Abstract
Oxidative stress is caused by the imbalance between the generation of free radicals/reactive oxygen species (ROS) and the antioxidant defense systems, which can activate various transcription factors and affect their transcriptional pathways. Oxidative stress plays an important role in the occurrence and development of leukemia and is closely related to the treatment and prognosis of leukemia. The standard chemotherapy strategies for the pre-treatment of leukemia have many drawbacks. Hence, the usage of antioxidants and oxidants in the treatment of leukemia is being explored and has been preliminarily applied. This article reviews the research progress of oxidative stress and leukemia. In addition, the application of antioxidants treatment in leukemia has been summarized.
Keywords: Reactive oxygen species, Oxidative stress, Leukemia, Antioxidant treatment
Introduction
Reactive oxygen species (ROS), such as superoxide ion , hydroxyl radical , and hydrogen peroxide (H2O2), are important products of cell metabolism, which participate in the processes of cell survival, proliferation, apoptosis, and lifecycle, thus maintaining intracellular homeostasis.[1] Most of the ROS are produced by the mitochondrial respiratory chain inside the cells. Oxidative stress is the change of cellular environment influenced by some pathological factors, due to the imbalance between free radicals and ROS production in the oxidation and antioxidant defense system, which can cause cell apoptosis and tissue damage, and may potentially affect the entire organism.[2] Recent studies have shown that redox disorders caused by ROS may promote the development of leukemia, closely related to the treatment and prognosis of leukemia.[3] However, there is still a lack of summary about the functions of oxidative stress in leukemia. Therefore, we summarized and discussed the relationship between oxidative stress and three types of leukemia: acute myeloid leukemia (AML), chronic myeloid leukemia (CML), and acute lymphoblastic leukemia (ALL). It deserves more researches in the development of oxidative stress mechanisms, oxidative stress-related transcription factors, and therapeutic approaches to combat leukemia.
ROS and Oxidative Stress
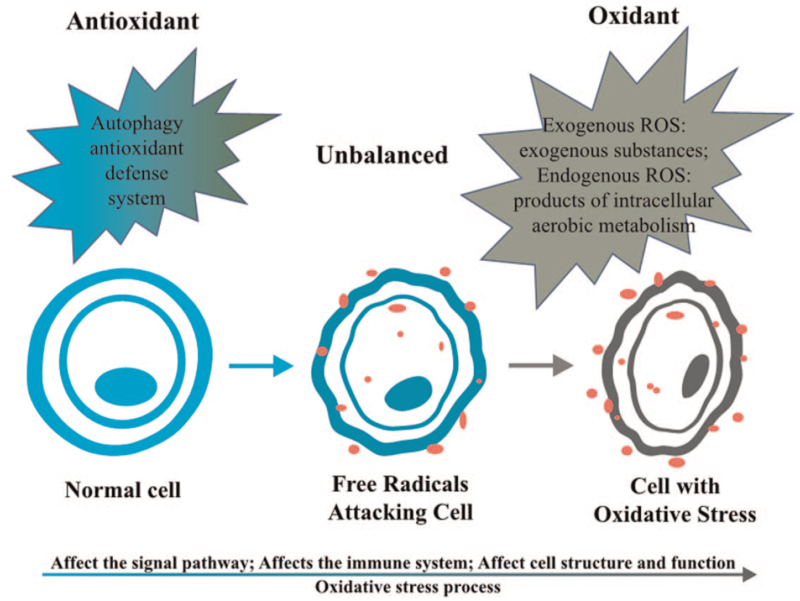
ROS are a key factor that can regulate cell homeostasis and metabolism and can also induce autophagy, control the level of ROS in cells, and reduce its toxic effects.[4] According to its source, ROS can be divided into exogenous and endogenous ROS. Exogenous ROS is mainly caused by exogenous substances, such as radiation and drugs; whereas endogenous ROS is the products of intracellular aerobic metabolism, among which the mitochondrial electron transport system and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase complex are the primary sources.[5] Meanwhile, to protect cells from oxidative damage, aerobic organisms also have an antioxidant defense system. The excessive production of ROS intermediates, exceeding the functional capacity of cellular antioxidants, may lead to the instability of important macromolecules, which is the molecular basis of inflammatory processes, cardiovascular diseases, cancer, and other diseases.[6] In addition, ROS can activate Nuclear Factor Kappa B (NF-κB), phosphatidylinositol-3-kinase (PI3K), mitogen-activated protein kinase (MAPK), and other tumor growth signaling pathways. Oxidative stress is caused by the imbalance between free radicals and ROS and the functions of oxidative stress are shown in Figure 1. First, oxidative stress can regulate the signaling pathways related to cell survival, proliferation, and apoptosis, and then it can promote tumor cell survival, induce cell proliferation, and protect cells from apoptosis further to promote cancers[7]; second, oxidative stress metabolic disorder participates in inflammation processes, thus promoting inflammation and affecting the immune system[8,9]; third, oxidative stress can also trigger secondary messengers between cells, thus destroying essential biomolecules (such as lipids, proteins, and DNA)[1] and damaging important structures and functions of cells.[7] It can stimulate ribosylation of poly adenosine diphosphate (ADP) of chromosome proteins, further to induce kinases activation, gene mutations, chromosome DNA degeneration, mitochondrial membrane potential changes, pro-apoptotic proteins activation and translocation, and anti-apoptotic proteins down-regulation, thus promoting carcinogenesis.[5] In conclusion, oxidative stress involves the process of oncogenesis and progression in cancers.
Figure 1.
Oxidative stress imbalance. The imbalance between oxidants and antioxidants will lead to oxidative stress and initiate carcinogenesis eventually. ROS: Reactive oxygen species.
Effect of Oxidative Stress on Hematopoietic Stem Cells
Hematopoietic stem cells (HSCs) are adult stem cells in the blood system, mainly found in the red bone marrow, which has the potential of long-term self-renewal and differentiation into different types of mature blood cells. Mature cells in the blood system often have a short lifespan, and HSCs need to proliferate and differentiate into progenitor cells of all kinds of blood cells, hematopoietic progenitor cells (HPCs), to replenish each mature cell component of the blood system in a timely manner, thus maintaining long-term dynamic balance. There are remarkable differences in metabolism and metabolic regulation among HSCs, HPCs, and differentiated hematopoietic cells.[10] Among them, oxidative stress is an important factor in regulating the homeostasis of HSCs. Under a certain degree of oxidative stress, HSCs can regulate and maintain the balance of various components in the blood system. However, high levels of ROS or continuous oxidative stress can cause severe damage to HSCs, thus altering their metabolic state and then incapacitating them.[5] ROS causes changes in the hematopoietic microenvironment, which in turn causes senescence or apoptosis of HSCs. Because HSCs that survive in niches have lower ROS levels than other general tissues, they are very sensitive to changes in ROS levels and gene mutations.[10] Besides, transcription factors such as forkhead box O3 (FOXO3) and ataxia-telangiectasia mutated kinase (ATM) also play an essential role in the regulation of ROS in HSCs. Studies have found that different ROS levels have different effects on HSCs: low levels of ROS can regulate the activity of adhesion molecules in the hematopoietic microenvironment and maintain HSCs in the quiescent phase; moderate levels of ROS can change molecular structures of oxidative stress-related molecules and regulate the cell cycle and improve cell proliferation of HSCs, whereas high levels of ROS can cause HSCs to age and change their metabolic status.[11] HSCs in the mature and normal differentiation stage will senesce under the stimulation of the high level of ROS, resulting in metabolic changes and loss of function of HSCs, and then develop to leukemia.
