Abstract
Understanding molecular alterations associated with peripheral inflammation is a critical factor in selectively controlling acute and persistent pain. The present report employs in situ hybridization of the 2 opioid precursor mRNAs coupled with quantitative measurements of 2 peptides derived from the prodynorphin and proenkephalin precursor proteins: dynorphin A 1-8 and [Met5]-enkephalin-Arg6-Gly7-Leu8. In dorsal spinal cord ipsilateral to the inflammation, dynorphin A 1-8 was elevated after inflammation, and persisted as long as the inflammation was sustained. Qualitative identification by high performance liquid chromatography and gel permeation chromatography revealed the major immunoreactive species in control and inflamed extracts to be dynorphin A 1-8. In situ hybridization in spinal cord after administration of the inflammatory agent, carrageenan, showed increased expression of prodynorphin (Pdyn) mRNA somatotopically in medial superficial dorsal horn neurons. The fold increase in preproenkephalin mRNA (Penk) was comparatively lower, although the basal expression is substantially higher than Pdyn. While Pdyn is not expressed in the dorsal root ganglion (DRG) in basal conditions, it can be induced by nerve injury, but not by inflammation alone. A bioinformatic meta-analysis of multiple nerve injury datasets confirmed Pdyn upregulation in DRG across different nerve injury models. These data support the idea that activation of endogenous opioids, notably dynorphin, is a dynamic indicator of persistent pain states in spinal cord and of nerve injury in DRG.
Keywords: Opioid, chronic pain, gene expression, neuropathic pain, allodynia, inflammation, morphine
Opioids are among the most effective analgesics in use, acting at receptors for endogenous opioid neuropeptides in spinal cord and in higher brain regions. These opioid peptides are also regulated in response to persistent pain states, participating in the complex plasticities that induce hyperalgesia, guarding, and other alterations associated with sustained nociceptive input. Enhancement of the opioid peptide precursor prodynorphin (Pdyn) mRNA levels31,38,62 in the dorsal spinal cord has been extensively described in response to persistent painful conditions,47,67 and is preceded by activation of transcriptional mechanisms regulating the Pdyn promoter.18,49–51,55 Similarly, regulation of enkephalin peptides in the spinal cord has been described in response to long-lasting nociceptive conditions.11,57 While these alterations have been demonstrated with a variety of molecular and neuroanatomical methods, examination of the corresponding functional roles has yielded a multifaceted picture with sometimes conflicting results. For example, dynorphin A 1-8 acts largely on the kappa opioid receptor,13 although other prodynorphin-derived peptides have activity at other targets such as the mu-opioid receptor or the nmethly-d-aspartate receptor.7,8 Intrathecal dynorphin administration, as well as stimulation of kappa opioid receptors, has been shown to produce both antinociceptive3,26 and pronociceptive effects,71 as well as suppression of itch.39 By contrast, proenkephalin peptides are generally analgesic, acting at the mu- and delta-opioid receptors.
In the present report, we examine the mRNA precursors and 2 opioid peptides that are representative of the 2 opioid peptide families found in the rat spinal cord: the preprodynorphin (PPD) and the preproenkephalin (PPE) systems. The neuroactive opioid peptides are formed by specific post-translational enzymatic cleavages of their representative protein precursors. The rat PPE precursor contains sequences corresponding to [Met5]-enkephalin-Arg6-Gly7-Leu8 (MERGL), as well as [Met5]-enkephalin, [Leu5]-enkephalin, and [Met5]-enkephalin-Arg6-Phe7 (MERF).28,75 Quantitative measurement and chromatographic characterization of MERGL, MERF and [Met5]-enkephalin have shown that each is present in spinal cord tissue with a similar distribution rostrocaudally and dorsoventrally, with the highest concentrations of all 3 molecules in the dorsal gray matter33,46 Similarly, dynorphin A 1-8 is one of several end products of prodynorphin post-translational processing.15,16,73 Other end products include dynorphin B, α/β-neoendorphin, and dynorphin A 1-17. Since dynorphin A 1-8 and MERGL are formed exclusively from their respective precursor molecules, changes in their content are likely to be representative of a general change in the PPD and PPE systems, respectively. The small C-terminal extensions of these 2 peptides allowed specific, C-terminally directed antibodies to be raised against each of the peptides, with essentially no cross reaction to each other or to the [Leu5]-enkephalin or [Met5]-enkephalin pentapeptide sequences. Thus, the 2 peptides can be extracted and independently measured from the same sample. In the present study, we examine spinal dynorphin after either complete Freunds-adjuvant (CFA) or carrageenan administration, 2 manipulations that drive peripheral inflammatory responses.31 Indeed, previous studies have compared at least 3 other injectable peripheral inflammatory agents including yeast, phorbol, and zymosan31,48 showing comparable results. We examine changes in dynorphin peptide or mRNA at the peak of inflammation-induced biochemical alterations in each model.
The neuroanatomical localization of the relevant PPE- and PPD-expressing neurons is an equally important component to understanding the functional roles of the 2 opioid systems. Multilabel fluorescent in situ hybridization for the 2 peptide precursor mRNAs was performed to explore somatotopy, coexpression, and issues of neuronal recruitment. Extending the investigation to regulation of PPD and PPE in dorsal root ganglion (DRG) after nerve injury indicates that during inflammation there is no contribution from DRG-derived PPE or PPD. Based on our findings, we suggest a dual spinal opioid system with high levels of potential on-demand enkephalinergic activity, but low levels of available dynorphinergic activity, except in those dorsal horn neurons undergoing upregulation of Pdyn.
Methods
Behavioral Assessments
Male Sprague-Dawley Rats (250–350 g, Envigo, Hackensack, NJ) were housed in pairs and tested under an approved animal protocol at the National Institutes of Health Clinical Center. Both the unilateral CFA and carrageenan inflammation modes were examined and they are used to some extent interchangeably in this report. For behavioral, inflammatory, and peptide characterizations, inflammation of 1 hind paw was induced by unilateral injection of 150 μL of a complete Freund’s adjuvant:sterile saline emulsion (1:1), containing 75 μg of killed mycobacterium tuberculosis cells (F5881, Millipore Sigma, St. Louis, MO), although for peptide content measurements in brain regions, the CFA was delivered bilaterally. Also, several doses of inflammatory material (as well as carrageenan vs CFA) were explored to extend the time period of inflammation, these are indicated in the text and figure legends where applicable. Hind paw edema was assessed by measuring the width of the thickest part of the paw (dorsum to plantaris) using a digital caliper. Thermal sensitivity was determined using a radiant thermal testing device (Plantar Test, Ugo Basille, Gemonio, Italy) calibrated with a thermistor wire (Omega Engineering, Inc, Stamford, CT). For assessment of spontaneous guarding behaviors, animals were habituated for 10 to 15 minutes while on a wire mesh table and spontaneous behaviors were recorded and scored over 6 consecutive 10-second epochs using the following scale: 0, normal weight bearing; 1, partial weight bearing; 2, no weight bearing; 3, spontaneous nocifensive behavior (licking, quivering, or excessive guarding). Subsequently, mechanical withdrawal thresholds were measured with von Frey filaments following the up-down method.12 After von Frey testing, animals were stimulated using a pinprick delivered to the plantar surface, and the duration of guarding behavior was recorded with a stopwatch. In control animals the response from stimulus application to return of the paw to the wire mesh platform occurs within .5 seconds, after inflammation the pin prick produces a more prolonged withdrawal/guarding response which can be timed. The inflammation did not interfere with locomotion or their ability to eat or groom normally. Although the rats tended to exhibit spontaneous guarding of the inflamed paw,31 at no time was veterinary treatment of the paw required.
Production of Antiserum
The dynorphin A 1-8 antibody from rabbit D5 was generated as described35 and the antibody properties and cross-reactivity of the antiserum have been characterized previously.38 Briefly, no cross-reactivity was observed with amounts of up to 500 pmols of dynorphin A 1-6, dynorphin B, [Leu5]- or [Met5]-enkephalin, [Met5]-enkephalin-[Lys6], [Met5]-enkephalin-[Arg6], and substance P. Negligible cross-reactivity was found with dynorphin A 1-7, α-neo-endorphin, dynorphin A 1-13, and dynorphin A 1-17. The characteristics of the MERGL and cholecystokinin (CCK) antisera have been previously described.23,33
Peptide Radioimmunoassay and Chromatography of Peripherally Inflamed Rats
Adult male Sprague-Dawley rats (300–400 g) were obtained from Zivic-Miller farms, Portersville, PA. Rats were housed using a 12-hour light-dark cycle and provided food and water ad libitum. To determine the effect of peripheral inflammation on spinal dynorphin and enkephalin peptide content, rats were unilaterally inflamed in the hind paw by injection of 200 μL of 1:1 emulsion of sterile saline and complete Freund’s adjuvant resulting in 100 μg of mycobacterium butyricum cells per injection in accordance with the manufacturer (344289, Sigma-Aldrich, St. Louis, MO). The rats were housed in pairs in cages with wood chip bedding to minimize the possibility of ulceration of the inflamed hind paw. In general, rats were euthanized 5 to 6 days after a single injection of Freund’s adjuvant-saline emulsion. However, 2 time-course experiments were performed. In the first experiment, rats were euthanized at 3, 5, 9, and 15 days after a single injection of the emulsion. Since sustained inflammation has been associated with an alteration in [Met5]-enkephalin,11 in the second experiment, rats were injected a second time on day 8 in order to sustain the peripheral inflammation, and that group was euthanized 7 days after the second injection. Both MERGL and dynorphin A 1-8 peptide contents were analyzed. For measurements in brain regions both hind paws were treated and brain regions were removed bilaterally at day 6 postinjection.
Tissue extraction and chromatographic procedures have been described previously.33 Briefly, tissues were extracted in 1 M acetic acid, .02 M HCl, and .1% β-mercaptoethanol. After boiling (10 minutes) and clarification by centrifugation (12,000 × g, 10 minutes), peptides were assayed in aliquots of the supernatant from which the acetic acid was removed by drying in a vacuum centrifuge. Prior to chromatography, samples were desalted on a C-18 Sep-Pak (Waters Associates, Milford, MA) and eluted with 60% acetonitrile in .1% trifluoracetic acid. Gel filtration was performed on a Bio Gel P-10 (Bio Rad, Hercules, CA) column (60 × .9 cm) equilibrated in and eluted with 1 M acetic acid. The column was pumped at 1 mL/20 min and 1 mL fractions were collected. High performance liquid chromatography (HPLC) was performed on a C-18 reverse phase column (.5 μ, 25 × .4 cm, Sulpeco, Sigma-Aldrich, St Louis, MO). The column was eluted with a linear gradient of 20% to 60% acetronitrile in .1% trifluoroacetic acid at a rate of 1 mL/min and 1 mL fractions were collected. For both types of chromatography, fractions were dried in a vacuum centrifuge prior to assay. Tissue peptide levels were normalized to protein content, which was measured by the dye binding assay of Bradford.6
Multiplex Fluorescent in situ Hybridization
To prepare sections for in situ hybrdization, male Sprague-Dawley rats (Envigo, Frederick, MD) were unilaterally inflamed with 150 μL of 4% lambda carrageenan (Sigma, Type IV, C-3889) in sterile saline. After 2 days, animals were deeply anesthetized and perfused intracardially with ice cold saline followed by ice cold 4% paraformaldehyde. The spinal cord lumbar enlargement was dissected divided into rostral and caudal halves and postfixed in 4% paraformaldehyde overnight. Samples were embedded in paraffin blocks at Histoserv Inc (Germantown, MD) and 6 μm sections were mounted on charged glass slides. After deparaffinization, in situ hybridization was performed with RNA-scope Multiplex Fluorescent assays v2 (Advanced Cell Diagnostics, Newark, CA) with Tyramide Signal Amplification (TSA Plus Fluorescein, Cyanine 3 and Cyanine 5 Systems; Perkin Elmer, Waltham, MA). Stained sections were imaged using an Axio Imager.Z2 slide scanning fluorescence microscope (Zeiss, Oberkochen, Germany) equipped with a 20X/0.8 Plan-Apochromat (Phase-2) nonimmersion objective (Zeiss, White Plains, NY), a high-resolution ORCA-Flash4.0 sCMOS digital camera (Hamamatsu, Shizuoka, Japan), a 200W X-Cite 200DC broad band lamp source (Excelitas Technologies, Waltham, MA), and 4 customized filter sets (Semrock, Rochester, NY) optimized to detect the following fluorophores: DAPI, FITC, CY3, and CY5. Image tiles (600 × 600 μm viewing area) were individually captured at .325 micron/pixel spatial resolution, and the tiles seamlessly stitched into whole specimen images using the ZEN 2 image acquisition and analysis software program (Zeiss, White Plains, NY), with an appropriate color table applied to each image channel to either match its emission spectrum or to set a distinguishing color balance. Pseudocolored stitched images were overlaid to create multicolored merged composites. Images were subsequently analyzed and quantified using Fiji image analysis software (v 1.0). Briefly, a threshold was determined for fluorescent puncta and intensity and area of the particles were analyzed using automatic particle counting. The paraffin sections were 6 microns thick, which resulted in relatively few fluorescently labeled neurons in each section. To obtain an indication of the overall staining patterns, images from 4 individual animals were warped into a common 2-dimensional registration of spinal cord laminae and gray and white matter compartments based on published literature.70 The individual section images were overlaid in Photoshop to show an aggregate projection image of stained neurons in all 4 sections.
