown that sympathetic activity is at least partially predicted by clinicodemographic characteristics, including age, height, weight, HR, blood pressure (BP), and urine specific gravity (Usg).[17,18] We hypothesized that the response to metoprolol treatment for children and adolescents with POTS might be associated with echocardiography-derived indexes (LVEF and LVFS), demographic characteristics, and/or hemodynamic parameters. This pilot study aimed to determine whether these pre-treatment factors could predict the response to metoprolol therapy and guide personalized treatment strategies in this setting.
Methods
Ethical approval
The study protocol was approved by the Ethics Committee of Peking University First Hospital, China (No. 2018 [202]). All parents or guardians of the patients were informed of the study's purpose and provided written informed consent.
Subjects
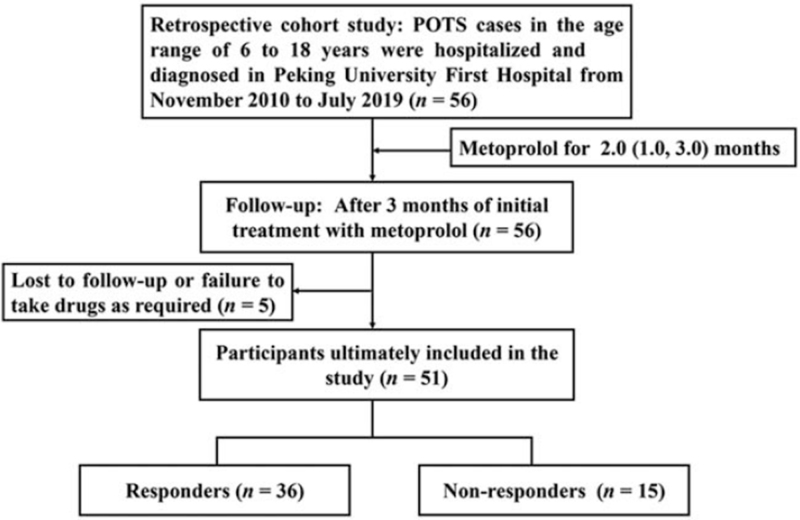
This retrospective study evaluated 56 children and adolescents with POTS who received metoprolol treatment as outpatients or inpatients at the Peking University First Hospital between November 2010 and July 2019. Follow-up was performed via telephone and inpatient/outpatient visits after 3 months to evaluate treatment response, although five patients (8.90%) were lost to follow-up. Thus, the study included 51 children and adolescents with POTS (28 males, median age: 12.0 ± 2.2 years). Figure 1 shows the study flowchart.
Figure 1.
Study flowchart of patients with postural tachycardia syndrome under metoprolol therapy. POTS: Postural tachycardia syndrome.
Diagnosis and data collection
The diagnosis of POTS had been based on the following factors: (1) the condition mainly affects older children; (2) the condition is often associated with predisposing factors, such as a rapid change from the supine position to the upright position or prolonged standing; (3) symptoms of OI, such as dizziness, headache, fatigue, blurred vision, chest tightness, palpitations, hand tremors, and syncope; (4) a HR increase of ≥40 beats per minute (bpm) or a maximum HR of ≥130 bpm (for 6–12-year-old children) or ≥125 bpm (for 13–18-year-old adolescents) without orthostatic hypotension (BP decrease of >20/10 mmHg) during the first 10 min of the standing test or basic head-up tilt test; and (5) exclusion of other diseases that can cause OI symptoms, such as organic cardiovascular diseases, metabolic diseases, neurological diseases, or mental illness.[14,19–21] Patients also underwent evaluations to collect data regarding their medical history, physical status, Usg index, electrocardiography echocardiography parameters, standing test, electroencephalography, cranial computed tomography or magnetic resonance imaging, basic head-up tilt test, and psychological tests. The treatment response was evaluated after 3 months of metoprolol treatment, based on the difference between the pre-treatment and post-treatment symptom scores (SS), as described below. Figure 1 displays a flow-chart explaining the inclusion of participants in this study.
Quality assurance
Before the start of the study, all researchers were required to complete professional training and a written assessment before they judged and entered data regarding the patients’ eligibility and treatment response.
