Abstract
SARS-CoV-2 is a global threat that influenced healthcare systems around the world. This virus caused an infection in humans with different clinical signs and syndromes, severity, and mortality.
The key components of the COVID-19 molecular pathogenesis are coronavirus entry and replication, antigen presentation, humoral and cellular immunity, cytokine storm, coronavirus immune evasion.
The analysis of recent literature displayed possible molecular targets in the key components of the COVID-19 pathogenesis. Some of these targets might have gene polymorphisms that influenced the COVID-19 course. Unfortunately, several findings are still putative or extrapolated from SARS and MERS experimental investigations or clinical trials.
We systematised original data about gene polymorphisms of possible molecular targets and associations with the COVID-19 course. Most data were obtained for angiotensin-converting enzymes 1 and 2, TMPRSS2 gene polymorphisms. Only a few results were found for gene polymorphisms of adhesion molecules, interferon system components, cytokines, and transcriptional factors, oxidative stress and metabolic molecules, as well as haemocoagulation.
Understanding the host gene variability and its associations with COVID-19 can provide insights into the disease pathogenesis, individual susceptibility to SARS-CoV-2 infection, severity, complications, and mortality prognosis for the disease. Besides, these data might help in the identification of appropriate targets for intervention.
Keywords: Gene, Polymorphism, Infection, SARS-CoV-2, COVID-19, Pathogenesis
Gene, Polymorphism, Infection, SARS-CoV-2, COVID-19, Pathogenesis
1. Introduction
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is a global threat that influenced health systems around the world [1, 2, 3]. This virus caused an infectious disease in humans with different clinical signs and syndromes, severity, and mortality [4, 5, 6]. These clinical features might depend on underlying chronic diseases and risk factors [7, 8, 9]. Moreover, the host genetic variability determines individual susceptibility to SARS-CoV-2 and the course of coronavirus disease 2019 (COVID-19) [10, 11, 12]. Several attempts were made to predict gene targets in polymorphism-associated studies on COVID-19 [13, 14, 15].
Understanding the host gene variability and its associations with COVID-19 can provide insights into the disease pathogenesis, individual susceptibility to SARS-CoV-2 infection, severity, complications, and mortality prognosis for the disease.
Thus, in this review, we evaluated the key components of the COVID-19 molecular pathogenesis to reveal possible molecular targets and their gene polymorphisms associated with the COVID-19 clinical characteristics.
2. Pathogenesis of COVID-19
Understanding the molecular immune pathogenesis of COVID-19 plays a pivotal role in exploring genetic-based individual susceptibility.
The key parts of molecular immune pathogenesis are determined as [16]:
-
−
Coronavirus entry and replication
-
−
Antigen presentation in coronavirus infection
-
−
Humoral and cellular immunity
-
−
Cytokine storm in COVID-19
-
−
Coronavirus immune evasion
Recently, SARS-CoV-2 entry and replication have been investigated quite sufficiently. Other parts of the COVID-19 pathogenesis are still putative or extrapolated from SARS and MERS experimental investigations or clinical trials. The pathological changes in the respiratory tract or endothelial dysfunction may be multifactorial and depend on direct viral infection, cytokine dysregulation, and coagulopathy [17].
Pulmonary pathological changes are the main features of severe COVID-19. These pathological findings embrace ground-glass opacities, lung oedema, and acute respiratory distress syndrome [18].
SARS-CoV-2 threatens target cells using SARS spike proteins (S-proteins) which bind to angiotensin (AT)-converting enzyme 2 (ACE2). As shown for SARS-CoV, these viruses affected pulmonary ACE-2 positive cells such as pneumocytes, airway epitheliocytes, as well as extrapulmonary ACE-2 positive cells [19, 20]. SARS-CoV-2 also used transmembrane protease, serine 2 (TMPRSS2) for viral proliferation in host cells. Additionally, this process involved cathepsins B and L [21].
SARS-CoV-2 also used a cell surface cluster of differentiation 147 (CD147) (basigin or extracellular matrix metalloproteinase inducer (EMMPRIN)) for cell entry [22].
The virus evasion depended on several factors such as:
-
−
avoiding the host innate antiviral defence due to the prevention of simultaneous activation of double-strand RNA (dsRNA) sensors melanoma differentiation-associated protein 5, 2′-5′-oligoadenylate synthetase 3, and protein kinase R (PKR) by coronavirus endonuclease [23];
-
−
a weak or absent interferon Type 1 ([IFN-1] i.e., IFNα and IFNβ) response [24];
-
−
suppression of the host gene expression (including IFN-1), degradation of host messenger RNA (mRNA) by viral nonstructural protein 1 with the subsequent inhibition of host protein translation [25];
-
−
inhibition of the cellular retinoic acid-inducible gene 1, Toll-like receptor 3 (TLR3)/TLR7 pathways by the viral papain-like protease with the subsequent decrease of proinflammatory cytokines and interferons [26, 27].
These events escalate the activation of macrophages and monocytes, as well as cellular and humoral immunity, leading to lung damage and impairment of the virus clearance [28, 29].
In COVID-19, immune dysregulation, followed by microvascular thrombi containing neutrophils, platelets, and neutrophil extracellular traps, led to severe polyorganic dysfunction [30].
Nowadays, there is abundant evidence that COVID-19 strongly depends on immunopathological reactions leading to immune dysregulation and asynchronicity.
SARS-CoV-2 replication and dsRNA formation resulted in the activation of PKR with the subsequent phosphorylation of IκB and activation of NF-κB in several cell types, including airway epithelial cells [31]. These intracellular pathways regulate the expression of p38 mitogen-activated protein kinase (MAPK) and the induction of dsRNA-mediated TNF-α production [32]. In turn, overproduction of IL-6, as well as activation of PKR-endoplasmic reticular kinase and transcription factor, stimulates PKR [33, 34, 35, 36].
In SARS, the binding of SARS-CoV to ACE2 activates serine protease, ADAM-17, which along with the TNF-α cleavage enzyme contributes to the increase of TNF-α [37] and the aggravation of cardiac injury [38].
PKR can induce apoptosis by activation of the FAS-associated death domain (FADD)/caspase-8/caspase-3 and caspase-9 apoptotic protease-activating factor-1 APAF-1 [39, 40].
PKR also induced phosphorylation of elongation initiation factor 2α, which blocks protein synthesis through mRNA translation. This factor also encodes for antiviral factors [41]. Unfortunately, SARS-CoV is not susceptible to PKR antiviral activities [39, 40]. In the course of the disease, the virus replication diminished and PKR expression decreased. In parallel, neutrophils released some serine proteases involved in IL-1 processing [42, 43, 44].
Ageing, diabetes, obesity, congestive heart failure, cancer, and genetic factors increased levels of PKR and were associated with severe courses of COVID-19 [45]. PKR increased the activity of inducible nitric oxide synthase (iNOS) leading to hypoxic pulmonary vasodilatation. Moreover, iNOS increased the activity of highly expressed cyclooxygenase-2 (COX-2) with the intensification of pulmonary and systemic inflammation in COVID-19 pneumonia [46, 47].
During passage through membranes of endoplasmic reticulum, SARS-CoV produced double-membrane vesicles (DMV) containing non-structural transmembrane proteins 3 and 4, and dsRNA. In DMV, the virus can replicate and avoid immune control. In part, this avoiding might be explained by microtubule-associated protein light chain 3. In COVID-19, this protein exists in the nonlipidated form of this protein and evades destruction [48, 49].
In addition to PKR independent IL-1β production, there was evidence of the selective activation of a caspase-1-dependent pathway in the paediatric population who suffered from SARS. The data suggested the involvement of the NLRP3 (cryopyrin) pathway in these processes [50].
Protein kinase R endoplasmic reticulum kinase (PERK) plays an important role in the immunopathogenesis of COVID-19. PERK reacts to the presence of dsRNA in the cytoplasm. Besides, SARS-CoV 3a protein activates PERK, and PERK also serves as a sensor for unfolded proteins. In such a situation, PERK indirectly stimulates the expression of molecular chaperones [51, 52].
In outline, the PKR/PERK pathway has high importance for SARS-CoV-2 invasion, proliferation, intracellular biology, and pathogenesis of COVID-19 as an immune-mediated disease.
CD147 can take part in the pathogenesis of COVID-19 as a key receptor for viral cellular entry. CD147 expression may be increased by IL-6 [53].
CD147 expression, in conjunction with cyclophilins, contributes to the recruitment of immune cells to inflammation sites due to chemokine-like activity. Cyclophilins may be a target in coronavirus disease. SARS-CoV increases signalling through the calcineurin/nuclear factor of activated T cells (NFAT) pathway with the subsequent IL-2 production [54].
CD147 and IL-6 overexpression can be responsible for the activation of inflammatory macrophages, leukocyte chemotaxis and adhesion, platelet activation, and thrombus formation. The prothrombotic phenotype in severe SARS depended on the intravascular expression of vitronectin, plasminogen activator inhibitor-1, and von Willebrand factor induced by IL-6 [55, 56, 57].
The pathogenesis of lung disease induced by SARS-CoV-2 also includes oxidative stress of the pulmonary microenvironment. The host-viral metabolism and host antiviral response increased the production of mitochondrial reactive oxygen species and induced hypoxic pulmonary vasoconstriction [58].
After the activation of neutrophils, monocytes and dendritic cells produced phospholipase A2 Group IID (PLA2G2D). This enzyme intensified the innate and adaptive immune responses by the mobilization of anti-inflammatory lipid mediators. For example, prostaglandin D2 inhibited dendritic cell migration and T-cell-driven antivirus response. SARS incurred this effect through oxidative stress [18, 59].
A high level of ATII induced important pathological responses in patients with COVID-19 [60]. ATII bound to the angiotensin I receptor and increased activity of various subtypes of NADPH oxidase. In the lung tissue, NADPH oxidase produced reactive oxygen species, enhanced phosphorylation, and activated PKR [61, 62].
Thus, precise immune mechanisms of neutrophil/macrophage/monocyte activation and cytokine hyperproduction are still unknown. In COVID-19, the dichotomy of synchronised cellular and molecular cascades, as well as the innate and adaptive immunity, is observed. Overall, theinnate immune hyperactivity system and ineffective adaptive immunity led to dramatic tissue damage without viral clearance and immunological resolution [63].
Immunothrombosis during defence reactions against viruses might be one of the main reasons for COVID-19 mortality [64]. In this process, complement system activation induced blood coagulation, as well as microvascular injury, especially in the pulmonary tissue [65, 66, 67]. Cytokine-driving coagulation is responsible for this immunothrombosis as supported by a positive correlation between the levels of D-dimer, fibrinogen, and IL-6 [68].
