Abstract
Management of ANCA-associated vasculitis (AAV) during the COVID-19 pandemic poses unique therapeutic challenges. An online survey was conducted to understand physician’s choices for treating AAV during the COVID-19 pandemic. Web-based survey featuring nineteen questions was circulated amongst physicians across various specialties. The responses regarding immunosuppressive therapy for remission induction and maintenance, COVID-19 testing, and preventive measures were recorded. A total of 304 responses were recorded. Most of the respondents were from India (83.9%) and comprised rheumatologists (66%) in practice for ≥ 5 years (71%). Though a majority preferred Rituximab or intravenous cyclophosphamide (CYC) as a remission induction agent, a significant proportion opted for oral CYC and mycophenolate mofetil (MMF) also. Only one-third wanted to test for COVID-19 before initiating immunosuppressive therapy in patients with organ/life-threatening manifestations. Rituximab was the most favored maintenance therapy (47%), followed by azathioprine, MMF, and methotrexate. The results of this focused survey of managing AAV patients depict the real-world dilemmas and physicians’ choices in this setting.
Keywords: ANCA-associated vasculitis, COVID-19, Agents, Immunosuppressive, Online survey
Introduction
The current era of the coronavirus disease 19 (COVID-19) pandemic poses a unique therapeutic challenge for managing patients with rheumatological disorders [1, 2]. The evidence for managing these patients in such a scenario is scarce. Immunosuppressive therapy (IST) initiation, escalation, and switch to other classes have been an area of active research amongst rheumatologists. Most of the data of COVID-19 in rheumatic patients has been collected from various registries and surveys but is largely restricted to more common rheumatic diseases, such as rheumatoid arthritis, psoriatic arthritis, systemic lupus erythematosus, and Sjogren syndrome. The data from the OpenSAFELY and TriNetX electronic record databases and from registries of the United Kingdom and Danish rheumatic disease population showed that patients with rheumatic diseases might have a worse outcome when compared with the general population [3–7].
Natural history of anti-neutrophil cytoplasmic antibody (ANCA) associated vasculitis (AAV) can vary from mild organ limited disease to severe life-threatening multisystem involvement with a course interrupted with relapses [8]. The effect of COVID-19 infection on AAV has been sparsely studied. The modifications of IST in such scenarios have to be carefully weighed considering the risk of disease flare and developing COVID-19. There is a lack of guidelines from any of the international rheumatology bodies/societies about the management of vasculitis during the current pandemic. Previous surveys had grouped all the rheumatological disorders and tried to understand the choices. In this survey, we focus on the only AAV to understand the physician’s choices regarding AAV management during the ongoing COVID-19 pandemic.
Methods
A case scenario-based survey featuring nineteen questions was presented to various medical practitioners from India and other regions of the world. Questions pertaining to demographic details, the initial dosage of steroids and taper strategy, choice of immunosuppressive agent for remission induction and maintenance periods, management of relapses, and COVID testing were communicated electronically. These questions were prepared by two practicing rheumatologists at tertiary center and revised several times after feedback intramurally, then by rheumatologists at other centers to establish face and content validity. For communicating the survey, electronic mail and other social media platforms such as Twitter were used. Participation was voluntary, and no incentives were provided to participants. Responses were recorded anonymously. Google forms were used for tabulating questions, recording, and analyses of responses. Ethics committee clearance was taken from the institute for conducting the survey (No: INT/IEC/2020/000431, 15/05/2020). Graphs and tables were downloaded from google forms, and descriptive statistics are being presented. Due to the limitations of google forms, IP addresses could not be established for participants. The uniqueness of each entry was established by checking similarity for responses to demographic variables between the different responses. The present survey conformed to the Checklist for reporting results of internet e-surveys (CHERRIES) reporting guidelines for online surveys [9].