Standing test and basic head-up tilt test
The standing test was performed in an environment with dim light. The patient rested in the supine position for 10 min and the HR and BP values were continuously monitored using a Dash 2000 multi-channel physiological monitor (General Electric Company, New York, NY, USA). The patient was then asked to stand upright for 10 min for additional monitoring of the HR and BP values.[20]
All drugs that could affect autonomic function were discontinued for at least five half-lives before the basic head-up tilt test and patients were required to fast for >4 h before the test. The patient was asked to rest in the supine position for 10 to 30 min on an adjustable bed (HUT-821; Beijing Juchi, Beijing, China) in a quiet, warm, and dimly lit environment. The HR, BP, and electrocardiography parameters were continuously evaluated using a multi-lead monitor (General Electric Company) until the HR and BP values stabilized. The bed was then tilted to 60° and the HR, BP, and electrocardiography parameters were again monitored until a positive reaction appeared or until the patient completed the 45 min examination.[19,22]
Measurement and calculation of LVEF and LVFS
The patients were confirmed that they had no vomiting, diarrhea, other fluid loss symptoms, iatrogenic supplements, rehydration, or diuretic use immediately before the echocardiographic measurements of LVEF and LVFS. The pre-treatment measures of LVEF and LVFS were performed with the patient in the supine position by professionally trained staff using Doppler color echocardiography (Aplio Artida SSH-880CV, TOSHIBA, Japan) with a linear 2.50 to 5.00 MHz transducer. The long-axis section of the parasternal ventricle was evaluated using M-mode echocardiography to determine the left ventricular end-diastolic diameter (LVDd) and the left ventricular end-systolic diameter (LVDs), which were then used to calculate the LVEF and LVFS values:
| LVEF = ([LVDd3 − LVDs3])/(LVDd3) |
| LVFS = ([LVDd − LVDs])/(LVDd) |
Evaluating treatment response using ΔSS
Each patient's SS was calculated for OI-related symptoms, which include syncope, dizziness, headache, chest tightness, nausea, palpitations, hand tremors, sweating, blurred vision, and inattention. Symptoms were scored as 0 point (no occurrence), 1 point (once per month), 2 points (2–4 times per month), 3 points (2–7 times per week), or 4 points (more than once per day), with the patient's total score calculated by adding the scores for each symptom. The pre-treatment baseline SS, the 3-month follow-up SS, and the change in the SSs (ΔSS) were evaluated. A decrease of ≥2 points was used to identify “responders” (ie, treatment was effective) and a decrease of <2 points was used to identify “non-responders.”[14,23]
Treatment and follow-up plan
After the initial diagnosis of POTS, the patients had received oral metoprolol treatment for 2.0 (1.0, 3.0) months (median [interquartile range, IQR]). The initial metoprolol dose was 0.5 mg·kg−1·day−1 (twice daily) and was gradually increased as tolerable and necessary to a maximum dose of 2.0 mg·kg−1·day−1.[19,24] After 3 months, the post-treatment SS scores were evaluated via telephone or inpatient/outpatient hospital visits. Drug adherence, OI symptom frequency, and adverse drug reactions were recorded during the follow-up.
Statistical analysis
All analyses were performed using SPSS software (version 23.0; IBM Corp., Armonk, NY, USA). Continuous data were reported as mean ± standard deviation or median (IQR), and the Shapiro-Wilk test was used to evaluate normality. Normally distributed data were analyzed using the independent sample t-test and non-normally distributed data were analyzed using the Mann-Whitney U test. Categorical data were reported as number (%) and analyzed using the Chi-squared test. Spearman correlation analysis was used to evaluate whether the ΔSS was correlated with LVEF or LVFS. Variables with univariate P values of <0.100 were selected to enter into a multivariable logistic regression model with conditional forward selection. Differences were considered statistically significant at P values of <0.050.
Results
Demographic and hemodynamic characteristics
A comparison of the responders (36 patients [21 males], mean age: 12.0 ± 2.3 years) and the non-responders (15 patients [7 males], mean age: 12.1 ± 2.1 years) revealed no significant differences in terms of sex, age, height, weight, and body mass index (BMI). Furthermore, no significant differences were observed in the pre-treatment baseline values for HR, systolic BP, diastolic BP, maximum HR during 10 min of standing, HR increase from the supine position to the upright position, and Usg [Table 1].
Table 1.