Regional Dissection and Peptide Measurement From Spinal Cord and Brain
Rats were decapitated and whole spinal cords were rapidly isolated by hydraulic ejection by forcing ice-cold saline through the spinal canal using a 12 mL syringe and a 19 gauge needle with the beveled end cut off and polished, inserted into the spinal canal at the coccygeal segments of the vertebral column.17 For peptide isolation, the caudal portions of the lumbar and cervical enlargements were dissected out of whole spinal cord and the left and right dorsal and ventral quadrants were removed and frozen on dry ice.
In addition to spinal cord, neuropeptide content was measured in periaqueductal gray (PAG), hypothalamus, septum, caudate, and substantia nigra (N = 4 rats). Since preserving lateralization is not as x0027 critical as in the spinal cord, rats were inflamed bilaterally with Freund’s adjuvant-sterile saline emulsion and tissues were collected at 6 days postinflammation, which was close to the peak in spinal cord. The dissection procedure is illustrated in.54 Brain regions were collected bilaterally where applicable from each animal and both sides were pooled. Tissues were extracted with 90% methanol, and then re-extracted with the acetic acid-HCl mixture to improve CCK-8 recovery. The resulting 2 supernatants were combined and aliquots were measured for CCK-8-like immunoreactivity, dynorphin A 1-8, and MERGL. The 5 brain regions were dissected for peptide radioimmunoassays as follows. First, the septum was removed after retracting the 2 cerebral hemispheres laterally on either side of the sagittal sinus and exposing the corpus collosum, which was then split midsagitally. The head of the caudate was removed from a coronal slice in which the posterior border was at the level of the anterior commissure, which was exposed upon removal of the septum. The hypothalamus was removed from the ventral surface of the brain, and the mesencephalon was isolated by 2 cuts: the first at the rostral border of the superior colliculus and the second at the caudal border of the inferior colliculus. The substantia nigrae, with adherent cerebral peduncles, were removed first from the ventral surface. Next, a block was cut around the perimeter of the aqueduct of sylvius which contained the PAG. All tissues were frozen on dry ice and stored at –80°C until assayed. Peptide concentration values are reported as pmoles/mg protein and represent mean ± SEM. Comparisons were made with Student’s t-test; *, P < .02.
Sciatic Nerve Axotomy
Rat sciatic nerve axotomy was used to examine transcriptional changes due to axon damage. A complete transection of the sciatic nerve was performed in male Sprague-Dawley rats (Harlan, 250–350 g) as follows: rats were anesthetized with 2.5 to 5% isoflurane; the common sciatic nerve was exposed by blunt dissection distal to the sciatic notch and freed of surrounding tissue, and a 5 mm section of the nerve was excised. On days 1, 3, 10, 30, and 90 after the axotomy, animals were euthanized and the L4 and L5 ganglia were dissected from individual rats after tracing the branches from the sciatic nerve. Total RNA was extracted from the 2 ganglia, and RNA-Seq was performed. For these experiments 4 animals were analyzed per time point (N = 4) except at the 90 day time point (N = 3), and each time point was compared to a naÿve control (N = 8). Contralateral DRGs were also quantified (N = 1 per time point), and are plotted, but statistics were performed by comparing the distribution of the axotomized animals to the distribution of the naÿve controls, but not to the single N = 1 observation from the contralateral side. These observations were further validated by reanalyzing sequence data from 4 published reports from which the raw sequence reads were deposited in National Center for Biotechnology Sequence Read Archive database (BioProjects PRJNA232819, PRJNA254183, PRJNA419222, PRJNA124389).1,24,43,56 These reports examined either rat lumbar spinal nerve transection or lumbar spinal nerve ligation, which have an axotomy component, and measured DRG mRNAs using RNA-Seq. Notably, several of these datasets contained sham operated animals which did not differ from naÿve animals with respect to Penk, Pdyn, or Gal peptide precursor transcripts in the DRG.
RNA-Seq Analyses of Opioid Precursor Genes Across Species
RNA-Seq datasets from DRG were aligned using the mRNA and Genome Intergrated Cooperative RNA-Seq analysis pipeline to generate significant fragments per kilobase per million reads, as described previously.42,76 The datasets for mouse,22 rat,63 dog,34,64 and human63 have been published previously, and were reanalyzed for this report. Tables containing the mRNA and Genome Intergrated Cooperative-aligned significant fragments per kilobase per million reads values for tissues reanalyzed in the present report are available in supplementary datafiles accompanying this manuscript.
Results
Behavioral Assessment of Hyperalgesia After Peripheral Inflammation With CFA
Male Sprague-Dawley rats were unilaterally injected with 150 μL of 1:1 saline:CFA emulsion and behavioral characterization was performed to assess hyperalgesia across several modalities. CFA injection induced substantial edema that was detectable to at least 28 days but with the strongest edema occurring between 4 hours and 3 days (Fig 1A). The inflamed paw showed marked evidence of thermal hyperalgesia, as measured by significantly shorter latencies of withdrawal to a radiant thermal stimulus, which was most pronounced at the earliest time point tested (4 hours) and resolved between 7 and 14 days (Fig 1B). Mechanical sensitivity was also enhanced. The inflamed paw showed mechanical hypersensitivity as demonstrated by significantly lower threshold responses to von Frey filament, which had an early and profound onset, similar to the thermal hyperalgesia, but remained significant until at least day 21, and largely resolved by day 28 (Fig 1C). To evaluate guarding behaviors, animals were given a pinprick stimulus (Fig 1D). This test of evoked mechanical sensitivity revealed long-lasting hypersensitivity (at least 21 days), similar to the von Frey test. Assessment of spontaneous guarding behavior on a wire mesh platform revealed spontaneous nocifensive behavior on the inflamed paw, which was most evident at the 4-hour time point, and returned slowly to control levels over ~21 days (Fig 1E). This indicates that spontaneous nocifensive behavior to a mildly agitating mechanical stimulus (standing on wire mesh) resolves more quickly than evoked mechanical responses (von Frey and pinprick withdrawal). Significance testing was performed using 2-way ANOVA (Fig 1A and B), multiple Mann-Whitney U-tests (Fig 1C and E), or exact binomial test (Fig 1D). For all results in Fig 1, Holm-šidák corrections were made to account for multiple comparisons.
Figure 1.
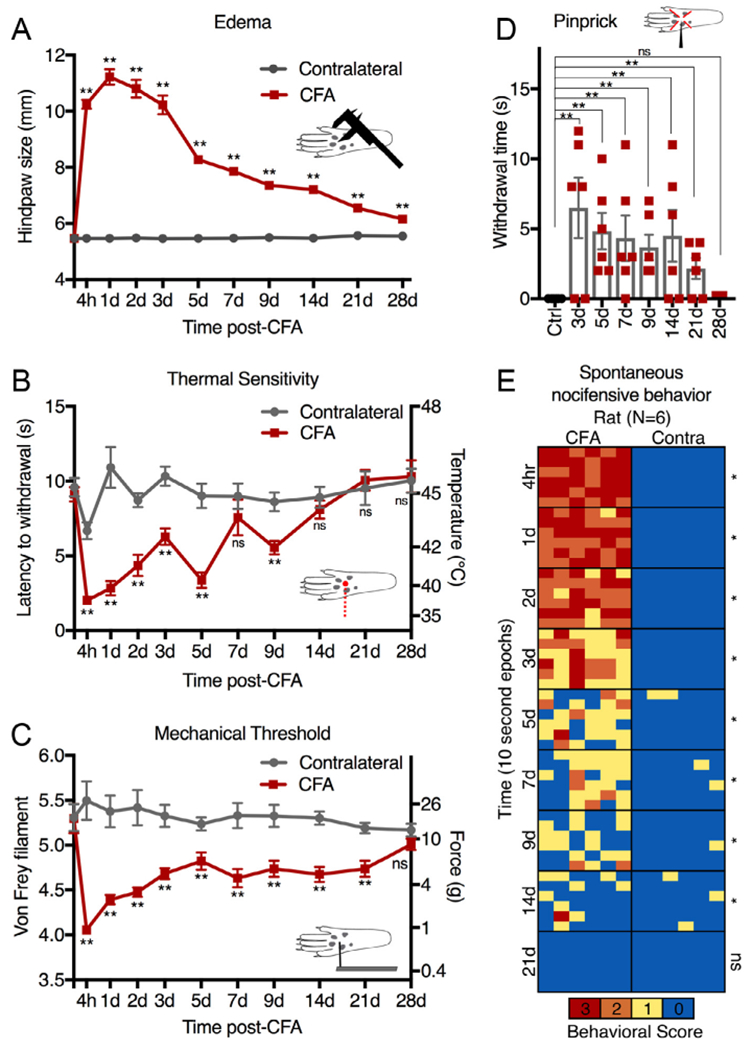
Behavioral assessment of hyperalgesia after peripheral inflammation with complete Freund’s adjuvant (CFA). Rats were unilaterally injected with 150 μL of 1:1 saline:CFA emulsion and behavioral characterization of several sensory modalities was performed. (A) Inflammation caused significant edema lasting at least 28 days; **, P < .01; 2-way ANOVA followed by Holm-šidák. (B) Thermal sensitivity was evaluated using radiant thermal stimuli. Significantly reduced withdrawal latencies occurred for the inflamed paw which resolved completely by 14 days. (C) Mechanical sensitivity was recorded as the threshold response to von Frey filament testing. Inflammation reduced mechanical force necessary to induce withdrawal and this effect largely resolved after 28 days. (D) Evoked guarding behavior was stimulated by plantar pinprick. No guarding occurred at baseline, but in inflamed animals prolonged guarding lasted at least 21 days. (E) Spontaneous guarding behavior and weight bearing was assessed by observation of the animals while standing on a wire mesh grid. Behavioral scores were assigned in 6 consecutive 10-second epochs. 0, normal weight bearing; 1, partial weight bearing; 2, no weight bearing; 3, spontaneous nocifensive behavior (licking, quivering, or excessive guarding). Animals showed significantly more spontaneous guarding on the inflamed paw for at least 2 weeks. Error bars show standard error of the mean (A, B) or standard deviation (C, D). Significance testing was performed using 2-way ANOVA (A, B), multiple Mann-Whitney U-tests (C, E), or exact binomial test (D). For all results, Holm-šidák corrections were made to account for multiple comparisons; *, P < .05; **, P < .01.
