Abstract
Manganese (Mn) is an abundant element in terrestrial and coastal ecosystems and an essential micronutrient in the metabolic processes of plants and animals. Mn is generally not considered a potentially toxic element due to its low content in both soil and water. However, in coastal ecosystems, the Mn dynamic (commonly associated with the Fe cycle) is mostly controlled by redox processes. Here, we assessed the potential contamination of the Rio Doce estuary (SE Brazil) by Mn after the world’s largest mine tailings dam collapse, potentially resulting in chronic exposure to local wildlife and humans. Estuarine soils, water, and fish were collected and analyzed seven days after the arrival of the tailings in 2015 and again two years after the dam collapse in 2017. Using a suite of solid-phase analyses including X-ray absorption spectroscopy and sequential extractions, our results indicated that a large quantity of MnII arrived in the estuary in 2015 bound to Fe oxyhydroxides. Over time, dissolved Mn and Fe were released from soils when FeIII oxyhydroxides underwent reductive dissolution. Due to seasonal redox oscillations, both Fe and Mn were then re-oxidized to FeIII, MnIII, and MnIV and re-precipitated as poorly crystalline Fe oxyhydroxides and poorly crystalline Mn oxides. In 2017, redox conditions (Eh: −47 ± 83 mV; pH: 6.7 ± 0.5) favorable to both Fe and Mn reduction led to an increase (~880%) of dissolved Mn (average for 2015: 66 ± 130 μg L−1; 2017: 582 ± 626 μg L−1) in water and a decrease (~75%, 2015: 547 ± 498 mg kg−1; 2017: 135 ± 80 mg kg−1) in the total Mn content in soils. The crystalline Fe oxyhydroxides content significantly decreased while the fraction of poorly ordered Fe oxides increased in the soils limiting the role of Fe in Mn retention. The high concentration of dissolved Mn found within the estuary two years after the arrival of mine tailings indicates a possible chronic contamination scenario, which is supported by the high levels of Mn in two species of fish living in the estuary. Our work suggests a high risk to estuarine biota and human health due to the rapid Fe and Mn biogeochemical dynamic within the impacted estuary.
Keywords: Estuarine soils, Manganese contamination, Iron oxides, Redox processes, Toxicity
1. Introduction
Manganese (Mn) is a widely distributed element in terrestrial and coastal ecosystems but usually occurs as trace amounts in most organisms (Levy and Nassetta, 2003; Pinsino et al., 2012). It is found in rocks (e.g., Mn content in Basalt: 1300 mg kg−1; Gneiss: 600 mg kg−1; Limestone: 550 mg kg−1; Graham et al., 1988), associated with different primary soil minerals (Mn content in amphiboles: 400–7000 mg kg−1; olivines: 100–6500 mg kg−1; pyroxenes: 600–8000 mg kg−1; Graham et al., 1988), and dissolved in natural waters (e.g., dissolved Mn in oceanic water ranges from 0.2 to 5.0 nmol kg−1; Graham et al., 1988), exhibiting unique redox dynamics (as MnII, MnIII, and MnIV) with MnIV being the most abundant form found in minerals (Burdige, 1993; Fischel et al., 2015).
For all living organisms, Mn is required in small amounts playing important roles in the maintenance of different biological functions and life (Arndt et al., 2014; Baly, 1989; Pinsino et al., 2012). For instance, in plants, Mn plays a key role in enzymatic activities and cell division (Broadley et al., 2012), while in humans and animals Mn acts as a protein transporter, is involved in neurological functions, and can also affect enzymatic activities (Fitsanakis et al., 2010). The required trace amounts of Mn considered beneficial to life are variable. In plants, such as soybean and corn, the critical quantity of Mn to reach toxic levels is 200 mg Mn kg−1 leaf dry weight (El-Jaoual and Cox, 1998). For humans, the World Health Organization, (WHO, 2011) set adequate intake levels for Mn at 2–3 mg day−1.
The many physiological roles of Mn have often masked the perception of its potential toxicity, hence studies are rare that focus on the severe toxic effects produced by this element in different environments such as water, soil, and air (Finkelstein and Jerrett, 2007; Huang et al., 2016; Li et al., 2007). Thus, Mn often remains unnoticed as a contaminant due to its role as a micronutrient for plants and animals and to its ubiquity in the environment (Pinsino et al., 2012; Sigel and Sigel, 2000). However, consumption of high Mn concentrations may cause severe adverse health effects such as a neurodegenerative disorder (Levy and Nassetta, 2003; Sandilyan and Kathiresan, 2014; Singh et al., 2010), cardiovascular toxicity (Jiang and Zheng, 2005), and liver damage (O’Neal and Zheng, 2015). In marine coastal ecosystems such as estuaries, few studies reported Mn as a potentially toxic element since the concentrations are generally low in these ecosystems (Hadlich et al., 2018; McKinley et al., 2019). However, recent studies worldwide, motivated by increasingly large inputs of Mn from human activities, such as mining activity, mining waste, and urban waste, have reported Mn as a potential contaminant for several aquatic species and, thus, its toxicity (Gabriel et al., 2020a; Harford et al., 2015; McKinley et al., 2019; Squadrone et al., 2016; Summer et al., 2019).
The risks associated with Mn are dynamic within estuaries since its bioavailability is driven by oscillating redox and acid-base conditions, leading to many possible fates (e.g., precipitation, adsorption, solubilization) and interactions with other mineral phases (carbonates, oxides, sulfates, and sulfides) (Du Laing et al., 2009b; Namgung et al., 2020; Otero et al., 2009; Thamdrup et al., 1994). Previous studies reported the precipitation of Mn with carbonates under anoxic conditions (Rhodochrosite; Lee et al., 2011; Zachara et al., 1991); at the same time, manganese sulfides (MnS), despite the restricted range of geochemical conditions favorable for their formation and stability, have also been reported under anoxic conditions (Lee et al., 2011; Stumm and Morgan, 1996). The oscillating redox conditions common in estuarine soils may also lead to Mn interactions with Fe oxyhydroxides (Mn associated with Fe; Burdige, 1993; Thamdrup et al., 1994) and formation of Mn oxides (e.g., birnessite; (Postma, 1985; Jacobsite; Burdige, 1993) during oxidizing periods. Thus, the Mn biogeochemical cycle is widely reported as closely associated with the Fe biogeochemical cycle (Burdige, 1993; Lewis and Landing, 1991; Slobodian and Badoz, 2019; Van Cappellen et al., 1998). In fact, these elements are involved in a wide spectrum of biogeochemical pathways such as mineral dissolution, microbial processes, flux-control of trace metals, the formation of a wide array of highly reactive solid phases (Fe and Mn oxy-hydroxides), and the biogeochemical cycles of other major elements (e.g. carbon, sulfur, and phosphorus; Borch et al., 2010; Duckworth et al., 2009). Therefore, coupled studies of Fe-Mn are crucial to advancing our understanding of a wide range of elemental cycles coupled with mechanisms that contribute to environmental contamination, particularly by manganese.
In 2015, a large-scale mine tailings dam disaster occurred in Brazil releasing 43 million m3 of Fe-rich tailings into the Rio Doce, one of the country’s largest river basins. The tailings were transported approximately 600 km downstream and reached the estuary and the ocean 16 d after the dam collapse (de Gomes et al., 2017; Queiroz et al., 2021). The disaster represents one of the largest failures of a tailings dam ever recorded and the largest environmental disaster in Brazil’s mining history (Carmo et al., 2017), also killing 19 people and causing extensive ecological (e.g., soil and water pollution; Bernardino et al., 2019; Gabriel et al., 2020b; Queiroz et al., 2018), economic, social and cultural damages (Fernandes et al., 2016).
In addition to the high content of Fe, previous studies have reported the presence of trace metals (e.g, Cu, Cr, Ni, and Zn) in the estuarine soils following the mine tailing contamination (Gomes et al., 2017; Queiroz et al., 2018). These metals arrived in the Rio Doce estuary associated with mine tailings, which are predominantly composed of Fe oxyhydroxides that have strong affinity with metals (Gabriel et al., 2020a; Queiroz et al., 2018). Among the reported elements, Mn does not have a threshold for soil quality according to Brazilian legislation for contaminated soil (CONAMA, 2009), but may pose different risks to the estuarine environment due to its naturally high affinity to Fe and its fast dynamics in redox active environments (Andreji and Stráňai, 2007; Gabriel et al., 2020b; Kennish, 2002; McKinley et al., 2019).
It is not surprising that Mn has not yet been reported as a contaminant in the Rio Doce estuary since Mn contamination in estuarine ecosystems is often overlooked (McKinley et al., 2019; Pinsino et al., 2012). We hypothesize that due to the redox environment in the Rio Doce estuarine soils, the Fe oxyhydroxides will act as sources of Mn leading to a potential risk of Mn contamination. Accordingly, the objective of this study was to evaluate the potential risk of Mn contamination in the Rio Doce estuary two years after the tailings arrival. We assessed the geochemical mechanisms controlling Mn bioavailability coupled to Fe dynamics in a redox active environment with Fe enrichment, to serve as a basis for public policies in coastal wetlands with potential risk of Mn contamination. Thus, the Rio Doce estuary offers a unique framework to evaluate the role of Fe oxyhydroxides controlling the Mn cycle and the environmental health in estuarine ecosystems.
2. Materials and methods
2.1. Site description
The Rio Doce estuary (19°37′51.45′′S, 39°48′54.62′′W) is located in SE-Brazil with a humid tropical climate classified as Am, according to the Köppen-Geiger climate classification system, presenting two distinct seasons including dry winters (from April to September) and wet summers (from October to March; Alvares et al., 2013; Bernardino et al., 2015). Eleocharis acutangular, Typha domingensis, and Hibiscus tiliaceus are the dominant local plant species. The Rio Doce basin is within the Brazilian Iron Quadrangle, a region rich in rocks such as itabirites with highly concentrated ores of Fe, Mn, and Al and where mining activity (e. g., iron, gold, bauxite, and manganese) is of great economic importance (ANA, 2020; de Rodrigues et al., 2014; Silva et al., 2017). In 2015, the Rio Doce estuary was the final destination of the Fe-rich mine tailings that were dumped into the river basin after the Mariana mining dam collapse (Gomes et al., 2017).
2.2. Sample collection
The estuarine soils were sampled in 2 periods: (i) in 2015, seven days after the arrival of the tailings to the estuary (for more details see Queiroz et al., 2018); and (ii) two years after the dam collapse, in 2017, to evaluate possible temporal variations. Samplings were performed during the same season in both campaigns (i.e., the wet season). Soil cores were collected using PVC tubes attached to a flooded soil sampler at four different sites in 2015. In 2017, cores were collected from eight sites, including the four sites sampled in 2015, to achieve a more comprehensive representation of sites affected by tailings deposition (Fig. 1). Additionally, a mine tailings sample collected inside the dam at the site of rupture located at Bento Rodrigues City, Minas Gerais, was donated by the Brazilian National Mining Agency (Aĝencia Nacional de Mineração – ANM) and analyzed to determine the total Mn contents.
Fig. 1.
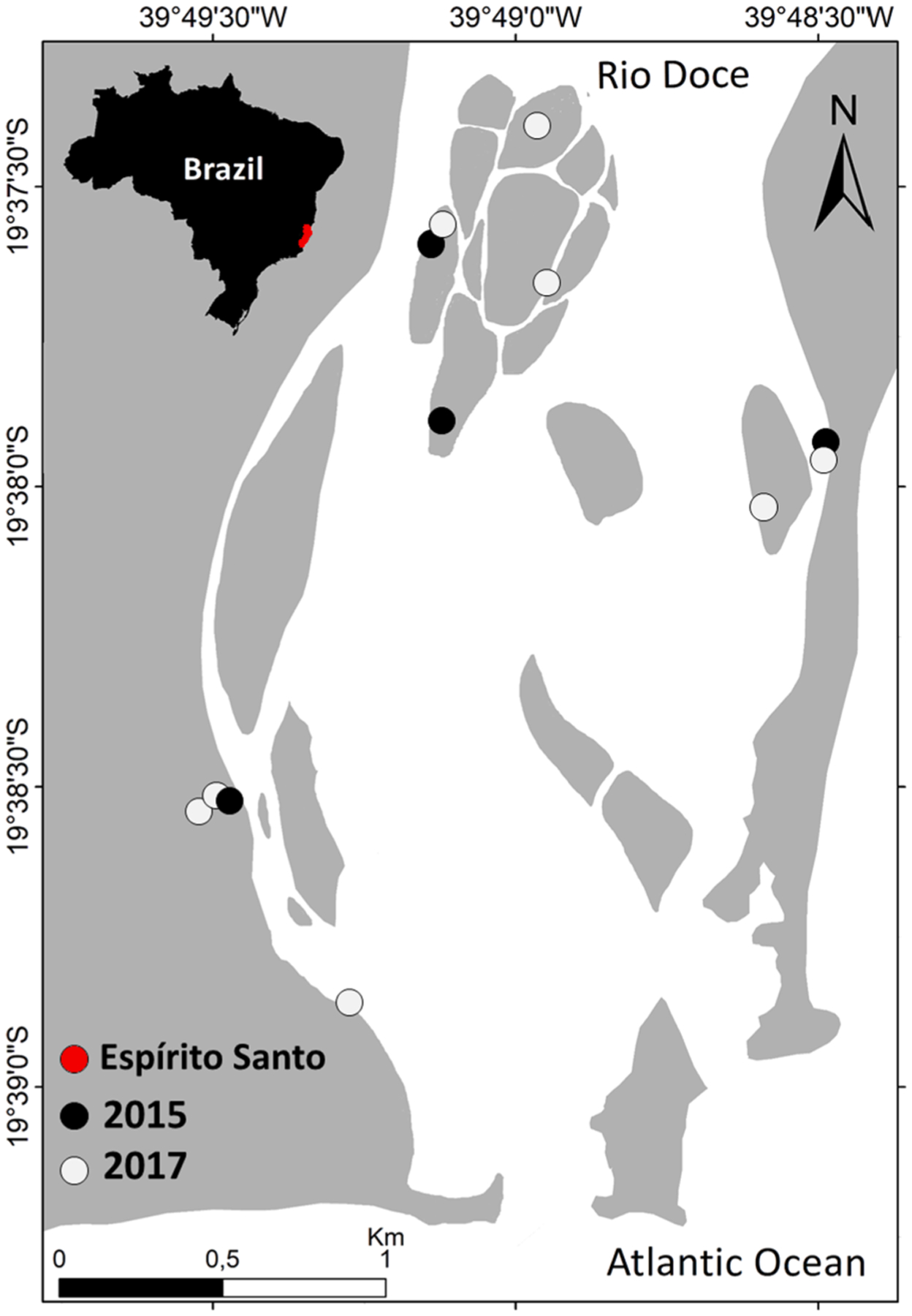
Location of soil sampling sites in 2015 and 2017 in the Rio Doce Estuary, Reĝencia, Espírito Santo, Brazil.
After sampling, cores were hermetically sealed and transported upright to the laboratory. In the laboratory, cores were sectioned at different depths depending on the year they were collected. In 2015 the samples were sectioned into 0–3, 3–5, 5–10, and 15–30 cm sections, totaling 21 samples (n = 21); whereas the samples collected in 2017 were sectioned into 0–3, 3–5, 5–10, 10–15, 15–20, 20–25, 25–30, and 30–35 cm intervals (total of 60 samples), to obtain a higher resolution of changes along the soil profiles.
Redox potential (Eh) and pH values of soils were determined in the field using portable meters and an electrode system using samples collected with a semi-open cylindrical soil auger. The pH meter was calibrated at pH 4.0 and 7.0 with standard solutions and the Eh meter measurements used a calomel reference electrode (+244 mV S.H.E.).
Water samples were collected in both years (n = 10 and n = 20 for the years 2015 and 2017, respectively), filtered (pore size 0.45 μm), and acidified with 0.45 mol L−1 HCl (trace metal grade) for the determination of dissolved Mn concentration. Water samples were collected from boreholes made with PVC tubes during soil core collection representing the pore water from the saturated soil zone that naturally drains towards the river. The total Mn content in all water samples was determined using ICP-OES (Thermo Scientific – iCAP 6200).