Comparison of demographic and hemodynamics parameters between POTS children with different responses to metoprolol.
| Items | Responders | Non-responders | Statistics | P value |
| Cases, n | 36 | 15 | – | – |
| Sex (M/F) | 21/15 | 7/8 | 0.206∗ | 0.650 |
| Age (years) | 12.0 ± 2.3 | 12.1 ± 2.1 | −0.195† | 0.847 |
| Height (cm) | 154.9 ± 14.4 | 158.2 ± 10.4 | −0.802† | 0.427 |
| Weight (kg) | 43.5 (40.0, 58.3) | 47.8 ± 13.6 | −0.207‡ | 0.836 |
| BMI (kg/m2) | 18.2 (16.7, 21.1) | 18.9 ± 3.6 | −0.434‡ | 0.664 |
| HR (bpm) | 78 ± 12 | 72 (65, 78) | −1.304‡ | 0.192 |
| SBP (mmHg) | 106 ± 13 | 104 ± 8 | 0.747† | 0.458 |
| DBP(mmHg) | 62 ± 10 | 61 ± 9 | 0.276† | 0.784 |
| ΔHR (bpm) | 48 (43, 52) | 48 ± 8 | −0.673‡ | 0.501 |
| HR max (bpm) | 126 ± 11 | 122 ± 11 | 1.360† | 0.180 |
| Pre-treatment SS (points) | 5 (3, 8) | 6 (4, 12) | −1.020‡ | 0.308 |
| Usg | 1.022 (1.017, 1.026) | 1.023 ± 0.007 | −0.777‡ | 0.437 |
∗χ2; †t; ‡Z. BMI: Body mass index; bpm: Beats per minute; DBP: Diastolic blood pressure; HR: Heart rate; ΔHR: HR increase from standing to the supine during 10 min; HR max: Maximum HR within 10 min of standing; POTS: Postural tachycardia syndrome; SS: Symptom scores; SBP: Systolic blood pressure; Usg: Urine specific gravity.
Baseline LVEF and LVFS according to responsiveness to the treatment
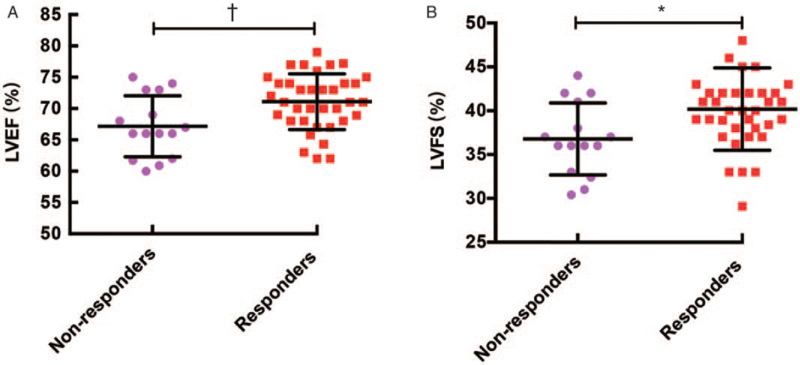
Relative to the non-responders, the responders had significantly higher pre-treatment baseline values for LVEF (71.09% ± 4.44% vs. 67.17% ± 4.88%, t = −2.789, P = 0.008) and LVFS (40.00 [38.00, 42.00]% vs. 36.79% ± 4.11%, Z = −2.542, P = 0.010) [Figure 2].
Figure 2.
Comparing LVEF and LVFS according to response to metoprolol therapy in children and adolescents with POTS. (A) LVEF; (B) LVFS. ∗P < 0.050; †P < 0.010. LVEF: Left ventricular ejection fraction; LVFS: Left ventricular fractional shortening; POTS: Postural tachycardia syndrome.
Correlations between the ΔSS and baseline LVEF or LVFS
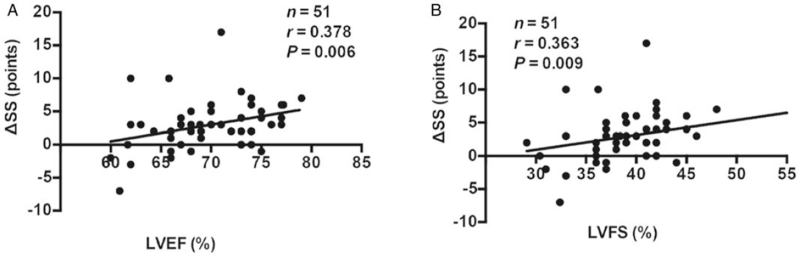
As a marker for treatment response, a ΔSS was weakly correlated with the baseline LVEF value (r = 0.378, P = 0.006) and the baseline LVFS value (r = 0.363, P = 0.009) [Figure 3].