Dynorphin A 1-8 and MERGL in Dorsal and Ventral Spinal Cord
Dynorphin A 1-8, and MERGL are processed peptides originating from the PPD and PPE precursor proteins, respectively (Fig 2A and B). Peptide measurements were made at days 0, 3, and 8 after unilateral hind paw inflammation using the 1:1 CFA:saline emulsion. In a second cohort of rats, a reinjection was performed on day 8 to maintain inflammation and these rats were euthanized at day 14 after the initial inflammation (day 6 after reinjection). Dynorphin A 1-8 was significantly elevated at day 3, day 8, and day 14 (day 6 after reinjection) in the lumbar dorsal spinal cord (ANOVA with post hoc Scheffé test; N = 5), but was not significantly altered in the ventral lumbar spinal cord (Fig 2C). The content of MERGL was not significantly altered in either dorsal or ventral lumbar spinal cord (Fig 2D).
Figure 2.
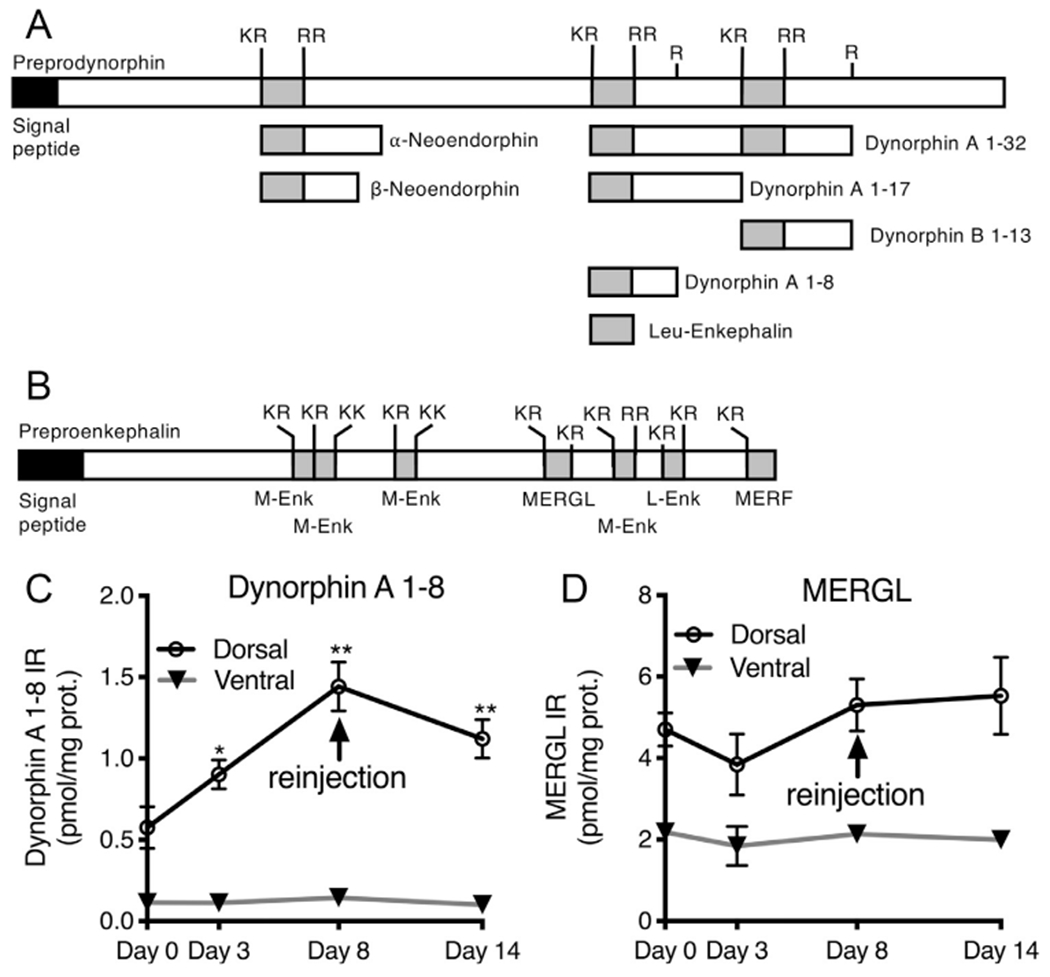
Time course of dynorphin A 1-8 and MERGL peptide content in dorsal and ventral spinal cord after inflammation. Peptide measurements were performed for 2 opioid peptides that act in the dorsal spinal cord. (A) Dynorphin A 1-8 (YGGFLRRI) is processed from the preprodynorphin precursor, where it is one of several neuroactive products. A simplified diagram, adapted from,69 shows some of the major products of this precursor protein. Met- and Leu-enkephalin-containing sequences are shown in gray shading. (B) A similar model is presented for preproenkephalin processing showing the location of the MERGL (YGGFMRGL) sequence. (C-D) Animals were injected with 200 μL 1:1 CFA saline emulsion, and reinjected with the same adjuvant at the end of day 8. Measurements were taken at day 0, 3, 8. A final sample was taken at day 14 after the initial inflammation, with reinjection performed on day 8. (C) Dynorphin A 1-8 was significantly elevated at day 3, day 8, and day 14 in the dorsal spinal cord in the lumbar dorsal spinal cord (ANOVA with post hoc Scheffé test; N = 5), but was not significantly altered in the ventral lumbar spinal cord. (D) Levels of MERGL were not altered in either dorsal or ventral lumbar spinal cord.
Segmental Specificity of Dynorphin A 1-8 Increase During Peripheral Inflammation
The time course following a single CFA injection was examined. One hind paw was injected with 200μL of a 1:1 emulsion of complete Freund’s adjuvant-sterile saline emulsion and dynorphin A 1-8 content was measured in lumbar dorsal spinal cord. Dynorphin A 1-8 was significantly elevated at days 3, 5, 9, and 15 after peripheral inflammation in the ipsilateral dorsal horn relative to the contralateral side (paired t-tests; N = 4; Fig 3A). Subsequently, an ANOVA was performed with 1 repeated measure followed by Scheffé post hoc testing, and showed significantly higher levels of dynorphin A 1-8 at day 5 relative to other the ipsilateral time points. The elevation in dynorphin A 1-8 was segmentally specific and confined to the lumbar enlargement. Dynorphin A 1-8 content was examined in lumbar and cervical segments in ipsilateral versus contralateral dorsal spinal cord of both inflamed and uninflamed animals at day 6. Dynorphin content was elevated on the ipsilateral side of inflamed animals in the lumbar, but not the cervical spinal cord, exemplifying the segmental specificity of the dynorphin A 1-8 increase (ANOVA, with post hoc Scheffé test, N = 4; Fig 3B).
Figure 3.
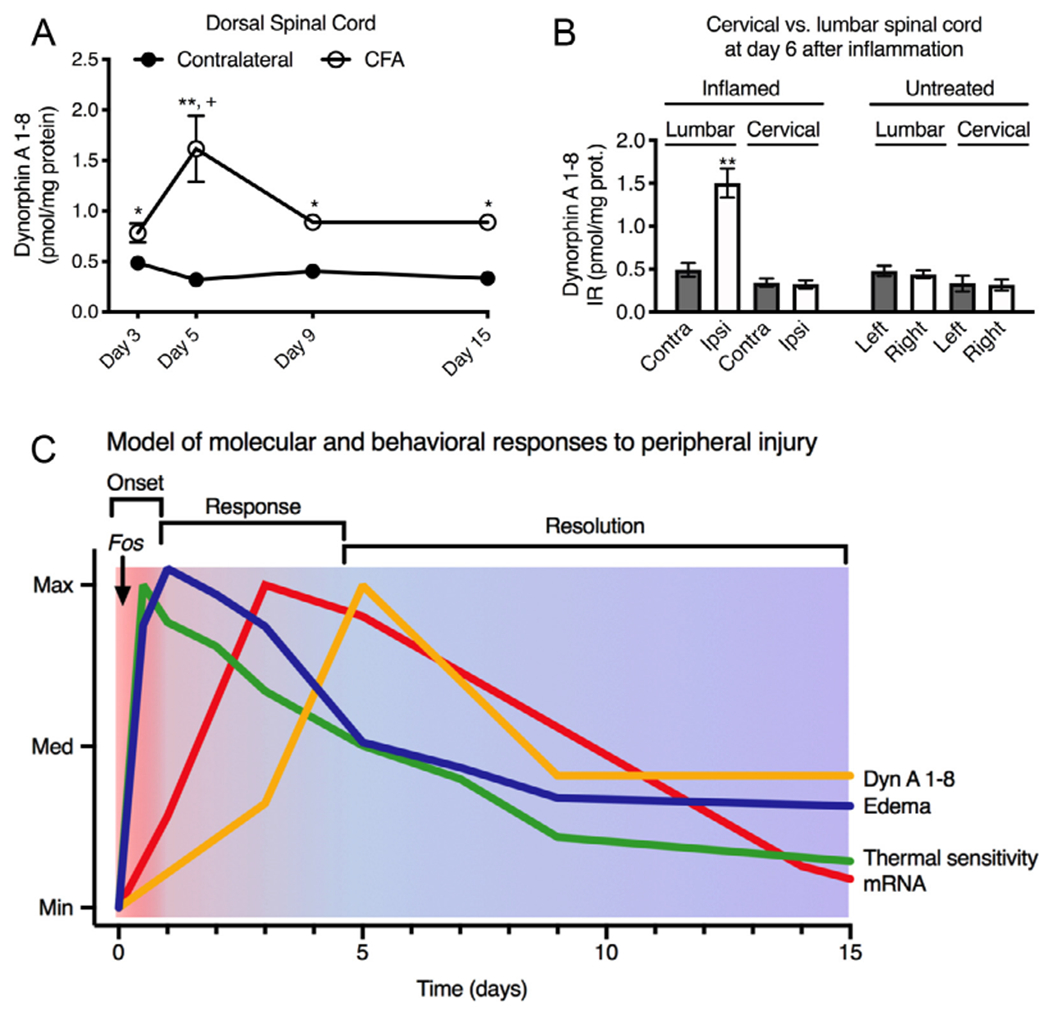
Time course segmental specificity and temporal model. To investigate the time course of a single CFA injection, 1 hind paw was injected at day 0 with 200μL of a 1:1 emulsion of complete Freund’s adjuvant and sterile saline. (A) Time course. The dynorphin content in spinal cord was significantly elevated at each time point relative to the contralateral side of the same animals using a paired t-test at each time point (N = 4; *, P < .02; **, P < .001). An ANOVA was performed with 1 repeated measure, using the Scheffé post hoc test, showing no difference in the levels of dynorphin A 1-8 on the contralateral side over time. The same test also revealed that the day 5 levels of dynorphin on the inflamed side were higher than at other time points (+, P < .05). (B) Segmental specificity. Segmental specificity of the increase in dynorphin content was assessed by comparing lumbar spinal cord, which receives innervation from the inflamed hind limb with cervical spinal cord, which does not. Only the ipsilateral inflamed dorsal spinal cord showed significant elevation of dynorphin peptide (1-way ANOVA with post hoc Scheffé test; **, P < .001). Error bars represent the standard error of the mean. (C) Multimodal temporal model. Based on the data in the present report, and previous literature, we modeled the time course of behavioral, inflammatory edema, and molecular events in response to peripheral inflammation. At onset phase, Fos mRNA spikes briefly with immediate edema and thermal hypersensitivity. Subsequently, Pdyn mRNA rises, which is then followed by Dyn A1-8 peptide content accumulation. Thermal sensitivity resolves faster than edema, while mechanical sensitivity resolves similarly to edema (as shown in Fig 1). We hypothesize that period up to ~day 4 represents a process of active dynorphin peptide release which keeps tissue levels from accumulating. Subsequently, as hyperalgesia resolves there is a overshoot in tissue peptide content.
