Abstract
Purpose
Asthma is a serious inflammatory disease of the respiratory system in which airway smooth muscle cells (ASMCs) play a key role. This study aimed to investigate the expression of SLC26A2 in human ASMCs (HASMCs) and the regulatory mechanism of SLC26A2 in the proliferation and inflammatory factor production of HASMCs.
Materials and Methods
We obtained the asthma-associated differential mRNA SLC26A2 by bioinformatics analysis in childhood acute asthma samples. To investigate its role in airway inflammation and airway remodeling, we treated HASMCs with platelet-derived growth factor (PDGF) in an in vitro model and determined SLC26A2 expression in cells using western blotting. Cell proliferation was detected by MTT and EdU assays, and cell contractile phenotype marker proteins were measured. Cell migration and production of inflammatory factors were determined by Transwell and ELISA assays. Additionally, the upstream regulatory miRNA and LncRNA of SLC26A2 were identified by bioinformatics, luciferase reporter gene, and RIP analyses.
Results
SLC26A2 was significantly upregulated in bioinformatics analysis of pediatric asthma-related sample. PDGF treatment up-regulated SLC26A2 expression in HASMCs, whereas the knockdown of SLC26A2 inhibited PDGF-stimulated proliferation, migration, and production of inflammatory factors, and enhanced the expression of cell contractile phenotype marker proteins in HASMCs. Luciferase reporter and RIP experiments validated that NEAT1 targeted miR-9-5p to regulate SLC26A2, thereby influencing the biological function of PDGF-induced HASMCs.
Conclusion
These findings indicate that NEAT1-mediated miR-9-5p targeting of SLC26A2 inhibits the PDGF-induced proliferation and production of inflammatory factors in HASMCs. These findings highlight potential therapeutic targets for asthma and airway inflammation.
Keywords: SLC26A2, miR-9-5p, NEAT1, asthma, airway smooth muscle cells, inflammatory factors, proliferation, platelet-derived growth factor
INTRODUCTION
Asthma is a chronic inflammatory disease of airway with intermittent and reversible episodes,1 including airway hyperresponsiveness, allergen-induced airway obstruction, airway remodeling, and airway inflammation. The pathogenesis of asthma is related to environmental, genetic, and infectious factors, and most often occurs in childhood.2,3 Airway smooth muscle cells (ASMCs) are effector cells for airway disorders, inflammation, and remodeling through hypertrophy.4 ASMCs have a strong capacity for switching between contractile and proliferative phenotype in response to stimulation, which contributes to the persistence of airway inflammation or remodeling.5 Platelet-derived growth factor (PDGF) is closely related to the phenotypic transformation of ASMCs, promoting proliferation, migration, and inflammatory responses.6,7
The solute carrier gene family 26 (SLC26) is the second largest membrane protein in the human genome.8 Among its family members, SLC26A9 modulates the airway stress response to stimulation.9 It has been reported that SLC26A2 mediates the exchange of electrically neutral anions, and participates in the exchange of SO42− and oxalic acid with 2 Cl−, 2 OH−, 1 Cl−/1 OH−, or with each other.10,11 Moreover, SLC26A2 is essential for the proliferation and differentiation of chondrocytes and the synthesis of proteoglycans,12 and it also regulates the secretion of aldosterone in the adrenal cortex.13 However, the function of SLC26A2 on airway remodeling and inflammation has not been investigated.
MiRNAs inhibit or degrade RNA by binding to complementary target mRNAs at the post-transcriptional level, and they also play an significant role in biological processes including cell growth, differentiation, and inflammatory response.14 It has been shown that miR-9-5p is down-regulated in asthmatic patients and associated with lung function and immune inflammation.15 Additionally, miR-9-5p has been found to be pro-inflammatory in TRAPM2.5-induced airway inflammation.16 More importantly, lncRNA could function as competitive endogenous RNA (ceRNA) via directly sponging of miRNA, which further regulates the expression of target mRNA.17 A previous study showed that LncRNA NEAT1 could sponge miR-9-5p to facilitate the cervical cancer cell growth as a ceRNA.18 Moreover, the knockdown of NEAT1 inhibits EMT by regulating miR-9-5p to modulate lung fibrosis.19
Here, we hypothesized that NEAT1/miR-9-5p regulates SLC26A2, thereby inhibiting the proliferation and inflammation in HASMCs. We detected the effect and molecular mechanisms of NEAT1/miR-9-5p/SLC26A2 in PDGF-induced HASMCs.
MATERIALS AND METHODS
Screening of candidate mRNA, miRNA, and lncRNA based on bioinformatics
We downloaded pediatric asthma-related expression profiling data from the GEO database, and obtained differential expressions of mRNAs by performing the difference analysis (|log2FC| >1, adj.P.Val<0.05) by the Limma package pair separately. Then, the upstream miRNA of SLC26A2 was predicted by TargetScan. In conjunction with the literature, miR-9-5p showed decreased expression level in asthmatic patients and had a target-regulatory relationship with SLC26A2. The starBase and ENCORI databases were then used to predict the upstream lncRNA of miR-9-5p, and we found that NEAT1 and miR-9-5p had base complementary sequence.
Cell culture
Human airway smooth muscle cells (HASMCs) (BeiNa Biological, Beijing, China) were incubated in DMEM medium with 10% FBS (Gibco, Grand Island, NY, USA), 1% penicillin-streptomycin (Invitrogen, Carlsbad, CA, USA), and placed in a humidified incubator at 37℃, 5% CO2. Cells were morphologically observed by optical microscopy (Olympus, Inc., Tokyo, Japan). Cells were stained by α-smooth muscle actin (α-SMA, a contractile phenotype protein fluorescence). After that, cells were stimulated with PDGF (20 ng/mL, Peprotech, Rocky Hill, NJ, USA) for 24 h.
Cell transfection
In order to overexpress the levels of LncRNA-NEAT and miR-9-5p, cells were transfected with miR-9-5p mimic or NEAT1 overexpression vector (GenePharma, Shanghai, China). Likewise, cells were transfected with NEAT1 siRNA, miR-9-5p inhibitor, and SLC26A2 siRNA (GenePharma) to knock down LncRNA-NEAT1, miR-9-5p, and SLC26A2 levels in cells. Transfection experiments were conducted with the Lipofectamine 2000 reagent (Invitrogen).
