Abstract
Background:
Sex differences in addiction have been described in humans and animal models. A key factor that influences addiction in both males and females is adolescent experience. Adolescence is associated with higher vulnerability to substance use disorders, and male rodents subjected to adolescent social isolation stress (SI) form stronger preferences for drugs of abuse in adulthood. However, little is known about how females respond to SI, and few studies have investigated the transcriptional changes induced by SI in the brain’s reward circuitry.
Methods:
We tested the hypothesis that SI alters the transcriptome in a persistent and sex-specific manner in prefrontal cortex (PFC), nucleus accumbens (NAc), and ventral tegmental area (VTA). Mice were isolated or group housed (GH) from postnatal day P22 - P42, then GH until ~P90. Transcriptome-wide changes were investigated by RNA-seq after acute or chronic cocaine or saline administration.
Results:
We found that SI disrupts sex-specific transcriptional responses to cocaine as well as reduces sex differences in gene expression across all three brain regions. Further, SI induces gene expression profiles in males that more closely resemble GH females, suggesting that SI “feminizes” the male transcriptome. Co-expression analysis reveals that such disruption of sex differences in gene expression alters sex-specific gene networks and identifies potential sex-specific key-drivers of these transcriptional changes.
Conclusions:
Together, these data show that SI has region-specific effects on sex-specific transcriptional responses to cocaine and provide a better understanding of reward-associated transcription that differs in males and females.
Keywords: RNA-sequencing, cocaine, gene expression, gene co-expression network analysis, sex differences
INTRODUCTION
Sex differences in cocaine use disorder are known for every stage of addiction. While men are more likely to use drugs of abuse, when women do use drugs they become addicted more quickly. Women also report greater craving during withdrawal, are more likely to relapse, and consume more drugs during relapse, which put them at greater risk for overdoses (1). These effects are documented in rodents as well (2), however, the underlying molecular mechanisms remain poorly understood.
Adolescence is a crucial development period for the reward circuitry and for the progression to addiction in particular. Adolescence is associated with increased vulnerability to several psychiatric disorders including substance use disorders (SUDs), and numerous studies show that adolescent rodents and humans are more sensitive to rewarding and stressful stimuli (3). The adolescent brain undergoes enormous structural and molecular changes, especially with regard to the mesocorticolimbic reward circuitry (4), suggesting that stimuli during this time may result in long-term behavioral consequences in adulthood. Indeed, adolescent social isolation (SI) affects anxiety- and depression-related behaviors long-term, and enhances preference for numerous drugs of abuse including cocaine in male rodents. Likewise, in humans, social stress during adolescence influences susceptibility for SUDs (5-7). Rodent adolescent SI has also been shown to disrupt dopamine signaling throughout the reward circuitry (8). However, few studies have investigated the transcriptional changes driven by adolescent SI in males, and no studies have investigated either phenomenon in females. An overarching goal of this study was to characterize the sex-specific response to cocaine under control conditions and determine if those sex differences are disrupted by SI.
Recently, we showed that adolescent SI decreases certain sex differences in behaviors, yet increases sex differences in cocaine place conditioning in mice (9). We also showed that these behavioral effects are reflected in transcriptional changes within medial amygdala, a brain region traditionally thought to be outside the reward circuitry (9). Therefore, in the present study, we tested the hypothesis that adolescent SI induces similar disruptions in transcriptional responses to cocaine in three brain reward regions: prefrontal cortex (PFC), nucleus accumbens (NAc), and ventral tegmental area (VTA). Our findings offer new insight into the lasting interactions between adolescent stress and cocaine exposure in adulthood at the transcriptional level in both sexes.
METHODS
Detailed descriptions of experimental design and approaches are provided in Supplemental Methods. All of the methods used have been published recently (9).
RESULTS
Adolescent SI disrupts sex-specific transcriptional responses to cocaine
To study the effect of adolescent SI on transcriptional responses to cocaine in an unbiased manner, we performed RNA-seq on PFC, NAc, and VTA (Figure 1A-B). We analyzed transcriptome-wide gene expression changes in response to acute (1 hr after the first dose; Figure 1C-H) or chronic (24 hr after the 10th dose; Suppl Fig 1C-H) experimenter-administered cocaine (7.5 mg/kg; Suppl Table 1). This approach can model differences in drug exposure observed in the progression of SUD, i.e., acute models the sensitivity to first drug dose and chronic models longer-term alterations taking place over time (7). While there are sex differences in many aspects of addiction, the most well characterized are differences in acquisition of cocaine self-administration: females acquire the task more quickly (10-12). Therefore, we focused on acute effects of cocaine in the main figures.
Figure 1: Sex-specific transcriptional responses to acute cocaine are disrupted by SI throughout the reward circuitry.
(A-B) Schematic of experimental design. Heatmaps show DEGs with at least a nominal p≤0.05 and a fold change (FC)≥1.3 in response to acute cocaine (AC Coc) for (C) PFC, (D) NAc, and (E) VTA (yellow = upregulated; blue = downregulated). Heatmaps are seeded by GHMs. All comparisons are made to the acute saline control for the group. Venn diagrams of DEGs in GH (closed circles) and (open circles) M/F after acute cocaine, (F) PFC, (G) NAc, and (H) VTA. There was very little overlap between males and females across all three brain regions. (I) IPA shows intracellular signaling, neurotransmitter, and other nervous system signaling pathways that are regulated by acute cocaine (activation z-scores≥2.0; p-value≤ 0.05; yellow = predicted activated; blue = predicted inhibited; black = no predicted activation/inhibition). *p<0.05; **corrected p-value<0.05.
