Abstract
Large quantities of bacteria, including Firmicutes, Actinobacteria, and Bacteroidetes, colonize the surface of the respiratory mucosa of healthy people. They interact and coexist with the local mucosal immune system of the human airway, maintaining the immune stability and balance of the respiratory system. While suffering from chronic respiratory diseases, the microbial population in the airway changes and the proportion of Proteobacteria is increased in patients with asthma. The abundance of the microbial population in patients with chronic obstructive pulmonary disease (COPD) is decreased, and conversely, the proportion of Firmicutes and Proteobacteria increased. The diversity of airway microorganisms in cystic fibrosis (CF) patients is decreased, while pathogenic bacteria and conditional pathogenic bacteria are proliferated in large numbers. The proportion of Firmicutes and Proteobacteria is increased in patients with upper airway cough syndrome (UACS), which replaces the dominance of Streptococcus and Neisseria in the pharynx of a normal population. Therefore, a clear understanding of the immune process of the airway flora and the immune dysfunction of the flora on the pathogenesis of chronic respiratory diseases can provide new ideas for the prevention and treatment of human respiratory diseases.
1. Introduction
The human body carries a huge group of microbial populations, which constitutes a complex and delicate ecosystem. The complex interaction between microbes and the human immune system determines the health of the human body. The airway is an open cavity connecting the human body with the outside world. It is always invaded by external microbes, so it has strong local immunity. For example, the airway contains not only motile cilia, strong secretory goblet cells, and secretory IgA on the mucosal surface but also abundant mucosal-associated lymphoid tissue under the mucosa, which is sufficient to cope with the invasion of external pathogens [1]. Once the local immunity of the airway is disturbed, acute and chronic respiratory diseases such as asthma, chronic obstructive pulmonary disease (COPD), cystic fibrosis (CF), and upper airway cough syndrome (UACS) are likely to be triggered (Figure 1). For a long time, the airway, especially the lower airway, has been considered amicrobic [2, 3]. The airway is a dynamic ecosystem full of microbial populations, which is closely related to the immunity and inflammatory response of the host [4]. As is in the intestine, there are certain types and quantities of microbial populations in the airway of healthy people, that is, normal flora. Under the influence of external factors, these normal florae continuously evolve to maintain the dynamic balance of respiratory microecology and resist and avoid the invasion and colonization of pathogens to the airway [5, 6]. However, under the influence of external factors, such as environmental pollution and antibiotic abuse, changes in respiratory flora may result in pathogenic infection. Even some strains in the normal flora may turn into conditional pathogenic bacteria, causing several respiratory diseases. The stability of the airway flora is greatly affected by the external environment, which affects the local airway immune balance and even leads to the immune dysfunction of the respiratory system, thereby adding lots of difficulties to the clinical treatment of respiratory diseases. From the microecological perspective, it is of great significance to pay attention to the local immunity of the airway in the prevention and treatment of respiratory diseases [7, 8]. In recent years, with the development and application of molecular biology techniques, the research on the relationship between respiratory microflora with respiratory system immunity and diseases has developed rapidly. Growing studies have interpreted the role of complex respiratory microflora in the process of immune dysfunction in various respiratory diseases, which provides new ideas for exploring the pathogenesis and treatment of respiratory diseases [9, 10].
Figure 1.
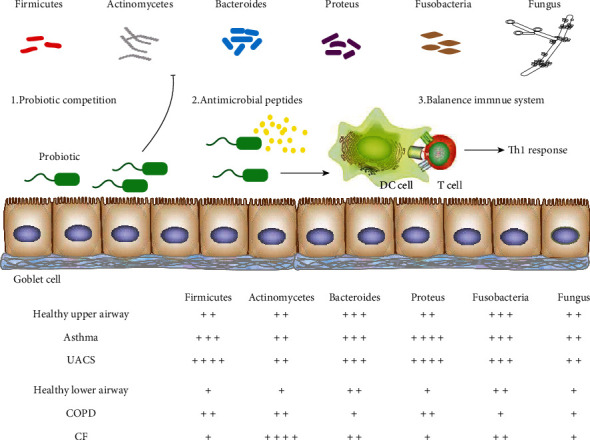
The profiles of respiratory flora in the occurrence and development of chronic respiratory diseases.
2. Flora Distribution and Local Immunity of the Airway in Healthy People
Large quantities of bacteria colonize the surface of the respiratory mucosa of healthy people, of which the main groups are Firmicutes, Actinobacteria, Bacteroidetes, Proteobacteria, and Fusobacteria [11]. Bacteria regulate the species and quantity through the quorum-sensing system and locally produced antimicrobial peptides to maintain dynamic balance and achieve peaceful coexistence with the human body. The floras in the upper and lower airways are highly homologous, and the quantity of upper airway flora is greater than that of the lower airway, which means that there are no specific microbes in the upper and lower airways [12]. This is different from the normal intestinal flora. The human airway is mainly defended by the local mucosal immune system. Mucosal epithelial cells act as a physical barrier separating the internal and external environments. They are the main structure of the local innate immune system and the main carrier of the airway flora. There are cilia on the surface of respiratory mucosal epithelial cells, which can transport bacteria of the lower airway upwards, while the mucus secreted by goblet cells and secretory IgA antibodies produced by submucosal lymphohistiocytes can further maintain the local chemical immune barrier function of the mucosa and prevent the invasion and colonization of pathogens [13, 14]. However, the local immune system of the airway neither equips with the strong acid and alkali on the surface of alimentary canal mucosa and the powerful germicidal mechanism of excellent enzyme system nor the extremely anaerobic environment, which determines that there is no significant difference in the species but only in the number of bacteria in the upper and lower airway. This is the evolutionary result of the interaction and symbiosis between the airway local immunity and the flora [15].