Oxidative Stress-Related Transcription Factors in Leukemia
Oxidative stress can activate various kinds of transcription factors, including NF-κB, Nuclear respiratory factor 1 (Nrf1), Nuclear factor erythroid 2-related factor 2 (Nrf2), Nuclear factor erythroid 2-related factor 2 (Nrf3), activating protein-1 (AP-1), p53, Hypoxia Inducible Factor 1 Subunit Alpha (HIF-1α), peroxisome proliferator-activated receptor gamma (PPAR-γ), signal transducer and activator of transcription 3 (STAT3), AKT Serine/Threonine Kinase (AKT), Forkhead box protein (FOXO), BTB and CNC homology 1 (Bach1), BTB and CNC homology 2 (Bach2), etc.[8,12–19] Some members of this family are identified as important regulating factors of oxidative stress in erythropoiesis. Some of these transcription factors are antioxidant stress factors, while others protect tumor cells from oxidative stress. Activation of oxidative stress-related transcription factors can influence the expression of more than 500 different genes, including growth factors, inflammatory cytokines, chemokines, cell cycle regulators, anti-inflammatory molecules, etc.[20] The relationship between ROS-dependent transcription factors and leukemia is shown Table 1. Transcription factors and targeted redox-sensitive pathways may provide great hope for cancer prevention and treatment.
Table 1.
ROS-dependent transcription factors related to leukemia.
| Transcription factors | Function | Reference |
| AKT and FOXO | Important transcription factors that resist oxidative stress Essential to the survival of HSCs AKT activation destroying redox homeostasis AKT inhibiting the activity of FOXO AKT and FOXO making cells more prone to apoptosis under oxidative stress | [2,11] |
| Nrf2 | Negatively regulated by Keap1 Controlling the expression of antioxidant and cytoprotective genes Participating in chemotherapy resistance in leukemia Protecting cells from ROS Protecting tumors from oxidative stress when cells undergo malignant transformation Playing a direct role in cell growth control Related to the apoptosis regulatory pathway Regulating glutathione (GSH) A future therapeutic target for AML with GSH disorders Promoting cell survival by regulating antioxidant and apoptosis pathways A therapeutic target for the prevention of some degenerative and malignant diseases | [12–14,65] |
| TRPM2 | Maintaining the viability of cancer cells Promoting tumorigenesis Maintaining mitochondrial function, cell bioenergetics, ATP production, and autophagy, reducing cell ROS level, and repairing DNA Promoting the expression of transcription factors, including HIF-1/2α, CREB, and Nrf2 Activating corresponding kinases and signaling pathways | [21] |
| STAT5 | Inhibiting the expression of catalase and glutathione-1 (glutaredoxin) Promoting ROS production Promoting leukemia cell proliferation | [16] |
| p53 | Helping the expression or activation of oncogenes Regulating metabolic pathways Resisting oxidative stress induced by oncogenes Playing a vital role in genome stability by inducing cell cycle arrest and DNA damage Limiting cell growth by inducing cell senescence, apoptosis, or proliferation | [18,19] |
AKT: AKT Serine/Threonine Kinase; AML: Acute myeloid leukemia; FOXO: Forkhead box protein; GSH: Glutathione; HSCs: Hematopoietic stem cells; Keap1: Kelch-like ECH-related protein 1; Nrf2: Nuclear factor E2-related factor 2; p53: Protein 53; PKB: Protein kinase B; ROS: Reactive oxygen species; STAT5: Signal transducer and activator of transcription 5; TRPM2: Transient receptor potential melastatin 2.
Traditional Treatment and Antioxidant Treatment in Different Types of Leukemia
Excessive production of ROS may lead to an imbalance of homeostasis and damage to important cell components when excessive oxidants fail to balance antioxidant defense with DNA repair mechanisms. Chronic oxidative stress can drive carcinogenesis by modifying the expression of cancer-related genes that cause mutation and transformation.[21] Some patients with leukemia are found to be in a state of chronic oxidative stress.[22] Leukemia, a life-threatening disease caused by malignant clonal hematopoiesis of stem and progenitor cells, is one of the top 10 cancers affecting human beings.[9] It is characterized by the accumulation of immature clonal hematopoietic cells in the bone marrow and other hematopoietic tissues, thus inhibiting normal hematopoietic function and infiltrating other tissues through enhanced self-renewal, resulting in uncontrolled proliferation, impaired differentiation, and cell apoptosis. According to the degree of differentiation, the natural course of the disease and the cells involved, leukemia can be divided into ALL, AML, CML, chronic lymphoblastic leukemia (CLL), and rare types of leukemia. Next, we will summarize the traditional treatment and antioxidant treatment strategies of these three kinds of leukemia.
Traditional treatment and antioxidant treatment for AML and its subtypes
AML is a malignant tumor that originates from bone marrow cells and develops rapidly if untreated. It is a kind of heterogeneous hematological tumor characterized by uncontrolled proliferation of clonal tumor HSCs, usually leading to impaired normal hematopoietic function.[23] Most AML is characterized by the expression of common acute lymphoblastic leukemia antigen (CALLA).[7] The subtypes of AML conclude promyelocytic leukemia (PML), acute promyelocytic leukemia (APL), etc. It has recently been reported that PML plays an important role in regulating oxidation and participates in the post-translational modification of key regulatory factors, such as p53, to be the key receptor of ROS.[24,25] And APL has become a curable subtype of AML.[26]
Treatments for AML include intensive chemotherapy, radiotherapy, stem cell transplantation, and immunotherapy.[27] Under the current chemotherapy treatments, about 70% of adult AML patients have achieved complete remission, while only about 20% of patients have achieved long-term disease-free survival, for most of the deceased patients have died of refractory or recurrent AML.[28] Therefore, it is necessary to develop new treatments that can prolong the disease-free survival of AML patients.
Arsenic trioxide (ATO) has been proved to be an effective drug for APL. While other studies show that Moringa oleifera leaf extract also has a protective effect on APL, and can significantly increase cell viability at a particular concentration. This kind of protective effect on APL is caused by the decrease of significant oxidative stress levels and the increase of enzyme activity in the antioxidant enzyme system, which can be observed in cells exposed to oxidative damage.[29] Besides, histone methyltransferase inhibitor can also induce substantial apoptosis of AML cell lines and primary AML cells, indicating its anti-leukemic effects on AML.[30] ROS-mediated interactions between c-Jun activation domain-binding protein-1 (JAB1) and thioredoxin (TRX) play a crucial role in the pathobiological and recurrence process of acute myeloid leukemia-M5 (AML-M5). Therefore, targeting the ROS/JAB1/TRX pathway may be a potential treatment strategy of AML-M5.[31,32] Oxidative stress can induce the abnormal expression of many genes.[29] The increase of oxidative stress levels plays an important role in the occurrence and development of AML.[33] Meanwhile, similar results also show a strong correlation between oxidative stress and disease recurrence, suggesting a potential prognostic value of AML patients.[34–36]
Recent researches find that histamine dihydrochloride (HDC) and low-dose interleukin-2 (IL-2) immunotherapy can reduce the risk of recurrence of AML patients after chemotherapy. HDC can suppress or inhibit the formation of oxygen radicals in mononuclear and polymorphonuclear myeloid cells, by targeting NADPH oxidase—the key enzyme in oxygen radical formation of myeloid cells.[37] The clinical phase III study has demonstrated that post-consolidation treatment with the combination of HDC and IL-2 effectively reduces relapse of AML patients.[38] Also, a randomized trial of HDC + IL-2 showed a statistically significant benefit for leukemia-free survival (LFS) compared with traditional treatments.[39] However, in view of the better therapeutic effect of AML by using HDC/IL-2 immunotherapy, there are no clinical trials with a single application of HDC to assess the effect of HDC compared with traditional treatments in leukemia. Hence, if possible, clinical trials of comparing HDC alone to traditional treatments and comparing HDC alone to HDC/IL-2 immunotherapy for the treatment of leukemia are hoped to be carried out in the future, further to explore the specific effectiveness of HDC in leukemia. Although antioxidant treatment is now approved for use for relapse prevention of AML within the EU and in Israel, it is still not a standard treatment strategy in clinical practice. At present, new clinical trials are proceeding for many kinds of antioxidants expected to be absorbed and become a new standard treatment strategy. It is worthy of exploring the combination of antioxidants and traditional treatments whether can enhance the therapeutic effect of traditional treatments, at the same time, and reduce side effects, further to establish a new standard treatment strategy of leukemia.