Gel Filtration and High-Performance Liquid Chromatographic Analyses of Dynorphin A 1-8 Immunoreactivity
Size exclusion gel chromatographic characterization in spinal cord extracts indicated that the immunoreactive material was composed mainly of a low molecular weight substance, which eluted in the position of dynorphin A 1-8 in extracts from control and inflamed dorsal spinal cord (Fig 4A). Qualitative identification of the immunoreactivity by reverse-phase HPLC established that the major peak of migrated in the position of the synthetic dynorphin A 1-8 standard (Fig 4B). Results from both chromatographic formats detected more immunoreactivity in the inflamed condition compared to control extracts. These results demonstrate that the antibody raised against dynorphin A 1-8 is highly specific for measurement of peptide levels from spinal cord lysates of both inflamed and uninflamed rats and that the elevated immunoreactive material is authentic dynorphin A 1-8.
Figure 4.
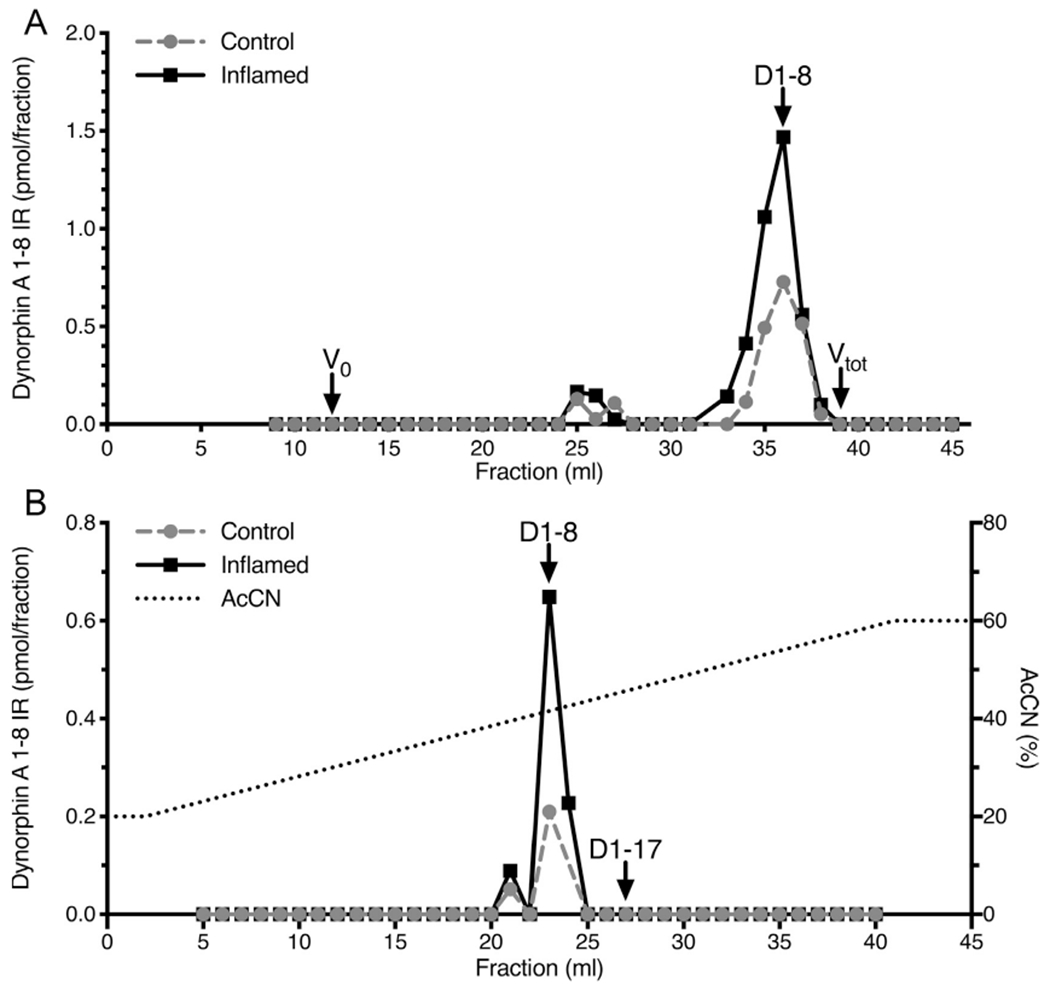
(A) Gel chromatographic and (B) high-performance liquid chromatographic analyses of dynorphin A 1-8 immunoreactivity. (A) Acidic extracts of lumbar dorsal cord from inflamed and control animals were adsorbed on a C-18 Sep-Pak, which was washed with water and then eluted with 60% acetonitrile, .1% TFA. The eluate was dried in a vacuum centrifuge. The sample was resuspended in 500 μL of 1 M acetic acid, which also was the mobile phase. One mL fractions were collected, lyophilized and analyzed for dynorphin A 1-8 immunoreactivity. One major peak of immunoreactivity was detected which eluted in the position of synthetic dynorphin A 1-8 standard. Note the greater amount of immunoreactivity in the extract from inflamed tissue. (B) Dorsal cord extracts were prepared as described for gel filtration, resuspended in 250 μL .1% TFA and injected into an Altex 5 μ octadecylsilane reversed phase column. Solvent delivery was 1 mL/min. The column was eluted with a linear gradient of 20 to 60% acetonitrile in .1% TFA over 40 minutes (dashed line). One mL fractions were collected and assayed for dynorphin A 1-8 after drying under reduced pressure. The elution positions of synthetic dynorphin A 1-8 and 1-17 are shown (arrows). One major peak eluting in the position of dynorphin A 1-8 was observed. As with size exclusion chromatography, a larger dynorphin A 1-8 peak was observed in extracts from inflamed spinal cord relative to control.
Neuropeptide Content of 5 Brain Regions After Peripheral Inflammation
Dynorphin and enkephalin peptides were also surveyed in several brain regions to examine the modulation of the peptide content in areas of the brain responsible for functions such as descending inhibition of nociception, and affective components of pain. The neuropeptide CCK was examined both as a control due to its high level of expression in several of the selected brain regions, and because it has been implicated in modulation of hyperalgesia. In the PAG there was an increase in MERGL peptide content, consistent with opioid inhibition increasing in response to inflammatory hyperalgesia. MERGL was also increased in the substantia nigra, suggesting modulation of opioid signaling in this brain region as well (Fig 5).
Figure 5.
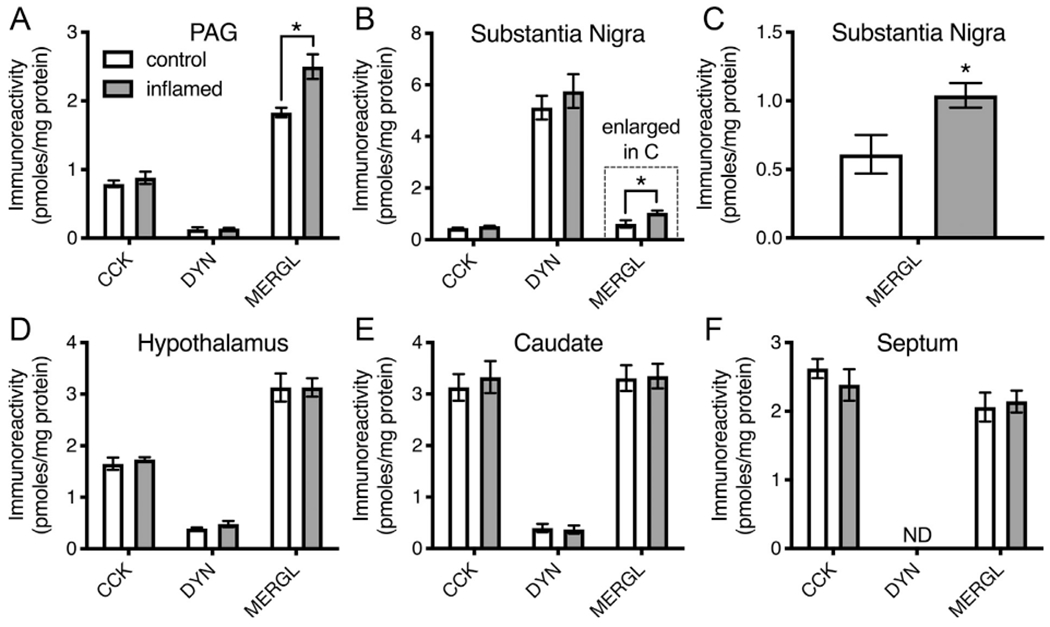
Neuropeptide content of 5 discrete brain regions after peripheral inflammation. Neuropeptide content (pmoles/mg protein) was measured in periaqueductal gray (PAG), hypothalamus, septum, caudate, and substantia nigra of 4 rats. The Freund’s adjuvant-saline emulsion was injected into both hind paws, and rats were euthanized after 6 days. Bilateral brain regions were pooled. Since sulfated cholecystokinin-8 (CCK-8) immunoreactivity is not extracted efficiently with acidic conditions, tissues were first extracted with 90% methanol and then re-extracted with acid mixture. The 2 supernatants were combined and aliquots were measured for CCK-8-like immunoreactivity, dynorphin A 1-8, and MERGL. Values represent mean ± SEM. Comparisons were made with Student’s t-test; *, P < .02.
Fluorescent in situ Hybridization of Prodynorphin and Proenkephalin mRNA 2 Days After Peripheral Inflammation
Animals were inflamed by a unilateral intraplantar injection of 6 mg of carrageenan suspended in 150 μL of sterile saline and lumbar spinal cord tissue was harvested 48 hours after inflammation. Neurons showing an increase in Pdyn mRNA were mainly located in the medial superficial layers (lamina I and II outer). The medial location is consistent with the somatotopic innervation of the dorsal horn whereby the distal paw afferents synapse medially and afferents from the proximal limb synapse more laterally. In the dorsal horn ipsilateral to the inflamed hind paw, both the area and intensity of Pdyn hybridization signal were increased after inflammation (Fig 6A–F), with very bright staining filling most of the cytoplasm of the labeled neurons (Fig 6A–D). The Pdyn in situ hybridization signal on the contralateral side was weaker than would be expected from peptide and RNA-Seq estimates of basal state dynorphin production in the dorsal horn (Fig. 2 and 7). The apparent accentuation of the induction of Pdyn may reflect technical issues with the fluorophore chosen and the in situ hybridization method. We observed that Penk intensity, but not area, was increased, indicating that the brightness of Penk+ cells increased with inflammation (Fig 6G). The area of the fluorescent signal for Penk staining was much greater than that of Pdyn, and this is also reflected by the larger number of Penk+ cells relative to Pdyn+ cells (Fig 6H) and transcriptomically by the higher expression level of Penk compared to Pdyn (Table 1). Within the medial dorsal horn, co-staining showed largely separate populations of neurons for the 2 transcripts (Fig 6C and D). However, systematic examination of the sections did reveal co-labeling of Penk and Pdyn within a small minority of neurons. Out of 55 Pdyn+ and 278 Penk+ neurons, 7 exhibited some degree of co-expression, (12.7% of Pdyn+ and 2.5% of Penk+ neurons, respectively; Fig 6H). Notably, these neurons were often faintly labeled for 1 of the 2 peptide precursor transcripts. A representative image of a Pdyn/Penk co-positive neuron is shown (Fig 6I–K).