Figure 3.
Correlations of LVEF and LVFS with ΔSS in children and adolescents with POTS. (A) LVEF; (B) LVFS. LVEF: Left ventricular ejection fraction; LVFS: Left ventricular fractional shortening; POTS: Postural tachycardia syndrome; ΔSS: Pre-treatment symptom score minus post-treatment symptom score.
Multivariable logistic regression analysis
The multivariable model was adjusted for sex, age, BMI, LVEF and LVFS, which revealed that LVEF was independently associated with the likelihood of response to metoprolol treatment in children and adolescents with POTS. Each 1% increase in the baseline LVEF value was associated with an approximately 21% higher likelihood of response to metoprolol treatment (odds ratio: 1.201, 95% confidence interval: 1.039–1.387, P = 0.013) [Table 2].
Table 2.
Variable analysis of multivariate logistic regression.
| Characteristic | Beta | SE | Wald | P value | OR (95% CI) |
| LVEF | 0.183 | 0.074 | 6.171 | 0.013 | 1.201 (1.039–1.387) |
| Constant | −11.784 | 5.059 | 5.426 | 0.020 | – |
The variables included in the logistic regression analysis are: gender, age, BMI, LVEF, and LVFS. BMI: Body mass index; CI: Confidence interval; LVEF: Left ventricular ejection fraction; LVFS: Left ventricular fractional shortening; OR: Odds ratio; SE: Standard error.
Discussion
This study indicates that pre-treatment baseline LVEF and LVFS values were associated with symptom improvement after metoprolol treatment for children and adolescents with POTS. Furthermore, the pre-treatment baseline LVEF value was independently associated with symptom improvement after metoprolol treatment.
Among children and adolescents, POTS is a common type of OI[25] that can seriously affect their physical health, mental health, and quality of life.[26] Therefore, active and effective treatment is important for children and adolescents with POTS. One potential treatment option is β-blockers, although previous studies have revealed conflicting findings regarding their efficacy, which may be related to the complex and diverse pathogenesis of POTS. For example, the pathogenesis of POTS mainly involves high catecholamine status,[11] overexcitement of sympathetic activity,[27] low central blood volume,[23,28] and excessive vasodilation.[29,30] Neuro-humoral factors, including hydrogen sulfide and sulfur dioxide, catecholamines and vasoactive peptides, siginificantly regulate cardiac structure and function. Metoprolol (a β-blocker) helps block the β-adrenoceptors of the myocardium, which suggests that metoprolol treatment for POTS may be effective only for children with sympathetic hyperactivity or β-receptor hypersensitivity.[31] Therefore, identifying sympathetic hyperactivity in children and adolescents with POTS might guide effective treatment selection. We have previously reported that response to metoprolol treatment was predicted by plasma concentrations of norepinephrine, copeptin, and C-type natriuretic peptide, as well as HR variability from a 24-h Holter evaluation.[11–14] However, these evaluations are invasive and/or time consuming, which can limit their clinical utility. Therefore, we attempted to identify relatively stable, non-invasive, and easily evaluable markers for predicting response to metoprolol treatment for POTS.
Values for LVEF and LVFS are relatively stable and readily obtainable using non-invasive echocardiography and are widely used to diagnose syncope in children. Furthermore, LVEF and LVFS values increase in humans and animals with sympathetic hyperactivity.[32–35] Moreover, previous studies have indicated that ventricular function indexes (LVEF, LVFS, or left ventricle volume) increased significantly during upright exercise in healthy humans (vs. the resting state) and that plasma catecholamine concentrations were positively correlated with LVEF changes or left ventricle volume.[36,37] However, the resting LVEF value decreased significantly after metoprolol treatment for coronary artery disease,[38] and children with vasovagal syncope experience symptom improvement after metoprolol treatment.[39] The above facts attracted us to design the present study to evaluate if LVEF might be associated with symptom improvement after metoprolol treatment for children and adolescents with POTS.
