Abstract
Purpose
We aimed to develop a novel mortality scoring system for inpatients with COVID-19 based on simple demographic factors and laboratory findings.
Materials and Methods
We reviewed and analyzed data from patients who were admitted and diagnosed with COVID-19 at 10 hospitals in Daegu, South Korea, between January and July 2020. We randomized and assigned patients to the development and validation groups at a 70% to 30% ratio. Each point scored for selected risk factors helped build a new mortality scoring system using Cox regression analysis. We evaluated the accuracy of the new scoring system in the development and validation groups using the area under the curve.
Results
The development group included 1232 patients, whereas the validation group included 528 patients. In the development group, predictors for the new scoring system as selected by Cox proportional hazards model were age ≥70 years, diabetes, chronic kidney disease, dementia, C-reactive protein levels >4 mg/dL, infiltration on chest X-rays at the initial diagnosis, and the need for oxygen support on admission. The areas under the curve for the development and validation groups were 0.914 [95% confidence interval (CI) 0.891–0.937] and 0.898 (95% CI 0.854–0.941), respectively. According to our scoring system, COVID-19 mortality was 0.4% for the low-risk group (score 0–3) and 53.7% for the very high-risk group (score ≥11).
Conclusion
We developed a new scoring system for quickly and easily predicting COVID-19 mortality using simple predictors. This scoring system can help physicians provide the proper therapy and strategy for each patient.
Keywords: COVID-19, mortality, scoring system
INTRODUCTION
Since the first case of pneumonia in December 2019,1 the new COVID-19 pandemic due to severe acute respiratory syndrome coronavirus 2 has spread worldwide, with an increase in critical illnesses and deaths, resulting in a lack of personal protective equipment and resources such as isolation rooms. In addition to remdesivir plus dexamethasone, new drugs such as monoclonal antibodies and baricitinib have been developed for the treatment of COVID-19; however, the optimal therapy for COVID-19 remains uncertain.2,3,4,5 Most patients with COVID-19 experience self-limiting and/or mild symptoms, whereas certain high-risk groups experience disease progression to critical illness or death. Therefore, predicting the severity and mortality of COVID-19 is crucial in making clinical decisions for its treatment.
Several articles have been published regarding the severity and mortality of COVID-19 in specific risk groups, with older adults more likely to experience disease progression and critical illness.6,7 Patients with one or more comorbidities, such as cardiovascular disease, diabetes, dementia, and malignancy, have shown more critical illness and higher mortality rates.8,9 Various laboratory findings, such as the neutrophil-lymphocyte ratio and C-reactive protein (CRP), ferritin, and lactate dehydrogenase (LDH) levels, are well-known risk factors associated with the progression and mortality of COVID-19.6,10,11
Several studies have employed pre-existing scoring systems for community-acquired pneumonia or sepsis to determine COVID-19 severity.12,13,14,15,16 However, a scoring system for COVID-19 mortality has not yet been established, and there have been several efforts to predict COVID-19. To our knowledge, no previous study has employed comorbidities and laboratory findings, such as CRP and routine inflammatory markers, to determine COVID-19 mortality.
Therefore, this study aimed to build a scoring system for predicting COVID-19 mortality using routine tests with large samples of hospitalized patients and based on demographics, clinical characteristics, and laboratory and radiological findings.
MATERIALS AND METHODS
Patient samples and data collection
The retrospective cohort study included COVID-19 patients who were admitted to 10 hospitals in the Daegu province of South Korea between February 18 and May 19, 2020. All patients were confirmed by real-time reverse-transcriptase polymerase chain reaction assay of nasopharyngeal swabs or sputum specimens. We excluded patients who lacked laboratory findings or chest radiological findings. We followed up with the admitted patients until their discharge or death, and the last monitored date was July 20, 2020.
From the patients' electronic medical records, we obtained data that included epidemiological characteristics, comorbidities, clinical symptoms, laboratory results, and radiological findings. Trained medical record administrators collected the data, which included laboratory findings on admission. The initial chest X-ray results were classified into two groups as no active lesion and unilateral or bilateral infiltration. The primary outcome was COVID-19 mortality during the follow-up period.
Definitions
Fever was defined as an axillary temperature above 37.5℃. All patients were classified as mild, moderate, severe, and death during hospitalization according to the worst severity score as defined in the Korean coronavirus disease 2019 response guideline version 9-5-1. Chronic heart disease included arrhythmia, cardiomyopathy, and requiring medication for coronary heart disease. Hypertension and heart failure were excluded. Neurological disease included cerebrovascular disease and hemorrhage.
Statistical analyses
We analyzed all statistical data using the R statistics ver. 3.1, and randomized and assigned the patients to the development and validation groups in a 70% to 30% ratio. Categorical variables are expressed as numbers and percentages, and were compared using the chi-squared test or Fisher's exact test. Continuous variables are expressed as means±standard deviations or medians with interquartile ranges (IQR), and were compared using the Student's t-test or Mann-Whitney U test. CRP cutoff value was defined according to the largest Youden index rounded up to first decimal places. We employed univariate regression analyses and a Cox proportional hazards model to determine the risk factors for COVID-19 mortality in the development group. By adjusting the parameters in the development group with the Cox proportional hazards model, we matched the risk score with the potential variables. We assessed the scoring system using data within the sensitivity of the development and validation groups. We determined the sensitivity and specificity using a receiver operating characteristic (ROC) curve for the two groups, and based the optimal cutoff score on the ROC curve with optimal sensitivity and specificity and on Youden's index (Supplementary Fig. 1, only online). We confirmed the prediction value for the new scoring system using the area under the curve (AUC). The ROC curve analysis as well as the criterion values and the coordinates of ROC curves were described using the MedCalc Statistical Software version 13.0.6 (MedCalc Software bvba, Ostend, Belgium; http://www.medcalc.org; 2014). We considered a p-value<0.05 to indicate statistical significance.