2.3. Total contents and sequential extraction of Fe and Mn
The total contents of Fe were obtained from estuarine soil samples and total Mn contents were obtained from both estuarine soil and mine tailings from inside the dam. The total contents were determined using ICP-OES (Thermo Scientific iCAP 6200) after tri-acid digestion in a microwave (HF + HCl + HNO3; USEPA, 1996).
Sequential extraction of Fe and Mn was performed on soil samples using a combination of methods proposed by Tessier et al. (1979), Huerta-Diaz and Morse (1990), and Fortin et al. (1993) to determine 6 operationally distinct fractions:
Exchangeable and soluble Fe and Mn (EX): extracted with 30 mL of 1 mol L−1 MgCl2 solution at pH 7.0 at 4 °C, agitated continuously for 30 min.
Fe and Mn bound to carbonates (CA): obtained with 30 mL of 1 mol L−1 NaOAc at pH 5.0, agitated for 5 h.
Fe and Mn bound to ferrihydrite (FR): extracted with 30 mL of 0.04 mol L−1 hydroxylamine + acetic acid (25% v/v) solution by shaking for 6 h at 30 °C.
Fe and Mn bound to lepidocrocite (LP): extracted with 30 mL of 0.04 mol L−1 hydroxylamine + acetic acid (25% v/v) solution by shaking for 6 h at 96 °C.
Fe and Mn bound to crystalline Fe oxyhydroxides (mainly goethite; CR): extracted with 20 mL of 0.25 mol L−1 sodium citrate + 0.11 mol L−1 sodium bicarbonate with 3 g sodium dithionite, agitated for 30 min at 75 °C.
Fe and Mn associated with pyrite (PY): extracted with concentrated HNO3 (2 h agitation) previously treated with 10 mol L−1 HF for silicates removal.
2.4. X-ray absorption spectroscopy
X-ray absorption spectra from the five most representative estuarine soil samples were obtained using beamline 7–3 at the Stanford Synchrotron Radiation Lightsource. Spectra were collected at the Mn K-edge using a Si (220) crystal set with orientation φ = 90°, with the beam detuned by 50% at 7500 eV. Soil samples had been previously dried in a 95% N2: 5% H2 atmosphere before being ground using a mortar and pestle. Samples were packed into aluminum sample holders and sealed with 0.5 mil Kapton tape prior to XAS analysis under ambient conditions. For each sample, two replicate scans were obtained, and beam damage was avoided by moving fresh sample into the beam path. An inline Mn foil was used as a reference for all scans.
Calibration, normalization, and merging of replicate scans was performed using the Demeter package (version 9.26) (Ravel and Newville, 2005) with Larch running as a backend (Newville, 2013) on Windows 10. The average Mn oxidation number (AMON) of the Mn in each sample was obtained through linear combination fitting analysis of the Mn X-ray absorption near-edge structure (XANES) spectra and was performed in Athena (Ravel and Newville, 2005) using the Combo method of Manceau et al. (2012). In all cases, reference spectra from 12 pure-valent Mn species were used to perform unconstrained linear fits. Any reference yielding a negative loading was progressively removed on a per-sample basis and re-added to the reference list before fitting the next sample.
The fraction of MnII, MnIII, and MnIV and AMON were calculated from the fits according to Manceau et al. (2012). A paired-sample t-test was used to assess the difference between the means of the AMON data corresponding to soil samples. For the assessment of the relative fraction of Mn distributed between adsorbed MnII and Mn oxide phases, linear combination fitting of EXAFS was used. For this technique, three MnII standards, 6 MnIII,IV oxides, and 2 Mn oxides containing MnII were used to fit the spectra. EXAFS references were a mixture of spectra collected in-house and those obtained by Santelli et al. (2011). The fractional weights for all oxide phases were combined and compared to the combined weight of the MnII standards prior to comparison of means through a paired-sample t-test. Fits were conducted between K = 3 and K = 11. XANES and EXAFS data from the standards used in all linear combination fitting, as well as the XANES linear combination fittings and data are available in the SI.
2.5. Diffuse reflectance spectroscopy
Diffuse reflectance spectroscopy (DRS) was used for the mineralogical characterization of soil samples. DRS spectra were measured from 300 to 800 nm at 1 nm increments with a 110 mm integrating sphere using a Varian Cary 5 Spectrophotometer. The results were transformed using the Kubelka Munk function to calculate the second derivative. Spectra from surface soil samples (0–3 cm, from 2015 and 2017) and subsurface samples (30–40 cm, depth with lower tailings deposition influence) from 2017 were obtained to compare the estuarine soil composition after tailings deposition as well as mineralogical changes over time.
2.6. Fish collection and analyses of metal contents in tissues
To assess the risk of Mn contamination to local wildlife, two fish species were collected in 2017 using a bottom Otter Trawl, at random locations in proximity to the soil sampling sites (Fig. 1). Cathorops spixii (Agassiz, 1829) (Madamango sea catfish; n = 15) and Genidens genidens (Valenciennes, 1839) (Guri sea catfish; n = 18) are estuarine species which spend their entire life cycle associated with bottom sediment. In addition, these species have been used previously as bioindicators of pollution and are an important food resources for the local population (Azevedo et al., 2009; Pinheiro and Joyeux, 2007).
After collection, fish were immediately frozen until dissection in the laboratory. Fish liver and axial muscle tissues were dissected and stored at −80 °C until quantification of total metal contents. The total contents of Mn and Fe were determined using approximately 100 mg of dried sample (muscle or liver) weighed in sterile polypropylene tubes, followed by the addition of 1.0 mL of double-distilled HNO3. The blanks containing only 1.0 mL of double-distilled HNO3 were prepared in triplicate. The DORM-4 (Dogfish muscle – National Research Council, Canada) Certified Reference Material (CRM) was used for quality control. The samples, blanks, and CRM were left in contact with HNO3 for approximately 12 h overnight then heated for digestions the following morning on a digester block for 4 h at approximately 100 °C. The closed tubes were monitored hourly with manual pressure relief when necessary. After heating, the samples, CRM, and blanks were left to cool until room temperature and made up to appropriate volumes with ultra-pure water (resistivity > 18.2 MΩ). The Mn and Fe quantification were performed by ICP-MS using an ICP-MS ELAN DRC II (Perkin-Elmer Sciex, Norwalk, CT, USA). 103Rh was used as the internal standard at 20 μg L−1.
2.7. Contamination factor determination
The contamination factor (Cf) was used to evaluate the Mn contamination at Rio Doce estuary, using as a background value the total Mn contents in the soils 11 d before the tailings arrival reported by Gomes et al., (2017). The Cf is a ratio between the content of an element in a soil sample and the background content of the same element at the studied site (Hakanson, 1980), following the equation Cf = Cs / Cb, where Cs is the soil content of Mn in 2017 and Cb is the Mn background value. According to Hakanson (1980), the following interpretations are suggested for the Cf value: Cf < 1, low; 1 to < 3, moderate; 3 to < 6, considerable; and > 6, high contamination.
2.8. Statistical analyses
The Fe and Mn total contents in soil and water samples were assessed with a non-parametric Kruskal–Wallis (p < 0.05) test to assess differences between 2015 and 2017, whereas the Fe and Mn contents in fish muscles and livers were analyzed with a non-parametric Friedman test at the 5% significance level with multiple pairwise comparisons (Reimann et al., 2008; XLSTAT version 2014.5.03). Non-parametric statistical tests are appropriate for non-normal distributions and rely on fewer assumptions, which make them more robust for environmental data (Reimann et al., 2008). The correlations between the total of Fe and Mn in soil were determined by calculating Spearman’s correlation coefficients (r) as this method does not assume a normal distribution.
3. Results
3.1. Physicochemical conditions, Fe and Mn total contents and fractionating
The Eh and pH values in 2015 were on average + 218 ± 116 mV and 6.2 ± 1.3, respectively. In 2017, the pH remained close to neutral (average 6.7 ± 0.5) but the Eh values decreased considerably with a mean of −47 ± 83 mV (Fig. 2).
Fig. 2.
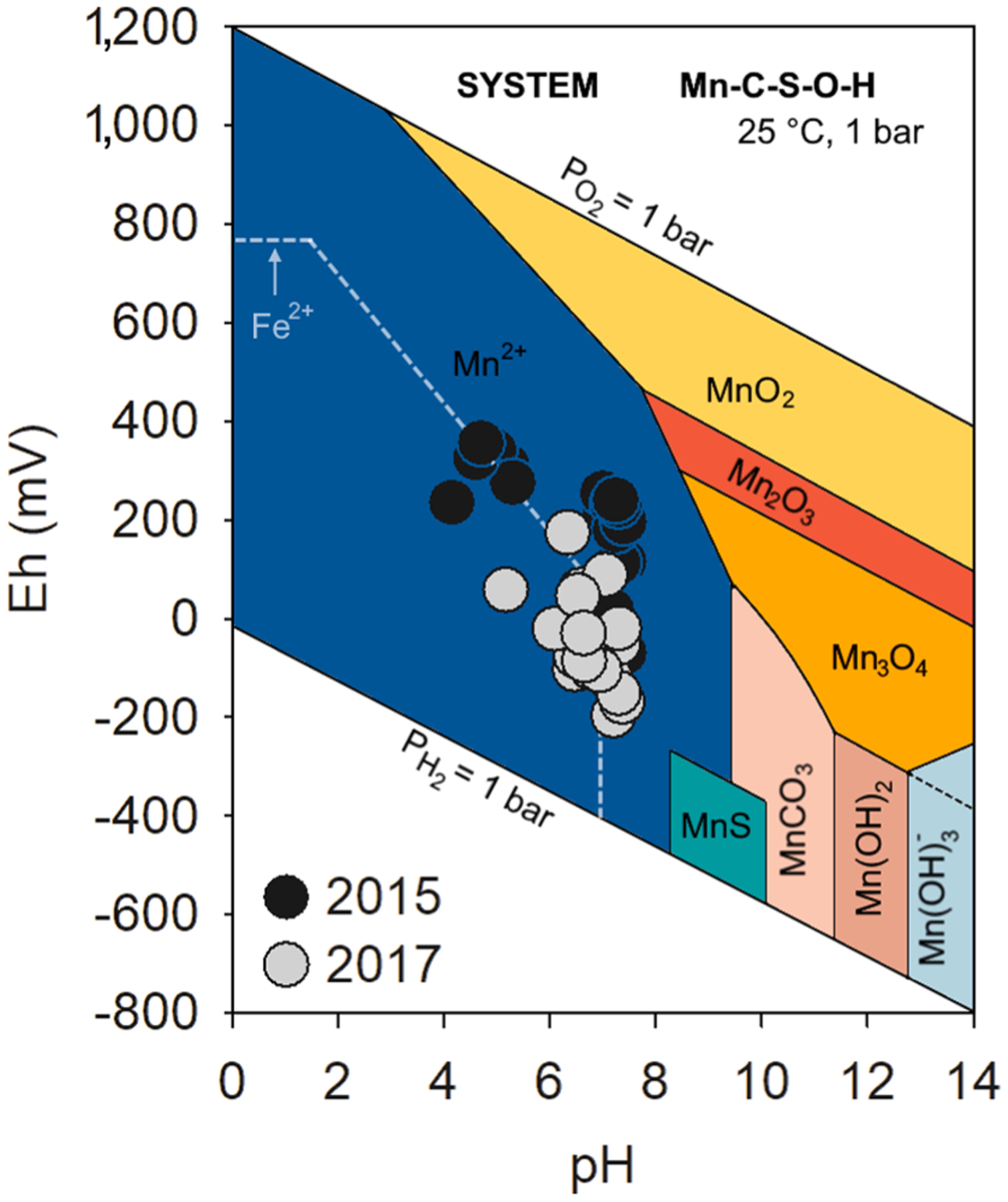
Eh-pH diagram (system Mn-C-S-O-H) with the data for the studied soils in both years. The gray dashed line indicates the Fe2+ stability field on the system Fe-C-O-H. The Eh-pH diagram was adapted from Brookins (1988).
The total Mn content in mine tailings from inside the Fundão Dam was on average 644 ± 241 mg kg−1 whereas in the estuarine soil, in 2015, higher total Fe (47,133 ± 16,538 mg kg−1) and Mn (704 ± 529 mg kg−1) contents were measured in the surface soil layers (0–3 cm), the soil layer most affected by tailings deposition (Fig. 3). Two years later, the mean total Fe and Mn concentrations decreased by 75% and 74% respectively across all soil depths. In 2017, the highest Fe and Mn contents were still found in the upper 0–3 cm (11,997 ± 8,239 mg kg−1 and 186 ± 120 mg kg−1 respectively), followed by a decrease of both elements with soil depth (depths > 3 cm; Fig. 3). The contamination factor (Cf) using the Mn content in the 0–3 soil layer just after the tailing arrival (in 2015) was 3.2 indicating considerable contamination levels, whereas in 2017 the Cf decreased to 0.84 indicating low contamination.
Fig. 3.
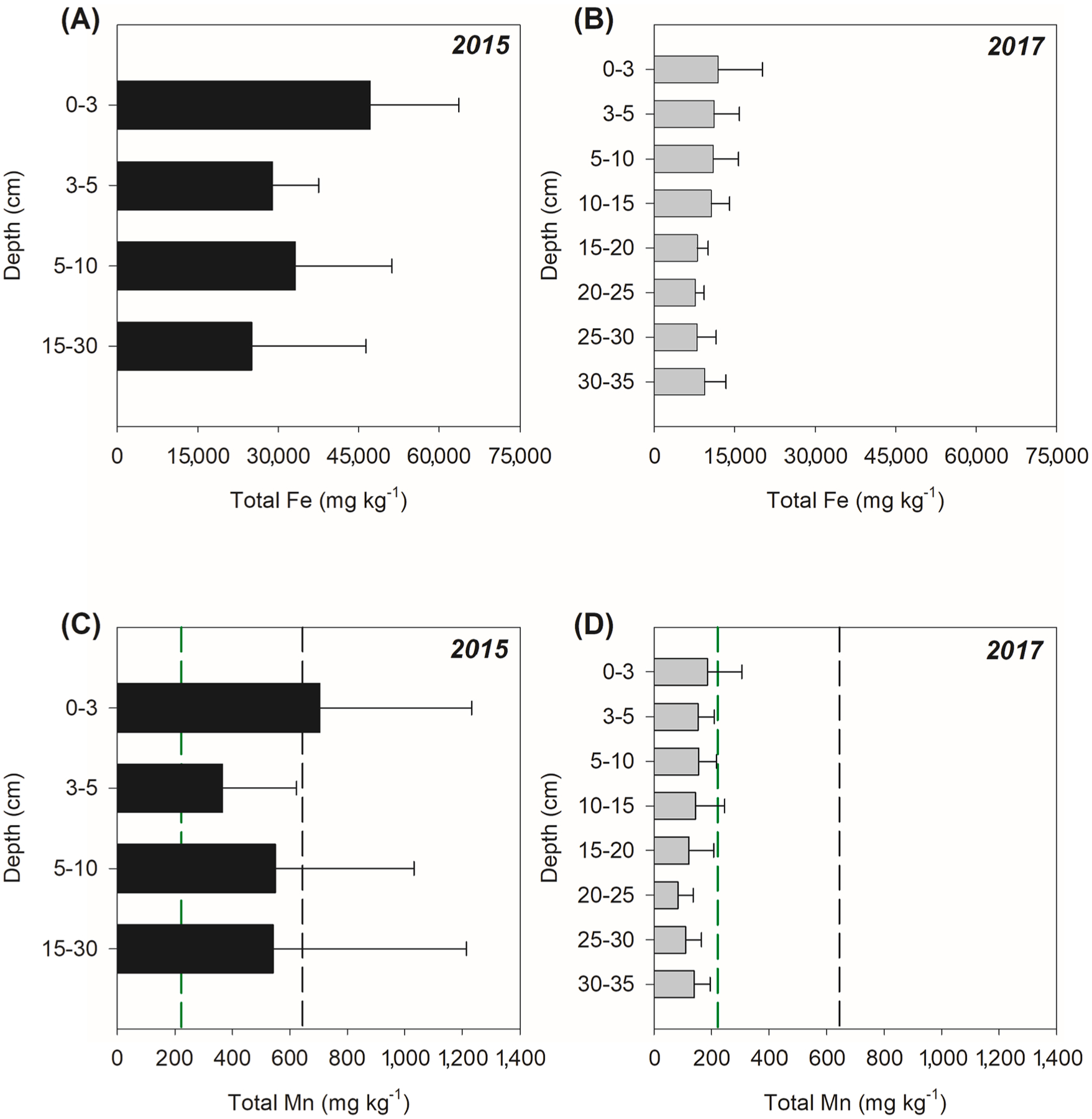
Total Fe and Mn contents of Rio Doce estuarine soils collected in 2015 and 2017. The black dashed line indicates the contents of the total Mn in tailings from inside the Fundão dam. The green dashed line indicates the Mn contents in Rio Doce estuarine soil, prior to mine tailings arrival according to Gomes et al., (2017).
