Abstract
Smaller, more affordable, and more portable MRI brain scanners offer exciting opportunities to address unmet research needs and long-standing health inequities in remote and resource-limited international settings. Field-based neuroimaging research in low- and middle-income countries (LMICs) can improve local capacity to conduct both structural and functional neuroscience studies, expand knowledge of brain injury and neuropsychiatric and neurodevelopmental disorders, and ultimately improve the timeliness and quality of clinical diagnosis and treatment around the globe. Facilitating MRI research in remote settings can also diversify reference databases in neuroscience, improve understanding of brain development and degeneration across the lifespan in diverse populations, and help to create reliable measurements of infant and child development. These deeper understandings can lead to new strategies for collaborating with communities to mitigate and hopefully overcome challenges that negatively impact brain development and quality of life. Despite the potential importance of research using highly portable MRI in remote and resource-limited settings, there is little analysis of the attendant ethical, legal, and social issues (ELSI). To begin addressing this gap, this paper presents findings from the first phase of an envisioned multi-staged and iterative approach for creating ethical and legal guidance in a complex global landscape. Section 1 provides a brief introduction to the emerging technology for field-based MRI research. Section 2 presents our methodology for generating plausible use cases for MRI research in remote and resource-limited settings and identifying associated ELSI issues. Section 3 analyzes core ELSI issues in designing and conducting field-based MRI research in remote, resource-limited settings and offers recommendations. We argue that a guiding principle for field-based MRI research in these contexts should be including local communities and research participants throughout the research process in order to create sustained local value. Section 4 presents a recommended path for the next phase of work that could further adapt these use cases, address ethical and legal issues, and co-develop guidance in partnership with local communities.
Keywords: Portable MRI, Neuroimaging, Neuroethics, Low- and middle-income countries (LMICs), International research
Introduction
The emergence of smaller, more affordable, and more portable MRI scanners (O’Reilly et al., 2021; Sarracanie et al., 2015; Wald 2019) offers exciting opportunities to address unmet research needs and long-standing health inequities in remote and resource-limited international settings (Cooley et al., 2020; Geethanath and Vaughan 2019). Field-based neuroimaging research in low- and middle-income countries (LMICs) can improve local capacity to conduct structural and functional neuroscience studies, expand knowledge of brain injury and neuropsychiatric and neurodevelopmental disorders, and ultimately improve the timeliness and quality of clinical diagnosis and treatment around the globe (Dasgupta et al., 2016; Hussain 2015; Illes et al., 2020; Mollura and Lungren 2019).
Portable MRI may be especially valuable for expanding research to remote and resource-limited settings because MRI currently remains costly and relatively immobile (World Health Organization 2017). Even large-scale international neuroimaging projects such as the Enhancing NeuroImaging Genetics through Meta-Analysis (ENIGMA) project (Palk et al., 2020; Thompson et al., 2020), the United Kingdom Biobank Imaging study (UK Biobank 2019), and MRI studies of young children in the Drakenstein Child Health Study (DCHS) include only participants who can travel to fixed scanners in urban medical centers (Wedderburn et al., 2020). Portable MRI research may provide sustained local benefits in multiple ways, including by improving local expertise for future introduction of accessible MRI for clinical uses.
Facilitating MRI research in remote and resource-limited settings can also diversify reference databases in neuroscience by enlarging the range of populations represented (Cirillo et al., 2020; ISMRM 2021), improving understanding of brain development and degeneration across the lifespan in diverse populations, and helping to create reliable and generalizable measurements of infant and childhood development (Katus et al., 2019; Wedderburn et al., 2020). These deeper understandings can lead to new strategies to help remote communities mitigate and hopefully overcome challenges that negatively impact brain development and quality of life. However, there is currently limited analysis of the ethical, legal, and social issues (ELSI) posed by field-based MRI research (Illes et al., 2020; Shen et al., 2020). The first Workshop on Accessible MRI for the World, hosted in 2019 by the International Society for Magnetic Resonance in Medicine (ISMRM) in New Delhi, India, concluded that ELSI guidance was urgently needed (Geethanath et al., 2019).
To advance work on these ELSI issues, we conducted a structured, year-long neuroethics analysis, embedded within a larger collaborative project funded by the U.S. National Institutes of Health (NIH) Brain Research through Advancing Innovative Neurotechnologies (BRAIN) Initiative (Imaging Human Brain Function with Minimal Mobility Restrictions, NIH 3U01EB025153-03S1). The primary focus of the ELSI analysis was on field-based MRI research in remote and resource-limited international settings, when that research is led, or involves significant participation from, research teams that are not based in the local community.
The goal of this first stage of ELSI analysis was to identify likely applications of this emerging highly portable MRI technology, the ELSI issues raised, and potential solutions. This work sets the stage for more expansive exploration with a broader set of stakeholders.
To conduct this first-step analysis, we assembled a multi-disciplinary Working Group including 10 scientists with experience conducting neuroscience research in remote or resource-poor communities, mostly outside the U.S., with limited access to nearby scientific and medical expertise. Many of these research projects have taken place in remote field settings far from a major health center. Many crucial perspectives need to be further incorporated to develop more formal guidelines and broad consensus documents. Future work will need to more directly involve prospective participants and their communities in the co-creation of ethical guidelines.
Our analysis builds on a related prior study of ELSI issues in field-based MRI research within the United States (Shen et al., 2020). Here we focus on field-based, structural and functional MRI research in remote and resource-limited international settings beyond the United States. These settings include LMICs, as defined by the World Bank (2020) and “resource-limited” contexts–communities with limited access to health care treatment and facilities, poor infrastructure, lack of trained health care professionals, and lack of adequate medical equipment (Vasco et al., 2019, Table 2).
Section 1 provides a brief introduction to the emerging technological developments for field-based MRI research. Section 2 presents our methodology for identifying plausible use cases and associated ELSI issues, as well as our survey results. Section 3 analyzes and provides recommendations for addressing core ELSI issues in designing and conducting field-based MRI research in remote, resource-limited settings. Section 4 presents recommendations for next steps in developing ethical and legal guidance.
1. Technological developments enabling field-based MRI research
Multiple highly portable MRI technologies are being developed to enable field-based structural and functional research (Cooley et al., 2020; Huang et al., 2019; Marques et al., 2019; O’Reilly et al., 2020; Sarracanie et al., 2015; Stopczynski et al., 2014; Ward et al., 2019). See Fig. 1. This next-generation MRI technology promises to allow MRI data acquisition at the push of button, and whole-brain structural scans in less than 10 minutes. The research teams developing these technologies are wrestling with a fundamental challenge: how to ensure sufficient field strength and satisfactory image resolution and signal-to-noise ratio, while simultaneously reducing magnet size, reconfiguring scanner design, minimizing safety risks, and lowering costs. To be deployable in remote geographical settings, the system must also be easy to set up, operate, take down, relocate, and maintain (Geethanath and Vaughan 2019), including when faced with infrastructure challenges such as power outages (Fatade 2021). Successful data management will require advances in MRI hardware and related data analytic methods, including transfer of data to cloud-based platforms for analysis often aided by artificial intelligence (AI), and innovations to allow for easier and even remote control of the MRI scanner.
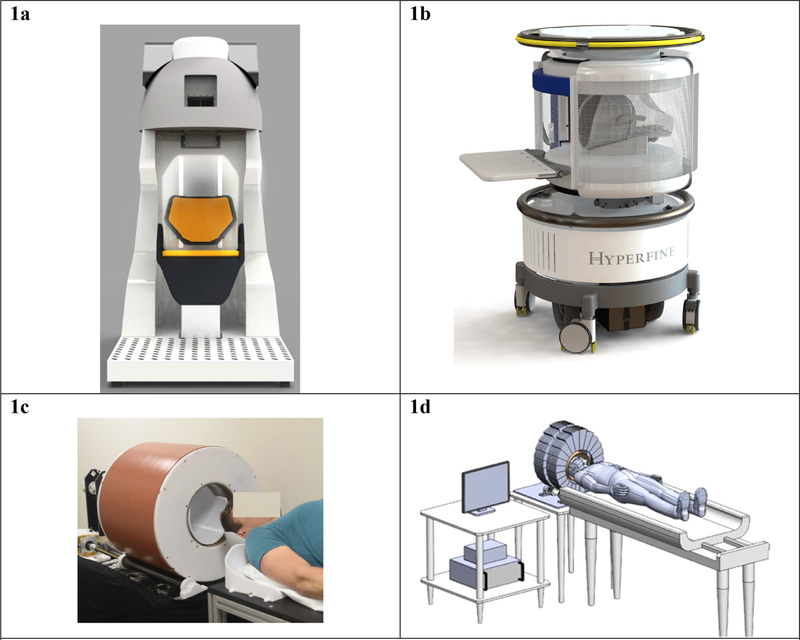
Fig. 1. Images of portable MRI scanners in use and in development.
These examples of portable MRI machines are representative, though not exhaustive, of the new MRI technology being developed by teams across the globe. Reproduction of these images here is not meant to be an endorsement of any particular technology, but instead illustrates the types of devices that are being developed. 1a Portable 1.5 Tesla MRI system technology developed by an international research team with support of NIH BRAIN (Imaging Human Brain Function with Minimal Mobility Restrictions, NIH #1U01EB025153–01). Source: Used with permission from Dr. Mailin Lemke and Ben Parksinon, Victoria University of Wellington. 1b Swoop™, the first FDA-cleared portable MRI scanner developed by Hyperfine Research Inc. Source: Reproduced with permission from Hyperfine, https://www.hyperfine.io/. 1c “A prototype portable brain MRI scanner based on the Halbach permanent magnet described in Cooley et al., (2018) and configured for rotational encoding as in Cooley et al., (2015). The magnet weighs ~125 kg and achieves an 80 mT B0 field.” Source: Used with permission from Dr. Lawrence Wald, as published in Wald et al. (2019). 1d Portable, low-field MRI head imager, with a permanent magnet array that generates strong magnetic fields inside the bore, but negligible magnetic fields outside the bore. This device uses an inward-outward ring array that supplies field in the axial direction (Ren et al., 2019a, 2019b). Source: Used with permission from Dr. Huang Shaoying, SUTD Singapore University of Technology and Design.
1.1. Innovations in MRI hardware and data analysis methods
A variety of innovative approaches can be used to facilitate structural and functional MRI research in the field. One approach would place low-cost, high-field fixed scanners in multiple locations in under-resourced remote settings, such as rural hospitals. Here we define “high field” as 1.5T–3.0T (with “ultra-high field” defined as > 3.0T). The high-field portable devices would require an RF-shielded room and infrastructure for cooling, but researchers could conduct MRI studies from a distant central location, with personnel using remote controls to operate the scanners in the field. Functional scanning techniques are also being developed that allow research participants to sit upright and move their limbs (Garwood et al., 2020, see Fig. 1a).