Ethics statement
The Institutional Review Boards of the 10 hospitals reviewed and approved our study protocol (approval no.: DGIRB 202007005). Considering the study's retrospective nature and the use of anonymous clinical data for analysis, the need for informed consent was waived.
RESULTS
Patient samples and clinical findings affected COVID-19 mortality
This study enrolled a total of 2254 patients hospitalized with COVID-19. The total case fatality rate was 7.94%, and 179 patients died during hospitalization. Of the total patients, 494 were excluded due to incomplete laboratory data; 1760 patients were ultimately included and analyzed in the study. Among them, 1232 patients were randomly assigned to the development group and 528 were assigned to the validation group. Table 1 lists the demographic and clinical characteristics of the survival and non-survival subgroups in the development and validation groups. The male sex was predominant in both development and validation groups (60.9% and 69.9%, respectively), and the two groups had similar median ages [61.0 (IQR 48.0–73.0) and 61.0 (IQR 49.0–74.0), respectively]. The median ages of the non-survival subgroups were significantly greater in both groups [development group: 59.0 (IQR 46.0–71.0) vs. 78.5 (IQR 71.0–85.0), p<0.001; validation group: 59.0 (IQR 47.0–70.0) vs. 81.0 (IQR 73.0–85.0), p<0.001]. The proportion of intensive care unit cases and the distribution of disease severity were similar in the two groups. In the development group, diabetes (p<0.001), heart failure (p<0.001), hypertension (p<0.001), chronic kidney disease (p<0.001), cancer (p=0.003), neurologic disease (p<0.001), and dementia (p<0.001) showed significantly higher rates in the non-survival subgroups than in the survival subgroups. In the validation group, diabetes (p<0.001), hypertension (p<0.001), chronic kidney disease (p=0.009), cancer (p=0.030), and dementia (p=0.004) showed significantly higher rates in the non-survival subgroups than in the survival subgroups.
Table 1. Clinical Characteristics and Outcomes of Patients Hospitalized for COVID-19 at 10 Hospitals (Core Cohort) in Daegu.
| Development group | Validation group | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total (n=1232) | Survival (n=1109) | Non-survival (n=123) | p value | Total (n=528) | Survival (n=483) | Non-survival (n=45) | p value | ||
| Sex, Female | 482 (39.1) | 410 (37.0) | 72 (58.5) | <0.001 | 159 (30.1) | 142 (29.4) | 17 (37.8) | 0.316 | |
| Age, yr | 61.0 (48.0–73.0) | 59.0 (46.0–71.0) | 78.5 (71.0–85.0) | <0.001 | 61.0 (49.0–74.0) | 59.0 (47.0–70.0) | 81.0 (73.0–85.0) | <0.001 | |
| Diabetes | <0.001 | <0.001 | |||||||
| No | 977 (79.3) | 912 (82.2) | 65 (52.8) | 443 (83.9) | 414 (85.7) | 29 (64.4) | |||
| Yes | 255 (20.7) | 197 (17.8) | 58 (47.2) | 85 (16.1) | 69 (14.3) | 16 (35.6) | |||
| Heart failure | <0.001 | 0.252 | |||||||
| No | 1206 (97.9) | 1093 (98.6) | 113 (91.9) | 513 (97.2) | 471 (97.5) | 42 (93.3) | |||
| Yes | 26 (2.1) | 16 (1.4) | 10 (8.1) | 15 (2.8) | 12 (2.5) | 3 (6.7) | |||
| Hypertension | <0.001 | <0.001 | |||||||
| No | 819 (66.5) | 771 (69.5) | 48 (39.0) | 359 (68.0) | 345 (71.4) | 14 (31.1) | |||
| Yes | 413 (33.5) | 338 (30.5) | 75 (61.0) | 169 (32.0) | 138 (28.6) | 31 (68.9) | |||
| Asthma | >0.999 | 0.016 | |||||||
| No | 1190 (96.6) | 1071 (96.6) | 119 (96.7) | 509 (96.4) | 469 (97.1) | 40 (88.9) | |||
| Yes | 42 (3.4) | 38 (3.4) | 4 (3.3) | 19 (3.6) | 14 (2.9) | 5 (11.1) | |||
| COPD | 0.142 | >0.999 | |||||||
| No | 1215 (98.6) | 1096 (98.8) | 119 (96.7) | 519 (98.3) | 475 (98.3) | 44 (97.8) | |||
| Yes | 17 (1.4) | 13 (1.2) | 4 (3.3) | 9 (1.7) | 8 (1.7) | 1 (2.2) | |||
| Chronic kidney disease | <0.001 | 0.009 | |||||||
