Abstract
Objective:
To examine rural-urban differences in temporal trends and risk of inappropriate antibiotic use by agent and duration among women with uncomplicated urinary tract infection (UTI).
Design:
Observational cohort study.
Methods:
Using the IBM® MarketScan® Commercial Database (2010–2015), we identified U.S. commercially-insured women aged 18–44 coded for uncomplicated UTI and prescribed an oral antibiotic agent. We classified antibiotic agents and durations as appropriate versus inappropriate based on clinical guidelines. Rural-urban status was defined by residence in a metropolitan statistical area. We used modified Poisson regression to determine the association between rural-urban status and inappropriate antibiotic receipt, accounting for patient- and provider-level characteristics. We used multivariable logistic regression to estimate trends in antibiotic use by rural-urban status.
Results:
Of 670,450 women with uncomplicated UTI, a large proportion received antibiotic prescriptions for inappropriate agents (46.7%) or durations (76.1%). Compared to urban women, rural women were more likely to receive prescriptions with inappropriately long durations (adjusted risk ratio 1.10, 95% CI, 1.10–1.10), which was consistent across subgroups. From 2011 to 2015, there was slight decline in the quarterly proportion of patients who received inappropriate agents (48.5% to 43.7%) and durations (78.3% to 73.4%). Rural-urban differences varied over time by agent (duration outcome only), geographic region, and provider specialty.
Conclusions:
Inappropriate antibiotic prescribing is quite common for the treatment of uncomplicated UTI. Rural women are more likely to receive inappropriately long antibiotic durations. Antimicrobial stewardship interventions are needed to improve outpatient UTI antibiotic prescribing and reduce unnecessary exposure to antibiotics, particularly in rural settings.
INTRODUCTION
Appropriate antibiotic prescribing is an important clinical and public health goal because inappropriate antibiotic use is associated with increased risk of treatment failure, adverse events, antibiotic resistance, and healthcare costs.1–6 Uncomplicated urinary tract infections (UTIs) account for approximately 10.5 million ambulatory visits annually in the U.S., and are a common reason for outpatient antibiotic use in otherwise healthy females.7 The international clinical practice guidelines for treatment of uncomplicated UTI in women, updated in 2010 by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases, recommend several first-line antibiotic agents and durations based on efficacy results from randomized clinical trials and known prevalence of antibiotic resistance.8 Guidelines recommend empiric use of nitrofurantoin or trimethoprim-sulfamethoxazole (TMP-SMX) as first-line agents, reserving fluoroquinolones for infections other than uncomplicated UTI because the risk of rising antibiotic resistance and serious adverse events generally outweighs the benefits.9,10 However, the majority of antibiotic prescriptions for uncomplicated UTI are suboptimal because they are written for non-recommended agents and durations.11,12
Given the high burden of inappropriate antibiotic prescribing among women with uncomplicated UTI in the U.S., research on contemporary patterns of treatment is needed to identify disparities in appropriate antibiotic use. Knowledge about current practice patterns is needed to inform antimicrobial stewardship programs, which could ultimately enhance outpatient antibiotic prescribing and improve health outcomes. It is important to identify settings characterized by higher inappropriate antibiotic prescribing, which could be targets for interventions designed to improve guideline adherence.
The rural health disparity has been a recent focus in the U.S., and its improvement is a priority of the Department of Health and Human Services.13 Previous studies on respiratory tract and pediatric infections have identified associations between inappropriate antibiotic prescribing and individual- and provider-level factors, including geographic region, rural-urban status, and provider specialty.14,15 However, no large-scale studies have evaluated rural-urban differences in inappropriate outpatient antibiotic prescribing for UTI.16
To address this knowledge gap, we analyzed rural-urban differences in real-world antibiotic treatment patterns for the outpatient treatment of uncomplicated UTI in a large cohort of U.S. commercially insured, premenopausal women. We explored rural-urban differences in temporal trends and risk of inappropriate antibiotic use by agent and duration, both overall and within subgroups, comparing to current IDSA guidelines to determine prescription appropriateness.
METHODS
Data Source
The IBM® MarketScan® Commercial Database (2006–2015) includes healthcare data for millions of commercially insured individuals from all 50 U.S. states and the District of Columbia. The database includes de-identified longitudinal paid physician, pharmacy, and hospital claims data linked to demographic information from large employers and health plans that insure employees, their spouses, and dependents.17 These data have been used widely in health services and pharmacoepidemiologic research due to their large size, national representation, longitudinal structure, and comprehensive capture of outpatient services, inpatient services, and outpatient drug claims. Drug information represents prescriptions filled at a pharmacy. Our study using deidentified data was considered exempt from human subject review by the Institutional Review Board at Washington University.
Study Design & Population
We constructed a cohort of women diagnosed and treated for uncomplicated UTI, defined in accordance with criteria outlined in current guidelines, using methods previously described 8,9. The cohort included premenopausal women aged 18–44 years who received an outpatient diagnosis of uncomplicated UTI (ICD-9-CM diagnosis codes 595.0, 595.9, or 599.0) with an accompanying antibiotic prescription from April 1, 2011 to June 30, 2015. The index date was defined as the date of a filled oral prescription for an antibiotic with activity against common uropathogens, which occurred on the day of or after the diagnosis of uncomplicated UTI. The 180-day baseline period prior to the index date was used to identify exclusion criteria and covariates. To ensure adequate ascertainment of medical history, women without continuous enrollment in a medical plan or prescription drug coverage during the 180-day baseline period were excluded. To meet the definition of uncomplicated UTI,8 women were excluded if they received diagnoses or prescription medications during the 180-day baseline period for pregnancy, urinary comorbidities or abnormalities, pyelonephritis, diabetes, systemic autoimmune conditions, spinal cord injuries, or hematologic or solid organ malignancies (Supplementary Tables 1–3). To restrict the study population to women with community-acquired UTI, women were excluded if they had been hospitalized within 90 days prior to the index date. To restrict the study population to new users of antibiotics, women were excluded if they had received an antibiotic in the 30 days prior to the index date or had an infection in the 30 days prior to or on the index date (Supplementary Table 4).