Results
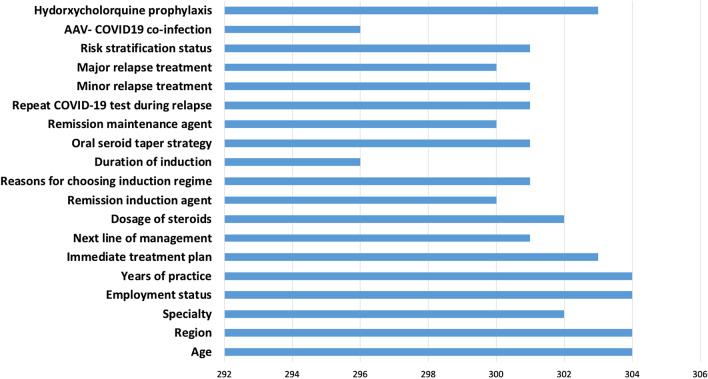
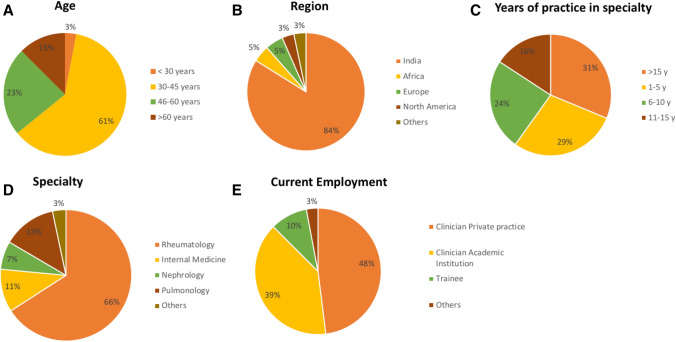
A total of 304 respondents across the globe completed the survey. Out of 304 respondents, 300 had answered completely to all the questions featured (Fig. 1). The majority of them were from India (83.9%), the rest were from Europe (4.9%), Africa (4.6%), and other regions of the world (6.6%), as shown in Fig. 2A. Most were from Rheumatology specialty (66%, Fig. 2B), and in practice for > 5 years (71%, Fig. 2C).
Fig. 1.
Showing featured questions and the number of respondents to each
Fig. 2.
Showing demographic data including age (A), region of practice (B), years of practice in their specialty (C), area of specialty (D), and current employment status (E)
Choice of immunosuppression therapy
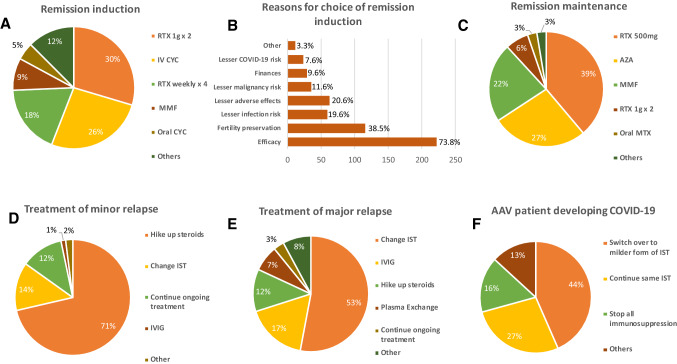
Irrespective of COVID-19 status, 64% of respondents opted to treat the patient presenting with life-threatening disease, while 34% wanted to test for COVID-19 before treatment. In the case scenario presented in the survey, the patient presented with subacute onset of shortness of breath and rapidly progressive renal failure of 6 week duration. On evaluation, she was found to have severe disease with diffuse alveolar hemorrhage and pauci-immune glomerulonephritis with anti-PR3 positivity. The majority (88%) opted to use combined glucocorticoids (GCs) and IST for remission induction, while 9% opted for GCs alone. Among those who opted for GCs, 83% opted to give intravenous methylprednisolone followed by oral prednisolone (1 mg/kg/day), while 10% opted to start high dose oral prednisolone (1 mg/kg/day). Rituximab was the most favored remission induction agent [48%; (30%: 1gm two doses biweekly and 18%: 375 mg/m2/week for 4 weeks)] followed by cyclophosphamide (CYC,31%;26%: intravenous, 5%: oral) and mycophenolate mofetil (MMF,9%) (Fig. 3A). The reasons for their preference are shown below (Fig. 3B). For GC taper, 46% favored prednisolone ≤ 10 mg/day at 3 months, 39% favored prednisolone ≤ 10 mg/day at 6 months, while 14% wanted to stop prednisolone at 6 months.