| No | 1206 (97.9) | 1094 (98.6) | 112 (91.1) | 521 (98.7) | 479 (99.2) | 42 (93.3) | |||
| Yes | 26 (2.1) | 15 (1.4) | 11 (8.9) | 7 (1.3) | 4 (0.8) | 3 (6.7) | |||
| Cancer | 0.003 | 0.030 | |||||||
| No | 1186 (96.3) | 1074 (96.8) | 112 (91.1) | 500 (94.7) | 461 (95.4) | 39 (86.7) | |||
| Yes | 46 (3.7) | 35 (3.2) | 11 (8.9) | 28 (5.3) | 22 (4.6) | 6 (13.3) | |||
| Chronic liver disease | 0.177 | 0.633 | |||||||
| No | 1214 (98.5) | 1095 (98.7) | 119 (96.7) | 517 (97.9) | 472 (97.7) | 45 (100.0) | |||
| Yes | 18 (1.5) | 14 (1.3) | 4 (3.3) | 11 (2.1) | 11 (2.3) | 0 (0.0) | |||
| Neurologic disease | <0.001 | >0.999 | |||||||
| No | 1220 (99.0) | 1103 (99.5) | 117 (95.1) | 526 (99.6) | 481 (99.6) | 45 (100.0) | |||
| Yes | 12 (1.0) | 6 (0.5) | 6 (4.9) | 2 (0.4) | 2 (0.4) | 0 (0.0) | |||
| Hematologic disease | |||||||||
| No | 1217 (98.8) | 1098 (99.0) | 119 (96.7) | 0.083 | 525 (99.4) | 480 (99.4) | 45 (100.0) | >0.999 | |
| Yes | 15 (1.2) | 11 (1.0) | 4 (3.3) | 3 (0.6) | 3 (0.6) | 0 (0.0) | |||
| Dementia | <0.001 | 0.004 | |||||||
| No | 1112 (90.3) | 1032 (93.1) | 80 (65.0) | 472 (89.4) | 438 (90.7) | 34 (75.6) | |||
| Yes | 120 (9.7) | 77 (6.9) | 43 (35.0) | 56 (10.6) | 45 (9.3) | 11 (24.4) | |||
| Psychiatric disease | 0.061 | 0.832 | |||||||
| No | 1139 (92.5) | 1031 (93.0) | 108 (87.8) | 491 (93.0) | 450 (93.2) | 41 (91.1) | |||
| Yes | 93 (7.5) | 78 (7.0) | 15 (12.2) | 37 (7.0) | 33 (6.8) | 4 (8.9) | |||
| Need for O2 supply at admission | <0.001 | <0.001 | |||||||
| No | 1005 (81.6) | 964 (86.9) | 41 (33.3) | 434 (82.2) | 416 (86.1) | 18 (40.0) | |||
| Yes | 227 (18.4) | 145 (13.1) | 82 (66.7) | 94 (17.8) | 67 (13.9) | 27 (60.0) | |||
| Body temperature ≥37.5°C | <0.001 | 0.192 | |||||||
| No | 964 (78.2) | 889 (80.2) | 75 (61.0) | 400 (75.8) | 370 (76.6) | 30 (66.7) | |||
| Yes | 268 (21.8) | 220 (19.8) | 48 (39.0) | 128 (24.2) | 113 (23.4) | 15 (33.3) | |||
| Hospitalization in intensive care unit | <0.001 | <0.001 | |||||||
| No | 1129 (91.6) | 1055 (95.1) | 74 (60.2) | 480 (90.9) | 453 (93.8) | 27 (60.0) | |||
| Yes | 103 (8.4) | 54 (4.9) | 49 (39.8) | 48 (9.1) | 30 (6.2) | 18 (40.0) | |||
| Worst severity during hospitalization* | <0.001 | <0.001 | |||||||
| Mild | 828 (67.2) | 828 (74.7) | 0 (0.0) | 362 (68.6) | 360 (74.5) | 2 (4.4) | |||
| Moderate | 271 (22.0) | 257 (23.2) | 14 (11.4) | 109 (20.6) | 106 (21.9) | 3 (6.7) | |||
| Severe | 33 (2.7) | 24 (2.2) | 9 (7.3) | 18 (3.4) | 17 (3.5) | 1 (2.2) | |||
| Death | 100 (8.1) | 0 (0.0) | 100 (81.3) | 39 (7.4) | 0 (0.0) | 39 (86.7) | |||
| Hemoglobin, g/dL | 12.6 (11.6–13.8) | 12.7 (11.7–13.9) | 11.9 (10.2–13.4) | <0.001 | 12.7 (11.8–13.6) | 12.8 (11.9–13.6) | 11.8 (10.3–13.1) | 0.002 | |
| Platelet, ×103/uL | 218.0 (168.0–273.0) | 222.0 (173.0–278.0) | 176.0 (123.5–224.5) | <0.001 | 229.0 (172.0–285.0) | 234.0 (176.0–286.0) | 168.0 (131.0–232.0) | <0.001 | |
| White blood cell, ×103/uL | 5.5 (4.3–7.0) | 5.4 (4.3– 6.8) | 6.7 (4.8– 9.4) | <0.001 | 5.7 (4.4–7.3) | 5.7 (4.4– 7.1) | 6.9 (4.4–12.0) | 0.007 | |
| Aspartate aminotransferase, U/L | 25.0 (20.0–36.0) | 25.0 (19.0–34.0) | 41.0 (26.0–63.0) | <0.001 | 25.0 (20.0–37.0) | 25.0 (20.0–35.0) | 46.0 (27.0–61.0) | <0.001 | |
| Alanine aminotransferase, U/L | 20.0 (14.0–31.0) | 20.0 (14.0–31.0) | 21.0 (14.0–36.0) | 0.244 | 20.0 (14.0–30.0) | 20.0 (14.0–30.0) | 20.0 (11.0–30.0) | <0.001 | |
| Blood urea nitrogen, mg/dL | 13.0 (10.1–17.1) | 13.0 (10.0–16.1) | 22.0 (15.0–33.5) | <0.001 | 13.0 (10.0–17.1) | 12.7 (10.0–16.2) | 20.1 (14.0–31.7) | <0.001 | |
| Creatinine, mg/dL | 0.8 (0.6–0.9) | 0.8 (0.6–0.9) | 1.0 (0.8–1.6) | <0.001 | 0.7 (0.6–0.9) | 0.7 (0.6–0.9) | 1.0 (0.8–1.4) | <0.001 | |
| Glucose, mg/dL | 111.0 (94.0–141.0) | 109.0 (94.0–135.0) | 142.5 (101.5–189.5) | <0.001 | 109.0 (93.0–136.0) | 107.0 (93.0–131.0) | 151.5 (115.0–204.5) | <0.001 | |
