Abstract
Chronic pain commonly occurs in people living with HIV (PLWH). Many PLWH in the United States (U.S.) continue to obtain opioids for chronic pain management. In addition to the known risk of opioid misuse, evidence from non-HIV populations suggest that opioid use is associated with poor sleep and negative mood. Whether insomnia severity and depressive symptoms might be exacerbated by chronic pain and opioid use in PLWH remains to be determined. This study examined insomnia severity and depressive symptoms in 85 PLWH with chronic pain and 35 PLWH without chronic pain. Among PLWH with chronic pain, reported opioid use was examined in relation to severity of insomnia and depressive symptoms. PLWH with chronic pain reported significantly greater severity of insomnia symptoms (p = .033) and greater depressive symptoms (p = .025) than PLWH without chronic pain. Among PLWH with chronic pain who reported opioid use (n = 36), insomnia severity was greater compared to those who denied opioid use (n = 49), even after controlling for and pain severity and number of comorbidities (p = .026). Greater pain severity was significantly associated with greater insomnia severity (p < .001) and possibly greater depressive symptoms (p = .048) among PLWH with chronic pain who reported opioid use; however, these same associations were not significant among those PLWH with chronic pain who denied opioid use. Findings suggest that PLWH with chronic pain are likely to experience poor sleep (i.e., insomnia) and depressed mood. Furthermore, poor sleep was associated with opioid use among PLWH with chronic pain.
Keywords: HIV, chronic pain, opioid use, depression, insomnia
Introduction
The introduction of antiretroviral therapy (ART) has led to longer life expectancies for people living with HIV (PLWH) (Antiretroviral Therapy Cohort, 2008). However, living longer is often accompanied by significant health comorbidity and threats to quality of life for many PLWH (Balderson et al., 2013; Rodriguez-Penney et al., 2013). Chronic pain is a common comorbidity in PLWH with some studies reporting prevalence estimates between 30% and 85% (Merlin et al., 2012; Miaskowski et al., 2011). This wide range is likely due to differences in chronic pain assessment methodologies and differences between clinical cohorts being studied. For example, chronic pain prevalence is closer to 30% in cohorts of patients engaged in HIV primary care (Jiao et al., 2016; Merlin et al., 2012), but closer to 85% in cohorts of homeless PLWH not appropriately engaged in HIV primary care (Miaskowski et al., 2011). Several additional factors may also contribute to the high prevalence of chronic pain in PLWH including the neurotoxic effects of HIV itself and ART side effects (Margolis et al., 2014), mental illness and substance use (Merlin et al., 2019), and chronic inflammation (Merlin et al., 2017).
Pain is considered chronic when it persists or recurs for more than 3 months and is associated with significant emotional distress and/or functional disability (Treede et al., 2019). In PLWH, chronic pain often coexists with other health conditions such as insomnia (Sabin et al., 2020; Taibi, 2013) and psychiatric illnesses including depression (de Souza et al., 2018; Scott et al., 2018). To illustrate, a recent study of 5,370 PLWH found that the most frequently reported symptoms in this cohort were fatigue (56%), insomnia (50%), pain (46%), and sadness (45%) (Webel et al., 2019). Other cross-sectional studies have shown significant associations among chronic pain severity, sleep disturbance, and depressive symptoms in PLWH (Redman et al., 2018; Sabin et al., 2020; Vosvick et al., 2004). Additional research is needed to better understand the reasons why chronic pain, disturbed sleep, and depressed mood so often occur together for PLWH.
In the United States (U.S.), opioid use for chronic pain management in PLWH remains common even though current consensus guidelines do not recommend opioid analgesics as a first-line treatment for the long-term management of chronic pain in this population (Bruce et al., 2017). PLWH with moderate-severe chronic pain have been found more likely to be depressed and taking prescribed opioids compared to those with mild or no chronic pain (Uebelacker et al., 2015). Emerging evidence suggests that the use of prescribed opioids for chronic pain management in PLWH is not reliably associated with clinically meaningful pain relief (Onen et al., 2012). However, prescribed opioid use is associated with an increased risk of developing sleep disorders such as insomnia (Serdarevic et al., 2017; Tran et al., 2009) and mood disorders such as depression (Salas et al., 2017; Scherrer et al., 2017). Given the frequent use of opioids to manage chronic pain in PLWH, opioid use may be associated with poor sleep and depressive symptoms in this population. However, research has not adequately addressed this possibility to date. It is critical to examine benefits and consequences of opioid use on sleep and psychological function in PLWH and chronic pain (Miller et al., 2018). Findings may help further inform chronic pain treatment guidelines and treatment options for PLWH.
The primary objectives of this study were to 1) examine differences in insomnia severity and depressive symptoms in PLWH with and without chronic pain, and 2) determine whether opioid use was associated with chronic pain severity, insomnia severity, and depressive symptoms. This study addressed the following three hypotheses. 1) PLWH with chronic pain will report greater insomnia severity and greater depressive symptoms compared to PLWH without chronic pain. 2) Among PLWH with chronic pain, those who report opioid use for pain management will have greater insomnia severity and depressive symptoms compared to those who denied any opioid use after controlling for covariates. 3) After controlling for covariates, insomnia severity and depressive symptoms will be significantly associated with pain severity in PLWH with chronic pain who report opioid use.