Fig. 3.
Showing physician treatment choices for remission induction (A), remission maintenance (C), minor (D) and major relapse (E), for development of COVID19 (F) and reasons for choosing remission induction (B for patient with ANCA associated vasculitis (AAV) during COVID-19 pandemic. AZA = Azathioprine; CYC = Cyclophosphamide; IST = Immunosuppressive therapy; IVIG = Intravenous Immunoglobulin; MMF = Mycophenolate Mofetil; MTX = Methotrexate; RTX = Rituximab
Rituximab was also the most favored remission maintenance therapy (47%) followed by azathioprine, MMF, and methotrexate (Fig. 3C). For minor relapses, 71% chose to hike GCs alone, 14% opted to change the IST, and 12% chose to continue the ongoing treatment in view of the COVID-19 risk (Fig. 3D). For major relapses, 53% opted to change IST, while 17% chose intravenous immunoglobulin, 12% chose to increase GCs alone, and 7% chose plasmapheresis, while 3% still chose to continue ongoing therapy because of COVID-19 risk (Fig. 3E).
49% wanted to test for COVID 19 at the time of relapse, 24% did not want it, and 28% were unsure. AAV patients on immunosuppression were considered as high risk for COVID-19 by 27% and at medium risk by 14%. For AAV developing COVID19, 45% wanted to switch over to milder IST, 28% wanted to continue the same IST, and 17% wanted to stop IST altogether (Fig. 3F). In addition, 55% were against hydroxychloroquine (HCQ) prophylaxis, whereas 28% favored this approach.
Discussion
In this web-based survey, we attempted to understand physician’s knowledge and practices about the management of AAV patients in the context of the COVID-19 pandemic. Although majority of the responders opted for a combination of GCs and IST, there were differences in the IST choice for remission induction and maintenance. First, the use of steroids for induction and maintenance should be based on risk versus benefit assessment. The majority of physician participants chose to use steroids (97%), and among that, IV pulse steroids were favored by 83% of them. However, data from the COVID-19 Global Rheumatology registry and French cohort shows that individuals receiving GC’s ≥ 10 mg/day had higher rates of hospitalization and worse outcomes [6, 10]. This contrasts with EULAR recommendations published initially, wherein no excess risk was stated [11]. The reason was that the guidelines were published initially at the start of the COVID-19 pandemic, and accumulating evidence suggests otherwise and should be incorporated. In the current survey, about half of the physician participants opted for rapid GC taper to ≤ 10 mg/day by 3 months, while the rest considered usual taper. In light of recent data, physicians may consider rapid tapering strategies and use the lowest possible steroids dose.