| C-reactive protein, mg/dL | 0.6 (0.1–3.8) | 0.4 (0.1–2.6) | 10.5 (5.3–17.7) | <0.001 | 0.6 (0.1–3.0) | 0.4 (0.1– 2.0) | 10.0 (4.7–14.9) | <0.001 | |
| Lactate dehydrogenase, U/L | 425.0 (356.0–531.0) | 435.0 (354.0–554.0) | 424.0 (348.0–538.0) | 582.0 (481.0–761.0) | <0.001 | ||||
| Total bilirubin, mg/dL | 0.5 (0.3–0.7) | 0.5 (0.3–0.6) | 0.7 (0.4–0.9) | <0.001 | 0.5 (0.3–0.7) | 0.5 (0.3–0.7) | 0.4 (0.3–0.7) | 0.880 | |
| Infiltration on the chest X-rays during initial diagnosis | <0.001 | <0.001 | |||||||
| No | 626 (50.8) | 594 (53.6) | 32 (26.0) | 256 (48.5) | 249 (51.6) | 7 (15.6) | |||
| Yes | 606 (49.2) | 515 (46.4) | 91 (74.0) | 272 (51.5) | 234 (48.4) | 38 (84.4) | |||
COPD, chronic obstructive pulmonary disease.
Data are presented as n (%) or median (IQR).
*According to the definition of Korean coronavirus disease 2019 response guidelines version 9-5-1, all cases were classified as follows. Mild: no limit of activity and limit of activity but no oxygen supplement; moderate: oxygen with nasal prong or facial mask; severe: oxygen with non-invasive ventilation, high flow oxygen, invasive ventilation, multi-organ failure, extracorporeal membrane oxygenation or continuous renal replacement therapy; and death.
Moreover, the laboratory and radiological findings on admission also showed a significant difference between the survival and non-survival subgroups (Table 1). In the development and validation groups, significant factors in the non-survival subgroups were leukocytosis, elevated CRP and LDH levels, and initial infiltration observed on the chest X-rays (p<0.001).
Severe risk factors predicting mortality
In the development group, we analyzed the various factors using a univariate logistic regression analysis and employed Cox proportional hazards regression for the total mortality during the follow-up period (Table 2). To predict mortality, we chose the following cutoff values: age ≥70 years and CRP >4 mg/dL. For the Cox hazards regression analyses, we employed the following factors: age ≥70 years [hazard ratio (HR) 2.490; 95% confidence interval (CI) 1.546–4.007; p<0.001], need for oxygen supply on admission (HR 2.538; 95% CI 1.661–3.878; p<0.001), diabetes (HR 2.116; 95% CI 1.462–3.065; p<0.001), chronic kidney disease (HR 2.348; 95% CI 1.245–4.426; p=0.008), dementia (HR 3.060; 95% CI 2.024–4.627; p<0.001), CRP >4 mg/dL (HR 3.893; 95% CI 2.407–6.296; p<0.001), and infiltration observed in the initial chest X-rays (HR 1.561; 95% CI 1.023–2.381; p=0.039).
Table 2. Mortality Risk Factors for Patients Admitted with COVID-19 in the Development Group.
| Variable | Univariate regression analysis | Cox proportional hazard analysis | Mortality score | ||
|---|---|---|---|---|---|
| OR (95% CI) | p value | HR (95% CI) | p value | ||
| Sex, Female | 2.299 (1.604–3.295) | <0.001 | 1.331 (0.092–1.963) | 0.149 | |
| Age ≥70 yr | 7.779 (5.041–12.002) | <0.001 | 2.490 (1.546–4.007) | <0.001 | 2 |
| Need for oxygen supply on admission | 8.610 (5.911–12.543) | <0.001 | 2.538 (1.661–3.878) | <0.001 | 3 |
| Diabetes | 3.577 (2.506–5.106) | <0.001 | 2.116 (1.462–3.065) | <0.001 | 2 |
| Heart failure | 4.345 (2.274–8.304) | <0.001 | 1.814 (0.938–3.508) | 0.077 | |
| Chronic kidney disease | 5.029 (2.705–9.350) | <0.001 | 2.348 (1.245–4.426) | 0.008 | 2 |
| Malignancy | 2.373 (1.269–4.440) | 0.068 | 1.653 (0.870–3.143) | 0.125 | |
| Dementia | 4.718 (3.243–6.864) | <0.001 | 3.060 (2.024–4.627) | <0.001 | 3 |
| C-reactive protein >4 mg/dL | 10.982 (7.160–16.844) | <0.001 | 3.893 (2.407–6.296) | <0.001 | 4 |
| Infiltration on initial chest X-ray | 2.875 (1.921–4.302) | <0.001 | 1.561 (1.023–2.381) | 0.039 | 2 |
OR, odds ratio; CI, confidence interval; HR, hazard radio.