Solid-phase Fe and Mn fractionation of soils collected in 2015 (Fig. 4) shows Fe mostly held in crystalline Fe oxyhydroxides representing 88% of total Fe (on average: 64,154 ± 45,104 mg kg−1), whereas poorly crystalline Fe oxyhydroxides represented only 11% (i.e., average: LP: 4,632 ± 3,635 mg kg−1 equivalent to 6%; and FR: 3,645 ± 3,573 mg kg−1 equivalent to 5%; Fig. 4). The sum of EX, CA, and PY fractions were approximately 1% of the total Fe.
Fig. 4.
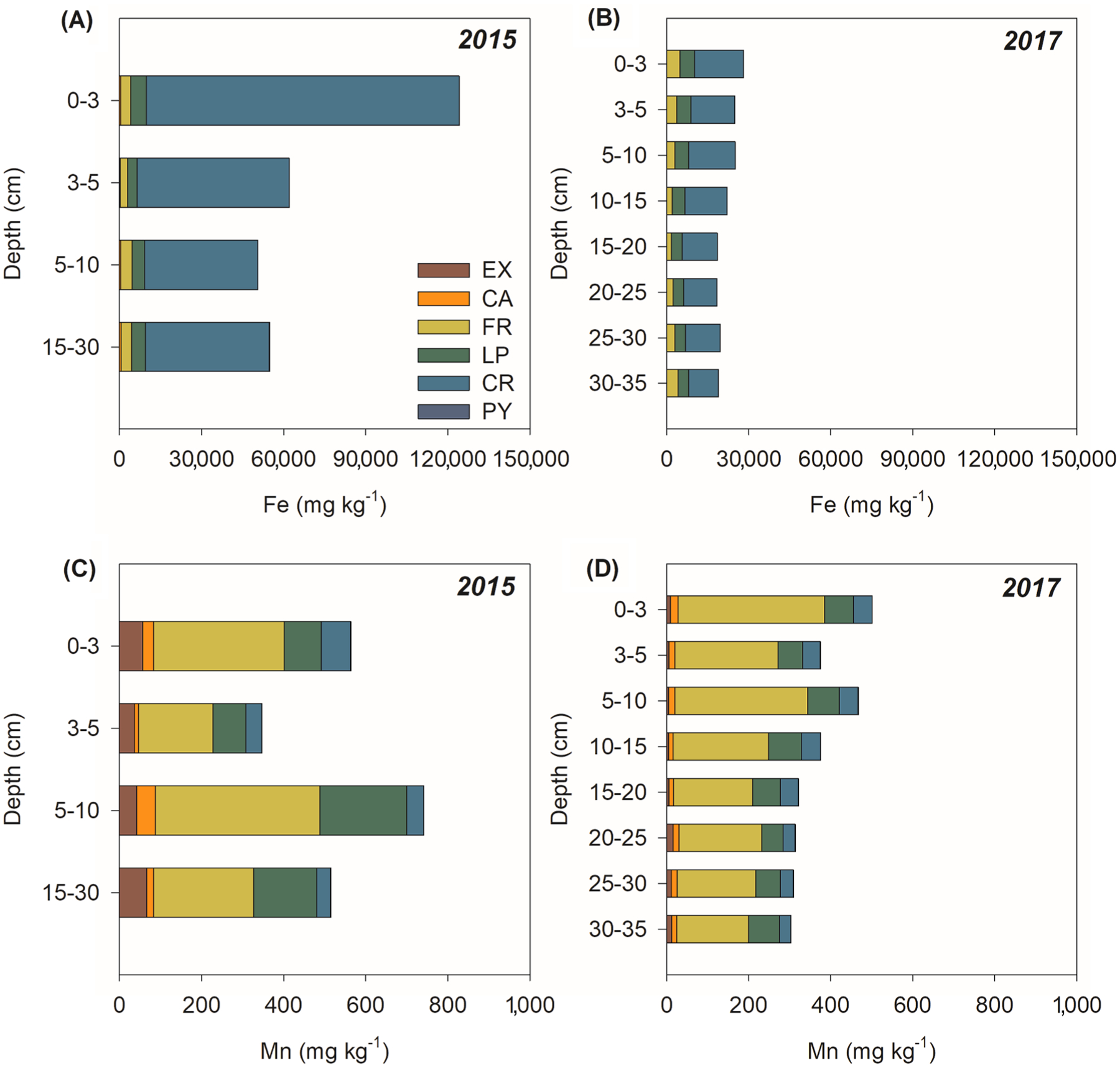
Fe and Mn solid-phase fractionation from Rio Doce estuarine soils in 2015 and 2017. EX: Soluble and exchangeable Fe and Mn; CA: Fe and Mn associated with carbonates; FR: Fe and Mn associated with ferrihydrite; LP: Fe and Mn associated with lepidocrocite; CR: Fe and Mn associated with crystalline oxides; PY: Fe and Mn associated with pyrite.
In contrast, Mn was mainly associated (78%) with poorly crystalline Fe oxyhydroxides (FR: 286 ± 352 mg kg−1; LP: 134 ± 171 mg kg−1) and to a lesser degree (9%) with the crystalline (CR) fraction (46 ± 27 mg kg−1). Soluble and exchangeable (EX) fractions represented 9% (50 ± 78 mg kg−1) and Mn associated with carbonates (CA: 25 ± 37 mg kg−1) and pyrite (PY: 25 ± 37 mg kg−1) represented about 5%.
In 2017, Fe showed a contrasting distribution to that of 2015, with a marked decrease in CR (14,326 ± 2,507 mg kg−1; equivalent to 65%) followed by a significant increase in LP (4,493 ± 732 mg kg−1; equivalent to 20%), and FR (3,042 ± 1,051 mg kg−1; equivalent to 20%). On average, the crystalline Fe oxyhydroxide contents decreased 49,828 mg kg−1 when compared to 2015 (Fig. 4). The other fractions (i.e., EX, CA, and PY) remained close to 1% of the sum of all Fe fractions. Similar to 2015, in 2017 Mn was mostly associated with poorly crystalline Fe oxyhydroxides (FR: 241 ± 67 mg kg−1; equivalent to 65%; LP: 68 ± 10 mg kg−1; equivalent to 18%) and to a lesser extent (11%) associated with crystalline Fe oxyhydroxide phases (CR: 39 ± 8 mg kg−1; Fig. 4). In contrast, the soluble and exchangeable Mn fraction decreased considerably (EX: 9 ± 4 mg kg−1; equivalent to 2% of total Mn) and Mn associated with carbonates and pyrite represented the less important Mn fractions found in the solid-phase in that year (14 ± 3 mg kg−1 and 0.4 ± 0.1 mg kg−1, respectively; representing about 4% of Mn).
3.2. Spectral reflectance characteristics
The DRS spectra corroborated the solid-phase fractionation and indicated a greater presence of both high- and low- crystallinity Fe oxyhydroxides in the surface soil layers (i.e., 0–3 cm) in both years. Deeper soil layers (30–35 cm) were less influenced by mine tailings with much smaller quantities of Fe oxyhydroxides (Fig. 5) as seen by the lower intensity of bands of iron oxides (Ji et al., 2002). It is noteworthy that band intensities changed over time, with a lower intensity of bands in 2017 corroborating a loss in Fe oxides (Figs. 3 and 4). In fact, the shift in the intensity of bands between 485 and 490 nm indicates a decrease of both lepidocrocite and goethite (absorption band at 488 nm) with time. The same patterns are observed for the absorption bands of ferrihydrite (seen between 484 and 499 nm; Scheinost, 1998) and hematite (absorption band shown at 533–588 nm; Hu et al., 2016). The Fe fractionation analyses (Fig. 4) can aid in distinguishing the relative contributions of the different iron forms to the DSR bands (Scheinost et al., 2001; Schwertmann and Taylor, 1989).
Fig. 5.
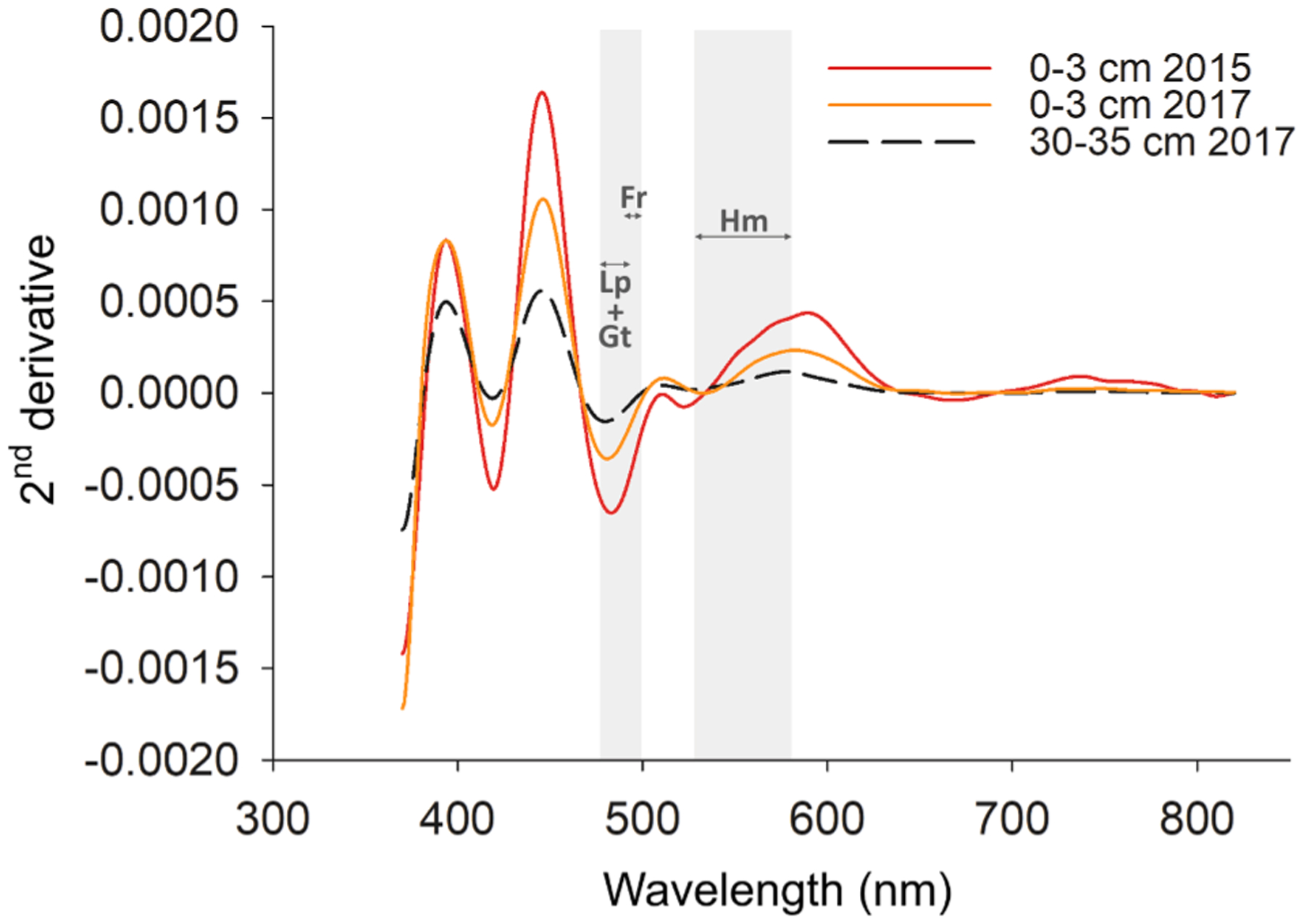
Second-derivative spectra of surface (0–3 cm depth from 2015 and 2017) and sub-superficial soil samples (30–40 cm depth from 2017). Range of crystal field band position for lepidocrocite (Lp) and goethite (Gt) (488 nm), ferrihydrite (Fr; 484–499 nm), and hematite (Hm; 533–588 nm).
3.3. XANES and EXAFS characterization
While Mn fractionation and acid digestions can quantify the total and relative mass of Mn in samples, X-ray absorption analyses including XANES and EXAFS provide Mn oxidation state and coordination information. Mn K-edge XANES showed that more than half of the Mn in the soil solid phase was reduced (52%; Table 1) in the soil surface samples (0–3 cm) from 2015 which represents the readily exchangeable or adsorbed Mn fractions (Tebo et al., 2004), while the abundance of MnIII and MnIV were 32% and 16%, respectively (Table 1).
Table 1.
The relative abundance of solid-phase MnII, MnIII, and MnIV within soil samples collected from multiple depths in 2017 and from the 0–3 cm depth in 2015 as determined by Mn K-edge XANES.
| Sample | Mn K-edg e XANES | |||
|---|---|---|---|---|
| MnII | MnIII | MnIV | AMON | |
| Rel. abundance (%)* | ||||
| 2015 (0–3 cm) | 52 | 32 | 16 | 2.626 |
| 2017 (0–3 cm) – R1 | 34 | 33 | 33 | 2.992 |
| 2017 (0–3 cm) – R2 | 36 | 48 | 15 | 2.789 |
| 2017 (30–35 cm) – R1 | 55 | 24 | 21 | 2.668 |
| 2017 (30–35 cm) – R2 | 77 | 17 | 6 | 2.290 |
AMON = Average Mn oxidation number; R1: Replicate 1; R2: Replicate 2.
Relative abundances determined using linear combination fitting.
In 2017, Mn EXAFS analysis shows that surface soil samples (0–3 cm) had a higher concentration of MnIII and MnIV than soils from 2015, where these oxidized forms of Mn were present as phyllo- and tectomanganates (Fig. 6). In contrast, the corresponding subsurface (i.e. 30–35 cm) soils showed greater concentrations of the MnII (Table 1).
Fig. 6.