Even though most of them opted to give rituximab or IV CYC, a significant proportion of physicians opted for oral CYC and MMF as a remission induction agents. Rituximab has emerged as an induction agent for AAV in parallel to cyclophosphamide in recent times [12–14]. However, some evidence suggests a greater risk of developing COVID-19 in patients treated with rituximab, and this might also have favoured other agents for remission induction. Data from the French cohort shows that vasculitis patients have a more severe disease (OR = 2.25, 95% CI 1.13–4.41) as compared to other rheumatic diseases, and for treatment, those who received rituximab (OR = 4.21, 95% CI 1.61–10.98) or MMF (OR = 6.6, 95% CI 1.47–29.62) had also severe disease [10][10]. Though rituximab has been used for treating hyperinflammation or hemophagocytic lymphohistiocytosis triggered by Epstein Barr virus (EBV), its role in COVID-19-related hyperinflammation suggests worse outcomes. The reason speculated that the EBV resides in B-cell, whereas SARS-CoV-2 do not, and antibodies may be protective against SARS-CoV-2 infection [16]. Therefore, extreme caution should be taken prior to initiation of rituximab or MMF in vasculitis patients. However, this also has to be seen in the context of the underlying disease, and ANCA-associated vasculitis constituted only 2.5% of the total French cohort. Recent studies have suggested impaired immune responses to COVID-19 vaccine when rituximab, MMF and methotrexate are used, which needs to be considered and may require modification[17]. All rheumatic diseases have a different prognosis, varied responses to immunosuppression, especially severe untreated AAV has very high mortality [18–20], hence withholding IST treatment in the context of COVID-19 needs to balanced weighing the risk versus benefit ratio.
Interestingly, about a third of physicians wanted to test the patient for COVID-19 before initiating therapy in organ/life-threatening manifestations. The survey had been conducted in the early part of the COVID-19 pandemic, when testing facilities to confirm COVID-19 were not as widely available as today. However, in light of newer data, we strongly suggest performing COVID-19 testing before induction therapy, use of rituximab, MMF, high dose steroids, change in IST, as we cannot exclude the risk of viral reactivation in asymptomatic carriers. American College of Rheumatology guidelines (version 3) suggests that all immunosuppressants except for IL-6 inhibitors should be withheld in the patients acquiring COVID-19 infection [21]. However, it may be deleterious for patients who have severe or difficult to treat disease to stop IST abruptly. IVIG as a bridge may have to be considered in such situations.
A significant number of physicians opted not to increase the dose of immunosuppression during disease flare due to the risk of COVID-19. There was no consensus on the continuation of immunosuppression in case a patient with AAV developed COVID-19. Despite the recommendation of compassionate use of HCQ prophylaxis by the FDA at the time of the survey, only 28% of physicians were in its favor. This was similar to the survey reported by Gupta et al. [22], where only 22.6% preferred HCQ prophylaxis. Our survey results depict the real-world dilemmas in treating AAV patients if they develop COVID-19 (Table 1). More data from various registries may provide answers to these questions. The strength of the survey is that we tried to answer problems specifically considering AAV rather than clubbing all the rheumatological disorders under common umbrella and homogenizing the results. More such specific surveys would make things more transparent and help put up things in perspective. Recommendations for the management of AAV from major Rheumatology societies [12, 23] are either wholly or predominantly based on data from the pre-COVID era. The findings of the present survey increase awareness about issues that require specific consideration for the treatment of AAV during the times of the COVID-19 pandemic. Many a times, patients with AAV are managed by different specialist including nephrologists for predominant renal involvement, pulmonologists for predominant lung involvement and emergency physicians when they present with acute life or organ threatening disease, apart from the rheumatologists. Hence, we included specialists from these fields as well in the survey as we wanted to gather information on the preferences and practices of any physician who manages AAV patients to simulate a real-life scenario. The timing of the survey predated the advent of vaccines for COVID-19. The timing of rituximab in AAV in the context of COVID-19 vaccines requires a balancing of risk–benefit ratio considering that humoral immune responses to COVID-19 vaccines could be dampened if such vaccines are administered in close proximity to the timing of rituximab [24]. The emergence of AAV following COVID-19 or AAV mimicking COVID-19 is also being increasingly recognized [25, 26]. This clinical situation poses a further challenge for the management of patients with AAV during the COVID-19 pandemic.
Table 1.