In addition to high-field approaches, several low-field MRI approaches are in development (Marques et al., 2019; McDaniel et al., 2019; O’Reilly 2020; Wald et al., 2019). We define “low-field” as 0.1T - 0.5T (with “ultra-low field” defined as < 0.1 T). Ultra-low field devices include the FDA pre-market approved Lucy Point-of-Care Magnetic Resonance Imaging Device (“Swoop™”) developed by the U.S. company Hyperfine (Mills 2020, see Fig. 1b) and the prototype “tabletop MRI” on a cart (Cooley et al., 2020, see Fig. 1c), while low-field scanners include the prototype ring-pair permanent magnet array portable scanner (Ren et al., 2019a, 2019b, see Fig. 1d). These three scanners are significantly more portable than high-field options, but these smaller devices have lower maximum field strengths (Sarracanie and Salameh 2020), limiting the resolution quality of resultant MRI images due to a lower signal-to-noise ratio. However, because the magnetic field is not as strong as with fixed scanners, shielding the scanner within a protected room or building is not necessary. Eliminating the requirement of an RF-shielded room permits the device to be deployed in new locations, and the reduced field strength decreases safety risks associated with metal objects and implants.
The challenges of data acquisition in traditional fixed MR scanning —such as too much head motion —remain challenges in the portable MRI context. One particularly vexing issue for portable MRI is ensuring that there are uniform data acquisition procedures across geographically dispersed study sites. This issue of harmonizing data across performance sites is presently being addressed by several large-scale multi-site imaging studies using fixed MRI, such as IMAGEN (Mascarell Maričić et al., 2020) and the Adolescent Brain Cognitive Development (ABCD) study (Casey et al., 2018). The data acquired by portable machines will have lower signal-to-noise ratio than fixed 1.5T and 3T scanners, and thus will require corresponding advances in data analysis methods to extract signal from the data (Geethanath and Vaughan 2018). For instance, iterative image reconstruction methods using cloud-based systems may be required (Wald et al., 2019).
1.2. Developments in MRI software for remote control of scanners
Portable MRI scanners are being designed so that they can be effectively operated in remote field settings, even with little or no prior local expertise performing MRI scanning. Two developments that may facilitate such use are (1) reducing the technical skills needed to operate the scanner, such as through an effective user-interface for the local technician acquiring the data; and (2) developing “autonomous MRI” (AMRI) software that can operate MR scanners remotely (Ravi and Geethanath 2020). AMRI remains in a proof-of-concept phase, but a combination of AMRI and open-source tools may soon allow an MRI scanner to be controlled via a remote web interface (Tong et al., 2019). In this way, specialists could interface directly with a scanner in a different location, even a location in a different country. Clearly, this scenario would raise questions regarding pragmatics, safety protocols, the nature of researcher interactions with participants, and how the ethics and law of both locations might apply.
1.3. Scanner setup in the field
There is presently “no consensus on the best approach for adapting MRI to portable and POC [point of care] use” (Salameh and Sarracanie 2020, p. 3) and no consensus guidance on how best to approach the reduced, but not eliminated, safety risks associated with highly portable low-field scanners. The American College of Radiology’s (ACR) five “safety zones” restrict access to fixed, RF-shielded MRI rooms (Expert Panel on MR Safety 2013). But the ACR zones as typically established in a medical/research facility are not applicable to portable MRI equipment with much different magnetic fields and Gauss lines. Indeed, one likely benefit of portable MRI scanners is reduced need to screen patients, family members, and medical staff prior to entering the portable MRI environment.
In fixed MRI scanning, Zone 3 is restricted access and requires a “privacy barrier so that unauthorized persons cannot view control panels.” Zone 4 is the scanner room, where the participant alone will be located during data acquisition. ACR guidance emphasizes that Zone 3 “should be physically restricted from general public access” and that there “should be no exceptions to this guideline.” But with portable MRI machines, others may be standing within mere feet of the machine. If safety protocols are not effectively communicated in local languages, there is a potential for onlookers to misunderstand or minimize safety requirements.
2. Identifying and addressing ELSI issues: project methods and survey results
2.1. Interdisciplinary workshop, follow-up survey, and development of recommendations
Approaches to the ethical development and deployment of new technology such as portable MRI emphasize the importance of identifying and addressing ethical and legal issues before a technology is fully developed (Guston 2014; Owen et al., 2012). Thus, while most of these new MRI technologies are still in prototype and proof-of-concept stages, we convened a collaborative workshop with participants who have expertise in neuroimaging, neuroscience, field-based research in resource-limited settings, engineering, physics, AI and machine learning (ML), neurodevelopment, psychology, ethics, law and regulation, electroencephalography, pediatrics, radiology, and neurology (see Appendix, Table A1). The 25 workshop participants were based in three continents (North America, Europe, and Asia). Participants included 10 who had conducted neuroscience research in resource-limited settings, mostly outside the U.S. Although many disciplines were represented, future work should draw on additional fields such as sociology, philosophy, international development, and social work. Moreover, as stated above, the full development of ethical guidance should proceed in consultation with local stakeholders. A key goal of the workshop was to chart a path toward that deeper engagement.
After the workshop was held in April 2020, a survey was designed by co-authors Shen and Wolf to elicit from workshop participants their views on key ELSI issues posed by field-based MRI research in remote and low-resource international settings. The University of Minnesota IRB determined that this activity was not research involving human subjects as defined by DHHS and FDA regulations. The survey included closed-response questions assessing technological feasibility and open-ended questions inviting feedback on use-case scenarios and ELSI issues. There was a 78% response rate for the survey (N = 18 of 23, excluding the two co-authors who designed the survey). Responses were received from May 28 - June 9, 2020. The survey results were utilized as the basis for two follow-up online meetings of the Working Group. See Appendix for details of the survey instrument.
The recommendations presented in Section 3 were developed using dialogic consensus methods well accepted in bioethics (Moreno 2004). Those methods involved extensive background research and syntheses shared with the participants prior to the workshop. Those materials reviewed relevant scholarly literature in neuroethics, the ethics and regulation of international research, and ethics of research with marginalized and vulnerable populations. Based on that shared background material, we convened the workshop and two follow-up meetings, iteratively developing our analyses through dialogue and progressive development of consensus. The survey results reported below were shared with the group before the first follow-up meeting and served as the basis for discussion and for the article’s analysis. We circulated successive drafts of the article for feedback.
2.2. Timeline for predicted emergence of field-based research with portable MRI scanners
Field-based research with portable MRI is currently at a nascent stage. We thus asked workshop participants to assess the technological feasibility of acquiring either structural or functional brain MRI scans in various locations outside the lab now, within 5 years, within 10 years, within 25 years, or never. It should be noted that the FDA 510k-cleared Hyperfine Swoop™ device (Fig. 1b) has already been introduced to acquire structural MRI in clinical use and research studies in multiple U.S. hospitals (Sheth et al., 2020), and to scan COVID-19 patients in multiple hospitals (Kremer et al., 2020; Turpin et al., 2020). In addition, Hyperfine scanners will be used to acquire structural MRI scans in children for research on infant asphyxia and nutritional brain development in remote and resource-limited settings, in a partnership with the Bill and Melinda Gates Foundation (Gates Foundation 2020). However, other portable MRI technologies have not yet been deployed in the field.
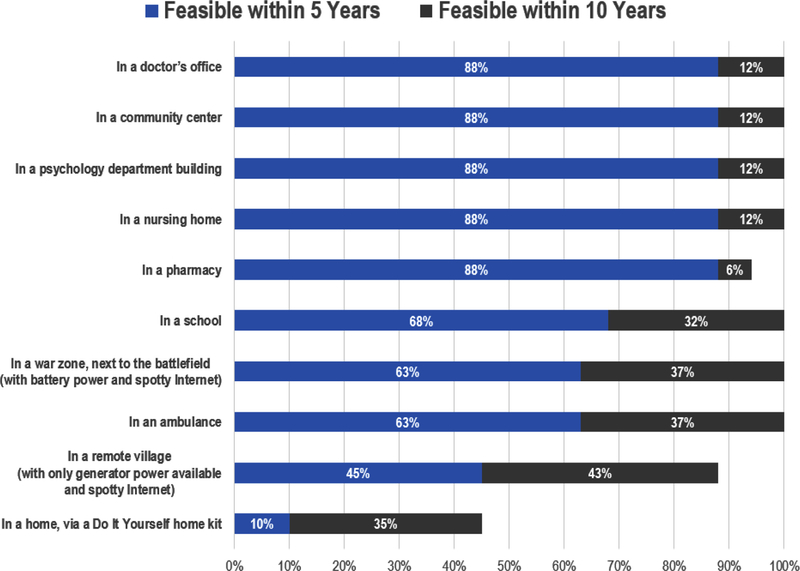
The workshop survey results (Fig. 2) suggest that portable MRI technology, at least to acquire structural images, could soon be available for use in locations such as doctors’ offices, community centers, psychology departments, nursing homes, and pharmacies. As noted above, the ability to acquire MRI scans in these field-based locations will re quire sacrificing image resolution. Thus, acquiring functional images from lower-field MRI scanners is likely to prove much more difficult than acquiring structural images, because of low signal-to-noise ratio and high field inhomogeneity for traditional gradient echo fMRI (see Buckenmaier et al., 2019 for technical discussion of fMRI with ultra-low field MRI). Finally, Fig. 2 shows that our Working Group saw a clear distinction between the feasibility of MRI acquisition inside stable institutional environments (such as nursing homes or pharmacies) versus more unpredictable or lower-resource environments (such as war zones, ambulances, or remote villages).
Fig. 2. Working Group assessment of technological feasibility and timeline for acquiring either structural or functional MRI data (N = 18).
This figure presents a summary of Working Group member responses to the survey question: “Please assess the earliest moment (if ever) that researchers will be able to acquire MRI data (structural or functional) in the following locations. Unless noted otherwise, assume that each location has (1) reliable access to power and (2) a stable internet connection.” For a discussion of the technical requirements of various portable MRI technologies in development, see Geethanath and Vaughan (2019); Sarracanie et al. (2015); Sarracanie and Salameh (2020); Wald et al. (2019).
2.3. Plausible use cases
Our workshop discussion and follow-up survey of workshop participants identified plausible use cases for field-based MRI research in remote and resource-limited international settings. Among the likely use cases are:
Studies of prevalence of brain disorders (such as stroke or hydrocephalus) and brain degeneration (such as that associated with Alzheimer’s disease) in communities in which such data are sparse or nonexistent.
Clinical research to examine how the introduction of low-cost MRI in remote communities could improve clinical care, for instance by implementing MRI screening for asphyxia and other perinatal complications in full-term and pre-term infants.
Research exploring the effects of nutritional, environmental, and psychosocial adversities including disease on brain development, for example by studying the effects of COVID-19 and other viruses on neural structure and function in individuals from marginalized communities.
While these are only some of the likely research use cases, they highlight the potential of field-based MRI research to advance knowledge on global brain health and disease. They also raise a series of challenging legal and ethical questions, to which we now turn.