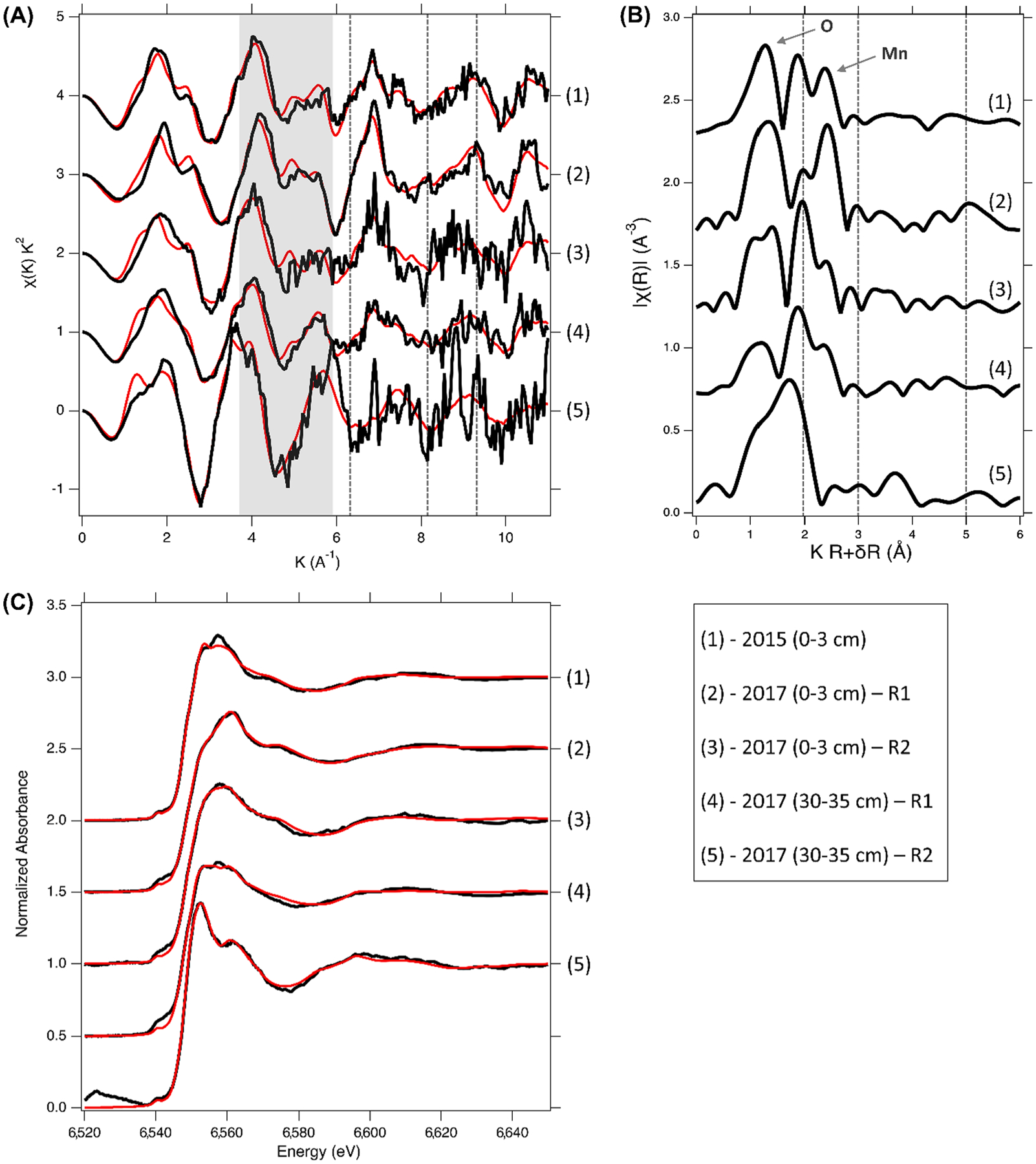
Mn K-edge EXAFS of soil samples from 2015 and 2017 collected at two depths (0–3 cm and 30–35 cm). (A) Shaded area highlights the characteristic “staircase” feature of the EXAFS indicative of phyllomanganate presence; the vertical dashed lines mark the shoulder feature at 6.5 Å−1 and the antinodes at 8.2 and 9 Å−1. (B) Pseudo-radial structure functions of the Mn EXAFS; vertical lines mark features at 2 Å, 3 Å and 5 Å; the arrows point to the nearest Mn-O and Mn-Mn shells. (C) A comparison of the Mn XANES from 2017 soil samples with the soil surface sample from 2015. Data is shown in black, model generated from linear combination fitting is shown in red.
Mn K-edge EXAFS of the surface soil samples from 2017 displayed the characteristic phyllomanganate (e.g. birnessite; δ-MnO2) “staircase” feature between 4 and 6 Å−1 (Fig. 6). Extensive corner-sharing cation octahedra dispersed in the interlayer region (i.e. MnII, MnIII, Zn, Ni) give rise to the shoulder development along the rising edge of the antinode at 6.4 Å−1 (Fig. 5); the corresponding peak at ~ 3 Å is also indicative of the presence of MnIII or MnIV (Manceau et al., 2002; Marcus et al., 2004; Toner et al., 2006). In addition, the absence of a defined antinode at 8.1 Å−1 in any of the samples suggests the presence of tectomanganates, such as pyrolusite (MnO2), as well as MnIII-rich octahedral sheets in the phyllosilicate minerals present in the 0–3 cm soil layer soil from 2017 (Marcus et al., 2004; Webb, 2005; Zhu et al., 2010).
3.4. Mn in water and in fish tissues
The mean total dissolved Mn concentration in water samples collected in 2015 was 66 ± 130 μg L−1, whereas in 2017 the average concentration increased 9-fold to 582 ± 626 μg L−1 (Fig. 7). The 2017 concentrations are 5 times higher than the threshold outlined by the Brazilian water quality guidelines for brackish waters (100 μg L−1 for inorganic constituents in brackish waters without chronic toxic effects on organisms; CONAMA, 2005). In comparison, the concentration of dissolved Mn in 2017 was higher than the threshold for chronic contamination in marine water according to the National Oceanic and Atmospheric Administration, USA (100 μg L−1; NOAA, 2008).
Fig. 7.
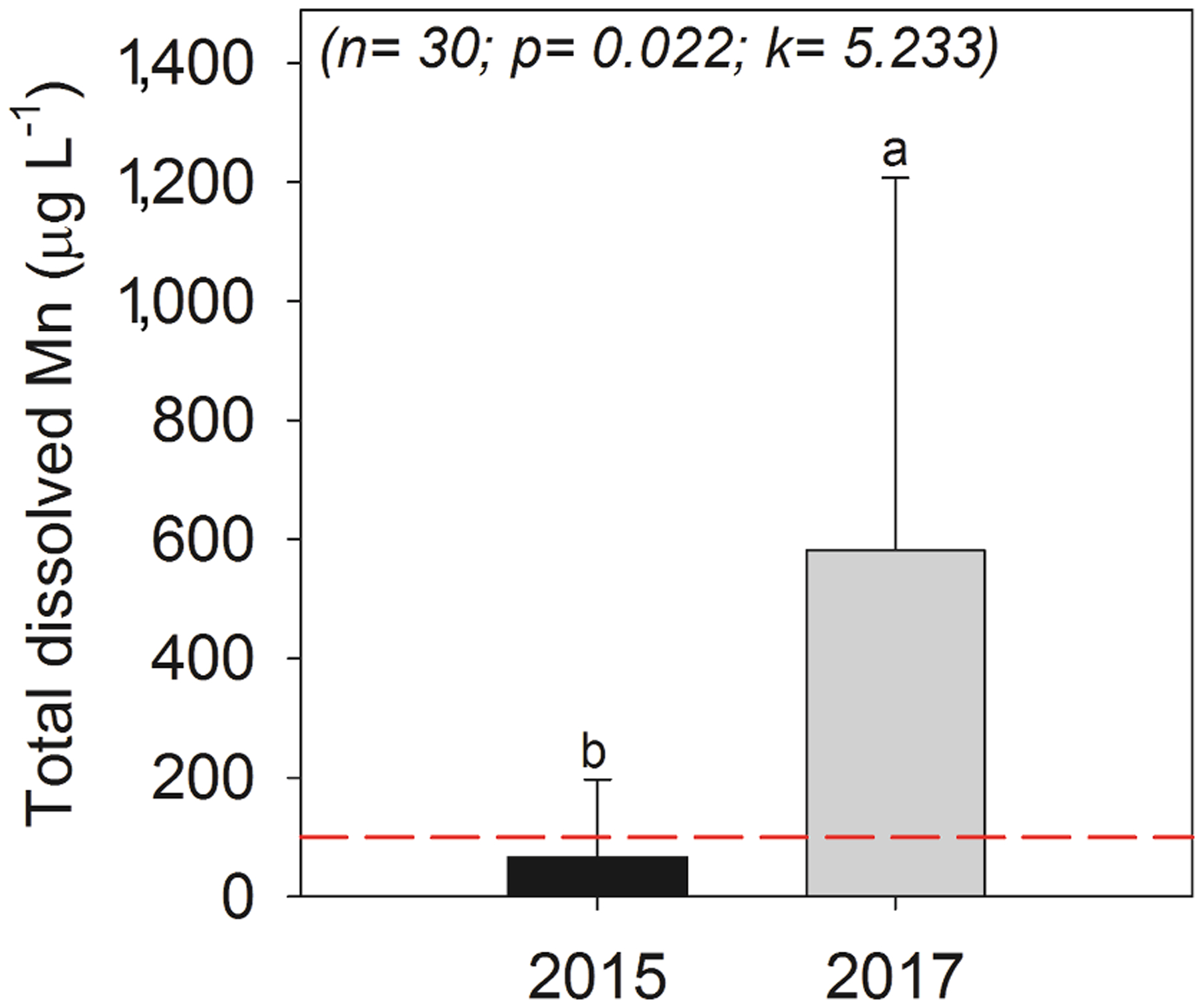
Total concentration of dissolved Mn in the Rio Doce estuary water sampled in 2015 and 2017. The red dashed line indicates the threshold according to the Brazilian water quality guidelines for brackish water (100 μg L−1; CONAMA, 2005). The different lowercase letters indicate a significant difference between the variables as determined by the Kruskal-Wallis test at the 5% probability level.
The Fe and Mn contents in fish liver and muscle tissues were significantly different, but did not differ between species (Fig. 8). Higher concentrations of Fe and Mn were observed in the liver than in the muscle (Fig. 8). The mean Fe content in the liver was 830.1 ± 638.0 mg kg−1 and 1,541.4 ± 1,725 mg kg−1 respectively in Cathoropus spixii and Genidens genidens, whereas the mean Mn contents were 3.2 ± 1.7 mg kg−1 and 2.1 ± 1.9 mg kg−1. In the muscle, Fe and Mn concentrations in Cathoropus spixii were 26.8 ± 26.4 mg kg−1 and 1.0 ± 1.0 mg kg−1, respectively; whereas in Genidens genidens mean Fe and Mn contents in muscles were 17.4 ± 11.4 mg kg−1 and 0.5 ± 0.2 mg kg−1, respectively (Fig. 8). There is no contamination threshold for either Mn or Fe for both studied fish species.
Fig. 8.
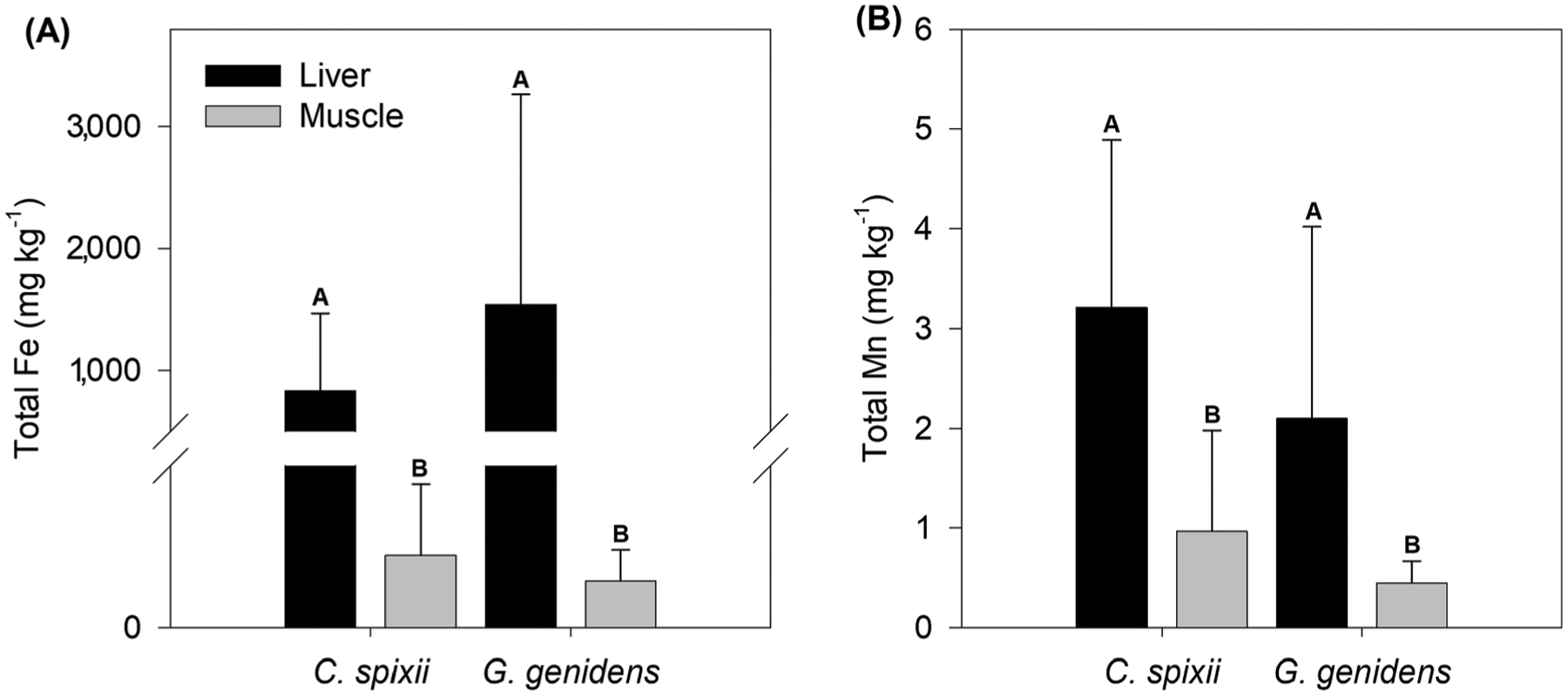
Total contents of Fe (A) and Mn (B) in the liver and muscle of Cathoropus spixii and Genidens genidens from Rio Doce. Labelled bars with uppercase letters (A and B) indicate groups between which statistical differences among species or tissues exist at the 5% probability level using the non-parametric Friedman test.
4. Discussion
4.1. Mining tailing disaster and its impacts on Mn geochemistry
In 2015, because of the Fundão dam rupture, Fe-rich tailings were dumped into the Rio Doce basin and traveled 688 km downriver toward the estuary (Gomes et al., 2017). The tailings were made mostly of crystalline Fe oxyhydroxides which have high metal retention capacity (Cornell and Schwertmann, 2003; Queiroz et al., 2018). Therefore, we hypothesized the tailings may have acted as a Mn sink until its ultimate deposition in the estuary. In addition to the high content of Mn (644 ± 241 mg kg−1) in the original tailings from inside the dam (Fig. 3), agricultural activities and pollution from large cities and villages along the basin may have acted as additional Mn sources to the mine tailings transported in the Rio Doce river on its way to the estuary (Queiroz et al., 2021). In the past, studies prior to the Mariana disaster also reported Mn contents ranging from 660 to 2,280 mg kg−1 in mine tailings from Samarco’s dams located in the same mining complex as Fundão dam (Pereira et al., 2008). Indeed, the Mn contents in the surface soil layers (0–3 cm) from 2015 (704 ± 529 mg kg−1), were on average 3-fold higher than Mn contents 11 d before the disaster (222 ± 13 mg kg−1) reported by Gomes et al., (2017) indicating the tailings deposition effects. In this sense, the Cf calculated (3.2) indicates a Mn enrichment in the soil soon after the disaster and considerable contamination. Moreover, the significant positive correlation between Fe and Mn (Fig. 9) in samples collected from 2015 support the arrival of Mn to the estuary in association with the Fe-rich mine-tailings (i.e. adsorbed to Fe oxyhydroxides).
Fig. 9.