Figure 6.
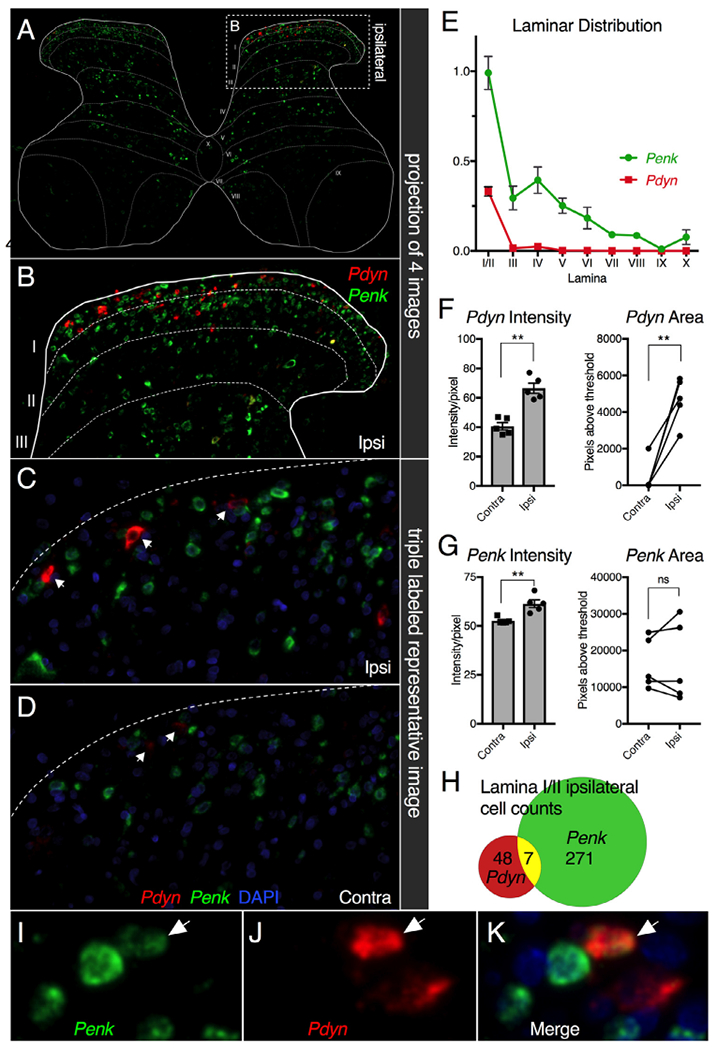
Fluorescent in situ hybridization of prodynorphin and proenkephalin mRNA 2 days after peripheral inflammation. Animals were inflamed by intraplantar injection of 6 mg of carrageenan in 150μL sterile saline and tissue was harvested 2 days later. Coronal sections of lumbar spinal cords from 4 unilaterally inflamed rats were stained using multiplex ISH using probes against Penk (green) and Pdyn (red) transcripts with DAPI as a nuclear marker. (A) All 4 stained images were scanned and spatially registered to show aggregate staining across all sections analyzed using a spinal laminar overlay template adapted from.70 (B) The upregulation of Pdyn in the ipsilateral dorsal horn is evident in the ipsilateral superficial laminae, where the Pdyn+ cells are concentrated. (C, D) Representative images of the medial aspect of the superficial laminae are shown for the (C) ipsilateral and (D) contralateral sides, red fluorescent Pdyn+ cells indicated by arrows. The signal is comparatively weak on the noninflamed control side. (E) Graph depicting the overall distribution of staining showed Penk in multiple laminae, with a concentration in lamina I/IIo, whereas Pdyn was mostly observed in lamina I/IIo. (F) Intensity and area of Pdyn staining was examined per pixel subsequent to thresholding. The average intensity of staining was significantly higher in the ipsilateral dorsal spinal cord compared to the contralateral side. The area (in pixels) of detectable Pdyn signal also was strongly increased on the ipsilateral side. (G) For Penk, the intensity measurement was increased significantly but by a much smaller magnitude, whereas the area was not different. (H) A small number (approximately 7) cells showed colocalization of Penk and Pdyn. (I-K) A representative image of a Pdyn+/Penk+ co-positive cell is shown (arrow). Statistics were performed using a 2-tailed Mann-Whitney U-test; **, P < .01. Error bars represent standard error of the mean. (Color version of figure is available online.)
Figure 7.
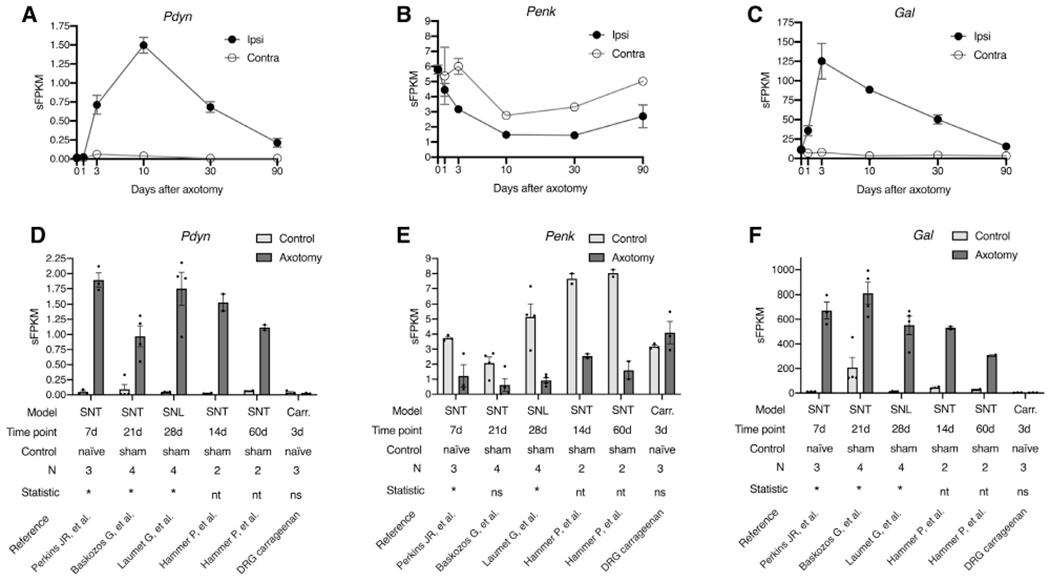
Modulation of prodynorphin (Pdyn), proenkephalin (Penk), and galanin (Gal) mRNA expression in the DRG after sciatic nerve transection or spinal nerve transection or spinal nerve ligation. (A-C) Sciatic transcetion. The sciatic nerve was transected and L4 and L5 DRGs were collected at 0d, 1d, 3d, 10d, 30d, and 90d after the axotomy. Both Pdyn and Gal were significantly elevated in the dorsal root ganglia, whereas Penk was significantly decreased. Statistics were performed in MAGIC with experimental time points compared to the naÿve control. The contralateral side is shown, but statistics were not performed for ipsilateral versus contralateral at each time point, because the contralateral sample for some time points were single replicates (10d, 30d, 90d). In the ipsilateral time points, some error bars are smaller than the marker size (not shown). (D-F) Molecular meta-analysis of spinal nerve injury studies. The experimental axotomy findings in A-C were also investigated by realigning and reanalyzing 4 RNA-Seq datasets from the SRA database from previously published reports. Also included is an examination the transcriptional effects of carrageenan inflammation on DRG gene expression. Statistics were performed to examine the overall statistical effect between all axotomy samples and all controls in aggregate (see methods). All 3 genes were significant when considering the data from all 5 datasets in aggregate. In contrast, carrageenan did not alter DRG expression of Pdyn, Penk, or Gal. Error bars show the standard error of the mean.
Table 1.
RNA-Seq Analyses of Peptide Precursor Genes in 3 Model Organisms and Human
| DRG |
|||||||
|---|---|---|---|---|---|---|---|
| Mouse |
Dorsal Spinal Cord |
||||||
| Trpv1L | Trpv1D | Rat | Dog | Human | Rat | Human | |
| n = 1 | n = 1 | n = 8 | n = 4 | n = 4 | n = 6 | n = 3 | |
| Pdyn | .13 | 0 | .03 ± .00 | .01 ± .00 | .01 ± .00 | 36.00 ± 1.02 | 33.89 ± 4.78 |
| Pnoc | .52 | 0 | .59 ± .12 | .04 ± .00 | .03 ± .00 | 21.93 ± 1.33 | 39.74 ± 5.48 |
| Pomc | 1.48 | 1.68 | 1.43 ± .18 | .07 ± .02 | .45 ± .13 | .31 ± .04 | 2.06 ± .21 |
| Penk | 3.63 | .95 | 5.97 ± .31 | 5.44 ± 1.32 | 5.57 ± 3.83 | 201.61 ± 5.01 | 265.45 ± 51.39 |
The levels of the mRNAs encoding proenkephalin (Penk), prodynorphin (Pdyn), pronociceptin (Pnoc), and Pro-opiomelanocortin (Pomc) were examined in DRG and dorsal spinal cord of rat and human, only in DRG of canines, and in 2 populations of DRG neurons in mouse. The latter were obtained from a cell sorting experiment in which Trpv1+ lineage DRG neurons (Trpv1L) were compared to whole DRG from animals expressing a toxin driven by the Trpv1 promoter, which removes all Trpv1 lineage neurons (Trpv1D). While the precise limit of quantitation is difficult to establish, .1 sFPKM or lower is very unlikely to be a consequential amount, especially for neuropeptides which are generally quite highly expressed because of a lack of a re-uptake mechanism for peptide transmitters (note levels of Penk, Pdyn, and Pnoc expression in the dorsal spinal cord of rat and human).
RNA-Seq Analysis of Basal Peptide Precursor Genes Expression in Mouse, Rat, Dog, and Human
In order to place our examination of dynorphin peptide in the dorsal horn in context and assess the potential for the DRG to contribute to dorsal horn dynorphin peptide content, we measured levels of opioid peptide precursor RNAs in the DRG and spinal cord using RNA-Seq. Proenkephalin (Penk), prodynorphin (Pdyn), pronociceptin (Pnoc), and Pro-opiomelanocortin (Pomc) were examined in DRG of mouse, rat, dog, and human, and dorsal spinal cord of rat and human (Table 1). In general, establishing a negative with RNA-Seq is difficult, however the inclusion of multiple species and a known positive result (Pdyn in the dorsal horn) makes us more confident that the levels of Pdyn in the DRG are unlikely to be contributory to the dynorphin peptide content measured in the dorsal horn (Table 1). In rat, dog, and human whole dorsal root ganglia were examined. In the mouse, a previously published cell sorting experiment22 was re-analyzed in which Trpv1+ lineage DRG neurons (Trpv1L) were compared to DRGs from animals expressing diptheria toxin driven by the Trpv1 promoter, which leads to the genetic deletion of all Trpv1 lineage neurons (Trpv1D), including populations which do not express Trpv1 in the adult. All 4 of these opioid peptides exhibited lower levels of expression in the 5 DRG datasets examined relative to Penk, and Pdyn in the spinal cord, indicating that the dorsal horn neurons are the major source of Penk- and Pdyn-derived peptides. These measurements are also consistent with the peptide measurements at baseline where the MERGL content is 5 to 10 times that of DynA1-8 (note y-axes in Fig 2C and D).
