Abstract
Tumor Necrosis Factor (TNF)-α is a proinflammatory cytokine (PIC) and has been implicated in a variety of illness including cardiovascular disease. The current study investigated the inflammatory response trigged by TNFα in both cultured brain neurons and the hypothalamic paraventricular nucleus (PVN), a key cardiovascular relevant brain area, of the Sprague Dawley (SD) rats. Our results demonstrated that TNFα treatment induces a dose- and time-dependent increase in mRNA expression of PICs including Interleukin (IL)-1β and Interleukin-6 (IL6); chemokines including C–C Motif Chemokine Ligand 5 (CCL5) and C–C Motif Chemokine Ligand 12 (CCL12), inducible nitric oxide synthase (iNOS), as well as transcription factor NF-kB in cultured brain neurons from neonatal SD rats. Consistent with this finding, immunostaining shows that TNFα treatment increases immunoreactivity of IL1β, CCL5, iNOS and stimulates activation or expression of NF-kB, in both cultured brain neurons and the PVN of adult SD rats. We further compared mRNA expression of the aforementioned genes in basal level as well as in response to TNFα challenge between SD rats and Dahl Salt-sensitive (Dahl-S) rats, an animal model of salt-sensitive hypertension. Dahl-S brain neurons presented higher baseline levels as well as greater response to TNFα challenge in mRNA expression of CCL5, iNOS and IL1β. Furthermore, central administration of TNFα caused significant higher response in CCL12 in the PVN of Dahl-S rats. The increased inflammatory response to TNFα in Dahl-S rats may be indicative of an underlying mechanism for enhanced pressor reactivity to salt intake in the Dahl-S rat model.
Keywords: TNFα, Inflammatory mediators, Neurons, Paraventricular nucleus
Introduction
Chronic Inflammation is a major health concern that serves as a risk factor for numerous comorbid health problems. It may also serve as a predictor for future cardiovascular risk and disease (Willerson and Ridker 2004) and has been shown to correlate with an increased risk of sudden cardiac death (Albert et al. 2002). Elevated proinflammatory cytokine (PIC) activity has been observed in essential hypertension patients (Dalekos et al. 1996, 1997), indicating its role in either the development or maintenance of essential hypertension. Furthermore, increased PIC activation has been reported in numerous cardiovascular disease models including heart failure (Kang et al. 2010) and Angiotensin-II infused hypertensive rat models (Kang et al. 2009; Shi et al. 2010; Sriramula et al. 2013). However, the direct mechanism underlying the role of inflammation in the development of hypertension remains contentious.
One primary proinflammatory cytokine that has recently been implicated in the development of hypertension is Tumor Necrosis Factor—alpha (TNFα). TNFα has been observed to be elevated in human hypertensive patients (Bautista et al. 2005; Cottone et al. 1998), and inhibition in humans with rheumatoid arthritis results in a reduced ambulatory blood pressure (Yoshida et al. 2014), indicating a translation to human models of chronic inflammation. In animal models, peripheral administration of TNFα through either intracarotid or intravenous catheters results in elevated blood pressure as well as sympathetic outflow (Zhang et al. 2003). Interestingly, vagotomy had no impact on the impact of TNFα on blood pressure and sympathetic outflow, while mid-collicular decerebration and Subfornical Organ (SFO) lesions attenuated this effect (Wei et al. 2013; Zhang et al. 2003), indicating mediation of the pressor effect of TNFα through interactions with the SFO. Interestingly, the SFO has numerous direct and indirect projections to the paraventricular nucleus (PVN), a major area of cardiovascular control with efferent projections innervating pre-sympathetic neurons. Wei et al. (2015) observed that microinjection of TNFα directly into the SFO of Sprague Dawley (SD) rats results in increased blood pressure and sympathetic outflow, as well as elevated renin-angiotensin-system components and inflammation within the SFO and PVN. These results indicate that the observed effects were primarily carried out through interactions of peripherally circulating TNFα acting upon the circumventricular organs, which lack a blood–brain barrier.
Intriguingly, this relationship has been sparsely explored in salt-sensitive models of hypertension, despite the high prevalence of impaired sodium handling among those individuals diagnosed with essential hypertension (Choi et al. 2015; Weinberger 1996). High salt intake has been observed to relate to inflammation in primary hypertensive patients (Yilmaz et al. 2012), while reducing sodium intake has shown promise as a treatment for resistant hypertensive patients (Pimenta et al. 2009). Previous researcher found that the Inadequate upregulation of Gαi2, a GPCR subunit important for salt-resistance (Moreira et al. 2019), may be responsible for the exaggerated pressor response to chronic salt intake in Dahl salt-sensitive (Dahl-S) rats (Wainford et al. 2015), a commonly used model of primary salt-sensitive hypertension. Previous work from our lab showed that in the Dahl-S model of essential hypertension, a high salt diet led to increased PIC within the PVN, and subsequent hypertension development (Jiang et al. 2018). Furthermore, our research showed an impaired blood–brain barrier buffering of sodium influx into the cerebrospinal fluid (Jiang et al. 2018), in agreement with previous research (Huang et al. 2004). While the peripheral activity of TNFα in the Dahl-S model has been shown to be elevated following a high salt diet (Huang et al. 2016), the translation to central inflammatory responsiveness has not been adequately explored.
The lack of protective mechanisms that aid in salt-resistance (Moreira et al. 2019; Wainford et al. 2015), as well as the impaired functioning of the blood–brain barrier in Dahl-S rats (Huang et al. 2004; Jiang et al. 2018) may be indicative of an augmented response to neural inflammation following salt intake, which may further exacerbate the development of hypertension in this animal model. However, it has yet to be elucidated if the neuroinflammatory response to TNFα is elevated in brain neurons as well as cardiovascular relevant regions of the Dahl-S rat brain when compared to SD rats. To this effect, the current study seeks to assess the impact of TNFα treatment on the expression of inflammatory markers in brain neuronal cultures as well as the PVN of both SD and Dahl-S rats. We hypothesize that TNFα administration and treatment will result in an augmented inflammatory response in Dahl-S rats compared to their normal SD controls, thus offering potential mechanistic insight into the observed pressor response to high salt intake.
Methods
Animals
Sprague Dawley (SD) rats and Dahl Salt-Sensitive (Dahl-S) rats (250–350 g) were purchased from Charles River Laboratories (Wilmington, MA, USA) and used in our breeding colony to generate pups. All rats were housed and kept on a 12:12-h light–dark cycle in a climate-controlled room. Chow and water were provided ad libitum. The adult SD and Dahl-S rats were used for intracerebroventricular (ICV) injection, and subsequent immunostaining and polymerase chain reaction (PCR) analysis. The 1-day-old pups were euthanized by administration of 5% isoflurane and used to prepare neuronal cultures as outlined below. All animal protocols were approved by the Michigan Technological University Institutional Animal Care and Use Committee.
