Abstract
Purpose
In this prospective study, we evaluated the association between the serum levels of antioxidants uric acid (UA), albumin, and total bilirubin and the risk of cancer in a Korean population.
Materials and Methods
A total of 15882 subjects were followed up for cancer development and cancer-related death. During the follow-up period, 1619 cancer diagnoses and 617 cancer-related deaths were recorded. Cox proportional regression was performed to calculate the hazard ratio (HR) per standard deviation (SD) increment and 95% confidence interval (CI). The model was adjusted for covariates such as the age, sex, smoking, alcohol consumption, physical activity, education level, body mass index, and family history. Sensitivity analyses using the study subjects with physiological serum levels of each indicator were also performed.
Results
UA levels were positively correlated with cancer risk (HR per SD increment 1.04; 95% CI, 1.01–1.09), and albumin levels were inversely associated with the overall cancer risk (HR, 0.92; 95% CI, 0.88–0.96) and cancer-related death (HR, 0.86; 95% CI, 0.80–0.93). Total bilirubin levels were negatively correlated with the risk of cancer-related death (HR, 0.91; 95% CI, 0.83–0.99). By cancer type, UA was positively associated with prostate cancer, total bilirubin was positively associated with liver cancer, and albumin was inversely associated with lung cancer.
Conclusion
The findings of this study support the role of antioxidants in carcinogenesis. Future large-cohort studies are needed to confirm the predictive value of albumin, UA, and total bilirubin levels in each type of cancer.
Keywords: Serum albumin, bilirubin, uric acid, neoplasms, antioxidants
INTRODUCTION
Endogenous antioxidants, including uric acid (UA), albumin, and bilirubin, have been shown to reduce the risk of cancer through detoxification of reactive oxygen species (ROS).1 ROS play a key role in cancer development and progression by promoting oxidative DNA damage, and also contributes to the introduction of mutations in oncogenes and tumor-suppressor genes.2 Although the effects of endogenous and dietary antioxidants on tumor initiation and progression have been extensively studied, the role of ROS in tumorigenesis remains poorly understood.1
Paradoxically, the endogenous antioxidant UA exerts antioxidant effects in the extracellular environment and pro-oxidative effects in the intracellular environment.3 Interestingly, a meta-analysis of five studies has shown a strong association between serum UA levels and cancer incidence in 456053 subjects.4 Consistently, the Swedish apolipoprotein-related mortality risk study (AMORIS) has demonstrated a similar association, although this association was sex- and cancer type-dependent.3 However, the European prospective investigation into cancer and nutrition (EPIC) study has shown a negative correlation between serum UA levels and the risk of breast cancer.5 The association between serum albumin levels and the risk of cancer is also poorly understood. A UK cohort study has shown that lower pre-diagnosis serum albumin levels were associated with an increased risk of cancer in 100122 subjects.6 In contrast, the AMORIS and EPIC-Heidelberg cohort studies have found a negative correlation between baseline serum albumin levels and the risk of colorectal and breast cancer, respectively.5,7 The inverse association between bilirubin levels and cancer incidence was well demonstrated;8 however, this association varied depending on the sex and cancer type. Among individuals, uridine diphosphoglucuronate glucuronosyltransferase 1A1 polymorphisms and high serum bilirubin levels were associated with an increased incidence of colorectal cancer in males, but not in females.9 Another prospective study reported no association between serum bilirubin levels and the risk of cancer.5
Despite the previous extensive investigations, the roles of UA, albumin, and bilirubin in different cancer types remain unclear. Additionally, the association between the risk of cancer and the levels of UA, albumin, and bilirubin in Asian populations is understudied. In this study, we prospectively evaluated the association between the levels of UA, albumin, and bilirubin and the risk of cancer in a Korean population.
MATERIALS AND METHODS
Study subjects
The study cohort consisted of community-dwelling adults aged ≥50 years who underwent baseline examinations as a part of two studies, the Namwon study (n=10667; 2004–2007) and the Don-gu study (n=9260; 2007–2010). Details of the study subjects and measurement methods have been previously reported.10 Subject follow-up for cancer development and death was conducted until the end of 2017 using the national cancer registry database of the National Cancer Center Korea and until the end of 2018 using the death registration database of the National Bureau of Statistics. Median follow-up period for cancer incidence and cancer death were 9.65 years (range 1.0 to 13.99) and 11.47 years (range 1.0 to 14.99), respectively. Among the participants of the two cohorts (n=19927), we excluded the subjects who had cancer at the time of baseline examination (n=814); subjects with missing information on UA, albumin, total bilirubin, or other baseline measurements (n=2980); and subjects who were diagnosed with cancer within 12 months after baseline examination (n=251). Our analyses included data from 15882 individuals (6094 males and 9788 females). During the follow-up period, 1619 subjects (938 males and 681 females) were diagnosed with cancer, and 617 subjects (403 males and 214 females) died due to cancer. All study participants provided informed consent, and the cohort studies were approved by the Institutional Review Board of Chonnam National University Hospital (IRB No. I-2008-05-056, I-2014-215).