Rural-Urban Definition
We classified individuals as urban if the primary beneficiary resided in a metropolitan statistical area; otherwise, the individual was classified as rural. IBM® mapped the 5-digit postal zip code of each primary beneficiary’s residence according to the U.S. Census Bureau’s mapping, which requires each metropolitan statistical area to have at least one urbanized area of ≥50,000 inhabitants and includes their nearby communities if they are socioeconomically joined together.17,18
Antibiotic Outcome Definitions
Table 1 presents the IDSA guideline-recommended first-line and non-first-line antibiotic agents as well as the recommended treatment durations for treatment of uncomplicated UTI.8 In accordance with these guidelines, we separately classified antibiotic prescriptions as appropriate or inappropriate based on antibiotic agent and duration, as described in our previous work.11 We classified first-line agents (nitrofurantoin, TMP-SMX, fosfomycin) as appropriate and non-first-line agents (fluoroquinolones, β-lactams) as inappropriate. In accordance with current guidelines, and because TMP monotherapy use was rare, we considered TMP monotherapy to be equivalent to TMP-SMX. For individuals who received multiple UTI-related antibiotic prescriptions, we classified the prescription as appropriate if they received only one first-line agent and inappropriate if they received multiple first-line agents or only non-first-line agents (on the same date). We classified antibiotic prescriptions as appropriate duration when the duration matched the recommended duration per current guidelines (Table 1).8 Specifically, the following regimens were classified as appropriate duration: nitrofurantoin 5-day regimen, TMP-SMX (including TMP monotherapy) 3-day regimen, fosfomycin 1-day regimen, fluoroquinolones 3-day regimen, and β-lactams 3 to 7-day regimen. All other regimens were classified as inappropriate duration. For individuals who received multiple UTI-related antibiotic prescriptions, we classified the duration as appropriate if each regimen was for the recommended duration; otherwise, we classified the duration as inappropriate if any regimen was not the recommended duration.
Table 1.
Guideline-recommended antibiotic therapy for acute uncomplicated cystitis by antibiotic agent and duration, adapted from the 2011 IDSA clinical practice guidelines for the treatment of acute uncomplicated cystitis in women.8
| Antibiotic agent | Recommended duration |
|---|---|
| First-line regimen | |
| Nitrofurantoin | 5 days |
| Trimethoprim-sulfamethoxazole | 3 days |
| Fosfomycin trometamol | 1 day |
| Non-first-line regimen | |
| Fluoroquinolones | 3 days |
| β-Lactams | 3–7 days |
| Trimethoprim monotherapy | 3 days |
Covariate Assessment
Covariate information was collected during the 180-day baseline period on age, year, state, geographic region, provider type, health insurance plan type, receipt of urinalysis at index, receipt of urine culture at index, and type of outpatient encounter (in-person or laboratory only).
Statistical Analysis
We summarized the distribution of antibiotic prescription agents and durations by rural-urban status. We plotted state-level maps of the proportion of antibiotic prescriptions with inappropriate agents or durations, stratified by rural-urban status. We calculated the standardized mean differences of baseline covariates to characterize imbalances of observed covariates.19 To examine the relationship between rural-urban status and inappropriate antibiotic receipt, we used modified Poisson regression to estimate risk ratios (RRs) and 95% confidence intervals (CIs), adjusting for age, year, geographic region, provider type, receipt of urinalysis at index, and receipt of urine culture at index.20 We built a separate model for each outcome (i.e., inappropriate agent and inappropriate duration). In sensitivity analyses, we also adjusted for health insurance plan type.
To describe temporal trends in inappropriate antibiotic use by calendar quarter, we used logistic regression models to generate adjusted estimates of the proportion of rural vs. urban patients that received inappropriate antibiotic agents or durations. Calendar quarter was treated as a categorical variable in the models to relax the assumption of linearity. We plotted the adjusted estimates, which are population marginal means that account for changes over time in age, geographic region, receipt of urinalysis at UTI diagnosis, receipt of urine culture at UTI diagnosis, and provider specialty. Trend lines were calculated as a smoothed conditional mean of the quarterly adjusted estimates. We performed subgroup analyses by stratifying models by antibiotic agent (duration outcome only), geographic region, and provider type.
RESULTS
Cohort characteristics
We identified 670,450 women who met study eligibility criteria from an original 1,349,276 women (Supplementary Figure 1). The majority of women (86.2%) were from urban areas and 13.8% were from rural areas. Table 2 presents characteristics of the study population stratified by rural-urban status. Rural and urban women had similar median age (30 years). Compared to urban women, rural women were more likely to reside in the South (52.2% vs. 40.2%) and the Midwest (26.1% vs. 21.3%), and less likely to reside in the Northeast (10.6% vs. 16.3%) or the West (11.2% vs. 22.3%). The distribution of provider type differed by rural-urban status. Compared to urban women, rural women were similarly likely to have been diagnosed with UTI by an emergency medicine physician, less likely to have been diagnosed by internal medicine or obstetrics/gynecology (OBGYN) physicians, and more likely from family medicine or pediatric physicians or non-physicians. Patterns of urine testing at index date differed by rural-urban status, as rural women were more likely than urban women to tested with a urinalysis (83.6% vs. 79.6%) but less likely to tested with a urine culture (44.3% vs. 51.8%).
Table 2.
