Abstract
This review summarizes advances in photoredox-mediated Giese reactions since 2013, with a focus on the breadth of methods that provide access to crucial carbon-centered radical intermediates that can engage in radical conjugate addition processes.
Keywords: Photoredox catalysis, Photocatalysis, Giese reaction, radical conjugate addition, radical Michael addition
Graphical Abstract

Photomediated Giese reactions are at the forefront of radical chemistry, much like the classical tin-mediated Giese reactions were nearly forty years ago. With the global recognition of organometallic photocatalysts for the mild and tunable generation of carbon-centered radicals, chemists have developed a torrent of strategies to form previously inaccesssible radical intermediates that are capable of engaging in intermolecular conjugate addition reactions. This review celebrates the many recent achievements to radical chemistry made possible by the Giese reaction.
Graphical Abstract

In the past several years, chemists have pushed the frontier of radical chemistry. The Giese reaction has played a significant role in the expansion of radical formation technologies. Owing to the efforts made to understand photocatalysis and visible light-mediated radical generation, chemists are no longer confined to halogenated alkyl precursors. The diversity of both prefunctionalized and non-prefunctionalized radical precursors has grown exponentially since the widespread application of organometallic photocatalysts to synthetic bond-forming reactions. This review highlights the recent use of photomediated-Giese reactions as a means of acknowledging the immense progress that has been made radical chemistry.
1. Introduction
Mainstream adoption of the Giese reaction1 as a strategy to form carbon–carbon bonds led to decades of investigations elucidating the principal factors2 that govern selectivity in radical-mediated processes (e.g. bond dissociation energies, radical polarity, radical propagation; Scheme 1).3 These insights have been used in concert with a modern understanding of mild radical generation technologies4 to develop photoinduced, intermolecular, radical conjugate addition reactions. 5
Scheme 1.
Until recently, most Giese reactions relied on tin hydride, or tin hydride and AIBN. Conditions were tuned to minimize potentially competitive off-pathway reactions.
Photoredox-mediated processes can employ mild reaction conditions, including tunable photocatalysts or photosensitizers to form synthetically versatile radical intermediates. Investigations into the synthetic potential of today’s well-known organometallic photocatalysts remained dormant for nearly 30 years,6 until concurrent publications7 attracted a spotlight to their applications to organic chemistry. Today, synthetic chemists rely on an array of organometallic and organic photocatalysts with tunable reactivity, owing to their varied photophysical and electrochemical properties (Figures 1-2). As a result, chemists can employ photoredox-based strategies to: 1) provide access to previously inaccessible radical intermediates, 2) enable novel bond connections and disconnections, and 3) exploit the practical advantages that arise when these processes rely on abundant and bench-stable reagents. Once again, the Giese reaction is serving as a useful forum for chemists to extend the boundaries of known reactivity and deepen our understanding of radical-mediated processes.
Figure 1.

Structures and electrochemical potentials of Ir/Ru/Rh complexes (in acetonitrile).a See ref 24. b See ref 25. c See ref 26. d See ref 27. e See ref 28. f See ref 29.
Figure 2.

Structures and electrochemical potentials or half potentials of organocatalysts and alternative transition metal photocatalysts (in acetonitrile). a See ref 151. b See ref 30 and 31. In CH3CN:H2O (1:1) c See ref 99. d See ref 32. e See ref 133. f See refs 33 and 134.
1.1. Until recently, most Giese reactions relied on tin hydride, or tin hydride and AIBN; often, photoredox-mediated Giese reactions offer practical advantages over these technologies
Giese reactions involving fine chemicals are formal conjugate addition reactions that rely on radical intermediates (Scheme 1A). Traditionally, in these reactions, a radical precursor is converted to a nucleophilic radical intermediate. This key, high-energy intermediate adds to an electron-deficient π-bond to forge a σ-bond and generate an electrophilic radical intermediate, which is trapped to form a second σ-bond. For decades, such radical mechanisms have offered advantages over organometallic conjugate addition reactions in cases where the corresponding organometallic reagent may be difficult to prepare or unstable, such as reactions involving secondary, tertiary, or heteroatom-stabilized substrates. These advantages are retained when using photoredox catalysts to initiate radical formation.
Until recently, Giese reactions have relied primarily on tin hydride, in combination with light, heat, or a radical initiator, such as azobis(isobutyronitrile) (AIBN) (Scheme 1B). Under these conditions, homolysis of the labile Sn─H bond (BDE = 78 kcal/mol)8 of a tin hydride initiates the reaction. In the productive reaction, tributyltin radical abstracts an atom (X) from the substrate. Unfortunately, off-pathway reactions can be competitive with this X-atom abstraction step (Scheme 1C). In particular, the tin radical itself may add to an electron deficient alkene, resulting in an undesired hydrostannylation reaction. Low alkene concentrations retard both this hydrostannylation reaction and the desired atom abstraction process. To outcompete hydrostannylation, the engaged X-atom needs to be a highly reactive atom donor, and is, therefore, typically an iodide, although bromides, selenides, nitro groups, and xanthate esters have been employed with varying degrees of efficiency.3, 9, 10, 11 This limitation on substrate scope ceases to be directly relevant for photoredox-mediated reactions that do not rely on X-atom abstraction to access a critical carbon-centered radical intermediate.
Once a nucleophilic carbon-centered radical forms, it can add to an electron-deficient alkene with a rate on the order of 106 M−1 s−1. 12 This rate is faster than that of unproductive atom-transfer reactions from sensitive functional groups such as alcohols, amines or addition to carbonyls, which are on the order of 102 M−1 s−1.13 This relative rate of productive versus unproductive reactions confers on these processes a substantial chemoselectivity advantage, proceeding in the presence of functional groups that might otherwise need to be masked by a protecting group. By limiting the need for protecting groups, both tin hydride- and photoredox-mediated reaction conditions streamline syntheses.
Unfortunately, alkyl radicals react more slowly with many potentially desirable unsaturated radical trapping agents than with tin hydride, which reacts with a rate on the order of 106 M−1 s−1 (Scheme 1C).14 To offset the kinetic bias favoring the undesired hydrogen-atom transfer reaction, chemists may use of a slight excess of alkene in the reaction, or may use a hydrogen-atom donor that is less reactive than a tin hydride. Nevertheless, the most common approach relies on an engineering strategy. To slightly elevate the local concentration of alkene relative to tin hydride, tin hydride may be added to the reaction over time by syringe pump.11 Such concentration-dependent kinetically-controlled process may prove challenging to scale-up, as local concentration effects do not scale linearly.
Notably, selectivity in the next step in this chain reaction would be enhanced by the use of reagents with the opposite features. Following radical trapping, the resultant electrophilic radical abstracts a hydrogen atom from tin hydride to form the desired alkyl product and regenerate a tributyltin radical, propagating the chain mechanism. This step would be accelerated with a more reactive hydrogen-atom donor. Optimal reactivity relies on a subtle balance in the relative rates of reactive processes – these are remarkable reactions. Some photoredox-mediated reactions are partially liberated from this balancing act: if the electrophilic radical intermediate is rapidly reduced to an anion in situ, then protonation can engage a strongly bound yet acidic hydrogen atom that is not prone to abstraction (see Section 2.1).
Tin hydride-mediated Giese reactions have driven meaningful research into the scientific underpinnings of radical-mediated reactions and remain synthetically useful. Nevertheless, these reactions present practical opportunities for improvement. The requisite tin hydrides are toxic, and form organotin residues that are neurotoxic, and difficult to separate from the targeted products. This limitation is partially addressed through the use of catalytic quantities of organotin halide in the presence of stoichiometric reducing agents (c.f. LiAlH4, NaBH4).15 Even with lower levels of organotin residues, these approaches remain impractical for process-scale industrial syntheses of medicines or other compounds intended for animal and human use.16 Fortunately, photoredox-mediated radical conjugate addition reactions do not generally furnish organotin byproducts, suggesting that they may be relevant to process-scale approaches.
Additional opportunities for practical improvement upon tin hydride-mediated reactions arise from properties of the most commonly employed radical initiator: AIBN. AIBN is an easily weighed white solid that decomposes into two initiating radicals with efficiencies as high as 80%.17 This efficiency is temperature-dependant, so it can be used in reactions between 60–120°C. In practical terms, AIBN is not ideal as it is potentially explosive, costly, and may be of variable quality, which can impact reaction reproducibility. Nevertheless, AIBN remains a preferred radical initiator because its use allows for a steady supply of initiating radicals under moderate reaction temperatures (70–85°C) .17 This is of particular benefit for slower intermolecular addition reactions, where a steady-state of initiator may minimize byproduct formation. Fortunately, photoredox catalysts can generate a similar advantage, as they can drive formation of key radical intermediates at low concentrations over the course of reactions.
Like the traditional Giese reaction, visible-light mediated photochemical radical conjugate addition reactions can rely on radical chain mechanisms.18 In these cases, photoredox complexes may behave as photosensitizers that do not need to turn over catalytically in order for the reaction to proceed. Some visible-light mediated photochemical reactions may involve both—chain propagation and catalytic—mechanisms.19 In order to characterize a prevalent mechanistic pathway, a combination of luminescence quenching studies20 and quantum yield measurements21 is required. Together, these measurements can be used to confirm the likelihood of, and describe the relative length of radical chain processes.18 Additionally, mechanistic data from quenching and quantum yield experiments can be used to design more efficient photoredox-mediated radical conjugate addition reactions. Absent data to distinguish between these mechanisms, it may be that several processes herein described as relying on "photoredox catalysts" are actually engaging these compounds as photosensitizers.
Fortunately, in either role, photoredox-active compounds have advanced Giese reaction technologies. The demand for safe, environmentally benign technologies with broad substrate tolerance has fuelled the development of photoredox-mediated processes which address many of the limitations of tin hydride-mediated reactions. Photoredox catalysts can furnish radical intermediates by harnessing low-energy visible-light, and are often non-toxic and bench-stable (Figures 1-2). Chemists can tune the photophysical and electrochemical properties of photoredox catalysts.5 Moreover, photoredox-mediated processes are as amenable to picomole-scale reactions for high-throughput screeing22 as they are to kilogram-scale syntheses, often with the use of flow technologies. 23 There remains room for improvement: photoredox-mediated reactions can rely on costly lamps or precious metal catalysts, and often require long reaction times. To surmount these limitations, chemists are developing continuous flow reactors that decrease reaction times by increasing light penetration, metal-free photocatalysts, and reagents that react efficiently with lower intensity light sources, such as compact fluorescent light bulbs.
Of paramount importance to the synthetic chemist, photoredox-mediated strategies have substantively broadened the range of substrates that can be used to access high-energy radical intermediates. Photocatalyst-driven approaches render as viable in intermolecular Giese reactions: (hetero)aryl halide substrates (Section 2), electronically varied benzylic radicals and some enolate radical anions (Sections 2 and 3), organotrifluoroborates (Section 4), and alkyl oxylates, hemioxylate salts, a broader range of carboxylates (Section 7). Furthermore, these reactions can offer previously unavailable selectivity features. For example, photodriven redox processes have resulted in several “firsts”, including effective enantio- or diastereoselecrive formal radical conjugate addition reactions by α-aminoalkyl and benzylic radicals (Section 5), and uses of α-aminyl radicals in reactions to prepare unnatural amino acids (Section 6). Additionally, efficiently accessed iminyl, amidyl, sulfamyl, and alkoxyl radicals can be used to direct cascade sequences that ultimately engage radical conjugate addition processes (Sections 8-11). In many of these cases, these photosensitized processes are the genesis of the documented reactions.
1.2. Scope of this Review
This review highlights photoredox-enabled strategies that have improved access to radical precursors for use in intermolecular Giese reactions, and documents progress in intermolecular photoredox-mediated radical conjugate addition reactions that have been disclosed since the 2013 publication of a comprehensive compendium on visible light-mediated photoredox catalysis by MacMillan and co-workers. 34 For each summarized technology, attention is paid to the method of carbon-centered radical generation, as well as to some synthetic advantages afforded by this method of generation. This review will serve the synthetic chemistry community by identifying mild approaches to carbon-centered radical generation and radical-mediated C─C bond formation.
2. Dehalogenative processes form carbon-centered radicals
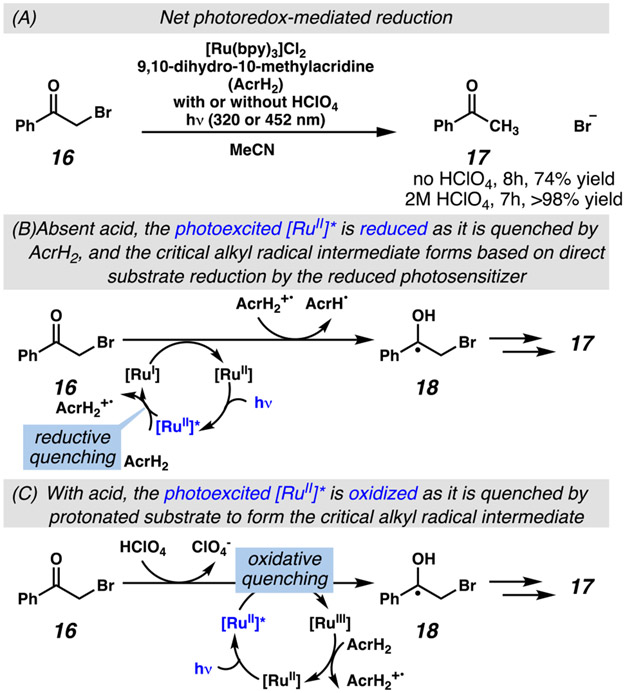
Seminal investigations by Fukuzumi and co-workers in the late 20th century are often cited as critical precedents for modern interest in photoredox-mediated synthetic organic transformations. In these ground-breaking reports, the reductive dehalogenation of phenacyl halides was employed to access carbon-centered radicals, which could be trapped in a net hydrodehalogenation process (Scheme 2A).35 Using this model system, Fukuzumi and co-workers demonstrated the more general point that ruthenium-based photocatalyst 7 could be engaged in either a reductive or oxidative quenching cycle depending on the presence of an acid co-catalyst (Scheme 2B-C). While so doing, these investigations suggested that photocatalysts could mediate more efficient synthetic transformations with fine-tuning of reaction conditions.
Scheme 2.
Key precedent: Fukuzumi and co-workers induce changes in the mechanism of photocatalyst quenching with an acid catalyst
More recently, renewed interest in photoredox-mediated formation of carbon-centered radicals has inspired the development of a broad range of methods for the dehalogenation of aryl and alkyl halides.36 Building on earlier reports of hydrogehalogenation reactions, tactics to install C─C bonds have emerged. Gagné and co-workers have extended photoredox-mediated dehalogenation processes in radical conjugate addition reactions to generate alkylated C-glycosides with exclusive α-selectivity (Scheme 3).37 This initiated a cascade of investigations into photocatalyzed dehalogenative techniques for radical conjugate addition reactions, processes described here with emphasis on their synthetic utility.
Scheme 3.
Photocatalytic reductive dehalogenation reaction enables diastereoselective synthesis of C-glycosides without the use of tributyl tin reagents
2.1. By relying on photocatalytic reductive dehalogenation, alkyl and (hetero)aryl halides become substrates for radical conjugate addition reactions to furnish unnatural amino acids and other fine chemicals
Photosensitized conjugate addition reactions have been developed to provide access to β-heteroaryl acids, including unnatural α-amino acids (Scheme 4).38 These reactions rely on heteroaryl radicals, which are not viable Giese reaction intermediates under tin-mediated conditions because heteroaryl radicals abstract hydrogen from tin hydrides rapidly, and do so in preference to adding intermolecularly to electron-deficient alkenes. Fortunately, heteroaryl radicals can be accessed through photoinduced electron transfer.35b Moreover, when generated through photochemical reduction of heteroaryl halides (Br, I) or esters in DMSO, nucleophilic heteroaryl radicals engage in productive radical conjugate addition reactions (Scheme 4A).
Scheme 4.
Photocatalytic dehalogenative carbon-radical formation streamlines access to heteroarylated unnatural amino acids, and expands radical precurosor scope to include alkyl iodides and alkyl bromides. NHPI = N-hydroxypthalimide
These transformations, along with a wide range of related radical conjugate addition processes, benefit from an already existant and extensive library of enantioenriched electrophilic olefins, 3, 39 designed and optimized to induce diastereoselective reactions. Notably, as the diastereoselectivlity-determining step, in contrast to processes that rely on hydrogen-atom abstraction to install a C─H bond, this Giese reaction relies on a polar mechanism to install the requisite C─H or C─D bond (Scheme 4B). Consequently, the product is almostly completely deuterated when the reaction is run with D2O as a co-solvent, in lieu of H2O. As this protonation process is the diastereodetermining step under these photoredox-mediated conditions, diastereoselectivity appears to be under thermodynamic control. Thereby, when enantioenriched methyleneoxazolidinones40, 41 are used as electrophilic trapping agents, the strategy developed by Jui and co-workers furnishes enantioenriched non-natural heteroaryl or alkyl amino acids (Scheme 4A).42 The products of these addition reactions to either racemic or enantioenriched methyleneoxazolidines can also be hydrolyzed to furnish non-natural amino acids. To the best of our knowledge, this research constitutes the first radical approach to heteroaryl amino acids.
Ultimately, photocatalytic reductive dehalogenation processes expand the scope of viable radical conjugate addition reactions, which can now rely on alkyl or (hetero)aryl halide substrates to furnish fine chemicals on small and large scales.
2.2. Halogen-atom abstraction remains a viable photosensitizer-mediated strategy to engage alkyl halide substrates in Giese reactions, albeit with greater tunability to achieve chemoselective reaction outcomes
As in tin hydride-mediated Giese reactions, in photoredox-mediated processes carbon-centered radicals can be prepared by atom-abstraction from otherwise unactivated alkyl bromides, sometimes based on intermediates generated indirectly based on reductive quenching conditions.43 Cyclic and acyclic alkyl bromides are appropriate precursors for radical conjugate addition reactions that rely on an iridium photosensitizer (Scheme 5).44 Imperative for the success of this transformation, tris(trimethylsilyl)silane serves a dual role as as a silyl radical precursor and as an exogenous terminal reductant. The use of tris(trimethylsilyl)silane as a terminal reductant in Giese reactions is not novel. 45 Tris(trimethylsilyl)silane is a known organotin hydride alternative, with previous applications using AIBN as a radical initiator requiring a modest excess of silane reagent. In this iridium photosensitized Giese reaction, to avoid competitive reduction of key alkyl radical intermediate 25b, tris(trimethylsilyl)silane must be used catalytically or in equimolar amounts. In its complementary role as a critical precursor to key tris(trimethylsilyl)silyl radical, tris(trimethylsilyl)silane selectively engages with the alkyl bromide substrates in halogen atom abstraction (Scheme 5). 43 Given these key mechanistic steps, this reaction seems likely to rely, at least in part, on a photoinduced chain-transfer reaction mechanism that benefits from the presence of a photosensitizer. Using this technology in Giese reactions, streamlines access to a synthetic precursor (i.e. 26) to the FDA approved oncology drug, Vorinostat.