Measurements
Venous blood samples were obtained after 12-hour (overnight) fasting. Blood processing, including the preparation of serum, plasma, and buffy coat, was performed on-site. Serum was isolated by high-speed centrifugation (4℃) within 30 min of blood withdrawal. The levels of UA, SA, total bilirubin, and high-density lipoprotein (HDL) cholesterol were determined by enzymatic assay using an automatic analyzer (Hitachi-7600; Hitachi, Japan). Smoking, alcohol consumption, education level, family history of cancer, and physical activity were recorded. Smoking and alcohol consumption status were recorded as current, former, and never smoker or drinker. Physical activity was classified as never, seldom, sometimes, often, and always. Education level was classified as uneducated, primary school, middle school, and post-high school. Height, waist circumference, and weight were registered to the nearest 0.1 cm and 0.1 kg, respectively. Cancer history was recorded at the time of baseline examination by both self-reporting and search in the cancer registry. Diabetes was defined using fasting glucose ≥126 mg/dL in baseline examination, and individuals who were under treatment of diabetes were also classified as having diabetes.
Statistical analysis
Baseline characteristics were compared between the subjects who were diagnosed with cancer during the follow-up period and those without a cancer diagnosis. Statistical significance was determined by chi-square test or Student's t-test as appropriate. The associations between the levels of endogenous antioxidants and the risk of cancer (stomach, colorectum, lung, breast, prostate, and thyroid cancer) and cancer-related death were assessed using Cox proportional hazards regression to calculate the hazard ratio (HR) per standard deviation (SD) increment and 95% confidence interval (CI) in each antioxidant. The levels of endogenous antioxidants were provided as continuous rather than quartiles. Age, sex, smoking, alcohol consumption, physical activity, education level, body mass index, family history of cancer, diabetes, and HDL cholesterol were adjusted as covariates in the model. Physiological levels of UA (males, <7 mg/dL; females, <6 mg/dL), albumin (>3.5 g/dL), and total bilirubin (<1.2 mg/dL) were used for sensitivity analysis. To evaluate the sex difference in the associations, sex-specific analysis was also performed for all cancer types. Penalized B-spline curves adjusted for covariates were fitted to evaluate the nonlinearity between cancer risk and the serum levels of antioxidants. Data points below the 0.5 percentile and above the 99.5 percentile were removed as outliers. p-values<0.05 were considered statistically significant. All analyses were performed using R (version 3.6.1, Vienna, Austria).
RESULTS
Baseline characteristics of the study participants are presented in Table 1. Cancer diagnosis (n=1619) was more frequent among male, older, educated individuals who smoked and consumed alcohol. Serum UA levels were significantly higher in cancer cases, whereas no significant differences were observed in the serum levels of total bilirubin and albumin (Table 1).
Table 1. Baseline Characteristics of Study Subjects (n=15882).
| No cancer (n=14263) | Cancer incidence (n=1619) | p value | ||
|---|---|---|---|---|
| Sex | <0.001 | |||
| Male | 5156 (36.1) | 938 (57.9) | ||
| Female | 9107 (63.9) | 681 (42.1) | ||
| Age (yr) | 62.9±8.2 | 65.0±7.5 | <0.001 | |
| Body mass index (kg/m2) | 24.4±3.0 | 24.4±3.0 | 0.558 | |
| Waist circumference (cm) | 87.4±8.4 | 87.9±8.4 | 0.094 | |
| Smoking | <0.001 | |||
| Never | 10004 (70.1) | 826 (51.0) | ||
| Former/current smoker | 4259 (29.9) | 793 (49.0) | ||
| Alcohol | <0.001 | |||
| Never | 6403 (44.9) | 570 (35.2) | ||
| Former/current drinker | 7860 (55.1) | 1049 (64.8) | ||
| Regular exercise | 0.232 | |||
| Never/seldom | 8777 (61.5) | 971 (60.0) | ||
| Sometimes and more | 5486 (38.5) | 648 (40.0) | ||
| Education levels | 0.218 | |||
| Primary school and under | 8267 (58.0) | 912 (56.3) | ||
| Middle school and higher | 5996 (42.0) | 707 (43.7) | ||
| Family cancer history | 0.927 | |||
| No | 10640 (74.6) | 1210 (74.7) | ||
| Yes | 3623 (25.4) | 409 (25.3) | ||
| Diabetes | 0.001 | |||
| No | 12066 (84.6) | 1316 (81.3) | ||
| Yes | 2197 (15.4) | 303 (18.7) | ||
| HDL cholesterol (mg/dL) | 49.6±11.9 | 48.2±12.1 | <0.001 | |
| Uric acid (mg/dL) | 4.6 (0.1–12.8) | 4.9 (1.6–10.5) | <0.001 | |
| Albumin (g/dL) | 4.5 (1.3–6.0) | 4.5 (0.7–5.8) | 0.057 | |
| Bilirubin (mg/dL) | 0.7 (0.1–5.3) | 0.7 (0.1–3.6) | 0.268 | |
HDL, high density lipoprotein.