To assess if SI disrupts sex-specific transcriptional responses to cocaine, we first focused on genes affected by cocaine in GH males (M) and females (F) after acute (Figure 1C-H) or chronic (Suppl Figure 1C-H) exposure. Union heatmaps and Venn diagrams of genes regulated by cocaine in GHMs and GHFs reveal that, in all 3 brain regions, GHMs regulate many more genes in response to acute (Figure 1C-H) but not chronic (Suppl Fig 1C-H) cocaine than GHFs, with very little overlap of those genes in the two sexes (Figure 1C-H; Suppl Figure 1C-H). Importantly, SI disrupts this pattern in PFC and VTA, but not NAc (Figure 1G; Suppl Figure 1G). It is noteworthy that in PFC expression patterns in SIMs mirror those of GHFs in response to both acute (Figure 1C) and chronic (Suppl Figure 1C) cocaine. In NAc and VTA, there is very little overlap in expression when comparing SI to their GH counterparts after acute (Figure 1D-E) or chronic (Suppl. Figure 1D-E) drug exposure. Notably, in VTA in response to acute cocaine, SIFs regulate similar genes as GHMs but in the opposite direction (Figure 1E). Another interesting pattern is the larger number of transcripts affected in PFC and VTA of GHMs vs GHFs, a difference not seen in NAc (Figure 1F-H). This pattern is reversed after SI, with many more transcripts regulated in SIM NAc. Together, these data suggest that SI disrupts sex-specific gene expression by cocaine in a brain region-specific manner with brain reward mechanisms likely being disrupted as a result.
We next used Ingenuity Pathway Analysis (IPA; Qiagen, Fredrick MD) to identify biological pathways that are affected by cocaine in GH and SI animals (activation z-scores >/= 2.0; p-value < 0.05). We focused on pathways that are associated with neurotransmitters and intracellular signaling because molecules in the two pathways overlap significantly and largely reflect neuronal signaling. Numerous signaling pathways were identified in gene expression profiles after acute cocaine in GHMs (Figure 1I), with minimal overlap in pathways predicted for GHFs, suggesting that pathways regulated in brain reward regions by the first cocaine dose differ greatly between males and females. By contrast, many of the same pathways were predicted to be regulated in SIMs and SIFs (Figure 1I), especially after chronic cocaine (Suppl Figure 1I). However, important region-specific differences in those pathways were observed. Together, these data suggest that adolescent SI reprograms both the acute and chronic response to cocaine in a sex- and region-specific manner.
Adolescent SI causes loss of sex differences in gene expression throughout the reward circuitry
The finding that SI disrupts sex-specific transcriptional responses to cocaine suggests that baseline sex differences might be altered by SI. We tested this hypothesis by evaluating sex differences in gene expression in GHMs vs GHFs after acute or chronic saline (Figure 2A-G) or cocaine (Suppl Figure 2A-G; Suppl Table 2). Union heatmaps and Venn Diagrams of sex differences in genes in GH animals under all conditions—acute/chronic saline (Figure 2) and cocaine (Suppl Figure 2)—reveal that baseline sex differences in gene expression are dramatically attenuated after SI in all three brain regions. The signaling pathways that are associated with those sex differences in genes overlap with those regulated by cocaine (Figure 2H-I; Suppl Figure 2 H-I), suggesting that SI disrupts sex-specific biological pathways associated with neuronal signaling in a region-specific manner.
Figure 2: Adolescent SI disrupts expression of baseline sex differences in the reward circuitry.
(A) Schematic of experimental design. (B-D) Union heatmaps of sex differences in DEGs in GHMs vs GHFs after acute or chronic saline. Blue represents genes upregulated in females, while yellow represents genes downregulated in females. (E-G) Venn diagrams of sex differences in genes in GH and SI animals. Sex differences in all three brain regions are lost after SI. IPA shows pathways composed of sex differences in genes overlap with pathways affected by cocaine after experiencing (H) acute and (I) chronic saline (activation z-scores≥2.0; p-value≤0.05). *p<0.05; **corrected p-value<0.05.