Methods
Study Overview
This study was part of a larger investigation that examined psychosocial risk and resilience factors in PLWH with and without chronic pain (Comprehensive HIV and Pain Study; CHIPS). The participants described in the current analysis were recruited between March 2017 and October 2019. A portion of the data generated from CHIPS has previously been published by our group (Gonzalez et al., 2019); however, the data published by Gonzalez and colleagues does not substantially overlap with the data presented in this study. The participants and measures described below are limited to those involved in the current study. PLWH with and without chronic pain were recruited via posted flyers from a large, urban HIV clinic in Alabama, U.S.A. that provides comprehensive medical, social, and behavioral services to approximately 3,500 adults (≥18 years) living with HIV. Interested individuals were assessed for eligibility via an initial telephone screening and review of medical records. Eligible participants subsequently presented to the laboratory to complete a single study session. At the beginning of the study session blood was then taken from each participant for determination of CD4+ count and viral load. Participants completed standardized self-report questionnaires that assessed pain severity and interference, opioid use, insomnia severity, and depressive symptoms. Sociodemographic information was collected from all participants including age, sex/gender, and ethnicity/race. Additional information gathered from medical records included prescribed medications (e.g., ART, opioids), duration of HIV infection, duration of chronic pain, and other documented health comorbidities. All procedures were approved by the local Institutional Review Board and carried out in accordance with guidelines for the ethical conduct of research.
Participants
A total of 120 PLWH were included in this study, 85 with chronic pain and 35 without chronic pain. We intentionally enrolled a greater number of PLWH and chronic pain to ensure inclusion of a sufficient proportion of individuals using opioids for chronic pain management. All PLWH with chronic pain reported that the pain had been present for at least three consecutive months, and was an ongoing problem for at least half of the days in the past six months (Treede et al., 2019). PLWH without chronic pain denied any current or previous history of persistent pain. Additional participant details, including participant inclusion and exclusion criteria, is provided in our previously published work (Gonzalez et al., 2019). Written informed consent was obtained from each participant prior to the study, and participants were compensated for their participation.
Measures
Brief Pain Inventory- Short Form is a 9-item questionnaire used to evaluate pain severity and the impact of pain on daily functioning (Cleeland & Ryan, 1994). Pain severity items and pain interference items are averaged to calculate scores, with higher scores (range 0 to 10) indicating greater pain severity and pain interference. This measure has been used in several populations including people with HIV/AIDS (Atkinson et al., 2011). Test-rest reliabilities for pain severity range from 0.81 to 0.98 for pain severity, and 0.72 to 0.97 for pain interference (Atkinson et al., 2011; Cleeland & Ryan, 1994; Mendoza et al., 2006).
Insomnia Severity Index (ISI).
The ISI is a 7-item questionnaire used to evaluate the severity and impact of insomnia. Participants used a 5-point Likert scale to rate severity of difficulties with sleep onset and sleep maintenance, as well as problems with early morning awakenings, sleep dissatisfaction, and interference of sleep difficulties with daytime functioning within the last month (Bastien et al., 2001). Items are summed to calculate a total score ranging from 0 to 28, thus indicating absence of insomnia (0-7), sub-threshold insomnia (8-14), moderate insomnia (15-21) and severe insomnia (22-28). Internal consistency is excellent (Cronbach α of 0.90 and 0.91) (Morin et al., 2011).
Center for Epidemiological Studies Depression Scale (CES-D).
The CES-D is a 20-item questionnaire used to assess for depressive symptoms (Radloff, 1977). Symptoms of depression measured by the CES-D include negative mood, guilt/worthlessness, helplessness/hopelessness, psychomotor retardation, loss of appetite, and sleep disturbance Respondents use a 4-point Likert scale to rate how frequently each symptom applied to them over the past week. Responses are summed (0-60), with higher scores indicating greater severity of depression. Reliability is excellent (r = 0.85 to 0.95) (Radloff, 1977).
Opioid use.
Participants answered the following question regarding opioid use for pain management, have you used opioid “painkillers” as a treatment for your chronic pain? For clarity sake, this question described opioid painkillers as prescription medications including morphine, codeine, hydrocodone, oxycodone, hydromorphone, oxymorphone, methadone, and tramadol. Only generic names of prescription opioids are listed here; however, participants were provided with generic names as well as their corresponding brand names for reference. Response options to this opioid use question included, no, yes, or not sure. We further asked participants to report the specific names of each opioid painkiller used. Medical records were reviewed for each participant to determine whether there was an active prescription for any opioid painkiller.
Data Analysis
A power analysis was conducted for the primary analyses examining the extent to which insomnia severity and depressive symptoms differed according to chronic pain status (hypothesis 1) and opioid use (hypothesis 2). A 3:1 allocation of PLWH with chronic pain to PLWH without chronic pain was incorporated to ensure a sufficient subsample of PLWH with chronic pain who reported opioid use was enrolled. The following parameters were specified for hypothesis 1: Cohen’s d = .6 (medium effect size), p < .05, power = .8. This resulted in a sample size of 120 total PLWH (90 with chronic pain and 30 without chronic pain), which very closely approximates the actual sample size in this study. The same parameters were specified for hypothesis 2, which resulted in a sample size of 84 PLWH with chronic pain (42 using opioids and 42 not using opioids). While our study included 85 PLWH with chronic pain, only 36 reported opioid use. To assess normality of the ISI, CES-D, and BPI-SF variables, we examined their skewness and kurtosis values. Acceptable values for skewness and kurtosis are ±1.5 or ± 2, respectively (Tabachnick & Fidell, 2013). Values in the current study ranged from .234 to .640 for skewness and −.1.730 to −.068 for kurtosis, which are well within the acceptable limits. Visual inspection of the histograms and Q-Q plots also supported normality. Therefore, parametric-based analyses were used to examine insomnia severity, depressive symptoms, and pain severity. Due to the unequal sample sizes (with chronic pain vs. without chronic pain and opioid use vs. no opioid use) in this study, the parametric assumption of homogeneity of variance was examined using Levene’s test. The homogeneity of variance assumption was not violated for any analysis given that Levene’s test was non-significant (all p’s > .05). All participants provided complete sociodemographic (e.g., sex/gender, race/ethnicity, age) and questionnaire (e.g., ISI, CES-D, and BPI-SF) data. Descriptive data for the sample are presented as percentages for categorical variables and as either means with standard deviations or medians with interquartile ranges for continuous variables. Differences in insomnia severity and depressive symptoms according to chronic pain status (i.e., with or without) and opioid use (i.e., opioid use or no opioid use) were examined after adjustment for covariates using analysis of covariance (ANCOVA). Effect sizes for the ANCOVA analyses used the following benchmarks for Cohen’s d: .2 (small), .5 (medium), and .8 (large). Partial correlation analysis controlling for covariates were completed to examine associations among pain severity, insomnia severity, and depressive symptoms. All data were analyzed using SPSS, version 25 (IBM; Chicago, IL).