Values are expressed as n (%), mean±standard deviation, or median (ranges), as appropriate.
The associations between cancer risk and the serum levels of UA, albumin, and total bilirubin are summarized in Table 2. UA levels were positively correlated with the overall risk of cancer (HR per 1 SD increment, 1.04; 95% CI, 1.01–1.09), as well as the risk of prostate cancer (HR, 1.33; 95% CI, 1.12–1.59). Albumin levels were negatively correlated with the overall risk of cancer incidence (HR, 0.92; 95% CI, 0.88–0.96), the risk of lung cancer (HR, 0.81; 95% CI, 0.73–0.91) and liver cancer (HR, 0.65; 95% CI, 0.59–0.72), as well as the risk of cancer-related death (HR, 0.86; 95% CI, 0.80–0.93). Total bilirubin levels were also associated with the risk of liver cancer (HR, 1.23; 95% CI, 1.11–1.37) and negatively correlated with the risk of cancer-related death (HR, 0.91; 95% CI, 0.83–0.99).
Table 2. Hazard Ratios Per 1 SD Increment (95% CI) of Cancer Incidence of UA, Albumin, and Total Bilirubin.
| UA | Albumin | Total bilirubin | |||||
|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | ||
| Cancer incidence (n=1619) | 1.05 (1.02–1.11) | 1.04 (1.01–1.09) | 0.92 (0.88–0.96) | 0.92 (0.88–0.96) | 0.95 (0.90–1.00) | 0.96 (0.91–1.01) | |
| Stomach (n=298) | 0.94 (0.83–1.07) | 0.92 (0.81–1.05) | 1.01 (0.90–1.13) | 0.97 (0.87–1.09) | 0.95 (0.85–1.07) | 0.96 (0.86–1.08) | |
| CRC (n=238) | 1.11 (0.98–1.27) | 1.08 (0.94–1.23) | 1.03 (0.91–1.17) | 0.99 (0.87–1.13) | 0.95 (0.83–1.08) | 0.96 (0.84–1.09) | |
| Lung (n=231) | 0.91 (0.79–1.04) | 0.93 (0.81–1.07) | 0.82 (0.73–0.92) | 0.81 (0.73–0.91) | 0.85 (0.73–0.98) | 0.88 (0.77–1.02) | |
| Thyroid (n=135) | 1.03 (0.84–1.28) | 0.97 (0.78–1.22) | 0.99 (0.83–1.17) | 1.06 (0.88–1.27) | 0.99 (0.81–1.20) | 0.98 (0.80–1.19) | |
| Prostate (n=116) | 1.36 (1.15–1.60) | 1.33 (1.12–1.59) | 1.07 (0.89–1.28) | 1.08 (0.90–1.30) | 0.96 (0.81–1.15) | 0.95 (0.80–1.14) | |
| Liver (n=95) | 1.07 (0.87–1.33) | 1.05 (0.84–1.30) | 0.67 (0.61–0.73) | 0.65 (0.59–0.72) | 1.24 (1.11–1.38) | 1.23 (1.11–1.37) | |
| Breast (n=52) | 1.00 (0.70–1.43) | 0.92 (0.63–1.35) | 1.12 (0.84–1.48) | 1.11 (0.82–1.49) | 1.21 (0.96–1.53) | 1.16 (0.89–1.52) | |
| Others (n=454) | 1.12 (1.02–1.24) | 1.11 (1.00–1.22) | 1.00 (0.91–1.10) | 0.98 (0.89–1.07) | 0.86 (0.77–0.95) | 0.86 (0.78–0.96) | |
| Cancer death (n=617) | 1.02 (0.94–1.11) | 1.03 (0.95–1.12) | 0.88 (0.82–0.95) | 0.86 (0.80–0.93) | 0.89 (0.81–0.97) | 0.91 (0.83–0.99) | |
SD, standard deviation; CI, confidence interval; UA, uric acid; N, number of cancer incidence or deaths; CRC, colorectal cancer.