Scheme 5.
Photocatalytic conditions rely on dual roles of (TMS)3SiH to streamline access to Vorinostat precursor.
Based on a complementary light-enhanced reaction, primary, secondary, and tertiary alkyl iodides can engage in productive Giese reactions in the presence of environmentally friendly and inexpensive manganese decacarbonyl photosensitizer (Scheme 6).46 To some extent, reaction limitations can be predicted based on the proposed mechanism for this transformation. This reaction is more efficient when light homolyzes the manganese–manganese bond and provides increased access to the active catalyst, [•Mn(CO)5]. 47 The active catalyst abstracts an iodide to begin the reaction. Consequently, this manganese-mediated reaction does not efficiently engage less readily abstracted halides, including alkyl bromides, alkyl chlorides, and aryl iodides. Fortunately, this limitation enables selective reaction of alkyl iodide in the presence of an aryl iodide (c.f. 27a → 27b). Once the critical carbon centered-radical 27b traps electrophilic olefin, furnishing stabilized electrophilic radical 27c, which is poised to abstract a hydrogen atom from Hantzsch ester to provide product 28. So, like tin-hydride-mediated processes, it is critical to this reaction that the organometallic radical abstract iodide in preference to abstracting a hydrogen atom from Hantzsch ester, the hydrogen-atom source for the reaction. Given the need to balance requisite atom-abstraction processes, these reactions provide complementary selectivity to that available through photocatalyst-mediated reductive dehalogenation reactions.
Scheme 6.
The putative reaction mechanism for a Mn-driven deiodinative Giese reaction parallels that for tin hydride-mediated processes. Consequently, these two non-photocatalysed processes feature similar substrate scopes.
2.3. A complex tertiary benzylic halide engages more effectively under photoredox-mediated conditions than tributyltin hydride conditions
Though tin hydride reagents are known to efficiently generate benzylic radicals—even of complex benzyl substrates48—subsequent radical conjugate addition is a historic challenge. 10, 11, 49, 50 Benzylic radicals appear to have relatively large deformation energies, so they do not readily undergo the pyrimidalization that is necessary to engage in reactions with alkenes.51a More importantly, relative to many alkyl radicals, benzylic radicals are weakly nucleophilic,52 and resonance-stabilized, lowering their energy by about 12 kcal/mol). 52 Likely as a consequence of this resonance stabilization, benzylic radicals react with electrophilic alkenes relatively slowly, with rates on the order of 4 x 102 to 2 x 103 M−1 s−1). 51b So, for tin-hydride mediated reactions relying on benzylic radicals, direct reduction often outcompetes the productive Giese reaction (c.f. Figure 1C). Photoredox-mediated processes indirectly surmount this limitation to the substrate scopes tolerated by traditional radical conjugate addition reactions.
Indeed, photoredox-mediated conditions enable reactivity unavailable under tributyltin hydride/AIBN conditions, as demonstrated in an intermolecular Giese reaction that is a critical step toward total syntheses of biologically active drimentine alkaloids (Scheme 7). 48 In the critical bond-forming step, a nucleophilic cyclotryptophan tertiary radical derived from bromide 29 reacts with sclareolide-derived enone 20c (Scheme 7). This reaction does not furnish product under traditional tributyl tin/AIBN conditions. Furthermore, only modest quantites of product are available when the concentration of tributyl tin in the reaction is lowered by employing syringe pump addition, and the concentration of bromide is increased (entries 1–2). Recognizing the importance of low radical intermediate concentrations, Li and co-workers turned to photoredox-mediated conditions (entry 3). Under the optimal conditions, the desired product forms in 91% yield, using synthetically more precious 20c as the limiting reagent. This example illuminates the potential for improve synthetic outcomes based on photoredox-mediated Giese reaction strategies.
Scheme 7.
Tertiary benzylic radical forms and reacts more effectively under conditions that are photoredox-mediated than tin hydride-mediated.
2.4. Primary benzylic halides undergo formal dehalogenation processes based on initial SN2 displacement and subsequent light-mediated homolysis to furnish benzylic radicals that are poised for conjugate addition
While the above types of alkyl halides have been shown to be appropriate substrates for photoinduced single-electron reductions, primary benzylic halides, mesylates and trifluoroacetates have proven more resistant to direct reaction in photoredox-mediated chemistries.
Fortunately, photoredox-mediated Giese reactions formally overcome this traditional substrate scope limitation, and provide access to primary benzylic radials via photoredox-mediated processes. An indirect dehalogenation strategy53 for radical conjugate addition has emerged that enables the generation of radical intermediates that would be otherwise challenging or impossible to access (Scheme 8). The key carbon-centered radical precursor is formed by an SN2 displacement of benzylic halide 31 or of a benzyl mesylate, trifluoroacetate, or Katritzky salt (not shown). When displaced by nucleophilic thiocarbamate anion 33, xanthate intermediate 34 forms containing a homolytically labile C─S bond (benzyl dimethydithiocarbamate C─S BDE = 31.3 kcal/mol). 54, 55 In the presence of light, this bond is homolyzed. Spectroscopic analyses and radical trapping experiments provide evidence of dithiocarbamate intermediates 34 and 35, respectively. Concurrently generated benzylic radical 36 traps electrophilic olefin 20d, and the formed electrophilic radical (i.e. 37) is poised to abstract a hydrogen atom from diene 38 and to provide product. The viability of this final hydrogen-atom abstraction step is supported by electrochemical half peak potentials (ethyl xanthogenate Ep/2 = +0.04 V vs. SCE in CH3CN; 54, 55 hydroxycylcohexyldienyl radical, Ep/2 = −0.1 V vs. SCE in N2O-saturated H2O). 56 The catalytic generation of the xanthate intermediate enables oxidatively sensitive substrates such as uracyl chloride 42 and adenine derivative 43 to participate in Giese reactions. Furthermore, these photocatalytic conditions are easily adjusted to facilitate radical trapping reactions that engage heteroaryl pyrrole derivatives such as Tolmetin precursor 44. These conditions can be adapted to continuous-flow photoreactors in multigram reactions.
Scheme 8.
Indirect dehalogenation enables photoredox-mediated radical generation from otherwise inert benzyl halides
Clearly, this photo-mediated reductive process expands the scope of viable radical conjugate addition reactions, to include those relying on primary benzylic halide, mesylate, and trifluoroacetate substrates.
3. Photocatalysts can be employed to access enolate radical anions and benzylic radicals based on photoreduction of enones or photooxidation of olefins or arenes
Under traditional tin hydride mediated conditions, allylic and benzylic radicals are challenging to engage in productive radical chain propagation processes. 57 Allylic radicals are characterized by their propensity to terminate radical chains through exclusive homocoupling,57a while benzylic radicals are more prone to competitive reduction by tin hydride reagents (see Section 2). 50 By taking adavantage of the electrochemical properties of these conjugated substrates; photoredox catalysts provide strategies to access formal allylic and benzylic radicals intermediates, and to engage these intermediates in Giese reactions.
3.1. Lewis acid co-catalysts can activate α,β-unsaturated carbonyl compounds to reduction, and the resultant radicals engage in formal Giese reactions
While few investigations had documented the use of metal polypyridal complexes as photocatalysts for synthetic organic transformations, 6 Yoon and co-workers7a discovered that these complexes could convert enone 45 to enolate radical anion 46 (Scheme 9). 7b, 58 Moreover, this process could be used to affect [2+2] cycloaddition reactions. 59 Yoon’s advance relies on the presence of LiBF4, and experimental results suggest that the lithium cation behaves as a Lewis acid co-catalyst to form a complex with the enone substrate. Upon photoreduction of or energy transfer to this complex, an intermediate can be trapped in both intra- and intermolecular [2+2] cycloaddition reactions. 60, 61 The intramolecular reaction relies on a radical chain propagation mechanism, as demonstrated by quantum yield experiments. 62 These transformations rely on olefins to be sufficiently electron deficient to either accept an electron or energy from photoexcited metal polypyridal complex or from another intermediate, so electron-rich olefins do not react under these conditions. For electron-deficient olefins, these pioneering investigations highlight the potential benefits of Lewis Acid co-catalysts to enable the formation and productive reaction of formally allylic radicals.
Scheme 9.
Lewis acid co-catalyst allows for the photochemically-mediated reduction of enones to generate allylic radical anions
3.2. With Lewis acid co-catalysts, enones can serve as precursors to enolate radicals anions that participate in enantioselective radical conjugate addition reactions
Enantioselective Giese reactions have been realized based on Lewis acid activation of enones to enable photocatalytic reduction processes. Specifically, Meggers and co-workers advance a family of chiral Lewis acidic bis-cyclometalated rhodium(III) complexes (c.f. Λ-RhO (8)) that also serve as photocatalysts (Scheme 10). 29, 63 During the reaction, the rhodium catalyst is chelated by an α,β-unsaturated acyl pyrazole (48a → 48b), thereby controlling the stereochemical outcome of the radical conjugate addition process. Upon coordination to the catalyst, complex 48b can be photochemically converted to enolate radical anion 49, which is trapped by allyl sulfone acceptor 20f, and generates a sulfonyl radical as a byproduct. This byproduct is trapped by a second equivalent of coordinated α,β-unsaturated pyrazole 48b in a parallel enantioselective sulfonylation reaction. These interdependent reactions rely on the use of two equivalents of unsaturated pyrazole 48a for every equivalent of allyl sulfone acceptor, and affect two complementary transformations.
Scheme 10.
Dual-acting chiral rhodium complexes serve as chiral template and photocatalyst for the asymmetric conjugate addition of enolate radicals
The efficiency of the enantioselective Giese reaction is not affected by the presence of additives 53, 54, and 55, which contain Lewis basic functionality that could unproductively coordinate the Lewis acidic photocatalyst. The diverse functional groups tolerated by the process suggest the possibility of applying this bond-forming reaction to the synthesis of complex small molecule targets. Nevertheless, there are limitations. A limited range of enones is viable in this reaction. For example, α,β-unsaturated β-aryl N-acyl pyrazoles do not engage productively in this reaction, and α,β-unsaturated N-acyl pyrazoles with shorter γ-alkyl substituents react with higher efficiencies than their longer-chain analogues. Even with these limitations, this method constitutes a creative approach and a rare example of enantioselective Giese reactions.
3.3. Direct oxidation of olefins and aromatic rings generates carbon-centered radical cations
Shortly after Yoon and co-workers shined light on the ability of photocatalysts to reduce enones to radical anions, they lay the conceptual foundations for another substantive advance in Giese technologies. In the foundational publication, Yoon and co-workers demonstrated that metal polypyridal complexes could serve as photooxidants to initiate conversion of styrenes to radical cation intermediates for use in [2+2] and [4+2] cycloaddition reactions (Scheme 11).64 Due to the limited oxidizing power of the metal polypyridal photocatalysts, these radical cation intermediates could only be prepared from more electron-rich styrene derivatives. Fortunately, a range of electronically varied alkenes reacts productively with the photogenerated radical cations. This discovery is at the heart of an oxidative strategy to surmount limitations tin hydride-based approaches to engage benzylic radicals in productive Giese reactions.
Scheme 11.

Key Precedent: Electron-rich alkenes can generate aryl radical cations in the presence of chemical oxidant additives
This photosensitizer-driven strategy to access benzylic radical cations is of broad utility in radical conjugate addition reactions. Coupled with Yoon’s insight, the discovery65 and application66, 67, 68, 69 of highly oxidizing acridinium organocatalyst 10 renders a broader range of alkenes and arenes70 viable precursors to radical cations71, 72, 73 (Scheme 12). Enabled by this class of photocatalyst, the Wu group demonstrates that allylic and benzylic C─H bonds can serve as precursors for the critical carbon-centered radical intermediates for Giese reactions. In the developed manifold, initial oxidation of the olefin or aromatic core of alkyl benzene derivatives renders a pendant allylic or benzylic C─H bond more acidic. This acidification is sufficient to enable facile deprotonation to generate the desired radical intermediate. In their initial report, Wu and co-workers used this strategy to enable the hydroalkylation of methylene-malonitriles with a broad range of alkene and arene substrates. When less activated Michael acceptors are employed, reactions proceed with diminished efficiency. Fortunately, the synthetic versatility of the malonitrile functional handle can be exploited for diverse product derivatization to generate alkyl amides, carboxylic acids and esters, as well as substituted pyrazoles, oxazoles, and piperazines.
Scheme 12.
Acridinium photocatalyst 10 enables the direct generation of allylic and benzylic radical cations for alkylation
In a subsequent report, 74 Wu, Chen and co-workers extend the range of effective Michael acceptor substrates (Scheme 12B). Accordingly, addition of Cu(OTf)2 enables efficient reaction between toluene derivatives and a range of aryl and alkyl enone substrates with diverse substitution at the β-position. For Michael acceptors bearing β-aryl substituents, the products of the hydroalkylation process can be converted into naphthalene derivatives by subsequent heating under air in an overall one-pot reaction sequence. The authors propose that the Cu(OTf)2 additive acts as a Lewis acid to activate the Michael acceptors, and support this proposal with DFT calculations.
Together, photocatalyst-driven approaches render electronically varied benzylic radicals and some enolate radical anions as viable intermediates in intermolecular Giese reactions. These radicals are accessed via reduction of benzylic halides, or their analogues (Section 2), or by arene oxidation, with some reactions proposed to be promoted by Lewis acidic additives that coordinate with radical trapping agents (Section 3). Thereby, these photocatalyst-mediated reactions overcome a historic limitation of radical conjugate addition technologies, processes for which benzylic and allylic radicals had not proven to be generally viable intermediates in intermolecular reactions.
4. Oxidation of organotrifluorborates leads to formation of carbon-centered radicals
Like olefins and aromatic rings, organotrifluoroborates offer a number of practical benefits as radical precursors. They are stable to air and moisture, relatively simple to prepare and can coexist in a molecule with a diversity of functional groups. As such, organotrifluoroborates have been developed as functional handles to install otherwise challenging, high-value functional groups in small molecule targets. 75 While initially popularized as substrates in transition metal-catalyzed cross-coupling reactions, organotrifluoroborates are known precursors of carbon-centered radicals. 76, 77, 78, 79 So, it may be unsurprising that photocatalytic conditions80 have been developed to convert organotrifluoroborates into carbon-centered radical intermediates for use in Giese reactions.
4.1. Organotrifluoroborates are substrates for radical conjugate addition reactions
Organotrifluoroborates can also be employed as precursors to α-aminoalkyl radicals (Scheme 13A). 81 Under photoredox-mediated conditions N-Boc protected aminomethyltrofluoroborates engage in facile single electron oxidation, 82 to generate an α-amino radical intermediate. This radical adds to a Michael acceptor or a styrene. Cleavage of the tert-butoxycarbonyl group affords access to primary alkyl amines. This method allows for a 2-step synthesis of baclofen•HCl salt (64a), a γ-aminobutric acid (GABA) analogue that is used as a muscle relaxer and an antispastic agent. These transformations highlight the utility of organotrifluoroborates as precursors to α-amino alkyl radicals.
Scheme 13.
Oxidation of aminotrifluoroborates offers a strategy for the installation of primary amines and sets the stage for the non-metal photoredox-mediated oxidation of a wider scope of organotrifluoroborates
Building on this research, Akita, Koike and co-workers83 demonstrate that a more strongly oxidizing photocatalyst can broaden the substrate tolerance of this reaction to include a range of primary, secondary and tertiary organotrifluoroborates (Scheme 13B). Notably, a pendant alkyl bromide can be carried through the reaction, evidencing chemoselectivity that would seem unlikely under traditional Giese reacton conditions. Here, viable substrates include potassium phenethyltrifuoroborate, which has a high electrochemical half potential outside the range of the previously employed organometallic photocatalyst. Despite the greater oxidative power of the organocatalyst, the new reaction conditions remain suitable for application to more oxidatively labile aminomethyltrofluoroborates.
4.2. Organotrifluoroborate salts engage in asymmetric Giese reactions
Radicals rapidly invert, so few strategies exist to affect light-mediated catalytic asymmetric conjugate addition reactions. Nevertheless, as early as 1995, chiral Lewis acid catalysts had been used to induce enantioselectivity in a tin-mediated radical conjugate addition reaction. 84 Bench-stable organotrifluoroborates offer a welcome alternative to the best-in-class tin-mediated85 asymmetric radical conjugate addition reaction for the installation of alkyl groups (Scheme 14). Under photoredox-mediated conditions, the approach of an in situ-generated radical can be dictated by a chiral rhodium-based Lewis acid catalyst. 86 This photosensitizer activates the Michael acceptor and determines the facial approach of the radical intermediate. Both alkyl and benzyl trifluoroborate-derived carbon-centered radicals react with excellent enantioselectivity to generate alkylated α,β-unsaturated N-acylpyrazoles and imidazoles.
Scheme 14.
Organotrifluoroborates expand the scope of enantioselective Giese reactions
5. Oxidation of α-silyl amines and organosilanes generate carbon-centered radicals
Primary, 87b secondary87 and tertiary88 amines have served as precursors for nucleophilic α-aminoalkyl radical intermediates that engage in Giese reactions (i.e., 68b + 20 → 68c, Scheme 15). Typically, the intermediates generated from these conjugate addition reactions cyclize in situ in the presence of base or oxidant, respectively, to form higher-value γ-lactam and pyrrole N-heterocycles 70–71.
Scheme 15.
Traditionally, amines have served as aminyl radical precursors in conjugate addition/cyclization cascade sequences.
Traditionally, to access the critical aminyl radical intermediates, these reactions have relied on photosensitizers excited by Ultraviolet irradiation. These conditions are not general, and prove ineffective with substrates that contain Ultraviolet-sensitive functional groups. Often, these processes are inefficient or plagued by byproduct formation, presumably owing to non-selective radical formation. With the advent of methods that rely on visible light-based excitation of photosensitizers, 89, 90 less specialized equipment is needed to run these related, some Ultraviolet-sensitive functional groups can be included in reactive substrates, and α-aminyl radical intermediates can be generated more selectively. Consequently, with visible-light photocatalysts, a broader range of nucleophilic α-aminyl radical intermediates can form and engage in radical conjugate addition reactions with synthetically useful levels of efficiency.