Results
Participant characteristics
Descriptive characteristics for the 85 PLWH with chronic pain and 35 PLWH without chronic pain are presented in Table 1. PLWH with chronic pain were significantly older than PLWH without chronic pain (p = .022). Both groups of PLWH with and without chronic pain were primarily comprised of men and African American individuals. The ethnicity/race (p = .066) and sex/gender (p = .085) compositions were not significantly different between groups. Over 97% of each group was receiving ART. CD4 count (p = .962), detectable viral load (p = .834), and duration of HIV infection (p = .056) were also not significantly different between the two groups. Among PLWH with chronic pain, the most frequently reported locations of chronic pain were low back/hips (46%), legs/feet (25%), widespread (2+ sites) (20%), arms/hands (6%), head (2%), and neck/shoulders (1%). Medical records indicated that 24% of the sample had a pain duration of >3 months but <1 year, 25% ≥1 year but <5 years, 23% ≥5 years but <10 years, and 28% ≥10 years. Pain severity over the past 24 hours was significantly greater for PLWH with chronic pain compared to PLWH without chronic pain on the 0-10 numeric rating scale of the BPI-SF (p < .001). Furthermore, PLWH with chronic pain possessed a significantly greater number of comorbidities relative to PLWH without chronic pain (p < .001).
Table 1.
Differences between PLWH with and without chronic pain (N = 120)
| Variable | PLWH with chronic pain (n = 85) |
PLWH without chronic pain (n = 35) |
|---|---|---|
| Age in Years, Median (IQR) | 50 (44.3 - 54) | 44 (30 – 54) |
| Race, N (%) | ||
| African American | 63 (74.1) | 30 (83.3) |
| Caucasian | 15 (17.6) | 5 (13.9) |
| American Indian/Alaskan Native | 1 (1.2) | 0 (0) |
| Latino | 0 (0) | 0 (0) |
| Multiracial | 6 (7.1) | 0 (0) |
| Gender, N (%) | ||
| Female | 27 (31.8) | 5 (14.3) |
| Male | 56 (65.9) | 27 (77.1) |
| Transgender | 2 (2.3) | 2 (5.7) |
| Other | 0 (0) | 1 (2.9) |
| Receiving ART, N (%) | 84 (98.8) | 34 (97.1) |
| Detectable Viral Load (>200), N (%) | 11 (12.9) | 4 (11.4) |
| Years with HIV, Mean (SD) | 16.6 (9.1) | 12.8 (10.1) |
| CD4+ Lymphocyte Count, Median (IQR) | 624 (430.3 – 793.5) | 624 (346.3 – 866.5) |
| CES-D, Mean (SD) | 21.2 (11.5) | 13.9 (10.9) |
| ISI, Mean (SD) | 12.3 (8.4) | 6.9 (6.7) |
| BPI-SF Pain Severity, Mean (SD) | 5.8 (2.3) | 1.1 (1.4) |
| Number of Comorbidities, Median (IQR) | 4 (2 – 5) | 1 (0 – 2) |
| Pain Duration, N (%) | ||
| No pain | 0 (0) | 35 (100) |
| >3 months but <1 year | 20 (24) | 0 (0) |
| ≥1 year but <5 years | 22 (25) | 0 (0) |
| ≥5 years but <10 years | 19 (23) | 0 (0) |
| ≥10 years | 24 (28) | 0 (0) |
| Opioid use, N (%) | 36 (42) | 0 (0) |
Note. PLWH = people living with HIV; IQR = interquartile range; SD = standard deviation; ART = antiretroviral therapy; CES-D = Center for Epidemiologic Studies Depression Scale; ISI = Insomnia Severity Index; BPI-SF = Brief Pain Inventory – Short Form
Hypothesis 1: Differences according to chronic pain status
After controlling for age and number of comorbidities, PLWH with chronic pain reported significantly greater severity of insomnia symptoms (F1,116 = 4.66, p = .033; Cohen’s d = .71) and greater depressive symptoms (F1,116 = 5.19, p = .025; Cohen’s d = .65) than PLWH without chronic pain. Figure 1 displays the difference in insomnia severity, while Figure 2 displays the difference in depressive symptoms.
Figure 1:
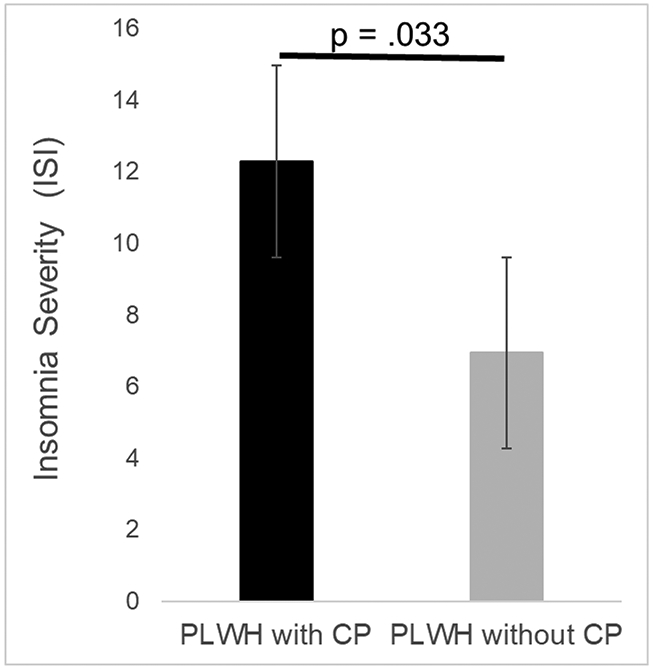
Difference in insomnia severity between PLWH with and without chronic pain. Bars represent means and standard error of the mean. The maximum possible score on the ISI is 28.