3. Immunity of Respiratory Flora and Asthma
Asthma is a kind of disease that is well-recognized as immune dysfunction of the respiratory system, which is defined as a clinical syndrome of intermittent respiratory symptoms caused by viral upper respiratory infections, environmental allergens, or other stimuli, characterized by nonspecific bronchial hyperresponsiveness and airway inflammation [16, 17]. Due to the low positive rate of colony culture in sputum in the past and the insignificant effect of antibiotic treatment in the early stage of asthma, it was believed that there was no relationship between bacteria and asthma attacks. An epidemiological study had disclosed that bronchial infection could be the basis of asthma attacks in the adult during the outbreaks of bronchitis and pneumonia. This was also in line with the previously reported efficacy of macrolide antibiotics in the treatment of patients with chronic infectious asthma [18]. With the application of serological testing and PCR-based research methods, it had found that Streptococcus pneumoniae and Chlamydia pneumoniae infections were common during acute asthma attacks [19]. Analysis of the microbiota in bronchoalveolar lavage (BAL), tracheal brushings, and sputum microorganisms revealed that the respiratory microbiota of asthmatic patients who had a significant increase in the abundance of Proteobacteria in the flora was significantly different from that of healthy people [20]. Through the cultivation of respiratory specimens (Figure 2), it was found that the proportions of asthma in children with bacteria, such as Moraxella catarrhalis, Haemophilus influenzae, or Streptococcus pneumoniae, were increased significantly, which were related to the severity and acute exacerbation of asthma [21, 22]. The results from 16S rRNA sequencing showed that the proportion of Proteobacteria, including Haemophilus influenzae, Pseudomonas aeruginosa, Klebsiella pneumonia, and other respiratory pathogens, in respiratory specimens of asthma patients was higher than that of the normal population. The increase in these pathogens was also found in patients with irregular inhaled hormone therapy, suggesting that this feature of respiratory flora was a characteristic of asthma itself and not simply a result of immunosuppression caused by inhaled hormones. In contrast, the proportion of Prevotella of Bacteroidetes in the airways of asthma patients was lower than that of the normal population [23–25].
Figure 2.
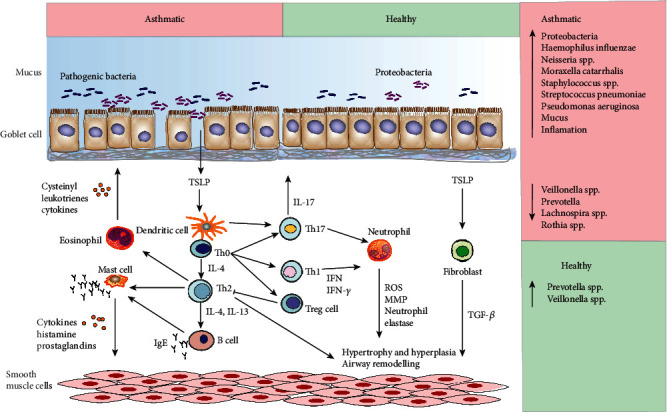
The roles of pathogens in the occurrence and development of asthma.
The distribution of different bacterial communities is also related to the features of asthma. Airway microbes and asthma patients also have certain effects on the responsiveness of glucocorticoids. In vitro studies have shown that monocytes and macrophages cocultured with Haemophilus influenzae and Haemophilus parainfluenza were impaired in response to dexamethasone, which was found in some patients with hormone-resistant asthma [26]. Studies have shown that dexamethasone can inhibit tumor necrosis factor (TNF) production by macrophages and promote the phagocytosis of microparticles by human monocytes, thereby played an important role in immune-mediated tissue damage or tissue repair after infection [27, 28]. The possible mechanism of the effect of flora changes on the sensitivity to corticosteroids is the release of superantigens to produce oxidative stress, the release of cytokines, or the activation of host p38 mitogen-activated protein kinase (MAPK) [29].
These studies indicated that changes in respiratory flora would increase types of pathogenic bacteria but reduce types of normal flora and biomass in asthma patients, suggesting that the immune dysregulation of the respiratory flora may be one of the causes of asthma attacks.
4. Immunity of Respiratory Flora and COPD
COPD is a highly heterogeneous disease that seriously affects human health. It is characterized by persistent airflow obstruction and increasing airway chronic inflammatory response to harmful particles or gases. Exposure to noxious smoke and particles is a major driver for the progression of COPD. At the same time, other factors also play a part, such as genetic predisposition, nutritional status, and respiratory infection [30]. The role of microbial infection in the onset and development of COPD is a hot topic in the research of the respiratory microbiome. COPD is associated with the colonization of early pathogenic pathogens in the bronchus. The possible effect of changes in respiratory microbes on the progression of COPD can be explained by the circular vicious hypothesis [31]. Once the innate self-protective mechanism of the lung affected by smoke exposure and the airway microbial homeostasis is broken, pathogenic microbes can induce an inflammatory response of COPD, leading to progressive airway obstruction as well as pulmonary parenchyma injury by disrupting the balance of proteases and antiproteases in the lung. Direct supporting evidence for this hypothesis came from conventional biological research. A large increase in local immune and inflammatory components such as inflammatory corpuscles, cytokines, chemotactic factors, and proteases in the airways of patients with COPD could be detected by bronchoscopy or sputum examination. These pathological changes were consistent with chronic infection. Meanwhile, COPD patients with bronchiectasis harbored more airway bacteria, showing worse clinical outcomes [32]. Microbiological research can help us better understand the impact of microbes on the pathogenesis and progression of COPD. But whether differences in airway microbes have an impact on COPD clinical symptoms (such as the presence or absence of bronchitis) deserves further exploration.
Unlike the early changes in the microbiota of patients with asthma, there is no significant difference in respiratory flora between mild and moderate COPD patients and normal people [33]. Changes in flora are present only in patients with severe COPD (Forced expiratory volume in one second (FEV1) is less than 40% to 50% of the expected value). Microbiological analysis of sputum samples showed that the abundance of bacteria in the airway of patients with COPD was decreased, the proportion of Proteobacteria such as Pseudomonadaceae, Burkholderiaceae, and Enterobacteriaceae was increased, and the relative abundance of Firmicutes was decreased [34]. However, some scholars believe that Firmicutes and Actinobacteria were the dominant flora in the lungs of COPD patients. By sampling and sequencing the BALF of patients with moderate and severe COPD, Pragman et al. found that the structure of the flora in the lungs of these two groups of patients was changed and that Firmicutes, Proteobacteria, and Actinobacteria were abundant in the lower airways of these patients [35]. Microbiological analysis of bronchial biopsy showed that this change was related to the increase in local heterogeneity of respiratory microbes. This change in respiratory microbes associated with disease severity had also been reported in patients with CF [36].
Microbiological studies showed that, at the acute exacerbation stage of COPD, there was no significant difference in respiratory microbes compared with the stationary phase [37]. The respiratory symptoms in the acute exacerbation stage of COPD are related to the colonization of new strains in the airway. Microbiological analysis showed a clear increase in the relative abundance of bacteria in one genus but without significant changes in other colonies. In the process of infection, the microbial pattern was completely different [38]. The latter had an obvious increase in the proportion of Firmicutes before the colonization of the dominant bacteria. This finding also supported the clinical features of acute exacerbation in both types. Taking into account the different microbial modes, different therapeutic measures are required. Although the composition of respiratory flora in patients with COPD has been clear through microbiological analysis, the role of respiratory flora in the pathogenesis of the disease remains to be further explored.