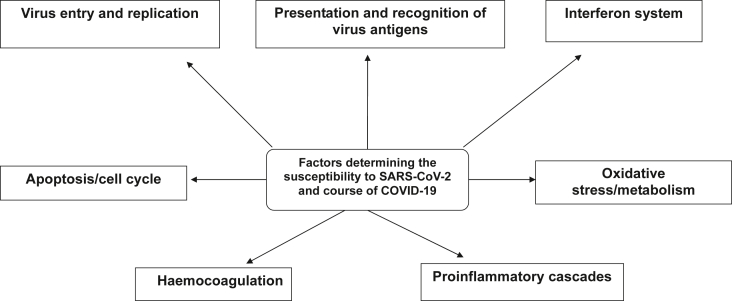
Possible targets for investigation of gene variability as factors determining the susceptibility to SARS-CoV-2 and course of COVID-19 are summarised in Figure 1.
Figure 1.
Possible targets for the investigation of gene variability as factors determining the susceptibility to SARS-CoV-2 and the course of COVID-19.
Figure 1 provides generalization and graphic expression of the scientific data presented above, highlighting the main links of the COVID-19 pathogenesis and the factors that determine susceptibility to SARS-CoV-2 and affect the COVID-19 course.
As the next part of this review, we analysed available publications from Springer Nature, ScienceDirect/Elsevier Connect, and PubMed that reported the results of gene variability studies in the context of possible targets.
3. Host gene variability and COVID-19
3.1. Host gene variability, virus entry, replication, and antigen presentation
Recent data display that single nucleotide polymorphisms (SNPs) in ACE2 may result in modulation of renin-angiotensin system pathway and associated cardiovascular and pulmonary conditions by altering the angiotensinogen-ACE2 interactions, such as polymorphism Arg514Gly in the African/African-American population. The ACE2 genomic variants may associate with susceptibilities to COVID-19 and cardiovascular complications by altering the AGT-ACE2 pathway (i.e., polymorphism Arg514Gly) [11].
Another mutation Leu584Ala in this gene promotes the entry of SARS-CoV-1 into host cells [69].
Polymorphism His378Arg decreases ACE2 activity, and polymorphism Ser19Pro could distort the most important helix to the S-protein. Other seven missense variants may affect secondary structures (i.e. polymorphisms Gly211Arg; Asp206Gly; Arg219Cys; Arg219His, Lys341Arg, Ile468Val, and Ser547Cys), whereas polymorphism Ile468Val with allele frequency 0.01 is only present in Asia [70].
Human ACE2 variants K26R, S16P, T27A, K31R, H34R, E35K, E37K, D38V, N51S, N64K, K68E, F72V, T921, Q102P, G326E, G352V, D355N, H378R, Q388L, and D509Y are predicted to increase the susceptibility of the individuals carrying these variations to SARS-CoV-2. It has been suggested that the T921I ACE2 variant will favour the improved viral S-protein binding, N90 and T92 ACE2 mutations are critical ACE2 residues that confer protection and are SARS-CoV host modifiers. Variants K31R, E35K, E37K, D38V, N33I, H34R, Q388L, and Y83H in ACE2 were found and are predicted to show a decreased binding to SARS-CoV-2 S-protein, thus protecting individuals corresponding to these genotypes [71].
Based on the genetic analysis, M. Bosso et al. found that the ACE2 polymorphisms associated with hypertension or with the efficacy of ACE1 or angiotensin-receptor blocker could have the potential to alter the binding of SARS-CoV-2 S-protein with ACE2 receptor [72].
The most investigated polymorphism of ACE1 is a genetic deletion/insertion (D/I) polymorphism in intron 16. The D allele is associated with a reduced expression of ACE2. D/I polymorphism has specific patterns of distribution in the European and Asian countries and this variability may be responsible for COVID-19 prevalence in different geographical regions. . About 38% of the prevalence variability can be explained by the relative frequency of the ACE1 D-allele [73]. Patients with the II genotype have lower mortality in comparison with ID and DD genotypes. In contrast, patients with the DD genotype had severe lung infections and high mortality [69].
The ACE I/D polymorphism is associated with many risk factors of COVID-19 severity such as obesity, diabetes, arterial hypertension, heart failure and cancer. The D allele might be associated with risk factor contributions as well as COVID-19 severity and progression [73]. This polymorphism could be important for the complications in COVID-19 patients such as severe lung injury due to the influence on the serum ACE concentration in the whole population. The patients without DD genotype had a lower risk of severe lung injury during COVID-19. Conversely, the presence of the ACE DD allele may be favourable for ACE inhibitors and AT1 receptor blockers therapy [74].
Supporting data were obtained by Gómez J. et al. they found that severe COVID-19 was associated with male gender, hypertension, hypercholesterolemia, and the ACE1 DD genotype [75].
The frequency of the ACE1 II genotype in the European population displays a significant negative correlation with the amount of SARS-CoV-2 cases. Similarly, the ACE1 II genotype is negatively correlated with the number of deaths from SARS-CoV-2 infection [76].
The prevalent polymorphisms in the TMPRSS2 gene, such as Val160Met (rs12329760), are responsible for the risk factor contribution and genetic susceptibility to COVID-19. These risk factors included the high-risk group of male patients and cancer [11]. Val160Met polymorphism influenced any post-translational modifications (e.g., proteolytic cleavage, acetylation, glycosylation, phosphorylation, and sulfation) and decreased the stability of the protein, which prevent the viral entry [77]. The expression quantitative trait loci (eQTLs) variant rs35074065 is linked to the overexpression of TMPRSS2 but with a low expression of the interferon (IFN)-α/β-inducible gene and under-expression of MX1 splicing isoform. This may give rise to enhanced susceptibility to viral infection or a decrease in cellular antiviral response [78, 79].
The involvement of the TMPRSS2 gene variants in the penetration of the virus into cells was described by Torre-Fuentes L. et al. The synonymous variants rs61735792 and rs61735794 showed a significant connection with infection [80]. The presence of nonsynonymous variants TMPRSS11 Arg290Gln (rs353163) and Lys48Arg (rs139010197) may influence the virus penetration into the cell and reduce the level of infection [77].
SARS-CoV-2 has a specific cleavage site between S1 and S2 domains of S protein. This site is critical for the cutting of S protein by the host protease – furin. Furin has some polymorphic variants and the Gly146Ser variant can modulate furin proteolytic activity especially its proprotein convertase activity and its ability to cleave S protein of SARS-CoV-2 [81].
The course and genetic susceptibility in COVID-19 patients may depend on polymorphisms of important regulatory genes – TMPRSS2, CD26 (dipeptidyl peptidase IV), and MX1. Genetic variants of TMPRSS2 (rs112657409, rs11910678, rs77675406, and rs713400) and CD26 (rs13015258) regulated the expression of these important genes during COVID-19. Epigenetic modification at C allele (rs13015258) induces CD26 overexpression which could explain a higher SARS-CoV-2 infected fatality rate in type 2 diabetes [82].
SARS-CoV-2 S protein binds to CD147/BSG receptor. This receptor had increased expression during inflammation and cancer. Inhibition of CD147/BSG can prevent diabetic complications, possibly involving severe lung injury triggers by COVID-19. CD147/BSG has one missense mutation F275V on the I-set domain. Presumably, this mutation might influence the severity of COVID-19 [83,84].
Cathepsin L and cathepsin B (CTSL/B) are determinants of the lysosomal pathway. Genetic changes in the locus of cathepsin L (CTSL1) associated with polymorphism (C-171A) affect the course of hypertension, the C allele associated with higher blood pressure [85]. Vargas et al. suggested a possible connection with SARS-CoV-2 infection of cathepsin gene polymorphisms since the minor allele of one of these polymorphisms (rs41307457) showed a high frequency only in the African population, and similarly, the minor allele rs41312184 was present with a high frequency only in the European population [77]. At the same time, there is insufficient information about the effect of cathepsin gene polymorphisms on the susceptibility to viral infections, their development, and course.
The killer cell lectin-like receptor C2, encoded by the KLRC2 gene, is one of the possible targets in the severity of COVID-19. This receptor had importance for the activation of natural killer cells. A study in a cohort of patients with mild to severe COVID-19 found that genetic variants KLRC2del and HLA-E∗0101 were independent risk factors for severe COVID-19 and may help to identify patients at high risk for severe COVID-19 [86].
The most important process in the recognition of SARS-CoV-2 is an antigen presentation of viral antigen peptides to T and B cells by major histocompatibility complex class I and II molecules (human leukocyte antigens (HLA) in humans).
At present, we have a lack of information about HLA allele polymorphism and arrangements of T and B cell antigen-recognizing receptors (TCRs and BCRs) in the pathogenesis of COVID-19.
In the Asian population, numerous studies have shown the importance of HLA gene polymorphisms for the SARS-CoV-1 induced infection process. The susceptibility and severity of SARS were significantly associated with several HLA class I polymorphisms – HLA-B∗46:01, HLA-B∗07:03, HLA-CW∗08:01 [87, 88, 89] as well as several HLA class II polymorphisms – HLA-DRB4∗01, HLA-DRB1∗12:02 [90, 91]. On the other hand, no evidence of an association between HLA polymorphisms and SARS was found in several studies [92, 93].
The presentation of SARS-CoV-2 antigen peptides to specific CD8+ T cells might be decreased in patients with HLA-A∗02:01 phenotypes in comparison with HLA-A∗11:01 and HLA-A∗24:02 [94, 95]. In COVID-19 patients, HLA class II polymorphisms can crucially influence the antigen presentation. HLA-DRB1∗08 was associated with low binding of viral antigen peptides and high mortality [96]. Important results were obtained for the identification of HLA-II peptides derived from SARS-CoV-2 spike glycoprotein. Researchers found 526 unique peptides from antigen presenting cells from 9 donors. HLA-II peptides had consensus HLA-II clusters recognized by CD4+ T cells [97].
Other players of SARS-CoV-2 antigen presentation are TCRs and BCRs. Currently there are limited data about the development of T and B cells immunity to SARS-CoV-2. In addition, little is known about the precise regulation of SARS-CoV-2 specific T and B cells differentiation and their persistence.
The key process for the virus-specific differentiation of T and B cells is the clonal V(D)J rearrangements of peripheral TCRs and BCRs. The bioinformatic approach allows comparing TCRs and BCRs from COVID-19 patients and healthy volunteers and to deduce virus-specific TCRs and BCRs.
Recent data showed the high diversity of TCRs in patients with mild COVID-19 and in patients with effective recovery from the disease. Thus, the achievement of huge TCR repertoires may be associated with appropriate immune response and successful outcome in COVID-19.
Likewise T cells, effective B cell response depends on the production of B cell clones with high BCR-antibody activity. Such high BCR-antibody activity might be achieved due to the maturation of somatic hypermutated BCRs during a germinal center reaction. It was shown, that SARS-CoV-2-antibody positive patients had a characteristic pattern of IGHV3 and IGHJ4/6. Unmutated BCRs profile was more characteristics for patients with severe course of COVID-19 [98].
HLA-I allele polymorphism has high importance in the recognition of antigen epitopes in SARS-CoV-2 S glycoprotein for TCRs. For example, HLA-A∗24:02 restricts recognizing by three epitopes S1208-1216, S448-456, and S193-201. Single-cell sequencing of TCR2β has shown that the A24/S448+ CD8+ T cell TCRαβ repertoire depended on a common TCRβ chain motif, while the A24/S1208+ CD8+ TCRαβ repertoire was diverse across COVID-19 patients [99].