Measurement of Prodynorphin (Pdyn), Proenkephalin (Penk), and Galanin (Gal) mRNA in the DRG After Sciatic Nerve Transection.
Several reports suggest that dynorphin peptides are produced in the DRG4,21,25,52 in nerve injury models of chronic pain. This possibility was investigated using RNA-Seq because the production of these peptides in DRG may contribute to peptide measurements made in the dorsal spinal cord in the hyperalgesic state as opposed to the basal state. These studies were performed in naÿve control and axotomized animals at 6 time points to examine the contribution of nerve injury. Both Pdyn and Gal were significantly elevated after axotomy (N = 4) in the dorsal root ganglia of axotomized animals relative to naÿve controls (N = 8, Fig 7). To confirm and extend the findings from our study, 4 datasets of rat axotomy1,24,43,56 (variants of the Chung model40 involving spinal nerve transection and/or spinal nerve ligation) were examined from the Sequence Read Archive database (BioProjects PRJNA232819, PRJNA254183, PRJNA419222, PRJNA124389). Despite using different models and time points, these 4 datasets showed induction of Pdyn and Gal, as well as a decrease in expression of Penk. Three of the 4 datasets used sham-operated controls, which corroborates that these gene changes are due to axotomy, as opposed to the surgical procedures (skin incision, etc). We also examined the effect of peripheral carrageenan injection (performed according to the methods described for Fig 6). Carrageenan inflammation did not affect expression of these 3 peptide precursor transcripts in the DRG. Overall these results corroborate that spinal dynorphin.
Discussion
The data presented in this report demonstrate that peripheral inflammation has a profound impact on the spinal dynorphin system. Inflammation induced by intraplantar injection of Freund’s adjuvant-saline emulsion produces a rapid increase in spinal cord dynorphin A 1-8 content. The peptide increase is strictly localized to the ipsilateral dorsal spinal cord segment innervated by the inflamed hind limb, with no increase observed contralateral to the inflammation, in the ventral spinal cord, or in more rostral spinal segments. This supports the idea that the increase in spinal dynorphin is triggered by, and dependent upon, afferent nociceptive inputs arising from the site of inflammation. The peptide changes are consistent with those previously reported for the upregulation of the dynorphin precursor mRNA (Pdyn) after unilateral peripheral inflammation with CFA and other inflammatory agents.31,62 High resolution reverse phase HPLC and gel filtration chromatography indicate that in extracts from both control and inflamed tissues, the dynorphin A 1-8 immunoreactivity elutes in the same position as an authentic standard. Thus, the signal cannot be attributed to a cross reaction with a high molecular weight precursor fragment that has a similar C-terminus as dynorphin A 1-8 as can occur with PPE.33 With both chromatographic methods there was more immunoreactivity in extracts from the dorsal spinal cord that received innervation from the inflamed limb (Fig 4). The present dataset also shows a ~20% nonsignificant trend towards elevation in MERGL peptide content, and analysis of the specificity of the antibody used to detect MERGL were examined in a previous study.33
The increase in dynorphin occurred relatively rapidly, although temporally delayed in comparison to hyperalgesia, which is maximal at 4 to 6 hours31,32 (Fig 1). We elaborate a summary diagram (Fig 3C) based on data from the present manuscript and previous studies. One of the first molecular events that has been described is the rapid elevation of Fos mRNA, which has been shown to occur within dynorphinergic neurons based on protein staining.18,55 This event indicates the immediate activation of a subpopulation of spinal dynorphinergic neurons. These neurons have also been shown to release dynorphin A 1-8 in response to mechanical stimuli in the inflamed state.60 However, transcriptionally, the mRNA for the dynorphin precursor does not show a measurable increase until ~day 1 depending on the severity of the inflammation, and does not reach a maximum until day 2 to 3. This reflects the fact that dynorphin peptide already exists within these neurons and is released rapidly in response to the inflammatory challenge. Subsequently, the mRNA increases to meet the demand over time. The Dyn A 1-8 peptide shows an even greater delay in elevation, which reflects the dynamic processes affecting the product being measured. When release slows, more dynorphin peptide is made than is secreted, leading to the late accumulation of peptide. Importantly, the measurements in the present report are indicative of accumulation, which begins in the resolution phase, whereas other endpoints such as transcription and peptide release have shown engagement early in the pain process.18,60 Dynorphin induction tracks the hyperalgesia in several pain models including peripheral inflammation, chronic constriction injury, painful peripheral neuropathy, and surgical incision.19,31,32,38,55,59,62 The time factor is elastic and the development of gene and peptide changes follow pathophysiological dimensions such that acute-onset injuries induce modulation of dynorphin more rapidly.31,59
Cellular-level characterization of PPD mRNA (Pdyn) expression in the dorsal spinal cord shows that it is expressed primarily by inhibitory interneurons.65 While the majority of the dynorphinergic neurons were inhibitory, Sardella, et al noted that in lamina I, only 50% of Pdyn+ neurons were GABA-immunoreactive, suggesting a mixed population of excitatory and inhibitory dynorphinergic neurons in lamina I. By contrast, 80% of dynorphinergic interneurons in lamina II were GABA immunoreactive.65 It has been shown that dynorphin induction in the superficial dorsal horn occurs in both projection and local circuit neurons.53 Notably, an excitatory population of dynorphinergic neurons has been identified in the medial aspect of lamina I/II, corresponding to the innervation from glabrous skin.5 It has previously been shown that a dense network of MERGL immunoreactive terminals and fibers are present in the substantia gelatinosa, with MERGL+ neurons found throughout the dorsal spinal cord.33 Release of enkephalin peptides has been shown in the spinal cord after painful mechanical and thermal stimulation.10,44 Intrathecal injection of MERGL exerts an analgesic action in the spinal cord, and MERGL is released in response to activation of spinal neurokinin 1 receptors that bind substance P, which is present at high levels in primary afferent nociceptors.36,45 Expression analysis and colocalization with Vglut2 revealed several populations of enkephalinergic neurons, including a large population of neurons that co-produce enkephalin peptides and glutamate, as well as inhibitory enkephalinergic interneurons.66 Using antibody-based immunofluorescence, no co-localization was observed between enkephalin and dynorphin precursors in the dorsal horn (Marvizon, et al, 2009). The present study largely confirms this result, although a small number of cells were observed that show colocalization of Penk and Pdyn mRNA. These cells were very sparse; we detected a total of ~7 co-positive cells in the 4 analyzed sections, out of 326 cells positive for one or the other. Additionally, co-positive cells were not among the brightest signals for one or both of the co-positive peptides, which may be why these have not been reported previously. Our measurements of peptide and mRNA, as well as cell counts in the dorsal spinal cord, strongly suggest that the induced dynorphinergic neurons express at least 2× more peptide than a typical MERGL-producing neuron (Supplementary Fig 1). However, given that enkephalinergic cells outnumber the dynorphinergic neurons and produce 6 other enkephalin peptides from proenkephalin (Fig 2B), it is likely that a dynorphin-dominant modulation of the persistent pain state is concentrated in the superficial laminae, in the region corresponding to the somatotopic peripheral innervation (medial lumbar, in the case of a hind paw injury). The result emphasizes the focal nature of hyperalgesia modulation.
Within the DRG, induction of Pdyn expression is apparently related to axonal injury (Fig 7).52 In the DRG, the Pdyn gene, like the Gal, Npy, and Vip27 genes, appears to be ectopically expressed during injury. While dynorphin peptides have been identified in DRG,4,21,25,52 several examinations have shown that the levels of the precursor mRNAs in DRG are negligible in basal conditions. Furthermore, Pdyn expression has been reported in immune cells, and we cannot exclude this possibility in the DRG, where the levels of expression are much lower than in the spinal cord, even at maximal induction after nerve injury. Assuming approximately 1 mg protein per 10 mg tissue, Botticelli, et al4 reported approximately 16 fmoles of dynorphin A 1-13 peptide per mg protein in the rat DRG, compared with 92 fmoles/mg protein in the ventral spinal cord, and 176 fmoles/mg protein in the dorsal spinal cord. The present study reports approximately 500 fmoles/mg protein of dynorphin A 1-8 in either contralateral or untreated dorsal spinal cord before inflammation, which is substantially greater than what has been previously reported in the DRG. Using RNA-Seq, we profiled the levels of mRNA encoding dynorphin peptide precursor, and found that in mouse, rat, dog, and human, the level of Pdyn mRNA was not prominent in the baseline even with relatively deep sequencing (~50–100 million paired-end reads). This is further evidence that in the basal state dynorphin is a not a prominent signaling peptide produced in DRG. While Herradon, et al25 were able to detect Pdyn mRNA in uninjured DRG using RT-PCR in 2 strains of rats, their estimated abundance in DRG was substantially less than what they estimated for the dorsal spinal cord, and the induction after chronic constriction nerve injury was approximately 4-fold. In order to investigate this further, we performed RNA-Seq in rat DRG after sciatic nerve transection. While very low levels of Pdyn were identified in naÿve control animals, there was an induction of Pdyn at 3 days, 10 days, and 30 days after axotomy (Fig 7A). Galanin (Gal) mRNA was examined as a positive control, and was significantly induced over a similar time course (Fig 7B). This result was also consistent in a meta-analysis of 4 different rat nerve injury studies (Fig 7D–F). Additionally, in a small (N = 3) evaluation of naÿve versus intraplantar carrageenan rat DRG samples, we did not note any alterations in these 3 peptide precursor transcripts in response to peripheral inflammation (Fig 7D–F). This supports the idea that the peptide changes observed in the spinal cord after peripheral inflammation are produced spinally, and not from the sensory afferents.
In our studies of peptide content, we examined 5 brain regions, each of which has been implicated in pain or pain control to some extent.2,29,37,41 These regions represent some of the most fundamental pain processing steps, with the PAG being a primary hub for descending pain control, while basal ganglia structures have been shown to encode intensity of noxious stimuli.14 In these experiments, we surveyed dynorphin A 1-8, MERGL, and an additional peptide, CCK. CCK is expressed at high levels in the brain regions examined and several recent studies point to CCK as a modulator of hyperalgesia, and of endogenous opioid signaling.74 Furthermore, CCK has recently shown to be analgesic in response to peripheral inflammation,61 a mechanism thought to involve CCK signaling in the amygdala, which modulates projections to the PAG. The PAG did not show a difference in CCK content. In these experiments, the only positive results were increases in MERGL content in the PAG and substantia nigra (Fig 5A–C), consistent with descending control and modulation of affective components of pain,20 respectively, in these brain areas.