Figure 2:
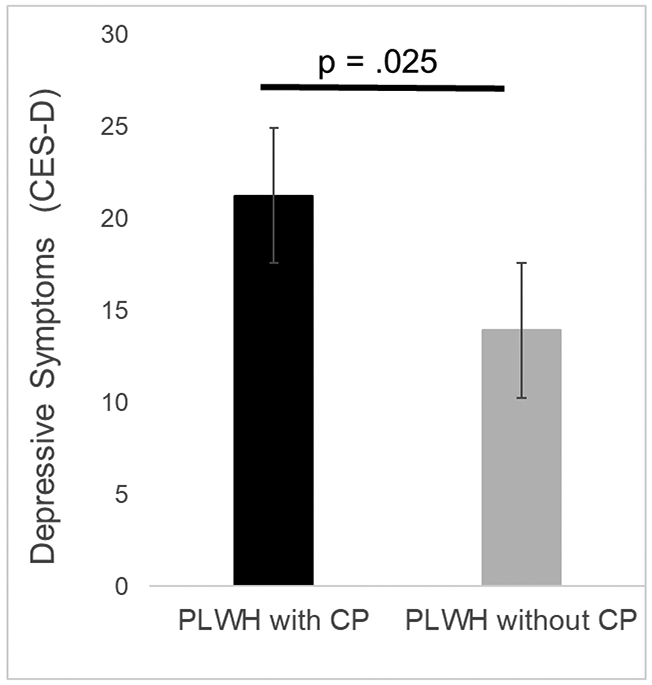
Difference in depressive symptoms between PLWH with and without chronic pain. Bars represent means and standard error of the mean. The maximum possible score on the CES-D is 60.
Hypothesis 2: Use of opioids in PLWH with chronic pain
As shown in Table 1, use of opioids as a treatment for chronic pain was self-reported by 36 out of 85 (42%) PLWH with chronic pain. The remaining 49 (58%) PLWH with chronic pain denied any opioid use. Of the 36 PLWH with chronic pain who reported opioid use, 16 had active opioid prescriptions verified upon medical record review. We were unable to verify via medical records whether the other 20 PLWH with chronic pain who self-reported opioid use had active prescriptions. None of the PLWH without chronic pain reported using opioid. For the analyses presented below, we focused exclusively on PLWH with chronic pain and compared the 36 who reported opioid use to the 49 who denied opioid use.
Hypothesis 2: Differences in PLWH with chronic pain according to opioid use
Among the 85 PLWH with chronic pain, pain severity was significantly greater for those using opioids in comparison to those not using opioids (F1,83 = 4.74, p = .032; Cohen’s d = .49). Number of comorbidities was significantly greater for those using opioids compared to those not using opioids (F1,83 = 4.28, p = .042; Cohen’s d = .45). Therefore, pain severity and number of comorbidities were selected as covariates and included in the analyses presented below. Neither age, sex/gender, ethnicity/race, duration of chronic pain, duration of HIV, CD4, or viral load significantly differed between those using opioids and those not using opioids. Therefore, none of these variables were selected as covariates. Insomnia severity was found to significantly differ between PLWH with chronic pain who reported opioid use and those who denied any opioid use (Figure 3). Specifically, the severity of insomnia was greater for those who reported opioid use. This difference in insomnia severity remained significant even after controlling for pain severity and number of comorbidities (F1,81 = 5.15, p = .026; Cohen’s d = .64). As shown in Figure 4, depressive symptoms did not significantly differ between those who reported opioid use and those who did not after controlling for pain severity and number of comorbidities (F1,81 = 1.16, p = .285; Cohen’s d = .31).
Figure 3:
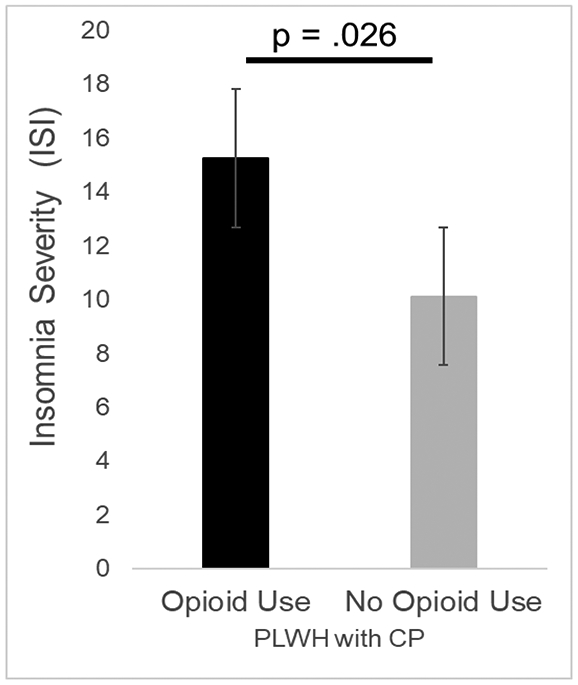
Difference in insomnia severity in PLWH with chronic pain according to opioid use. Bars represent means and standard error of the mean. The maximum possible score on the ISI is 28.