In a similar study, the TCRs were compared between healthy volunteers and patients with COVID-19. Single-cell V(D)J analysis revealed 6 VJ pairs (such as TRAV12-2-J27-TRBV7-9-J2-3) significantly increased in patients with COVID-19 [100].
Also, it was shown the monitoring of TCR diversity might be used for the prediction of COVID-19 progression and recovery [101].
The use of TCR repertoire analysis in clinical practice for the prediction of the COVID-19 course is restricted by a large amount of sequencing and data analysis. An open-source software package, tcrdist3, may be used for distance-based analysis and it resolved this problem during SARS-CoV-2 infection [102].
3.2. Interferon system
Interferons are part of the body's antiviral defences and their gene variability influences the susceptibility and severity of COVID-19. It was shown IFN-γ in combination with IFN-β inhibits the replication of SARS-CoV. IFN-γ polymorphic allele +874A was strongly associated with SARS-CoV infection [103].
The single nucleotide polymorphism rs12252-C/C in the IFITM3 gene (which encodes interferon-induced transmembrane protein 3) was detected in patients with COVID-19 and is a risk factor for severe influenza [104]. Y. Zhang et al. also report the relationship between the presence of homozygous polymorphism C of the rs12252 allele in IFITM3 and the development of a more severe course of COVID-19 [105].
The IFITM3 protein showed a potent antiviral capacity to a wide range of viruses, including influenza A viruses, Ebola virus, Marburg virus, SARS-CoV, dengue virus, West Nile virus, Zika virus, and foot-and-mouth disease virus. A strong correlation between the case fatality rate of COVID-19 and the minor allele frequency of the rs6598045 SNP of the IFITM3 gene was identified [106].
Interferon lambda 3 (IFNL3) rs1297860 C/T and INFL4 rs368234815 TT/ΔG gene polymorphisms could affect the ability of the host to modulate viral infection without a clear impact on the outcome of COVID-19 [107].
The large-scale epidemiological data indicate the role of polymorphism A946T (rs1990760) of interferon-induced helicase 1 (IFIH1) in SARS-CoV-2 infection and T allele–carrying individuals may be more resistant to SARS-CoV-2 infection [108].
3.3. Proinflammatory cascades
Recently there are limited data about the polymorphisms of several cytokine genes (IFN γ +874A allele, IL12RB1, etc.) and their association with SARS. Other important players in antiviral defence are Toll-like receptor (TLR), which recognise viral components and initiate proinflammatory cascades.
Gene polymorphism of Ticam2, TLR adaptor protein, and its knock out in mice increased susceptibility to SARS-CoV infection [109]. Ticam2 participates in the formation of neutrophil extracellular traps (NETs) [110].
TLRs of the cell surface, especially TLR4, are involved in the recognition of SARS-CoV-2 molecular patterns, causing inflammatory reactions [111].
TLR3 is a receptor for double-strand RNA (dsRNA), and it is associated with the pathogenesis of severe viral infections such as HSV and influenza virus. On the other hand, TLR3 protects against HIV. Replication of many viruses results in the production of dsRNA which induces IFN response [112]. Genetic polymorphisms in other TLRs such as TLR7 and TLR9 led to decreased production of type 1 IFN [113].
TLR3 protein has two polymorphic loci in the extracellular region - N284I and L412F, which modify the receptor response and decrease NF-κB activity. Moreover, human TLR3 has specific residues (His39, His60, His108, His539, and Asn541) which interact with dsRNA, and mutations in these residues might be critical for dsRNA binding and TLR3 signalling [114].
Distribution and prognostic value of Arg753Gln TLR2, Leu412Рhe TLR3, Asp299Gly TLR4 genes polymorphisms in case of grippe have been studied. It has been established that there is an increased risk of grippe development for persons with Asp/Gly TLR4 genotype and TLR2, TLR3, TLR4 mutant genotypes combinations; there is an increased risk of grippe-associated pneumonia for patients with mutant homozygous genotype Phe/Phe TLR3 [115].
In the context of COVID-19 severity interleukin 6 (IL-6) is the most discussed proinflammatory cytokine. IL-6 has clinically important SNP at rs180079 associated with lung diseases, such as chronic obstructive pulmonary disease (COPD), pneumonia. Thus, this IL-6 polymorphism might be relevant to COVID-19 susceptibility and severity [116].
3.4. Oxidative stress/metabolism
Glutathione S-transferase genes T1 (GSTT1, MIM: 600436) and M1 (GSTM1, MIM: 138350) show deletion polymorphism, allele zero. Homozygosity for the null alleles results in a lack of appropriate enzyme activity, which increases the risk of pulmonary fibrosis, one of the most serious complications of COVID-19 disease. Thus, the null genotype GSTT1 can be considered as a predictor of morbidity and mortality from COVID-19 in different geographic regions [117].
The difference in susceptibility to and mortality from COVID-19 might be explained by the presence of vitamin D deficiency due to a different vitamin D metabolism, orchestrated by the DBP polymorphisms of rs7041 and rs4588 [118]. Karcioglu Batur L. described the genetic predisposition to viral infection in vitamin D deficiency and also found a significant correlation between the rs7041 polymorphism, the prevalence of COVID-19, and mortality rates. However, no significant correlation was found between the prevalence (per million) and mortality (per million) at the rs4588 locus [119].
The heme oxygenase-1 (HO-1) is a potential target in COVID-19. Singh D. et al., based on the studies by other authors, suggested a pivotal role of HO-1 induction in inflammation-induced coagulation, observed in COVID-19 patients. The HO-1 genetic polymorphisms, specifically the GT dinucleotide repeat in the promoter region, regulate the inducibility (i.e., transcription) of HO-1 to reactive oxygen species (ROS). Individuals with larger (GT)n repeats have been found to be more susceptible to diseases that involve the endothelium of the cardiovascular system, especially in diabetes and obesity. Given the relationship between the presence of GT repeats in the HO-1 promoter region and the severity of disease in various conditions such as acute lung injury, thromboembolism, and diabetes, it is necessary to determine the length of GT repeats in patients with severe COVID-19 [120, 121].
3.5. Apoptosis/cell cycle
We observed the lack of data about gene polymorphisms concerned with apoptosis/cell cycle machinery.
3.6. Haemocoagulation
Janssen R. proposed a hypothesis that the vitamin K epoxide reductase complex 1 (VKORC1) −1639 A allele had a protective property against inflammation-induced coagulation and decreased the mortality in COVID-19 patients. VKORC1 gene polymorphisms influencing vitamin K metabolism may partially explain the imbalance in morbidity and mortality from COVID-19 in different geographical regions [122].
Karst M et al. have proposed that hyperhomocysteinemia can initiate severe lung injury in COVID-19 patients. This homocysteinemia can be triggered by the presence of the C677T polymorphism of the methylenetetrahydrofolic acid reductase (MTHFR) gene [123].
A separate clinical case showed a positive result for ApoE e3/e4 genes, MTHFR A1298C heterozygous genotype, ACE D/I heterozygous genotype, angiotensinogen M235 heterozygous genotype, and factor XIII (zymogen) Val34Leu heterozygous genotype. A thorough clinical trial has been reported, aiming to validate the hypothesis on the involvement of thrombophilic genetic profiles in the COVID-19 [124].
Rare variants in the gene of plasminogen (PLG), such as Arg261His and Ala494Val, may be recognised as potential markers of inter-individual differences in susceptibility to coronavirus [81].
Recently, the new Dendritic Cell-Specific Intercellular adhesion molecule-3-Grabbing Non-integrin (DC-SIGN) was described. DC-SIGN bound to different viruses and to S glycoprotein of SARS-CoV. Thus, DC-SIGN can mediate ACE2 independent binding of SARS-CoV-2 to the surface of human cells. Similar properties were characteristic for macrophage galactose lectin (MGL). DC-SIGN gene polymorphism associated with increased concentration of lactate dehydrogenase (LDH) displaying the high level of systemic inflammation in COVID-19. Other members of the lectin family are also associated with the severity and mortality of COVID-19, for example, mannose-binding lectin. Thus, polymorphic alleles of C-lectin genes might determine the severity of COVID-19 [125].
We summarised the integrating data of possible gene variability as factors of the susceptibility to SARS-CoV-2 and course of COVID-19 with relevant bibliography in Table 1.
Table 1.
Integrating data on possible gene variability as factors determining the susceptibility to SARS-CoV-2 and the course of COVID-19.
| Possible targets | Relevant bibliography |
|---|---|
|
Virus entry and replication | |
| ACE2 | [11, 19, 20, 69, 70, 71, 72] |
| TMPRSS2 | [11, 77, 78, 79, 80, 82] |
| Furin | [81] |
| Angiotensin I receptor | [60, 61, 62] |
| Cathepsins B and L | [21, 77, 85] |
| Protein kinase R | [23, 39, 40] |
| Protein kinase R endoplasmic reticulum kinase (PERK) | [51, 52] |
| [23] | |
| dsRNA sensor melanoma differentiation-associated protein 5, 2′-5′-oligoadenylate synthase 3 | [23] |
| [41] | |
| [28] | |
| Elongation initiation factor 2α | [22, 83, 84] |
| CD11 | [54] |
| CD147 | |
| Cyclophilins |
|
|
Presentation and recognition of virus antigens | |
| HLA class I and II molecules | [87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 99] |
| TCRs | [99, 100, 101, 102] |
| BCRs |
[98] |
|
Haemocoagulation | |
| Vitronectin | [55] |
| Plasmonogen | [81] |
| Plasminogen activator inhibitor 1 | [56] |
| Von Willebrand factor | [57] |
| Factors of complement | [65, 66, 67] |
| Methylenetetrahydrofolate reductase | [123, 124] |
| Factor XIII | [124] |
| Vitamin K epoxide reductase complex I |
[122] |
|
Apoptosis/cell cycle | |
| FAS-associated death domain (FADD)/caspase-8/caspase-3, caspase-9 apoptotic protease-activating factor-1 (APAF-1) |
[39, 40] |
|
Oxidative stress/metabolism | |
| Phospholipase A2G2D | [18, 59] |
| D-type prostanoid receptor | [58] |
| Various types of NADPH oxidase | [61, 62] |
| iNOS, COX-2 | [46, 47] |
| Hemoxigenase-1 (HO-1) | [120, 121] |
| Glutation S-transferase |
[117] |
|
Interferon system | |
| Interferon regulatory factor 1 | [78, 79] |
| IFNα | [24] |
| IFNβ, IFNg |
[103] |
|
Proinflammatory cascades | |
| IκB | [31] |
| NFκB | [31, 114] |
| p38 MAPK | [32] |
| Activating transcription factor 4 | [36] |
| TLR3 – signaling pathway | [112, 114, 115] |
| TLR7 – signaling pathway | [113] |
| TLR4 | [111] |
| Retinoic acid-inducible gene 1 signaling pathway | [26, 27] |
| NLRP3 (cryopyrin) pathway | [50] |
| IL-6 | [116] |
| IL-1α/1β | [42, 43] |
| IL-2 | [54] |
| TNF-α | [37] |
4. Conclusion
Thus, the conducted analysis showed that the majority of reports were published in the prospective trials and are not the result of genetic association studies of patients with COVID-19. It is necessary to search and analyse the genetic determinants of susceptibility, severity, and outcomes of the disease using genetic data from patients with this pathology. These data might help in the identification of appropriate targets for intervention in COVID-19 patients.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This work was supported by Ministry of Public Health of Ukraine (0121U107440).