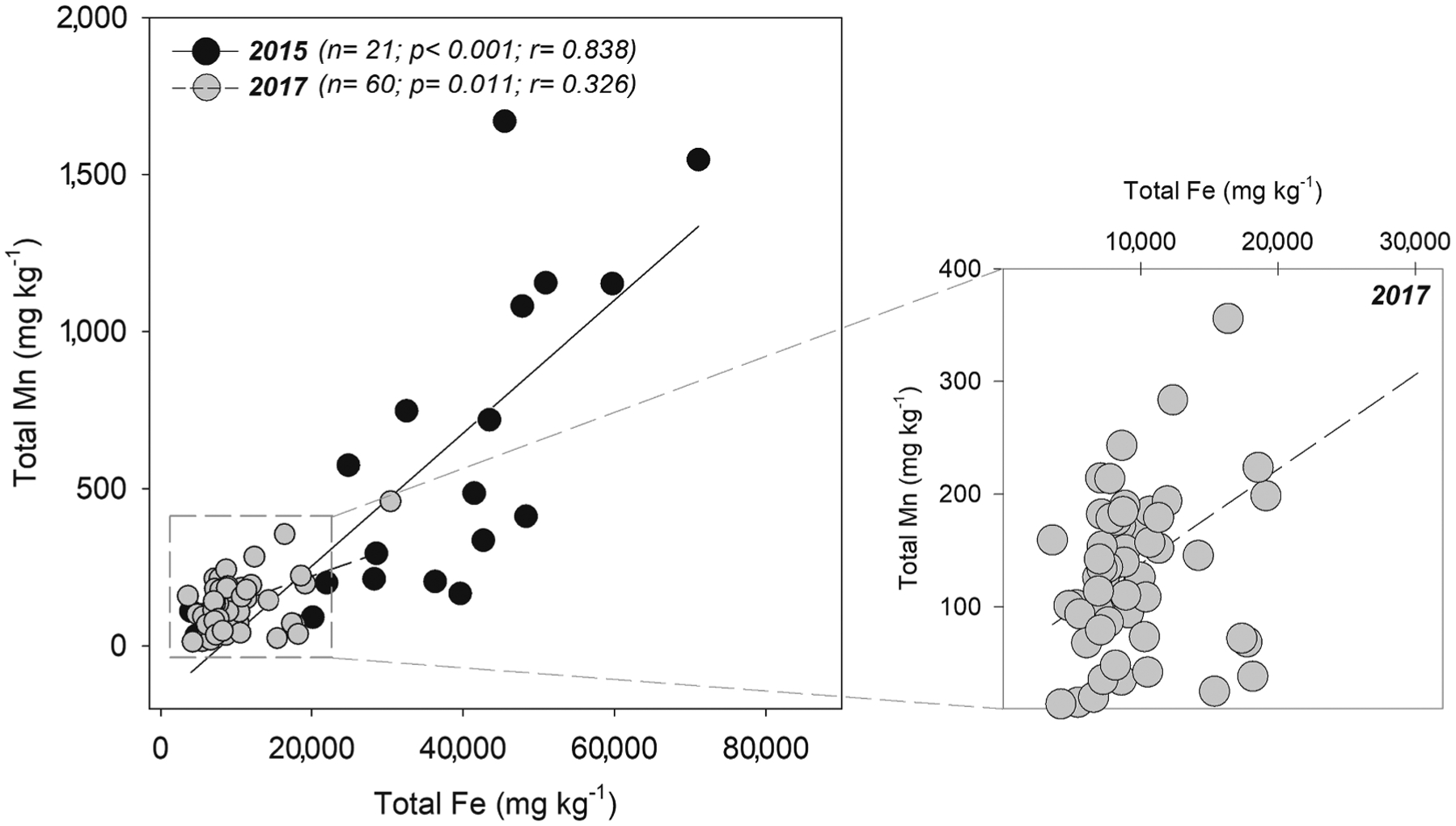
Spearman correlations between total Fe and Total Mn in 2015 and 2017. The right panel shows in detail the spearman correlation between total Fe and total Mn content from 2017 highlighting the loss of correlation with time.
The interaction of Mn with Fe oxyhydroxides in soils has been widely reported due to the energetic favorability of Mn forming mono- and binuclear inner-sphere complexes through reacting with excess structural OH− groups on the Fe oxyhydroxide surface (Ugwu and Igbokwe, 2019; Zhu et al., 2020). In general, Fe oxyhydroxides uptake the MnII forming virtually irreversible complexes (Coughlin and Stone, 1995; Namgung et al., 2020). Indeed, the EXAFS results showed higher abundance of MnII in the 0–3 cm depth range from 2015 (Table 1), as well as in the Fe fractionation, which indicates that Mn was mostly associated with Fe oxyhydroxides (FR: 53%; LP: 25%; and CR: 9%). In addition, circumneutral pH and Eh values above + 100 mV recorded in 2015 (Fig. 2) indicate suboxic conditions that are favorable to Fe oxyhydroxide formation (Reddy and DeLaune, 2008).
Once the Fe-rich tailings arrived and were deposited on the soils of the Rio Doce estuary, the tailings were then exposed to redox oscillating conditions caused by tidal flooding and plant activity (Bianchi, 2007; Du Laing et al., 2009a). By 2017, a sharp decrease in Eh (−46 ± 83 mV, on average) was observed compared to measurements in 2015 (+218 ± 116 mV, on average) indicating increasingly anoxic conditions (Fig. 2) (Reddy and DeLaune, 2008; Søndergaard, 2009). The marked decrease in Eh over time is likely due to estuarine plants (i.e., Eleocharis acutangula, Typha domingensis, and Hibiscus tiliaceus) that serve as direct inputs of organic matter (e.g. via dead leaves and roots) while also efficiently trapping particulate organic matter (OM) that is transported downstream. These plants contributing OM inputs coupled with tidal flooding stimulate anaerobic OM degradation and FeIII reduction (Badarudeen et al., 1996; Kristensen et al., 1995; Marín-Muñiz et al., 2014). Additionally, the plant growth enhances the maintenance of settled tailings since plant stems reduce the turbulence kinetics that could lead to physical tailings removal (Jay et al., 2007; Mudd et al., 2010).
Thus, Fe oxyhydroxides from the tailings were subjected to a biogeochemical environment highly favorable towards FeIII reduction to FeII and its subsequent solubilization (Cummings et al., 2000; Xia et al., 2019). In estuaries, the fate of solubilized FeII following dissimilatory Fe reduction may vary, for instance as precipitation of poorly crystalline Fe oxyhydroxide, uptake by plants, or removal from the estuary into the ocean (Canfield and Kristensen, 2005; Johnston et al., 2011; da Richard et al., 2020). The significant decrease in total Fe in soils collected in 2017 (r < 0.001; Fig. 10), mainly at the soil surface, which was the soil layer most affected by tailings deposition, clearly corroborates a massive loss of total Fe through reduction (i.e. reductive dissolution). Additionally, the total Fe loss reflected the significant loss of the CR Fe fraction (Fig. 4) which was also supported by a clear decrease in the goethite and hematite bands of the DRS spectra likely due to their dissolution (Canfield et al., 1993; Lovley et al., 2004).
Fig. 10.
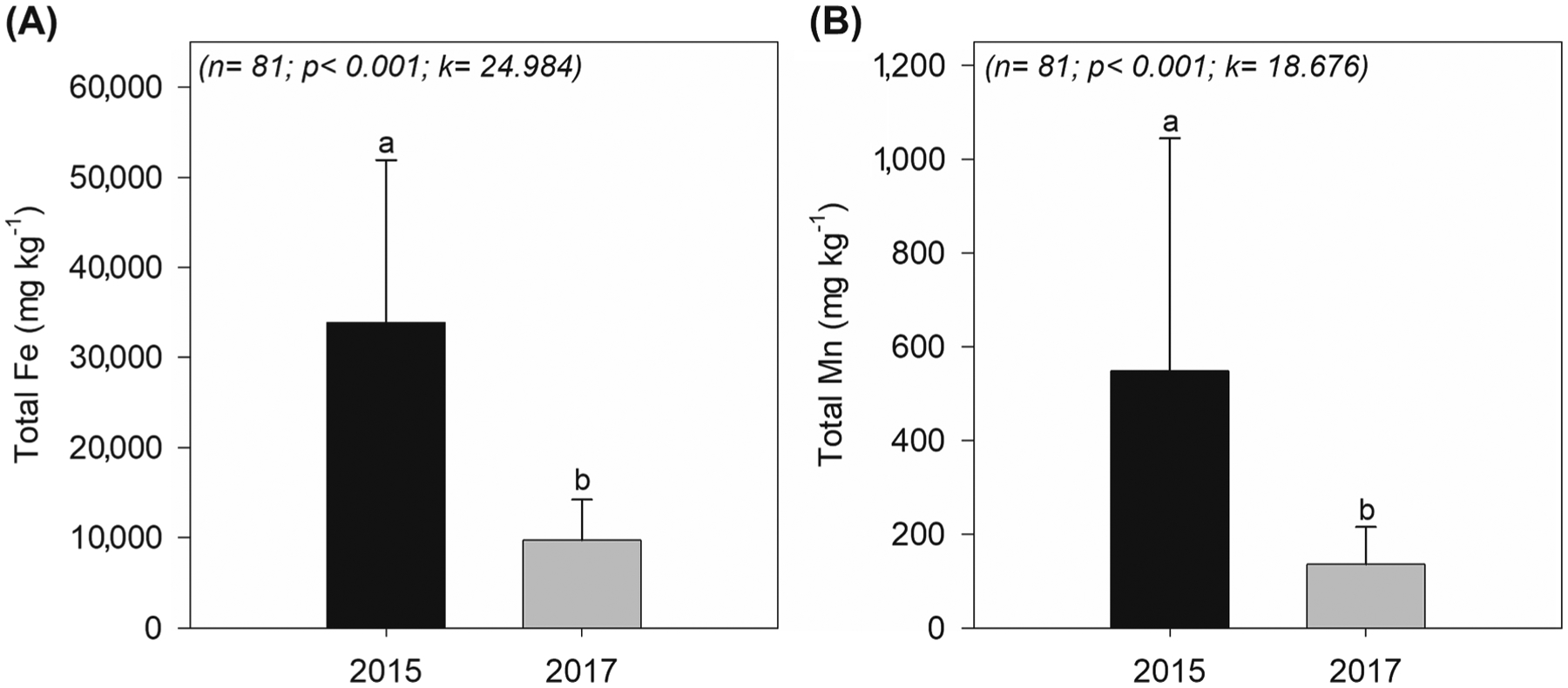
Mean Fe (A) and Mn (B) total soil contents from 2015 and 2017. The different lowercase letters indicate significant differences among the variables using the Kruskal-Wallis test at the 5% probability level.
The total Mn content in the soil from 2017 also showed a significant decrease (p-value < 0.001; about 75%) when compared to 2015 data (Fig. 10). Furthermore, the solid-phase fractionation showed that Mn associated with both high and low crystallinity Fe oxyhydroxides decreased on average 25% in 2017 (Fig. 4). Likely, the decrease of soil Mn contents was associated with its release as MnII in response to Fe oxyhydroxides dissolution. In fact, the association of Mn with high and low crystallinity Fe oxyhydroxides was clearly shown by the Mn K-edge XANES data (Table 1).
Following release and diffusion out of soils, dissolved MnII had a number of possible fates: 1) be transported further downstream and washed out from the estuary (particularly during the rainy season); 2) be retained on Fe oxyhydroxides; or 3) undergo oxidation followed by precipitation as Mn oxides (Chaudry and Zwolsman, 2008; Otero et al., 2009; Sundby et al., 2003). XANES analysis of soil samples from 2017 (Table 1) shows that a high proportion of Mn had been oxidized (i.e., MnIII and MnIV) in the soil surface layers (i.e. 0–3 cm) corresponding to EXAFS spectra with characteristics attributable to phyllo- and tectomanganates (i.e., Mn oxides; Fig. 5). According to Oldham et al. (2019) MnII can be quickly oxidized to Mn oxides in estuarine surface soils because O2 diffusion in the surface layers is more rapid than at depth.
It is likely during the first two years (2015 to 2017) after the arrival of the tailings that the prevailing conditions in the estuarine soils favored the release of MnII through reduction of Fe oxyhydroxides, with subsequent transformation of MnII into poorly crystalline Mn oxides due to redox fluctuations (Fig. 2). Previous studies reported that both dissolved Mn and Fe may re-oxidize or co-precipitate with a variety of different soil minerals (e.g. oxides, carbonates, sulfides) (Du Laing et al., 2009b; Otero et al., 2009). However, by 2017, the redox potential observed in Rio Doce estuarine soils indicated geochemical conditions (i. e., Eh and pH) had become favorable for anaerobic processes including both Fe and Mn dissimilatory reduction (Fig. 2) (Canfield et al., 1993; Lovley et al., 2004). It is widely known that anaerobic microorganisms through microbial reduction of both Fe and Mn may use MnIII, MnIV, and FeIII present on minerals as electron acceptors for anaerobic respiration under anoxic conditions (Otero et al., 2009; Patrick and Jugsujinda, 1992). Thus, our results indicate that Mn released during 2017 onwards might have occurred through reduction of both Fe oxyhydroxides and poorly crystalline Mn oxides (Lovley et al., 2004; Postma and Appelo, 2000).
Therefore, an increase in dissolved Mn concentrations is expected given that MnII is generally more stable against abiotic oxidation by O2 than FeII, which can be rapidly oxidized under Eh conditions above + 100 mV and circumneutral pH (Burdige, 1993; Frohne et al., 2011). Although Mn oxides have been formed due to O2 diffusion in surface layers, the Mn oxides required very strong oxic conditions (approximately + 900 to + 1000 mV at pH 5) to reach stability against reductive processes (Frohne et al., 2011). In our study, the highest Eh value measured in 2017 was + 174 mV. The FeII oxidation, however, occurred rapidly according to the Eh measured in samples from 2017, leading to the formation of poorly crystalline Fe oxyhydroxides such as ferrihydrite and lepidocrocite (Frohne et al., 2011; Yu et al., 2007), both of which increased in 2017 representing 40% of Fe minerals (Fig. 4). Contrarily long-term the contents of FeCR will mostly decrease since its formation is very low in environments with redox oscillations (Winkler et al., 2018). It should be noted, however, that poorly crystalline Fe minerals (i.e., FeLP and FeFR) phases are more susceptible to reduction within redox-oscillating environments (Nealson and Myers, 1992; Patrick and Jugsujinda, 1992; Reddy and DeLaune, 2008).
Thus, results showing the significant loss of Fe in the tailings-affected estuarine soils and the higher susceptibility of poorly crystallinity Fe oxyhydroxides to undergo reductive dissolution under transitory/cyclic anoxic conditions both point toward a decreasing capacity of poorly crystalline Fe minerals to control future Mn retention. The decrease in the significance of the Spearman correlation coefficient between total Fe and Mn in 2017 reinforces a less marked capacity of Fe oxyhydroxides in Mn retention (Fig. 9).
4.2. Environmental consequences
Metals can be absorbed into fish tissues through direct contact (i.e. through gills, skin or ingestion) such as with metals dissolved in the water column and associated with bottom sediments (Olsson et al., 1998; Weber et al., 2013). While the Cf in 2017 indicates low contamination levels of Mn in the soil at the studied site, high concentrations of Mn and other trace metals in the Rio Doce estuary suggested a high ecological risk for marine life (Bernardino et al., 2019; Gabriel et al., 2020a). Thus, the high contents of Mn in fish livers is indicative of chronic or acute exposure given the liver’s role in storage, redistribution, and metabolism of contaminants (Al-Yousuf et al., 2000). For these reasons, metal concentrations in the liver are useful indicators of bioaccumulation and are a bioindicator of Mn exposure in the Rio Doce estuary (Azevedo et al., 2009; Hauser-Davis et al., 2014). In fact, a recent study in the Rio Doce estuary indicated bioaccumulation of metals, including Mn, in tissues of Cathoropus spixii and Genidens genidens resulting in physiological effects due to chronic exposure to metal contaminants (Gabriel et al., 2020b).
The presence of Mn in fish muscle tissue poses a high risk to human health for the local community because the fish muscles are consumed by humans (Gabriel et al., 2020b; Gusso-Choueri et al., 2018). According to Gusso-Choueri et al. (2018), the consumption of metal-contaminated fish is one of the main routes of exposure for riverside communities. In most cases, the health risk is aggravated for those who depend on fish from the estuary to meet their daily dietary needs, which is the case for many people living near the Rio Doce estuary (Gabriel et al., 2020a).
According to the World Health Organization (WHO, 2011), food consumption is the primary route of exposure to Mn for humans, with the average concentration of Mn in staple protein sources such as beef, poultry, and fish ranging from 0.10 to 3.99 mg kg−1. Estimates for adequate daily consumption of Mn varies from 2 to 3 mg day−1 for adults (WHO, 2011). Therefore, according to the average Mn content found in fish muscles and the adequate daily consumption of Mn, the daily threshold consumption of C. spixii and G. genidens for an adult would be 2.5 kg and 5 kg, respectively, which may pose a real risk to the local population over the long-term due to presence of other sources of Mn exposure, such as drinking water, dust, fruit, vegetables, and dairy (Jolly et al., 2013; O’Neal and Zheng, 2015).