3. Core ELSI issues in designing and conducting field-based MRI research in remote and resource-limited international settings
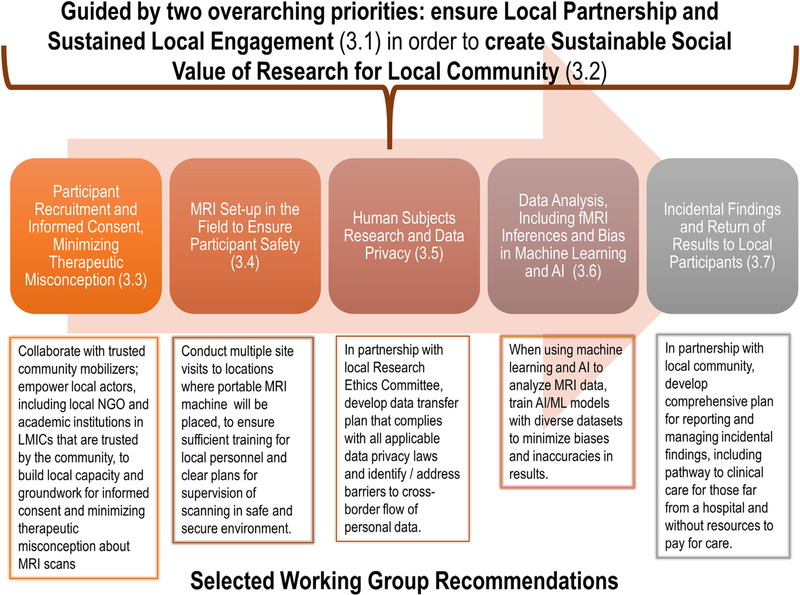
Leading ethical guidance and codes of conduct for international research in resource-poor settings emphasize the paramount importance of two, overarching principles: ensuring that local communities are partners in the research enterprise, and ensuring the local social value of the research (Schroeder et al., 2019; WHO and CIOMS 2016). As compared to general social value, defined as “important generalizable knowledge from the research,” local social value refers to the idea that “populations that host research also ought to benefit from the results of the research” (Barsdorf and Millum 2017). Guided by these two overarching principles, we discuss in this section five core ELSI issues that should be addressed in developing guidance for field-based MRI research studies in resource-limited contexts (see Fig. 3).
Fig. 3. Core ELSI issues, with key Working Group recommendations for addressing them, in the life cycle of field-based MRI research in remote and resource-limited communities.
Note: The ELSI issues presented in this Figure were identified through the Working Group process described in Part 2 and detailed in the Appendix.
3.1. Overarching priority #1 – local partnership and sustained local engagement
Multiple ethics guidance documents make clear that when research projects involve external research teams, the local community should be an equal partner in the research endeavor (Amadio et al., 2018; Brownsword et al., 2008, Guidance point 3; Global Code of Conduct for Research in Resource-Poor Settings (GCC) 2018, Articles 2, 4; Greely et al., 2018; WHO and CIOMS 2016, Guideline 8; WHO 2011).
Putting this principle into practice in the context of field-based MRI research requires opening a dialogue with local care providers, educators, and researchers as well as potential participants and their families. We interpret “local community” to refer to both the residents living and working in the community, as well as the local researchers; both should be engaged in sustained dialogue. Defining the “community” can be challenging; community could refer to individuals in neighborhoods, towns, cities, municipalities, states, or even the entire country. How “local” and “community” are defined will have implications for forging partnerships to facilitate co-creation of social value. But however “local community” is defined, consultation with key stakeholders and focus group discussions, prior to launching the research and continuing throughout, as well as “multi-level stakeholder engagement and multisectoral coordination,” provide avenues for meaningful engagement (Thondoo et al., 2020). Such engagement will also help the research team better define, prioritize, and operationalize the research questions.
Current training for MRI researchers is inadequate for field-based MRI studies because the training places little emphasis on relationship- and capacity-building with the local community. Such training is particularly important to address cultural differences and avoid bias. While each field-based MRI study will require engagement tailored to local context, some general lessons can be drawn from a growing body of research using EEG in resource-limited communities, especially on infants and children (Lockwood Estrin et al., 2019; Tarullo et al., 2017) and fNIRS (e.g., Begus et al., 2016; Blasi et al., 2019; Katus et al., 2019; Lloyd-Fox et al., 2016, 2019).
A lesson learned from this EEG field research is that an effective strategy for strengthening local partnerships is to build in adequate time for “formative work” such as community gatherings, question-and-answer sessions, and consultations with both community members and local research institutions prior to data acquisition (Lockwood Estrin et al., 2019). In EEG research conducted by co-authors Lockwood Estrin and Bhavnani in India, the recruitment strategy was informed by such formative work, and led to engaging the community to participate in research through “community mobilizers” who understand the research benefits and risks, before seeking individual informed consent. Pilot work also showed that logistics such as distance to the research site would serve as a barrier to participation, so the decision was made to move testing sites to be nearer the community (Lockwood Estrin et al., 2019).
Collaboration with community members can provide opportunities to build local capacity and ensure that benefits are bi-directional. Bidirectional learning refers to international researchers learning about local community concerns, and the community learning about the nature and purpose of the study (Harris et al., 2020; Skopec et al., 2019). Engagement can help to build understanding of the technology, facilitating informed decision-making about accepting or declining participation in the research.
A major challenge for community engagement with field-based MRI is the power of MRI brain imaging —both perceived and real (Jones et al., 2009). Participants may perceive MRI brain imaging as having exceptional power to reveal brain functioning and abnormalities, leading them to mistake research for clinical care in what is known as the “therapeutic misconception” (Appelbaum et al., 1982). Yet the power of MRI to reveal incidental findings (IFs) is also a challenge. Compared to other modalities such as EEG and fNIRS, MRI imposes on researchers greater responsibilities to manage IFs because EEG and fNIRS do not produce images of brain structure. We address below the responsibilities incumbent on researchers to manage IFs.
3.2. Overarching priority #2 – sustainable social value of research for local community
Prominent guidance for research in remote and resource-limited international contexts emphasizes that the research study should produce both general scientific value and local social value, as noted above (GCC 2018, Article 1; WMA 2001, Article 20; WHO and CIOMS 2016, Neuroethics Questions for Neuroscientists [NeQN] 5b). The social value of research can be generally defined as “knowledge that can lead to improvements in health” (Emanuel et al., 2000). While all researchers must justify their studies by showing that the social benefit outweighs the risks (Nuremberg Code 1947; Office for Human Research Protections [OHRP] 2017), research in remote and resource-limited settings also requires significant local social value (Lairumbi et al., 2011; Wenner 2017, 2018; Wertheimer 2015). This is in contrast to customary fMRI studies outside of resource-limited settings (for example, studies enrolling college students), where participants are explicitly told that there is no personal benefit (beyond compensation) for participation. Conducting research in a resource-limited setting requires a different, more beneficial, and longer lasting relationship with the local community and participants in the study.
To produce local social value, research priorities can be determined in collaboration with the local community (GCC 2018, Article 1), recognizing that the priorities of a low-resource community may differ from those in the researcher’s home community or country (Barsdorf and Millum 2017; London 2008). As discussed above, “local” can be understood at multiple levels such as community/town, district/state, and nation. Engagement at different levels will vary across research studies, but a constant should be pursuing co-creation of social value “which matches innovative tools to the needs of the population” (DePasse and Celi 2016). An example of a model that embraces co-creation of social value with new technology is the Consortium for Affordable Medical Technology (CAMTech) Co-Creation laboratories. As applied to portable MRI, this approach would suggest that rather than investigators developing hypotheses and research question by themselves prior to community engagement, the community —local researchers and residents —should be directly involved in developing the research plan. These “collaborations will require deliberate and thoughtful effort that may run counter to the intensely individualistic entrepreneurial spirit of many innovators who wish to lead global health improvement” (DePasse and Celi 2016).
Field-based MRI research may contribute to local social value by: focusing on research questions and health conditions of high priority to the community, using portable MRI as a teaching tool (Wald et al., 2019) for local scientists, establishing partnerships with major hospital systems to improve training of clinicians, and capacity building (WHO and CIOMS 2016, Guidelines 6, 8) such as contributing to a center for excellence (Franzen et al., 2017) or allowing local clinicians to utilize the portable MRI machine when it is not being used for the research study. These types of investment in the community’s future health infrastructure represent one means of elevating the local social value of the research.
3.3. Participant recruitment and informed consent, minimizing therapeutic misconception
As MRI research moves out of the lab and into the community, lessons from “community-based participatory research” (CBPR) and “integrated knowledge translation” (IKT) may prove useful. CBPR and IKT “are research approaches that emphasize the importance of creating partnerships between researchers and the people for whom the research is ultimately meant to be of use (‘knowledge users’)” (Jull et al., 2017). From this perspective, and consistent with the international research ethics standards referred to earlier, participant recruitment and informed consent procedures should be co-developed in consultation with the local community (GCC 2018, Articles 4, 12; WHO and CIOMS 2016, Guideline 8). Research on informed consent in international contexts suggests the importance of sensitivity to cultural and linguistic differences, as well as socioeconomic context in resource-limited communities (Colom and Rohloff 2018). Community assent may also be required (GCC 2018, Article 9). These ethical concerns speak to the need for the field of neuroethics to consider how “social relationships will be altered by continuing neuroscientific advances” (Chiong 2020).
In field-based research with portable MRI, explaining MRI data acquisition and analysis to prospective participants and local research partners will be important. Studies may become more difficult to explain if new technologies are combined or “stacked” on one another (Wolf et al., 2003). For example, if cloud storage of data and AI-enabled data analysis are involved, explaining the full set of technologies becomes more challenging. With technology this complex, having a translator who understands the technology enough to explain it in their own words and can communicate this information in multiple local languages (which may not themselves have specific words to describe the technology) may be especially helpful. An additional issue emerging during study recruitment is the possibility of coercion, undue influence, and exploitation due to incentives offered for participation (Largent and Fernandez 2017; Nyangulu et al., 2019). It is also possible that “structural coercion” can emerge when “the broader social, economic and political context compels individuals to enroll in research” (Fisher 2013). To address these issues, sociocultural context should be carefully considered, consultation with the local Research Ethics Committees (REC) (see Section 3.5 below) can help to clarify renumeration policies, and in some instances offering incentives to participate may be ill-advised (Gordon et al., 2018).
Also of concern is the therapeutic misconception (TM) (Appelbaum et al., 1982), especially in settings where access to health care is limited and participants have not previously had access to MRI scanning and other health interventions associated with the study. TM exists when participation in research is confused with medical care (Lema 2009; Lidz et al., 2004). Previous research suggests that there is a risk that MRI research will be perceived as, or used in place of, medical care (Hadskis et al., 2008). This issue is more likely to arise in settings that lack clinical MRI services. TM may also be more likely for patients who live far from a hospital or medical clinic with an MRI scanner.
As an example illustrating the risk of TM, it is possible that in field-based research with children using portable brain imaging, parents may enroll their children in hopes that this will lead to medical benefits for their child. There is “no single or simple answer” to improving informed consent and participant knowledge about the research process (Pickersgill 2011). But to begin addressing potential misconceptions about the goals of the study, researchers can offer increased opportunities for parents to ask questions, can explain what is meant by “research” in the study, and can communicate that the study will not confer medical benefits or substitute for health care.
3.4. MRI setup in the field to ensure participant safety
Given that portable MRI machines may not require the same level of physical distancing as fixed MRI machines (see Section 1.3), third-parties may be closer to portable MRI scanning. Field-based MRI studies must therefore be responsible for participant safety in the scanner and the safety of others who may be observing nearby. This could include a parent holding a child’s hand, a friend looking on, or a curious onlooker who enters the scanner area.