Characteristics of the study population.
| Characteristic | Total (N=670,450) |
Urban (N=578,117) |
Rural (N=92,333) |
Standardized mean differencea |
|---|---|---|---|---|
| Age, median (IQR), y | 30.0 (23.0–38.0) | 30.0 (23.0–38.0) | 30.0 (22.0–38.0) | 0.01 |
| Age group, y | 0.07 | |||
| 18–24 | 215,624 (32.2) | 184,517 (31.9) | 31,107 (33.7) | |
| 25–29 | 103,181 (15.4) | 90,633 (15.7) | 12,548 (13.6) | |
| 30–34 | 108,616 (16.2) | 94,040 (16.3) | 14,576 (15.8) | |
| 35–39 | 116,458 (17.4) | 100,164 (17.3) | 16,294 (17.6) | |
| 40–44 | 126,571 (18.9) | 108,763 (18.8) | 17,808 (19.3) | |
| Year | 0.07 | |||
| 2011 | 150,138 (22.4) | 128,083 (22.2) | 22,055 (23.9) | |
| 2012 | 179,757 (26.8) | 153,963 (26.6) | 25,794 (27.9) | |
| 2013 | 141,138 (21.1) | 122,451 (21.2) | 18,687 (20.2) | |
| 2014 | 147,823 (22.0) | 128,647 (22.3) | 19,176 (20.8) | |
| 2015 | 51,594 (7.7) | 44,973 (7.8) | 6,621 (7.2) | |
| Region of residence | 0.39 | |||
| Northeast | 103,752 (15.5) | 94,007 (16.3) | 9,745 (10.6) | |
| Midwest | 147,362 (22.0) | 123,285 (21.3) | 24,077 (26.1) | |
| South | 280,343 (41.8) | 232,172 (40.2) | 48,171 (52.2) | |
| West | 138,993 (20.7) | 128,653 (22.3) | 10,340 (11.2) | |
| Provider type | 0.33 | |||
| Emergency medicine | 56,573 (8.4) | 48,621 (8.4) | 7,952 (8.6) | |
| Internal medicine | 92,589 (13.8) | 83,268 (14.4) | 9,321 (10.1) | |
| Family medicine & Pediatrics NEC | 239,628 (35.7) | 202,039 (34.9) | 37,589 (40.7) | |
| OBGYN | 41,014 (6.1) | 37,057 (6.4) | 3,957 (4.3) | |
| MD / DO NEC | 42,301 (6.3) | 35,627 (6.2) | 6,674 (7.2) | |
| NP / PA | 45,401 (6.8) | 34,571 (6.0) | 10,830 (11.7) | |
| Other | 133,545 (19.9) | 119,774 (20.7) | 13,771 (14.9) | |
| Unknown | 19,399 (2.9) | 17,160 (3.0) | 2,239 (2.4) | |
| Receipt of urine tests at index | ||||
| Urinalysis | 537,258 (80.1) | 460,102 (79.6) | 77,156 (83.6) | 0.10 |
| Culture | 340,643 (50.8) | 299,733 (51.8) | 40,910 (44.3) | 0.15 |
| Health Plan Type | 0.32 | |||
| Basic, comprehensive or high-deductible | 41,617 (6.2) | 36,592 (6.3) | 5,025 (5.4) | |
| CDHP | 50,967 (7.6) | 43,924 (7.6) | 7,043 (7.6) | |
| EPO or PPO | 416,495 (62.1) | 351,874 (60.9) | 64,621 (70.0) | |
| HMO | 87,588 (13.1) | 82,962 (14.4) | 4,626 (5.0) | |
| POS or POS with capitation | 46,669 (7.0) | 40,354 (7.0) | 6,315 (6.8) | |
| Unknown | 27,114 (4.0) | 22,411 (3.9) | 4,703 (5.1) |
Note: CDHP, consumer-driven health plans; DO, doctor of osteopathic medicine; EPO, exclusive provider organization; HMO, health maintenance organization; IQR, interquartile range; MD, medical doctor; NEC, not elsewhere classified; NP, nurse practitioner; OBGYN, obstetrics and gynecology; PA, physician’s assistant; POS, point of service; PPO, Preferred provider organization.
Standardized mean differences <0.10 indicate balance in observed covariates between rural and urban women.
Risk of Receipt of Inappropriate Antibiotic Agent
Figure 1 and Supplementary Table 5 present the distribution of antibiotic prescriptions by agent, overall and by rural-urban status. The most commonly prescribed agents were fluoroquinolones and the first-line agents, TMP-SMX and nitrofurantoin. A similar proportion of antibiotic prescriptions were inappropriate agents for rural (45.9%) and urban (46.9%) women, with similar percentages for individual agents. The use of inappropriate agents was similar between rural and urban women: fluoroquinolones (41.0% vs. 41.7%), β-lactams (4.8% vs. 5.0%), multiple agents without first-line agents (0.1%), and multiple agents with ≥2 first-line agents (0.1%). Rural women received more TMP-SMX (33.3% vs. 26.4%) and less nitrofurantoin (20.8% vs. 26.7%).
Figure 1.
