Abstract
Three genes encoding AfsK, AfsR, AfsS homologues in Streptomyces pristinaespiralis were studied, respectively, to investigate regulatory role of AfsKRS system for pristinamycin biosynthesis. Transcription change and gene inactivation analysis indicated that these genes had active transcription and positive regulation for the improvement of pristinamycin production in S. pristinaespiralis. The analysis of AfsKRS-defective mutagenesis indicated that there might be a positive correlation between the product of afsK and pristinamycin I biosynthesis, and a negative correlation to pristinamycin II biosynthesis. However, both afsR and afsS might have negative correlation to pristinamycin I production and positive correlation to pristinamycin II production. The effects on pristinamycin production of AfsKRS disruptants by protein kinase inhibitor K252a indicated that AfsR, both not AfsK and AfsS, was the inhibition target of K252a in S. pristinaespiralis, and AfsR should serve as a pleiotropic regulator to have differential regulation on biosynthesis of pristinamycin I and II components. Based on above study, it might be deduced that different signal transduction patterns via AfsK, AfsR, AfsS of AfsKRS system should be involved in respective regulation for biosynthesis of pristinamycin I and II in S. pristinaespiralis. In conclusion, the investigation could give some valuable clues for exploring furtherly regulatory function of AfsKRS system in S. pristinaespiralis.
Keywords: Streptomyces pristinaespiralis, Pristinamycin, Biosynthesis, AfsKRS system, Regulation
Introduction
Streptomyces pristinaespiralis produces pristinamycin, which is a clinically important mixture of two types of chemically diverse antibiotics: the cyclohexadepsipeptide pristinamycin I (PI) and the polyunsaturated macrolactone pristinamycin II (PII). PI and PII are coproduced in a 30:70 ratio, and pristinamycin IA (PIA) and pristinamycin IIA (PIIA) are its two main components (Mast and Wohlleben 2014). It has been found most of genes for PI and PII biosynthesis are organized together in a large supercluster (Mast et al. 2011). As mutli-component antibiotics, each component of pristinamycin has different biosynthesis pathways, and these production process can be regulated by different regulators (Martin and Liras 2010). At least seven different cluster-situated regulatory genes exist complex signaling cascades, which are responsible for the fine-tuned regulation of pristinamycin production in S. pristinaespiralis (Mast et al. 2015; Wang et al. 2015). However, whether AfsKRS system regulates pristinamycin biosynthesis remains poorly understood.
AfsKRS is a type of regulatory system related to antibiotic production in some species of Streptomyces, which consists of three proteins: AfsK, AfsR and AfsS (Horinouchi 2003; Liu et al. 2013). AfsK and AfsR belong to serine/threonine kinase family. The autokinase activity of AfsK can activate AfsR, and then the phosphorylated AfsR binds to afsS as a transcriptional factor for activating its transcription in the model species S. coelicolor (Horinouchi 2003). In our previous study, it was found that the AfsK homologue (spy1) had a positively effect on PI biosynthesis but a negative effect on PII biosynthesis, which implied that AfsKRS regulatory system might play a differential role for the production of two different components of pristinamycin in S. pristinaespiralis (Jin et al. 2012). So, a further investigation of three homologous proteins (GenBank Accession No. ACT34021 for AfsK, QMU14830 for AfsR and QMU14831 for AfsS) of AfsKRS regulatory system from S. pristinaespiralis were performed for understanding whether the complex signaling cascade is responsible for the regulation of pristinamycin production in this study.
K252a can inhibit phosphorylation of some members of serine/threonine kinase family by competing with the binding of ATP to the catalytic domain in some species of Streptomyces (Hong and Horinouchi 1998; Hong et al. 1993). In this study, K252a as protein kinase inhibitor also was used for the functional exploration of AfsK, AfsR and AfsS during pristinamycin production in S. pristinaespiralis.
Materials and methods
Bacterial strains, plasmids, genes and culture
Escherichia coli DH5α was used as a host for routine cloning strategies. E. coli ET12567, harbouring the conjugative plasmid pUZ8002, was used to perform the intergeneric conjugation from E. coli to S. pristinaespiralis (Bierman et al. 1992). Streptomyces pristinaespiralis F618 was used the generation of gene inactivation mutants. E. coli-Streptomyces shuttle plasmid pKC1139 was used to construct the plasmids for gene inactivation. High pristinamycin-producing recombinants F618, F303 were obtained by genome shuffling of the ancestral strain ATCC25486 of S. pristinaespiralis (Xu et al. 2008). Frozen mycelia from one single batch culture was maintained in 20% (v/v) glycerol at − 70 °C.