New scale for predicting mortality in COVID-19
We set up the new scale with 10 risk factors for mortality: age ≥70, need for oxygen supply at admission, diabetes, chronic kidney disease, dementia, CRP >4 mg/dL, and infiltration observed in the chest X-rays at the initial diagnosis (Table 2). The scale was established according to the coefficients in the Cox regression analyses. The risk scores ranged from 0 to 16, from lowest to highest risk in the development group. A total of 395 (32.1%) patients scored 0 point on the scale. As the score increased, the number of patients decreased and the mortality rate increased (Supplementary Fig. 1, only online).
Predictive value of the new scale for COVID-19 mortality
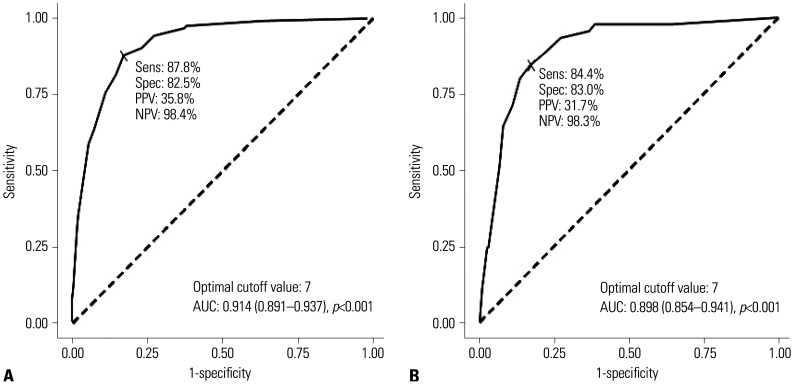
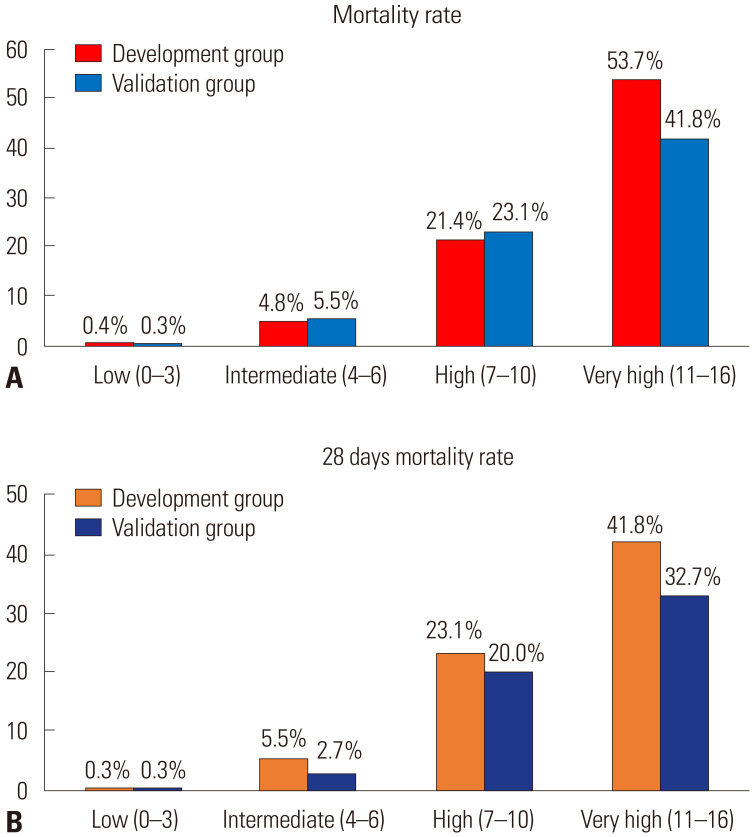
Supplementary Table 1 (only online) lists the cutoff scores and their corresponding sensitivity and specificity in the development group. The ROC curve showed a cutoff score of >6 points, and the largest AUC was 0.914 (95% CI 0.891–0.937; p<0.001) (Fig. 1). With a cutoff score of >6, the sensitivity and specificity of the score were 87.8% and 82.5%, respectively. The positive predictive value of the scoring system was 35.8%, and the negative predictive value was 98.4%. We classified 0–3 points as low-risk, 4–6 points as intermediate-risk, 7–10 points as high-risk, and >11 points as very high-risk (Table 3). The mortality rates for low-risk were 0.4% and 0.3% in the development and validation groups, respectively, and 53.7% and 41.8% for very high-risk, respectively (Fig. 2).
Fig. 1. Discrimination of new scoring system for mortality among COVID-19 patients. (A) Development group. (B) Validation group. AUC, area under the curve; Sens, sensitivity; Spec, specificity; PPV, positive predictive value; NPV, negative predictive value.