Traditional treatment and antioxidant treatment for CML
CML is a common hematological malignancy that mainly involves the myeloid system. The pathophysiological features of CML stem from the uncontrolled enzymatic activity of a fusion protein called Breakpoint Cluster Region-Abelson (BCR-ABL),[40] which induces granulocytes in peripheral blood further to increase in number and differentiate immaturely. Ph chromosome or BCR-ABL fusion genes can induce the accumulation of ROS in leukemic cells by activating NADPH oxidase and mitochondrial respiratory chain complex III (rac2/MRC-CIII).[34] The level of oxidative stress in patients with acute CML is significantly higher than that in patients with chronic CML.[41] Furthermore, in patients with a high level of ROS, the BCR-ABL gene is prone to mutation, resulting in a poor prognosis for CML.[40]
Tyrosine kinase inhibitors (TKI) is a kind of traditional treatment for CML, which triggers CML cells apoptosis by the signal cascade unfolded protein response (UPR) induced by endoplasmic reticulum (ER) stress.[41,42] Besides, imatinib mesylate (Gleevec), a BCR-ABL inhibitor, is also used as a first-line drug for the treatment of CML.[23] BCR-ABL transformation is associated with genomic instability, so that most patients who receive continuous treatment using all-trans retinoic acid and imatinib mesylate are easy to develop disease resistance. Therefore, researchers are actively looking for new drugs that can stimulate cell differentiation and induce apoptosis of acute leukemia and multiple myeloma cells. ROS are expected to be the ideal targets for therapeutic intervention, for high ROS levels are particularly relevant to BCR/ABL kinase inhibitor resistance.[4,43]
Antioxidants that regulate the level of oxidative stress are the primary defense line to regulate the overall state of health. Ivermectin is a new anti-cancer drug targeted to mitochondria, which can selectively kill CML cells by inducing oxidative stress and mitochondrial dysfunction, being a promising candidate drug for the treatment of CML.[44] In addition, BCR-ABL-induced high-activity glucose metabolism is essential for ROS production. Based on it, new targeted therapies by identifying this kind of molecular mechanisms can be developed.[40] Besides, AML is propagated by subpopulations of leukemic stem cells (LSCs). Many intrinsic factors can affect the survival of human LSCs, including the regulators of cell cycle and survival signaling pathways (such as NF-κB and AKT), the regulators of oxidative stress pathways, and specific molecular components promoting cell self-renewal. Regulating the survival of LSCs is a critical step for improving treatment regimens for leukemia.[45] Moreover, the plasma lipid peroxidation and non-enzymatic antioxidant status can reflect the oxidative stress status of patients with CML, using as the indicators of disease progression and early response to different treatment strategies.[46]
Traditional treatment and antioxidant treatment for ALL
ALL is a malignant neoplastic disease that arises from the abnormal proliferation of B or T cells in the bone marrow. ALL is the most common type of leukemia in children, especially in 0 to 9 years old. The antioxidant defense capacity of ALL patients is significantly higher than that of healthy people.[2] Currently, the main treatment strategy of ALL is systemic chemotherapy, while other treatment strategies also include targeted therapy, biological immunotherapy, and HSC transplantation.
Recent studies have demonstrated that traditional chemotherapy, combined with antioxidant therapy, is of great help to ALL patients.[47]Mycobacterium Michaeli snake venom and a new l-amino acid oxidase (LAAO) selectively induce apoptosis of T-ALL cells through the H2O2-mediated signaling pathway.[48] Microtubule inhibitors are the potential treatment for B-ALL. Bone marrow mesenchymal stem cells can become tumor-associated fibroblasts that survive B-ALL cells from ROS-induced chemotherapy, while microtubule inhibitors can overcome the rescue effect on B-ALL cells.[49] Besides, myelogenic suppressor cells (MDSCs) are one of the main drivers of the immune tolerance of tumor cells. The number of granulocytic myeloid-derived suppressor cells (G-MDSC) is closely associated with disease progression and clinical treatment response. STAT3 signaling-mediated ROS generation drives the activation of B-ALL-derived MDSCs. Therefore, targeting MDSCs is a promising strategy for the treatment of B-ALL patients.[50] B-cell lymphoma protein-2 (BCL-2) is one of the most studied proteins, playing a vital role in regulating apoptosis and autophagy. It has been found that BCL-2 protein is significantly increased and the total antioxidant status is significantly decreased in ALL patients, indicating the potential effectiveness of antioxidant therapy for ALL.[51] Hopefully, in the future, there may be more effective antioxidant treatments for ALL.
Traditional treatment and antioxidant treatment for CLL
CLL, characterized by clonal proliferation of highly differentiated lymphocytes with immunodeficiency, mainly occurs in the elderly. CLL mostly involves lymph nodes and the spleen, causing bone marrow hyperplasia. Recent studies suggest that ROS and oxidative stress can promote the formation and development of CLL.[52] The level of blood oxidation in CLL patients was significantly higher than that of the control group, while the level of antioxidant capacity was significantly lower than that of the control group,[53] which indicates that oxidative stress level of metabolic activity may reflect the progress stage of the CLL.[54]
Traditional treatment for CLL is mostly palliative, including chemotherapy, immunotherapy, and HSC transplantation, with the main purpose of reducing tumor load and improving symptoms. In recent years, antioxidant adjuvant therapy has become a new treatment strategy for CLL. In some CLL subclones, the increase of oxidative stress caused may lead to a mutation rate increase, leading to the disease progression. From a clinical perspective, oxidants are tested in combination with other p53 independent drugs, such as monoclonal antibodies, to determine the most effective treatment for CLL patients with ATM deficiency.[52] Moreover, lenalidomide has been shown to reverse the abnormal immunologic synapse formation in CLL and modulate the microenvironment through monocyte and Natural Killer (NK) cell activation.[55]
Significance of Oxidative Stress in the Treatment and Prognosis of Patients with Leukemia
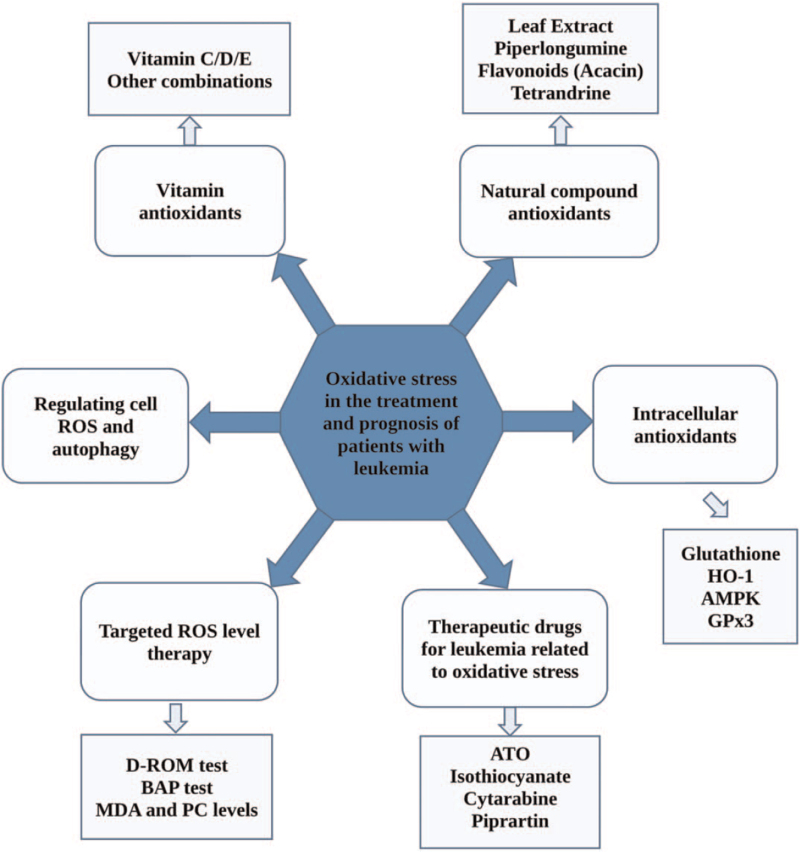