Streptomyces pristinaespiralis strains were activated, cultured and fermented respectively on slant, seed, fermentation medium and the cultural conditions were described as reported previously (Jia et al. 2006). The medium for intergeneric conjugation and Luria–Bertani (LB) medium was used as described (Kieser et al. 2000; Sambrook and Russell 2001). Where needed, the bacterial strains were incubated in the presence of ampicillin (100 µg/ml), apramycin (50 µg/ml) or kanamycin (50 µg/ml). Protein kinase inhibitor K252a from MedChemExpress was used to study effect on pristinamycin production.
For cultivation and harvesting of genomic DNA, about 0.5 ml frozen mycelia suspension of S. pristinaespiralis strains were inoculated to 25 ml seed medium and cultured for 48 h as described previously (Jin et al. 2008). Plasmid DNA from E. coli and genomic DNA from S. pristinaespiralis were isolated using standard protocols (Kieser et al. 2000; Sambrook and Russell 2001). E. coli competent cells were prepared as the standard description (Sambrook and Russell 2001). Taq DNA polymerase, T4 DNA Ligase and restriction enzymes were obtained from Promega.
The gene sequence of AfsK (GenBank Accession No. ACT34021) was obtained from S. pristinaespiralis F618 in our previous study (Jin et al. 2012), and the gene sequences of AfsR (GenBank Accession No. QMU14830) and AfsS (GenBank Accession No. QMU14831) were obtained from the genome of S. pristinaespiralis Pr11 in the GenBank database (https://www.ncbi.nlm.nih.gov/nuccore/CP059696.1).
Quantitative real-time PCR analysis
To determine the transcription of each gene in AfsKRS regulatory system, total RNA was prepared, respectively, from the mycelia of two recombinants F618, F303 and the ancestor ATCC25486 collected after 60 h fermentation with Trizol reagent (Invitrogen) according to the instruction. The contaminating genomic DNA was removed by treatment with RNase-free DNase (Promega). RNA concentration and purity were determined by measuring the absorbance at 260 nm and 280 nm in a BioPhotometer spectrophotometer (Eppendorf).
Quantitative real-time PCR was used to monitor transcription changes of afsK, afsR, afsS genes in the recombinants F618, F303 and the ancestor ATCC25486 as the control. Primers of these genes were designed with the software PRIMER v5.0 to generate PCR products of appropriate sizes (Table 1). RNA samples were reverse transcribed using SuperScriptTM II RNase H- Reverse Transcriptase (Invitrogen) as cDNA templates. Transcript level of each gene was quantified using iQTM SYBR Green Supermix (Bio-Rad). Each reaction was performed in a total volume of 25 μl with the following components: 50 mM KCl, 20 mM Tris–Cl of pH 8.4, 200 μM each dNTP, 25 units iTaq DNA polymerase, 3 mM MgCl2, 10 nM SyBR green I (Molecular Probes), 300 nM each primer, and the cDNA template. PCR was performed on the iCycler instrument (Bio-Rad) with the following cycling program: 93 °C for 1 min followed by 50 cycles of 10 s at 93 °C, 20 s at 60 °C, and 10 s at 72 °C. The products were analyzed by melting-curve analysis by applying 93 °C for 1 min, 60 °C for 2 min, and 50 °C for 10 s, followed by an increase in temperature from 50 to 85 °C (0.5 °C/s) and continuous fluorescence recording. This quantifying program was followed by a melting program that allows the detection of the melting points for every PCR product. All experiments were repeated three times. Gene transcription levels obtained by the real-time PCR were normalized with the rDNA gene as an inner control to quantify the relative expression of all the genes and water as a negative control.
Table 1.
