Abstract
The vinegar fly Drosophila melanogaster is a pivotal model for invertebrate development, genetics, physiology, neuroscience, and disease. The whole family Drosophilidae, which contains over 4,400 species, offers a plethora of cases for comparative and evolutionary studies. Despite a long history of phylogenetic inference, many relationships remain unresolved among the genera, subgenera, and species groups in the Drosophilidae. To clarify these relationships, we first developed a set of new genomic markers and assembled a multilocus data set of 17 genes from 704 species of Drosophilidae. We then inferred a species tree with highly supported groups for this family. Additionally, we were able to determine the phylogenetic position of some previously unplaced species. These results establish a new framework for investigating the evolution of traits in fruit flies, as well as valuable resources for systematics.
Keywords: Drosophilidae, phylogenomics, systematics
Introduction
The vinegar fly Drosophila melanogaster is a well-established and versatile model system in biology (Hales et al. 2015). The story began at the start of the 20th century when the entomologist Charles Woodworth bred D. melanogaster in captivity, paving the way to William Castle’s seminal work at Harvard in 1901 (Sturtevant 1959). But it is undoubtedly with Thomas Hunt Morgan and his colleagues that D. melanogaster became a model organism in genetics (Morgan 1910). Nowadays, D. melanogaster research encompasses diverse fields, such as biomedicine (Ugur et al. 2016), developmental biology (Hales et al. 2015), growth control (Wartlick et al. 2011), gut microbiota (Trinder et al. 2017), innate immunity (Buchon et al. 2014), behavior (Cobb 2007), and neuroscience (Bellen et al. 2010).
By the mid-20th century, evolutionary biologists have widened Drosophila research by introducing many new species of Drosophilidae in comparative studies. For example, the mechanisms responsible for morphological differences of larval denticle trichomes (Sucena et al. 2003; McGregor et al. 2007), adult pigmentation (Jeong et al. 2008; Yassin, Delaney, et al. 2016), sex combs (Tanaka et al. 2009), and genital shape (Glassford et al. 2015; Peluffo et al. 2015) have been thoroughly investigated across Drosophilidae. Comparative studies brought new insights into the evolution of ecological traits, such as host specialization (Lang et al. 2012; Yassin et al. 2016), niche diversification (Chung et al. 2014), species distribution (Kellermann et al. 2009), pathogen virulence (Longdon et al. 2015), and behavior (Dai et al. 2008; Karageorgi et al. 2017).
More than 150 genomes of Drosophila species are now sequenced (Adams et al. 2000; Clark et al. 2007; Wiegmann and Richards 2018; Kim et al. 2021), allowing the comparative investigation of gene families (Sackton et al. 2007; Almeida et al. 2014; Finet et al. 2019) as well as global comparison of genome organization (Bosco et al. 2007; Bhutkar et al. 2008). For all these studies, a clear understanding of the historical relationships between species is necessary to interpret the results in an evolutionary context. A robust phylogeny is then crucial to confidently infer ancestral states, identify synapomorphic traits, and reconstruct the history of events during the evolution and diversification of Drosophilidae.
Fossil-based divergence time estimation suggest that the family Drosophilidae originated at least 30–50 Ma (Throckmorton 1975; Grimaldi 1987; Wiegmann et al. 2011). To date, the family comprises more than 4,400 species (DrosWLD-Species 2021; Available from: https://bioinfo.museum.hokudai.ac.jp/db/index.php; last accessed June 29, 2021) classified into two subfamilies, the Drosophilinae Rondani and the Steganinae Hendel. Each of these subfamilies contains several genera, which are traditionally subdivided into subgenera, and are further composed of species groups. Nevertheless, the monophyletic status of each of these taxonomic units is frequently controversial or unassessed. Part of this controversy is related to the frequent detection of paraphyletic taxa within Drosophilidae (Throckmorton 1975; Katoh et al. 2000, 2017; Robe et al. 2005; Da Lage et al. 2007; Robe, Loreto, et al. 2010; Van Der Linde et al. 2010; Russo et al. 2013; Yassin 2013; Gautério et al. 2020), although the absence of a consistent phylogenetic framework for the entire family makes it difficult to assess alternative scenarios.
Despite the emergence of the Drosophila genus as a model system to investigate the molecular genetics of functional evolution, relationships within the family Drosophilidae remain poorly supported. The first modern phylogenetic trees of this family relied on morphological characters (Throckmorton 1962, 1975, 1982), followed by a considerable number of molecular phylogenies that mainly focused on individual species groups (reviewed in Markow and O’Grady [2006], O’Grady and DeSalle [2018]). For the last decade, only a few large-scale studies have attempted to resolve the relationships within Drosophilidae as a whole. For example, supermatrix approaches brought new insights, such as the identification of the earliest branches in the subfamily Drosophilinae (Van Der Linde et al. 2010; Yassinet al. 2010), the paraphyly of the subgenus Drosophila (Sophophora) (Gao et al. 2011), the placement of Hawaiian clades (O’Grady et al. 2011; Lapoint et al. 2013; Katoh et al. 2017), and the placement of Neotropical Drosophilidae (Robe et al. 2010). Most of the aforementioned studies have suffered from limited taxon or gene sampling. Recent studies improved the taxon sampling and the number of loci analyzed (Morales-Hojas and Vieira 2012; Russo et al. 2013; Izumitani et al. 2016). To date, the most taxonomically broad study is a revision of the Drosophilidae that includes 30 genera in Steganinae and 43 in Drosophilinae, but only considering a limited number of genomic markers (Yassin 2013).
To clarify the phylogenetic relationships in the Drosophilidae, we built a comprehensive data set of 704 species that include representatives from most of the major genera, subgenera, and species groups in this family. We developed new genomic markers and compiled available ones from previously published phylogenetic studies. We then inferred well-supported trees at the group- and species-level for this family. Additionally, we were able to determine the phylogenetic position of several species of uncertain affinities. Our results establish a new framework for investigating the systematics and diversification of fruit flies and provide a valuable genomic resource for the Drosophila community.
Results and Discussion
A Multigene Phylogeny of 704 Drosophilid Species
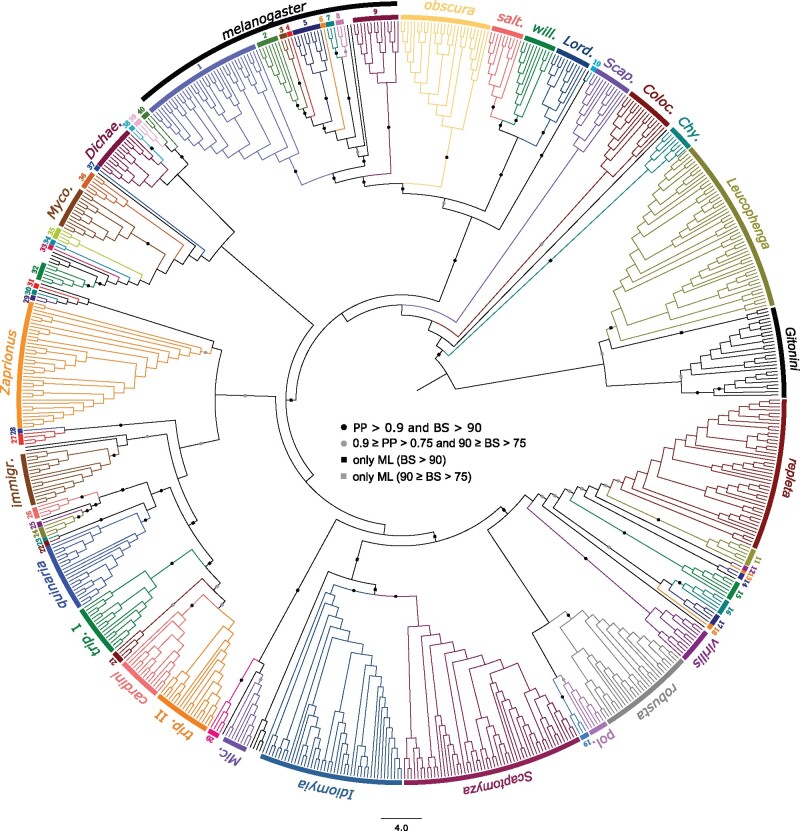
We assembled a multilocus data set of 17 genes (14,961 unambiguously aligned nucleotide positions) from 704 species of Drosophilidae. Our phylogeny recovers many of the clades or monophyletic groups previously described in the Drosophilidae (fig. 1). Although the branching of the species groups is generally well-supported, we observe that some of the deepest branches of the phylogenic tree remain poorly supported or unresolved, especially in Bayesian analyses (supplementary figs. S1 and S2, Supplementary Material online). This observation prompted us to apply a composite taxon strategy that has been used to resolve challenging phylogenetic relationships (Finet et al. 2010; Campbell and Lapointe 2011; Sigurdsen and Green 2011; Charbonnier et al. 2015; Mengual et al. 2017; Fan et al. 2020). This approach limits branch lengths in selecting slow-evolving sequences, and decreases the percentage of missing data, improving phylogenetic reconstruction for sparse data matrices (Campbell and Lapointe 2009). We defined 63 composite groups as the monophyletic groups identified in the 704-taxon analysis (fig. 1 and supplementary table S1, Supplementary Material online), and added these to the sequences of 20 other ungrouped taxa to perform additional phylogenetic evaluations. The overall bootstrap values and posterior probabilities were higher for the composite tree (fig. 2A and supplementary figs. S3 and S4, Supplementary Material online). In addition, we applied the summary method ASTRAL to our composite data set to infer a species tree from a collection of input trees. However, the resulting tree is less resolved than the one obtained by concatenation (supplementary fig. S5, Supplementary Material online).