5. Immunity of Respiratory Flora and CF
CF is a familial autosomal recessive inherited congenital disease, which is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR), resulting in the structural and functional alterations of the transmembrane protein in pulmonary CF, thereby causing chronic pulmonary infection and pulmonary function deterioration in patients, leading to early deaths [39]. CF is characterized by defective mucociliary clearance and chronic infection of complex microbial flora [40]. Infection, persistent inflammation, and acute periodic attacks lead to an irreversible decline in lung function. Although little is known about the factors contributing to acute exacerbation, antibiotic treatment can temporarily resolve lung symptoms and partially restore lung function. Acute exacerbations may be associated with changes in microbial density and the acquisition of new microbial strains.
Fodor et al. used massive pyrosequencing technology to identify changes in the airway microbial community structure of 23 CF patients during CF exacerbations and stabilities and obtained more than 350,000 sequences, representing nearly 170 distinct microbial floras [41]. Approximately 60% of the sequences obtained were from the known CF pathogenic bacteria Pseudomonas aeruginosa and Burkholderiaceae. Although antibiotic treatment and species richness declined slightly, the microbial community structure hardly changed. Moreover, the microbial composition in acute exacerbation was highly similar to that in the stationary phase, suggesting that exacerbations may be due to the spread of infection in the lungs, rather than changes in the composition of the microbial community.
The research through in vitro bacterial culture of the patients' sputa revealed that the predominant pathogenic bacteria were Pseudomonas aeruginosa, Haemophilus influenzae, and Staphylococcus aureus. Meanwhile, other strains such as Burkholderia, Stenotrophomonas maltophilia, and Achromobacteria xyloxydans were also present in elderly patients [42, 43]. The diversity of microbial communities in the lungs of CF patients was decreased, and the degree of pulmonary inflammation was related to the decrease in microbial diversity. Moreover, Bacteroidetes and Fusobacteria were the dominant strains in healthy individuals while Actinobacteria accounted for a larger proportion in CF patients. The structure of pulmonary florae was destroyed, pathogenic bacteria and conditional pathogens were multiplied, and florae were out of balance, which may be one of the causes of CF.
6. Immunity of Respiratory Flora and UACS
UACS, formerly known as postnasal drip syndrome (PNDS), is a syndrome of chronic cough caused by the reflux of allergic or nonallergic inflammatory secretions from the nasal cavity and nasopharynx into the pharynx [44]. It can be accompanied by a series of symptoms such as pharyngeal foreign body sensation, itchy pharynx, blocking sensation, and sputum adhesion in the pharynx. It is considered to be one of the most common causes of chronic cough. The pathogenesis of UACS remains unclear. Physicians in different countries have different definitions as well as treatments for UACS. The pathogenetic theories of UACS include the earliest theory of postnasal drip irrigation, the subsequent theory of chronic airway inflammation, and the more recent theory of sensory nerve hypersensitivity. Furthermore, some researchers believed that UACS was the clinical phenotype of irritable cough syndrome [45].
There is a slight difference in the types of pharyngeal flora between healthy people and patients with UACS. Firmicutes and Proteobacteria are the dominant flora in UACS that are different from the normal flora. Among these, Lactobacillus of Firmicutes and Pseudomonas aeruginosa of Proteobacteria have significantly increased and replaced the dominant position of original Streptococcus and Neisseria in the pharynx of normal people. Moreover, TRPV1 and TGF-β2 were increased significantly in UACS patients [46]. TRPV1 is a nonselective cation channel associated with cough sensitivity and widely distributed in sensory nerves from the upper to lower airways [47]. TRPV1 is regulated by multiple physicochemical factors, e.g., increased lactate secretion and the stimulation of inflammatory factors will lead to the increase of TRPV1. And it is highly expressed in pathological conditions such as chronic cough and high airway responsiveness. Another study showed significant elevation of both TGF-β1 and β2 in mammary secretions of dairy cows infected with Pseudomonas aeruginosa compared to those uninfected, demonstrating the correlation between Pseudomonas aeruginosa and TGF-β1 and β2. Meanwhile, Pseudomonas aeruginosa could also stimulate airway inflammation through a variety of known inflammatory pathways, leading to an increase in the prevalence of UACS [48].
7. Immunity of Respiratory Flora and Infection of Respiratory Fungal
Fungal colonization in the human body refers to the presence of a large number of fungi growing in the form of spores at a site where the human body communicates with the outside world, such as the alimentary canal, the upper airway, and urogenital tract, but without local tissues damage or symptoms [49]. Colonization is generally the final step in a long-lasting symbiotic or innocuous relationship between the fungus and the host and also is the first step in the conversion to fungal infection and the development of related diseases [50].
Contamination and colonization with Candida and Aspergillus in the upper airway are very common [51]. The results of ICU investigation in domestic general hospitals showed that Candida was the most common fungus colonizing in the lower airway, among which the infection rate of Candida albicans was the highest (37.91%), followed by Aspergillus (16.99%) [52]. Systemic or local immunosuppression caused by granulocytopenia in leukemia chemotherapy is an important cause of airway fungal infection. Pathogenic fungi can be colonized in patients, with infection and colonization by Aspergillus fumigatus and Candida albicans being the most common [53].
8. Inhaled Corticosteroids (ICSs) and Airway Flora Immunity
Glucocorticoid has a potent anti-inflammatory and immunosuppression function, which eliminates most pathogenic bacteria and improves the airway microenvironment by effectively reducing the levels of proinflammatory cytokines and airway inflammation. Inhaled corticosteroids, without obvious systemic side effects, can easily form an effective concentration in the airway and work directly, which is quickly destroyed by enzymes in alveoli and inactivated by the liver after entering the blood circulation.
Glucocorticoids can enter into the nucleus by binding with glucocorticoid receptor in the cytoplasm through the cell membrane and form hormone-hormone receptor complex, which combines with special DNA sequence to produce biological effect through the following three ways: (1) inhibiting the release of inflammatory factors such as TNF-α and leukotrienes by promoting the production of enzyme-linked protein I, resulting in the decrease of phospholipase A2-α; (2) inhibiting the production of inflammatory protein and its phospholipase A2-α by inactivating MAPK; (3) inhibiting the expression of cytokines, chemokines, cell adhesion factors, and their receptors by activating NF-κB pathway [54]. Glucocorticoids not only can directly inhibit inflammatory cells but also reduce the exudation of capillary and the formation of sputum. Studies have found that dexamethasone reduced lung inflammation by promoting the secretion of IL-12 and inhibiting the expression of IL-13 in rats [55]. However, there are still side effects in the long-term repeated use of inhaled corticosteroids to control respiratory diseases, such as dryness, hoarseness, and Candida infection in the oral and pharyngeal [56]. Furthermore, long-term use of glucocorticoids may also cause immunosuppression and aggravate the development of the disease by destroying the local mucosal barrier and microenvironment of the airway, resulting in the unbalance of the microbial flora. These will lead to changes in the microbiome and increase the risk of some systemic reactions such as airway infections (pneumonia and mycobacterial disease). Thus, rationally controlling the time of using hormones may reduce the incidence of adverse reactions.