The present study revealed that responders had considerably high LVEF and LVFS values (vs. non-responders), which suggests that patients with sympathetic hyperactivity would respond well to metoprolol treatment. We also found that the baseline LVEF and LVFS values were positively correlated with the ΔSS, which is an indicator of treatment response. Furthermore, the logistic regression analysis revealed that the baseline LVEF value predicted response to metoprolol treatment in children and adolescents with POTS. These results suggest that patients with a low baseline LVEF value would be less likely to experience symptom improvement after metoprolol treatment. Therefore, evaluation of the pre-treatment LVEF may be useful for predicting the response to metoprolol therapy in children and adolescents with POTS, which may guide more personalized treatment selection.
The present study still has several limitations, however. The follow-up period was short. The sample size was relatively small. Also, direct blood volume measurements were not performed in the study. In the future, large-size based multicenter studies with prolonged follow-up period are needed.
Funding
This study was supported by the Science and Technology Program of Beijing (Z171100001017253), Beijing Natural Science Foundation (7182168), Peking University Clinical Scientist Program (BMU2019LCKXJ001, Beijing, China), and the Fundamental Research Funds for the Central Universities.
Conflicts of interest
None.
Footnotes
How to cite this article: Wang YY, Han ZH, Wang YL, Liao Y, Zhang CY, Liu P, Tang CS, Du JB, Jin HF, Huang YQ. Baseline left ventricular ejection fraction associated with symptom improvements in both children and adolescents with postural tachycardia syndrome under metoprolol therapy. Chin Med J 2021;134:1977–1982. doi: 10.1097/CM9.0000000000001698
References
- 1.Medow MS, Stewart JM. The postural tachycardia syndrome. Cardiol Rev 2007; 15:67–75. doi: 10.1097/01.crd.0000233768.68421.40. [DOI] [PubMed] [Google Scholar]
- 2.Jarjour IT. Postural tachycardia syndrome in children and adolescents. Semin Pediatr Neurol 2013; 20:18–26. doi: 10.1016/j.spen.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Liao Y, Qi JG, Yan H, Zhang QY, Ji TY, Chang XZ, et al. A report on comorbidity of chronic fatigue syndrome, postural tachycardia syndrome, and narcolepsy with 5,10-methylenetetrahydrofolate reductase (MTHFR) mutation in an adolescent. Chin Med J (Engl) 2021; 134:1495–1497. doi: 10.1097/CM9.0000000000001387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin J, Han Z, Li X, Ochs T, Zhao J, Zhang X, et al. Risk factors for postural tachycardia syndrome in children and adolescents. PLoS One 2014; 9:e113625.doi: 10.1371/journal.pone.0113625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bagai K, Song Y, Ling JF, Malow B, Black BK, Biaggion I, et al. Sleep disturbances and diminished quality of life in postural tachycardia syndrome. J Clin Sleep Med 2011; 7:204–210. doi: 10.1016/j.jocn.2010.07.104. [PMC free article] [PubMed] [Google Scholar]
- 6.Raj V, Haman KL, Raj SR, Byrne D, Blakely RD, Biaggioni I, et al. Psychiatric profile and attention deficits in postural tachycardia syndrome. J Neurol Neurosurg Psychiatry 2009; 80:339–344. doi: 10.1136/jnnp.2008.144360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bryarly M, Phillips LT, Fu Q, Vernino S, Levine BD. Postural orthostatic tachycardia syndrome: JACC focus seminar. J Am Coll Cardiol 2019; 73:1207–1228. doi: 10.1016/j.jacc.2018.11.059. [DOI] [PubMed] [Google Scholar]
- 8.Tobias J, Kernan S. Perioperative care of an adolescent with postural orthostatic tachycardia syndrome. Saudi J Anaesth 2010; 4:23–27. doi: 10.4103/1658-354X.62611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen L, Wang L, Sun J, Qin J, Tang C, Jin H, et al. Midodrine hydrochloride is effective in the treatment of children with postural orthostatic tachycardia syndrome. Circ J 2011; 75:927–931. doi: 10.1253/circj.cj-10-0514. [DOI] [PubMed] [Google Scholar]