Figure 4:
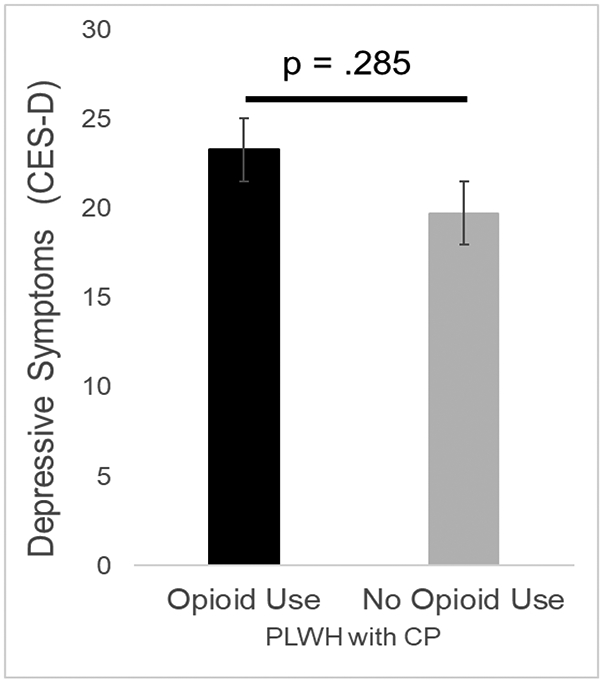
Difference in depressive symptoms in PLWH with chronic pain according to opioid use. Bars represent means and standard error of the mean. The maximum possible score on the CES-D is 60.
Hypothesis 3: Associations among pain severity, insomnia severity, and depressive symptoms
Chronic pain duration (r = .440, p < .001), but not number of comorbidities (r = .081, p = .464), was significantly associated with greater pain severity. Therefore, separate partial correlation analyses controlling for chronic pain duration were carried out for PLWH with chronic pain according to who reported opioid use and who denied opioid use. Among those who reported opioid use, greater pain severity was significantly associated with greater insomnia severity (r = .573, p < .001) and greater depressive symptoms (r = .337, p = .048). Further, greater insomnia severity was significantly associated with greater depressive symptoms (r = .569, p < .001). Interestingly, among those who denied any opioid use, pain severity was not significantly associated with insomnia severity (r = .018, p = .904) or depressive symptoms (r = −.178, p = .226). However, greater insomnia severity was significantly associated with greater depressive symptoms for those who denied opioid use (r = .646, p < .001).
Discussion
This study aimed to examine insomnia severity and depressive symptoms in PLWH with and without chronic pain, as well as whether insomnia severity and depressive symptoms were associated with opioid use. Findings supported our first hypothesis, such that greater insomnia severity and depressive symptoms were reported among PLWH and chronic pain compared to PLWH without chronic pain. These group differences are consistent with a recent meta-analysis indicating that depression and sleep disturbance are both commonly associated with chronic pian in (Scott et al., 2018). There is strong evidence from non-HIV populations that chronic pain can bring about and exacerbate sleep disturbances (Finan et al., 2013) as well as depression (Bair et al., 2003). Chronic pain may also contribute to the development of sleep and mood disorders such as insomnia and depression, respectively, for PLWH. Conversely, established insomnia and depression might also be risk factors for subsequent chronic pain development in PLWH. Additional research is needed to address these issues of causality and directionality.
Findings from this study partially supported our second hypothesis. Among PLWH with chronic pain, there was significantly greater insomnia severity for those who reported opioid use compared to those who denied opioid use, even after controlling for covariates. The current literature addressing whether prescription opioids improves or worsens the sleep of people dealing with chronic pain is currently mixed. For example, in a comprehensive review of randomized trials for patients with chronic pain due to osteoarthritis, prescription opioid medications were superior to placebo in improving diverse sleep outcomes (Turk & Cohen, 2010). Conversely, a more recent review of the literature addressing prescribed opioid effects on sleep concluded that opioids are not viable sleep aids, and may actually worsen the sleep (i.e., greater disturbances) of people with chronic pain (Tang et al., 2019). Furthermore, additional research suggests that opioid-induced respiratory depression and sleep-disordered breathing (e.g., obstructive sleep apnea) may exacerbate sleep disturbance in patients with chronic pain (Cheatle & Webster, 2015; Chung et al., 2019). Depressive symptoms did not significantly differ between PLWH with chronic pain who reported opioid use and those who denied opioid use. These results suggest, that while opioid use is not associated with greater depressive symptoms, insomnia severity in PLWH with chronic pain may be worse in the context of opioid use. We cannot fully determine whether use of opioids indeed worsened insomnia in PLWH with chronic pain given the cross-sectional nature of this study. It may be that PLWH with chronic pain who also demonstrate a sleep disorder like insomnia are more likely to receive and use a prescribed opioid. Both of these seem plausible, and additional research is required to address this matter. Given that opioid medications are not a recommended first-line treatments for chronic pain (Bruce et al., 2017) or sleep (Tang et al., 2019), non-pharmacological evidence-based treatments such as cognitive-behavioral therapy for chronic pain and insomnia may be preferable for PLWH (Finan et al., 2014).
Our findings also generally supported our third hypothesis. Greater pain severity was significantly associated with greater insomnia severity and greater depressive symptoms for PLWH with chronic pain who reported opioid use, yet these same associations were not significant for PLWH with chronic pain who denied opioid use. One explanation for this finding is that severe chronic pain is more detrimental to sleep and mood than mild chronic pain (Blagestad et al., 2016), thereby resulting in greater insomnia severity and depressive symptoms. PLWH with severe chronic pain are more likely to be prescribed and use opioid medications for pain management (Uebelacker et al., 2015). Indeed, in this study, PLWH with chronic pain who reported opioid use had significantly greater pain severity than their counterparts who denied opioid use. Another explanation is related to previous research demonstrating that sleep disturbance (Smith et al., 2020) and depressed mood (Pecina et al., 2019; Zubieta et al., 2003) attenuate opioid analgesia and endogenous opioid neurotransmission in humans. Therefore, it may be that greater insomnia severity and depressive symptoms diminishes the analgesic effects of opioids in PLWH, which in turn promotes greater pain severity. Both of these explanations are speculative and meant to generate hypotheses for future research. Each explanation is beyond the scope of conclusions that can be drawn from the data derived from this cross-sectional and correlational study.