Table 3. Stratified Risk Group by Score and Mortality in the Development and Validation Groups.
| Score | Risk group | Development group | Validation group | ||
|---|---|---|---|---|---|
| Number of patients (%) | Number of deaths (%) | Number of patients (%) | Number of deaths (%) | ||
| 0–3 | Low | 682 (55.4) | 9 (0.6) | 298 (56.4) | 1 (0.3) |
| 4–6 | Intermediate | 248 (20.1) | 20 (18.4) | 110 (20.8) | 6 (5.5) |
| 7–10 | High | 168 (13.6) | 44 (19.4) | 65 (12.3) | 15 (23.1) |
| ≥11 | Very high | 134 (10.9) | 106 (50.0) | 55 (10.4) | 23 (41.8) |
| Overall | 1232 | 179 | 528 | 45 | |
Fig. 2. Clinical outcome in risk categories of the study population. (A) Mortality of COVID-19 in risk categories using the prediction model. (B) 28-day mortality for COVID-19 in risk categories using the prediction model.
DISCUSSION
The present study analyzed 1760 patients who were admitted and diagnosed with COVID-19, with a case mortality of 9.54%.17 This study defined a scoring system for predicting mortality using seven variables: age, diabetes, chronic kidney disease, dementia, CRP >4 mg/dL, infiltration observed on chest X-rays at the initial diagnosis, and need for oxygen supply at admission. We stratified the four risk groups according to the score, identified that the very high-risk group was associated with high mortality, and showed high discrimination using ROC curves (AUC 0.914, p<0.001). We suggest a novel scale for predicting COVID-19 mortality based on demographics, CRP levels, and chest X-ray findings.
As a routine inflammatory marker, CRP has already been shown to be a remarkable predictor of severity in COVID-19. Chen, et al.18 reported that CRP levels >2.0 mg/dL (20 mg/L) were associated with severe COVID-19 and computed tomography grading, and that CRP levels helped stratify patients. Luo, et al.19 reported that CRP could be the high discriminating independent predictor for disease severity in COVID-19 (cutoff 41.4 mg/L, sensitivity 90.5%, specificity 77.6, AUC 0.783). Satici, et al.20 were unable to show that CRP levels were a remarkable predictor of 30-day mortality, given that adding CRP levels to the pneumonia severity index did not improve the 30-day mortality; however, the authors were able to show a relationship between CRP levels and radiological grading through multivariate logistic regression analysis. As shown in previous studies, CRP was one of the independent risk factors in the Cox hazard regression analysis in this study. High CRP levels reflect inflammation and suggest longer hospital stays and poorer prognosis in COVID-19.18 However, previous studies have shown that the CRP trend is more closely related to the prognosis in community-acquired pneumonia compared to the initial CRP level.21 Therefore, further research on mortality and CRP follow-up in early hospitalizations is needed.
In our study, infiltration observed on chest X-rays as a risk factor was consistent with a previous report.22 Researchers have demonstrated that the Brixia score based on lesions observed in chest X-rays in COVID-19 pneumonia was likely to show high-risk severity.23 A worsening Brixia score and a Brixia score of >3 on admission are correlated with disease severity and death.24 Feng, et al.25 found that age, neutrophil-lymphocyte ratio, and chest computed tomography severity score corresponded with severe pneumonia in COVID-19, and developed a nomogram on the risk of severe pneumonia. Given its predictor of severity and mortality, chest X-rays were nonspecific, but low in cost and easy available. Previous studies have supported the use of chest X-rays in scoring systems such as ours, since X-rays have an advantage of easy availability and low cost in predicting COVID-19 severity.
We highlighted each comorbidity as severity predictor by weighting the points according to Cox hazard regression analyses. Guan, et al.26 stated that having one or more comorbidity was correlated to poor clinical outcomes (one comorbidity vs. two or more comorbidities: HR 1.79, 95% CI 1.16–2.77 vs. HR 2.59, 95% CI 1.61–4.17). Given the results of various studies from weighting each score for separate medical conditions, individualizing the variables will likely help predict mortality in COVID-19.14,27 Our study results showed that diabetes and chronic kidney disease were associated with COVID-19 severity, which was similar to the findings of previous studies.27,28,29 Wang, et al.28 demonstrated that a high glycosylated hemoglobin level could lead to hyperinflammation and hypercoagulability. Given that the data collection period included an outbreak at the psychiatric hospital, the dementia score was 3 points in our study, which might have biased the results to a larger proportion of dementia cases. The mechanism behind dementia and disease progression in COVID-19 remains unclear, but we believe that the patients with dementia had difficulty accessing medical facilities and were living in an enclosed environment.30
Our study's main strengths are the high sensitivity and specificity values as well as the high accuracy using easily accessible variables. There have been several studies exploring new scoring systems. The CALL scoring system for predicting disease progression employs age, comorbidities, lymphocyte counts, and serum LDH levels with an AUC of 0.91 (95% CI 0.86–0.94),31 but it has shown certain limitations due to its relatively low accuracy for determining disease progression in a validation study.32 A previous study in the Bronx, New York, divided 4711 patients into derivation and validation groups, and then created a scoring system based on age, oxygen saturation, mean arterial pressure, blood urea nitrogen and CRP levels, and the international normalized ratio.33 Due to the COVID-19 surge, the study showed a higher mortality rate (23%) than the 7.9% in our study. Gue, et al.34 suggested a novel score using a modified sepsis-induced coagulopathy score, age, and sex. The authors showed that a score of ≥4 indicated a 7.6-fold greater risk for 30-day mortality. The scale is a simple and easy-to-use calculator, but it tends to have limitations with small samples. The GRAM score, which was applied to 1590 patients hospitalized with COVID-19, was similar to our scoring system in that it employed comorbidities, CRP levels, and chest X-rays to predict critical illness.35 However, since the GRAM score is a web-based scoring system, accessibility is limited. In contrast, our scoring system has the advantage of being highly accessible, as it uses routine laboratory and radiological results and calculates the points corresponding to each variable.