In addition, previous studies have proved that the diversity of oral microflora was decreased, while the proportion of firmicutes and Bacteroidetes was increased in normal obese children [57]. Because the diversity of flora is affected by many factors, maintaining the balance of flora is the main measure to reduce the occurrence of adverse reactions when infants are given Budesonide atomization therapy. Therefore, it is necessary to strengthen the detection of flora and maintain the balance of respiratory flora in clinical treatment to reduce the occurrence of adverse reactions [58].
Although ICSs have been widely used in the clinical treatment of respiratory diseases because of the strong anti-inflammatory activities, they could not effectively improve the inflammatory response of airway remodeling and proliferation. Most clinical reports have shown that excessive inhalation of ICSs may cause drug dependence or resistance and other adverse reactions, especially in children with asthma in the early stage, which will affect children's immune system and result in hormone-dependent. Therefore, exploring new anti-inflammatory drugs to combine or even replace hormones is the leading direction of drug development.
9. Probiotic Preparation and Improvement of Respiratory Flora Immunity
Probiotic refers to live biotherapeutic products (LBP) containing enough viable organisms with well-defined composition. It can change the composition of the flora at a certain site of the host through colonization, thereby improving the microecological balance of the host and playing a beneficial role [59]. On the one hand, probiotics can regulate the adaptive immune response of the host by promoting the release of cytokines from dendritic cells (DCs), altering the balance of Th1/Th2, shifting the immune response in the direction of Th1 cells, and inhibiting the response of Th2 cells. On the other hand, it can also play a role in the biological barrier by competing with pathogens and secreting antimicrobial peptides and other metabolites [60]. At present, probiotics have been used to treat diseases associated with immune dysregulation of the intestinal flora, such as diarrhea, obesity, type 2 diabetes, inflammatory bowel disease, and other diseases [61, 62]. In recent years, a probiotic composition has been invented, including Lactobacillus rhamnosus and Lactobacillus plantarum. This supplement inhibits the expression of inflammatory factors in the lung by activating TLR3 and RIG-1 signaling pathways in alveolar macrophages to promote IFN-β and also regulates lung flora and intestinal flora to alleviate respiratory syncytial virus (RSV) infection. Over-the-counter probiotics have been used in clinical trials to investigate their potential effects in various disease conditions, but it is required more stringent quality control to ensure the purity and efficacy of products. The challenge of detecting unwanted microbial contaminants is that the sensitivity of detection may be reduced in the presence of the required probiotic microorganisms. One of the strategies under study was to reduce or eliminate the growth of bacteria in the product for improving the sensitivity of detecting the contaminating microbes. FDA scientists developed and used recombinant phage lysine as a reagent to improve LBP purity detection through “mock” purity assays (“test-tube” studies) where Lactobacillus jensenii represented the probiotic's product strain. However, the type of strains, dosage, and course of probiotic can affect the outcome of clinical application, and even the same flora will have different effects at different ages. Therefore, the prevention and treatment of respiratory infection with probiotics deserves further research.
10. Protective Effects of Natural Products against Various Stimuli-Induced Lung Dysbiosis
Natural products or traditional Chinese medicine have been reported to improve airway immunity, ameliorate the function of the blood-air barrier, inhibit inflammation, and reduce growth of pathogenic bacteria due to its advantages of multicomponent, multichannel, and multitarget (Table 1), which had the potential for the prevention and treatment of several respiratory diseases including COPD, asthma, and UACS [63]. Baicalin, a kind of flavonoid derived from Scutellaria baicalensis (Huangqin in Chinese), with broad-spectrum antibacterial activity and obvious anti-inflammatory activity, which can effectively improve the respiratory system inflammation and treat respiratory disease by inhibiting a variety of bacteria and fungi [64, 65]. Baicalin has an inhibitory effect on Candida albicans and Staphylococcus aureus, which was correlated with the concentration of baicalin, that is, the DNA synthesis of Candida albicans was decreased with the increase of drug concentration [66, 67]. Cryptotanshinone has antiasthmatic effect, the mechanism of which may be related to downregulating Th2 cytokines and reducing inflammatory cell infiltration [68]. The Yinqiao powder is equipped with the effect of clearing away heat and toxin by inhibiting Escherichia coli, Staphylococcus aureus, and Pseudomonas aeruginosa [69]. Xiaochaihu decoction, a famous herbal medicine exerting antiallergic and antitussive effects, has been widely used for the treatment of asthma in clinics [70]. It can effectively reduce the frequency of asthma attacks and airway infection in children with asthma and alleviate airway inflammation by enhancing the immune function and antiallergic ability of airway mucosa. However, the current research is still insufficient. Most studies focus on in vitro antibacterial (Streptococcus pneumoniae, Staphylococcus aureus, etc.) activities of the agents, but few considered the antibacterial effect in vivo. And due to the complexity of pharmacodynamics, it is difficult to draw the conclusion whether antibacterial effects of drugs or anti-inflammatory effects play the decisive role in airway inflammation.
Table 1.
The protective effects of traditional Chinese medicine on asthma.
| Mechanism | Traditional Chinese medicine | Reference |
|---|---|---|
| Regulating Th1/Th2 balance | Bulleyaconitine A | [71] |
| Ligustrazine | [72] | |
| Selaginella uncinata flavonoids | [67] | |
| Turmeric (Curcuma longa) | [73] | |
|
| ||
| Regulating Th17/Treg balance | Bufei Yishen formula | [74, 75] |
| Baicalin | [76] | |
| Gu-Ben-Fang-Xiao-Tang | [77] | |
| Louqin Zhisou decoction | [78] | |
|
| ||
| Regulating antigen presenting cells | Atractylodin | [79] |
| Oligomeric proanthocyanidins | [80] | |
| Tilianin | [81] | |
| Wuhu decoction | [82] | |
|
| ||
| Inhibiting oxidative stress | Citrus flavonoids | [83] |
| Platycodi Radix | [84] | |
| Quercetin | [85] | |
| Saikosaponin A | [86] | |
| Wedelolactone | [87] | |
|
| ||
| Inhibiting NLRP3 inflammasome | Abscisic acid | [88] |
| Andrographolide | [89] | |
| EGCG | [90] | |
There is still a lack of robust research on pharmacological mechanisms. Therefore, further efforts are needed to elucidate the underlying mechanism in germ-free mice.