This study possessed several limitations. First, we were unable to confirm through medical records whether 20 PLWH with chronic pain who reported opioid use indeed had an active prescription for this medication. This may likely have been because they had a provider outside of the investigator-affiliated healthcare system who was prescribing the medication, or they were receiving these medications via other means such as purchasing “on the street”. Regardless whether opioid use was self-reported or verified with an active prescription in medical records, our study design did not allow for determination of actual patterns or volume of opioid used for chronic pain management. Second, our study sample was comprised primarily of middle-aged, African American/Black, cisgender men. Therefore, our study findings may not generalize well to Caucasian/White populations of cisgender women or transgender individuals. In the U. S., risk of opioid analgesic use is elevated in PLWH (Edelman et al., 2013), and opioid overdose is a leading cause of accidental death (Cunningham, 2018). However, use and misuse of prescribed opioids for chronic pain management is not as problematic in other countries outside the U.S.; for example see (Klein et al., 2020). Therefore, our opioid-related findings may not be as relevant for populations of PLWH with chronic pain living in other countries. Third, our study was cross-sectional in design. As a result, no conclusions can be drawn regarding causality or directionality pertaining to the associations among pain severity, insomnia severity, and depressive symptoms in this cohort of PLWH. Fourth, our power analysis indicated that 42 PLWH with chronic pain who use opioids was necessary to detect differences with a medium effect size; however, this study only included 36 PLWH with chronic pain who reported opioid use. Therefore, the non-significant difference in depressive symptoms between participants who reported opioid use and those who denied opioid use may have been due to lack of sufficient statistical power, rather than the lack of an actual difference. Lastly, this study did not include any formal sleep or mood assessments. It is unclear whether participants met formal diagnostic criteria for insomnia or depression, or other related disorders such as sleep apnea or bipolar disorder. Future research in PLWH is needed to better appreciate the extent to which diagnosable sleep and mood disorders co-occur along with chronic pain.
Conclusion
Chronic pain appears to be a highly prevalent comorbidity that negative impacts the quality of life among PLWH (Madden et al., 2020). The evidence supporting the associations of insomnia and depression with chronic pain in PLWH is increasing; however, the number of clinical studies investigating the intersection of these factors is limited. Further research is necessary to examine the benefits and consequences of opioid use on chronic pain, insomnia, and depressive symptoms in PLWH. Challenges with chronic pain management and insomnia are expected to persist given that adults with HIV are living longer. It is unclear whether some PLWH are using opioids for other comorbidities such as sleep disorders (i.e., insomnia) and/or depression. Targeted interventions for insomnia and depression among PLWH with chronic pain may lead to reductions in opioid use while also promoting improvements in pain severity, sleep quality and mood.
Acknowledgments
None of the authors have any conflicts of interest to report. This research was supported by a research supplement to promote diversity in health-related research (S.L.C.) under award number R01HL147603 (B.R.G.), as well as the Creative and Novel Ideas in HIV Research Program (B.R.G.) through a supplement to the University of Alabama at Birmingham Center for AIDS Research under award number P30AI027767. Additional support was also provided by the UAB Deep South Resource Center for Minority Aging Research under award number P30AG031054 (S.L.C.) from the NIH National Institute on Aging and UAB CTSA Grant UL1TR001417 from the NIH Center for Advancing Translational Sciences.
References
- Antiretroviral Therapy Cohort, C. (2008). Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet, 372(9635), 293–299. 10.1016/S0140-6736(08)61113-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson TM, Rosenfeld BD, Sit L, Mendoza TR, Fruscione M, Lavene D, Shaw M, Li Y, Hay J, Cleeland CS, Scher HI, Breitbart WS, & Basch E (2011). Using confirmatory factor analysis to evaluate construct validity of the Brief Pain Inventory (BPI). J Pain Symptom Manage, 41(3), 558–565. 10.1016/j.jpainsymman.2010.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bair MJ, Robinson RL, Katon W, & Kroenke K (2003). Depression and pain comorbidity: a literature review. Arch Intern Med, 163(20), 2433–2445. 10.1001/archinte.163.20.2433 [DOI] [PubMed] [Google Scholar]
- Balderson BH, Grothaus L, Harrison RG, McCoy K, Mahoney C, & Catz S (2013). Chronic illness burden and quality of life in an aging HIV population. AIDS Care, 25(4), 451–458. 10.1080/09540121.2012.712669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastien CH, Vallieres A, & Morin CM (2001). Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med, 2(4), 297–307. 10.1016/s1389-9457(00)00065-4 [DOI] [PubMed] [Google Scholar]