Primer sequences
| Experiment | Gene | Amplicon size (bp) | Forward primer (5′–3′) | Reverse primer (5′–3′) |
|---|---|---|---|---|
| Real-time PCR | afsK | 338 | CTCCACCCTTGCTCTGCT | CGTCATCTCCCATCTCCTTC |
| afsR | 219 | TGCTGAACCTGTGGGACG | GGGCGAGCATCAGCAGT | |
| afsS | 186 | GCACCAACAAGCAGGACC | TGGTCGTGATGGTGGTGTC | |
| rDNA | 697 | TGGCGAACGGGTGAGTAA | CGTGGACTACCAGGGTATCTAA | |
| Gene inactivation | afsR | 975 | TTGGAATTCGCAGGAACTGGGCGGTCAA | GCTCTAGAAAGTCCAGCAGGTGGGAGACG |
| afsS | 539 | TTGGAATTCGGGCAGACGGATCACTCG | GCTCTAGACAGTTGCGCTCGGTTTCT |
The underlined words indicate the restriction enzyme sites of EcoRI and XbaI, respectively
Gene inactivation
To further analyze regulation role of the AfsKRS system in pristinamycin biosynthesis, the genes encoding AfsR and AfsS in S. pristinaespiralis were inactivated by gene insertion mutagenesis as the construction strategy of AfsK mutant with F618 as original strain as described previously (Jin et al. 2012).
To inactivate the afsR and afsS, the procedure of gene inactivation mutagenesis was performed, respectively, according to that of spy1 (afsK) as described previously (Jin et al. 2012). About 1 kb internal fragment of afsR and 0.5 kb fragment including afsS was amplified, respectively, from the genomic DNA of S. pristinaespiralis F618 with oligonucleotide primers with restriction enzyme sites of EcoRI and XbaI (Table 1). The PCR products were digested with the enzymes and cloned into pKC1139 digested with the same enzymes. The recombinant plasmid afsR::pKC1139 and afsS::pKC1139 was obtained and introduced into E. coli DH5α prior to introduction into ET12567 (pUZ8002), respectively. As donor strains, E. coli ET12567/pUZ8002/afsR::pKC1139 and E. coli ET12567/pUZ8002/afsS::pKC1139 were grown in the presence of 50 µg/ml apramycin and 50 µg/ml kanamycin, and then used, respectively, for intergeneric conjugation with S. pristinaespiralis F618. Finally, mutants were screened in the plates containing 50 µg/ml nalidixic acid and 50 µg/ml apramycin.
The mutants were identified by PCR analysis. PCR products were amplified from genomic DNA of the mutants and the parent strain F618 as the control using oligonucleotides primers (5′-CTGGTCCACAGCTCCTTCG-3′ and 5′-ATTCTTCGCATCCCGCCTCT-3′) of aac(3)IV gene contained in pKC1139.
Analytical procedure
The seed culture of mutants and wild type were inoculated at 6% (v/v) into Erlenmeyer flasks in the production medium containing different concentrations of K252a. The fermentation was carried out at 28 °C with shaking at 220 rpm. Mediums for culturing and antibiotic production of S. pristinaespiralis strains can be found in previous study (Jia et al. 2006).
For sampling of fermentation, a 2.0 ml fermentation broth was extracted with 4.0 ml of methanol for 1 h and centrifuged at 4000 rpm for 10 min, and then the supernatant was directly applied for HPLC analysis. The column packed with Hypersil ODS2 (5 µm; 4.6 mm × 250 mm) was developed with acetonitrile-0.1 M potassium phosphate buffer (pH2.9; v/v = 45:55), at a flow of 1.0 ml/min. Pristinamycin IA (PIA) and pristinamycin IIA (PIIA) were detected in UV absorption at 206 nm. In this paper, production of pristinamycin was examined based on peak areas of PIA and PIIA as previous description (Jia et al. 2006). Commercial pristinamycin from Rhone-Poulenc Rorer Co. (Montrouge) was used as a reference standard.
Results and discussion
Transcription changes of AfsKRS system in high pristinamycin-producing recombinants
To investigate relation of transcription of AfsKRS system to pristinamycin biosynthesis, afsK (spy1) from S. pristinaespiralis F618 (Jin et al. 2012), and the putative genes afsR and afsS from S. pristinaespiralis PR11 in the GenBank database were used for transcription change analysis by quantitative real-time PCR. Two high pristinamycin-producing recombinants F618 and F303 with about ten times higher than that of the ancestral strain ATCC25486 in previous study (Xu et al. 2008), were selected out and used as templates of quantitative real-time PCR. It was found that all afsK, afsR and afsS had higher transcription abundance in these two high-yield recombinants than the ancestor at 60 h fermentation (Fig. 1). It indicated that the active transcription AfsKRS system might have positive correlation to the yield improvement of pristinamycin production in the two high-yield recombinants as well as in the ancestor of S. pristinaespiralis.
Fig. 1.