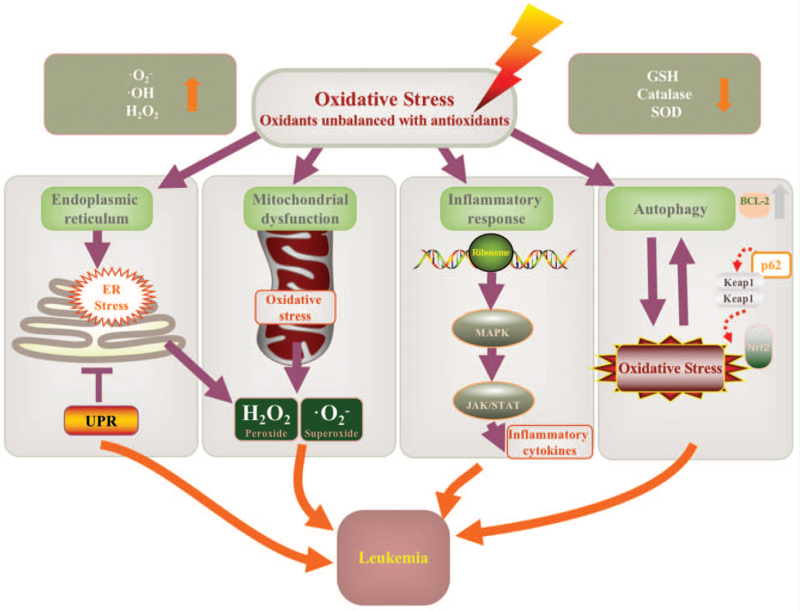
Oxidative stress plays an important role in the process of immune escape, proliferation, differentiation, anti-apoptosis, and drug resistance of leukemic cells by regulating the expression of many genes.[56,57] At present, the general treatment for leukemia is chemotherapy, which aims to attack the DNA replication process in malignant tumor cells for achieving complete remission. However, the theory of chemotherapy is based on the concept of “whole cell-killing,”[23] which does not distinguish malignant and non-malignant cells. So, it may lead to serious side effects and complications, such as severe infection and bleeding, and recurrent attacks may lead to refractory or chemotherapy-resistant diseases. In terms of treatment, some leukemias such as myeloid leukemia are still facing tremendous challenges, and the prognosis of some patients treated with chemotherapy is usually very poor.[58] This piece of clinical evidence clearly shows the limitations of chemotherapy in leukemia, so it is necessary to explore new effective treatment strategies of leukemia based on the pathogenesis and important targeted molecules. Recent studies show that ROS plays a vital role in the process of leukemia. The oncogenes regulating the production of ROS and the expression of antioxidant can affect the apoptosis pathway and control the progression of leukemia.[59] The antioxidant mechanism is to balance out the adverse effects of oxidation and reduce the inhibition of the tumor cell cycle by reducing ROS.[7] As research continues to identify and describe the biomarkers of oxidative stress biology, it will be ultimately possible to determine an individual's susceptibility to oxidative stress and the treatment's impact on clinical outcomes and symptom severity.[4] The oxidative stress biomarkers make it possible to focus on the individualized treatment of diseases.[60] In the near future, targeted oxidative stress therapy combined with chemotherapy or other strategies may become a kind of useful clinical treatment for various blood diseases.[61,62] However, at the present stage, the application of antioxidants in leukemia is still in the clinical trail stage. More studies and clinical trials should be carried out as soon as possible for antioxidants able to become the new standard treatment strategies of leukemia in the near future. The next part only reviews the existing research results of antioxidants application in leukemia, not to guide the treatment for leukemia patients. Major types of combination treatment are shown in Figure 2. The specific mechanisms of oxidation in leukemia are shown in Figure 3.
Figure 2.
ROS-related treatments of patients with leukemia. Oxidative stress treatment of leukemia includes vitamin antioxidants, natural plant-derived antioxidants, endogenous antioxidants (including enzymatic and non-enzymatic antioxidants), targeted oxidative stress therapy, induced autophagy therapy, etc. Antioxidant therapy in combination with chemotherapy or other strategies may be a promising treatment for leukemia. AMPK: 5′ adenosine-monophosphate (AMP)-activated protein kinase; ATO: Arsenic trioxide; BAP: Biological antioxidant potential; D-ROM: Derived active oxygen metabolites; GPx3: Glutathione peroxidase 3; HO-1: Heme oxygenase-1; MDA: Malondialdehyde; PC: Phosphatidylcholine; ROS: Reactive oxygen species.
Figure 3.
The mechanism map to summarize the mechanism of ROS in leukemia. ROS plays an important role in the process of ER stress, mitochondrial dysfunction, inflammatory response, autophagy, etc. BCL-2: B-cell lymphoma protein-2; ER: Endoplasmic reticulum; GSH: Glutathione; JAK: Janus kinase; Keap: Kelch-like ECH-associated protein; MAPK: Mitogen-activated protein kinase; Nrf2: Nuclear factor, erythroid 2 like 2; ROS: Reactive oxygen species; SOD: Superoxide dismutase; STAT: Signal transducer and activator of transcription; UPR: Unfolded protein response.
Application of vitamin antioxidants in leukemia
Vitamin antioxidants have the functions of scavenging free radicals and reducing DNA damage, further reducing oxidative stress. It has been reported that vitamin D3 and vitamin C have potential effects in improving the pharmacological effects of ATO. Vitamin D3 can enhance the antitumor effect of ATO on HL-60 human acute myelogenous leukemia cells. In the future, vitamin C/vitamin D3/ATO combination therapy may become a kind of good treatment strategy for patients with APL. Combination of vitamin C and other oxidative antioxidants such as vitamin E can also effectively protect the body from the damage of ROS and free radicals. Meanwhile, patients with CML who received vitamin A combined with standard chemotherapy can prolong clinical progression-free survival and overall survival.[7] In a word, vitamin antioxidants combined with traditional chemotherapy are expected to be a new treatment strategy in leukemia.
Application of natural compound antioxidants in leukemia
Antioxidants extracted from natural ingredients and biological products can neutralize free radicals produced by cell metabolism and induce tumor cell apoptosis. Natural compounds with the advantage of low toxicity can be discovered as effective anticancer drugs for the treatment of hematological malignancies, especially for elderly and immunocompromised patients.[8] Recent studies suggest that natural compound antioxidants, such as Butterolide, Toona sinensis, gallic acid, curcumin, resveratrol, cinnamaldehyde, and artesunate all reveal their anti-leukemia effects, and therefore they are used in cancer chemotherapy to reverse, inhibit, or prevent cancer progression.[2] For example, M. oleifera leaf extract can significantly improve cell viability, having the best effect at the concentration of 800 μg/mL.[29] Piperlongumine, a kind of plant-derived compound existing in some piper plants, is a kind of new potential antitumor drug. Tetrandrine, a natural product isolated from a Chinese plant Stephania tetrandra, can induce autophagy of leukemia cells. Low dose of tetrandrine can inhibit cell proliferation, but has no significant effect on cell viability; while high dose of tetrandrine can significantly increase the apoptosis of leukemia cells for the treatment of leukemia. Tetrandrine, whether used alone or in combination with other autophagy inhibitors, may be a therapeutic strategy for leukemia, with the underlying molecular mechanism by stimulation of ROS-dependent Notch1 and Akt signaling pathway.[63] Certainly, more natural compound antioxidants are hopeful of applying in clinical treatment for leukemia in the future.