Dilemmas in treating patients with ANCA vasculitis during the COVID-19 pandemic
| 1. Choice of induction and maintenance agent (since rituximab associates with higher risk of COVID) |
| 2. Use of corticosteroids for induction in a patient with concomitant ANCA vasculitis flare and COVID-19 |
| 3. Testing for COVID-19 routinely in patients with vasculitic flares in the absence of clinical symptoms for COVID-19 |
The current study had certain limitations. The majority of the participants were from India; hence the results may not truly reflect choices of physicians around the globe. It is difficult to draw definitive treatment recommendations for AAV in the context of the COVID-19 pandemic from the study. However, it sensitizes the physicians about the umbrella of choices that could be there and facilitates their decision. A significant number of the respondents were from non-rheumatological specialties (n = 103, 34%); therefore, the practice of managing AAV might be heterogenous and given the rarity of AAV incidence, it would difficult to interpret the responses of those who have managed very few cases in their practice. The anonymity of the study responses would keep the bias low; however, the post-hoc analysis cannot be conducted due to this very reason of anonymity, such as specialtywise and other inter-group responses and comparison. The use of unique IP addresses in removing duplicates would be a better way of removing duplicates than checking similarity for responses to demographic variables. The survey was circulated via email and social media platforms (Twitter); therefore, the denominator of how many respondents received the survey and how many actually responded from these could not be computed. Responses from rheumatologists and other physicians could not be compared due to the online nature of the survey. Information derived from cohort studies and registries of AAV would complement the information from the present survey to better understand the prognosis of patients with AAV with COVID-19, any differences in such prognosis and response to therapy with respect to autoantibody status (anti-proteinase 3 or anti-myeloperoxidase antibody) and the differences between treatment regimens with respect to risk of developing COVID-19.
Conclusion
This AAV specific survey results depict the real-world dilemmas regarding choice of Immunosuppressive therapy in treating patients. Case-based specific and focused surveys like this can facilitate rheumatologists to consider these pragmatic clinical scenarios in the setting of COVID-19.
Acknowledgements
Nil
Author contributions
The conception and design of the study: AS, GN, DPM, VA; acquisition of data, analysis and interpretation of data: CRK, GN, PD, RSJ, KM, AM. Drafting the article: CRK, GN, DPM; Revising it critically for important intellectual content: KM, AM, VA, AS. Final approval of the version to be submitted: CRK, GN, DPM, PD, RSJ KM, AM, VA, AS. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: CRK, GN, DPM, PD, RSJ, KM, AM, VA, AS.
Funding
Nil.
Declarations
Conflicts of interest
Chirag Rajkumar Kopp has no conflicts of interest to declare, including no relationship with pharmaceutical companies. GSRSNK Naidu has no conflicts of interest to declare, including no relationship with pharmaceutical companies. Durga Prasanna Misra has no conflicts of interest to declare, including no relationship with pharmaceutical companies. Prateek Deo has no conflicts of interest to declare, including no relationship with pharmaceutical companies. Roopesh Sai Jakulla has no conflicts of interest to declare, including no relationship with pharmaceutical companies. Kavita Makan has no conflicts of interest to declare, including no relationship with pharmaceutical companies. Ajesh Maharaj has no conflicts of interest to declare, including no relationship with pharmaceutical companies. Vikas Agarwal has no conflicts of interest to declare, including no relationship with pharmaceutical companies. Aman Sharma has no conflicts of interest to declare, including no relationship with pharmaceutical companies.
Ethics Approval
Ethics committee clearance was taken from the institute for conducting the survey (No: INT/IEC/2020/000431, 15/05/2020).
