Dear Editor,
Gut microorganisms process food in animal guts and release many metabolic by-products, which are predicted to influence host physiological processes such as energy and lipid metabolism. Here, we investigate how succinate, a TCA cycle intermediate that is a major predicted release product of gut bacteria in Drosophila, influences the nutritional physiology of its Drosophila host. We administered succinate as a dietary supplement to microbe-free Drosophila, and quantified key nutritional indices. Dietary succinate significantly reduced fly lipid levels by up to ~50%. This response was not replicated in parallel experiments conducted with dietary fumarate supplement, indicating that it could not be attributed to a general effect of TCA intermediates. We hypothesize that microbe-derived succinate may contribute to the reduced lipid content of Drosophila bearing gut bacteria, relative to axenic Drosophila. More generally, this study highlights the importance of microbial derived metabolites as regulators of host metabolism.
The focus of this study, succinate, is predicted to be released at substantial rates by several of the key bacterial members of the Drosophila gut microbiota (Ankrah et al., 2021). Succinate is a TCA cycle intermediate and it has been implicated as a modulator of host metabolism in mammalian systems. Specifically, microbial-derived metabolites from the TCA cycle play important roles in regulating host glucose homeostasis (De Vadder et al., 2016; Connors et al., 2019; Fernández-Veledo & Vendrell, 2019) and are correlated with the onset and progression of metabolic diseases such as obesity (Serena et al., 2018; Wang et al., 2019). To investigate the possible role of succinate in the nutritional physiology of Drosophila, we supplemented axenic (microbe-free) Drosophila diet with different concentrations of succinate and monitored changes to fly weight, triacylglyceride (TAG), glucose, and protein levels. (The basis for the succinate concentrations selected for this study is provided in Supplementary methods).
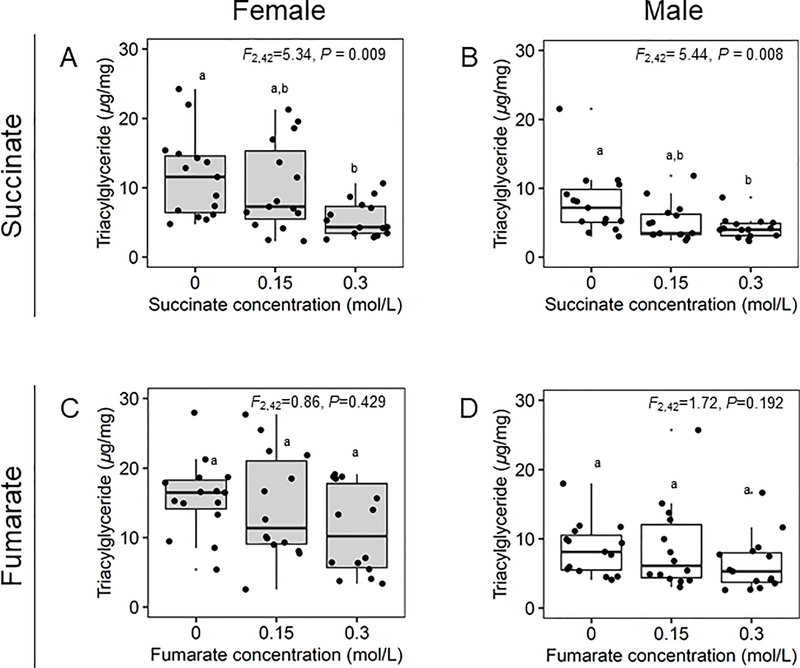
Drosophila fed on diet supplemented with succinate over 4 days displayed significantly reduced TAG levels in both female and male flies, with the TAG content halved on average in flies fed on diet containing 0.3 mol/L succinate relative to the succinate-free diet (Fig. 1A, B). Further analyses revealed that the effect of succinate was specific to TAG, and not mirrored by any significant effect on the glucose or protein contents of the flies, or on fly weight (Fig. S1). In addition, we observed a minimal negative effect of the dietary succinate supplement on survival of male flies and no effect on female flies over the 4-day experiment after Bonferroni correction for multiple comparisons (Fig. S1, Table S1).
Fig. 1.