5.1. Oxidation activates α-silyl amines so they can serve as precursors to secondary and primary α-amino alkyl radicals that participate in conjugate addition reactions
In 2012, Nishibayashi and co-workers recognized that, under photochemical conditions, Giese reactions could rely on tertiary α-silylamines as precursors to α-aminyl radical intermediates.90a Relative to amines, α-silylamines have slightly lower oxidation potentials (~0.4–0.8 V vs SCE in MeCN). 91α-Silylamines have long been exploited as radical precursors as a result of their relatively low oxidation potentials and their propensity to undergo selective desilylation to generate neutral carbon-centered radical intermediates. 92 These characteristics make α-silylamines exceptional candidates for selective Giese reactions. More recently, this approach has proven applicable secondary amines, and been extended to afford cyclization products (Scheme 16). 93 Overall, the use of secondary and tertiary α-silylamines as precursors to α-aminyl radical intermediates has proven more broadly viable than prior approaches, presumably owing to the relatively low oxidation potentials of α-silylamines.
Scheme 16.
Conjugate addition of α-silylaminyl radicals provide access to high value N-heterocycles
5.2. α-Silyl amines and organosilanes are precursors to radicals that engage in enantioselective Giese reactions
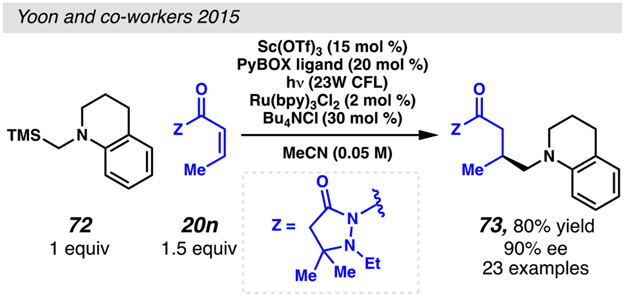
The first report of an asymmetric conjugate addition reaction involving an α-aminyl radical relies on a chiral hydrogen-bonding photosensitizer to catalyze intramolecular conjugate addition reactions.94 More recently, catalytic quantities of photoredox complexes have been used in concert with chiral Lewis acids to achieve intermolecular reactions. In these transformations, the chiral Lewis Acid catalyst controls the facial selectivity of α-aminyl radical addition reactions to pyrazilidinone-based Michael acceptors (Scheme 17).95 These Michael acceptors96 coordinate Sc(III)-PyBOX complexes, activating them to radical conjugate addition reactions. The radical components for these reactions can be generated from a variety of tertiary α-silylamine radical precursors to enable conjugate addition reactions with high enantioselectivity.
Scheme 17.
α-silylamines engage in enantioselective photocatalyzed Giese reactions with the aid of Lewis acid co-catalysts
In a complementary approach, in the presence of a catalytic photosensitizer, superstoichiometric quantites of chiral hydrogen-bond donor can induce enantioselective conjugate addition reactions involving α-aminyl radical intermediates (Scheme 18). This research builds on decades of experiments that show that intramolecular hydrogen bonding can be used to induce facially selective hydrogen-atom abstraction as the diastereoselectivity-determining step of Giese reactions (Scheme 18A). 3, 97 More recently, Bach and co-workers have invented chiral hydrogen-bonding scaffolds that can be used in conjunction with a ruthenium photosensitizer to impart stereocontrol of α-aminyl radical additions to rigid Michael acceptors intra-94 and intermolecularly98 (Scheme 18B). A model to explain the facial selectivity of the radial addition reaction relies on hydrogen-bonding interactions between the chiral template and a 3-alkylidene indolin-2-one substrate, which is postulated to form a 1:1 complex under the reaction conditions, and thereby induce enantioselectivity.
Scheme 18.
Hydrogen-bonding strategies can induce enantioselective Giese reactions, even those involving of α-silylamine radicals
Other asymmetry-inducing reactions also rely on organosilane substrates (Scheme 19). Like α-silylamines, other organosilanes are labile to oxidative conditions and readily generate silane radical cations. These silyl radical cations can undergo C─Si bond cleavage to furnish carbon-centered radicals. This phenomenon is particularly well documented for benzyltrialkyl silanes where oxidation forms a neutral benzyl radical and a trialkylsilyl cation. While resonance stabilized benzyl radicals do not typically engage in conjugate addition reactions, recent advances in photoredox-chemistry have enabled these otherwise challenging bond-forming reactions.
Scheme 19.
Photoxidation of α-organosilanes can generate carbon-centered radicals that participate in enantioselective Giese reactions in the presence of a chiral organocatalyst
A photodriven organocatalytic strategy enables α,β-unsaturated aldehydes to act as formal acceptors in enantioselective conjugate addition reactions (Scheme 19). This strategy relies on condensation of an achromatic enal substrate (i.e. 20q) with an organocatalyst that incorporates a chiral secondary amine (12). Generated iminium ion 78 is photoexcitable. Ultimately, the key bond-forming event involves a formal net conjugate addition reaction (i.e. enaminyl radical 82 + benzylic radical 83).
To realize this vision, Melchiorre and co-workers99 developed a bulky, highly oxidizing and chiral perfluoroisopropyl substituted diarylprolinol catalyst (i.e. 12) capable of condensing onto α,β-unsaturated aldehydes. Not only does this strategy furnish synthetically useful β-benzylated aldehyde building blocks with excellent enantioselectivity, this method also employs benzyl radicals as effective trapping agents for C─C bond formation. 100 Indeed, photodriven redox processes have resulted in the first effective enantio- or diastereoselecrive formal radical conjugate addition reactions by α-aminoalkyl and benzylic radicals.
6. Amine oxidation can lead to formation of carbon-centered radicals
Amines have a rich history as precursors to versatile radical cation intermediates. 101, 102 Aminium radical cation intermediates are employed in a number of chemical transformations; including hydrogen-atom transfer processes (i.e. Hofmann-Loffler-Freytag reactions) 103 and amination reactions that proceed by additions across π-systems, including olefins104 and aromatic groups. 105 Additionally, aminium radical cations can be deprotonated to generate neutral α-aminyl carbon-centered radical intermediates, which can react to form new carbon-carbon bonds (for a discussion of traditional methods for their generation, see Section 5). 106 Following the development of a visible light-mediated photoredox-catalyzed method to generate α-aminyl radicals for conjugate addition reactions, 90a,b a number of complementary reactions were introduced that harness the reactivity of the amine radical cation intermediates. 91 Collectively, these investigations broaden the variety of amine substrates that are known to participate in single electron transfer (SET) processes and streamline synthesis of biologically relevant nitrogen-containing motifs.
6.1. α-Aminyl radicals can form based on initial oxidation of tertiary amines
As an alternative strategy for site-selective peptide conjugation to generate unnatural amino acid derivatives, Jui and co-workers107 reacted tertiary amines with readily accessible dehydroalanine derivatives (Scheme 20). Giese reactions have been used to prepare unnatural amino acids, employing a tin hydride approach for particularly reactive substrates, 108 or with alkylmercuryhalides109 as radical precursors. Photoredox-mediated reactions offer the advantage of avoiding organotin and alkylmercuryhalides as radical precursors, which can limit the biological application of these products. Additionally, to the best of our knowledge, photoredox catalysts mediate the first uses of α-aminyl radicals in reactions to prepare unnatural amino acids. The ability to react α-aminyl radicals easily introduces amine functional groups to complex molecules. Conjugate addition of radicals derived from complex tertiary amines, such as bioactive dextromethorphan 87a with enantioenriched methyleneoxazolidinones40, 41 affords unnatural amino acid derivatives as single diastereomers. This method enables the installation of new carbon–carbon bonds to dehydroalanine-containing tripeptide 87b. Even in the presence of a number of sensitive functional groups, conjugate addition occurs with high chemoselectivity in synthetically useful yields.
Scheme 20.
α-aminyl radicals react with dehydroalanine derivatives to generate unnatural amino acid derivatives
Extending the relevance of this approach to carbonylated amine derivatives, Nicewicz and co-workers employ a highly oxidizing acridinium-derived organophotocatalyst 10 (Scheme 21). 110 Diastereoselective examples of this transformation are rationalized based on a Fürst-Plattner-like transition state. 111 With the ability to induce otherwise unactivated amines, such as carbonylated 88, to participate in Giese reactions, subsequent decarbonylation can provide rapid access to bio-relevant small molecules. For example, pheromone (+)-monomorine I (91) can be prepared in three steps and 51% overall yield from 88. 112 Moreover, the method is readily scalable and can be adapted to photochemical flow reactor systems for large-scale preparation of alkylated amines.
Scheme 21.
Giese reactions engaging tert-butylcarbamoylated compounds as radical precursors provide simplified syntheses of alkylated amines
With analogous sequences in which conjugate addition is followed by cleavage of an electron-deficient nitrogen substituent, it is possible to access the formal products of conjugate addition by primary amines (Scheme 22).113 Nitrogen-centered radicals derived from typical primary amines engage in intramolecular 1,5-HAT processes. By contrast, if a primary amine is masked with a strongly electron-withdrawing N-trifluoromethanesulfonyl group, an α-aminyl radical is instead accessed and can engage in a radical conjugate addition reaction. Based on this realization, a variety of alkylated primary amine-derivatives have been prepared, a subset of which contain oxidatively sensitive heteroaromatic groups (i.e. 93a).
Scheme 22.
Photomediated α-C(sp3)─H alkylation of electron deficient primary amine derivatives
7. Decarboxylation generates carbon-centered radicals
For more than a third of a century, N-(acyloxy)-phthalimides114, oxalates115 and carboxylic acids116 have been used as carbon-centered radical precursors in radical conjugate addition reactions (Scheme 23). More recently, the utility of N-(acyloxy)-phthalimides has been highlighted as photoredox-mediated decarboxylative Giese reactions have streamlined syntheses of complex natural products. 117 Furthermore, with modern photoredox technologies, decarboxylation reactions proceed under mild conditions, so these reactions can engage a broader variety of stable and user-friendly substrates, including alkyl oxylates and carboxylates. Modern approaches have used visible light to promote decarboxylative Giese reactions involving arylacetic acids, 118 amino acids, 119 and can include aliphatic carboxylic acid derivatives. 120 These tactics accelerate stereoselective syntheses of biologically relevant small molecules.
Scheme 23.
Key precedent: N-(acyloxy)-phthalimides (A), oxalates (B), and carboxylic acids (C) are used as radical precursors for conjugate addition reactions for dating back over 25 years
7.1. Carbon-centered radicals generated by reduction of N-(acyloxy)-phthalimides and tert-alkyl N-phthalimidoyl oxalates react with diastereoselectivity that complements the selectivity available through polar conjugate addition reactions
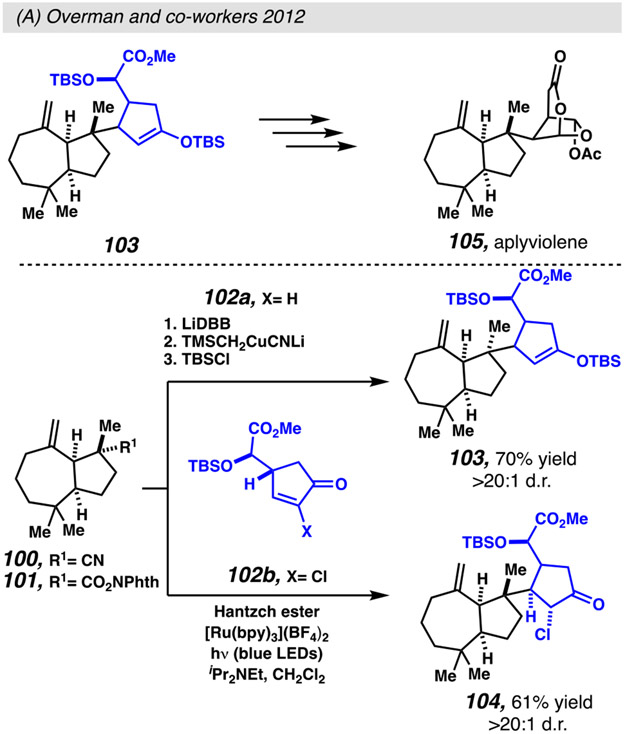
Interest in the application of N-(acyloxy)-phthalamides as radical precursors resurfaced during an attempt to streamline the synthesis of diterpene target, (−)-aplyviolene 105 (Scheme 24).117a Initial attempts to obtain desired intermediate 104 via conjugate addition based on organolithium and organocuprate intermediates resulted in exclusive production of undesired epimer 103. As an alternative, Overman and co-workers turned to radical chemistry. Fortunately, using modified Okada conditions, 114 a radical conjugate addition reaction offers access to desired product epimer 104, detected as a single diastereomer. This is among the first demonstrations of the stereoselective formation of quaternary carbon centers via photoredox catalysis.
Scheme 24.
Radical addition resulting from the decarboxylation of N-(acyloxy)-phthalamides offers complimentary stereoselectivity to traditional organocuprate-mediated conjugate addition reaction for the formation of quaternary carbon centers
Moreover, the diastereoselectivity afforded by radical conjugate addition technologies continues to offer broad benefit within synthetic campaigns. Recently, N-(acyloxy)-phthalimides have been developed as radical precursors in an approach to the syntheses of trans-clerodane diterpenoids (Scheme 25).117b In this transformation, the radical trapping agent reacts from the less-hindered equatorial face of the decalin system to give the penultimate intermediate en route to (−)-solidagolactone.
Scheme 25.
N-(acyloxy)-phthalamide radical precursors streamline the synthesis of trans-clerodane diterprenoids
To expand the selection of methods for constructing quaternary centers from tertiary center carbon-radicals, Overman and co-workers121 developed N-phthalimidoyl oxalate reagents that are capable of undergoing reductive decarboxylation to generate reactive tertiary carbon-centered radical intermediates (Scheme 26). Easily prepared and isolable N-phthalimidoyl oxalate reagents 109, derived from abundant tertiary alcohol starting materials, constitute an improvement over Barton and co-worker’s mixed oxalate esters with N-hyroxypyridine-2-thione (Scheme 23B-C) in terms of stability and performance. Many tertiary carbon-centered radical intermediates can be generated from the decarboxylative reduction of N-phthalimidoyl oxalates. When intercepted, the Giese reaction tolerates cyclic and acyclic Michael acceptors.
Scheme 26.
N-phthalimidoyl oxalates are readily prepared, user-friendly radical precursors for the generation of quaternary carbon-centers
Similar synthetic operations can be carried out using the abundant and cheap dye, Eosin Y, as a photocatalyst (Scheme 27). 122 To highlight the sustainability of this approach, the authors evaluate a photoredox-mediated decarboxylation of N-(acyloxy)phthalimides derived only from natural carboxylic acids that can be produced from renewable biomass. Among these substrates N-Boc protected amino acids (111) and saturated and unsaturated fatty acids reacted in modest to good yields, and reactions with N-Boc-protected proline tolerated a range of alkyl and aryl Michael acceptors. These conditions rely on the use of higher concentrations of acceptor.
Scheme 27.
Metal-free decarboxylation of biomass-derived N-(acyloxy)phthalimides offers an environmentally sustainable method for derivitizing amino acid among other sustainably produced carboxylic acids.
7.2. Reduction of N-(acyloxy)-phthalimides generates carbon-centered radicals that can be employed in the enantioselective syntheses of γ-aminobutyric acid derivatives
Following the above examples of N-(acyloxy)phthalimides as substrates for diastereoselective Giese reactions, Meggers and co-workers disclosed that these molecules can engage similarly in enantioselective reactions (Scheme 28). 123 The addition of N-(acyloxy)-phthalimide-derived α-aminoalkyl radicals can occur efficiently with excellent enantioselectivity as a result of the incorporation of a chiral Rh-based Lewis acid catalyst. In these reactions, the Rh-based catalyst serves to control the available face of an α,β-unsaturated N-acylpyrazole Michael acceptor. This transformation affords access to α-aminobutyric acid (GABA) analogues, such as drug precursors 114a–b.
Scheme 28.
Application of Rh-based chiral Lewis acid enables enantioselective Giese reaction using N-(acyloxy)-phthalamide radical precursors
7.3. Carbon-centered radicals generated from N-(acyloxy)-phthalimides and carboxylic acids react diastereoselectively to generate enantiopure β-thiolated/selenolated amino acids
N-(Acyloxy)-phthalimides 115a and 115b, carboxylic acids 115c, and alkyl iodides (not depicted) serve as percursors to primary, secondary, and tertiatry alkyl radicals 124 generated via photochemically-driven reduction or decarboxylation. When this reaction proceeds in the presence of enantioenriched β-thiolated/selenolated amino acid derivatives, 39 diastereoselective Giese reactions proceed. To showcase the synthetic utility of the photoredox-mediated asymmetric Giese method, thusly prepared enantioenriched unnatural amino acids have been incorporated into polypeptides using a one-pot ligation and dechalocogenation strategy. This approach improves access to nonnatural amino acids.
7.4. Oxidative decarboxylation can furnish secondary, tertiary, and benzylic radicals for addition across electron-deficient olefins and vinyl arenes
With photoredox catalysts, carboxylic acids are appropriate precursors to carbon-centered radicals at secondary, tertiary centers, as well as primary centers that are α-oxygenated, or adjacent to nitrogen atoms. 120, 125 These radicals can be generated in transformations that rely on strongly oxidizing iridium photocatalyst 1, and a mild inorganic base (Scheme 30A). To demonstrate its synthetic utility, this method has been used in the key C─C bond forming step in a 3-step total synthesis of the commercial anticonvulsant drug (±)-Lyrica (pregabalin). Synthetically, use of carboxylic acids as substrates may offer benefits over the use of N-(acyloxy)phthalimides as these processes do not require the use of an external reductant and obviate the need for installation of the N-hydroxyphthalimide activating group.
Scheme 30.
Iridium photocatalyst enables oxidative decarboxylation to generate alky, α-oxy, and α-aminoalkyl radicals for conjugate addition
Extending this technology, 1,3-dithiane126 2-carboxylic acids (E1/2 ~ +0.39 V versus SCE in MeCN) have been developed as a class of precursors in decarboxylative radical reactions (Scheme 30B). Notably, this reaction can also serve as a strategy for the formal addition of a methyl group to an α,β-unsaturated compound when this photo-driven decarboxylative Giese reaction is paired with desulfurization with raney-nickel. Similar to its function in classical synthetic strategies, the 1,3-dithiane radical precursor is particularly useful for the installation of aldehydes, ketones, and carboxylic acids through dithiane cleavage. As a complementary method for the introduction of protected aldehydes via radical conjugate addition, Xu and co-workers127 demonstrate that glyoxylic acid acetals (E1/2 = +0.95 V versus SCE in CH3CN) 128 also serve as effective carbon-centered radical precursors under reductively quenching catalytic cycles. Notably, this reaction can affect Giese addition, or radical addition reactions to vinyl arenes, preferentially engaging in addition across electron-deficient olefins (Scheme 30C).