11. Conclusion
Changes in the immunity of respiratory flora play an important role in the development of multiple chronic respiratory diseases. In recent years, with the application of various new techniques in the detection of microbes in respiratory specimens, growing evidence showed that respiratory microbial population and its related local mucosal immunity were associated with the clinical manifestations, acute exacerbation, and prognosis of chronic respiratory immune disorders, such as asthma and COPD. Through studying the microbiological basis of the progression of human respiratory diseases, we can make a better understanding of the local immunological mechanism of the progression of these diseases, facilitate the judgment of disease types, predict the responsiveness to treatment, and evaluate the therapeutic effect. It provided a new idea for the clinical diagnosis, treatment, prediction, and prognosis of respiratory diseases.
Acknowledgments
This work is supported by the Zhejiang Public Technology Application Project (No. LGD19C040004), Zhejiang Medical Science and Technology Project (No. 2020KY527), and Zhejiang Disciplinary Construction of Laboratory Animal Genetic Engineering (No. 201604).
Data Availability
The data used to support the findings of this study is included within the article.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- 1.Yang L., Li C., Tang X. The impact of PM2.5 on the host defense of respiratory system. Frontiers in Cell and Development Biology. 2020;8:p. 91. doi: 10.3389/fcell.2020.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lanaspa M., Bassat Q., Medeiros M. M., Munoz-Almagro C. Respiratory microbiota and lower respiratory tract disease. Expert Review of Anti-Infective Therapy. 2017;15(7):703–711. doi: 10.1080/14787210.2017.1349609. [DOI] [PubMed] [Google Scholar]
- 3.Bond S. L., Timsit E., Workentine M., Alexander T., Leguillette R. Upper and lower respiratory tract microbiota in horses: bacterial communities associated with health and mild asthma (inflammatory airway disease) and effects of dexamethasone. Bmc Microbiology. 2017;17(1):p. 184. doi: 10.1186/s12866-017-1092-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sato S., Kiyono H. The mucosal immune system of the respiratory tract. Current Opinion in Virology. 2012;2(3):225–232. doi: 10.1016/j.coviro.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 5.Dai W., Wang H., Zhou Q., et al. An integrated respiratory microbial gene catalogue to better understand the microbial aetiology of Mycoplasma pneumoniae pneumonia. GigaScience. 2019;8(8):p. giz093. doi: 10.1093/gigascience/giz093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marsland B. J., Gollwitzer E. S. Host-microorganism interactions in lung diseases. Nature Reviews Immunology. 2014;14(12):827–835. doi: 10.1038/nri3769. [DOI] [PubMed] [Google Scholar]
- 7.Man W. H., de Steenhuijsen Piters W. A. A., Bogaert D. The microbiota of the respiratory tract: gatekeeper to respiratory health. Nature Reviews Microbiology. 2017;15(5):259–270. doi: 10.1038/nrmicro.2017.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanshew A. S., Jette M. E., Rosen S. P., Thibeault S. L. Integrating the microbiota of the respiratory tract with the unified airway model. Respiratory Medicine. 2017;126:68–74. doi: 10.1016/j.rmed.2017.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dickson R. P., Erb-Downward J. R., Martinez F. J., Huffnagle G. B. The microbiome and the respiratory tract. Annual Review of Physiology. 2016;78(1):481–504. doi: 10.1146/annurev-physiol-021115-105238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumpitsch C., Koskinen K., Schopf V., Moissl-Eichinger C. The microbiome of the upper respiratory tract in health and disease. Bmc Biology. 2019;17(1):p. 87. doi: 10.1186/s12915-019-0703-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dickson R. P., Erb-Downward J. R., Huffnagle G. B. Homeostasis and its disruption in the lung microbiome. American Journal of Physiology. Lung Cellular and Molecular Physiology. 2015;309(10):L1047–L1055. doi: 10.1152/ajplung.00279.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee J. T., Kim C. M., Ramakrishnan V. Microbiome and disease in the upper airway. Current Opinion in Allergy and Clinical Immunology. 2019;19(1):1–6. doi: 10.1097/ACI.0000000000000495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baschal E. E., Larson E. D., Bootpetch R. T., et al. Identification of novel genes and biological pathways that overlap in infectious and nonallergic diseases of the upper and lower airways using network analyses. Frontiers in Genetics. 2019;10:p. 1352. doi: 10.3389/fgene.2019.01352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agujetas R., Barrio-Perotti R., Ferrera C., Pandal-Blanco A., Walters D. K., Fernandez-Tena A. Construction of a hybrid lung model by combining a real geometry of the upper airways and an idealized geometry of the lower airways. Computer Methods and Programs in Biomedicine. 2020;196:p. 105613. doi: 10.1016/j.cmpb.2020.105613. [DOI] [PubMed] [Google Scholar]
- 15.Dickson R. P., Huffnagle G. B. The lung microbiome: new principles for respiratory bacteriology in health and disease. PLoS Pathogens. 2015;11(7):p. e1004923. doi: 10.1371/journal.ppat.1004923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mims J. W. Asthma: definitions and pathophysiology. International Forum of Allergy and Rhinology. 2015;5(Suppl 1):S2–S6. doi: 10.1002/alr.21609. [DOI] [PubMed] [Google Scholar]
- 17.Hering T. Update of the GINA-recommendations. MMW Fortschritte der Medizin. 2017;159(10):63–64. doi: 10.1007/s15006-017-9710-6. [DOI] [PubMed] [Google Scholar]