Dynorphinergic peptide synthesis serves as a marker for hyperalgesia, but the kappa opioid system also has a functional role in this process. Like the mu and delta opioid systems, kappa opioid agonists have been manipulated to produce analgesic and antihyperalgesic actions, and kappa receptor signaling at various steps through the nociceptive process has demonstrated analgesic actions.26,58,68 This signaling may also act to control the transition from normal behavior to a state of guarding and protective hyper-responsiveness aimed at preventing further injury. However, the role of kappa opioid signaling at the level of the spinal cord remains a subject of active investigation given the conflicting evidence as to whether kappa signaling is analgesic at the level of the spinal cord.30,72 As a technical note, the actions of exogenous dynorphin A 1-17 can be influenced by nonopioid actions mediated by the polybasic region in this C-terminally extended peptide, which has been reported to produce an nmethly-d-aspartatereceptor mediated neurotoxicity.7 Certainly, there is a dynamic interplay between the mu, kappa, and delta opioid systems driven by focused spinal segmental and somatotopic alterations in enkephalin and dynorphin peptide signaling. This interplay may be a key factor in modulating G-protein opioid receptor signaling to influence processes of analgesia and hyperalgesia.9
Supplementary Material
Perspective:
This is a systematic, quantitative assessment of dynorphin and enkephalin peptides and mRNA in dorsal spinal cord and DRG neurons in response to peripheral inflammation and axotomy. These studies form the foundational framework for understanding how endogenous spinal opioid peptides are involved in nociceptive circuit modulation.
Acknowledgments
The authors would like to acknowledge Joshua Starrost for assistance with quantification of multiplex in situ sections.
Disclosures:
This work was supported by the intramural research program at the National Institutes of Health Clinical Center, the National Institute of Neurological Disorders and Stroke and the National Library of Medicine. Additional funding was provided by the NIH Office of Behavioral and Social Sciences Research and the National Institute of Complementary and Integrative Health.
Abbreviations:
- MERGL
[Met5]-enkephalin-Arg6-Gly7-Leu8
- MERF
[Met5]-enkephalin-Arg6-Phe7
- Pdyn
mRNA encoding the preprodynorphin precursor protein
- Penk
mRNA encoding the preproenkephalin precursor protein
- Pomc
mRNA encoding the prepro-opiomelanocortin precursor protein
- Pnoc
mRNA encoding the prepronociceptin precursor protein
- PPD
preprodynorphin
- PPE
preproenkephalin
- HPLC
High performance liquid chromatography
- TRPV1
Transient receptor potential family member V1
Footnotes
Conflict of Interest: The authors have no other conflicts of interest to report.
Supplementary Data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jpain.2020.01.001.
References
- 1.Baskozos G, Dawes JM, Austin JS, Antunes-Martins A, McDermott L, Clark AJ, Trendafilova T, Lees JG, McMahon SB, Mogil JS, Orengo C, Bennett DL: Comprehensive analysis of long noncoding RNA expression in dorsal root ganglion reveals cell-type specificity and dysregulation after nerve injury. Pain 160:463–485, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baumeister AA, Anticich TG, Hawkins MF, Liter JC, Thibodeaux HF, Guillory EC: Evidence that the substantia nigra is a component of the endogenous pain suppression system in the rat. Brain Res 447:116–121, 1988 [DOI] [PubMed] [Google Scholar]
- 3.Beyer A, Schafer M, Stein C: Antinociceptive effects of dynorphin peptides in a model of inflammatory pain. Pain 70:141–147, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Botticelli LJ, Cox BM, Goldstein A: Immunoreactive dynorphin in mammalian spinal cord and dorsal root ganglia. Proc Natl Acad Sci 78:7783–7786, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyle KA, Gutierrez-Mecinas M, Polgar E, Mooney N, O’Connor E, Furuta T, Watanabe M, Todd AJ: A quantitative study of neurochemically defined populations of inhibitory interneurons in the superficial dorsal horn of the mouse spinal cord. Neuroscience 363:120–133, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradford MM: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254, 1976 [DOI] [PubMed] [Google Scholar]
- 7.Caudle RM, Dubner R: Ifenprodil blocks the excitatory effects of the opioid peptide dynorphin 1-17 on NMDA receptor-mediated currents in the CA3 region of the guinea pig hippocampus. Neuropeptides 32:87–95, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Caudle RM, Isaac L: A novel interaction between dynorphin(1-13) and an N-methyl-D-aspartate site. Brain Res 443:329–332, 1988 [DOI] [PubMed] [Google Scholar]
- 9.Caudle RM, Mannes AJ, Benoliel R, Eliav E, Iadarola MJ: Intrathecally administered cholera toxin blocks allodynia and hyperalgesia in persistent pain models. J Pain 2:118–127, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Cesselin F, Le Bars D, Bourgoin S, Artaud F, Gozlan H, Clot AM, Besson JM, Hamon M: Spontaneous and evoked release of methionine-enkephalin-like material from the rat spinal cord in vivo. Brain Res 339:305–313, 1985 [DOI] [PubMed] [Google Scholar]
- 11.Cesselin F, Montastruc JL, Gros C, Bourgoin S, Hamon M: Met-enkephalin levels and opiate receptors in the spinal cord of chronic suffering rats. Brain Res 191:289–293, 1980 [DOI] [PubMed] [Google Scholar]
- 12.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL: Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53:55–63, 1994 [DOI] [PubMed] [Google Scholar]
- 13.Chavkin C, James IF, Goldstein A: Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science 215:413–415, 1982 [DOI] [PubMed] [Google Scholar]
- 14.Chudler EH, Dong WK: The role of the basal ganglia in nociception and pain. Pain 60:3–38, 1995 [DOI] [PubMed] [Google Scholar]
- 15.Civelli O, Douglass J, Goldstein A, Herbert E: Sequence and expression of the rat prodynorphin gene. Proc Natl Acad Sci 82:4291–4295, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cone RI, Weber E, Barchas JD, Goldstein A: Regional distribution of dynorphin and neo-endorphin peptides in rat brain, spinal cord, and pituitary. J Neurosci 3:2146–2152, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Sousa BN, Harrocks LA: Development of rat spinal cord. Dev Neurosci 2:115–121, 1979 [Google Scholar]
- 18.Draisci G, Iadarola MJ: Temporal analysis of increases in C-Fos, preprodynorphin and preproenkephalin mRNAs in rat spinal cord. Brain Res Mol Brain Res 6:31–37, 1989 [DOI] [PubMed] [Google Scholar]
- 19.Draisci G, Kajander KC, Dubner R, Bennett GJ, Iadarola MJ: Up-regulation of opioid gene expression in spinal cord evoked by experimental nerve injuries and inflammation. Brain Res 560:186–192, 1991 [DOI] [PubMed] [Google Scholar]
- 20.Fields HL: Neuroscience. More pain; less gain. Science 345:513–514, 2014 [DOI] [PubMed] [Google Scholar]
- 21.Gibbins IL, Furness JB, Costa M: Pathway-specific patterns of the co-existence of substance P, calcitonin gene-related peptide, cholecystokinin and dynorphin in neurons of the dorsal root ganglia of the guinea-pig. Cell Tissue Res 248:417–437, 1987 [DOI] [PubMed] [Google Scholar]
- 22.Goswami SC, Mishra SK, Maric D, Kaszas K, Gonnella GL, Clokie SJ, Kominsky HD, Gross JR, Keller JM, Mannes AJ, Hoon MA, Iadarola MJ: Molecular signatures of mouse TRPV1-lineage neurons revealed by RNA-Seq transcriptome analysis. J Pain 15:1338–1359, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Govoni S, Yang HY, Bosio A, Pasinetti G, Costa E: Possible interaction between cholecystokinin and dopamine. Adv Biochem Psychopharmacol 33:437–444, 1982 [PubMed] [Google Scholar]
- 24.Hammer P, Banck MS, Amberg R, Wang C, Petznick G, Luo S, Khrebtukova I, Schroth GP, Beyerlein P, Beutler AS: mRNA-seq with agnostic splice site discovery for nervous system transcriptomics tested in chronic pain. Genome Res 20:847–860, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herradon G, Ezquerra L, Nguyen T, Wang C, Siso A, Franklin B, Dilorenzo L, Rossenfeld J, Silos-Santiago I, Alguacil LF: Noradrenergic and opioidergic alterations in neuropathy in different rat strains. Neurosci Lett 438:186–189, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Ho J, Mannes AJ, Dubner R, Caudle RM: Putative kappa-2 opioid agonists are antihyperalgesic in a rat model of inflammation. J Pharmacol Exp Ther 281:136–141, 1997 [PubMed] [Google Scholar]
- 27.Hokfelt T, Wiesenfeld-Hallin Z, Villar M, Melander T: Increase of galanin-like immunoreactivity in rat dorsal root ganglion cells after peripheral axotomy. Neurosci Lett 83:217–220, 1987 [DOI] [PubMed] [Google Scholar]
- 28.Howells RD, Kilpatrick DL, Bhatt R, Monahan JJ, Poonian M, Udenfriend S: Molecular cloning and sequence determination of rat preproenkephalin cDNA: Sensitive probe for studying transcriptional changes in rat tissues. Proc Natl Acad Sci 81:7651–7655, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsieh JC, Stahle-Backdahl M, Hagermark O, Stone-Elander S, Rosenquist G, Ingvar M: Traumatic nociceptive pain activates the hypothalamus and the periaqueductal gray: A positron emission tomography study. Pain 64:303–314, 1996 [DOI] [PubMed] [Google Scholar]
- 30.Hylden JL, Thomas DA, Iadarola MJ, Nahin RL, Dubner R: Spinal opioid analgesic effects are enhanced in a model of unilateral inflammation/hyperalgesia: Possible involvement of noradrenergic mechanisms. Eur J Pharmacol 194:135–143, 1991 [DOI] [PubMed] [Google Scholar]
- 31.Iadarola MJ, Brady LS, Draisci G, Dubner R: Enhancement of dynorphin gene expression in spinal cord following experimental inflammation: Stimulus specificity, behavioral parameters and opioid receptor binding. Pain 35:313–326, 1988 [DOI] [PubMed] [Google Scholar]
- 32.Iadarola MJ, Douglass J, Civelli O, Naranjo JR: Differential activation of spinal cord dynorphin and enkephalin neurons during hyperalgesia: Evidence using cDNA hybridization. Brain Res 455:205–212, 1988 [DOI] [PubMed] [Google Scholar]
- 33.Iadarola MJ, Panula P, Majane EA, Yang YT: The opioid octapeptide Met5-enkephalin-Arg6-Gly7-Leu8: Characterization and distribution in rat spinal cord. Brain Res 330:127–134, 1985 [DOI] [PubMed] [Google Scholar]
- 34.Iadarola MJ, Sapio MR, Raithel SJ, Mannes AJ, Brown DC: Long-term pain relief in canine osteoarthritis by a single intra-articular injection of resiniferatoxin, a potent TRPV1 agonist. Pain 159:2105–2114, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iadarola MJ, Shin C, McNamara JO, Yang HY: Changes in dynorphin, enkephalin and cholecystokinin content of hippocampus and substantia nigra after amygdala kindling. Brain Res 365:185–191, 1986 [DOI] [PubMed] [Google Scholar]