Application of intracellular antioxidants in leukemia
Intracellular antioxidants are composed of various substances, including glutathione and heme oxygenase-1 (HO-1). Glutathione is an important antioxidant that can improve the symptoms of adult cancer patients.[64] HO-1, a cytoprotective protein in the heme oxygenase (HO) family, potentially plays a key role in oxidative stress and inhibits apoptosis of AML cells by activating the JNK/c-Jun signaling pathway.[5]HO-1 gene can be transcribed by Nrf2, usually occurring after oxidative stress and cell injury. HO-1 can reduce oxidative stress and inflammation, protect cells from apoptosis, and alter cell cycle, thus improving the prognosis of patients with leukemia.[65] In addition, the glucose pathway has the potential of targeted therapy for leukemia. The potential of regulating the target pathway of ROS and investigating new drugs with higher specificity can facilitate treating leukemia associated with activated ABL. The glucose pathway has significant effects on cell growth, viability, tyrosine phosphorylation, and intracellular ROS. Since the enhancement of glucose metabolism is a common feature of active hematopoietic cells, the therapeutic effect can be enhanced by combining intracellular antioxidants with standard therapy (such as imatinib mesylate).[66]
Some enzymes are also associated with oxidative stress, participating in the treatment and prognosis of leukemia. 5′ adenosine-monophosphate (AMP)-activated protein kinase (AMPK), a metabolic checkpoint kinase, confers metabolic stress resistance to leukemia-initiating cells and promotes the development of leukemia. Targeting AMPK can inhibit myeloid leukemia by interfering with glucose metabolism and reveal the different metabolic needs of leukemia residing in the bone marrow and the spleen.[67] The expression of glutathione peroxidase 3 (GPx3), a scavenger of ROS, is positively correlated with the frequency of LSCs. Compared with leukemia with low LSC frequency, leukemia with high LSC frequency expresses high levels of GPx3 and low levels of ROS. In human primary AML samples, the expression level of GPx3 is directly related to poor prognosis, revealing potential targets for LSC eradication.[68]
Therapeutic drugs based on oxidative stress for treatment of leukemia
The main target of leukemia drugs is a small number of mitochondria within HSCs. Disturbance and attenuation of respiratory function further enhance the initial pro-oxidative state of cells and can easily lead to serious oxidative stress status, creating the necessary conditions for the induction of leukemia. Respiratory function degradation is thought to be the leading cause of the changes of oxidative stress-related genes in cells and the development of diseases. ROS, reactive nitrogen species (RNS), and peroxides, acting as signaling molecules, can significantly influence the expression of redox-sensitive transcription factors, enzymes, oncogenes, and other effectors. Furthermore, it can influence the process and direction of proliferation, differentiation, and apoptosis of leukemia cells, leading to leukemia occurrence.[57]
ATO, a pro-oxidant leukemia drug, is the first choice for the treatment of newly diagnosed and relapsed APL,[27] which has attracted extensive attention over the past decades for its remarkable therapeutic efficacy on APL. ATO has an excellent inhibitory effect on signal transduction pathways and transcription factors in APL cells.[69] It exerts cytotoxic effects by increasing oxidative stress and inhibiting the normal functions of the glutathione/glutathione system.[20] ATO may also suppress APL by inhibiting thioredoxin, inducing ROS production, or activating NOX.[70] However, ATO resistance remains a significant obstacle for treating APL. Recent studies have shown that the PI3K pathway is involved in ATO resistance, so the effect of PI3K inhibitors on reversing ATO resistance has been explored. Among the selective PI3K small molecule inhibitors, PAN-PI3K inhibitors have shown a wide range of anticancer activities in many kinds of tumors with a range of molecular changes. Some approaches, such as JAK2/STAT3, can reduce the antitumor activity of ATO by reducing the content of ROS and then protect cells from apoptosis. The combination of ATO and ruxolitinib (a JAK2 inhibitor) can reduce the metabolic activity, proliferation, and survival rate of AML cells. Due to the increase of ROS and the decrease of glutathione (GSH), the cell cycle of AML cells is arrested in G1/S cells. It follows that the synergistic antitumor effect of ATO and ruxolitinib in AML cells is mediated by the increase of ROS and DNA damage. Besides, targeting catalase, a new CML treatment method, may significantly improve the effectiveness of ATO in the treatment of CML.[65] In conclusion, the combination therapy of AML is more effective than the single targeted therapy, contributing to the treatment of AML patients.[36]
There are still some oxidative stress-related drugs using in clinical practice. Another kind of drug, isothiocyanate, works by depleting the glutathione pool, effectively killing fludarabine-resistant CLL cells and imatinib-resistant CML cells, without attacking healthy hematopoietic cells.[7] Cytarabine is an important antimetabolite for the treatment of acute leukemia. It can activate some tumor suppressor genes, such as NF-κB and p53 by increasing the production of ROS, leading to apoptosis of leukemic cells.[5] Piprartin can inhibit the proliferation and survival of different kinds of leukemia cells. It can inhibit the proliferation of B-cell acute lymphocytic leukemia cells without affecting normal B-cells, which is achieved by increasing ROS-induced apoptosis.[71] Flavonoids, composed of some chemical subunits, are a class of widely distributed phytochromes. Acacetin (5,7-dihydroxy-4′-methoxy flavone) is a kind of flavonoid with anti-inflammatory, anti-peroxidation, anti-plasmodium, anti-mutation, and anti-cancer effects. Acacetin has also been shown to play an anti-proliferative role in CLL by inducing cell apoptosis and preventing cell cycle progression. It can selectively kill B lymphocytes in CLL by targeting the formation of cancerous mitochondria and ROS, thus having a good therapeutic effect on CLL.[5] Certainly, more therapeutic drugs based on oxidative stress for the treatment of leukemia are waiting to study.
Targeted to ROS level therapy
ROS is a double-edged sword in the treatment of tumors. Low levels of ROS can promote the growth of tumor cells. Whereas high levels of ROS can not only cause tumor cell death but also cause oxidative damage to organelles, proteins, and DNA, resulting in genomic instability and abnormal cell functions.[28] Therefore, it has become a major task to monitor the concentration of ROS in chemotherapy for controlling the dose of chemotherapeutic drugs, so that the best antitumor effect can be achieved at a certain intracellular ROS concentration. Some laboratory tests have developed suitable tools for rapidly measuring oxidative stress assessment of blood, especially by these two kinds of photometric analysis: derived active oxygen metabolites (D-ROM) test and biological antioxidant potential (BAP) test. These two kinds of analysis tools have been proved with significant predictive value, and they have been routinely used to measure oxidative stress and evaluate its role in the pathogenesis of leukemia.[1] Certainly, the appearance of some new markers of oxidative stress damage benefit patients with leukemia. It is worth mentioning that new markers such as glutathione hemoglobin are highly sensitive and specific, and can provide early warning signals of oxidative damage. It can ameliorate or delay the development of oxidative stress state so as to prevent cancers. Malondialdehyde (MDA) and phosphatidylcholine (PC) levels are used as biomarkers for assessing oxidative stress in patients with cancer and certain hematological malignancies, for the levels of MDA and PC in plasma are stable and easy to measure. The elevation of their levels may suggest cancer progression. Therefore, ROS indicators, such as glutathione, MDA, and PC, may be regarded as biomarkers of oxidative stress and disease progression in leukemia patients.[42]
The treatment scheme of regulating cell ROS and autophagy
The balance between ROS and autophagy plays an essential role in maintaining cell homeostasis and in the occurrence and development of leukemia.[58] Autophagy, a common cellular activity which is related to cell proliferation and differentiation, has two sides in tumor cells. On one hand, autophagy may have a protective effect in the early stages of cancer development. It can remove protein aggregates and damaged organelles, reduce oxidative stress and local inflammation, and maintain chromosome instability, thus inhibiting tumor development.[21] On the other hand, autophagy can improve the tolerance of tumor cells to stress in adverse environments and maintain tumor cell survival, thus promoting tumor development. Nowadays, many kinds of chemotherapy drugs applied in the clinical treatment of leukemia can induce leukemic cells autophagy and promote programmed death of leukemic cells by increasing the production of ROS. Specifically, the elevation of ROS levels induces autophagy through various signaling pathways, which in turn reduces ROS-induced damage of cells and tissues. ROS and autophagy can coordinate with each other to maintain intracellular homeostasis in leukemia.