Western blot
Western blot was conducted as previously described.20 In brief, SLC26A2 (Thermo Fisher Scientific Inc., Waltham, MA, USA), PCNA, MMP-9, α-SMA, and calponin (Cell Signaling Technology, Boston, MA, USA) expressions were determined by western blot using the corresponding primary antibody. Antibodies were incubated with HRP-conjugated secondary antibody followed by ECL western blot detection reagent (Beyotime, Shanghai, China). The protein expressions were indexed to GAPDH.
Quantitative real-time PCR
Total RNA in cells was extracted by TRIzol reagent (Invitrogen), and reversely transcribed into cDNA via High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). qRT-PCR was conducted by a miScript SYBR Green PCR Kit (Qiagen, Hilden, Germany) on the 7900 H T Fast Real-Time PCR system (Applied Biosystems). For miR-9-5p, reverse transcription was conducted by miScript II Reverse Transcription Kit (Qiagen), and the miScript SYBR Green PCR Kit with miScript Primer Assay Kit (Qiagen) was used to detect the miR-9-5p expression. MiR-9-5p, NEAT1, and SLC26A2 mRNA levels were calculated by the 2−ΔΔCt method, and GAPDH served as the endogenous control of NEAT1 and SLC26A2, and U6 for miR-9-5p. The primer sequences used were as follows:
MiR-9-5p: 5′-GCG TCT TTG GTT ATC TAG CTG TA-3′(forward), 5′-AGT GCA GGG TCC GAG GTA TT-3′(reverse); NEAT1: 5′-TGG CTA GCT CAG GGC TTC AG-3′(forward), 5′-TCT CCT TGC CAA GCT TCC TTC-3′(reverse); SLC26A2: 5′-CCA GAT GTG GAG GAT TAG CAG AAT GG-3′(forward), 5′-ACA GCT TCA TAA TCT CTG CGA ACT TCT TTC AGT GT-3′(reverse); U6: 5′-GCT TCG GCA GCA CAT ATA CTA AAA T-3′(forward), 5′-CGC TTC ACG AAT TTG CGT GTC AT-3′(reverse); GAPDH: 5′-CAT GGC CTT CCG TGT CCC CA-3′(forward), 5′-TGC TTC ACC ACC TTC TTG ATG-3′(reverse).
Measurement of HASMC proliferation
Cell proliferation assays were performed by MTT and EdU analyses. After cell treatment, MTT assay was performed according to the instructions. Cells were incubated in 96-well plates with 200 µL of 5 mg/mL MTT (Sigma, St. Louis, MO, USA). After 4 h, 150 µL of DMSO was added into the plates after the medium was aspirated.
For EdU assay, the treated cells were exposed to 10 nM of EdU solution (RiboBio, Guangzhou, China). After 2 h, cells were then treated by 4% formaldehyde and 0.5% Triton X-100. After 30 min of treatment with Apollo cocktail, Hoechst was applied to stain cells for 30 min. The fluorescence microscopy was used to observe and record the images.
HASMC migration analysis
Cell migration ability was analyzed by Transwell. The treated cells were incubated in the upper chamber containing serum-free medium, and cell migration was induced by the lower chamber containing 20% FBS. After 24 h, cells adhering to the upper surface were wiped off using cotton swabs. While cells on the lower surface were treated with 10% methanol and 0.1% crystal violet solution. The number of stained cells were recorded with a microscopy.
ELISA assay
Inflammatory factors IL-4, IL-6, and IL-13 in supernatants of cells were assayed with ELISA Kits (ExCell Bio, Shanghai, China) according to the instructions.
Immunofluorescence
Immunofluorescence experiments were performed as reported previously in the literature.21 Briefly, after fixation and blocking, cells were incubated with α-SMA antibodies overnight and FITC-labeled secondary antibodies for 1 h. DAPI (Beyotime) was applied to stain the nucleus. The results were observed using a fluorescence microscope (Olympus).
Luciferase assay
Cells were co-treated with vector harboring wild or mutated NEAT1 or SLC26A2 and miR-9-5p mimic for 48 h. The dual-luciferase reporter assay system (Promega, Madison, WI, USA) was used to test luciferase activity.
RIP assay
RIP assay was conducted by the Magna RIP kit (Millipore, Bedford, MA, USA) as described in previous literature.22 Magnetic beads conjugated with human anti-Ago2 antibody (Millipore) or anti-IgG (negative control) were used to treat lysed cells at 4℃ for 24 h. Finally, the beads were collected and digested with Dnase and Proteinase K for sebsequent qRT-PCR analysis.
Statistics
All experimental treatments were repeated three times. The results are presented as mean±SD, and were plotted by Prism software (Graphpad Software Inc., La Jolla, CA, USA). Statistical comparisons were conducted by Student's t test or one-way analysis of variance, and a p-value<0.05 was considered a statistically significant difference.