While the Gauss lines around portable MRI technologies will vary considerably depending on the design and magnet strength of each scanner, there will remain at least some concerns about metal shrapnel or implants within the participant’s body, as well as metal objects carried into the room. Traditional MRI screening questionnaires and procedures that are in resource-rich environments will need to be adapted to ensure that the requisite safety questions are asked and understood and that participants can answer safety questions accurately. Fortunately, it is likely that portable MRI scanners will have a much lower risk profile due to significantly reduced field strength.
To address safety concerns, supervision of local personnel as well as quality control across multiple scanning field sites are a priority. Moreover, researchers must ensure that the equipment can be securely stored when not in use, securely transported from site to site, and set up/taken down in ways that are minimally disruptive to the community.
3.5. Human subjects research and data privacy
The collection of brain data through MRI in the field may trigger the application of a variety of regulations governing research with human participants and data privacy. For instance, the research will be subject to the Federal Policy for the Protection of Human Subjects (the “Common Rule”) if the research is conducted or supported by a U.S. federal department or agency that has adopted the Common Rule, such as the U.S. Department of Health and Human Services (45 C.F.R. § 46.101(a)). For research conducted abroad, the Common Rule does not displace local law but rather provides that its requirements will apply to the research in addition to the requirements of applicable local law (45 C.F.R. § 46.101(g)). Because portable neuroimaging technology is regulated by the FDA, FDA-specific regulations protecting human subjects may also apply (21 C.F.R. part 50). These regulations are similar, but not identical to the Common Rule. For those researchers who wish to submit internationally-collected data as part of an investigational device exemption (IDE) application, a premarket approval submission, or another type of research or marketing permit submission to FDA, the FDA regulations require that the data have been collected in accordance with Good Clinical Practice (21 C.F.R. § 812.28), which includes review and approval by a research ethics committee (REC) and informed consent from participants (FDA 2018, 83 FR 7366).
International research ethics guidance emphasizes that local ethical review, by a REC and relevant agencies, is vital to field-based research (GCC 2018, Articles 10, 11; WHO 2011; WMA 2001, Article 23). Understanding and complying with the applicable laws of the country within which the research is taking place will likely require both consultation with country-level officials and partnership with local researchers and their institutions (GCC 2018). If a remote community does not have a local REC, the research team will need to understand the local regulatory framework to determine how best to obtain REC review and oversight of the research.
Similarly, although participants in field-based neuroimaging research may be thousands of miles away from the researcher’s home institution, if any part of the research is based in the U.S. and involves a “covered entity” subject to the Health Insurance Portability and Accountability Act of 1996 (HIPAA), privacy and security regulations issued under HIPAA will apply to the use and disclosure of participants’ protected health information (PHI) during the portion of the research conducted at the “covered entity.” Determining the applicability of HIPAA will require understanding how data are flowing from field to researcher.
Data privacy laws of the host country are also likely to apply. These laws will vary by country and region, but some foreign privacy laws, such as the European Union’s General Data Protection Regulation (GDPR), place limitations on the cross-border flow of personal data, an important barrier for conducting field-based neuroimaging research (GDPR 2019, Chapter V). Many data privacy laws, such as the GDPR, require that vendors that process data, referred to as “processors,” be subject to stringent limitations (GDPR 2019, Article 28). Accordingly, if portable MRI technology utilizes cloud-based vendors to process participant data, the third-party will likely need to be bound by terms that apply specifically to processors of data. Relatedly, field-based neuroimaging may occur in locations where an Internet connection is not reliable, requiring a backup plan for secure data storage and delivery.
Finally, a legally relevant distinction can be made between where the data are collected, where they are stored, and who is accessing the data. If the data will be accessed by individuals in a country different from the one in which the data were collected and where the data are stored, then depending on the data privacy regulations in place, this may be considered a cross-border transfer of data, and restrictions on the cross-border transfer of data may apply (Information Commissioner’s Office 2019).
3.6. Data analysis, including fMRI inferences and bias in machine learning and AI
As MRI research moves into the field, it brings with it the still-unresolved issues of traditional fixed MRI. Analyses of structural data are generally simpler and more straightforward, but if functional data are acquired, it is important to recognize that the fMRI community has long struggled with developing reproducible and valid inferences from such data (Carp 2012; Poldrack et al., 2017). Multiple labs analyzing the same fMRI data may develop substantially different interpretations (Botvinik-Nezer et al., 2020). Reasons for this variation across labs include the relatively low signal-to-noise ratio of fMRI data, different assumptions in statistical models used to analyze the data, and the general challenge of understanding how the brain carries out cognitive functions (Poldrack 2018). Furthermore, while AI-based analytic procedures might have the appeal of providing usable results without time-consuming examination of the data, these should be approached with caution at this early stage of development (Langlotz et al., 2019). The use of AI in studies in remote and resource-limited communities also raises concerns about whether the sample data on which the AI model was trained were sufficiently diverse to allow the AI model to make accurate out-of-sample predictions on brain data acquired in culturally, economically, and environmentally diverse populations (Schiff 2021). Researchers can find guidance on the use of AI and big data analytics in low-resource settings in expert reports published by the U.S. Agency for International Development (USAID) (2019), the World Health Organization (2019), and the UN Secretary-General (2014). As the USAID report observes, “to be accurate in new geographies, AI tools need millions of historical health data-points to train their algorithms to provide accurate outputs appropriate to the geography and population —and this type of broader health data are generally absent in LMICs” (USAID 2019, p. 18). This problem counsels conducting an “AI audit” to assess potential bias in the system (Zou and Schiebinger 2018).
3.7. Incidental findings and return of results to local participants
Estimates of incidental findings (IF) rates in structural brain MRI vary by study population and scan acquisition method, but have been described by one meta-analysis as common (Morris et al., 2009). Another study estimated the overall rate at 34% (Shoemaker et al., 2011), with the likelihood of IFs increasing with participant age (Morris et al., 2009). A recent study, analyzing an Adolescent Brain Cognitive Development (ABCD) dataset of nearly 12,000 children ages 9 to 10 years, found an IF rate of 21% (Li et al., 2021). Analyses of IFs in MRI research (Illes 2006) have revealed that recommended policies range from having every scan read by a radiologist (Milstein 2008), to having findings reviewed by an expert only if the researcher flags a brain abnormality (Cramer et al., 2011), to having no scans read by a radiologist (Royal and Peterson 2008).
Portable MRI research in remote and under-resourced field settings will exacerbate the issues raised by IFs. Radiological expertise to confirm the presence of concerning findings may be unavailable or available only remotely. Referral to clinicians for clinical analysis may be equally challenging when research is done in a remote setting far from a major health care center and participants may lack health insurance and reliable access to health care.
The Common Rule in the United States (45 C.F.R. part 46), as well as the Additional Protocol to the Convention on Human Rights and Biomedicine Concerning Biomedical Research in Europe (Council of Europe Treaty Series No. 195, 2007), call for research participants to be informed of how the research team plans to address IFs in the study. There is a range of approaches to IFs in MRI research (Borget et al., 2013; Underwood 2012), as well as a literature on the legal and ethical requirements for identifying and returning IFs in brain imaging research (Brown and Hasso 2008; Illes et al., 2006; King 2018; Wolf et al., 2008).
In the context of portable MRI, a significant challenge will be identifying local site-specific leadership to communicate IFs to research participants. Such communication is a key aspect of IF policy in fixed-scanner research projects. For example, the Adolescent Brain Cognitive Development (ABCD) study, a national Consortium study in the United States that involves 21 data collection sites and a sample of nearly 12,000 youth, combines local leadership with a centralized radiology team (Auchter et al., 2018). In the ABCD study, MRI data are sent by each site to a radiology team at the University of California, San Diego (UCSD) (the Coordinating Center for the Consortium), and the radiology team members rate the scans on a four-point scale (1 = no anomalies; 4 = urgent clinical care needed) (Clark et al., 2018). Then those numbers and a description of the findings are relayed to the local-site PI, who must have a procedure in place for notifying the participants if the readings are 3s or 4s (Clark et al., 2018). In field-based MRI research in remote and resource-limited settings, a radiology team to analyze scans at a central location could be established, even if this central location is far from local scanning sites. Teleradiology has, for instance, proven effective in a variety of settings (Hanna et al., 2020). However, that centralized team would need established procedures (including technology to communicate with local participants or a local team member) to facilitate the communication of IFs, offer counseling if needed, and recommend follow-up with a clinical MRI scan, and address barriers to obtaining a clinical scan, consistent with the overarching goal to ensure local partnership (see Section 3.1).
Identification of IFs raises the specter of stigmatizing participants by suggesting that something may be wrong with their brain. While alerting participants to potential findings of health importance may advance their well-being and on occasion even save lives, researchers must consider how to offer the information without causing harm. Multiple ethics guidance documents remind researchers to consider the issue of social stigma and self-stigma (Amadio et al., 2018, Question 1a; GCC 2018, Article 16). Self-stigma is the internalization of stigma experience resulting in “diminished self-esteem and self-efficacy” (Corrigan et al., 2006). Similar to concerns raised about stigma associated with genetic diagnoses (Academy of Science of South Africa 2018) and mental illness in LMICs (Mascayano et al., 2015), brain data suggesting a mental illness or neurodegeneration might be stigmatizing for research participants. For instance, a recent meta-analysis of seven studies from 1985 to 2018 on patients younger than 21 found a 16% IFs rate in MRI research with children, including many instances of cysts (Dangouloff-Ros et al., 2019). Telling parents that there is a cyst —even one that may be benign —on their child’s brain may be seen as marking that child as less healthy or desirable. Similarly, breast and cervical cancer screening in some LMICs has led to the discovery that detection of cancer —rather than producing a favorable response focused on treatment —can lead to social stigma (Suwankhong and Liamputtong 2016; Nyblade et al., 2017). There is also evidence that in some LMIC contexts children diagnosed with a neurocognitive disability can face parental neglect and even sometimes abandonment (Paget et al., 2016; Namazzi et al. 2020). Guidance on returning IFs recognizes that “cultural norms surrounding certain diseases may be different in low-resource settings” (Sullivan and Berkman 2018) and thus the plan for managing IFs needs to address social and cultural stigma that might emerge if abnormalities are detected and communicated.
For field-based MRI research in remote settings, a challenging problem is how to provide support and clinical referral when an IF is discovered. In consultation with the local community, the study protocol should specify what sort of support and referral services will be available to the participants so that they can obtain follow-up clinical care as warranted. Such follow-up care may be difficult to obtain if local medical facilities are far away or not adequate. But a “hands off” approach, such as simply communicating the IF without clinical referral, is not ethically acceptable (CIOMS 2018, Guideline 6; Dickert and Wendler 2009).
A related question arises about the provision of ancillary care (Bright and Nelson 2012; Pratt et al., 2013). Ancillary care is “health care that is not required for either the scientific validity of a study or redressing study-related harms” (Pratt et al., 2013). One view, emerging from the Georgetown University Workshop on the Ancillary-Care Obligations of Medical Researchers Working in Developing Countries, is that researchers in resource-limited settings have a “positive moral obligation to provide some ancillary care to their study participants” (Brownsword et al., 2008). MRI research teams can work with local partners to reach agreement on what such an obligation should look like in a particular research setting.