Informed consent
Consent to participate was taken at the time of filling the online survey.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Avouac J, Drumez E, Hachulla E, Seror R, Georgin-Lavialle S, El Mahou S, et al. COVID-19 outcomes in patients with inflammatory rheumatic and musculoskeletal diseases treated with rituximab: a cohort study. Lancet Rheumatol. 2021;3:e419–e426. doi: 10.1016/S2665-9913(21)00059-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chattopadhyay A, Mishra D, Sharma V, Naidu K, G, Sharma A, Coronavirus disease-19 and rheumatological disorders: A narrative review. Indian J Rheumatol. 2020;15:122–129. doi: 10.4103/injr.injr_73_20. [DOI] [Google Scholar]
- 3.Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cordtz R, Lindhardsen J, Soussi BG, Vela J, Uhrenholt L, Westermann R, et al. Incidence and severeness of COVID-19 hospitalization in patients with inflammatory rheumatic disease: a nationwide cohort study from Denmark. Rheumatology (Oxford) 2020 doi: 10.1093/rheumatology/keaa897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D’Silva KM, Jorge A, Cohen A, McCormick N, Zhang Y, Wallace ZS, Choi HK. COVID-19 outcomes in patients with systemic autoimmune rheumatic diseases compared to the general population: a US multicenter, comparative cohort study. Arthritis Rheumatol. 2021;73:914–920. doi: 10.1002/art.41619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gianfrancesco M, Hyrich KL, Al-Adely S, Carmona L, Danila MI, Gossec L, et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 global rheumatology alliance physician-reported registry. Ann Rheum Dis. 2020;79:859–866. doi: 10.1136/annrheumdis-2020-217871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peach E, Rutter M, Lanyon P, Grainge MJ, Hubbard R, Aston J, et al. Risk of death among people with rare autoimmune diseases compared with the general population in England during the 2020 COVID-19 pandemic. Rheumatology (Oxford) 2021;60:1902–1909. doi: 10.1093/rheumatology/keaa855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma A, Naidu GSRSNK, Rathi M, Verma R, Modi M, Pinto B, et al. Clinical features and long-term outcomes of 105 granulomatosis with polyangiitis patients: a single center experience from north India. Int J Rheum Dis. 2018;21:278–284. doi: 10.1111/1756-185X.13071. [DOI] [PubMed] [Google Scholar]
- 9.Eysenbach G. Improving the quality of Web surveys: the Checklist for reporting results of internet E-Surveys (CHERRIES) J Med Internet Res. 2004;6:e34. doi: 10.2196/jmir.6.3.e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.FAI2R, SFR, SNFMI, SOFREMIP, CRI, IMIDIATE Consortium and Contributors Severity of COVID-19 and survival in patients with rheumatic and inflammatory diseases: data from the French RMD COVID-19 cohort of 694 patients. Ann Rheum Dis. 2021;80:527–538. doi: 10.1136/annrheumdis-2020-218310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Landewé RBM, Machado PM, Kroon F, Bijlsma HWJ, Burmester GR, Carmona L, et al. EULAR provisional recommendations for the management of rheumatic and musculoskeletal diseases in the context of SARS-CoV-2. Ann Rheum Dis. 2020;79:851–858. doi: 10.1136/annrheumdis-2020-217877. [DOI] [PubMed] [Google Scholar]
- 12.Yates M, Watts RA, Bajema IM, Cid MC, Crestani B, Hauser T, Hellmich B, Holle JU, Laudien M, Little MA, Luqmani RA, Mahr A, Merkel PA, Mills J, Mooney J, Segelmark M, Tesar V, Westman K, Vaglio A, Yalçındağ N, Jayne DR, Mukhtyar C. EULAR/ERA-EDTA recommendations for the management of ANCA-associated vasculitis. 2016;75:1583–1594. doi: 10.1136/annrheumdis-2016-209133. [DOI] [PubMed] [Google Scholar]