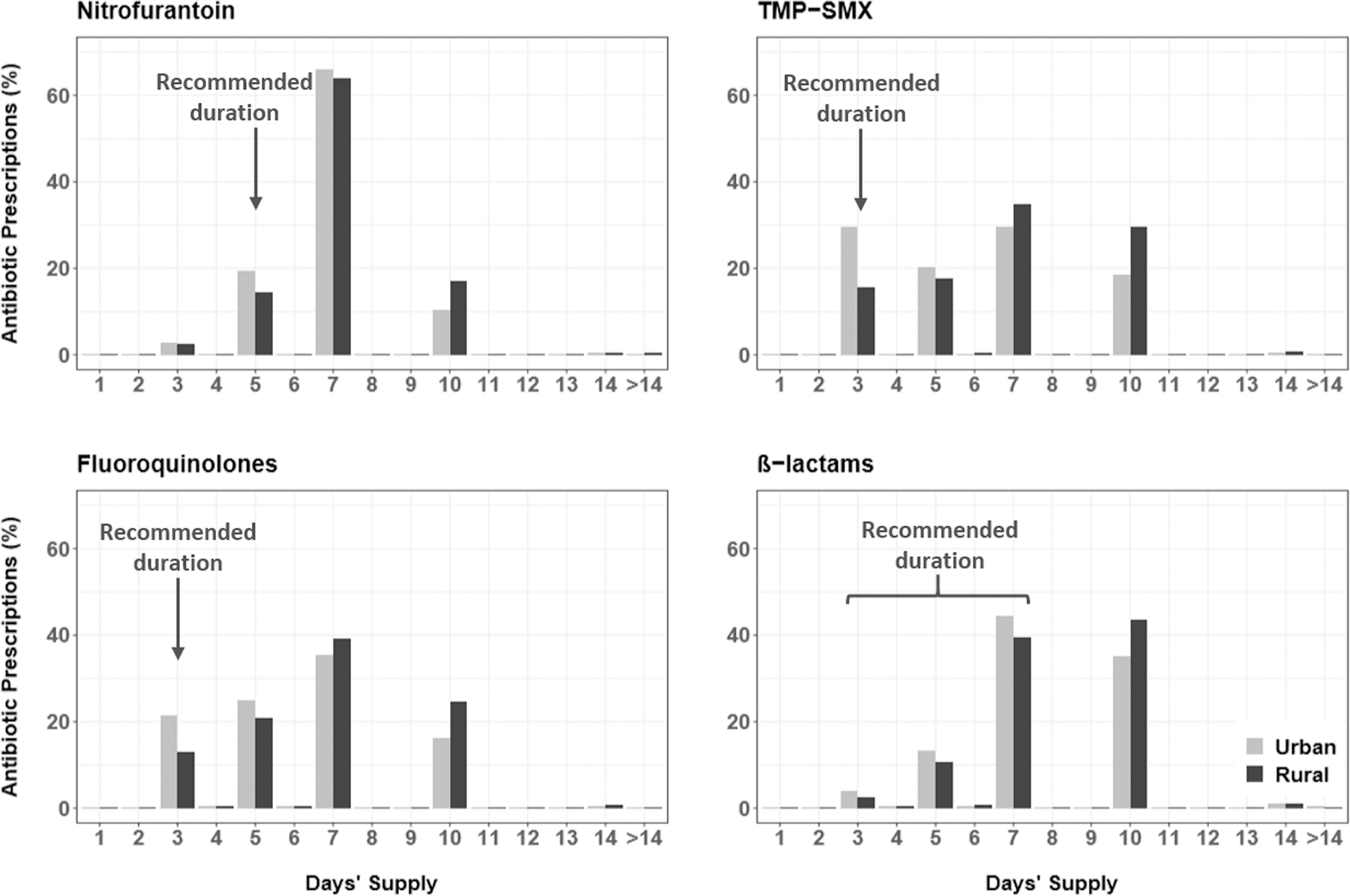
Distribution of the antibiotic prescription duration by rural-urban status for recipients of nitrofurantoin (n=153,098 urban and 19,079 rural women), TMP-SMX (n=152,053 urban and 30,568 rural women), fluoroquinolones (n=240,982 urban and 37,854 rural women), and β-lactams (n=29,023 urban and 4,411 rural women).
Over the study period, the proportion of patients that received inappropriate agents by quarter declined from 48.5% in mid-2011 to 43.7% in mid-2015 (Figure 2A). The use of inappropriate agents declined slightly among both urban (48.8% to 43.5%) and rural women (46.6% to 44.8%). State-level maps illustrated that women were more likely to receive inappropriate agents in the South and West, irrespective of rural-urban status (Figure 3). Subgroup analyses demonstrated that rural-urban differences in inappropriate agent use varied over time within geographic regions (e.g., Northeast, West) and provider specialties (e.g., internal medicine, OBGYN) (Supplementary Figures 2–3). For example, among internal medicine physicians, inappropriate agent use was higher in rural areas in 2011, similar by 2013, and higher again by 2015.
Figure 2.
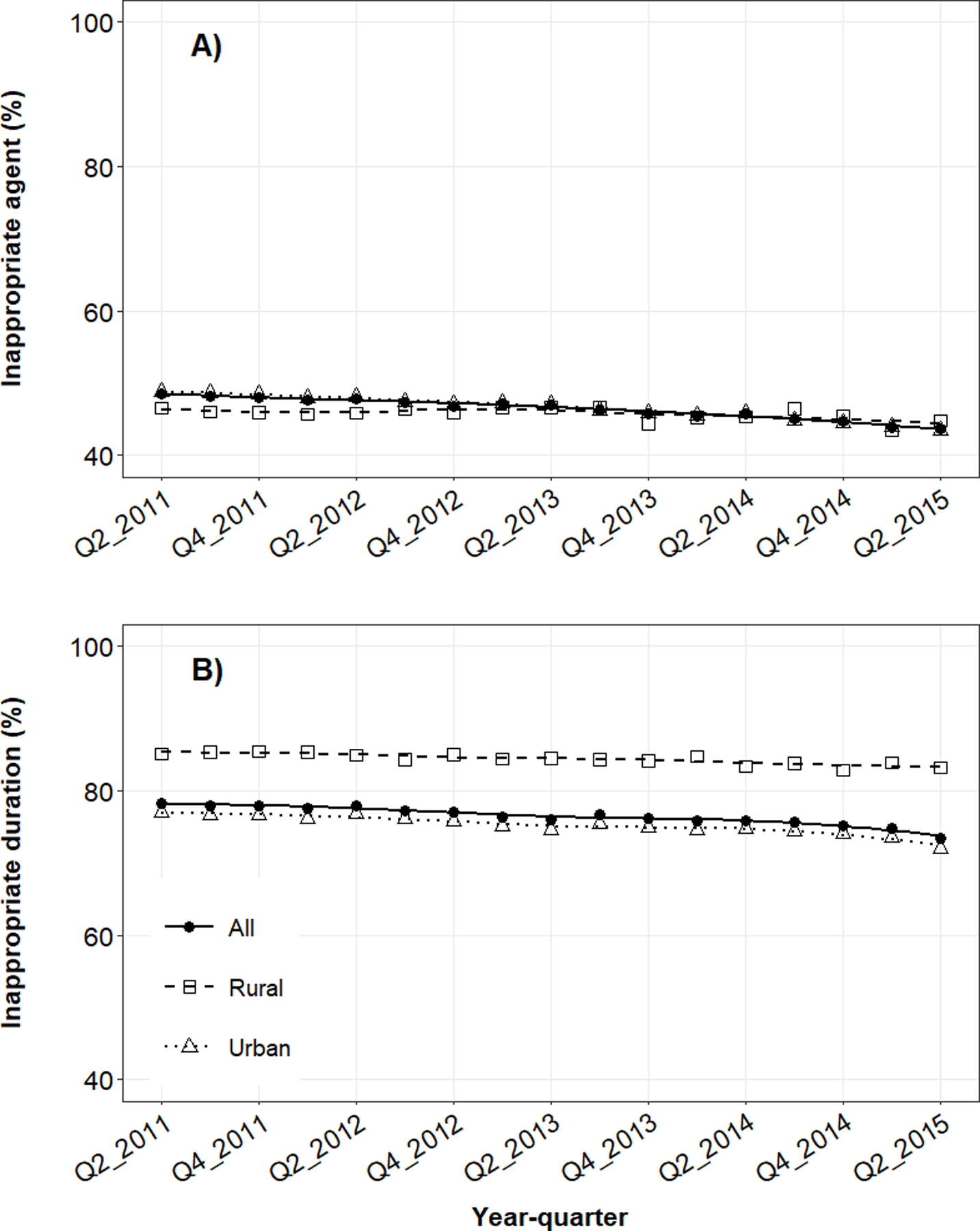
Mean quarterly use of UTI-related antibiotic prescriptions with A) inappropriate agents or B) inappropriate durations in the outpatient setting by rural-urban status. Quarterly estimates were adjusted for age, geographic region, provider type, receipt of urinalysis at index, and receipt of urine culture at index. Trend lines represent smoothed conditional means.