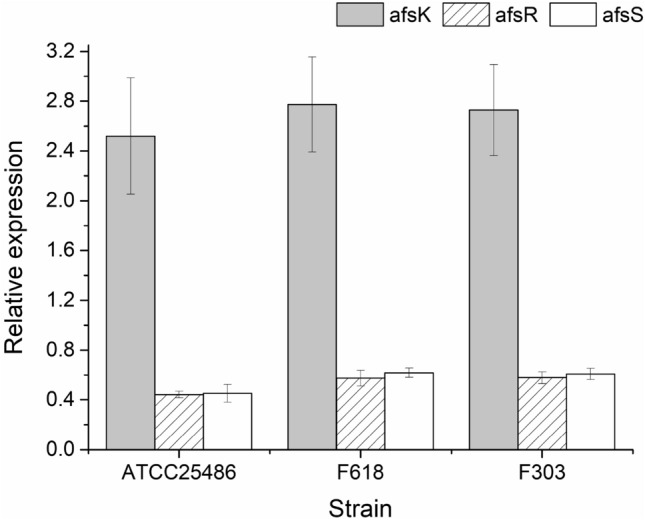
Quantitative real-time PCR analysis of afsK, afsR and afsS involved in the AfsKRS system in S. pristinaespiralis. Total mycelia RNA extracted from two recombinants F618, F303 and the ancestor ATCC25486, was used as template in quantitative real-time PCR. Then quantitative PCR was used to perform relative quantity of tested genes after normalization with control 16S rRNA. Mean and standard error were calculated from three independent replicates
Gene inactivation analysis of AfsKRS system
To further analyze regulation role of the AfsKRS system in pristinamycin biosynthesis, the putative genes encoding AfsR and AfsS in S. pristinaespiralis were inactivated by gene insertion mutagenesis as the construction strategy of AfsK mutant with F618 as original strain as described previously(Jin et al. 2012).
Pristinamycin production in ΔafsK, ΔafsR and ΔafsS mutants was determined respectively by fermentation experiments and HPLC analysis. It was shown that respective disruption of afsK, afsR and afsS could not disrupt completely the production of pristinamycin compared with that in original strain F618, but the knockout of three genes had different influences on yield change of PI and PII components in three disruptants (Fig. 2).
Fig. 2.
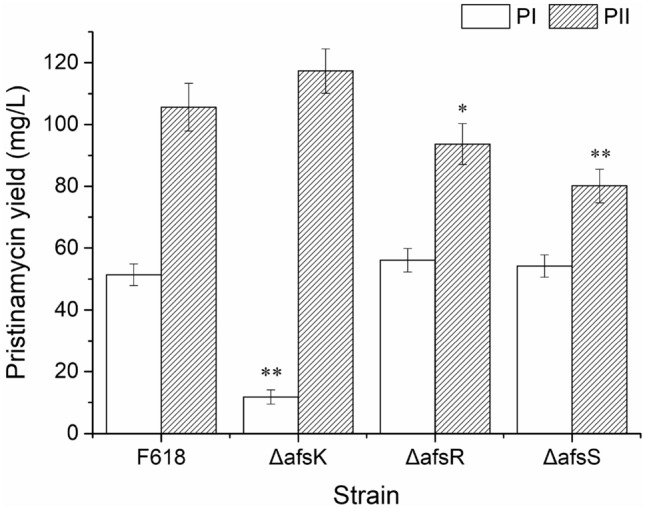
Pristinamycin I and II production of the AfsKRS-defective mutants and the original strain F618 after 60 h fermentation in S. pristinaespiralis. Mean and standard error were calculated from three independent replicates. Symbol * and ** represents respectively statistically difference (p < 0.05) and significant difference (p < 0.01) between the pristinamycin yield of three mutants and the original strain F618
For ΔafsK mutant, there was a drastic decrease of PIA [about 77% (**) less than F618], whereas a slight increase of PIIA (about 11% more than F618; Fig. 2). From the result it was concluded that afsK was involved in the regulation of secondary metabolism, and there might be a positive correlation between the product of afsK and PI biosynthesis, and a negative correlation to PII biosynthesis. It has been known that the signal transduction of AfsK was much complex, and it can phosphorylate not only AfsR but also other multiple kinases, and AfsK was also modulated by some repressors such as KbpA for actinorhodin biosynthesis in S. coelicolor (Matsumoto et al. 1994; Umeyama and Horinouchi 2001). As observed in Fig. 2, the production change of PI and PII between the afsK- and afsR-disruptant compared with that in original strain F618 was inconsistent. It might indicate that AfsK appears to phosphorylate not only afsR but also some other protein kinases in S. pristinaespiralis.