To sum up, these results deepen the researcher's understanding of promising treatments related to oxidative stress in the field of leukemia.[72–74] Perhaps, within a few years, direct intervention with oxidative stress mediators or their molecular targets will be better understood, either alone or in combination with other drugs. It will be effective in the clinical treatment of various hematological diseases and benefit leukemia patients in the future. However, there are still some limitations of antioxidants to become a real part of the treatment of leukemia. At present, the study and clinical trials of them in the clinical practice are still in the primary stage. Large-scale multicenter trials of different kinds of antioxidants compared with the traditional treatment in different types of leukemia should be carried out to screen out the effective antioxidant application for every type of leukemia. Expanded samples in the clinical may contribute to a more realistic result, guiding clinical application in the future. Certainly, it is better to compare the effectiveness of different kinds of antioxidants in the same type of leukemia, aiming to establish a kind of new treatment strategy for leukemia patients.
Conclusion
Recent studies have shown that oxidative stress caused by the imbalance between the generation of free radicals/ROS and the antioxidant defense systems can activate various transcription factors, further affecting their transcriptional pathways. Oxidative stress plays an important role in the occurrence, development, treatment, and prognosis of leukemia. At present, the standard chemotherapy strategies for the pre-treatment of leukemia still have many limitations. Hence, new treatment strategies of using antioxidants and oxidants in the treatment of leukemia still need to be explored and have been preliminarily applied in clinical practice. The article reviews the latest research progress of oxidative stress in leukemia. In addition, non-invasive treatment with combined oxidative stress is proposed as well. However, the specific mechanism of oxidative stress in the pathogenesis and development of leukemia is still not fully understood. The value of antioxidant therapy in leukemia lacks reliable multicenter clinical trials and prospective studies, which may be the focus of future research.
Conflicts of Interest
None.
Footnotes
How to cite this article: Dong C, Zhang NJ, Zhang LJ. Oxidative stress in leukemia and antioxidant treatment. Chin Med J 2021;134:1897–1907. doi: 10.1097/CM9.0000000000001628
References
- 1.D’Arena G, Seneca E, Migliaccio I, De Feo V, Giudice A, La Rocca F, et al. Oxidative stress in chronic lymphocytic leukemia: Still a matter of debate. Leuk Lymphoma 2019; 60:867–875. doi: 10.1080/10428194.2018.1509317. [DOI] [PubMed] [Google Scholar]
- 2.Ben Mahmoud L, Mdhaffar M, Ghozzi H, Ammar M, Hakim A, Atheymen R, et al. Oxidative stress in tunisian patients with acute lymphoblastic leukemia and its involvement in leukemic relapse. J Pediatr Hematol Oncol 2017; 39:e124–e130. doi: 10.1097/MPH.0000000000000793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dizdaroglu M. Oxidatively induced DNA damage: mechanisms, repair and disease. Cancer Lett 2012; 327:26–47. doi: 10.1016/j.canlet.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 4.Chen YF, Liu H, Luo XJ, Zhao Z, Zou ZY, Li J, et al. The roles of reactive oxygen species (ROS) and autophagy in the survival and death of leukemia cells. Crit Rev Oncol Hematol 2017; 112:21–30. doi: 10.1016/j.critrevonc.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Ammar M, Ben Mahmoud L, Medhaffar M, Ghozzi H, Sahnoun Z, Hakim A, et al. Relationship of oxidative stress in the resistance to imatinib in Tunisian patients with chronic myeloid leukemia: aretrospective study. J Clin Lab Anal 2020; 34:e23050.doi: 10.1002/jcla.23050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zelen I, Djurdjevic P, Popovic S, Stojanovic M, Jakovljevic V, Radivojevic S, et al. Antioxidant enzymes activities and plasma levels of oxidative stress markers in B-chronic lymphocytic leukemia patients. J BUON 2010; 15:330–336. [PubMed] [Google Scholar]
- 7.Udensi UK, Tchounwou PB. Dual effect of oxidative stress on leukemia cancer induction and treatment. J Exp Clin Cancer Res 2014; 33:106.doi: 10.1186/s13046-014-0106-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med 2010; 49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie W, Ma W, Liu P, Zhou F. Overview of thioredoxin system and targeted therapies for acute leukemia. Mitochondrion 2019; 47:38–46. doi: 10.1016/j.mito.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 10.Testa U, Labbaye C, Castelli G, Pelosi E. Oxidative stress and hypoxia in normal and leukemic stem cells. Exp Hematol 2016; 44:540–560. doi: 10.1016/j.exphem.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 11.Samimi A, Kalantari H, Lorestani MZ, Shirzad R, Saki N. Oxidative stress in normal hematopoietic stem cells and leukemia. APMIS 2018; 126:284–294. doi: 10.1111/apm.12822. [DOI] [PubMed] [Google Scholar]
- 12.Gañán-Gómez I, Wei Y, Yang H, Boyano-Adánez MC, García-Manero G. Oncogenic functions of the transcription factor Nrf2. Free Radic Biol Med 2013; 65:750–764. doi: 10.1016/j.freeradbiomed.2013.06.041. [DOI] [PubMed] [Google Scholar]
- 13.Ge C, Huang H, Huang F, Yang T, Zhang T, Wu H, et al. Neurokinin-1 receptor is an effective target for treating leukemia by inducing oxidative stress through mitochondrial calcium overload. Proc Natl Acad Sci U S A 2019; 116:19635–19645. doi: 10.1073/pnas.1908998116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdul-Aziz A, MacEwan DJ, Bowles KM, Rushworth SA. Oxidative stress responses and NRF2 in human leukaemia. Oxid Med Cell Longev 2015; 2015:454659.doi: 10.1089/ars.2008.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu RP, Hayashi T, Cottam HB, Jin G, Yao S, Wu CCN, et al. Nrf2 responses and the therapeutic selectivity of electrophilic compounds in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A 2010; 107:7479–7484. doi: 10.1073/pnas.1002890107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bourgeais J, Ishac N, Medrzycki M, Brachet-Botineau M, Desbourdes L, Gouilleux-Gruart V, et al. Oncogenic STAT5 signaling promotes oxidative stress in chronic myeloid leukemia cells by repressing antioxidant defenses. Oncotarget 2017; 8:41876–41889. doi: 10.18632/oncotarget.11480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du W, Adam Z, Rani R, Zhang X, Pang Q. Oxidative stress in Fanconi anemia hematopoiesis and disease progression. Antioxid Redox Signal 2008; 10:1909–1921. doi: 10.1089/ars.2008.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romeo M, Hutchison T, Malu A, White A, Kim J, Gardner R, et al. The human T-cell leukemia virus type-1 p30(II) protein activates p53 and induces the TIGAR and suppresses oncogene-induced oxidative stress during viral carcinogenesis. Virology 2018; 518:103–115. doi: 10.1016/j.virol.2018.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niwa-Kawakita M, Ferhi O, Soilihi H, Le Bras M, Lallemand-Breitenbach V, de Thé H. PML is a ROS sensor activating p53 upon oxidative stress. J Exp Med 2017; 214:3197–3206. doi: 10.1084/jem.20160301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imbesi S, Musolino C, Allegra A, Saija A, Morabito F, Calapai G, et al. Oxidative stress in oncohematologic diseases: an update. Expert Rev Hematol 2013; 6:317–325. doi: 10.1586/ehm.13.21. [DOI] [PubMed] [Google Scholar]