Minor adjustments to the more sustainable, organocatalyst-mediated decarboxylative conditions, 83 originally limited to tri- and disubstituted alkyl carboxylic acids, made a major impact to the reaction efficiency and substrate scope. Suspecting an unfavorable thermodynamic parameters for the final hydrogen-atom abstraction step of the proposed catalytic cycle (not shown), Gonzalez-Gomez and co-workers129 modified the reaction conditions to favor a fast protonation, by introducting H2O as a solvent in the reaction (Scheme 30D). Deuterium studies confirmed water-mediated protonation over hydrogen abstraction. Under the amended conditions, the organocatalyst-mediated decarboxylative Giese reaction could now transform a variety of α-heteroatom containing substrates including α-keto acids as well as monosubstuted carboxylic acids in good to excellent yield. Moreover, this Giese reaction could be applied to diastereoselective cyclizations of lactone via a tandem decarboxylation-lacontization sequence.
Fang, Jin, Li and co-workers have disclosed photo-driven decarboxylative radical conjugate addition reactions to vinyl phosphonates as a means of installing α-aryl phosphonate moieties in organic compounds (Scheme 30E). 130 Remarkably, vinyl phosphonates are viable traps for benzylic radicals, which do not typically react productively in Giese reactions. 10, 11, 131
Vintage organocatalyst tetrapropylpyrimidopteridine N-oxide (PrPPTNO, 14a, 132 E1/2 = 2.29 V versus SCE in MeCN) and its deoxygenated analogue tetrapropylpyrimidopteridine (PrPPT, 14b, E1/2 = 2.08 V versus SCE in MeCN) are productive in visible-light photoinitiated Giese reactions (Scheme 30F).133 Both species have very high reduction potentials, rivalling that of the current best-in-class organocatalyst [Acr-Mes]ClO4. Unlike the chemoselectivity observed in classic Giese reactions, electron-dense functional groups like alcohols and amines may competitively quench the catalyst and hamper reaction initiation. Interestingly, PrPPTNO-mediated Giese reactions are tolerant of substrates containing free alcohols, as well as oxidizable aromatic groups. Giese transformations of benzylic carboxylic acids and phenylcarbamic acid do not generate synthetically useful yields of product, highlighting an opportunity for future technological improvements.
While few examples of Giese reactions rely on benzylic radicals, Ravelli and co-workers have identified conditions to affect formal conjugate addition reactions that engage benzylic radicals. In this case, benzylic radicals form through oxidative decarboxylation of arylacetic acid substrates (E1/2 = 1–1.6 V versus SCE in MeCN) (Scheme 31). 134
Scheme 31.
Oxidative decarboxylation generates benzylic carbon-centered radicals for conjugate addition
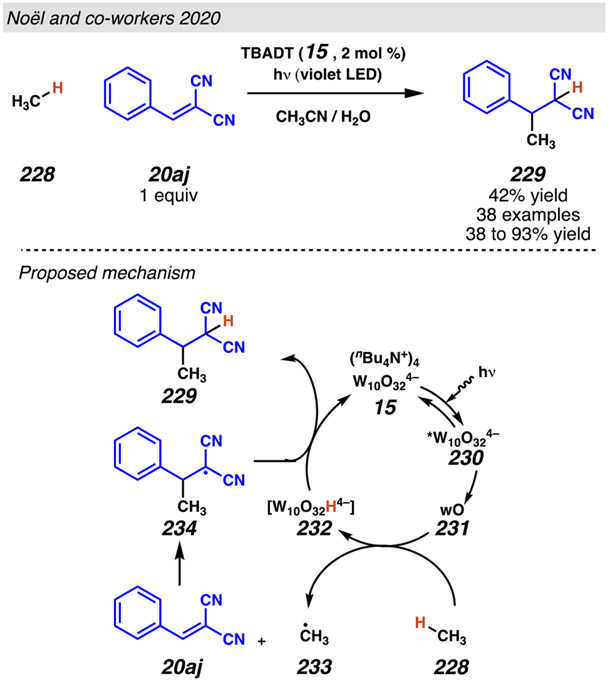
These benzylic radicals engage in formal conjugate addition reactions with extremely electron-deficient olefins. As in other formal Giese reactions involving benzylic radicals (see Section 2), it has been proposed that these reactions are feasible because the photocatalyst reduces the electron deficient olefin to a radical anion that can rapidly trap the decarboxylatively generated benzylic radical. 99, 100a To affect this transformation, this method takes advantage of a relatively cheap tetrabutylammonium decatungstate (TBADT; (nBu4N)4[W10O32]) photocatalyst (15) in combination with readily oxidizable biphenyl additive. By making secondary, tertiary, and benzylic carboxylic acids directly useful as radical precursors, photoredox-mediated processes broaden access to readily available radical precursors.
7.5. Hemioxylate salts are appropriate precursors to carbon-centered radicals that can engage in Giese reactions
Methods based on carboxylic acid decarboxylation have gained importance in part due to the broad accessibility of carboxylic acid substrates. Alcohols are a complementary functional group that offer the advantages arising from ubiquity – when used directly, or as anchors for other functional groups. To leverage alcohols as precursors to carbon-centered radicals, hemioxalate salts have been developed. These salts are bench stable and readily oxidizable (E1/2 = +1.28 V vs SCE in MeCN) (Scheme 32). 135 The synthetic power of this method is evident in efficient racemic135 and enantioselective136 total syntheses of trans-clerodane (136, Scheme 32A-B).
Scheme 32.
Oxalate radical precursors engage in selective 1,6-conjugate addition reactions
These conditions are sufficiently mild to enable selective 1,6-conjugate addition reactions to proceed (Scheme 32C). 137 The exclusive formation of 1,6-addition products constitutes a significant achievement in selectivity over traditional tin-mediated Giese conditions, which are known to give both 1,4- and 1,6-addition product, among other byproducts. 138
7.6. Oxidation of zinc sulfinates generates carbon-centered radicals
Sulfinates are easily accessible and commercially available carbon-centered radical precursors, 139 which have been employed photoredox-mediated carbon-carbon bond-forming reactions. 140 More recently, a variety of substituted benzylic and alkyl zinc sulfinates (E1/2 ≈ +0.9 V versus SCE in MeCN) have been used as radical precursors in Giese reactions (Scheme 33).141
Scheme 33.
Zinc sulfinates generate carbon-centered radicals under reductive quenching conditions
8. Iminyl radicals are appropriate precursors to remote carbon-centered radicals
Iminyl radicals are important intermediates. 142 Recently, chemists have identified precursors to these radicals that are reactive under mild photo-driven conditions, and have developed these precursors in the context of Giese reactions.
8.1. Redox-active oximes are appropriate precursors to iminyl radicals that can trigger a cascade sequence involving a serial cyclization and Giese reaction
Recently, Jiang and Studer, 143 and Leonori and co-workers144 concurrently identified mild conditions that rely on a photoredox catalyst and base to convert α-imino-oxy acids into iminyl radicals (Schemes 34, 35). This strategy builds on foundational research from Forrester and co-workers who identifed α-imino-oxy acids as precursors to iminyl radicals, albeit under relatively harsh conditions. 145-148 Moreover, the recent innovations recapitulate a formal reaction cascade first demonstrated by Boivin, Fouquet, and Zard. 149 Specifically, both the recent and prior cascades use α-imino-oxy acids as substrates, with more recent research focusing on the more accessible and bench-stable oximes as radical precursors (c.f. Scheme 34A, 141). Once the parent oxime is converted to iminyl radical intermediate 142, the generated iminyl radical participates in a cascade sequence, trapping an olefin intramolecularly in a 5-exo-trig cyclization reaction to form pyrroline 143. This sequence results in an intermediate which incorporates a pendant carbon-centered radical, which subsequently participates in an intermolecular Giese reaction to form products 144. Under the conditions developed by Leonori and co-workers, the imino-Giese reaction can proceed with modest diastereoselectivity to generate a bicyclic system (i.e. 144a). Moreover, this approach tolerates a range of electrophilic radical trapping agents, including diethyl (E)-diazene-1,2-dicarboxylate, to furnish products such as pyridine-containing 144c and theovinone-derived 144d. 150 Overall, this cascade sequence developed by Leonori and co-workers installs the new carbon–nitrogen bond of the pyrroline, and a carbon–carbon bond or carbon–nitrogen bond selectively.
Scheme 34.
Hydroxyacid-derived oximes engage in oxidative decarboxylation to generate iminyl radicals to initiate serial cyclization /Giese reactions
Scheme 35.
Hydroxyacid-derived oximes engage in oxidative decarboxylation to generate iminyl radicals to initiate serial cyclization /Giese reactions
Leonori and co-workers' identify an optimal iminyl radical precursor by strategically analyzing the electrochemical half potentials for a series of carboxylates (Scheme 34B).144 The simpler glycolate analogue 145d is less readily oxidized than α, α-dimethylated carboxylate 145a.148 Accordingly, Leonori and co-workers focus on development of α, α-dimethylated carboxylate analogues, such as 141, anticipating that carboxylates in this series would be readily oxidized by photoexcited *[Mes-Acr]ClO4 (E*1/2 = +2.08 vs. SCE in CH3CN)151 to facilitate decarboxylation en route to critical iminyl radical intermediates.
Interestingly, Jiang and Studer find similar photocatalyst *[Mes-Acr]BF4 to be ineffective in their iminofunctionalization reaction (Scheme 35, entry 1), and identify optimal conditions that rely on photosensitization of [Ir(dF(CF3)ppy)2(dtbbpy)]PF6, which has a less oxidizing excited state potential. Their conditions are effective with a range of radical trapping agents, including esters, and amides (not depicted), as well as sulfones, phosphates, and ketones, and the conditions tolerate heterocycles. Additionally, under these conditions, fused bicyclic 147d can be prepared with excellent diastereoselectivity.
8.2. Redox-active cyclic oximes generate carbon-centered radicals upon β-scission to enable remote Giese reactions
As an alternative to the direct addition to π-systems, iminyl radical intermediates can be used to induce ring opening of cyclobutyliminoradicals via β-scission processes. Such pathways reveal nitriles and carbon-centered radicals which, in turn, are poised to be trapped in radical conjugate addition processes. More than twenty years ago, Zard and co-workers demonstrated that, in the presence of tributylstannane reagents, O-benzoyl oximes, 150 S-aryl sulphenylimines152 (not shown), and N-hydroxy-2-thiopyridones, 153 such as 148, are among the appropriate precursors for use in β-scission / radical conjugate addition cascade reactions (Scheme 36). While these transformations found some early utility, they require incorporation of the activating group within the product scaffold (c.f. 152). To avoid this limitation, chemists have developed traceless activating groups that serve as precursors to iminyl radicals under modern mild, photoredox-mediated conditions.
Scheme 36.
Key precedent: Oximes are iminyl-radical precursors capable of inducing β-scission / radical conjugate addition cascade sequences
More than twenty years after Zard’s pioneering disclosure, came the first demonstration that photoredox catalysts can activate cyclic O-benzoyl oximes for iminyl radical formation / β-scission / radical conjugate addition reactions (Scheme 37). O-benzoyl oxime 153a (Ep/2 = −0.99 V vs SCE in CH2Cl2) 154 is susceptible to reduction in the presence of the strongly reducing Ir photocatalyst 5 (E1/2 (IrIV/IrIII*) = −1.73 V vs SCE in CH3CN). Oxime 153a undergoes β-scission, with subsequent radical conjugate addition to ethyl cinnamate, and, ultimately, etherifcation involving solvent to give methyl ether 154a. Under slightly modified conditions, 3-napthyl O-acyl oximes, such as 153b, engage in iminyl radical formation / β-scission / radical conjugate addition cascades. 155 This cyclization can be extended to generate complex 1,2,3,4-tetrahydrophenanthrenes, owing to omission of a hydrogen-atom donor from the reaction.
Scheme 37.
Cyclic oximes engage in photoredox-mediated β-scission / radical conjugate addition cascade reactions
To complement photoredox-mediated C─C scission of 4-membered ring oxime derivatives, a recent investigation156 has established photocatalytic conditions that generate remote carbon-centered radicals from 5- and 6-membered ring O-4-methoxybenzoyl oximes (Scheme 37). Rather than relying on strain energy, these reactions benefit from the relative ease of β-scission reactions that generate α-oxylyl radicals. Overall, these strategies provide access to nitriles with distal ethers, which are building blocks for common structural motifs. 157
9. Nitrogen-centered radicals enable hydrogen atom transfer (HAT) processes to generate remote carbon-centered radicals
Nitrogen-centered radicals have been known to promote site-selective C(sp3)─H functionalization for over 100 years. The discovery of the Hofmann-Löffler-Freytag (HLF) reaction103 established the conceptual framework for what is now the flourishing arena of radical-mediated C(sp3)─H functionalization technologies. Traditionally, N-haloamines have served as radical precursors in these reactions (Scheme 38). Upon homolysis of the labile N─Cl bond, the resultant N-centered radical guides a 1,5-hydrogen atom transfer (HAT) processes via a 6-membered ring transition state (157). Until very recently, the utility of this remarkably selective reaction for diverse C(sp3)─H functionalization was limited to atom- and group-transfer reactions because the initial radical formation step relied on prior installation of a group or atom that would be transferred. 158 Photoredox catalysis has allowed access to N-centered radicals from previously inert precursors. 159 As a result, site-selective Giese reactions at remote C(sp3)─H centers, which were historically unrealized, have now been rendered viable (Scheme 40).
Scheme 38.
Key precedent: Hofmann-Löffler-Freytag reaction enables oxidative functionalization at remote C(5) position via 1,5-HAT
Scheme 40.
Amides, sulfonamides, and carbamates undergo PCET and SET processes, and the resultant amidyl radicals direct Giese reactions
9.1. Amide, sulphonamide, and carbamate-derived N-centered radicals guide 1,5-HAT processes to enable functionalization of remote C─H bonds.
Knowles and co-workers160 set the stage for the direct use of amides as N-centered radical precursors in 1,5-HAT processes in a report describing a photocatalyzed alkene carboamination process (Scheme 39). Importantly, this investigation demonstrates that, for the first time, a strong N─H bond of an amide (BDFE ~ 107–110 kcal/mol), 161, 162 could be homolyzed in the presence of a strongly oxidizing photocatalyst and a weak hydrogen-bonding phosphaste base. With this base, deprotonation of an electron rich benzamide (ΔpKa ≈ 20 in MeCN) is not kinetically competitive with the luminescence decay of the excited state iridium catalyst. 163 Thus, it is proposed that the hydrogen bonding interaction between the phosphate base and the benzamide sufficiently weakens the N─H bond, making it susceptible to oxidation in the presence of a strong Ir oxidant (Scheme 39A). This proton-coupled electron transfer (PCET) process provides mild conditions for homolytic N─H bond activation, and enables previously infeasible transformations.
Scheme 39.
PCET enables amides to serve as direct precursors to amidyl radicals in the course of alkene carboaminiation reactions.
Simultaneous reports from the laboratories of Knowles25 and Rovis164 establish that amidyl radicals accessed under photochemical conditions are capable of directing Giese reactions with traditional HLF selectivity (Scheme 40). By using strongly oxidizing photocatalysts, both investigations develop strategies for C(5)─H alkylation via in situ generation of N-centered radicals without the need to prefunctionalize the amide with a labile N─X bond. The ability to bypass this prefunctionalization step resolves a century old limitation of the HLF reaction. With this innovation, amine derivatives can finally be employed to effectively direct reactions other than atom-transfer reactions. These publications ignited a renaissance of hydrogen atom transfer processes and modified HLF reactions. 165
The key difference between these two strategies for forming N-centered radicals originates in the proposed mechanisms for amide oxidation to amidyl radicals (Scheme 41). Knowles and co-workers’ propose that their method converts an amide to an amidyl radical directly through a concerted PCET process (i.e. 164 → 169). By contrast, Chu and Rovis rely on a more acidic N─H bond (pKa = ~13.8), and a stronger base. Consequently, deprotonation of the amide substrate is kinetically feasible (Scheme 41A). 166 Additionally, oxidation of the generated amide anion 165 by photocatalyst 167 is both kinetically and thermodynamically feasible, and amidyl radical 169 is proposed to form via a stepwise SET process. These groundbreaking approaches to access amidyl radicals are mechanistically complementary.
Scheme 41.
Photocatalysis enables non-prefunctionalized amidyl radical formation via PCET or SET processes
Once generated, amidyl radical intermediates 169 direct remote carbon-centered radical formation prior to intermolecular Giese reactions (Scheme 41B). The amidyl radicals adopt kinetically preferred six-membered transition states to affect a 1,5-hydrogen atom transfer (HAT) processes. The resultant nucleophilic alkyl radicals 170 are poised for radical conjugate addition to furnish more readily reduced electrophilic alkyl radicals 171, which are proposed to be reduced through SET by [IrII], thereby regenerating [IrIII] catalyst with concurrent formation of anions 172. These anions are poised for protonation to complete the guided Giese reaction.
Both of these strategies were demonstrated to guide Giese reactions at tertiary and secondary centers, albeit with lower efficiency when reactions at secondary C─H bonds were required (Scheme 40). Furthermore, Knowles and co-workers demonstrate that PCET can be used to induce a sulfamide (163d) and a carbamate (163e) to direct Giese reactions based on 1,5-HAT processes. In a follow-up report, Rovis and co-workers166 extend their method to the remote, site-specific alkylation of imides (Scheme 40). As an extension of this strategy, Flechsig & Wang and co-workers demonstrate that an organic photocatalyst can drive a range of directed Giese reactions (Scheme 42). 167 Under the disclosed conditions, a diversity of N-substituted N-acetyl, N-isobutryl, N-pivaloyl, and N-cyclopentylcarbonyl substrates react in synthetically useful yields. The reaction can be used to generate new C─C bonds at secondary and tertiary centers.
Scheme 42.