- 10.Lai CC, Fischer PR, Brands CK, Fisher JL, Porter CJ, Driscoll SW, et al. Outcomes in adolescents with postural orthostatic tachycardia syndrome treated with midodrine and β-blockers. Pacing Clin Electrophysiol 2009; 32:234–238. doi: 10.1111/j.1540-8159.2008.02207.x. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Q, Chen X, Li J, Du J. Orthostatic plasma norepinephrine level as a predictor for therapeutic response to metoprolol in children with postural tachycardia syndrome. J Transl Med 2014; 12:249.doi: 10.1186/s12967-014-0249-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao J, Du S, Yang J, Lin J, Tang C, Du J, et al. Usefulness of plasma copeptin as a biomarker to predict the therapeutic effectiveness of metoprolol for postural tachycardia syndrome in children. Am J Cardiol 2014; 114:601–615. doi: 10.1016/j.amjcard.2014.05.039. [DOI] [PubMed] [Google Scholar]
- 13.Lin J, Han Z, Li H, Chen SY, Li X, Wang Y, et al. Plasma C-type natriuretic peptide as a predictor for therapeutic response to metoprolol in children with postural tachycardia syndrome. PLoS One 2015; 10:e0121913.doi: 10.1371/journal.pone.0121913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Zhang C, Chen S, Liu P, Tang C, Jin H, et al. Heart rate variability predicts therapeutic response to metoprolol in children with postural tachycardia syndrome. Front Neurosci 2019; 13:1214.doi: 10.3389/fnins.2019.01214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelbaek H, Christensen NJ, Godtfredsen J. Left ventricular volumes during graded upright exercise in healthy untrained subjects. Clin Physiol 1988; 8:51–56. doi: 10.1111/j.1475-097x.1988.tb00261.x. [DOI] [PubMed] [Google Scholar]
- 16.Garcia MIM, Jian Z, Settels JJ, Hunley C, Cecconi M, Hatib F, et al. Determinants of left ventricular ejection fraction and a novel method to improve its assessment of myocardial contractility. Ann Intensive Care 2019; 9:48.doi: 10.1186/s13613-019-0526-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan AA, Lip GYH, Shantsila A. Heart rate variability in atrial fibrillation: the balance between sympathetic and parasympathetic nervous system. Eur J Clin Invest 2019; 49:e13174.doi: 10.1111/eci.13174. [DOI] [PubMed] [Google Scholar]
- 18.Song JJ, Ma Z, Wang J, Chen LX, Zhong JC. Gender differences in hypertension. J Cardiovasc Transl Res 2020; 13:47–54. doi: 10.1007/s12265-019-09888-z. [DOI] [PubMed] [Google Scholar]
- 19.Wang C, Li Y, Liao Y, Tian H, Huang M, Dong X, et al. 2018 Chinese Pediatric Cardiology Society (CPCS) guideline for diagnosis and treatment of syncope in children and adolescents. Sci Bull 2018; 63:1558–1564. doi: 10.1016/j.scib.2018.09.019. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Zhang C, Chen S, Li X, Jin H, Du J. Frequency domain indices of heart rate variability are useful for differentiating vasovagal syncope and postural tachycardia syndrome in children. J Pediatr 2019; 207:59–63. doi: 10.1016/j.jpeds.2018.11.054. [DOI] [PubMed] [Google Scholar]
- 21.Sheldon RS, Grubb BP, Olshansky B, Shen WK, Calkins H, Brignole M, et al. 2015 heart rhythm society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal ayncope. Heart Rhythm 2015; 12:e41–e63. doi: 10.1016/j.hrthm.2015.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin J, Wang Y, Ochs T, Tang C, Du J, Jin H. Tilt angles and positive response of head-up tilt test in children with orthostatic intolerance. Cardiol Young 2015; 25:76–80. doi: 10.1017/S1047951113001601. [DOI] [PubMed] [Google Scholar]
- 23.Li H, Wang Y, Liu P, Chen Y, Feng X, Tang C, et al. Body mass index (BMI) is associated with the therapeutic response to oral rehydration solution in children with postural tachycardia syndrome. Pediatr Cardiol 2016; 37:1313–1318. doi: 10.1007/s00246-016-1436-1. [DOI] [PubMed] [Google Scholar]