Model 1 was adjusted for age and sex. Model 2 was adjusted for age, sex, smoking, alcohol drinking, physical activity, education levels, body mass index, family history of diabetes, and serum high density lipoprotein cholesterol levels.
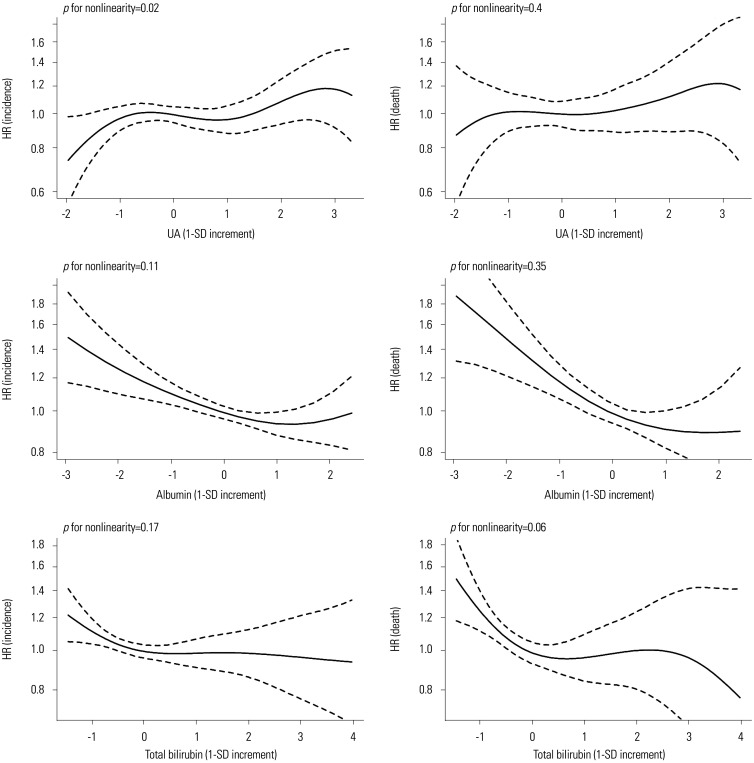
Sensitivity analysis results of the subjects with physiological serum levels of UA, albumin, and total bilirubin are presented in Table 3. UA levels were not associated with the overall cancer risk or prostate cancer risk. However, the negative correlation between albumin levels and the overall risk of cancer, the risk of lung cancer and liver cancer, and cancer-related mortality were maintained. In addition, a significant inverse association between total bilirubin levels and the overall risk of cancer incidence and cancer death were observed (Table 3). Sex-specific analysis results are presented in Table 4. The inverse association between albumin and the risk of overall cancer (HR, 0.92; 95% CI, 0.86–0.99) and cancer-related death (HR, 0.89; 95% CI, 0.80–0.99) was maintained in male, but not in female. No association between cancer risk and serum bilirubin levels was found in both sexes, but an inverse association between serum bilirubin and risk of cancer death was maintained in male (HR, 0.89; 95% CI, 0.80–0.99) (Table 4). The differences between the goodness-of-fit and non-linear models were not significant (p>0.05) (Fig. 1).
Table 3. Hazard Ratios Per 1 SD Increment (95% CI) of Cancer Incidence of UA, Albumin, and Total Bilirubin within the Normal Range.
| UA | Albumin | Total bilirubin | |||||
|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | ||
| Cancer incidence (n=1348) | 1.04 (0.98–1.10) | 1.02 (0.96–1.08) | 0.93 (0.89–0.98) | 0.93 (0.88–0.98) | 0.94 (0.89–1.00) | 0.95 (0.90–1.00) | |
| Stomach (n=250) | 0.95 (0.83–1.09) | 0.93 (0.81–1.07) | 0.99 (0.88–1.12) | 0.97 (0.86–1.10) | 0.91 (0.80–1.03) | 0.92 (0.81–1.05) | |
| CRC (n=197) | 1.14 (0.98–1.34) | 1.13 (0.96–1.32) | 1.03 (0.90–1.19) | 1.00 (0.87–1.15) | 0.88 (0.76–1.01) | 0.89 (0.77–1.03) | |
| Lung (n=198) | 0.91 (0.78–1.06) | 0.94 (0.81–1.10) | 0.83 (0.72–0.96) | 0.82 (0.72–0.94) | 0.85 (0.74–0.98) | 0.89 (0.77–1.03) | |
| Thyroid (n=123) | 1.07 (0.86–1.33) | 1.01 (0.80–1.26) | 1.01 (0.84–1.21) | 1.07 (0.89–1.28) | 1.06 (0.88–1.27) | 1.05 (0.87–1.27) | |
| Prostate (n=83) | 1.26 (0.99–1.59) | 1.23 (0.97–1.57) | 1.06 (0.86–1.30) | 1.07 (0.86–1.32) | 1.09 (0.89–1.34) | 1.08 (0.87–1.33) | |
| Liver (n=69) | 1.03 (0.79–1.33) | 0.99 (0.76–1.29) | 0.54 (0.43–0.68) | 0.53 (0.42–0.66) | 1.16 (0.92–1.46) | 1.19 (0.94–1.50) | |
| Breast (n=49) | 1.06 (0.75–1.50) | 0.96 (0.67–1.38) | 1.07 (0.81–1.43) | 1.06 (0.79–1.43) | 1.34 (1.02–1.77) | 1.27 (0.95–1.70) | |
| Others (n=379) | 1.09 (0.97–1.22) | 1.07 (0.95–1.20) | 1.00 (0.91–1.11) | 0.99 (0.89–1.10) | 0.89 (0.81–0.99) | 0.89 (0.80–0.99) | |
| Cancer death (n=509) | 0.99 (0.90–1.08) | 1.01 (0.91–1.11) | 0.89 (0.82–0.97) | 0.88 (0.80–0.95) | 0.87 (0.79–0.95) | 0.89 (0.82–0.98) | |
SD, standard deviation; CI, confidence interval; UA, uric acid; N, number of cancer incidence or deaths; CRC, colorectal cancer.