Site-selective, remote allylation of prefunctionalized aliphatic amides allows for C─C bond formation at secondary centers
9.2. Amidyl radicals guide 1,5-HAT processes for enantioselective functionalization at remote C─H bonds
Following Knowles’ and Rovis’ pioneering investigations25, 164, Meggers and co-workers168 disclosed a dual chiral Lewis acid/photoredox platform to achieve enantioselective remote alkylations of aliphatic amides via 1,5-HAT processes (Scheme 43). Site-selective alkylation reactions with a variety of substituted α,β-unsaturated N-imidazole Michael acceptors occurred in moderate to good yields with good to excellent enatioselectivity.
Scheme 43.
Photoredox-mediated site-selective, remote alkylation of aliphatic amides occurs enantioselectively in the presence of a chiral Lewis acid
9.3. Iminyl radicals direct Giese reactions.
Studer and co-workers disclosed the first photoredox-mediated strategy143 to engage iminyl radicals in a 1,5-HAT process, and used it to induce Giese reactions (Scheme 44). 169 As a demonstration of synthetic value, subsequent hydrolysis of the imine to the ketone provides technology for obtaining γ-C(sp3)─H functionalized ketones. Alternatively, the versatile imine functionality can be retained through a 2-step, one-pot protection with benzoyl chloride.
Scheme 44.
Iminyl radicals guide alkylation reactions at C(5)─H bonds and can be used to access γ-C(sp3)─H functionalized ketones.
9.4. Sulfamyl radicals guide 1,6-HAT processes for functionalization at remote C─H bonds.
Cognizant of the emergence of sulfamyl radical-directed halogen170, 171 and group-transfer reactions, 172-174 Roizen and co-workers, 175 Duan and co-workers, 176 and Shu et al177 have demonstrated that sulfamate esters178 can serve as direct precursors to radicals under photochemical conditions (Scheme 45). The accessed sulfamyl radicals guide Giese reactions based on remarkable 1,6-HAT processes, which proceed with site-selectivity that has been historically inaccessible.
Scheme 45.
Sulfamyl radicals guide Giese reactions
These conceptually similar publications develop different detailed reaction insights. In terms of substrate scope, while Roizen and co-workers do not detect efficient reactions at benzylic centers, Duan and co-workers do. More broadly, Roizen and co-workers focus on substrates that should be prone to competitive off-site reactions, and substrates where the desired reactions would proceed at secondary centers (Scheme 46). For example, the methylene center in sulfamate ester 184 can engage in a single alkylation event to form Giese product 185, which incorporates a tertiary C─H center at the reactive site. This tertiary C─H bond is weaker than the substrate’s reactive secondary C─H center, so it might be expected to be susceptible to a second alkylation event. Roizen and co-workers determine that substrates incorporating a less challenging steric environment at the site of hydrogen atom abstraction can undergo bis-alkylation events (c.f. 186a), while slightly encumbered systems engage principally in monoalkylation events at secondary centers (c.f. 185b). Furthermore, as expected, the sulfamyl radical-guided Giese reaction proceeds at secondary centers with modest diastereoselectivity when functionalizing commonly occurring fused-ring systems of natural products (c.f. 185C─D).
Scheme 46.
Secondary centers undergo productive Giese reactions
To date, data supports initial sulfamyl radical formation, either through a PCET (i.e. 187 → 192) or SET (i.e. 187 → 188 → 192) process (Scheme 47). Initial sulfamyl radical formation via SET is thermodynamically feasible, as sodiated 5-methylhexyl N-tert-butyl sulfamate ester anion 197 has a half-peak potential (Ep/2) of +0.753 V versus SCE (in acetonitrile), 175 and should be readily oxidizable by either of the engaged excited state iridium catalysts. Moreover, this process should be kinetically feasible, as demonstrated by Stern-Volmer analysis.175 The generated sulfamyl radical is poised to guide an intramolecular 1,6-HAT process to furnish carbon-centered radical 193. This step is irreversible, 175 and rate-determining. 176 Resultant nucleophilic radical 193 traps electron-deficient olefin 20ag in a Giese reaction. At this point, reduction of ester stabilized radical 194 by a strong reductant, such as an IrIII species, should be thermodynamically favorable.175 Finally, protonation of resultant anion 195 is consistent with deuteration in the presence of D2O, 176 and proceeds under standard reaction conditions to generate Giese product 196.
Scheme 47.
Multiple possible reaction mechanisms are consistent with data for sulfamate ester directed Giese reactions
10. Alkoxyl radicals enable 1,5-HAT processes generate to carbon-centered radicals
Alkoxyl radicals179 are well known intermediates in radical transposition chemistry, and preferentially affect 1,5-HAT processes. 180 Prior to the application of photoredox catalysts, two methods for generating alkoxyl radicals as intermediates for site-selective Giese reactions had been reported (Scheme 48). 181 Both methods rely on a labile O─X bond (X = S, or N) that can be homolyzed under irradiation conditions. Unfortunately, these methods require unstable substrates or harsh conditions with toxic reagents, which has limited their broad application. With recent advances in photocatalysis, alkoxyl radicals from easy to handle bench-stable precursors have become accessible under conditions that are compatible with a wide variety of functional groups.
Scheme 48.
Alkoxyl radicals generated by UV irradiation engage in 1,5-HAT processes for remote alkylation reactions with modest success
10.1. Alkoxyl radicals guide 1,5-HAT processes for site-selective functionalization.
The instability of alkoxyl radical precursors limited early efforts to engage alkoxyl radicals in guided Giese reactions. To overcome this limitation, Chen and co-workers demonstrate that, under photoredox-mediated conditions, N-alkoxyphthalamides can serve as precursors for alkoxyl radicals (Scheme 49). 182 In the presence of a strongly oxidizing Ir(III) photocatalyst and Hantszch ester as an external reductant, the N-alkoxyphthalamide can be reduced to liberate an alkoxyl radical. This alkoxyl radical engages in 1,5-HAT to generate a carbon-centered radical that traps an electron-deficient olefin, ultimately introducing a new terminal olefin with excellent site-selectivity, even with a complex steroid substrate. The use of masked alcohols as radical precurors is particularly attractive for late-stage functionalization processes, as many natural products contain alcohol groups. With this method, the alcohol functional group is restored without the need for additional synthetic manipulations.
Scheme 49.
N-alkoxyphthalamides serve as user friendly alkoxyl radical precurosors to efficiently engage aliphatic alcohols in site-selective Giese reactions
10.2. Alkoxyl radicals guide 1,5-HAT processes in directed enantioselective Giese reactions.
Meggers and co-workers merge this strategy for alkoxyl radical generation with their approach to asymmetric induction during conjugate addition reactions. 183 Using N-alkoxyphthalamides as alkoxyl radical precursors, a chiral rhodium(III) Lewis acid catalyst activates the radical acceptor, and determines the face of the radical conjugate addition (Scheme 50). Crystallographic analysis of a ent-Λ-RhS (ent-9) complex (not depicted) is used to support this model for asymmetric induction. To support their mechanistic proposal (not depicted), luminescence quenching experiments show that an excited state iridium complex is quenched more efficiently by Hantzsch ester than by an N-alkoxyphthalamide, or by α,β-unsaturated 20 (R = Me; not depicted) (Scheme 50). The method contributes to the mechanistic understanding of these complex reactions. More importantly, it induces site-selectivity and enantioselectivity, demonstrating that photredox-mediated HAT processes can be merged with asymmetric catalysis.
Scheme 50.
N-alkoxyphthalamides engage in enantioselective Giese reactions under photoredox-mediated conditions
10.3. Alkoxyl radicals are HAT agents for the alkylation of cyclohexane via cerium-catalyzed Giese reactions.
The direct generation of alkoxy radicals from free alcohols is thermodynamically challenging owing to the very high oxidation potentials of free alcohols. Recently, photocatalytic strategies for the direct generation of alkoxy radicals from free alcohols via Ir-catalyzed PCET have been developed184 and employed in a number of C─C bond β-scission transformations (Scheme 51A). 185 Concurrently, an abundant and inexpensive cerium trichloride photocatalyst186 has been advanced to affect similar bond scission transformations (Scheme 51B). 187 By merging these technologies, Zuo and co-workers188 have developed a strategy for alcohol-directed Giese reactions (Scheme 52) and innately selective C─H animation reactions (not pictured).
Scheme 51.

Light-mediated strategies for the catalytic homolysis of free alcohols to generate alkoxy radicals
Scheme 52.
Proposed reaction mechanism by which alkoxy radicals generated under light-mediated cerium-catalyzed conditions are utilized as HAT agents to engage cyclohexane in Giese reactions
In the proposed catalytic mechanism, in situ generated Ce(IV)-methoxide complex 215 undergoes photoinduced ligand-to-metal charge-transfer (LMCT) to generate the methoxy radical 216 and Ce(III) species. The generated methoxy radical is poised to abstract a C─H bond from cyclohexane. Resultant nucleophilic carbon-centered radical 218 traps enone 20ah to produce stabilized electrophilic radical 219. Finally, this radical can be reduced by excited-state DPA co-catalyst 220 to furnish desired alkylated product 212. Co-catalyst 13 accelerates product formation, suggesting that the oxidation of Ce(III) to the active Ce(IV) is the rate-limiting step of the catalytic cycle. The proposed mechanism is supported by steady-state homolysis experiments and transient absorption spectroscopic studies, which describe a fast bond homolysis process and rule out the LMCT even as the rate-determining step in the catalytic cycle. The light-mediated cerium-catalyzed Giese reaction efficiently alkylates enones. The reaction is not as effective at trapping other commonly used electron-deficient alkenes such as acrylates, or heterocyclic enones.
11. Direct hydrogen atom abstraction of non-reactive C(sp3)─H bonds generates carbon-centered radicals
Many photoredox-mediated strategies for forming carbon-centered radicals take advantage of labile bonds like alkyl halides and organoboranes, or other redox-active functional groups that can readily engage in electron transfer. Methods for the cleavage of non-reactive aliphatic C─H bonds typically require C─H activation, 189 or involve intramolecular hydrogen atom transfer (HAT) processes (see Sections 9-10). 158d,e Recently, a method has emerged that invokes photosensitizers/substrate interactions as directly enabling the C─H abstraction event to affect the cleavage of inert C(sp3)─H bonds (Scheme 53). This strategy does not rely on the presence of redox-active functional groups in the substrate, and benefits from innate site-selectivity.
Scheme 53.
Coordination between Ir catalyst and phosphate base engages inert C(sp3)─H bonds in PCET to enable innately selective Giese reactions
A novel mechanism for achieving direct homolysis of unactivated C(sp3)─H was recently disclosed by Knowles and Alexanian and co-workers190 (Scheme 53). The development of this method was prompted by an unexpected control reaction, in which alkylation of tetrahydrofuran proceeded in good yield absent reagent 223, which had been expected to affect C─H abstraction. Subsequent mechanistic experiments are consistent with a mechanism that relies on trimolecular PCET to achieve C─H abstraction. Specificially, evaluation of the redox half-potentials of the phosphate base and the iridium photocatalyst, leads to the conclusion that HAT mediated by an oxygen-centered radical is improbable. Titration experiments reveal a prominent shift in the proton signals at the 3,3’-position of the dCF3 bipyridine ligand in the presence of increasing concentrations of base. The base-ligand coordination is further supported by evaluations of binding kinetics. The alkylation reaction can be effectively shut down in the presence of substitutions at the 3,3’-position which disrupt hydrogen bonding interactions between the ligand and base, such as the installation of fluorine atoms. Additionally, Stern Volmer quenching studies support the hypothesis that both the alkane and phosphate base are needed to promote electron transfer in order to generate the carbon-centered radical intermediate. KIE studies provide evidence that Ir catalyst quenching occurs as a result of C─H bond cleavage. This PCET-mediated Giese reaction proceeds with innate selectivity by generating carbon-centered radicals at the most electron-rich C(sp3)─H bonds (225–227). This method expands the scope of radical acceptors to include vinyl ketones (226), and demonstrates that the direct C(sp3)─H cleavage can be used in for the late-stage functionalization of complex molecules such as ibuprofen-derivative 227.
Less mild methods have been proposed to affect direct C─H abstraction in much simpler systems. Recently, Noël and co-workers191 developed a light-mediated, decatungstate (TBADT)-catalyzed method for the direct C─H homolysis of gaseous hydrocarbons under flow-reaction conditions (Scheme 54). Under near UV irradiation, it is plausible that the photoexcited TBADT anion 230 (*[W10O32]4−) relaxes to the reactive species, wO (231), characterized by highly electrophilic oxygen centers, 192 which can abstract a hydrogen atom from a light-chain alkane (isobutene, propane, ethane, or methane) to generate nucleophilic carbon-centered radical 233. These alkyl radicals trap electron-deficient alkenes to produce electrophilic radical 234, which can be converted to product. The reaction with aforementioned gaseous hydrocarbons proceeded efficiently with a range of benzylidenemalonitriles (i.e. 20aj). With the exception of methane, the generated alkyl radicals can also form C─C bonds with N-phenylmaleiminde and norboranone radical traps (not pictured).
Scheme 54.
Photoredox-mediated TBADT-catalyzed direct C─H homolysis of light-chain, gaseous hydrocarbons generate alkyl radicals for Giese reactions
Flow-reaction conditions are critical to the successful generation of key carbon-centered radical intermediates from gaseous reagents. As opposed to batch conditions, the flow reactor can take advantage of the safe regulation of pressure to increase contact between the gaseous substrate and the liquid reaction mixture. Additionally, flow conditions improve irradiation, which often shortens reaction times. 23 While these benefits can enable more efficient and safer photoredox-mediated reactions of gaseous reagents, flow-reactors can be complicated to set up. Similar to some tin hydride-mediated Giese reactions, flow-reactors typically require syringe pumps, if not even more costly equipment.
12. Summary and Outlook
Giese reactions have served and will continue to serve as a useful platform for the discovery of photochemical technologies. New photoredox-mediated radical conjugate addition reactions herald the development of improved photocatalysts and radical precursors, as well as novel approaches to achieve site-selectivity and even asymmetric induction. These reactions streamline syntheses of therapeutically-relevant targets, and move the chemical industry toward reliance on sustainable sources of energy and reagents. Importantly, photocatalyzed Giese reactions advance our understanding of the exponentially growing field of photochemical synthesis, and serve to demonstate its power.
Scheme 29.
Diastereoselective Giese reactions produce enantioenriched β-thiolated/selenolated amino acids
Acknowledgements
We gratefully acknowledge financial support from the National Institutes of Health (R35GM128741-01), Kitae Kwon for the artistic and chemical vision that inspired Schemes 31 and 34, and Dr. J. Miles Blackburn for insightful comments.
Biography

A.L. Gant Kanegusuku earned a B.A. from Smith College where she carried out research with Prof. Laura Katz to investigate the evolutionary relationships among single-celled eukaryotes. Subsequently, Anastasia moved to Lima, Perú as a Fulbright Fellow with Prof. Helena Maruenda at Pontifica Universidad Católica del Perú where she helped to develop and assess an analytical method for the evaluation of coffee quality markers. Anastasia returned to the states to earn a Ph.D. in Chemistry at Duke University. Her research in Prof. Jennifer Roizen’s laboratory focused on the development of photochemically-mediated strategies for C─C and C─N bond-forming reactions.

Prof. Roizen’s laboratory advances cross-coupling and C─H functionalization technologies. She first entered a synthetic chemistry laboratory while an undergraduate at Williams College. There, Prof. J. Hodge Markgraf and Thomas E. Smith patiently guided her as she pursued syntheses of benzoisocanthenones and of hennoxazole A. Driven by a passion for total synthesis, she advanced approaches to access the core of ineleganolide as she earned a Ph.D. with Prof. Brian M. Stoltz at the California Institute of Technology. By graduation, Dr. Roizen had become interested in the amination technologies developed by Prof. Justin Du Bois at Stanford University. She advanced these technologies as both an NIH and a CMAD Postdoctoral Fellow. These strategies continue to inspire her ongoing research.