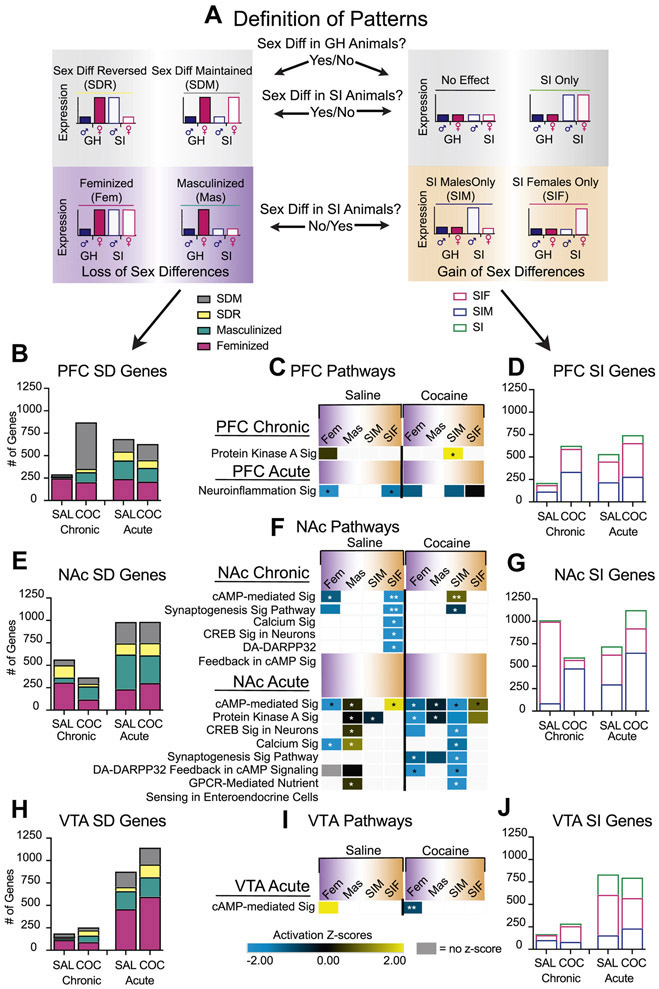
Pattern analysis indicates that sex-specific gene expression changes are driven by a loss and gain of baseline sex differences
We next used pattern analysis, a technique used previously (13), to account for the finding that SI disrupts both baseline sex-specific expression and transcriptional responses to cocaine. All differentially expressed gene (DEG) data were compared to the same baseline (GHF chronic saline) and expression changes were categorized based on the patterns of expression across the 4 groups (GHM, GHF, SIM, and SIF). This dimensional reduction technique identified many patterns (Supple Table 2). We focused on two, based on the finding that sex differences in gene expression were lost (different between GHMs and GHFs but not after SI) or gained (not different between GHMs and GHFs but different after SI) (Figure 3A). For both patterns, genes were first categorized by their differential expression between GHMs and GHFs and then characterized by how they changed after SI. For example, if the loss of sex difference after SI was driven by the fact that SIM expression looked like GHF expression it was categorized as “feminized” (Figure 3A). Pattern analysis revealed that the loss of sex differences in expression in all three brain regions and under all treatment conditions was driven by feminization of expression in SIMs: ~40% of sex differences in genes were feminized by SI. Masculinization of sex differences in genes did not make up a large proportion of the patterns observed under baseline condition (chronic saline). However, after SIFs were exposed to another stimulus, male-typical expression patterns were observed across all 3 brain regions (Figure 3B,E,H). Similar effects were observed in the patterns representing a gain of sex differences (Figure 3D,G,J). Genes only differentially expressed in SIMs made up a large proportion of the genes categorized as a “gain of sex difference” under all treatment conditions and all brain regions. The one exception to this was in NAc, where under baseline conditions (chronic saline), genes are disproportionally altered in SIFs (Figure 3G). These data suggest that many of the sex-specific transcriptional changes observed after SI are driven by changes in SIMs and point to a more responsive transcriptional state in SIMs throughout the reward circuitry. Finally, this analysis revealed that animals regulate many more transcripts in response to acute stimuli (saline or cocaine) in a sex-specific manner. This occurs even after several injections meant to induce habituation, suggesting that habituation is a transcriptionally active event and differs between the sexes. Indeed, we showed previously that stress resilience is associated with greater changes in transcription than stress susceptibility in males (27). The present data suggest a similar phenomenon regulating habituation to handling and injection stress.
Figure 3: Pattern analysis reveals sex differences that are lost or gained after SI.
(A) Schematic of decision-tree for categorizing genes in pattern analysis. Pattern analysis of (B) PFC, (E) NAc, and (H) VTA sex differences in genes in all four conditions. Gray = sex difference maintained (SDM), yellow = sex difference reversed (SDR), blue = masculinized (MAS), and pink = feminized (FEM). IPA of PFC (C), NAc (F), and VTA (I) Pathway analysis reveals same pathways that are affected by cocaine and sex-specific gene expression. Only those pathways that overlap with those regulated by cocaine are included (C,F & I). See Suppl Figure 3 for complete list of the predicted pathways associated with the genes identified through pattern analysis. (activation z-scores≥2.0; p-value≤ 0.05; yellow = predicted activated; blue = predicted inhibited; black = no predicted activation/inhibition). Pattern analysis of SI effects after all four treatment conditions in (D) PFC, (G) NAc, and (J) VTA. Translucent pink = SIF effects only, translucent blue = SIM effects only, and translucent green = SI effects. *p<0.05; **corrected p-value<0.05.
We next focused on pathways predicted to be regulated by cocaine (Figure 1) or sex-specific expression (Figure 2) to determine if the pathways reflect a loss or gain of sex-specific expression. Pathways that were predicted to be feminized under saline conditions were predicted to be regulated by cocaine only in SIMs in NAc and PFC (Figure 3C,F). This was not the case for pathways predicted to be regulated in SIFs, where most were predicted to be regulated under saline conditions (both acute and chronic). These data suggest that neuronal signaling pathways are disrupted by SI more in males than in females.
Co-expression analysis reveals that sex-specific gene networks are disrupted by SI
We next asked if sex-specific disruption of transcription altered transcriptomic structure using multi-scale embedded gene co-expression network analysis (MEGENA). This approach is unique in that, unlike other co-expression analyses, MEGENA identifies modules of genes at different compactness scales, leading to a hierarchical module structure (14). We hypothesized that disruption of sex-specific transcription disrupts co-expression modules on a global scale, providing insight into hubs that might be important for regulating sex-specific transcription in the reward circuitry. Each brain region was analyzed separately. Because we hypothesized that SI disrupts sex-specific transcriptional regulation under various conditions, modules were identified for each of the four groups (GHM/F, SIM/F) by collapsing data from all four treatment paradigms (acute/chronic cocaine and saline). We first focused on sex-specific co-expression networks between GHMs and GHFs and then determined how SI disrupted sex-specific co-expression on a global scale in each brain region (Figures 4-6). By comparing the number of parent modules we identified disruptions to transcriptomic structure across the groups (Figure 4-6E). Because parent modules are the largest groups of co-expressed genes identified within the root module, a transcriptome with fewer parent modules has more “structure.”
Figure 4: Sex-specific co-expression networks are disrupted by SI in PFC.