RESULTS
SLC26A2 was overexpressed in PDGF-stimulated HASMCs, and SLC26A2 knockdown restrained cell proliferation and migration
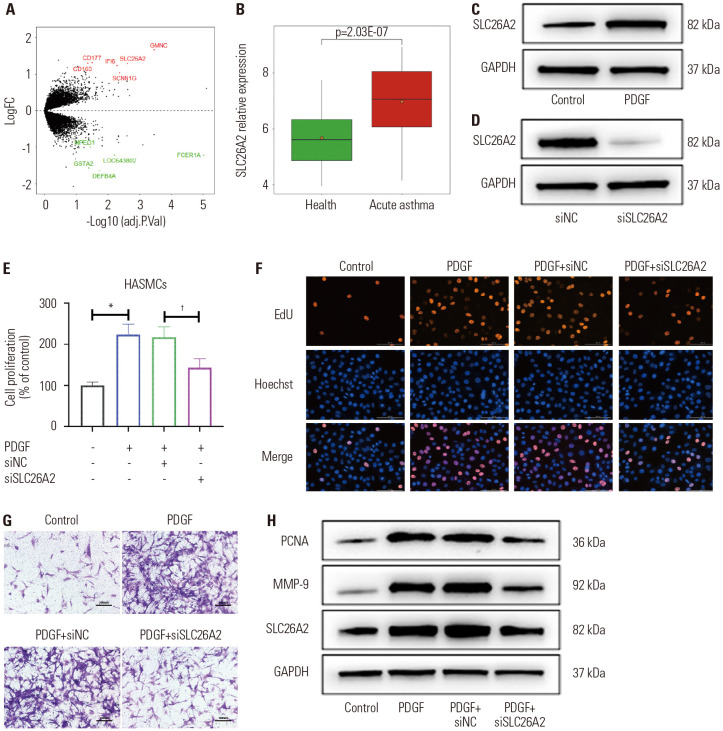
Childhood acute asthma-related expression profiling data downloaded from the GEO database (GSE103166) were analyzed. Difference analysis was performed by the Limma package. A total of 13 differently expressed genes were obtained; among them, seven were up-regulated and six were down-regulated (Fig. 1A). Analysis of the expression of differently expressed genes in the samples showed that SLC26A2 was significantly up-regulated in the samples with childhood acute asthma compared to healthy samples (Fig. 1B). These findings suggested that SLC26A2 is of great significance in asthma. To validate the effect of SLC26A2 on asthma, we cultured HASMCs and treated them with PDGF to promote the proliferation and migration of HASMCs (Supplementary Fig. 1, only online). As shown in Fig. 1C, PDGF-induced HASMCs remarkably upregulated the expression of SLC26A2 compared to the control, suggesting that SLC26A2 was involved in regulating the biological function of PDGF on HASMCs. To investigate the effect of SLC26A2 on PDGF-induced HASMCs, we transfected HASMCs with siSLC26A2 and verified its silencing efficiency by western blotting (Fig. 1D). The proliferative and migratory capacity of the cells was assessed by MTT, EdU, and Transwell analysis. The abovementioned experimental results indicated that PDGF markedly induced HASMCs proliferation and migration, and SLC26A2 deficiency significantly lightened the effect of PDGF on cells (Fig. 1E-G). Moreover, western blotting revealed that PDGF treatment noticeably elevated the level of proliferation-related protein PCNA and the migration-related protein MMP-9, whereas this trend was reversed by siSLC26A2 (Fig. 1H).
Fig. 1. SLC26A2 was overexpressed in PDGF-stimulated HASMCs, and SLC26A2 knockdown restrained cell proliferation and migration. (A) Volcano plot of differently expressed genes in the GEO database. Red dots indicate highly expressed genes and green dots represent poorly expressed genes. (B) The expression of SLC26A2 in samples with acute asthma (n=56) and healthy samples (n=50). (C) Western blotting showed SLC26A2 expression in PDGF-induced HASMCs. (D) Western blotting showed SLC26A2 expression in PFGF-induced HASMCs transfected with siSLC26A2. (E and F) The effect of SLC26A2 on the proliferation of PDGF-induced HASMC was assessed by MTT and EdU analyses. (G) The effect of SLC26A2 on the migration of PDGF-induced HASMC was evaluated by Transwell analysis (scale bar: 100 µm). (H) Western blotting showed PCNA and MMP-9 expressions in PDGF-induced HASMCs transfected with siSLC26A2. Data are presented as the mean±SD of three independent experiments. *P<0.05, compared to scrambled control group, †P<0.05, compared to PDGF+si-NC group. HASMCs, human airway smooth muscle cells; PGDF, platelet-derived growth factor; NC, negative control.
SLC26A2 knockdown enhanced contractile phenotype marker protein expression and restrained inflammatory factor production in PDGF-stimulated HASMCs
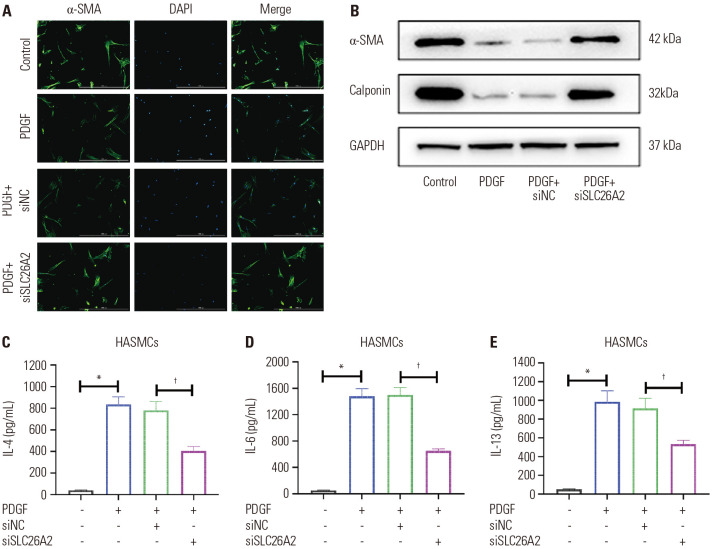
PDGF stimulation induces a decrease in cell contractility, accompanied by a decrease in the expression of contractile proteins (α-SMA, calponin).23 Therefore, we further explored whether SLC26A2 affected the PDGF-induced hypotonicity of HASMCs. Consistent with previous research, PDGF significantly decreased α-SMA expression in cells, and the knockdown of SLC26A2 reversed this trend (Fig. 2A and B). Similarly, Calponin expression level was inhibited by PDGF, and it was increased after knocking down SLC26A2 (Fig. 2B). Furthermore, PDGF significantly up-regulated interleukin (IL)-4, IL-6, and IL-13 levels compared to the control. However, knocking down SLC26A2 significantly decreased the levels of inflammatory factors (Fig. 2C-E). In summary, the knockdown of SLC26A2 promoted PDGF-stimulated contractile phenotype marker protein expression of HASMCs, and inhibited the production of inflammatory factors.