References
- [1].(a) For the seminal publications establishing the synthetic utility of radical conjugate addition reactions, see: Giese B, Lachhein S, Angew. Chem. Int. Ed 1981, 20, 967 [Google Scholar]; Angew. Chem 1981, 93, 1016. [Google Scholar]; (b) Giese B, Dupuis J, Angew. Chem. Int. Ed 1983, 22, 622–623 [Google Scholar]; Angew. Chem 1983, 95, 633–634. [Google Scholar]; (c) Giese B, Gonzalez-Gomez JA, Witzel T, Angew. Chem. Int. Ed 1984, 23, 69–70 [Google Scholar]; Angew. Chem 1984, 96, 51–52. [Google Scholar]
- [2].Studer A, Curran DP, Angew. Chem. Int. Ed 2016, 55, 58–102 [DOI] [PubMed] [Google Scholar]; Angew. Chem 2016, 128, 58–106. [Google Scholar]
- [3].(a) For reviews demonstrating the scope and selectivity of the Giese reaction, see: Giese B, Angew. Chem. Int. Ed 1983, 22, 753–764 [Google Scholar]; Angew. Chem 1983, 95, 771–782. [Google Scholar]; (b) Giese B, Angew. Chem. Int. Ed 1985, 24, 553–565 [Google Scholar]; Angew. Chem 1983, 97, 555–567. [Google Scholar]; (c) Sibi MK, Venkatraman L, Liu M, J. Am. Chem. Soc 2001, 123, 8444–8445. [DOI] [PubMed] [Google Scholar]; (d) Bar G, Parsons AF, Chem. Soc. Rev 2003, 32, 251–263. [DOI] [PubMed] [Google Scholar]; (e) Srikanth GSC, Castle SL, Tetrahedron, 2005, 61, 10377–10441. [Google Scholar]
- [4].(a) For seminal examples of the application of metal polypyridal complexes in synthetic transformations, see: Hedstrand DM, Kruizinga WM, Kellogg RM, Tetrahedron Lett. 1978, 19, 1255–1258. [Google Scholar]; (b) Pac C, Ihama M, Yasuda M, Miyauchi Y, Sakurai H, J. Am. Chem. Soc 1981, 103, 6495–6497. [Google Scholar]; (c) Hironaka K, Fukuzumi S, Tanaka T, J. Chem. Soc., Perkin Trans. 2 1984, 1705–1709. [Google Scholar]; (d) Cano-Yelo H, Deronzier A, J. Chem. Soc. Perkin Trans. II, 1984, 1093–1098. [Google Scholar]
- [5].(a) For select reviews on photoredox-mediated processes published since 2013, see: Staveness D, Bosque I, Stephenson CRJ, Acc. Chem. Res 2016, 49, 2295–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Uygur M, Mancheño OG, Org. Biomol. Chem 2019, 17, 5475–5489. [DOI] [PubMed] [Google Scholar]; (c) Romero NA, Nicewicz DA, Chem. Rev 2016, 116, 10075–10166. [DOI] [PubMed] [Google Scholar]; (d) Schultz DM, Yoon TP, Science, 2014, 343, 1239176. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Michelin C, Hoffmann N, ACS Catal. 2018, 8, 12046–12055 [Google Scholar]; (f) McAtee RC, McCLain EJ, Stephenson CRJ, Trends in Chemistry, 2019, 1, 111–125. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Teegardin K, Day JI, Chan J, Weaver J, Or. Process Res. Dev 2016, 20, 1156–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Cavedon C, Seeberger PH, Pieber B, Eur. J. Org. Chem 2019, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Skubi KL, Blum TR, Yoon TP, Chem. Rev 2016, 116, 10035–10074. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Fensterbank L, Goddard J-P, Ollivier C in Visible Light Photocatalysis in Organic Chemistry; (Eds.; Stephenson CRJ, Yoon TP, MacMillan DWC)Wiley, Weinheim, Germany, 2018, pp 25–59. [Google Scholar]; (h) Akita M, Kioke T. C.R. Chimie, 2015, 18, 742–751. [Google Scholar]
- [6].(a) For prior applications of metal polypyridal complexes in organic synthesis, see: Hedstrand DM, Kruizinga WH, Kellogg RM, Tetrahedron Lett. 1978, 19, 1255–1258. [Google Scholar]; (b) Cano-Yelo H, Deronzier A, J. Chem. Soc. Perkin Trans. II, 1984, 1093–1098. [Google Scholar]; (c) Zen J-M, Liou S-L, Kumar AS, Hsia M-S, Angew. Chem., Int. Ed 2003, 42, 577–579 [DOI] [PubMed] [Google Scholar]; Angew. Chem 2003, 115, 597–599. [Google Scholar]
- [7].(a) Ischay MA, Anzovino ME, Du J, Yoon TP, J. Am. Chem. Soc 2008, 130, 12886–12887. [DOI] [PubMed] [Google Scholar]; (b) Nicewicz DA, MacMillan DWC, Science 2008, 322, 77–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Luo YR, Handbook of bond dissociation energies in organic compounds. CRC Press: Boca Raton, Florida, 2002. [Google Scholar]
- [9].Barton DHR, McCombie SW, J. Chem. Soc., Perkin Trans. 1 1975, 1574–1585. [Google Scholar]
- [10].Giese B, Radicals in Organic Synthesis: Formation of Carbon–Carbon Bonds, Pergamon Press, Oxford, 1986. [Google Scholar]
- [11].Curran DP, Radical Addition Reactions. In Comprehensive Organic Synthesis, Vol 4, (Eds.; Trost BM, Fleming I) Pergamon Press, Oxford,1991, pp 715–777. [Google Scholar]
- [12].Giese B, Angew. Chem. Int. Ed 1983, 22, 753–764 [Google Scholar]; Angew. Chem 1983, 95, 771–782. [Google Scholar]
- [13].(a) Ingold KU, Kochi J, Free Radicals, Vol 1, Wiley, New York, 1973, pp 37. [Google Scholar]; (b) Knoll H, Z. Chem 1982, 22, 245–252. [Google Scholar]
- [14].Chatgilialoglu C, Ingold KU, Scaiano JC, J. Am. Chem. Soc 1981, 103, 7738–7742. [Google Scholar]
- [15].(a) Kuivila HG, J. Org. Chem 1963, 28, 2165–2167. [Google Scholar]; (b) Gerth DB, Giese B, J. Org. Chem 1986, 51, 3726–3729. [Google Scholar]
- [16].Baguley PA, Walton JC, Angew. Chem. Int. Ed 1998, 37, 3072–3082 [DOI] [PubMed] [Google Scholar]; Angew. Chem 1998, 110, 3271–3283. [Google Scholar]
- [17].Walling C, Tetrahedron 1985, 41, 3887–3900. [Google Scholar]
- [18].Cismesia MA, Yoon TP. Chem. Sci 2015, 6, 5426–5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Majek M, Filace F, Jacobi von Wangelin A. Beilstein J. Org. Chem 2014, 10, 981–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Fraij LK, Hayes DM, Werner TC. J. Chem. Ed 1992, 69, 424–428. [Google Scholar]
- [21].Julliard M, Chanon M, Chem. Rev 1983, 83, 425–506. [Google Scholar]
- [22].Cline ED, Bernhard S, Chimia 2009, 63, 709–713. [Google Scholar]
- [23].Choi GJ, Zhu Q, Miller DC, Gu CJ, Knowles RR, Nature 2016, 539, 268–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Flamigni L, Barbieri A, Sabatini C, Ventura B, Barigelletti F in Photochemistry and Photophysics of Coordination Compounds II. Topics in Current Chemistry vol 281, (Eds.; Balzani V, Campagna S) Springer, Berlin, Heidelberg: 2007, pp. 143–203. [Google Scholar]
- [25].Gualandi A, Matteucci E, Monti F, Baschieri A, Armaroli N, Sambri L, Cozzi PG, Chem. Sci 2017, 8, 1613–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].(a) Kalyanasundaram K, Coord. Chem. Rev 1982, 46, 159. [Google Scholar]; (b) Juris A, Balzani V, Belser P, von Zelewsky A, Helv. Chim. Acta 1981, 64, 2175–2182. [Google Scholar]
- [27].Huang X, Luo S, Burghaus O, Webster RD, Harms K, Meggers E, Chem. Sci 2017, 8, 7126–7131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sun AC, Steyer DJ, Allen AR, Payne EM, Kennedy RT, Stephenson CRJ, Nat Commun 2020, 11, 6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].(a) Tucker JW, Zhang Y, Jamison TF, Stephenson CRJ, Angew. Chem. Int. Ed 2012, 51, 4144–4147 [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem 2012, 124, 4220–4223. [Google Scholar]; (b) Harper KC, Moschetta EG, Bordaweker SV, Wittenberger SJ, ACS Cent. Sci 2019, 5, 109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lazarides T, McCormick T, Du P, Luo G, Lindley B, Eisenberg R, J. Am. Chem. Soc 2009, 131, 9192–9194. [DOI] [PubMed] [Google Scholar]
- [31].Neumann N, Füldner S, König B, Zeitler K, Angew. Chem. Int. Ed 2011, 50, 951–954 [DOI] [PubMed] [Google Scholar]; Angew. Chem 2011, 123, 981–985. [Google Scholar]
- [32].Sioda RE, J. Phys. Chem 1968, 72, 2322–2330. [Google Scholar]
- [33].Texier I, Delaire JA, Giannotti C, Phys. Chem. Chem. Phys 2000, 2, 1205–1212 [Google Scholar]
- [34].Prier CK, Rankic DA, MacMillan DWC, Chem. Rev 2013, 113, 5322–5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].(a) Fukuzumi S, Mochizuki S, Tanaka T, J. Phys. Chem 1990, 94, 722–726. [Google Scholar]; (b) For an earlier example of a photochemically induced hydrodehalogenation reaction, see: Beckwith ALJ, Goh SH, J. Chem. Soc. Chem. Commun 1983, 905–906. [Google Scholar]
- [36].(a) Nguyen JD, D'Amato EM, Narayanam JMR, Stephenson CRJ, Nat. Chem 2012, 4, 854–859 [DOI] [PubMed] [Google Scholar]; (b) Devery JJ III, Nguyen JD, Dai C, Stephenson CRJ, ACS Catal. 2016, 6, 5962–5967. [Google Scholar]; (c) Poelma SO, Burnett GL, Discekici EH, Mattson KM, Treat NJ, Luo Y, Hudson ZM, Shankel SL, Clark PG, Kramer JW, Hawker CJ, de Alaniz JR, J. Org. Chem 2016, 81, 7155–7160 [DOI] [PubMed] [Google Scholar]; (d) Discekici EH, Treat NJ, Poelma SO, Mattson KM, Hudson ZM, Luo Y, Hawker CJ and de Alaniz JR, Chem. Commun 2015, 51, 11705–11708 [DOI] [PubMed] [Google Scholar]; (e) Arora A, Teegardin KA, Weaver JD, Org. Lett 2015, 17, 3722–3725 [DOI] [PubMed] [Google Scholar]; (f) Arora A, Weaver JD, Org. Lett 2016, 18, 3996–3999 [DOI] [PubMed] [Google Scholar]; (g) Singh A, Kubik JJ, Weaver JD, Chem. Sci 2015, 6, 7206–7212 [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Singh A, Fennell CJ, Weaver JD, Chem. Sci 2016, 7, 6796–6802 [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Senaweera S, Weaver JD, J. Am. Chem. Soc 2016, 138, 2520–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Ghosh I, König B, Angew. Chem., Int. Ed 2016, 55, 7676–7679 [DOI] [PubMed] [Google Scholar]; Angew. Chem 2016, 128, 7994. [Google Scholar]; (k) Ghosh I, Ghosh T, Bardagi JI, König B, Science 2014, 346, 725–728 [DOI] [PubMed] [Google Scholar]; (l) Marzo L, Ghosh I, König B, ACS Catal. 2016, 6, 6780–6784. [Google Scholar]
- [37].(a) Andrews SR, J Becker J, Gagné MR, Angew. Chem., Int. Ed 2010, 49, 7274–7276 [DOI] [PubMed] [Google Scholar]; Angew. Chem 2010, 122, 7432–7434. [Google Scholar]; (b) Andrews SR, Becker JJ, Gagné MR, Org. Lett 2011, 13, 2406–2409. [DOI] [PubMed] [Google Scholar]
- [38].Aycock RA, Wang H, Jui NT, Chem. Sci, 2017, 8, 3121–3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].For examples of strategies for inducing asymmetry in free- radical conjugate addition, see: Porter NA, Giese B, Curran DP, Acc. Chem. Res 1991, 24, 296–304. [Google Scholar]
- [40].Axon JR, Beckwith ALJ, J. Chem. Soc., Chem. Commun 1995, 549–550. [Google Scholar]
- [41].(a) The development of this radical trapping agent relies on prior innovations, see: Hiskey RG, Jung JM, J. Am. Chem. Soc 1963, 85, 578–582. [Google Scholar]; (b) Karady S, Amto JS, Weinstock LM, Tetrahedron Lett. 1984, 25, 4337–4340. [Google Scholar]; (c) Seebach D, Fadel AN, Helv. Chim. Acta 1985, 68, 1243–1250. [Google Scholar]; (d) Beckwith ALJ, Chai CLL, J. Chem. Soc., Chem. Commun 1990, 1087–1088. [Google Scholar]
- [42].Aycock RA, Vogt DB, Jui NT, Chem. Sci, 2017, 8, 7998–8003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zhang P, Le CC, MacMillan DWC, J. Am. Chem. Soc, 2016, 138, 8084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].ElMarrouni A, Ritts CB, Balsells J, Chem. Sci 2018, 9, 6639–6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Giese B, Kopping B, Chatgilialoglu C, Tetrahedron Lett. 1989, 30, 681–684. [Google Scholar]
- [46].Dong J, Wang X, Wang Z, Song H, Lui Y, Wang Q, Chem. Commun, 2019, 55, 11707–11710. [DOI] [PubMed] [Google Scholar]
- [47].Hudson A, Lappert MF, Nicholson BK, J. Chem. Soc., Dalton Trans, 1977, 551–554. [Google Scholar]
- [48].Sun Y, Li R, Zhang W, Li A, Angew. Chem. Int. Ed 2013, 52, 9201–9204 [DOI] [PubMed] [Google Scholar]; Angew. Chem 2013, 125, 9371–9374. [Google Scholar]
- [49].(a) Bruncko M, Crich D, Raghu S. J. Org. Chem 1994, 59, 5543–5549. [Google Scholar]; (b) Movassaghi M, Schmidt MA, Angew. Chem. Int. Ed 2007, 46, 3725–3728 [DOI] [PubMed] [Google Scholar]; Angew. Chem 2007, 119, 3799–3802. [Google Scholar]
- [50].Depew KM, Marsden SP, Zatorska D, Zatorski A, J. Am. Chem. Soc 1999, 121, 11953–11963 [Google Scholar]
- [51].(a) Heberger K, Walbiner M, Fischer H, Angew. Chem., Int. Ed 1992, 31, 635–636 [Google Scholar]; Angew. Chem 1992, 104, 651–653. [Google Scholar]; (b) Fischer H, Radom L, Angew. Chem., Int. Ed 2001, 40, 1341–1371 [DOI] [PubMed] [Google Scholar]; Angew. Chem 2001, 113, 1380–1414. [Google Scholar]
- [52].(a) Conradi MS, Zeldes H, Livingston R, J. Phys. Chem 1979, 83, 2160–2161. [Google Scholar]; (b) de AM Nicholas P, Arnold DR, Can. J. Chem 1986, 64, 270–276. [Google Scholar]
- [53].Schweitzer-Chaput B, Horwitz MA, de Pedro Beato R, Melchiorre P, Nat. Chem 2019, 11, 129–135. [DOI] [PubMed] [Google Scholar]
- [54].Lalevée J, Blanchard N, El-Roz M, Allonas X, Fouassier JP, Macromolecules 2008, 41, 2347–2352. [Google Scholar]
- [55].Dag O, Yaman SO, Onal AM, Isci H, J. Chem. Soc. Dalton Trans 2001, 2819–2824. [Google Scholar]
- [56].Bahtia K, Schuler RH, J. Phys. Chem 1974, 78, 2335–2338. [Google Scholar]
- [57].(a) Klein R, Kelley RD, J. Phys. Chem 1975, 79, 1780–1784. [Google Scholar]; (b) Ingold KU, Bowry VW, J. Org. Chem 2015, 80, 1321–1331 [DOI] [PubMed] [Google Scholar]
- [58].For contemporaneous publications, see: Narayanam JMR, Tucker JW, Stephenson CRJ, J. Am. Chem. Soc 2009, 131, 8756–8757. [DOI] [PubMed] [Google Scholar]
- [59].(a) Prior to these investigations, Kriche and co-workers had reported that cobalt and copper complexes could mediate one-electron reduction of enones to initiate [2+2] cyclization reactions. For the most relevant publications, see: Baik T-G, Luiz AL, Wang L-C, Krische MJ, J. Am. Chem. Soc 2001, 123, 6716–6717.11439068 [Google Scholar]; (b) Wang L-C, Jang H-Y, Roh Y, Lynch V, Schultz AJ, Wang X, Krische MJ, J. Am. Chem. Soc 2002, 124, 9448–9453. [DOI] [PubMed] [Google Scholar]; (c) Yang J, Cauble DF, Berro AJ, Bauld NL, Krische MJ, J. Org. Chem 2004, 69, 7979–7984. [DOI] [PubMed] [Google Scholar]; (d) Roh Y, Jang H-Y, Lynch V, Bauld NL, Krische MJ, Org. Lett 2002, 4, 611–613. [DOI] [PubMed] [Google Scholar]; (e) Yang J, Felton GAN, Bauld NL, Krische MJ, J. Am. Chem. Soc 2004, 126, 1634–1635. [DOI] [PubMed] [Google Scholar]
- [60].Du J, Yoon TP, J. Am. Chem. Soc 2009, 131, 14604–14605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].For recent advances of the photocatalyzed, anion mediated [2+2] reaction, see: Du J, Skubi KL, Schultz DM, Yoon TP. Science 2014, 344, 392–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Cismesia MA, Yoon TP, Chem. Sci 2015, 6, 5426–5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].(a) For relevant publications which inspired application of asymmetric LA catalysis by chiral metal-centered catalysts to enantioselective Giese, see: Zhang L, Meggers E, Acc. Chem. Res 2017, 50, 320–330. [DOI] [PubMed] [Google Scholar]; (b) Wang C, Chen L-A, Huo H, Shen X, Harms K, Gong L, Meggers E, Chem. Sci 2015, 6, 1094–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Ma J, Shen X, Harms K, Meggers E, Dalton Trans. 2016, 45, 8320–8323. [DOI] [PubMed] [Google Scholar]
- [64].(a) Ischay MA, Lu Z, Yoon TP, J. Am. Chem. Soc 2010, 132, 8572–8574. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Lin S, Ischay MA, Fry CG, Yoon TP, J. Am. Chem. Soc 2011, 133, 19350–19353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Fukuzumi S, Kotani H, Ohkubo K, Ogo S, Tkachenko NV, Lemmetyinen H, J. Am. Chem. Soc 2004, 126, 1600–1601. [DOI] [PubMed] [Google Scholar]