- Blagestad T, Pallesen S, Gronli J, Tang NK, & Nordhus IH (2016). How Perceived Pain Influence Sleep and Mood More Than The Reverse: A Novel, Exploratory Study with Patients Awaiting Total Hip Arthroplasty. Front Psychol, 7, 1689. 10.3389/fpsyg.2016.01689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce RD, Merlin J, Lum PJ, Ahmed E, Alexander C, Corbett AH, Foley K, Leonard K, Treisman GJ, & Selwyn P (2017). 2017 HIVMA of IDSA Clinical Practice Guideline for the Management of Chronic Pain in Patients Living With HIV. Clin Infect Dis, 65(10), e1–e37. 10.1093/cid/cix636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheatle MD, & Webster LR (2015). Opioid Therapy and Sleep Disorders: Risks and Mitigation Strategies. Pain Med, 16 Suppl 1, S22–26. 10.1111/pme.12910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung F, Wong J, Bellingham G, Lebovic G, Singh M, Waseem R, Peng P, George CFP, Furlan A, Bhatia A, Clarke H, Juurlink DN, Mamdani MM, Horner R, Orser BA, Ryan CM, & Op-Safe I (2019). Predictive factors for sleep apnoea in patients on opioids for chronic pain. BMJ Open Respir Res, 6(1), e000523. 10.1136/bmjresp-2019-000523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleeland CS, & Ryan KM (1994). Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singap, 23(2), 129–138. https://www.ncbi.nlm.nih.gov/pubmed/8080219 [PubMed] [Google Scholar]
- Cunningham CO (2018). Opioids and HIV Infection: From Pain Management to Addiction Treatment. Top Antivir Med, 25(4), 143–146. https://www.ncbi.nlm.nih.gov/pubmed/29689538 [PMC free article] [PubMed] [Google Scholar]
- de Souza A, Caumo W, Calvetti PU, Lorenzoni RN, da Rosa GK, Lazzarotto AR, & Dussan-Sarria JA (2018). Comparison of pain burden and psychological factors in Brazilian women living with HIV and chronic neuropathic or nociceptive pain: An exploratory study. PLoS One, 13(5), e0196718. 10.1371/journal.pone.0196718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman EJ, Gordon K, Becker WC, Goulet JL, Skanderson M, Gaither JR, Brennan Braden J, Gordon AJ, Kerns RD, Justice AC, & Fiellin DA (2013). Receipt of opioid analgesics by HIV-infected and uninfected patients. J Gen Intern Med, 28(1), 82–90. 10.1007/s11606-012-2189-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan PH, Buenaver LF, Coryell VT, & Smith MT (2014). Cognitive-Behavioral Therapy for Comorbid Insomnia and Chronic Pain. Sleep Med Clin, 9(2), 261–274. 10.1016/j.jsmc.2014.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan PH, Goodin BR, & Smith MT (2013). The association of sleep and pain: an update and a path forward. J Pain, 14(12), 1539–1552. 10.1016/j.jpain.2013.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez CE, Okunbor JI, Parker R, Owens MA, White DM, Merlin JS, & Goodin BR (2019). Pain-Specific Resilience in People Living With HIV and Chronic Pain: Beneficial Associations With Coping Strategies and Catastrophizing. Front Psychol, 10, 2046. 10.3389/fpsyg.2019.02046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao JM, So E, Jebakumar J, George MC, Simpson DM, & Robinson-Papp J (2016). Chronic pain disorders in HIV primary care: clinical characteristics and association with healthcare utilization. Pain, 157(4), 931–937. 10.1097/j.pain.0000000000000462 [DOI] [PubMed] [Google Scholar]
- Klein A, Patwardhan S, & Loglo MGA (2020). Divergences and commonalities between the US opioid crisis and prescription medicine mis/use in West Africa. Int J Drug Policy, 76, 102640. 10.1016/j.drugpo.2019.102640 [DOI] [PubMed] [Google Scholar]
- Madden VJ, Parker R, & Goodin BR (2020). Chronic pain in people with HIV: a common comorbidity and threat to quality of life. Pain Manag, 10(4), 253–260. 10.2217/pmt-2020-0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis AM, Heverling H, Pham PA, & Stolbach A (2014). A review of the toxicity of HIV medications. J Med Toxicol, 10(1), 26–39. 10.1007/s13181-013-0325-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza T, Mayne T, Rublee D, & Cleeland C (2006). Reliability and validity of a modified Brief Pain Inventory short form in patients with osteoarthritis. Eur J Pain, 10(4), 353–361. 10.1016/j.ejpain.2005.06.002 [DOI] [PubMed] [Google Scholar]
- Merlin JS, Samet JH, Cheng DM, Lira MC, Tsui JI, Forman LS, Colasanti J, Walley AY, Del Rio C, & Liebschutz JM (2019). Marijuana Use and Its Associations With Pain, Opioid Dose, and HIV Viral Suppression Among Persons Living With HIV on Chronic Opioid Therapy. J Acquir Immune Defic Syndr, 82(2), 195–201. 10.1097/QAI.0000000000002119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlin JS, Westfall AO, Heath SL, Goodin BR, Stewart JC, Sorge RE, & Younger J (2017). Brief Report: IL-1beta Levels Are Associated With Chronic Multisite Pain in People Living With HIV. J Acquir Immune Defic Syndr, 75(4), e99–e103. 10.1097/QAI.0000000000001377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlin JS, Westfall AO, Raper JL, Zinski A, Norton WE, Willig JH, Gross R, Ritchie CS, Saag MS, & Mugavero MJ (2012). Pain, mood, and substance abuse in HIV: implications for clinic visit utilization, antiretroviral therapy adherence, and virologic failure. J Acquir Immune Defic Syndr, 61(2), 164–170. 10.1097/QAI.0b013e3182662215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miaskowski C, Penko JM, Guzman D, Mattson JE, Bangsberg DR, & Kushel MB (2011). Occurrence and characteristics of chronic pain in a community-based cohort of indigent adults living with HIV infection. J Pain, 12(9), 1004–1016. 10.1016/j.jpain.2011.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MB, Chan WS, Curtis AF, Boissoneault J, Robinson M, Staud R, Berry RB, & McCrae CS (2018). Pain intensity as a moderator of the association between opioid use and insomnia symptoms among adults with chronic pain. Sleep Med, 52, 98–102. 10.1016/j.sleep.2018.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin CM, Belleville G, Belanger L, & Ivers H (2011). The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep, 34(5), 601–608. 10.1093/sleep/34.5.601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onen NF, Barrette EP, Shacham E, Taniguchi T, Donovan M, & Overton ET (2012). A review of opioid prescribing practices and associations with repeat opioid prescriptions in a contemporary outpatient HIV clinic. Pain Pract, 12(6), 440–448. 10.1111/j.1533-2500.2011.00520.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecina M, Karp JF, Mathew S, Todtenkopf MS, Ehrich EW, & Zubieta JK (2019). Endogenous opioid system dysregulation in depression: implications for new therapeutic approaches. Mol Psychiatry, 24(4), 576–587. 10.1038/s41380-018-0117-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS (1977). The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1(3), 385–401. [Google Scholar]