Fig. 1.
Phylogram of the 704-taxon analyses. IQ-TREE maximum-likelihood analysis was conducted under the GTR+R+FO model. Support values obtained after 100 bootstrap replicates are shown for selected supragroup branches, and infragroup branches within the melanogaster group (all the support values are shown online). Black dots indicate support values of PP>0.9 and BP>90; gray dots 0.9 ≥ PP>0.75 and 90 ≥ BP>75; black squares only BP>90; gray squares only 90 ≥ BP>75. Scale bar indicates the number of changes per site. Groups and subgroups are numbered or abbreviated as follows: (1) montium, (2) takahashii sgr, (3) suzukii sgr, (4) eugracilis sgr, (5) melanogaster sgr, (6) ficusphila sgr, (7) elegans sgr, (8) rhopaloa sgr, (9) ananassae, (10) Collessia, (11) mesophragmatica, (12) dreyfusi, (13), coffeata, (14) canalinea, (15) nannoptera, (16) annulimana, (17) flavopilosa, (18) flexa, (19) angor, (20) Dorsilopha, (21) ornatifrons, (22) histrio, (23) macroptera, (24) testacea, (25) bizonata, (26) funebris, (27) Samoaia, (28) quadrilineata sgr, (29) Liodrosophila, (30) Hypselothyrea, (31) Sphaerogastrella, (32) Zygothrica I, (33) Paramycodrosophila, (34) Hirtodrosophila III, (35) Hirtodrosophila II, (36) Hirtodrosophila I, (37) Dettopsomyia, (38) Mulgravea, (39) Hirtodrosophila IV, (40) Zygothrica II, Chy, Chymomyza; Colo, Colocasiomyia; Dichae, Dichaetophora; immigr, immigrans; Lord, Lordiphosa; Mic, Microdrosophila; Myco, Mycodrosophila; pol, polychaeta; salt, saltans; Scap, Scaptodrosophila; trip, tripunctata; will, willistoni.
Fig. 2.
(A) Phylogram of the 83-taxon analyses. The overall matrix represents 14,961 nucleotides and 83 taxa, including 63 composite ones. Support values obtained after 100 bootstrap replicates and Bayesian posterior probabilities are shown for selected branches and mapped onto the ML topology (all the support values are shown in supplementary fig. S1, Supplementary Material online). The dotted line indicates that the placement of Dettopsomyia varies between ML and Bayesian trees. Scale bar indicates the number of changes per site. (B–H) Photos of species of particular interest in this article. (B) Drosophila oshimai female (top) and male (bottom) (Japan, courtesy of Japan Drosophila Database), (C and D) Collessia kirishimana (Japan, courtesy of Masafumi Inoue), (E and F) Drosophila annulipes (Japan, courtesy of Yasuo Hoshino), (G) Drosophila pruinosa (São Tomé, courtesy of Stéphane Prigent), (H) Drosophila adamsi (Cameroun, courtesy of Stéphane Prigent).
Incongruence among phylogenetic markers can be related to incomplete lineage sorting, introgression, hybridization, or other processes and can be detrimental to accurate species tree reconstruction (Jeffroy et al. 2006; Kapli et al. 2020). In order to estimate the presence of incongruent signal in our data set, we first investigated the qualitative effect of single marker removal on the topology of the composite tree (supplementary fig. S6, Supplementary Material online). We found the overall topology is very robust to marker sampling, with only a few minor changes for each data set. For instance, the melanogaster subgroup sometimes clusters with the eugracilis subgroup instead of branching off prior to the eugracilis subgroup (fig. 2 and supplementary fig. S6, Supplementary Material online). The position of the genus Dettopsomyia and that of the angor and histrio groups is also very sensitive to single marker removal, which could explain the low support values obtained (fig. 2 and supplementary fig. S6, Supplementary Material online). To a lesser extent, the position of Drosophila fluvialis can vary as well depending on the removed marker (fig. 2 and supplementary fig. S6, Supplementary Material online). We also quantitatively investigated the incongruence present in our data set by calculating genealogical concordance. The gene concordance factor is defined as the percentage of individual gene trees containing that node for every node of the reference tree. Similarly, the fraction of nodes supported by each marker can be determined. The markers we developed in this study show concordance rates ranging from 46.2% to 90.9% (fig. 3 and table 1). With an average concordance rate of 65%, these new markers appear as credible phylogenetic markers, without significantly improving the previous markers (average concordance rate of 64.8%).
Fig. 3.

Concordance versus mutational saturation of the phylogenetic markers. The y axis indicates the percentage of concordant nodes, and the x axis indicates the saturation level. In comparison with published markers (black dots), the markers developed in this study (orange dots) generally show moderate saturation levels and satisfying concordance.
Table 1.
Data Set Statistics
| Name | No. Sequences | No. Sites | Informative Sites (%) | Inferred Distance | Observed Distance | Saturation | No. Concording Nodes | No. Missing Nodes | Concordance (%) |
|---|---|---|---|---|---|---|---|---|---|
| 28S | 49/83 | 848 | 18.4 | 0.200 | 0.189 | 0.700 | 25/80 | 44 | 69.4 |
| Adh | 53/83 | 724 | 54.4 | 0.886 | 0.331 | 0.430 | 28/80 | 35 | 62.2 |
| Amyrel | 48/83 | 1475 | 53.5 | 2.458 | 0.545 | 0.290 | 18/80 | 44 | 50.0 |
| COI | 51/83 | 1438 | 33.8 | 1.119 | 0.666 | 0.191 | 35/80 | 40 | 87.5 |
| COII | 57/83 | 688 | 37.8 | 1.004 | 0.169 | 0.185 | 40/80 | 33 | 85.1 |
| Gpdh | 26/83 | 859 | 35.0 | 0.784 | 0.286 | 0.400 | 9/80 | 64 | 56.3 |
| Sod | 22/83 | 574 | 49.3 | 1.072 | 0.333 | 0.373 | 4/80 | 68 | 33.3 |
| Xdh | 19/83 | 2088 | 42.4 | 0.919 | 0.314 | 0.368 | 9/80 | 68 | 75.0 |
| Ddc | 52/83 | 1162 | 42.3 | 1.003 | 0.262 | 0.358 | 27/80 | 39 | 65.9 |
| Dll | 56/83 | 377 | 30.8 | 0.629 | 0.229 | 0.463 | 40/80 | 36 | 90.9 |
| eb | 67/83 | 891 | 46.7 | 1.247 | 0.318 | 0.380 | 32/80 | 21 | 54.2 |
| en | 51/83 | 1119 | 51.1 | 1.009 | 0.307 | 0.371 | 18/80 | 41 | 46.2 |
| eve | 66/83 | 806 | 48.6 | 1.083 | 0.303 | 0.367 | 40/80 | 22 | 69.0 |
| hh | 63/83 | 486 | 62.6 | 1.203 | 0.352 | 0.400 | 29/80 | 27 | 54.7 |
| Notum | 51/83 | 672 | 62.6 | 1.005 | 0.352 | 0.417 | 18/80 | 45 | 51.4 |
| ptc | 60/83 | 430 | 55.8 | 1.076 | 0.323 | 0.413 | 42/80 | 29 | 82.4 |
| wg | 57/83 | 324 | 51.5 | 1.223 | 0.321 | 0.352 | 33/80 | 33 | 70.2 |
Multiple substitutions at the same position is another classical bias in phylogenetic reconstruction, capable of obscuring the genuine phylogenetic signal (Jeffroy et al. 2006). We quantified the mutational saturation for each phylogenetic marker. On an average, the newly developed markers are moderately saturated (fig. 3, supplementary fig. S7, Supplementary Material online, and table 1). These markers are indeed less saturated than the Amyrel, COI, and COII genes that have been commonly applied for phylogenetic inference in Drosophilidae (Baker and Desalle 1997; O’Grady et al. 1998, 2011; Remsen and O’Grady 2002; Bonacum et al. 2005; Da Lage et al. 2007; Robe et al. 2010; Gao et al. 2011; Russo et al. 2013; Yassin 2013).