Fig. 2. SLC26A2 knockdown enhanced contractile phenotype marker protein expression and restrained inflammatory factor production in PDGF-stimulated HASMCs. (A) Immunofluorescent staining of α-SMA (green) in HASMCs (scale bar: 50 µm). (B) Western blotting showed α-SMA and Calponin expressions in HASMC. ELISA assays of (C) IL-4, (D) IL-6, and (E) IL-13 levels in HASMC culture supernatant. Data are presented as the mean±SD of three independent experiments. *P<0.05, compared to scrambled control group, †P<0.05, compared to PDGF+si-NC group. α-SMA, α-smooth muscle actin; PGDF, platelet-derived growth factor; HASMCs, human airway smooth muscle cells; IL, interleukin; NC, negative control.
The upstream regulatory miRNA of SLC26A2 is miR-9-5p
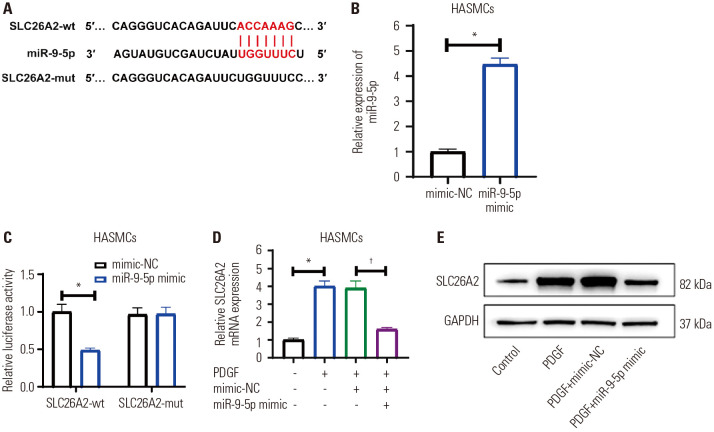
To further investigate the effect mechanisms of SLC26A2 on PDGF-stimulated HASMCs, we predicted the upstream miRNA (miR-9-5p) for SLC26A2 using TargetScan (Fig. 3A). We first verified the direct targeting effect of miR-9-5p and SLC26A2 (Fig. 3B). Dual luciferase reporter gene analysis revealed that miR-9-5p overexpression obviously inhibited the luciferase activity of the reporter vector containing SLC26A2-wt, but not the reporter vector containing SLC26A2-mut (Fig. 3C). These results validated that miR-9-5p targeted SLC26A2 directly. Next, qRT-PCR and western blot were applied to measure SLC26A2 mRNA and protein expressions in PDGF-induced HASMCs transfected with miR-9-5p mimic. The results in Fig. 3D and E showed that PDGF stimulation significantly up-regulated SLC26A2 levels, and the up-regulated levels were remarkably attenuated by miR-9-5p overexpression. Such findings showed that miR-9-5p targeted and negatively regulated SLC26A2 level.
Fig. 3. The upstream regulatory miRNA of SLC26A2 is miR-9-5p. (A) Schematic representation of the miR-9-5p putative binding site in the SLC26A2 3′-UTR. (B) qRT-PCR showed miR-9-5p level in miR-9-5p mimic-transfected HASMCs. (C) Luciferase reporter assays showed that miR-9-5p overexpression remarkably reduced the luciferase activity of the reporter vector containing SLC26A2-wt, but not the reporter vector containing SLC26A2-mut. (D and E) MiR-9-5p mimic was transfected into HASMCs induced by PDGF, qRT-PCR and Western blotting were used to detect the mRNA and protein expression of SLC26A2. Data are presented as the mean±SD of three independent experiments. *P<0.05, compared to their respective NCs or scrambled control group, †P<0.05, compared to PDGF+mimic-NC group. HASMCs, human airway smooth muscle cells; PGDF, platelet-derived growth factor; NC, negative control.
Knockdown of miR-9-5p significantly reversed the effect of SLC26A2 knockdown on PDGF-stimulated HASMCs
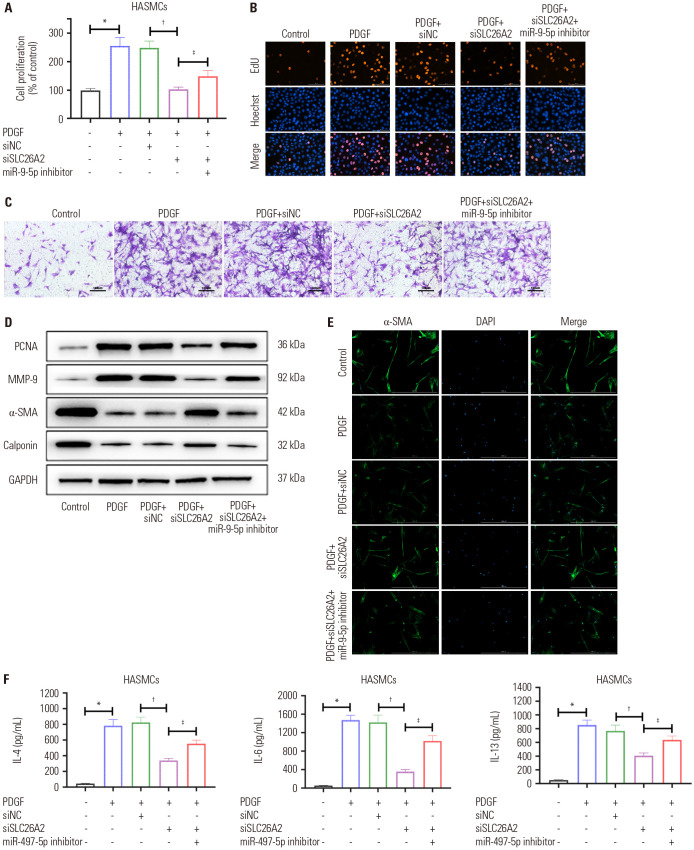
Based on the negative regulation of miR-9-5p on SLC26A2, we assumed that the function of SLC26A2 on HASMCs could be inhibited by miR-9-5p. We transfected HASMCs with siNC, siSLC26A2, or siSLC26A2+miR-9-5p inhibitor, and then performed MTT, EDU, and Transwell analyses. A series of experimental results showed that the inhibition of proliferation and migration of HASMCs caused by siSLC26A2 was restored by the miR-9-5p inhibitor (Fig. 4A-D). Similarly, the upregulation of cell contractile proteins caused by siSLC26A2 was also inhibited by the miR-9-5p inhibitor (Fig. 4D and E). The decline in inflammatory factor levels caused by the knockdown of SLC26A2 was also restored by the treatment of miR-9-5p inhibitor (Fig. 4F).