(A-D) Sunburst plots in PFC of (A) GHMs, (B) GHFs, (C) SIMs, and (D) SIFs. Plots are organized with parent modules on the inner-most rings, followed by children, grandchildren, modules, etc. Modules with asterisks are of interest due to conservation across all four groups and a loss or gain of sex difference. A variety of colors indicate enrichment of pattern genes; gray = no enrichment. (E) Bar graph of number of parent modules shows transcriptomic structure is conserved. (F) Module Differential Connectivity (MDC) plots show comparison of module connectivity across parent modules. Numbers = MDC value for comparison; purple = differentially connected, and gray = not differentially connected. (G) Enrichment plot of conserved hub genes across differentially connected parent modules. Significant enrichment is indicated by purple, number of genes in each module is in parentheses, and number of overlapping genes is in each box. (H) Bar graph of the number of connections of the hub gene Gpr37 in GH and SI males and females. (I) Heatmap showing the relative expression of the hub gene Gpr37 in response to acute and chronic saline and cocaine. (J) Pathway analysis of the genes in each module reveals sex-specific pathways associate with co-expression modules is disrupted by SI; purple = enrichment of genes associated with pathways; gray = no enrichment. *=p<0.05; **corrected p-value<0.05.
Figure 6: Sex-specific co-expression networks are disrupted by SI in VTA.
(A-D) Sunburst plots in VTA of (A) GHM, (B) GHF, (C) SIM, and (D) SIF. Plots are organized with parent and submodules as in Figure 4, where asterisks and colors are also defined. (E) Bar graph of number of parent module shows loss of transcriptomic structure in SI animals. (F) Module Differential Connectivity (MDC) plots show comparison of module connectivity across parent modules. Numbers and colors are defined in Figure 4. (G) Enrichment plot of conserved hub genes across differentially connected parent modules. Numbers and colors are defined in Figure 4. (H) Bar graph of the number of connections of the hub gene Ash1 in GH and SI males and females. (I) Heatmap showing the relative expression of the hub gene Ash1 in response to acute and chronic saline and cocaine. (J) Comparison analysis of pathway enrichment of gene modules in each condition show no overlap between males and females independent of SI. *p<0.05; **corrected p-value<0.05.
Using module differential connectivity (MDC) analysis, we identified parent modules that were differentially connected between GHMs and GHFs, with the predicted sex differences in MDC disrupted after SI (Figures 4-6F). We next used Fisher’s exact tests to find parent modules composed of the same genes in GHMs and GHFs (Figure 4-6G; Suppl Figure 5B) and then focused on parent modules enriched in genes categorized by our pattern analysis. This approach identified conserved modules that might be important for driving the region- and sex-specific transcriptomic effects observed across the three brain regions (Figures 4-6H,I). Within those parent modules we highlighted “children” and “grandchildren” submodules with conserved hubs across all groups but displaying sex-specific connectivity (Figures 4-6H) and expression patterns that fall into our categories identified by pattern analysis (Figures 4-6I). We focused on key drivers that met these criteria because previous work from our laboratory showed that manipulation of a key driver that is conserved across all groups results in predicted behavioral effects, whereas manipulation of a key driver that is not conserved across all 4 groups has no impact on behavior (9). This approach yielded more than one possible key driver for follow-up investigation in each brain region (Suppl Table 4); we highlight one key driver per brain region as an illustration (Figure 4-6; Suppl Fig 4-6).
Co-expression analysis reveals that SI disrupts differential connectivity in brain reward regions
We observed that SI disrupted transcriptome structure in NAc and VTA but not PFC (Figures 4-6A,B). In PFC, we identified 4 parent modules in GHMs vs 7 in GHFs, numbers that were similar to those observed in SIMs and SIFs (Fisher’s Exact Test (FET) p = 0.219; Figure 4E). Even though the number of modules was not disrupted by SI, we hypothesized that the parent modules would be differentially connected in GHMs vs GHFs and that SI would disrupt this sex-specific connectivity (Figure 4F). Modules were constructed in all 4 groups such that the same genes did not always comprise the same modules across comparisons. Therefore, MDC analysis was conducted by comparing the connectivity of the genes within a module constructed in one group (e.g., GHMs or GHFs) to the connectivity of the same genes in the other groups. Interestingly, every parent module identified in GH animals was differentially connected when compared to connectivity of genes in the GH animal of the opposite sex (e.g., GHM modules vs GHF gene co-expression). However, SI disrupted module connectivity in a sex-specific manner, especially modules constructed in GHMs (Figure 4F). This suggests that SI disrupts sex-specific co-expression of a majority of the parent modules representing the global PFC transcriptome.
Unlike PFC, NAc transcriptomic structure is disrupted by SI. A large sex difference was observed in the number of parent modules in GH (GHM = 18; GHF = 2; FET p < 0.001; chi-square between GHM vs GHF p<0.001) but not SI (SIM = 63 vs SIF 53; Figure 5A-E). Notably, we only identified 2 parent modules in GHFs and one of those modules contains ~80% of the genes in the transcriptome. Modules this large generally offer limited insight. Therefore, we focused on the second layer of modules that compose module 2 for GHFs. Again, all parent modules identified in GHMs are differentially connected when compared to GHFs which was disrupted after SI. This effect does not translate to GHF modules where the vast majority of the modules that make up parent module 2 are differentially connected when compared to GHMs and SIMs but not SIFs (Suppl Figure 5A). This suggests that the SI effects in NAc may be driven by changes in SIMs to a greater extent than SIFs.
Figure 5: Sex-specific co-expression networks are disrupted by SI in NAc.