In the following sections of the article, we will highlight and discuss some of the most interesting results we obtained. Our analyses either confirm or challenge previous phylogenies and shed light on several unassessed questions, contributing to an emerging picture of phylogenetic relationships in Drosophilidae.
The Steganinae Subfamily
To avoid long-branch attraction due to some divergent steganine sequences, we compiled a more specific and comprehensive data set from 164 taxa of Steganinae (vs. 80 taxa in the 704-taxon analysis). Whereas morphology-based studies suggest the monophyly of Steganinae (Okada 1989; Grimaldi 1990), molecular phylogenetic have led to contradictory results (Remsen and O’Grady 2002; Otranto et al. 2008; Van Der Linde et al. 2010; Russo et al. 2013; Yassin 2013). Our study identifies the Steganinae as monophyletic for both data sets (fig. 1 and supplementary fig. S8, Supplementary Material online) and supports a recent phylogenomic study of Steganinae (Dias et al. 2020). The topology within the Steganinae substantially differs from the division of the subfamily into two monophyletic tribes: Steganini and Gitonini (Yassin 2013). Our study does not recover the monophyly of the genera Leucophenga and Parastegana, only due to the placement of the two species Leucophenga maculata and Parastegana femorata. Future studies are needed to disentangle possible contamination and true phylogenetic position. We also found the branching of some Colocasiomyia species within the Steganinae (supplementary fig. S8, Supplementary Material online). This finding, which challenges previous published cladograms of Colocasiomyia (Grimaldi 1991; Sultana et al. 2006) and our 704-taxon analysis (fig. 1), is likely an artifact of reconstruction.
The Sophophora Subgenus and Closely Related Taxa
We found that the obscura–melanogaster clade is the sister group of the lineages formed by the Neotropical saltans and willistoni groups, and the Lordiphosa genus (bootstrap percentage [BP]=73) (fig. 2A and supplementary fig. S3, Supplementary Material online). Thus, our study recovers the relationship between the groups of the Sophophora subgenus (Gao et al. 2011; Russo et al. 2013; Yassin 2013) and supports the paraphyletic status of Sophophora regarding Lordiphosa (Katoh et al. 2000). However, we noted substantial changes within the topology presented for the melanogaster species group. The original description of Drosophila oshimai noted a likeness to Drosophila unipectinata, thus classifying D. oshimai into the suzukii species subgroup (Choo and Nakamura 1973). The phylogenetic tree we obtained does not support this classification (fig. 2A). It rather defines D. oshimai as the representative of a new subgroup (Bayesian posterior probability [PP]=1, BP=96) that diverged immediately after the split of the montium group. The position of D. oshimai therefore challenges the monophyly of the suzukii subgroup. Interestingly, the paraphyly of the suzukii subgroup has also been suggested in previous studies (Lewis et al. 2005; Russo et al. 2013). Another interesting case is the positioning of the denticulata subgroup that has never been tested before. Our analysis convincingly places its representative species Drosophila denticulata as the fourth subgroup to branch off within the melanogaster group (PP=1, BP=82). Last, the topology within the montium group drastically differs from the most recent published phylogeny (Conner et al. 2021). Despite substantial sampling in the subgenus Sophophora, our study would benefit from the addition of representatives of the dentissima, dispar, fima, populi, setifemur groups, as well as the genus Zapriothrica, to draw a more complete picture of the relationships within Sophophora.
The genus Collessia comprises five described species that can be found in Australia, Japan, and Sri Lanka, but its phylogenetic status was so far quite ambiguous (Okada 1967, 1988; Bock 1982). In addition, Grimaldi (1990) proposed that Tambourella ornata should belong to the genus Collessia. These two genera are similar in the wing venation and pigmentation pattern (Okada 1984).
Our phylogenetic analysis identifies Collessia as sister group to the species Hirtodrosophila duncani (PP=1, BP=100). Interestingly, this branching is also supported by morphological similarities shared between the genera Collessia and Hirtodrosophila. The species Collessia kirishimana and Collessia hiharai were indeed initially described as Hirtodrosophila species (Okada 1967) but later assigned to the genus Collessia (Okada 1984), based on the similarity in wing coloration with Collessia superba. However, the affiliation of Collessia kirishimana to Collessia would require further investigations. The species H. duncani is morphologically disparate for Hirtodrosophila and might be removed from this genus in the future (Grimaldi 2018). The clade Collessia–H. duncani is sister to the Sophophora–Lordiphosa lineage in the ML inference (BP=100) but to the Neotropical Sophophora–Lordiphosa clade in the Bayesian inference (PP=0.92).
The Early Lineage of Microdrosophila and Dorsilopha
Within the tribe Drosophilini, all the remaining taxa (composite taxa+ungrouped species) other than those of the Sophophora–Lordiphosa and Collessia–H. duncani lineage form a large clade (PP=1, BP=100). Within this clade, the genus Microdrosophila, the subgenus Dorsilopha, and Drosophila ponera group into a lineage (PP=0.97, BP=82) that appears as an early offshoot in our composite tree (fig. 2), reminiscent of the placement of Dorsilopha found in Yassin (2013). It is nevertheless noteworthy that the placement of the Dorsilopha+Microdrosophila clade differs in our supermatrix tree (fig. 1) and resembles the placement of Microdrosophila in Yassin (2013). In spite of scarce genomic data, we added the genus Styloptera which has been previously found close to the genus Dorsilopha (Yassin 2013). The position of Styloptera varies according to the analysis (supplementary fig. S9 and tree files, Supplementary Material online) without grouping with Dorsilopha. Generating genomic data for the genus Styloptera will be necessary to unambiguously place this genus. Drosophila ponera is an enigmatic species collected in La Réunion (David and Tsacas 1975), whose phylogenetic position has never or rarely been investigated. In spite of morphological similarities with the quinaria group, the authors suggested to keep D. ponera as ungrouped with respect to a divergent number of respiratory egg filaments (David and Tsacas 1975). To our knowledge, our study is the first attempt to phylogenetically position this species. We found that D. ponera groups with the Dorsilopha subgenus (PP=0.99, BP=75) within this early-diverging lineage.
The Hawaiian Drosophilid Clade and the Siphlodora Subgenus
The endemic Hawaiian Drosophilidae contain approximately 1,000 species that split into the genera Idiomyia (or Hawaiian Drosophila according to Grimaldi [1990]) and the genus Scaptomyza (O’Grady et al. 2009). Generally considered as sister to the Siphlodora subgenus (Robe, Loreto, et al. 2010; Russo et al. 2013; Yassin 2013), these lineages represent a remarkable framework to investigate evolutionary radiation and subsequent diversification of morphology (Stark and O’Grady 2010), pigmentation (Edwards et al. 2007), ecology (Magnacca et al. 2008), and behavior (Kaneshiro 2001). Although the relationships within the Siphlodora clade are generally in agreement with previous studies (Tatarenkov et al. 2001; Robe et al. 2010; Russo et al. 2013; Yassin 2013), its sister clade does not seem to be restricted to the Hawaiian Drosophilidae. In fact, according to our phylogenies, it also includes at least four other species of the genus Drosophila (fig. 2A and supplementary fig. S3 and tree files, Supplementary Material online). We propose that this broader clade, rather than the Hawaiian clade sensu stricto, should be seen as a major lineage of Drosophilidae.