Fig. 4. Knockdown of miR-9-5p significantly reversed the effect of SLC26A2 knockdown on PDGF-stimulated HASMCs. (A and B) The proliferation of HASMC was evaluated by MTT and EdU analyses. (C) The migration of HASMC was evaluated by Transwell analysis (scale bar: 100 µm). (D) Western blotting showed related proteins in HASMC. (E) Immunofluorescent staining of α-SMA (green) in HASMCs (scale bar: 50 µm). (F) ELISA assays of IL-4, IL-6, and IL-13 levels in HASMC culture supernatant. Data are presented as the mean±SD of three independent experiments. *p<0.05, compared to scrambled control group, †P<0.05, compared to PDGF+si-NC group, ‡P<0.05, compared to PDGF+si-SLC26A2 group. HASMCs, human airway smooth muscle cells; PGDF, platelet-derived growth factor; α-SMA, α-smooth muscle actin; IL, interleukin; NC, negative control.
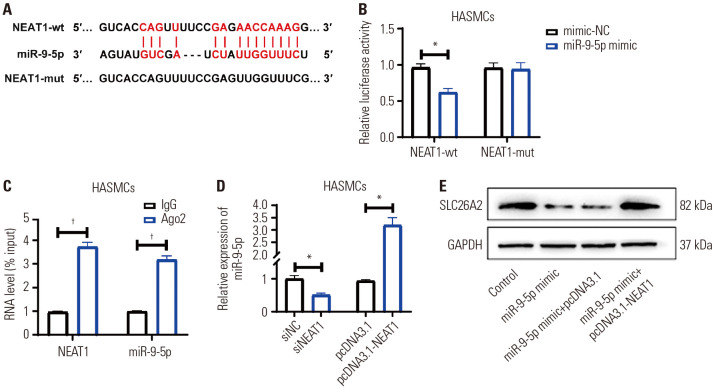
NEAT1 directly regulated miR-9-5p/SLC26A2
There has been growing evidence that lncRNA sponges miRNAs, thereby down-regulating miRNA expression and acting as ceRNA. To explore the lncRNA-regulating miR-9-5p/SLC26A2, we confirmed the upstream lncRNA of miR-9-5p by starBase and ENCORI databases, which showed that NEAT1 has a base complementary sequence to miR-9-5p (Fig. 5A). The binding relationship between NEAT1 and miR-9-5p was validated by dual luciferase reporter gene and RIP experiments. As shown in Fig. 5B, miR-9-5p mimic treatment markedly reduced the luciferase activity of NEAT1-wt-containing vector, whereas it had no effect on NEAT1-mut-containing vector. RIP experiments also validated that miR-9-5p was a bona fide NEAT1-targeting miRNA (Fig. 5C). qRT-PCR assay showed that overexpressing NEAT1 down-regulated the miR-9-5p levels, while knocking down NEAT1 significantly up-regulated the miR-9-5p levels (Fig. 5D). Rescue experiments showed that miR-9-5p inhibited SLC26A2 expression, but oe-NEAT1 transfection mitigated this inhibition (Fig. 5E). These results validated that NEAT1 directly targeted miR-9-5p to regulate the level of SLC26A2.
Fig. 5. NEAT1 directly regulated miR-9-5p/ SLC26A2. (A) Schematic representation of the miR-9-5p putative binding site in the NEAT1 3′-UTR. (B) Luciferase reporter assays showed that miR-9-5p overexpression remarkably reduced the luciferase activity of NEAT1-wt-containing vector, whereas it had no effect on the NEAT1-mut-containing vector. (C) The expressions of NEAT1 and miR-9-5p bound to Ago2 or IgG were detected by RIP and qRT-PCR. (D) qRT-PCR showed miR-9-5p level in PDGF-induced HASMCs transfected with siNEAT1or oe-NEAT1. (E) Western blotting showed SLC26A2 expression in PDGF-induced HASMCs treated with miR-9-5p mimic or miR-9-5p mimic+oe-NEAT1. Data are presented as the mean±SD of three independent experiments. *p<0.05, compared to their respective NCs, †p<0.05, compared to IgG group. HASMCs, human airway smooth muscle cells; PGDF, platelet-derived growth factor; NC, negative control.
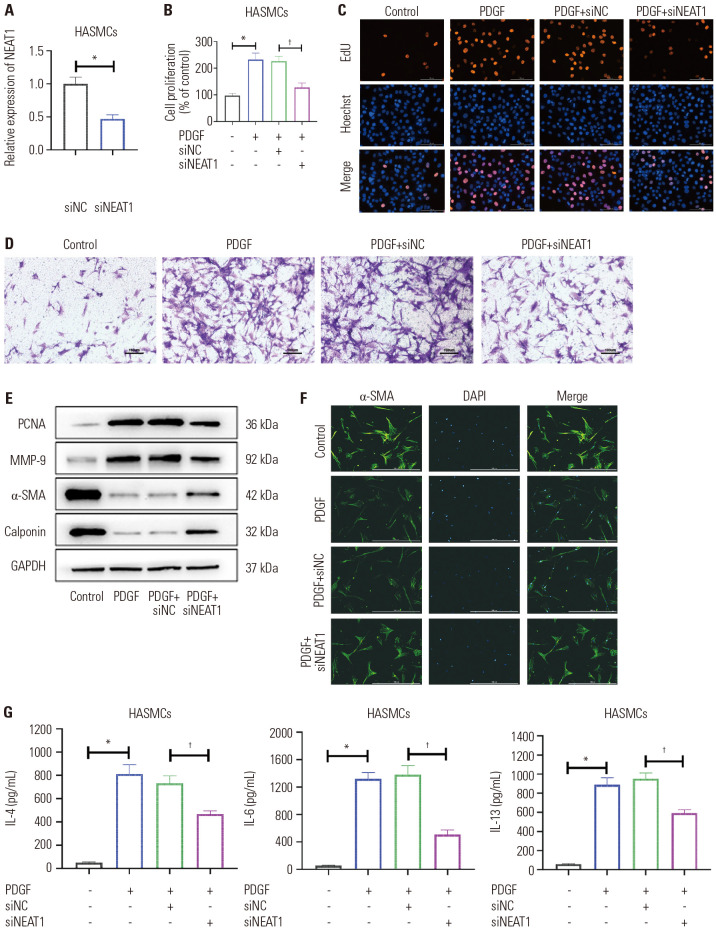
Knockdown of NEAT1 regulated miR-9-5p/SLC26A2 to inhibit the effect of PDGF on HASMCs
To further validate that NEAT1 regulates the miR-9-5p/SLC26A2 signaling to affect the function of PDGF-treated HASMCs in terms of proliferation, migration, contractile phenotype marker protein expression, and inflammatory factor production, we firstly synthesized siNEAT1 for cell transfection (Fig. 6A). A series of assays revealed that NEAT1 knockdown regulated miR-9-5p/SLC26A2 to inhibit PDGF-stimulated HASMC proliferation (Fig. 6B, C, and E), migration (Fig. 6D and E), and inflammatory factor production (Fig. 6G), while enhancing the cell contractile phenotype marker protein expression (Fig. 6E and F).