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The study was a part of research No. 0121U107440 “Genetic variants and their potential link with COVID-19 in the Ukrainian population” funded by the Ministry of Public Health of Ukraine.
References
- 1.Ohannessian R., Duong T.A., Odone A. Global telemedicine implementation and integration within health systems to fight the COVID-19 pandemic: a call to action. JMIR Public Health Surveill. 2020 Apr 2;6(2) doi: 10.2196/18810. PMID: 32238336; PMCID: PMC7124951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iserson K.V. SARS-CoV-2 (COVID-19) vaccine development and production: an ethical way forward. Camb. Q. Healthc. Ethics. 2021 Jan;30(1):59–68. doi: 10.1017/S096318012000047X. Epub 2020 Jun 5. PMID: 32498742; PMCID: PMC7327159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chatterjee P., Nagi N., Agarwal A., Das B., Banerjee S., Sarkar S., Gupta N., Gangakhedkar R.R. The 2019 novel coronavirus disease (COVID-19) pandemic: a review of the current evidence. Indian J. Med. Res. 2020 Feb & Mar;151(2 & 3):147–159. doi: 10.4103/ijmr.IJMR_519_20. PMID: 32362642; PMCID: PMC7357405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang X., Wei F., Hu L., Wen L., Chen K. Epidemiology and clinical characteristics of COVID-19. Arch. Iran. Med. 2020 Apr 1;23(4):268–271. doi: 10.34172/aim.2020.09. PMID: 32271601. [DOI] [PubMed] [Google Scholar]
- 5.Meo S.A., Alhowikan A.M., Al-Khlaiwi T., Meo I.M., Halepoto D.M., Iqbal M., Usmani A.M., Hajjar W., Ahmed N. Novel coronavirus 2019-nCoV: prevalence, biological and clinical characteristics comparison with SARS-CoV and MERS-CoV. Eur. Rev. Med. Pharmacol. Sci. 2020 Feb;24(4):2012–2019. doi: 10.26355/eurrev_202002_20379. PMID: 32141570. [DOI] [PubMed] [Google Scholar]
- 6.Weiss P., Murdoch D.R. Clinical course and mortality risk of severe COVID-19. Lancet. 2020 Mar 28;395(10229):1014–1015. doi: 10.1016/S0140-6736(20)30633-4. Epub 2020 Mar 17. PMID: 32197108; PMCID: PMC7138151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grasselli G., Greco M., Zanella A., Albano G., Antonelli M., Bellani G., Bonanomi E., Cabrini L., Carlesso E., Castelli G., Cattaneo S., Cereda D., Colombo S., Coluccello A., Crescini G., Forastieri Molinari A., Foti G., Fumagalli R., Iotti G.A., Langer T., Latronico N., Lorini F.L., Mojoli F., Natalini G., Pessina C.M., Ranieri V.M., Rech R., Scudeller L., Rosano A., Storti E., Thompson B.T., Tirani M., Villani P.G., Pesenti A., Cecconi M., COVID-19 Lombardy ICU Network Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med. 2020 Oct 1;180(10):1345–1355. doi: 10.1001/jamainternmed.2020.3539. PMID: 32667669; PMCID: PMC7364371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jordan R.E., Adab P., Cheng K.K. COVID-19: risk factors for severe disease and death. BMJ. 2020 Mar 26;368:m1198. doi: 10.1136/bmj.m1198. PMID: 32217618. [DOI] [PubMed] [Google Scholar]
- 9.Liu X., Zhou H., Zhou Y., Wu X., Zhao Y., Lu Y., Tan W., Yuan M., Ding X., Zou J., Li R., Liu H., Ewing R.M., Hu Y., Nie H., Wang Y. Risk factors associated with disease severity and length of hospital stay in COVID-19 patients. J. Infect. 2020 Jul;81(1):e95–e97. doi: 10.1016/j.jinf.2020.04.008. Epub 2020 Apr 17. PMID: 32305490; PMCID: PMC7162771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gemmati D., Bramanti B., Serino M.L., Secchiero P., Zauli G., Tisato V. COVID-19 and individual genetic susceptibility/receptivity: role of ACE1/ACE2 genes, immunity, inflammation and coagulation. Might the double X-chromosome in females Be protective against SARS-CoV-2 compared to the single X-chromosome in males? Int. J. Mol. Sci. 2020 May 14;21(10):3474. doi: 10.3390/ijms21103474. PMID: 32423094; PMCID: PMC7278991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hou Y., Zhao J., Martin W., Kallianpur A., Chung M.K., Jehi L., Sharifi N., Erzurum S., Eng C., Cheng F. New insights into genetic susceptibility of COVID-19: an ACE2 and TMPRSS2 polymorphism analysis. BMC Med. 2020 Jul 15;18(1):216. doi: 10.1186/s12916-020-01673-z. PMID: 32664879; PMCID: PMC7360473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giudicessi J.R., Roden D.M., Wilde A.A.M., Ackerman M.J. Genetic susceptibility for COVID-19-associated sudden cardiac death in African Americans. Heart Rhythm. 2020 Sep;17(9):1487–1492. doi: 10.1016/j.hrthm.2020.04.045. Epub 2020 May 5. PMID: 32380288; PMCID: PMC7198426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Severe COVID-19 GWAS Group. Ellinghaus D., Degenhardt F., Bujanda L., Buti M., Albillos A., Invernizzi P., Fernández J., Prati D., Baselli G., Asselta R., Grimsrud M.M., Milani C., Aziz F., Kässens J., May S., Wendorff M., Wienbrandt L., Uellendahl-Werth F., Zheng T., Yi X., de Pablo R., Chercoles A.G., Palom A., Garcia-Fernandez A.E., Rodriguez-Frias F., Zanella A., Bandera A., Protti A., Aghemo A., Lleo A., Biondi A., Caballero-Garralda A., Gori A., Tanck A., Carreras Nolla A., Latiano A., Fracanzani A.L., Peschuck A., Julià A., Pesenti A., Voza A., Jiménez D., Mateos B., Nafria Jimenez B., Quereda C., Paccapelo C., Gassner C., Angelini C., Cea C., Solier A., Pestaña D., Muñiz-Diaz E., Sandoval E., Paraboschi E.M., Navas E., García Sánchez F., Ceriotti F., Martinelli-Boneschi F., Peyvandi F., Blasi F., Téllez L., Blanco-Grau A., Hemmrich-Stanisak G., Grasselli G., Costantino G., Cardamone G., Foti G., Aneli S., Kurihara H., ElAbd H., My I., Galván-Femenia I., Martín J., Erdmann J., Ferrusquía-Acosta J., Garcia-Etxebarria K., Izquierdo-Sanchez L., Bettini L.R., Sumoy L., Terranova L., Moreira L., Santoro L., Scudeller L., Mesonero F., Roade L., Rühlemann M.C., Schaefer M., Carrabba M., Riveiro-Barciela M., Figuera Basso M.E., Valsecchi M.G., Hernandez-Tejero M., Acosta-Herrera M., D'Angiò M., Baldini M., Cazzaniga M., Schulzky M., Cecconi M., Wittig M., Ciccarelli M., Rodríguez-Gandía M., Bocciolone M., Miozzo M., Montano N., Braun N., Sacchi N., Martínez N., Özer O., Palmieri O., Faverio P., Preatoni P., Bonfanti P., Omodei P., Tentorio P., Castro P., Rodrigues P.M., Blandino Ortiz A., de Cid R., Ferrer R., Gualtierotti R., Nieto R., Goerg S., Badalamenti S., Marsal S., Matullo G., Pelusi S., Juzenas S., Aliberti S., Monzani V., Moreno V., Wesse T., Lenz T.L., Pumarola T., Rimoldi V., Bosari S., Albrecht W., Peter W., Romero-Gómez M., D'Amato M., Duga S., Banales J.M., Hov J.R., Folseraas T., Valenti L., Franke A., Karlsen T.H. Genomewide association study of severe COVID-19 with respiratory failure. N. Engl. J. Med. 2020 Oct 15;383(16):1522–1534. doi: 10.1056/NEJMoa2020283. Epub 2020 Jun 17. PMID: 32558485; PMCID: PMC7315890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen Z., Xiao Y., Kang L., Ma W., Shi L., Zhang L., Zhou Z., Yang J., Zhong J., Yang D., Guo L., Zhang G., Li H., Xu Y., Chen M., Gao Z., Wang J., Ren L., Li M. Genomic diversity of severe acute respiratory syndrome-coronavirus 2 in patients with coronavirus disease 2019. Clin. Infect. Dis. 2020 Jul 28;71(15):713–720. doi: 10.1093/cid/ciaa203. PMID: 32129843; PMCID: PMC7108196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tabibzadeh A., Zamani F., Laali A., Esghaei M., Safarnezhad Tameshkel F., Keyvani H., Jamshidi Makiani M., Panahi M., Motamed N., Perumal D., Khoonsari M., Ajdarkosh H., Sohrabi M., Ghanbari B., Savaj S., Mosavi-Jarrahi A., Karbalaie Niya M.H. SARS-CoV-2 Molecular and Phylogenetic analysis in COVID-19 patients: a preliminary report from Iran. Infect. Genet. Evol. 2020 Oct;84:104387. doi: 10.1016/j.meegid.2020.104387. Epub 2020 May 30. PMID: 32485332; PMCID: PMC7832360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X., Geng M., Peng Y., Meng L., Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J. Pharm Anal. 2020 Apr;10(2):102–108. doi: 10.1016/j.jpha.2020.03.001. Epub 2020 Mar 5. PMID: 32282863; PMCID: PMC7104082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cevik M., Kuppalli K., Kindrachuk J., Peiris M. Virology, transmission, and pathogenesis of SARS-CoV-2. BMJ. 2020 Oct 23;371 doi: 10.1136/bmj.m3862. PMID: 33097561. [DOI] [PubMed] [Google Scholar]