There is little guidance for returning to participants either individual or aggregate study results on the variables under study in field-based MRI research (Shen et al., 2020). Even in research with fixed MRI scanners, there is variation in current practice. Many MRI researchers do not routinely return individual-specific study results to their participants, but at least one study found that neuroimaging research participants overwhelmingly desire to receive all of their research brain scans, along with the accompanying report (Shoemaker et al., 2016). In anticipation of likely demand, researchers can work with the community to plan how to make individual-level and aggregate results readily available in a form that is understandable, with the necessary explanation and counseling.
4. Recommended next steps
The ELSI issues identified above call for new work to establish adequate guidance and processes. While much work has been done over the last 15 years to develop guidance for fixed MRI in urban hospitals and similar institutional settings, our Working Group concluded that the emergence of highly portable MRI for deployment in remote and low-resource international field settings requires additional guidance. In 2005, the NIMH Council Workgroup on MRI Research Practices addressed the evolution of MRI from “a tool used primarily for medical diagnosis” to a tool for “clinical and basic cognitive and affective neuroscience research” (NIMH 2005). The NIMH Workgroup recognized that there was a “lack … of any comprehensive guidance” for researchers in “non-medical settings” NIMH (2005). But in 2005 the idea of field-based neuroimaging around the globe was not mentioned. Fifteen years later, technological developments once again compel a revision to existing ethical guidance.
In the context of field-based MRI research in remote and resource-limited contexts, we return to two overarching goals: (1) ensuring that local communities are ongoing partners in the research enterprise, and (2) ensuring that the research is designed to produce sufficient, local social value to justify the risks of the research. As suggested above (Fig. 3), achieving these goals must involve collaboration with the local community in which the research will occur, empowering local actors, investing in site visits and training of local personnel, partnering with local Research Ethics Committees, minimizing bias in AI and other data analytic models, and collaboratively developing comprehensive plans for reporting and managing incidental findings, including a pathway to clinical care for those far from a hospital and without resources to pay for care. To facilitate this bi-directional learning, we recommend several concrete next steps. See Table 1.
Table 1.
Recommended next steps
| 1. Establish more robust platforms for engagement between community representatives, local researchers and trainees, neuroimaging research teams, and local NGOs that work within communities that might be included in the research. |
| 2. Work with local communities to update current guidance for MRI to address field-based MRI research with portable scanners. |
| 3. Consult with experts in international research ethics and international standard-setting bodies such as the World Health Organization (WHO), World Medical Association (WMA), and United Nations Educational, Scientific and Cultural Organization (UNESCO) to determine how guidelines such as the CIOMS International Ethical Guidelines for Biomedical Research Involving Human Subjects (2016), Declaration of Helsinki (2013), and the Global Code of Conduct for Research in Resource-Poor Settings (GCC) (2019) apply to field-based MRI research in international contexts. |
| 4. Ensure that ELSI guidance for portable MRI is developed in collaboration with experts using other portable imaging modalities such as mobile PET, mobile MEG, fNIRS, and HD-DOT. |
| 5. Analyze and compare ELSI issues associated with clinical use of portable MRI in remote and limited-resource settings. |
First, more robust platforms are needed for engagement between community representatives, local researchers and trainees, neuroimaging research teams, and local NGOs who work within communities that might be included in the research. One model for such engagement is the U.S. NIH Common Fund’s Harnessing Data Science for Health Discovery and Innovation in Africa (DS-I Africa) (NIH 2020), which includes virtual (and eventually in-person) symposia for cross-national networking between research groups and communities that would not otherwise engage one another. The networking allows for community representatives to voice their own priorities. Integrating neuroimaging-specific networking into these and similar events would allow for the engagement required to build strong relationships between local communities and external research teams. Similarly, an international workshop hosted in an LMIC, with representation from resource-limited communities, would be of great value. Such a workshop could facilitate engagement both across and within countries. Even in resource-rich countries, it is important to design highly portable MRI research so that it can reach populations previously excluded from research and begin to address inequity (Cooley et al., arXiv). While our focus in this article has primarily been on communities outside of resource-rich countries, the analysis could also be adapted and applied to low-income and marginalized communities within richer countries (Meyers and Hunt 2014).
Second, current guidance for MRI should be updated to address field-based MRI research with portable scanners. This effort should involve community leaders, government agencies, professional societies, industry partners, NGOs, academics, ethicists, legal experts, and the international MRI research community. As discussed above, guidance exists but is not yet sufficiently specific about application to portable MRI technology. A clear example of a standard in need of revision is the ACR’s demarcation of four safety zones for setting up an MRI facility. These safety zones make assumptions about magnet strength and Gauss lines that are not applicable in the case of newer MRI technology. Because it was not previously possible to set up an MRI scanner outside a research facility, there is no guidance as yet from standard-setting organizations on how to do it. To develop standards for use in remote and limited-resource settings, the neuroimaging community might consider the successful model created by the World Federation of Pediatric Imaging and the InterSociety working group on MR safety to help standardize MR practice across regions. These groups have established best practices for careful, inclusive development of rigorous standards (Calamante et al., 2016; Otero et al., 2020).
Third, consultation with experts in international research ethics and international standard-setting bodies such as the World Health Organization (WHO), World Medical Association (WMA), and United Nations Educational, Scientific and Cultural Organization (UNESCO) is required to determine how guidelines such as the CIOMS International Ethical Guidelines for Biomedical Research Involving Human Subjects (2012), Declaration of Helsinki (2013), and Global Code of Conduct for Research in Resource-Poor Settings (GCC) (2019) apply to field-based MRI research in international contexts. In this article, we begin to address these codes and the accompanying literature, but further discussion —with more diverse representation of interests and organizations —is required.
Fourth, further dialogue is required with experts using other portable imaging modalities. We have focused here on mobile MRI, but future research should also consider advances in mobile positron emission tomography (PET) (Bauer et al., 2016), mobile magnetoencephalography (MEG) (Bosso 2020; Boto et al., 2018, 2019), functional near-infrared spectroscopy (fNIRS) (Baker et al., 2017a,b; Blasi et al., 2019), and high-density diffuse optical tomography (DOT) (Fishell et al., 2020). There is a growing body of LMIC research, especially on infants and children, utilizing EEG (Lockwood Estrin et al., 2019; Tarullo et al., 2017) and fNIRS (e.g., Begus et al., 2016; Blasi et al., 2019; Katus et al., 2019; Lloyd-Fox et al., 2019, 2016; Wijeakumar et al., 2019). MRI is not being used because it is costly and generally not yet feasible in the field (Gossé 2018). MRI can be a complementary tool to EEG and fNIRS, and researchers in traditional MRI labs are already experimenting with simultaneous EEG/fMRI recording in order to combine the better spatial resolution of MRI with the better temporal resolution of EEG (Mele et al., 2019). The potential use of multiple modalities, either in tandem or simultaneously, in field-based research may raise additional ELSI issues not explored here.
Fifth, while we have focused exclusively on research use of MRI, further work is needed to examine the ELSI issues associated with clinical use of portable MRI in remote and limited-resource settings. Global access to MRI for clinical purposes is addressed elsewhere (Inalegwu et al., 2018; Mollura and Lungren 2019), but there are overlapping ELSI issues (including management of incidental findings) that would benefit from joint analysis by clinicians and researchers.
5. Conclusion
The advent of portable MRI scanners offers the potential for communities in remote, resource-limited settings to partner with neuroimagers on research that addresses health inequities, builds local capacity for better brain health, improves understanding of brain development and degeneration in diverse populations, and ultimately improves clinical care in the local community. A necessary step to achieve this potential is an interdisciplinary, global effort to address the ethical and legal issues identified in this article.
In addressing familiar ELSI issues, as well as those novel issues raised by MRI scanning in remote locations, a key to success is early, frequent, and meaningful engagement with the local community, as well as with the relevant IRB and Research Ethics Committees, regulatory agencies, and funding agencies. Compared to a study carried out in the familiar confines of a university research facility, field-based work in remote locations will require significantly more engagement with the local community before, during, and after MRI data acquisition.
Investing time to address ELSI issues can unlock the tremendous possibilities of field-based MRI research. Both historical and contemporary experiences of marginalized and low-resourced communities point to a real concern that the benefits of this technology will not flow primarily to the communities in which the resource is conducted. To address this concern, and to ensure that the benefits to be gained from research with field-based MRI flow to communities that have not previously participated in neuroimaging research, collaboration between researchers and participating communities must be strengthened.
Supplementary Material
Acknowledgments
Thank you to all participants in the project’s virtual workshop on The Ethical, Legal, and Social Implications of Rapidly Expanding Global Access to MRI, held on April 6, 2020. For research assistance, we thank Warren Cormack, Jacob Hauschild, and Caroline Sell. For administrative and logistics support, we thank Audrey Boyle and the University of Minnesota’s Consortium on Law and Values in Health, Environment & the Life Sciences.
Funding
Research reported in this publication was supported by a Bioethics Supplement from the National Institute of Biomedical Imaging and Bioengineering (NIBIB) of the National Institutes of Health (NIH) under Award Number U01EB025153–03S1. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIBIB or NIH. GLE is funded by a Sir Henry Wellcome Post-doc fellowship (Wellcome Trust grant number: 204706/Z/16/Z).
Declaration of Competing Interest
FXS and SMW disclose NIH/NIMH grant RF1MH123698 on “Highly Portable and Cloud-Enabled Neuroimaging Research: Confronting Ethics in Field Research with New Populations” (Shen, Wolf, Lawrenz, PIs). SD receives grant support from Nestlé Nutrition. DF is a patent holder on the Framewise Integrated Real-Time Motion Monitoring (FIRMM) software. He is also a co-founder of Nous Imaging Inc. The nature of this financial interest and the design of the study have been reviewed by two committees at the University of Minnesota. They have put in place a plan to help ensure that this research study is not affected by the financial interest. KR discloses that she is funded by The Kavli Foundation. KS is an employee of Hyperfine Research, Inc.
Footnotes
Human subjects review
The University of Minnesota Institutional Review Board determined that the workshop and survey research activity described in this article were not research involving human subjects as defined by DHHS and FDA regulations (STUDY00009902, May 15, 2020).
Disclaimer
The authors are responsible for the views expressed in this article. Those views do not necessarily represent the views, decisions, or policies of the institutions with which they are affiliated. Institutions are listed for author identification only.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi: 10.1016/j.neuroimage.2021.118210.