To our knowledge, there has been no new scoring system that includes separate comorbidities and CRP levels. With its high sensitivity, specificity, and accuracy, the scale classifies low-risk and high-risk patients and allocates them to the appropriate medical centers. As a result, the use of medical resources can be optimized, and the unnecessary transfer and transport of patients can be reduced.
This study had certain limitations. First, given that the patients were recruited in Daegu, South Korea, this study should be carefully considered for generalization, and its scoring system should be tested with large samples and further validation. Second, since this was a retrospective cohort study, we cannot rule out information bias and confounders. However, the selection bias should be minimal as we included all confirmed patients who contracted the disease in an urban setting for a specific period. Third, given that the guidelines for COVID-19 treatment were uncertain and the fact that consistent treatment was not applied, the mortality rates might differ depending on each hospital's treatment method. Nevertheless, we believe that this study can help predict mortality by using demographic and simple radiological findings, thereby helping to manage inpatients with COVID-19.
In conclusion, we propose a new risk scoring system to predict COVID-19 mortality based on clinical characteristics, CRP levels, and chest X-ray results. Predicting the clinical outcomes for mortality and discharge could help assign patients to the appropriate medical facilities and match each patient with the appropriate management, for as long as the pandemic persists.
Footnotes
Parts of these data were presented at the KSAT 2021 Spring Conference (p18).
The authors have no potential conflicts of interest to disclose.
- Conceptualization: Sohyun Bae and Shin-Woo Kim.
- Data curation: Sohyun Bae, Hyun-Ha Chang, and Shin-Woo Kim.
- Formal analysis: Sohyun Bae, Hyun-Ha Chang, and Shin-Woo Kim.
- Funding acquisition: Shin-Woo Kim.
- Investigation: Yoonjung Kim, Soyoon Hwang, and Ki Tae Kwon.
- Methodology: Ki Tae Kwon and Hyun-Ha Chang.
- Project administration: all authors.
- Resources: Shin-Woo Kim.
- Software: Shin-Woo Kim.
- Supervision: Shin-Woo Kim.
- Validation: Yoonjung Kim and Soyoon Hwang.
- Visualization: Sohyun Bae and Shin-Woo Kim.
- Writing—original draft: Sohyun Bae.
- Writing—review & editing: Hyun-Ha Chang and Shin-Woo Kim.
- Approval of final manuscript: all authors.
SUPPLEMENTARY MATERIALS
Calibration of the mortality prediction model of COVID-19 in the development group.
Calibration of the Mortality Prediction Model of COVID-19 in the Development Group
References
- 1.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of COVID-19-final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen MS. Monoclonal antibodies to disrupt progression of early COVID-19 infection. N Engl J Med. 2021;384:289–291. doi: 10.1056/NEJMe2034495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.RECOVERY Collaborative Group. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghazaryan V, et al. Baricitinib plus remdesivir for hospitalized adults with COVID-19. N Engl J Med. 2021;384:795–807. doi: 10.1056/NEJMoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X, Xu S, Yu M, Wang K, Tao Y, Zhou Y, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146:110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee JY, Kim HA, Huh K, Hyun M, Rhee JY, Jang S, et al. Risk factors for mortality and respiratory support in elderly patients hospitalized with COVID-19 in Korea. J Korean Med Sci. 2020;35:e223. doi: 10.3346/jkms.2020.35.e223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection. Lancet Respir Med. 2020;8:e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harrison SL, Fazio-Eynullayeva E, Lane DA, Underhill P, Lip GYH. Comorbidities associated with mortality in 31,461 adults with COVID-19 in the United States: a federated electronic medical record analysis. PLoS Med. 2020;17:e1003321. doi: 10.1371/journal.pmed.1003321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kermali M, Khalsa RK, Pillai K, Ismail Z, Harky A. The role of biomarkers in diagnosis of COVID-19: a systematic review. Life Sci. 2020;254:117788. doi: 10.1016/j.lfs.2020.117788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shang Y, Liu T, Wei Y, Li J, Shao L, Liu M, et al. Scoring systems for predicting mortality for severe patients with COVID-19. EClinicalMedicine. 2020;24:100426. doi: 10.1016/j.eclinm.2020.100426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang C, Qin L, Li K, Wang Q, Zhao Y, Xu B, et al. A novel scoring system for prediction of disease severity in COVID-19. Front Cell Infect Microbiol. 2020;10:318. doi: 10.3389/fcimb.2020.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mancilla-Galindo J, Vera-Zertuche JM, Navarro-Cruz AR, Segura-Badilla O, Reyes-Velázquez G, Tepepa-López FJ, et al. Development and validation of the patient history COVID-19 (PH-Covid19) scoring system: a multivariable prediction model of death in Mexican patients with COVID-19. Epidemiol Infect. 