- 18.Gattinoni L., Chiumello D., Caironi P., Busana M., Romitti F., Brazzi L., Camporota L. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020 Jun;46(6):1099–1102. doi: 10.1007/s00134-020-06033-2. Epub 2020 Apr 14. PMID: 32291463; PMCID: PMC7154064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 Apr 16;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. e8 Epub 2020 Mar 5. PMID: 32142651; PMCID: PMC7102627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sims A.C., Baric R.S., Yount B., Burkett S.E., Collins P.L., Pickles R.J. Severe acute respiratory syndrome coronavirus infection of human ciliated airway epithelia: role of ciliated cells in viral spread in the conducting airways of the lungs. J. Virol. 2005 Dec;79(24):15511–15524. doi: 10.1128/JVI.79.24.15511-15524.2005. PMID: 16306622; PMCID: PMC1316022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iwata-Yoshikawa N., Okamura T., Shimizu Y., Hasegawa H., Takeda M., Nagata N. TMPRSS2 contributes to virus spread and immunopathology in the airways of murine models after coronavirus infection. J. Virol. 2019 Mar 5;93(6):e01815–e01818. doi: 10.1128/JVI.01815-18. PMID: 30626688; PMCID: PMC6401451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Z., Mi L., Xu J., Yu J., Wang X., Jiang J., Xing J., Shang P., Qian A., Li Y., Shaw P.X., Wang J., Duan S., Ding J., Fan C., Zhang Y., Yang Y., Yu X., Feng Q., Li B., Yao X., Zhang Z., Li L., Xue X., Zhu P. Function of HAb18G/CD147 in invasion of host cells by severe acute respiratory syndrome coronavirus. J. Infect. Dis. 2005 Mar 1;191(5):755–760. doi: 10.1086/427811. Epub 2005 Jan 25. PMID: 15688292; PMCID: PMC7110046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kindler E., Gil-Cruz C., Spanier J., Li Y., Wilhelm J., Rabouw H.H., Züst R., Hwang M., V'kovski P., Stalder H., Marti S., Habjan M., Cervantes-Barragan L., Elliot R., Karl N., Gaughan C., van Kuppeveld F.J., Silverman R.H., Keller M., Ludewig B., Bergmann C.C., Ziebuhr J., Weiss S.R., Kalinke U., Thiel V. Early endonuclease-mediated evasion of RNA sensing ensures efficient coronavirus replication. PLoS Pathog. 2017 Feb 3;13(2) doi: 10.1371/journal.ppat.1006195. PMID: 28158275; PMCID: PMC5310923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A., Niemeyer D., Jones T.C., Vollmar P., Rothe C., Hoelscher M., Bleicker T., Brünink S., Schneider J., Ehmann R., Zwirglmaier K., Drosten C., Wendtner C. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020 May;581(7809):465–469. doi: 10.1038/s41586-020-2196-x. Epub 2020 Apr 1. Erratum in: Nature. 2020 Dec;588(7839):E35. PMID: 32235945. [DOI] [PubMed] [Google Scholar]
- 25.Yuan L., Chen Z., Song S., Wang S., Tian C., Xing G., Chen X., Xiao Z.X., He F., Zhang L. p53 degradation by a coronavirus papain-like protease suppresses type I interferon signaling. J. Biol. Chem. 2015 Jan 30;290(5):3172–3182. doi: 10.1074/jbc.M114.619890. Epub 2014 Dec 10. PMID: 25505178; PMCID: PMC4317044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Totura A.L., Whitmore A., Agnihothram S., Schäfer A., Katze M.G., Heise M.T., Baric R.S. Toll-like receptor 3 signaling via TRIF contributes to a protective innate immune response to severe acute respiratory syndrome coronavirus infection. mBio. 2015 May 26;6(3) doi: 10.1128/mBio.00638-15. e00638-15 PMID: 26015500; PMCID: PMC4447251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao J., Zhao J., Van Rooijen N., Perlman S. Evasion by stealth: inefficient immune activation underlies poor T cell response and severe disease in SARS-CoV-infected mice. PLoS Pathog. 2009 Oct;5(10) doi: 10.1371/journal.ppat.1000636. Epub 2009 Oct 23. PMID: 19851468; PMCID: PMC2762542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Channappanavar R., Fehr A.R., Vijay R., Mack M., Zhao J., Meyerholz D.K., Perlman S. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe. 2016 Feb 10;19(2):181–193. doi: 10.1016/j.chom.2016.01.007. PMID: 26867177; PMCID: PMC4752723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cameron M.J., Ran L., Xu L., Danesh A., Bermejo-Martin J.F., Cameron C.M., Muller M.P., Gold W.L., Richardson S.E., Poutanen S.M., Willey B.M., DeVries M.E., Fang Y., Seneviratne C., Bosinger S.E., Persad D., Wilkinson P., Greller L.D., Somogyi R., Humar A., Keshavjee S., Louie M., Loeb M.B., Brunton J., McGeer A.J., Canadian SARS Research Network. Kelvin D.J. Interferon-mediated immunopathological events are associated with atypical innate and adaptive immune responses in patients with severe acute respiratory syndrome. J. Virol. 2007 Aug;81(16):8692–8706. doi: 10.1128/JVI.00527-07. Epub 2007 May 30. PMID: 17537853; PMCID: PMC1951379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicolai L., Leunig A., Brambs S., Kaiser R., Weinberger T., Weigand M., Muenchhoff M., Hellmuth J.C., Ledderose S., Schulz H., Scherer C., Rudelius M., Zoller M., Höchter D., Keppler O., Teupser D., Zwißler B., von Bergwelt-Baildon M., Kääb S., Massberg S., Pekayvaz K., Stark K. Immunothrombotic dysregulation in COVID-19 pneumonia is associated with respiratory failure and coagulopathy. Circulation. 2020 Sep 22;142(12):1176–1189. doi: 10.1161/CIRCULATIONAHA.120.048488. Epub 2020 Jul 28. PMID: 32755393; PMCID: PMC7497892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zamanian-Daryoush M., Mogensen T.H., DiDonato J.A., Williams B.R. NF-kappaB activation by double-stranded-RNA-activated protein kinase (PKR) is mediated through NF-kappaB-inducing kinase and IkappaB kinase. Mol. Cell Biol. 2000 Feb;20(4):1278–1290. doi: 10.1128/mcb.20.4.1278-1290.2000. PMID: 10648614; PMCID: PMC85265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meusel T.R., Kehoe K.E., Imani F. Protein kinase R regulates double-stranded RNA induction of TNF-alpha but not IL-1 beta mRNA in human epithelial cells. J. Immunol. 2002 Jun 15;168(12):6429–6435. doi: 10.4049/jimmunol.168.12.6429. PMID: 12055262. [DOI] [PubMed] [Google Scholar]
- 33.Nakamura M., Kanda T., Sasaki R., Haga Y., Jiang X., Wu S., Nakamoto S., Yokosuka O. MicroRNA-122 inhibits the production of inflammatory cytokines by targeting the PKR activator PACT in human hepatic stellate cells. PloS One. 2015 Dec 4;10(12) doi: 10.1371/journal.pone.0144295. PMID: 26636761; PMCID: PMC4670168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deshmane S.L., Kremlev S., Amini S., Sawaya B.E. Monocyte chemoattractant protein-1 (MCP-1): an overview. J. Interferon Cytokine Res. 2009 Jun;29(6):313–326. doi: 10.1089/jir.2008.0027. PMID: 19441883; PMCID: PMC2755091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arendt B.K., Velazquez-Dones A., Tschumper R.C., Howell K.G., Ansell S.M., Witzig T.E., Jelinek D.F. Interleukin 6 induces monocyte chemoattractant protein-1 expression in myeloma cells. Leukemia. 2002 Oct;16(10):2142–2147. doi: 10.1038/sj.leu.2402714. PMID: 12357369. [DOI] [PubMed] [Google Scholar]
- 36.Huang H., Jing G., Wang J.J., Sheibani N., Zhang S.X. ATF4 is a novel regulator of MCP-1 in microvascular endothelial cells. J. Inflamm. 2015 Apr 17;12:31. doi: 10.1186/s12950-015-0076-1. PMID: 25914608; PMCID: PMC4409760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haga S., Yamamoto N., Nakai-Murakami C., Osawa Y., Tokunaga K., Sata T., Yamamoto N., Sasazuki T., Ishizaka Y. Modulation of TNF-alpha-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-alpha production and facilitates viral entry. Proc. Natl. Acad. Sci. U. S. A. 2008 Jun 3;105(22):7809–7814. doi: 10.1073/pnas.0711241105. Epub 2008 May 19. PMID: 18490652; PMCID: PMC2409424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rose N.R. Critical cytokine pathways to cardiac inflammation. J. Interferon Cytokine Res. 2011 Oct;31(10):705–710. doi: 10.1089/jir.2011.0057. Epub 2011 Aug 23. PMID: 21861699; PMCID: PMC3189548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.García M.A., Meurs E.F., Esteban M. The dsRNA protein kinase PKR: virus and cell control. Biochimie. 2007 Jun-Jul;89(6-7):799–811. doi: 10.1016/j.biochi.2007.03.001. Epub 2007 Mar 12. PMID: 17451862. [DOI] [PubMed] [Google Scholar]
- 40.Hoang H.D., Graber T.E., Alain T. Battling for ribosomes: translational control at the forefront of the antiviral response. J. Mol. Biol. 2018 Jul 6;430(14):1965–1992. doi: 10.1016/j.jmb.2018.04.040. Epub 2018 May 7. PMID: 29746850. [DOI] [PubMed] [Google Scholar]
- 41.Wang X., Xu W., Hu G., Xia S., Sun Z., Liu Z., Xie Y., Zhang R., Jiang S., Lu L. Retracted article: SARS-CoV-2 infects T lymphocytes through its spike protein-mediated membrane fusion. Cell. Mol. Immunol. 2020 Apr 7:1–3. doi: 10.1038/s41423-020-0424-9. Epub ahead of print. Retraction in: Cell Mol Immunol. 2020 Aug;17(8):894. PMID: 32265513; PMCID: PMC7136698. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Döring G. The role of neutrophil elastase in chronic inflammation. Am. J. Respir. Crit. Care Med. 1994 Dec;150(6 Pt 2):S114–S117. doi: 10.1164/ajrccm/150.6_Pt_2.S114. PMID: 7952645. [DOI] [PubMed] [Google Scholar]
- 43.Guma M., Ronacher L., Liu-Bryan R., Takai S., Karin M., Corr M. Caspase 1-independent activation of interleukin-1beta in neutrophil-predominant inflammation. Arthritis Rheum. 2009 Dec;60(12):3642–3650. doi: 10.1002/art.24959. PMID: 19950258; PMCID: PMC2847793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alfaidi M., Wilson H., Daigneault M., Burnett A., Ridger V., Chamberlain J., Francis S. Neutrophil elastase promotes interleukin-1β secretion from human coronary endothelium. J. Biol. Chem. 2015 Oct 2;290(40):24067–24078. doi: 10.1074/jbc.M115.659029. Epub 2015 Aug 12. PMID: 26269588; PMCID: PMC4591798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gal-Ben-Ari S., Barrera I., Ehrlich M., Rosenblum K. PKR: A kinase to remember. Front. Mol. Neurosci. 2019 Jan 9;11:480. doi: 10.3389/fnmol.2018.00480. PMID: 30686999; PMCID: PMC6333748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yan X., Hao Q., Mu Y., Timani K.A., Ye L., Zhu Y., Wu J. Nucleocapsid protein of SARS-CoV activates the expression of cyclooxygenase-2 by binding directly to regulatory elements for nuclear factor-kappa B and CCAAT/enhancer binding protein. Int. J. Biochem. Cell Biol. 2006;38(8):1417–1428. doi: 10.1016/j.biocel.2006.02.003. Epub 2006 Mar 3. PMID: 16546436; PMCID: PMC7108415. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Kim S.F., Huri D.A., Snyder S.H. Inducible nitric oxide synthase binds, S-nitrosylates, and activates cyclooxygenase-2. Science. 2005 Dec 23;310(5756):1966–1970. doi: 10.1126/science.1119407. PMID: 16373578. [DOI] [PubMed] [Google Scholar]