This broader clade is strongly supported (PP=1, BP=100) and divided into two subclades, one comprises the genera Idiomyia and Scaptomyza (PP=0.99, BP=97) and the other includes Drosophila annulipes, Drosophila adamsi, Drosophila maculinotata, and Drosophila nigrosparsa (PP=0.99, BP=75). The latter subclade, also suggested by Katoh et al. (2007) and Russo et al. (2013), is interesting with respect to the origin of Hawaiian drosophilids. Of the four component species, D. annulipes was originally described as a member of the subgenus Spinulophila, which was synonymized with Drosophila and currently corresponds to the immigrans group, although Wakahama et al. (1983) and Zhang and Toda (1992) cast doubt on its systematic position. The fact that D. annulipes does not belong to the immigrans species group implies that the subgenus Drosophila is paraphyletic rather than polyphyletic. As for D. adamsi, Da Lage et al. (2007) suggested it may be close to the Idiomyia–Scaptomyza clade, which is supported by our analyses. On the other hand, Prigent et al. (2013) based on morphological characters and Prigent et al. (2017) based on DNA barcoding have proposed that D. adamsi defines a new species group along with Drosophila acanthomera and an undescribed species. Drosophila adamsi resembles D. annulipes in the body color pattern (fig. 2F, E, and H), suggesting their close relationship: Adams (1905) described, “mesonotum with five longitudinal, brown vittae, the central one broader than the others and divided longitudinally by a hair-like line, …; scutellum yellow, with two sublateral, brownish lines, …; pleurae with three longitudinal brownish lines,” for Drosophila quadrimaculataAdams, 1905, which is a homonym of Drosophila quadrimaculata Walker, 1856 and has been replaced with the new specific epithet “adamsi” by Wheeler (1959). Another species, D. nigrosparsa, belongs to the nigrosparsa species group, along with D. secunda, D. subarctica, and D. vireni (Bächli et al. 2004). Moreover, Máca (1992) pointed out the close relatedness of D. maculinotata to the nigrosparsa group. It is noteworthy that the nigrosparsa species group is thought to be basal to Siphlodora in regard to the morphology of male genitalia (Yassin 2013).
The Drosophila Subgenus and Closely Related Taxa
Although general relationships within the Drosophila subgenus closely resemble those recovered by previous studies (Hatadani et al. 2009; Robe et al. 2010; Robe et al. 2010; Izumitani et al. 2016), there are some outstanding results related to other genera or poorly studied Drosophila species.
Samoaia is a small genus of seven described species endemic to the Samoan Archipelago (Malloch 1934; Wheeler and Kambysellis 1966), particularly studied for their body and wing pigmentation (Dufour et al. 2020). In our analysis, the genus Samoaia is found to group with the quadrilineata species subgroup of the immigrans group. This result is similar to conclusions formulated by some previous studies (Tatarenkov et al. 2001; Robe et al. 2010; Yassin et al. 2010; Yassin 2013), but differs from other published phylogenies in which Samoaia is sister to most other lineages in the subgenus Drosophila (Russo et al. 2013). It is noteworthy that our sampling is the most substantial with four species of Samoaia.
The two African species Drosophila pruinosa and Drosophila pachneissa, which were assigned to the loiciana species complex because of shared characters such as a glaucous-silvery frons and rod-shaped surstylus (Tsacas 2002), are placed together with the immigrans group (PP=1, BP=94). In previous large-scale analyses, D. pruinosa was suggested to group with Drosophila sternopleuralis into the sister clade of the immigrans group (Da Lage et al. 2007; Russo et al. 2013).
Among other controversial issues, the phylogenetic position of Drosophila aracea was previously found to markedly change according to the phylogenetic reconstruction methods (Da Lage et al. 2007). This anthophilic species lives in Central America (Heed and Wheeler 1957). Its name comes from the behavior of females that lay eggs on the spadix of plants in the family Araceae (Heed and Wheeler 1957; Tsacas and Chassagnard 1992). Our analysis places D. aracea as the sister taxon of the bizonata–testacea clade with high confidence (PP=1, BP=85). No occurrence of flower-breeding behavior has been reported in the bizonata–testacea clade, reinforcing the idea that D. aracea might have recently evolved from a generalist ancestor (Tsacas and Chassagnard 1992).
The Zygothrica Genus Group
The fungus-associated genera Hirtodrosophila, Mycodrosophila, Paraliodrosophila, Paramycodrosophila, and Zygothrica contain 449 identified species (DrosWLD-Species 2021; https://bioinfo.museum.hokudai.ac.jp/db/index.php; last accessed June 29, 2021) and have been associated with the Zygothrica genus group (Grimaldi 1990). Although the Zygothrica genus group was recurrently recovered as paraphyletic (Da Lage et al. 2007; Van Der Linde et al. 2010; Russo et al. 2013; Yassin 2013), two recent studies suggest, on the contrary, its monophyly (Gautério et al. 2020; Zhang et al. 2021). Our study does not support the monophyly of the Zygothrica genus group in virtue of the polyphyletic status of Hirtodrosophila and Zygothrica: some representatives (e.g., H. duncani) cluster with Collessia, whereas others (e.g., Hirtodrosophila IV and Zygothrica II) appear closely related to the genera Dichaetophora and Mulgravea. Furthermore, the placement of the Zygothrica genus group recovered in our study also differs from some previous estimates. In fact, the broadly defined Zygothrica genus group, which includes Dichaetophora and Mulgravea (PP=0.95, BP=64), appears as sister to the clade composed of the subgenus Drosophila and the Hypselothyrea/Liodrosophila+Sphaerogastrella+Zaprionus clade (PP=1, BP=56) (fig. 2A and supplementary fig. S3, Supplementary Material online). This placement is similar to the ones obtained in different studies (Van Der Linde et al. 2010; Russo et al. 2013), but contrasts with the close relationship of the Zygothrica genus group to the subgenus Siphlodora+Idiomyia/Scaptomyza proposed in two recent studies (Gautério et al. 2020; Zhang et al. 2021). Given the moderate bootstrap value, the exact status of the Zygothrica genus group remains as an open question.
Furthermore, within the superclade of the broadly defined Zygothrica genus group (figs. 1 and 2A), the genus Hirtodrosophila is paraphyletic and split into four independent lineages, reinforcing previous suggestions based on multilocus approaches (Van Der Linde et al. 2010; Gautério et al. 2020; Zhang et al. 2021). This also occurred with the genus Zygothrica, which split into two independent clades (fig. 2A). The leptorostra subgroup (Zygothrica II) clusters with the subgroup Hirtodrosophila IV (PP=1, BP=100), whereas the Zygothrica I subgroup clusters with the species Hirtodrosophila levigata (PP=0.99, BP=98).
DrosoPhyla: A Powerful Tool for Systematics
Besides bringing an updated and improved phylogenetic framework to Drosophilidae, our approach also addresses several questions that were previously unassessed or controversial at the genus, subgenus, group, or species level. We are therefore confident that it may become a powerful tool for future drosophilid systematics. According to diversity surveys (O’Grady and DeSalle 2018), ∼25% of drosophilid species remain to be discovered, potentially a thousand species to place in the tree of Drosophilidae. Although whole-genome sequencing is becoming widespread, newly discovered species often come down to a few specimens pinned or stored in ethanol—nonoptimal conditions for subsequent genome sequencing and whole-genome studies (Korlević et al. 2021). An alternative promising approach to PCR is exome capture using baits to hybridize to genomic regions of interest, which has been used with other insects (Branstetter et al. 2017). Nevertheless, based on a few short genomic markers, our approach is compatible with taxonomic work, and gives good resolution.