Preparation of Neuronal Cultures
Brain tissues including cortex, hippocampus and hypothalamus of 1-day-old rat pups (n = 3 per preparation) were harvested and combined. The brain tissues were homogenized and neuronal cells were isolated using papain dissociation system (Worthington Biochemical Cooperation) following manufacture’s instruction. Then neuronal cells were plated in poly-d-lysine precoated 12-well cell culture plates (105 cells/well) in Neurobasal -A medium (Fisher Scientific) supplemented with 2% B27+ (Fisher scientific) and 1% antibiotic-penicillin/streptomycin (Invitrogen). The cell cultures were incubated at 37 °C in a 5% CO2 incubator for 10–14 days and then received TNFα treatment.
TNFα Neuronal Culture Treatment
10–14-day-old SD or Dah-S pup primary neuronal cultures were used to test the effect of TNFα treatment on inflammatory mediators’ expression. One day before the treatment, neuronal cells were removed full culture medium and incubated with Neurobasal-A only medium. 16 h following Neurobasal -A incubation, vehicle control or TNFα were added to the culture medium and then cells were incubated in a 5% CO2 incubator for differing time periods (3, 6, 24 h), at differing doses (0.2–20 ng/mL) of TNFα. Real-time PCR was then performed to test TNFα-induced inflammatory responses in the brain neurons.
Intracerebroventricular Injections
ICV infusion was performed as detailed in our previous publication (Fan et al. 2018; Huber et al. 2017). In brief, adult male SD rats (250–350 g) and age as well as sex-matched Dahl-S rats were anesthetized with 3% isoflurane in O2 and received either TNFα (250 ng dissolved in 2.5 µl 0.9% NaCl) or vehicle (2.5 µl 0.9% NaCl) solution into the left lateral ventricle using the following stereotaxic coordinates: 0.8–0.9 mm caudal to bregma, 1.4–1.8 mm lateral to midline, and 3.2–3.8 mm ventral to dura. Either solution was administered at the rate of 1 μl/min using a UltraMicroPump3 (WPI). Three hours following ICV injection, rats were euthanized with overdose of isoflurane and then were used for either mRNA expression measurement or immunostaining. The brains of those rats used for PVN mRNA expression assay were removed and instantly frozen in liquid nitrogen and then kept in − 80 °C freezer until use. The rats used for immunostaining were transcardially perfused with ice-cold 4% PFA. Following successful perfusion, the brains were removed and kept in 4% PFA overnight at 4 °C. The next day, the brains were transferred to 30% sucrose in PBS and kept in the solution until they sank to the bottom of the container. Upon successful treatment, the brains were cut into 25 μm thick coronal sections, and were subjected to immunostaining for proteins of interest as outlined below.
Real-time PCR mRNA Measurement of Inflammatory Markers
mRNA levels of the genes of interest in cultured brain neurons and the PVN tissues were assessed using real-time PCR as detailed previously (Fan et al. 2018; Huber et al. 2017). Briefly, RNAs were isolated from cultured neurons or PVN tissues using RNeasy Mini kit (Qiagen, CA, USA) following the manufacturer's instructions. About 200 ng of RNA was converted to complementary DNA (cDNA) using iScript™ cDNA Synthesis Kit (Bio-Rad) in 20-µl PCR system. The cDNAs were then used as templates, and Real-time PCR was performed to measure mRNA levels of IL-1β, IL6, CCL5, CCL12, iNOS, and NF-kB subunit nfkb1 using Taqman primers and probe in the Step One Plus Real-Time PCR System (Applied Biosystems). Data were normalized to GAPDH mRNA.
Immunoreactivity Assessment of Inflammatory Markers
Immunostaining of IL1β, CCL5, iNOS and NF-kB subunit P65 or phosphorylated P65 (P-P65) was carried out in both brain neuronal cultures and the PVN tissues. Cell cultures were first fixed with 4% PFA in the PBS for 10 min followed by washed with PBS three times for 10 min each, then the cells were either directly used for immunostaining or kept in 4 °C until used. Brain coronal sections (25 µm) containing the PVN were first washed with PBS three times for 10 min each. Then, neuronal cultures or brain sections were incubated with 5% horse serum in the PBS for 30 min, and then incubated with either mouse anti-IL1β (1:100, Santa Cruz Biotechnology), or rabbit anti-CCL5 ( 1:250, Invitrogen), or rabbit anti-iNOS (1:100, Invitrogen), or abbit anti-P65 (1:400, Cell Signaling Technology), or rabbit anti-P-P65 (1:1000, Cell Signaling Technology) in PBS containing 0.5% Triton X-100 and 5% horse serum overnight (for cell cultures) or for 72 h (brain sections) at 4 °C. Afterward, cells or brain sections were washed with PBS three times for 10 min each. They were then incubated with secondary antibody Alexa Fluor® 488 donkey anti-mouse IgG (1:500) or Alexa Fluor® 488 donkey anti-rabbit IgG (1:500) overnight at 4 °C. Afterward, they were again washed with PBS three times, and cell cultures were taken images using ZOE Fluorescent Cell Imager (Bio-Rad). Brain sections were mounted in Vectashield mounting medium and images were taken with an Olympus BX51 TRF Microscope (Olympus, Japan).
Chemicals and Reagents
Real-time PCR master mix, primers and probes for IL-1β (Rn00580432_m1), IL6 (Rn01410330_m1), CCL5 Rn00579590_m1), CCL12 (Rn01464638_m1), iNOS (Rn00561646_m1), nfkb1(Rn01399572_m1) and GAPDH (Rn01775763_g1) were all purchased from Applied Biosystems (Foster City, CA). Recombinant Rat TNF-alpha Protein was purchased from R& D systems.
Statistical Analysis
All data are expressed as means ± SEM. Statistical significance was evaluated with the use of two-way ANOVA and unpaired Students t test. Differences were considered to be significant at P < 0.05.
Results
TNFα Treatment Induces Dose and Time-dependent Increases in the mRNA Levels of Inflammatory Mediators in the Brain Neurons from SD Neonatal Pups
Primary neuronal cultures prepared from the brains of SD neonatal pups were incubated with 2 ng/mL TNFα for different time periods (3, 6, 24 h), and then subjected to RNA isolation and subsequently real-time PCR to test relevant genes of interest. The results showed that TNFα treatment significantly increased mRNA levels of IL1β, IL6, CCL5, CCL12, iNOS and nfkb1 in a time-dependent manner (P < 0.05). The highest increase occurred after 3 h of TNFα incubation in nfkb1 (6.3-fold), 6 h of TNFα incubation in IL1β (70.9-fold), IL6 (5.7-fold) and iNOS (457-fold), and 24 h of TNFα incubation in CCL5 (330-fold) and CCL12 (tenfold) when compared to vehicle controls (which were assigned to arbitrary unit 1) (Fig. 1).
Fig. 1.