- 48.Reggiori F., Monastyrska I., Verheije M.H., Calì T., Ulasli M., Bianchi S., Bernasconi R., de Haan C.A., Molinari M. Coronaviruses Hijack the LC3-I-positive EDEMosomes, ER-derived vesicles exporting short-lived ERAD regulators, for replication. Cell Host Microbe. 2010 Jun 25;7(6):500–508. doi: 10.1016/j.chom.2010.05.013. PMID: 20542253; PMCID: PMC7103375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oostra M., te Lintelo E.G., Deijs M., Verheije M.H., Rottier P.J., de Haan C.A. Localization and membrane topology of coronavirus nonstructural protein 4: involvement of the early secretory pathway in replication. J. Virol. 2007 Nov;81(22):12323–12336. doi: 10.1128/JVI.01506-07. Epub 2007 Sep 12. PMID: 17855519; PMCID: PMC2168994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.He Y., Hara H., Núñez G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem. Sci. 2016 Dec;41(12):1012–1021. doi: 10.1016/j.tibs.2016.09.002. Epub 2016 Sep 23. PMID: 27669650; PMCID: PMC5123939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu Z., Lv Y., Zhao N., Guan G., Wang J. Protein kinase R-like ER kinase and its role in endoplasmic reticulum stress-decided cell fate. Cell Death Dis. 2015 Jul 30;6(7) doi: 10.1038/cddis.2015.183. PMID: 26225772; PMCID: PMC4650730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang X.Z., Lawson B., Brewer J.W., Zinszner H., Sanjay A., Mi L.J., Boorstein R., Kreibich G., Hendershot L.M., Ron D. Signals from the stressed endoplasmic reticulum induce C/EBP-homologous protein (CHOP/GADD153) Mol. Cell Biol. 1996 Aug;16(8):4273–4280. doi: 10.1128/mcb.16.8.4273. PMID: 8754828; PMCID: PMC231426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hu J., Lei L., Wang Y., Wang K., Hu X., Wang A., Vanderkerken K. Interleukin-6 drives multiple myeloma progression by up-regulating of CD147/emmprin expression. Blood. 2016;128(22):5632. [Google Scholar]
- 54.Pfefferle S., Schöpf J., Kögl M., Friedel C.C., Müller M.A., Carbajo-Lozoya J., Stellberger T., von Dall'Armi E., Herzog P., Kallies S., Niemeyer D., Ditt V., Kuri T., Züst R., Pumpor K., Hilgenfeld R., Schwarz F., Zimmer R., Steffen I., Weber F., Thiel V., Herrler G., Thiel H.J., Schwegmann-Wessels C., Pöhlmann S., Haas J., Drosten C., von Brunn A. The SARS-coronavirus-host interactome: identification of cyclophilins as target for pan-coronavirus inhibitors. PLoS Pathog. 2011 Oct;7(10) doi: 10.1371/journal.ppat.1002331. Epub 2011 Oct 27. PMID: 22046132; PMCID: PMC3203193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seizer P., Ungern-Sternberg S.N., Schönberger T., Borst O., Münzer P., Schmidt E.M., Mack A.F., Heinzmann D., Chatterjee M., Langer H., Malešević M., Lang F., Gawaz M., Fischer G., May A.E. Extracellular cyclophilin A activates platelets via EMMPRIN (CD147) and PI3K/Akt signaling, which promotes platelet adhesion and thrombus formation in vitro and in vivo. Arterioscler. Thromb. Vasc. Biol. 2015 Mar;35(3):655–663. doi: 10.1161/ATVBAHA.114.305112. Epub 2014 Dec 30. PMID: 25550208. [DOI] [PubMed] [Google Scholar]
- 56.Elvers M., Herrmann A., Seizer P., Münzer P., Beck S., Schönberger T., Borst O., Martin-Romero F.J., Lang F., May A.E., Gawaz M. Intracellular cyclophilin A is an important Ca(2+) regulator in platelets and critically involved in arterial thrombus formation. Blood. 2012 Aug 9;120(6):1317–1326. doi: 10.1182/blood-2011-12-398438. Epub 2012 Jun 27. PMID: 22740452. [DOI] [PubMed] [Google Scholar]
- 57.Wu Y.P., Wei R., Liu Z.H., Chen B., Lisman T., Ren D.L., Han J.J., Xia Z.L., Zhang F.S., Xu W.B., Preissner K.T., de Groot P.G. Analysis of thrombotic factors in severe acute respiratory syndrome (SARS) patients. Thromb. Haemostasis. 2006 Jul;96(1):100–101. doi: 10.1160/TH05-12-0827. Erratum in: Thromb Haemost. 2006 Oct;96(4):543. Zhang, Fu Sheng [corrected to Zhang, Fu Sen]. PMID: 16807662. [DOI] [PubMed] [Google Scholar]
- 58.Schumacker P.T. Lung cell hypoxia: role of mitochondrial reactive oxygen species signaling in triggering responses. Proc. Am. Thorac. Soc. 2011 Nov;8(6):477–484. doi: 10.1513/pats.201103-032MW. PMID: 22052923; PMCID: PMC3359072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 2017 Jul;39(5):529–539. doi: 10.1007/s00281-017-0629-x. Epub 2017 May 2. PMID: 28466096; PMCID: PMC7079893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu Y., Yang Y., Zhang C., Huang F., Wang F., Yuan J., Wang Z., Li J., Li J., Feng C., Zhang Z., Wang L., Peng L., Chen L., Qin Y., Zhao D., Tan S., Yin L., Xu J., Zhou C., Jiang C., Liu L. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 2020 Mar;63(3):364–374. doi: 10.1007/s11427-020-1643-8. Epub 2020 Feb 9. PMID: 32048163; PMCID: PMC7088566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nguyen Dinh Cat A., Montezano A.C., Burger D., Touyz R.M. Angiotensin II, NADPH oxidase, and redox signaling in the vasculature. Antioxidants Redox Signal. 2013 Oct 1;19(10):1110–1120. doi: 10.1089/ars.2012.4641. Epub 2012 Jun 11. PMID: 22530599; PMCID: PMC3771549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li G., Scull C., Ozcan L., Tabas I. NADPH oxidase links endoplasmic reticulum stress, oxidative stress, and PKR activation to induce apoptosis. J. Cell Biol. 2010 Dec 13;191(6):1113–1125. doi: 10.1083/jcb.201006121. Epub 2010 Dec 6. PMID: 21135141; PMCID: PMC3002036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou T., Su T.T., Mudianto T., Wang J. Immune asynchrony in COVID-19 pathogenesis and potential immunotherapies. J. Exp. Med. 2020 Oct 5;217(10) doi: 10.1084/jem.20200674. PMID: 32910820; PMCID: PMC7481961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bohn M.K., Hall A., Sepiashvili L., Jung B., Steele S., Adeli K. Pathophysiology of COVID-19: mechanisms underlying disease severity and progression. Physiology. 2020 Sep 1;35(5):288–301. doi: 10.1152/physiol.00019.2020. PMID: 32783610; PMCID: PMC7426542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020 Jun 4;135(23):2033–2040. doi: 10.1182/blood.2020006000. PMID: 32339221; PMCID: PMC7273827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Subramaniam S., Jurk K., Hobohm L., Jäckel S., Saffarzadeh M., Schwierczek K., Wenzel P., Langer F., Reinhardt C., Ruf W. Distinct contributions of complement factors to platelet activation and fibrin formation in venous thrombus development. Blood. 2017 Apr 20;129(16):2291–2302. doi: 10.1182/blood-2016-11-749879. Epub 2017 Feb 21. PMID: 28223279; PMCID: PMC5399485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Magro C., Mulvey J.J., Berlin D., Nuovo G., Salvatore S., Harp J., Baxter-Stoltzfus A., Laurence J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl. Res. 2020 Jun;220:1–13. doi: 10.1016/j.trsl.2020.04.007. Epub 2020 Apr 15. PMID: 32299776; PMCID: PMC7158248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ranucci M., Ballotta A., Di Dedda U., Bayshnikova E., Dei Poli M., Resta M., Falco M., Albano G., Menicanti L. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J. Thromb. Haemostasis. 2020 Jul;18(7):1747–1751. doi: 10.1111/jth.14854. Epub 2020 May 6. PMID: 32302448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Al-Eitan L.N., Alahmad S.Z. Pharmacogenomics of genetic polymorphism within the genes responsible for SARS-CoV-2 susceptibility and the drug-metabolising genes used in treatment. Rev. Med. Virol. 2020 Nov 17 doi: 10.1002/rmv.2194. Epub ahead of print. PMID: 33205496; PMCID: PMC7744885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guo X., Chen Z., Xia Y., Lin W., Li H. Investigation of the genetic variation in ACE2 on the structural recognition by the novel coronavirus (SARS-CoV-2) J. Transl. Med. 2020 Aug 24;18(1):321. doi: 10.1186/s12967-020-02486-7. PMID: 32831104; PMCID: PMC7443814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stawiski E.W., Diwanji D., Suryamohan K., Gupta R., Fellouse F.A., Sathirapongsasuti J.F., Liu J., Jiang Y.-P., Ratan A., Mis M., Santhosh D., Somasekar S., Mohan S., Phalke S., Kuriakose B., Antony A., Junutula J.R., Schuster S.C., Jura N., Seshagiri S. Human ACE2 receptor polymorphisms predict SARS-CoV-2 susceptibility. BioRxiv. 2020 doi: 10.1038/s42003-021-02030-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bosso M., Thanaraj T.A., Abu-Farha M., Alanbaei M., Abubaker J., Al-Mulla F. The two faces of ACE2: the role of ACE2 receptor and its polymorphisms in hypertension and COVID-19. Mol. Ther. Methods Clin. Dev. 2020 Jun 25;18:321–327. doi: 10.1016/j.omtm.2020.06.017. PMID: 32665962; PMCID: PMC7314689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Delanghe J.R., Speeckaert M.M., De Buyzere M.L. The host's angiotensin-converting enzyme polymorphism may explain epidemiological findings in COVID-19 infections. Clin. Chim. Acta. 2020 Jun;505:192–193. doi: 10.1016/j.cca.2020.03.031. Epub 2020 Mar 24. PMID: 32220422; PMCID: PMC7102561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zheng H., Cao J.J. Angiotensin-converting enzyme gene polymorphism and severe lung injury in patients with coronavirus disease 2019. Am. J. Pathol. 