(A-D) Sunburst plots in NAc of (A) GHM, (B) GHF, (C) SIM, and (D) SIF. Plots are organized with parent and submodules as in Figure 4, where asterisks and colors are also defined. (E) Bar graph of number of parent module shows loss of transcriptomic structure. (F) Module Differential Connectivity (MDC) plots show comparison of module connectivity across parent modules. Numbers and colors are defined in Figure 4. (G) Enrichment plot of conserved hub genes across differentially connected parent modules. Numbers and colors are defined in Figure 4. (H) Bar graph of the number of connections of the hub gene Ash1 in GH and SI males and females. (I) Heatmap showing the relative expression of the hub gene Ash1 in response to acute and chronic saline and cocaine. (J) Comparison analysis of pathways associated with the genes modules reveals sex- and SI-specific pathways associate with co-expression modules. *=p<0.05; **corrected p-value<0.05.
In VTA, GHFs have more modules, and thus less co-expression structure, than GHMs (FET p<0.001; chi-square GHM vs GHF p < 0.001; Figure 6A,B,E) which is disrupted by SI. Both SIM and SIF transcriptomes are composed of more modules suggesting disruption of global co-expression (Figure 6C-E). Analogous to PFC and NAc, parent modules are differentially connected between GHMs and GHFs and disrupted by SI (Figure 6F). Together, these data suggest that global transcriptomic structure and connectivity are different in GHMs vs GHFs across all three brain regions and that this sex difference is disrupted by SI in a region-specific manner.
Identification of sex-specific hub genes in brain reward regions
We used the criteria discussed above to identify key hub or driver genes in each brain region that might be important for regulating sex-specific gene expression patterns. In PFC, this approach identified 9 conserved hubs, 3 of which displayed sex-specific connectivity ±30% connections between GHMs and GHFs within the modules of interest. Two key drivers lost the sex difference in connectivity after SI: Cnn3 and Gpr37 (Figure 4H). Analysis of their expression profiles revealed that only Gpr37 is also a pattern gene under multiple conditions in PFC (Figure 4I). Gpr37 encodes an orphan G protein-coupled receptor that is associated with Parkinson’s disease and has been proposed to interact with dopamine receptor 2 (15), as knockout of Gpr37 alters dopamine dynamics (16). In humans, GPR37 has been identified as a sex-biased gene across development and throughout the brain (17). Mice lacking Gpr37 fail to form a preference for cocaine and amphetamine (18) and display altered cocaine-induced corticostriatal long-term depression (19). In our dataset, Gpr37 is different between the sexes in PFC of GH animals (M>F) and feminized by SI after chronic saline. A gain of sex difference in cocaine responses is induced in that Gpr37 is upregulated by acute and chronic cocaine in SIM only. Thus, behavioral and transcriptional effects of SI are reflected in expression profiles of Gpr37 and give credence to this molecule as an important regulator of the sex-specific transcriptional effects of cocaine in PFC. Pathway analysis of PFC modules containing Gpr37 as a hub display a similar pattern of regulation, with minimal overlap of predicted pathways observed between GHM and GHF modules. Interestingly, the neuronal signaling pathways associated with the modules are overrepresented in the pathways associated with sex differences in genes in GH and SI animals after exposure to chronic cocaine (Suppl Figure 2I).
In NAc, there are very few shared hubs across the four groups (GHM/F and SIM/F; Suppl Table 4). However, we identified 2 conserved hubs in parent modules GHM M3 and GHF M17 or their children and grandchildren submodules. Only one of those key drivers, Ash1-like histone lysine methyltransferase (Ash1l), displayed sex-specific connectivity within the GH modules (Figure 5H). Ash1l encodes an activity-dependent histone methyltransferase that is important for maintenance of H3K36me2 at intergenic regions (20) and has been associated with neuronal development (21). Ash1l deletion abolished activity-dependent repression of neurexin1a, a cell adhesion molecule important for synaptic plasticity (22). Finally, ASH1L is associated with autism in human males (23). Ash1l was not captured in our original pattern analysis approach because its expression did not change >30% (Suppl Table 4). However, because only 1 hub met our criteria, we characterized its expression changes using our pattern analysis criteria (Figure 3). Sex differences in Ash1l expression are reversed by SI (p-value <0.05) and only SIFs decrease expression in response to acute cocaine (p<0.05; Figure 5I). Pathway analysis of NAc modules that contain Ash1l as a hub (Figure 5L) reveal little overlap of neuronal signaling pathways across the modules. However, integrin signaling and RhoGDI signaling pathways are overrepresented in sex differences in gene expression after chronic cocaine in SI animals (Suppl Figure 5I), and genes associated with RhoGDI signaling are enriched in SIF and SIM pattern genes after chronic saline and cocaine, respectively (Suppl Figure 3C).
Finally, we identified brain-specific angiogenesis inhibitor I-associated protein 3 (Baiap3) as a sex-specific hub in VTA. Baiap3 encodes a transmembrane protein that is important for recycling secretory vesicles: its knockout causes vesicle accumulation (24-26). Baiap3 is also associated with dopaminergic (27) and GABAergic (25) signaling. Inactivation of this gene induces sex-specific effects on anxiety and reward in humans and mice. Females lacking BAIAP3 display increased anxiety, while males display increased benzodiazepine dependence (28). Pathways associated with the modules containing Baiap3 do not overlap between GHMs and GHFs (Figure 6I), but do overlap with pathways associated with sex-specific expression after acute cocaine in SI animals (Suppl Figure 2H).
Together, these data suggest that integrating all levels of our bioinformatic analyses identified several sex-specific hub genes previously implicated in key neurobiological actions associated with stress and addiction, and support our interpretation that coherence of gene expression patterns throughout the reward circuitry is disrupted by adolescent SI.