Fig. 6. Knockdown of NEAT1 regulated miR-9-5p/SLC26A2 to inhibit the effect of PDGF on HASMCs. (A) qRT-PCR showed NEAT1 level in cells. (B and C) The proliferation of HASMC was evaluated by MTT and EdU analyses. (D) The migration of HASMC was evaluated by Transwell analysis (scale bar: 100 µm). (E) Western blotting showed related proteins in HASMC. (F) Immunofluorescent staining of α-SMA (green) in HASMCs (scale bar: 50 µm). (G) ELISA assays of IL-4, IL-6, and IL-13 levels in HASMC culture supernatant. Data are presented as the mean±SD of three independent experiments. *p<0.05, compared to si-NC or scrambled control group, †p<0.05, compared to PDGF+si-NC group. HASMCs, human airway smooth muscle cells; PGDF, platelet-derived growth factor; α-SMA, α-smooth muscle actin; IL, interleukin; NC, negative control.
DISCUSSION
As effector cells involved in the abnormal constriction and narrowing of asthmatic airways, the ASMCs are of great significance in airway remodeling.24 Furthermore, ASMCs have been reported to have contractile, proliferative/synthetic phenotypes. In response to stimulation, ASMCs switches from one phenotype to another.25 The different phenotypes exhibit different biological functions. Growth factors, such as PDGF and transforming growth factor, are involved in the phenotypic transformation.26 Among them, PDGF has been shown to be associated with changes in airway structure and function.25,27 PDGF has also been reported to induce the proliferation, synthesis, and migration of ASMCs, as well as the production of inflammatory factors.23,28,29,30 In our study, PDGF remarkably increased the proliferation, migration, and inflammation of HASMCs, and was applied to treat ASMCs in vitro for subsequent experiments.
In addition, SLC26A2 was found to be very highly expressed in PDGF-induced HASMCs, and the deficiency of SLC26A2 inhibited proliferation, migration, and inflammatory factor production in HASMCs. SLC26A9 could also affect the proliferation and migration of colon cancer cells as a member of the SLC26 family.9 However, SLC26A2 has not been studied in cell models of asthma. Next, we further investigated the molecular mechanisms of SLC26A2 in modulating PDGF-induced HASMCs.
MicroRNAs are a class of small non-coding RNAs containing approximately 18 to 24 nucleotides. According to the most recent findings, miRNAs are of great significance in the regulation of asthma pathology. MiR-29c plays a vital role in children with asthma by affecting Th2/Th17 cell differentiation.31 MiR-142-3p is associated with abnormal WNT signaling during asthmatic airway remodeling.32 MiR-221-3p in airway epithelial cells correlates with airway eosinophilic inflammation.33 LncRNAs target and down-regulate miRNA expression via a ceRNA mechanism, thereby regulating the function of mRNA in asthmatic ASMCs. For example, LncRNA-PVT1 targets miR-203a/E2F3 to inhibit the proliferation and migration of ASMCs in RSV-infected rats.34 LncRNA GAS5 induces the proliferation of ASMCs via miR-10a/BDNF.35 In this paper, we performed bioinformatic identification of LncRNA NEAT1 and miR-9-5p. We hypothesized that the regulation of SLC26A2 by LncRNA NEAT1/miR-9-5p affects PDGF-induced proliferation, migration, and production of inflammatory factor production of HASMCs. To verify our assumptions, dual luciferase reporter gene and RIP analysis were used to confirm the binding relationship between miR-9-5p and NEAT1 or SLC26A2. Rescue experiments indicated that the inhibitory role of SLC26A2 deficiency on the proliferation, migration, and production of inflammatory factors in HASMCs were blocked by miR-9-5p inhibitor. Knockdown of NEAT1 inhibited PDGF-stimulated HASMC proliferation, migration, and inflammatory factors production, and enhanced cell contractile phenotype marker protein expression via miR-9-5p/SLC26A2.
In summary, our study is the first to identify and validate that SLC26A2 is significantly upregulated in PDGF-induced HASMCs. Also, SLC26A2 knockdown inhibits proliferation, migration, and inflammatory factor production of HASMCs via NEAT1/miR-9-5p. The findings provide a theoretical basis for exploring the inhibition of airway inflammation and remodeling in asthma.
ACKNOWLEDGEMENTS
This work was supported by grants from the Zhejiang Medical and Health Science and Technology Plan Project (2021KY928).
Footnotes
The authors have no potential conflicts of interest to disclose.
- Conceptualization: Xiangying Wang.
- Data curation: Ruju Xu.
- Formal analysis: Di Chi.
- Funding acquisition: Xiangying Wang.
- Investigation: Chufeng Dai.
- Methodology: Xiangying Wang and Ruju Xu.
- Project administration: Meiling Sheng.
- Resources: Chufeng Dai.