Effect of succinate (A, B) and fumarate (C, D) on Drosophila triacylglyceride levels. Significantly different (P < 0.025) samples by Tukey’s HSD post hoc test are indicated by different letters. For each boxplot, the center line displays the median, and the lower and upper hinges correspond to the 25th and 75th percentiles; all data points are shown. Female and male flies are represented by grey and white boxes respectively.
To check whether the effect of succinate on fly lipid content was general to dicarboxylic acids we then analyzed flies fed on diets supplemented with fumarate, the product of succinate oxidation via the TCA enzyme succinate dehydrogenase and a metabolite that is not predicted to be released from Drosophila gut microorganisms (Ankrah et al., 2021). In contrast to flies fed on succinate, flies fed on fumarate displayed no statistically significant reductions in TAG levels compared to flies on the fumarate-free controls (Fig. 1C, D). The sole significant effect of fumarate-supplemented diets on the flies was a significantly reduced glucose levels in male flies, specifically ~41% decrease in average glucose levels in flies fed on 0.3 mol/L fumarate compared to male flies fed on the control diet (Fig. S1). Similar to succinate-supplemented diets we observed no significant changes in glucose levels for female flies (Fig. S1). The protein contents, fly weight and survival (Fig. S1, Table S1) remained unchanged in both female and male flies feeding on fumarate or the fumarate-free controls.
As part of these experiments, we quantified food intake by the flies. Food consumption did not vary significantly with diet composition for either female or male flies (Fig. S2), indicating that the lower TAG content of flies on diets containing succinate could not be explained by an antifeedant effect of the dietary supplement. Additional statistical analysis performed to quantify variation between experimental replicates indicated that, apart from glucose content, the variation across experimental replicates was not statistically significant for both metabolites (Fig. S3).
Previous studies have demonstrated that the lipid content of Drosophila is significantly elevated by elimination of the gut microbiota (Shin et al., 2011; Huang & Douglas, 2015; Sommer & Newell, 2019). The capacity of certain individual bacterial taxa and bacterial communities, particularly of Acetobacter and Lactobacillus species, to lower fly lipid content has been attributed to two processes (which are not mutually exclusive): bacterial competition for dietary sugars, which are the principal source of stored lipid in Drosophila (Chaston et al., 2014; Huang & Douglas, 2015), and bacterial production of acetic acid, which modulates insulin signaling and reduces nutrient allocation patterns to lipid (Shin et al., 2011; McMullen et al., 2020). In this study, we demonstrate that succinate, predicted from metabolic models to be released at high rates from Drosophila gut bacteria, also functions to reduce Drosophila TAG content. The effect of succinate on fly lipid is specific in two ways: (i) other nutritional indices were not significantly affected by dietary succinate; and (ii) fumarate, a metabolically related TCA cycle intermediate, did not reduce fly lipid content. The effect of succinate on Drosophila lipid content is reminiscent of its effect on brown adipose tissue in mammals, where oxidation of accumulated succinate by the enzyme succinate dehydrogenase initiates production of reactive oxygen species which induces adipocyte damage and promotes lipolysis resulting in reduced lipid levels (Ayala et al., 2014; Mills et al., 2018).
Nevertheless, the global effect of succinate supplement on Drosophila is different from mammals, where increases in succinate levels have been associated with weight gain and obesity (Serena et al., 2018; Keiran et al., 2019; Wan et al., 2020). This effect has been attributed to the metabolic response of white adipose tissue to succinate, mediated by succinate binding to its cognate receptor succinate receptor 1 (SUCNR1/GPR91) to activate downstream signaling pathways that inhibit lipolysis (McCreath et al., 2015). Although the fat body of insects is, in some respects, functionally similar to mammalian white adipose tissue (Canavoso et al., 2001; Trinh & Boulianne, 2013), our data raise the possibility that the Drosophila fat body and mammalian adipose respond differently to succinate.
A priority for future research is to investigate the molecular basis of succinate transport across the gut epithelium and the effect of succinate metabolism on lipid synthesis and mobilization in the fat body. Identifying the molecular players such as transporters and receptor proteins involved in succinate assimilation and signaling will aid in understanding the mechanism by which succinate affects lipid homeostasis in insects. Altogether, our data reinforce the growing evidence that specific metabolites produced by gut microbiota play important roles in modulating host metabolism, and they specifically point to a physiological role for succinate in regulating insect lipid metabolism.