High concentrations of dissolved Mn measured in 2017 resulting from reducing conditions in the estuary is likely to have caused Mn accumulation in the tissues of the studied fishes (Arndt et al., 2014; Gabriel et al., 2020b; Pinsino et al., 2012). Our results from fish muscle and liver analyses show that Mn content in local fish species selected for this study are higher than Mn concentrations measured in other economically important fish species collected from areas that have also reported high concentrations of dissolved Mn (Table 2). Our findings suggest that fish living in the Rio Doce estuary may pose a chronic health risk for humans due to the elevated levels of tissue-bound Mn.
Table 2.
Mean values of Fe and Mn in liver and muscle for Cathoropus spixii and Genidens genidens collected 2017 from the Rio Doce estuary and for different commercial fish species worldwide.
| Species | Total Fe | Total Mn | Reference | ||
|---|---|---|---|---|---|
| Liver | Muscle | Liver | Muscle | ||
| mg kg−1 | |||||
| Cathoropus spixii | 830 ± 638 | 26.75 ± 26.35 | 3.2 ± 1.78 | 1.0 ± 1.0 | This study |
| Genidens genidens | 1,541 ± 1,725 | 17.36 ± 11.4 | 2.1 ± 1.9 | 0.5 ± 0.2 | This study |
| Silurus triostegus | 35.3 ± 6.6 | n.d | n.d | n.d | Karadede et al. (2004) |
| Lethrinus lentjan | n.d | n.d | 1.4 ± 0.2 | 0.1 ± 0.0 | Al-Yousuf et al. (2000) |
| Genidens barbus | 181 ± 100 | 3.87 ± 0.82 | n.d | n.d | Avigliano et al. (2019) |
| Chiloscyllium plagiosum | n.d | n.d | 0.2 ± 0.1 | 0.1 ± 0.0 | Cornish et al. (2007) |
| Mullus barbatus | 161.00 ± 25.30 | 29.20 ± 7.96 | 0.9 ± 0.2 | 0.4 ± 0.1 | Tepe et al. (2008) |
| Merlangius merlangus | 49.90 ± 7.16 | 21.90 ± 3.26 | 1.6 ± 0.2 | 0.4 ± 0.0 | Tepe et al. (2008) |
| Silurus glanis | 54.48 ± 17.59 | 10.17 ± 4.66 | 1.1 ± 1.4 | 0.5 ± 0.3 | Andreji and Stráňai, (2007) |
n.d: not determined.
It is likely that the continued release of Mn from the estuarine soils will lead to Mn accumulation in other species of fish (Rather et al., 2019), crabs (Zhang et al., 2019), plants (Intawongse and Dean, 2006), and oysters (Silva et al., 2003), all of which are likely important food sources for the local population. The risks of Mn within the food chain are often overlooked in estuarine ecosystems because information on the toxic effect of Mn in aquatic organisms from these ecosystems is poorly studied despite recent studies that have suggested Mn induces oxidative stress, damage to tissues, inflammation and neurodegeneration in fish and crabs (Barrio-Parra et al., 2018; Vieira et al., 2012). Thus, this unnoticed toxicity of Mn increases the risk of bioaccumulation for the local population.
Moreover, a constant uptake of Mn trough food with high Mn concentrations a long-term may expose to local population to adverse human health effects promoted by high Mn accumulation as neurodegenerative disorder (Levy and Nassetta, 2003), cardiovascular toxicities (Jiang and Zheng, 2005), and liver damage (O’Neal and Zheng, 2015). In this sense, additional in vitro bio accessibility tests may be beneficial to provide toxicological issues that were not reported so far (Luo et al., 2012).
The continued downstream transport of the mine tailings accumulated along the Rio Doce basin will serve as a long-term source of Mn and other trace metals, and potentially maintain the continued bioaccumulation risks of Mn into the estuary. Therefore, chronic Mn contamination is expected to persist along with other trace metals, as a result of biogeochemical soil conditions that favor seasonal Fe and Mn oxide reduction, and the absence of other mineral fractions that can retain and immobilize Mn (except for poorly crystallinity Fe oxyhydroxides which exert ephemeral control; Fig. 11).
Fig. 11.
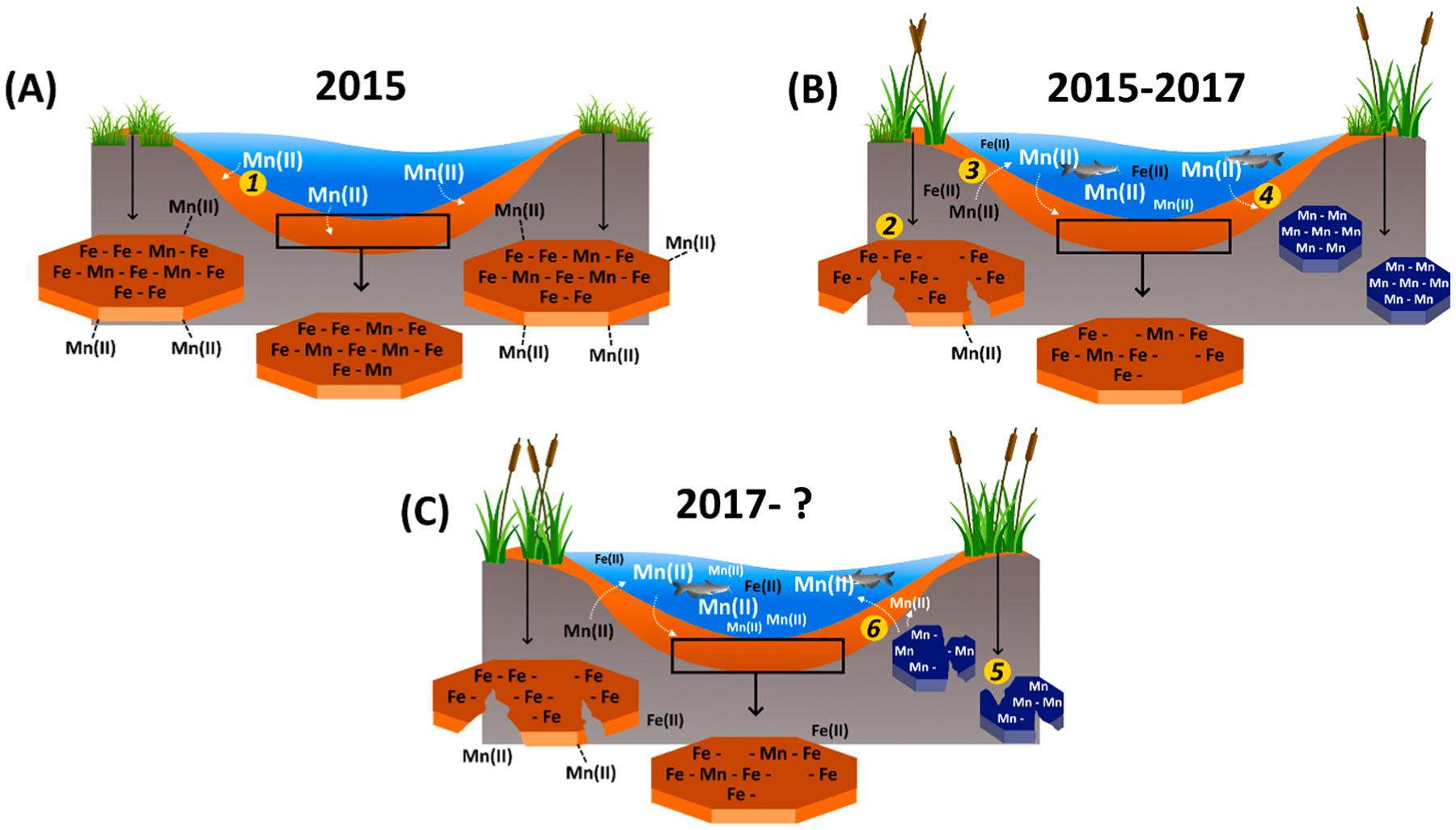
Schematic summary of the sequence of processes (order provided in yellow circles) leading to Fe and Mn mobilization in the Doce River estuary. The deposited mine tailings in 2015 from the Fundão dam rupture (Samarco mining company) led to an Fe oxyhydroxide and Mn enrichment in the estuary. Initially, Mn that arrived was predominantly immobilized on Fe oxyhydroxides (1). Between 2015 and 2017, plant growth promoted organic matter input to estuarine soils which stimulated the reduction of Fe oxyhydroxides (2) and subsequent release of dissolved Fe and Mn to estuarine waters, exposing fish and other wildlife to high concentrations of the metals (3). Redox oscillations caused by seasonal changes in precipitation and water levels, tidal flooding, plant activity, and fauna, has favored precipitation of poorly crystalline Fe oxyhydroxides and poorly crystalline Mn oxides (4). Mn oxides are easily reducible (5) which can contribute to an increase of Mn in estuarine water (6). Poorly crystalline Fe oxyhydroxides are also susceptible to reduction limiting their role in Mn retention in the future.
5. Conclusions
The Fe-rich mine tailings deposited from the Samarco disaster in the Rio Doce estuarine soils largely increased Mn concentrations in soil, water, and fish. The Fe minerals exert a temporary control on Mn bioavailability because crystalline Fe oxyhydroxides are gradually solubilized and replaced by poorly crystalline Fe oxides which can be easily reduced. Although the Mn released from Fe minerals may be reoxidized into poorly crystalline Mn oxides over time, the redox conditions in the Rio Doce estuary are highly conducive to reductive dissolution of MnIII and MnIV containing poorly crystalline oxides, such as phyllomanganate and tectomanganates, which then ultimately leads to the continued increase in dissolved Mn.
Although Mn is considered an important micronutrient for all flora and fauna, the concentration of Mn found in the pore waters of the impacted estuary drastically exceeds the concentrations necessary for biological function leading to chronic Mn exposure. In this study, we found elevated Mn concentrations in liver and muscle tissues of multiple fish species that are regularly consumed by the local population. This discovery demonstrates that Mn sourced from the mine tailings can ultimately be impacting human health through long-term ecosystem contamination. Moreover, other animals and plants that are also consumed by the local population may accumulate Mn, worsening the risk to human health in the area.
Supplementary Material
Acknowledgments
This work was funded by grants to AFB and TOF from Fundação de Amparo do Espirito Santo (FAPES/CNPq/CAPES Rio Doce 77683544/2017), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior CAPES - Finance Code 001 and CNPq (grant numbers, AFB: 301161/2017-8, TOF: 305996/2018-5; GNN: 409593/2018-4). The authors are grateful for the financial support provided by São Paulo Research Foundation (FAPESP, HMQ grant number 2018/04259-2 and 2019/17413-2; DB grant number 2019/02855-0; TOF grant numbers 2019/ 19987-6 and 2018/08408-2). Xunta de Galicia-Consellería de Educación e Ordeanción Universitaria de Galicia (Consolidation of competitive groups of investigation; GRC GI 1574) and CRETUS strategic group (AGRUP2015/02). Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (GNN, JCNE Grant E-26/202.757/2019). Use of the Stanford Synchrotron Radiation Lightsource, SLAC National Accelerator Laboratory, is supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences under Contract No. DEAC02-76SF00515. The SSRL Structural Molecular Biology Program is supported by the DOE Office of Biological and Environmental Research, and by the National Institutes of Health, National Institute of General Medical Sciences (P41GM103393). MJA was supported by a fellowship from the National Institute of Health T32 Training Grant (T32 ES018827) and SCY was supported by USDA NIFA Hatch Project CA-RENS-5151-H and Fulbright Award awarded by the J. William Fulbright Commission.
Footnotes
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envint.2020.106284.
References
- Al-Yousuf M, El-Shahawi M, Al-Ghais S, 2000. Trace metals in liver, skin and muscle of Lethrinus lentjan fish species in relation to body length and sex. Sci. Total Environ 256, 87–94. 10.1016/S0048-9697(99)00363-0. [DOI] [PubMed] [Google Scholar]
- Alvares CA, Stape JL, Sentelhas PC, de Moraes Gonçalves JL, Sparovek G, 2013. Köppen’s climate classification map for Brazil. Meteorol. Zeitschrift 22, 711–728. 10.1127/0941-2948/2013/0507. [DOI] [Google Scholar]
- ANA A.N. de Á., 2020. Rio Doce - Sala de Situação [WWW Document]. Sala Situação da Agência Nac. Águas URL https://www.ana.gov.br/sala-de-situacao/rio-doce/riodoce-saiba-mais# (accessed7.3.20). [Google Scholar]
- Andreji J, Stráňai I, 2007. A contamination of tissues from fish originated from the lower part of Nitra river with some metals (Fe, Mn, Zn, Pb, Cu Co, Ni, Cr, Cd). Slovak J. Anim. Sci 40, 146–156. [Google Scholar]
- Arndt A, Borella MI, Espósito BP, 2014. Toxicity of manganese metallodrugs toward Danio rerio. Chemosphere 96, 46–50. 10.1016/j.chemosphere.2013.07.014. [DOI] [PubMed] [Google Scholar]
- Avigliano E, Maichak de Carvalho B, Invernizzi R, Olmedo M, Jasan R, Volpedo AV, 2019. Arsenic, selenium, and metals in a commercial and vulnerable fish from southwestern Atlantic estuaries: distribution in water and tissues and public health risk assessment. Environ. Sci. Pollut. Res 26, 7994–8006. 10.1007/s11356-019-04258-3. [DOI] [PubMed] [Google Scholar]
- Azevedo JS, Fernandez WS, Farias LA, Fávaro DTI, Braga ES, 2009. Use of Cathorops spixii as bioindicator of pollution of trace metals in the Santos Bay, Brazil. Ecotoxicology 18, 577–586. 10.1007/s10646-009-0315-4. [DOI] [PubMed] [Google Scholar]
- Badarudeen A, Damodaran KT, Sajan K, Padmalal D, 1996. Texture and geochemistry of the sediments of a tropical mangrove ecosystem, southwest coast of India. Environ. Geol 27, 164–169. 10.1007/BF00770428. [DOI] [Google Scholar]
- Baly DL, 1989. Manganese in Metabolism and Enzyme Function. J. Nutr 10.1093/jn/119.2.327. [DOI] [Google Scholar]
- Barrio-Parra F, Elío J, De Miguel E, García-Gonźalez JE, Izquierdo M, Álvarez R, 2018. Environmental risk assessment of cobalt and manganese from industrial sources in an estuarine system. Environ. Geochem. Health 40, 737–748. 10.1007/s10653-017-0020-9. [DOI] [PubMed] [Google Scholar]
- Bernardino AF, Netto SA, Pagliosa PR, Barros F, Christofoletti RA, Rosa Filho JS, Colling A, Lana PC, 2015. Predicting ecological changes on benthic estuarine assemblages through decadal climate trends along Brazilian Marine Ecoregions. Estuar. Coast. Shelf Sci 166, 74–82. 10.1016/j.ecss.2015.05.021. [DOI] [Google Scholar]
- Bernardino AF, Pais FS, Oliveira LS, Gabriel FA, Ferreira TO, Queiroz HM, Mazzuco ACA, 2019. Chronic trace metals effects of mine tailings on estuarine assemblages revealed by environmental DNA. PeerJ 7, e8042. 10.7717/peerj.8042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi TS, 2007. Biogeochemistry of Estuaries, Eos, Transactions American Geophysical Union. Oxford University Press, Oxford; New York. 10.1029/2007EO520011. [DOI] [Google Scholar]
- Borch T, Kretzschmar R, Skappler A, Van Cappellen P, Ginder-Vogel M, Voegelin A, Campbell K, 2010. Biogeochemical redox processes and their impact on contaminant dynamics. Environ. Sci. Technol 44, 15–23. 10.1021/es9026248. [DOI] [PubMed] [Google Scholar]
- Broadley M, Brown P, Cakmak I, Rengel Z, Zhao F, 2012. Function of Nutrients, in: Marschner’s Mineral Nutrition of Higher Plants. Elsevier, pp. 191–248. 10.1016/B978-0-12-384905-2.00007-8. [DOI] [Google Scholar]
- Brookins DG, 1988. Eh-pH diagrams for geochemistry, 1st ed.Springer-Verlag Berlin Heidelberg. 10.1007/978-3-642-73093-1. [DOI] [Google Scholar]
- Burdige DJ, 1993. The biogeochemistry of manganese and iron reduction in marine sediments. Earth-Science Rev. 35, 249–284. 10.1016/0012-8252(93)90040-E. [DOI] [Google Scholar]
- Canfield DE, Erik Kristensen, Bo Thamdrup, 2005. The Iron and Manganese Cycles, in: Aquatic Geomicrobiology. pp. 269–312. 10.1016/S0065-2881(05)48008-6. [DOI] [Google Scholar]
- Canfield DE, Thamdrup B, Hansen JW, 1993. The anaerobic degradation of organic matter in Danish coastal sediments: Iron reduction, manganese reduction, and sulfate reduction. Geochim. Cosmochim. Acta 57, 3867–3883. 10.1016/0016-7037(93)90340-3. [DOI] [PubMed] [Google Scholar]
- Carmo Fĺavio Fonseca do, Kamino LHY, Junior RT, Campos I.C. de, Carmo Felipe Fonseca do, Silvino G, Castro K.J. da S.X. de, Mauro ML, Rodrigues NUA, Miranda M.P. de S., Pinto CEF, 2017. Fundão tailings dam failures: the environment tragedy of the largest technological disaster of Brazilian mining in global context. Perspect. Ecol. Conserv 15, 145–151. 10.1016/j.pecon.2017.06.002. [DOI] [Google Scholar]
- Chaudry MA, Zwolsman JJG, 2008. Seasonal Dynamics of Dissolved Trace Metals in the Scheldt Estuary: Relationship with Redox Conditions and Phytoplankton Activity. Estuaries Coasts 31, 430–443. 10.1007/s12237-007-9030-7. [DOI] [Google Scholar]
- CONAMA, 2009. Resolução No 420, de 28 de Dezembro de 2009, in: Brazilian National Environment Council (Ed.), Diário Oficial Da União. Ministério do Meio Ambiente, Brasília, pp. 81–84. [Google Scholar]
- CONAMA, 2005. Brazilian water quality guidelines. Resolution 357.