- 36.Iadarola MJ, Tang J, Costa E, Yang HY: Analgesic activity and release of [MET5]enkephalin-Arg6-Gly7-Leu8 from rat spinal cord in vivo. Eur J Pharmacol 121:39–48, 1986 [DOI] [PubMed] [Google Scholar]
- 37.Jaaskelainen SK, Rinne JO, Forssell H, Tenovuo O, Kaasinen V, Sonninen P, Bergman J: Role of the dopaminergic system in chronic pain—A fluorodopa-PET study. Pain 90:257–260, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Kajander KC, Sahara Y, Iadarola MJ, Bennett GJ: Dynorphin increases in the dorsal spinal cord in rats with a painful peripheral neuropathy. Peptides 11:719–728, 1990 [DOI] [PubMed] [Google Scholar]
- 39.Kardon AP, Polgar E, Hachisuka J, Snyder LM, Cameron D, Savage S, Cai X, Karnup S, Fan CR, Hemenway GM, Bernard CS, Schwartz ES, Nagase H, Schwarzer C, Watanabe M, Furuta T, Kaneko T, Koerber HR, Todd AJ, Ross SE: Dynorphin acts as a neuromodulator to inhibit itch in the dorsal horn of the spinal cord. Neuron 82:573–586, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim SH, Chung JM: An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain 50:355–363, 1992 [DOI] [PubMed] [Google Scholar]
- 41.Koyama T, Kato K, Mikami A: During pain-avoidance neurons activated in the macaque anterior cingulate and caudate. Neurosci Lett 283:17–20, 2000 [DOI] [PubMed] [Google Scholar]
- 42.LaPaglia DM, Sapio MR, Burbelo PD, Thierry-Mieg J, Thierry-Mieg D, Raithel SJ, Ramsden CE, Iadarola MJ, Mannes AJ: RNA-Seq investigations of human post-mortem trigeminal ganglia. Cephalalgia 38:912–932, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laumet G, Garriga J, Chen SR, Zhang Y, Li DP, Smith TM, Dong Y, Jelinek J, Cesaroni M, Issa JP, Pan HL: G9a is essential for epigenetic silencing of K(+) channel genes in acute-to-chronic pain transition. Nat Neurosci 18:1746–1755, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Le Bars D, Bourgoin S, Clot AM, Hamon M, Cesselin F: Noxious mechanical stimuli increase the release of Metenkephalin-like material heterosegmentally in the rat spinal cord. Brain Res 402:188–192, 1987 [DOI] [PubMed] [Google Scholar]
- 45.Lindberg I, Yang HY, Costa E: Release of high molecular weight forms of met5-enkephalin-arg6-gly7-leu8 from rat brain. Neuropeptides 5:541–544, 1985 [DOI] [PubMed] [Google Scholar]
- 46.Majane EA, Iadarola MJ, Yang HY: Distribution of Met5-enkephalin-Arg6, Phe7 in rat spinal cord. Brain Res 264:336–339, 1983 [DOI] [PubMed] [Google Scholar]
- 47.Malan TP, Ossipov MH, Gardell LR, Ibrahim M, Bian D, Lai J, Porreca F: Extraterritorial neuropathic pain correlates with multisegmental elevation of spinal dynorphin in nerve-injured rats. Pain 86:185–194, 2000 [DOI] [PubMed] [Google Scholar]
- 48.Meller ST, Gebhart GF: Intraplantar zymosan as a reliable, quantifiable model of thermal and mechanical hyperalgesia in the rat. Eur J Pain 1:43–52, 1997 [DOI] [PubMed] [Google Scholar]
- 49.Messersmith DJ, Gu J, Dubner R, Douglass J, Iadarola MJ: Basal and inducible transcriptional activity of an upstream AP-1/CRE element (DYNCRE3) in the prodynorphin promoter. Mol Cell Neurosci 5:238–245, 1994 [DOI] [PubMed] [Google Scholar]
- 50.Messersmith DJ, Kim DJ, Gu J, Dubner R, Iadarola MJ: c-Jun activation of the DYNCRE3 site in the prodynorphin promoter. Brain Res Mol Brain Res 40:15–21, 1996 [DOI] [PubMed] [Google Scholar]
- 51.Messersmith DJ, Kim DJ, Iadarola MJ: Transcription factor regulation of prodynorphin gene expression following rat hindpaw inflammation. Brain Res Mol Brain Res 53:260–269, 1998 [DOI] [PubMed] [Google Scholar]
- 52.Mika J, Obara I, Przewlocka B: The role of nociceptin and dynorphin in chronic pain: Implications of neuro-glial interaction. Neuropeptides 45:247–261, 2011 [DOI] [PubMed] [Google Scholar]
- 53.Nahin RL, Hylden JL, Iadarola MJ, Dubner R: Peripheral inflammation is associated with increased dynorphin immunoreactivity in both projection and local circuit neurons in the superficial dorsal horn of the rat lumbar spinal cord. Neurosci Lett 96:247–252, 1989 [DOI] [PubMed] [Google Scholar]
- 54.Naranjo JR, Iadarola MJ, Costa E: Changes in the dynamic state of brain proenkephalin-derived peptides during amygdaloid kindling. J Neurosci Res 16:75–87, 1986 [DOI] [PubMed] [Google Scholar]
- 55.Noguchi K, Kowalski K, Traub R, Solodkin A, Iadarola MJ, Ruda MA: Dynorphin expression and Fos-like immunoreactivity following inflammation induced hyperalgesia are colocalized in spinal cord neurons. Brain Res Mol Brain Res 10:227–233, 1991 [DOI] [PubMed] [Google Scholar]
- 56.Perkins JR, Antunes-Martins A, Calvo M, Grist J, Rust W, Schmid R, Hildebrandt T, Kohl M, Orengo C, McMahon SB, Bennett DL: A comparison of RNA-seq and exon arrays for whole genome transcription profiling of the L5 spinal nerve transection model of neuropathic pain in the rat. Mol Pain 10:7, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pohl M, Ballet S, Collin E, Mauborgne A, Bourgoin S, Benoliel JJ, Hamon M, Cesselin F: Enkephalinergic and dynorphinergic neurons in the spinal cord and dorsal root ganglia of the polyarthritic rat—In vivo release and cDNA hybridization studies. Brain Res 749:18–28, 1997 [DOI] [PubMed] [Google Scholar]
- 58.Porreca F, Mosberg HI, Hurst R, Hruby VJ, Burks TF: Roles of mu, delta and kappa opioid receptors in spinal and supraspinal mediation of gastrointestinal transit effects and hot-plate analgesia in the mouse. J Pharmacol Exp Ther 230:341–348, 1984 [PubMed] [Google Scholar]
- 59.Raithel SJ, Sapio MR, LaPaglia DM, Iadarola MJ, Mannes AJ: Transcriptional changes in dorsal spinal cord persist after surgical incision despite preemptive analgesia with peripheral resiniferatoxin. Anesthesiology 128:620–635, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Riley RC, Zhao ZQ, Duggan AW: Spinal release of immunoreactive dynorphin A(1-8) with the development of peripheral inflammation in the rat. Brain Res 710:131–142, 1996 [DOI] [PubMed] [Google Scholar]
- 61.Roca-Lapirot O, Fossat P, Ma S, Egron K, Trigilio G, Lopez-Gonzalez MJ, Covita J, Bouali-Benazzouz R, Faver-eaux A, Gundlach AL, Landry M: Acquisition of analgesic properties by the cholecystokinin (CCK)/CCK2 receptor system within the amygdala in a persistent inflammatory pain condition. Pain 160:345–357, 2019 [DOI] [PubMed] [Google Scholar]
- 62.Ruda MA, Iadarola MJ, Cohen LV, Young WS 3rd: In situ hybridization histochemistry and immunocytochemistry reveal an increase in spinal dynorphin biosynthesis in a rat model of peripheral inflammation and hyperalgesia. Proc Natl Acad Sci 85:622–626, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sapio MR, Goswami SC, Gross JR, Mannes AJ, Iadarola MJ: Transcriptomic analyses of genes and tissues in inherited sensory neuropathies. Exp Neurol 283:375–395, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sapio MR, Neubert JK, LaPaglia DM, Maric D, Keller JM, Raithel SJ, Rohrs EL, Anderson EM, Butman JA, Caudle RM, Brown DC, Heiss JD, Mannes AJ, Iadarola MJ: Pain control through selective chemo-axotomy of centrally projecting TRPV1+ sensory neurons. J Clin Invest 128:1657–1670, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sardella TC, Polgar E, Garzillo F, Furuta T, Kaneko T, Watanabe M, Todd AJ: Dynorphin is expressed primarily by GABAergic neurons that contain galanin in the rat dorsal horn. Mol Pain 7:76, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schneider SP, Walker TM: Morphology and electrophysiological properties of hamster spinal dorsal horn neurons that express VGLUT2 and enkephalin. J Comp Neurol 501:790–809, 2007 [DOI] [PubMed] [Google Scholar]
- 67.Schwei MJ, Honore P, Rogers SD, Salak-Johnson JL, Finke MP, Ramnaraine ML, Clohisy DR, Mantyh PW: Neurochemical and cellular reorganization of the spinal cord in a murine model of bone cancer pain. J Neurosci 19:10886–10897, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Snyder LM, Chiang MC, Loeza-Alcocer E, Omori Y, Hachisuka J, Sheahan TD, Gale JR, Adelman PC, Sypek EI, Fulton SA, Friedman RL, Wright MC, Duque MG, Lee YS, Hu Z, Huang H, Cai X, Meerschaert KA, Nagarajan V, Hirai T, Scherrer G, Kaplan DH, Porreca F, Davis BM, Gold MS, Koerber HR, Ross SE: Kappa opioid receptor distribution and function in primary afferents. Neuron 99, 2018. 1274–1288 e1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Spampinato S, Bedini A, Baiula M: Opioid peptides: Prodynorphin-derived Peptides. Handbook of Biologically Active Peptides; 20061596–1601 [Google Scholar]
- 70.Todd AJ: Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci 11:823–836, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vanderah TW, Ossipov MH, Lai J, Malan TP Jr., Porreca F: Mechanisms of opioid-induced pain and antinociceptive tolerance: Descending facilitation and spinal dynorphin. Pain 92:5–9, 2001 [DOI] [PubMed] [Google Scholar]
- 72.Wang Z, Gardell LR, Ossipov MH, Vanderah TW, Brennan MB, Hochgeschwender U, Hruby VJ, Malan TP Jr., Lai J, Porreca F: Pronociceptive actions of dynorphin maintain chronic neuropathic pain. J Neurosci 21:1779–1786, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weber E, Evans CJ, Barchas JD: Predominance of the amino-terminal octapeptide fragment of dynorphin in rat brain regions. Nature 299:77–79, 1982 [DOI] [PubMed] [Google Scholar]
- 74.Wiesenfeld-Hallin Z, Xu XJ, Hokfelt T: The role of spinal cholecystokinin in chronic pain states. Pharmacol Toxicol 91:398–403, 2002 [DOI] [PubMed] [Google Scholar]
- 75.Yoshikawa K, Williams C, Sabol SL: Rat brain preproenkephalin mRNA. cDNA cloning, primary structure, and distribution in the central nervous system. J Biol Chem 259:14301–14308, 1984 [PubMed] [Google Scholar]
- 76.Zhang W, Yu Y, Hertwig F, Thierry-Mieg J, Zhang W, Thierry-Mieg D, Wang J, Furlanello C, Devanarayan V, Cheng J, Deng Y, Hero B, Hong H, Jia M, Li L, Lin SM, Nikolsky Y, Oberthuer A, Qing T, Su Z, Volland R, Wang C, Wang MD, Ai J, Albanese D, Asgharzadeh S, Avigad S, Bao W, Bessarabova M, Brilliant MH, Brors B, Chierici M, Chu TM, Zhang J, Grundy RG, He MM, Hebbring S, Kaufman HL, Lababidi S, Lancashire LJ, Li Y, Lu XX, Luo H, Ma X, Ning B, Noguera R, Peifer M, Phan JH, Roels F, Rosswog C, Shao S, Shen J, Theissen J, Tonini GP, Vande-sompele J, Wu PY, Xiao W, Xu J, Xu W, Xuan J, Yang Y, Ye Z, Dong Z, Zhang KK, Yin Y, Zhao C, Zheng Y, Wolfinger RD, Shi T, Malkas LH, Berthold F, Wang J, Tong W, Shi L, Peng Z, Fischer M: Comparison of RNA-seq and microarray-based models for clinical endpoint prediction. Genome Biol 16:133, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