- 24.Tao C, Liu X, Zhang C, Chen Y, Huang Y. Comments on 2018 CPCS guideline for diagnosis and treatment of syncope in children and adolescents. Sci Bull 2019; 64:291–292. doi: 10.1016/j.scib.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 25.Stewart JM. Mechanisms of sympathetic regulation in orthostatic intolerance. J Appl Physiol 2012; 113:1659–1668. doi: 10.1152/japplphysiol.00266.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kizilbash SJ, Ahrens SP, Bruce BK, Chelimsky G, Driscoll SW, Harbeck-Weber C, et al. Adolescent fatigue, POTS, and recovery: a guide for clinicians. Curr Probl Pediatr Adolesc Health Care 2014; 44:108–133. doi: 10.1016/j.cppeds.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shannon JR, Flattem NL, Jordan J, Jacob G, Black BK, Biaggioni I, et al. Orthostatic intolerance and tachycardia associated with norepinephrine-transporter deficiency. N Engl J Med 2000; 324:541–548. doi: 10.1056/NEJM200002243420803. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Q, Liao Y, Tang C, Du J, Jin H. Twenty-four-hour urinary sodium excretion and postural orthostatic tachycardia syndrome. J Pediatr 2012; 161:281–284. doi: 10.1016/j.jpeds.2012.01.054. [DOI] [PubMed] [Google Scholar]
- 29.Liao Y, Yang J, Zhang F, Chen S, Liu X, Zhang Q, et al. Flow-mediated vasodilation as a predictor of therapeutic response to midodrine hydrochloride in children with postural orthostatic tachycardia syndrome. Am J Cardiol 2013; 112:816–820. doi: 10.1016/j.amjcard.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 30.Medow MS, Minson CT, Stewart JM. Decreased microvascular nitric oxide-dependent vasodilation in postural tachycardia syndrome. Circulation 2005; 112:2611–2618. doi: 10.1161/CIRCULATIONAHA.104.526764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thieben MJ, Sandroni P, Sletten DM, Benrud-Larson LM, Fealey RD, Vernino S, et al. Postural orthostatic tachycardia syndrome: the Mayo clinic experience. Mayo Clin Proc 2007; 82:308–313. doi: 10.4065/82.3.308. [DOI] [PubMed] [Google Scholar]
- 32.Stratton JR, Preifer MA, Ritchie JL, Halter JB. Hemodynamic effects of epinephrine: concentration-effect study in humans. J Appl Physiol 1985; 58:1199–1206. doi: 10.1152/jappl.1985.58.4.1199. [DOI] [PubMed] [Google Scholar]
- 33.Stephens J, Ead H, Spurrell R. Haemodynamic effects of dobutamine with special reference to myocardial blood flow. Br Heart J 1979; 42:43–50. doi: 10.1136/hrt.42.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Plante E, Lachance D, Drolet MC, Roussel E, Couet J, Arsenault M. Dobutamine stress echocardiography in healthy adult male rats. Cardiovasc Ultrasound 2005; 3:34.doi: 10.1186/1476-7120-3-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dubin A, Lattanzio B, Gatti L. The spectrum of cardiovascular effects of dobutamine - from healthy subjects to septic shock patients. Rev Bras Ter Intensiva 2017; 29:490–498. doi: 10.5935/0103-507X.20170068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kanstrup IL, Marving J, Gadsboll N, Lonborg-Jensen H, Hoilund-Carlsen PF. Left ventricle haemodynamics and vaso-activehormones during graded supine exercise in healthy male subjects. Eur J Appl Physiol Occup Physiol 1995; 72:86–94. doi: 10.1007/bf00964120. [DOI] [PubMed] [Google Scholar]
- 37.Poliner LR, Dehmer GJ, Lewis SE, Parkey RW, Blomqvist CG, Willerson JT. Left ventricular performance in normal subjects: a comparison of the responses to exercise in the upright and supine positions. Circulation 1980; 62:528–534. doi: 10.1161/01.cir.62.3.5. [DOI] [PubMed] [Google Scholar]
- 38.Silke B, Verma SP, Frais MA, Reynolds G, Taylor SH. Comparative effects of metoprolol and celiprolol on cardiac hemodynamics and left ventricular volume at rest and during exercise-induced angina. Clin Pharmacol Ther 1986; 39:5–13. doi: 10.1038/clpt.1986.2. [DOI] [PubMed] [Google Scholar]
- 39.Song J, Li H, Wang Y, Liu P, Li X, Tang C, et al. Left ventricular ejection fraction and fractional shortening are useful for the prediction of the therapeutic response to metoprolol in children with vasovagal syncope. Pediatr Cardiol 2018; 39:1366–1372. doi: 10.1007/s00246-018-1904-x. [DOI] [PubMed] [Google Scholar]