Materials and Methods
Taxon Sampling
The species used in this study were sampled from different locations throughout the world (supplementary table S1, Supplementary Material online). The specimens were field-collected by the authors, purchased from the National Drosophila Species Stock Center (http://blogs.cornell.edu/drosophila/; last accessed January 2021) and the Kyoto Stock Center (https://kyotofly.kit.jp/cgi-bin/stocks/index.cgi; last accessed January 2021), or obtained from colleagues. Individual flies were preserved in 100% ethanol and identified based on morphological characters.
Data Collection
Ten genomic markers were amplified by PCR using degenerate primers developed for the present study (table 2). Genomic DNA was extracted from a single adult fly as follows: the fly was placed in a 0.5-ml tube and mashed in 50 µl of squishing buffer (Tris–HCl pH = 8.2 10 mM, EDTA 1 mM, NaCl 25 mM, proteinase K 200 µg/ml) for 20–30 s, the mix was incubated at 37 °C for 30 min, then the proteinase K was inactivated by heating at 95 °C for 1–2 min. A volume of 1 µl was used as template for PCR amplification. Nucleotide sequences were also retrieved from the NCBI database for the five nuclear markers 28S ribosomal RNA (28S), alcohol dehydrogenase (Adh), glycerol-3-phosphate dehydrogenase (Gpdh), superoxide dismutase (Sod), xanthine dehydrogenase (Xdh), and the two mitochondrial markers cytochrome oxidase subunit 1 (COI) and cytochrome oxidase subunit 2 (COII). The sequences reported in this article have been deposited in GenBank under specific accession numbers: Amyrel (MW392482–MW392524), Ddc (MW403139–MW403307), Dll (MW403308–MW403483), eb (MW415022–MW415267), en (MW418945–MW419079), eve (MW425034–MW425273), hh (MW385549–MW385782), Notum (MW429853–MW430003), ptc (MW442160–MW442361), and wg (MW392301–MW392481).
Table 2.
List of PCR Primers Used in This Study
| Genomic Locus | Primer | Primer Sequence (5′–3′) | Annealing (°C) | Size (bp) | References |
|---|---|---|---|---|---|
| Amyrel | zone2bis | GTAAATNGGNNCCACGCGAAG | 53 | 1,000 | Da Lage et al. (2007) |
| relrev+ | GTTCCCCAGCTCTGCAGCC | ||||
| reludir | TGGATGCNGCCAAGCACATGGC | 1,000 | |||
| relavbis | GCATTTGTACCGTTTGTGTCGTTATCG | ||||
| Distal-less | dll-F | TGATACCAATACTGSGGCACATA | 56 | 600 | This study |
| dll-R | ATGATGAARGCMGCTCAGGG | ||||
| Dopa decarboxylase | ddc-F | TTCCASGAGTACTCCATGTCCTCG | 58 | 1,200 | This study |
| ddc-R | GGCAGGATGTKATGAAGGACATTGAG | ||||
| ebony | eb-F | CCCATSACCTCKGTGGAGCCGTA | 59 | 900 | This study |
| eb-R | CTGCATCGCATCTTYGAGGAGCA | ||||
| engrailed | en-F | AATCAGCGCCCAGTCCACCAG | 65 | 1,500 | This study |
| en-R | GCCACATCTCGTTCTTGCCGC | ||||
| even-skipped | eve-F | TGCCTVTCCAGTCCRGAYAACTC | 55 | 1,000 | This study |
| eve-R | TACGCCTCAGTCTTGTAGGG | ||||
| hedgehog | hh-F | ACCTTGTABARGGCATTGGCATACCA | 56 | 600 | This study |
| hh-R | ATCGGWGATCGDGTGCTRAGCATG | ||||
| Notum | not-F | TGGAACTAYATHCAYGADATGGGCGG | 56 | 800 | This study |
| not-R | GAGCAGYTCVAGRAADCGCATCTC | ||||
| patched | ptc-F1 | ACCCAGCTGCGCATSAGRAAGG | 54 | 600 | This study |
| ptc-F2 | ACCCAGCTGCGCATSAGRAACG | ||||
| ptc-R | GCTGACGGCSGCSTATGCGG | ||||
| wingless | wg-F | AGCACGTYCARGCRGAGATGCG | 58 | 400 | This study |
| wg-R | ACTGTTKGGCGAYGGCATRTTGGG |
Phylogenetic Reconstruction
Alignments for each individual gene were generated using MAFFT 7.45 (Katoh and Standley 2013) assuming a gap opening penalty of 1.53 and other default parameters (no offset and extra round of refinement). Unreliably aligned positions were excluded using trimAl with parameters -gt 0.5 and -st 0.001 (Capella-Gutiérrez et al. 2009). The possible contamination status was verified by inferring independent trees for each gene using RAxML 8.2.4 under the GTR+Γ4 model (Stamatakis 2014). Thus, any sequence leading to the suspicious placement of a taxonomically well-assigned species, in terms of both topology and bootstrap value, was removed from the data set. Moreover, almost identical sequences leading to very short tree branches were carefully examined and excluded if involving nonclosely related taxa. In-house Python scripts were used to concatenate the aligned and filtered sequences, and the resulting data set was used for phylogenetic reconstruction. Maximum-likelihood (ML) searches were performed using IQ-TREE 2.0.6 (Minh, Schmidt, et al. 2020) under the GTR model, with the FreeRate model of rate heterogeneity across sites with four categories, and ML estimation of base frequencies from the data (GTR+R+FO). The edge-linked proportional partition model was used with one partition for each gene.
Composite Taxa
This strategy started from clustering the species by unambiguous monophyletic genera, groups, or subgroups identified in the 704-taxon analysis. After this, the least diverging sequence or species recovered for each taxonomic unit for each marker was selected to ultimately yield a unique composite taxon by concatenation. The composite matrix was also used for conducting ML and Bayesian phylogenetic inference using IQ-TREE under a partitioned GTR+R+FO model (parameters: -m GTR+FO+R -B 1000 -bnni -p), and PhyloBayes under a GTR+Γ model (parameters: -ncat 1 -gtr) (Lartillot et al. 2009), respectively.
Saturation and Concordance Analysis
For each marker gene, the saturation was computed by performing a simple linear regression of the percent identity for each pair of taxa (observed distance) onto the ML patristic distance (inferred distance) (Philippe et al. 1994) estimated using the ETE 3 library (Huerta-Cepas et al. 2016). We also calculated per gene and per site concordance factors using IQ-TREE under the GTR+R+FO model as recently described (Minh, Hahn, et al. 2020). We also applied ASTRAL to estimate species tree from individual species tree, using default parameters and the same input single gene trees (Zhang et al. 2018).
Supplementary Material
Supplementary data are available at Genome Biology and Evolution online.
Supplementary Material
Acknowledgments
We thank Jean-Luc Da Lage, Beatriz Goni, John Jaenike, Louis Bernard Klaczko, Adriana Ludwig, Suzana Vaz, and Carlos Vilela for providing fly specimens. We thank Virginie Orgogozo and Noah Whiteman for giving early access to the genome of Drosophila pachea and Scaptomyza flava, respectively. We thank Masafumi Inoue, Stéphane Prigent, Yasuo Hoshino, and the Japan Drosophila Database for providing photos. We thank Amir Yassin for fruitful discussions and comments on the manuscript. We thank the Sean Carroll laboratory for discussions and financial support. This article is dedicated to the memory of the French biologist Jean David and his great legacy to the biology of Drosophila.
Author Contributions
C.F. and H.D.D. initiated the project. M.J.T. provided most of the specimens. C.F. and F.M. established the methodological approaches. The generation of new sequences is primarily attributable to C.F., V.A.K., H.D.D., then to most authors of the article. C.F. gathered and formatted the data. F.M. conducted all analyses. C.F., M.J.T., L.J.R., and F.M. wrote the first version of the manuscript, and all authors contributed edits and further elaborations.
Data Availability
The data underlying this article are available on Zenodo (10.5281/zenodo.5091961).