Tumor Necrosis Factor-α (TNFα) treatment results in a time-dependent increase in mRNA expression of inflammatory mediators in brain neurons of neonatal Sprague Dawley (SD) rats. Neuronal cells were obtained from the cortex, hippocampus, and hypothalamus and incubated with 2 ng/mL TNFα in Neurobasal-A medium for 3, 6 and 24 h, respectively. Cells were collected and their mRNA expression of inflammatory mediators were measured using real-time Polymerase Chain Reaction (PCR). Each treatment using 2–3 well of neuronal cells, and each experiment was repeated using 3 different batch of cells, the results were combined. Data were normalized to the housekeeping gene, GAPDH (n = 6–9/group, *P < 0.05)
We further investigated whether TNFα induced increases in the aforementioned gene expression were in a dose-dependent manner. Brain neuronal cultures from SD neonatal pups were incubated with following doses of TNFα: 0.2 ng/mL, 2 ng/mL and 20 ng/mL, for 6 h, respectively. Cells were then collected, and real-time PCR was performed to test mRNA levels of the aforementioned 6 genes. The results showed that TNFα treatment results in dose-dependent increases in mRNA levels of CCL5, CCL12, IL1β, IL6, iNOS and nfkb1. The maximum increase occurred in the 20 ng/mL TNFα-treated groups (CCL5: 40-fold: CCL12: 4.3-fold, IL1β: 80.9-fold; IL6: 7.9-fold; iNOS: 659-fold; nfkb1: 6.7-fold) (Fig. 2).
Fig. 2.
Tumor Necrosis Factor-α (TNFα) treatment results in a dose-dependent increase in mRNA expression of inflammatory mediators in brain neurons of neonatal Sprague Dawley (SD) rats. Neuronal cells were obtained from the cortex, hippocampus, and hypothalamus and were treated with differing doses of TNFα (0.2 ng/mL, 2 ng/ml, and 20 ng/ml) for 6 h. Cells were collected and mRNA levels of inflammatory mediators were measured using real-time Polymerase Chain Reaction (PCR). Each treatment using 2–3 well of neuronal cells, and each experiment was repeated using 3 different batch of cells, the results were combined. Data were normalized to the housekeeping gene, GAPDH (n = 6–9/group, *P < 0.05)
TNFα Treatment Drastically Increases the Immunoreactivities of IL1β, CCL5 and iNOS as Well as Activation of NF-kB
The results described above showed that TNFα treatment dramatically increased mRNA expressions in varieties of inflammatory mediators including PICs, chemokines, iNOS and transcription regulator NF-kB subunit nfkb1. NF-kB is a potent transcription regulator which activation can stimulate PIC and chemokines’ expression. We then further performed immunofluorescent staining to test whether TNFα treatment also stimulates NF-kB activation and increase protein expression of those inflammatory mediators. Instead of testing all genes, we choose to test several representatives including IL1β, CCL5 and iNOS as well as P-P65, an activation form of NF-kB subunit.
Brain neurons from neonatal SD pups were incubated with either TNFα (20 ng/mL) or vehicle control for 6 h, then cells were fixed with 4% PFA and subjected to immunostaining of genes of interest. The results showed that TNFα treatment strongly increased immunoreactivities of IL1β, CCL5 (Fig. 3b) and iNOS, as well as P-P65 (Fig. 3c).
Fig. 3.
Tumor Necrosis Factor-α (TNFα) treatment increases immunoreactivities of inflammatory mediators in brain neurons of neonatal Sprague Dawley (SD) rats. Neuronal cultures were taken from the cortex, hippocampus, and hypothalamus of SD rats and incubated with 20 ng/mL TNFα or vehicle control for 6 h. After treatment, culture medium was removed and cells were fixed with 4% paraformaldehyde (PFA) and then probe to primary antibodies anti-Interleukin 1β (IL1β) (a), anti- C–C Motif Chemokine Ligand 5 (CCL5) (b), anti- inducible Nitric Oxide Synthase (iNOS) (c), and anti-P-P65 (d), respectively. Images were taken using ZOE Fluorescent Cell Imager (Bio-Rad). In each group of images, lower panels are representative fluorescent images showed the immunoreactivities of target genes in the control (left) or TNF treatment (right) cells. Upper panels are the brightfield images taken from the identical area as associated immunofluorescent images
Central Administration of TNFα Increases Immunoreactivities of Inflammatory Mediators in the PVN of Adult SD rats and Dahl-S Rats
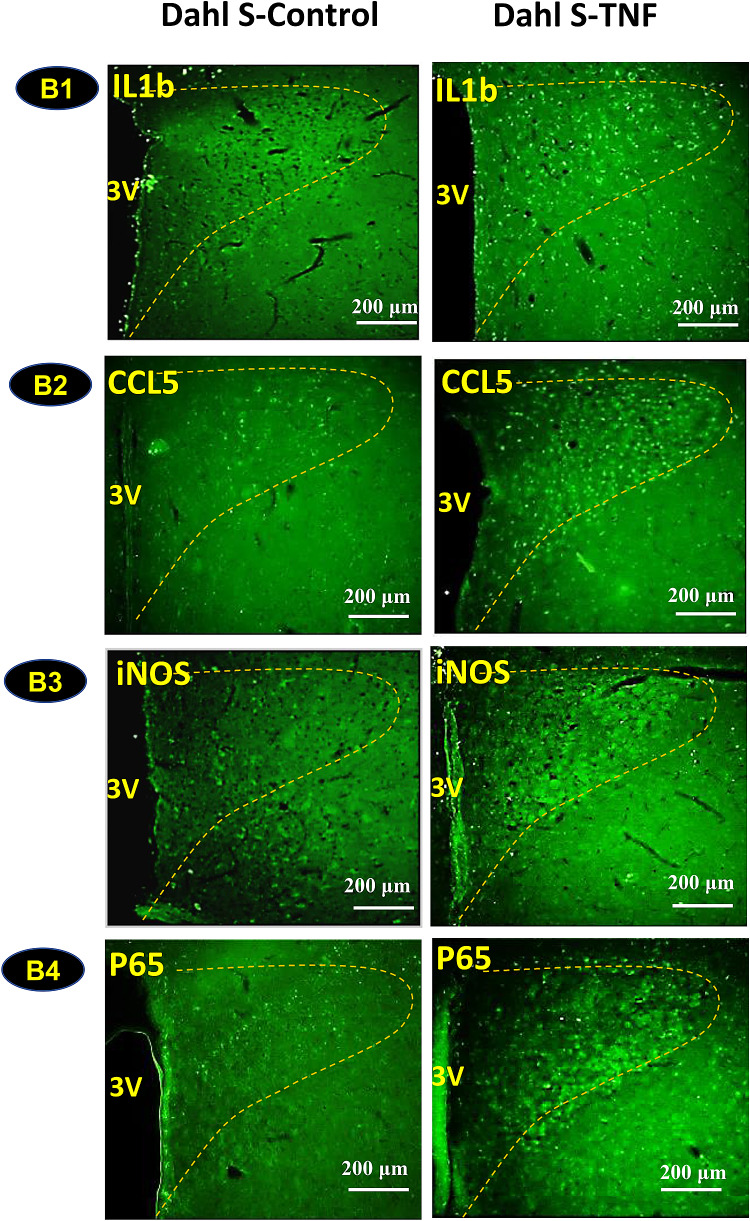
The above experiments were performed using brain neurons from various brain areas. We further wanted to test whether central administration of TNFα can increase expression of inflammatory mediator genes within the PVN of SD rats and Dahl-S rats, given the implications for cardiovascular modulation. TNFα (250 ng dissolved in 2.5 µl saline) or vehicle control (2.5 µl saline) was microinjected into the lateral ventricle of rats. Three hours following injection, rats were euthanized and their brain coronal sections were cut and subjected to immunostaining to probe for IL1β, CCL5, iNOS and NF-kB subunit P65. The results showed that central administration of TNFα significantly increased immunoreactivities of IL1β (Fig. 4A1, B1), CCL5 (Fig. 4A2, B2), iNOS (Fig. 4A3, B3) and P65 (Fig. 4A4, B4) in the PVN of both SD rats (Fig. 4a) and Dahl-S rats (Fig. 4b). The rationale to administrate 250 ng TNFα per rats is based on the dose used in a previous study (Stefferl et al. 1996), the study showed that ICV injection of TNFα at 0.4–1.2 µg/kg body weight (133–399 ng/rat) resulted in a significant increase in body temperature in rats, with the higher dose caused stronger response. We therefore choose an intermediate dose in our study.