- Software: Di Chi.
- Supervision: Meiling Sheng.
- Validation: Xiangying Wang and Ruju Xu.
- Visualization: Di Chi and Chufeng Dai.
- Writing—original draft: Xiangying Wang.
- Writing—review & editing: Meiling Sheng.
- Approval of final manuscript: all authors.
SUPPLEMENTARY MATERIAL
The identification of HASMCs. (A) Morphological and phenotypic identification of cultured HASMCs (original magnification: ×100). The typical peak and valley growth pattern of HASMCs. (B) Immunofluorescence staining of positive expression of α-SMA (green-fluorescence) in HASMCs. HASMCs, human airway smooth muscle cells; α-SMA, α-smooth muscle actin.
References
- 1.Schwartz SP, Rehder KJ. Quality improvement in pediatrics: past, present, and future. Pediatr Res. 2017;81:156–161. doi: 10.1038/pr.2016.192. [DOI] [PubMed] [Google Scholar]
- 2.Fleming L, Wilson N, Bush A. Difficult to control asthma in children. Curr Opin Allergy Clin Immunol. 2007;7:190–195. doi: 10.1097/ACI.0b013e3280895d0c. [DOI] [PubMed] [Google Scholar]
- 3.Bellin MH, Osteen P, Kub J, Bollinger ME, Tsoukleris M, Chaikind L, et al. Stress and quality of life in urban caregivers of children with poorly controlled asthma: a longitudinal analysis. J Pediatr Health Care. 2015;29:536–546. doi: 10.1016/j.pedhc.2015.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perry MM, Baker JE, Gibeon DS, Adcock IM, Chung KF. Airway smooth muscle hyperproliferation is regulated by microRNA-221 in severe asthma. Am J Respir Cell Mol Biol. 2014;50:7–17. doi: 10.1165/rcmb.2013-0067OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dragon S, Hirst SJ, Lee TH, Gounni AS. IL-17A mediates a selective gene expression profile in asthmatic human airway smooth muscle cells. Am J Respir Cell Mol Biol. 2014;50:1053–1063. doi: 10.1165/rcmb.2012-0267OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlin SM, Roth M, Black JL. Urokinase potentiates PDGF-induced chemotaxis of human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2003;284:L1020–L1026. doi: 10.1152/ajplung.00092.2002. [DOI] [PubMed] [Google Scholar]
- 7.Hirst SJ, Barnes PJ, Twort CH. Quantifying proliferation of cultured human and rabbit airway smooth muscle cells in response to serum and platelet-derived growth factor. Am J Respir Cell Mol Biol. 1992;7:574–581. doi: 10.1165/ajrcmb/7.6.574. [DOI] [PubMed] [Google Scholar]
- 8.Mount DB, Romero MF. The SLC26 gene family of multifunctional anion exchangers. Pflugers Arch. 2004;447:710–721. doi: 10.1007/s00424-003-1090-3. [DOI] [PubMed] [Google Scholar]
- 9.Dorwart MR, Shcheynikov N, Wang Y, Stippec S, Muallem S. SLC26A9 is a Cl− channel regulated by the WNK kinases. J Physiol. 2007;584:333–345. doi: 10.1113/jphysiol.2007.135855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heneghan JF, Akhavein A, Salas MJ, Shmukler BE, Karniski LP, Vandorpe DH, et al. Regulated transport of sulfate and oxalate by SLC26A2/DTDST. Am J Physiol Cell Physiol. 2010;298:C1363–C1375. doi: 10.1152/ajpcell.00004.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohana E, Shcheynikov N, Park M, Muallem S. Solute carrier family 26 member a2 (Slc26a2) protein functions as an electroneutral SO4(2-)/OH-/Cl- exchanger regulated by extracellular Cl- J Biol Chem. 2012;287:5122–5132. doi: 10.1074/jbc.M111.297192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park M, Ohana E, Choi SY, Lee MS, Park JH, Muallem S. Multiple roles of the SO4(2-)/Cl-/OH- exchanger protein Slc26a2 in chondrocyte functions. J Biol Chem. 2014;289:1993–2001. doi: 10.1074/jbc.M113.503466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spyroglou A, Bozoglu T, Rawal R, De Leonardis F, Sterner C, Boulkroun S, et al. Diastrophic dysplasia sulfate transporter (SLC26A2) is expressed in the adrenal cortex and regulates aldosterone secretion. Hypertension. 2014;63:1102–1109. doi: 10.1161/HYPERTENSIONAHA.113.02504. [DOI] [PubMed] [Google Scholar]
- 14.Hogg DR, Harries LW. Human genetic variation and its effect on miRNA biogenesis, activity and function. Biochem Soc Trans. 2014;42:1184–1189. doi: 10.1042/BST20140055. [DOI] [PubMed] [Google Scholar]
- 15.Francisco-Garcia AS, Garrido-Martín EM, Rupani H, Lau LCK, Martinez-Nunez RT, Howarth PH, et al. Small RNA species and microRNA profiles are altered in severe asthma nanovesicles from broncho alveolar lavage and associate with impaired lung function and inflammation. Noncoding RNA. 2019;5:51. doi: 10.3390/ncrna5040051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao J, Pu J, Hao B, Huang L, Chen J, Hong W, et al. LncRNA RP11-86H7.1 promotes airway inflammation induced by TRAPM2.5 by acting as a ceRNA of miRNA-9-5p to regulate NFKB1 in HBECS. Sci Rep. 2020;10:11587. doi: 10.1038/s41598-020-68327-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang H, Su X, Wu Q, Shan H, Lv L, Yu T, et al. LncRNA 28104 03D21Rik/Mirf promotes ischemic myocardial injury by regulating autophagy through targeting Mir26a. Autophagy. 2020;16:1077–1091. doi: 10.1080/15548627.2019.1659610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie Q, Lin S, Zheng M, Cai Q, Tu Y. Long noncoding RNA NEAT1 promotes the growth of cervical cancer cells via sponging miR-9-5p. Biochem Cell Biol. 2019;97:100–108. doi: 10.1139/bcb-2018-0111. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Yao XH, Wu Y, Cao GK, Han D. LncRNA NEAT1 regulates pulmonary fibrosis through miR-9-5p and TGF-β signaling pathway. Eur Rev Med Pharmacol Sci. 2020;24:8483–8492. doi: 10.26355/eurrev_202008_22661. [DOI] [PubMed] [Google Scholar]