Figure 3.
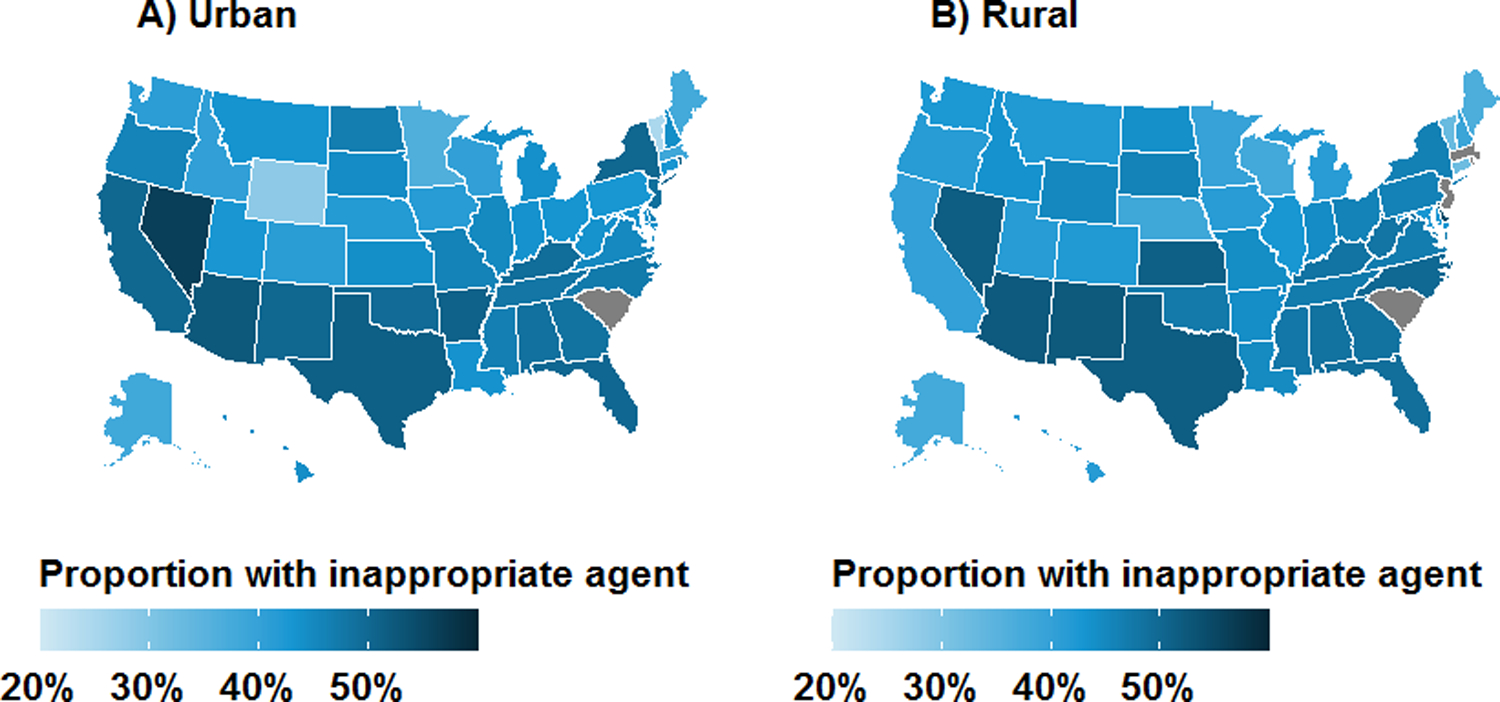
Geographic distribution of antibiotic prescriptions with inappropriate agents for treatment of uncomplicated UTI in the outpatient setting by rural-urban status: A) urban; and B) rural. Results are unadjusted and are not presented for grey states due to business agreement with IBM.
In the multivariable model, rural women had a slightly lower risk of receipt of an inappropriate antibiotic agent (adjusted RR, 0.98, 95% CI, 0.98–0.99) compared to urban women (Table 3). In the sensitivity analysis, results did not change appreciably in the model that also accounted for health insurance plan type. The association between rural-urban status and inappropriate agent varied across geographic regions (Supplementary Figure 4). Compared to urban women, risk of receipt of an inappropriate agent among rural women was similar in the South and Midwest, but lower in the West (adjusted RR 0.92, 95% CI, 0.90–0.95) and Northeast (adjusted RR 0.94, 95% CI, 0.92–0.97). The association between rural-urban status and inappropriate agent also varied across provider specialties (Supplementary Figure 4). Compared to urban women, the risk of receipt of an inappropriate agent among rural women was higher for OBGYN providers (adjusted RR 1.09, 95% CI, 1.04–1.14), similar for emergency medicine providers, and lower for internal medicine (adjusted RR 0.93, 95% CI, 0.91–0.95) and family medicine and pediatric providers (adjusted RR 0.96, 95% CI, 0.95–0.97) providers.