Threshold-free analyses reveal that sex-specific expression is maintained across brain regions in GH animals
Because the three brain regions studied are interconnected, we investigated if disruption of transcriptomic structure by SI is observed across brain regions. We used rank rank hypergeometric overlay (RRHO), which compares two large datasets in a threshold-free manner, to determine if sex-specific expression across the transcriptome is conserved across brain regions in GH animals and if this is disrupted by SI. We found strong co-regulation of genes in the same direction in response to acute cocaine between all brain regions (Suppl Figure 7A-C), indicating sex differences align across these regions in GHMs and GHFs. SI disrupts this pattern in VTA, with opposite regulation observed for many of the same genes (Suppl Figure 7D-F). Similar patterns of sex-specific expression are seen across all brain regions after acute saline (Suppl Figure 8A-C), chronic saline (Suppl Figure 8D-F), and chronic cocaine (Suppl Figure 8G-I). These findings suggest that SI profoundly disrupts sex-specific expression in VTA which may impact signaling between its connected regions.
DISCUSSION
We utilized adolescent SI, which has been used for decades to induce susceptibility to addiction-related behaviors in males, to understand how SI alters transcriptional responses to cocaine. Including females enabled us to determine that adolescent SI disrupts sex-specific behaviors and gene expression in several brain reward regions. By use of multiple bioinformatic techniques, we identify potential regulators of sex-specific transcriptional responses to cocaine in these regions and highlight biological pathways that appear critical for sex differences in SUDs. Together, these data serve as an important resource in the study of SUDs, as this is the first unbiased study of the transcriptional effects of acute and chronic cocaine in both males and females in the brain’s reward circuitry.
Sex-specific transcriptional responses to cocaine are disrupted by adolescent SI
A prominent finding of this study is that transcriptional responses to cocaine are vastly different between males and females. This is in line with recent findings for other psychiatric disorders, both in humans (29, 30) and rodent models (31, 32). We found very little overlap between the sexes in the specific transcripts regulated by cocaine (Figure 1C-H; Suppl Figure 1C-H), the biological pathways associated with those transcripts (Figure 1I; Suppl Figure 1I), or transcriptome-wide co-expression patterns (Figures 4-6) across the reward circuitry. This finding is surprising partly because we observed a similar preference for cocaine in males vs females (6), suggesting that very different sex-specific transcriptional profiles yield similar behavioral phenotypes. This too is similar to the effects observed for other psychiatric disorders including depression and anxiety (29-32). We hypothesize that this reflects the fact that sex differences in reward are necessary for normal reproductive behaviors and strategies (33) such that different molecular and cellular mechanisms are utilized by the two sexes for adaptation. Our data are thus important for understanding how these mechanisms are hijacked differently by drugs of abuse in males and females. For instance, our pathway analysis sheds light on sex-specific processes and cell types that influence behaviors related to stress and cocaine in males and females. We identify, for example, pathways associated with dopamine signaling in males, but not females, exposed to cocaine. Others have shown that psychostimulant-induced dopamine release is reportedly lower in females than males (34), suggesting that cocaine-induced dopamine signaling may be sex-specific.
We next leveraged the sex-specific disruption induced by adolescent SI to better understand sex-specific transcription in brain. We found that adolescence SI reduces sex differences in the transcriptome at baseline and increases sex differences in response to cocaine in PFC, NAc, and VTA (Figure 2; Suppl Figure 2). These effects are reflected in results from our pattern analysis, which indicates that both the loss and gain of sex differences are driven overwhelmingly by changes within SIMs rather than SIFs. This effect was observed across all 3 brain regions and under almost all treatment groups (Figure 3).
Co-expression analysis reveals that sex-specific disruptions are transcriptome-wide
We used co-expression analysis to determine how transcriptomic structure is altered by adolescent SI. We observed in NAc and VTA, but not PFC, a loss of transcriptomic structure after adolescent SI. This finding is crucial for understanding how sex-specific disruptions might affect the entire transcriptome and suggests that altered expression of a small number of sex-specific genes leads to important changes in the entire transcriptomic network (Figure 4-6A-E). In addition to transcriptomic structure, we found that parent modules are differentially connected between GHMs and GHFs across all three brain regions, which is also disrupted by SI. In many cases, SI resulted in genes with connectivity that is similar to the GH module of the opposite sex comparison rather than their same-sex counterpart (i.e., genes in SIMs were not differentially connected when compared to GHF modules but were when compared to GHM modules). While our study is the first to investigate how cocaine influences sex-specific module differential connectivity, others have found similar effects in response to stress (31). Importantly, these effects were reflected in our RRHO analyses (Suppl Figures 7 and 8) and especially in the disruption of the sex-specific alignment of expression after acute cocaine that is strikingly disrupted in VTA and to a lesser extent in NAc (Suppl Figure 7). Together, our findings suggest that disruption of sex-specific gene expression in the reward circuitry substantially impacts organization of the transcriptome and alignment of sex differences in multiple brain regions.
Co-expression analysis identified conserved hubs that are associated with sex-specific expression and dopamine regulation
Co-expression analysis identifies key driver genes associated with behavioral phenotypes (29, 35). We identified 3 hubs that are conserved in each of the three brain regions studied across all four groups of animals. While the function of these molecules are quite different, they have all been identified as sex-specific genes in rodents (28) or humans (17, 23). Additionally, even though the network analysis identified hubs based on gene co-expression and not function, it is worth noting that all 3 of these key driver genes are implicated in dopamine function (15, 16, 27, 36).