- Cornell RM, Schwertmann U, 2003. The Iron Oxides: Structure Reactions, Occurences and Uses. WILEY-VCH. 10.1002/3527602097.ch1. [DOI] [Google Scholar]
- Cornish AS, Ng WC, Ho VCM, Wong HL, Lam JCW, Lam PKS, Leung KMY, 2007. Trace metals and organochlorines in the bamboo shark Chiloscyllium plagiosum from the southern waters of Hong Kong. China. Sci. Total Environ 376, 335–345. 10.1016/j.scitotenv.2007.01.070. [DOI] [PubMed] [Google Scholar]
- Coughlin BR, Stone AT, 1995. Nonreversible Adsorption of Divalent Metal Ions (MnII, CoII, NiII, CuII, and PbII) onto Goethite: Effects of Acidification, FeII Addition, and Picolinic Acid Addition. Environ. Sci. Technol 29, 2445–2455. 10.1021/es00009a042. [DOI] [PubMed] [Google Scholar]
- Cummings DE, March AW, Bostick B, Spring S, Caccavo F, Fendorf S, Rosenzweig RF, Rosenzweig R Frank, 2000. Evidence for microbial Fe(III) reduction in anoxic, mining-impacted lake sediments (Lake Coeur d’Alene, Idaho). Appl. Environ. Microbiol 66, 154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Laing G, Meers E, Dewispelaere M, Rinklebe J, Vandecasteele B, Verloo MG, Tack FMG, 2009a. Effect of Water Table Level on Metal Mobility at Different Depths in Wetland Soils of the Scheldt Estuary (Belgium). Water Air Soil Pollut. 202, 353–367. 10.1007/s11270-009-9982-2. [DOI] [Google Scholar]
- Du Laing G, Rinklebe J, Vandecasteele B, Meers E, Tack FMG, 2009b. Trace metal behaviour in estuarine and riverine floodplain soils and sediments: A review. Sci. Total Environ 407, 3972–3985. 10.1016/j.scitotenv.2008.07.025. [DOI] [PubMed] [Google Scholar]
- Duckworth OW, Bargar JR, Sposito G, 2009. Coupled biogeochemical cycling of iron and manganese as mediated by microbial siderophores. Biometals 22, 605–613. 10.1007/s10534-009-9220-9. [DOI] [PubMed] [Google Scholar]
- El-Jaoual T, Cox DA, 1998. Manganese toxicity in plants. J. Plant Nutr 21, 353–386. 10.1080/01904169809365409. [DOI] [Google Scholar]
- Fernandes GW, Goulart FF, Ranieri BD, Coelho MS, Dales K, Boesche N, Bustamante M, Carvalho FA, Carvalho DC, Dirzo R, Fernandes S, Galetti PM, Millan VEG, Mielke C, Ramirez JL, Neves A, Rogass C, Ribeiro SP, Scariot A, Soares-Filho B, 2016. Deep into the mud: ecological and socio-economic impacts of the dam breach in Mariana. Brazil. Nat. e Conserv 14, 35–45. 10.1016/j.ncon.2016.10.003. [DOI] [Google Scholar]
- Finkelstein MM, Jerrett M, 2007. A study of the relationships between Parkinson’s disease and markers of traffic-derived and environmental manganese air pollution in two Canadian cities. Environ. Res 104, 420–432. 10.1016/j.envres.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Fischel JS, Fischel MH, Sparks DL, 2015. Advances in Understanding Reactivity of Manganese Oxides with Arsenic and Chromium in Environmental Systems. In: ACS Symposium Series. Oxford University Press, Washington, DC, pp. 1–27. 10.1021/bk-2015-1197.ch001. [DOI] [Google Scholar]
- Fitsanakis VA, Zhang N, Garcia S, Aschner M, 2010. Manganese (Mn) and Iron (Fe): Interdependency of Transport and Regulation. Neurotox. Res 18, 124–131. 10.1007/s12640-009-9130-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin D, Leppard GG, Tessier A, 1993. Characteristics of lacustrine diagenetic iron oxyhydroxides. Geochim. Cosmochim. Acta 57, 4391–4404. 10.1016/0016-7037(93)90490-N. [DOI] [Google Scholar]
- Frohne T, Rinklebe J, Diaz-Bone RA, Du Laing G, 2011. Controlled variation of redox conditions in a floodplain soil: Impact on metal mobilization and biomethylation of arsenic and antimony. Geoderma 160, 414–424. 10.1016/j.geoderma.2010.10.012. [DOI] [Google Scholar]
- Gabriel FA, Silva AG, Queiroz HM, Ferreira TO, Hauser-Davis RA, Bernardino AF, 2020a. Ecological Risks of Metal and Metalloid Contamination in the Rio Doce Estuary. Integr. Environ. Assess. Manag 16, 655–660. 10.1002/ieam.4250. [DOI] [PubMed] [Google Scholar]
- Gabriel FA, Hauser-Davis RA, Soares L, Mazzuco ACA, Rocha RCC, Saint Pierre TD, Saggioro E, Correia FV, Ferreira TO, Bernardino AF, 2020b. Contamination and oxidative stress biomarkers in estuarine fish following a mine tailing disaster. PeerJ 8, e10266. 10.7717/peerj.10266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes L.E. de O., Correa LB, Sá F, Neto RR, Bernardino AF, 2017. The impacts of the Samarco mine tailing spill on the Rio Doce estuary, Eastern Brazil. Mar. Pollut. Bull 120, 28–36. 10.1016/j.marpolbul.2017.04.056. [DOI] [PubMed] [Google Scholar]
- Graham RD, Hannam RJ, Uren NC, 1988. Manganese in Soils and Plants, Manganese in Soils and Plants. Springer Netherlands, Dordrecht. 10.1007/978-94-009-2817-6. [DOI] [Google Scholar]
- Gusso-Choueri PK, Araújo G.S. de, Cruz ACF, Stremel T.R. de O., Campos S.X. de, Abessa D.M. de S., Oliveira Ribeiro C.A. de, Choueri RB, 2018. Metals and arsenic in fish from a Ramsar site under past and present human pressures: Consumption risk factors to the local population. Sci. Total Environ 628–629, 621–630. 10.1016/j.scitotenv.2018.02.005. [DOI] [PubMed] [Google Scholar]
- Hadlich HL, Venturini N, Martins CC, Hatje V, Tinelli P, Gomes L.E. de O., Bernardino AF, 2018. Multiple biogeochemical indicators of environmental quality in tropical estuaries reveal contrasting conservation opportunities. Ecol. Indic 95, 21–31. 10.1016/j.ecolind.2018.07.027. [DOI] [Google Scholar]
- Hakanson L, 1980. An ecological risk index for aquatic pollution control.a sedimentological approach. Water Res. 10.1016/0043-1354(80)90143-8. [DOI] [Google Scholar]
- Harford AJ, Mooney TJ, Trenfield MA, van Dam RA, 2015. Manganese toxicity to tropical freshwater species in low hardness water. Environ. Toxicol. Chem 34, 2856–2863. 10.1002/etc.3135. [DOI] [PubMed] [Google Scholar]
- Hauser-Davis RA, Bastos FF, Tuton B, Chávez Rocha R, Pierre T Saint Ziolli, R.L., Arruda MAZ, 2014. Bile and liver metallothionein behavior in copper-exposed fish. J. Trace Elem. Med Biol 28, 70–74. 10.1016/j.jtemb.2013.09.003. [DOI] [PubMed] [Google Scholar]
- Hu P, Jiang Z, Liu Q, Heslop D, Roberts AP, Torrent J, Barrón V, 2016. Estimating the concentration of aluminum-substituted hematite and goethite using diffuse reflectance spectrometry and rock magnetism: Feasibility and limitations. J. Geophys. Res. Solid Earth 121, 4180–4194. 10.1002/2015JB012635. [DOI] [Google Scholar]
- Huang Z, Tang Y, Zhang K, Chen Y, Wang Y, Kong L, You T, Gu Z, 2016. Environmental risk assessment of manganese and its associated heavy metals in a stream impacted by manganese mining in South China. Hum. Ecol. Risk Assess. An Int. J 22, 1341–1358. 10.1080/10807039.2016.1169915. [DOI] [Google Scholar]
- Huerta-Diaz MA, Morse JW, 1990. A quantitative method for determination of trace metal concentrations in sedimentary pyrite. Mar. Chem 29, 119–144. 10.1016/0304-4203(90)90009-2. [DOI] [Google Scholar]
- Intawongse M, Dean JR, 2006. Uptake of heavy metals by vegetable plants grown on contaminated soil and their bioavailability in the human gastrointestinal tract. Food Addit. Contam 23, 36–48. 10.1080/02652030500387554. [DOI] [PubMed] [Google Scholar]
- Jay DA, Orton PM, Chisholm T, Wilson DJ, Fain AMV, 2007. Particle trapping in stratified estuaries: Consequences of mass conservation. Estuaries Coasts 30, 1095–1105. 10.1007/BF02841399. [DOI] [Google Scholar]
- Ji J, Balsam W, Chen JU, Liu L, 2002. Rapid and Quantitative Measurement of Hematite and Goethite in the Chinese Loess-paleosol Sequence by Diffuse Reflectance Spectroscopy. Clays Clay Miner. 50, 208–216. 10.1346/000986002760832801. [DOI] [Google Scholar]
- Jiang Y, Zheng W, 2005. Cardiovascular Toxicities Upon Manganese Exposure. Cardiovasc. Toxicol 5, 345–354. 10.1385/CT:5:4:345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston SG, Keene AF, Bush RT, Burton ED, Sullivan LA, Isaacson L, McElnea AE, Ahern CR, Smith CD, Powell B, 2011. Iron geochemical zonation in a tidally inundated acid sulfate soil wetland. Chem. Geol 280, 257–270. 10.1016/j.chemgeo.2010.11.014. [DOI] [Google Scholar]
- Jolly YN, Islam A, Akbar S, 2013. Transfer of metals from soil to vegetables and possible health risk assessment. Springerplus 2, 385. 10.1186/2193-1801-2-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karadede H, Oymak SA, Ünlü E, 2004. Heavy metals in mullet, Liza abu, and catfish, Silurus triostegus, from the Atatürk Dam Lake (Euphrates). Turkey. Environ. Int 30, 183–188. 10.1016/S0160-4120(03)00169-7. [DOI] [PubMed] [Google Scholar]
- Kennish MJ, 2002. Environmental threats and environmental future of estuaries. Environ. Conserv 29, 78–107. 10.1017/S0376892902000061. [DOI] [Google Scholar]
- Kristensen E, Ahmed SI, Devol AH, 1995. Aerobic and anaerobic decomposition of organic matter in marine sediment: Which is fastest? Limnol. Oceanogr 40, 1430–1437. 10.4319/lo.1995.40.8.1430. [DOI] [Google Scholar]
- Lee J-H, Kennedy DW, Dohnalkova A, Moore DA, Nachimuthu P, Reed SB, Fredrickson JK, 2011. Manganese sulfide formation via concomitant microbial manganese oxide and thiosulfate reduction. Environ. Microbiol 13, 3275–3288. 10.1111/j.1462-2920.2011.02587.x. [DOI] [PubMed] [Google Scholar]
- Levy BS, Nassetta WJ, 2003. Neurologic Effects of Manganese in Humans: A Review. Int. J. Occup. Environ. Health 9, 153–163. 10.1179/oeh.2003.9.2.153. [DOI] [PubMed] [Google Scholar]
- Lewis BL, Landing WM, 1991. The biogeochemistry of manganese and iron in the Black Sea. Deep Sea Res. Part A. Oceanogr. Res. Pap 38, S773–S803. 10.1016/S0198-0149(10)80009-3. [DOI] [Google Scholar]
- Li MS, Luo YP, Su ZY, 2007. Heavy metal concentrations in soils and plant accumulation in a restored manganese mineland in Guangxi, South China. Environ. Pollut 147, 168–175. 10.1016/j.envpol.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Lovley DR, Holmes DE, Nevin KP, 2004. Dissimilatory Fe(III) and Mn(IV) Reduction. Adv. Microb. Physiol 219–286. 10.1016/S0065-2911(04)49005-5. [DOI] [PubMed] [Google Scholar]
- Luo XS, Ding J, Xu B, Wang YJ, Li HB, Yu S, 2012. Incorporating bioaccessibility into human health risk assessments of heavy metals in urban park soils. Sci. Total Environ 424, 88–96. 10.1016/j.scitotenv.2012.02.053. [DOI] [PubMed] [Google Scholar]
- Manceau A, Marcus MA, Grangeon S, 2012. Determination of Mn valence states in mixed-valent manganates by XANES spectroscopy. Am. Mineral 97, 816–827. 10.2138/am.2012.3903. [DOI] [Google Scholar]
- Manceau A, Marcus MA, Tamura N, 2002. Quantitative Speciation of Heavy Metals in Soils and Sediments by Synchrotron X-ray Techniques. Rev. Mineral. Geochemistry 49, 341–428. 10.2138/gsrmg.49.1.341. [DOI] [Google Scholar]
- Marcus MA, Manceau A, Kersten M, 2004. Mn, Fe, Zn and As speciation in a fast-growing ferromanganese marine nodule. Geochim. Cosmochim. Acta 68, 3125–3136. 10.1016/j.gca.2004.01.015. [DOI] [Google Scholar]
- Marín-Muñiz JL, Hernández ME, Moreno-Casasola P, 2014. Comparing soil carbon sequestration in coastal freshwater wetlands with various geomorphic features and plant communities in Veracruz, Mexico. Plant Soil 378, 189–203. 10.1007/s11104-013-2011-7. [DOI] [Google Scholar]
- McKinley K, McLellan I, Gagńe F, Quinn B, 2019. The toxicity of potentially toxic elements (Cu, Fe, Mn, Zn and Ni) to the cnidarian Hydra attenuata at environmentally relevant concentrations. Sci. Total Environ 665, 848–854. 10.1016/j.scitotenv.2019.02.193. [DOI] [PubMed] [Google Scholar]
- Mudd SM, D’Alpaos A, Morris JT, 2010. How does vegetation affect sedimentation on tidal marshes? Investigating particle capture and hydrodynamic controls on biologically mediated sedimentation. J. Geophys. Res. Earth Surf 115, 1–14. 10.1029/2009JF001566. [DOI] [Google Scholar]
- Namgung S, Guo B, Sasaki K, Lee SS, Lee G, 2020. Macroscopic and microscopic behaviors of Mn(II) (ad)sorption to goethite with the effects of dissolved carbonates under anoxic conditions. Geochim. Cosmochim. Acta 277, 300–319. 10.1016/j.gca.2020.03.036. [DOI] [Google Scholar]
- Nealson KH, Myers CR, 1992. Microbial reduction of manganese and iron: New approaches to carbon cycling. Appl. Environ. Microbiol 58, 439–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newville M, 2013. Larch: An Analysis Package for XAFS and Related Spectroscopies. J. Phys. Conf. Ser 430, 012007. 10.1088/1742-6596/430/1/012007. [DOI] [Google Scholar]
- NOAA, 2008. Screening Quick Reference Tables. National Oceanic and Atmospheric Administration, Seattle, WA. [Google Scholar]