Table 3.
Risk ratio estimates for the association between rural-urban status and receipt of an inappropriate antibiotic prescription.
| Inappropriate antibiotic prescription No. (%) |
Appropriate antibiotic prescription No. (%) |
Unadjusted RR (95% CI) |
Adjusted RR a (95% CI) |
|
|---|---|---|---|---|
| By agent | ||||
| Rural | 42,391 (13.5) | 49,942 (14.0) | 0.98 (0.97 to 0.99) | 0.98 (0.98 to 0.99) |
| Urban | 270,897 (86.5) | 307,220 (86.0) | 1.00 | 1.00 |
| By duration | ||||
| Rural | 77,497 (15.2) | 14,836 (9.3) | 1.12 (1.12 to 1.12) | 1.10 (1.10 to 1.10) |
| Urban | 433,356 (84.8) | 144,761 (90.7) | 1.00 | 1.00 |
Risk of Receipt of Inappropriate Antibiotic Duration
Figure 1 and Supplementary Table 6 present the distribution of antibiotic prescriptions by duration, overall and by rural-urban status. The majority of prescriptions (76.1%) were written for inappropriate durations. Of 507,737 prescriptions with inappropriate duration, almost all were written for a duration that was longer (N=501,496; 98.8%) than recommended, rather than shorter (N=6,241; 1.2%). For all antibiotic agents combined, rural women were prescribed inappropriate antibiotic durations more frequently than urban women (83.9% vs. 74.9%). Similarly, within analyses stratified by antibiotic agent, rural women received more prescriptions with inappropriate durations than urban women: nitrofurantoin (85.4% vs. 80.7%), TMP-SMX (84.4% vs. 70.4%), fluoroquinolones (87.1% vs. 78.5%), and β-lactams (45.8% vs. 37.4%).
Over the study period, the quarterly proportion of patients who received inappropriate durations declined from 78.3% in mid-2011 to 73.4% in mid-2015 (Figure 2B). The use of inappropriate agents declined among urban women (77.1% to 72.0%) and less so among rural women (85.1% to 83.2%). State-level maps illustrate ubiquitous use of antibiotic prescriptions with inappropriate durations in all regions, particularly the South and Midwest (Figure 4). Rural-urban differences in inappropriate duration use remained generally constant over time within subgroups of antibiotic agent, geographic region, and provider specialty (Supplementary Figures 5–7).
Figure 4.
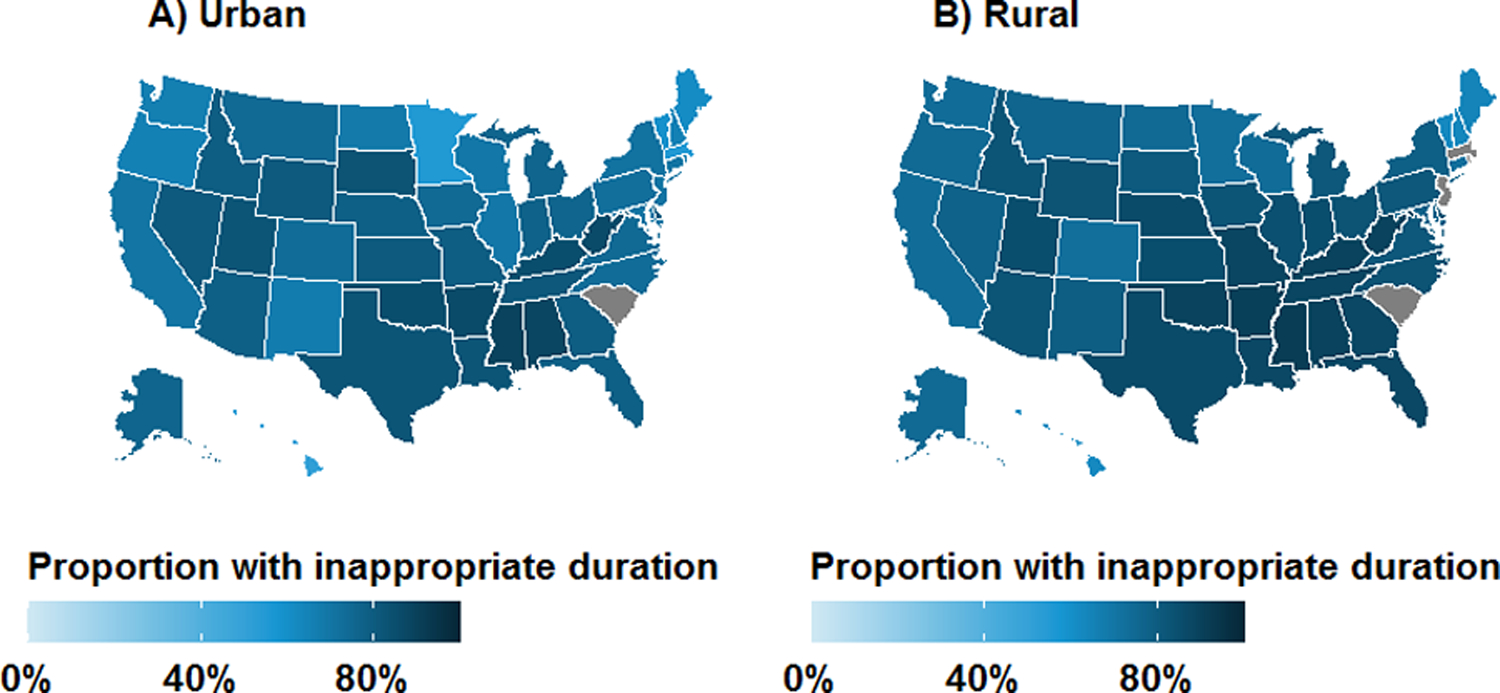
Geographic distribution of antibiotic prescriptions with inappropriate duration for treatment of uncomplicated UTI in the outpatient setting by rural-urban status: A) urban; and B) rural. Results are unadjusted and are not presented for grey states due to business agreement with IBM.