Literature Cited
- Adams CF.1905. Diptera Africana, I. Kansas Univ Sci Bull. 3:149–188. [Google Scholar]
- Adams MD, et al. 2000. The genome sequence of Drosophila melanogaster. Science 287:2185–2195. [DOI] [PubMed] [Google Scholar]
- Almeida FC, Sánchez-Gracia A, Campos JL, Rozas J.. 2014. Family size evolution in Drosophila chemosensory gene families: a comparative analysis with a critical appraisal of methods. Genome Biol Evol. 6(7):1669–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BächliG, , Vilela CR, Escher AS, Saura A. . 2004. The Drosophilidae (Diptera) of Fennoscandia and Denmark. Fauna Entomologica Scandinavica. 39:1–362. [Google Scholar]
- Baker RH, Desalle R.. 1997. Multiple sources of character information and the phylogeny of Hawaiian Drosophilids. Syst Biol. 46(4):654–673. [DOI] [PubMed] [Google Scholar]
- Bellen HJ, Tong C, Tsuda H.. 2010. 100 years of Drosophila research and its impact on vertebrate neuroscience: a history lesson for the future. Nat Rev Neurosci. 11(7):514–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhutkar A, et al. 2008. Chromosomal rearrangement inferred from comparisons of 12 Drosophila genomes. Genetics 179(3):1657–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock I.1982. Drosophilidae of Australia V. Remaining genera and synopsis (Insecta: Diptera). Aust J Zoo Supps. 30(89):1–164. [Google Scholar]
- Bonacum J, O’Grady PM, Kambysellis M, DeSalle R.. 2005. Phylogeny and age of diversification of the Planitibia species group of the Hawaiian Drosophila. Mol Phylogenet Evol. 37(1):73–82. [DOI] [PubMed] [Google Scholar]
- Bosco G, Campbell P, Leiva-Neto JT, Markow TA.. 2007. Analysis of Drosophila species genome size and satellite DNA content reveals significant differences among strains as well as between species. Genetics 177(3):1277–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branstetter MG, et al. 2017. Phylogenomic insights into the evolution of stinging wasps and the origins of ants and bees. Curr Biol. 27(7):1019–1025. [DOI] [PubMed] [Google Scholar]
- Buchon N, Silverman N, Cherry S.. 2014. Immunity in Drosophila melanogaster – from microbial recognition to whole-organism physiology. Nat Rev Immunol. 14(12):796–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell V, Lapointe FJ.. 2009. The use and validity of composite taxa in phylogenetic analysis. Syst Biol. 58(6):560–572. [DOI] [PubMed] [Google Scholar]
- Campbell V, Lapointe FJ.. 2011. Retrieving a mitogenomic mammal tree using composite taxa. Mol Phylogenet Evol. 58(2):149–156. [DOI] [PubMed] [Google Scholar]
- Capella-Gutiérrez S, Silla-Martínez JM, Gabaldón T.. 2009. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25(15):1972–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbonnier S, et al. 2015. Phylogeny of fossil and extant glypheid and litogastrid lobsters (Crustacea, Decapoda) as revealed by morphological characters. Cladistics 31(3):231–249. [DOI] [PubMed] [Google Scholar]
- Choo J, Nakamura K.. 1973. On a new species of Drosophila (Sophophora) from Japan (Diptera). Kontyû 41:305–306. [Google Scholar]
- Chung H, et al. 2014. A single gene affects both ecological divergence and mate choice in Drosophila. Science 343(6175):1148–1151. [DOI] [PubMed] [Google Scholar]
- Clark AG, et al. 2007. Evolution of genes and genomes on the Drosophila phylogeny. Nature 450(7167):203–218. [DOI] [PubMed] [Google Scholar]
- Cobb M.2007. A gene mutation which changed animal behaviour: Margaret Bastock and the yellow fly. Anim Behav. 74(2):163–169. [Google Scholar]
- Conner WR, et al. 2021. A phylogeny for the Drosophila montium species group: a model clade for comparative analyses. Mol Phylogenet Evol. 158:107061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai H, et al. 2008. The evolution of courtship behaviors through the origination of a new gene in Drosophila. Proc Natl Acad Sci U S A. 105(21):7478–7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Lage JL, et al. 2007. A phylogeny of Drosophilidae using the Amyrel gene: questioning the Drosophila melanogaster species group boundaries. J Zool Syst. 45(1):47–63. [Google Scholar]
- David J, Tsacas L.. 1975. Les Drosophilidae (Diptera) de l’Ile de la Réunion et de l’Ile Maurice. I. Deux nouvelles espèces du genre Drosophila. Bull Mens Soc Linn Lyon. 44(5):134–143. [Google Scholar]
- Dias GR, Dupim EG, Vanderlinde T, Mello B, Carvalho AB.. 2020. A phylogenomic study of Steganinae fruit flies (Diptera: Drosophilidae): strong gene tree heterogeneity and evidence for monophyly. BMC Evol Biol. 20(1):141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour HD, Koshikawa S, Finet C.. 2020. Temporal flexibility of gene regulatory network underlies a novel wing pattern in flies. Proc Natl Acad Sci U S A. 117(21):11589–11596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards KA, Doescher LT, Kaneshiro KY, Yamamoto D.. 2007. A database of wing diversity in the Hawaiian Drosophila. PLoS One 2(5):e487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L, et al. 2020. Phylogenetic analyses with systematic taxon sampling show that mitochondria branch within Alphaproteobacteria. Nat Ecol Evol. 4(9):1213–1219. [DOI] [PubMed] [Google Scholar]
- Finet C, Slavik K, Pu J, Carroll SB, Chung H.. 2019. Birth-and-death evolution of the fatty acyl-CoA reductase (FAR) gene family and diversification of cuticular hydrocarbon synthesis in Drosophila. Genome Biol Evol. 11(6):1541–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finet C, Timme RE, Delwiche CF, Marlétaz F.. 2010. Multigene phylogeny of the green lineage reveals the origin and diversification of land plants. Curr Biol. 20(24):2217–2222. [DOI] [PubMed] [Google Scholar]
- Gao JJ, Hu YG, Toda MJ, Katoh T, Tamura K.. 2011. Phylogenetic relationships between Sophophora and Lordiphosa, with proposition of a hypothesis on the vicariant divergences of tropical lineages between the Old and New Worlds in the family Drosophilidae. Mol Phylogenet Evol. 60(1):98–107. [DOI] [PubMed] [Google Scholar]
- Gautério TB, Machado S, Loreto EL da S, Gottschalk MS, Robe LJ.. 2020. Phylogenetic relationships between fungus-associated Neotropical species of the genera Hirtodrosophila, Mycodrosophila and Zygothrica (Diptera, Drosophilidae), with insights into the evolution of breeding sites usage. Mol Phylogenet Evol. 145:106733. [DOI] [PubMed] [Google Scholar]
- Glassford WJ, et al. 2015. Co-option of an ancestral Hox-regulated network underlies a recently evolved morphological novelty. Dev Cell. 34(5):520–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi D.1987. Amber fossil Drosophilidae (Diptera), with particular reference to the Hispaniolan taxa. Am Museum Novit. 2880:1–23. [Google Scholar]
- Grimaldi D.1991. Systematics of the genus Colocasiomyia de Meijere (Diptera: Drosophilidae): cladistics, a new generic synonym, new records, and a new species from Nepal. Insect Syst Evol. 22(4):417–426. [Google Scholar]
- Grimaldi DA.1990. A phylogenetic, revised classification of genera in the Drosophilidae (Diptera). Bull Am Museum Nat Hist. 197:139. [Google Scholar]
- Grimaldi DA.2018. Hirtodrosophila of North America (Diptera: Drosophilidae). Bull Am Museum Nat Hist. 421(421):1–75. [Google Scholar]
- Hales KG, Korey CA, Larracuente AM, Roberts DM.. 2015. Genetics on the fly: a primer on the Drosophila model system. Genetics 201(3):815–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatadani LM, et al. 2009. Molecular phylogeny of the Drosophila tripunctata and closely related species groups (Diptera: Drosophilidae). Mol Phylogenet Evol. 51(3):595–600. [DOI] [PubMed] [Google Scholar]