- 18.Li X. Y., She J., Zhu L. The application of macrolide antibiotics in asthma. Zhonghua Jie He He Hu Xi Za Zhi. 2019;42(1):33–36. doi: 10.3760/cma.j.issn.1001-0939.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 19.Huang Y. J., Boushey H. A. The microbiome in asthma. The Journal of Allergy and Clinical Immunology. 2015;135(1):25–30. doi: 10.1016/j.jaci.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barcik W., Boutin R., Sokolowska M., Finlay B. B. The role of lung and gut microbiota in the pathology of asthma. Immunity. 2020;52(2):241–255. doi: 10.1016/j.immuni.2020.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al B. M., Hamoudi R. A., Dash N. R., et al. Altered respiratory microbiota composition and functionality associated with asthma early in life. Bmc Infectious Diseases. 2020;20(1):p. 697. doi: 10.1186/s12879-020-05427-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Earl C. S., An S. Q., Ryan R. P. The changing face of asthma and its relation with microbes. Trends in Microbiology. 2015;23(7):408–418. doi: 10.1016/j.tim.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang Y. J., Nariya S., Harris J. M., et al. The airway microbiome in patients with severe asthma: associations with disease features and severity. The Journal of Allergy and Clinical Immunology. 2015;136(4):874–884. doi: 10.1016/j.jaci.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li N., Qiu R., Yang Z., et al. Sputum microbiota in severe asthma patients: relationship to eosinophilic inflammation. Respiratory Medicine. 2017;131:192–198. doi: 10.1016/j.rmed.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 25.Sobieraj D. M., Weeda E. R., Nguyen E., et al. Association of inhaled corticosteroids and long-acting β-Agonists as controller and quick relief therapy with exacerbations and symptom control in persistent Asthma. JAMA. 2018;319(14):1485–1496. doi: 10.1001/jama.2018.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mennini M., Dahdah L., Artesani M. C., Fiocchi A., Martelli A. Probiotics in asthma and allergy prevention. Frontiers in Pediatrics. 2017;5:p. 165. doi: 10.3389/fped.2017.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boulet L. P. Airway remodeling in asthma: update on mechanisms and therapeutic approaches. Current Opinion in Pulmonary Medicine. 2018;24(1):56–62. doi: 10.1097/MCP.0000000000000441. [DOI] [PubMed] [Google Scholar]
- 28.Fang L., Sun Q., Roth M. Immunologic and non-immunologic mechanisms leading to airway remodeling in asthma. International Journal of Molecular Sciences. 2020;21(3):p. 757. doi: 10.3390/ijms21030757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goleva E., Jackson L. P., Harris J. K., et al. The effects of airway microbiome on corticosteroid responsiveness in asthma. American Journal of Respiratory and Critical Care Medicine. 2013;188(10):1193–1201. doi: 10.1164/rccm.201304-0775OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh D., Agusti A., Anzueto A., et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. European Respiratory Journal. 2019;53(5):p. 1900164. doi: 10.1183/13993003.00164-2019. [DOI] [PubMed] [Google Scholar]
- 31.Ren L., Zhang R., Rao J., et al. Transcriptionally active lung microbiome and its association with bacterial biomass and host inflammatory status. MSystems. 2018;3(5) doi: 10.1128/mSystems.00199-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sze M. A., Dimitriu P. A., Hayashi S., et al. The lung tissue microbiome in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 2012;185(10):1073–1080. doi: 10.1164/rccm.201111-2075OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tangedal S., Nielsen R., Aanerud M., et al. Sputum microbiota and inflammation at stable state and during exacerbations in a cohort of chronic obstructive pulmonary disease (COPD) patients. PLoS One. 2019;14(9):p. e222449. doi: 10.1371/journal.pone.0222449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leung R. K., Zhou J. W., Guan W., Li S. K., Yang Z. F., Tsui S. K. Modulation of potential respiratory pathogens by pH1N1 viral infection. Clinical Microbiology and Infection. 2013;19(10):930–935. doi: 10.1111/1469-0691.12054. [DOI] [PubMed] [Google Scholar]
- 35.Pragman A. A., Kim H. B., Reilly C. S., Wendt C., Isaacson R. E. The lung microbiome in moderate and severe chronic obstructive pulmonary disease. PLoS One. 2012;7(10):p. e47305. doi: 10.1371/journal.pone.0047305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Filkins L. M., Hampton T. H., Gifford A. H., et al. Prevalence of streptococci and increased polymicrobial diversity associated with cystic fibrosis patient stability. Journal of Bacteriology. 2012;194(17):4709–4717. doi: 10.1128/JB.00566-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Millares L., Perez-Brocal V., Ferrari R., et al. Functional metagenomics of the bronchial microbiome in COPD. PLoS One. 2015;10(12):p. e144448. doi: 10.1371/journal.pone.0144448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang Y. J., Boushey H. A. The sputum microbiome in chronic obstructive pulmonary disease exacerbations. Annals of the American Thoracic Society. 2015;12(Supplement 2):S176–S180. doi: 10.1513/AnnalsATS.201506-319AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klimova B., Kuca K., Novotny M., Maresova P. Cystic fibrosis revisited - a review study. Medicinal Chemistry. 2017;13(2):102–109. doi: 10.2174/1573406412666160608113235. [DOI] [PubMed] [Google Scholar]
- 40.Scott A. Cystic fibrosis. Radiologic Technology. 2013;84(5):493–513. [PubMed] [Google Scholar]
- 41.Fodor A. A., Klem E. R., Gilpin D. F., et al. The adult cystic fibrosis airway microbiota is stable over time and infection type, and highly resilient to antibiotic treatment of exacerbations. PLoS One. 2012;7(9):p. e45001. doi: 10.1371/journal.pone.0045001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parkins M. D., Floto R. A. Emerging bacterial pathogens and changing concepts of bacterial pathogenesis in cystic fibrosis. Journal of Cystic Fibrosis. 2015;14(3):293–304. doi: 10.1016/j.jcf.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 43.Lipuma J. J. The changing microbial epidemiology in cystic fibrosis. Clinical Microbiology Reviews. 2010;23(2):299–323. doi: 10.1128/CMR.00068-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu L., Xu X., Lv H., Qiu Z. Advances in upper airway cough syndrome. Kaohsiung Journal of Medical Sciences. 2015;31(5):223–228. doi: 10.1016/j.kjms.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 45.Yu X., Kong L., Jiang W., et al. Etiologies associated with chronic cough and its clinical characteristics in school-age children. Journal of Thoracic Disease. 2019;11(7):3093–3102. doi: 10.2103/jtd.2019.07.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lucanska M., Hajtman A., Calkovsky V., Kunc P., Pecova R. Upper airway cough syndrome in pathogenesis of chronic cough. Physiological Research. 