- 21.Miller BA. TRPM2 in cancer. Cell Calcium 2019; 80:8–17. doi: 10.1016/j.ceca.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kruk J, Aboul-Enein HY. Reactive oxygen and nitrogen species in carcinogenesis: implications of oxidative stress on the progression and development of several cancer types. Mini Rev Med Chem 2017; 17:904–919. doi: 10.2174/1389557517666170228115324. [DOI] [PubMed] [Google Scholar]
- 23.Kizaki M, Xian M, Sagawa M, Ikeda Y. Induction of apoptosis via the modulation of reactive oxygen species (ROS) production in the treatment of myeloid leukemia. Curr Pharm Biotechnol 2006; 7:323–329. doi: 10.2174/138920106778521541. [DOI] [PubMed] [Google Scholar]
- 24.Tessier S, Martin-Martin N, de Thé H, Carracedo A, Lallemand-Breitenbach V. Promyelocytic leukemia protein, a protein at the crossroad of oxidative stress and metabolism. Antioxid Redox Signal 2017; 26:432–444. doi: 10.1089/ars.2016.6898. [DOI] [PubMed] [Google Scholar]
- 25.Fan XY, Chen XY, Liu YJ, Zhong HM, Jiang FL, Liu Y, et al. Oxidative stress-mediated intrinsic apoptosis in human promyelocytic leukemia HL-60 cells induced by organic arsenicals. Sci Rep 2016; 6:29865.doi: 10.1038/srep29865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vivas-Mejía PE, Ozpolat B, Chen X, Lopez-Berestein G. Downregulation of the c-MYC target gene, peroxiredoxin III, contributes to arsenic trioxide-induced apoptosis in acute promyelocytic leukemia. Int J Cancer 2009; 125:264–275. doi: 10.1002/ijc.24341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aurelius J, Thorén FB, Akhiani AA, Brune M, Palmqvist L, Hansson M, et al. Monocytic AML cells inactivate antileukemic lymphocytes: Role of NADPH oxidase/gp91(phox) expression and the PARP-1/PAR pathway of apoptosis. Blood 2012; 119:5832–5837. doi: 10.1182/blood-2011-11-391722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jevtovic-Stoimenov T, Cvetkovic T, Despotovic M, Basic J, Cvetkovic J, Marjanovic G, et al. The influence of TNF alpha -308 G/A polymorphism on oxidative stress in patients with chronic lymphocytic leukemia. Leuk Res 2017; 54:66–72. doi: 10.1016/j.leukres.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 29.Dilworth LL, Stennett D, Omoruyi FO. Effects of Moringa oleifera leaf extract on human promyelocytic leukemia cells subjected to oxidative stress. J Med Food 2020; 23:728–734. doi: 10.1089/jmf.2019.0192. [DOI] [PubMed] [Google Scholar]
- 30.Zhou J, Bi C, Cheong LL, Mahara S, Liu SC, Tay KG, et al. The histone methyltransferase inhibitor, DZNep, up-regulates TXNIP, increases ROS production, and targets leukemia cells in AML. Blood 2011; 118:2830–2839. doi: 10.1182/blood-2010-07-294827. [DOI] [PubMed] [Google Scholar]
- 31.Mimura K, Kua LF, Shimasaki N, Shiraishi K, Nakajima S, Siang LK, et al. Upregulation of thioredoxin-1 in activated human NK cells confers increased tolerance to oxidative stress. Cancer Immunol Immunother 2017; 66:605–613. doi: 10.1007/s00262-017-1969-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou F, Pan Y, Wei Y, Zhang R, Bai G, Shen Q, et al. Jab1/Csn5-Thioredoxin signaling in relapsed acute monocytic leukemia under oxidative stress. Clin Cancer Res 2017; 23:4450–4461. doi: 10.1158/1078-0432.CCR-16-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hole PS, Darley RL, Tonks A. Do reactive oxygen species play a role in myeloid leukemias? Blood 2011; 117:5816–5826. doi: 10.1182/blood-2011-01-326025. [DOI] [PubMed] [Google Scholar]
- 34.Zhou F, Shen Q, Claret FX. Novel roles of reactive oxygen species in the pathogenesis of acute myeloid leukemia. J Leukoc Biol 2013; 94:423–429. doi: 10.1189/jlb.0113006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou FL, Zhang WG, Wei YC, Meng S, Bai GG, Wang BY, et al. Involvement of oxidative stress in the relapse of acute myeloid leukemia. J Biol Chem 2010; 285:15010–15015. doi: 10.1074/jbc.M110.103713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mesbahi Y, Zekri A, Ghaffari SH, Tabatabaie PS, Ahmadian S, Ghavamzadeh A. Blockade of JAK2/STAT3 intensifies the anti-tumor activity of arsenic trioxide in acute myeloid leukemia cells: Novel synergistic mechanism via the mediation of reactive oxygen species. Eur J Pharmacol 2018; 834:65–76. doi: 10.1016/j.ejphar.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 37.Romero AI, Thorén FB, Aurelius J, Askarieh G, Brune M, Hellstrand K. Post-consolidation immunotherapy with histamine dihydrochloride and interleukin-2 in AML. Scand J Immunol 2009; 70:194–205. doi: 10.1111/j.1365-3083.2009.02303.x. [DOI] [PubMed] [Google Scholar]
- 38.Nilsson MS, Hallner A, Brune M, Nilsson S, Thorén FB, Martner A, et al. Immunotherapy with HDC/IL-2 may be clinically efficacious in acute myeloid leukemia of normal karyotype. Hum Vaccin Immunother 2020; 16:109–111. doi: 10.1080/21645515.2019.1636598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berry SM, Broglio KR, Berry DA. Addressing the incremental benefit of histamine dihydrochloride when added to interleukin-2 in treating acute myeloid leukemia: a Bayesian meta-analysis. Cancer Invest 2011; 29:293–299. doi: 10.3109/07357907.2011.568563. [DOI] [PubMed] [Google Scholar]
- 40.Rodrigues MS, Reddy MM, Sattler M. Cell cycle regulation by oncogenic tyrosine kinases in myeloid neoplasias: From molecular redox mechanisms to health implications. Antioxid Redox Signal 2008; 10:1813–1848. doi: 10.1089/ars.2008.2071. [DOI] [PubMed] [Google Scholar]
- 41.Ahmad R, Tripathi AK, Tripathi P, Singh S, Singh R, Singh RK. Malondialdehyde and protein carbonyl as biomarkers for oxidative stress and disease progression in patients with chronic myeloid leukemia. In Vivo 2008; 22:525–528. [PubMed] [Google Scholar]
- 42.Cao ZH, Wu Z, Hu C, Zhang M, Wang WZ, Hu XB. Endoplasmic reticulum stress and destruction of pancreatic β cells in type 1 diabetes. Chin Med J 2020; 133:68–73. doi: 10.1097/CM9.0000000000000583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bazi A, Keramati MR, Gholamin M. Role of oxidative stress in modulating unfolded protein response activity in chronic myeloid leukemia cell line. Iran Biomed J 2016; 20:63–67. doi: 10.7508/ibj.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang J, Xu Y, Wan H, Hu J. Antibiotic ivermectin selectively induces apoptosis in chronic myeloid leukemia through inducing mitochondrial dysfunction and oxidative stress. Biochem Biophys Res Commun 2018; 497:241–247. doi: 10.1016/j.bbrc.2018.02.063. [DOI] [PubMed] [Google Scholar]
- 45.Konopleva MY, Jordan CT. Leukemia stem cells and microenvironment: Biology and therapeutic targeting. J Clin Oncol 2011; 29:591–599. doi: 10.1200/JCO.2010.31.0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ahmad R, Tripathi AK, Tripathi P, Singh R, Singh S, Singh RK. Studies on lipid peroxidation and non-enzymatic antioxidant status as indices of oxidative stress in patients with chronic myeloid leukaemia. Singapore Med J 2010; 51:110–115. [PubMed] [Google Scholar]
- 47.Cole PD, Finkelstein Y, Stevenson KE, Blonquist TM, Vijayanathan V, Silverman LB, et al. Polymorphisms in genes related to oxidative stress are associated with inferior cognitive function after therapy for childhood acute lymphoblastic leukemia. J Clin Oncol 2015; 33:2205–2211. doi: 10.1200/JCO.2014.59.0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bedoya-Medina J, Mendivil-Perez M, Rey-Suarez P, Jimenez-Del-Rio M, Núñez V, Velez-Pardo C. l-amino acid oxidase isolated from Micrurus mipartitus snake venom (MipLAAO) specifically induces apoptosis in acute lymphoblastic leukemia cells mostly via oxidative stress-dependent signaling mechanism. Int J Biol Macromol 2019; 134:1052–1062. doi: 10.1016/j.ijbiomac.2019.05.174. [DOI] [PubMed] [Google Scholar]