- Heed WB, Wheeler MR.. 1957. Thirteen new species in the genus Drosophila from the Neotropical region. Univ Texas Publ. 5721:17–38. [Google Scholar]
- Huerta-Cepas J, Serra F, Bork P.. 2016. ETE 3: reconstruction, analysis, and visualization of phylogenomic data. Mol Biol Evol. 33(6):1635–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumitani HF, Kusaka Y, Koshikawa S, Toda MJ, Katoh T.. 2016. Phylogeography of the subgenus Drosophila (Diptera: Drosophilidae): evolutionary history of faunal divergence between the old and the new worlds. PLoS One 11(7):e0160051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffroy O, Brinkmann H, Delsuc F, Philippe H.. 2006. Phylogenomics: the beginning of incongruence? Trends Genet. 22(4):225–231. [DOI] [PubMed] [Google Scholar]
- Jeong S, et al. 2008. The evolution of gene regulation underlies a morphological difference between two Drosophila sister species. Cell 132(5):783–793. [DOI] [PubMed] [Google Scholar]
- Kaneshiro KY.2001. Sexual selection and speciation in Hawaiian Drosophila (Drosophilidae): a model system for research in Tephritidae. In: Aluja M, Norrbom A, editors. Fruit Flies (Tephritidae). Boca Raton: CRC Press. [Google Scholar]
- Kapli P, Yang Z, Telford MJ.. 2020. Phylogenetic tree building in the genomic age. Nat Rev Genet. 21(7):428–444. [DOI] [PubMed] [Google Scholar]
- Karageorgi M, et al. 2017. Evolution of multiple sensory systems drives novel egg-laying behavior in the fruit pest Drosophila suzukii. Curr Biol. 27(6):847–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Standley DM.. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 30(4):772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh T, Izumitani HF, Yamashita S, Watada M.. 2017. Multiple origins of Hawaiian Drosophilids: phylogeography of Scaptomyza hardy (Diptera: Drosophilidae). Entomol Sci. 20(1):33–44. [Google Scholar]
- Katoh T, Nakaya D, Tamura K, Aotsuka T.. 2007. Phylogeny of the Drosophila immigrans species group (Diptera: Drosophilidae) based on Adh and Gpdh sequences. Zoolog Sci. 24(9):913–921. [DOI] [PubMed] [Google Scholar]
- Katoh T, Tamura K, Aotsuka T.. 2000. Phylogenetic position of the subgenus Lordiphosa of the genus Drosophila (Diptera: Drosophilidae) inferred from alcohol dehydrogenase (Adh) gene sequences. J Mol Evol. 51(2):122–130. [DOI] [PubMed] [Google Scholar]
- Kellermann V, Van Heerwaarden B, Sgrò CM, Hoffmann AA.. 2009. Fundamental evolutionary limits in ecological traits drive Drosophila species distributions. Science 325(5945):1244–1246. [DOI] [PubMed] [Google Scholar]
- Kim BY, et al. 2021. Highly contiguous assemblies of 101 Drosophilid genomes. eLife 10:e66405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korlević P, et al. 2021. A minimally morphologically destructrive approach for DNA retrieval and whole genome shotgun sequencing of pinned historic Dipteran vector species. BioRxiv. [DOI] [PMC free article] [PubMed]
- Lang M, et al. 2012. Mutations in the neverland gene turned Drosophila pachea into an obligate specialist species. Science 337(6102):1658–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapoint RT, O’Grady PM, Whiteman NK.. 2013. Diversification and dispersal of the Hawaiian Drosophilidae: the evolution of Scaptomyza. Mol Phylogenet Evol. 69:95–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lartillot N, Lepage T, Blanquart S.. 2009. PhyloBayes 3: a Bayesian software package for phylogenetic reconstruction and molecular dating. Bioinformatics 25(17):2286–2288. [DOI] [PubMed] [Google Scholar]
- Lewis RL, Beckenbach AT, Mooers A.. 2005. The phylogeny of the subgroups within the melanogaster species group: likelihood tests on COI and COII sequences and a Bayesian estimate of phylogeny. Mol Phylogenet Evol. 37(1):15–24. [DOI] [PubMed] [Google Scholar]
- Longdon B, et al. 2015. The causes and consequences of changes in virulence following pathogen host shifts. PLoS Pathog. 11(3):e1004728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Máca J.1992. Addition to the fauna of Drosophilidae, Camillidae, Curtonotidae, and Campichoetidae (Diptera) of Soviet Middle Asia. Annotationes Zoologicae Et Botanicae. 210:1–8. [Google Scholar]
- Magnacca KN, Foote D, O’Grady PM.. 2008. A review of the endemic Hawaiian Drosophilidae and their host plants. Zootaxa 1728(1):1–58. [Google Scholar]
- Malloch JR.1934. Part VI. Diptera. In: Insects of Samoa and other Samoan terrestrial arthropoda. London: British Museum Natural History. p. 267–328. [Google Scholar]
- Markow TA, O’Grady PM.. 2006. Drosophila: a guide to species identification and use. London: Academic Press. [Google Scholar]
- McGregor AP, et al. 2007. Morphological evolution through multiple cis-regulatory mutations at a single gene. Nature 448(7153):587–590. [DOI] [PubMed] [Google Scholar]
- Mengual X, et al. 2017. Phylogenetic relationships of the tribe Toxotrypanini (Diptera: Tephritidae) based on molecular characters. Mol Phylogenet Evol. 113:84–112. [DOI] [PubMed] [Google Scholar]
- Minh BQ, Hahn MW, Lanfear R.. 2020. New methods to calculate concordance factors for phylogenomic datasets. Mol Biol Evol. 37(9):2727–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minh BQ, Schmidt HA, et al. 2020. IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Mol Biol Evol. 37(5):1530–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Hojas R, Vieira J.. 2012. Phylogenetic patterns of geographical and ecological diversification in the subgenus Drosophila. PLoS One 7(11):e49552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan TH.1910. Sex limited inheritance in Drosophila. Science 32(812):120–122. [DOI] [PubMed] [Google Scholar]
- O’Grady PM, Clark JB, Kidwell MG.. 1998. Phylogeny of the Drosophila saltans species group based on combined analysis of nuclear and mitochondrial DNA sequences. Mol Biol Evol. 15(6):656–664. [DOI] [PubMed] [Google Scholar]
- O’Grady PM, DeSalle R.. 2018. Phylogeny of the genus Drosophila. Genetics 209(1):1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Grady PM, et al. 2011. Phylogenetic and ecological relationships of the Hawaiian Drosophila inferred by mitochondrial DNA analysis. Mol Phylogenet Evol. 58:244–256. [DOI] [PubMed] [Google Scholar]
- O’Grady PM, Magnacca K, Lapoint RT.. 2009. Drosophila. In:Gillespie R, Clague D, editors. Encyclopedia of Islands. Berkeley (CA: ): University of California Press. p. 232–235. [Google Scholar]
- Okada T.1967. A revision of the subgenus Hirtodrosophila of the Old World, with descriptions of some new species and subspecies (Diptera, Drosophilidae, Drosophila). Mushi 41:1–36. [Google Scholar]
- Okada T.1984. The genus Collessia of Japan (Diptera: Drosophilidae). Proc Jpn Soc Syst Zool. 29:57–58. [Google Scholar]
- Okada T.1988. Family Drosophilidae (Diptera) from the Lund University Ceylon Expedition in 1962 and Borneo collections in 1978–1979. Entomol Scand. 30:109–149. [Google Scholar]
- Okada T.1989. A proposal of establishing tribes for the family Drosophilidae with key to tribes and genera (Diptera): taxonomy and systematics. Zool Sci. 6:391–399. [Google Scholar]
- Otranto D, Stevens JR, Testini G, Cantacessi C, Máca J.. 2008. Molecular characterization and phylogenesis of Steganinae (Diptera, Drosophilidae) inferred by the mitochondrial cytochrome c oxidase subunit 1. Med Vet Entomol. 22(1):37–47. [DOI] [PubMed] [Google Scholar]