References
- Academy of Science of South Africa (ASSAf), 2018. Human genetics and genomics in South Africa: Ethical, legal and social implications DOI: 10.17159/assaf.2018/0033. [DOI]
- Amadio J, Bi GQ, Boshears PF, Carter A, Devor A, et al. , 2018. Neuroethics questions to guide ethical research in the international brain initiatives. Neuron 100, 19–36. doi: 10.1016/j.neuron.2018.09.021. [DOI] [PubMed] [Google Scholar]
- Appelbaum PS, Roth LH, Lidz C, 1982. The therapeutic misconception: Informed consent in psychiatric research. Int. J. Law Psychiatry 5, 319–329. doi: 10.1016/0160-2527(82)90026-7. [DOI] [PubMed] [Google Scholar]
- Auchter AM, Hernandez Mejia M, Heyser CJ, Shilling PD, Jernigan TL, et al. , 2018. A description of the ABCD organizational structure and communication framework. Dev. Cogn. Neurosci. 32, 8–15. doi: 10.1016/j.dcn.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DA, Ware JM, Schweitzer NJ, Risko EF, 2017a. Making sense of research on the neuroimage bias. Public Underst. Sci. 26, 251–258. doi: 10.1177/0963662515604975. [DOI] [PubMed] [Google Scholar]
- Baker JM, Rojas-Valverde D, Gutiérrez R, Winkler M, Fuhrimann S, et al. , 2017b. Portable functional neuroimaging as an environmental epidemiology tool: A how-to guide for the use of fNIRS in field studies. Environ. Health Persp. 125. doi: 10.1289/EHP2049,094502-1-094502-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsdorf N, Millum J, 2017. The social value of health research and the worst off. Bioethics 31 (2), 105–115. doi: 10.1111/bioe.12320. [DOI] [PubMed] [Google Scholar]
- Bauer CE, Brefczynski-Lewis J, Marano G, Mandich MB, Stolin A, et al. , 2016. Concept of an upright wearable positron emission tomography imager in humans. Brain Behav. 6, e00530. doi: 10.1002/brb3.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begus K, Lloyd-Fox S, Halliday D, Papademetriou M, Darboe MK, et al. , 2016. Using fNIRS to study working memory of infants in rural Africa. In: Elwell CE, Leung TS, Harrison DK (Eds.), Oxygen Transport to Tissue XXXVII. Springer, New York, pp. 273–279. [DOI] [PubMed] [Google Scholar]
- Blasi A, Lloyd-Fox S, Katus L, Elwell CE, 2019. fNIRS for tracking brain development in the context of global health projects. Phonetics 6, Article 89. DOI: 10.3390/photonics6030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgelt E, Anderson JA, Illes J, 2013. Managing incidental findings: Lessons from neuroimaging. Am. J. Bioeth. 13 (2), 46–47. doi: 10.1080/15265161.2012.754069. [DOI] [PubMed] [Google Scholar]
- Boto E, Holmes N, Leggett J, Roberts G, Shah V, et al. , 2018. Moving magnetoencephalography towards real-world applications with a wearable system. Nature 555, 657–661. doi: 10.1038/nature26147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boto E, Seedat ZA, Holmes N, Leggett J, Hill RM, et al. , 2019. Wearable neuroimaging: Combining and contrasting magnetoencephalography and electroencephalography. NeuroImage 201, 116099. doi: 10.1016/j.neuroimage.2019.116099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinik-Nezer R, Holzmeister F, Camerer CF, Dreber A, Huber J, et al. , 2020. Variability in the analysis of a single neuroimaging dataset by many teams. Nature 582, 84–88. doi: 10.1038/s41586-020-2314-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright PL, Nelson RM, 2012. A capacity-based approach for addressing ancillary care needs: Implications for research in resource limited settings. J. Med. Ethics 38, 672–676. doi: 10.1136/medethics-2011-100205. [DOI] [PubMed] [Google Scholar]
- Brown DA, Hasso AN, 2008. Toward a uniform policy for handling incidental findings in neuroimaging research. AJNR Am. J. Neuroradiol. 29, 1425–1427. doi: 10.3174/ajnr.A1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownsword R, Cermak A, Chaisson R, Clayman MD, Corr PB, et al. , 2008. The ancillary-care obligations of medical researchers working in developing countries. PLoS Med. 5, 709–713. doi: 10.1371/journal.pmed.0050090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckenmaier K, Pedersen A, SanGiorgio P, Scheffler K, Clarke J, et al. , 2019. Feasibility of functional MRI at ultralow magnetic field via changes in cerebral blood volume. NeuroImage 186, 185–191. doi: 10.1016/j.neuroimage.2018.10.071. [DOI] [PubMed] [Google Scholar]
- Calamante F, Ittermann B, Kanal E, Inter-Society Working Group on MR Safety, Norris D, 2016. Recommended responsibilities for management of MR safety. J Magn. Reson. Imaging 44, 1067–1069. DOI: 10.1002/jmri.25282. [DOI] [PubMed] [Google Scholar]
- Carp J, 2012. On the plurality of (methodological) worlds: Estimating the analytic flexibility of fMRI experiments. Front. Neurosci. 6, 1–13. doi: 10.3389/fnins.2012.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, et al. , 2018. The Adolescent Brain Cognitive Development (ABCD) study: Imaging acquisition across 21 sites. Dev. Cogn. Neurosci. 32, 43–54. doi: 10.1016/j.dcn.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Council for International Organizations of Medical Sciences (CIOMS), 2016. International Ethical Guidelines for Health-related Research Involving Humans: Fourth Edition Available at: https://cioms.ch/wp-content/uploads/2017/01/WEB-CIOMS-EthicalGuidelines.pdf.
- Cirillo A, Diniz E, Gadelha A, Asevedo E, Axelrud LK, et al. , 2020. Population neuroscience: Challenges and opportunities for psychiatric research in low- and middle-income countries. Braz. J. Psychiatry 42, 442–448. doi: 10.1590/1516-4446-2019-0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DB, Fisher CB, Brookheimer S, Brown SA, Evans JH, et al. , 2018. Biomedical ethics and clinical oversight in multisite observational neuroimaging studies with children and adolescents: The ABCD experience. Dev. Cogn. Neurosci. 32, 143–154. doi: 10.1016/j.dcn.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colom M, Rohloff P, 2018. Cultural considerations for informed consent in paediatric research in low/middle-income countries: A scoping review. BMJ Paediatr. Open 2, e000298. doi: 10.1136/bmjpo-2018-000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooley CZ, Haskell MW, Cauley SF, Sappo C, Lapierre CD, et al. , 2018. Design of sparse Halbach magnet arrays for portable MRI using a genetic algorithm. IEEE Trans. Magn. 54, 5100112. doi: 10.1109/TMAG.2017.2751001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooley CZ, Stockmann JP, Armstrong BD, Sarracanie M, Lev MH, et al. , 2015. Two-dimensional imaging in a lightweight portable MRI scanner without gradient coils. Magn. Reson. Med. 73, 872–883. doi: 10.1002/mrm.25147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooley CZ, Stockmann JP, Witzel T, LaPierre C, Mareyam A, et al. , 2020. Design and implementation of a low-cost, tabletop MRI scanner for education and research prototyping. J. Magn. Reson. 310, 106625. doi: 10.1016/j.jmr.2019.106625. [DOI] [PubMed] [Google Scholar]
- Corrigan PW, Watson AC, Barr L, 2006. The self–stigma of mental illness: Implications for self–esteem and self–efficacy. J. Soc. Clin. Psychol. 25, 875–884. doi: 10.1521/jscp.2006.25.8.875. [DOI] [Google Scholar]
- Cramer SC, Wu J, Hanson JA, Nouri S, Karnani D, et al. , 2011. A system for addressing incidental findings in neuroimaging research. NeuroImage 55, 1020–1023. doi: 10.1016/j.neuroimage.2010.11.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangouloff-Ros V, Roux CJ, Boulouis G, Levy N, Nicolas C, et al. , 2019. Incidental brain MRI findings in children: A systematic review and meta-analysis. AJNR Am. J. Neuroradiol. 40, 1818–1823. doi: 10.3174/ajnr.A6281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta J, Bhavnani S, Estrin GL, Mukherjee D, Banerjee A, et al. , 2016. Translating neuroscience to the front lines: Point-of-care detection of neuropsychiatric disorders. Lancet Psychiatry 3, 915–917. doi: 10.1016/S2215-0366(16)30186-9. [DOI] [PubMed] [Google Scholar]
- DePasse J, Celi LA, 2016. Collaboration, capacity building and co-creation as a new mantra in global health. Int. J. Qual. Health Care 28, 536–537. doi: 10.1093/intqhc/mzt077. [DOI] [PubMed] [Google Scholar]
- Dickert N, Wendler D, 2009. Ancillary care obligations of medical researchers. JAMA 302, 424–428. doi: 10.1001/jama.2009.1076. [DOI] [PubMed] [Google Scholar]
- Emanuel EJ, Wendler D, Grady C, 2000. What makes clinical research ethical? JAMA 283, 2701–2711. doi: 10.1016/S0002-9394(00)00691-7. [DOI] [PubMed] [Google Scholar]
- Expert Panel on MR Safety, Kanal E, Barkovich AJ, Bell C, Borgstede JP, Bradley WG Jr., et al. , 2013. ACR guidance document on MR safe practices. J. Magn. Reson. Imaging 37, 501–530. doi: 10.2214/AJR.06.1616. [DOI] [PubMed] [Google Scholar]
- Fatade A, 2021. MRI in Practice in Africa https://cds.ismrm.org/protected/public_videos/20210225Africa/.
- Fishell AK, Arbeláez AM, Valdés CP, Burns-Yocum TM, Sherafati A, et al. , 2020. Portable, field-based neuroimaging using high-density diffuse optical tomography. NeuroImage 215, 116541. doi: 10.1016/j.neuroimage.2020.116541. [DOI] [PubMed] [Google Scholar]
- Fisher JA, 2013. Expanding the frame of ‘voluntariness’ in informed consent: Structural coercion and the power of social and economic context. Kennedy Inst. Ethics J. 23, 355–379. doi: 10.1353/ken.2013.0018. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration, HHS, 2018. Human subject protection; acceptance of data from clinical investigations for medical devices. Final rule. Federal Register 83, 7366–7388. https://www.govinfo.gov/app/details/FR-2018-02-21/2018-03244. [PubMed] [Google Scholar]
- Franzen SRP, Chandler C, Lang T, 2017. Health research capacity development in low and middle income countries: Reality or rhetoric? A systematic meta-narrative review of the qualitative literature. BMJ Open 7, e012332. doi: 10.1136/bmjopen-2016-012332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garwood M, Mullen M, Kobayashi N, delaBarre L, et al. , 2020. A compact vertical 1.5T human head scanner with shoulders outside the bore and window for studying motor coordination. Proc. ISMRM. Session PP-30 https://www.ismrm.org/20/program_files/PP30.htm. (accessed 5 October 2020).
- Geethanath S, Vaughan JT Jr., 2019. Accessible magnetic resonance imaging: A review. J. Magn. Reson. Imaging 49, e65–e77. doi: 10.1002/jmri.26638. [DOI] [PubMed] [Google Scholar]
- Global Code of Conduct for Research in Resource-Poor Settings (GCC), 2018. Available at: https://www.globalcodeofconduct.org/wp-content/uploads/2018/05/Global-Code-of-Conduct-Brochure.pdf (accessed 8 September 2020).