In the multivariable model, rural women were 10% more likely to receive an antibiotic prescription for an inappropriate duration (adjusted RR 1.10, 95% CI, 1.10–1.10) compared to urban women (Table 3). Results were robust to additional adjustment for health insurance plan type. Results of subgroup analyses were consistent across geographic regions, provider specialties, and antibiotic agents (Supplementary Figure 8).
DISCUSSION
We conducted a cohort study using a large claims database to examine rural-urban differences in real-world antibiotic treatment patterns in a population of uncomplicated UTI patients with commercial insurance in the United States. Of 670,450 patients in our study, nearly half received inappropriate agents and almost three-quarters received prescriptions with inappropriately long treatment durations. We observed little rural-urban difference in the receipt of inappropriate agents in the overall population, but results varied across geographic regions, provider specialties, and calendar time. We observed rural-urban differences in receipt of antibiotic prescriptions for inappropriate durations, wherein rural women were more likely to receive inappropriately long durations. These differences in inappropriate duration were consistent across antibiotic agents, geographic regions, and provider specialties. Overall, we observed slight decline in inappropriate UTI antibiotic use–both by agent and duration–over time.
Our observations of overall or subgroup variability in rural-urban differences in appropriate use of antibiotic agents and durations have several possible explanations. Regional variability in agent use may be related to local patterns of uropathogen resistance, especially for Escherichia coli, the predominant bacterial cause of UTIs.21 However, we are not aware of regional uropathogen resistance data in the U.S.to compare with our findings. It is recommended that first-line TMP-SMX be avoided in settings with known local resistance; first-line nitrofurantoin is an appropriate alternative choice for therapy due to minimal resistance,8,10 though studies of antibiotic prescriptions for other indications and patient populations demonstrate that providers frequently prescribe non-first-line agents.22–26
In addition, rural-urban differences in use of appropriate antibiotic durations may be related to patient- and provider-level factors. For example, distance to healthcare (a patient-level factor) is associated with lower adherence to treatment and poorer outcomes in oncology patients 27,28. In the context of our study on antibiotic prescribing, rural women were more likely to receive longer treatment durations, possibly in an effort to avoid treatment failure-related healthcare encounters that require travel. These rural-urban differences may also be associated with provider-level factors. Late-career physicians – more prevalent in rural locations29 – are more likely to prescribe antibiotics with longer durations.30
Our study has several potential limitations. First, our guideline-based definition of inappropriate duration may be too lenient given recent meta-analytic evidence that shorter course regimens of some agents have similar effects on clinical response31; however, the impact of possible misclassification on the results would be minimal because the proportion of prescriptions with inappropriate durations were overwhelmingly longer (98.8%) rather than shorter (1.2%) than recommended. Second, the study population is limited to commercially insured women from a database that oversamples residents of the South and under-samples residents of the West. Thus the results may not be generalizable to other populations such as Medicaid-insured or uninsured women, and may not directly generalize to the commercially-insured population in the U.S. Third, our definition of rural-urban status was based on zip-code level data rather than distance to urbanized areas/clusters or access to healthcare.18 Individuals classified as rural residents in our study reside in a wide variety of settlements, from densely settled small towns and exurbs on the fringes of urban areas, to more sparsely populated and remote areas.13 Fourth, the database lacked information on race/ethnicity or income-level; thus, we were not able to account for these important disparity-related variables in the analysis, which may result in confounding bias. Lastly, test results from urine cultures were not available in the database, therefore we were not able to account for prescribing decisions influenced by susceptibility results. However, we expect the impact of test results on prescribing practices to be minimal because guidelines recommend empiric treatment of uncomplicated UTI.
Despite these limitations, our study identified rural-urban differences in antibiotic prescribing, including an actionable disparity in the duration of antibiotics that disproportionately affects women who live in rural locations. In recent years, little effective progress has been achieved to reduce inappropriate antibiotic prescribing for uncomplicated UTI. Given the large quantity of inappropriate prescriptions annually in the U.S., as well as the negative patient- and society-level consequences of unnecessary exposure to antibiotics, antimicrobial stewardship interventions are needed to improve outpatient UTI antibiotic prescribing, particularly in rural settings. Existing recommendations for the promotion of outpatient antibiotic stewardship include establishing personal and policy commitment to change, reporting progress, and enhancing education around best practices.32 Future research is needed to effectively identify, disseminate, and implement guideline-concordant antibiotic prescribing in rural settings.
Supplementary Material
ACKNOWLEDGEMENTS
Financial support. This work was supported by a grant from the NCATS (NIH) under award number KL2 TR002346 (A.M.B. and M.J.D). Data programming for this study was conducted by the Center for Administrative Data Research, which is supported in part by the Washington University Institute of Clinical and Translational Sciences grant UL1 TR002345 from the National Center for Advancing Translational Sciences at the NIH, Grant Number R24 HS19455 through the Agency for Healthcare Research and Quality.
Footnotes
Previous presentation of the data or findings: Preliminary findings were presented at the 2020 American College of Physicians Missouri Chapter annual meeting and ID Week 2020.
Potential conflicts of interest. M.A.O. reported receiving investigator-initiated research funds from Sanofi Pasteur, Pfizer, and Merck and serving as a consultant for Pfizer.