Fig. 4.
Intracerebroventricular (ICV) injection of Tumor Necrosis Factor-α (TNFα) induces increases in inflammatory mediators in the Paraventricular Nucleus (PVN) of Sprague Dawley (SD) rats (a) and Dahl Salt-Sensitive (Dahl-S) rats (b). Adult SD and Dahl-S rats were divided into two groups of each strain (n = 3 per group), and received ICV injection of either TNFα (100 ng/µl, 2.5 µl) or vehicle control (0.9% NaCl, 2.5 µl). Three hours following injection, rats were transcranial perfused with 4% paraformaldehyde (PFA). Brain coronal sections containing the PVN (delineated by the dashed yellow outline) were used to perform immunostaining against primary antibodies anti- Interleukin 1β (IL1β), anti- C–C Motif Chemokine Ligand 5 (CCL5), anti-inducible Nitric Oxide Synthase (iNOS) and anti-P65, a NF-kB subunit. Images were taken with an Olympus BX51 TRF Microscope (Olympus, Japan). Representative images showing immunoactivities of IL1β, CCL5, iNOS, and P65 in SD (A1–A4) and Dahl-S rats (B1–B4) in response to ICV injection of TNFα (right panels) or vehicle controls (left panels). (3 V: third ventricle, The PVN area included in the image is delineated by the dashed line)
Inflammatory Response to TNFα Challenge is Upregulated in the Brain Neurons of Dahl-S Rats Compared to SD Rats
Neuronal cell cultures from SD and Dahl-S neonatal pups were incubated with TNFα (20 ng/mL) for 6 h. Then mRNA expression of inflammatory mediator genes was compared in both baseline and post TNFα treatment between the two strains. In order to alleviate experimental variation, each experimental procedure including neuronal preparation, TNFα treatment, RNA isolation, and cDNA conversion was carried out in the same time for two strains of rats. Also, mRNA assessment for each gene of two strains’ samples were completed in the same PCR plate. The baseline mRNA level of each gene of SD rats were assigned an arbitrary unit of 1, and the baselines in Dahl-S as well as post-treatment mRNA levels in both SD and Dahl-S rats were compared to SD base value. The results showed that the baseline mRNA levels of CCL5 (3.8-fold), IL1β (17-fold), iNOS (54-fold), nfkb1 (1.7-fold) are statistically greater in Dahl-S rats. Furthermore, when comparing the gene expression responsiveness to TNFα, Dahl-S rat neurons showed an exaggerated increase in CCL5 (113-fold vs. 28-fold; P < 0.05), IL1β (152-fold vs. 40-fold; P < 0.05), iNOS (1492-fold vs. 658-fold; *P < 0.05) compared to SD rats (Fig. 5).
Fig. 5.
Comparison of the inflammatory response in response to Tumor Necrosis Factor-α (TNFα) challenge in the brain neurons from Sprague Dawley (SD) and Dahl Salt-Sensitive (Dahl-S) rats. 10-day-old brain neuronal cultures obtained from the cortex, hippocampus, and hypothalamus of neonatal SD and Dahl-S rats were incubated with either 20 ng/mL TNFα or vehicle control for 6 h, then cell cultures were collected and subjected to real-time Polymerase Chain Reaction (PCR) to measure the mRNA expression of Interleukin 1β (IL1β), Interleukin-6 (IL6), C–C Motif Chemokine Ligand 5 (CCL5), C–C Motif Chemokine Ligand 12 (CCL12), and NF-kappa- p105 (nfkb1). Data were normalized to the housekeeping gene, GAPDH. Each treatment using 2 ~ 3 well of neuronal cells, and each experiment was repeated using 3 different batch of cells, the results were combined. (n = 6 ~ 9/group, *P < 0.05 in TNF treatment groups compared to their own control groups; &P < 0.05 in Dahl-S control group compared to SD control group; #P < 0.05 in Dahl-S TNF treatment group compared to the SD TNFα treatment group)
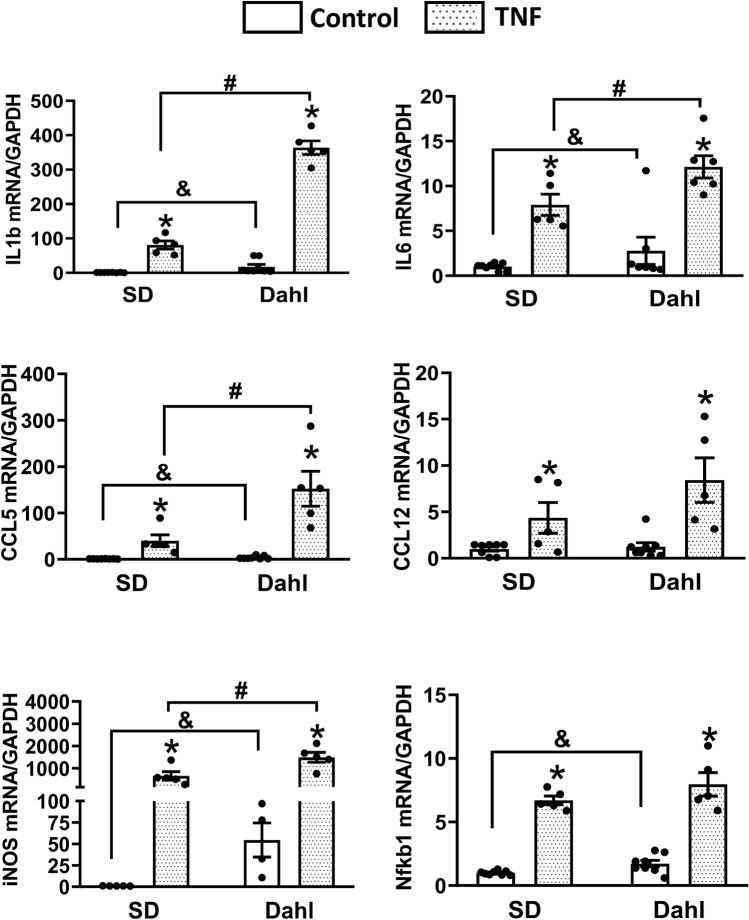
ICV Injection of TNFα Causes an Augmented Increase of CCL12 Expression in Dahl-S Rats
In this experiment, we aimed to investigate whether central administration of TNFα can trigger stronger increases in inflammatory mediator genes in Dahl-S rats than SD rats as observed in their neonatal pups’ brain neurons. Three hours following central administration of TNFα (250 ng) into to the lateral ventricle of the adult SD rats and age- and sex-matched Dahl-S rats, PVN tissues were punched out and real-time PCR was performed to compare the mRNA levels of inflammatory mediators between two strains of rats. While there was no significant difference between baseline mRNA levels of the two animal strains (P > 0.05), ICV injection of TNFα significantly increased mRNA levels of CCL5, CCL12, IL1β, IL6, iNOS and nfkb1 in both SD rats and Dahl-S rats (P < 0.05) (Fig. 6). However, the increase in CCL12 mRNA was greater in Dahl-S rats compared to SD rats (Dahl: 115-fold vs. SD 24-fold; n = 5 ~ 6, #P < 0.05). The increase in IL1β mRNA was also higher in Dahl-S compared to SD rats, but did not reach significance (Dahl: 52-fold vs. SD: 31-fold; n = 5–6, P = 0.087). No significant difference in other genes including CCL5, IL6 and nfkb1 in the PVN of two strains were observed in response to ICV TNFα injection (Fig. 6).