- 13.Misra DP, Naidu GSRSNK, Sharma A. Recent advances in the management of antineutrophil cytoplasmic antibody-associated vasculitis. Indian J Rheumatol. 2019;14:218–228. doi: 10.4103/injr.injr_141_19. [DOI] [Google Scholar]
- 14.Mittal S, Naidu GSRSNK, Jha S, Rathi M, Nada R, Minz RW, et al. Experience with similar biologic rituximab in 77 patients of granulomatosis with polyangiitis-a real-life experience. Clin Rheumatol. 2021;40:645–651. doi: 10.1007/s10067-020-05261-7. [DOI] [PubMed] [Google Scholar]
- 15.Loarce-Martos J, García-Fernández A, López-Gutiérrez F, García-García V, Calvo-Sanz L, del Bosque-Granero I, et al. High rates of severe disease and death due to SARS-CoV-2 infection in rheumatic disease patients treated with rituximab: a descriptive study. Rheumatol Int. 2020;40:2015–2021. doi: 10.1007/s00296-020-04699-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehta P, Porter JC, Chambers RC, Isenberg DA, Reddy V. B-cell depletion with rituximab in the COVID-19 pandemic: where do we stand? Lancet Rheumatol. 2020;2:e589–e590. doi: 10.1016/S2665-9913(20)30270-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Furer V, Eviatar T, Zisman D, Peleg H, Paran D, Levartovsky D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021 doi: 10.1136/annrheumdis-2021-220647. [DOI] [PubMed] [Google Scholar]
- 18.Yates M, Watts R. ANCA-associated vasculitis. Clin Med. 2017;17:60–64. doi: 10.7861/clinmedicine.17-1-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoffman GS, Kerr GS, Leavitt RY, Hallahan CW, Lebovics RS, Travis WD, et al. Wegener Granulomatosis: An Analysis of 158 Patients. Ann Intern Med. 1992;116:488–498. doi: 10.7326/0003-4819-116-6-488. [DOI] [PubMed] [Google Scholar]
- 20.Zimba O, Doskaliuk B, Yatsyshyn R, Bahrii M, Hrytsevych M. Challenges in diagnosis of limited granulomatosis with polyangiitis. Rheumatol Int. 2021;41:1337–1345. doi: 10.1007/s00296-021-04858-8. [DOI] [PubMed] [Google Scholar]
- 21.Mikuls TR, Johnson SR, Fraenkel L, Arasaratnam RJ, Baden LR, Bermas BL, et al. American College of Rheumatology Guidance for the management of rheumatic disease in adult patients during the COVID-19 pandemic: version 3. Arthritis Rheumatol. 2021;73:e1–e12. doi: 10.1002/art.41596. [DOI] [PubMed] [Google Scholar]
- 22.Gupta L, Misra DP, Agarwal V, Balan S, Agarwal V. Management of rheumatic diseases in the time of covid-19 pandemic: perspectives of rheumatology practitioners from India. Ann Rheum Dis. 2021;80:e1. doi: 10.1136/annrheumdis-2020-217509. [DOI] [PubMed] [Google Scholar]
- 23.Chung SA, Langford CA, Maz M, Abril A, Gorelik M, Guyatt G, et al. 2021 American College of Rheumatology/vasculitis foundation guideline for the management of antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheumatol. 2021;73(8):1366–1383. doi: 10.1002/art.41773. [DOI] [PubMed] [Google Scholar]
- 24.Bruchfeld A, Kronbichler A, Alberici F, Fervenza FC, Jayne DRW, Segelmark M, et al. COVID-19 and ANCA-associated vasculitis: recommendations for vaccine preparedness and the use of rituximab. Nephrol Dial Transplant. 2021 doi: 10.1093/ndt/gfab174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Izci Duran T, Turkmen E, Dilek M, Sayarlioglu H, Arik N. ANCA-associated vasculitis after COVID-19. Rheumatol Int. 2021;41:1523–1529. doi: 10.1007/s00296-021-04914-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Özdemir B, Erden A, Güven SC, Armagan B, Apaydin H, Karakas Ö, et al. COVID-19 and eosinophilic granulomatosis with polyangiitis or COVID-19 mimicking eosinophilic granulomatosis with polyangiitis? Rheumatol Int. 2021;41:1515–1521. doi: 10.1007/s00296-021-04896-2. [DOI] [PMC free article] [PubMed] [Google Scholar]