Manuscript preparation. The funding agencies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
REFERENCES
- 1.CDC. Antibiotic Resistance Threats in the United States, 2019 Atlanta, GA: U.S. Department of Health and Human Services, CDC;2019. [Google Scholar]
- 2.Costelloe C, Metcalfe C, Lovering A, Mant D, Hay AD. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ 2010;340:c2096. [DOI] [PubMed] [Google Scholar]
- 3.Falagas ME, Kotsantis IK, Vouloumanou EK, Rafailidis PI. Antibiotics versus placebo in the treatment of women with uncomplicated cystitis: a meta-analysis of randomized controlled trials. The Journal of infection 2009;58:91–102. [DOI] [PubMed] [Google Scholar]
- 4.Geller AI, Lovegrove MC, Shehab N, Hicks LA, Sapiano MRP, Budnitz DS. National Estimates of Emergency Department Visits for Antibiotic Adverse Events Among Adults-United States, 2011–2015. J Gen Intern Med 2018;33:1060–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shehab N, Lovegrove MC, Geller AI, Rose KO, Weidle NJ, Budnitz DS. US Emergency Department Visits for Outpatient Adverse Drug Events, 2013–2014. JAMA 2016;316:2115–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shehab N, Patel PR, Srinivasan A, Budnitz DS. Emergency department visits for antibiotic-associated adverse events. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2008;47:735–743. [DOI] [PubMed] [Google Scholar]
- 7.Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol 2015;13:269–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 2011;52:e103–120. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi M, Shapiro DJ, Hersh AL, Sanchez GV, Hicks LA. Outpatient Antibiotic Prescribing Practices for Uncomplicated Urinary Tract Infection in Women in the United States, 2002–2011. Open forum infectious diseases 2016;3:ofw159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanchez GV, Babiker A, Master RN, Luu T, Mathur A, Bordon J. Antibiotic Resistance among Urinary Isolates from Female Outpatients in the United States in 2003 and 2012. Antimicrob. Agents Chemother 2016;60:2680–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durkin MJ, Keller M, Butler AM, et al. An Assessment of Inappropriate Antibiotic Use and Guideline Adherence for Uncomplicated Urinary Tract Infections. Open forum infectious diseases 2018;5:ofy198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grigoryan L, Zoorob R, Wang H, Trautner BW. Low Concordance With Guidelines for Treatment of Acute Cystitis in Primary Care. Open Forum Infect Dis 2015;2:ofv159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall SA, Kaufman JS, Ricketts TC. Defining urban and rural areas in U.S. epidemiologic studies. J Urban Health 2006;83:162–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barlam TF, Soria-Saucedo R, Cabral HJ, Kazis LE. Unnecessary Antibiotics for Acute Respiratory Tract Infections: Association With Care Setting and Patient Demographics. Open Forum Infect Dis 2016;3:ofw045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fleming-Dutra KE, Demirjian A, Bartoces M, Roberts RM, Taylor TH Jr., Hicks LA. Variations in Antibiotic and Azithromycin Prescribing for Children by Geography and Specialty-United States, 2013. Pediatr Infect Dis J 2018;37:52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shapiro DJ, Hicks LA, Pavia AT, Hersh AL. Antibiotic prescribing for adults in ambulatory care in the USA, 2007–09. J Antimicrob Chemother 2014;69:234–240. [DOI] [PubMed] [Google Scholar]
- 17.Hansen LG. White Paper: IBM MarketScan Research Databases for life sciences researchers Somers, NY: IBM Watson Health;2018. [Google Scholar]
- 18.Office of Management and Budget. 2010 Standards for Delineating Metropolitan and Micropolitan Statistical Areas Federal Register 2010;75:37245–37252. [Google Scholar]
- 19.Yang D-S, Dalton JE. A unified approach to measuring the effect size between two groups using SAS 2012.
- 20.Zou G A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004;159:702–706. [DOI] [PubMed] [Google Scholar]
- 21.Sannes MR, Kuskowski MA, Johnson JR. Geographical distribution of antimicrobial resistance among Escherichia coli causing acute uncomplicated pyelonephritis in the United States. FEMS Immunol Med Microbiol 2004;42:213–218. [DOI] [PubMed] [Google Scholar]
- 22.Bishop JL, Schulz TR, Kong DCM, James R, Buising KL. Similarities and differences in antimicrobial prescribing between major city hospitals and regional and remote hospitals in Australia. Int J Antimicrob Agents 2019;53:171–176. [DOI] [PubMed] [Google Scholar]
- 23.Gonzales R, Steiner JF, Sande MA. Antibiotic prescribing for adults with colds, upper respiratory tract infections, and bronchitis by ambulatory care physicians. JAMA 1997;278:901–904. [PubMed] [Google Scholar]
- 24.Handy LK, Bryan M, Gerber JS, Zaoutis T, Feemster KA. Variability in Antibiotic Prescribing for Community-Acquired Pneumonia. Pediatrics 2017;139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yaeger JP, Temte JL, Hanrahan LP, Martinez-Donate P. Roles of Clinician, Patient, and Community Characteristics in the Management of Pediatric Upper Respiratory Tract Infections. Ann Fam Med 2015;13:529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steinman MA, Landefeld CS, Gonzales R. Predictors of broad-spectrum antibiotic prescribing for acute respiratory tract infections in adult primary care. JAMA 2003;289:719–725. [DOI] [PubMed] [Google Scholar]
- 27.Ambroggi M, Biasini C, Del Giovane C, Fornari F, Cavanna L. Distance as a Barrier to Cancer Diagnosis and Treatment: Review of the Literature. Oncologist 2015;20:1378–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campbell NC, Elliott AM, Sharp L, Ritchie LD, Cassidy J, Little J. Rural and urban differences in stage at diagnosis of colorectal and lung cancers. Br J Cancer 2001;84:910–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fordyce MA, Doescher MP, Skillman SM. The Aging of the Rural Primary Care Physician Workforce: Will Some Locations Be More Affected than Others? Seattle, WA: WWAMI Rural Health Research Center, University of Washington School of Medicine, Department of Family Medicine;2013. [Google Scholar]
- 30.Fernandez-Lazaro CI, Brown KA, Langford BJ, Daneman N, Garber G, Schwartz KL. Late-career Physicians Prescribe Longer Courses of Antibiotics. Clin Infect Dis 2019;69:1467–1475. [DOI] [PubMed] [Google Scholar]
- 31.Kim DK, Kim JH, Lee JY, et al. Reappraisal of the treatment duration of antibiotic regimens for acute uncomplicated cystitis in adult women: a systematic review and network meta-analysis of 61 randomised clinical trials. Lancet Infect Dis 2020. [DOI] [PubMed]
- 32.Sanchez GV, Fleming-Dutra KE, Roberts RM, Hicks LA. Core Elements of Outpatient Antibiotic Stewardship. MMWR Recomm Rep 2016;65:1–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