Supplementary Material
Table S1 Discrete survival analysis summary statistics for each metabolite administered to each sex. For significant P-values below 0.025, the text is displayed in bold font. For the post hoc Tukey’s test, fly survival was compared among concentrations by day with results displayed as the estimated probability (95% confidence intervals) and letter ranking.
Fig. S1 Effect of succinate (A) and fumarate (B) on Drosophila nutritional indices and survival. Changes in glucose, protein and fly weight after 4-day incubation on succinate and fumarate supplemented diets and fly survival over 4-day incubation period. For boxplots, the center line displays the median, and the lower and upper hinges correspond to the 25th and 75th percentiles; all data points are shown. Female and male flies are represented by grey and white boxes respectively. Significantly different (P < 0.025) samples by Tukey’s HSD post hoc test are indicated by different letters. For line and dot plots, concentrations of succinate and fumarate used are indicated by red (0 mol/L), black (0.15 mol/L) and purple (0.3 mol/L) lines and dots. Dots represent individual data points and lines represent the mean for all data points for each day. Statistical analyses for fly survival are provided in Table S1.
Fig. S2 Drosophila food intake on succinate (A, B) and fumarate (C, D) supplemented diets. Concentrations of succinate and fumarate used are indicated by red (0 mol/L), black (0.15 mol/L) and purple (0.3 mol/L) boxes.
Fig. S3 Among-trial variation in nutritional indices for (A) female and (B) male flies fed on control diet without metabolite added. Effect size means and standard deviation for best linear unbiased predictors are displayed. The dashed line indicates the grand mean. For F statistics, the degrees of freedom for the treatment and residuals are 1 and 4, respectively, and 1 for χ2 statistics. The open triangle indicates a significant difference by non-overlapping 95% confidence intervals with grand mean as determined by simulated posterior distributions. Note: no differences were detected for between-trial variation for male glucose content. NS: P > 0.025; **: P < 0.01; ****: P < 0.0001.
Acknowledgments
This research was funded by an NIH grant R01GM095372 to AED. We thank Eduardo Bueno for assistance with setting up experiments.
Footnotes
Disclosure
The authors declare no competing interests.
Data availability
The datasets supporting this article have been uploaded as part of the electronic supplementary material.
References
- Ankrah NY, Barker BE, Song J, Wu C, McMullen JG II and Douglas AE (2021) The predicted metabolic function of the gut microbiota of Drosophila melanogaster. bioRxiv, DOI: 10.1101/2021.01.20.427455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala A, Muñoz MF and Argüelles S (2014) Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Medicine and Cellular Longevity, 2014, 360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canavoso LE, Jouni ZE, Karnas KJ, Pennington JE and Wells MA (2001) Fat metabolism in insects. Annual Review of Nutrition, 21, 23–46. [DOI] [PubMed] [Google Scholar]
- Chaston JM, Newell PD and Douglas AE (2014) Metagenome-wide association of microbial determinants of host phenotype in Drosophila melanogaster. mBio, 5, e01631–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors J, Dawe N and Van Limbergen J (2019) The role of succinate in the regulation of intestinal inflammation. Nutrients, 11(1), 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vadder F, Kovatcheva-Datchary P, Zitoun C, Duchampt A, Bäckhed F and Mithieux G (2016) Microbiota-produced succinate improves glucose homeostasis via intestinal gluconeogenesis. Cell Metabolism, 24, 151–157. [DOI] [PubMed] [Google Scholar]
- Fernández-Veledo S and Vendrell J (2019) Gut microbiota-derived succinate: Friend or foe in human metabolic diseases? Reviews in Endocrine and Metabolic Disorders, 20, 439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JH and Douglas AE (2015) Consumption of dietary sugar by gut bacteria determines Drosophila lipid content. Biology Letters, 11, 20150469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiran N, Ceperuelo-Mallafré V, Calvo E, Hernández-Alvarez MI, Ejarque M, Núñez-Roa C, et al. (2019) SUCNR1 controls an anti-inflammatory program in macrophages to regulate the metabolic response to obesity. Nature Immunology, 20, 581–592. [DOI] [PubMed] [Google Scholar]