Overall, this work provides a genome-wide map of transcriptomic effects of stress x cocaine interactions that occur in three brain reward regions and highlights dramatic sex differences. While it is beyond the scope of this study to determine the mechanisms by which adolescent stress reprograms the transcriptome, other studies have found that early life experience influences serum hormone concentrations and motivated behaviors (37) and epigenetic mechanisms (38). These, along with cell-type specific analyses, will be important factors to investigate in the future. Our study serves as a guide to better understand the influence of adolescent stress on SUDs and to develop sex-specific, precision treatments for these conditions.
Supplementary Material
KEY RESOURCES TABLE
| Resource Type | Specific Reagent or Resource | Source or Reference | Identifiers | Additional Information |
|---|---|---|---|---|
| Add additional rows as needed for each resource type | Include species and sex when applicable. | Include name of manufacturer, company, repository, individual, or research lab. Include PMID or DOI for references; use “this paper” if new. | Include catalog numbers, stock numbers, database IDs or accession numbers, and/or RRIDs. RRIDs are highly encouraged; search for RRIDs at https://scicrunch.org/resources. | Include any additional information or notes if necessary. |
| Antibody | N/A | |||
| Bacterial or Viral Strain | N/A | |||
| Biological Sample | RNA from c57Bl6 wild-type mice | |||
| Cell Line | N/A | |||
| Chemical Compound or Drug | N/A | |||
| Commercial Assay Or Kit | RNAeasy mini kit | Qiagen | Catalog#: 74104 | slight adjusted protocol - see methods for details |
| Deposited Data; Public Database | In the process of uploading to NCBI website | |||
| Genetic Reagent | N/A | |||
| Organism/Strain | N/A | |||
| Peptide, Recombinant Protein | ||||
| Recombinant DNA | N/A | |||
| Sequence-Based Reagent | TruSeq Stranded mRNA HT Sample Prep Kit | Illumina | Catalog #: 20020595 | |
| Software; Algorithm | Pattern Analysis Algorithm: https://gist.github.com/aartrama/c94bf11c0be40dfb0093c9be4bd0c53d | |||
| Transfected Construct | N/A | |||
| Other |
Significance Statement:
We leveraged a sex-specific model of disrupted adolescent development to understand basic mechanisms of sex-differences in transcription within the brain’s reward circuitry. We show that sex-specific transcriptional responses to cocaine and baseline sex differences are disrupted in three brain reward regions by adolescent stress, which is driven mainly by changes within stressed males. By use of differential gene expression and gene co-expression network analyses, we identified key driver genes that may be critical for sex-specific reward processing within the three brain regions examined. This study is the first of its kind to investigate cocaine-induced transcriptome-wide changes in both sexes and provides valuable insight into the mechanisms underlying sex differences in substance use disorders in humans.
ACKNOWLEDGMENTS AND FINANCIAL DISCLOSURES
This work was funded by grants from the National Institute on Drug Abuse (NIDA) P01DA0047233 (EJN), R01DA007359 (EJN), R01MH051399 (EJN) and K99DA042100 (DMW), U01AG046170 (BZ) and RF1AG054014 (BZ). The authors reported no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Bobzean SA, DeNobrega AK, Perrotti LI (2014): Sex differences in the neurobiology of drug addiction. Exp Neurol. 259:64–74. [DOI] [PubMed] [Google Scholar]
- 2.Becker JB, Perry AN, Westenbroek C (2012): Sex differences in the neural mechanisms mediating addiction: a new synthesis and hypothesis. Biol Sex Differ. 3:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romeo RD (2013): The Teenage Brain: The Stress Response and the Adolescent Brain. Curr Dir Psychol Sci. 22:140–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walker DM, Bell MR, Flores C, Gulley JM, Willing J, Paul MJ (2017): Adolescence and Reward: Making Sense of Neural and Behavioral Changes Amid the Chaos. J Neurosci. 37:10855–10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mates D, Allison KR (1992): Sources of stress and coping responses of high school students. Adolescence. 27:461–474. [PubMed] [Google Scholar]
- 6.Wagner EF, Myers MG, McIninch JL (1999): Stress-coping and temptation-coping as predictors of adolescent substance use. Addict Behav. 24:769–779. [DOI] [PubMed] [Google Scholar]
- 7.Wills TA (1986): Stress and coping in early adolescence: relationships to substance use in urban school samples. Health Psychol. 5:503–529. [DOI] [PubMed] [Google Scholar]
- 8.Walker DM, Cunningham AM, Gregory JK, Nestler EJ (2019): Long-Term Behavioral Effects of Post-weaning Social Isolation in Males and Females. Front Behav Neurosci. 13:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walker DM, Zhou X, Ramakrishnan A, Cates HM, Cunningham AM, Peña CJ, et al. (2020): Adolescent Social Isolation Reprograms the Medial Amygdala: Transcriptome and Sex Differences in Reward. bioRxiv.2020.2002.2018.955187. [Google Scholar]
- 10.Hu M, Crombag HS, Robinson TE, Becker JB (2004): Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology. 29:81–85. [DOI] [PubMed] [Google Scholar]
- 11.Jackson LR, Robinson TE, Becker JB (2006): Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology. 31:129–138. [DOI] [PubMed] [Google Scholar]
- 12.Lynch WJ, Carroll ME (1999): Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology (Berl). 144:77–82. [DOI] [PubMed] [Google Scholar]