- Gordon SB, Chinula L, Chilima B, et al. , 2018. A Malawi guideline for research study participant remuneration. Wellcome Open Res. 3, 1–17. doi: 10.12688/wellcomeopenres.14668.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossé L, 2018. Shining a light on brain development. Birkbeck University of London Blog http://blogs.bbk.ac.uk/research/tag/bill-and-melinda-gates-foundation/. [Google Scholar]
- Greely HT, Grady C, Ramos KM, Chiong W, Eberwine J, et al. , 2018. Neuroethics guiding principles for the NIH BRAIN initiative. J. Neurosci. 28, 10586–10588. doi: 10.1523/JNEUROSCI.2077-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guston DH, 2014. Understanding ‘anticipatory governance’. Soc. Stud. Sci. 44, 218–242. doi: 10.1177/0306312713508669. [DOI] [PubMed] [Google Scholar]
- Hadskis M, Kenny N, Downie J, Schmidt M, D’Arcy R, 2008. The therapeutic misconception: A threat to valid parental consent for pediatric neuroimaging research. Account. Res. 15, 133–151. doi: 10.1080/08989620801946917. [DOI] [PubMed] [Google Scholar]
- Hanna TN, Steenburg SD, Rosenkrantz AB, Pyatt RS Jr, Duszak R Jr, Friedberg EB, 2020. Emerging challenges and opportunities in the evolution of teleradiology. AJR Am. J. Roentgenol. 215, 1411–1416. 10.2214/AJR.20.23007. [DOI] [PubMed] [Google Scholar]
- Harris M, Dadwal V, Syed SB, 2020. Review of the reverse innovation series in globalization and health: Where are we and what else is needed? Glob. Health 16 doi: 10.1186/s12992-020-00555-6, Article 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Insurance Portability and Accountability Act of 1996 (HIPAA), 1996. P.L. No. 104–191, 110Stat. 1938. [Google Scholar]
- Huang SZ, Ren ZH, Obruchkov S, Gong J, Dykstra R, et al. , 2019. Portable low-cost MRI system based on permanent magnets/magnet arrays. Investig. Magn. Reson. Imaging 23, 179–201. doi: 10.13104/imri.2019.23.3.179. [DOI] [Google Scholar]
- Hussain S, 2015. Welcome to the Journal of Global Radiology. J. Glob. Radiol. 1, 1–2. doi: 10.7191/jgr.2015.1006. [DOI] [Google Scholar]
- Illes J, De Vries JR, Cho MK, Schraedley-Desmond P, 2006. ELSI priorities for brain imaging. Am. J. Bioeth. 6 (2), W24–W31. doi: 10.1080/15265160500506274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inalegwu OG, Adeyomoye AO, Augustina BP, Yaw M, Nzeh DA, 2018. Survey of magnetic resonance imaging availability in West Africa. Pan. Afr. Med. J. 30, 1–9. doi: 10.11604/pamj.2018.30.240.14000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Information Commissioner’s Office, 2019. Guide to the General Data Protection Regulation, Available at: https://ico.org.uk/for-organisations/guide-to-data-protection/guide-to-the-general-data-protection-regulation-gdpr/ (accessed 9 October 2020).
- International Society for Magnetic Resonance in Medicine, 2021. ISMRM Spotlights Africa: Doing Much with Little https://cds.ismrm.org/protected/public_videos/2021-02-25-Africa/.
- Jones OD, Buckholtz JW, Schall JD, Marois R, 2009. Brain imaging for legal thinkers: A guide for the perplexed. Stanford Technol. Law Rev. 5. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=1563612. [Google Scholar]
- Jull J, Giles A, Graham ID, 2017. Community-based participatory research and integrated knowledge translation: Advancing the co-creation of knowledge. Implement. Sci. 12, 1–9. doi: 10.1155/2017/1478541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katus L, Hayes NJ, Mason L, Blasi A, McCann S, et al. , 2019. Implementing neuroimaging and eye tracking methods to assess neurocognitive development of young infants in low- and middle-income countries. Gates Open Res. 3, 1–26. doi: 10.12688/gatesopenres.12951.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King ML, 2018. A social constructivism decision-making approach to managing incidental findings in neuroimaging research. Ethics Behav. 28, 393–410. doi: 10.1080/10508422.2017.1306445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer S, Lersy F, de Sèze J, Ferré JC, Maamar A, Carsin-Nicol B, Collange O, Bonneville F, Adam G, Martin-Blondel G, Rafiq M, 2020. Brain MRI findings in severe COVID-19: A retrospective observational study. Radiology 297, E242–E251. doi: 10.1148/radiol.2020202222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lairumbi GM, Michael P, Fitzpatrick R, English MC, 2011. Ethics in practice: The state of the debate on promoting the social value of global health research in resource poor settings particularly Africa. BMC Med. Ethics 12, 22. doi: 10.1186/1472-6939-12-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlotz CP, Allen B, Erickson BJ, Kalpathy-Cramer J, Bigelow K, 2019. A roadmap for foundational research on artificial intelligence in medical imaging: From the 2018 NIH/RSNA/ACR/The Academy Workshop. Radiology 291, 781–791. doi: 10.1148/radiol.2019190613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Largent EA, Fernandez LH, 2017. Paying research participants: Regulatory uncertainty, conceptual confusion, and a path forward. Yale J. Health Policy Law Ethics 17, 61–141. https://digitalcommons.law.yale.edu/yjhple/vol17/iss1/2/. [PMC free article] [PubMed] [Google Scholar]
- Lema VM, 2009. Therapeutic misconception and clinical trials in sub-Saharan Africa: A review. East Afr. Med. J. 86, 291–299. doi: 10.4314/eamj.v86i6.54142. [DOI] [PubMed] [Google Scholar]
- Lidz CW, Appelbaum PS, Grisso T, Renaud M, 2004. Therapeutic misconception and the appreciation of risks in clinical trials. Soc. Sci. Med. 58, 1689–1697. doi: 10.1016/S0277-9536(03)00338-1. [DOI] [PubMed] [Google Scholar]
- Li Y, Thompson WK, Reuter C, Nillo R, Jernigan T, Anders D, Sugrue LP, ABCD Consortium, 2021. Rates of incidental findings in brain magnetic resonance imaging in children. JAMA Neurol. 78, 578–587. doi: 10.1001/jamaneurol.2021.0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Fox S, Blasi A, McCann S, Rozhko M, Katus L, et al. , 2019. Habituation and novelty detection fNIRS brain responses in 5- and 8-month-old infants: The Gambia and UK. Dev. Sci. 22, e12817. doi: 10.1111/desc.12817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Fox S, Moore S, Darboe M, Prentice A, Papademetriou M, et al. , 2016. fNIRS in Africa & Asia: An objective measure of cognitive development for global health settings. The FASEB J. 30, 1149.18. doi: 10.1096/fasebj.30.1_supplement.1149.18. [DOI] [Google Scholar]
- Lockwood Estrin G, Bhavnani S, Jones E, Mason L, Goodwin A, et al. , 2019. From the lab to the field: Measuring brain activity and eye movements to assess neurodevelopment. In: Proceedings of The Road to Global Mental Health Conference. [Google Scholar]
- London A, et al. , 2008. Responsiveness to host community health needs. In: Emanuel E, Grady C, Crouch R, Lie R, Miller, et al. (Eds.), The Oxford Textbook of Clinical Research Ethics. Oxford University Press, New York, pp. 737–744. [Google Scholar]
- Marques JP, Simonis FFJ, Webb AG, 2019. Low-field MRI: An MR physics perspective. J Magn. Reson. Imaging 49, 1528–1542. doi: 10.1002/jmri.26637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascarell Maričić L, Walter H, Rosenthal A, et al. , 2020. The IMAGEN study: A decade of imaging genetics in adolescents. Mol. Psychiatry 25, 2648–2671. doi: 10.1038/s41380-020-0822-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascayano F, Armijo JE, Yang LH, 2015. Addressing stigma relating to mental illness in low- and middle-income countries. Front. Psychiatry 6, 1–4. doi: 10.3389/fpsyt.2015.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel PC, Cooley CZ, Stockmann JP, Wald LL, 2019. The MR Cap: A single-sided MRI system designed for potential point-of-care limited field-of-view brain imaging. Magn. Reson. Med. 82, 1946–1960. doi: 10.1002/mrm.27861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers T, Hunt NR, 2014. The other global South. Lancet 384, 1921–1922. doi: 10.1016/S0140-6736(14)62270-4. [DOI] [PubMed] [Google Scholar]
- Mills TT, 2020. 510(k) Premarket notification of Lucy Point-of-Care Magnetic Resonance Imaging device. U.S. Food and Drug Administration https://www.accessdata.fda.gov/cdrh_docs/pdf19/K192002.pdf. [Google Scholar]
- Milstein AC, 2008. Research malpractice and the issue of incidental findings. J. Law Med. Ethics 36, 356–360. doi: 10.1111/j.1748-720X.2008.00280.x. [DOI] [PubMed] [Google Scholar]
- Mollura D, Lungren M, 2019. Radiology in Global Health, 2nd ed. Springer, New York. [Google Scholar]
- Moreno J, 2004. Consensus, role and authority of. In: Post SG (Ed.), Encyclopedia of Bioethics, 3rd ed. Macmillan Reference, New York, pp. 520–523. [Google Scholar]
- Morris Z, Whiteley WN, Longstreth WT Jr, Weber F, Lee YC, et al. , 2009. Incidental findings on brain magnetic resonance imaging: Systematic review and meta-analysis. BMJ 339, b3016. doi: 10.1136/bmj.b3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namazzi G, et al. , 2020. Caring for children with neurodevelopmental disability: Experiences from caretakers and health workers in rural eastern Uganda. PLOS One 15, e0236488. doi: 10.1371/journal.pone.0236488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health, 2020. NIH to invest $58M to catalyze data science and health research innovation in Africa https://www.nih.gov/news-events/news-releases/nih-invest-58m-catalyze-data-science-health-research-innovation-africa.
- NIMH Council Workgroup on MRI Research Practices, 2005. MRI Research Safety and Ethics: Points to Consider https://www.nimh.nih.gov/about/advisory-boards-and-groups/namhc/reports/mri-research-safety-ethics_33826.pdf.