2020;148:e286. doi: 10.1017/S0950268820002903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jang JG, Hur J, Hong KS, Lee W, Ahn JH. Prognostic accuracy of the SIRS, qSOFA, and NEWS for early detection of clinical deterioration in SARS-CoV-2 infected patients. J Korean Med Sci. 2020;35:e234. doi: 10.3346/jkms.2020.35.e234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kodama T, Obinata H, Mori H, Murakami W, Suyama Y, Sasaki H, et al. Prediction of an increase in oxygen requirement of SARSCoV-2 pneumonia using three different scoring systems. J Infect Chemother. 2021;27:336–341. doi: 10.1016/j.jiac.2020.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim SW, Kim SM, Kim YK, Kim JY, Lee YM, Kim BO, et al. Clinical characteristics and outcomes of COVID-19 cohort patients in Daegu metropolitan city outbreak in 2020. J Korean Med Sci. 2021;36:e12. doi: 10.3346/jkms.2021.36.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen W, Zheng KI, Liu S, Yan Z, Xu C, Qiao Z. Plasma CRP level is positively associated with the severity of COVID-19. Ann Clin Microbiol Antimicrob. 2020;19:18. doi: 10.1186/s12941-020-00362-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo X, Zhou W, Yan X, Guo T, Wang B, Xia H, et al. Prognostic value of c-reactive protein in patients with coronavirus 2019. Clin Infect Dis. 2020;71:2174–2179. doi: 10.1093/cid/ciaa641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Satici C, Demirkol MA, Sargin Altunok E, Gursoy B, Alkan M, Kamat S, et al. Performance of pneumonia severity index and CURB-65 in predicting 30-day mortality in patients with COVID-19. Int J Infect Dis. 2020;98:84–89. doi: 10.1016/j.ijid.2020.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo S, Mao X, Liang M. The moderate predictive value of serial serum CRP and PCT levels for the prognosis of hospitalized community-acquired pneumonia. Respir Res. 2018;19:193. doi: 10.1186/s12931-018-0877-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borghesi A, Zigliani A, Golemi S, Carapella N, Maculotti P, Farina D, et al. Chest X-ray severity index as a predictor of in-hospital mortality in coronavirus disease 2019: a study of 302 patients from Italy. Int J Infect Dis. 2020;96:291–293. doi: 10.1016/j.ijid.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borghesi A, Maroldi R. COVID-19 outbreak in Italy: experimental chest X-ray scoring system for quantifying and monitoring disease progression. Radiol Med. 2020;125:509–513. doi: 10.1007/s11547-020-01200-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maroldi R, Rondi P, Agazzi GM, Ravanelli M, Borghesi A, Farina D. Which role for chest x-ray score in predicting the outcome in COVID-19 pneumonia. Eur Radiol. 2021;31:4016–4022. doi: 10.1007/s00330-020-07504-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng Z, Yu Q, Yao S, Luo L, Zhou W, Mao X, et al. Early prediction of disease progression in COVID-19 pneumonia patients with chest CT and clinical characteristics. Nat Commun. 2020;11:4968. doi: 10.1038/s41467-020-18786-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55:2000547. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.King JT, Jr, Yoon JS, Rentsch CT, Tate JP, Park LS, Kidwai-Khan F, et al. Development and validation of a 30-day mortality index based on pre-existing medical administrative data from 13,323 COVID-19 patients: the Veterans Health Administration COVID-19 (VACO) index. PLoS One. 2020;15:e0241825. doi: 10.1371/journal.pone.0241825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Z, Du Z, Zhu F. Glycosylated hemoglobin is associated with systemic inflammation, hypercoagulability, and prognosis of COVID-19 patients. Diabetes Res Clin Pract. 2020;164:108214. doi: 10.1016/j.diabres.2020.108214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen D, Hu C, Su F, Song Q, Wang Z. Exposure to SARS-CoV-2 in a high transmission setting increases the risk of severe COVID-19 compared with exposure to a low transmission setting. J Travel Med. 2020;27:taaa094. doi: 10.1093/jtm/taaa094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ji D, Zhang D, Xu J, Chen Z, Yang T, Zhao P, et al. Prediction for progression risk in patients with COVID-19 pneumonia: the CALL score. Clin Infect Dis. 2020;71:1393–1399. doi: 10.1093/cid/ciaa414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grifoni E, Valoriani A, Cei F, Vannucchi V, Moroni F, Pelagatti L, et al. The CALL score for predicting outcomes in patients with COVID-19. Clin Infect Dis. 2021;72:182–183. doi: 10.1093/cid/ciaa686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Altschul DJ, Unda SR, Benton J, de la Garza Ramos R, Cezayirli P, Mehler M, et al. A novel severity score to predict inpatient mortality in COVID-19 patients. Sci Rep. 2020;10:16726. doi: 10.1038/s41598-020-73962-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gue YX, Tennyson M, Gao J, Ren S, Kanji R, Gorog DA. Development of a novel risk score to predict mortality in patients admitted to hospital with COVID-19. Sci Rep. 2020;10:21379. doi: 10.1038/s41598-020-78505-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liang W, Liang H, Ou L, Chen B, Chen A, Li C, et al. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern Med. 2020;180:1081–1089. doi: 10.1001/jamainternmed.2020.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Calibration of the mortality prediction model of COVID-19 in the development group.
Calibration of the Mortality Prediction Model of COVID-19 in the Development Group