- 13.Walker DM, Cates HM, Loh YE, Purushothaman I, Ramakrishnan A, Cahill KM, et al. (2018): Cocaine Self-administration Alters Transcriptome-wide Responses in the Brain's Reward Circuitry. Biol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song WM, Zhang B (2015): Multiscale Embedded Gene Co-expression Network Analysis. PLoS Comput Biol. 11:e1004574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hertz E, Terenius L, Vukojevic V, Svenningsson P (2019): GPR37 and GPR37L1 differently interact with dopamine 2 receptors in live cells. Neuropharmacology. 152:51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marazziti D, Golini E, Mandillo S, Magrelli A, Witke W, Matteoni R, et al. (2004): Altered dopamine signaling and MPTP resistance in mice lacking the Parkinson's disease-associated GPR37/parkin-associated endothelin-like receptor. Proc Natl Acad Sci U S A. 101:10189–10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi L, Zhang Z, Su B (2016): Sex Biased Gene Expression Profiling of Human Brains at Major Developmental Stages. Sci Rep. 6:21181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marazziti D, Mandillo S, Di Pietro C, Golini E, Matteoni R, Tocchini-Valentini GP (2007): GPR37 associates with the dopamine transporter to modulate dopamine uptake and behavioral responses to dopaminergic drugs. Proc Natl Acad Sci U S A. 104:9846–9851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rial D, Morato X, Real JI, Goncalves FQ, Stagljar I, Pereira FC, et al. (2017): Parkinson's disease-associated GPR37 receptor regulates cocaine-mediated synaptic depression in corticostriatal synapses. Neurosci Lett. 638:162–166. [DOI] [PubMed] [Google Scholar]
- 20.Huang C, Zhu B (2018): Roles of H3K36-specific histone methyltransferases in transcription: antagonizing silencing and safeguarding transcription fidelity. Biophys Rep. 4:170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bilodeau ML, Boulineau T, Greulich JD, Hullinger RL, Andrisani OM (2001): Differential expression of sympathoadrenal lineage-determining genes and phenotypic markers in cultured primary neural crest cells. In Vitro Cell Dev Biol Anim. 37:185–192. [DOI] [PubMed] [Google Scholar]
- 22.Zhu T, Liang C, Li D, Tian M, Liu S, Gao G, et al. (2016): Histone methyltransferase Ash1L mediates activity-dependent repression of neurexin-1alpha. Sci Rep. 6:26597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Li N, Li C, Zhang Z, Teng H, Wang Y, et al. (2020): Genetic evidence of gender difference in autism spectrum disorder supports the female-protective effect. Transl Psychiatry. 10:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Man KN, Imig C, Walter AM, Pinheiro PS, Stevens DR, Rettig J, et al. (2015): Identification of a Munc13-sensitive step in chromaffin cell large dense-core vesicle exocytosis. Elife. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varoqueaux F, Sigler A, Rhee JS, Brose N, Enk C, Reim K, et al. (2002): Total arrest of spontaneous and evoked synaptic transmission but normal synaptogenesis in the absence of Munc13-mediated vesicle priming. Proc Natl Acad Sci U S A. 99:9037–9042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X, Jiang S, Mitok KA, Li L, Attie AD, Martin TFJ (2017): BAIAP3, a C2 domain-containing Munc13 protein, controls the fate of dense-core vesicles in neuroendocrine cells. J Cell Biol. 216:2151–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lauridsen JB, Johansen JL, Rekling JC, Thirstrup K, Moerk A, Sager TN (2011): Regulation of the Bcas1 and Baiap3 transcripts in the subthalamic nucleus in mice recovering from MPTP toxicity. Neurosci Res. 70:269–276. [DOI] [PubMed] [Google Scholar]
- 28.Wojcik SM, Tantra M, Stepniak B, Man KN, Muller-Ribbe K, Begemann M, et al. (2013): Genetic markers of a Munc13 protein family member, BAIAP3, are gender specifically associated with anxiety and benzodiazepine abuse in mice and humans. Mol Med. 19:135–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Labonte B, Engmann O, Purushothaman I, Menard C, Wang J, Tan C, et al. (2017): Sex-specific transcriptional signatures in human depression. Nat Med. 23:1102–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seney ML, Huo Z, Cahill K, French L, Puralewski R, Zhang J, et al. (2018): Opposite Molecular Signatures of Depression in Men and Women. Biol Psychiatry. 84:18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barko K, Paden W, Cahill KM, Seney ML, Logan RW (2019): Sex-Specific Effects of Stress on Mood-Related Gene Expression. Mol Neuropsychiatry. 5:162–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hodes GE, Pfau ML, Purushothaman I, Ahn HF, Golden SA, Christoffel DJ, et al. (2015): Sex Differences in Nucleus Accumbens Transcriptome Profiles Associated with Susceptibility versus Resilience to Subchronic Variable Stress. J Neurosci. 35:16362–16376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simerly RB (2002): Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci. 25:507–536. [DOI] [PubMed] [Google Scholar]
- 34.Justice AJ, de Wit H (1999): Acute effects of d-amphetamine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl). 145:67–75. [DOI] [PubMed] [Google Scholar]
- 35.Bagot RC, Cates HM, Purushothaman I, Lorsch ZS, Walker DM, Wang J, et al. (2016): Circuit-wide Transcriptional Profiling Reveals Brain Region-Specific Gene Networks Regulating Depression Susceptibility. Neuron. 90:969–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu S, Tian M, He F, Li J, Xie H, Liu W, et al. (2020): Mutations in ASH1L confer susceptibility to Tourette syndrome. Mol Psychiatry. 25:476–490. [DOI] [PubMed] [Google Scholar]
- 37.Eck SR, Bangasser DA (2020): The effects of early life stress on motivated behaviors: A role for gonadal hormones. Neurosci Biobehav Rev. 119:86–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCarthy MM, Nugent BM (2015): At the frontier of epigenetics of brain sex differences. Front Behav Neurosci. 9:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.