2020 Oct;190(10):2013–2017. doi: 10.1016/j.ajpath.2020.07.009. Epub 2020 Jul 29.PMID: 32735889; PMCID: PMC7387924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gómez J., Albaiceta G.M., García-Clemente M., López-Larrea C., Amado-Rodríguez L., Lopez-Alonso I., Hermida T., Enriquez A.I., Herrero P., Melón S., Alvarez-Argüelles M.E., Boga J.A., Rojo-Alba S., Cuesta-Llavona E., Alvarez V., Lorca R., Coto E. Angiotensin-converting enzymes (ACE, ACE2) gene variants and COVID-19 outcome. Gene. 2020 Dec 15;762:145102. doi: 10.1016/j.gene.2020.145102. Epub 2020 Aug 31. PMID: 32882331; PMCID: PMC7456966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yamamoto N., Ariumi Y., Nishida N., Yamamoto R., Bauer G., Gojobori T., Shimotohno K., Mizokami M. SARS-CoV-2 infections and COVID-19 mortalities strongly correlate with ACE1 I/D genotype. Gene. 2020 Oct 20;758:144944. doi: 10.1016/j.gene.2020.144944. Epub 2020 Jul 3. PMID: 32628976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vargas-Alarcón G., Posadas-Sánchez R., Ramírez-Bello J. Variability in genes related to SARS-CoV-2 entry into host cells (ACE2, TMPRSS2, TMPRSS11A, ELANE, and CTSL) and its potential use in association studies. Life Sci. 2020 Nov 1;260:118313. doi: 10.1016/j.lfs.2020.118313. Epub 2020 Aug 21. PMID: 32835700; PMCID: PMC7441892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Singh H., Choudhari R., Nema V., Khan A.A. ACE2 and TMPRSS2 polymorphisms in various diseases with special reference to its impact on COVID-19 disease. Microb. Pathog. 2021 Jan;150:104621. doi: 10.1016/j.micpath.2020.104621. Epub 2020 Dec 2. PMID: 33278516; PMCID: PMC7709597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Russo R., Andolfo I., Lasorsa V.A., Iolascon A., Capasso M. Genetic analysis of the coronavirus SARS-CoV-2 host protease TMPRSS2 in different populations. Front. Genet. 2020 Aug 4;11:872. doi: 10.3389/fgene.2020.00872. PMID: 32849840; PMCID: PMC7417663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Torre-Fuentes L., Matías-Guiu J., Hernández-Lorenzo L., Montero-Escribano P., Pytel V., Porta-Etessam J., Gómez-Pinedo U., Matías-Guiu J.A. ACE2, TMPRSS2, and Furin variants and SARS-CoV-2 infection in Madrid, Spain. J. Med. Virol. 2021 Feb;93(2):863–869. doi: 10.1002/jmv.26319. Epub 2020 Jul 28. PMID: 32691890; PMCID: PMC7404937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Klaassen K., Stankovic B., Zukic B., Kotur N., Gasic V., Pavlovic S., Stojiljkovic M. Functional prediction and comparative population analysis of variants in genes for proteases and innate immunity related to SARS-CoV-2 infection. Infect. Genet. Evol. 2020 Oct;84:104498. doi: 10.1016/j.meegid.2020.104498. Epub 2020 Aug 7. PMID: 32771700; PMCID: PMC7410821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Senapati S., Kumar S., Singh A.K., Banerjee P., Bhagavatula S. Assessment of risk conferred by coding and regulatory variations of TMPRSS2 and CD26 in susceptibility to SARS-CoV-2 infection in human. J. Genet. 2020;99(1):53. doi: 10.1007/s12041-020-01217-7. PMID: 32661206; PMCID: PMC7280172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ulrich H., Pillat M.M. CD147 as a target for COVID-19 treatment: suggested effects of azithromycin and stem cell engagement. Stem Cell Rev. Rep. 2020 Jun;16(3):434–440. doi: 10.1007/s12015-020-09976-7. PMID: 32307653; PMCID: PMC7167302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ilikci Sagkan R., Akin-Bali D.F. Structural variations and expression profiles of the SARS-CoV-2 host invasion genes in lung cancer. J. Med. Virol. 2020 Nov;92(11):2637–2647. doi: 10.1002/jmv.26107. Epub 2020 Jun 19. PMID: 32492203; PMCID: PMC7300553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mbewe-Campbell N., Wei Z., Zhang K., Friese R.S., Mahata M., Schork A.J., Rao F., Chiron S., Biswas N., Kim H.S., Mahata S.K., Waalen J., Nievergelt C.M., Hook V.Y., O'Connor D.T. Genes and environment: novel, functional polymorphism in the human cathepsin L (CTSL1) promoter disrupts a xenobiotic response element (XRE) to alter transcription and blood pressure. J. Hypertens. 2012 Oct;30(10):1961–1969. doi: 10.1097/HJH.0b013e328356b86a. PMID: 22871890; PMCID: PMC3478326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vietzen H., Zoufaly A., Traugott M., Aberle J., Aberle S.W., Puchhammer-Stöckl E. Deletion of the NKG2C receptor encoding KLRC2 gene and HLA-E variants are risk factors for severe COVID-19. Genet. Med. 2021 Jan 26:1–5. doi: 10.1038/s41436-020-01077-7. Epub ahead of print. PMID: 33500568; PMCID: PMC7835668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lin M., Tseng H.K., Trejaut J.A., Lee H.L., Loo J.H., Chu C.C., Chen P.J., Su Y.W., Lim K.H., Tsai Z.U., Lin R.Y., Lin R.S., Huang C.H. Association of HLA class I with severe acute respiratory syndrome coronavirus infection. BMC Med. Genet. 2003 Sep 12;4:9. doi: 10.1186/1471-2350-4-9. PMID: 12969506; PMCID: PMC212558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ng M.H., Lau K.M., Li L., Cheng S.H., Chan W.Y., Hui P.K., Zee B., Leung C.B., Sung J.J. Association of human-leukocyte-antigen class I (B∗0703) and class II (DRB1∗0301) genotypes with susceptibility and resistance to the development of severe acute respiratory syndrome. J. Infect. Dis. 2004 Aug 1;190(3):515–518. doi: 10.1086/421523. Epub 2004 Jul 7. PMID: 15243926; PMCID: PMC7109646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen Y.M., Liang S.Y., Shih Y.P., Chen C.Y., Lee Y.M., Chang L., Jung S.Y., Ho M.S., Liang K.Y., Chen H.Y., Chan Y.J., Chu D.C. Epidemiological and genetic correlates of severe acute respiratory syndrome coronavirus infection in the hospital with the highest nosocomial infection rate in Taiwan in 2003. J. Clin. Microbiol. 2006 Feb;44(2):359–365. doi: 10.1128/JCM.44.2.359-365.2006. PMID: 16455884; PMCID: PMC1392693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ng M.H., Cheng S.H., Lau K.M., Leung G.M., Khoo U.S., Zee B.C., Sung J.J. Immunogenetics in SARS: a case-control study. Hong Kong Med. J. 2010;16(5 Suppl 4):29–33. PMID: 20864745. [PubMed] [Google Scholar]
- 91.Keicho N., Itoyama S., Kashiwase K., Phi N.C., Long H.T., Ha L.D., Ban V.V., Hoa B.K., Hang N.T., Hijikata M., Sakurada S., Satake M., Tokunaga K., Sasazuki T., Quy T. Association of human leukocyte antigen class II alleles with severe acute respiratory syndrome in the Vietnamese population. Hum. Immunol. 2009 Jul;70(7):527–531. doi: 10.1016/j.humimm.2009.05.006. Epub 2009 May 13. PMID: 19445991; PMCID: PMC7132661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yuan F.F., Velickovic Z., Ashton L.J., Dyer W.B., Geczy A.F., Dunckley H., Lynch G.W., Sullivan J.S. Influence of HLA gene polymorphisms on susceptibility and outcome post infection with the SARS-CoV virus. Virol. Sin. 2014 Apr;29(2):128–130. doi: 10.1007/s12250-014-3398-x. PMID: 24643938; PMCID: PMC7090670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xiong P., Zeng X., Song M.S., Jia S.W., Zhong M.H., Xiao L.L., Lan W., Cai C., Wu X.W., Gong F.L., Wang W. Lack of association between HLA-A, -B and -DRB1 alleles and the development of SARS: a cohort of 95 SARS-recovered individuals in a population of Guangdong, southern China. Int. J. Immunogenet. 2008 Feb;35(1):69–74. doi: 10.1111/j.1744-313X.2007.00741.x. PMID: 18186801; PMCID: PMC7165669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Habel J.R., Nguyen T.H.O., van de Sandt C.E., Juno J.A., Chaurasia P., Wragg K., Koutsakos M., Hensen L., Jia X., Chua B., Zhang W., Tan H.X., Flanagan K.L., Doolan D.L., Torresi J., Chen W., Wakim L.M., Cheng A.C., Doherty P.C., Petersen J., Rossjohn J., Wheatley A.K., Kent S.J., Rowntree L.C., Kedzierska K. Suboptimal SARS-CoV-2-specific CD8+ T cell response associated with the prominent HLA-A∗02:01 phenotype. Proc. Natl. Acad. Sci. U. S. A. 2020 Sep 29;117(39):24384–24391. doi: 10.1073/pnas.2015486117. Epub 2020 Sep 10. PMID: 32913053; PMCID: PMC7533701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tomita Y., Ikeda T., Sato R., Sakagami T. Association between HLA gene polymorphisms and mortality of COVID-19: an in silico analysis. Immun. Inflamm. Dis. 2020 Dec;8(4):684–694. doi: 10.1002/iid3.358. Epub 2020 Oct 13. PMID: 33047883; PMCID: PMC7654404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Amoroso A., Magistroni P., Vespasiano F., Bella A., Bellino S., Puoti F., Alizzi S., Vaisitti T., Boros S., Grossi P.A., Trapani S., Lombardini L., Pezzotti P., Deaglio S., Brusaferro S., Cardillo M., Italian Network of Regional Transplant Coordinating Centers HLA and AB0 polymorphisms may influence SARS-CoV-2 infection and COVID-19 severity. Transplantation. 2021 Jan 1;105(1):193–200. doi: 10.1097/TP.0000000000003507. PMID: 33141807. [DOI] [PubMed] [Google Scholar]