Fig. 6.
Comparison of inflammatory response to Tumor Necrosis Factor-α (TNFα) intracerebroventricular (ICV) injection in the Paraventricular Nucleus (PVN) of Sprague Dawley (SD) and Dahl Salt-Sensitive (Dahl-S) rats. Adult male SD rats received either ICV injection of TNFα (250 ng in 2.5 µl) (n = 6) or vehicle control (2.5 µl 0.9% NaCl) (n = 5). Similarly, age- and sex-matched Dahl-S rats received ICV injection of TNFα (250 ng in 2.5 µl) (n = 5) or vehicle control (2.5 µl 0.9% NaCl) (n = 5). Three hours following microinjection, rats were euthanized, their brain PVN were punched out and subjected to real-time Polymerase Chain Reaction (PCR) to test mRNA elves of Interleukin 1β (IL1β), Interleukin-6 (IL6), C–C Motif Chemokine Ligand 5 (CCL5), C–C Motif Chemokine Ligand 12 (CCL12), inducible Nitric Oxide Synthase (iNOS), and NF-kappa- p105 (nfkb1). Data were normalized to the endogenous housekeeping gene, GAPDH. (*P < 0.05 in TNF treatment groups compared to their own control groups. &P < 0.05 in Dahl-S TNF treatment group compared to the SD TNFα treatment group)
Discussion
Elevation of TNFα is a hallmark of neuroinflammation and has been implicated in a number of pathological conditions including neurodegenerative disease (Frankola et al. 2011; Kinney et al. 2018) and cardiovascular dysfunction (Bautista et al. 2005; Willerson and Ridker 2004). However, the inflammatory response in brain neurons under TNFα exposure as well as the differential responsiveness between hypertensive and normotensive animal models are not well known. The current protocol sought to assess the inflammatory gene expression triggered by TNFα in brain neurons as well as the differences in those gene expression levels in both neuronal culture and brain PVN of Dahl-S rats compared to normal SD rats. We report three novel findings: (1) TNFα stimulates NF-kB activation and triggers strong expression of inflammatory mediator genes in brain neurons. (2) The inflammatory response to TNFα administration is augmented in Dahl-S cultured neurons compared to SD neurons; (3) Central injection of TNFα elicits an exacerbated response of CCL12, and a trend toward augmented expression of IL1b in the PVN of Dahl-S rat when compared to the SD rat. Together, these results show that TNFα acts as a driver for inflammatory responsiveness, and that this responsiveness is augmented in the Dahl-S rat, an animal model of salt sensitive hypertension.
Inflammation, while necessary as an acute response to infection, can become detrimental to physiological processes if chronically present. Excessive inflammation has been implicated in the development of numerous pathological disorders including neurodegenerative diseases (Kinney et al. 2018) and hypertension (Bautista et al. 2005). One of the key proinflammatory cytokines responsible for mediating, at least in part, the development of these disorders is TNFα. Wei and colleagues showed through a series of experiments that TNFα released in the periphery interacts with the circumventricular organs of the brain to elicit a neural inflammatory response (Wei et al. 2013, 2015; Zhang et al. 2003). This interaction at an area of the brain that lacks a rigid blood–brain barrier helps explain how peripherally circulating TNFα can impact the central inflammatory response. Furthermore, the overexpression of TNFα has been shown to play a role in the development of salt-sensitive hypertension. Our own research has shown that the expression of proinflammatory cytokines is elevated in the PVN of Dahl-S rats following a high salt intake (Jiang et al. 2018). Moreira et al. observed that knockdown of the Gαi2 GPCR subunit within the brain of normal rats results in elevated PIC activity (Moreira et al. 2019). This is important, as it has also been observed that the Gαi2 subunit is essential in countering the pressor effects of high salt intake, which Dahl-S rats may lack (Wainford et al. 2015). It is worthwhile to consider that Dahl-S rats may have an underlying disposition toward excessive neuroinflammatory responsiveness due to an impaired blood–brain barrier in addition to lack of essential salt-resistant mechanistic subunits within the brain. The combination of these abnormalities may underly the hypertensive tendencies of the Dahl-S rat strain.