- 20.Lou L, Tian M, Chang J, Li F, Zhang G. MiRNA-192-5p attenuates airway remodeling and autophagy in asthma by targeting MMP-16 and ATG7. Biomed Pharmacother. 2020;122:109692. doi: 10.1016/j.biopha.2019.109692. [DOI] [PubMed] [Google Scholar]
- 21.Huo Y, Guan L, Xu J, Zhou L, Chen R. Tiotropium inhibits methacholine-induced extracellular matrix production via β-catenin signaling in human airway smooth muscle cells. Int J Chron Obstruct Pulmon Dis. 2018;13:1469–1481. doi: 10.2147/COPD.S158552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu YP, Jin YP, Wu XS, Yang Y, Li YS, Li HF, et al. LncRNA-HGBC stabilized by HuR promotes gallbladder cancer progression by regulating miR-502-3p/SET/AKT axis. Mol Cancer. 2019;18:167. doi: 10.1186/s12943-019-1097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang X, Wang J, Chen W, Ma X, Wang Y, Nagao N, et al. Inhibition of airway remodeling and inflammation by isoforskolin in PDGF-induced rat ASMCs and OVA-induced rat asthma model. Biomed Pharmacother. 2017;95:275–286. doi: 10.1016/j.biopha.2017.08.063. [DOI] [PubMed] [Google Scholar]
- 24.Oliver MN, Fabry B, Marinkovic A, Mijailovich SM, Butler JP, Fredberg JJ. Airway hyperresponsiveness, remodeling, and smooth muscle mass: right answer, wrong reason? Am J Respir Cell Mol Biol. 2007;37:264–272. doi: 10.1165/rcmb.2006-0418OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wright DB, Trian T, Siddiqui S, Pascoe CD, Johnson JR, Dekkers BG, et al. Phenotype modulation of airway smooth muscle in asthma. Pulm Pharmacol Ther. 2013;26:42–49. doi: 10.1016/j.pupt.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 26.Hoshino M, Takahashi M, Aoike N. Expression of vascular endothelial growth factor, basic fibroblast growth factor, and angiogenin immunoreactivity in asthmatic airways and its relationship to angiogenesis. J Allergy Clin Immunol. 2001;107:295–301. doi: 10.1067/mai.2001.111928. [DOI] [PubMed] [Google Scholar]
- 27.Hirota JA, Ask K, Farkas L, Smith JA, Ellis R, Rodriguez-Lecompte JC, et al. In vivo role of platelet-derived growth factor-BB in airway smooth muscle proliferation in mouse lung. Am J Respir Cell Mol Biol. 2011;45:566–572. doi: 10.1165/rcmb.2010-0277OC. [DOI] [PubMed] [Google Scholar]
- 28.Dai Y, Cheng R, Gao J, Li Y, Lou C, Li Y. Casticin inhibits PDGF-induced proliferation and migration of airway smooth muscle cells. Eur J Pharmacol. 2018;830:39–46. doi: 10.1016/j.ejphar.2018.04.016. [DOI] [PubMed] [Google Scholar]
- 29.Cheng W, Yan K, Chen Y, Zhang W, Ji Z, Dang C. ABCA1 inhibits PDGF-induced proliferation and migration of rat airway smooth muscle cell through blocking TLR2/NF-κB/NFATc1 signaling. J Cell Biochem. 2018;119:7388–7396. doi: 10.1002/jcb.27046. [DOI] [PubMed] [Google Scholar]
- 30.Xu YD, Wang Y, Yin LM, Peng LL, Park GH, Yang YQ. S100A8 inhibits PDGF-induced proliferation of airway smooth muscle cells dependent on the receptor for advanced glycation end-products. Biol Res. 2017;50:23. doi: 10.1186/s40659-017-0128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang X, Zhao X, Sun H, Yan Y, Huang L, Gu W, et al. The role of miR-29c/B7-H3 axis in children with allergic asthma. J Transl Med. 2018;16:218. doi: 10.1186/s12967-018-1590-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bartel S, Carraro G, Alessandrini F, Krauss-Etschmann S, Ricciardolo FLM, Bellusci S. miR-142-3p is associated with aberrant WNT signaling during airway remodeling in asthma. Am J Physiol Lung Cell Mol Physiol. 2018;315:L328–L333. doi: 10.1152/ajplung.00113.2018. [DOI] [PubMed] [Google Scholar]
- 33.Zhang K, Liang Y, Feng Y, Wu W, Zhang H, He J, et al. Decreased epithelial and sputum miR-221-3p associates with airway eosinophilic inflammation and CXCL17 expression in asthma. Am J Physiol Lung Cell Mol Physiol. 2018;315:L253–L264. doi: 10.1152/ajplung.00567.2017. [DOI] [PubMed] [Google Scholar]
- 34.Yu X, Zhe Z, Tang B, Li S, Tang L, Wu Y, et al. α-Asarone suppresses the proliferation and migration of ASMCs through targeting the lncRNA-PVT1/miR-203a/E2F3 signal pathway in RSV-infected rats. Acta Biochim Biophys Sin (Shanghai) 2017;49:598–608. doi: 10.1093/abbs/gmx048. [DOI] [PubMed] [Google Scholar]
- 35.Zhang XY, Tang XY, Li N, Zhao LM, Guo YL, Li XS, et al. GAS5 promotes airway smooth muscle cell proliferation in asthma via controlling miR-10a/BDNF signaling pathway. Life Sci. 2018;212:93–101. doi: 10.1016/j.lfs.2018.09.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The identification of HASMCs. (A) Morphological and phenotypic identification of cultured HASMCs (original magnification: ×100). The typical peak and valley growth pattern of HASMCs. (B) Immunofluorescence staining of positive expression of α-SMA (green-fluorescence) in HASMCs. HASMCs, human airway smooth muscle cells; α-SMA, α-smooth muscle actin.