2020;69(Suppl 1):S35–S42. doi: 10.33549/physiolres.934400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alawi K., Keeble J. The paradoxical role of the transient receptor potential vanilloid 1 receptor in inflammation. Pharmacology & Therapeutics. 2010;125(2):181–195. doi: 10.1016/j.pharmthera.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 48.Dabrowska M., Arcimowicz M., Grabczak E. M., et al. Chronic cough related to the upper airway cough syndrome: one entity but not always the same. European Archives of Oto-Rhino-Laryngology. 2020;277(10):2753–2759. doi: 10.1007/s00405-020-06071-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gow N. A., Netea M. G. Medical mycology and fungal immunology: new research perspectives addressing a major world health challenge. Philosophical Transactions of The Royal Society B Biological Sciences. 2016;371(1709):p. 20150462. doi: 10.1098/rstb.2015.0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nguyen L. D., Viscogliosi E., Delhaes L. The lung mycobiome: an emerging field of the human respiratory microbiome. Frontiers in Microbiology. 2015;6:p. 89. doi: 10.3389/fmicb.2015.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fisher M. C., Henk D. A., Briggs C. J., et al. Emerging fungal threats to animal, plant and ecosystem health. Nature. 2012;484(7393):186–194. doi: 10.1038/nature10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Woerden H. C., Gregory C., Brown R., Marchesi J. R., Hoogendoorn B., Matthews I. P. Differences in fungi present in induced sputum samples from asthma patients and non-atopic controls: a community based case control study. BMC Infectious Diseases. 2013;13(1) doi: 10.1186/1471-2334-13-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perez-Losada M., Castro-Nallar E., Bendall M. L., Freishtat R. J., Crandall K. A. Dual transcriptomic profiling of host and microbiota during health and disease in pediatric asthma. PLoS One. 2015;10(6):p. e131819. doi: 10.1371/journal.pone.0131819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cutrera R., Baraldi E., Indinnimeo L., et al. Management of acute respiratory diseases in the pediatric population: the role of oral corticosteroids. Italian Journal of Pediatrics. 2017;43(1):p. 31. doi: 10.1186/s13052-017-0348-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matsuda K., Narita M., Sera N., et al. Gene and cytokine profile analysis of macrolide-resistant Mycoplasma pneumoniaeinfection in Fukuoka, Japan. BMC Infectious Diseases. 2013;13(1) doi: 10.1186/1471-2334-13-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lewis M., Williams D. W. Diagnosis and management of oral candidosis. British Dental Journal. 2017;223(9):675–681. doi: 10.1038/sj.bdj.2017.886. [DOI] [PubMed] [Google Scholar]
- 57.Greenhill C. Childhood weight gain and oral microbiota. Nature Reviews Endocrinology. 2018;14(12):p. 689. doi: 10.1038/s41574-018-0107-0. [DOI] [PubMed] [Google Scholar]
- 58.Hardy J., Baggott C., Fingleton J., et al. Budesonide-formoterol reliever therapy versus maintenance budesonide plus terbutaline reliever therapy in adults with mild to moderate asthma (PRACTICAL): a 52-week, open-label, multicentre, superiority, randomised controlled trial. Lancet. 2019;394(10202):919–928. doi: 10.1016/S0140-6736(19)31948-8. [DOI] [PubMed] [Google Scholar]
- 59.Hill C., Guarner F., Reid G., et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nature Reviews. Gastroenterology & Hepatology. 2014;11(8):506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 60.Pascal M., Perez-Gordo M., Caballero T., et al. Microbiome and allergic diseases. Frontiers in Immunology. 2018;9(1584) doi: 10.3389/fimmu.2018.01584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oka A., Sartor R. B. Microbial-based and microbial-targeted therapies for inflammatory bowel diseases. Digestive Diseases and Sciences. 2020;65(3):757–788. doi: 10.1007/s10620-020-06090-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yadav H., Lee J. H., Lloyd J., Walter P., Rane S. G. Beneficial Metabolic Effects of a Probiotic via Butyrate-induced GLP-1 Hormone Secretion. Journal of Biological Chemistry. 2013;288(35):25088–25097. doi: 10.1074/jbc.M113.452516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu C., Yin Z., Feng T., Zhang M., Zhou Z., Zhou Y. An integrated network pharmacology and RNA-Seq approach for exploring the preventive effect of Lonicerae japonicae flos on LPS-induced acute lung injury. Journal of Ethnopharmacology. 2021;264:p. 113364. doi: 10.1016/j.jep.2020.113364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen J., Wang J. B., Yu C. H., Chen L. Q., Xu P., Yu W. Y. Total flavonoids of _Mosla scabra_ leaves attenuates lipopolysaccharide- induced acute lung injury via down-regulation of inflammatory signaling in mice. Journal of Ethnopharmacology. 2013;148(3):835–841. doi: 10.1016/j.jep.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 65.Yu W., Li L., Wu F., et al. _Moslea Herba_ flavonoids alleviated influenza A virus-induced pulmonary endothelial barrier disruption _via_ suppressing NOX4/NF- κB/MLCK pathway. Journal of Ethnopharmacology. 2020;253:p. 112641. doi: 10.1016/j.jep.2020.112641. [DOI] [PubMed] [Google Scholar]
- 66.Ge G. F., Shi W. W., Yu C. H., et al. Baicalein attenuates vinorelbine-induced vascular endothelial cell injury and chemotherapeutic phlebitis in rabbits. Toxicology and Applied Pharmacology. 2017;318:23–32. doi: 10.1016/j.taap.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 67.Yu B., Cai W., Zhang H. H., et al. _Selaginella uncinata_ flavonoids ameliorated ovalbumin-induced airway inflammation in a rat model of asthma. Journal of Ethnopharmacology. 2017;195:71–80. doi: 10.1016/j.jep.2016.11.049. [DOI] [PubMed] [Google Scholar]
- 68.Wu Y. H., Wu Y. R., Li B., Yan Z. Y. Cryptotanshinone: a review of its pharmacology activities and molecular mechanisms. Fitoterapia. 2020;145:p. 104633. doi: 10.1016/j.fitote.2020.104633. [DOI] [PubMed] [Google Scholar]
- 69.Guo R., Zhao M., Liu H., et al. Uncovering the pharmacological mechanisms of Xijiao Dihuang decoction combined with Yinqiao powder in treating influenza viral pneumonia by an integrative pharmacology strategy. Biomedicine & Pharmacotherapy. 2021;141:p. 111676. doi: 10.1016/j.biopha.2021.111676. [DOI] [PubMed] [Google Scholar]