It is well known that Nuclear Factor Kappa Beta (NF-kB) is a potent transcription regulator, and its activation stimulates expression of numerous inflammatory mediators including cytokines and chemokines. Our study showed that TNFα exposure stimulates NF-kB activation (increased P-P65, Fig. 3d), and results in robust increases in the mRNA expression of inflammation mediators including IL1β, IL6, CCL5, CCL12, iNOS and NF-kB subunit nfkb1 in cultured SD brain neurons. It is known that increased IL1β, IL6, CCL5, and excessive iNOS are implicated in many pathological conditions such as Alzheimer’s disease, Parkinson's disease, depression, and cardiovascular diseases (Agudelo et al. 2014; Shaftel et al. 2008; Shi et al. 2010; Tang et al. 2014; Yuste et al. 2015; Zubcevic et al. 2013). Our results suggest that TNFα insult through activation of NF-kB further induces neuroinflammation and ultimately leads to health problems. To test whether central TNFα activity may cause a greater response in Dahl-S rats compared to normal SD rats, we compared the mRNA levels of inflammatory genes and NF-kB prior to and post TNFα treatment between cultured SD and Dahl-S neurons. At baseline, Dahl-S rat neurons expressed higher mRNA levels of CCL5, IL1b, iNOS, and NF-kB subunit nfkb1. The elevated levels of baseline NF-kB may be indicative of a higher sensitivity of Dahl-S neurons to TNFα activity. This idea is strengthened as the response to TNFα administration was shown to be exaggerated in the Dahl-S rat. TNFα treatment caused a significantly augmented PIC response in the Dahl-S rat neuron cultures compared to the SD rat, specifically through upregulation of CCL5, IL1b, IL16, and iNOS. These results confirm that the neuroinflammatory response in Dahl-S rats is augmented, specifically through TNFα mediation. These observations also suggest that in addition to high salt sensitivity, Dahl-S brain neurons may also more vulnerable to other neuroinflammation induced disease and brain dysfunction.
In order to compare the impact of central TNFα on the PVN, a key cardiovascular relevant region in the brain, between SD and Dahl-S rat models, we performed ICV injection of TNFα in both strains. Opposite to the results in primary neuronal cultures, we did not observe any baseline differences in PVN PIC expression between SD and Dahl-S rats. The difference in baseline observed in brain neuronal cultures could be due to the fact that neurons were isolated from hypothalamic, hippocampal, and cortex areas of the brain, whereas in whole animal study, gene expression was only assessed on PVN area. Furthermore, the Dahl-S rats were not on a high salt diet, which may be necessary to facilitate the previously observed increases in PVN inflammation (Jiang et al. 2018). Similar to the results observed in primary neuron cultures, TNFα ICV injection elicited an increase in all tested PIC mRNA expression within the PVN regardless of rat strain. However, CCL12 was elevated significantly more in the Dahl-S strain. CCL12 is an agonist of C–C chemokine receptor type 2 (CCR2). A previous study showed that CCR2, CCL12 and macrophage numbers were significantly increased in the aorta of deoxycorticosterone acetate (DOCA)–salt-treated hypertensive mice, and antagonism of CCR2 prevents macrophage accumulation in the vessel wall and markedly reverses DOCA/salt-induced increases in blood pressure (Chan et al. 2012). This evidence suggests that CCL12 through activation of CCR2, recruiting macrophages, which in turn initiate inflammatory response, and contributed to hypertension development. The evidence also suggests that increased TNFα in the PVN may thorough stimulating CCL12 secretion to increase blood pressure in Dahl-S rats. As we mentioned earlier, high salt diet can increase TNFα expression in the PVN (Jiang et al. 2018) of Dahl-S rats. The increased TNFα can bind with its receptors and triggers activation of transcription factor NF-kB. Activated NF-kB binds to the responsive elements in the promoter of CCL12 gene and stimulate CCL12 expression and secretion. The increased CCL2 may directly modulate neuronal activity and/or through activation of CCR2, leading to further inflammatory response in the PVN, ultimately leads to blood pressure increase.
Interestingly, IL1β trended toward a significantly elevated response to TNFα central infusion in the Dahl-S rat. IL1β has been observed to be elevated in humans with essential hypertension (Dalekos et al. 1997). This is important to note as IL1β has been shown to significantly impact blood pressure regulation along with TNFα (Shi et al. 2011; Wei et al. 2013, 2015). Shi and colleagues observed that direct microinjection of IL1β into the PVN of SD rats elicits a sympathomimetic response (Shi et al. 2011). Wei et al. further showed that TNFα microinjection into the SFO elevated PVN levels of IL1β in the SD rat model in concert with blood pressure and sympathetic outflow (Wei et al. 2015). This may indicate heightened sensitivity in the Dahl-S rat model in response to TNFα action in the PVN, resulting in enhanced production of IL1β in this strain, leading to the development of hypertension in this model.
Contrary to our hypothesis, we did not observe similarly elevated responsiveness in all of the same PICs in Dahl-S rats following ICV injection as we did post TNFα treatment of neuronal cells. One reason for this discrepancy may simply lie in the idea that neuronal cultures do not offer the same complex interactions that the brain does. The brain consists of multiple cell types including neurons, astrocytes, and microglia. All cell types are capable of producing cytokines, although microglia are the primary source of cytokines. While neuronal studies isolate the effective response to the action of neurons, in vivo studies reflect responsiveness of the numerous different cell types to TNFα treatment. The combined effects of these cell types to the same physiological stressor may result in interactions that could not be accounted for in our study. Despite this discrepancy, it is worthwhile to re-emphasize the observed elevated responsiveness of CCL12 and IL1β in the Dahl-S rat model following TNFα ICV injection, as these two cytokines are having potential in the development of cardiovascular complications as eluded to earlier.
It is also important to note that, while the current study was performed in Dahl-S rats, a model of salt-sensitive hypertension, the results of our study extend into numerous disciplines of pathophysiology, including neurodegeneration. Human patients with Alzheimer’s disease, for example, have been observed to have elevated levels of TNFα within the brain as well as the plasma (Chang et al. 2017). Mice lacking the TNFR1 showed a reduction in cognitive decline, and reduced plaque aggregation (He et al. 2007). Our model of neurons combined samples from areas including not only the hypothalamus, but also the cortex and hippocampus. Thus, the elevated inflammatory response observed in our neuronal cultures may be representative of a key role for circulating TNFα in the progression and maintenance of neurodegeneration. IL1β has also been implicated for its potential role in the early development of Alzheimer’s disease, and IL1β inhibition has been shown to reduce the onset of neurodegeneration (Basu et al. 2004). Our results showed an augmented increase in IL1β in Dahl-S neuron cultures, and a trend toward augmented reactivity in the PVN following ICV injection of TNFα. This may act to strengthen the key role that TNFα levels play in the development and maintenance of neurodegenerative disorders, specifically through signaling with IL1β. Although it is beyond the scope of this paper, further studies should be performed in differing animal models to fully elucidate mechanistic differences in action and susceptibility to neurodegeneration, through mediation of TNFα.