Model 1 was adjusted for age and sex. Model 2 was adjusted for age, sex, smoking, alcohol drinking, physical activity, education levels, body mass index, family history of diabetes, and serum high density lipoprotein cholesterol levels.
Table 4. Hazard Ratios Per 1 SD Increment (95% CI) of Cancer Incidence of UA, Albumin, and Total Bilirubin within the Normal Range Stratified by Sex.
| Sex | UA | Albumin | Bilirubin | ||
|---|---|---|---|---|---|
| Male (n=716) | |||||
| Cancer incidence (n=716) | 1.01 (0.93–1.09) | 0.92 (0.86–0.99) | 0.94 (0.87–1.01) | ||
| Stomach (n=149) | 0.91 (0.77–1.08) | 1.03 (0.88–1.20) | 0.93 (0.79–1.09) | ||
| CRC (n=110) | 1.08 (0.88–1.32) | 0.96 (0.80–1.15) | 0.96 (0.80–1.15) | ||
| Lung (n=137) | 0.93 (0.78–1.11) | 0.85 (0.72–0.99) | 0.87 (0.74–1.03) | ||
| Thyroid (n=11) | 0.92 (0.48–1.77) | 0.87 (0.49–1.53) | 1.73 (0.98–3.05) | ||
| Prostate (n=83) | 1.23 (0.97–1.57) | 1.07 (0.86–1.32) | 1.08 (0.87–1.33) | ||
| Liver (n=42) | 0.93 (0.67–1.28) | 0.50 (0.37–0.66) | 1.21 (0.90–1.61) | ||
| Others (n=184) | 1.06 (0.91–1.25) | 0.98 (0.85–1.12) | 0.84 (0.73–0.97) | ||
| Cancer death (n=316) | 0.99 (0.88–1.11) | 0.87 (0.78–0.96) | 0.89 (0.80–0.99) | ||
| Female (n=628) | |||||
| Cancer incidence (n=628) | 1.03 (0.93–1.13) | 0.96 (0.89–1.04) | 0.96 (0.88–1.05) | ||
| Stomach (n=99) | 0.94 (0.74–1.20) | 0.88 (0.72–1.08) | 0.90 (0.72–1.12) | ||
| CRC (n=88) | 1.16 (0.90–1.50) | 1.06 (0.85–1.31) | 0.80 (0.63–1.02) | ||
| Lung (n=59) | 0.96 (0.70–1.31) | 0.81 (0.62–1.05) | 0.98 (0.74–1.30) | ||
| Thyroid (n=111) | 1.00 (0.79–1.27) | 1.09 (0.89–1.33) | 0.98 (0.80–1.21) | ||
| Breast (n=49) | 0.96 (0.67–1.38) | 1.06 (0.79–1.43) | 1.27 (0.95–1.70) | ||
| Liver (n=27) | 1.06 (0.67–1.68) | 0.54 (0.37–0.77) | 1.12 (0.76–1.66) | ||
| Others (n=195) | 1.08 (0.90–1.28) | 1.02 (0.88–1.18) | 0.96 (0.82–1.12) | ||
| Cancer death (n=191) | 1.03 (0.87–1.23) | 0.90 (0.78–1.04) | 0.91 (0.78–1.07) | ||
SD, standard deviation; CI, confidence interval; UA, uric acid; N, number of cancer incidence or deaths; CRC, colorectal cancer.