- Redman KN, Karstaedt AS, & Scheuermaier K (2018). Increased CD4 counts, pain and depression are correlates of lower sleep quality in treated HIV positive patients with low baseline CD4 counts. Brain Behav Immun, 69, 548–555. 10.1016/j.bbi.2018.02.002 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Penney AT, Iudicello JE, Riggs PK, Doyle K, Ellis RJ, Letendre SL, Grant I, Woods SP, & Group, H. I. V. N. R. P. H. (2013). Co-morbidities in persons infected with HIV: increased burden with older age and negative effects on health-related quality of life. AIDS Patient Care STDS, 27(1), 5–16. 10.1089/apc.2012.0329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabin CA, Harding R, Doyle N, Redline S, de Francesco D, Mallon PWG, Post FA, Boffito M, Sachikonye M, Geressu A, Winston A, & Kunisaki KM (2020). Associations Between Widespread Pain and Sleep Quality in People With HIV. J Acquir Immune Defic Syndr, 85(1), 106–112. 10.1097/QAI.0000000000002410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas J, Scherrer JF, Schneider FD, Sullivan MD, Bucholz KK, Burroughs T, Copeland LA, Ahmedani BK, & Lustman PJ (2017). New-onset depression following stable, slow, and rapid rate of prescription opioid dose escalation. Pain, 158(2), 306–312. 10.1097/j.pain.0000000000000763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer JF, Salas J, Schneider FD, Bucholz KK, Sullivan MD, Copeland LA, Ahmedani BK, Burroughs T, & Lustman PJ (2017). Characteristics of new depression diagnoses in patients with and without prior chronic opioid use. J Affect Disord, 210, 125–129. 10.1016/j.jad.2016.12.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott W, Arkuter C, Kioskli K, Kemp H, McCracken LM, Rice ASC, & de CWAC (2018). Psychosocial factors associated with persistent pain in people with HIV: a systematic review with meta-analysis. Pain, 159(12), 2461–2476. 10.1097/j.pain.0000000000001369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serdarevic M, Osborne V, Striley CW, & Cottler LB (2017). The association between insomnia and prescription opioid use: results from a community sample in Northeast Florida. Sleep Health, 3(5), 368–372. 10.1016/j.sleh.2017.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MT, Mun CJ, Remeniuk B, Finan PH, Campbell CM, Buenaver LF, Robinson M, Fulton B, Tompkins DA, Tremblay JM, Strain EC, & Irwin MR (2020). Experimental sleep disruption attenuates morphine analgesia: findings from a randomized trial and implications for the opioid abuse epidemic. Sci Rep, 10(1), 20121. 10.1038/s41598-020-76934-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabachnick T, & Fidell L (2013). Using multivariate statistics (6th Edition ed.). Pearson Education. [Google Scholar]
- Taibi DM (2013). Sleep disturbances in persons living with HIV. J Assoc Nurses AIDS Care, 24(1 Suppl), S72–85. 10.1016/j.jana.2012.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang NKY, Stella MT, Banks PDW, Sandhu HK, & Berna C (2019). The effect of opioid therapy on sleep quality in patients with chronic non-malignant pain: A systematic review and exploratory meta-analysis. Sleep Med Rev, 45, 105–126. 10.1016/j.smrv.2019.03.005 [DOI] [PubMed] [Google Scholar]
- Tran A, Fuller JM, Wong KK, Krass I, Grunstein R, & Saini B (2009). The development of a sleep disorder screening program in Australian community pharmacies. Pharm World Sci, 31(4), 473–480. 10.1007/s11096-009-9301-4 [DOI] [PubMed] [Google Scholar]
- Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, Cohen M, Evers S, Finnerup NB, First MB, Giamberardino MA, Kaasa S, Korwisi B, Kosek E, Lavand'homme P, Nicholas M, Perrot S, Scholz J, Schug S, Smith BH, Svensson P, Vlaeyen JWS, & Wang SJ (2019). Chronic pain as a symptom or a disease: the IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain, 160(1), 19–27. 10.1097/j.pain.0000000000001384 [DOI] [PubMed] [Google Scholar]
- Turk DC, & Cohen MJ (2010). Sleep as a marker in the effective management of chronic osteoarthritis pain with opioid analgesics. Semin Arthritis Rheum, 39(6), 477–490. 10.1016/j.semarthrit.2008.10.006 [DOI] [PubMed] [Google Scholar]
- Uebelacker LA, Weisberg RB, Herman DS, Bailey GL, Pinkston-Camp MM, & Stein MD (2015). Chronic Pain in HIV-Infected Patients: Relationship to Depression, Substance Use, and Mental Health and Pain Treatment. Pain Med, 16(10), 1870–1881. 10.1111/pme.12799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosvick M, Gore-Felton C, Ashton E, Koopman C, Fluery T, Israelski D, & Spiegel D (2004). Sleep disturbances among HIV-positive adults: the role of pain, stress, and social support. J Psychosom Res, 57(5), 459–463. 10.1016/j.jpsychores.2004.03.003 [DOI] [PubMed] [Google Scholar]
- Webel AR, Willig AL, Liu W, Sattar A, Boswell S, Crane HM, Hunt P, Kitahata M, Matthews WC, Saag MS, Lederman MM, & Rodriguez B (2019). Physical Activity Intensity is Associated with Symptom Distress in the CNICS Cohort. AIDS Behav, 23(3), 627–635. 10.1007/s10461-018-2319-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta JK, Ketter TA, Bueller JA, Xu Y, Kilbourn MR, Young EA, & Koeppe RA (2003). Regulation of human affective responses by anterior cingulate and limbic mu-opioid neurotransmission. Arch Gen Psychiatry, 60(11), 1145–1153. 10.1001/archpsyc.60.11.1145 [DOI] [PubMed] [Google Scholar]