The present research study does yield some limitations worth mentioning. First, ICV injection does not specifically impact only the PVN area. Numerous other brain areas may have been impacted by the increased amounts of central TNFα. However, we chose to focus on the PVN given our previous findings implicating increased inflammatory responsiveness in Dahl-S rats (Jiang et al. 2018). Other brain areas, including the Rostral Ventrolateral Medulla, Nucleus Tractus Solitarius, and the Caudal Ventrolateral Medulla are equally important for cardiovascular regulation, and may have been impacted by TNFα, offering a future line of inquiry. Secondly, IL6 is known to elicit both pro and anti-inflammatory responses depending on how it is bound (Scheller et al. 2011). Specifically, when IL6 binds to soluble IL6R to facilitate IL6 trans-signaling, proinflammatory processes occur devoid of any membrane bound IL6R interaction. This means that in our sample, the observed increase in IL6 mRNA may have translated to either a pro or anti-inflammatory response. Since we measured only mRNA levels, we cannot say with confidence which receptor type the excess IL6 acted upon. However, we would like to note that the IL6 response to TNFα was not different between SD and Dahl rats, while CCL12 and IL1β, both implicated in proinflammatory processes with links to hypertension development, appeared to be elevated. Further, IL6 is consistently elevated in humans with hypertension (Chamarthi et al. 2011; Sesso et al. 2007) implicating it in pathology in humans. Research from our lab has shown that IL6 is elevated in the PVN of Dahl-S rats following a HS diet (Jiang et al. 2018), while others have shown that inhibition of IL6 in Dahl-S rats results in an attenuation of hypertension (Hashmat et al. 2016). The combination of these results presents IL6 as a pertinent target in the development of hypertension, specifically in the Dahl-S rat model.
In conclusion, the current study outlines a key role for central TNFα signaling in mediating the inflammatory response in both SD and Dahl-S rats. Furthermore, our results show an augmented PIC responsiveness in both neuronal and in vivo Dahl-S rats following TNFα, elucidating a higher sensitivity in this rat model. These results may explain the mechanistic underpinnings regarding Dahl-S rats’ susceptibility to hypertension. Lastly, these results may have far-reaching implications in the field of neurodegeneration, further elucidating the complex interplay between TNFα and PIC signaling cascades, which have shown importance in the development of neurodegenerative disorders.
Acknowledgements
This work was supported by NIH R15HL150703 (Shan) and Portage Health Foundations Mid-Career (Shan).
Author Contributions
HG and ZS designed the experiments and participated in data collection and analysis, YF participated in data analysis and Figure editing, JB and ZS wrote the paper. EJ, QC and BC supervised HG for her research and participated in helpful discussion and data analysis. All Authors read and approved the manuscript.
Data Availability
Data will be available upon reasonable request to the corresponding author.
Compliance with Ethical Standards
Conflict of interest
There are no conflicts of interest to report.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Agudelo LZ et al (2014) Skeletal muscle PGC-1alpha1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell 159:33–45. 10.1016/j.cell.2014.07.051 [DOI] [PubMed] [Google Scholar]
- Albert CM, Ma J, Rifai N, Stampfer MJ, Ridker PM (2002) Prospective study of C-reactive protein, homocysteine, and plasma lipid levels as predictors of sudden cardiac death. Circulation 105:2595–2599. 10.1161/01.cir.0000017493.03108.1c [DOI] [PubMed] [Google Scholar]
- Basu A, Krady JK, Levison SW (2004) Interleukin-1: a master regulator of neuroinflammation. J Neurosci Res 78:151–156. 10.1002/jnr.20266 [DOI] [PubMed] [Google Scholar]
- Bautista LE, Vera LM, Arenas IA, Gamarra G (2005) Independent association between inflammatory markers (C-reactive protein, interleukin-6, and TNF-alpha) and essential hypertension. J Hum Hypertens 19:149–154. 10.1038/sj.jhh.1001785 [DOI] [PubMed] [Google Scholar]
- Chamarthi B et al (2011) Inflammation and hypertension: the interplay of interleukin-6, dietary sodium, and the renin-angiotensin system in humans. Am J Hypertens 24:1143–1148. 10.1038/ajh.2011.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CT et al (2012) Reversal of vascular macrophage accumulation and hypertension by a CCR2 antagonist in deoxycorticosterone/salt-treated mice. Hypertension 60:1207–1212. 10.1161/HYPERTENSIONAHA.112.201251 [DOI] [PubMed] [Google Scholar]
- Chang R, Yee KL, Sumbria RK (2017) Tumor necrosis factor alpha Inhibition for Alzheimer’s Disease. J Cent Nerv Syst Dis 9:1179573517709278. 10.1177/1179573517709278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HY, Park HC, Ha SK (2015) Salt sensitivity and hypertension: a paradigm shift from kidney malfunction to vascular endothelial dysfunction. Electrolyte Blood Press 13:7–16. 10.5049/EBP.2015.13.1.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottone S, Vadala A, Vella MC, Mule G, Contorno A, Cerasola G (1998) Comparison of tumour necrosis factor and endothelin-1 between essential and renal hypertensive patients. J Hum Hypertens 12:351–354. 10.1038/sj.jhh.1000596 [DOI] [PubMed] [Google Scholar]
- Dalekos GN, Elisaf MS, Papagalanis N, Tzallas C, Siamopoulos KC (1996) Elevated interleukin-1 beta in the circulation of patients with essential hypertension before any drug therapy: a pilot study. Eur J Clin Investig 26:936–939. 10.1111/j.1365-2362.1996.tb02141.x [DOI] [PubMed] [Google Scholar]
- Dalekos GN, Elisaf M, Bairaktari E, Tsolas O, Siamopoulos KC (1997) Increased serum levels of interleukin-1beta in the systemic circulation of patients with essential hypertension: additional risk factor for atherogenesis in hypertensive patients? J Lab Clin Med 129:300–308. 10.1016/s0022-2143(97)90178-5 [DOI] [PubMed] [Google Scholar]
- Fan Y, Jiang E, Hahka T, Chen QH, Yan J, Shan Z (2018) Orexin A increases sympathetic nerve activity through promoting expression of proinflammatory cytokines in Sprague Dawley rats. Acta Physiol (Oxford). 10.1111/apha.12963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankola KA, Greig NH, Luo W, Tweedie D (2011) Targeting TNF-alpha to elucidate and ameliorate neuroinflammation in neurodegenerative diseases. CNS Neurol Disord Drug Targets 10:391–403. 10.2174/187152711794653751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashmat S, Rudemiller N, Lund H, Abais-Battad JM, Van Why S, Mattson DL (2016) Interleukin-6 inhibition attenuates hypertension and associated renal damage in Dahl salt-sensitive rats. Am J Physiol Renal Physiol 311:F555–F561. 10.1152/ajprenal.00594.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He P et al (2007) Deletion of tumor necrosis factor death receptor inhibits amyloid beta generation and prevents learning and memory deficits in Alzheimer’s mice. J Cell Biol 178:829–841. 10.1083/jcb.200705042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang BS, Van Vliet BN, Leenen FH (2004) Increases in CSF [Na+] precede the increases in blood pressure in Dahl S rats and SHR on a high-salt diet. Am J Physiol Heart Circ Physiol 287:H1160–H1166. 10.1152/ajpheart.00126.2004 [DOI] [PubMed] [Google Scholar]