HRs were adjusted for age, sex, smoking, alcohol drinking, physical activity, education levels, body mass index, family history of diabetes, and serum high density lipoprotein cholesterol levels.
Fig. 1. HRs of cancer incidence and cancer death of UA, albumin, and total bilirubin levels using penalized B-spline curves. HR, hazard ratio; SD, standard deviation; UA, uric acid.
DISCUSSION
In this prospective cohort study, we evaluated the association between the serum levels of endogenous antioxidants (UA, albumin, and bilirubin) and the risk of cancer and cancer-related death in a Korean population. To the best of our knowledge, this is the most extensive prospective study to evaluate the relationship between the levels of UA and albumin and the risk of different cancers in an Asian population. We found that the overall risk of cancer was positively correlated with serum UA levels and negatively correlated with albumin and bilirubin levels. The risks of gastric cancer and prostate cancer were positively correlated with serum UA levels, whereas lung cancer risk was negatively correlated with serum albumin levels. Furthermore, albumin and bilirubin serum levels were negatively correlated with the risk of cancer-related death.
Growing evidence suggests a protective role of endogenous antioxidants in ROS-induced carcinogenesis;1 however, findings vary depending on the cancer type, and the precise mechanisms underlying the protective effects of different antioxidants remain elusive. ROS play a key role in cancer development and progression by promoting oxidative DNA damage, and also contributes to the introduction of mutations in oncogenes or tumor-suppressor genes.2 By preventing ROS-induced DNA oxidation and DNA damage, antioxidants inhibit tumor progression. However, the protective effect of antioxidants against ROS-induced tumor initiation remains unclear.1
We found that the serum levels of UA were positively correlated with the overall risk of cancer, as well as the risk of prostate cancer. However, these associations were not observed when only subjects with physiological serum UA levels were taken into consideration. Consistent with our findings, a recent meta-analysis and the AMORIS study showed a strong association between serum UA levels and the risk of cancer.3,4
In contrast to some previous studies,5,11,12 we found a significant association between serum UA levels and prostate cancer risk. Among Japanese male in Hawaii, increased incidence of prostate cancer was observed in individuals with high serum UA levels compared to those with low serum UA levels.11 However, two recent large-cohort prospective studies reported no association between serum UA levels and the risk of prostate cancer.5,12 Consistent with the findings of a recent meta-analysis,13 we found a significant association between serum UA levels and the risk of urological and digestive cancers, but not breast cancer. The AMORIS study reported a negative correlation between serum UA levels and the risk of breast and lung cancers.3 Xanthine, a precursor of UA, is catabolized by xanthine oxidoreductase, which produces ROS as by-products,14 providing a potential link between UA and carcinogenesis. Additionally, hyperuricemia is closely related to chronic inflammation, type 2 diabetes mellitus, obesity, insulin resistance, and hypertension, all of which may contribute to carcinogenesis.15
In this study, we found a negative correlation between albumin levels and the overall risk of cancer, the risk of lung cancer, and cancer-related death. These associations were significant in individuals with physiological serum levels of albumin. A previous prospective cohort study showed an increased cancer risk in individuals with hypoalbuminemia.6 However, several prospective studies reported no association between serum albumin levels and the risk of lung cancer,5,16 whereas a negative correlation was observed between albumin levels and the risk of breast and colorectal cancers.5,7 In contrast to these previous findings, we found a significant association between albumin levels and the risk of lung cancer. Additionally, we found an inverse association between albumin levels and the risk of cancer-related death, in line with previous findings.5,17 Most previous studies on the relationship between albumin levels and the risk of cancer involved the measurement of albumin levels in subjects who were already diagnosed with cancer; therefore, further prospective investigations are required to elucidate the relationship between pre-diagnosis albumin levels and the risk of cancer. Additionally, the negative association between albumin and liver cancer outcomes is likely to be findings due to reverse-causality.