For ΔafsR and ΔafsS mutants, they have similar changes of pristinamycin production between each mutant. There was a slight increase of PIA (up by 9 and 5%, respectively, for ΔafsR, ΔafsS), whereas a drastic decrease of PIIA (down by 11% (*) and 24% (**), respectively, for ΔafsR, ΔafsS; Fig. 2). It was concluded that afsR, and afsS was involved in the regulation of secondary metabolism, and there might be negative correlation between the product of the two gene and PI biosynthesis, and positive correlation to PII biosynthesis. The afsS-disruptant produced smaller amounts of PIA and PIIA than afsR-disruptant. Especially, the degree of reduction for the PIIA component in the afsS-disruptant was much greater than the afsR-disruptant, and reached significant difference. This is probably because transcriptional activation of afsS depends on AfsR, but AfsR appears to activate not only afsS but also some other genes in S. pristinaespiralis. The similar phenomena had been observed in the production of actinorhodin and undecylprodigiosin in S. coelicolor (Lee et al. 2002).
Effects of K252a on pristinamycin production in AfsKRS mutant
Because the inhibitor K252a can affect the phosphorylation of protein kinases in some species of Streptomyces, and it can inhibit the production of actinorhodin in S. coelicolor and streptomycin biosynthesis in S. griseus (Lee et al. 2002). We also examined the effects of the inhibitor on pristinamycin production in ΔafsK, ΔafsR and ΔafsS mutants of S. pristinaespiralis with defective phosphorylation.
The original strain F618 was used to determine optimal concentration of K252a on pristymaycin production. K252a at an optimum concentration of 50 nM showed maximum but not complete inhibition on both PI and PII production in fermentation of S. pristinaespiralis (Fig. 3).
Fig. 3.
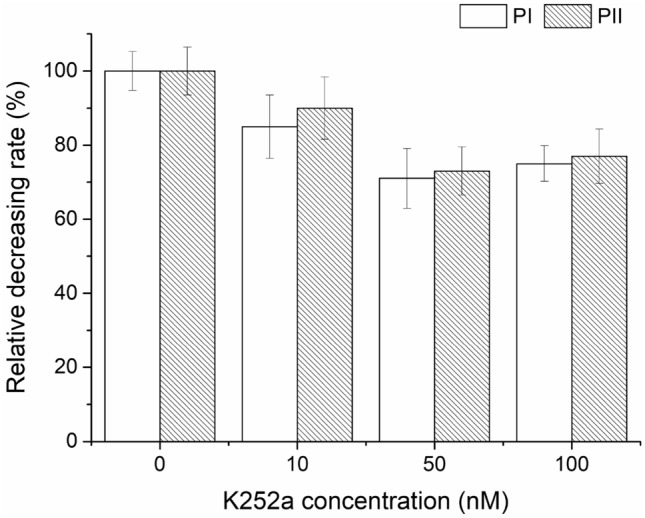
Inhibition effect on the production of pristinamycin I and II of the original strain F618 by different concentration of the inhibitor K252a. Mean and standard error were calculated from three independent replicates
The fermentation process of pristinamycin is mainly divided into initial synthesis stage and stable synthesis stage. For the original strain F618 (Fig. 4A), the initial synthesis stage of the antibiotic was 0–36 h, and production of PI and PII increased gradually. After 36 h, there was the stable synthesis stage of pristinamycin, and the highest yield arrived. After addition of 50 nM K252a, the production of pristinamycin was inhibited in different degree during the whole fermentation process, both PI and PII components were most significantly inhibited by K252a at 24 h, and the inhibition rate is 15 and 26%, respectively. Therefore, for the fermentation of F618, K252a had the most significant inhibitory effect at the initial stage of the production of pristinamycin, and there was a more obvious inhibition on production of PII than PI component.
Fig. 4.