- Huang B et al (2016) Renal tumor necrosis factor alpha contributes to hypertension in dahl salt-sensitive rats. Sci Rep 6:21960. 10.1038/srep21960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber MJ et al (2017) Increased activity of the orexin system in the paraventricular nucleus contributes to salt-sensitive hypertension. Am J Physiol Heart Circ Physiol 313:H1075–H1086. 10.1152/ajpheart.00822.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang E et al (2018) Expression of proinflammatory cytokines is upregulated in the hypothalamic paraventricular nucleus of dahl salt-sensitive hypertensive rats. Front Physiol 9:104. 10.3389/fphys.2018.00104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang YM, Ma Y, Zheng JP, Elks C, Sriramula S, Yang ZM, Francis J (2009) Brain nuclear factor-kappa B activation contributes to neurohumoral excitation in angiotensin II-induced hypertension. Cardiovasc Res 82:503–512. 10.1093/cvr/cvp073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang YM et al (2010) TNF-alpha in hypothalamic paraventricular nucleus contributes to sympathoexcitation in heart failure by modulating AT1 receptor and neurotransmitters. Tohoku J Exp Med 222:251–263. 10.1620/tjem.222.251 [DOI] [PubMed] [Google Scholar]
- Kinney JW, Bemiller SM, Murtishaw AS, Leisgang AM, Salazar AM, Lamb BT (2018) Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement (New York) 4:575–590. 10.1016/j.trci.2018.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira JD et al (2019) Inhibition of microglial activation in rats attenuates paraventricular nucleus inflammation in Galphai2 protein-dependent, salt-sensitive hypertension. Exp Physiol 104:1892–1910. 10.1113/EP087924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimenta E, Gaddam KK, Oparil S, Aban I, Husain S, Dell’Italia LJ, Calhoun DA (2009) Effects of dietary sodium reduction on blood pressure in subjects with resistant hypertension: results from a randomized trial. Hypertension 54:475–481. 10.1161/HYPERTENSIONAHA.109.131235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S (2011) The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta 1813:878–888. 10.1016/j.bbamcr.2011.01.034 [DOI] [PubMed] [Google Scholar]
- Sesso HD, Wang L, Buring JE, Ridker PM, Gaziano JM (2007) Comparison of interleukin-6 and C-reactive protein for the risk of developing hypertension in women. Hypertension 49:304–310. 10.1161/01.HYP.0000252664.24294.ff [DOI] [PubMed] [Google Scholar]
- Shaftel SS, Griffin WS, O’Banion MK (2008) The role of interleukin-1 in neuroinflammation and Alzheimer disease: an evolving perspective. J Neuroinflamm 5:7. 10.1186/1742-2094-5-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi P et al (2010) Brain microglial cytokines in neurogenic hypertension. Hypertension 56:297–303. 10.1161/HYPERTENSIONAHA.110.150409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z et al (2011) Inflammatory cytokines in paraventricular nucleus modulate sympathetic activity and cardiac sympathetic afferent reflex in rats. Acta Physiol (Oxford) 203:289–297. 10.1111/j.1748-1716.2011.02313.x [DOI] [PubMed] [Google Scholar]
- Sriramula S, Cardinale JP, Francis J (2013) Inhibition of TNF in the brain reverses alterations in RAS components and attenuates angiotensin II-induced hypertension. PLoS ONE 8:e63847. 10.1371/journal.pone.0063847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefferl A, Hopkins SJ, Rothwell NJ, Luheshi GN (1996) The role of TNF-alpha in fever: opposing actions of human and murine TNF-alpha and interactions with IL-beta in the rat. Br J Pharmacol 118:1919–1924. 10.1111/j.1476-5381.1996.tb15625.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang P, Chong L, Li X, Liu Y, Liu P, Hou C, Li R (2014) Correlation between serum RANTES levels and the severity of Parkinson’s disease. Oxid Med Cell Longev 2014:208408. 10.1155/2014/208408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainford RD, Carmichael CY, Pascale CL, Kuwabara JT (2015) Galphai2-protein-mediated signal transduction: central nervous system molecular mechanism countering the development of sodium-dependent hypertension. Hypertension 65:178–186. 10.1161/HYPERTENSIONAHA.114.04463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei SG, Zhang ZH, Beltz TG, Yu Y, Johnson AK, Felder RB (2013) Subfornical organ mediates sympathetic and hemodynamic responses to blood-borne proinflammatory cytokines. Hypertension 62:118–125. 10.1161/HYPERTENSIONAHA.113.01404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei SG, Yu Y, Zhang ZH, Felder RB (2015) Proinflammatory cytokines upregulate sympathoexcitatory mechanisms in the subfornical organ of the rat. Hypertension 65:1126–1133. 10.1161/HYPERTENSIONAHA.114.05112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger MH (1996) Salt sensitivity of blood pressure in humans. Hypertension 27:481–490. 10.1161/01.hyp.27.3.481 [DOI] [PubMed] [Google Scholar]
- Willerson JT, Ridker PM (2004) Inflammation as a cardiovascular risk factor. Circulation 109:II2–II10. 10.1161/01.CIR.0000129535.04194.38 [DOI] [PubMed] [Google Scholar]
- Yilmaz R, Akoglu H, Altun B, Yildirim T, Arici M, Erdem Y (2012) Dietary salt intake is related to inflammation and albuminuria in primary hypertensive patients. Eur J Clin Nutr 66:1214–1218. 10.1038/ejcn.2012.110 [DOI] [PubMed] [Google Scholar]
- Yoshida S et al (2014) Infliximab, a TNF-alpha inhibitor, reduces 24-h ambulatory blood pressure in rheumatoid arthritis patients. J Hum Hypertens 28:165–169. 10.1038/jhh.2013.80 [DOI] [PubMed] [Google Scholar]
- Yuste JE, Tarragon E, Campuzano CM, Ros-Bernal F (2015) Implications of glial nitric oxide in neurodegenerative diseases. Front Cell Neurosci 9:322. 10.3389/fncel.2015.00322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZH, Wei SG, Francis J, Felder RB (2003) Cardiovascular and renal sympathetic activation by blood-borne TNF-alpha in rat: the role of central prostaglandins. Am J Physiol Regul Integr Comp Physiol 284:R916-927. 10.1152/ajpregu.00406.2002 [DOI] [PubMed] [Google Scholar]
- Zubcevic J et al (2013) Nucleus of the solitary tract (pro)renin receptor-mediated antihypertensive effect involves nuclear factor-kappaB-cytokine signaling in the spontaneously hypertensive rat. Hypertension 61:622–627. 10.1161/HYPERTENSIONAHA.111.199836 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be available upon reasonable request to the corresponding author.