- O’Neal SL, Zheng W, 2015. Manganese Toxicity Upon Overexposure: a Decade in Review. Curr. Environ. Heal. Reports 2, 315–328. 10.1007/s40572-015-0056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldham VE, Siebecker MG, Jones MR, Mucci A, Tebo BM, Luther GW, 2019. The Speciation and Mobility of Mn and Fe in Estuarine Sediments. Aquat. Geochemistry 25, 3–26. 10.1007/s10498-019-09351-0. [DOI] [Google Scholar]
- Olsson P-E, Kling P, Hogstrand C, 1998. Mechanisms of heavy metal accumulation and toxicity in fish, in: Metal Metabolism in Aquatic Environments. 10.1007/978-1-4757-2761-6_10. [DOI] [Google Scholar]
- Otero XL, Ferreira TO, Huerta-Díaz MA, Partiti CSM, Souza V, Vidal-Torrado P, Macías F, 2009. Geochemistry of iron and manganese in soils and sediments of a mangrove system, Island of Pai Matos (Cananeia — SP, Brazil). Geoderma 148, 318–335. 10.1016/j.geoderma.2008.10.016. [DOI] [Google Scholar]
- Patrick WH, Jugsujinda A, 1992. Sequential Reduction and Oxidation of Inorganic Nitrogen, Manganese, and Iron in Flooded Soil. Soil Sci. Soc. Am. J 56, 1071. 10.2136/sssaj1992.03615995005600040011x. [DOI] [Google Scholar]
- Pereira AA, Van Hattum B, Brouwer A, Van Bodegom PM, Rezende CE, Salomons W, 2008. Effects of iron-ore mining and processing on metal bioavailability in a tropical coastal lagoon. J. Soils Sediments 8, 239–252. 10.1007/s11368-008-0017-1. [DOI] [Google Scholar]
- Pinheiro HT, Joyeux J-C, 2007. Pescarias multi-específicas na regĩao da foz do Rio Doce, ES, Brasil: características, problemas e opções para um futuro sustentável. Brazilian J. Aquat. Sci. Technol 11, 15. 10.14210/bjast.v11n2.p15-23. [DOI] [Google Scholar]
- Pinsino A, Matranga V, Roccheri MC, 2012. Manganese: A New Emerging Contaminant in the Environment, in: Environmental Contamination. InTech 10.5772/31438. [DOI] [Google Scholar]
- Postma D, 1985. Concentration of Mn and separation from Fe in sediments—I. Kinetics and stoichiometry of the reaction between birnessite and dissolved Fe(II) at 10°C. Geochim. Cosmochim. Acta 49, 1023–1033. 10.1016/0016-7037(85)90316-3. [DOI] [Google Scholar]
- Postma D, Appelo CAJ, 2000. Reduction of Mn-oxides by ferrous iron in a flow system: column experiment and reactive transport modeling. Geochim. Cosmochim. Acta 64, 1237–1247. 10.1016/S0016-7037(99)00356-7. [DOI] [Google Scholar]
- Queiroz HM, Ferreira TO, Barcellos D, Nóbrega GN, Antelo J, Otero XL, Bernardino AF, 2021. From sinks to sources: The role of Fe oxyhydroxide transformations on phosphorus dynamics in estuarine soils. J. Environ. Manage 278, 111575. 10.1016/j.jenvman.2020.111575. [DOI] [PubMed] [Google Scholar]
- Queiroz HM, Nóbrega GN, Ferreira TO, Almeida LS, Romero TB, Santaella ST, Bernardino AF, Otero XL, 2018. The Samarco mine tailing disaster: A possible time-bomb for heavy metals contamination? Sci. Total Environ 637–638, 498–506. 10.1016/j.scitotenv.2018.04.370. [DOI] [PubMed] [Google Scholar]
- Rather MY, Tilwani YM, Dey A, 2019. Assessment of heavy metal contamination in two edible fish species Carassius carassius and Triplophysa kashmirensis of Dal Lake, Srinagar, Kashmir, India. Environ. Monit. Assess 191 10.1007/s10661-019-7382-7. [DOI] [PubMed] [Google Scholar]
- Ravel B, Newville M, 2005. Athena, artemis, hephaestus : data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat 12, 537–541. 10.1107/S0909049505012719. [DOI] [PubMed] [Google Scholar]
- Reddy KR, DeLaune RD, 2008. Biogeochemistry of wetlands: science and applications, 1st ed.CRC Press. [Google Scholar]
- Reimann C, Filzmoser P, Garrett RG, Dutter R, 2008. Statistical Data Analysis Explained, Statistical Data Analysis Explained: Applied Environmental Statistics with R. John Wiley & Sons, Ltd, Chichester, UK, UK. 10.1002/9780470987605. [DOI] [Google Scholar]
- Richard E. da C., Estrada GCD, Bechtold J, Aguiar Duarte H, Maioli BG, Freitas AHA, Warner KE, Figueiredo LHM, 2020. Water and Sediment Quality in the Coastal Zone Around the Mouth of Doce River After the Fundão Tailings Dam Failure. Integr. Environ. Assess. Manag 16, 643–654. 10.1002/ieam.4309. [DOI] [PubMed] [Google Scholar]
- Rodrigues A.S. de L., Malafaia G, Costa AT, Nalini Júnior HA, 2014. Iron ore mining promotes iron enrichment in sediments of the Gualaxo do Norte River basin, Minas Gerais State, Brazil. Environ. Earth Sci 71, 4177–4186. 10.1007/s12665-013-2808-y. [DOI] [Google Scholar]
- Sandilyan S, Kathiresan K, 2014. Decline of mangroves – A threat of heavy metal poisoning in Asia. Ocean Coast. Manag 102, 161–168. 10.1016/j.ocecoaman.2014.09.025. [DOI] [Google Scholar]
- Santelli CM, Webb SM, Dohnalkova AC, Hansel CM, 2011. Diversity of Mn oxides produced by Mn(II)-oxidizing fungi. Geochim. Cosmochim. Acta 75, 2762–2776. 10.1016/j.gca.2011.02.022. [DOI] [Google Scholar]
- Scheinost AC, 1998. Use and Limitations of Second-Derivative Diffuse Reflectance Spectroscopy in the Visible to Near-Infrared Range to Identify and Quantify Fe Oxide Minerals in Soils. Clays Clay Miner. 46, 528–536. 10.1346/CCMN.1998.0460506. [DOI] [Google Scholar]
- Scheinost AC, Stanjek H, Schulze DG, Gasser U, Sparks DL, 2001. Structural environment and oxidation state of Mn in goethite-groutite solid-solutions. Am. Mineral 86, 139–146. 10.2138/am-2001-0115. [DOI] [Google Scholar]
- Schwertmann U, Taylor RM, 1989. Iron Oxides. Minerals Soil Environ. 379–438. 10.2136/sssabookser1.2ed.c8. [DOI] [Google Scholar]
- Sigel A, Sigel H, 2000. Manganese and Its Role in Biological Processes. In: Metal Ions in Biological Systems. CRC Press, New York, NY. 10.1021/jm000216b, 2969–2969. [DOI] [Google Scholar]
- Silva AC, Cavalcante LCD, Fabris JD, Júnior RF, Barral UM, Farnezi M.M. de M., Viana AJS, Ardisson JD, Fernandez-Outon LE, Lara LRS, Stumpf HO, Barbosa JBS, Silva L.C. da, 2017. Características químicas, mineralógicas e físicas do material acumulado em terraços fluviais, originado do fluxo de lama proveniente do rompimento de barragem de rejeitos de mineração de ferro em Bento Rodrigues, Minas Gerais, Brasil. Rev. Espinhaço | UFVJM; Rev. Espinhaço #9. [Google Scholar]
- Silva CAR, Rainbow PS, Smith BD, 2003. Biomonitoring of trace metal contamination in mangrove-lined Brazilian coastal systems using the oyster Crassostrea rhizophorae: Comparative study of regions affected by oil, salt pond and shrimp farming activities. Hydrobiologia 501, 199–206. 10.1023/A:1026242417427. [DOI] [Google Scholar]
- Singh N, Singh A, Das D, Mohan ML, 2010. Redox Control of Prion and Disease Pathogenesis. Antioxid. Redox Signal 12, 1271–1294. 10.1089/ars.2009.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slobodian L, Badoz L, 2019. Tangled roots and changing tides: mangrove governance for conservation and sustainable use, 1st ed.WWF Germany, Berlin, Germany and IUCN, Gland, Switzerland, Berlin, Germany; Gland, Switzerland. [Google Scholar]
- Søndergaard M, 2009. Redox Potential, in: Encyclopedia of Inland Waters. Elsevier, pp. 852–859. 10.1016/B978-012370626-3.00115-0. [DOI] [Google Scholar]
- Squadrone S, Brizio P, Stella C, Prearo M, Pastorino P, Serracca L, Ercolini C, Abete MC, 2016. Presence of trace metals in aquaculture marine ecosystems of the northwestern Mediterranean Sea (Italy). Environ. Pollut 215, 77–83. 10.1016/j.envpol.2016.04.096. [DOI] [PubMed] [Google Scholar]
- Stumm W, Morgan JJ, 1996. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters, 3rd ed.John Wiley & Sons Inc., Danvers. [Google Scholar]
- Summer K, Reichelt-Brushett A, Howe P, 2019. Toxicity of manganese to various life stages of selected marine cnidarian species. Ecotoxicol. Environ. Saf 167, 83–94. 10.1016/j.ecoenv.2018.09.116. [DOI] [PubMed] [Google Scholar]
- Sundby B, Vale C, Caetano M, Luther GW III, 2003. Redox Chemistry in the Root Zone of a Salt Marsh Sediment in the Tagus Estuary. Portugal. Aquat. Geochemistry 9, 257–271. 10.1023/B:AQUA.0000022957.42522.9a. [DOI] [Google Scholar]
- Tebo BM, Bargar JR, Clement BG, Dick GJ, Murray KJ, Parker D, Verity R, Webb SM, 2004. Biogenic manganese oxides: Properties and mechanisms of formation. Annu. Rev. Earth Planet. Sci 32, 287–328. 10.1146/annurev.earth.32.101802.120213. [DOI] [Google Scholar]
- Tepe Y, Türkmen M, Türkmen A, 2008. Assessment of heavy metals in two commercial fish species of four Turkish seas. Environ. Monit. Assess 146, 277–284. 10.1007/s10661-007-0079-3. [DOI] [PubMed] [Google Scholar]
- Tessier A, Campbell PGC, Bisson M, 1979. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem 51, 844–851. 10.1021/ac50043a017. [DOI] [Google Scholar]
- Thamdrup B, Fossing H, Jørgensen BB, 1994. Manganese, iron and sulfur cycling in a coastal marine sediment, Aarhus bay. Denmark. Geochim. Cosmochim. Acta 58, 5115–5129. 10.1016/0016-7037(94)90298-4. [DOI] [Google Scholar]
- Toner B, Manceau A, Webb SM, Sposito G, 2006. Zinc sorption to biogenic hexagonal-birnessite particles within a hydrated bacterial biofilm. Geochim. Cosmochim. Acta 70, 27–43. 10.1016/j.gca.2005.08.029. [DOI] [Google Scholar]
- Ugwu IM, Igbokwe OA, 2019. Sorption of Heavy Metals on Clay Minerals and Oxides: A Review, in: Advanced Sorption Process Applications. IntechOpen, pp. 1–23. 10.5772/intechopen.80989. [DOI] [Google Scholar]
- USEPA, 1996. Method 3052, Microwave assisted acid digestion of siliceous and organically based matrices. Usepa 1–20. 10.1017/CBO9781107415324.004. [DOI] [Google Scholar]
- Van Cappellen P, Viollier E, Roychoudhruy A, Clark L, Ingall E, Lowe K, Dichristina T, 1998. Biogeochemical cycles of manganese and iron at the oxicanoxic transoition of a stratified marine basin. Env Sci Technol 32, 2931–2939. [Google Scholar]
- Vieira MC, Torronteras R, Córdoba F, Canalejo A, 2012. Acute toxicity of manganese in goldfish Carassius auratus is associated with oxidative stress and organ specific antioxidant responses. Ecotoxicol. Environ. Saf 78, 212–217. 10.1016/j.ecoenv.2011.11.015. [DOI] [PubMed] [Google Scholar]
- Webb SM, 2005. Structural characterization of biogenic Mn oxides produced in seawater by the marine bacillus sp. strain SG-1. Am. Mineral 90, 1342–1357. 10.2138/am.2005.1669. [DOI] [Google Scholar]
- Weber P, Behr ER, Knorr CDL, Vendruscolo DS, Flores EMM, Dressler VL, Baldisserotto B, 2013. Metals in the water, sediment, and tissues of two fish species from different trophic levels in a subtropical Brazilian river. Microchem. J 106, 61–66. 10.1016/j.microc.2012.05.004. [DOI] [Google Scholar]
- WHO, 2011. Manganese in Drinking-water. Manganese Drink. Backgr. Doc. Dev. WHO Guidel. Drink. water Qual [Google Scholar]
- Winkler P, Kaiser K, Thompson A, Kalbitz K, Fiedler S, Jahn R, 2018. Contrasting evolution of iron phase composition in soils exposed to redox fluctuations. Geochim. Cosmochim. Acta 10.1016/j.gca.2018.05.019. [DOI] [Google Scholar]
- Xia D, Yi X, Lu Y, Huang W, Xie Y, Ye H, Dang Z, Tao X, Li L, Lu G, 2019. Dissimilatory iron and sulfate reduction by native microbial communities using lactate and citrate as carbon sources and electron donors. Ecotoxicol. Environ. Saf 174, 524–531. 10.1016/j.ecoenv.2019.03.005. [DOI] [PubMed] [Google Scholar]
- Yu K, Böhme F, Rinklebe J, Neue H-U, DeLaune RD, 2007. Major Biogeochemical Processes in Soils-A Microcosm Incubation from Reducing to Oxidizing Conditions. Soil Sci. Soc. Am. J 71, 1406–1417. 10.2136/sssaj2006.0155. [DOI] [Google Scholar]
- Zachara JM, Cowan CE, Resch CT, 1991. Sorption of divalent metals on calcite. Geochim. Cosmochim. Acta 55, 1549–1562. 10.1016/0016-7037(91)90127-Q. [DOI] [Google Scholar]
- Zhang Z, Fang Z, Li J, Sui T, Lin L, Xu X, 2019. Copper, zinc, manganese, cadmium and chromium in crabs from the mangrove wetlands in Qi’ao Island, South China: Levels, bioaccumulation and dietary exposure. Watershed Ecol. Environ 1, 26–32. 10.1016/j.wsee.2019.09.001. [DOI] [Google Scholar]
- Zhu M, Ginder-Vogel M, Parikh SJ, Feng X-H, Sparks DL, 2010. Cation Effects on the Layer Structure of Biogenic Mn-Oxides. Environ. Sci. Technol 44, 4465–4471. 10.1021/es1009955. [DOI] [PubMed] [Google Scholar]
- Zhu S, Zhang P, Liang Y, Wang M, Xiong J, Tan W, 2020. Effects of aluminum substitution on the surface charge of colloidal goethite particles: experiments and MUSIC modeling. Environ. Sci. Pollut. Res 10.1007/s11356-020-07793-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