- [66].(a) Hamilton DS, Nicewicz DA, J. Am. Chem. Soc 2012, 134, 18577–18580. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Romero NA, Margrey KA, Tay NE, Nicewicz DA, Science, 2015, 349, 1326–1330. [DOI] [PubMed] [Google Scholar]
- [67].(a) For a seminal reports of photoinduced oxidation of olefins to generate radical cation intermediates, see: Neunteufel RA, Arnold DR, J. Am. Chem. Soc 1973, 95, 4080–4081. [Google Scholar]; (b) Gassman PG, Bottorff KJ, Tetrahedron Lett. 1987, 29, 5449–5452. [Google Scholar]
- [68].(a) For seminal reports on catalytic photoredox-mediated hydrofunctionalization methods, see: Kazuhiko M, Isao N, Nobuyuki I, Yoshio O, Chem. Lett 1989, 18, 1095–1098. [Google Scholar]; (b) Hamilton DS, Nicewicz DA, J. Am. Chem. Soc 2012, 134, 18577–18580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].For a review on recent photocatalyzed methods for the direct oxidation of olefins, see: Margrey KA, Nicewicz DA, Acc. Chem. Res 2016, 49, 1997–2006. [DOI] [PubMed] [Google Scholar]
- [70].For a resource on the relative redox potentials of relevant unactivated olefins, see: Roth H, Romero N, Nicewicz DA, Synlett 2016, 27, 714–723. [Google Scholar]
- [71].Zhou R, Liu H, Tao H, Yu X, Wu J, Chem. Sci 2017, 8, 4654–4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].(a) Kamijo S, Takao G, Kamijo K, Tsuno T, Ishiguro K, Murafuji T, Org. Lett 2016, 18, 4912–4915. [DOI] [PubMed] [Google Scholar]; (b) Qrareya H, Ravelli D, Fagnoni M, Albini A, Adv. Synth. Catal 2013, 355, 2891–2899. [Google Scholar]; (b) Ueda M, Kondoh E, Ito Y, Shono H, Kakiuchi M, Ichii Y, Kimura T, Miyoshi T, Naito T, Miyata O, Org. Biomol. Chem 2011, 9, 2062–2064. [DOI] [PubMed] [Google Scholar]
- [73].(a) Some longstanding challenges to the development of these proceses include: competitive radical ion pair back electron transfer as well as additional oxidation or dimerization of the radical cation, see: Schmittel M, Burghart A, Angew. Chem. Int. Ed 1997, 36, 2550–2589 [Google Scholar]; Angew. Chem 1997, 109, 2658–2699. [Google Scholar]; (b) Mella M, Freccero M, Albini A, Tetrahedron, 1996, 52, 5533–5548. [Google Scholar]; (c) Vanossi M, Mella M, Albini A, J. Am. Chem. Soc 1994, 116, 10070–10075. [Google Scholar]
- [74].Liu H, Ma L, Zhou R, Chen X, Fang W, Wu J, ACS Catal. 2018, 8, 6224–6229. [Google Scholar]
- [75].(a) For select reviews of the reactivity of organotrifluoroborates, see: Stefani HA, Cella R, Vieira AS, Tetrahedron, 2007, 63, 3623–3658. [Google Scholar]; (b) Molander GA, Ellis N, Acc. Chem. Res 2007, 40, 275–286. [DOI] [PubMed] [Google Scholar]; (c) Darses S, Genet J-P, Chem. Rev 2008, 108, 288–325 [DOI] [PubMed] [Google Scholar]; (d) Molander GA, J. Org. Chem, 2015, 80, 7837–7848. [DOI] [PubMed] [Google Scholar]
- [76].For a detailed study of a reaction involving triaryl borate oxidation, see: Schuster GB, Pure Appl. Chem 1990, 62, 1565–1572. [Google Scholar]
- [77].For a review of boranes as radial precursors, see: Ollivier C, Renaud P, Chem. Rev 2001, 101, 3415–3434. [DOI] [PubMed] [Google Scholar]
- [78].Electrochemical oxidation of polyphenyltrifluoroborate anions has been studied, see: Shundrin LA, Bardin VV, Frohn H-J, Z. Anorg. Allg. Chem 2004, 630, 1253–1257. [Google Scholar]
- [79].(a) Prior to the development of photoredox-mediated reactions to oxidize organotrifluoroborate salts, organotrifluoroborate salts had been used as precursors to radical-intermediates in synthetically useful reactions. For a copper-mediated Ritter reaction of trifluoroborates, see: Carzola C, Métay E, Andrioletti B, Lemaire M, Tetrahedron Lett. 2009, 50, 6855–6857. [Google Scholar]; (b) For a light-initiated process involving allyl- and benzyltrifluoroborates, see: Nishigaichi Y, Orimi T, Takuwa A, J. Organomet. Chem 2009, 694, 3837–3839. [Google Scholar]; For a copper-mediated process, see: (c) Sorin G, Malloquin RM, Contie Y, Baralle A, Malacria M, Goddard J-P, Fensterbank L, Angew. Chem., Int. Ed 2010, 49, 8721–8723 [DOI] [PubMed] [Google Scholar]; Angew. Chem 2010, 122, 8903–8905. [Google Scholar]; For a manganese-mediated reaction, see: (d) Molander GA, Colombel V, Braz VA, Org. Lett 2011, 13, 1852–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]; For a silver-mediated reaction, see: (e) Lockner JW, Dixon DD, Risgaard R, Baran PS, Org. Lett 2011, 13, 5628–5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].(a) Yasu Y, Koike T, Akita M, Adv. Synth. Catal 2012, 354, 3414–3420. [Google Scholar]; (b) Miyazawa K, Yasu Y, Koike T, Akita M, Chem. Commun 2013, 49, 7249–7251. [DOI] [PubMed] [Google Scholar]
- [81].Miyazawa K, Koike T, Akita M, Adv. Synth. Catal 2014, 356, 2749–2755. [Google Scholar]
- [82].(a) Previous applications of protected aminotrifluoroborates as electron donors under photoredox-mediated reductive quenching catalytic cycle: Yasu Y, Koike T, Akita M, Adv. Synth. Catal 2012, 354, 3414–3420. [Google Scholar]; (b) Miyazawa K, Yasu Y, Koike T, Akita M, Chem. Commun 2013, 49, 7249–7251. [DOI] [PubMed] [Google Scholar]; (c) Koike T, Akita M, Synlett 2013, 24, 2492–2505. [Google Scholar]; (d) Molander GA, Fleury-BrØgeot N, Hiebel M-A, Org. Lett 2011, 13, 1694–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Molander GA, Shin I, Org. Lett 2011, 13, 3956–3959. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Molander GA, Shin I, Org. Lett 2012, 14, 3138–3141. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Murai N, Miyano M, Yonaga M, Tanaka K, Org. Lett 2012, 14, 2818–2821. [DOI] [PubMed] [Google Scholar]; (g) Molander GA, Shin I, Org. Lett 2012, 14, 4458–4461. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Devulapally R, Fleury-Brøgeot N, Molander GA, Seapy DG, Tetrahedron Lett. 2012, 53, 1051–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Molander GA, Shin I, Org. Lett 2013, 15, 2534–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Chinzei T, Miyazawa K, Yasu Y, Kioke T, Akita M, RSC Adv. 2015, 5, 21297–21300 [Google Scholar]
- [84].(a) For a pioneering example, see: Sibi MP, Ji J, J. Org. Chem 1997, 62, 3800–3801. [Google Scholar]; For a review, see: Sibi MP, Manyem S, Zimmerman J, Chem. Rev 2003, 103, 3263–3296. [DOI] [PubMed] [Google Scholar]
- [85].(a) Huo H, Harms K, Meggers E, J. Am. Chem. Soc 2016, 138, 6936–6939. [DOI] [PubMed] [Google Scholar]; (b) Tutkowski B, Meggers E, Wiest O, J. Am. Chem. Soc 2017, 139, 8062–8065. [DOI] [PubMed] [Google Scholar]
- [86].(a) For pioneering examples of Giese reactions that use Ultraviolet irradiation to access nucleophilic α-aminyl radical intermediates from secondary, or secondary and primary amines, see: Das S, Dileep Kumar JS, Shivaramayya K, George MV, J. Chem. Soc. Perkins Trans. 1 1995, 1797–1799. [Google Scholar]; (b) Das S, Dileep Kumar JS, Shivaramayya K, George MV, J. Photochem. Photobiol. A 1996, 97, 139–150. [Google Scholar]
- [87].(a) For pioneering examples of Giese reactions that use Ultraviolet irradiation to access nucleophilic α-aminyl radical intermediates from tertiary amines, see: Bertrand S, Glapski C, Hoffmann N, Pete J-P, Tetrahedron Lett. 1999, 40, 3169–3172. [Google Scholar]; (b) Bertrand S, Hoffmann N, Pete J-P, Eur. J. Org. Chem 2000, 2227–2238. [DOI] [PubMed] [Google Scholar]
- [88].(a) For early examples in which secondary amines are converted to nucleophilic α-aminyl radical intermediates and used in C-arylation reactions that rely on visible light photoredox catalysts, see: McNally A, Prier CK and MacMillan DWC, Science, 2011, 334, 1114.22116882 [Google Scholar]; (b) For examples in which tertiary amines are converted to nucleophilic α-aminyl radical intermediates and used in C─C bond formation reactions that rely on visible light photoredox catalysts, see: Miyake Y Ashida Y, Nakajima K, Nishibayashi Y, Chem. Eur. J, 2014, 20, 6120–6125. [DOI] [PubMed] [Google Scholar]; (c) Miyake Y, Nakajima K, Nishibayashi Y, Chem. Eur. J, 2012, 18, 16473–16477. [DOI] [PubMed] [Google Scholar]; (d) Zhou H, Lu P, Gu X, Li P, Org. Lett, 2013, 15, 5646–5649 [DOI] [PubMed] [Google Scholar]
- [89].(a) For earlier examples in which tertiary amines are converted to nucleophilic α-aminyl radical intermediates and used in Giese reactions that rely on visible light photoredox catalysts, see: Kohls P, Jadhav D, Pandey G, Reiser O, Org. Lett, 2012, 14, 672–675. [DOI] [PubMed] [Google Scholar]; (b) Miyake Y, Ashida Y, Nakajima K, Nishibayashi Y, Chem. Commun, 2012, 48, 6966–6968. [DOI] [PubMed] [Google Scholar]; (c) Miyake Y, Nakajima K, Nishibayashi Y, J. Am. Chem. Soc, 2012, 134, 3338–3341. [DOI] [PubMed] [Google Scholar]; (d) Ju X, Li D, Li W, Yu W, Bian F, Adv. Synth. Catal, 2012, 354, 3561–3567. [Google Scholar]; (e) Espelt LR, Wiensch EM, Yoon TP, J. Org. Chem 2013, 78, 4107–4114. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Zhu S, Das A, Bui L, Zhou H, Curran DP, Rueping M, J. Am. Chem. Soc, 2013, 135, 1823–1829. [DOI] [PubMed] [Google Scholar]
- [90].(a) Yoshida J, Maekawa T, Murata T, Matsunaga S, Isoe S, J. Am. Chem. Soc 1990, 112, 1962–1970. [Google Scholar]; (b) Jouikov VV Russ. Chem. Rev 1997, 66, 509–540. [Google Scholar]
- [91].(a) Cooper BE, Owen WJ, J. Organomet. Chem, 1971, 29, 33–40. [Google Scholar]; (b) Cho DW, Yoon U-C, Mariano PS, Acc. Chem. Res 2011, 44, 204–215. [DOI] [PubMed] [Google Scholar]; (c) Yoon U-C, Mariano PS, Acc. Chem. Res 1992, 25, 233–240. [Google Scholar]; (d) Hasegawa E, Xu W, Mariano PS, Yoon U-C and Kim J-U, J. Am. Chem. Soc 1988, 110, 8099–8111. [Google Scholar]; (e) Yoon U-C, Kim J-U, Hasegawa E, Mariano PS, J. Am. Chem. Soc 1987, 109, 4421–4423. [Google Scholar]
- [92].Kakajima K, Kitagawa M, Ashida Y, Miyake Y, Nishibayashi Y, Chem. Commun 2014, 50, 8900–8903. [DOI] [PubMed] [Google Scholar]
- [93].Wu JH, Radinov R, Porter NA, J. Am. Chem. Soc 1995, 117, 11029–11030. [Google Scholar]
- [94].Bauer A, Westkamper F, Grimme S, Bach T, Nature 2005, 436, 1139–1140. [DOI] [PubMed] [Google Scholar]
- [95].Espelt LR, McPherson IS, Wiench EM, Yoon TP, J. Am. Chem. Soc 2015, 137, 2452–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].(a) For select examples of pyrazolidinone-based Michael acceptors used in chiral-relay reactions, see: Sibi MP, Stanley LM, Nie X, Venkatraman L, Liu M, Jasperse CP, J. Am. Chem. Soc 2007, 129, 395–405. [DOI] [PubMed] [Google Scholar]; (b) Sibi MK, Venkatraman L, Liu M, Jasperse CP, J. Am. Chem. Soc 2001, 123, 8444–8445. [DOI] [PubMed] [Google Scholar]
- [97].(a) For select examples of tin hydride-mediated stereoselective carbon-carbon bond formation resulting from intramolecular hydrogen-bonding, see: Waldner A, Mesmaeker AD, Hoffmann P, Mindt T, Winkler T, Synlett 1991, 101–104. [Google Scholar]; (b) Hanessian S, Yang H, Schaum R, J. Am. Chem. Soc 1996, 118, 2507–2508. [Google Scholar]; (c) Mase N, Wake S, Wantanabe Y, Toru T, Tetrahedron Lett. 1998, 39, 5553–5556. [Google Scholar]
- [98].Lenhart D, Bauer A, Pöthig A, Bach T, Chem. Eur. J 2016, 22, 6519–6523. [DOI] [PubMed] [Google Scholar]
- [99].Silvi M, Varrier C, Rey PY, Buzzetti L, Melchiorre P, Nat. Chem 2017, 9, 868–873. [DOI] [PubMed] [Google Scholar]
- [100].(a) Benzyl radical addition to electron-poor alkenes rely on SET reduction of the acceptor to generate alkene radical anions, see: Montanaro S, Ravelli D, Merli D, Fagnoni M, Albini A, Org. Lett 2012, 14, 4218–4221. [DOI] [PubMed] [Google Scholar]; (b) Borg RM, Heuckeroth RO, Lan AJY, Quillen SL, Mariano PS, J. Am. Chem. Soc 1987, 109, 2728–2737. [Google Scholar]; (c) Chen C, Chang V, Cai X, Duesler E, Mariano PS, J. Am. Chem. Soc 2001, 123, 6433–6434. [DOI] [PubMed] [Google Scholar]
- [101].Chow YL, Danen WC, Nelsen SF, Rosenblatt DH, Chemical Reviews, 1978, 78, 243–274. [Google Scholar]
- [102].(a) For select reviews demonstrating photoinduced access to amine radical cations, see: Yoon UC, Mariano PS, Acc. Chem. Res 1992, 25, 233–240. [Google Scholar]; (b) Pienta NJ, in Photoinduced Electron Transfer, Part C (Eds Fox MA, Chanon M) Elsevier: New York, 1988, p. 42. [Google Scholar]; (c) Hu J, Wang J, Nguyen TH, Zheng N, Beilstein J. Org. Chem 2013, 9, 1977–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].(a) Hofmann AW, Chem. Ber 1883, 16, 558–560. [Google Scholar]; (b) Löffler K, Freytag C, Chem. Ber 1909, 42, 3427–3431. [Google Scholar]
- [104].Neale RS, Synthesis, 1971, 1–15. [Google Scholar]
- [105].Minisci F, Synthesis, 1973, 1–24. [Google Scholar]
- [106].(a) For pioneering examples of photochemically-mediated carbon–carbon bond formation employing aminium cations, see: Lewis FD, Ho TI, J. Am. Chem. Soc 1977, 99, 7991–7996. [Google Scholar]; (b) Cookson RC, Hudec J, Mirza NA, J. Chem. Soc., Chem. Commun 1968, 180. [Google Scholar]; (c) Pienta NJ, McKimmey JE, J. Am. Chem. Soc 1982, 104, 5501–5502. [Google Scholar]
- [107].Aycock RA, Pratt CJ, Jui NT, ACS Catal. 2018, 8, 9115–9119. [Google Scholar]
- [108].Kessler H, Wittmann V, Köck M, Kottenhahn M, Angew. Chem., Int. Ed. Engl 1992, 31, 902–904 [Google Scholar]; Angew. Chem 1992, 104, 874–877. [Google Scholar]
- [109].(a) For a pioneering example of peptide synthesis via the Giese reaction, see: Crich D, Davies JW, Negron G, Quintero L, J. Chem. Res., Synop 1988, 140–141. [Google Scholar]; (b) For applications to di- and tripeptide syntheses, see: Crich D, Davies JW, Tetrahedron 1989, 45, 5641–5654. [Google Scholar]
- [110].McManus JB, Onuska NPR, Nicewicz DA, J. Am. Chem. Soc 2018, 140, 9056–9060. [DOI] [PubMed] [Google Scholar]
- [111].Fürst A, Plattner PA, Helv. Chim. Acta 1949, 32, 275–278. [DOI] [PubMed] [Google Scholar]
- [112].Monomorine I had been prepared by an alternative two-step route: Le TQ, Oliver RM, Arcari JT Mitton-Fry MJ Total synthesis of (±)-monomorine. Tetrahedron Lett. 2012, 53, 5660–5662. [Google Scholar]
- [113].Ashley MA, Yamauchi C, Chu JCK, Otsuka S, Yorimitsu H, Rovis T, Angew. Chem. Int. Ed 2019, 58, 4002–4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].For the pioneering development of N-(acyloxy)phthalimides for forming carbon radicals, see: Okada K, Okamoto K, Morita N, Okubo K, Oda M, J. Am. Chem. Soc 1991, 113, 9401–9402. [Google Scholar]
- [115].(a) Barton DHR, Crich D, Tetrahedron Lett. 1985, 26, 757–760. [Google Scholar]; (b) Oxalates as radical precursors are inspired by: Barton DHR, Crich D, J. Chem. Soc., Perkin Trans. 1 1986, 1603–1611. [Google Scholar]
- [116].For a pioneering example using carboxylic acids as carbon-radical precursors for conjugate addition, see: Barton DHR, Crich D, Kretzschmar G, Tetrahedron Lett. 1984, 25, 1055–1058. [Google Scholar]
- [117].(a) For the use of N-(acyloxy)phthalimides in a complex natural product synthesis, see: Schnermann MJ, Overman LE, Angew. Chem., Int. Ed 2012, 51, 9576–9580. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Müller DS, Untiedt NL, Dieskau AP, Lackner GL, Overman LE, J. Am. Chem. Soc 2015, 137, 660–663. [DOI] [PubMed] [Google Scholar]
- [118].Miyake Y, Nakajima K, Nishibayashi Y, Chem. Commun 2013, 49, 7854–7856. [DOI] [PubMed] [Google Scholar]