- 70.Xuan X., Sun Z., Yu C., et al. Network pharmacology-based study of the protective mechanism of conciliatory anti-allergic decoction on asthma. Allergol Immunopathol (Madr) 2020;48(5):441–449. doi: 10.1016/j.aller.2019.12.011. [DOI] [PubMed] [Google Scholar]
- 71.Liu L., Wang S., Xing H., Sun Y., Ding J., He N. Bulleyaconitine A inhibits the lung inflammation and airway remodeling through restoring Th1/Th2 balance in asthmatic model mice. Bioscience, Biotechnology, and Biochemistry. 2020;84(7):1409–1417. doi: 10.1080/09168451.2020.1752140. [DOI] [PubMed] [Google Scholar]
- 72.Ji N. F., Xie Y. C., Zhang M. S., et al. Ligustrazine corrects Th1/Th2 and Treg/Th17 imbalance in a mouse asthma model. International Immunopharmacology. 2014;21(1):76–81. doi: 10.1016/j.intimp.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 73.Shin H. S., See H. J., Jung S. Y., et al. Turmeric (_Curcuma longa_) attenuates food allergy symptoms by regulating type 1/type 2 helper T cells (Th1/Th2) balance in a mouse model of food allergy. Journal of Ethnopharmacology. 2015;175:21–29. doi: 10.1016/j.jep.2015.08.038. [DOI] [PubMed] [Google Scholar]
- 74.Zhao P., Liu X., Dong H., et al. Bufei Yishen formula restores Th17/Treg balance and attenuates chronic obstructive pulmonary disease via activation of the adenosine 2a receptor. Frontiers in Pharmacology. 2020;11:p. 1212. doi: 10.3389/fphar.2020.01212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhao P., Li J., Tian Y., et al. Restoring Th17/Treg balance via modulation of STAT3 and STAT5 activation contributes to the amelioration of chronic obstructive pulmonary disease by Bufei Yishen formula. Journal of Ethnopharmacology. 2018;217:152–162. doi: 10.1016/j.jep.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 76.Xu L., Li J., Zhang Y., Zhao P., Zhang X. Regulatory effect of baicalin on the imbalance of Th17/Treg responses in mice with allergic asthma. Journal of Ethnopharmacology. 2017;208:199–206. doi: 10.1016/j.jep.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 77.Ruan G., Tao B., Wang D., Li Y., Wu J., Yin G. Chinese herbal medicine formula Gu-Ben-Fang-Xiao-Tang attenuates airway inflammation by modulating Th17/Treg balance in an ovalbumin-induced murine asthma model. Experimental and Therapeutic Medicine. 2016;12(3):1428–1434. doi: 10.3892/etm.2016.3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Feng F., Du J., Meng Y., Guo F., Feng C. Louqin Zhisou decoction inhibits mucus hypersecretion for acute exacerbation of chronic obstructive pulmonary disease rats by suppressing EGFR-PI3K-AKT signaling pathway and restoring Th17/Treg balance. Evidence-Based Complementary and Alternative Medicine. 2019;2019:14. doi: 10.1155/2019/6471815.6471815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lin Y. C., Yang C. C., Lin C. H., Hsia T. C., Chao W. C., Lin C. C. Atractylodin ameliorates ovalbumin-induced asthma in a mouse model and exerts immunomodulatory effects on Th2 immunity and dendritic cell function. Molecular Medicine Reports. 2020;22(6):4909–4918. doi: 10.3892/mmr.2020.11569. [DOI] [PubMed] [Google Scholar]
- 80.Li Y., Yu Q., Zhao W., et al. Oligomeric proanthocyanidins attenuate airway inflammation in asthma by inhibiting dendritic cells maturation. Molecular Immunology. 2017;91:209–217. doi: 10.1016/j.molimm.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 81.Park S. J., Lee K., Kang M. A., et al. Tilianin attenuates HDM-induced allergic asthma by suppressing Th2-immune responses via downregulation of IRF4 in dendritic cells. Phytomedicine. 2021;80:p. 153392. doi: 10.1016/j.phymed.2020.153392. [DOI] [PubMed] [Google Scholar]
- 82.Chen X., Luo Y., Wang M., et al. Wuhu decoction regulates dendritic cell autophagy in the treatment of respiratory syncytial virus (RSV)-induced mouse asthma by AMPK/ULK1 signaling pathway. Medical Science Monitor. 2019;25:5389–5400. doi: 10.12659/MSM.917692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang W. L., Chen S. Y., Ho C. Y., Yen G. C. Citrus flavonoids suppress IL-5 and ROS through distinct pathways in PMA/ionomycin-induced EL-4 cells. Food & Function. 2020;11(1):824–833. doi: 10.1039/C9FO02815C. [DOI] [PubMed] [Google Scholar]
- 84.Lee H. Y., Lee G. H., Kim H. K., Chae H. J. Platycodi Radix and its active compounds ameliorate against house dust mite- induced allergic airway inflammation and ER stress and ROS by enhancing anti- oxidation. Food and Chemical Toxicology. 2019;123:412–423. doi: 10.1016/j.fct.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 85.Hayashi Y., Matsushima M., Nakamura T., et al. Quercetin protects against pulmonary oxidant stress via heme oxygenase-1 induction in lung epithelial cells. Biochemical and Biophysical Research Communications. 2012;417(1):169–174. doi: 10.1016/j.bbrc.2011.11.078. [DOI] [PubMed] [Google Scholar]
- 86.Chen R. J., Guo X. Y., Cheng B. H., Gong Y. Q., Ying B. Y., Lin M. X. Saikosaponin a inhibits cigarette smoke-induced oxidant stress and inflammatory responses by activation of Nrf2. Inflammation. 2018;41(4):1297–1303. doi: 10.1007/s10753-018-0778-7. [DOI] [PubMed] [Google Scholar]
- 87.Ding S., Hou X., Yuan J., et al. Wedelolactone protects human bronchial epithelial cell injury against cigarette smoke extract-induced oxidant stress and inflammation responses through Nrf2 pathway. International Immunopharmacology. 2015;29(2):648–655. doi: 10.1016/j.intimp.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 88.Zhao C. C., Xu J., Xie Q. M., Zhang H. Y., Fei G. H., Wu H. M. Abscisic acid suppresses the activation of NLRP3 inflammasome and oxidative stress in murine allergic airway inflammation. Phytotherapy Research. 2021;35(6):3298–3309. doi: 10.1002/ptr.7051. [DOI] [PubMed] [Google Scholar]
- 89.Li J., Yang X., Yang P., et al. Andrographolide alleviates bleomycin-induced NLRP3 inflammasome activation and epithelial-mesenchymal transition in lung epithelial cells by suppressing AKT/mTOR signaling pathway. Annals of Translational Medicine. 2021;9(9):p. 764. doi: 10.21037/atm-20-7973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Luo Z. L., Sun H. Y., Wu X. B., Cheng L., Ren J. D. Epigallocatechin-3-gallate attenuates acute pancreatitis induced lung injury by targeting mitochondrial reactive oxygen species triggered NLRP3 inflammasome activation. Food & Function. 2021;12(12):5658–5667. doi: 10.1039/D1FO01154E. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study is included within the article.