- McCreath KJ, Espada S, Gálvez BG, Benito M, de Molina A, Sepúlveda P, et al. (2015) Targeted disruption of the SUCNR1 metabolic receptor leads to dichotomous effects on obesity. Diabetes, 64, 1154–1167. [DOI] [PubMed] [Google Scholar]
- McMullen JG, Peters-Schulze G, Cai J, Patterson AD and Douglas AE (2020) How gut microbiome interactions affect nutritional traits of Drosophila melanogaster. Journal of Experimental Biology, 223(19), jeb227843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills EL, Pierce KA, Jedrychowski MP, Garrity R, Winther S, Vidoni S, et al. (2018) Accumulation of succinate controls activation of adipose tissue thermogenesis. Nature, 560, 102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell PD and Douglas AE (2014) Interspecies interactions determine the impact of the gut microbiota on nutrient allocation in Drosophila melanogaster. Applied and Environmental Microbiology, 80, 788–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serena C, Ceperuelo-Mallafré V, Keiran N, Queipo-Ortuño MI, Bernal R, Gomez-Huelgas R, et al. (2018) Elevated circulating levels of succinate in human obesity are linked to specific gut microbiota. Multidisciplinary Journal of Microbial Ecology, 12, 1642–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin SC, Kim SH, You H, Kim B, Kim AC, Lee KA, et al. (2011) Drosophila microbiome modulates host developmental and metabolic homeostasis via insulin signaling. Science, 334, 670–674. [DOI] [PubMed] [Google Scholar]
- Sommer AJ and Newell PD (2019) Metabolic basis for mutualism between gut bacteria and its impact on the Drosophila melanogaster host. Applied and Environmental Microbiolology, 85, e01882–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh I and Boulianne GL (2013) Modeling obesity and its associated disorders in Drosophila. Physiology, 28, 117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y, Yuan JH, Li J, Li H, Yin KH, Wang FL, et al. (2020) Overweight and underweight status are linked to specific gut microbiota and intestinal tricarboxylic acid cycle intermediates. Clinical Nutrition, 39(10), 3189–3198 [DOI] [PubMed] [Google Scholar]
- Wang K, Liao MF, Zhou N, Bao L, Ma K, Zheng ZY, et al. (2019) Parabacteroides distasonis alleviates obesity and metabolic dysfunctions via production of succinate and secondary bile acids. Cell Reports, 26, 222–235. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Discrete survival analysis summary statistics for each metabolite administered to each sex. For significant P-values below 0.025, the text is displayed in bold font. For the post hoc Tukey’s test, fly survival was compared among concentrations by day with results displayed as the estimated probability (95% confidence intervals) and letter ranking.
Fig. S1 Effect of succinate (A) and fumarate (B) on Drosophila nutritional indices and survival. Changes in glucose, protein and fly weight after 4-day incubation on succinate and fumarate supplemented diets and fly survival over 4-day incubation period. For boxplots, the center line displays the median, and the lower and upper hinges correspond to the 25th and 75th percentiles; all data points are shown. Female and male flies are represented by grey and white boxes respectively. Significantly different (P < 0.025) samples by Tukey’s HSD post hoc test are indicated by different letters. For line and dot plots, concentrations of succinate and fumarate used are indicated by red (0 mol/L), black (0.15 mol/L) and purple (0.3 mol/L) lines and dots. Dots represent individual data points and lines represent the mean for all data points for each day. Statistical analyses for fly survival are provided in Table S1.
Fig. S2 Drosophila food intake on succinate (A, B) and fumarate (C, D) supplemented diets. Concentrations of succinate and fumarate used are indicated by red (0 mol/L), black (0.15 mol/L) and purple (0.3 mol/L) boxes.
Fig. S3 Among-trial variation in nutritional indices for (A) female and (B) male flies fed on control diet without metabolite added. Effect size means and standard deviation for best linear unbiased predictors are displayed. The dashed line indicates the grand mean. For F statistics, the degrees of freedom for the treatment and residuals are 1 and 4, respectively, and 1 for χ2 statistics. The open triangle indicates a significant difference by non-overlapping 95% confidence intervals with grand mean as determined by simulated posterior distributions. Note: no differences were detected for between-trial variation for male glucose content. NS: P > 0.025; **: P < 0.01; ****: P < 0.0001.