Total bilirubin levels were negatively correlated with the risk of cancer-related death, both in the entire cohort and in individuals with physiological total bilirubin levels (<1.2 mg/dL). In the latter, bilirubin levels were also negatively correlated with the overall risk of cancer, possibly since patients with elevated bilirubin levels due to hepatobiliary disease were excluded from the analysis. Although the EPIC study reported no significant association between bilirubin levels and the risk of breast, prostate, colorectal, and lung cancers,5 other studies reported an inverse association between bilirubin levels and lung cancer risk.18,19 A causal relationship between low bilirubin levels and the risk of lung cancer was suggested in a Mendelian randomization study using two variants (rs887829 and rs4149056) as instrumental variables that together explain nearly 40% of the variability in bilirubin levels.20 The risk of cancer-related death was also inversely associated with bilirubin levels, consistent with previous findings;21,22 however, the mechanisms underlying the protective effects of bilirubin remain unclear. Bilirubin is a metabolic end-product of heme catabolism. Upon oxidization, bilirubin is converted to biliverdin by biliverdin reductase. Moreover, bilirubin has been shown to inhibit the activity of nicotinamide adenine dinucleotide phosphate oxidase, a primary source of ROS in the vasculature.23
This study has several limitations. First, the levels of UA, albumin, and total bilirubin might have been affected by the subjects' physical condition and underlying illnesses, such as gout and hepatobiliary disease; however, we could not adjust for these factors due to the lack of information regarding the subjects' underlying conditions. To overcome this limitation, we performed sensitivity analyses focusing on the subjects with physiological serum levels of UA, albumin, and total bilirubin. Second, the study subjects were not representative of the general population, as the participants of the Namwon and the Dong-gu studies were aged between 45 and 74 years, and over 50 years, respectively. Third, the statistical power of subgroup analyses based on cancer type was low due to the small number of cases. Despite these limitations, this study has numerous strengths. First, it was a community-based prospective cohort study, which is a more accurate real-world approach than hospital-based case-control studies. Second, the follow-up period of participants was relatively long. Third, the association between serum UA levels and gastric cancer risk was found for the first time. Gastric cancer is known as the most common type of cancer among Koreans; therefore, Koreans would be more epidemiologically suitable as study subjects than the Western population, which has a lower incidence of gastric cancer. Lastly, the current study offers a novel finding suggesting the role of albumin in lung cancer carcinogenesis.
In conclusion, this study assessed the relationship of the serum levels of UA, albumin, and bilirubin with the risk of cancer and cancer-related death. Consistent associations were observed between antioxidants and cancer incidence or cancer death, except between UA and cancer incidence. As a result of an exploratory analysis of the association for each cancer type, some novel findings were observed, such as an inverse association between albumin levels and lung cancer risk. However, due to the weak association and inconsistencies, the results are still limited evidence. Future large-cohort studies with longer follow-up periods are needed to confirm the predictive value of albumin, UA, and total bilirubin levels in each cancer type.
ACKNOWLEDGEMENTS
This study was financially supported by Chonnam National University Hwasun Hospital (grant number: HCRI18007-1).
Footnotes
The authors have no potential conflicts of interest to disclose.
- Conceptualization: Sun-Seog Kweon.
- Data curation: Chang Kyun Choi.
- Formal analysis: Ye Rim Kim.
- Funding acquisition: Sun-Seog Kweon.
- Investigation: Young-Hoon Lee, Seong-Woo Choi, and Hye-Yeon Kim.
- Methodology: Sun-Seog Kweon.
- Project administration: Sun-Seog Kweon.
- Resources: Sun-Seog Kweon and Min-Ho Shin.
- Software: Chang Kyun Choi.
- Supervision: Sun-Seog Kweon.
- Validation: Sun-Seog Kweon.
- Visualization: Chang Kyun Choi.
- Writing—original draft: Ye Rim Kim.
- Writing—review & editing: Sun-Seog Kweon.
- Approval of final manuscript: all authors.
References