Effect of the inhibitor K252a on the pristinamycin I and II production of the AfsKRS-defective mutants and the original strain after 60 h fermentation in S. pristinaespiralis. A detection of PIA and PIIA represented yield level of PI and PII by HPLC. Mean and standard error were calculated from three independent replicates. Symbol * and ** represents, respectively, statistically difference (p < 0.05) and significant difference (p < 0.01) at sampling time during the pristinamycin fermentation in S. pristinaespiralis after the addition of K252a
During the fermentation of the mutants ΔafsK and ΔafsS (Fig. 4B, D), they showed similar inhibitory pattern on the production of pristinamycin to the original strain F618 by the addition of K252a, indicating the defective afsK or afsS did not change obviously inhibition pattern in the two disruptants compared with that in F618 by the addition of K252a. In other words, both AfsK and AfsS might not be inhibition targets of K252a. It is worth mentioning that K252a inhibited more obviously PII than PI production both in the afsK- and afsS-disruptants, and the highest inhibition rate for PII components is 24% at 24 h for ΔafsK and 30% at 36 h for ΔafsS. It might hint that K252a probably inhibit unknown phosphorylation of other protein kinase related to afsK or afsS responsible for the production of PII component in the two mutants.
For the mutant ΔafsR (Fig. 4C), K252a inhibited more obviously production of PI component, but inhibited slightly PII production. It indicated that K252a had a different inhibitory pattern with F618 in the fermentation of the mutant. It has been known that the phosphorylation of AfsR was significantly inhibited by K252a in secondary metabolism of S. coelicolor and S. griseus (Hong and Horinouchi 1998; Hong et al. 1993). The different inhibitory pattern between the pristinamycin production of ΔafsR and the original strain F618 might indicate that K252a could inhibit the phosphorylation of AfsR in S. pristinaespiralis, and AfsR should serve as a pleiotropic regulator to affect biosynthesis of different pristinamycin components. In addition, the defective afsR affect more obviously the inhibition of K252a on the production of PI component, and the inhibition rate is above 10% after 24 h fermentation in the mutant. It might hint that K252a had a more obvious inhibition on production of PI than PII component in the AfsKRS system-defective mutant via AfsR site.
It has been known that regulation of antibiotic biosynthesis via AfsKRS system is universal in different Streptomyces strains, and its regulation mechanism may be relatively diverse (Liu et al. 2013). The investigation in this study could give some valuable clues for exploring furtherly regulatory function of AfsKRS system in S. pristinaespiralis. In S. pristinaespiralis, some signal transduction patterns via AfsKRS system should be involved in regulation for biosynthesis of pristinamycin I and II. As mutli-component antibiotics, it has been known that each of PI and PII has different biosynthesis pathways influenced by apparently independent regulatory systems (Martin and Liras 2010). Based on our study, regulations of AfsK, AfsR and AfsS for the production of each pristinamycin component was unidirectional and significant difference. It might be very complex and exist in probable crosstalk among regulating biosynthesis of PI, PII or other metabolism pathways, especially for the crosstalk of pleiotropic regulations of AfsK and AfsR sensed different signals. Some of the signals sensed by the AfsKRS system should be transferred to AfsS, which might affect, respectively, some pathway-specific regulators for the production of PI or PII component.
Acknowledgements
This work was financially supported by National Natural Science Foundation of China (No. 21376217 and No. 81502421), Natural Science Foundation of Ningbo, China (No. 2019A610205), and Industrial Science and Technology Major Project of Ningbo, China (No. 2017C110017).
Declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Footnotes
Qingchao Jin and Haipeng Liao contributed equally to this work.