- Peluffo AE, et al. 2015. A major locus controls a genital shape difference involved in reproductive isolation between Drosophila yakuba and Drosophila santomea. G3 (Bethesda) 5:2893–2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippe H, et al. 1994. Comparison of molecular and paleontological data in diatoms suggests a major gap in the fossil record. J Evol Biol. 7(2):247–265. [Google Scholar]
- Prigent SR, Le Gall P, Mbunda SW, Veuille M.. 2013. Seasonal and altitudinal structure of drosophilid communities on Mt Oku (Cameroon volcanic line). Comptes Rendus Geosci. 345(7–8):316–326. [Google Scholar]
- Prigent SR, Suwalski A, Veuille M.. 2017. Connecting systematic and ecological studies using DNA barcoding in a population survey of Drosophilidae (Diptera) from Mt Oku (Cameroon). Eur J Taxon. 287. doi:10.5852/ejt.2017.287. [Google Scholar]
- Remsen J, O’Grady P.. 2002. Phylogeny of Drosophilinae (Diptera: Drosophilidae), with comments on combined analysis and character support. Mol Phylogenet Evol. 24:249–264. [DOI] [PubMed] [Google Scholar]
- Robe LJ, Cordeiro J, Loreto ELS, Valente VLS.. 2010. Taxonomic boundaries, phylogenetic relationships and biogeography of the Drosophila willistoni subgroup (Diptera: Drosophilidae). Genetica 138:601–617. [DOI] [PubMed] [Google Scholar]
- Robe LJ, Loreto ELS, Valente VLS.. 2010. Radiation of the “Drosophila” subgenus (Drosophilidae, Diptera) in the neotropics. J Zool Syst Evol Res. 48:310–321. [Google Scholar]
- Robe LJ, Valente VLS, Budnik M, Loreto ÉLS.. 2005. Molecular phylogeny of the subgenus Drosophila (Diptera, Drosophilidae) with an emphasis on Neotropical species and groups: a nuclear versus mitochondrial gene approach. Mol Phylogenet Evol. 36(3):623–640. [DOI] [PubMed] [Google Scholar]
- Robe LJ, Valente VLS, Loreto ELS.. 2010. Phylogenetic relationships and macro-evolutionary patterns within the Drosophila tripunctata “radiation” (Diptera: Drosophilidae). Genetica 138(7):725–735. [DOI] [PubMed] [Google Scholar]
- Russo CAM, Mello B, Frazão A, Voloch CM.. 2013. Phylogenetic analysis and a time tree for a large drosophilid data set (Diptera: Drosophilidae). Zool J Linn Soc. 169(4):765–775. [Google Scholar]
- Sackton TB, et al. 2007. Dynamic evolution of the innate immune system in Drosophila. Nat Genet. 39(12):1461–1468. [DOI] [PubMed] [Google Scholar]
- Sigurdsen T, Green DM.. 2011. The origin of modern amphibians: a re-evaluation. Zool J Linn Soc. 162(2):457–469. [Google Scholar]
- Stamatakis A.2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30(9):1312–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark JB, O’Grady PM.. 2010. Morphological variation in the forelegs of the Hawaiian Drosophilidae. I. The AMC clade. J Morphol. 271(1):86–103. [DOI] [PubMed] [Google Scholar]
- Sturtevant AH.1959. Thomas Hunt Morgan. Biogr Mem Natl Acad Sci. 33:283–325. [Google Scholar]
- Sucena E, Delon I, Jones I, Payre F, Stern DL.. 2003. Regulatory evolution of shavenbaby/ovo underlies multiple cases of morphological parallelism. Nature 424(6951):935–938. [DOI] [PubMed] [Google Scholar]
- Sultana F, Hu YG, Toda MJ, Takenaka K, Yafuso M.. 2006. Phylogeny and classification of Colocasiomyia (Diptera, Drosophilidae), and its evolution of pollination mutualism with aroid plants. Syst Entomol. 31(4):684–702. [Google Scholar]
- Tanaka K, Barmina O, Kopp A.. 2009. Distinct developmental mechanisms underlie the evolutionary diversification of Drosophila sex combs. Proc Natl Acad Sci U S A. 106(12):4764–4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatarenkov A, Zurovcová M, Ayala FJ.. 2001. Ddc and amd sequences resolve phylogenetic relationships of Drosophila. Mol Phylogenet Evol. 20(2):321–325. [DOI] [PubMed] [Google Scholar]
- Throckmorton L.1962. The problem of phylogeny in the genus Drosophila. Univ Texas Publ. 2:207–343. [Google Scholar]
- Throckmorton L.1975. The phylogeny, ecology and geography of Drosophila. In: King R, editor. Handbook of genetics. New York: Plenum Publishing Corporation. p. 421–469. [Google Scholar]
- Throckmorton L.1982. Pathways of evolution in the genus Drosophila and the founding of the repleta group. In: Barker J, Starmer W, editors. Ecological genetics and evolution: the Cactus-Yeast-Drosophila model system. New York: Academic Press. p. 33–47. [Google Scholar]
- Trinder M, Daisley BA, Dube JS, Reid G.. 2017. Drosophila melanogaster as a high-throughput model for host-microbiota interactions. Front Microbiol. 8:751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsacas L.2002. Le nouveau complexe africain Drosophila loiciana et l’espèce apparentée D. matileana n. sp. (Diptera: Drosophilidae). Ann Soc Entomol Fr. 38:57–70. [Google Scholar]
- Tsacas L, Chassagnard M-T.. 1992. Les relations Araceae-Drosophilidae. Drosophila aracea une espèce anthophile associée à l’aracée Xanthosoma robustum au Mexique (Diptera: Drosophilidae). Ann Soc Entomol Fr. 28:421–439. [Google Scholar]
- Ugur B, Chen K, Bellen HJ.. 2016. Drosophila tools and assays for the study of human diseases. Dis Model Mech. 9(3):235–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Linde K, Houle D, Spicer GS, Steppan SJ.. 2010. A supermatrix-based molecular phylogeny of the family Drosophilidae. Genet Res (Camb). 92(1):25–38. [DOI] [PubMed] [Google Scholar]
- Wakahama K-I, Shinohara T, Hatsumi M, Uchida S, Kitagawa O.. 1983. Metaphase chromosome configuration of the immgrans species group of Drosophila. Jpn J Genet. 58(4):315–326. [Google Scholar]
- Wartlick O, Mumcu P, Jülicher F, Gonzalez-Gaitan M.. 2011. Understanding morphogenetic growth control – lessons from flies. Nat Rev Mol Cell Biol. 12(9):594–604. [DOI] [PubMed] [Google Scholar]
- Wheeler MR, Kambysellis MP.. 1966. Notes on the Drosophilidae (Diptera) of Samoa. Univ Texas Publ. 6615:533–565. [Google Scholar]
- Wiegmann BM, et al. 2011. Episodic radiations in the fly tree of life. Proc Natl Acad Sci U S A. 108(14):5690–5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegmann BM, Richards S.. 2018. Genomes of Diptera. Curr Opin Insect Sci. 25:116–124. [DOI] [PubMed] [Google Scholar]
- Yassin A.2013. Phylogenetic classification of the Drosophilidae Rondani (Diptera): the role of morphology in the postgenomic era. Syst Entomol. 38(2):349–364. [Google Scholar]
- Yassin A, et al. 2016. Recurrent specialization on a toxic fruit in an island Drosophila population. Proc Natl Acad Sci U S A. 113(17):4771–4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassin A, Delaney EK, et al. 2016. The pdm3 locus is a hotspot for recurrent evolution of female-limited color dimorphism in Drosophila. Curr Biol. 26(18):2412–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassin A, et al. 2010. Polyphyly of the Zaprionus genus group (Diptera: Drosophilidae). Mol Phylogenet Evol. 55(1):335–339. [DOI] [PubMed] [Google Scholar]
- Zhang C, Rabiee M, Sayyari E, Mirarab S.. 2018. ASTRAL-III: polynomial time species tree reconstruction from partially resolved gene trees. BMC Bioinformatics 19(Suppl 6):153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Toda MJ.. 1992. A new species-subgroup of the Drosophila immigrans species group (Diptera, Drosophilidae) with description of two new species from China and revision of taxonomic terminology. Jpn J Entomol. 60:839–850. [Google Scholar]
- Zhang Y, et al. 2021. Phylogeny and evolution of mycophagy in the Zygothrica genus group (Diptera: Drosophilidae). Mol Phylogenet Evol. 163:107257. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available on Zenodo (10.5281/zenodo.5091961).