- 97.Knierman M.D., Lannan M.B., Spindler L.J., McMillian C.L., Konrad R.J., Siegel R.W. The human leukocyte antigen class II immunopeptidome of the SARS-CoV-2 spike glycoprotein. Cell Rep. 2020 Dec 1;33(9):108454. doi: 10.1016/j.celrep.2020.108454. Epub 2020 Nov 13. PMID: 33220791; PMCID: PMC7664343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schultheiß C., Paschold L., Simnica D., Mohme M., Willscher E., von Wenserski L., Scholz R., Wieters I., Dahlke C., Tolosa E., Sedding D.G., Ciesek S., Addo M., Binder M. Next-generation sequencing of T and B cell receptor repertoires from COVID-19 patients showed signatures associated with severity of disease. Immunity. 2020 Aug 18;53(2):442–455. doi: 10.1016/j.immuni.2020.06.024. e4 Epub 2020 Jun 30. PMID: 32668194; PMCID: PMC7324317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rowntree L.C., Petersen J., Juno J.A., Chaurasia P., Wragg K., Koutsakos M., Hensen L., Wheatley A.K., Kent S.J., Rossjohn J., Kedzierska K., Nguyen T.H. SARS-CoV-2-specific CD8+ T-cell responses and TCR signatures in the context of a prominent HLA-A∗24:02 allomorph. Immunol. Cell Biol. 2021 Jun 4 doi: 10.1111/imcb.12482. Epub ahead of print. PMID: 34086357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang P., Jin X., Zhou W., Luo M., Xu Z., Xu C., Li Y., Ma K., Cao H., Huang Y., Xue G., Jin S., Nie H., Jiang Q. Comprehensive analysis of TCR repertoire in COVID-19 using single cell sequencing. Genomics. 2021 Mar;113(2):456–462. doi: 10.1016/j.ygeno.2020.12.036. Epub 2020 Dec 28. PMID: 33383142; PMCID: PMC7833309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Niu X., Li S., Li P., Pan W., Wang Q., Feng Y., Mo X., Yan Q., Ye X., Luo J., Qu L., Weber D., Byrne-Steele M.L., Wang Z., Yu F., Li F., Myers R.M., Lotze M.T., Zhong N., Han J., Chen L. Longitudinal analysis of T and B cell receptor repertoire transcripts reveal dynamic immune response in COVID-19 patients. Front. Immunol. 2020 Sep 30;11:582010. doi: 10.3389/fimmu.2020.582010. Erratum in: Front Immunol. 2020 Dec 21;11:633815. PMID: 33117392; PMCID: PMC7561365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mayer-Blackwell K., Schattgen S., Cohen-Lavi L., Crawford J.C., Souquette A., Gaevert J.A., Hertz T., Thomas P.G., Bradley P., Fiore-Gartland A. TCR meta-clonotypes for biomarker discovery with tcrdist3: quantification of public, HLA-restricted TCR biomarkers of SARS-CoV-2 infection. bioRxiv. 2020 Dec 26:2020. doi: 10.7554/eLife.68605. [Preprint] PMID: 33398288; PMCID: PMC7781332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chong W.P., Ip W.K., Tso G.H., Ng M.W., Wong W.H., Law H.K., Yung R.W., Chow E.Y., Au K.L., Chan E.Y., Lim W., Peiris J.S., Lau Y.L. The interferon gamma gene polymorphism +874 A/T is associated with severe acute respiratory syndrome. BMC Infect. Dis. 2006 May 4;6:82. doi: 10.1186/1471-2334-6-82. PMID: 16672072; PMCID: PMC1468415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Thevarajan I., Nguyen T.H.O., Koutsakos M., Druce J., Caly L., van de Sandt C.E., Jia X., Nicholson S., Catton M., Cowie B., Tong S.Y.C., Lewin S.R., Kedzierska K. Breadth of concomitant immune responses prior to patient recovery: a case report of non-severe COVID-19. Nat. Med. 2020 Apr;26(4):453–455. doi: 10.1038/s41591-020-0819-2. PMID: 32284614; PMCID: PMC7095036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang Y., Qin L., Zhao Y., Zhang P., Xu B., Li K., Liang L., Zhang C., Dai Y., Feng Y., Sun J., Hu Z., Xiang H., Knight J.C., Dong T., Jin R. Interferon-induced transmembrane protein 3 genetic variant rs12252-C associated with disease severity in coronavirus disease 2019. J. Infect. Dis. 2020 Jun 16;222(1):34–37. doi: 10.1093/infdis/jiaa224. PMID: 32348495; PMCID: PMC7197559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kim Y.C., Jeong B.H. Strong correlation between the case fatality rate of COVID-19 and the rs6598045 single nucleotide polymorphism (SNP) of the interferon-induced transmembrane protein 3 (IFITM3) gene at the population-level. Genes (Basel) 2020 Dec 30;12(1):42. doi: 10.3390/genes12010042. PMID: 33396837; PMCID: PMC7824003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Amodio E., Pipitone R.M., Grimaudo S., Immordino P., Maida C.M., Prestileo T., Restivo V., Tramuto F., Vitale F., Craxì A., Casuccio A. SARS-CoV-2 viral load, IFNλ polymorphisms and the course of COVID-19: an observational study. J. Clin. Med. 2020 Oct 15;9(10):3315. doi: 10.3390/jcm9103315. PMID: 33076493; PMCID: PMC7602550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Maiti A.K. The African-American population with a low allele frequency of SNP rs1990760 (T allele) in IFIH1 predicts less IFN-beta expression and potential vulnerability to COVID-19 infection. Immunogenetics. 2020 Sep;72(6-7):387–391. doi: 10.1007/s00251-020-01174-6. Epub 2020 Jul 31. PMID: 32737579; PMCID: PMC7394703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Debnath M., Banerjee M., Berk M. Genetic gateways to COVID-19 infection: implications for risk, severity, and outcomes. Faseb. J. 2020 Jul;34(7):8787–8795. doi: 10.1096/fj.202001115R. Epub 2020 Jun 11. PMID: 32525600; PMCID: PMC7300732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Thierry A.R. Host/genetic factors associated with COVID-19 call for precision medicine. Precis. Clin. Med. September 2020;3(3):228–234. doi: 10.1093/pcmedi/pbaa026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Choudhury A., Mukherjee S. In silico studies on the comparative characterization of the interactions of SARS-CoV-2 spike glycoprotein with ACE-2 receptor homologs and human TLRs. J. Med. Virol. 2020 Oct;92(10):2105–2113. doi: 10.1002/jmv.25987. Epub 2020 May 17. PMID: 32383269; PMCID: PMC7267663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Oshiumi H., Matsumoto M., Funami K., Akazawa T., Seya T. TICAM-1, an adaptor molecule that participates in Toll-like receptor 3-mediated interferon-beta induction. Nat. Immunol. 2003 Feb;4(2):161–167. doi: 10.1038/ni886. Epub 2003 Jan 21. PMID: 12539043. [DOI] [PubMed] [Google Scholar]
- 113.Kawasaki T., Kawai T. Toll-like receptor signaling pathways. Front. Immunol. 2014 Sep 25;5:461. doi: 10.3389/fimmu.2014.00461. PMID: 25309543; PMCID: PMC4174766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Trejo-de la O.A., Hernández-Sancén P., Maldonado-Bernal C. Relevance of single-nucleotide polymorphisms in human TLR genes to infectious and inflammatory diseases and cancer. Gene Immun. 2014 Apr;15(4):199–209. doi: 10.1038/gene.2014.10. Epub 2014 Mar 13. PMID: 24622688. [DOI] [PubMed] [Google Scholar]
- 115.Dubynska H.M., Pryimenko N.O., Kaidashev I.Р., Pokhylko V.I., Chub K.F. The role of TLR-2, TLR-3, TLR-4 genes polymorphism of grippe. Georgian Med. News. 2014 Jul-Aug;(232-233):51–55. Russian. PMID: 25214272. [PubMed] [Google Scholar]
- 116.Kirtipal N., Bharadwaj S. Interleukin 6 polymorphisms as an indicator of COVID-19 severity in humans. J. Biomol. Struct. Dyn. 2020 Jun 12:1–3. doi: 10.1080/07391102.2020.1776640. Epub ahead of print. PMID: 32490733. [DOI] [PubMed] [Google Scholar]
- 117.Saadat M. An evidence for correlation between the glutathione S-transferase T1 (GSTT1) polymorphism and outcome of COVID-19. Clin. Chim. Acta. 2020 Sep;508:213–216. doi: 10.1016/j.cca.2020.05.041. Epub 2020 May 23. PMID: 32454047; PMCID: PMC7245315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Speeckaert M.M., De Buyzere M.L., Delanghe J.R. Vitamin D binding protein polymorphism and COVID-19. J. Med. Virol. 2021 Feb;93(2):705–707. doi: 10.1002/jmv.26508. Epub 2020 Sep 28. PMID: 32918506. [DOI] [PubMed] [Google Scholar]
- 119.Karcioglu Batur L., Hekim N. The role of DBP gene polymorphisms in the prevalence of new coronavirus disease 2019 infection and mortality rate. J. Med. Virol. 2020 Aug 8 doi: 10.1002/jmv.26409. Epub ahead of print. PMID: 32770768; PMCID: PMC7436554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Singh D., Wasan H., Reeta K.H. Heme oxygenase-1 modulation: a potential therapeutic target for COVID-19 and associated complications. Free Radic. Biol. Med. 2020 Dec;161:263–271. doi: 10.1016/j.freeradbiomed.2020.10.016. Epub 2020 Oct 19. PMID: 33091573; PMCID: PMC7571447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fakhouri E.W., Peterson S.J., Kothari J., Alex R., Shapiro J.I., Abraham N.G. Genetic polymorphisms complicate COVID-19 therapy: pivotal role of HO-1 in cytokine storm. Antioxidants (Basel) 2020 Jul 18;9(7):636. doi: 10.3390/antiox9070636. PMID: 32708430; PMCID: PMC7402116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Janssen R., Walk J. Vitamin K epoxide reductase complex subunit 1 (VKORC1) gene polymorphism as determinant of differences in COVID-19-related disease severity. Med. Hypotheses. 2020 Nov;144:110218. doi: 10.1016/j.mehy.2020.110218. Epub 2020 Aug 25. PMID: 33254525; PMCID: PMC7446614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Karst M., Hollenhorst J., Achenbach J. Life-threatening course in coronavirus disease 2019 (COVID-19): is there a link to methylenetetrahydrofolic acid reductase (MTHFR) polymorphism and hyperhomocysteinemia? Med. Hypotheses. 2020 Nov;144:110234. doi: 10.1016/j.mehy.2020.110234. Epub 2020 Sep 2. PMID: 33254541; PMCID: PMC7467063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Burlacu A., Artene B., Crisan-Dabija R., Popa I.V., Covic A. Is thrombophilic genetic profile responsible for an acute ischemic stroke in a COVID-19 male patient? Clin. Appl. Thromb. Hemost. 2020 Jan-Dec;26 doi: 10.1177/1076029620967107. 1076029620967107:PMID: 33054360; PMCID: PMC7573713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Elhabyan A., Elyaacoub S., Sanad E., Abukhadra A., Elhabyan A., Dinu V. The role of host genetics in susceptibility to severe viral infections in humans and insights into host genetics of severe COVID-19: a systematic review. Virus Res. 2020 Nov;289:198163. doi: 10.1016/j.virusres.2020.198163. Epub 2020 Sep 9. PMID: 32918943; PMCID: PMC7480444. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.