References
- Bierman M, Logan R, O'Brien K, Seno ET, Rao RN, Schoner BE. Plasmid cloning vectors for the conjugal transfer of DNA from Escherichia coli to Streptomyces spp. Gene. 1992;116:43–49. doi: 10.1016/0378-1119(92)90627-2. [DOI] [PubMed] [Google Scholar]
- Hong SK, Horinouchi S. Effects of protein kinase inhibitors on in vitro protein phosphorylation and on secondary metabolism and morphogenesis in Streptomyces coelicolor A3(2) J Microbiol Biotechnol. 1998;8:325–332. [Google Scholar]
- Hong SK, Matsumoto A, Horinouchi S, Beppu T. Effects of protein-kinase inhibitors on in vitro protein-phosphorylation and cellular-differentiation of Streptomyces-griseus. Mol Gen Genet. 1993;236:347–354. doi: 10.1007/BF00277132. [DOI] [PubMed] [Google Scholar]
- Horinouchi S. AfsR as an integrator of signals that are sensed by multiple serine/threonine kinases in Streptomyces coelicolor A3(2) J Ind Microbiol Biotechnol. 2003;30:462–467. doi: 10.1007/s10295-003-0063-z. [DOI] [PubMed] [Google Scholar]
- Jia B, Jin ZH, Lei YL, Mei LH, Li NH. Improved production of pristinamycin coupled with an adsorbent resin in fermentation by Streptomyces pristinaespiralis. Biotechnol Lett. 2006;28:1811–1815. doi: 10.1007/s10529-006-9157-9. [DOI] [PubMed] [Google Scholar]
- Jin QC, Jin ZH, Xu B, Wang Q, Lei YL, Yao SJ, Cen PL. Genomic variability among high pristinamycin-producing recombinants of Streptomyces pristinaespiralis revealed by amplified fragment length polymorphism. Biotech Lett. 2008;30:1423–1429. doi: 10.1007/s10529-008-9701-x. [DOI] [PubMed] [Google Scholar]
- Jin QC, Yin HL, Hong XW, Jin ZH. Isolation and functional analysis of spy1 responsible for pristinamycin yield in Streptomyces pristinaespiralis. J Microbiol Biotechnol. 2012;22:793–799. doi: 10.4014/jmb.1111.11031. [DOI] [PubMed] [Google Scholar]
- Kieser T, Bibb MJ, Buttner MJ, Chater K, Hopwood DA. Practical Streptomyces genetics. Norwich: Joho Innes Foundation; 2000. [Google Scholar]
- Lee PC, Umeyama T, Horinouchi S. afsS is a target of AfsR, a transcriptional factor with ATPase activity that globally controls secondary metabolism in Streptomyces coelicolor A3(2) Mol Microbiol. 2002;43:1413–1430. doi: 10.1046/j.1365-2958.2002.02840.x. [DOI] [PubMed] [Google Scholar]
- Liu G, Chater KF, Chandra G, Niu GQ, Tan HR. Molecular regulation of antibiotic biosynthesis in Streptomyces. Microbiol Mol Biol Rev. 2013;77:112–143. doi: 10.1128/MMBR.00054-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JF, Liras P. Engineering of regulatory cascades and networks controlling antibiotic biosynthesis in Streptomyces. Curr Opin Microbiol. 2010;13:263–273. doi: 10.1016/j.mib.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Mast Y, Wohlleben W. Streptogramins—two are better than one! Int J Med Microbiol. 2014;304:44–50. doi: 10.1016/j.ijmm.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Mast Y, Weber T, Golz M, Ort-Winklbauer R, Gondran A, Wohlleben W, Schinko E. Characterization of the ‘pristinamycin supercluster’ of Streptomyces pristinaespiralis. Microb Biotechnol. 2011;4:192–206. doi: 10.1111/j.1751-7915.2010.00213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mast Y, Guezguez J, Handel F, Schinko E. A complex signaling cascade governs pristinamycin biosynthesis in Streptomyces pristinaespiralis. Appl Environ Microbiol. 2015;81:6621–6636. doi: 10.1128/AEM.00728-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto A, Hong SK, Ishizuka H, Horinouchi S, Beppu T. Phosphorylation of the Afsr protein involved in secondary metabolism in Streptomyces species by a eukaryotic-type protein-kinase. Gene. 1994;146:47–56. doi: 10.1016/0378-1119(94)90832-X. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Russell DW. Molecular cloning: a laboratory manual. New York: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- Umeyama T, Horinouchi S. Autophosphorylation of a bacterial serine/threonine kinase, AfsK, is inhibited by KbpA, an AfsK-binding protein. J Bacteriol. 2001;183:5506–5512. doi: 10.1128/JB.183.19.5506-5512.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WF, Tian JZ, Li L, Ge M, Zhu H, Zheng GS, Huang H, Ruan LJ, Jiang WH, Lu YH. Identification of two novel regulatory genes involved in pristinamycin biosynthesis and elucidation of the mechanism for AtrA-p-mediated regulation in Streptomyces pristinaespiralis. Appl Microbiol Biotechnol. 2015;99:7151–7164. doi: 10.1007/s00253-015-6638-6. [DOI] [PubMed] [Google Scholar]
- Xu B, Jin Z, Wang H, Jin Q, Jin X, Cen P. Evolution of Streptomyces pristinaespiralis for resistance and production of pristinamycin by genome shuffling. Appl Microbiol Biotechnol. 2008;80:261–267. doi: 10.1007/s00253-008-1540-0. [DOI] [PubMed] [Google Scholar]