- [119].(a) Yoshimi Y, Masuda M, Mizunashi T, Nishikawa K, Maeda K, Koshida N, Itou T, Morita T, Hatanaka M, Org. Lett 2009, 11, 4652–4655. [DOI] [PubMed] [Google Scholar]; (b) Chen L, Chao CS, Pan Y, Dong S, Teo YC, Wang J, Tan C-H, Org. Biomol. Chem 2013, 11, 5922–5925. [DOI] [PubMed] [Google Scholar]
- [120].Chu L, Ohta C, Zuo Z, MacMillan DWC, J. Am. Chem. Soc 2014, 136, 10886–10889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Lackner GL, Quasdorf KW, Overman LE, J. Am. Chem. Soc 2013, 135, 15342–15345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Schwarz J, König B, Green Chem. 2016, 18, 4743–4749. [Google Scholar]
- [123].Ma J, Lin J, Zhao L, Harms K, Marsch M, Xie X, Meggers E, Angew. Chem. Int. Ed 2018, 57, 11193–11197 [DOI] [PubMed] [Google Scholar]; Angew. Chem 2018, 130, 11363–11367. [Google Scholar]
- [124].Yin H, Zheng M, Chen H, Wang S, Zhou Q, Zhang Q, Wang P, J. Am. Chem. Soc 2020, 142, 14201–14209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Prior to the development of this technology, a decarboxylatve arylation of α-amino acids was disclosed: Zuo Z, MacMillan DWC, J. Am. Chem. Soc 2014, 136, 5257–5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].(a) Nishida A, Nishida M, Yonemitsu O, Tetrahedron Lett. 1990, 31, 7035–7048. [Google Scholar]; (b) Curran DP, Shen W, J. Am. Chem. Soc 1993, 115, 6051–6059. [Google Scholar]; (c) Roberts BP, Chem. Soc. Rev 1999, 28, 25–35. [Google Scholar]; (d) Dang H-S, Roberts BP, Tetrahedron Lett. 1999, 40, 8929–8933. [Google Scholar]
- [127].Zhang S, Tan Z, Zhang H, Liu J, Xu W, Xu K, Chem. Commun 2017, 53, 11642–11645. [DOI] [PubMed] [Google Scholar]
- [128].Huang H, Li X, Yu C, Zhang Y, Mariano PS, Wang W, Angew Chem. Int. Ed, 2017, 56, 1500–1505 [DOI] [PubMed] [Google Scholar]; Angew. Chem 2017, 129, 1522–1527. [Google Scholar]
- [129].Ramirez NP, Gonzalez-Gomez JC, Eur. J. Org 2017, 2154–2163. [Google Scholar]
- [130].Guo T, Zhang L, Fang Y, Jin X, Li Y, Li R, Li X, Cen W, Liu X, Tian Z, Adv. Synth. Catal 2018, 360, 1352–1357. [Google Scholar]
- [131].Depew KM, Marsden SP, Zatorska D, Zatorski A, Bornmann WG, Danishefsky SJ, J. Am. Chem. Soc 1999, 121, 11953–11963. [Google Scholar]
- [132].Maki Y, Oyabu I, Ohara S, Sako M, Kitade Y, Hirota K. Chem. Pharm. Bull 1989, 37, 3229–3242. [Google Scholar]
- [133].El-Hage F, Schöll C, Pospech J, J. Org. Chem 2020, 85, 13853–13867. [DOI] [PubMed] [Google Scholar]
- [134].Capaldo L, Buzzetti L, Merli D, Fagnoni M, Ravelli D. J. Org. Chem 2016, 81, 7102–7109. [DOI] [PubMed] [Google Scholar]
- [135].Nawrat CC, Jamison CR, Slutskyy Y, MacMillan DWC, J. Am. Chem. Soc 2015, 137, 11270–11273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Slutskyy Y, Jamison CR, Lackner GL, Müller DS, Dieskau AP, Untiedt NL, Overman LE, J. Org. Chem 2016, 81, 7029–2035. [DOI] [PubMed] [Google Scholar]
- [137].Abbas SY, Zhao P, Overman LE, Org. Lett 2018, 20, 868–871. [DOI] [PubMed] [Google Scholar]
- [138].(a) Ohno M, Ishizaki K, Eguchi SJ, Org. Chem 1988, 53, 1285–1288. [Google Scholar]; (b) Mao S, Fang X, Ba L, Wu F, J. Fluorine Chem 2007, 128, 5–11. [Google Scholar]; (c) Gong H, Andrews RS, Zuccarello JL, Lee SJ, Gagne MR,´ Org. Lett 2009, 11, 879–882. [DOI] [PubMed] [Google Scholar]; (c) Liu X-K, Zheng X, Ruan Y-P, Ma J, Huang P-Q, Org. Biomol. Chem 2012, 10, 1275–1284. [DOI] [PubMed] [Google Scholar]
- [139].(a) Gui J, Zhou Q, Pan C-M, Yabe Y, Burns AC, Collins MR, Ornelast MA, Hishihara Y and Baran PS, J. Am. Chem. Soc 2014, 136, 4853–4856. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Minisci F, Fontana F, Vismara EJ, Heterocycl. Chem 1990, 27, 79–96. [Google Scholar]; (c) Fujiwara Y, Dixon JA, O’Hara F, Funder ED, Dixon DD, Rodriguez RA, Baxter RD, Herle B, Sach N, Collins MR, Ishihara Y, Baran PS, Nature 2012, 492, 95–99. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Ni C, Hu M, Hu J, Chem. Rev 2015, 115, 765–825. [DOI] [PubMed] [Google Scholar]; (e) Meesin J, Katrun P, Pareseecharoen C, Pohmakotr M, Reutrakul V, Soorukram D, Kuhakarn C, J. Org. Chem 2016, 81, 2744–2752. [DOI] [PubMed] [Google Scholar]; (f) Li Z, Cui Z, Liu Z-Q, Org. Lett 2013, 15, 406–409. [DOI] [PubMed] [Google Scholar]
- [140].(a) Meyer AU, Jager S, Hari DP, König B, Adv. Synth. Catal 2015, 357, 2050–2054. [Google Scholar]; (b) Meyer AU, Lau VWH, König B, Lotsch BV, Eur. J. Org. Chem. 2017, 2179–2185. [Google Scholar]; (c) Hering T, Meyer AU, König B, J. Org. Chem 2016, 81, 6927–6936. [DOI] [PubMed] [Google Scholar]
- [141].Gualandi A, Mazzarella D, Ortega-Martinez A, Mengozzi L, Calcinelli F, Matteucci E, Monti F, Armaroli N, Sambri L, Cozzi PG, ACS Catal. 2017, 7, 5357–5362. [Google Scholar]
- [142].(a) For reviews on iminyl radicals, see: Zhu X, Chiba S, Chem. Soc. Rev 2016, 45, 4504–4523. [DOI] [PubMed] [Google Scholar]; (b) Walton JC, Acc. Chem. Res 2014, 47, 1406–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Zard SZ, Chem. Soc. Rev 2008, 37, 1603–1618. [DOI] [PubMed] [Google Scholar]; (d) Kitamura M, Narasaka K, Bull. Chem. Soc. Jpn 2008, 81, 539–547. [Google Scholar]; (e) Narasaka K, Kitamura M, Eur. J. Org. Chem 2005, 4505–4519. [Google Scholar]; (f) Fallis AG, Brinza IM, Tetrahedron, 1997, 53, 17543–17954. [Google Scholar]; (g) Zard SZ, Synlett, 1996, 1148–1154. [Google Scholar]
- [143].Jiang H, Studer A, Angew. Chem. Int. Ed 2017, 56, 12273–12276 [DOI] [PubMed] [Google Scholar]; Angew. Chem 2017, 129, 12441–12444. [Google Scholar]
- [144].Davies J, Sheikh NS, Leonori D, Angew. Chem. Int. Ed 2017, 56, 13361–13365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Forrester AR, Gill M, Sadd JS, Thomson RH, J Chem. Soc. Chem. Comm 1975, 291–291. [Google Scholar]
- [146].Forrester AR, Gill M, Thomson RH, J. Chem. Soc. Chem. Commun 1976, 677–678. [Google Scholar]
- [147].Forrester AR, Gill M, Napier RJ, Thomson RH, J. Chem. Soc. Perkin 1 1978, 632–636. [Google Scholar]
- [148].For pioneering research that employs α,α-dimethylated carboxylate as iminyl radical precursor, see: Forrester AR, Gill M, Meyer CJ, J.S., Thomson RH, J. Chem. Soc. Perkin 1 1979, 606–611. [Google Scholar]
- [149].(a) Boivin J, Fouquet E, Zard SZ, Tetrahedron Lett. 1991, 32, 4299–4302. [Google Scholar]; (b) Boivin J, Fouquet E, Zard SZ, Tetrahedron Lett. 1990, 31, 3545–3548. [Google Scholar]
- [150].For strong precedent involving theovinine-derivative hydroimination, see: Boivin J, Callier-Dublanchet A-C, Quiclet-Sire B, Schiano A-M, Zard SZ, Tetrahedron 1995, 51, 6517–6528. [Google Scholar]
- [151].[Mes-Acr]ClO4 excited redox potential calculated from the Rehms-Weller equation, where E*red = E0-0 - Ered using the E0-0 and Ered values from: Benniston AC, Harriman A, Li P, Rostron JP, van Ramesdonk HJ, Groeneveld MM, Zhang H, Verhoeven JW, J. Am. Chem. Soc 2005, 127, 16054–16064. [DOI] [PubMed] [Google Scholar]
- [152].Boivin J, Fouquet E, Zard SZ, J. Am. Chem. Soc 1991, 113, 1055–1057. [Google Scholar]
- [153].Barton DHR, Zard SZ, Janssen Chim. Acta 1986, 4, 3–9. [Google Scholar]
- [154].Li L, Chen H, Mei M, Zhou L, Chem. Commun 2017, 53, 11544–11547. [DOI] [PubMed] [Google Scholar]
- [155].Wang P-Z, Yu X-Y, Li C-Y, He B-Q, Chen J-R, Xiao W-J, Chem. Commun 2018, 54, 9925–9928. [DOI] [PubMed] [Google Scholar]
- [156].Wang P-Z, He B-Q, Cheng Y, Chen J-R, Xiao W-J, Org. Lett 2019, 21, 6924–6929. [DOI] [PubMed] [Google Scholar]
- [157].(a) Oxo nitriles are important building block for the synthesis of isoquinoline derivatives: Zdrojewski T, Jonćzyk A, Tetrahedron 1995, 51, 12439–12444. [Google Scholar]; (b) Grunewald GL, Paradkar VM, Bioorg. Med. Chem. Lett 1991, 1, 59–60. [Google Scholar]; Benzonitrile derivatives (c) Bradsher CK, Wallis TG, J. Org. Chem 1978, 43, 3817–3820. [Google Scholar]; As key intermediates for the synthesis of alkeneitriles: (d) Fleming FF, Funk LA, J. Org. Chem 2002, 67, 9414–9416. [DOI] [PubMed] [Google Scholar]
- [158].(a) For reviews on N-centerd radials, see: Zard SZ, Chem. Soc. Rev 2008, 37, 1603–1618. [DOI] [PubMed] [Google Scholar]; (b) Neale RS, Synthesis, 1971, 1–15. [Google Scholar]; (c) Mackiewicz P, Furstoss R, Tetrahedron, 1978, 34, 3241–3260. [Google Scholar]; For a review on 1,5-HAT, see: (d) Cekovic Z, J. Serb. Chem. Soc 2005, 70, 287–318. [Google Scholar]; (e) Capaldo L, Ravelli D, Eur. J. Org. Chem 2017, 2056–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].(a) For a recent review on photocatalyzed access to N-centered radicals, see: Jiang H, Studer A, CCS Chem. 2019, 1, 38–49. [Google Scholar]; (b) Davies J, Morcillo SP, Douglas JJ, Leonori D, Chem. Eur. J 2018, 24, 12154–12163. [DOI] [PubMed] [Google Scholar]; (c) Kärkäs MD, ACS Catal. 2017, 7, 4999–5022. [Google Scholar]
- [160].Choi GJ, Knowles RR. J. Am. Chem. Soc 2015, 137, 9226–9229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Bordwell FG, Zhang S, Zhang X-M, Liu W-Z, J. Am. Chem. Soc 1995, 117, 7092–7096. [Google Scholar]
- [162].Bordwell FG, Ji G-Z, J. Am. Chem. Soc 1991, 113, 8398–8401. [Google Scholar]
- [163].Chiong HA “Potassium Phosphate” can be found under 10.1002/047084289X.rn01172, 2010. [DOI]
- [164].Chu JCK, Rovis T, Nature 2016, 539, 272–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].(a) For the recent reviews highlighting subsequent advances in HLF-inspired chemistry, see: Chen H, Yu S, Org. Biomol. Chem 2020, 18, 4519–4532. [DOI] [PubMed] [Google Scholar]; (b) Wu W, Chen Z, CCS Chem. 2020, 2, 813–828. [Google Scholar]; (c) Kumar G, Pradhan S, Chatterjee I, Chem. Asian J 2020, 15, 651–672. [DOI] [PubMed] [Google Scholar]
- [166].Chen D, Chu JCK, Rovis T, J. Am. Chem. Soc 2017, 139, 14897–14900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Wu K, Eang L, Colón-Rodríguez S, Flechsig G-U, Wang T, Angew. Chem. Int. Ed 2019, 58, 1774–1778 [DOI] [PubMed] [Google Scholar]; Angew. Chem 2019, 131, 1788–1792. [Google Scholar]
- [168].Yuan W, Zhou Z, Gong L, Meggers E, E. Chem Commun 2017, 53, 8964–8967. [DOI] [PubMed] [Google Scholar]
- [169].Jiang H, Studer A, Angew. Chem. Int. Ed 2018, 57, 1692–1696 [DOI] [PubMed] [Google Scholar]; Angew. Chem 2018, 130, 1708–1712. [Google Scholar]
- [170].Short MA, Blackburn JM, Roizen JL, Angew. Chem., Int. Ed 2018, 57, 296–299 [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem 2018, 130, 302–305. [Google Scholar]
- [171].Sathyamoorthi S, Banerjee S, Du Bois J, Burns N, Zare RN, Chem. Sci 2018, 9, 100–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Ayer SK, Roizen JL, J. Org. Chem, 2019, 84, 3508–3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].These investigations have been reviewed: Short MA, Blackburn JM, Roizen JL, Synlett 2020, 31, 102–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].For related studies of sulfamides as directing groups for exogenous atom-transfer, see: Short MA, Shehata MF, Sanders MA, Roizen JL, Chem. Sci 2020, 11, 217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].(a) Kanegusuku ALG, Castanheiro T, Ayer SK, Roizen JL, Org. Lett 2019, 21, 6089–6095. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) A preprint of this manuscript was published on May 31, 2019: Kanegusuku ALG, Castanheiro T, Ayer SK, Roizen JL ChemRxiv 2019, Preprint. [Google Scholar]; The bulk of this research was initially disclosed in August 19, 2018: (c) Kanegusuku ALG, Castanheiro T, Roizen JL Sulfamate esters guide selective alkylation at traditionally non-reactive gamma-C(sp3)─H bonds. ABSTRACTS OF PAPERS OF THE AMERICAN CHEMICAL SOCIETY 256. [Google Scholar]
- [176].Ma Z-Y, Guo L-N, You Y, Yang F, Hu M, Duan X-H, Org. Lett 2019, 21, 5500–5504. [DOI] [PubMed] [Google Scholar]
- [177].Shu W, Zhang H, Huang Y, Org. Lett 2019, 21, 6107–6111. [DOI] [PubMed] [Google Scholar]
- [178].Publications describing these Giese reactions rely at least in part on sulfamate ester preparation methods disclosed in Blackburn JM, Short MA, Castanheiro T, Ayer SK, Muellers TD, Roizen JL, Org. Lett 2017, 19, 6012–6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].(a) For additional reports of alkoxy radical precursors, see: Barton DHR, Beaton JM, Geller LE, Pechet MM, J. Am. Chem. Soc 1960, 82, 2640–2641. [Google Scholar]; (b) Vite GD, Fraser-Reid B, Synth. Commun 1988, 18, 1339–1342. [Google Scholar]; (c) Walling C, Clark RT, J. Am. Chem. Soc 1974, 96, 4530–4534. [Google Scholar]; (d) Beckwith ALJ, Hay BP, Williams GM, J. Chem. Soc. Chem. Commun 1989, 1202–1203. [Google Scholar]
- [180].Cekovic Z, Tetrahedron, 2003, 59, 8073–8090. [Google Scholar]
- [181].Petrovic G, Cekovic Z. Tetrahedron Lett. 1997, 38, 627–630. [Google Scholar]; (a) For additional applications, see: Petrovic G, Cekovic Z. Tetrahedron, 1999, 55, 1377–1390. [Google Scholar]; (b) Petrovic G, Cekovic Z. Org. Lett 2000, 2, 3769–3772. [DOI] [PubMed] [Google Scholar]; (c) Petrovic G, Cekovic Z. Synthesis, 2004, 1671–1679. [Google Scholar]
- [182].Zhang J, Li Y, Zhang F, Hu C, Chen Y, Angew. Chem. Int. Ed 2016, 55, 1872–1875 [DOI] [PubMed] [Google Scholar]; Angew. Chem 2016, 128, 1904–1907. [Google Scholar]
- [183].Wang C, Harms K, Meggers E, Angew. Chem. Int. Ed 2016, 55, 13495–13498 [DOI] [PubMed] [Google Scholar]; Angew. Chem 2016, 128, 13693–13696. [Google Scholar]
- [184].Yayla HG, Wang HJ, Tarantino KT, Orbe HS, Knowles RR, J. Am. Chem. Soc 2016, 138, 10794–10797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].(a) Wu X, Wang M, Huan L, Wang D, Wang J, Zhu C, Angew. Chem., Int. Ed 2018, 57, 1640–1644 [DOI] [PubMed] [Google Scholar]; Angew. Chem 2018, 130, 1656–1660. [Google Scholar]; (b) Wang D, Mao J, Zhu C, Chem. Sci 2018, 9, 5805–5809. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Ota E, Wang H, Frye NL, Knowles RR, J. Am. Chem. Soc 2019, 141, 1457–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Zhao K, Yamashita K, Carpenter JE, Sherwood TC, Ewing WR, Cheng PTW, Knowles RR, J. Am. Chem. Soc 2019, 141, 8752–8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].(a) Yin H, Carrol PJ, Anna JM, Schelter EJ, J. Am. Chem. Soc 2015, 137, 9234–9237. [DOI] [PubMed] [Google Scholar]; (b) Yin H, Carrol PJ, Manor BC, Anna JM, Schelter EJ, J. Am. Chem. Soc 2016, 138, 5984–5993. [DOI] [PubMed] [Google Scholar]
- [187].Guo J-J, Hu A, Chen Y, Sun J, Tang H, Zuo Z, Angew. Chem., Int. Ed 2016, 55, 15319–15322 [DOI] [PubMed] [Google Scholar]; Angew. Chem 2016, 128, 15545–15548. [Google Scholar]
- [188].An Q, Wang Z, Chen Y, Wang X, Zhang K, Pan H, Liu W, Zuo Z, J. Am. Chem. Soc 2020, 142, 6216–6226. [DOI] [PubMed] [Google Scholar]
- [189].Bergman RG, Nature, 2007, 446, 391–393. [DOI] [PubMed] [Google Scholar]; (b) Chen MS, White MCA, Science, 2007, 318, 783–787. [DOI] [PubMed] [Google Scholar]
- [190].Morton CM, Zhu Q, Ripberger H, Troian-Gautier L, D Toa ZS, Knowles RR, Alexanian EJ. J. Am. Chem. Soc 2019, 141, 13253–13260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Luadadio G, Deng Y, van der Wal K, Ravelli D, Nuño M, Fagnoni M, Guthrie D, Sun Y, Noël T, Science 2020, 369, 92–96. [DOI] [PubMed] [Google Scholar]
- [192].Tanielian C, Coord. Chem. Rev 1998, 178–180, 1165–1181. [Google Scholar]