- 49.Burt R, Dey A, Aref S, Aguiar M, Akarca A, Bailey K, et al. Activated stromal cells transfer mitochondria to rescue acute lymphoblastic leukemia cells from oxidative stress. Blood 2019; 134:1415–1429. doi: 10.1182/blood.2019001398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu YF, Chen YY, He YY, Wang JY, Yang JP, Zhong SL, et al. Expansion and activation of granulocytic, myeloid-derived suppressor cells in childhood precursor B cell acute lymphoblastic leukemia. J Leukoc Biol 2017; 102:449–458. doi: 10.1189/jlb.5MA1116-453RR. [DOI] [PubMed] [Google Scholar]
- 51.Tahir IM, Iqbal T, Jamil A, Saqib M. Association of BCL-2 with oxidative stress and total antioxidant status in pediatric acute lymphoblastic leukemia. J Biol Regul Homeost Agents 2017; 31:1023–1027. [PubMed] [Google Scholar]
- 52.Navrkalova V, Kafkova LR, Divoky V, Pospisilova S. Oxidative stress as a therapeutic perspective for ATM-deficient chronic lymphocytic leukemia patients. Haematologica 2015; 100:994–996. doi: 10.3324/haematol.2015.130260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salimi A, Roudkenar MH, Sadeghi L, Mohseni A, Seydi E, Pirahmadi N, et al. Selective anticancer activity of acacetin against chronic lymphocytic leukemia using both in vivo and in vitro methods: key role of oxidative stress and cancerous mitochondria. Nutr Cancer 2016; 68:1404–1416. doi: 10.1080/01635581.2016.1235717. [DOI] [PubMed] [Google Scholar]
- 54.Koczula KM, Ludwig C, Hayden R, Cronin L, Pratt G, Parry H, et al. Metabolic plasticity in CLL: adaptation to the hypoxic niche. Leukemia 2016; 30:65–73. doi: 10.1038/leu.2015.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lanasa MC. Novel insights into the biology of CLL. Hematology Am Soc Hematol Educ Program 2010; 2010:70–76. doi: 10.1182/asheducation-2010.1.70. [DOI] [PubMed] [Google Scholar]
- 56.Vinnai JR, Cumming RC, Thompson GJ, Timoshenko AV. The association between oxidative stress-induced galectins and differentiation of human promyelocytic HL-60 cells. Exp Cell Res 2017; 355:113–123. doi: 10.1016/j.yexcr.2017.03.059. [DOI] [PubMed] [Google Scholar]
- 57.Lyu BN, Ismailov SB, Ismailov B, Lyu MB. Mitochondrial concept of leukemogenesis: key role of oxygen-peroxide effects. Theor Biol Med Model 2008; 5:23.doi: 10.1186/1742-4682-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu SY, Wen YC, Ku CC, Yang YC, Chow JM, Yang SF, et al. Penfluridol triggers cytoprotective autophagy and cellular apoptosis through ROS induction and activation of the PP2A-modulated MAPK pathway in acute myeloid leukemia with different FLT3 statuses. J Biomed Sci 2019; 26:63.doi: 10.1186/s12929-019-0557-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen YC, Lu MC, El-Shazly M, Lai KH, Wu TY, Hsu YM. Breaking down leukemia walls: Heteronemin, a sesterterpene derivative, induces apoptosis in leukemia Molt4 cells through oxidative stress, mitochondrial dysfunction and induction of talin expression. Mar Drugs 2018; 16:212.doi: 10.3390/md16060212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hockenberry MJ, Pan W, Scheurer ME, Hooke MC, Taylor O, Koerner K, et al. Influence of inflammatory and oxidative stress pathways on longitudinal symptom experiences in children with leukemia. Biol Res Nurs 2019; 21:458–465. doi: 10.1177/1099800419863160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang J, Lei W, Chen X, Wang S, Qian W. Oxidative stress response induced by chemotherapy in leukemia treatment. Mol Clin Oncol 2018; 8:391–399. doi: 10.3892/mco.2018.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Swords RT, Kelly KR, Smith PG, Garnsey JJ, Mahalingam D, Medina E, et al. Inhibition of NEDD8-activating enzyme: a novel approach for the treatment of acute myeloid leukemia. Blood 2010; 115:3796–3800. doi: 10.1182/blood-2009-11-254862. [DOI] [PubMed] [Google Scholar]
- 63.Liu T, Zhang Z, Yu C, Zeng C, Xu X, Wu G, et al. Tetrandrine antagonizes acute megakaryoblastic leukaemia growth by forcing autophagy-mediated differentiation. Br J Pharmacol 2017; 174:4308–4328. doi: 10.1111/bph.14031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rodgers C, Sanborn C, Taylor O, Gundy P, Pasvogel A, Moore IM, et al. Fatigue and oxidative stress in children undergoing leukemia treatment. Biol Res Nurs 2016; 18:515–520. doi: 10.1177/1099800416647794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barrera LN, Rushworth SA, Bowles KM, MacEwan DJ. Bortezomib induces heme oxygenase-1 expression in multiple myeloma. Cell Cycle 2012; 11:2248–2252. doi: 10.4161/cc.20343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim JH, Chu SC, Gramlich JL, Pride YB, Babendreier E, Chauhan D, et al. Activation of the PI3K/mTOR pathway by BCR-ABL contributes to increased production of reactive oxygen species. Blood 2005; 105:1717–1723. doi: 10.1182/blood-2004-03-0849. [DOI] [PubMed] [Google Scholar]
- 67.Saito Y, Chapple RH, Lin A, Kitano A, Nakada D. AMPK protects leukemia-initiating cells in myeloid leukemias from metabolic stress in the bone marrow. Cell Stem Cell 2015; 17:585–596. doi: 10.1016/j.stem.2015.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Herault O, Hope KJ, Deneault E, Mayotte N, Chagraoui J, Wilhelm BT, et al. A role for GPx3 in activity of normal and leukemia stem cells. J Exp Med 2012; 209:895–901. doi: 10.1084/jem.20102386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sumi D, Shinkai Y, Kumagai Y. Signal transduction pathways and transcription factors triggered by arsenic trioxide in leukemia cells. Toxicol Appl Pharmacol 2010; 244:385–392. doi: 10.1016/j.taap.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 70.Dugo EB, Yedjou CG, Stevens JJ, Tchounwou PB. Therapeutic potential of arsenic trioxide (ATO) in treatment of hepatocellular carcinoma: Role of oxidative stress in ATO-induced apoptosis. Ann Clin Pathol 2017; 5:1101. [PMC free article] [PubMed] [Google Scholar]
- 71.Song LL, Tu YY, Xia L, Wang WW, Wei W, Ma CM, et al. Targeting catalase but not peroxiredoxins enhances arsenic trioxide-induced apoptosis in K562 cells. PLoS One 2014; 9:e104985.doi: 10.1371/journal.pone.0104985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.de Oliveira MS, Barbosa MIF, de Souza TB, Moreira DRM, Martins FT, Villarreal W, et al. A novel platinum complex containing a piplartine derivative exhibits enhanced cytotoxicity, causes oxidative stress and triggers apoptotic cell death by ERK/p38 pathway in human acute promyelocytic leukemia HL-60 cells. Redox Biol 2019; 20:182–194. doi: 10.1016/j.redox.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Turrini E, Laurita R, Stancampiano A, Catanzaro E, Calcabrini C, Maffei F, et al. Cold atmospheric plasma induces apoptosis and oxidative stress pathway regulation in T-Lymphoblastoid leukemia cells. Oxid Med Cell Longev 2017; 2017:4271065.doi: 10.1155/2017/4271065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ghaffari S. Oxidative stress in the regulation of normal and neoplastic hematopoiesis. Antioxid Redox Signal 2008; 10:1923–1940. doi: 10.1089/ars.2008.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]