- 1.Harris IS, DeNicola GM. The complex interplay between antioxidants and ROS in cancer. Trends Cell Biol. 2020;30:440–451. doi: 10.1016/j.tcb.2020.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Waris G, Ahsan H. Reactive oxygen species: role in the development of cancer and various chronic conditions. J Carcinog. 2006;5:14. doi: 10.1186/1477-3163-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yiu A, Van Hemelrijck M, Garmo H, Holmberg L, Malmström H, Lambe M, et al. Circulating uric acid levels and subsequent development of cancer in 493281 individuals: findings from the AMORIS study. Oncotarget. 2017;8:42332–42342. doi: 10.18632/oncotarget.16198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yan S, Zhang P, Xu W, Liu Y, Wang B, Jiang T, et al. Serum uric acid increases risk of cancer incidence and mortality: a systematic review and meta-analysis. Mediators Inflamm. 2015;2015:764250. doi: 10.1155/2015/764250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kühn T, Sookthai D, Graf ME, Schübel R, Freisling H, Johnson T, et al. Albumin, bilirubin, uric acid and cancer risk: results from a prospective population-based study. Br J Cancer. 2017;117:1572–1579. doi: 10.1038/bjc.2017.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merriel SW, Carroll R, Hamilton F, Hamilton W. Association between unexplained hypoalbuminaemia and new cancer diagnoses in UK primary care patients. Fam Pract. 2016;33:449–452. doi: 10.1093/fampra/cmw051. [DOI] [PubMed] [Google Scholar]
- 7.Ghuman S, Van Hemelrijck M, Garmo H, Holmberg L, Malmström H, Lambe M, et al. Serum inflammatory markers and colorectal cancer risk and survival. Br J Cancer. 2017;116:1358–1365. doi: 10.1038/bjc.2017.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagner KH, Wallner M, Mölzer C, Gazzin S, Bulmer AC, Tiribelli C, et al. Looking to the horizon: the role of bilirubin in the development and prevention of age-related chronic diseases. Clin Sci (Lond) 2015;129:1–25. doi: 10.1042/CS20140566. [DOI] [PubMed] [Google Scholar]
- 9.Bajro MH, Josifovski T, Panovski M, Jankulovski N, Nestorovska AK, Matevska N, et al. Promoter length polymorphism in UGT1A1 and the risk of sporadic colorectal cancer. Cancer Genet. 2012;205:163–167. doi: 10.1016/j.cancergen.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 10.Kweon SS, Shin MH, Jeong SK, Nam HS, Lee YH, Park KS, et al. Cohort profile: the Namwon Study and the Dong-gu Study. Int J Epidemiol. 2014;43:558–567. doi: 10.1093/ije/dys244. [DOI] [PubMed] [Google Scholar]
- 11.Kolonel LN, Yoshizawa C, Nomura AM, Stemmermann GN. Relationship of serum uric acid to cancer occurrence in a prospective male cohort. Cancer Epidemiol Biomarkers Prev. 1994;3:225–228. [PubMed] [Google Scholar]
- 12.Wang A, Barber JR, Tin A, De Marzo AM, Kottgen A, Joshu CE, et al. Serum urate, genetic variation, and prostate cancer risk: Atherosclerosis Risk in Communities (ARIC) study. Cancer Epidemiol Biomarkers Prev. 2019;28:1259–1261. doi: 10.1158/1055-9965.EPI-19-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang W, Xu D, Wang B, Yan S, Wang X, Yin Y, et al. Increased risk of cancer in relation to gout: a review of three prospective cohort studies with 50358 subjects. Mediators Inflamm. 2015;2015:680853. doi: 10.1155/2015/680853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Battelli MG, Polito L, Bortolotti M, Bolognesi A. Xanthine oxidoreductase in cancer: more than a differentiation marker. Cancer Med. 2016;5:546–557. doi: 10.1002/cam4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fini MA, Elias A, Johnson RJ, Wright RM. Contribution of uric acid to cancer risk, recurrence, and mortality. Clin Transl Med. 2012;1:16. doi: 10.1186/2001-1326-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sprague BL, Trentham-Dietz A, Klein BE, Klein R, Cruickshanks KJ, Lee KE, et al. Physical activity, white blood cell count, and lung cancer risk in a prospective cohort study. Cancer Epidemiol Biomarkers Prev. 2008;17:2714–2722. doi: 10.1158/1055-9965.EPI-08-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Umeki Y, Adachi H, Enomoto M, Fukami A, Nakamura S, Nohara Y, et al. Serum albumin and cerebro-cardiovascular mortality during a 15-year study in a community-based cohort in Tanushimaru, a cohort of the seven countries study. Intern Med. 2016;55:2917–2925. doi: 10.2169/internalmedicine.55.6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim JE, Kimm H, Jee SH. Combined effects of smoking and bilirubin levels on the risk of lung cancer in Korea: the Severance cohort study. PLoS One. 2014;9:e103972. doi: 10.1371/journal.pone.0103972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horsfall LJ, Rait G, Walters K, Swallow DM, Pereira SP, Nazareth I, et al. Serum bilirubin and risk of respiratory disease and death. JAMA. 2011;305:691–697. doi: 10.1001/jama.2011.124. [DOI] [PubMed] [Google Scholar]
- 20.Horsfall LJ, Burgess S, Hall I, Nazareth I. Genetically raised serum bilirubin levels and lung cancer: a cohort study and Mendelian randomisation using UK Biobank. Thorax. 2020;75:955–964. doi: 10.1136/thoraxjnl-2020-214756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vitek L, Hubacek JA, Pajak A, Doryn´ska A, Kozela M, Eremiasova L, et al. Association between plasma bilirubin and mortality. Ann Hepatol. 2019;18:379–385. doi: 10.1016/j.aohep.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Temme EH, Zhang J, Schouten EG, Kesteloot H. Serum bilirubin and 10-year mortality risk in a Belgian population. Cancer Causes Control. 2001;12:887–894. doi: 10.1023/a:1013794407325. [DOI] [PubMed] [Google Scholar]
- 23.Maruhashi T, Kihara Y, Higashi Y. Bilirubin and endothelial function. J Atheroscler Thromb. 2019;26:688–696. doi: 10.5551/jat.RV17035. [DOI] [PMC free article] [PubMed] [Google Scholar]