- Nyangulu W, Mungwira R, Nampota N, Nyirenda O, Tsirizani L, et al. , 2019. Compensation of subjects for participation in biomedical research in resource-limited settings: A discussion of practices in Malawi. BMC Med. Ethics 20, 1–5. doi: 10.1186/s12910-019-0422-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyblade L, Stockton M, Travasso S, Krishnan S, 2017. A qualitative exploration of cervical and breast cancer stigma in Karnataka, India. BMC Women’s Health 17, 1–15. doi: 10.1186/s12905-017-0407-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Office for Human Research Protections, 2017. Federal policy for the protection of human subjects. Federal Register 82, 7149–7274. [PubMed] [Google Scholar]
- O’Reilly T, Teeuwisse WM, de Gans D, Koolstra K, Webb AG, 2021. In vivo 3D brain and extremity MRI at 50 mT using a permanent magnet Halbach array. Magn. Reson. Med. 85, 495–505. doi: 10.1002/mrm.28396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero HJ, Andronikou S, Grassi DC, Silva CT, 2020. Providing expert pediatric teleradiology services around the globe: The World Federation of Pediatric Imaging experience. J. Am. Coll. Radiol. 17, 53–55. doi: 10.1016/j.jacr.2019.06.006. [DOI] [PubMed] [Google Scholar]
- Owen R, Macnaghten P, Stilgoe J, 2012. Responsible research and innovation: From science in society to science for society, with society. Sci. Publ. Policy 39, 751–760. doi: 10.1093/scipol/scs093. [DOI] [Google Scholar]
- Paget A, Mallewa M, Chinguo D, Mahebere-Chirambo C, Gladstone M, 2016. “It means you are grounded”–caregivers’ perspectives on the rehabilitation of children with neurodisability in Malawi. Disabil. Rehabil. 38, 223–234. doi: 10.3109/09638288.2015.1035458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palk A, Illes J, Thompson PM, Stein DJ, 2020. Ethical issues in global neuroimaging genetics collaborations. NeuroImage 221, 117208. doi: 10.1016/j.neuroimage.2020.117208. [DOI] [PubMed] [Google Scholar]
- Pickersgill MD, 2011. Research, engagement and public bioethics: Promoting socially robust science. J. Med. Ethics 37, 698–701. doi: 10.1136/jme.2010.041954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, 2018. The New Mind Readers: What Neuroimaging Can and Cannot Reveal About Our Thoughts. Princeton University Press. [Google Scholar]
- Poldrack RA, Baker CI, Durnez J, Gorgolewski KJ, Matthews PM, et al. , 2017. Scanning the horizon: Towards transparent and reproducible neuroimaging research. Nat. Rev. Neurosci. 18, 115–126. doi: 10.1038/nrn.2016.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt B, Zion D, Lwin KM, Cheah PY, Nosten F, et al. , 2013. Ancillary care: From theory to practice in international clinical research. Public Health Ethics 6, 154–169. doi: 10.1093/phe/pht015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravi KS, Geethanath S, 2020. Autonomous magnetic resonance imaging. Magn. Reson. Imaging 73, 177–185. doi: 10.1016/j.mri.2020.08.010. [DOI] [PubMed] [Google Scholar]
- Ren ZH, Gong J, Huang SY, 2019a. An irregular-shaped inward-outward ring-pair magnet array with a monotonic field gradient for 2D head imaging in low-field portable MRI. IEEE Access 7, 48715–48724. doi: 10.1109/ACCESS.2019.2909834. [DOI] [Google Scholar]
- Ren ZH, Mu WC, Huang SY, 2019b. Design and optimization of a ring-pair permanent magnet array for head imaging in a low-field portable MRI system. IEEE Trans. Magn. 55, 1–8. doi: 10.1109/TMAG.2018.2876679. [DOI] [Google Scholar]
- Royal JM, Peterson BS, 2008. The risks and benefits of searching for incidental findings in MRI research scans. J. Law Med. Ethics 36, 305–314. doi: 10.1111/j.1748-720X.2008.00274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarracanie M, LaPierre C, Salameh N, Waddington DEJ, Witzel T, et al. , 2015. Low-cost high-performance MRI. Sci. Rep. 5, 15177. doi: 10.1038/srep15177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarracanie M, Salameh N, 2020a. Low-field MRI: how low can we go? A fresh view on an old debate. Front. Phys. 8, 1–14. doi: 10.3389/fphy.2020.00172. [DOI] [Google Scholar]
- Sarracanie M, Salameh N, 2020b. Re-envisioning low-field MRI. MAGNETOM Flash 76, 8–13. https://cdn0.scrvt.com/39b415fb07de4d9656c7b516d8e2d907/1800000007101589/58dd6ebe0e0f/Salameh_Sarracanie_Lower-Field-MRI_ISMRM_2020_1800000007101589.pdf. (accessed 9 October 2020). [Google Scholar]
- Schiff SA, What is “good”? Recasting image quality as clinical utility. Proc ISMRM, Session MIS-03. https://www.ismrm.org/21/program-files/MIS-03.htm (accessed 1 June 2021).
- Schroeder D, Chatfield K, Singh M, Chennells R, Herissone-Kelly P, 2019. Equitable Research Partnerships: A Global Code of Conduct to Counter Ethics Dumping. Springer Nature; doi: 10.1007/978-3-030-15745-6. [DOI] [Google Scholar]
- Shen FX, Wolf SM, Gonzalez RG, Garwood M, 2020. Ethical issues posed by field research using highly portable and cloud-enabled neuroimaging. Neuron 105, 771–775. doi: 10.1016/j.neuron.2020.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth K, Cahn B, Salehi S, Shah J, By S, et al. , 2020. First deployment of a portable, bedside, low-field magnetic resonance imaging solution for artificial intelligence based application. Neurology 94, 2771. https://n.neurology.org/content/94/15_Supplement/2771. [Google Scholar]
- Shoemaker JM, Cole C, Petree LE, Helitzer DL, Holdsworth MT, et al. , 2016. Evolution of universal review and disclosure of MRI reports to research participants. Brain Behav. 6, 1–15. doi: 10.1002/brb3.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker JM, Holdsworth MT, Aine C, Calhoun VD, de La Garza R, et al. , 2011. A practical approach to incidental findings in neuroimaging research. Neurology 77, 2123–2127. doi: 10.1212/WNL.0b013e31823d7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skopec M, Hamdi I, Matthew H, 2019. Delivering cost effective healthcare through reverse innovation. BMJ 367, l6205. doi: 10.1136/bmj.l6205. [DOI] [PubMed] [Google Scholar]
- Stopczynski A, Stahlhut C, Petersen MK, Larsen JE, Jensen CF, et al. , 2014. Smartphones as pocketable labs: Visions for mobile brain imaging and neurofeedback. Int. J. Psychophysiol. 91, 54–66. doi: 10.1016/j.ijpsycho.2013.08.007. [DOI] [PubMed] [Google Scholar]
- Sullivan HK, Berkman BE, 2018. Incidental findings in low-resource settings. Hastings Center Rep. 48, 20–28. doi: 10.1002/hast.851. [DOI] [PubMed] [Google Scholar]
- Suwankhong D, Liamputtong P, 2016. Breast cancer treatment: Experiences of changes and social stigma among Thai women in southern Thailand. Cancer Nursing 39, 213–220. doi: 10.1097/NCC.0000000000000255. [DOI] [PubMed] [Google Scholar]
- Tarullo AR, Obradović J, Keehn B, Rasheed MA, Siyal S, et al. , 2017. Gamma power in rural Pakistani children: Links to executive function and verbal ability. Dev. Cogn. Neurosci. 26, 1–8. doi: 10.1016/j.dcn.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Nuremberg Code, 1947. BMJ 313, 1448. doi: 10.1136/bmj.313.7070.1448. [DOI] [Google Scholar]
- Thompson PM, Jahanshad N, Ching CRK, Salminen LE, Thomopoulos SI, et al. , 2020. ENIGMA and global neuroscience: A decade of large-scale studies of the brain in health and disease across more than 40 countries. Transl. Psychiatry 10, 1–28. doi: 10.1038/s41398-020-0705-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thondoo M, et al. , 2020. Framework for participatory quantitative health impact assessment in low-and middle-income countries. Int. J. Environ. Res. Public Health 17, 7688. doi: 10.3390/ijerph17207688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong G, Geethanath S, Qian E, Ravi KS, Jimeno Manso M, et al. , 2019. Virtual MR scanner software. Proc. ISMRM Poster 4583. https://www.ismrm.org/19/program_files/DP22.htm. (accessed 5 October 2020). [Google Scholar]
- Turpin J, Unadkat P, Thomas J, Kleiner N, Khazanehdari S, Wanchoo S, Samuel K, Moclair BO, Black K, Dehdashti AR, Narayan RK, 2020. Portable magnetic resonance imaging for ICU patients. Crit. Care Expl. 2, e0306. doi: 10.1097/CCE.0000000000000306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UN Secretary-General’s Independent Expert Advisory Group, 2014. A World That Counts: Mobilizing the Data Revolution for Sustainable Development https://www.undatarevolution.org/.
- U.S. Agency for International Development (USAID), 2019. Artificial intelligence in global health: Defining a collective path forward https://www.usaid.gov/cii/ai-in-global-health.
- Vasco M, Pandya S, Van Dyk D, Bishop DG, Wise R, et al. , 2019. Maternal critical care in resource-limited settings. Narrative review. Int. J. Obstet. Anesth. 37, 86–95. doi: 10.1016/j.ijoa.2018.09.010. [DOI] [PubMed] [Google Scholar]
- Wald LL, 2019. Ultimate MRI. J. Magn. Reson. 306, 139–144. doi: 10.1016/j.jmr.2019.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald LL, McDaniel PC, Witzel T, Stockmann JP, Cooley CZ, 2019. Low-cost and portable MRI. J. Magn. Reson. Imaging 52, 686–696. doi: 10.1002/jmri.26942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JA, Pinti P, Amft O, Van Laerhoven K, 2019. Wearables and the brain. IEEE Pervasive Comput. 18, 94–100. doi: 10.1109/MPRV.2019.2898536. [DOI] [Google Scholar]
- Wedderburn CJ, Subramoney S, Yeung S, Fouche JP, Joshi SH, et al. , 2020. Neuroimaging young children and associations with neurocognitive development in a South African birth cohort study. NeuroImage 219, 116846. doi: 10.1016/j.neuroimage.2020.116846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenner DM, 2017. The social value of knowledge and the responsiveness requirement for international research. Bioethics 31, 97–104. doi: 10.1111/bioe.12316. [DOI] [PubMed] [Google Scholar]
- Wenner DM, 2018. The social value requirement in research: From the transactional to the basic structure model of stakeholder obligations. Hastings Center Rep. 48, 25–32. doi: 10.1002/hast.934. [DOI] [PubMed] [Google Scholar]
- Wertheimer A, 2015. The social value requirement reconsidered. Bioethics 29, 301–308. doi: 10.1111/bioe.12128. [DOI] [PubMed] [Google Scholar]
- Wijeakumar S, Kumar A, Delgado Reyes LM, Tiwari M, Spencer JP, 2019. Early adversity in rural India impacts the brain networks underlying visual working memory. Develop. Sci. 22, e12822. doi: 10.1111/desc.12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf SM, Kahn JP, Wagner JE, 2003. Using preimplantation genetic diagnosis to create a stem cell donor: Issues, guidelines & limits. J. Law Med. Ethics 31, 327–339. doi: 10.1111/j.1748-720x.2003.tb00097.x. [DOI] [PubMed] [Google Scholar]
- Wolf SM, Lawrenz FP, Nelson CA, Kahn JP, Cho MK, et al. , 2008. Managing incidental findings in human subjects research: Analysis and recommendations. J. Law Med. Ethics 36, 211–219. doi: 10.1111/j.1748-720X.2008.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Bank, 2020. How does the World Bank classify countries? https://datahelpdesk.worldbank.org/knowledgebase/articles/378834-how-does-the-world-bank-classify-countries (accessed 9 October 2020).
- World Health Organization (WHO), 2011. Standards and operational guidance for ethics review of health-related research with human participants. https://www.who.int/ethics/publications/9789241502948/en/ (accessed 9 October 2020). [PubMed]
- World Health Organization (WHO), 2017. Global atlas of medical devices. WHO medical devices technical series; https://www.who.int/medical_devices/publications/global_atlas_meddev2017/en/. [Google Scholar]
- World Health Organization (WHO), 2019. Global strategy on digital health 2020–2024. https://extranet.who.int/dataform/upload/surveys/183439/files/Draft%20Global%20Strategy%20on%20Digital%20Health.pdf (accessed 9 October 2020).
- World Medical Association (WMA), 2001. World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. Bull. World Health Organ. 79, 373–374. [PMC free article] [PubMed] [Google Scholar]
- Zou J, Schiebinger L, 2018. AI can be sexist and racist —it’s time to make it fair. Nature 559, 324–326. doi: 10.1038/d41586-018-05707-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.